Insight into the Structure–Activity Relationship of Delafossite Catalysts for Enhanced Peroxymonosulfate Activation and Pollutant Degradation
Abstract
1. Introduction
2. Results and Discussion
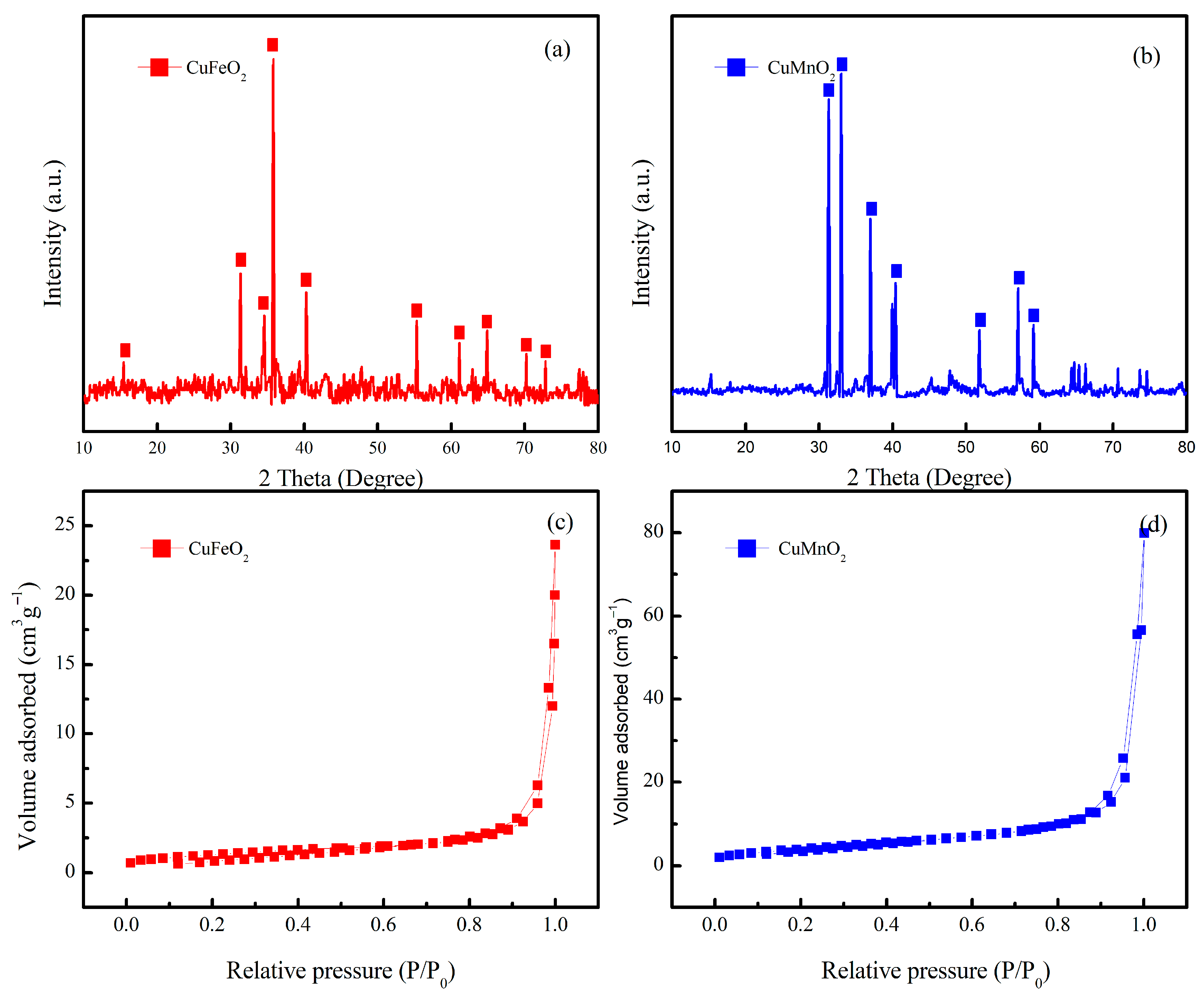
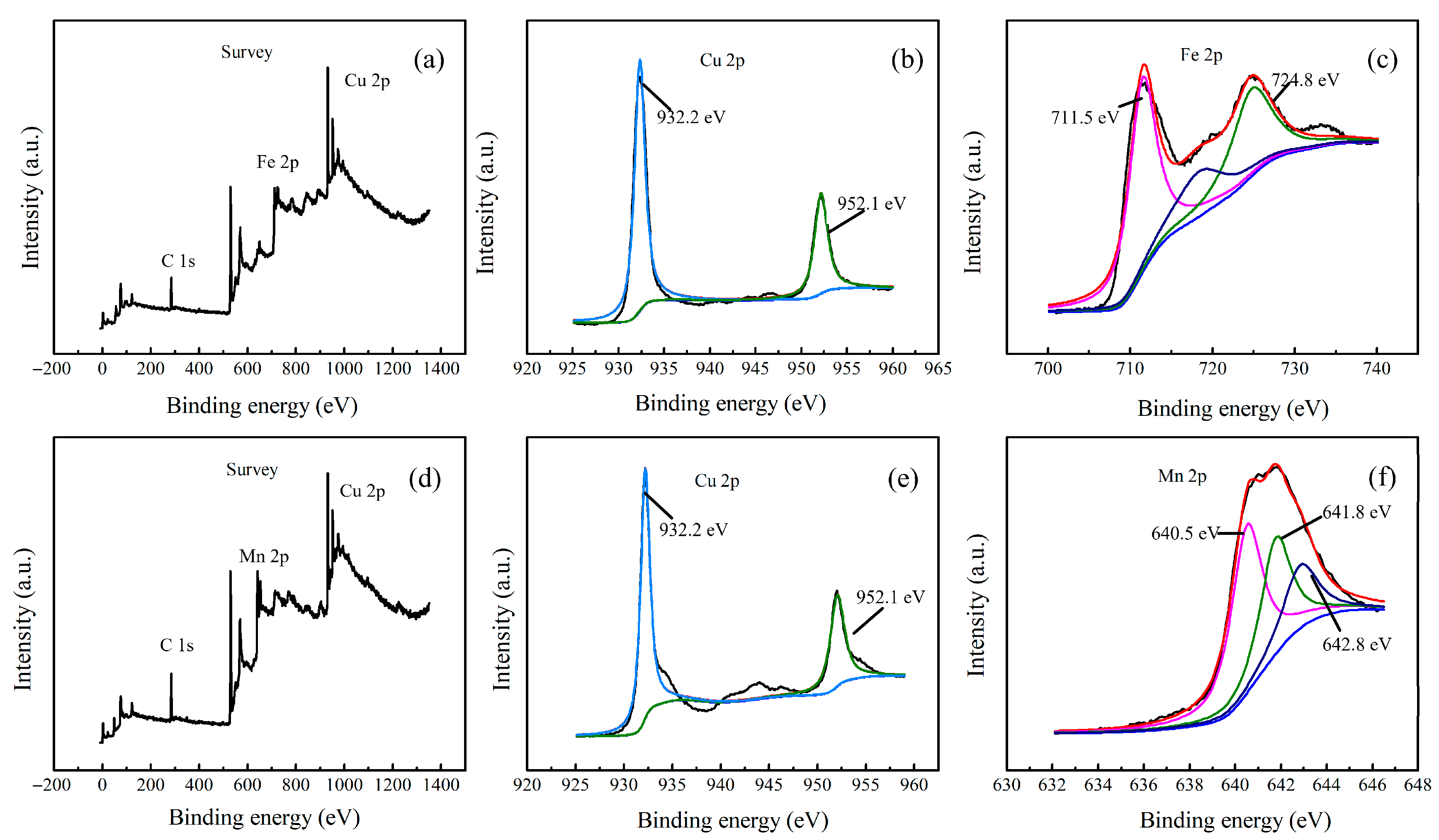
2.1. Structural Analysis of CuMnO2 and CuFeO2 Catalysts
2.2. Catalytic Performance Analysis
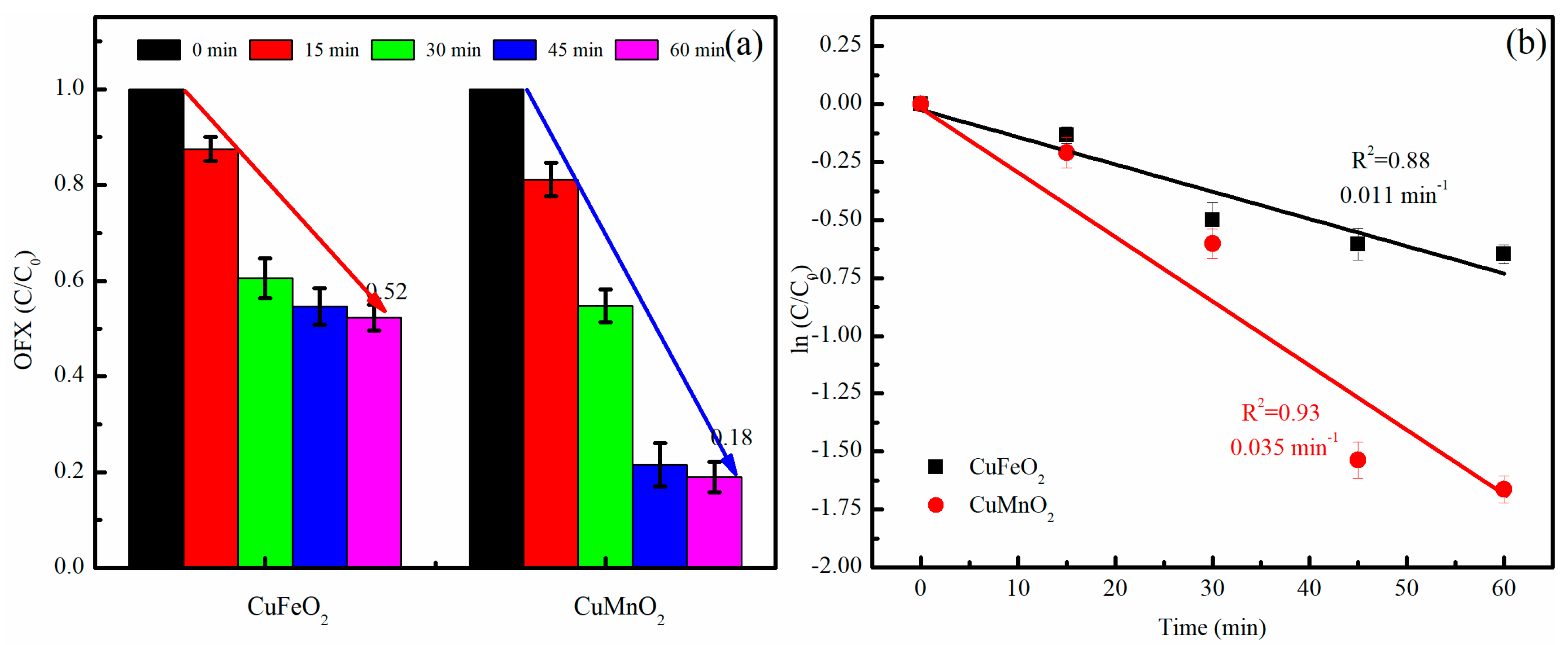
2.2.1. Performance Tests of CuMnO2 and CuFeO2 Catalysts
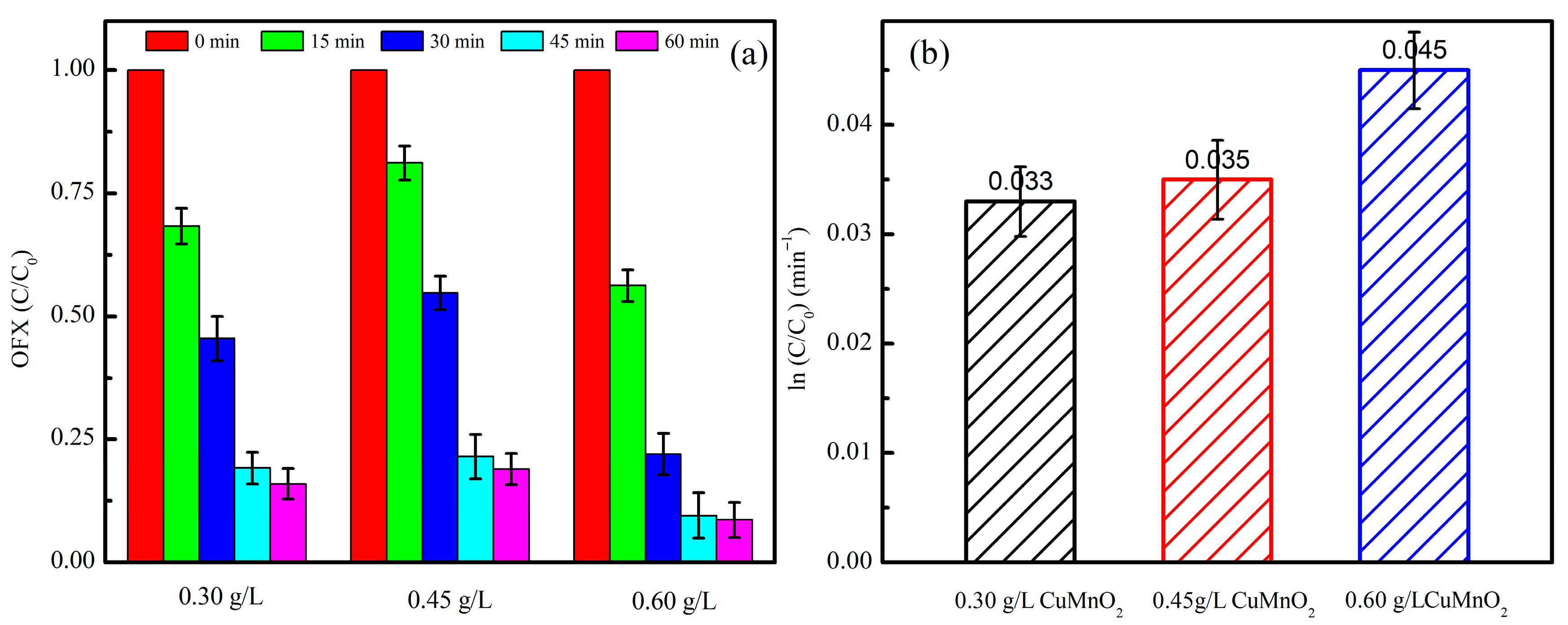
2.2.2. Effect of Catalyst Amount
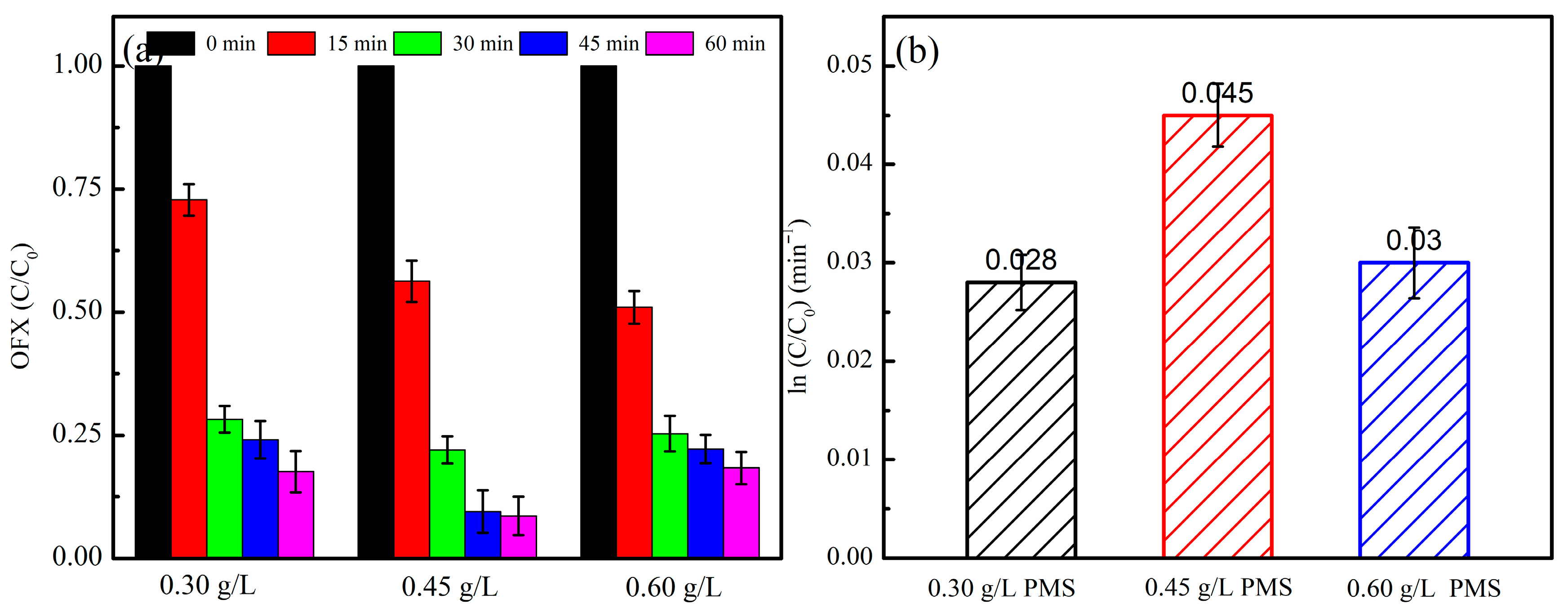
2.2.3. Effect of PMS Dosage
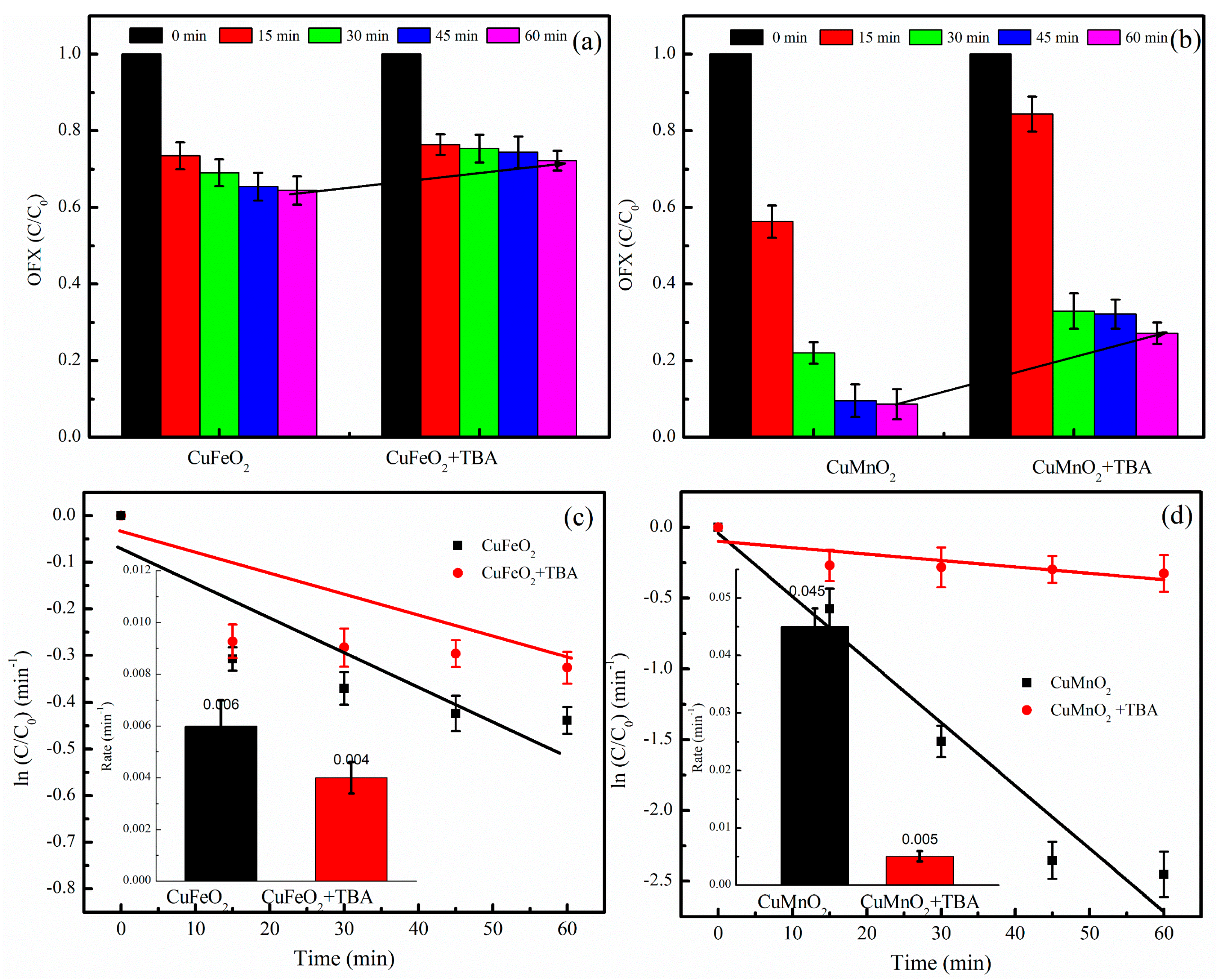
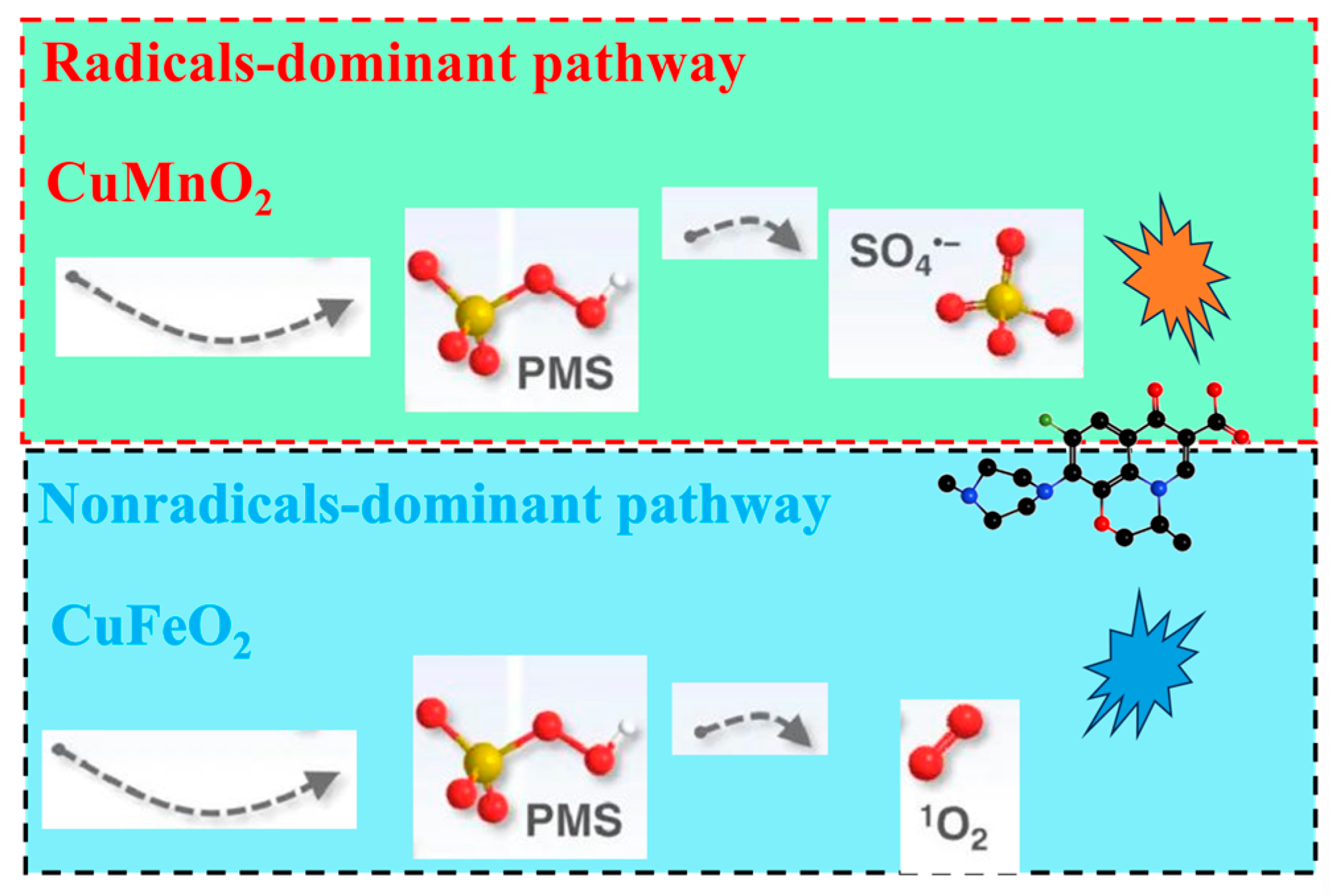
2.3. Catalytic Mechanism Analysis
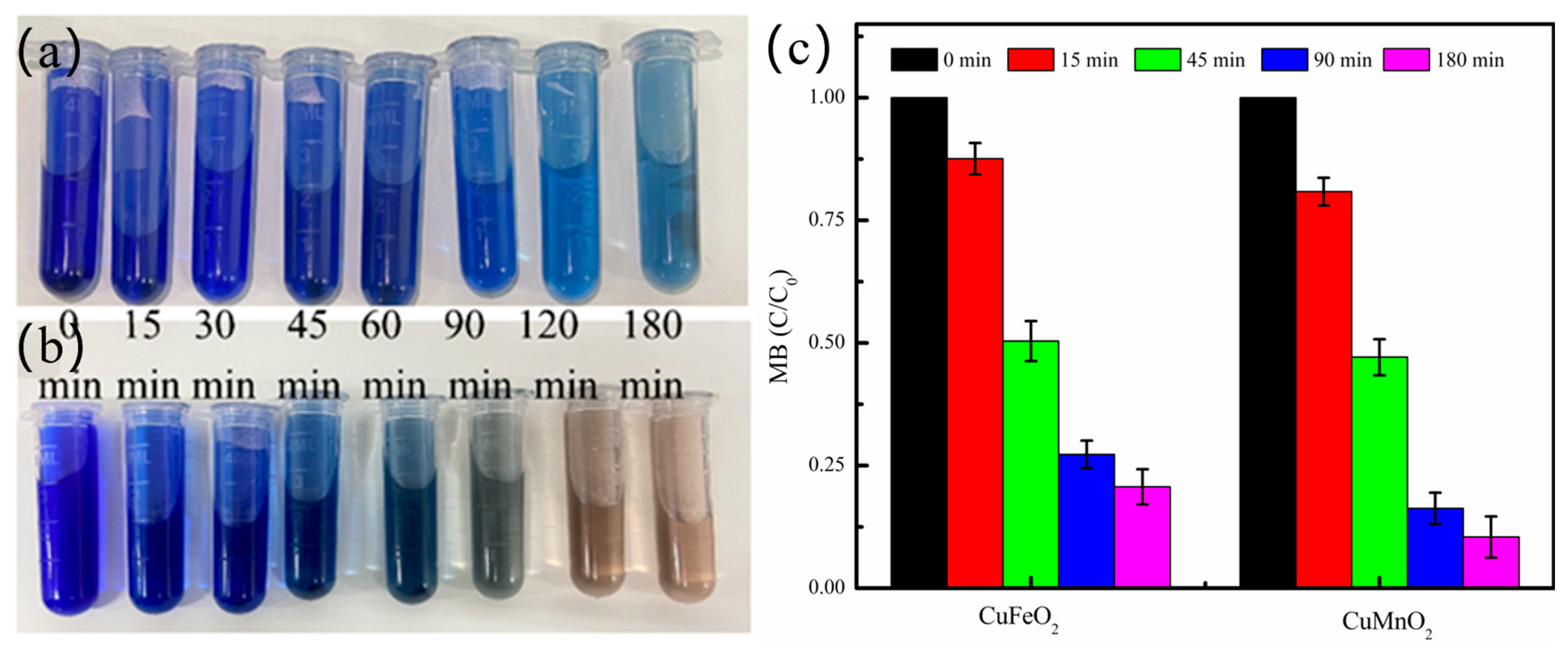
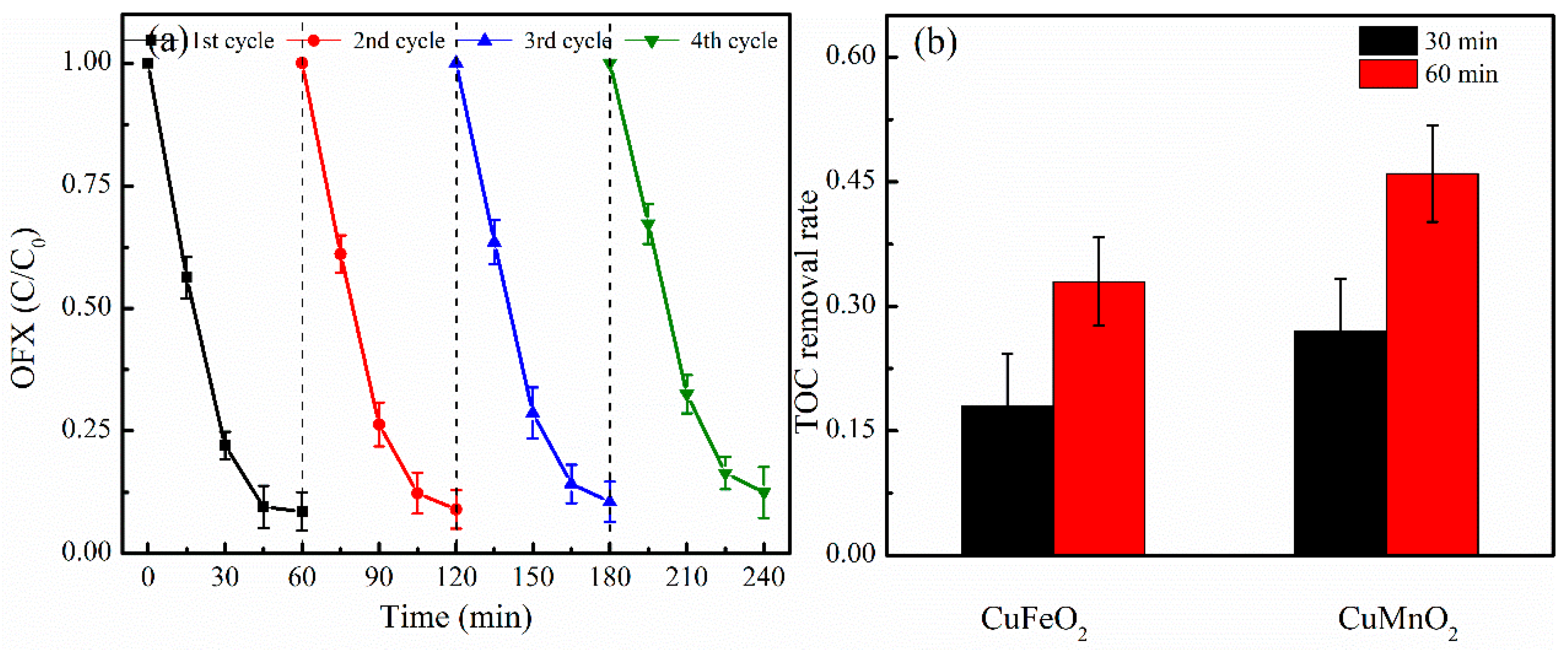
2.4. Potential Application of CuMnO2 Catalyst
3. Materials and Methods
3.1. Materials
3.2. Preparation of CuMnO2 and CuFeO2
3.3. Characterization of the Prepared Catalysts
3.4. Catalytic Experiments and Analysis Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, S.; Yu, D.; Zhou, S.; Zhang, C.; Wang, L.; Fan, X.; Yu, X.; Zhao, Z. Construction of cerium-based oxide catalysts with abundant defects/vacancies and their application to catalytic elimination of air pollutants. J. Mater. Chem. A Mater. Energy Sustain. 2023, 11, 34. [Google Scholar] [CrossRef]
- Wang, W.; Mo, T.; Wang, Y. Better self and better us: Exploring the individual and collective motivations for China’s Generation Z consumers to reduce plastic pollution. Resour. Conserv. Recycl. 2022, 179, 106111. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, Y.; Sui, C.; Ding, J.; Wang, J. Recent advances on photocatalytic degradation of phthalate ester plasticizers using nanomaterial photocatalysts. Environ. Res. 2025, 276, 121497. [Google Scholar] [CrossRef]
- Islam, N.; Dihingia, A.; Khare, P.; Saikia, B.K. Atmospheric particulate matters in an Indian urban area: Health implications from potentially hazardous elements, cytotoxicity, and genotoxicity studies. J. Hazard. Mater. 2020, 384, 121472. [Google Scholar] [CrossRef]
- Shen, Q.C.; Song, X.S.; Fan, J.S.; Chen, C.; Guo, Z.L. Degradation of humic acid by UV/PMS: Process comparison, influencing factors, and degradation mechanism. RSC Adv. 2024, 14, 22988. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Cao, J.Y.; Lu, Y.; Zhang, H.; Zhang, J.; Shi, Y.; Lai, B. Cytotoxicity and Genotoxicity toward Mammalian Cells Induced by Organic Iodine in Peroxymonosulfate (PMS) Processes: Activated PMS Is Better than Nonactivated PMS in Mitigating Toxicity. Environ. Sci. Technol. 2025, 59, 5925–5935. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Liu, Y.; Wang, Y.; Wang, H.; Yin, W.; Xu, G. Recyclable Fe/S co-doped nanocarbon derived from metal–organic framework as a peroxymonosulfate activator for efficient removal of 2,4-dichlorophenol. Environ. Sci. Pollut. Res. 2023, 30, 6906–6918. [Google Scholar] [CrossRef]
- Du, Y.; Wang, W.L.; Wang, Z.W.; Yuan, C.J.; Ye, M.Q.; Wu, Q.Y. Overlooked Cytotoxicity and Genotoxicity to Mammalian Cells Caused by the Oxidant Peroxymonosulfate during Wastewater Treatment Compared with the Sulfate Radical-Based Ultraviolet/Peroxymonosulfate Process. Environ. Sci. Technol. 2023, 57, 3311–3322. [Google Scholar] [CrossRef]
- Xiao, R.; Luo, Z.; Wei, Z.; Luo, S.; Spinney, R.; Yang, W.; Dionysiou, D.D. Activation of peroxymonosulfate/persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies. Curr. Opin. Chem. Eng. 2018, 19, 51–58. [Google Scholar] [CrossRef]
- Qi, X.; Xu, S.; Zhang, L.; Cao, Q.; Zhang, L.; Shi, X.; Gu, Y.; Wang, C. NiFe2O4@MoS2 heterojunction induces the changes of PMS activation mode in PMS/Vis system for the directed generation of 1O2. Sep. Purif. Technol. 2025, 363, 132175. [Google Scholar] [CrossRef]
- Khamis, A.; Mahmoud, A.S.; Naga, A.O.A.E.; Shaban, S.A.; Youssef, N.A. Activation of peroxymonosulfate with ZIF-67-derived Co/N-doped porous carbon nanocubes for the degradation of Congo red dye. Sci. Rep. 2024, 14, 12313. [Google Scholar] [CrossRef]
- Li, X.; Liu, K.; Ren, Z.; Du, Z.; Xiao, R.; Jiang, R.; Li, X.; Chen, T. Effect of peroxymonosulfate pre-oxidation coupled with subsequent Fe-based coagulation on the mitigation of organic matter and the formation of disinfection by-products. Environ. Sci. Water Res. Technol. 2025, 11, 972–981. [Google Scholar] [CrossRef]
- Xiao, K.; Liang, F.; Liang, J. Magnetic bimetallic Fe, Ce-embedded N-enriched porous biochar for peroxymonosulfate activation in metronidazole degradation: Applications, mechanism insight and toxicity evaluation. Chem. Eng. J. 2022, 433, 134387. [Google Scholar] [CrossRef]
- Sun, C.; Li, M.; Wang, Q.; Liu, R.; Li, M.; Chen, F.; Xu, S.; Wang, G. Enhanced degradation of rhodamine B by UV/Co3O4@BC/PMS system: Performance and mechanism. J. Water Process Eng. 2025, 73, 107719. [Google Scholar] [CrossRef]
- Fu, W.; Huo, S.; Zhang, M.; Song, L.; Zhao, Q.; Wu, X.; Gao, M. Efficient degradation of oxytetracycline by glucose modified CuFeO2 in visible-light-assisted heterogeneous activation of peroxymonosulfate system:Performance, mechanism and DFT calculation. J. Environ. Chem. Eng. 2023, 11, 111225. [Google Scholar] [CrossRef]
- Fu, S.W.; Yang, J.; Zhao, Z.Y.; Shan, B.F.; Zhang, J.X.; Zhang, J.; Liu, Q.; Feng, J.; Li, Z.; Zou, Z. Band-Edge Electronic Structure on Photo(electro)catalytic Performance of ABO2 (A = Cu, Ag; B = Al, Ga, In): Elucidating the Role of Valence Electron States. Chem. Mater. 2024, 36, 3177–3190. [Google Scholar] [CrossRef]
- Seshadri, R.; Felser, C.; Thieme, K.; Tremel, W. MetalMetal Bonding and Metallic Behavior in Some ABO2 Delafossites. Chem. Mater. 1998, 10, 2189–2196. [Google Scholar] [CrossRef]
- John, M.; Heuss-Assbichler, S.; Ullrich, A.; Rettenwander, D. Purification of heavy metal loaded wastewater from electroplating industry under synthesis of delafossite (ABO2) by “Lt-delafossite process”. Water Res. 2016, 100, 98–104. [Google Scholar] [CrossRef]
- Kumar, S.; Deng, Z.; Liu, S.; Meng, G. Recent advances in p-type delafossite ABO2 based chemiresistive gas sensors. Sens. Actuators B Chem. 2025, 435, 137606. [Google Scholar] [CrossRef]
- Pu, Y.; Liu, Y.; Liu, D.; Zhou, Z.; Ding, S.; Xia, Z.; Li, M. First-principles screening visible-light active delafossite ABO2 structures for photocatalytic application. Int. J. Hydrogen Energy 2018, 43, 17271–17282. [Google Scholar] [CrossRef]
- Yang, X.; Zou, Y.; Hu, C.; Su, S.; Wang, Z.; Dong, H.; Teng, W.; Teng, B.; Zhang, B.; Zhong, D. Realizing Ultrabroadband NIR-II Emission and Wide-Range Wavelength Tuning in Cr4+-activated ABO2 (A = Li, Na; B = Al, Ga) Phosphors. Inorg. Chem. 2024, 63, 10. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, D.; Zhang, X.; Wang, S.; Sun, Z.; Liu, C.; Ma, J.; Ren, Y. Regulating B-Site Metals in Delafossite to Reach Efficient and Selective Peroxymonosulfate Activation for Water Remediation. ACS ES T Eng. 2023, 3, 2109–2121. [Google Scholar] [CrossRef]
- Chang, Y.H.; Wang, H.; Siao, T.F.; Lee, Y.H.; Bai, S.Y.; Liao, C.W.; Zhuang, J.K.; Chiu, T.W.; Kuo, C.H. A new solution route for the synthesis of CuFeO2 and Mg-doped CuFeO2 as catalysts for dye degradation and CO2 conversion. J. Alloys Compd. 2021, 854, 157235. [Google Scholar] [CrossRef]
- Jiang, C.M.; Reyeslillo, S.E.; Liang, Y.; Liu, Y.S.; Liu, G.; Toma, F.M.; Prendergast, D.; Sharp, I.D.; Cooper, J.K. Electronic Structure and Performance Bottlenecks of CuFeO2 Photocathodes. Chem. Mater. 2019, 31, 2524–2534. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, G. Light-Mediated Modulation of Enzyme-Mimetic Activity of CuMnO2 Nanosheets. J. Phys. Chem. Lett. 2022, 13, 11770–11777. [Google Scholar] [CrossRef]
- Qasim, M.; Atta, M.S.; Safra, I.; Makasana, J.; Rekha, M.M.; Kumar, G.S.; Al-Anber, M.A.; Das, S.N.; Chaudhary, R.; Kumar, A. Enhancement in performance of CuMnO2 anchored over rGO for water splitting. J. Phys. Chem. Solids 2025, 206, 112838. [Google Scholar] [CrossRef]
- Tsai, W.T.; Yang, J.M.; Hsu, H.C.; Lin, C.M.; Lin, K.Y.; Chiu, C.H. Development and characterization of mesoporosity in eggshell ground by planetary ball milling. Microporous Mesoporous Mater. 2008, 111, 379–386. [Google Scholar] [CrossRef]
- Mendhe, V.A.; Mishra, S.; Varma, A.K.; Kamble, A.D.; Bannerjee, M.; Singh, B.D.; Sutay, T.M.; Singh, V.P. Geochemical and petrophysical characteristics of Permian shale gas reservoirs of Raniganj Basin, West Bengal, India. Int. J. Coal Geol. 2018, 188, 1–24. [Google Scholar] [CrossRef]
- Gopinath, S.C.B.; Wang, L.; Rajapaksha, R.D.A.A.; Anbu, P.; Velusamy, P.; Pandian, K.; Arshad, M.K.M.; Lakshmipriya, T.; Lee, C.G. Photovoltaic and Antimicrobial Potentials of Electrodeposited Copper Nanoparticle. Biochem. Eng. J. 2019, 142, 97–104. [Google Scholar] [CrossRef]
- Saroj, S.K.; Singh, P.; Nagarajan, R. Perovskite (ACuF3) to double perovskite (A3CuF6) (A = K, Rb) transformation by a simple shaking procedure with hydrogen peroxide. Solid State Sci. 2018, 83, 137–142. [Google Scholar] [CrossRef]
- Rakovan, J.; Becker, U.; Hochella, M.F. Aspects of goethite surface microtopography, structure, chemistry, and reactivity. American Mineralogist 1999, 84, 884–894. [Google Scholar] [CrossRef]
- Chen, G.T.; Wang, C.H.; Yan, W.X.; Shu, K.; Ji, Z.L.; Luo, F. Crystal Structure and Electrochemical Behaviors of the New Sodium Cathode Material NaFe(SeO3)2. J. Phys. Chem. C 2024, 128, 10. [Google Scholar] [CrossRef]
- Luo, X.X.; Peng, C.Y.; Shao, P.H.; Tang, A.P.; Huang, A.P.; Wu, Q.; Sun, L.H.; Yang, L.M.; Shi, H.; Luo, X.B. Enhancing nitrate removal from wastewater by integrating heterotrophic and autotrophic denitrification coupled manganese oxidation process (IHAD-MnO): Internal carbon utilization performance. Environ. Res. 2021, 194, 110744. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.; Yim, J.H. High temperature-functioning ceramic-based ionic liquid electrolyte engraved planar HAp/PVP/MnO2@MnCO3 supercapacitors on carbon cloth. J. Mater. Chem. A 2021, 9, 14319–14330. [Google Scholar] [CrossRef]
- Tian, Y.Y.; Tian, X.K.; Zeng, W.B.; Nie, Y.L.; Yang, C.; Dai, C.; Li, Y.; Lu, L.Q. Enhanced peroxymonosulfate decomposition into •OH and 1O2 for sulfamethoxazole degradation over Se doped g-C3N4 due to induced exfoliation and N vacancies formation. Sep. Purif. Technol. 2021, 267, 118664. [Google Scholar] [CrossRef]
- Tian, Y.Y.; He, X.Y.; Chen, W.; Tian, X.K.; Nie, Y.L.; Han, B.; Lin, H.M.; Yang, C.; Wang, Y.X. Significant enhancement of photo-Fenton degradation of ofloxacin over Fe-Dis@Sep due to highly dispersed FeC6 with electron deficiency. Sci. Total Environ. 2020, 723, 138144. [Google Scholar] [CrossRef]
- Gao, P.P.; Yan, S.L.; Tian, X.K.; Nie, Y.L.; Wang, Y.X.; Deng, Y.; Tu, J.J. Identification and manipulation of active centers on perovskites to enhance catalysis of peroxymonosulfate for degradation of emerging pollutants in water. J. Hazard. Mater. 2022, 424, 127384. [Google Scholar] [CrossRef]
- Gao, P.P.; Tian, X.K.; Fu, W.; Wang, Y.X.; Nie, Y.L.; Yang, C.; Deng, Y. Copper in LaMnO3 to promote peroxymonosulfate activation by regulating the reactive oxygen species in sulfamethoxazole degradation. J. Hazard. Mater. 2021, 411, 125163. [Google Scholar] [CrossRef]
- Dai, C.; Tian, X.; Nie, Y.; Fu, W.; Wang, J. Effect of the interaction mode of H2O2 over CuMnO2 surface on •OH generation for efficient degradation of ofloxacin: Activity and mechanism. Chem. Eng. J. 2023, 451, 138749. [Google Scholar] [CrossRef]
- Dai, C.; Sheng, Z.; Tian, X.; Nie, Y. Chalcogen Elements in Regulating the Local Electron Density of Cu2X for an Efficient Heterogeneous Fenton-like Process. ACS Appl. Mater. Interfaces 2023, 15, 11324–11332. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, S.; Qie, H.; Zhu, S.; Zhang, C.; Li, X.; Wang, W.; Ma, J.; Sun, Z. Selective activation of peroxymonosulfate govern by B-site metal in delafossite for efficient pollutants degradation: Pivotal role of d orbital electronic configuration. Water Res. 2023, 236, 9. [Google Scholar] [CrossRef] [PubMed]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2020, 411, 127957. [Google Scholar] [CrossRef]
- Shahzada, A.; Alia, J.; Ifthikarb, J.; Aregayb, G.G.; Zhua, J.Y.; Chen, Z.L.; Chen, Z.Q. Non-radical PMS activation by the nanohybrid material with periodic confinement of reduced graphene oxide (rGO) and Cu hydroxides. J. Hazard. Mater. 2020, 392, 122316. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Deng, J. Insight into the Structure–Activity Relationship of Delafossite Catalysts for Enhanced Peroxymonosulfate Activation and Pollutant Degradation. Catalysts 2025, 15, 869. https://doi.org/10.3390/catal15090869
Li L, Deng J. Insight into the Structure–Activity Relationship of Delafossite Catalysts for Enhanced Peroxymonosulfate Activation and Pollutant Degradation. Catalysts. 2025; 15(9):869. https://doi.org/10.3390/catal15090869
Chicago/Turabian StyleLi, Liya, and Jiang Deng. 2025. "Insight into the Structure–Activity Relationship of Delafossite Catalysts for Enhanced Peroxymonosulfate Activation and Pollutant Degradation" Catalysts 15, no. 9: 869. https://doi.org/10.3390/catal15090869
APA StyleLi, L., & Deng, J. (2025). Insight into the Structure–Activity Relationship of Delafossite Catalysts for Enhanced Peroxymonosulfate Activation and Pollutant Degradation. Catalysts, 15(9), 869. https://doi.org/10.3390/catal15090869





