Abstract
Bismuth oxyiodide (BiOI) microspheres were synthesized by a microwave-assisted solvothermal method at 126 °C in only 4 min, a significantly shorter reaction time than in previously reported works. The structural, surface, morphological, and optical properties of BiOI were analyzed and correlated with the photocatalytic activity during the degradation of gallic acid (GA) and the photo-oxidation of nitric oxide (NO) in the aqueous and gas phases, respectively. The BiOI microspheres exhibited higher first-order apparent rate constants for GA and NO (0.188 min−1 and 0.230 min−1) than the benchmark TiO2 P25 (0.101 min−1 and 0.066 min−1). In addition, in steady-state reaction conditions (after 10 min), BiOI achieved 86% degradation of GA instead of the 63% degradation observed with TiO2 P25. Furthermore, at the same point in the reaction, the BiOI microspheres showed up to 65% NO conversion, while TiO2 P25 only achieved 15%. Accordingly, the results suggest that the microwave-assisted solvothermal method provides significant advantages for rapid, low-cost, and eco-friendly synthesis of BiOI microspheres for photocatalytic remediation of polluted water and air.
1. Introduction
BiOI microspheres have recently been widely studied in water and air remediation processes [1,2,3,4,5,6,7,8]. These semiconductors are characterized by a narrow gap (ca. 1.7–1.9 eV), enabling efficient absorption of visible light and making them promising candidates for solar-driven photocatalytic applications. For instance, BiOI exhibits superior photocatalytic performance compared to the widely used semiconductor TiO2 P25 [5,9], and it has been reported [5,7,8,9,10,11,12,13,14] that BiOI displays increased photocatalytic activity in the degradation of various pollutants and hazardous substances, including dyes, pharmaceuticals, pesticides, and even microorganisms in aqueous environments under simulated solar irradiation. In addition, BiOI has also shown potential for air purification applications, particularly in volatile organic compound (VOC) degradation and NOx removal [10]. The high photoefficiency of BiOI has been attributed to its unique layered structure and high surface area, which reduce the recombination rate of photogenerated electron–hole pairs [6]. Furthermore, the presence of iodine within the crystalline structure promotes the formation of oxygen vacancies, enhancing charge transport and surface reactivity [7].
In contrast, solvothermal synthesis has been used [11,12] as an efficient and low-cost method to synthesize BiOI with a controlled morphology and crystallinity. However, it has important limitations, such as long durations and high synthesis temperatures, resulting in high energy consumption. In this sense, our group has reported [15,16] that solvothermal synthesis of photoactive semiconductors, including Ti/SiO2 [15] and TiO2/ZnO [16], can be optimized by microwave-assisted irradiation. This method retains the advantages of the traditional solvothermal route while offering several advantages, such as rapid and uniform heating, yielding more homogeneous materials, higher yields, improved reproducibility, and significant reductions in synthesis time and energy consumption [17]. Furthermore, the microwave synthesis route promotes crystal nucleation and growth of the materials, resulting in a controlled particle size distribution [17]. Combining microwave-assisted irradiation and solvothermal synthesis is a reproducible and sustainable method to produce BiOI for environmental photocatalytic applications [18,19,20].
This work aimed to prepare BiOI microspheres using a microwave-assisted solvothermal method. Solvothermal synthesis on its own usually requires several hours at elevated temperatures, while combining both methods permits a reduction in synthesis time. The present work introduced a synthesis duration of only 4 min using ethylene glycol (EG) as a solvent. It played the role of a structural templating agent. To our knowledge, previous research has not reported such a rapid synthesis route for BiOI microspheres. The efficiency of BiOI microspheres was evaluated following the photodegradation of gallic acid (GA) and the photo-oxidation of nitric oxide (NO). Gallic acid was selected due to its presence in agroindustrial effluents generated by the wine industry [21]. NO was selected because it is an atmospheric pollutant generated by vehicle emissions and is responsible for producing nitrogen oxides (NOx), which affect air quality and human health [22]. Photocatalytic tests were performed under simulated solar irradiation to evaluate the scaling potential of BiOI microspheres in water and air remediation, and their results were compared against TiO2 P-25.
2. Results and Discussion
2.1. Characterization of BiOI
2.1.1. Crystalline Structure and Morphology
The crystalline structure of BiOI was analyzed using X-ray diffraction patterns (XRD). As shown in Figure 1a, the diffraction peaks can be indexed to the tetragonal crystal structure of BiOI (JCPDS No. 01-073-202). The Rietveld refinement [23] included in Figure 1a confirms the formation of a pure phase of BiOI. In addition, it should be mentioned that Figure 1a shows broad peaks, suggesting a lower crystallinity in the material [18,24].
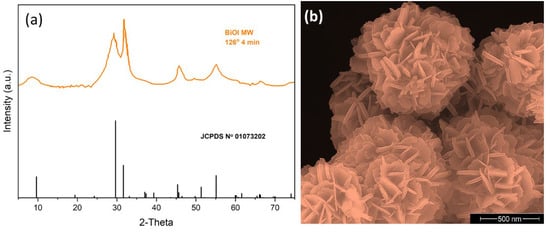
Figure 1.
Crystal structure and morphology of BiOI. (a) XRD pattern; (b) SEM image.
The SEM image in Figure 1b shows that the BiOI microspheres, formed by self-assembled BiOI nanosheets, exhibit an interesting flower-like morphology with a rough surface. The spherical structures have an approximate diameter of 2.80 μm. This cluster-like morphology, combined with the broad peaks observed in the XRD pattern, suggests that the synthesized BiOI is nanostructured and has a relatively low crystallinity. Therefore, the evaluation of crystallinity was based on XRD, while SEM provided insights into the morphological features relevant to light harvesting and photocatalytic performance.
As reported by Liao et al. [25], this flower-shaped morphology offers a high specific surface area and high photocatalytic activity. In agreement with Smith and Lee [26], microwave-assisted solvothermal synthesis is efficient in controlling the spherical morphology and crystallinity of semiconductors, as in the present case. Our group has reported [17,27,28] that the roughness on the surfaces of spherical particles enhances light harvesting efficiency by inhibiting light scattering. As expected, this feature enhances photocatalytic activity, as discussed in Section 2.2.
2.1.2. Surface Composition
The XPS survey spectra in Figure 2a show that the prepared material mainly comprises Bi, O, and I. A small content of C was also observed and was attributed to the adsorption of CO2 on the surface of the semiconductor material [29]. Figure 2b shows the XPS spectra of Bi at the 4f7/2 level with a binding energy (BE) of ca. 159.5 eV assigned to Bi3+ [30], characteristic of the Bi in BiOI.
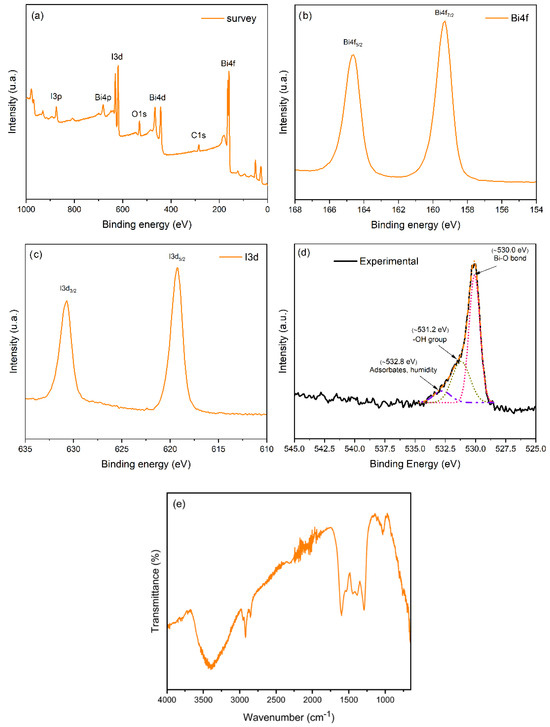
Figure 2.
The surface composition of BiOI. (a) XPS survey spectra; (b) XPS at the Bi 4f level; (c) XPS at the I 3d level; (d) XPS at the O 1s level; (e) FTIR spectra.
Figure 2c shows that the XPS spectra of I at the 3d5/2 level appear at ca. 619.5 eV [31], confirming the presence of iodide ions (I−), which correspond to the I in the layered BiOI structure [32,33,34]. The XPS spectrum of O at the 1s level in Figure 2d shows a peak at ~530.0 eV, which in this material is assigned to the Bi–O bond, typical of bismuth oxides such as BiOI [35,36]. This is the main and most intense component, indicating that most of the oxygen in the sample is in the form of bismuth oxides. The peak at ~531.2 eV is associated with hydroxyl groups (-OH) on the material’s surface [37]. These hydroxyl species suggest that the present BiOI materials may have a hydrophilic nature. A signal at ~532.8 eV is related to physically adsorbed water molecules or atmospheric pollutants (such as CO2) [35].
Fourier transform infrared spectroscopy (FTIR) analysis of the material in Figure 2e shows a broad band at ca. 3500 cm−1, confirming that surface hydroxyl groups are linked to the BiOI [34]. The band observed at ca. 1600 cm−1 (H-O-H bending) is due to water adsorbed by the material’s surface [34]. The noise observed in the FTIR spectra between 2200 and 2400 cm−1 may be associated with adsorbed CO2 [38], as suggested above in the analysis of the XPS spectra at the O 1s level.
2.1.3. Textural and Optical Properties
Figure 3a shows the N2 adsorption–desorption isotherm of BiOI. According to the recommendation of the IUPAC [39], Figure 3a shows a type IV isotherm, indicating that the BiOI structure mainly comprises a mesoporous framework. However, it is important to note that micropores could also be detected in the material.
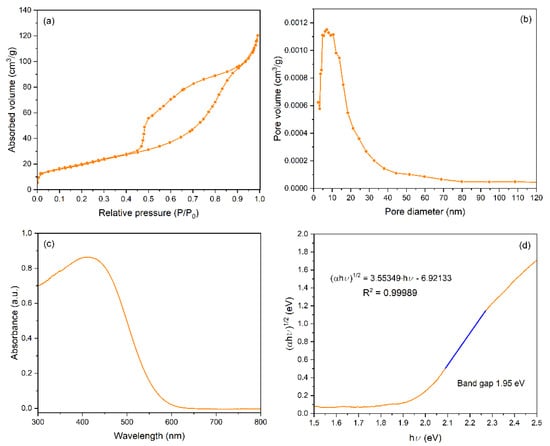
Figure 3.
Textural and optical properties of BiOI. (a) N2 adsorption–desorption isotherm; (b) pore size distribution; (c) UV–Vis absorbance spectra; (d) Tauc plot of data in Figure 3c.
The H3-type hysteresis loop [40] observed in Figure 3a suggests the material is composed of laminar-shaped aggregates [25], as observed in the SEM image (Figure 1b). Table 1 shows a summary of the main textural properties obtained for BiOI compared to the benchmark semiconductor TiO2 P-25, including the BET surface area (SBET), total pore volume (Vtot), micropore volume (Vmicro), mesopore volume (Vmeso), and mean pore diameter (dpore).
Figure 3b shows the pore size distribution (PSD) obtained by applying BJH analysis. This BiOI is characterized by an important proportion of mesopores, in agreement with its moderate BET surface area, and the total volume of pores is ca. 71 m2·g−1 and ca. 0.183 cm3·g−1. The micropore volume is ca. 8% of the total volume of pores, so it can be concluded that the synthesized material mainly comprises small BiOI mesopores in the 2.5–12.5 nm range. The Gurvich rule [41,42] assumes the mean pore diameter (dpore) of an adsorbent material can be estimated by Equation (1) using the data in Table 1.
dpore = 4 (VTot/SBET)

Table 1.
Summary of textural and optical properties.
Table 1.
Summary of textural and optical properties.
| Material | SBET a (m2·g−1) | Vtot b (cm3·g−1) | Vmicro c (cm3·g−1) | Vmeso d (cm3·g−1) | dpore e (nm) | Ebg f (eV) | cut-off (nm) |
|---|---|---|---|---|---|---|---|
| BiOI | 71 | 0.183 | 0.015 | 0.168 | 10.3 | 1.95 | 632 |
| TiO2 P25 | 54 g | 0.100 g | -- | -- | 8.6 | 3.00 | 385 |
a BET surface area. b Total volume of pores is estimated at P/P° = 0.98. c Micropore volume obtained from BJH analysis. d Vmeso = Vtot−Vmicro. e Mean pore diameter. f Energy band gap data taken from [43]. g Data for commercial TiO2 P25 obtained from [44].
According to this equation, the dpore of BiOI is ca. 10.3 nm, which agrees with the PSD observed in Figure 3b. However, when this treatment is applied to TiO2 P25, the dpore for the benchmark semiconductor is ca. 8.6 nm, which is not acceptable, as it is well known that TiO2 is not a porous material [43]. The mesopore volume reported is a consequence of the empty spaces between the nanometric particles [44]. It can be seen in Table 1 that BiOI presents significant textural differences compared to the data reported for TiO2 P25 [40]. The BiOI semiconductor presents a higher specific surface area and a much higher total volume of pores than TiO2 P25 [44], which can enhance the adsorption of pollutants.
Figure 3c shows the UV–visible absorbance spectrum of the BiOI material, and Figure 3d displays the corresponding Tauc plot formalism [45]. This method was used to estimate the band gap energy (Ebg) of BiOI using Equation (2), where α is the absorption coefficient, hν is the photon energy, and n is an exponent that depends on the nature of the electronic transition. For BiOI, an indirect allowed transition is assumed (n = 2) [46], especially for BiOI samples synthesized by solvothermal methods [47,48,49,50].
(αhν)1/n = A(hν−Ebg)
In the case of TiO2 P25, it is frequently reported in the literature that the Tauc exponent (1/n) is equal to 1/2 due to an indirect transition (n = 2), which is due to the joint density of states and is dependent on the (hv−Eg)1/2 for a bulk intrinsic semiconductor [13,15]. Thus, it is widely recognized that TiO2 is fundamentally an indirect band gap semiconductor. Furthermore, TiO2 P25 comprises a mixture of anatase (~80%) and rutile (~20%) phases, each exhibiting distinct optical transitions and band gap values. Consequently, the band gap value of ~3.0 eV typically attributed to TiO2 P25 reflects its mixed phase composition, consisting of anatase (Eg ≈ 3.2 eV) and rutile (Eg ≈ 3.0 eV) phases [51,52,53]. In this study, a more accurate estimation was made based on the absorption edge observed in the UV–Vis spectrum, which appeared at approximately 413 nm. This corresponds to an effective band gap of ca. 3.00 eV, calculated using the relation Eg = 1240/λ, in agreement with values from the literature [43]. This value is physically reasonable and suggests a dominant contribution from the anatase phase, consistent with its indirect-transition character [46].
The BiOI obtained in this work exhibits an indirect band gap of approximately 1.95 eV, as determined by the Tauc method (1/n = ½), consistent with the literature for BiOI with a layered structure [54]. This relatively narrow band gap allows for strong absorption in the visible region, with a cut-off wavelength around 632 nm, making BiOI a suitable candidate for visible-light-driven photocatalysis [54,55].
Figure 4 illustrates a representative comparison of the band structures of BiOI and TiO2 P25, a material commonly used as a reference (see the caption). BiOI typically exhibits a valence band (VB) located at approximately +2.3 eV and a conduction band (CB) around +0.4 eV compared to a standard hydrogen electrode (NHE) [53,54]. This configuration enables the photogenerated holes (h+) in the VB to act as potent oxidizing agents for the degradation of organic compounds. At the same time, the electrons in the CB can reduce molecular oxygen to generate superoxide radicals (•O2−). Additionally, the internal electric fields arising from the alternating [Bi2O2]2+ and I− layers in the BiOI crystal structure further enhance the separation of photogenerated charge carriers and suppress electron–hole recombination [56].
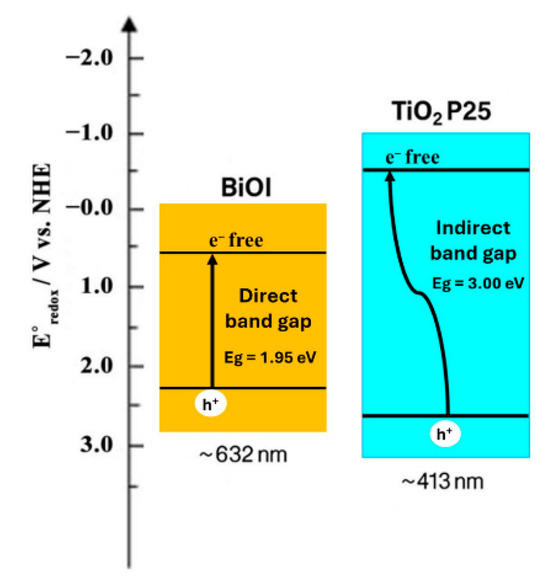
Figure 4.
An energy band diagram of BiOI and TiO2 P25. Band edge positions are shown compared to an NHE, highlighting differences in light activation and charge carrier behavior. BiOI is frequently described in the literature as a direct band gap semiconductor; however, this study employed an indirect band gap model for consistency with our previous report on solvothermal BiOI [57], allowing for a direct comparison of its optical and photocatalytic properties.
In comparison, TiO2 P25 (Degussa), a widely used benchmark photocatalyst, possesses a band gap of approximately 3.00 eV, composed of mixed anatase (~80%) and rutile (~20%) crystalline phases [57,58,59]. This mixed phase structure facilitates charge transfer across phase junctions, contributing to more effective charge separation under UV light. TiO2 exhibits a conduction band at approximately −0.5 eV and a valence band near +2.7 eV compared to an NHE [51] (Figure 4). Although these positions provide strong redox capabilities, the wide band gap limits light absorption to the near-UV spectrum and the edge of the visible spectrum (λ ≈ 413 nm), accounting for only ~5% of solar irradiance [56,60].
Therefore, despite the established efficiency of TiO2 P25 under UV radiation, its limited photoresponse under visible light significantly reduces its performance under simulated solar radiation. In contrast, the narrower band gap and favorable band edge positions of BiOI enable it to harvest a larger portion of the solar spectrum and facilitate efficient charge separation [12,13,45].
These features explain the superior photocatalytic activity of BiOI under visible-light irradiation compared to TiO2 P25, particularly in the degradation of gallic acid and photo-oxidation of NO demonstrated in this study.
2.2. Photocatalytic Activity
Regarding the photocatalytic activity, it is noteworthy that the adsorption of gallic acid (GA) under dark conditions differed significantly between the tested materials. Specifically, the BiOI semiconductor exhibited a higher adsorption capacity, reaching 39%, whereas TiO2 P25 achieved only 16% adsorption from the aqueous solution. Additionally, as shown in Figure 5a, direct photolysis of GA in the absence of any photocatalyst led to minimal degradation, with a removal efficiency of approximately 10%, highlighting the necessity of using a photocatalyst to initiate and sustain the degradation process under simulated solar radiation.
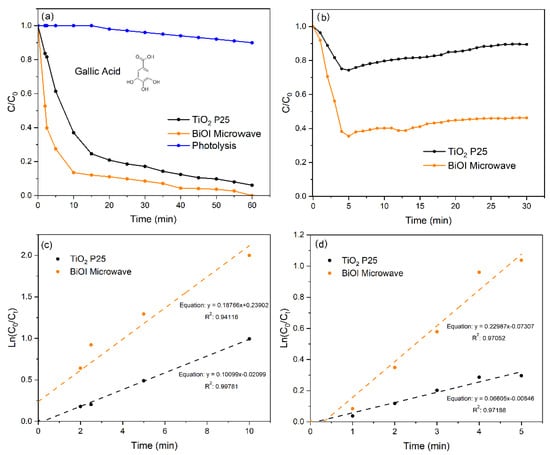
Figure 5.
The photoactivity of BiOI under simulated solar irradiation. (a) The kinetics of catalytic degradation of GA. (b) The kinetics of catalytic oxidation of NO. (c) A first-order linear regression of the kinetic data from (a), using the initial 10 min. (d) A first-order linear regression of the kinetic data from (b), using the initial 5 min.
In addition, Figure 5a shows the kinetics of the photocatalytic degradation of GA under simulated solar irradiation. The synthesized BiOI exhibited remarkable activity with ca. 87 and 100% degradation of GA after 10 and 60 min of reaction, respectively. These values show that BiOI microspheres are clearly more efficient than TiO2 P25, which achieved photocatalytic degradation of ca. 63 and 94% of GA after 10 and 60 min, respectively.
Figure 5b shows the kinetics of NO photo-oxidation under artificial solar irradiation. BiOI exhibited faster NO conversion than TiO2 P25. After 5 min, 65% of the NO was oxidized on BiOI, while TiO2 P25 could only convert 25%. However, it is fascinating that after this time, the photoactivity was monotonically lost, achieving values of ca. 55 and 10% using BiOI and TiO2 P25, respectively, after 30 min reactions. The loss of photoactivity is due to competitive adsorption between NO and NOx produced from NO and is highly influenced by the relative humidity in the photoreactor [61]. Another explanation for this deactivation trend is the loss of active sites due to the formation of mineral acids like HNO3, which can also be responsible for inhibiting the formation of reactive oxygenated species (ROS), as reported by our group [62] for solar-irradiated Fe/C photocatalysts in the presence of phosphates. In addition, the slight increase in the NO concentration between 5 and 30 min after an initial drop can be attributed to NO desorption or photoreduction of intermediate anions and nitrogen species (e.g., NO2− or NO3−) adsorbed on the catalyst surface. These intermediates may be re-emitted as NO during prolonged irradiation, a phenomenon reported in the literature for various photocatalysts under UV and visible light [63,64,65]. However, in spite of these inconveniences, BiOI still proved to be a more efficient photocatalyst than TiO2 P25 under simulated solar irradiation.
The photocatalytic degradation kinetics of gallic acid and NO oxidation were analyzed using the pseudo-first-order model, which is commonly employed for heterogeneous photocatalysis. In this approach, only the initial stage of the reaction was considered (the first 10 min for gallic acid and the first 5 min for NO), when the pollutant concentration was significantly higher than the catalyst surface coverage and mass transfer effects did not limit the reaction rate. This methodology allows more accurate estimations of intrinsic reaction-rate constants under linear conditions [66,67]. The corresponding kinetic plots and regression equations are shown in Figure 5c,d.
The first approach to estimating the photoactivity of the BiOI catalyst was to assume that the GA photodegradation and NO photo-oxidation followed a first-order reaction mechanism [28,43,67,68,69,70] described by Equation (3), where Ceq is the concentration of the pollutant after the preliminary period of adsorption in the dark, kapp is the first-order apparent rate constant (min−1), and t is the time of the reaction.
kapp is a valuable kinetic parameter for analyzing the photoactivity of BiOI compared to TiO2 P25. Figure 5c,d show the linear regression form, Equation (4), of the kinetic data from the GA degradation and NO photo-oxidation.
(dCeq/dt) = −kapp·t
Ln(Ceq/Ct) = kapp·t
Aiming to obtain the best possible fit for Equation (4), kapp values were obtained from the linear regression slopes of the kinetic data in the first 10 and 5 min of the GA degradation and NO photo-oxidation reactions, respectively. The R2kapp values from the linear regressions were above 0.94 in all cases, suggesting that the first-order kinetic model correctly describes the kinetics of both reactions, at least in the time ranges selected for the estimations of kapp.
The calculated photoactivity factor (IF) shows that BiOI’s degradation of gallic acid (GA) relative to TiO2 P-25 was approximately 1.9, and its NO photo-oxidation was 3.5. These findings demonstrate that this BiOI material exhibits significantly superior photocatalytic performance under simulated solar radiation, confirming its greater effectiveness as a semiconductor for environmental remediation applications. Table 2 summarizes the kinetic parameters calculated for GA photodegradation and NO photo-oxidation using BiOI and TiO2 P25.

Table 2.
A summary of the kinetic parameters obtained for GA photodegradation and NO photo-oxidation.
The photocatalytic performance of a semiconductor is inherently linked to its ability to generate, separate, and transport photogenerated charge carriers under light irradiation. Although direct photocurrent responses and electrochemical impedance spectroscopy (EIS) measurements were not obtained in this study, the observed photocatalytic behavior of BiOI can be interpreted based on its structural and morphological properties, as well as insights from the literature on BiOI synthesized via microwave-assisted solvothermal methods. This synthetic route facilitates rapid nucleation and crystallization, typically yielding microspherical architectures composed of nanosheets. Such hierarchical structures enhance the exposure and reduce charge carrier migration distances, favoring effective charge separation.
Several studies have confirmed [71,72,73] that BiOI materials prepared by microwave-assisted methods exhibit enhanced charge separation efficiency, attributed to their improved crystallinity and layered morphology. For instance, BiOI synthesized through microwave-assisted solvothermal techniques has shown significantly higher transient photocurrent responses and reduced charge transfer resistance compared to conventionally hydrothermally synthesized counterparts. These characteristics have been correlated with superior visible-light photocatalytic activity. In comparison, TiO2 P25, a benchmark photocatalyst widely studied for UV-driven applications, benefits from high crystallinity and a mixed composition of anatase (ca. 80%) and rutile (ca. 20%) phases, which enhances electron–hole separation through interfacial charge transfer mechanisms [69]. This synergistic effect improves photocatalytic efficiency under UV light. However, TiO2 P25 has a wide band gap (ca. 3.2 eV), which restricts its absorption to the UV region (<400 nm), thus limiting its performance under visible-light- or solar-driven conditions [74,75]. In contrast, BiOI exhibits a narrower band gap (ca. 1.9–2.0 eV) and a unique layered structure that induces internal electric fields, facilitating charge separation and activity under visible light [76].
These findings support the assumption that the BiOI material synthesized in the present work—characterized by flower-like microspheres composed of self-assembled nanosheets—likely benefits from favorable charge separation dynamics and enhanced visible-light photocatalytic activity, in contrast to TiO2 P25, whose performance is strongly dependent on UV excitation.
To further contextualize the photocatalytic performance of BiOI synthesized via the microwave-assisted solvothermal method, we compared the results obtained in the present work with values reported in previous studies on BiOI prepared through conventional solvothermal synthesis. Table 3 summarizes this comparison with results reported [77,78,79,80,81,82] in the literature.

Table 3.
Comparison of synthesis conditions and photocatalytic performance of BiOI microspheres prepared by conventional and microwave-assisted solvothermal methods.
It is important to note that Ref. [77] corresponds to a previous study conducted by our research group, in which BiOI microspheres synthesized at 126 °C for 18 h by the conventional solvothermal method using a response surface methodology (RSM) demonstrated superior photocatalytic activity compared to TiO2 P25 for oxidation of gallic acid under simulated solar radiation, reaching up to 60% degradation in 30 min [77]. To highlight the advantages of the microwave-assisted solvothermal route, Table 4 presents a comparative summary of the properties of both materials. Both methods yielded BiOI with a tetragonal structure and similar morphological characteristics. However, the BET surface area of the BiOI synthesized via the microwave-assisted method (71 m2/g) was considerably higher compared to the material obtained through conventional solvothermal synthesis (47 m2/g). The band gap values were equivalent for both materials (1.9 eV), indicating a good capacity for visible-light absorption. Furthermore, the photocatalytic activity of the microwave-assisted BiOI achieved 86% pollutant removal, outperforming its solvothermal counterpart (60%). Although the semiconductors obtained by both routes exhibited similar properties, marked differences were observed in the surface area and the photocatalytic efficiency of the removal of the phenolic compound from water. Nevertheless, the main advantage of the microwave-assisted solvothermal method lies in a drastic reduction in synthesis time. The changes are highlighted in yellow in the revised manuscript.

Table 4.
Comparison of properties of BiOI synthesized by microwave-assisted and solvothermal methods [77,78].
Similar photocatalytic behavior was also observed under gas-phase conditions for NO photo-oxidation [79]. For example, hollow BiOI microspheres synthesized by the solvothermal method at 160 °C for 12 h were evaluated for NO removal under visible light, showing representative photocatalytic activity of up to 60% in the photo-oxidation of NOx at 35 min under experimental conditions equivalent to those used in this work [78].
In contrast, the material reported in this study achieved a significantly higher degradation efficiency for gallic acid (≥86%) in 30 min and higher NO photo-oxidation of up to 65% in less time (ca. 5 min) than most of the results found in the literature [80,81,82].
The present results confirm the advantages of the microwave-assisted method used in this work, mainly those referred to as enhancements of photocatalytic performance for environmental remediation, the lower synthesis time, and the improved material characteristics. However, TiO2 P25 shows better fit values in the first-order kinetic model, indicating a more predictable photocatalytic mechanism or one less influenced by parallel reactions. Parallel reactions affect the oxidation mechanism, and it is well known that they are highly influenced by the generation of distinct reactive oxygenated species (ROS). For instance, highly reactive hydroxyl radicals (•OH) are generated on the TiO2 surface. Under visible-light irradiation, BiOI systems predominantly generate photogenerated holes (h+) and superoxide radicals (•O2−). In contrast, the formation of hydroxyl radicals (OH) is less favorable due to the insufficient oxidation potential of the valence band in this semiconductor [7,79,80,81].
We also suggest that the inhibition observed in the kinetics of NO photo-oxidation could be due to this fact. Accordingly, studies on the influence of molecular oxygen, relative humidity, catalyst loading, and the NO concentration are currently being conducted on BiOI catalysts.
Table 3 shows various synthesis parameters and photocatalytic efficiencies for BiOI microspheres prepared via microwave-assisted methods that have been reported in the literature. In this context, the present study demonstrates successful synthesis of BiOI microspheres through a microwave-assisted solvothermal route at 126 °C for only 4 min—a considerably shorter reaction time than in previous reports. Remarkably, despite the reduced synthesis duration and moderate temperature, the resulting BiOI microspheres exhibited high photocatalytic activity, highlighting the efficiency and potential of this rapid synthesis approach.
Specifically, the material we obtained showed 86% gallic acid (GA) degradation after just 20 min under simulated solar radiation, and complete degradation (100%) was achieved in 60 min. This result significantly exceeds the photocatalytic efficiencies described in previously reported studies (Table 3), where similar or lower degradation percentages were achieved only after considerably longer irradiation.
This yield significantly exceeds previously reported values for microwave-synthesized BiOI. For example, lower NO conversion efficiencies have been reported using BiOI synthesized with longer microwave irradiation times [82,83,84]. Similarly, only 61% NO removal was achieved under comparable irradiation conditions [83]. In contrast, 80% Rhodamine B (RhB) degradation was achieved with just 480 min of microwave synthesis at 170 °C [81]. However, the present study employed an even shorter synthesis time and achieved complete organic compound degradation in 60 min under simulated solar irradiation. Additionally, the synthesized BiOI microspheres exhibited excellent performance in NO photo-oxidation, reaching a 65% conversion rate and significantly surpassing values obtained with BiOI materials synthesized using similar methods and reference photocatalysts such as TiO2 P-25. In other reports, only 43% degradation of methylene blue was observed after 120 min of irradiation [85].
Our process significantly shortens the synthesis time compared to conventional methods (typically 8 to 20 min) and generates a photocatalyst that affects both liquid and gaseous contaminants. The results in Table 3 suggest that the short synthesis time and moderate temperature employed in the present study did not compromise but instead improved the properties of the material. Rapid microwave heating promotes uniform nucleation and defect formation, which improves charge carrier dynamics and translates into better photocatalytic activity [6,14,86,87,88].
Recent advances have also demonstrated that integrating bismuth oxyhalides (BiOX, where X = Cl, Br, or I) with other functional components or carbonaceous supports can significantly enhance photocatalytic efficiency. For instance, core–shell Bi-MOF@BiOX heterostructures synthesized through a one-pot strategy have shown remarkable performance in the degradation of both single and mixed pollutants, owing to improved charge separation at the interface and accelerated charge transport [89]. Similarly, the use of cellulose-derived carbon (CDC) to induce the formation of flower-like BiOX morphologies has been reported to increase the surface area and promote visible-light-driven Cr+6 reduction, achieving efficiencies far superior to those of pristine BiOX [90]. These findings reinforce the importance of hierarchical structures and interfacial engineering strategies—elements that are inherently present in our microwave-assisted BiOI microspheres—further validating the effectiveness of this synthesis approach for environmental photocatalysis.
Finally, though the goal of the present work was to demonstrate the effectiveness of microwave-assisted solvothermal synthesis in producing a material with high activity under simulated solar radiation and to compare its performance in different reaction phases, the importance of assessing the photocatalyst’s long-term stability and reusability in practical applications is recognized. Although cycling experiments were not performed, previous studies on BiOI materials synthesized by similar microwave-assisted methods [91,92] have reported good stability over multiple photocatalytic cycles. Detailed cycling tests will be performed in the future to evaluate the material’s structural integrity and activity retention during repeated use using the lab’s scale photoreactor and semi-scale pilot plants.
3. Materials and Methods
3.1. Synthesis of BiOI Microspheres
The synthesis temperature of 126 °C was selected based on prior optimization studies in which BiOI microspheres with high crystallinity and photocatalytic efficiency were obtained via a conventional solvothermal treatment at 126 °C for 18 h [93,94] To adapt these conditions to a microwave-assisted solvothermal approach—aiming to reduce the synthesis time and energy consumption—a series of preliminary experiments were conducted, varying the reaction time between 1 and 5 min while maintaining the temperature at 126 °C. It was observed that BiOI powders did not form in less than 4 min. Thus, 4 min was established as the minimum time necessary to reproducibly obtain well-defined BiOI microspheres under microwave irradiation.
In this study, BiOI microspheres were synthesized using a microwave-assisted solvothermal method. First, a solution was prepared by dissolving 1 mmol of potassium iodide (DEQ, 99.5%) in 10 mL of ethylene glycol (EG) (CTR Scientific, 99.7%). The solution was then added dropwise to 10 mL of another solution containing 1 mmol of bismuth nitrate pentahydrate (Sigma-Aldrich, ≥98.0%) dissolved in EG and stirred at room temperature for 30 min.
Subsequently, the mixture was transferred into a 30 mL borosilicate glass vial and placed in a microwave reactor (Anton Paar Monowave 300). The temperature was increased from 25 °C at a controlled heating rate of approximately 30 °C·min−1 (0.5 °C·s−1) until reaching 126 °C and was then maintained at this temperature for 4 min under continuous stirring (600 rpm). This well-defined time of 4 min refers strictly to the isothermal holding stage and does not include the heating and cooling phases. After the reaction, the system was rapidly cooled to 30 °C using compressed air.
3.2. Characterization of BiOI Microspheres
The crystalline structure of BiOI was analyzed using X-ray powder diffraction (XRD) with a Bruker D8 Advance diffractometer and CuKα radiation (λ = 1.5418 Å) over a 2θ scan range of 10–70°. The morphology of the BiOI materials was studied by scanning electron microscopy (SEM) using an FEI Nova NanoSEM 200 instrument. Before analysis, samples were sputter-coated with a thin layer of gold to enhance surface conductivity and improve image quality. X-ray photoelectron spectroscopy (XPS) was performed to analyze the surface composition of the materials. A Thermo Scientific K-Alpha Surface Analyzer apparatus was used, and all binding energy values were calibrated at 284.8 eV based on the C 1s signal. The power source was a monochromatic Al Kα X-ray source (1486.6 eV).
Functional groups linked to the BiOI surface were identified by Fourier transform infrared (FTIR) spectroscopy using a Nicolet Nexus spectrometer. Samples were finely ground and mixed with spectroscopic-grade KBr, then pressed into pellets using a hydraulic press to ensure adequate transparency for infrared analysis. The specific surface area was determined by the Brunauer–Emmett–Teller (BET) method using N2 adsorption–desorption isotherm data at 77 K, which were obtained with a BEL Japan Minisorp II surface area and pore size analyzer. Pore size distributions were calculated using the Barrett–Joyner–Halenda (BJH) method. The total pore volume was estimated from the amount of N2 adsorbed at a relative pressure (P/P0) of approximately 0.98, while the micropore volume was calculated using the Gurvich rule, based on the adsorption data in the low-relative-pressure region. Finally, the diffuse reflectance UV–visible spectrum (DRS) was obtained using an Agilent Cary 4500 Series spectrophotometer equipped with an integrating sphere using BaSO4 as the reference standard.
3.3. Photocatalytic Tests
3.3.1. Photocatalytic Degradation of GA in Water
The photocatalytic efficiency of the BiOI microspheres was first evaluated following the degradation of gallic acid (GA) in the aqueous phase. A Xe lamp was used as a solar-simulating light source (VIPHID, 6000 K, 12 V, 35 W). It emitted a broad and continuous spectrum ranging from approximately 300 to 900 nm, with a primary intensity peak around 600 nm and additional contributions in the UV-A (ca. 370 nm) and near-infrared (ca. 800 nm) regions. This emission profile closely resembles the solar spectral distribution and is commonly used for photocatalytic applications. The corresponding emission spectrum of the lamp is provided in Figure S1 in the Supplementary Materials. First, 80 mg of BiOI was suspended in 250 mL of GA (giving an initial concentration of 10 ppm) for a constant catalyst loading of ca. 0.32 g·L−1. The mixture was stirred for 40 min until an equilibrium condition was observed in the adsorption of GA. The measurements were carried out by UV–vis spectroscopy using a Thermo Scientific Evolution 220 spectrophotometer, monitoring the absorbance at a wavelength of 264.5 nm, corresponding to the maximum absorption peak of GA. For instance, Figure S2 in the Supplementary Materials shows a classical UV–visible spectrum of GA as a function of the reaction time.
3.3.2. Photo-Oxidation of Nitric Oxide (NO)
The photocatalytic performance of the synthesized BiOI microspheres was evaluated through gas-phase photo-oxidation of nitric oxide (NO) under simulated solar irradiation. Experiments were conducted in a stainless-steel photoreactor (internal volume: 0.5 L) equipped with a quartz window to allow irradiation from a UV–visible lamp (Philips Actinic BL, 24 W, DELIGHT SPL-24 W, MidView, Singapore). This lamp’s representative emission spectrum covers the range 360–740 nm, with a distribution that includes the UV-A spectrum and a significant portion of the visible-light spectrum, reaching its maximum intensity near 550 nm. Although the emission is not identical to natural sunlight, it provides sufficient intensity within the photocatalytic active region (particularly 400–600 nm) to simulate solar-driven processes in environmental applications [90]. The corrected emission spectrum corresponding to the lamp used in this study is clearly presented in Figure S3 in the Supplementary Materials, with an appropriate reference attribution. Figure 6 provides a schematic representation of the reactor configuration employed.
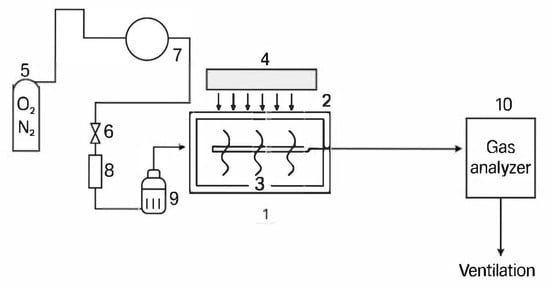
Figure 6.
A schematic representation of the photocatalytic reactor system used for the photo-oxidation of NOx experiments. (1) The photocatalytic reactor chamber; (2) the optical window; (3) the photocatalyst support (sample holder); (4) the light source; (5) the synthetic air source (O2/N2 mixture); (6) the control valve; (7) and (8) mass flow controllers; (9) the humidifier; (10) the gas analyzer.
For each run, 200 mg of the photocatalyst was suspended in 2 mL of absolute ethanol and uniformly applied to a 50 cm2 glass substrate using a brush, forming a homogeneous thin film. The NO feed was prepared by diluting a certified gas mixture (3 ppmv NO in N2) to an inlet concentration of 1 ppmv using synthetic air composed of 20.5 vol% O2 and 79.5 vol% N2. The total gas flow rate was maintained at 1.0 L·min−1 (although relatively high, this flow rate ensured sufficient residence time within the reactor and consistent NO exposure across the photocatalyst surface). The outlet NO concentration was continuously monitored using a chemiluminescence-based analyzer (EcoPhysics CLD88p) at a sampling rate of 0.5 L·min−1. Once adsorption–desorption equilibrium was achieved (typically within 15–20 min), the LED lamp was turned on to initiate the photocatalytic reaction. NO concentrations were recorded at 1 min intervals over a 30 min irradiation period.
4. Conclusions
This study shows the successful and highly efficient synthesis of BiOI microspheres using a microwave-assisted solvothermal method. The BiOI material was crystallized entirely in just 4 min at 126 °C, one of the shortest reaction times recorded for this material to date. The use of ethylene glycol as a structuring solvent facilitated the formation of a hierarchical 3D morphology composed of self-assembled nanosheets, resulting in a high specific surface area (71 m2 g−1) and a mesoporous network favorable for the adsorption of pollutants present in water and air.
Structural (XRD) and surface (XPS) analyses confirmed the high phase purity of the synthesized BiOI and a surface rich in hydroxyl groups, characteristics that enhance interactions with polar species in both aqueous and gaseous media. The material exhibited a narrow band gap of 1.95 eV that favored visible-light absorption, substantially improving visible-light capture compared to TiO2 P-25, which was used as a reference.
Photocatalytic assays under simulated solar radiation revealed superior performance of the obtained BiOI with respect to TiO2 P25 in gallic acid degradation (kapp = 0.188 min−1) and NO photo-oxidation (kapp = 0.230 min−1), achieving 100% degradation of the phenolic compound after 60 min and 65% NO conversion in 30 min. These values correspond to photoactivity factors (IFs) that are 1.9 and 3.5 times higher than those of TiO2 P25, respectively. The high performance of the obtained material is attributed to the synergistic effects of its structural and surface properties, including improved charge separation, higher initial adsorption, and the probable participation of alternative reactive species (h+ and O2•−) in addition to •OH radicals.
This study demonstrated that microwave-assisted solvothermal synthesis of BiOI enables preparation of the material with significantly shorter reaction times compared to the conventional solvothermal method. Despite the drastic reduction in the synthesis time (4 min vs. 18 h), the obtained BiOI exhibited properties comparable to its conventionally synthesized counterpart. However, the BiOI synthesized using microwaves exhibited a higher specific surface area, resulting in enhanced photocatalytic efficiency in the degradation of the phenolic contaminant. These results highlight the microwave-assisted method as an energy-efficient and highly promising strategy for producing photocatalysts.
Overall, this work highlights the potential for microwave-assisted synthesis to provide a rapid, reproducible, and energy-efficient route for producing BiOI microspheres with excellent photocatalytic properties. These characteristics position BiOI obtained with short synthesis times as a promising photocatalyst for environmental remediation applications, particularly water and air purification under solar radiation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15090868/s1, Figure S1. Emission spectrum of the Xe lamp (VIPHID 6000 K, 12 V, 35 W) used during the photocatalytic degradation of gallic acid in the aqueous phase. Figure S2. UV-Vis absorption spectra of gallic acid (10 ppm) during photocatalytic degradation with BiOI microspheres synthesized via solvothermal method (126 °C and 18 h). Figure S3. Emission spectrum of the UV-Visible LED (360 to 740 nm) lamp Philips Actinic BL, DELIGHT SPL-24 W, MidView, Singapore (line color black) used during the gas-phase photooxidation of nitric oxide (NO) [94].
Author Contributions
Conceptualization, A.C.M.; Methodology, C.A.V.; Software, A.A.A.; Validation, A.C.M. and J.M.; Formal analysis, A.C.M., J.M. and C.A.V.; Investigation, A.C.M.; Resources, A.C.M. and A.A.A.; Data curation, C.A.V.; Writing—original draft, A.C.M.; Writing—review & editing, A.C.M., J.M., C.A.V. and A.A.A.; Supervision, A.C.M., J.M. and A.A.A.; Project administration, J.M.; Funding acquisition, J.M. All authors have read and agreed to the published version of the manuscript.
Funding
A.C.M. is thankful for funds from ANID-ANILLO ATE220014. J.M. is thankful for funds from the ANID-ANILLO ATE220014, ANID-FONDECYT 1220228, and ANID-FONDEF ID23I10085 projects.
Data Availability Statement
Data and materials are available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhu, M.; Kurniawan, T.A.; Duan, L.; Song, Y.; Hermanowicz, S.W.; Othman, M.H.D. Advances in BiOX-Based Ternary Photocatalysts for Water Technology and Energy Storage Applications: Research Trends, Challenges, Solutions, and Ways Forward. Rev. Environ. Sci. Biotechnol. 2022, 21, 331–370. [Google Scholar] [CrossRef]
- Hassan, J.Z.; Raza, A.; Qumar, U.; Li, G. Recent advances in engineering strategies of Bi-based photocatalysts for environmental remediation. Sustain. Mater. Technol. 2022, 33, e00478. [Google Scholar] [CrossRef]
- Zhu, G.; Hojamberdiev, M.; Zhang, S.; Din, S.T.U.; Yang, W. Enhancing the visible-light-induced photocatalytic activity of BiOI microspheres for NO removal by synchronous coupling with Bi-metal and graphene. Appl. Surf. Sci. 2019, 467–468, 968–978. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, M.; Huang, T.; Huang, Y.; Cao, J.; Li, H.; Ho, W.; Lee, S.C. Oxygen vacancy-dependent photocatalytic activity of well-defined Bi2Sn2O7−x hollow nanocubes for NOx removal. Environ. Sci. Nano 2021, 8, 1927–1933. [Google Scholar] [CrossRef]
- Nzaba, S.K.M.; Mmelesi, O.K.; Malefane, M.E.; Mafa, P.J.; Mamba, B.B.; Kuvarega, A.T. Comparative study of visible-light active BiOI and N,Pd-TiO2 photocatalysts: Catalytic ozonation for dye degradation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133167. [Google Scholar] [CrossRef]
- Huang, S.; Zhong, J.; Li, J.; Chen, J.; Xiang, Z.; Li, M.; Liao, Q. Charge separation and photocatalytic properties of BiOI prepared by ionic liquid-assisted hydrothermal method. Mater. Lett. 2016, 183, 248–250. [Google Scholar] [CrossRef]
- Zhan, F.; Wen, G.; Li, R.; Feng, C.; Liu, Y.; Liu, Y.; Zhu, M.; Zheng, Y.; Zhao, Y.; La, P. A comprehensive review of oxygen vacancy modified photocatalysts: Synthesis, characterization, and applications. Phys. Chem. Chem. Phys. 2024, 26, 11182–11207. [Google Scholar] [CrossRef]
- Castillo-Cabrera, G.X.; Espinoza-Montero, P.J.; Alulema-Pullupaxi, P.; Mora, J.R.; Villacís-García, M.H. Bismuth Oxyhalide-Based Materials (BiOX: X = Cl, Br, I) and Their Application in Photoelectrocatalytic Degradation of Organic Pollutants in Water: A Review. Front. Chem. 2022, 10, 900622. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Ibañez, P.F.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewater. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yao, H.; Dang, L.; Li, Z. Efficient decomposition of organic compounds and reaction mechanism with BiOI photocatalyst under visible light irradiation. J. Mol. Catal. A Chem. 2011, 334, 116–122. [Google Scholar] [CrossRef]
- Sun, L.; Xiang, L.; Zhao, X.; Jia, C.-J.; Yang, J.; Jin, Z.; Cheng, X.; Fan, W. Enhanced visible-light photocatalytic activity of BiOI/BiOCl heterojunctions: Key role of crystal facet combination. ACS Catal. 2015, 5, 3540–3551. [Google Scholar] [CrossRef]
- Narenuch, T.; Senasu, T.; Chankhanittha, T.; Nanan, S. Sunlight-Active BiOI Photocatalyst as an Efficient Adsorbent for the Removal of Organic Dyes and Antibiotics from Aqueous Solutions. Molecules 2021, 26, 5624. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, T.-T.; Ren, H.-T.; Zhang, X.; Shen, B.; Lin, J.-H.; Lou, C.-W. Construction of BiOI/TiO2 Flexible and Hierarchical S-Scheme Heterojunction Nanofibers Membranes for Visible-Light-Driven Photocatalytic Pollutants Degradation. Sci. Total Environ. 2022, 806, 150698. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, T.; Li, K.; Huang, P.; Li, W.; Zhuo, Y.; Liu, K.; Yang, Z.; Han, D. Photocatalytic Enhancement and Recyclability in Visible-Light-Responsive 2D/2D g-C3N4/BiOI p-n Heterojunctions via a Z-Scheme Charge Transfer Mechanism. Molecules 2024, 29, 5418. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; García, A.; Park, S.-E. Ti-Containing Mesoporous Silica for Methylene Blue Photodegradation. Appl. Catal. A Gen. 2011, 393, 359–366. [Google Scholar] [CrossRef]
- Rangel-Mendez, J.R.; Matos, J.; Cházaro-Ruiz, L.F.; González-Castillo, A.C.; Barrios-Yáñez, G. Microwave-assisted synthesis of C-doped TiO2 and ZnO hybrid nanostructured materials as quantum-dot sensitized solar cells. Appl. Surf. Sci. 2018, 434, 744–755. [Google Scholar] [CrossRef]
- Chin, C.D.-W.; Treadwell, L.J.; Wiley, J.B. Microwave synthetic routes for shape-controlled catalyst nanoparticles and nanocomposites. Molecules 2021, 26, 3647. [Google Scholar] [CrossRef]
- Dai, W.-W.; Zhao, Z.-Y. Structural and electronic properties of low-index stoichiometric BiOI surfaces. Mater. Chem. Phys. 2017, 193, 164–176. [Google Scholar] [CrossRef]
- Niu, J.; Dai, P.; Wang, K.; Zhang, Z.; Zhang, Q.; Yao, B.; Yu, X. Microwave-assisted synthesis of highly efficient α-Fe2O3/BiOI composites and its performance in photocatalytic degradation of organic pollutants. Adv. Powder Technol. 2020, 31, 2327–2336. [Google Scholar] [CrossRef]
- Jamil, S.; Sabir, M.I.; Jing, X.; Wang, J.; Ge, L.; Wang, J.; Zhang, M. Microwave assisted solvothermal synthesis of magnetic Fe3O4 micro spheres and spherical aggregates at low temperature. Integr. Ferroelectr. 2011, 127, 193–198. [Google Scholar] [CrossRef]
- Rodrigues, R.A.; Machado de Campos, M.B.; Tonello, P.S. Degradation of Phenolic Compounds and Organic Matter from Real Winery Wastewater by Fenton and Photo-Fenton Processes Combined with Ultrasound. Water 2025, 17, 763. [Google Scholar] [CrossRef]
- Gómez-García, M.A.; Pitchon, V.; Kiennemann, A. Pollution by nitrogen oxides: An approach to NOx abatement by using sorbing catalytic materials. Environ. Int. 2005, 31, 445–467. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Zhao, H.; Dai, Z.; Cheng, G.; Chen, R. Mediation of valence band maximum of BiOI by Cl incorporation for improved oxidation power in photocatalysis. Ind. Eng. Chem. Res. 2016, 55, 4969–4978. [Google Scholar] [CrossRef]
- Long, Y.; Wang, Y.; Zhang, D.; Ju, P.; Sun, Y. Facile synthesis of BiOI in hierarchical nanostructure preparation and its photocatalytic application to organic dye removal and biocidal effect of bacteria. J. Colloid Interface Sci. 2016, 481, 47–56. [Google Scholar] [CrossRef]
- Liao, H.; Li, Z.; Luo, L.; Zhong, J.; Li, J. Water hyacinth powder-assisted preparation of defects-rich and flower-like BiOI/Bi5O7I heterojunctions with excellent visible light photocatalytic activity. Surf. Interfaces 2021, 27, 101470. [Google Scholar] [CrossRef]
- Khan, A.; Gao, L.; Numan, A.; Khan, S.; Hussain, I.; Sajjad, M.; Zhao, G. Recent advancements in the tailoring of nanomaterials via microwave-assisted synthesis: A comprehensive review. Crit. Rev. Solid State Mater. Sci. 2025, 1–24. [Google Scholar] [CrossRef]
- Praxedes, F.R.; Nobre, M.A.L.; Olean-Oliveira, A.; Portugal, M.L.; Poon, P.S.; Teixeira, M.F.S.; Lanfredi, S.; Matos, J. Photoelectrocatalytic oxygen evolution reaction on visible-light irradiated W-doped alkali niobate-based perovskite. Appl. Catal. A Gen. 2023, 659, 119171. [Google Scholar] [CrossRef]
- Praxedes, F.R.; Nobre, M.A.L.; Lanfredi, S.; Poon, P.S.; Matos, J. Influence of the structural properties and W/Nb ratio upon the photocatalytic activity of tungsten-doped potassium sodium niobate-based perovskites. Mater. Res. Bull. 2025, 184, 113256. [Google Scholar] [CrossRef]
- Luo, L.; Zhong, J.; Li, J. Photocatalytic property of MWCNTs/BiOI with rich oxygen vacancies. Mater. Res. Bull. 2022, 150, 111763. [Google Scholar] [CrossRef]
- Hitha, H.; Mathew, J.; Jose, J.A.; Kuriakose, S.; Varghese, T. Influence of Bi3+ doping on structural, optical, and photocatalytic degradation properties of NiWO4 nanocrystals. J. Solid State Chem. 2021, 295, 121892. [Google Scholar] [CrossRef]
- Li, K.; Zhao, Y.; Zhang, P.; He, C.; Deng, J.; Ding, S.; Shi, W. Combined DFT and XPS investigation of iodine anions adsorption on the sulfur-terminated (001) chalcopyrite surface. Appl. Surf. Sci. 2016, 390, 412–421. [Google Scholar] [CrossRef]
- Lan, H.; Zhang, G.; Zhang, H.; Liu, H.; Liu, R.; Qu, J. Solvothermal synthesis of BiOI flower-like microspheres for efficient photocatalytic degradation of BPA under visible light irradiation. Catal. Commun. 2017, 98, 9–12. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y. XPS characterization of BiOI: Insights into the oxidation states of bismuth and iodine. J. Surf. Sci. Technol. 2022, 60, 250–261. [Google Scholar]
- Lee, W.W.; Lu, C.-S.; Chuang, C.-W.; Chen, Y.-J.; Fu, J.-Y.; Siao, C.-W.; Chen, C.-C. Synthesis of bismuth oxyiodides and their composites: Characterization, photocatalytic activity, and degradation mechanisms. RSC Adv. 2015, 5, 23450–23463. [Google Scholar] [CrossRef]
- Yan, T.; Sun, M.; Liu, H.; Wu, T.; Liu, X.; Yan, Q.; Xu, W.; Du, B. Fabrication of hierarchical BiOI/Bi2MoO6 heterojunction for degradation of bisphenol A and dye under visible light irradiation. J. Alloys Compd. 2015, 634, 223–231. [Google Scholar] [CrossRef]
- Sultana, S.; Mansingh, S.; Parida, K.M. Facile Synthesis of CeO2 Nanosheets Decorated upon BiOI Microplate: A Surface Oxygen Vacancy Promoted Z-Scheme-Based 2D-2D Nanocomposite Photocatalyst with Enhanced Photocatalytic Activity. J. Phys. Chem. C 2018, 122, 808–819. [Google Scholar] [CrossRef]
- Ajin, V.C.A.; Lenus, A.J. Engineering the Role of Oxygen Vacancies in Photocatalysts for Environmental Remediation and Energy Conversion Applications: A Comprehensive Review. Mater. Sci. Semicond. Process. 2025, 197, 109705. [Google Scholar] [CrossRef]
- Smal, I.M.; Yu, Q.; Veneman, R.; Fränzel-Luiten, B.; Brilman, D.W.F. TG-FTIR Measurement of CO2-H2O co-adsorption for CO2 air capture sorbent screening. Energy Procedia 2014, 63, 6834–6841. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Thommes, M.; Smarsly, B.; Groenewolt, M.; Ravikovitch, P.I.; Neimark, A.V. Adsorption hysteresis of nitrogen and argon in pore networks and characterization of novel micro- and mesoporous silicas. Langmuir 2006, 22, 756–764. [Google Scholar] [CrossRef]
- Helmich, M.; Luckas, M.; Pasel, C.; Bathen, D. Characterization of microporous activated carbons using molecular probe method. Carbon 2014, 74, 22–31. [Google Scholar] [CrossRef]
- Matos, J.; Samudio-González, D.; Blanco, E.; Poon, P.S.; Escalona, N. Alkali-driven selectivity of products on carbon-supported Ni-based catalysts during the HDO of guaiacol. Fuel 2024, 374, 132442. [Google Scholar] [CrossRef]
- Wang, X.; Pehkonen, S.O.; Rämö, J.; Väänänen, M.; Highfield, J.G.; Laasonen, K. Experimental and computational studies of nitrogen doped Degussa P25 TiO2: Application to visible-light driven photo-oxidation of As(III). Catal. Sci. Technol. 2012, 2, 784–793. [Google Scholar] [CrossRef]
- Fernández de Cordoba, M.C.; Matos, J.; Montaña, R.; Poon, P.S.; Lanfredi, S.; Praxedes, F.R.; Hernández-Garrido, J.C.; Calvino, J.J.; Rodríguez-Aguado, E.; Rodríguez-Castellón, E.; et al. Sunlight photoactivity of rice husks-derived biogenic silica. Catal. Today 2019, 328, 125–135. [Google Scholar] [CrossRef]
- Pourshirband, N.; Nezamzadeh-Ejhieh, A. A Z-scheme AgI/BiOI binary nanophotocatalyst for the Eriochrome Black T photodegradation: A scavenging agents study. Mater. Res. Bull. 2022, 148, 111689. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Shi, X.; Chen, X.; Chen, X.; Zhou, S.; Lou, S. Solvothermal synthesis of BiOI hierarchical spheres with homogeneous sizes and their high photocatalytic performance. Mater. Lett. 2012, 68, 296–299. [Google Scholar] [CrossRef]
- Kwolek, P.; Szaciłowski, K. Photoelectrochemistry of n-Type Bismuth Oxyiodide. Electrochim. Acta 2013, 104, 448–453. [Google Scholar] [CrossRef]
- Dai, W.-W.; Zhao, Z.-Y. Electronic Structure and Optical Properties of BiOI as a Photocatalyst Driven by Visible Light. Catalysts 2016, 6, 133. [Google Scholar] [CrossRef]
- Lal, S.; Righetto, M.; Ulatowski, A.M.; Motti, S.G.; Sun, Z.; MacManus-Driscoll, J.L.; Hoye, R.L.Z.; Herz, L.M. Bandlike Transport and Charge-Carrier Dynamics in BiOI Films. J. Phys. Chem. Lett. 2023, 14, 6620–6629. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S.; Wei, Q. Porous BiOI Sonocatalysts: Hydrothermal Synthesis, Characterization, Sonocatalytic, and Kinetic Properties. Ind. Eng. Chem. Res. 2012, 51, 1193–1197. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Hurum, D.C.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Recombination Pathways in the Degussa P25 Formulation of TiO2: Surface versus Lattice Mechanisms. J. Phys. Chem. B 2005, 109, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yu, J.C.; Fan, C.; Wen, H.; Hu, S. Synthesis and characterization of Pt/BiOI nanoplate catalyst with enhanced activity under visible light irradiation. Mater. Sci. Eng. B 2010, 166, 213–219. [Google Scholar] [CrossRef]
- He, R.; Cao, S.; Yu, J.; Yang, Y. Microwave-Assisted Solvothermal Synthesis of Bi4O5I2 Hierarchical Architectures with High Photocatalytic Performance. Catal. Today 2016, 264, 221–228. [Google Scholar] [CrossRef]
- Gong, J.; Imbault, A.; Farnood, R. The promoting role of bismuth for the enhanced photocatalytic oxidation of lignin on Pt-TiO2 under solar light illumination. Appl. Catal. B 2017, 204, 296–303. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Byrne, J.A.; O’Shea, K.; Entezari, M.H.; et al. A Review on the Visible Light Active Titanium Dioxide Photocatalysts for Environmental Applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Mikhaylov, R.V.; Lisachenko, A.A.; Titov, V.V. Investigation of photostimulated oxygen isotope exchange on TiO2 Degussa P25 surface upon UV–Vis irradiation. J. Phys. Chem. C 2012, 116, 23332–23341. [Google Scholar] [CrossRef]
- Diebold, U. The Surface Science of Titanium Dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Tao, H.; Liu, Y. Dynamic adsorption/desorption of NOx on MFI zeolites: Effects of relative humidity and Si/Al ratio. Nanomaterials 2023, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Arcibar-Orozco, J.; Poon, P.S.; Pecchi, G.; Rangel-Mendez, J.R. Influence of phosphorous upon the formation of DMPO-•OH and POBN-O2•¯ spin-trapping adducts in carbon-supported P-promoted Fe-based photocatalysts. J. Photochem. Photobiol. A Chem. 2020, 391, 112362. [Google Scholar] [CrossRef]
- Dalton, J.S.; Janes, P.A.; Jones, N.G.; Nicholson, J.A.; Hallam, K.R.; Allen, G.C. Photocatalytic Oxidation of NOx Gases Using TiO2: A Surface Spectroscopic Approach. Environ. Pollut. 2002, 120, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Shen, B.; Adwek, G.; Xiong, L.; Liu, L.; Yuan, P.; Gao, H.; Liang, C.; Guo, Q. Review on the NO Removal from Flue Gas by Oxidation Methods. J. Environ. Sci. 2021, 101, 49–71. [Google Scholar] [CrossRef]
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Mater. 2017, 3, 3–16. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous Photocatalysis: Fundamentals and Applications to the Removal of Various Types of Aqueous Pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Edelmannová, M.; Reli, M.; Kočí, K.; Papailias, I.; Todorova, N.; Giannakopoulou, T.; Dallas, P.; Devlin, E.; Ioannidis, N.; Trapalis, C. Photocatalytic Reduction of CO2 over Iron-Modified g-C3N4 Photocatalysts. Photochem 2021, 1, 462–476. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Microporous Mesoporous Mater. 2005, 82, 1–78. [Google Scholar] [CrossRef]
- Xie, W.; Li, R.; Xu, Q. Enhanced Photocatalytic Activity of Se-Doped TiO2 under Visible Light Irradiation. Sci. Rep. 2018, 8, 8752. [Google Scholar] [CrossRef]
- Niu, J.; Dai, P.; Zhang, Q.; Yao, B.; Yu, X. Microwave-assisted solvothermal synthesis of novel hierarchical BiOI/rGO composites for efficient photocatalytic degradation of organic pollutants. Appl. Surf. Sci. 2018, 430, 165–175. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, J.; Di, J.; Zhao, D.; Ji, M.; Xia, J.; Li, H. Facile microwave-assisted ionic liquid synthesis of sphere-like BiOBr hollow and porous nanostructures with enhanced photocatalytic performance. Green Energy Environ. 2017, 2, 124–133. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Visible-light active titanium dioxide nanomaterials with bactericidal properties. Nanomaterials 2020, 10, 124. [Google Scholar] [CrossRef]
- Pawar, T.J.; Contreras López, D.; Olivares Romero, J.L.; Vallejo Montesinos, J. Surface modification of titanium dioxide. J. Mater. Sci. 2023, 58, 6887–6930. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, W.-D. Hydrothermal synthesis and photocatalytic performance of hierarchical Bi2MoO6 microspheres using BiOI microspheres as self-sacrificing templates. J. Solid State Chem. 2015, 227, 247–254. [Google Scholar] [CrossRef]
- Mera, A.C.; Rodríguez, C.A.; Meléndrez, M.F.; Valdés, H. Synthesis and Characterization of BiOI Microspheres under Standardized Conditions. J. Mater. Sci. 2017, 52, 944–954. [Google Scholar] [CrossRef]
- Dong, G.; Ho, W.; Zhang, L. Photocatalytic NO Removal on BiOI Surface: The Change from Nonselective Oxidation to Selective Oxidation. Appl. Catal. B Environ. 2015, 168–169, 490–496. [Google Scholar] [CrossRef]
- Nie, Q.; Jia, L.; Zhang, G.; Xie, J.; Liu, J. Micro-spherical BiOI photocatalysts for efficient degradation of residual xanthate and gaseous nitric oxide. Nanomaterials 2024, 14, 576. [Google Scholar] [CrossRef]
- Mera, A.C.; Martínez-de la Cruz, A.; Pérez-Tijerina, E.; Meléndrez, M.F.; Valdés, H. Nanostructured BiOI for air pollution control: Microwave-assisted synthesis, characterization and photocatalytic activity toward NO transformation under visible light irradiation. Mater. Sci. Semicond. Process 2018, 88, 20–27. [Google Scholar] [CrossRef]
- Zhang, B.; Ji, G.; Gondal, M.A.; Liu, Y.; Zhang, X.; Chang, X.; Li, N. Rapid adsorption properties of flower-like BiOI nanoplates synthesized via a simple EG-assisted solvothermal process. J. Nanopart. Res. 2013, 15, 1773. [Google Scholar] [CrossRef]
- Nava Núñez, M.Y.; Martínez-de la Cruz, A.; López-Cuéllar, E. Preparation of BiOI microspheres in 2-propanol/ethylene glycol by microwave method with high visible-light photocatalytic activity. Res. Chem. Intermed. 2019, 45, 1475–1492. [Google Scholar] [CrossRef]
- Ao, Y.; Xu, J.; Wang, P.; Wang, C.; Hou, J.; Qian, J. Enhanced Photocatalytic Activity of BiOI Microspheres by Coupling with Graphene Oxide under Visible Light Irradiation. Appl. Surf. Sci. 2013, 276, 390–396. [Google Scholar] [CrossRef]
- Montoya-Zamora, J.M.; Martínez-de la Cruz, A.; López-Cuéllar, E. Synthesis of BiOI photocatalyst by microwave method using EDTA as retarder of the reaction. Res. Chem. Intermed. 2017, 43, 2545–2563. [Google Scholar] [CrossRef]
- Hu, P.; Hou, D.; Shi, H.; Chen, C.; Huang, Y.; Hu, X. Microwave-Assisted Synthesis of Self-Assembled BiO1.84H0.08 Hierarchical Nanostructures as a New Photocatalyst. Appl. Surf. Sci. 2014, 319, 244–249. [Google Scholar] [CrossRef]
- Gulab, H.; Fatima, N.; Tariq, U.; Gohar, O.; Irshad, M.; Khan, M.Z.; Saleem, M.; Ghaffar, A.; Hussain, M.; Jan, A.K.; et al. Advancements in Zinc Oxide Nanomaterials: Synthesis, Properties, and Diverse Applications. Nano-Struct. Nano-Objects 2024, 39, 101271. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Pereira, L.O.; Lelo, R.V.; Coelho, G.C.M.; Magalhães, F. Degradation of textile dyes from synthetic and wastewater samples using TiO2/C/Fe magnetic photocatalyst and TiO2. J. Iran. Chem. Soc. 2019, 16, 2281–2289. [Google Scholar] [CrossRef]
- Dai, D.; Qiu, J.; Xia, G.; Tang, Y.; Wu, Z.; Yao, J. Competitive Coordination Initiated One-Pot Synthesis of Core–Shell Bi-MOF@BiOX (X = I, Br and Cl) Heterostructures for Photocatalytic Elimination of Mixed Pollutants. Sep. Purif. Technol. 2023, 316, 123819. [Google Scholar] [CrossRef]
- Fang, B.; Qiu, J.; Xia, G.; Wang, M.; Dai, D.; Tang, Y.; Li, Y.; Yao, J. Carboxylated Cellulose-Derived Carbon Mediated Flower-Like Bismuth Oxyhalides for Efficient Cr(VI) Reduction under Visible Light. J. Colloid Interface Sci. 2025, 678, 125–133. [Google Scholar] [CrossRef]
- Wu, H.; Yuan, C.; Chen, R.; Wang, J.; Dong, F.; Li, J.; Sun, Y. Mechanisms of Interfacial Charge Transfer and Photocatalytic NO Oxidation on BiOBr/SnO2 p–n Heterojunctions. ACS Appl. Mater. Interfaces 2020, 12, 43741–43749. [Google Scholar] [CrossRef]
- Das, T.K.; Jesionek, M.; Mistewicz, K.; Nowacki, B.; Kępińska, M.; Zubko, M.; Godzierz, M.; Gawron, A. Ultrasonic-Assisted Conversion of Micrometer-Sized BiI3 into BiOI Nanoflakes for Photocatalytic Applications. Int. J. Mol. Sci. 2024, 25, 10265. [Google Scholar] [CrossRef]
- Mera, A.C.; Moreno, Y.; Contreras, D.; Escalona, N.; Meléndrez, M.F.; Mangalaraja, R.V.; Mansilla, H.D. Improvement of the BiOI Photocatalytic Activity Optimizing the Solvothermal Synthesis. Solid State Sci. 2017, 63, 84–92. [Google Scholar] [CrossRef]
- Nava-Núñez, M.Y.; Jimenez-Relinque, E.; Martínez-de la Cruz, A.; Castellote, M. Photocatalytic NOx Removal in Bismuth-Oxyhalide (BiOX, X = I, Cl) Cement-Based Materials Exposed to Outdoor Conditions. Catalysts 2022, 12, 982. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).