Abstract
The mechanism of the electrophilic addition between phenylacetylene and N-heterocyclic carbene borane (NHC–borane), initiated by di-tert-butyl peroxide (DTBP), was elucidated at the M06-2X-D3/ma-def2-TZVP level to yield the Z-configured product. The computational results show that DTBP undergoes homolysis to generate two t-BuO· radicals; subsequently, it undergoes an H-shift reaction with N-heterocyclic carbene borane to form the N-heterocyclic carbene boron radical. Then, it is added to phenylacetylene to obtain the product radical intermediate. Finally, the product is yielded via an H-shift reaction. Meanwhile, this paper also explores the formation pathways of relevant byproducts. Structural analysis of the reaction reveals that weak interactions have a significant impact on the selectivity of the Z-configuration of the product. In addition, electron spin density contour maps are used to explain the electron distribution and reaction sites during the reaction process. This paper will provide relevant theoretical support for this type of addition reaction.
1. Introduction
Organoboron compounds have extensive applications in synthetic chemistry, life sciences, and materials science; for example, vinyl boronate esters can serve as substrates in Suzuki–Miyaura cross-coupling reactions to generate products with a valuable boronate esters group [1], and alkenyl borides can also provide an efficient synthetic route for the rapid acquisition of γ-aminoboronate esters in the field of organic synthesis [2]. In addition, organoboron compounds are critical precursors in pharmaceutical development, frequently participating in enzyme inhibitor synthesis for disease treatment [3,4,5].
A persistent focus has been developing efficient methods for constructing carbon–boron (C-B) bonds in synthetic chemistry. Unsaturated systems’ borylation reactions offer critical approaches to synthesize boron-containing compounds with unique structures and properties, forming the material foundation for related fields.
While the hydroboration reaction between trivalent boranes and alkynes makes it possible to form enynylboron compounds [6,7,8,9,10,11], only cis-adducts are readily accessible due to the syn-selective nature of typical trivalent borane-induced concerted hydroborations. Miyaura and colleagues first demonstrated the rhodium- and iridium-catalyzed hydroboration of terminal alkynes, achieving trans-addition products with (Z)-1-alkenylboron compounds. Subsequent studies explored other transition metals (e.g., ruthenium, iridium, cobalt) for alkyne borylation, yet most yielded cis-structured products [12,13,14,15,16,17,18,19] (Scheme 1). Notably, Wang Suning’s group later achieved the trans-hydroboration of 2-pyridyl alkynes with 9-borabicyclo [3.3.1]nonane (9-BBN) under metal-free conditions [20], revealing that pyridyl groups alter stereochemical outcomes. Independently, Ingleson’s team reported trans-borylation using 9-BBN and terminal alkynes with B(C6F5)3 N-heterocyclic carbene complexes [21], leveraging strong electrophilic boron reagents to bypass metal catalysis [22,23,24,25,26].
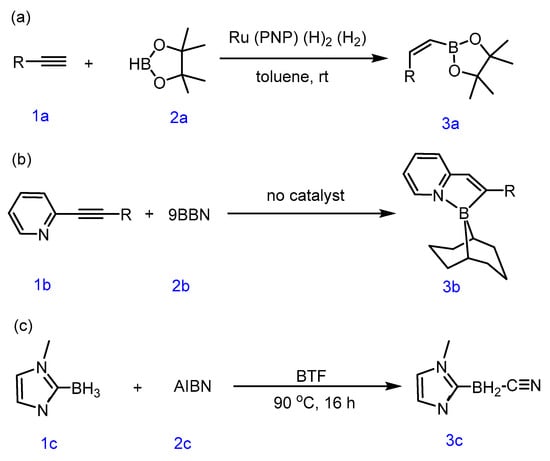
Scheme 1.
Examples of three synthetic methods, (a) Metal-catalyzed; (b) without catalyst; (c) BTF-catalyzed.
Subsequently, a highly attractive approach was proposed, namely the radical-mediated hydroboration process. Since radical participation could alter the regioselectivity of reactions, this approach holds unique advantages. However, this method is not applicable to most boranes due to the strong B-H bonds in free boranes, which typically prevent them from acting as hydrogen donors. Notably, borane complexes such as amine boranes, phosphine boranes, and NHC-BH3 can function as hydrogen donors under specific conditions to generate radicals. This hypothesis has been experimentally validated, with density functional theory (DFT) calculations providing further theoretical support [27,28,29,30,31]. In 2018, the Shimoi group demonstrated the trans-hydroboration of phenylacetylene using NHC-BH3 as the boron source and a radical initiator (e.g., di-tert-butyl peroxide) [32] (Scheme 2). The reaction proceeds via hydrogen abstraction by the initiator to form a boron-centered radical intermediate, which subsequently participates in anti-Markovnikov hydroboration. Despite experimental success, the detailed mechanistic pathway remains unclear, necessitating further investigation to elucidate the origin of stereochemical control [33,34,35,36,37,38,39] and guide future reaction design.
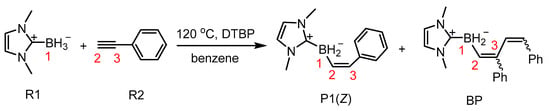
Scheme 2.
Total reactions from R1 + R2 → P1(Z) (BP2(ZZ), BP3(ZE), BP4(ZZ), BP5(ZE), or BP6).
2. Results and Discussion
This paper focuses on investigating the reaction process of R1 (N-heterocyclic carbene borane) with R2 (phenylacetylene) in benzene solvent at 120 °C, initiated by DTBP (di-tert-butyl peroxide), which yields the Z-isomer product P1((Z)-N,N-Dimethylimidazolylborane-Styrene) (Scheme 3). A detailed discussion of the underlying mechanism is provided in the subsequent sections.
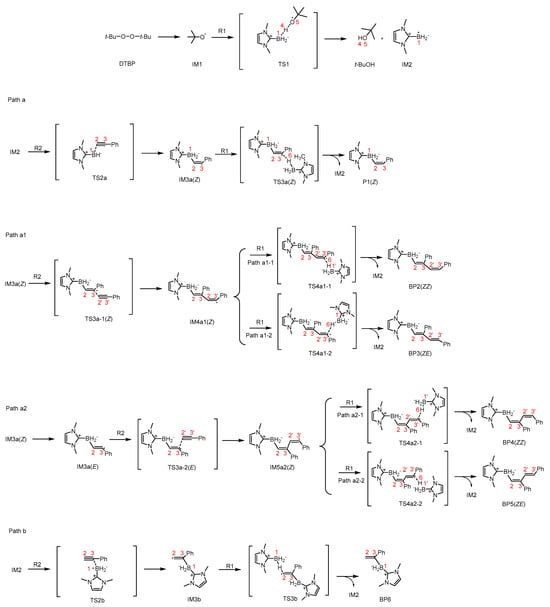
Scheme 3.
The detailed reaction processes from R1 + R2 to P1(Z) (BP2(ZZ), BP3(ZE), BP4(ZZ), BP5(ZE), or BP6).
2.1. DTBP→IM1
DTBP, a commonly used radical initiator, readily undergoes homolytic cleavage to generate radicals (Scheme 3). As indicated by the scanning process in Figure 1, this homolytic process proceeds without a transition state, directly forming two IM1 ((Z)-N,N-Dimethylimidazolylborane-Styrene) radicals. The homolysis is one endothermic process, and the energy of DTBP is at the zero point to draw the Gibbs-free energy profiles. The electron spin density contour map of IM1 (Figure 2a) reveals that the single electron is predominantly localized on the O atom.
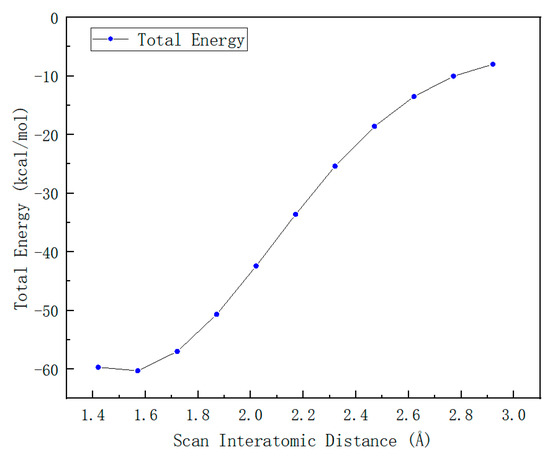
Figure 1.
Scanning of the energy change process during DTBP homolysis.
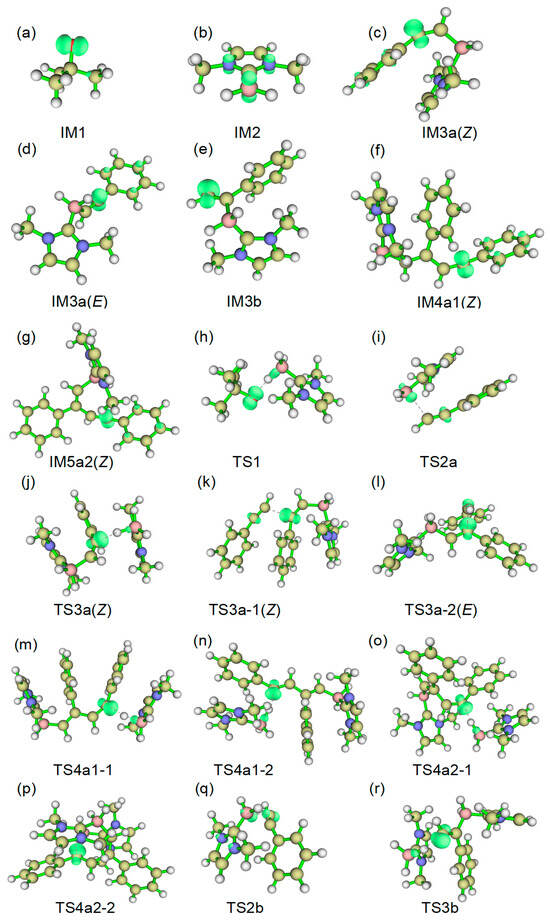
Figure 2.
The electron spin density isosurface graphs of some structures in Path a and Path b; the threshold for generating the isosurfaces is 0.02000 e/Å3, (a) IM1; (b) IM2; (c) IM3a(Z); (d) IM3a(E); (e) IM3b; (f) IM4a1(Z); (g) IM5a2(Z); (h) TS1; (i) TS2a; (j) TS3a(Z); (k) TS3a-1(Z); (l) TS3a-2(E); (m) TS4a1-1; (n) TS4a1-2; (o) TS4a2-1; (p) TS4a2-2; (q) TS2b; (r) TS3b.
2.2. IM1→IM2
The obtained IM1, acting as a radical, can undergo hydrogen atom transfer with R1, as shown in Figure 3. According to the calculation results in Figure 3, IM1 and R1 absorb 10.9 kcal/mol energy to overcome the energy barrier, proceeding through transition state TS1 to generate intermediate IM2 (N-heterocyclic carbene boron radical) and byproduct t-BuOH (tert-butanol). Furthermore, Figure 2a,b,g indicate that during this process, the unpaired electron transfers from the O5 atom in IM1 to the B1 atom in IM2, with partial unpaired electron distribution also observed on the imidazole ring. The plausible reason is that the B atom forms a planar structure with the imidazole ring, which facilitates the delocalization of the unpaired electron. The total energy of IM2 and t-BuOH is 25.5 kcal/mol lower than that of IM1 and R1, indicating that this process is exothermic and also indirectly confirming that the unpaired electron is more stable in IM2 than in IM1. The calculation of the potential energy surface and the relevant data can be found in the Supplementary Materials.
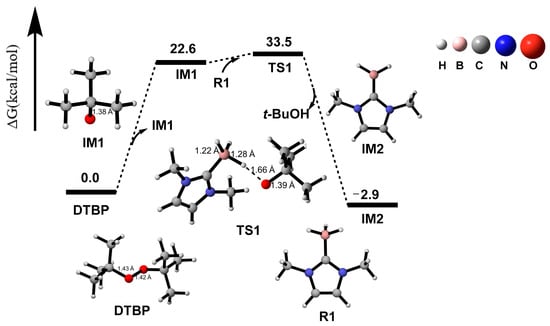
Figure 3.
Gibbs-free energy surfaces from DTBP to IM2.
2.3. Path a
Based on the structure of R2, its reaction sites are primarily concentrated on the C2 and C3 atoms of C2≡C3 triple bond. Hence, two paths (a and b) were designed. In Path a, the B1 atom of IM2 reacts with the C2 atom of R2 via transition state TS2a to form the addition product intermediate IM3a(Z)((Z)-1-(2-phenylethenyl)-1H-imidazole radical). The calculated results show that this process requires 12.3 kcal/mol of energy (Figure 4). In the analysis of IRI, the blue region represents notable attraction and the red region represents notable repulsion in Figure 5. IRI analysis (Figure 5) of the IM3a(Z) structure reveals that there are large and relatively deep green regions between the imidazole ring and the benzene ring, indicating the presence of numerous weak interactions that enable IM3a(Z) to maintain the Z configuration and resist E/Z conformational conversion. This is consistent with experimental observations where only the Z-configured product was detected. Additionally, this addition process is exothermic, suggesting that the addition product intermediate is relatively stable. Electron spin density analysis of IM3a(Z) indicates that the unpaired electron successfully transfers to the C3 atom in IM3a(Z), with a certain probability of distribution also observed on the benzene ring. Finally, IM3a(Z) undergoes a hydrogen atom transfer reaction with R1 to generate product P1(Z), a process that requires the absorption of 17.2 kcal/mol of energy via transition state TS3a(Z). This process not only achieves the formation of product P1(Z) but also simultaneously activates R1, facilitating the continuation of subsequent reactions.
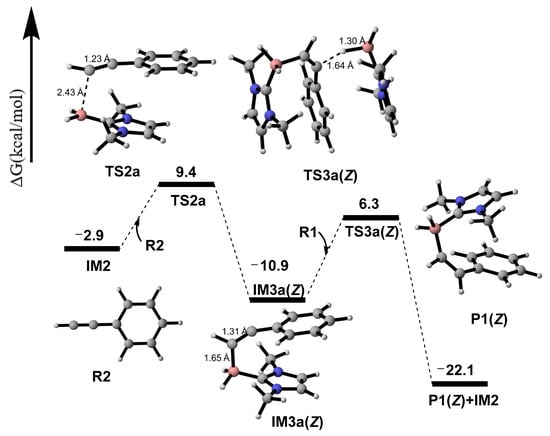
Figure 4.
Gibbs-free energy surfaces from R2 + IM2 to P1(Z).
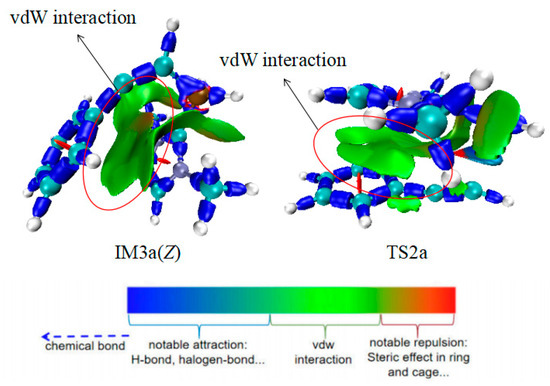
Figure 5.
IRI of IM3a(Z) and TS2a; the red-lineed circle represents the vdW interaction.
2.3.1. Path a1
However, according to relevant literature, byproducts are also generated in this process. From the structure of IM3a(Z), it is known that the presence of weak interactions enables it to maintain the Z-configuration, while the unpaired electron is distributed on the C3 atom. Meanwhile, it may also undergo further bonding reactions with the electron-rich R2 structure. Thus, Path a1 is designed for the addition reaction between IM3a(Z) and R2, which requires absorbing 17.6 kcal/mol energy to overcome the barrier. In the transition state TS3a1(Z), the distance between C3 and C2′ is 2.31 Å. The energy of the addition intermediate IM4a1(Z) is 20.1 kcal/mol lower than that of IM3a(Z)+R2, indicating that this step is an exothermic process. Subsequently, IM4a1(Z) forms byproducts BP2(ZZ) and BP3(ZE) via Paths a1-1 and a1-2, respectively. The calculation results show that the Gibbs energy barrier for the reaction pathway IM4a1(Z) + R1 → TS4a1-1(Z) → BP2(ZZ) + IM2 is 17.3 kcal/mol, whereas the pathway IM4a1(Z) + R1 → TS4a1-2(Z) → BP3(ZE) + IM2 exhibits a higher barrier of 19.2 kcal/mol. Although the BP2(ZZ)-forming pathway has a lower energy barrier, the energy of byproduct BP3(ZE) is 9.4 kcal/mol lower than that of BP2(ZZ). If the reaction is thermodynamically controlled, BP3(ZE) would dominate due to its lower energy, whereas kinetic control would favor BP2(ZZ) accumulation. Considering that both pathways have relatively low energy barriers, both byproducts BP2(ZZ) and BP3(ZE) are expected to coexist.
2.3.2. Path a2
While in path a2, the intermediate IM3a(Z) would absorb 1.5 kcal/mol of energy to break the weak interaction between imidazole ring and benzene ring to generate intermediate IM3a(E) ((E)-1-(2-phenylethenyl)-1H-imidazole), which goes on to have an addition reaction to react with R2. The calculation in Figure 6 displays that this addition reaction has an energy barrier of 19.0 kcal/mol, and the IM3a(E)+R2→TS3a-2(E)→IM5a2(E) process releases about 18.5 kcal/mol of energy. Finally, the obtained IM5a2(E) has two possible paths (a2-1 and a2-2) to yield the byproducts BP4(ZZ) and BP5(ZE). Comparing the IM5a2(E)→BP4(ZZ) process and IM5a2(E)→BP5(ZE) process, it is clear that IM5a2(E)→BP4(ZZ) has an energy barrier of 17.4 kcal/mol, which is 0.8 kcal/mol lower than that of the IM5a2(E)→BP5(ZE) process. So, the dynamic factor tells us that BP4(ZZ) is favorable.
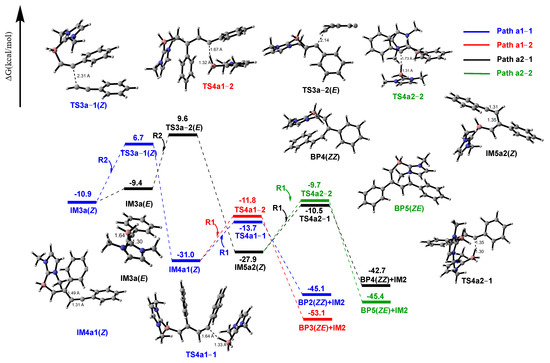
Figure 6.
Gibbs-free energy surfaces from R2+IM3a(Z) to BP2(ZZ) + BP3(ZE) + BP4(ZZ) + BP5(ZE).
All the Gibbs-free energy surfaces in Figure 6 show that the highest point for the E-configuration is 9.6 kcal/mol, 2.9 kcal/mol higher than that for Z-configuration, suggesting that IM3a(Z) has the priority to react with R2 with a lower energy barrier (blue line). While the analysis of Figure 4 and Figure 6 points out that the IM3a(Z) and R1 has an energy barrier of 17.2 kcal/mol, IM3a(Z) and R2 (blue line) have an energy barrier of 17.6 kcal/mol, implying that the process for P1(Z) in Figure 4 is favorable and P1(Z) is the main product. Moreover, the laboratory measures that the productivity of P1(Z) is 15%, but the productivity of the byproducts (BP2, BP3, BP4, and BP5 are isomers) was not measured. The productivity of P1(Z) is low, which is consistent with the computational results that the energy barriers (17.2 kcal/mol (Path a), 17.6 kcal/mol,(Path a1), 20.5 kcal/mol (Path a2)) in Figure 4 and Figure 6 are relatively close to each other within the error margin.
2.4. Path b
When the addition site becomes the C3 atom from the C2 atom in R2 in Scheme 3 the computational results show that the energy barrier would increase to 21.0 kcal/mol via transition state TS2b in Figure 7, 8.7 kcal/mol higher than that of TS2a in Figure 4, implying that the C2 atom of R2 is the first choice for the addition reaction. Moreover, the IM2+R2→IM3b process is an endothermic step, and 4.0 kcal/mol energy is required. Meanwhile, the IM2+R2 → IM3a(Z) process in Figure 4 is an exothermic procedure. The reason for this could be that the single electron in IM3a(Z) could have a wider motion range in Figure 2c than that of IM3b in Figure 2e. The formed intermediate IM3b continues through an H-shift reaction to obtain the BP6. The calculated energy barrier of the IM3b+R1→BP6 procedure is 13.7 kcal/mol. Comparing Path a in Figure 4 with Path b in Figure 7, it is obvious that Path a is favorable and P1(Z) is the main product. The dynamic and thermodynamic analyses conclude that the productivity of BP6 does not exist.
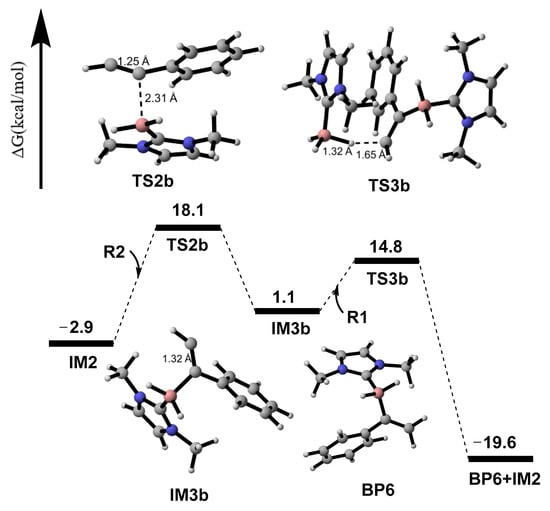
Figure 7.
Gibbs-free energy surfaces from R2 + IM2 → BP6.
3. Computational Details
All the structural calculations in this paper were performed using the Gaussian 09 [40] program package. The reactive sites and other atoms were calculated at the level of M06-2X-D3 [41,42,43,44]/ma-def2-SVP and M06-2X-D3 [41,42,43,44]/6-31G(d), respectively, to obtain accurate energies. In particular, all structures were calculated at 120 °C with the M06-2X-D3/ma-def2-SVP and M06-2X-D3/6-31G (d) methods to obtain accurate thermal free energy corrections. The M06-2X-D3 [41,42,43,44]/ma-def2-TZVP method/basis set was used to calculate the single point energy for all structures, which has been proved to be a powerful method to obtain accurate results in the process for investigating the mechanisms of organic chemistry. The SMD (solvation model based on solute electron density) model [42] was adopted to simulate the solvent effect of benzene. The calculation results show that each transition state has only one imaginary frequency, while no imaginary frequencies were observed for reactants, intermediates, or products. Intrinsic reaction coordinate (IRC) [45,46] calculations were performed for each transition state to confirm their correctness. The 3D structures presented in this paper were visualized using CYLview (version 1.0b) [47] (Claude Legault). All electron spin density contour maps were generated using Multiwfn (version 3.8) [48,49] software. In this study, the colored green isosurfaces denote the regions of vdW interactions, and the configuration attains relatively high stability owing to these weak interactions. The IRI [50] graphs were formed with Multiwfn (version 3.8) and VMD software (version 1.9.3) [51].
4. Conclusions
In summary, this study systematically investigated the activation and selective addition reaction mechanism of phenylacetylene with N-heterocyclic carbene boranes, using di-tert-butyl peroxide (DTBP) as a radical initiator, at the M06-2X-D3/ma-def2-TZVP level. The computational results revealed that DTBP undergoes homolytic cleavage to generate tert-butoxy radicals (t-BuO·), which subsequently participate in an H-shift reaction with N-heterocyclic carbene boranes to form N-heterocyclic carbene boron radical. The obtained radical undergoes electrophilic addition to the C2≡C3 of phenylacetylene to form intermediate products, which has a hydrogen atom transfer to yield the final products. The formation of different configurational products (e.g., Z/E isomers) arises from variations in the addition sites and hydrogen atom attack directions. The experimental results show that the main product P1(Z) has been yielded, and the byproducts (BP2, BP3, BP4, and BP5) have also been yielded. The computational Gibbs-free energy surface demonstrated that the major product P1(Z) has a rather low energy barrier, consistent with the experimental observations. Meanwhile, the formations of byproducts BP2-5 have rather higher energy. However, these energy barriers are close to each other within the error margin, which means that P1(Z) and byproducts BP2-5 could exist in the system, agreeing with the experimental phenomena. Moreover, the yield of BP6 has the highest energy barrier, suggesting that it has the lowest possibility to happen. Conformational analysis revealed that weak interactions between the imidazole ring of the N-heterocyclic carbene and the phenyl ring of phenylacetylene significantly influence the Z-selectivity of the products. The electron spin density contour maps provided insights into the radical transfer sites during the reaction. This mechanistic investigation not only elucidates the reaction essence theoretically but also guides experimental design for similar radical-mediated additions. Future work could extend this strategy to functionalize other unsaturated hydrocarbons.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15090867/s1, Table S1: With benzene as the solvent, the number of imaginary frequencies, Thermal Free Energy Correction, and Electronic Energy of transition states in the reaction of DTBP to IM2; Table S2: With benzene as the solvent, the number of Thermal Free Energy Correction, and Electronic Energy of reactants, intermediates and product in the reaction of DTBP to IM2; Table S3: With benzene as the solvent, the number of imaginary frequencies, Thermal Free Energy Correction, and Electronic Energy of transition states in the reaction of IM2+R2 to P1(Z)(path a); Table S4: With benzene as the solvent, the number of Thermal Free Energy Correction, and Electronic Energy of reactants, intermediates and product in the reaction of IM2+R2 to P1(Z)(path a); Table S5: With benzene as the solvent, the number of imaginary frequencies, Thermal Free Energy Correction, and Electronic Energy of transition states in the reaction of IM3a(Z)+R2 to BP2(ZZ) and BP3(ZE)(path a1); Table S6: With benzene as the solvent, the number of Thermal Free Energy Correction, and Electronic Energy of reactants, intermediates and product in the reaction of IM3a(Z)+R2 to BP2(ZZ) and BP3(ZE)(path a1); Table S7: With benzene as the solvent, the number of imaginary frequencies, Thermal Free Energy Correction, and Electronic Energy of transition states in the reaction of IM3a(Z)+R2 to BP4(ZZ) and BP5(ZE)(path a2); Table S8: With benzene as the solvent, the number of Thermal Free Energy Correction, and Electronic Energy of reactants, intermediates and product in the reaction of IM3a(Z)+R2 to BP4(ZZ) and BP5(ZE)(path a2); Table S9: With benzene as the solvent, the number of imaginary frequencies, Thermal Free Energy Correction, and Electronic Energy of transition states in the reaction of IM2+ R2 to BP6(path b); Table S10: With benzene as the solvent, the number of Thermal Free Energy Correction, and Electronic Energy of reactants, intermediates and product in the reaction of IM2+ R2 to BP6(path b).
Author Contributions
Investigation, data curation, and writing—original draft preparation, H.-W.-X.W. and X.-M.L.; writing—review and editing, L.-J.Z. and T.-T.F.; supervision and funding acquisition, D.-G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kischkewitz, M.; Okamoto, K.; Mück-Lichtenfeld, C.; Studer, A. Radical-polar crossover reactions of vinylboron ate com-plexes. Science 2017, 355, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.; Mega, R.S.; Pflästerer, D.; Myers, E.L.; Aggarwal, V.K. Visible-Light-Mediated Decarboxylative Radical Additions to Vinyl Boronic Esters: Rapid Access to γ-Amino Boronic Esters. Angew. Chem. Int. Ed. 2018, 57, 2155–2159. [Google Scholar] [CrossRef]
- Bassini, E.; Gazzotti, S.; Sannio, F.; Presti, L.L.; Sgrignani, J.; Docquier, J.D.; Grazioso, G.; Silvani, A. Isonitrile-Based Multi-component Synthesis of β-Amino Boronic Acids as β-Lactamase Inhibitors. Antibiotics 2020, 9, 21. [Google Scholar] [CrossRef]
- Dos Santos, E.M.; Silva, N.; Gonçalves, K.G.; Vale, A.A.M.; De Azevedo-Santos, A.P.S.; França, T.C.C.; LaPlante, S.R.; Resende, J.; Romeiro, N.C.; Lima, J.A.; et al. Arylboronic acids as safe and specific human butyrylcholines-terase inhibitors. J. Mol. Struct. 2023, 1290, 135932. [Google Scholar] [CrossRef]
- Tan, J.; Cognetta, A.B., III; Diaz, D.B.; Lum, K.M.; Adachi, S.; Kundu, S.; Cravatt, B.F.; Yudin, A.K. Multicomponent mapping of boron chemotypes furnishes selective enzyme inhibitors. Nat. Commun. 2017, 8, 1760. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Zhang, J.; Krummenacher, I.; Braunschweig, H.; Lin, Z. DFT Studies on the Reactions of Boroles with Alkynes. Chem. Eur. J. 2018, 24, 9612–9621. [Google Scholar] [CrossRef]
- Procter, R.J.; Uzelac, M.; Cid, J.; Rushworth, P.J.; Ingleson, M.J. Low-Coordinate NHC–Zinc Hydride Complexes Catalyze Alkyne C–H Borylation and Hydroboration Using Pinacolborane. ACS Catal. 2019, 9, 5760–5771. [Google Scholar] [CrossRef]
- Romero, E.A.; Jazzar, R.; Bertrand, G. (CAAC)CuX-catalyzed hydroboration of terminal alkynes with pinacolborane directed by the X-ligand. J. Organomet. Chem. 2016, 829, 11–13. [Google Scholar] [CrossRef]
- Ito, S.; Fukazawa, M.; Takahashi, F.; Nogi, K.; Yorimitsu, H. Sodium-Metal-Promoted Reductive 1,2-syn-Diboration of Al-kynes with Reduction-Resistant Trimethoxyborane. Bull. Chem. Soc. Jpn. 2020, 93, 1171–1179. [Google Scholar]
- Dong, W.K.; Liu, Z.M.; Zhang, M.H.; Zhang, Z.Y.; Wei, Y.H.; Liu, G.L.; Zhao, W.X. Copper-Catalyzed Regioselective Dihy-droboration of Alkynes to Construct gem-Diborylalkanes. Org. Lett. 2025, 27, 3101–3106. [Google Scholar] [CrossRef]
- Zhong, M.L.; Zhang, J.; Lu, Z.P.; Xie, Z.W. Diboration of alkenes and alkynes with a carborane-fused four-membered bora-cycle bearing an electron-precise B-B bond. Dalton Trans. 2021, 50, 17150–17155. [Google Scholar]
- Zhou, J.; Lee, C.I.; Ozerov, O.V. Computational Study of the Mechanism of Dehydrogenative Borylation of Terminal Al-kynes by SiNN Iridium Complexes. ACS Catal. 2018, 8, 536–545. [Google Scholar]
- Mao, L.; Bose, S.K. Hydroboration of Enynes and Mechanistic Insights. Adv. Synth. Catal. 2020, 362, 4174–4188. [Google Scholar] [CrossRef]
- Huang, Z.; Zuo, Z.; Wen, H.; Liu, G. Cobalt-Catalyzed Hydroboration and Borylation of Alkenes and Alkynes. Synlett 2018, 29, 1421–1429. [Google Scholar] [CrossRef]
- Foley, B.J.; Bhuvanesh, N.S.; Zhou, J.; Ozerov, O.V. Combined Experimental and Computational Studies of the Mechanism of Dehydrogenative Borylation of Terminal Alkynes Catalyzed by PNP Complexes of Iridium. ACS Catal. 2020, 10, 9824–9836. [Google Scholar] [CrossRef]
- Chen, Z.S.; Nie, B.; Li, X.N.; Liu, T.; Li, C.S.; Huang, J.Z. Ligand-controlled regiodivergent Ni-catalyzed hydroboration/carboboration of internal alkynes with B2pin2. Chem. Sci. 2024, 15, 2236–2242. [Google Scholar] [CrossRef]
- Chen, J.P.; Shen, X.Z.; Lu, Z. Cobalt-Catalyzed Markovnikov-Type Selective Hydroboration of Terminal Alkynes. Angew. Chem. Int. Ed. 2021, 60, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Corpas, J.; Mauleón, P.; Arrayas, R.G.; Carretero, J.C. Transition-Metal-Catalyzed Functionalization of Alkynes with Or-ganoboron Reagents: New Trends, Mechanistic Insights, and Applications. ACS Catal. 2021, 11, 7513–7551. [Google Scholar] [CrossRef]
- Zuo, Z.; Yang, J.; Huang, Z. Cobalt-Catalyzed Alkyne Hydrosilylation and Sequential Vinylsilane Hydroboration with Markovnikov Selectivity. Angew. Chem. Int. Ed. 2016, 128, 10997–11001. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, S. Trans-Aminoboration across Internal Alkynes Catalyzed by B(C6F5)3 for the Synthesis of Borylated In-doles. Org. Lett. 2017, 19, 1462–1465. [Google Scholar] [CrossRef]
- Fasano, V.; Radcliffe, J.E.; Ingleson, M.J. Mechanistic Insights into the B(C6F5)3-Initiated Aldehyde-Aniline-Alkyne Reaction to Form Substituted Quinolines. Organometallics 2017, 36, 1623–1629. [Google Scholar] [CrossRef]
- Liu, Y.L.; Kehr, G.; Daniliuc, C.G.; Erker, G. Metal-Free Arene and Heteroarene Borylation Catalyzed by Strongly Electro-philic Bis-boranes. Chem. Eur. J. 2017, 23, 12141–12144. [Google Scholar] [CrossRef]
- Teng, S.; Zhou, J.S.; Huang, W. New chemistry of alkynyl trifluoroborates under transition metal catalyst-free conditions. Org. Chem. Front. 2024, 11, 5985–6003. [Google Scholar] [CrossRef]
- Zhang, M.; Shan, J.R.; Xie, Y.K.; Wei, L.E.; Xiong, H.G.; Xie, G.N.; Qi, T.; Shi, Q.Q.; Houk, K.N.; Huang, H. General Base-Free Suzuki-Miyaura Cross-Coupling Reaction via Electrophilic Substitution Transmetalation. Angew. Chem. Int. Ed. 2025, 11, e202512496. [Google Scholar]
- Glasspoole, B.W.; Ghozati, K.; Moir, J.; Crudden, C.M. Suzuki–Miyaura cross-couplings of secondary allylic boronic esters. Chem. Commun. 2011, 48, 1230–1232. [Google Scholar] [CrossRef]
- Wen, Y.; Deng, C.; Xie, J.; Kang, X. Recent Synthesis Developments of Organoboron Compounds via Metal-Free Catalytic Borylation of Alkynes and Alkenes. Molecules 2018, 24, 101. [Google Scholar] [CrossRef]
- Vuckovic, S.; Song, S.; Kozlowski, J.; Sim, E.; Burke, K. Density Functional Analysis: The Theory of Density-Corrected DFT. J. Chem. Theory Comput. 2019, 15, 6636–6646. [Google Scholar] [CrossRef]
- Butera, V. Density functional theory methods applied to homogeneous and heterogeneous catalysis: A short review and a practical user guide. Phys. Chem. Chem. Phys. 2024, 26, 7950–7970. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, H. Comparison of DFT methods for molecular structure and vibration spectra of ofloxacin calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 85, 303–309. [Google Scholar] [CrossRef]
- Bartlett, R.J. Adventures in DFT by a wavefunction theorist. J. Chem. Phys. 2019, 151, 19. [Google Scholar] [CrossRef]
- Bursch, M.; Mewes, J.; Hansen, A.; Grimme, S. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry**. Angew. Chem. Int. Ed. 2022, 61, 27. [Google Scholar] [CrossRef]
- Shimoi, M. Radical Hydroboration of Alkynes with N-Heterocyclic Carbene Boranes. Chem. Soc. Jpn. 2018, 57, 9485–9490. [Google Scholar]
- Poredoš, T.; Trampuž, M.; Gornik, T.; Naveršnik, K.; Tisnikar, M.S.; Pirc, S.; Časar, Z. Why and How to Control P-Chirality in Phosphorothioated Therapeutic Oligonucleotides: Analytical Challenges Associated with Determination of Stereochemical Composition. Org. Process. Res. Dev. 2024, 28, 4194–4214. [Google Scholar] [CrossRef]
- Sha, Y.; Zhang, Y.; Qiu, Y.; Xu, Z.; Li, S.; Feng, X.; Wang, M.; Xu, H. Efficient Biosynthesis of Low-Molecular-Weight Poly-γ-glutamic Acid by Stable Overexpression of PgdS Hydrolase in Bacillus amyloliquefaciens NB. J. Agric. Food Chem. 2018, 67, 282–290. [Google Scholar] [CrossRef]
- Xie, H.; Li, Y.; Wang, L.V.; Kuang, J.; Lei, Q.; Fang, W. Why different ligands can control stereochemistry selectivity of Ni-catalyzed Suzuki–Miyaura cross-coupling of benzylic carbamates with arylboronic esters: A mechanistic study. Dalton Trans. 2017, 46, 13010–13019. [Google Scholar] [CrossRef]
- Mann, S.G.A.; Paz-Galeano, M.; Shahsavarani, M.; Perley, J.O.; Guo, J.; Garza-Garcia, J.J.O.; Qu, Y. Stereochemical insights into sarpagan and akuammiline alkaloid biosynthesis. New Phytol. 2025, 247, 1335–1351. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, D.; Ronson, T.K.; Tarzia, A.; Lu, Z.; Jelfs, K.E.; Nitschke, J.R. Sterics and Hydrogen Bonding Control Stereochemistry and Self-Sorting in BINOL-Based Assemblies. J. Am. Chem. Soc. 2021, 143, 9009–9015. [Google Scholar] [CrossRef]
- Bismillah, A.; Johnson, T.; Hussein, B.; Turley, A.; Wong, H.C.; Aguilar, J.; Yufit, D.; McGonigal, P. Control of dynamic sp3-C stereochemistry. Nat. Chem. 2023, 15, 615–624. [Google Scholar] [CrossRef]
- Nakamura, K.; Kitayama, T.; Inoue, Y.; Ohno, A. Stereochemical Control in Microbial Reduction. 12. (S)-4-Nitro-2-butanol as a Source to Synthesize Natural Products. Bull. Chem. Soc. Jpn. 1990, 63, 91–96. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.C.; Scalmani, J.R.; Barone, G.; Mennucci, V.; Peters-son, B.; Nakatsuji, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2007. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2007, 120, 215–241. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Contin-uum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Hou, X.-X.; Wei, D. Mechanism and Origin of Stereoselectivity for the NHC-Catalyzed Desymmetrization Reaction for the Synthesis of Axially Chiral Biaryl Aldehydes. J. Org. Chem. 2024, 89, 3133–3142. [Google Scholar] [CrossRef]
- Jia, J.J.; Wang, Q.Q.; Li, J.Y.; Xu, Z.W.; Li, H.; Wei, D.H.; Yuan, B.X. Liquid-Assisted Grinding Accelerating the Defluorina-tive Coupling of gem-Difluoroalkenes under Ball-Milling Conditions. ACS Sustain. Chem. Eng. 2024, 12, 111–119. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical reactions–the IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Legault, C.Y. CYLview, v1. 0b; Universite de Sherbrooke: Sherbrooke, QC, Canada, 2009.
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 82503. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Interaction Region Indicator: A Simple Real Space Function Clearly Revealing Both Chemical Bonds and Weak Interactions**. Chem. Methods. 2021, 1, 231–239. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).