Comparative Thermal and Supramolecular Hydrothermal Synthesis of g-C3N4 Toward Efficient Photocatalytic Degradation of Gallic Acid
Abstract
1. Introduction
2. Results and Discussion
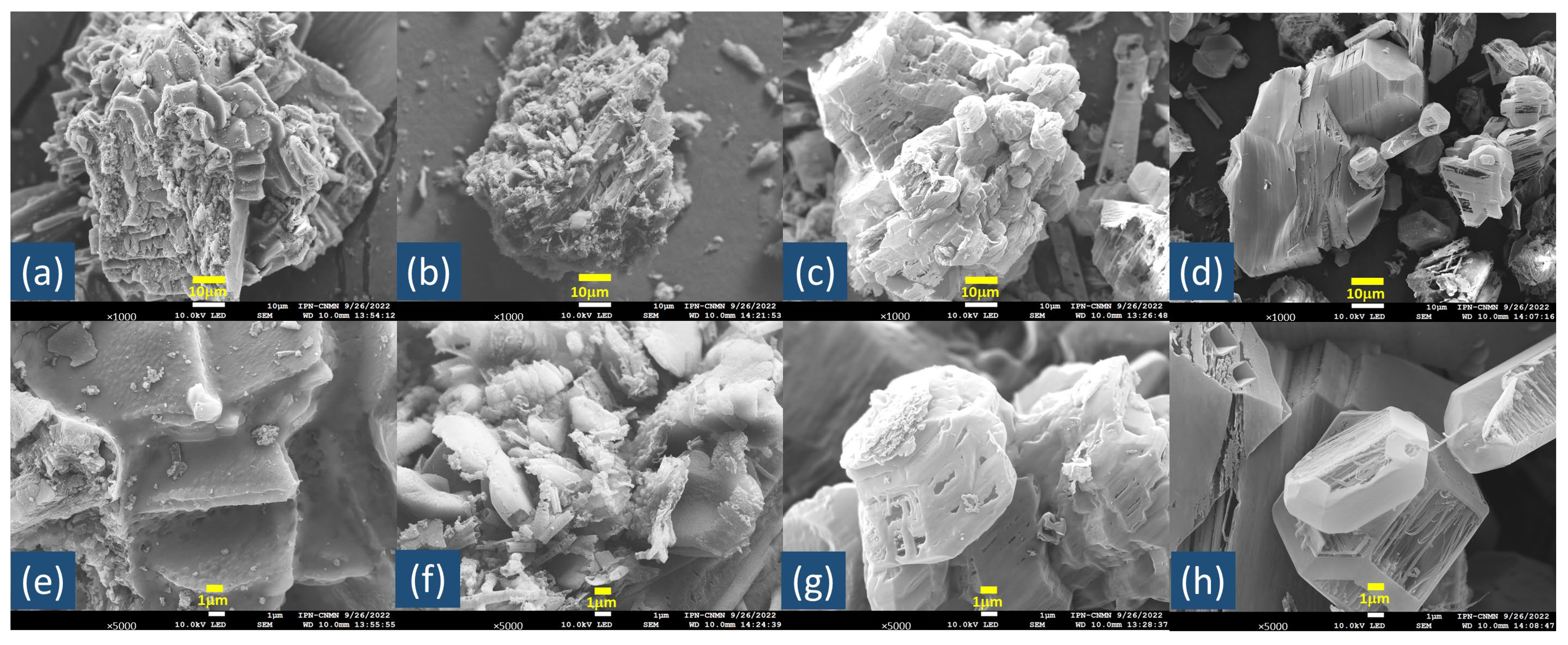
2.1. Catalyst Characterization
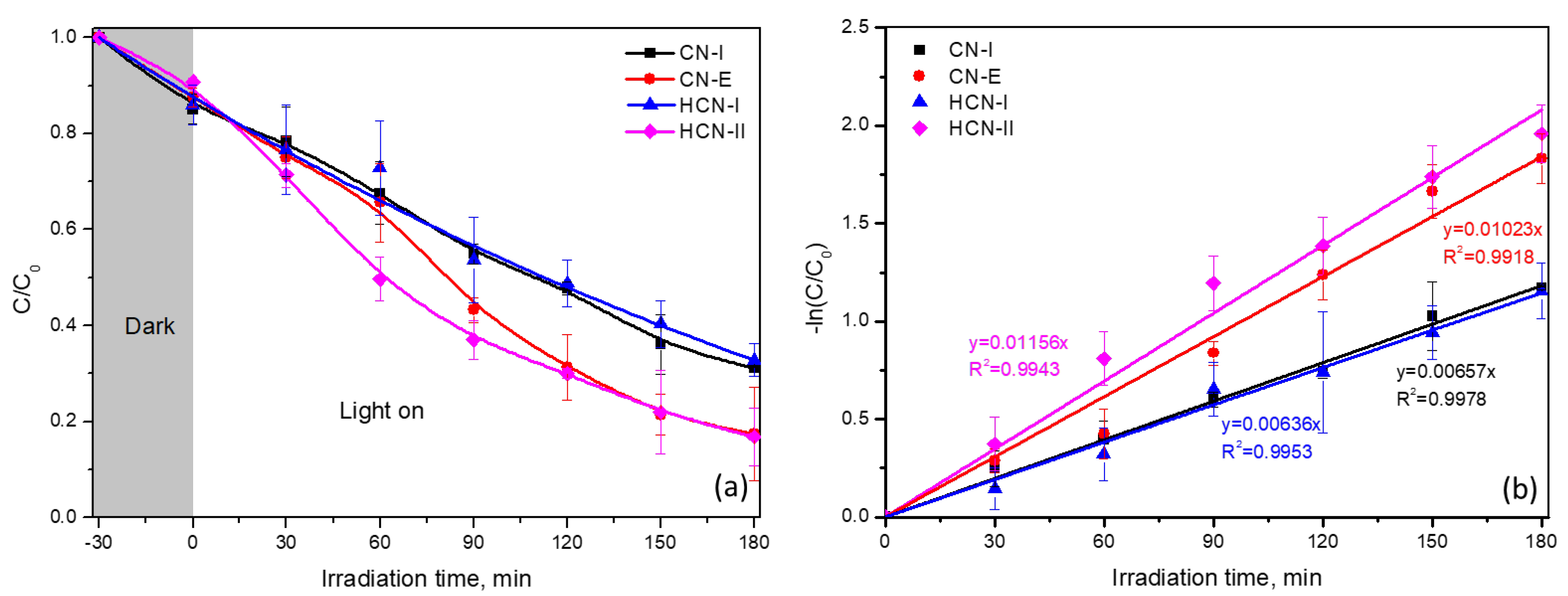
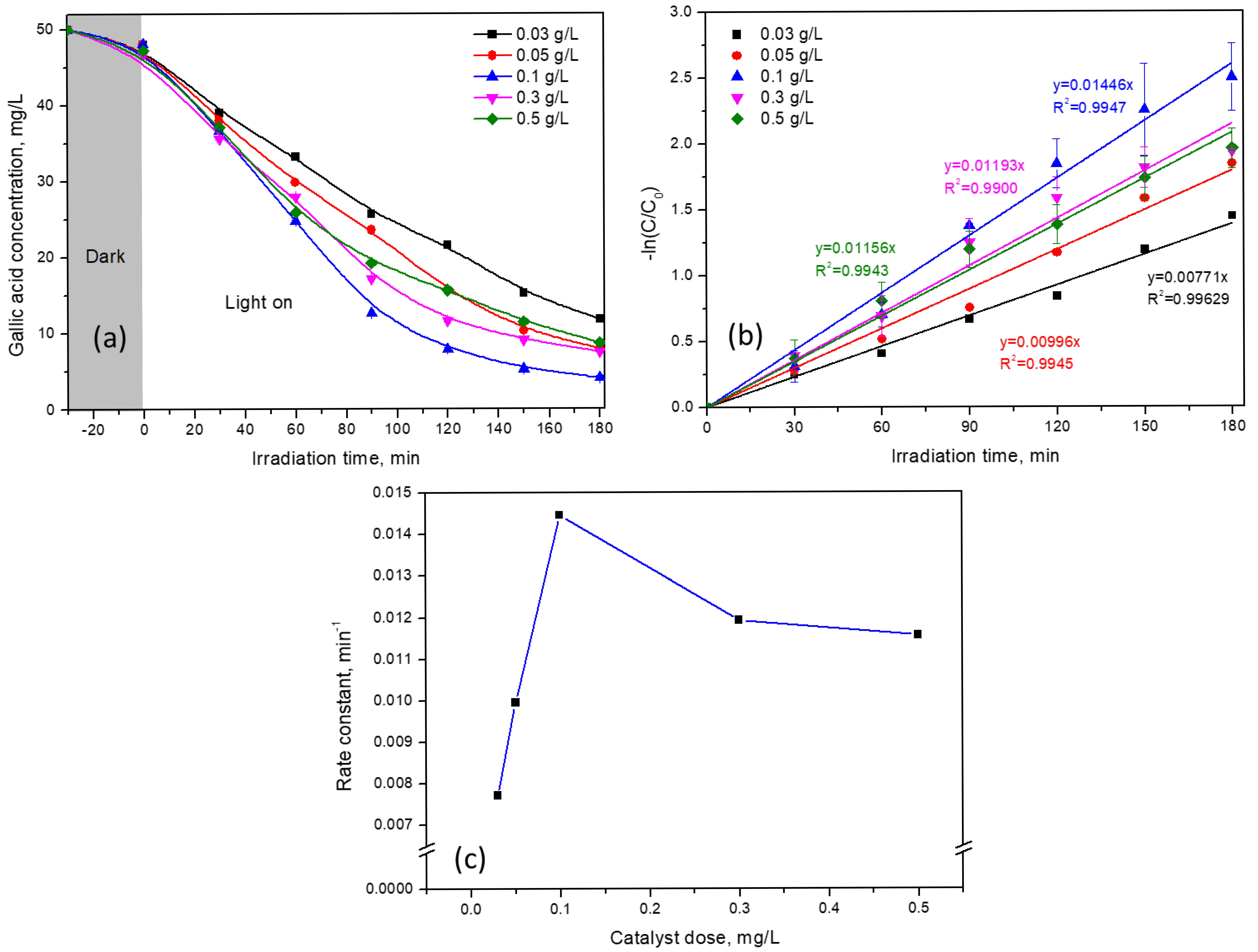
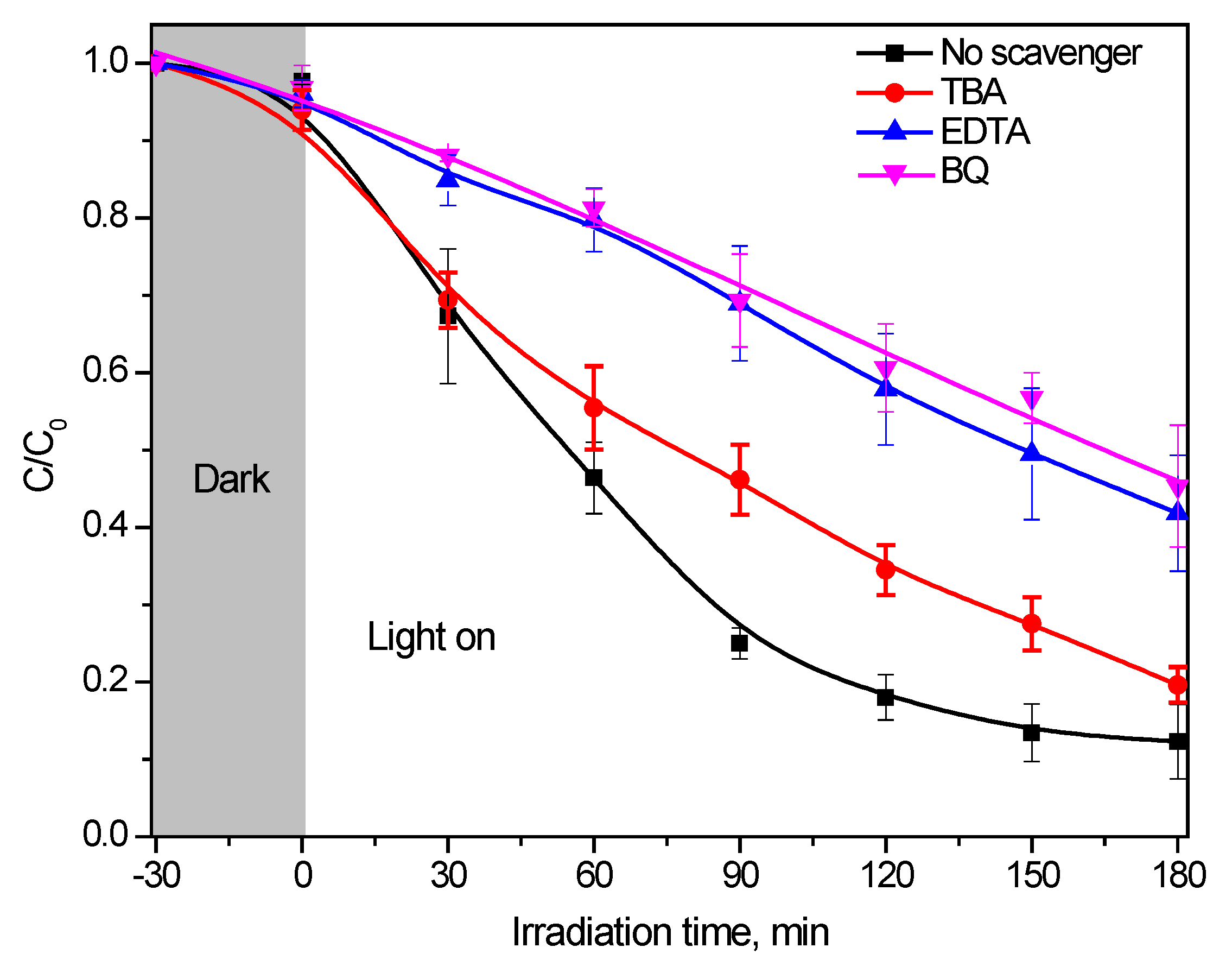
2.2. Photocatalytic Performance of g-C3N4 Samples for GA Removal
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of Catalysts
3.3. Characterization
3.4. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohod, A.V.; Teixeira, A.C.S.C.; Bagal, M.V.; Gogate, P.R.; Giudici, R. Degradation of organic pollutants from wastewater using hydrodynamic cavitation: A review. J. Environ. Chem. Eng. 2023, 11, 109773. [Google Scholar] [CrossRef]
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-Ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A review on emerging water contaminants and the application of sustainable removal technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- Varjani, S.J.; Gnansounou, E.; Pandey, A. Comprehensive review on toxicity of persistent organic pollutants from petroleum refinery waste and their degradation by microorganisms. Chemosphere 2017, 188, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Kumar, A. ADVANCED OXIDATION PROCESS: A remediation technique for organic and non-biodegradable pollutant. Results Surf. Interfaces 2023, 11, 100122. [Google Scholar] [CrossRef]
- Liu, S.; Tang, J.; Yu, X.-f.; Cheng, J.; Zhu, Y.; Zhu, B.; Cao, Q.; Cai, H.; Yang, X. Engineering Co(OH)2/mesoporous g-C3N4 composites for synergistic radical/non-radical antibiotic degradation via electron-bridged peroxymonosulfate activation. J. Environ. Chem. Eng. 2025, 13, 118457. [Google Scholar] [CrossRef]
- Costa, L.N.; Nobre, F.X.; Lobo, A.O.; de Matos, J.M.E. Photodegradation of ciprofloxacin using Z-scheme TiO2/SnO2 nanostructures as photocatalyst. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100466. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, F.; Khaing, A.; Yang, D.; Jiang, Z. Acetic acid-assisted supramolecular assembly synthesis of porous g-C3N4 hexagonal prism with excellent photocatalytic activity. Appl. Surf. Sci. 2019, 479, 757–764. [Google Scholar] [CrossRef]
- Lin, H.; Tang, X.; Wang, J.; Zeng, Q.; Chen, H.; Ren, W.; Sun, J.; Zhang, H. Enhanced visible-light photocatalysis of clofibric acid using graphitic carbon nitride modified by cerium oxide nanoparticles. J. Hazard. Mater. 2021, 405, 124204. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, C.; Liu, J. Guanidine carbonate assisted supramolecular self-assembly for synthesizing porous g-C3N4 for enhanced photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 19939–19947. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, Z.; Tang, C.; Zhou, Y.; Zeng, L.; Huang, L. Enhancement of photocatalytic hydrogen evolution activity of porous oxygen doped g-C3N4 with nitrogen defects induced by changing electron transition. Appl. Catal. B Environ. 2019, 240, 30–38. [Google Scholar] [CrossRef]
- Khan, M.A.; Mutahir, S.; Shaheen, I.; Qunhui, Y.; Bououdina, M.; Humayun, M. Recent advances over the doped g-C3N4 in photocatalysis: A review. Coord. Chem. Rev. 2025, 522, 216227. [Google Scholar] [CrossRef]
- Ghosh, U.; Majumdar, A.; Pal, A. Photocatalytic CO2 reduction over g-C3N4 based heterostructures: Recent progress and prospects. J. Environ. Chem. Eng. 2021, 9, 104631. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, G.; Zhang, H.; Li, Z.; Wen, Y. One-pot synthesis of porous g-C3N4 nanosheets with enhanced photocatalytic activity under visible light. Diam. Relat. Mater. 2021, 116, 108416. [Google Scholar] [CrossRef]
- Deng, P.; Gan, M.; Zhang, X.; Li, Z.; Hou, Y. Non-noble-metal Ni nanoparticles modified N-doped g-C3N4 for efficient photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 30084–30092. [Google Scholar] [CrossRef]
- Tay, Q.; Kanhere, P.; Ng, C.F.; Chen, S.; Chakraborty, S.; Huan, A.C.H.; Sum, T.C.; Ahuja, R.; Chen, Z. Defect Engineered g-C3N4 for Efficient Visible Light Photocatalytic Hydrogen Production. Chem. Mater. 2015, 27, 4930–4933. [Google Scholar] [CrossRef]
- Wen, Y.; Qu, D.; An, L.; Gao, X.; Jiang, W.; Wu, D.; Yang, D.; Sun, Z. Defective g-C3N4 Prepared by the NaBH4 Reduction for High-Performance H2 Production. ACS Sustain. Chem. Eng. 2019, 7, 2343–2349. [Google Scholar] [CrossRef]
- Yu, G.; Zhao, H.; Xing, C.; Guo, L.; Li, X. Creation of carbon defects and in-plane holes with the assistance of NH4Br to enhance the photocatalytic activity of g-C3N4. Catal. Sci. Technol. 2021, 11, 5349–5359. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, L.; Liao, Y.; Xiang, Q. Effect of Functional Group Modifications on the Photocatalytic Performance of g-C3N4. Small 2023, 19, 2300109. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Z.; Wei, G.; Wu, S.; Huang, L.; Li, D.; Ruan, X.; Liu, Y.; Jiang, C.; Ren, F. Defective High-Crystallinity g-C3N4 Heterostructures by Double-End Modulation for Photocatalysis. ACS Energy Lett. 2024, 9, 1915–1922. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Yang, Q.; Zhang, Z.; Fang, X. Mesoporous g-C3N4 nanosheets prepared by calcining a novel supramolecular precursor for high-efficiency photocatalytic hydrogen evolution. Appl. Surf. Sci. 2018, 450, 46–56. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A critical review on graphitic carbon nitride (g-C3N4)-based materials: Preparation, modification and environmental application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Liu, C.; Dong, X.; Hao, Y.; Wang, X.; Ma, H.; Zhang, X. A novel supramolecular preorganization route for improving g-C3N4/g-C3N4 metal-free homojunction photocatalysis. New J. Chem. 2017, 41, 11872–11880. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Zheng, K.; Xu, C. A supramolecule-based shape-controllable preparation of carbon nitride nanotubes for the visible light driven photodegradation. Surf. Interfaces 2022, 30, 101894. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, C.; Jin, Y.; Jing, Q.; Yang, Y.; Shen, X.; Tang, Q.; Mu, Y.; Du, A.-K. Facile synthesis of 3D porous thermally exfoliated g-C3N4 nanosheet with enhanced photocatalytic degradation of organic dye. J. Colloid Interface Sci. 2016, 468, 211–219. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, H.; Quan, X.; Chen, S.; Zhao, H.; Wang, H. Atomic single layer graphitic-C3N4: Fabrication and its high photocatalytic performance under visible light irradiation. RSC Adv. 2014, 4, 624–628. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Athanasekou, C.P.; Dimotikali, D.; Trapalis, C. Chemical vs thermal exfoliation of g-C3N4 for NOx removal under visible light irradiation. Appl. Catal. B Environ. 2018, 239, 16–26. [Google Scholar] [CrossRef]
- Lucas, M.S.; Dias, A.A.; Bezerra, R.M.; Peres, J.A. Gallic acid photochemical oxidation as a model compound of winery wastewaters. J. Environ. Sci. Health Part A 2008, 43, 1288–1295. [Google Scholar] [CrossRef]
- Wu, K.; Chen, D.; Lu, S.; Fang, J.; Zhu, X.; Yang, F.; Pan, T.; Fang, Z. Supramolecular self-assembly synthesis of noble-metal-free (C, Ce) co-doped g-C3N4 with porous structure for highly efficient photocatalytic degradation of organic pollutants. J. Hazard. Mater. 2020, 382, 121027. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Shen, Y.; Zhou, Z.; Yu, J.; Li, Y.; Wei, W.; Liu, S.; Zhang, Y. Environment-friendly preparation of porous graphite-phase polymeric carbon nitride using calcium carbonate as templates, and enhanced photoelectrochemical activity. J. Mater. Chem. A 2015, 3, 5126–5131. [Google Scholar] [CrossRef]
- Tang, D.; Chen, Y.; Yin, M.; Yang, Q.; Zhou, Y.; Zhou, L. Supramolecular self-assembly production of porous carbon nitride nanosheets with excellent photocatalytic activity by a melamine derivative as doping molecule. Mater. Sci. Semicond. Process. 2020, 105, 104735. [Google Scholar] [CrossRef]
- Eroglu, Z.; Ozer, M.S.; Metin, Ö. Nitrogen-Based Imperfections in Graphitic Carbon Nitride—New Trend for Enhancing Photocatalytic Activity? ChemCatChem 2024, 16, e202301560. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, L.; Wang, P.; Guo, Y.; Shi, Z.; Guo, X.; Zhang, L. Synthesis of nitrogen vacancies g-C3N4 with increased crystallinity under the controlling of oxalyl dihydrazide: Visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2020, 505, 144576. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, K.; Deng, W.; Li, J.; Yan, Y.; Li, Y.; Quan, X.; Wang, T. Nitrogen vacancy mediated exciton dissociation in carbon nitride nanosheets: Enhanced hydroxyl radicals generation for efficient photocatalytic degradation of organic pollutants. J. Hazard. Mater. 2020, 387, 122023. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, S.; Chen, Y.; Shen, Y.; Liao, X.; Zhou, X.; Xu, H.; Tan, Y.; Zhong, J. Nitrogen vacancies enriched g-C3N4 nanosheets for photocatalytic CO2 reduction. Int. J. Hydrogen Energy 2025, 158, 150571. [Google Scholar] [CrossRef]
- Pattnaik, S.P.; Behera, A.; Martha, S.; Acharya, R.; Parida, K. Facile synthesis of exfoliated graphitic carbon nitride for photocatalytic degradation of ciprofloxacin under solar irradiation. J. Mater. Sci. 2019, 54, 5726–5742. [Google Scholar] [CrossRef]
- Jiang, X.; Li, X.; Liu, Y.; Zhai, H.; Wang, G. The oxidative thermal exfoliation-dependent microstructure and photocatalytic performance of g-C3N4 nanosheets. Diam. Relat. Mater. 2024, 143, 110918. [Google Scholar] [CrossRef]
- Xue, Y.; Ma, C.; Yang, Q.; Wang, X.; An, S.; Zhang, X.; Tian, J. Construction of g-C3N4 with three coordinated nitrogen (N3C) vacancies for excellent photocatalytic activities of N2 fixation and H2O2 production. Chem. Eng. J. 2023, 457, 141146. [Google Scholar] [CrossRef]
- Yang, L.; Hu, C.; Nie, Y.; Qu, J. Catalytic Ozonation of Selected Pharmaceuticals over Mesoporous Alumina-Supported Manganese Oxide. Environ. Sci. Technol. 2009, 43, 2525–2529. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, S.; Duan, X.; Wang, Y.; Wu, D.; Pang, J.; Wang, X.; Wang, S. Catalytic oxidation of sulfachloropyridazine by MnO2: Effects of crystalline phase and peroxide oxidants. Chemosphere 2021, 267, 129287. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, J.; Sun, Z.-Z. Oxidation products and pathway of ceramic honeycomb-catalyzed ozonation for the degradation of nitrobenzene in aqueous solution. Appl. Catal. B Environ. 2008, 79, 244–253. [Google Scholar]
- Wang, J.; Guo, H.; Liu, Y.; Li, W.; Yang, B. Peroxymonosulfate activation by porous BiFeO3 for the degradation of bisphenol AF: Non-radical and radical mechanism. Appl. Surf. Sci. 2020, 507, 145097. [Google Scholar]
- Kumar, J.E.; Sahoo, M.K. A review on effect of operational parameters for the degradation of azo dyes by some advanced oxidation processes. Sustain. Chem. Environ. 2025, 11, 100274. [Google Scholar] [CrossRef]
- Hübner, U.; Spahr, S.; Lutze, H.; Wieland, A.; Rüting, S.; Gernjak, W.; Wenk, J. Advanced oxidation processes for water and wastewater treatment—Guidance for systematic future research. Heliyon 2024, 10, e30402. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.-C.; Fatema, K.N.; Liu, Y.; Lim, C.S.; Cho, K.Y.; Jung, C.-H.; Biswas, R.U.D. Sonochemical synthesis of quaternary LaNiSbWO4-G-PANI polymer nanocomposite for photocatalytic degradation of Safranin-O and gallic acid under visible light irradiation. J. Photochem. Photobiol. A Chem. 2020, 394, 112484. [Google Scholar] [CrossRef]
- Mera, A.C.; Contreras, D.; Escalona, N.; Mansilla, H.D. BiOI microspheres for photocatalytic degradation of gallic acid. J. Photochem. Photobiol. A Chem. 2016, 318, 71–76. [Google Scholar] [CrossRef]
- Araujo, P.Z.; Morando, P.J.; Martínez, E.; Blesa, M.A. Time evolution of surface speciation during heterogeneous photocatalysis: Gallic acid on titanium dioxide. Appl. Catal. B Environ. 2012, 125, 215–221. [Google Scholar] [CrossRef]
- Luna, A.L.; Valenzuela, M.A.; Colbeau-Justin, C.; Vázquez, P.; Rodriguez, J.L.; Avendaño, J.R.; Alfaro, S.; Tirado, S.; Garduño, A.; De la Rosa, J.M. Photocatalytic degradation of gallic acid over CuO–TiO2 composites under UV/Vis LEDs irradiation. Appl. Catal. A: Gen. 2016, 521, 140–148. [Google Scholar] [CrossRef]
- Ashfaq, M.; Talreja, N.; Chauhan, D.; Rodríguez, C.A.; Mera, A.C.; Ramalinga Viswanathan, M. Synthesis of reduced graphene oxide incorporated bimetallic (Cu/Bi) nanorods based photocatalyst materials for the degradation of gallic acid and bacteria. J. Ind. Eng. Chem. 2022, 110, 447–455. [Google Scholar] [CrossRef]
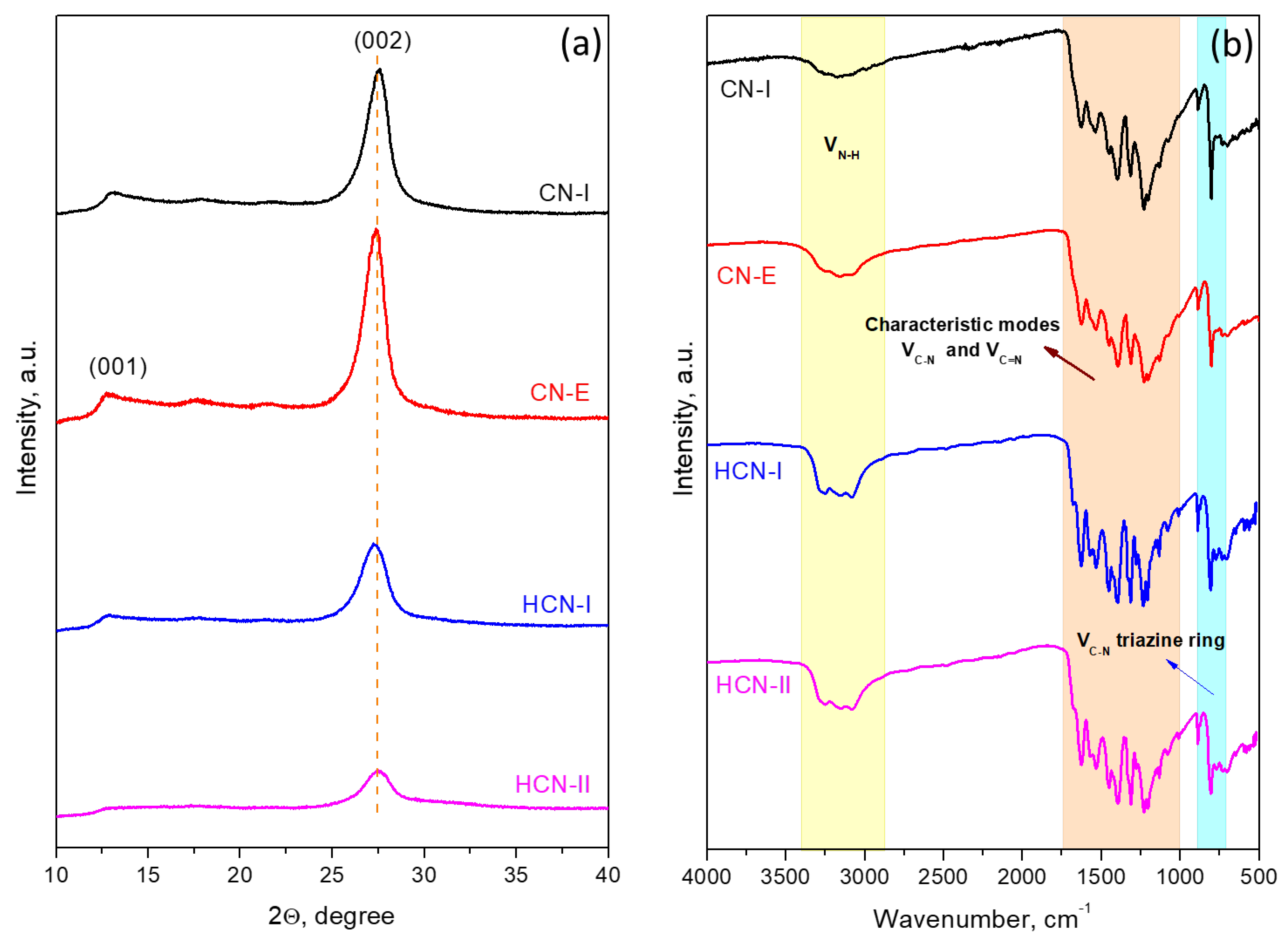
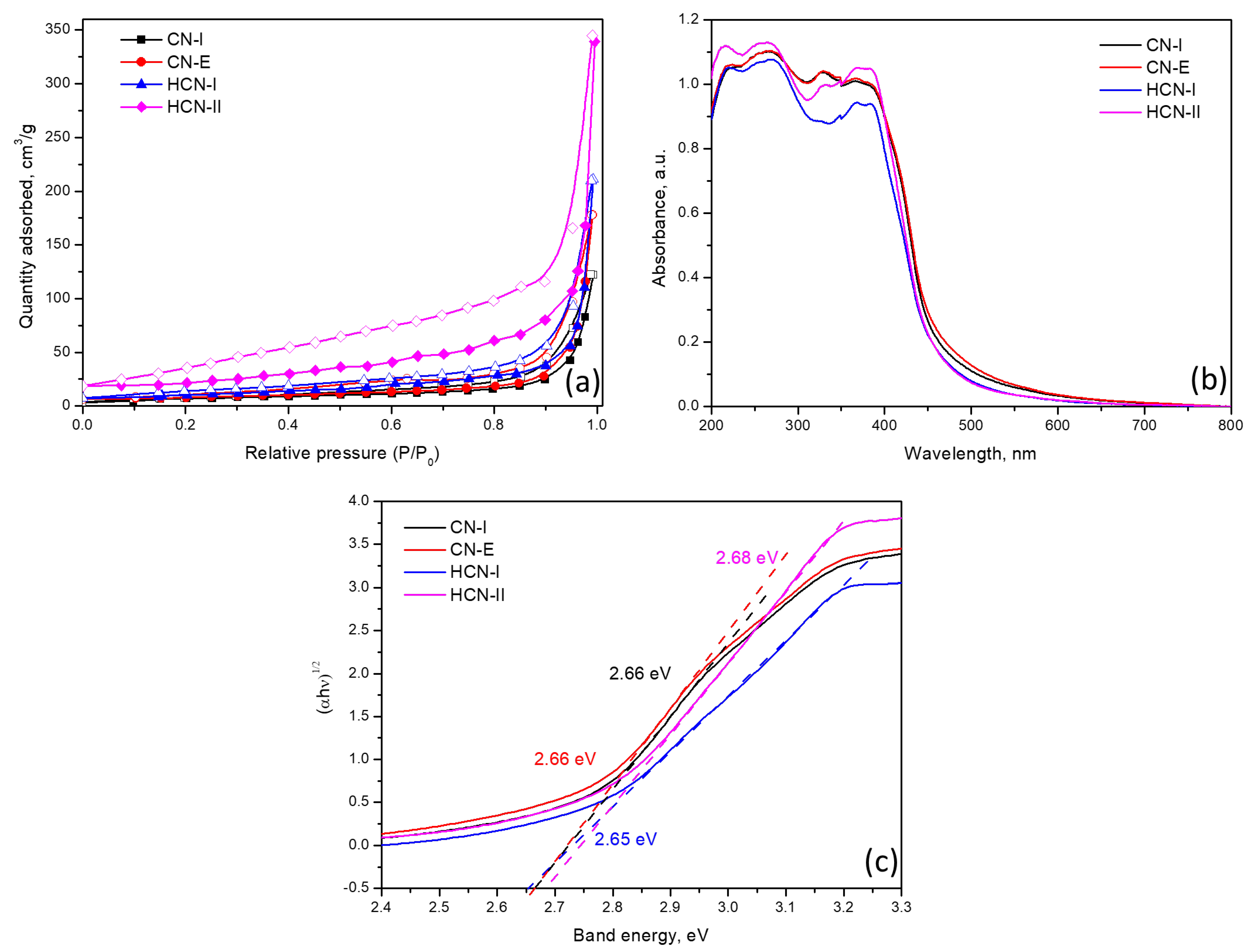
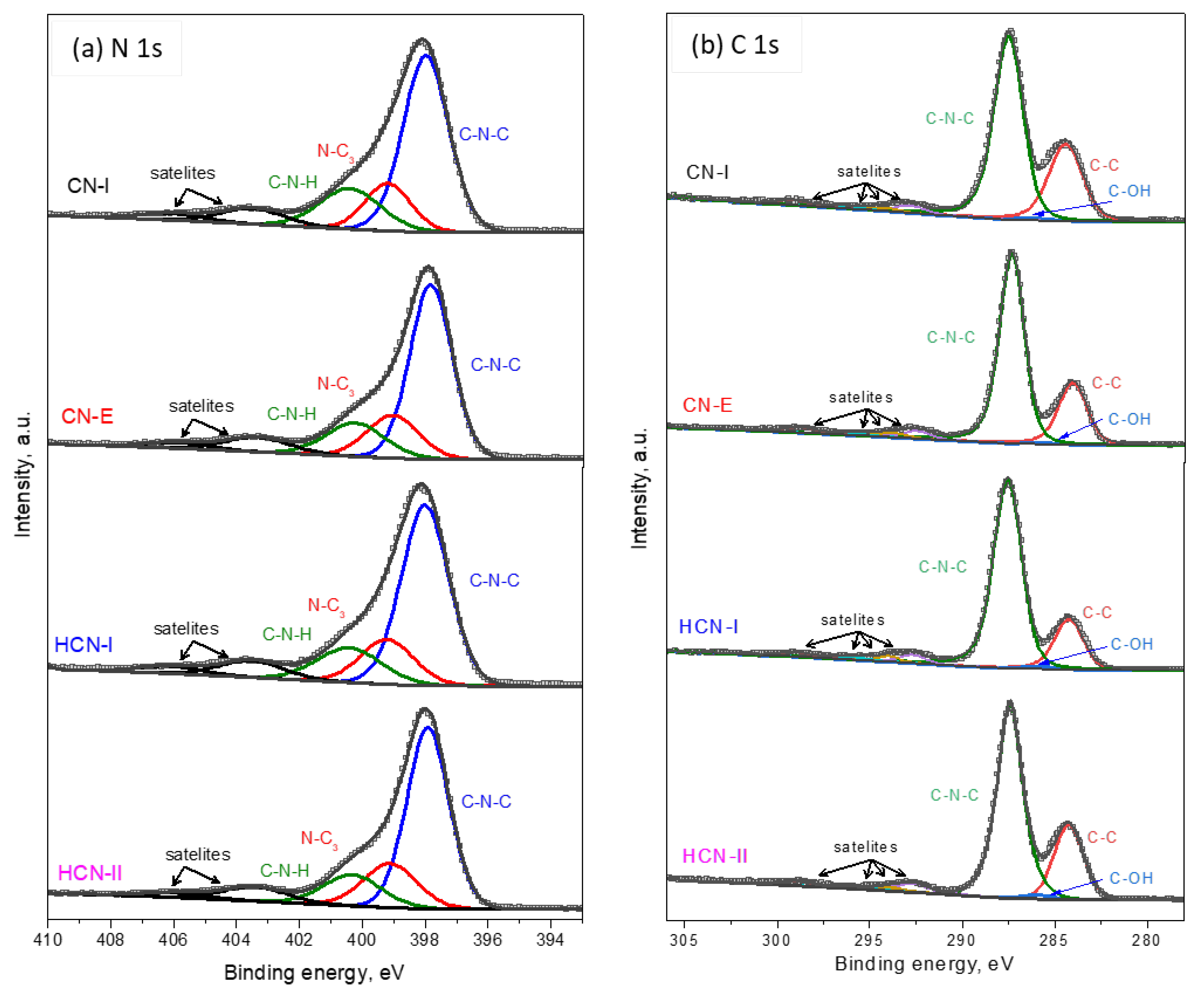

| Catalyst | 2ϴ, ° | d002, nm * | BET, m2/g | Pore Diameter, nm | Band Gap, eV |
|---|---|---|---|---|---|
| CN-I | 27.40 | 0.3248 | 24.3 | 3.90 | 2.68 |
| CN-E | 27.27 | 0.3268 | 28.1 | 3.89 | 2.66 |
| HCN-I | 27.24 | 0.3272 | 36.4 | 3.88 | 2.64 |
| HCN-II | 27.48 | 0.3243 | 81.7 | 3.91 | 2.67 |
| Sample | C=N-C, % | N-(C)3, % | C=N-C/N-(C)3 |
|---|---|---|---|
| CN-I | 57.29 | 17.19 | 3.33 |
| CN-E | 58.05 | 16.25 | 3.57 |
| HCN-I | 56.92 | 15.37 | 3.70 |
| HCN-II | 58.58 | 18.16 | 3.22 |
| Photocatalyst | Experimental Conditions | Irradiation Source and Exposure Time | % Degradation | Reference |
|---|---|---|---|---|
| g-C3N4 (Thermal exfoliation and hydrothermal synthesis) | V = 200 mL [GA] = 50 mg/L [g-C3N4] = 0.1 g/L | A LED Chip lamp (50 W) 180 min | 82 | This work |
| LaNiSbWO4-G-PANI (Sonochemical process) | V = 100 mL [GA] = 17 mg/L [LaNiSbWO4-G-PANI] =1 g/L | Xe lamp (500 W) 180 min | 90 | [44] |
| BiOI microspheres (Solvothermal synthesis) | V = 250 mL [GA] = 20 mg/L [BiOI]= 0.2–0.6 g/L | Xe lamp (12 W) 5 min | 82 | [45] |
| TiO2 (P25, Degussa) | V = 250 mL [GA] = 10 mg/L [TiO2] = 0.2 g/L | Solar simulator (300 W) 60 min | 70 | [46] |
| 40%wt CuO–TiO2 (Impregnation method followed by calcination at 400 °C in air) | V = 150 mL [GA] = 50 mg/L [40%wt CuO–TiO2] = 0.5 g/L | LEDs (λ = 375 nm and λ = 470 nm) 150 min | 70 | [47] |
| r-GO-Cu/Bi-NRs (Co-precipitation) | V = 250 mL [GA] = 1–20 mg/L [catalyst] = 100–600 mg/L | Xe lamp (12 W) 60 min | 96 | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantor Pérez, F.; Rodríguez Santillán, J.L.; Santillán Peréz, R.; Fuentes Camargo, I.; Romero Ibarra, I.C.; Guzmán Castañeda, J.I.; Vazquez-Arce, J.L.; Tiznado, H.; Martínez Gutiérrez, H. Comparative Thermal and Supramolecular Hydrothermal Synthesis of g-C3N4 Toward Efficient Photocatalytic Degradation of Gallic Acid. Catalysts 2025, 15, 858. https://doi.org/10.3390/catal15090858
Cantor Pérez F, Rodríguez Santillán JL, Santillán Peréz R, Fuentes Camargo I, Romero Ibarra IC, Guzmán Castañeda JI, Vazquez-Arce JL, Tiznado H, Martínez Gutiérrez H. Comparative Thermal and Supramolecular Hydrothermal Synthesis of g-C3N4 Toward Efficient Photocatalytic Degradation of Gallic Acid. Catalysts. 2025; 15(9):858. https://doi.org/10.3390/catal15090858
Chicago/Turabian StyleCantor Pérez, Fernando, Julia Liliana Rodríguez Santillán, Ricardo Santillán Peréz, Iliana Fuentes Camargo, Issis C. Romero Ibarra, Jesús I. Guzmán Castañeda, Jorge L. Vazquez-Arce, Hugo Tiznado, and Hugo Martínez Gutiérrez. 2025. "Comparative Thermal and Supramolecular Hydrothermal Synthesis of g-C3N4 Toward Efficient Photocatalytic Degradation of Gallic Acid" Catalysts 15, no. 9: 858. https://doi.org/10.3390/catal15090858
APA StyleCantor Pérez, F., Rodríguez Santillán, J. L., Santillán Peréz, R., Fuentes Camargo, I., Romero Ibarra, I. C., Guzmán Castañeda, J. I., Vazquez-Arce, J. L., Tiznado, H., & Martínez Gutiérrez, H. (2025). Comparative Thermal and Supramolecular Hydrothermal Synthesis of g-C3N4 Toward Efficient Photocatalytic Degradation of Gallic Acid. Catalysts, 15(9), 858. https://doi.org/10.3390/catal15090858






