Copper Active Sites in Metal–Organic Frameworks Advance CO2 Adsorption and Photocatalytic Conversion
Abstract
1. Introduction
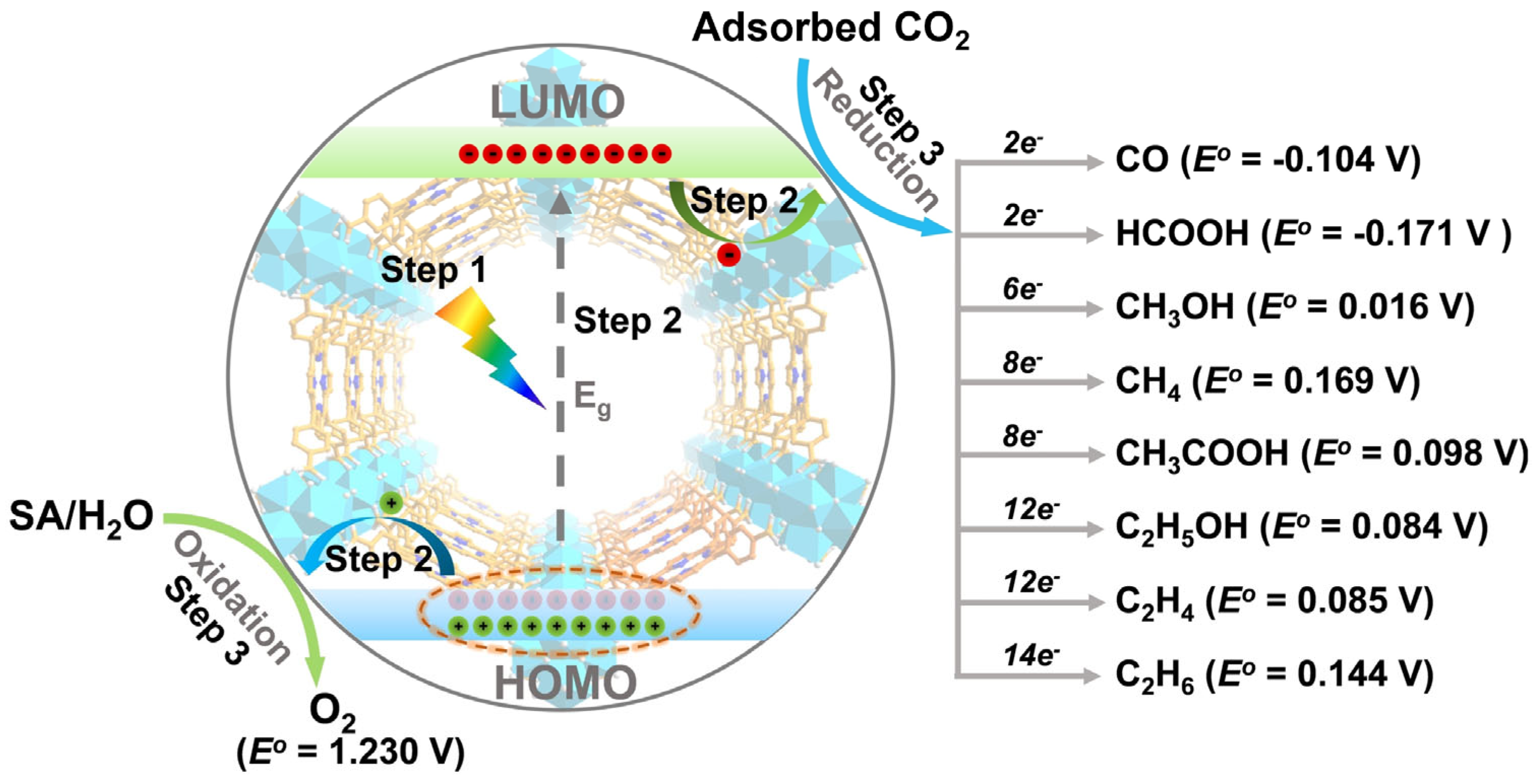
2. Principles of MOF Photocatalysts for CO2 Conversion
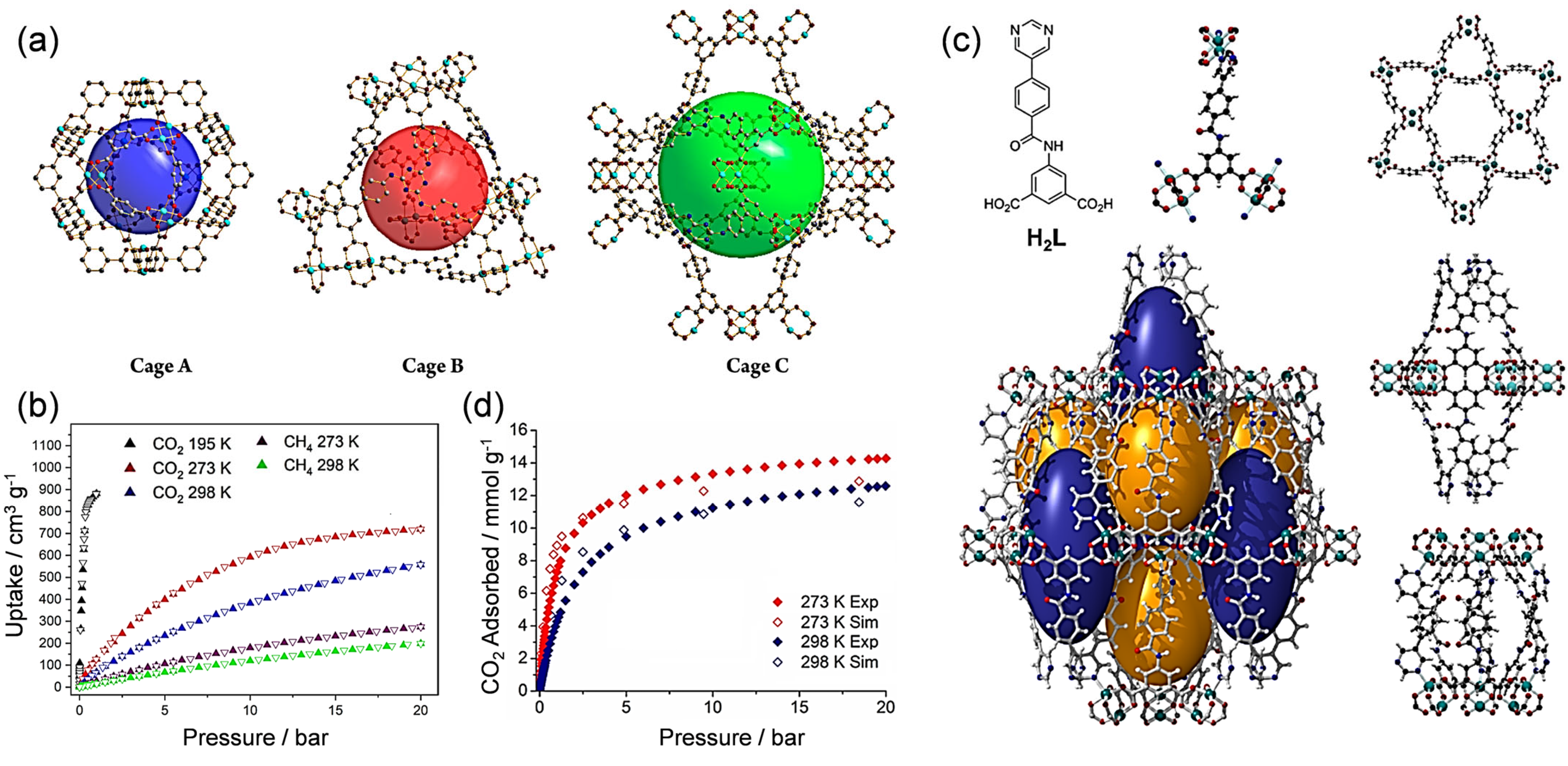
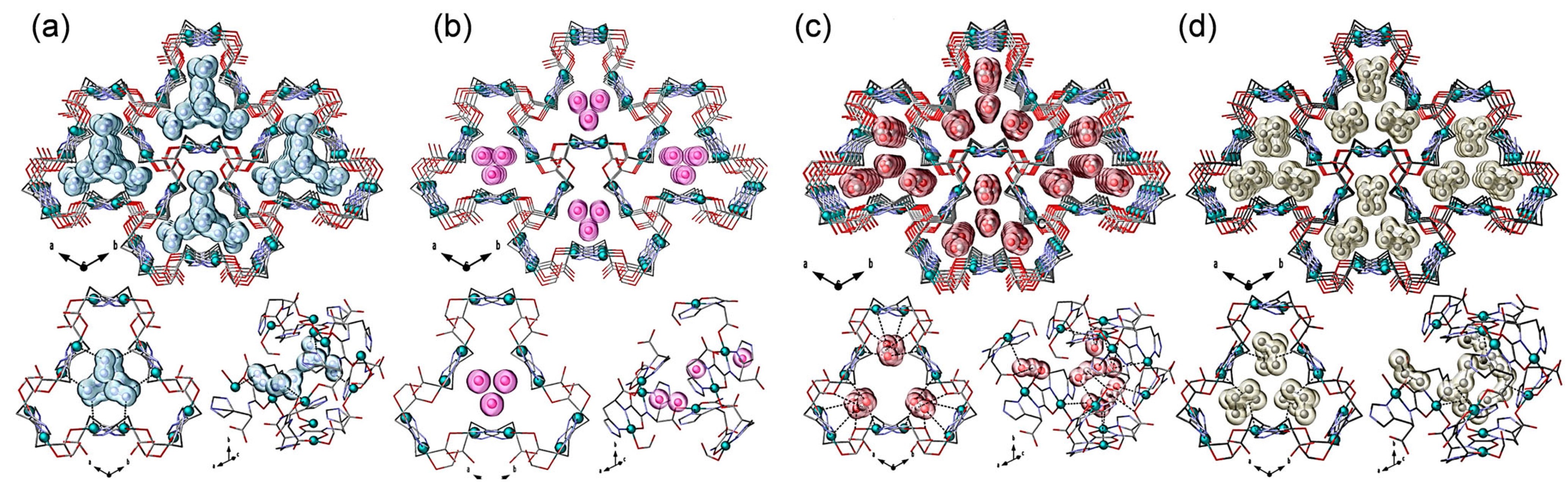
3. Cu Sites in MOFs for CO2 Adsorption
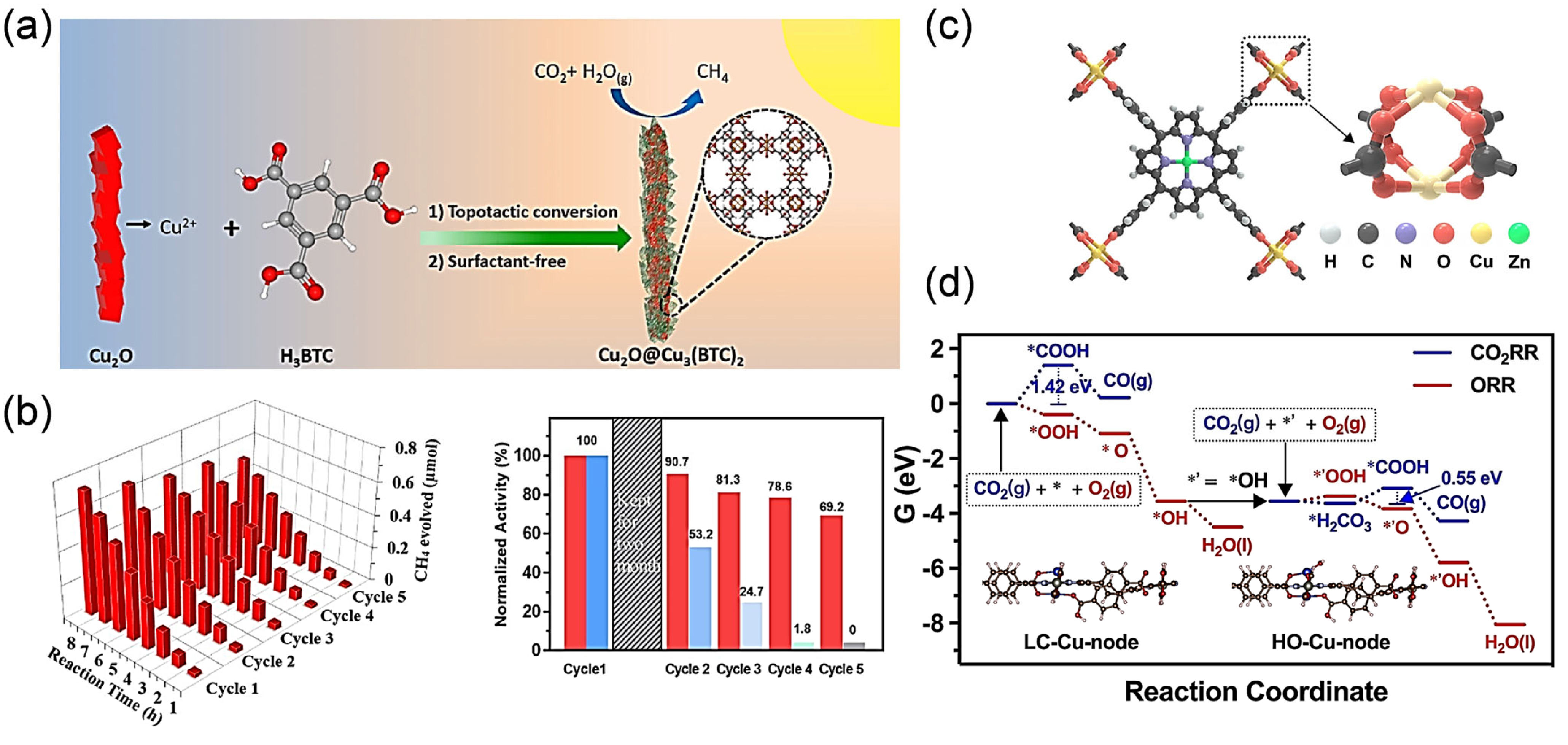
3.1. Cu (I) Sites in MOFs
3.2. Cu (II) Sites in MOFs
3.3. Cu Single-Atom Sites in MOFs
3.4. Pros and Cons of Cu (I, II, and Single-Atom)-Based MOFs
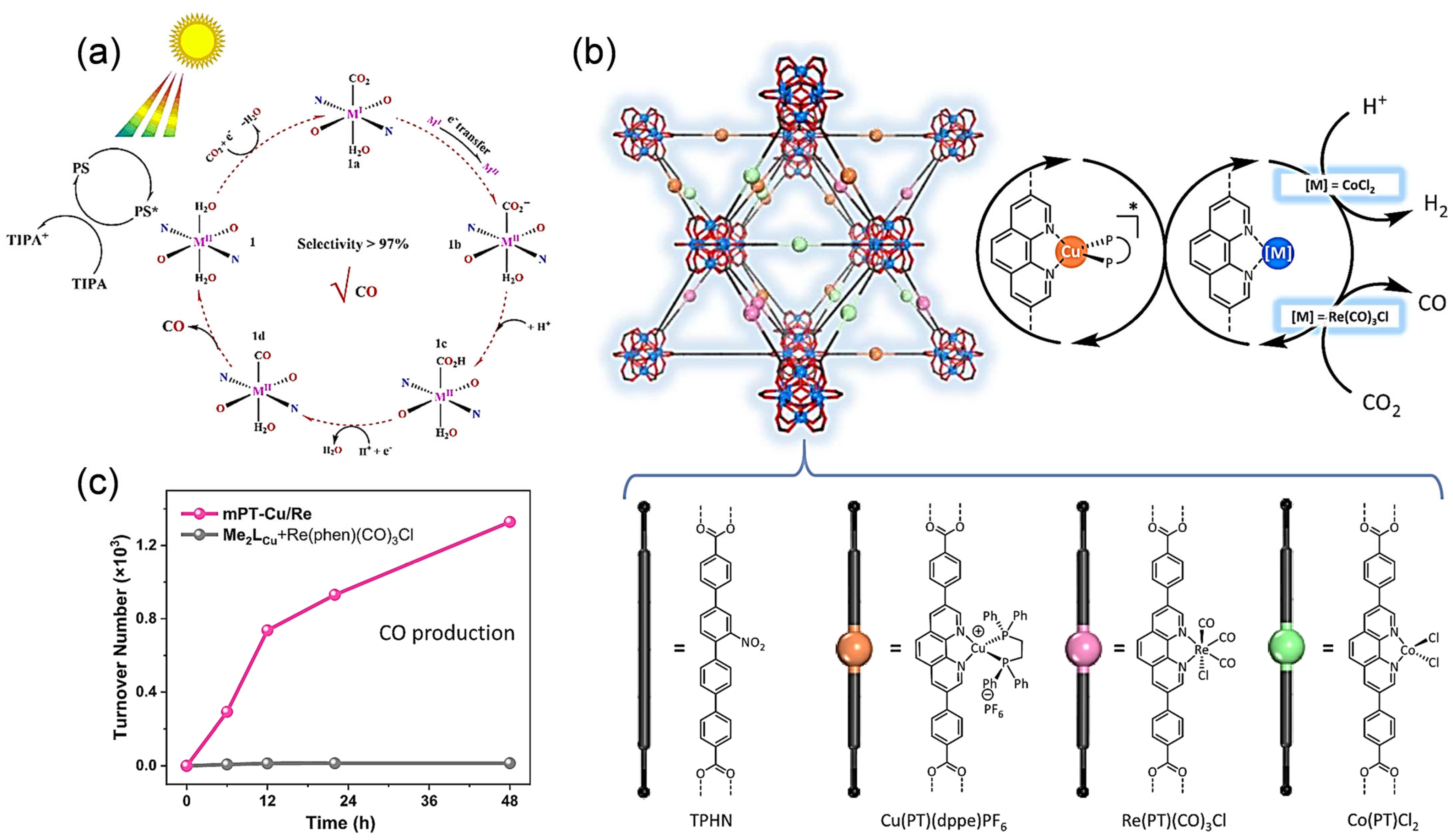
4. Photocatalytic CO2 Reduction on Cu-MOFs
4.1. Two-Electron Reduction Process
4.2. Six-Electron Reduction Process
4.3. Eight-Electron Reduction Process
4.4. Twelve-/Fourteen-Electron Reduction Process
4.5. Electronic and Geometric Factors of CO2 Conversion Mechanism
5. Advanced Characterization
6. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jones, M.W.; Peters, G.P.; Gasser, T.; Andrew, R.M.; Schwingshackl, C.; Gütschow, J.; Houghton, R.A.; Friedlingstein, P.; Pongratz, J.; Quéré, C.L. National contributions to climate change due to historical emissions of carbon dioxide, methane, and nitrous oxide since 1850. Sci. Data 2023, 10, 1–23. [Google Scholar] [CrossRef]
- Fernández, J.; Sotenko, M.; Derevschikov, V.; Lysikov, A.; Rebrov, E.V. A radiofrequency heated reactor system for post-combustion carbon capture. Chem. Eng. Process. Process. Intensif. 2016, 108, 17–26. [Google Scholar] [CrossRef]
- Chronopoulos, T.; Fernandez-Diez, Y.; Maroto-Valer, M.M.; Ocone, R.; Reay, D.A. CO2 desorption via microwave heating for post-combustion carbon capture. Microporous Mesoporous Mater. 2014, 197, 288–290. [Google Scholar] [CrossRef]
- Nagireddi, S.; Agarwal, J.R.; Vedapuri, D. Carbon Dioxide Capture, Utilization, and Sequestration: Current Status, Challenges, and Future Prospects for Global Decarbonization. ACS Eng. Au 2023, 4, 22–48. [Google Scholar] [CrossRef]
- Geweda, A.E.; Zayed, M.E.; Khan, M.Y.; Alquaity, A.B.S. Mitigating CO2 emissions: A review on emerging technolo-gies/strategies for CO2 capture. J. Energy Inst. 2025, 118, 101911. [Google Scholar] [CrossRef]
- Kumagai, H.; Tamaki, Y.; Ishitani, O. Photocatalytic Systems for CO2 Reduction: Metal-Complex Photocatalysts and Their Hybrids with Photofunctional Solid Materials. Accounts Chem. Res. 2022, 55, 978–990. [Google Scholar] [CrossRef]
- Fang, S.; Rahaman, M.; Bharti, J.; Reisner, E.; Robert, M.; Ozin, G.A.; Hu, Y.H. Photocatalytic CO2 reduction. Nat. Rev. Methods Primers 2023, 3, 1–21. [Google Scholar] [CrossRef]
- Wu, H.; Han, D.; Hu, B.; Liang, J.; Tang, X.; Zhu, Z.; Huo, P. Insights into enhanced photocatalytic CO2 reduction on carbon nitride: A strategy of simultaneous O, S co-doping. J. Environ. Chem. Eng. 2025, 13, 116121. [Google Scholar] [CrossRef]
- Wang, J.; Jia, C.-S.; Li, C.-J.; Peng, X.-L.; Zhang, L.-H.; Liu, J.-Y. Thermodynamic Properties for Carbon Dioxide. ACS Omega 2019, 4, 19193–19198. [Google Scholar] [CrossRef]
- Yanagi, R.; Zhao, T.; Solanki, D.; Pan, Z.; Hu, S. Charge Separation in Photocatalysts: Mechanisms, Physical Parameters, and Design Principles. ACS Energy Lett. 2021, 7, 432–452. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, J.; García, H. Charge-transfer dynamics in S-scheme photocatalyst. Nat. Reviews Chem. 2025, 9, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhou, J.; An, X. Recent advances in single-atom catalysts (SACs) for photocatalytic applications. Mater. Rep. Energy 2024, 4, 100285. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, S.; Liu, Q.; Wang, W.; Hao, N.; Wang, Y.; Li, Z.; Luo, D. Recent advances in novel materials for photocatalytic carbon dioxide reduction. Carbon Neutralization 2024, 3, 142–168. [Google Scholar] [CrossRef]
- Kovačič, A.; Likozar, B.; Huš, M. Photocatalytic CO2 Reduction: A Review of Ab Initio Mechanism, Kinetics, and Multiscale Modeling Simulations. ACS Catal. 2020, 10, 14984–15007. [Google Scholar] [CrossRef]
- Kim, Y.; Smith, J.G.; Jain, P.K. Harvesting multiple electron–hole pairs generated through plasmonic excitation of Au nanoparticles. Nat. Chem. 2018, 10, 763–769. [Google Scholar] [CrossRef]
- Shen, W.; Qi, Q.; Hu, B.; Zhu, Z.; Huo, P.; Jiang, J.; Tang, X. Studying bimetals copper-indium for enhancing PCN photocatalytic CO2 reduction activity and selectivity mechanism. Ind. Eng. Chem. 2025, 145, 384–394. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, H.; Qian, J.; Su, H.; Lee, K.-J.; Cheng, T.; Xiao, H.; Yano, J.; Goddard, W.A.; Crumlin, E.J. Dramatic differences in carbon dioxide adsorption and initial steps of reduction between silver and copper. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Álvarez, A.; Borges, M.; Corral-Pérez, J.J.; Olcina, J.G.; Hu, L.; Cornu, D.; Huang, R.; Stoian, D.; Urakawa, A. CO2 Activation over Catalytic Surfaces. ChemPhysChem 2017, 18, 3135–3141. [Google Scholar] [CrossRef]
- Han, C.; Kundu, B.K.; Liang, Y.; Sun, Y. Near-Infrared Light-Driven Photocatalysis with an Emphasis on Two-Photon Excitation: Concepts, Materials, and Applications. Adv. Mater. 2024, 36, 2307759. [Google Scholar] [CrossRef]
- Navarro-Jaén, S.; Virginie, M.; Bonin, J.; Robert, M.; Wojcieszak, R.; Khodakov, A.Y. Highlights and challenges in the selective reduction of carbon dioxide to methanol. Nat. Rev. Chem 2021, 5, 564–579. [Google Scholar] [CrossRef]
- Jiao, X.; Zheng, K.; Hu, Z.; Sun, Y.; Xie, Y. Broad-Spectral-Response Photocatalysts for CO2 Reduction. ACS Cent. Sci. 2020, 6, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’kEeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Zhu, Z.; Xing, X.; Qi, Q.; Li, H.; Han, D.; Song, X.; Tang, X.; Ng, Y.H.; Huo, P. Regulation CN reduction of CO2 products selectivity by adjusting the number of V sites and mechanism exploration. Fuel 2025, 388, 134509. [Google Scholar] [CrossRef]
- Xu, W.; Pei, X.; Diercks, C.S.; Lyu, H.; Ji, Z.; Yaghi, O.M. A Metal-Organic Framework of Organic Vertices and Polyoxometalate Linkers as a Solid-State Electrolyte. J. Am. Chem. Soc. 2019, 141, 17522–17526. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal-organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Senkovska, I.; Bon, V.; Mosberger, A.; Wang, Y.; Kaskel, S. Adsorption and Separation by Flexible MOFs. Adv. Mater. 2025, 2414724. [Google Scholar] [CrossRef]
- Li, D.; Yadav, A.; Zhou, H.; Roy, K.; Thanasekaran, P.; Lee, C. Advances and Applications of Metal-Organic Frameworks (MOFs) in Emerging Technologies: A Comprehensive Review. Glob. Challenges 2023, 8, 2300244. [Google Scholar] [CrossRef]
- Stanley, P.M.; Ramm, V.; Fischer, R.A.; Warnan, J. Analysis of metal-organic framework-based photosynthetic CO2 reduction. Nat. Synth. 2024, 3, 307–318. [Google Scholar] [CrossRef]
- Zhu, C.; Hou, J.; Wang, X.D.; Wang, S.; Xu, H.; Hu, J.; Jing, L.; Wang, S. Optimizing ligand-to-metal charge transfer in metal-organic frameworks to enhance photocatalytic performance. Chem. Eng. J. 2024, 499, 156527. [Google Scholar] [CrossRef]
- Behera, D.; Priyadarshini, P.; Parida, K. ZIF-8 metal–organic frameworks and their hybrid materials: Emerging photocatalysts for energy and environmental applications. Dalton Trans. 2025, 54, 2681–2708. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P.M.; Haimerl, J.; Shustova, N.B.; Fischer, R.A.; Warnan, J. Merging molecular catalysts and metal-organic frameworks for photocatalytic fuel production. Nat. Chem. 2022, 14, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Guo, Q.; Wang, H.; Liu, M.; Zuo, C. Research progress of MOF-based materials in the photocatalytic CO2 reduction. Carbon Resour. Convers. 2024, 7, 100211. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Q. MOF-based materials for photo-and electrocatalytic CO2 reduction. EnergyChem 2020, 2, 100033. [Google Scholar] [CrossRef]
- Li, T.; Wang, P.; He, M.; Zhang, T.; Yang, C.; Li, Z. Metal-organic frameworks for photocatalytical carbon dioxide reduction reaction. Coord. Chem. Rev. 2024, 521, 216179. [Google Scholar] [CrossRef]
- Zhou, Q.; Guo, Y.; Zhu, Y. Reticular copper dual sites embedded with semiconductor particles for selective CO2-to-C2H4 photoreduction. Nat. Catalysis 2025, 8, 728–739. [Google Scholar] [CrossRef]
- De Almeida, J.C.; Wang, Y.J.; Rodrigues, T.A.; Nunes, P.H.H.; De Mendonca, V.R.; Falsetti, P.H.E.; Savazi, L.V.; He, T.; Bardakova, A.V.; Rudakova, A.V.; et al. Copper-based Materials for Photo and Electrocatalytic Process: Advancing Renewable Energy and Environmental Applications. Adv. Funct. Mater. 2025, 2502901. [Google Scholar] [CrossRef]
- Liu, X.; Huang, S.; Li, G.; Chen, X.; Peng, J. Construction of molecular compartments on the HKUST-1 for space-limited enhancement of visible light CO2 reduction. J. Colloid Interface Sci. 2025, 690, 137347. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Sun, Z.C.; Peng, C.; Wang, A.J. Microenvironment Regulation, Promoting CO2 Conversion to Mono-and Multi-carbon Products over Cu-Based Catalysts. Ind. Eng. Chem. Res. 2024, 63, 21613–21644. [Google Scholar] [CrossRef]
- Fan, L.; Li, F.; Liu, T.; Huang, J.E.; Miao, R.K.; Yu, Y.; Feng, S.; Tai, C.; Hung, S.; Tsai, H.-J.; et al. Atomic-level Cu active sites enable energy-efficient CO2 electroreduction to multicarbon products in strong acid. Nat. Synthesis 2024, 4, 262–270. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Yang, C.; Li, S.; Long, C.; Zhuang, Z.; Li, Q.; Guo, Z.; Huang, X.; Tang, Z.; et al. Ultralow Coordination Copper Sites Compartmentalized within Ordered Pores for Highly Efficient Electrosynthesis of n-Propanol from CO2. J. Am. Chem. Soc. 2025, 147, 6688–6697. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zhao, Z.-J.; Zhang, G.; Yang, P.; Li, L.; Gao, H.; Liu, S.; Chang, X.; Chen, S.; Wang, T.; et al. The nature of active sites for carbon dioxide electroreduction over oxide-derived copper catalysts. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Cairnie, D.R.; Troya, D.; Kumar, N.; Yang, X.; Morris, A.J. Photoinduced Dynamic Ligation in Metal-Organic Frameworks. J. Am. Chem. Soc. 2023, 146, 101–105. [Google Scholar] [CrossRef]
- Li, D.; Kassymova, M.; Cai, X.; Zang, S.; Jiang, H. Photocatalytic CO2 reduction over metal-organic framework-based materials. Coord. Chem. Reviews 2020, 412, 213262. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Yang, S.; García, H. Metal-organic framework heterojunctions for photocatalysis. Chem. Soc. Reviews 2024, 53, 3002–3035. [Google Scholar] [CrossRef]
- Huang, N.-Y.; Zheng, Y.-T.; Chen, D.; Chen, Z.-Y.; Huang, C.-Z.; Xu, Q. Reticular framework materials for photocatalytic organic reactions. Chem. Soc. Rev. 2023, 52, 7949–8004. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, P.; Qian, Y.; Xiao, J.; Chao, Z.; Jiang, H. Metal-organic frameworks for organic transformations by photocatalysis and photothermal catalysis. Chem. Soc. Reviews 2024, 53, 9378–9418. [Google Scholar] [CrossRef]
- Chang, X.-L.; Yan, T.; Pan, W.-G. Toward Tailoring Metal-Organic Frameworks for Photocatalytic Reduction of CO2 to Fuels. Cryst. Growth Des. 2024, 24, 2619–2644. [Google Scholar] [CrossRef]
- Huang, N.Y.; Li, B.; Wu, D.J.; Chen, Z.Y.; Shao, B.; Chen, D.; Zheng, Y.T.; Wang, W.J.; Yang, C.Z.; Gu, M.; et al. Crystal Engineering of MOF-Derived Bimetallic Oxide Solid Solution Anchored with Au Nanoparticles for Photocatalytic CO2 Reduction to Syngas and C2 Hydrocarbons. Angew. Chem. Int. Ed. 2024, 63, e202319177. [Google Scholar] [CrossRef]
- Huang, T.; Yang, H.; Xu, W.; Sun, Y.; Pang, H. Research progress of MOF-based materials in photocatalytic reduction of CO2 and N2. Chem. Catal. 2024, 4, 100211. [Google Scholar] [CrossRef]
- Song, B.; Song, W.T.; Liang, Y.H.; Liu, Y.; Li, B.W.; Li, H.; Zhang, L.; Ma, Y.H.; Ye, R.Q.; Tang, B.Z.; et al. Direct Synthesis of Topology-Controlled BODIPY and CO2-Based Zirconium Metal-Organic Frameworks for Efficient Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2025, 64, e202421248. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, X.; Sun, Y.T.; Chen, K.Z.; Zhao, H.Y.; Lin, Q.Q.; Li, R.; Li, J.R. Modular MOF-red phosphorus hetero-structure for synergistic capture and photocatalytic reduction of dilute CO2 into ethane. Appl. Catal. B Environ. 2025, 378, 125568. [Google Scholar] [CrossRef]
- Wu, Y.; Qu, Y.; Su, C.; Yang, X.; Yang, Y.; Zhang, Y.; Huang, W. Enhanced Photoinduced Carrier Separation in Fe-MOF-525/CdS for Photocatalytic Hydrogen Evolution: Improved Catalytic Dynamics with Specific Active Sites. Inorg. Chem. 2023, 62, 21290–21298. [Google Scholar] [CrossRef]
- Hamad, S.; Hernandez, N.C.; Aziz, A.; Ruiz-Salvador, A.R.; Calero, S.; Grau-Crespo, R. Electronic structure of porphyrin-based metal–organic frameworks and their suitability for solar fuel production photocatalysis. J. Mater. Chem. A 2015, 3, 23458–23465. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Z.; Xie, L.; Liu, C.; Cai, L.; Wu, X.; Liu, T. Delocalized Orbitals Over Metal Clusters and Organic Linkers Enable Boosted Charge Transfer in Metal-Organic Framework for Overall CO2 Photoreduction. Angew. Chemie 2024, 136, e202411508. [Google Scholar] [CrossRef]
- Li, K.; Peng, B.; Peng, T. Recent Advances in Heterogeneous Photocatalytic CO2 Conversion to Solar Fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Grau-Crespo, R.; Aziz, A.; Collins, A.W.; Crespo-Otero, R.; Hernández, N.C.; Rodríguez-Albelo, L.M.; Ruiz-Salvador, A.R.; Calero, S.; Hamad, S. Modelling a Linker Mix-and-Match Approach for Controlling the Optical Excitation Gaps and Band Alignment of Zeolitic Imidazolate Frameworks. Angew. Chemie 2016, 128, 16246–16250. [Google Scholar] [CrossRef]
- Sun, K.; Qian, Y.; Jiang, H.L. Metal-Organic Frameworks for Photocatalytic Water Splitting and CO2 Reduction. Angew. Chem. Int. Ed. Engl. 2023, 62, e202217565. [Google Scholar] [CrossRef]
- White, J.L.; Baruch, M.F.; Pander, J.E.; Hu, Y.; Fortmeyer, I.C.; Park, J.E.; Zhang, T.; Liao, K.; Gu, J.; Yan, Y.; et al. Light-Driven Heterogeneous Reduction of Carbon Dioxide: Photocatalysts and Photoelectrodes. Chem. Rev. 2015, 115, 12888–12935. [Google Scholar] [CrossRef]
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; García-Martínez, J. Mesoporous materials for clean energy technologies. Chem. Soc. Rev. 2014, 43, 7681–7717. [Google Scholar] [CrossRef]
- Karmakar, S.; Barman, S.; Rahimi, F.A.; Rambabu, D.; Nath, S.; Maji, T.K. Confining charge-transfer complex in a metal-organic framework for photocatalytic CO2 reduction in water. Nat. Commun. 2023, 14, 4508. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.X.; Yu, H.; Shi, J.J.; Li, B.; Wu, L.X.; Wang, M. Dislocated Bilayer MOF Enables High-Selectivity Photocatalytic Reduction of CO2 to CO. Adv. Mater. 2023, 35, 2209814. [Google Scholar] [CrossRef]
- Liu, K.-G.; Bigdeli, F.; Panjehpour, A.; Larimi, A.; Morsali, A.; Dhakshinamoorthy, A.; Garcia, H. Metal organic framework composites for reduction of CO2. Coord. Chem. Rev. 2023, 493, 493. [Google Scholar] [CrossRef]
- Qian, Z.; Zhang, R.; Xiao, Y.; Huang, H.; Sun, Y.; Yang, C.; Ma, T.; Sun, X. Trace to the Source: Self-Tuning of MOF Photocatalysts. Adv. Energy Materials 2023, 13, 2300086. [Google Scholar] [CrossRef]
- Li, D.; Wang, K.; Li, J.; Li, Z.; Wang, H.; Wang, Y. Strategies for optimizing the efficiency and selectivity of photocatalytic aqueous CO2 reduction: Catalyst design and operating conditions. Nano Energ. 2024, 133, 110460. [Google Scholar] [CrossRef]
- Gao, C.-Y.; Tian, H.-R.; Ai, J.; Li, L.-J.; Dang, S.; Lan, Y.-Q.; Sun, Z.-M. A microporous Cu-MOF with optimized open metal sites and pore spaces for high gas storage and active chemical fixation of CO2. Chem. Commun. 2016, 52, 11147–11150. [Google Scholar] [CrossRef]
- Gupta, A.K.; Guha, N.; Krishnan, S.; Mathur, P.; Dhirendra, K. A Three-Dimensional Cu(II)-MOF with Lewis acid−base dual functional sites for Chemical Fixation of CO2 via Cyclic Carbonate Synthesis. J. CO2 Util. 2020, 39, 101173. [Google Scholar] [CrossRef]
- Stolar, T.; Prasnikar, A.; Martinez, V.; Karadeniz, B.; Bjelic, A.; Mali, G.; Friscic, T.; Likozar, B.; Uzarevic, K. Scalable Mech-anochemical Amorphization of Bimetallic Cu-Zn MOF-74 Catalyst for Selective CO2 Reduction Reaction to Methanol. ACS Appl. Mater. Interfaces 2021, 13, 3070–3077. [Google Scholar] [CrossRef] [PubMed]
- Tapiador, J.; Leo, P.; Gándara, F.; Calleja, G.; Orcajo, G. Robust Cu-URJC-8 with mixed ligands for mild CO2 cycloaddition reaction. J. CO2 Util. 2022, 64, 102166. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, Y.; Chen, G.L.; Xu, H.; Yin, W.; Wang, B.; Zhu, X.; Ning, X.; Chu, P.K.; Wang, X.; et al. Enhanced CO2 Photoreduction in Pure Water Systems by Surface-Reconstructed Asymmetric Mn−Cu Sites. Appl. Catalysis B Environ. Energy 2024, 361, 124617. [Google Scholar] [CrossRef]
- Heine, T.; Dinca, M.; Zhu, G. Physical Phenomena in Porous Frameworks. Accounts Chem. Res. 2025, 58, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kang, M.; Ha, H.; Hong, C.S.; Kim, M. Multiple functional groups in metal–organic frameworks and their positional regioisomerism. Coord. Chem. Rev. 2021, 438, 213892. [Google Scholar] [CrossRef]
- Ren, D.; Xia, H.; Zhou, K.; Wu, S.; Liu, X.; Wang, X.; Li, J. Tuning and Directing Energy Transfer in the Whole Visible Spectrum through Linker Installation in Metal–Organic Frameworks. Angew. Chem. Int. Ed. Engl. 2021, 60, 25048–25054. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Bose, S.; Wang, X.; Schweitzer, N.M.; Malliakas, C.D.; Xie, H.; Duncan, J.; Kirlikovali, K.O.; Yildirim, T.; Farha, O.K. Integrated CO2 Capture and Conversion by a Robust Cu(I)-Based Metal-Organic Framework. J. Am. Chem. Soc. 2024, 146, 27006–27013. [Google Scholar] [CrossRef]
- Miller, R.G.; Warren, M.R.; Allan, D.R.; Brooker, S. Direct Crystallographic Observation of CO2 Captured in Zig Zag Channels of a Copper(I) Metal-Organic Framework. Inorg. Chem. 2020, 59, 6376–6381. [Google Scholar] [CrossRef]
- Trenholme, W.J.F.; Kolokolov, D.I.; Bound, M.; Argent, S.P.; Gould, J.A.; Li, J.; Barnett, S.A.; Blake, A.J.; Stepanov, A.G.; Besley, E.; et al. Selective Gas Uptake and Rotational Dynamics in a (3, 24)-Connected Metal–Organic Framework Material. J. Am. Chem. Soc. 2021, 143, 3348–3358. [Google Scholar] [CrossRef]
- Benson, O.; Da Silva, I.; Argent, S.P.; Cabot, R.; Savage, M.; Godfrey, H.G.W.; Yan, Y.; Parker, S.F.; Manuel, P.; Lennox, M.J.; et al. Amides Do Not Always Work: Observation of Guest Binding in an Amide–Functionalized Porous Metal-Organic Framework. J. Am. Chem. Soc. 2016, 138, 14828–14831. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Rungtaweevoranit, B.; Parlińska-Wojtan, M.; Pei, X.; Yaghi, O.M.; Behm, R.J. Highly Active and Stable Single–Atom Cu Catalysts Supported by a Metal–Organic Framework. J. Am. Chem. Soc. 2019, 141, 5201–5210. [Google Scholar] [CrossRef]
- Chen, S.; Li, W.; Jiang, W.; Yang, J.; Zhu, J.; Wang, L.; Ou, H.; Zhuang, Z.; Chen, M.; Sun, X.; et al. MOF Encapsulating N-Heterocyclic Carbene-Ligated Copper Single-Atom Site Catalyst towards Efficient Methane Electrosynthesis. Angew. Chemie Int. Ed. 2021, 61, e202114450. [Google Scholar] [CrossRef]
- Rosado, A.; Popa, I.-M.; Markeb, A.A.; Moral-Vico, J.; Naughton, E.M.; Eckhardt, H.-G.; Ayllon, J.A.; López-Periago, A.M.; Domingo, C.; Negahdar, L. Multifunctionalized zirconium-based MOF as a novel support for dispersed copper: Application in CO2 adsorption and catalytic conversion. J. Mater. Chem. A 2024, 12, 21758–21771. [Google Scholar] [CrossRef]
- Zhang, Q.; Tsai, H.J.; Li, F.; Wei, Z.; He, Q.; Ding, J.; Liu, Y.; Lin, Z.-Y.; Yang, X.; Chen, Z.; et al. Boosting the Proton-coupled Electron Transfer via Fe−P Atomic Pair for Enhanced Electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2023, 62, e202311550. [Google Scholar] [CrossRef]
- Wang, X.-K.; Liu, J.; Zhang, L.; Dong, L.-Z.; Li, S.-L.; Kan, Y.-H.; Li, D.-S.; Lan, Y.-Q. Monometallic Catalytic Models Hosted in Stable Metal-Organic Frameworks for Tunable CO2 Photoreduction. ACS Catal. 2019, 9, 1726–1732. [Google Scholar] [CrossRef]
- Feng, X.; Pi, Y.; Song, Y.; Brzezinski, C.; Xu, Z.; Li, Z.; Lin, W. Metal-Organic Frameworks Significantly Enhance Photocatalytic Hydrogen Evolution and CO2 Reduction with Earth-Abundant Copper Photosensitizers. J Am Chem Soc 2020, 142, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Baluk, M.A.; Pieczyńska, A.; Mazierski, P.; Kroczewska, M.; Nikiforow, K.; Mikołajczyk, A.; Dołżonek, J.; Łuczak, J.; Zaleska-Medynska, A. Effect of copper and silver modification of NH2-MIL-125(Ti) on the photoreduction of carbon dioxide to formic acid over this framework under visible-light irradiation. Appl. Catalysis B Environ. Energy 2024, 354, 124107. [Google Scholar] [CrossRef]
- Guo, S.; Kong, L.; Wang, P.; Yao, S.; Lu, T.; Zhang, Z. Switching Excited State Distribution of Metal-Organic Framework for Dramatically Boosting Photocatalysis. Angew. Chemie Int. Ed. 2022, 61, e202206193. [Google Scholar] [CrossRef]
- Goyal, S.; Shaharun, M.S.; Kait, C.F.; Abdullah, B.; Ameen, M. Photoreduction of Carbon Dioxide to Methanol over Copper Based Zeolitic Imidazolate Framework-8: A New Generation Photocatalyst. Catalysts 2018, 8, 1–19. [Google Scholar] [CrossRef]
- Wu, H.; Kong, X.Y.; Wen, X.; Chai, S.P.; Lovell, E.C.; Tang, J.; Ng, Y.H. Metal-Organic Framework Decorated Cuprous Oxide Nanowires for Long-lived Charges Applied in Selective Photocatalytic CO2 Reduction to CH4. Angew. Chem. Int. Ed. Engl. 2021, 60, 8455–8459. [Google Scholar] [CrossRef]
- Xie, S.; Deng, C.; Huang, Q.; Zhang, C.; Chen, C.; Zhao, J.; Sheng, H. Facilitated Photocatalytic CO2 Reduction in Aerobic Environment on a Copper-Porphyrin Metal-Organic Framework. Angew. Chem. Int. Ed. Engl. 2023, 62, e202216717. [Google Scholar] [CrossRef]
- Chen, D.; Chu, B.; Li, F.; Zheng, Y.T.; Lu, Y.; Shao, B.; Li, L.; Huang, N.Y.; Xu, Q. Synergistic Catalysis by Cu Single Atoms and Atomically Cu-Doped Au Nanoparticles in a Metal-Organic Framework for Photocatalytic CO2 Reduction to C2H6. J. Am. Chem. Soc. 2025, 147, 22705–22713. [Google Scholar] [CrossRef]
- Qin, C.; Li, X.; Wang, T.; Xu, Z.; Chen, K.-J.; Pan, F. Metal-Organic Frameworks-Based Copper Catalysts for CO2 Electroreduction Toward Multicarbon Products. Exploration 2025, 5, 270011. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Wang, B.; Huang, C.; Chu, P.K. Progress in Cu-Based Catalyst Design for Sustained Electrocatalytic CO2 to C2+ Conversion. Adv. Sci. 2025, 12, 2416597. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ji, S.; Chen, Y.; Wang, D. Synthetic strategies for MOF-based single-atom catalysts for photo-and electro-catalytic CO2 reduction. IScience 2022, 25, 104177. [Google Scholar] [CrossRef]
- Scatena, R.; Guntern, Y.T.; Macchi, P. Electron Density and Dielectric Properties of Highly Porous MOFs: Binding and Mobility of Guest Molecules in Cu3(BTC)2 and Zn3(BTC)2. J. Am. Chem. Soc. 2019, 141, 9382–9390. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, U.; Siahrostami, S. Copper-based metal-organic frameworks for CO2 reduction: Selectivity trends, design paradigms, and perspectives. Catalysis Sci. Technol. 2023, 13, 3740–3761. [Google Scholar] [CrossRef]
- Wang, D.; Huang, R.; Liu, W.; Sun, D.; Li, Z. Fe-Based MOFs for Photocatalytic CO2 Reduction: Role of Coordination Un-saturated Sites and Dual Excitation Pathways. ACS Catal. 2014, 4, 4254–4260. [Google Scholar] [CrossRef]
- Zhang, L.; Ran, J.; Qiao, S.-Z.; Jaroniec, M. Characterization of semiconductor photocatalysts. Chem. Soc. Rev. 2019, 48, 5184–5206. [Google Scholar] [CrossRef]
- Mon, M.; Bruno, R.; Tiburcio, E.; Grau-Atienza, A.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V.; Fuoco, A.; Esposito, E.; Monteleone, M.; Jansen, J.C.; et al. Efficient Gas Separation and Transport Mechanism in Rare Hemilabile Metal-Organic Framework. Chem. Mater. 2019, 31, 5856–5866. [Google Scholar] [CrossRef]
- Saadh, M.J.; Mustafa, M.A.; Altalbawy, F.M.A.; Kaur, M.; Sharma, R.; Juraev, N.; Ghazy, H.; Abdulwahid, A.S.; Muften, N.F.; Saud, H.R.; et al. Incorporation anthracene and Cu to NH2-Zr-UiO-67 metal-organic framework: Introducing the simul-taneous selectivity and efficiency in photocatalytic CO2 reduction to ethanol. J. Mol. Struct. 2024, 1318, 139329. [Google Scholar] [CrossRef]
- Batoo, K.M.; Ali, E.; Hussein, B.A.; Al-khalidi, A.; Altimari, U.S.; Hussain, S.; Kareem, S.H.; Abid, M.K.; Alawadi, A.; Ali, I. The cooperative performance of iodo and copper in a Zr-based UiO-67 metal-organic framework for highly selective photocatalytic CO2 reduction to methanol. J. Mol. Struct. 2024, 1307, 137927. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, L.; Feng, H.; Liu, H.; Wang, Y. Improved CO2 Photoreduction Activity and Selectivity Using an Indirect Z-Scheme Heterojunction of ZIF-67 and Bi4O5Br2 Nanostructures. ACS Appl. Nano Mater. 2024, 7, 27846–27857. [Google Scholar] [CrossRef]
- Ling, J.; Zhou, A.; Wang, W.; Jia, X.; Ma, M.; Li, Y. One-Pot Method Synthesis of Bimetallic MgCu-MOF-74 and Its CO2 Adsorption under Visible Light. ACS Omega 2022, 7, 19920–19929. [Google Scholar] [CrossRef]
- Khalilzadeh, A.; Shariati, A. Fe-N-TiO2/CPO-Cu-27 nanocomposite for superior CO2 photoreduction performance under visible light irradiation. Sol. Energy 2019, 186, 166–174. [Google Scholar] [CrossRef]
- Li, J.; Li, K.; Du, J.; Yang, H.; Song, C.; Guo, X. Impact of transition metal incorporation on the photocatalytic CO2 reduction activity of polymeric carbon nitride. J. CO2 Util. 2022, 64, 101173. [Google Scholar] [CrossRef]
- Yong, C.; Lu, G.; Wang, X.; Shi, G.; Wang, Y.; Xie, X.; Sun, J. Alkali-Metal-Modified Al-PMOF for Enhanced CO2 Adsorption and Photocatalytic Reduction. ACS Appl. Eng. Mater. 2025, 3, 1513–1521. [Google Scholar] [CrossRef]
- Batoo, K.M.; Ali, E.A.; Hussein, S.A.; Chandra, S.; Abdulwahid, A.S.; Kareem, S.; Hussain, S.; Omran, A.A.; Abid, M.K.; Alawadi, A. UiO-67 metal-organic frameworks with dual amino/iodo functionalization, and mixed Zr/Ce clusters: Highly selective and efficient photocatalyst for CO2 transformation to methanol. J. Mol. Struct. 2024, 1312, 138492. [Google Scholar] [CrossRef]
- Ding, L.; Bai, F.; Borjigin, B.; Li, Y.; Li, H.; Wang, X. Embedding Cs2AgBiBr6 QDs into Ce-UiO-66-H to in situ construct a novel bifunctional material for capturing and photocatalytic reduction of CO2. Chem. Eng. J. 2022, 446, 137102. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Kafizas, A.; Zafeiratos, S.; Petit, C. CO2 capture and photocatalytic reduction using bifunctional TiO2/MOF nanocomposites under UV–vis irradiation. Appl. Catal. B Environ. 2017, 210, 131–140. [Google Scholar] [CrossRef]





| Materials | CO2 Capacity | Conditions for Adsorption | Photocatalytic Conversion Product (μmol/g/h) | Conditions for Photocatalysis | Ref. |
|---|---|---|---|---|---|
| Cu/An (1:2)@NZU67 | 71.6 cm3 g−1 | 298 K 1 bar | EtOH: 624.17 Selectivity: 100% | Light: 450 nm, TEOA (0.3 M), CH3CN/H2O (4:1 v/v) | [98] |
| Cu/I2-Zr-BPDC/BPyDC | 48.6 cm3 g−1 | 298 K 1 bar | MeOH: 9.4 | Light: 450 nm, Ru(Bipy)3Cl2 (4 mg), TEOA (0.3 M) | [99] |
| Cu-Zr-BPyDC | 31.1 cm3 g−1 | MeOH: 1.88 | |||
| Zr-I2-BPDC | 28.7 cm3 g−1 | / | |||
| Zr-BPyDC | 27.3 cm3 g−1 | / | |||
| 1% ZIF-67CoCu(1:1)/ Bi4O5Br2 | 4.22 cm3 g−1 | 273 K 750 mmHg | CO: 6469.88 Selectivity: 97% | UV−visible light, TEOA (0.3 M), BIH (10 mg), CH3CN (4 mL) H2O (1 mL) | [100] |
| Mg0.4Cu0.6-MOF-74 | 4.58 mmol·g−1 | 298 K 1 bar | CO: 6.18 | Visible light, H2O (2 mL) | [101] |
| Fe-N-TiO2/CPO-Cu | 1.07 mmol·g−1 | 298 K 1 bar | CH4: 47.52 MeOH: 2.17 | Light: 350–600 nm, H2O | [102] |
| CuCN | 7.4 cm3 g−1 | 298 K 1 bar | CO: 246 Selectivity: 88% | Visible light, TEOA (1 mL), bipyridine (100 μL), CH3CN (4 mL), CoCl2 (2 μmol), H2O (1 mL) | [103] |
| 1-K-Al-PMOF | 3.46 mmol·g−1 | 273.15 K 1 bar | CO: 587.2 | Visible light, CH3CN/H2O/TEOA (8:2:1 v/v) | [104] |
| Zr/Ce29%-UiO-67(NH 2, I) | 78.4 cm3 g−1 | 298 K 1 bar | MeOH: 44.7 Selectivity: 100% | Light: 450 nm, TEOA (0.3 M), CH3CN/H2O (4:1 v/v) | [105] |
| 20Cs2AgBiBr6/Ce-UiO-66-H | 58.8 cm3 g−1 | 298 K 1 bar | CO: 309.01 | 300 W Xe lamp, H2O (50 mL) | [106] |
| NH2-UiO-66 | 1.3 mmol·g−1 | 298 K 1 bar | CO: 1.5 | UV−visible light, gas/solid reactor | [107] |
| 1-TiMOF | 0.32 mmol·g−1 | 298 K 1 bar | CO: 3.74 | ||
| 2-TiMOF | 0.36 mmol·g−1 | 298 K 1 bar | CO: 4.24 | ||
| 3-TiMOF | 0.47 mmol·g−1 | 298 K 1 bar | CO: 3.37 | ||
| 4-TiMOF | 0.56 mmol·g−1 | 298 K 1 bar | CO: 2.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, E.; Yan, Y.; Yan, Y. Copper Active Sites in Metal–Organic Frameworks Advance CO2 Adsorption and Photocatalytic Conversion. Catalysts 2025, 15, 856. https://doi.org/10.3390/catal15090856
Jiang E, Yan Y, Yan Y. Copper Active Sites in Metal–Organic Frameworks Advance CO2 Adsorption and Photocatalytic Conversion. Catalysts. 2025; 15(9):856. https://doi.org/10.3390/catal15090856
Chicago/Turabian StyleJiang, Enhui, Yan Yan, and Yongsheng Yan. 2025. "Copper Active Sites in Metal–Organic Frameworks Advance CO2 Adsorption and Photocatalytic Conversion" Catalysts 15, no. 9: 856. https://doi.org/10.3390/catal15090856
APA StyleJiang, E., Yan, Y., & Yan, Y. (2025). Copper Active Sites in Metal–Organic Frameworks Advance CO2 Adsorption and Photocatalytic Conversion. Catalysts, 15(9), 856. https://doi.org/10.3390/catal15090856






