Anodic Catalytic Oxidation of Sulfamethoxazole: Efficiency and Mechanism on Co3O4 Nanowire Self-Assembled CoFe2O4 Nanosheet Heterojunction
Abstract
1. Introduction
2. Results and Discussion
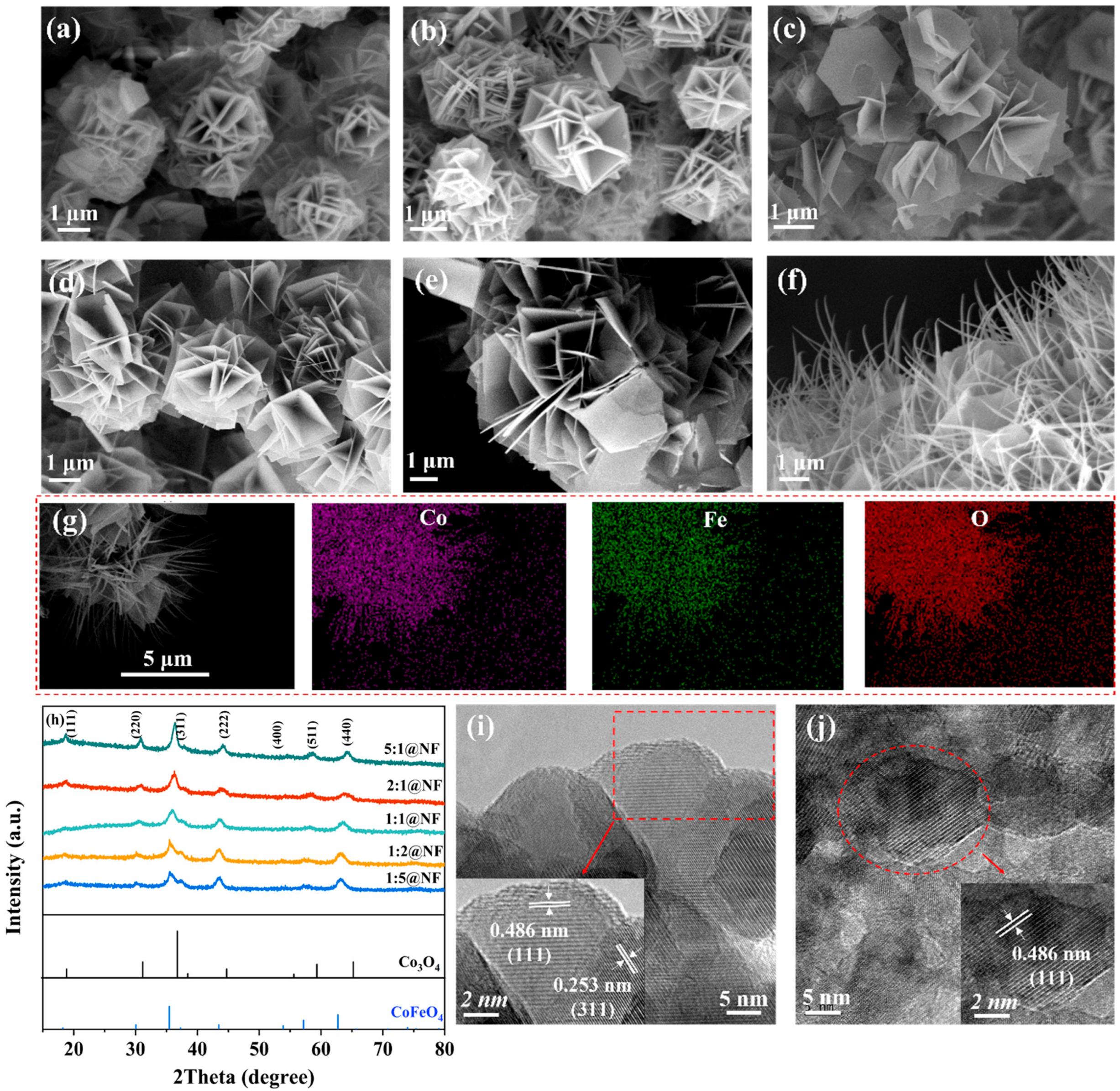
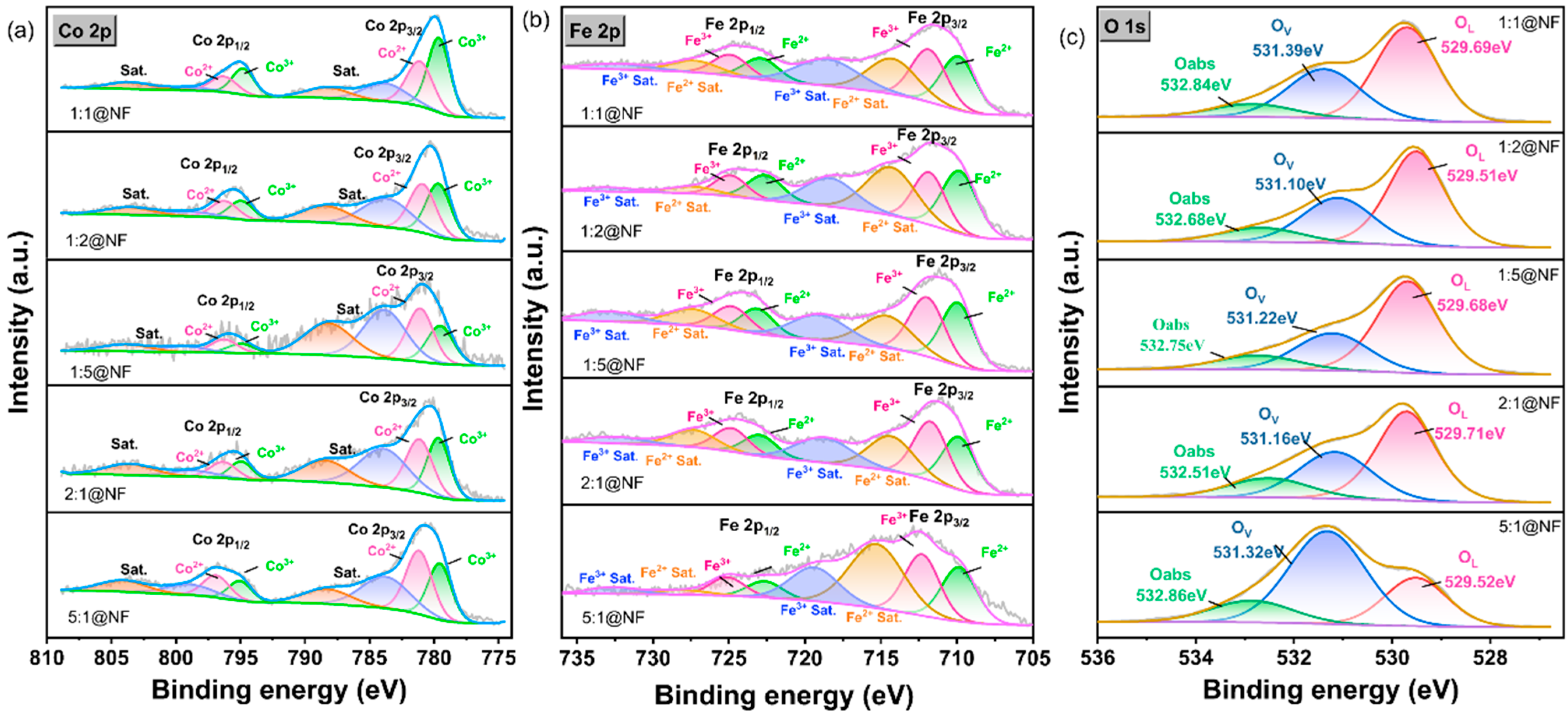
2.1. Physical Characterization
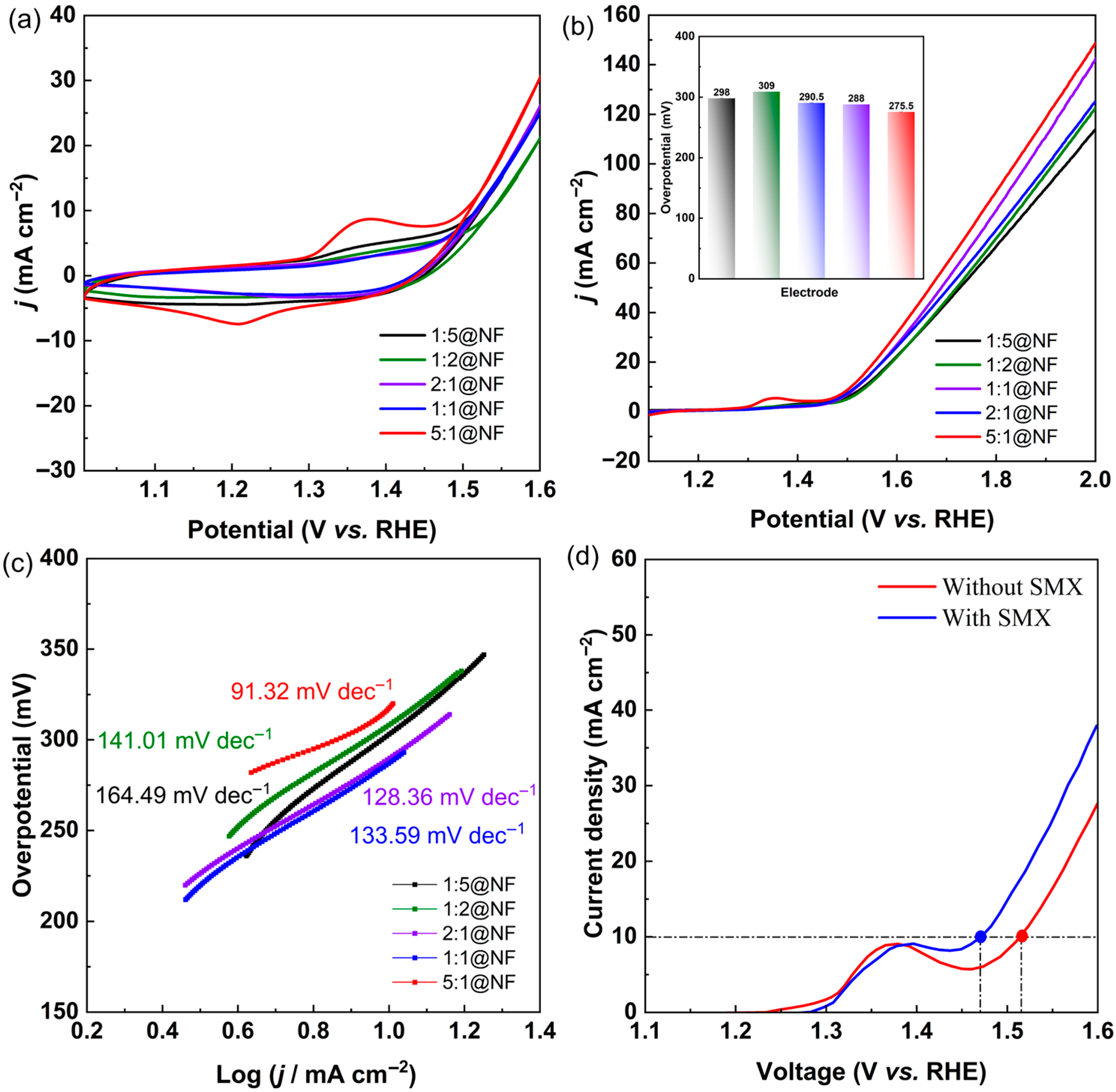
2.2. Electrochemical Characterization
2.3. SMX Degradation via Anodic Oxidation
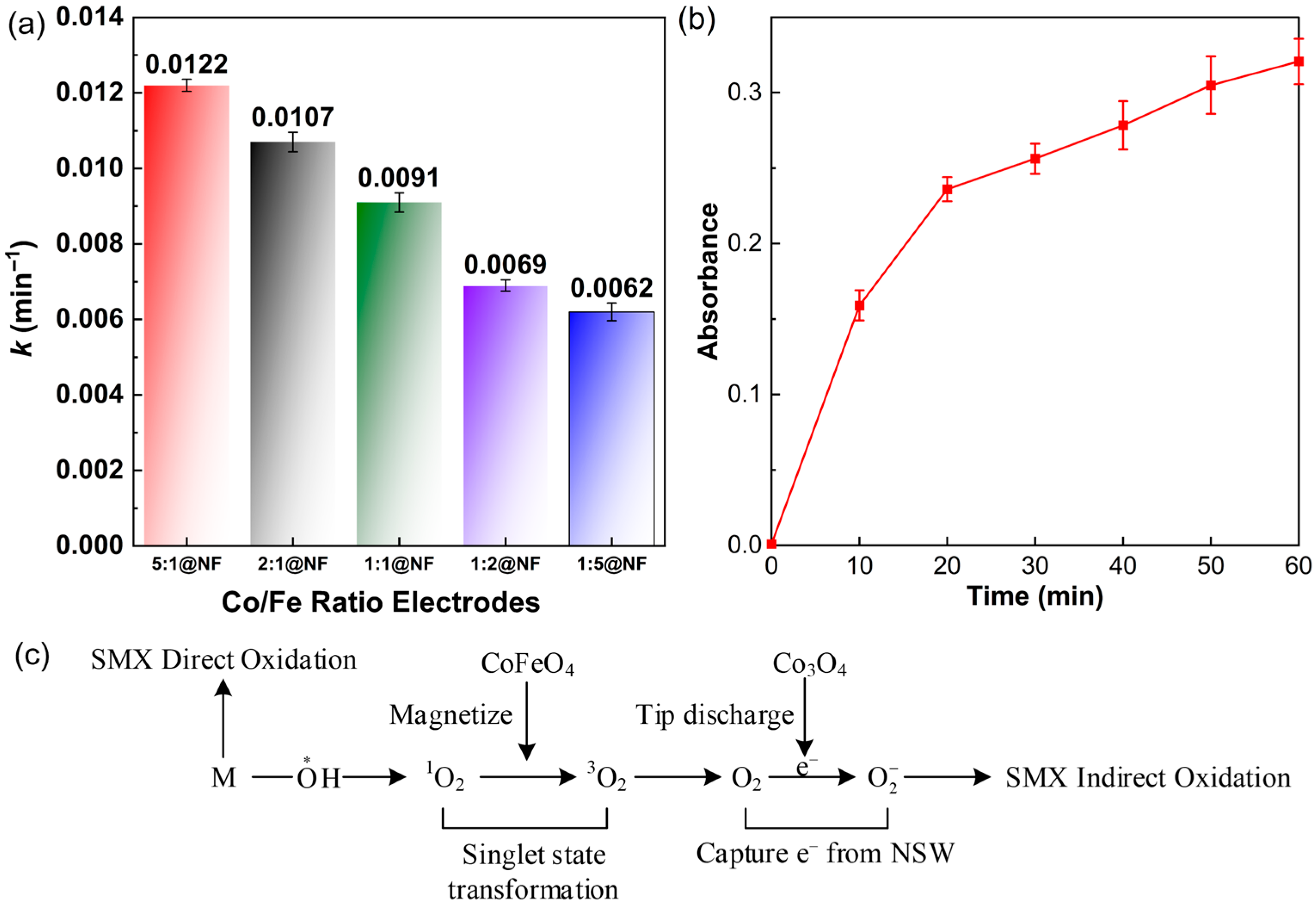
2.4. Mechanism and Kinetics of Generation of ·O2−
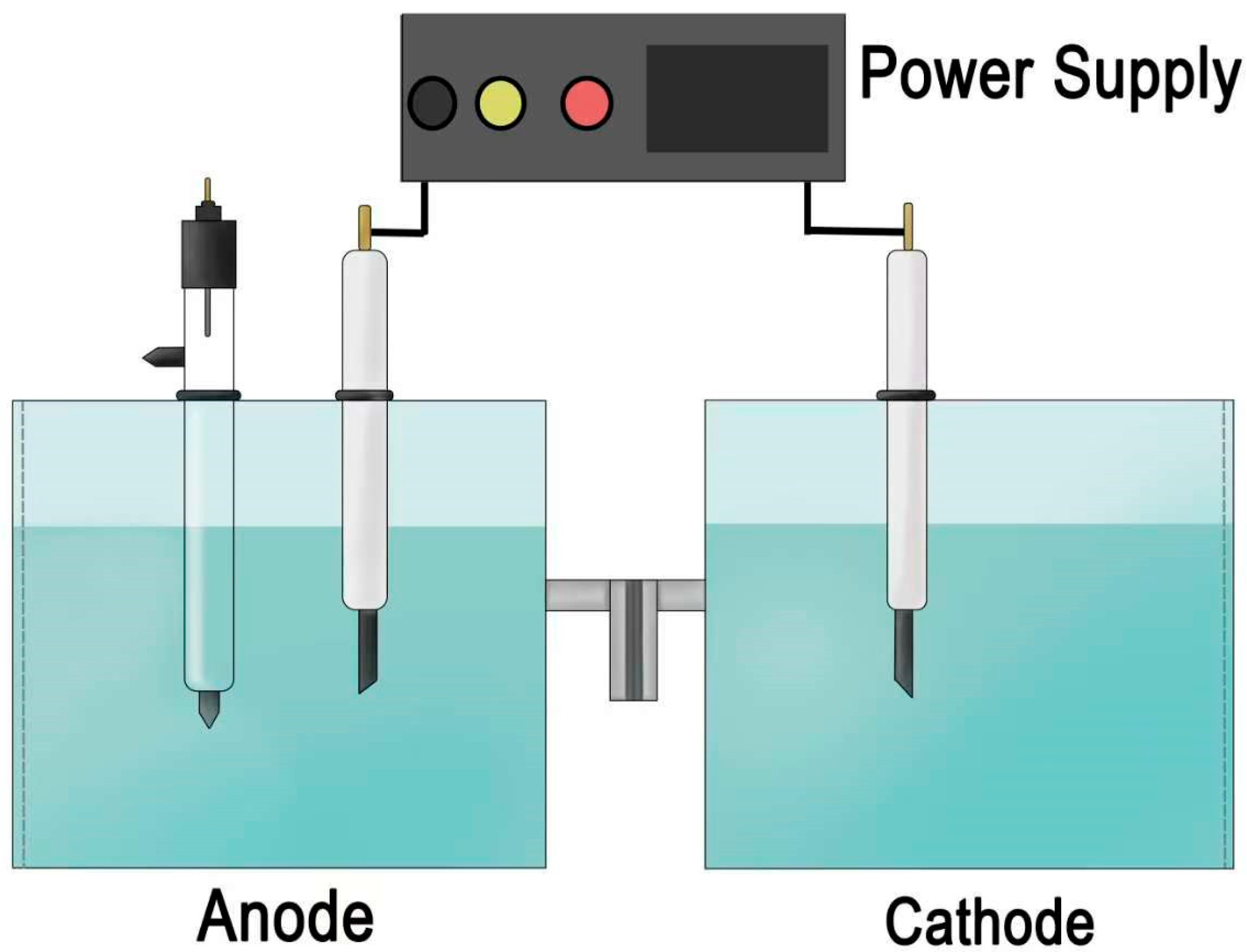
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Synthesis of CoFe2O4@NF Electrodes in Different Ratios of Co/Fe
3.3. Characterizations
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, J. Chemical-assisted hydrogen electrocatalytic evolution reaction (CAHER). J. Mater. Chem. A 2018, 6, 13538–13548. [Google Scholar] [CrossRef]
- Wang, Z.; Goddard, W.A.; Xiao, H. Potential-dependent transition of reaction mechanisms for oxygen evolution on layered double hydroxides. Nat. Commun. 2023, 14, 4228. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cao, X.; Jiao, L. Progress in hydrogen production coupled with electrochemical oxidation of small molecules. Angew. Chem. 2022, 134, e202213328. [Google Scholar] [CrossRef]
- Lidasan, J.J.B.; Iguchi, S.; Yamanaka, I. Pure hydrogen production by aqueous ethanol electrolysis on Pt–Ru–O anodes in a solid polymer electrolyte electrolysis cell. ACS Sustain. Chem. Eng. 2022, 10, 2921–2929. [Google Scholar] [CrossRef]
- Bambagioni, V.; Bevilacqua, M.; Bianchini, C.; Filippi, J.; Lavacchi, A.; Marchionni, A.; Vizza, F.; Shen, P.K. Self-sustainable production of hydrogen, chemicals, and energy from renewable alcohols by electrocatalysis. ChemSusChem 2010, 3, 851–855. [Google Scholar] [CrossRef]
- Zhang, Q.; Cui, C.; Wang, Z.; Deng, F.; Qiu, S.; Zhu, Y.; Jing, B. Mott Schottky CoSx-MoOx@NF heterojunctions electrode for H2 production and urea-rich wastewater purification. Sci. Total Environ. 2023, 858, 160170. [Google Scholar] [CrossRef]
- Zhang, Q.; Jing, B.; Qiu, S.; Cui, C.; Zhu, Y.; Deng, F. A mechanism in boosting H2 generation: Nanotip-enhanced local temperature and electric field with the boundary layer. J. Colloid Interface Sci. 2023, 629, 755–765. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, Y.; Wang, Z.; Jing, B.; Zhu, Y.; Qiu, S.; Cui, C.; Deng, F. Improved alkaline water electrolysis system for green energy: Sulfonamide antibiotic-assisted anodic oxidation integrated with hydrogen generation. J. Mater. Chem. A 2023, 11, 6129–6143. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, G.; Shao, P.; Ren, W.; Li, M.; Hu, Y.; Yang, L.; Shi, H.; Luo, X. A comparison of SMX degradation by persulfate activated with different nanocarbons: Kinetics, transformation pathways, and toxicity. Appl. Catal. B Environ. 2022, 310, 121345. [Google Scholar] [CrossRef]
- Jamesh, M.-I.; Harb, M. Tuning the electronic structure of the earth-abundant electrocatalysts for oxygen evolution reaction (OER) to achieve efficient alkaline water splitting—A review. J. Energy Chem. 2021, 56, 299–342. [Google Scholar] [CrossRef]
- Zhu, K.; Shi, F.; Zhu, X.; Yang, W. The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction. Nano Energy 2020, 73, 104761. [Google Scholar] [CrossRef]
- Jamesh, M.-I.; Kuang, Y.; Sun, X. Constructing earth-abundant 3D nanoarrays for efficient overall water splitting—A review. ChemCatChem 2019, 11, 1550–1575. [Google Scholar] [CrossRef]
- Li, X.; Hao, X.; Abudula, A.; Guan, G. Nanostructured catalysts for electrochemical water splitting: Current state and prospects. J. Mater. Chem. A 2016, 4, 11973–12000. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Duan, X.; Li, Y.; Fan, X.; Zhang, G.; Zhang, F.; Peng, W. Fine-tuning radical/nonradical pathways on graphene by porous engineering and doping strategies. ACS Catal. 2021, 11, 4848–4861. [Google Scholar] [CrossRef]
- Dong, H.; Qiang, Z.; Lian, J.; Qu, J. Promoted oxidation of diclofenac with ferrate (Fe(VI)): Role of ABTS as the electron shuttle. J. Hazard. Mater. 2017, 336, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Wang, H.; Fang, Y.; Li, Z.; Lu, S.; Wang, A. Performance and mechanism of SMX removal in an electrolysis-integrated tidal flow constructed wetland at low temperature. Chem. Eng. J. 2022, 434, 134494. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, G.; Liu, Y.; Liu, S.; Wang, S.; Fu, Y. Activated peracetic acid by Mn3O4 for sulfamethoxazole degradation: A novel heterogeneous advanced oxidation process. Chemosphere 2022, 306, 135506. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, F.; Da Silva, S.W.; Garg, V.K.; Oliveira, A.C.; Morais, P.C.; Franco Júnior, A.; Lima, E.C.D. The influence of cobalt population on the structural properties of CoxFe3−xO4. J. Appl. Phys. 2007, 101, 09M514. [Google Scholar] [CrossRef]
- Gil, A.F.; Benavides, O.; Vargas, S.M.; May, L.D.L.C.; Carachure, C.P. Synthesis and characterization of cobalt ferrite CoxFe3-xO4 nanoparticles by raman spectroscopy and X-Ray diffraction. Int. J. Metall. Met. Phys. 2020, 5, 47. [Google Scholar]
- Duan, J.-J.; Zhang, R.-L.; Feng, J.-J.; Zhang, L.; Zhang, Q.-L.; Wang, A.-J. Facile synthesis of nanoflower-like phosphorus-doped Ni3S2/CoFe2O4 arrays on nickel foam as a superior electrocatalyst for efficient oxygen evolution reaction. J. Colloid Interface Sci. 2021, 581, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, Y.; Liu, C.; Yu, Y.; Zhang, B. Integrating hydrogen production with aqueous selective semi-dehydrogenation of tetrahydroisoquinolines over a Ni2P bifunctional electrode. Angew. Chem. 2019, 131, 12142–12145. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, D.; Chen, G.; Zhang, N.; Wan, H.; Liu, X.; Ma, R. Microcrystallization and lattice contraction of NiFe LDHs for enhancing water electrocatalytic oxidation. Carbon Energy 2022, 4, 901–913. [Google Scholar] [CrossRef]
- Ahmed, S.; Yoon, W.; Jo, H.; Irshad, M.; Khan, M.K.; Kim, J. Structure-activity relationship and deactivation behavior of iron oxide during CO2 hydrogenation. Chem. Eng. J. 2024, 499, 156104. [Google Scholar] [CrossRef]
- Wang, W.P.; Yang, H.; Xian, T.; Jiang, J.L. XPS and magnetic properties of CoFe2O4 nanoparticles synthesized by a polyacrylamide gel route. Mater. Trans. 2012, 53, 1586–1589. [Google Scholar] [CrossRef]
- Shao, L.; Sun, A.; Zhang, Y.; Yu, L.; Suo, N.; Zuo, Z. Microstructure, XPS and magnetic analysis of Al-doped nickel–manganese–cobalt ferrite. J. Mater. Sci. Mater. Electron. 2021, 32, 20474–20488. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, Z.; Yang, N.; Zhao, G. An overall reaction integrated with highly selective oxidation of 5-Hydroxymethylfurfural and efficient hydrogen evolution. Adv. Funct. Mater. 2021, 31, 2102886. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, L.; Jiang, R.; Zhang, H. Dielectric relaxation and magnetic resonance in microwave absorption performance of CoxFe3-xO4 (0 ≤ x ≤ 1) nanocrystals. Ceram. Int. 2019, 45, 18347–18355. [Google Scholar] [CrossRef]
- Xiang, W.; Yang, N.; Li, X.; Linnemann, J.; Hagemann, U.; Ruediger, O.; Heidelmann, M.; Falk, T.; Aramini, M.; Debeer, S.; et al. 3D atomic-scale imaging of mixed Co-Fe spinel oxide nanoparticles during oxygen evolution reaction. Nat. Commun. 2022, 13, 179. [Google Scholar] [CrossRef]
- He, W.; Wang, J.; Shao, H.; Zhang, J.; Cao, C.-N. Novel KOH electrolyte for one-step electrochemical synthesis of high purity solid K2FeO4: Comparison with NaOH. Electrochem. Commun. 2005, 7, 607–611. [Google Scholar] [CrossRef]
- Lapicque, F.; Valentin, G. Direct electrochemical preparation of solid potassium ferrate. Electrochem. Commun. 2002, 4, 764–766. [Google Scholar] [CrossRef]
- Lee, Y.; Yoon, J.; Von Gunten, U. Spectrophotometric determination of ferrate (Fe(VI)) in water by ABTS. Water Res. 2005, 39, 1946–1953. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, F.; Qiu, S.; Ma, F.; Zheng, Y.; Gao, L. A self-sufficient electro-Fenton system with enhanced oxygen transfer for decontamination of pharmaceutical wastewater. Chem. Eng. J. 2022, 429, 132176. [Google Scholar] [CrossRef]
- Rao, P.S.; Hayon, E. Experimental determination of the redox potential of the superoxide radical •O2−. Biochem. Biophys. Res. Commun. 1973, 51, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K. Oxidation of inorganic compounds by ferrate(VI) and ferrate(V): One-electron and two-electron transfer steps. Environ. Sci. Technol. 2010, 44, 5148–5152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, M.; Wang, X.; Wang, C.; Lu, D.; Ma, W.; Kube, S.A.; Ma, J.; Elimelech, M. Janus electrocatalytic flow-through membrane enables highly selective singlet oxygen production. Nat. Commun. 2020, 11, 6228. [Google Scholar] [CrossRef]
- Guo, Y.; Long, J.; Huang, J.; Yu, G.; Wang, Y. Can the commonly used quenching method really evaluate the role of reactive oxygen species in pollutant abatement during catalytic ozonation? Water Res. 2022, 215, 118275. [Google Scholar] [CrossRef] [PubMed]

| Electrode | 5:1@NF | 2:1@NF | 1:5@NF |
|---|---|---|---|
| ·O2− spin concentration (spins/mm3) | 3.518 × 1011 | 5.361 × 1011 | 6.068 × 1012 |
| Electrode | Direct-Oxidize Percentage (%) | Fe(VI) Percentage (%) | ·O2− Percentage (%) | Other Radicals Percentage (%) |
|---|---|---|---|---|
| 5:1@NF | 45.99 | 9.37 | 38.53 | 6.11 |
| 2:1@NF | 66.27 | 11.79 | Undetected | 21.94 |
| 1:1@NF | 76.27 | 11.29 | Undetected | 12.44 |
| 1:5@NF | 79.85 | 11.46 | Undetected | 8.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Zhang, Q.; Qiu, S. Anodic Catalytic Oxidation of Sulfamethoxazole: Efficiency and Mechanism on Co3O4 Nanowire Self-Assembled CoFe2O4 Nanosheet Heterojunction. Catalysts 2025, 15, 854. https://doi.org/10.3390/catal15090854
Cui H, Zhang Q, Qiu S. Anodic Catalytic Oxidation of Sulfamethoxazole: Efficiency and Mechanism on Co3O4 Nanowire Self-Assembled CoFe2O4 Nanosheet Heterojunction. Catalysts. 2025; 15(9):854. https://doi.org/10.3390/catal15090854
Chicago/Turabian StyleCui, Han, Qiwei Zhang, and Shan Qiu. 2025. "Anodic Catalytic Oxidation of Sulfamethoxazole: Efficiency and Mechanism on Co3O4 Nanowire Self-Assembled CoFe2O4 Nanosheet Heterojunction" Catalysts 15, no. 9: 854. https://doi.org/10.3390/catal15090854
APA StyleCui, H., Zhang, Q., & Qiu, S. (2025). Anodic Catalytic Oxidation of Sulfamethoxazole: Efficiency and Mechanism on Co3O4 Nanowire Self-Assembled CoFe2O4 Nanosheet Heterojunction. Catalysts, 15(9), 854. https://doi.org/10.3390/catal15090854






