Plasma-Assisted CO2 Conversion to Methanol in Energy Systems: Parameter Optimization and Synergistic Effects
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characteristics of Hydrogen Reduction of CO2 Under Plasma Conditions
2.1.1. Input Voltage
2.1.2. Discharge Gap
2.1.3. Gas Flow Rate
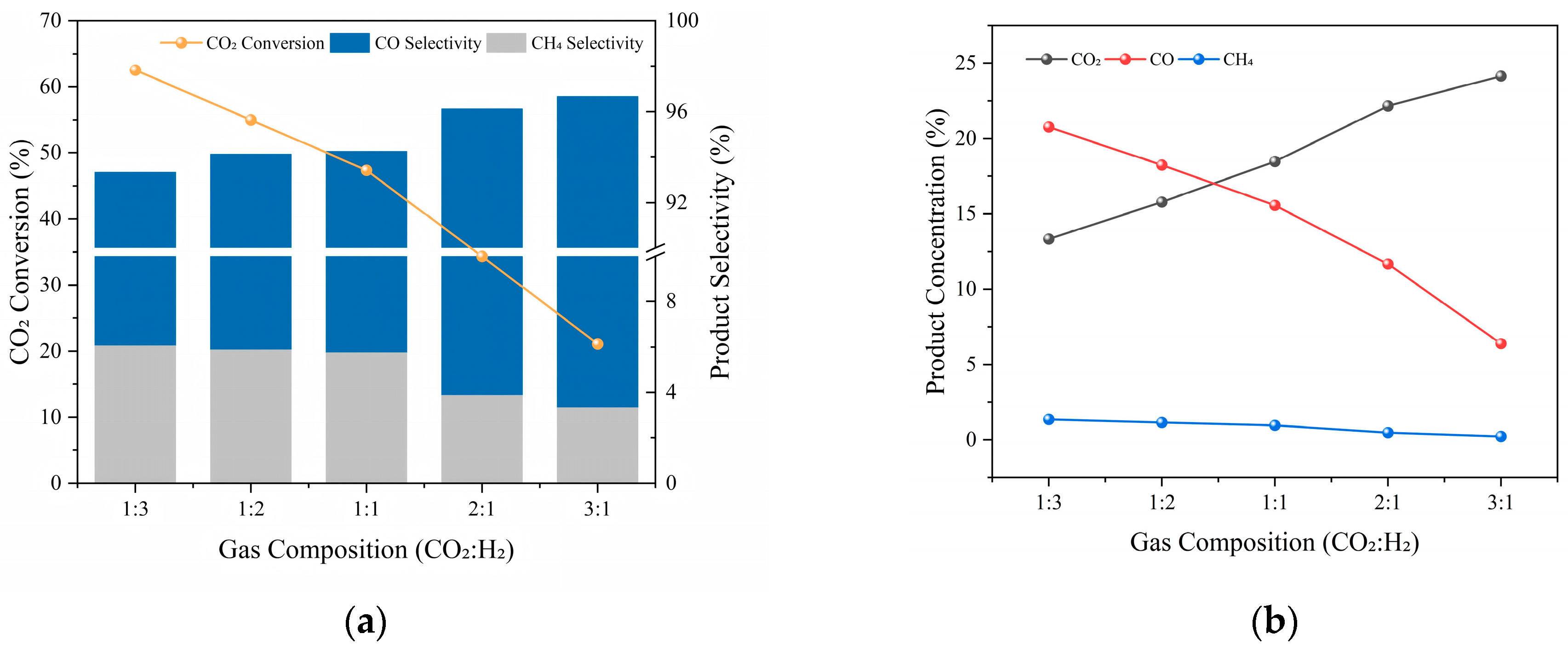
2.1.4. Gas Composition
2.2. Research on Thermocatalytic Hydrogenation of CO/CO2 Mixtures for Methanol Synthesis
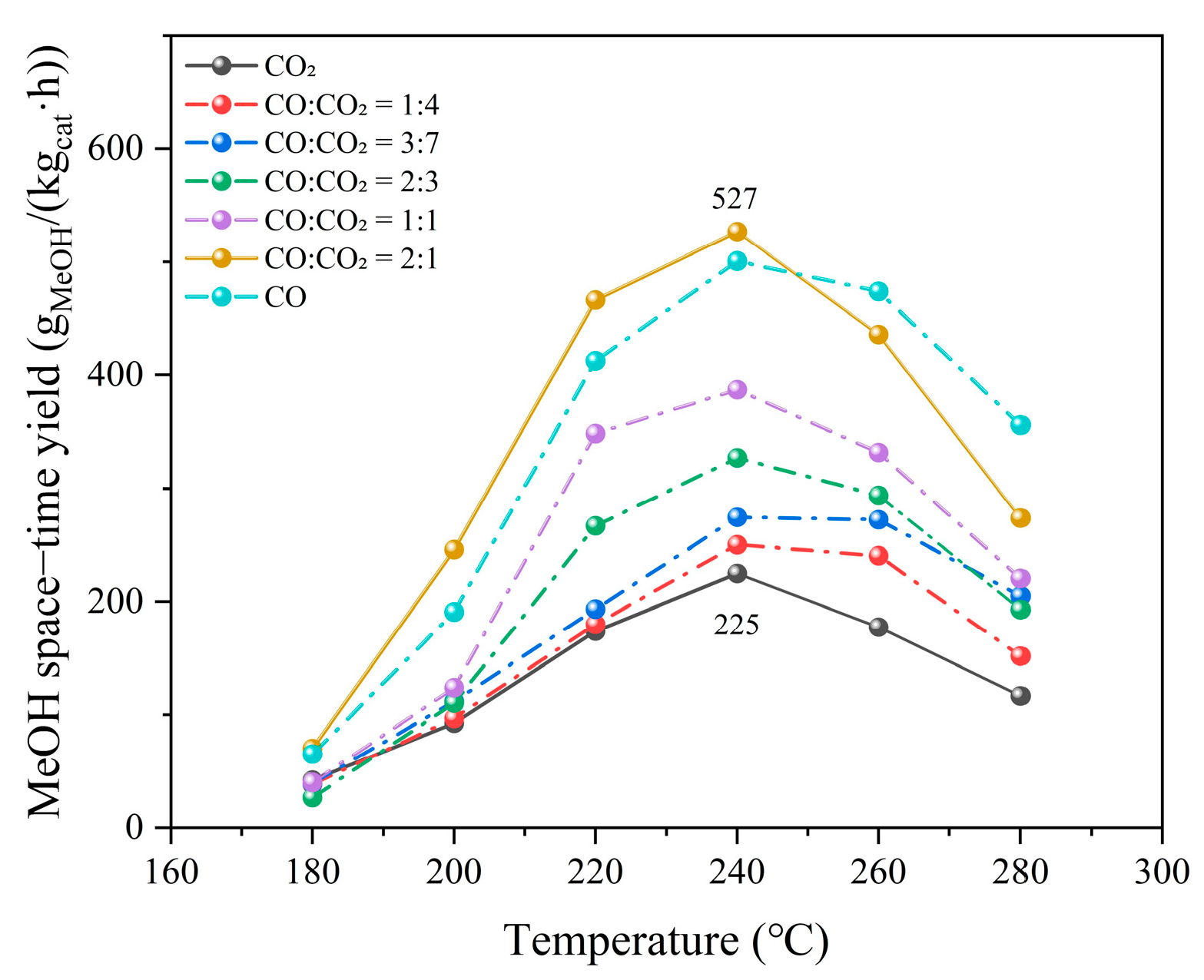
2.2.1. CO/CO2
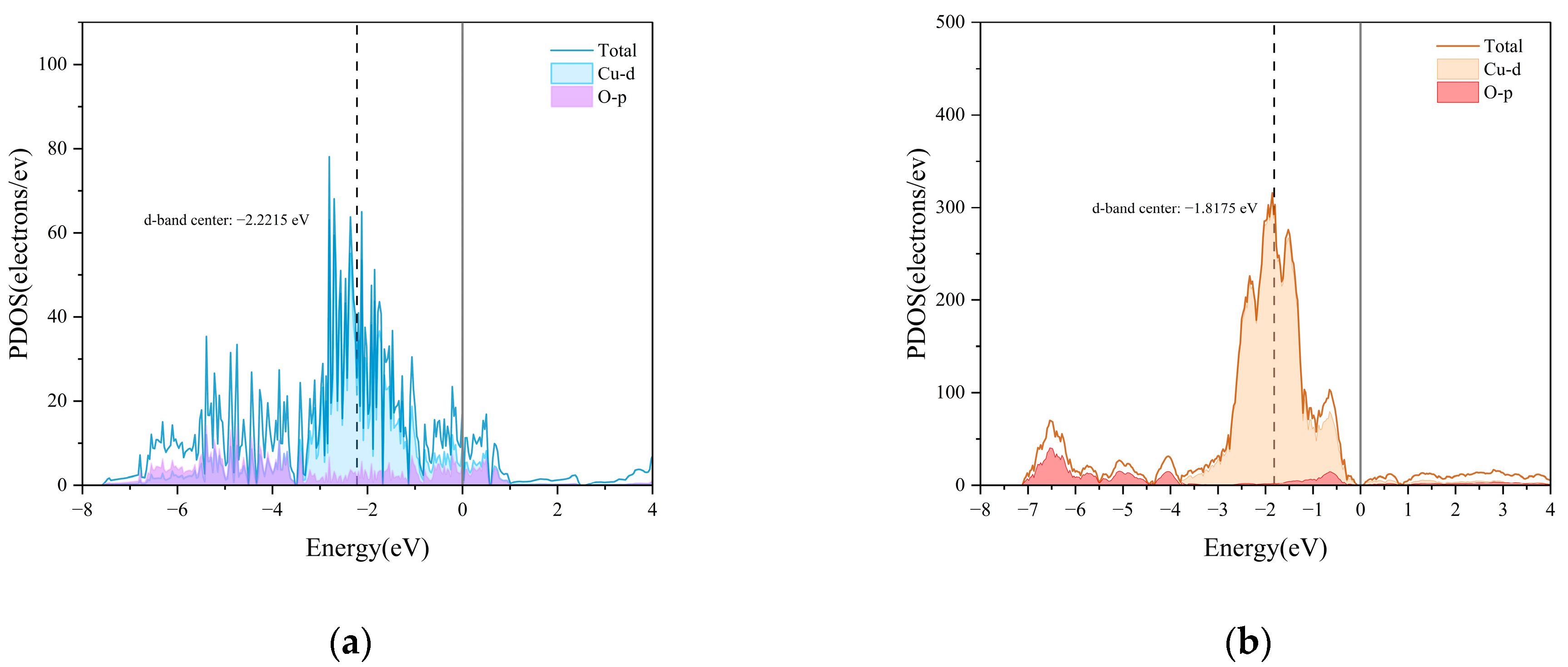
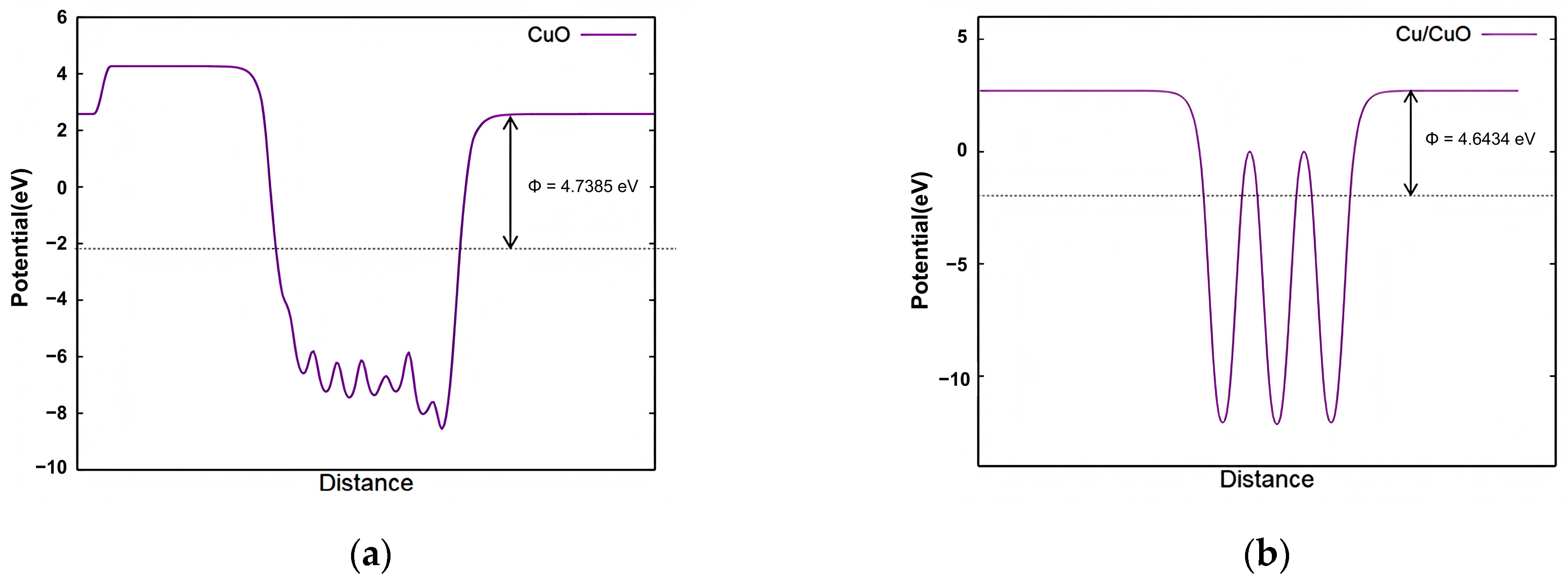
2.2.2. Surface Chemical State Analysis
2.2.3. DFT
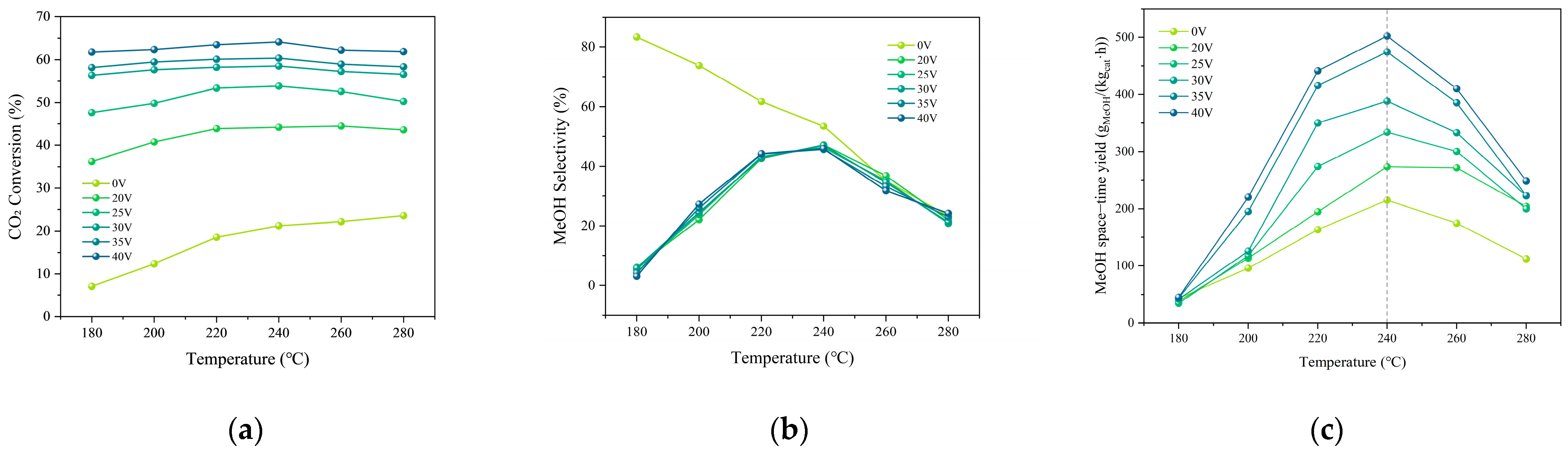
2.3. Non-Thermal Plasma–Thermal Catalysis Synergistic System Experiment
3. Materials and Methods
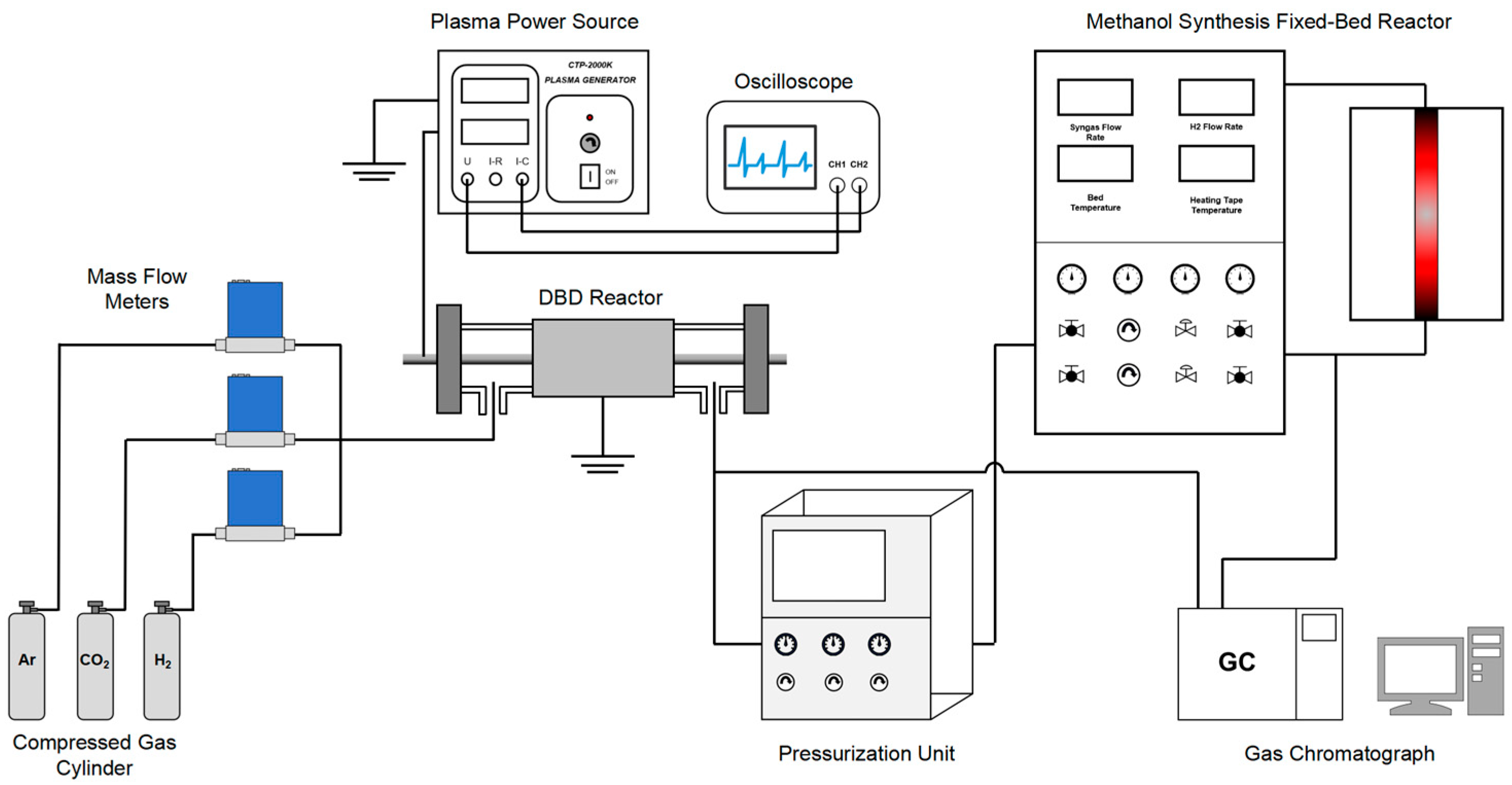
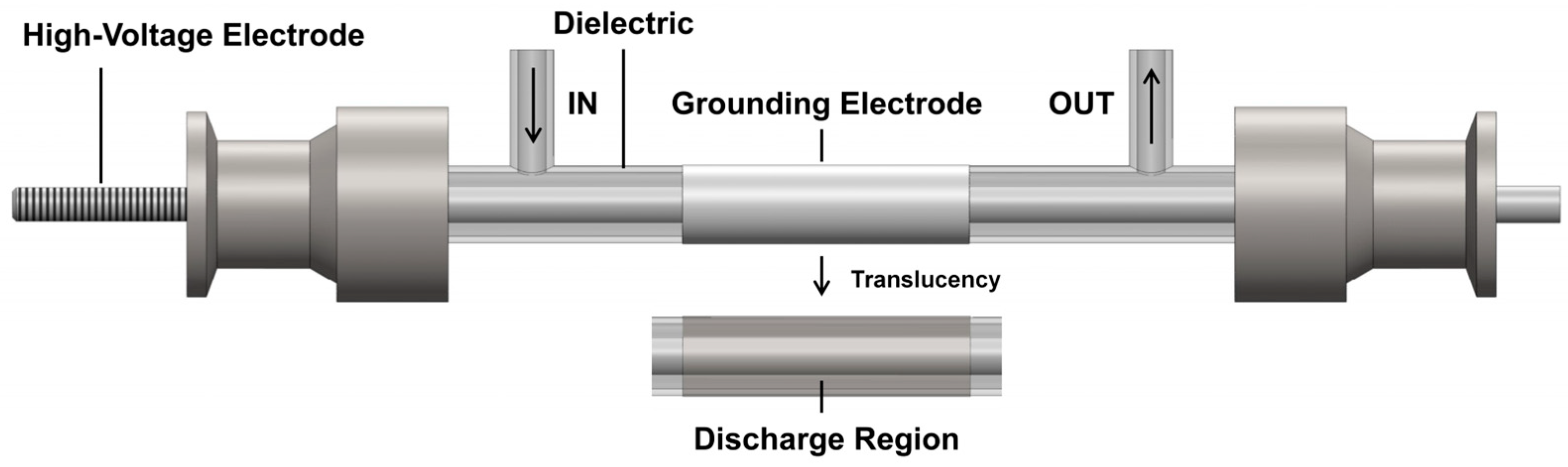
3.1. Experimental Setup
3.2. Experimental Test
3.3. Catalyst Preparation
3.4. Evaluation Methodology
3.4.1. DBD Plasma Module Evaluation Metrics
3.4.2. Evaluation Metrics for Syngas Thermocatalytic Methanol Synthesis Module
3.5. DFT Settings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DBD | Dielectric Barrier Discharge |
| CCUS | Carbon Capture, Utilization, and Storage |
| RWGS | Reverse Water–Gas Shift |
| NTP | Non-Thermal Plasma |
| STY | Space–Time Yield |
| DFT | Density Functional Theory |
References
- Alexander, M.; Eissa, M.; McDermott-Levy, R.; Osborne, R.; Pleuss, E.; Prabhakaran, P.; Sorensen, C. COP26: Looking Forward from Glasgow by Placing Health at the Center of Climate Action. J. Clim. Change Health 2022, 5, 100117. [Google Scholar] [CrossRef]
- Chen, X.; Shuai, C.; Wu, Y.; Zhang, Y. Analysis on the Carbon Emission Peaks of China’s Industrial, Building, Transport, and Agricultural Sectors. Sci. Total Environ. 2020, 709, 135768. [Google Scholar] [CrossRef]
- Storrs, K.; Lyhne, I.; Drustrup, R. A Comprehensive Framework for Feasibility of CCUS Deployment: A Meta-Review of Literature on Factors Impacting CCUS Deployment. Int. J. Greenh. Gas Control. 2023, 125, 103878. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Zhao, Q.; Fang, X. Status and prospects for CO2 capture, utilization and storage technology in China. Pet. Sci. Bull. 2023, 4, 391–397. [Google Scholar] [CrossRef]
- Kianfar, E.; Hajimirzaee, S.; Mousavian, S.; Mehr, A.S. Zeolite-Based Catalysts for Methanol to Gasoline Process: A Review. Microchem. J. 2020, 156, 104822. [Google Scholar] [CrossRef]
- Wang, C.; Yang, L.; Gao, M.; Shao, X.; Dai, W.; Wu, G.; Guan, N.; Xu, Z.; Ye, M.; Li, L. Directional Construction of Active Naphthalenic Species within SAPO-34 Crystals toward More Efficient Methanol-to-Olefin Conversion. J. Am. Chem. Soc. 2022, 144, 21408–21416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, T.; Ouyang, Y.; Zhong, J.; Zhang, Y.; Xiong, X.; Hu, Q.; Deng, J.; Sun, H.; Yan, Z. Novel-Ordered Hierarchical ZSM-5 Zeolite with Interconnected Macro–Meso–Microporosity for the Enhanced Methanol to Aromatics Reaction. Catal. Sci. Technol. 2024, 14, 2461–2469. [Google Scholar] [CrossRef]
- Meca, V.L.; d’Amore Domenech, R.; Crucelaegui, A.; Leo, T.J. Large-Scale Maritime Transport of Hydrogen: Economic Comparison of Liquid Hydrogen and Methanol. ACS Sustain. Chem. Eng. 2022, 10, 4300–4311. [Google Scholar] [CrossRef]
- Olah, G.A. Beyond Oil and Gas: The Methanol Economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar] [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef]
- Kiani, M.R.; Kamandi, R.; Nozarian, K.; Rahimpour, M.R. Introducing a Novel Catalyst for Efficient Conversion of CO2 into Syngas through Reverse-Water-Gas-Shift (RWGS) Reactions Based on Highly Mesoporous Structures MCM-41: Influence of the Fe Incorporation. Energy Convers. Manag. 2024, 304, 118247. [Google Scholar] [CrossRef]
- Chen, H.; Mu, Y.; Xu, S.; Xu, S.; Hardacre, C.; Fan, X. Recent Advances in Non-Thermal Plasma (NTP) Catalysis towards C1 Chemistry. Chin. J. Chem. Eng. 2020, 28, 2010–2021. [Google Scholar] [CrossRef]
- Chen, S.; Liu, T.; Niu, J.; Huang, J.; Peng, X.; Zhou, H.; Chen, H.; Fan, X. Plasma-Catalytic CO2 Methanation over Ni Supported on MCM-41 Catalysts: Effect of Metal Dispersion and Process Optimization. Carbon Capture Sci. Technol. 2024, 11, 100194. [Google Scholar] [CrossRef]
- Jwa, E.; Lee, S.B.; Lee, H.W.; Mok, Y.S. Plasma-Assisted Catalytic Methanation of CO and CO2 over Ni–Zeolite Catalysts. Fuel Process. Technol. 2013, 108, 89–93. [Google Scholar] [CrossRef]
- Arita, K.; Iizuka, S. Production of CH4 in a Low-Pressure CO2/H2 Discharge with Magnetic Field. J. Mater. Sci. Chem. Eng. 2015, 3, 69–77. [Google Scholar] [CrossRef]
- Nizio, M.; Albarazi, A.; Cavadias, S.; Amouroux, J.; Galvez, M.E.; Da Costa, P. Hybrid Plasma-Catalytic Methanation of CO2 at Low Temperature over Ceria Zirconia Supported Ni Catalysts. Int. J. Hydrog. Energy 2016, 41, 11584–11592. [Google Scholar] [CrossRef]
- Zeng, Y.; Tu, X. Plasma-Catalytic CO2 Hydrogenation at Low Temperatures. IEEE Trans. Plasma Sci. 2016, 44, 405–411. [Google Scholar] [CrossRef]
- Zeng, Y.; Tu, X. Plasma-Catalytic Hydrogenation of CO2 for the Cogeneration of CO and CH4 in a Dielectric Barrier Discharge Reactor: Effect of Argon Addition. J. Phys. D Appl. Phys. 2017, 50, 184004. [Google Scholar] [CrossRef]
- Lan, L.; Wang, A.; Wang, Y. CO2 Hydrogenation to Lower Hydrocarbons over ZSM-5-Supported Catalysts in a Dielectric-Barrier Discharge Plasma Reactor. Catal. Commun. 2019, 130, 105761. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.; Guo, H.; Tu, X. Atmospheric Pressure and Room Temperature Synthesis of Methanol through Plasma-Catalytic Hydrogenation of CO2. ACS Catal. 2017, 8, 90–100. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.; Ma, Y.; Lin, X.; Zou, X.; Zhang, H.; Li, X.; Lin, Q.; Qu, M.-L.; Ma, Z.; et al. Investigation of Highly Efficient CO2 Hydrogenation at Ambient Conditions Using Dielectric Barrier Discharge Plasma. Green Energy Resour. 2024, 2, 100102. [Google Scholar] [CrossRef]
- Ashford, B.; Poh, C.-K.; Ostrikov, K.; Chen, L.; Tu, X. Plasma-Catalytic CO2 Hydrogenation to Ethane in a Dielectric Barrier Discharge Reactor. J. CO2 Util. 2022, 57, 101882. [Google Scholar] [CrossRef]
- Meloni, E.; Cafiero, L.; Renda, S.; Martino, M.; Pierro, M.; Palma, V. Ru- and Rh-Based Catalysts for CO2 Methanation Assisted by Non-Thermal Plasma. Catalysts 2023, 13, 488. [Google Scholar] [CrossRef]
- Araújo, T.P.; Hergesell, A.H.; Faust-Akl, D.; Büchele, S.; Stewart, J.A.; Mondelli, C.; Pérez-Ramírez, J. Methanol Synthesis by Hydrogenation of Hybrid CO2–CO Feeds. ChemSusChem 2021, 14, 2914–2923. [Google Scholar] [CrossRef]
- Duyar, M.S.; Tsai, C.; Snider, J.L.; Singh, J.A.; Gallo, A.; Yoo, J.S.; Medford, A.J.; Abild-Pedersen, F.; Studt, F.; Kibsgaard, J.; et al. A Highly Active Molybdenum Phosphide Catalyst for Methanol Synthesis from CO and CO2. Angew. Chem. Int. Ed. 2018, 57, 15045–15050. [Google Scholar] [CrossRef]
- Shi, P.; Han, J.; Yan, Z.; Luo, P.; Wang, J.; Ban, H.; Zhang, X.; Li, C. Insight into the Correlation between Cu Species and Methanol Selectivity from CO2 Hydrogenation over Cu-Based Catalyst. Mol. Catal. 2024, 563, 114278. [Google Scholar] [CrossRef]
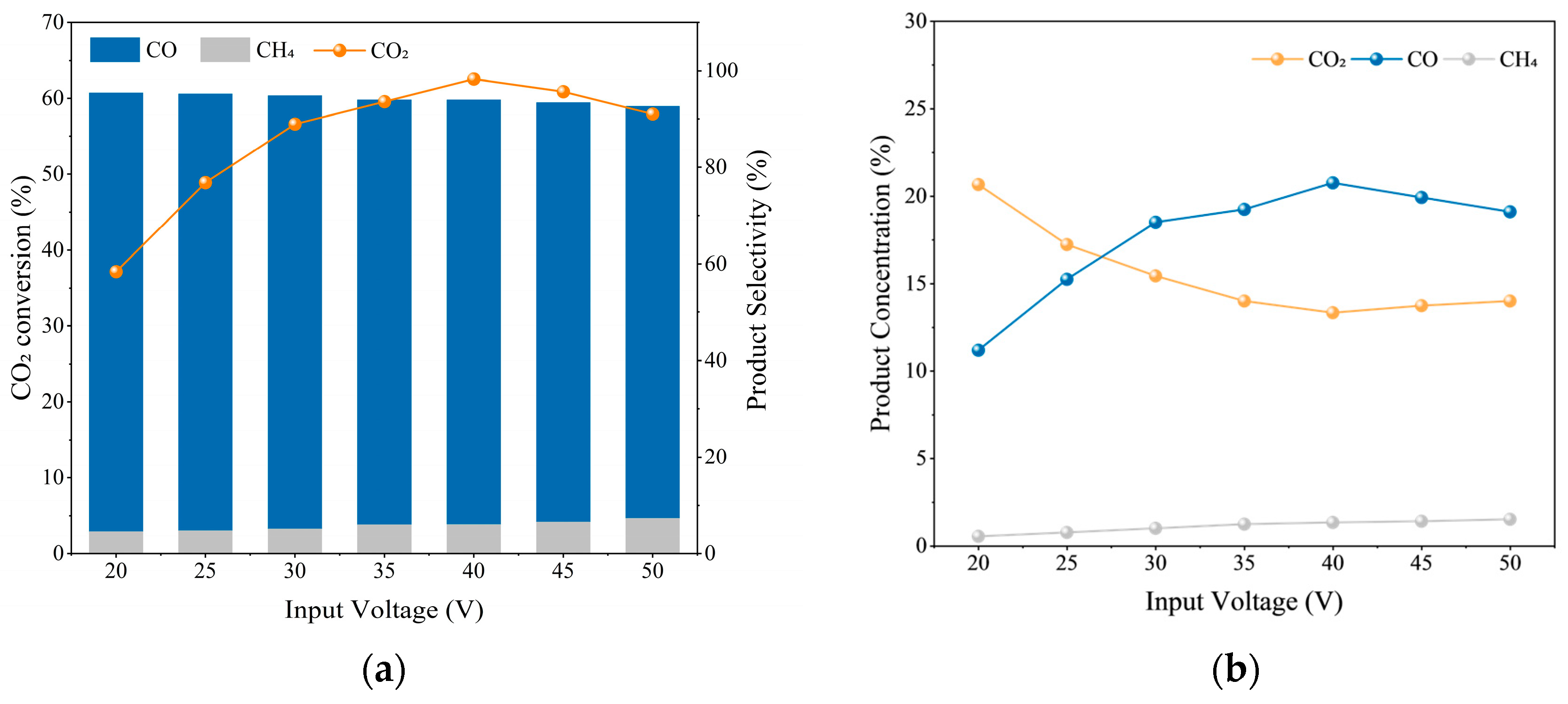
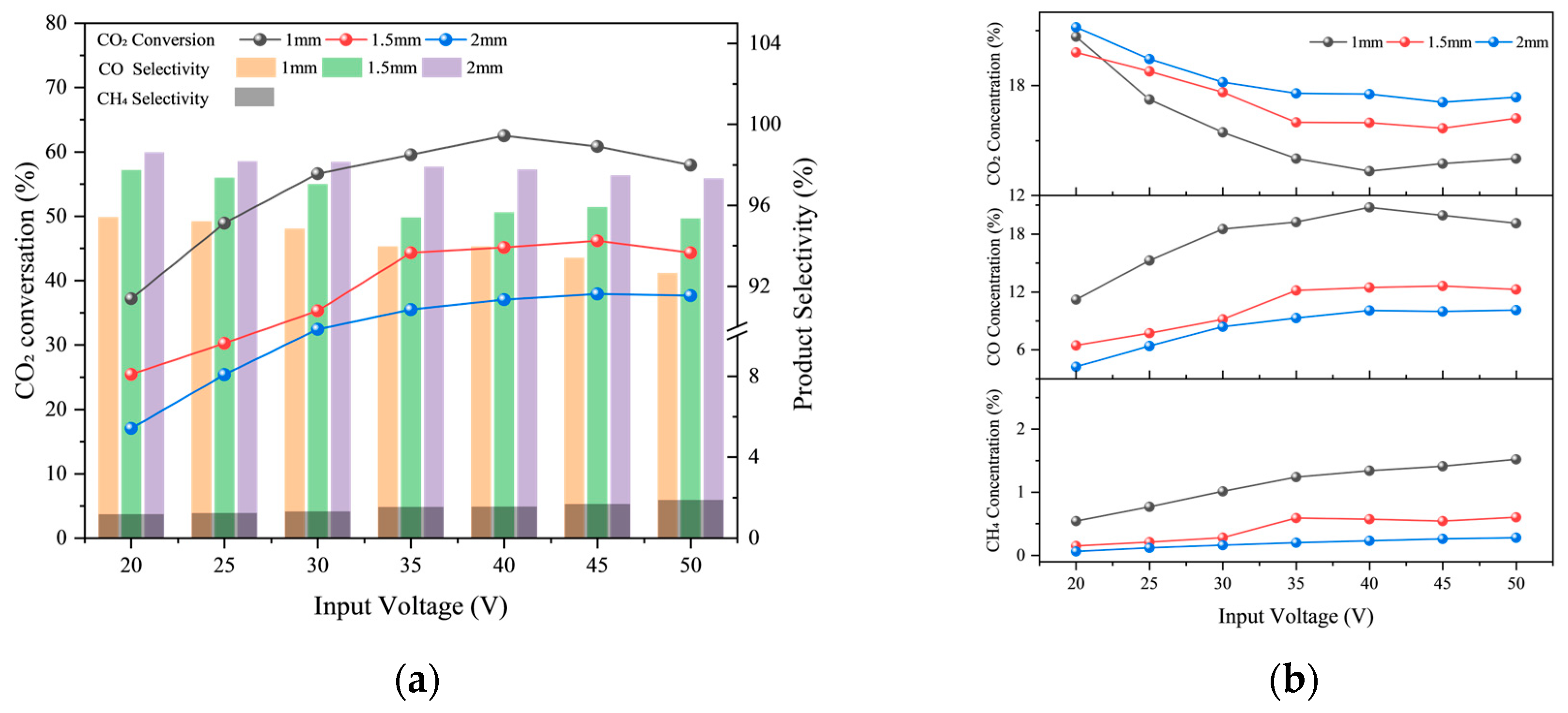
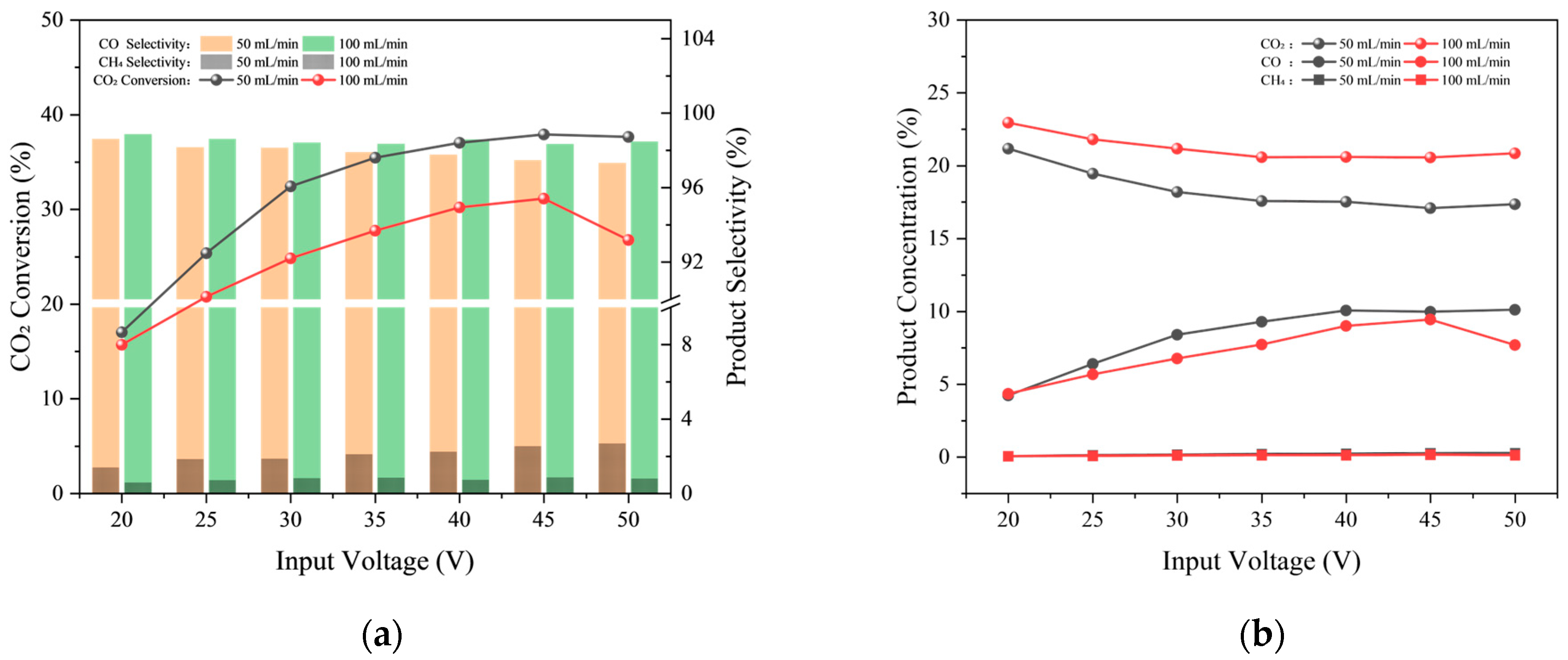
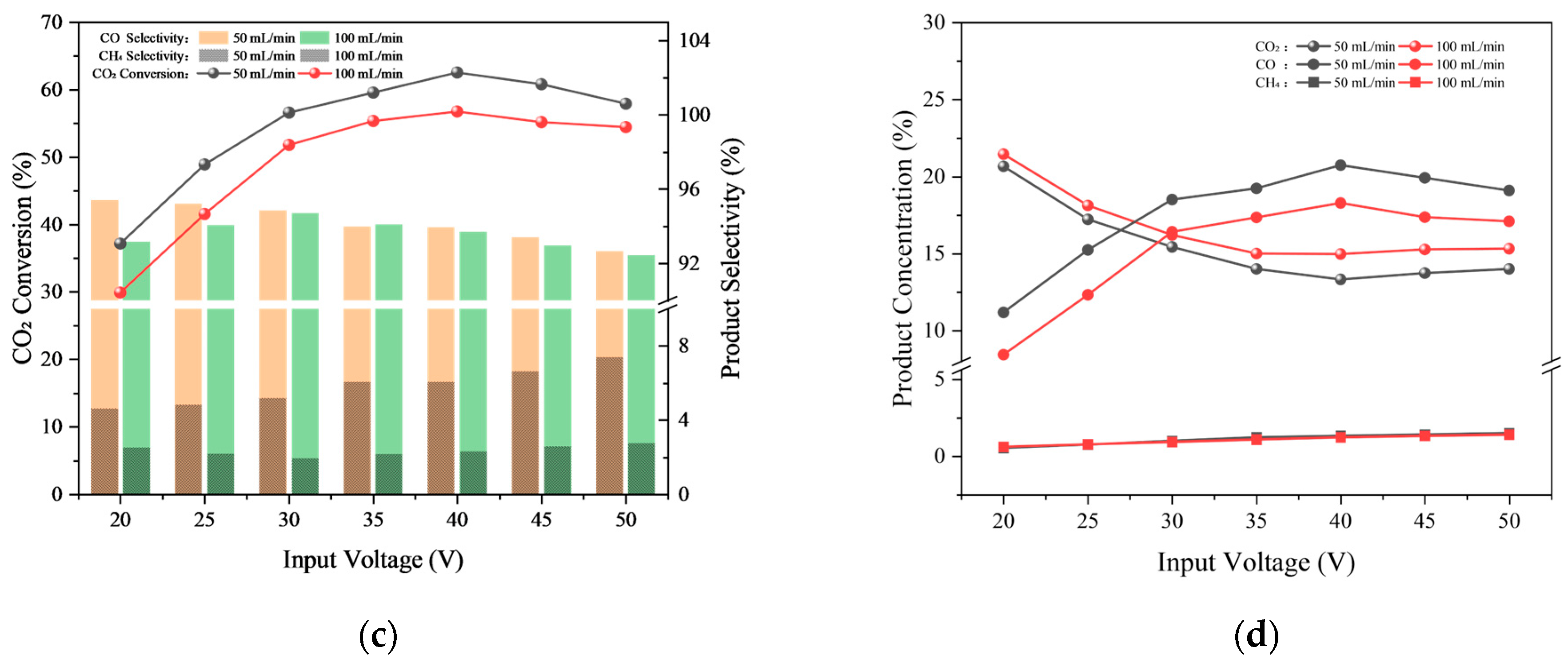


| Test Condition | Discharge gap/mm | Input Voltage/V | Gas Flow Rate/sccm | CO2/H2 Ratio |
|---|---|---|---|---|
| TC-A | 1.0 | 20–50 | 50 | 1:3 |
| TC-B | 1.0 | 20–50 | 50 | 1:3 |
| 1.5 | ||||
| 2.0 | ||||
| TC-C | 1.0 | 20–50 | 50 | 1:3 |
| 1.0 | 100 | |||
| 2.0 | 50 | |||
| 2.0 | 100 | |||
| TC-D | 1.0 | 40 | 50 | 1:3 |
| 1:2 | ||||
| 1:1 | ||||
| 2:1 | ||||
| 3:1 |
| Chemical Formula | Specifications | Manufacturer |
|---|---|---|
| CO2 | 99.999% | Jingong Special Materials Co., Ltd., Hangzhou, China |
| CO | 99.999% | |
| H2 | 99.999% | |
| Ar | 99.999% | |
| Cu(NO3)2·3H2O | AR, ≥99% | China National Pharmaceutical Group Co., Ltd., Beijing, China |
| Zn(NO3)2·6H2O | AR, ≥99% | |
| Al(NO3)2·9H2O | AR, ≥99% | |
| Na2CO3 | AR, ≥99.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, X.; Ma, Y.; Ma, Y.; Qin, S.; Chen, C.; Chen, G.; Shen, Z.; Wu, A.; Lin, X. Plasma-Assisted CO2 Conversion to Methanol in Energy Systems: Parameter Optimization and Synergistic Effects. Catalysts 2025, 15, 846. https://doi.org/10.3390/catal15090846
Zou X, Ma Y, Ma Y, Qin S, Chen C, Chen G, Shen Z, Wu A, Lin X. Plasma-Assisted CO2 Conversion to Methanol in Energy Systems: Parameter Optimization and Synergistic Effects. Catalysts. 2025; 15(9):846. https://doi.org/10.3390/catal15090846
Chicago/Turabian StyleZou, Xiangbo, Yunfei Ma, Yunfeng Ma, Shiwei Qin, Chuangting Chen, Gongda Chen, Zirong Shen, Angjian Wu, and Xiaoqing Lin. 2025. "Plasma-Assisted CO2 Conversion to Methanol in Energy Systems: Parameter Optimization and Synergistic Effects" Catalysts 15, no. 9: 846. https://doi.org/10.3390/catal15090846
APA StyleZou, X., Ma, Y., Ma, Y., Qin, S., Chen, C., Chen, G., Shen, Z., Wu, A., & Lin, X. (2025). Plasma-Assisted CO2 Conversion to Methanol in Energy Systems: Parameter Optimization and Synergistic Effects. Catalysts, 15(9), 846. https://doi.org/10.3390/catal15090846








