PdII Catalysis: Recent Advances in the Intramolecular Wacker-Type Reaction of Alkenols and Related Domino Reactions
Abstract
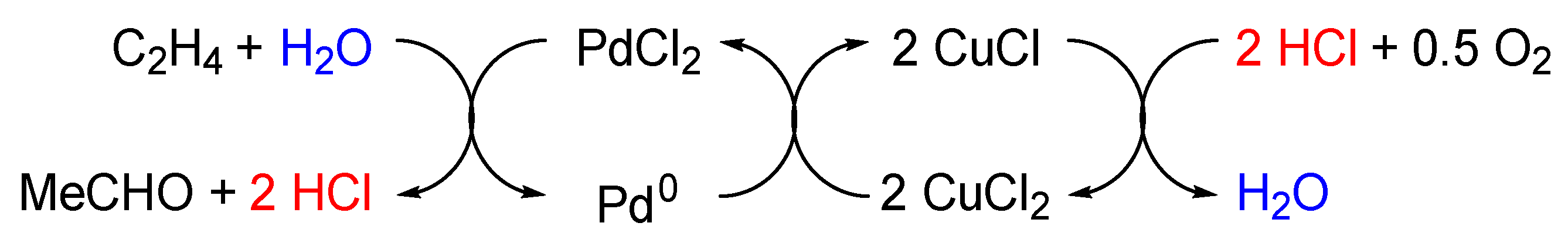
1. Introduction
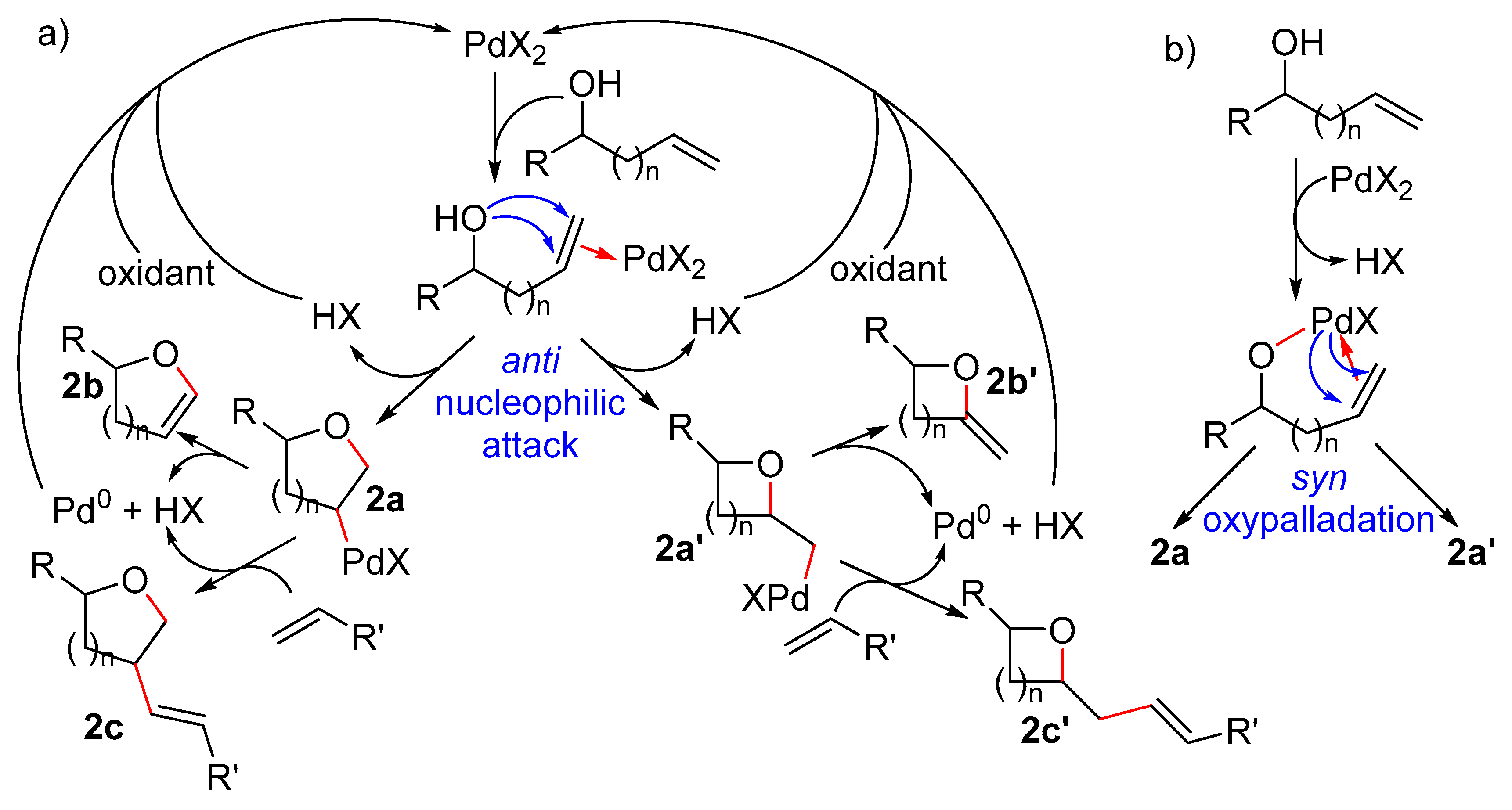
2. Intramolecular Wacker-Type Reaction
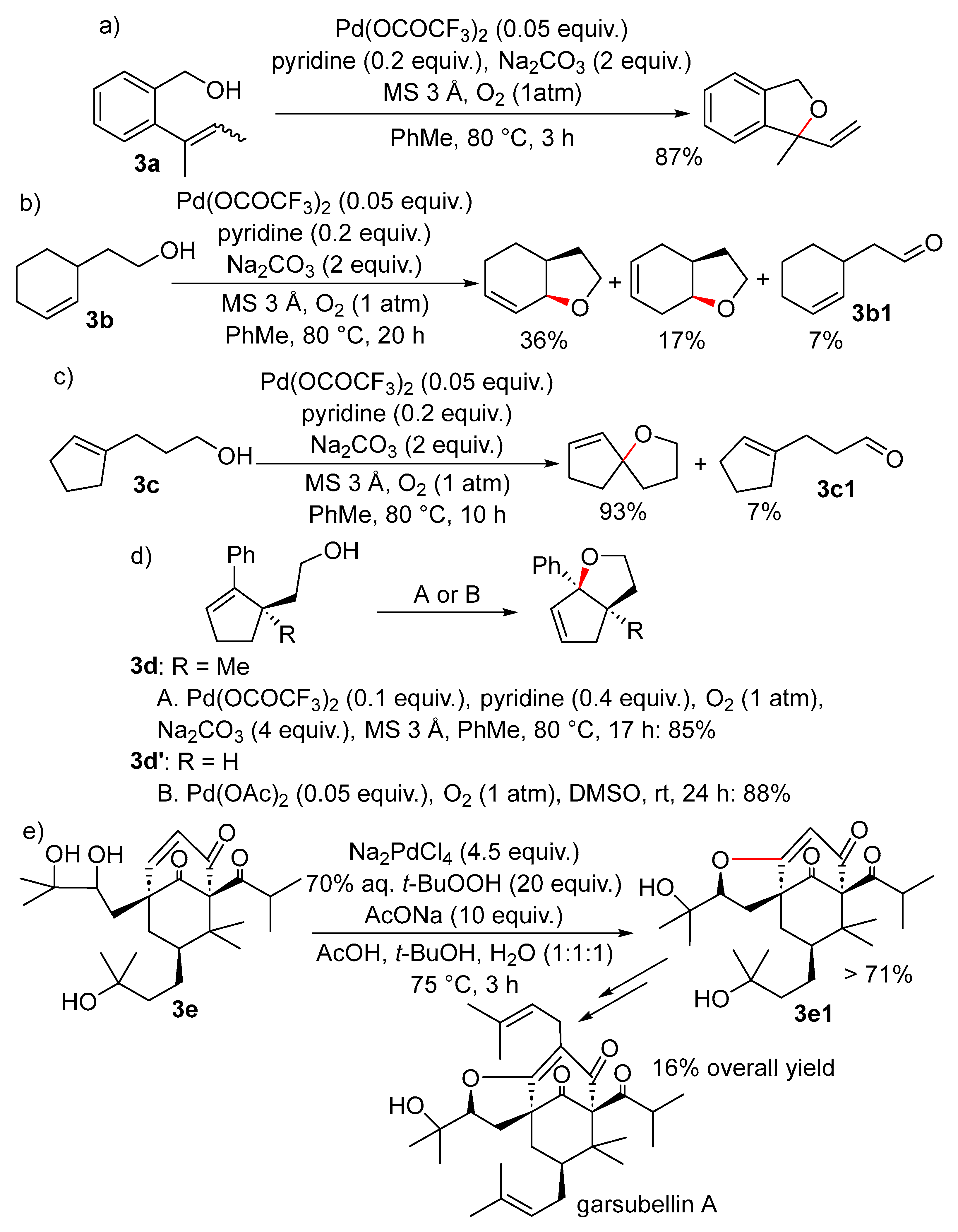
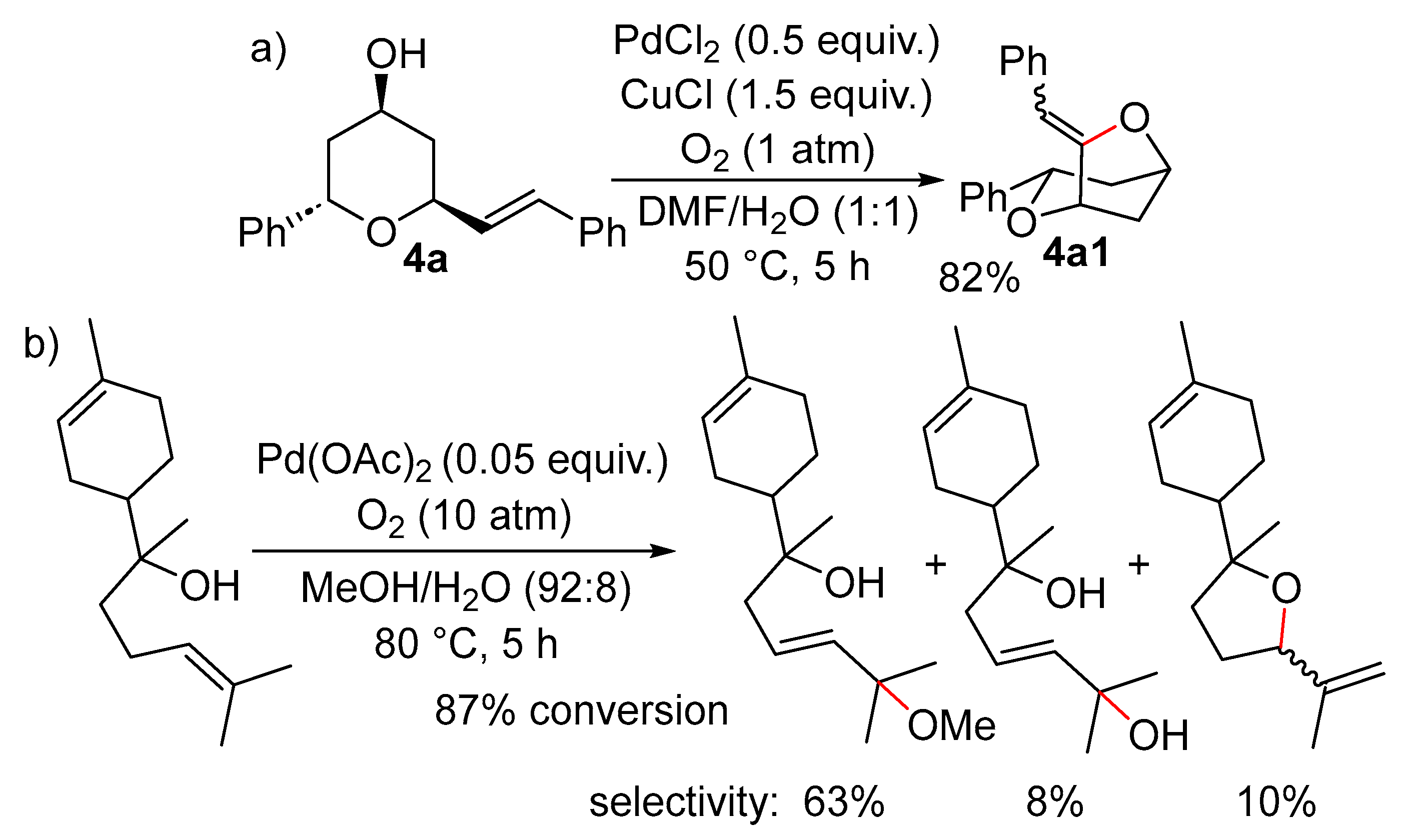
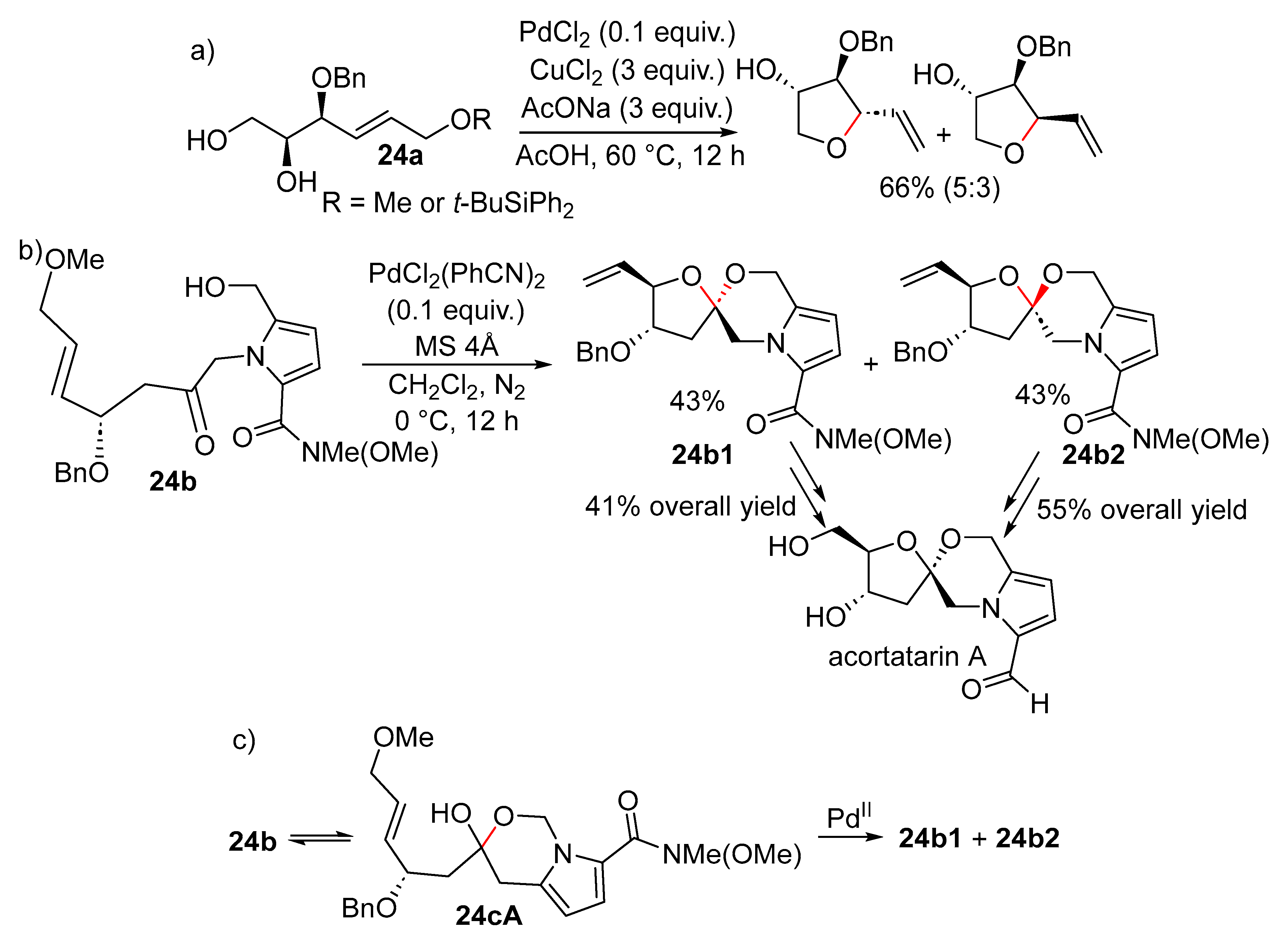
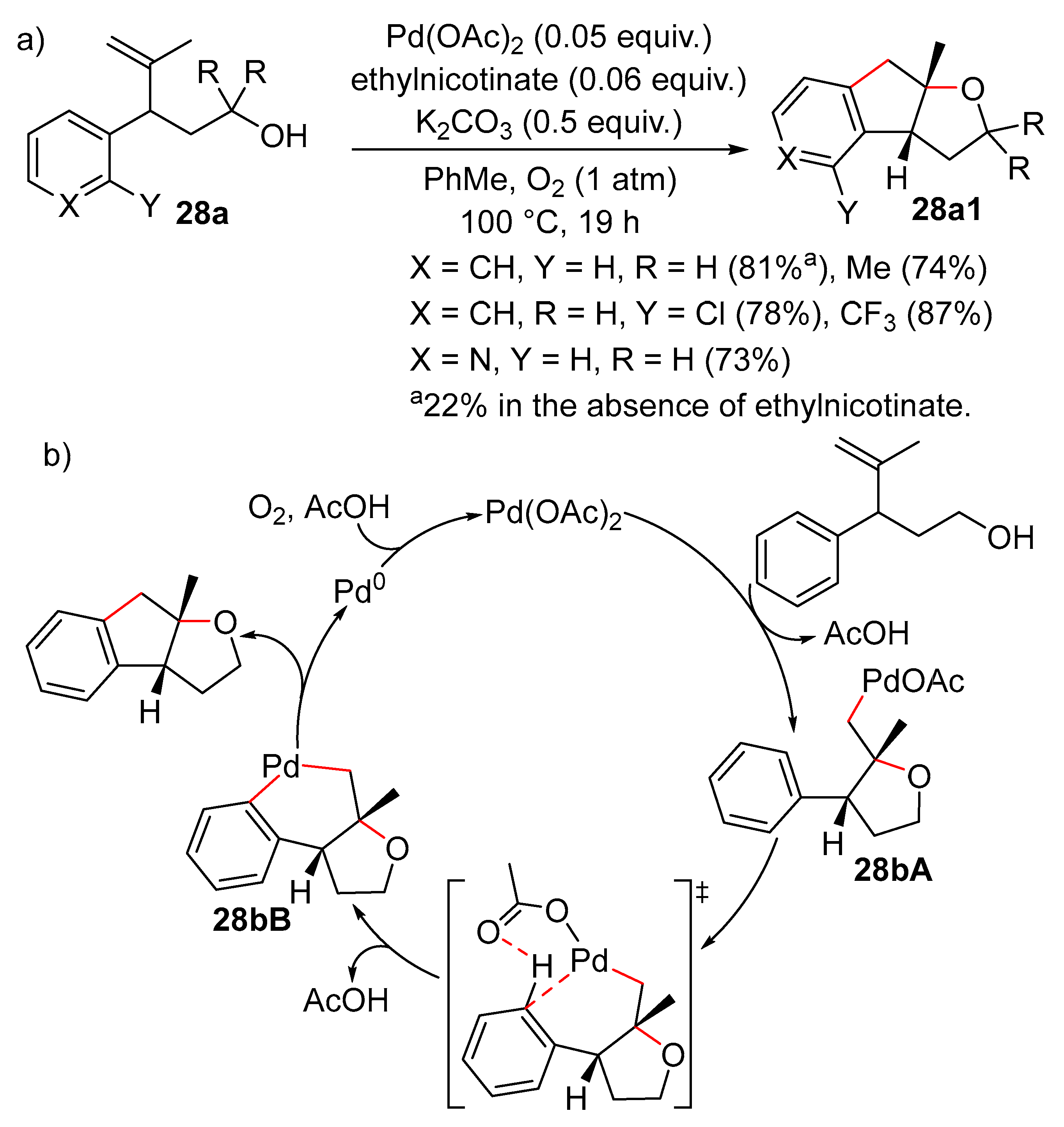
2.1. Formation of Dihydro- or Tetrahydrofurans
2.2. Formation of Dihydro- or Tetrahydropyrans
2.3. Formation of Dihydro-1,4-Oxazines
2.4. Formation of a Tetrahydrooxocine
2.5. Formation of Dihydropyranones or Furanones
3. Intramolecular Wacker-Type Reaction/Dehydration
4. Intramolecular Wacker-Type Reaction/Nucleophilic Addition
5. Intramolecular Wacker-Type Reaction/Hydroalkoxylation or Hydroaryloxylation
6. Intramolecular Wacker-Type Reaction/Dehydrogenation (Dehydration)/Palladation/Oxidation
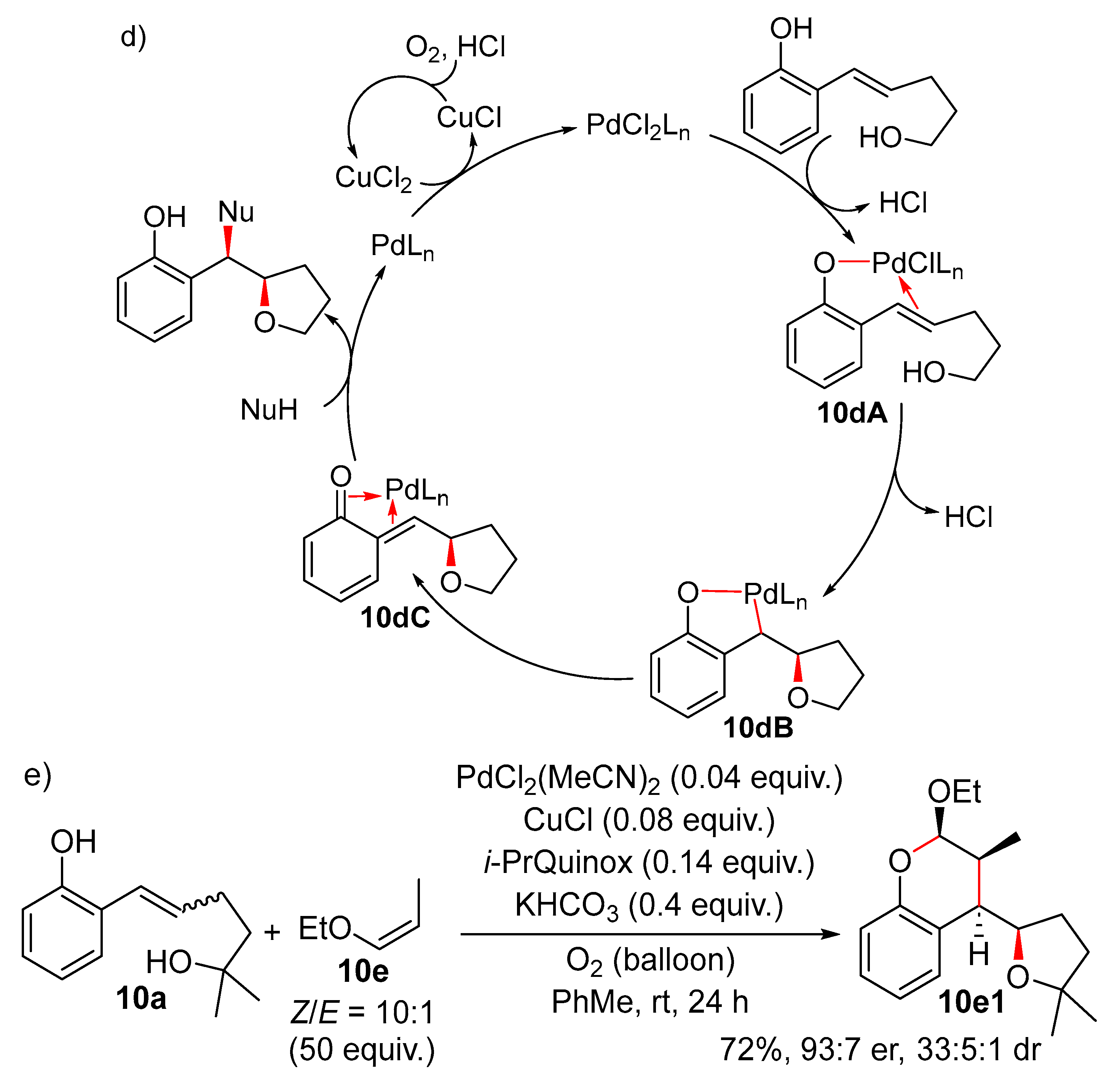
7. Intra/Intermolecular Wacker-Type Reaction
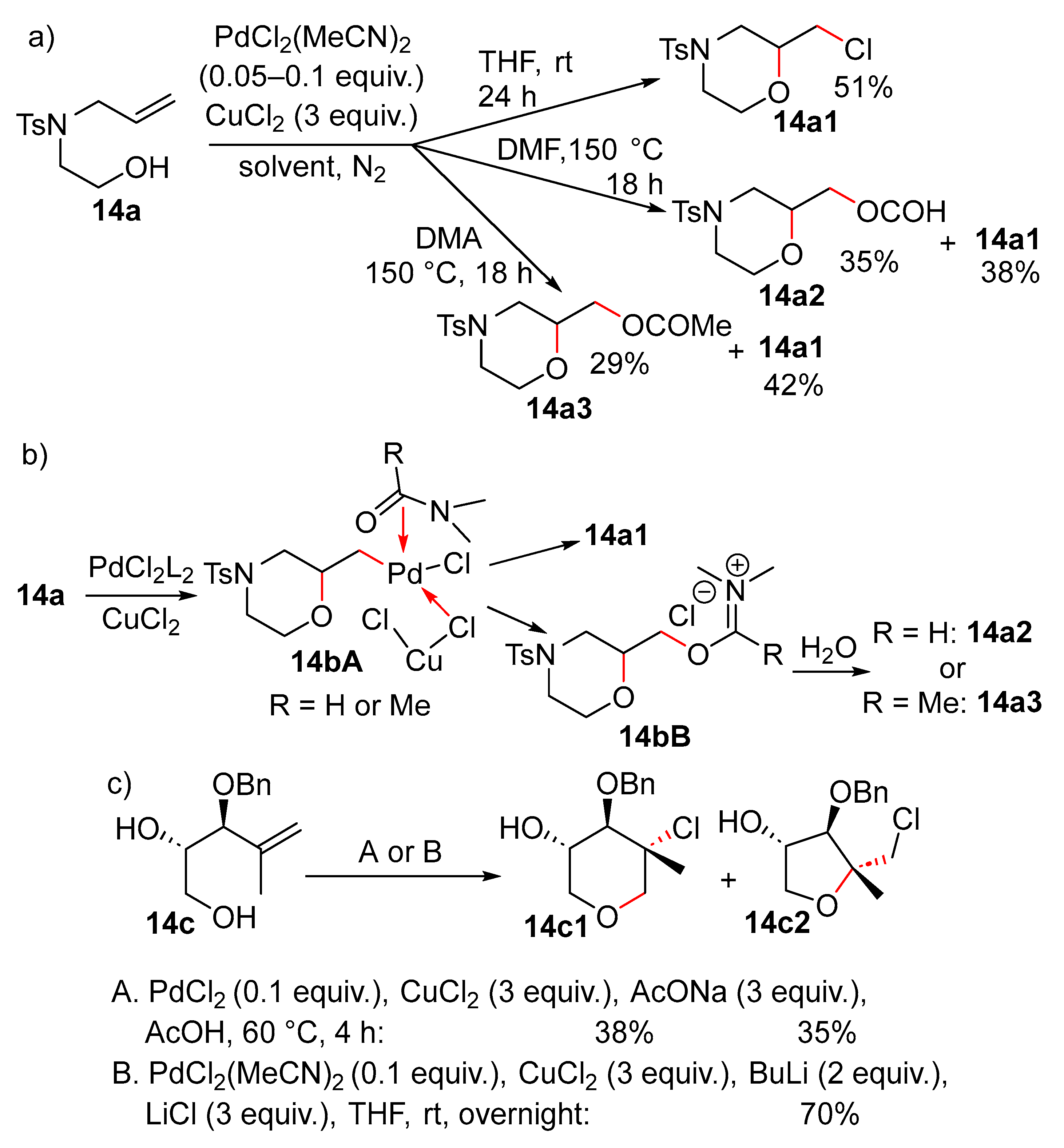
8. Cyclisation/Chlorination or Esterification
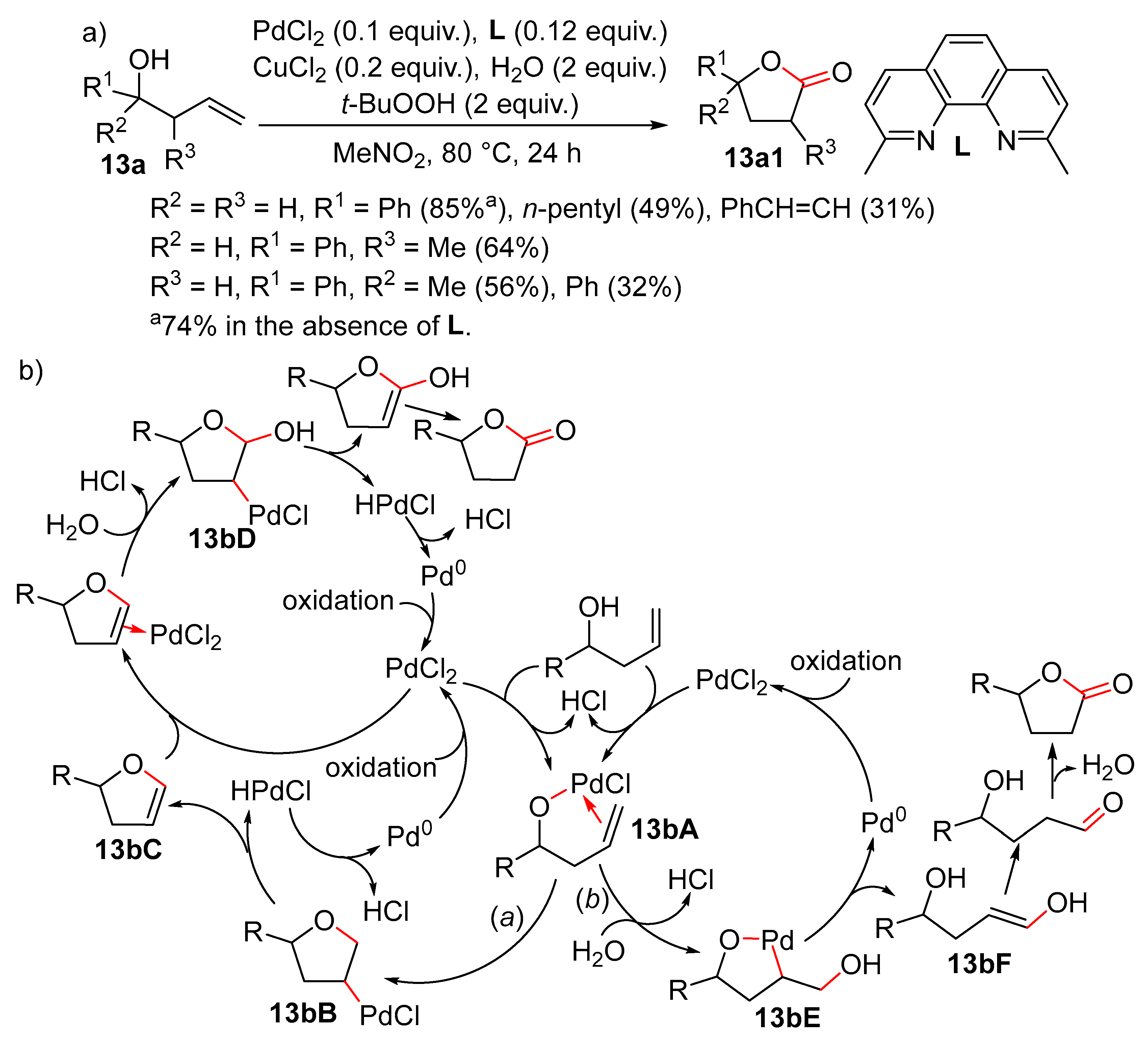
9. Cyclisation/Alkoxylation
10. Cyclisation/Dehydrogenation or Isomerisation
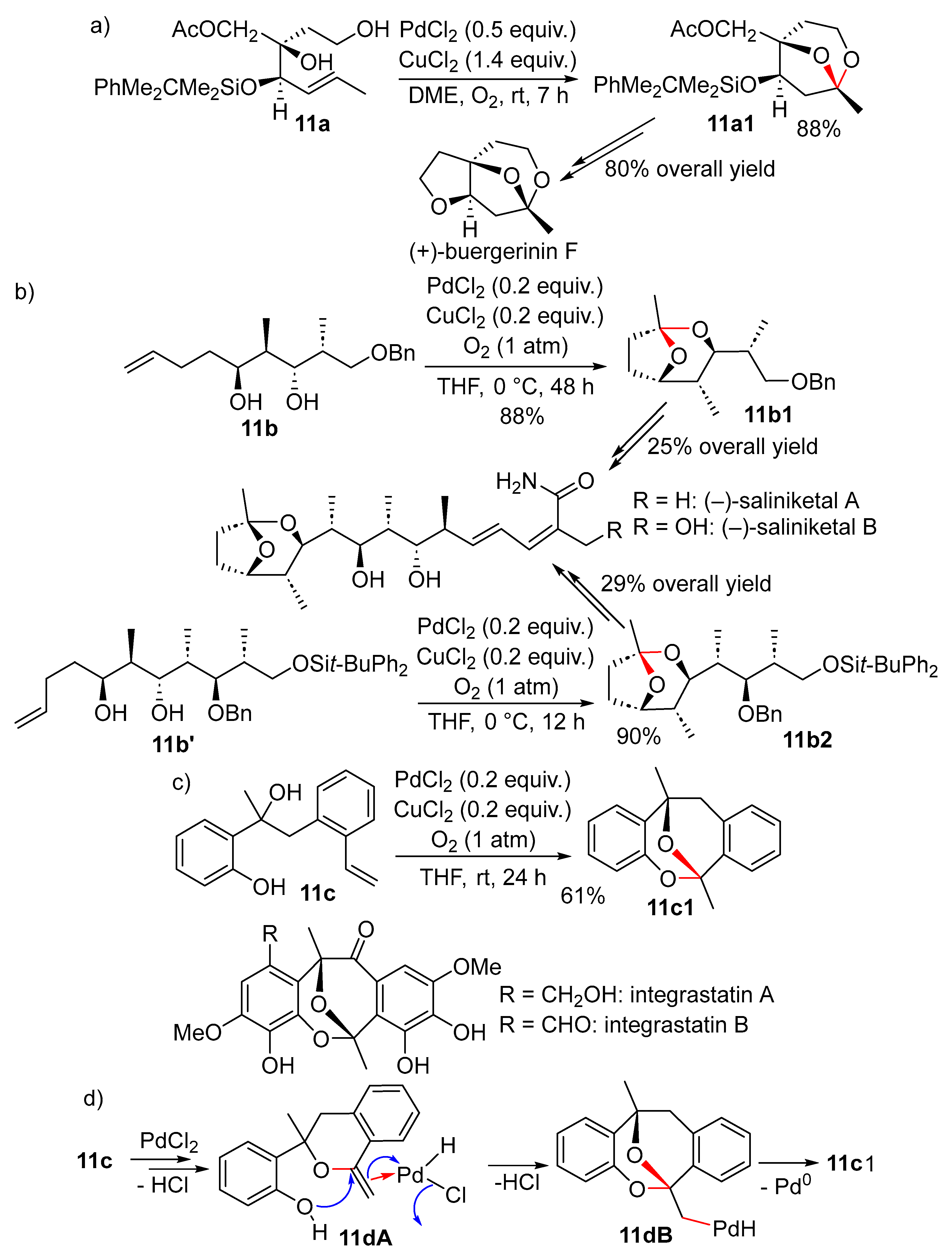
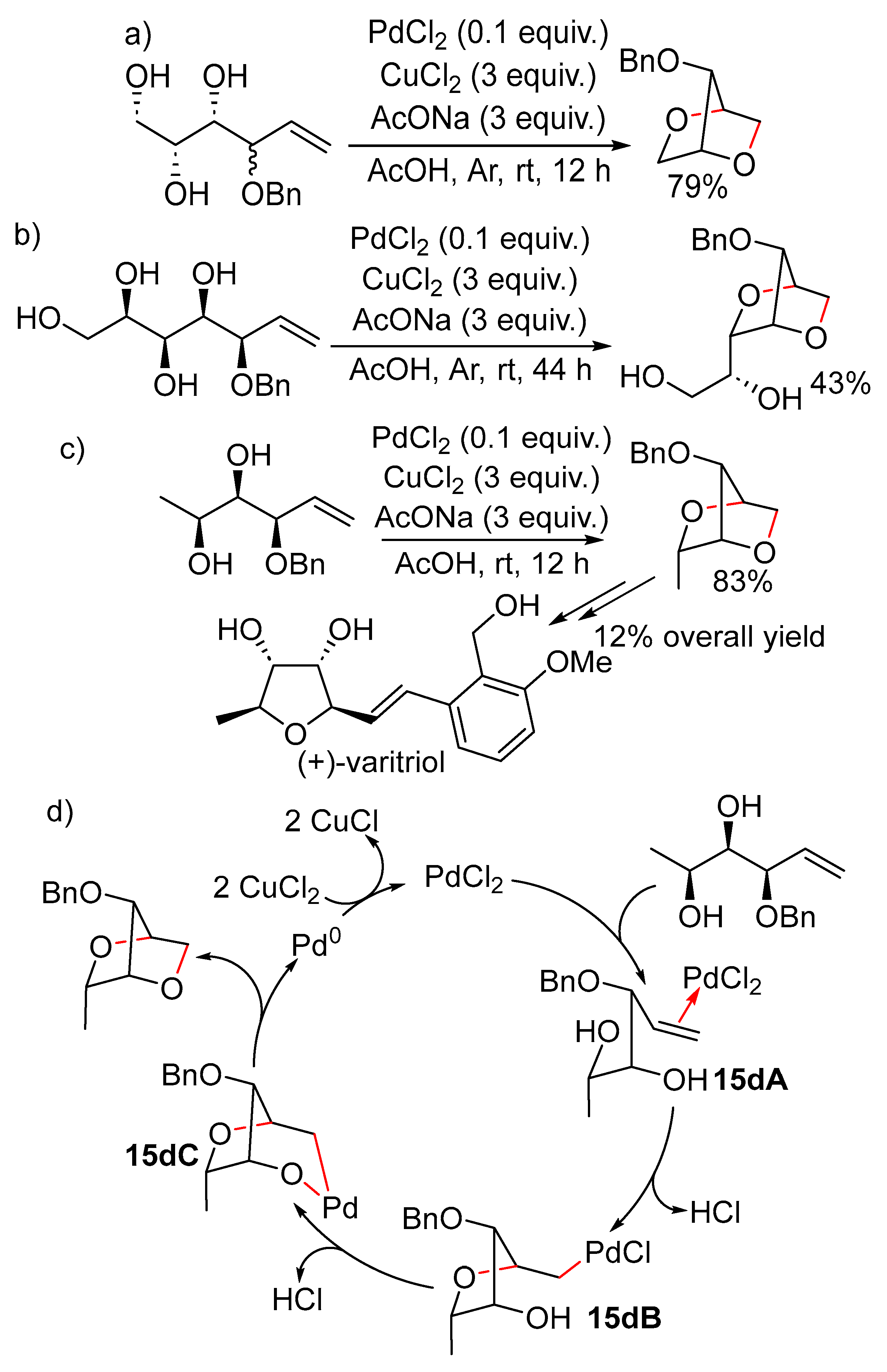
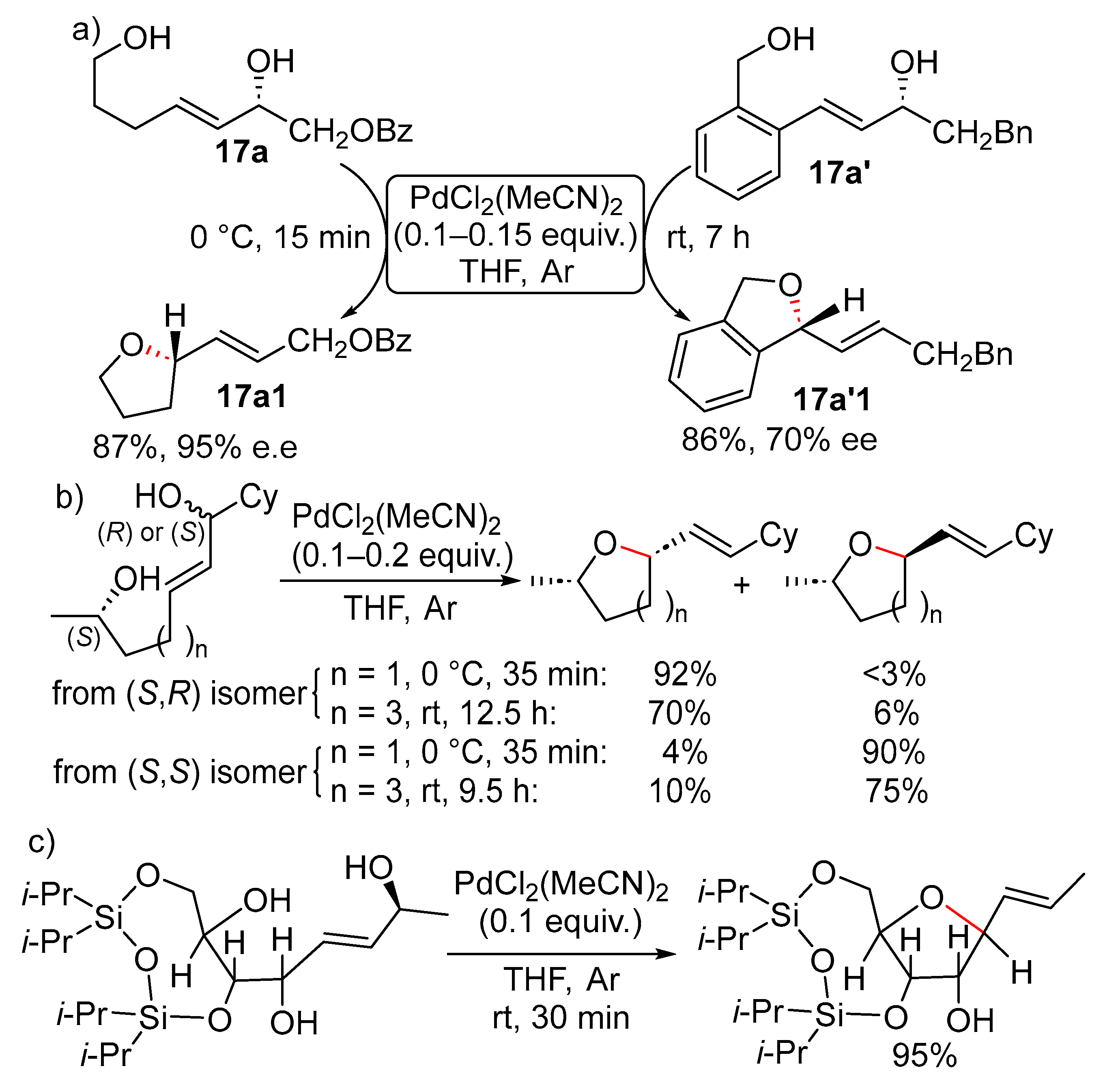
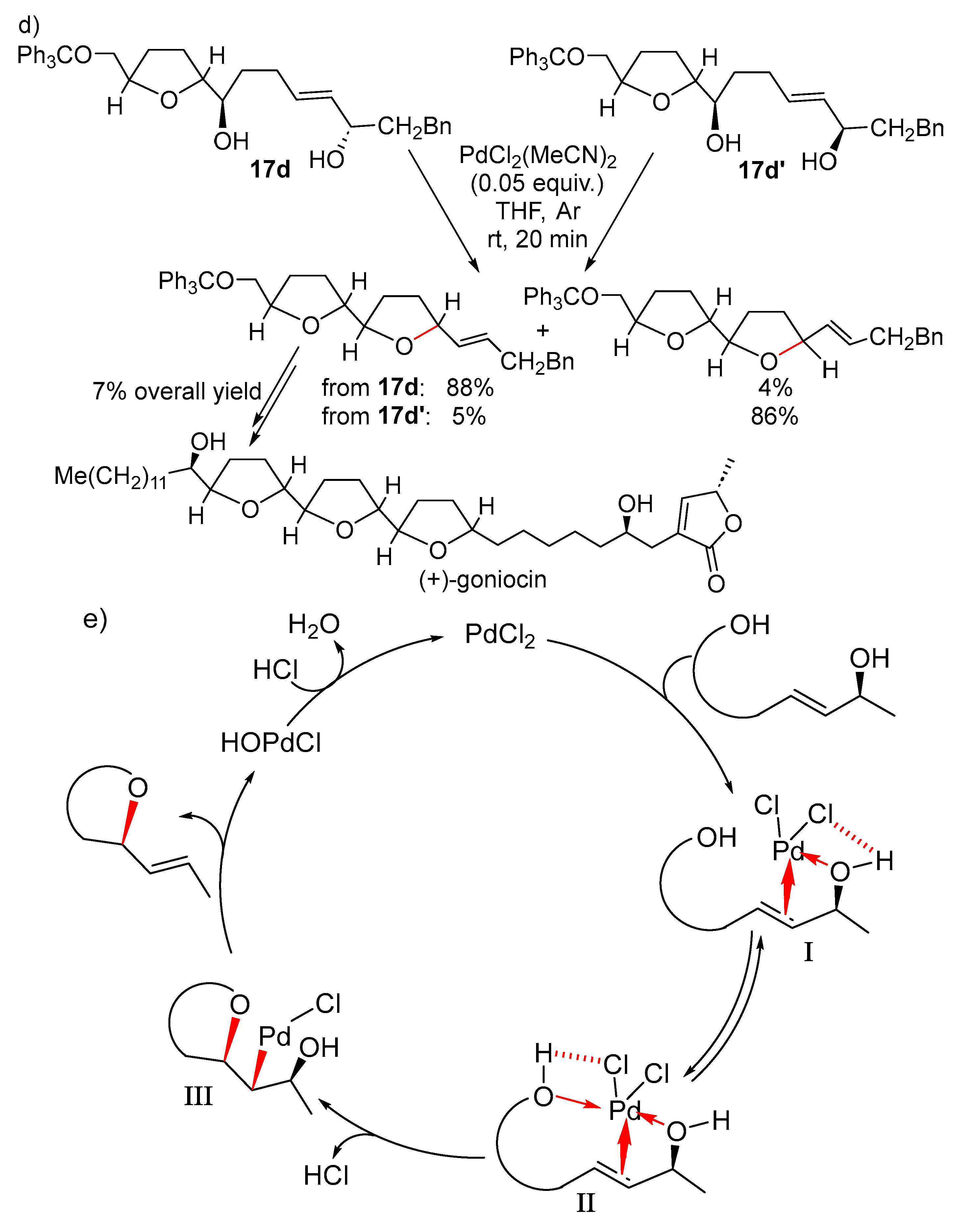
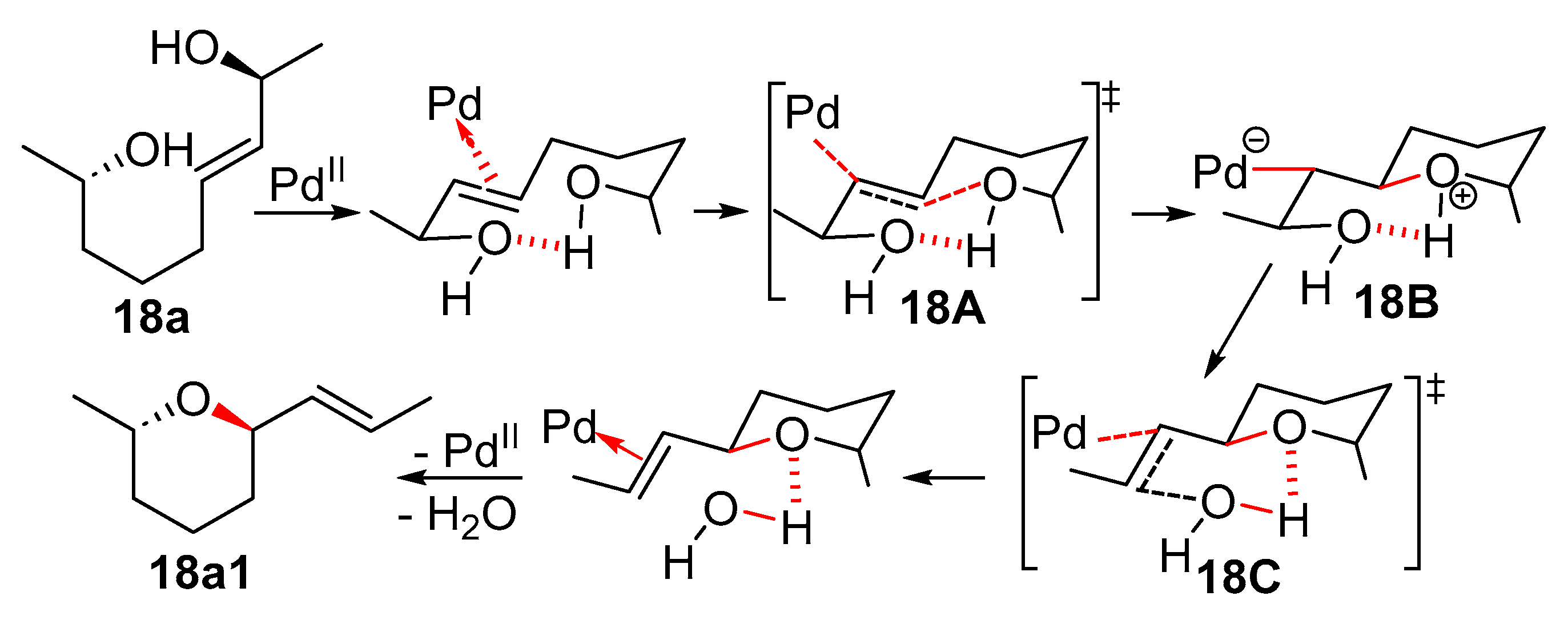
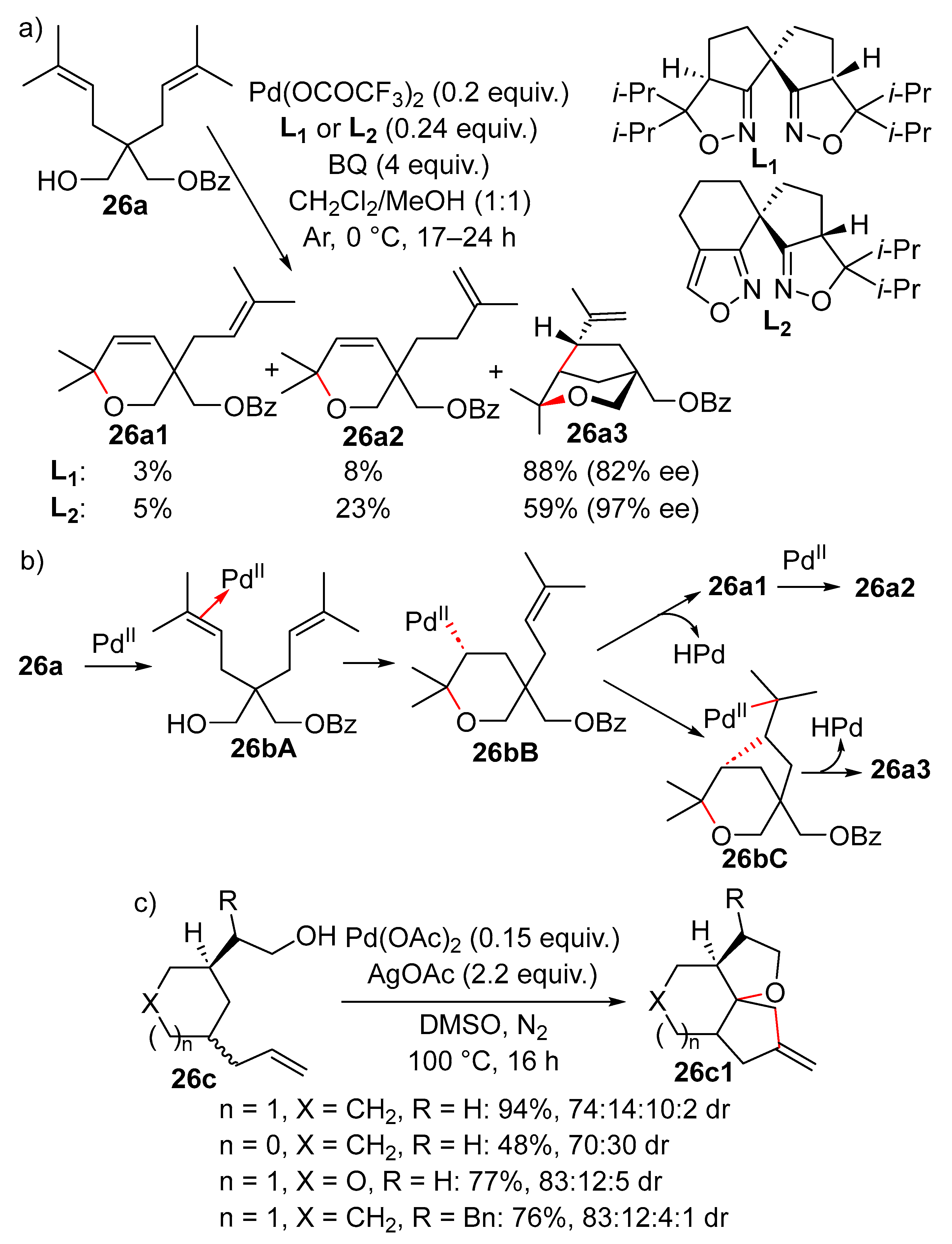
11. Cyclisation with β-Heterounit Elimination
11.1. β-OH Elimination
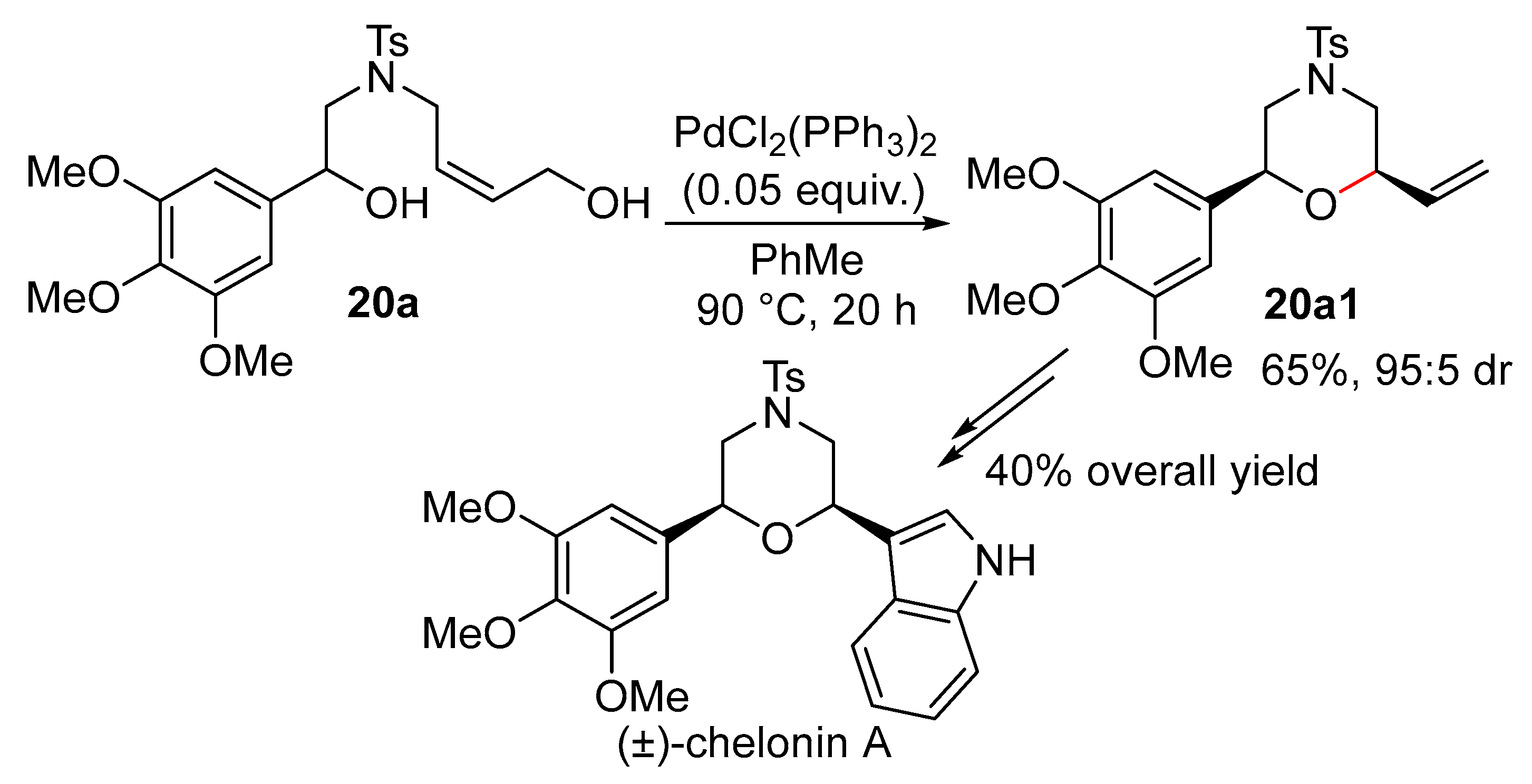
11.1.1. With 1,3-Chirality Transfer
11.1.2. Without Chirality Transfer
11.2. β-OCOR Elimination
11.3. β-OR Elimination
12. Cyclisation/Intramolecular Heck Reaction
13. Cyclisation/Intermolecular Heck Reaction
14. Cyclisation/Dehydrogenative Heck Reaction
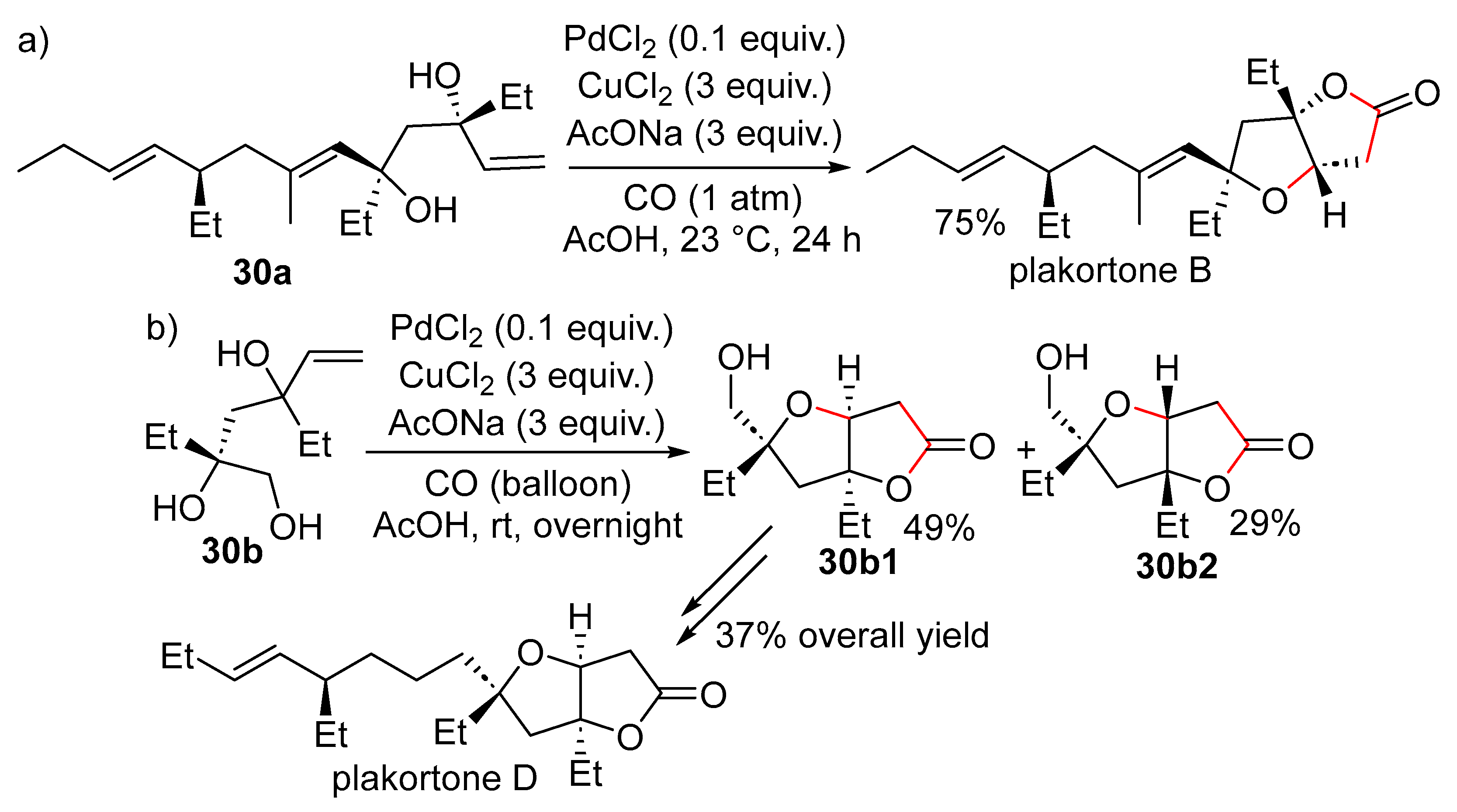
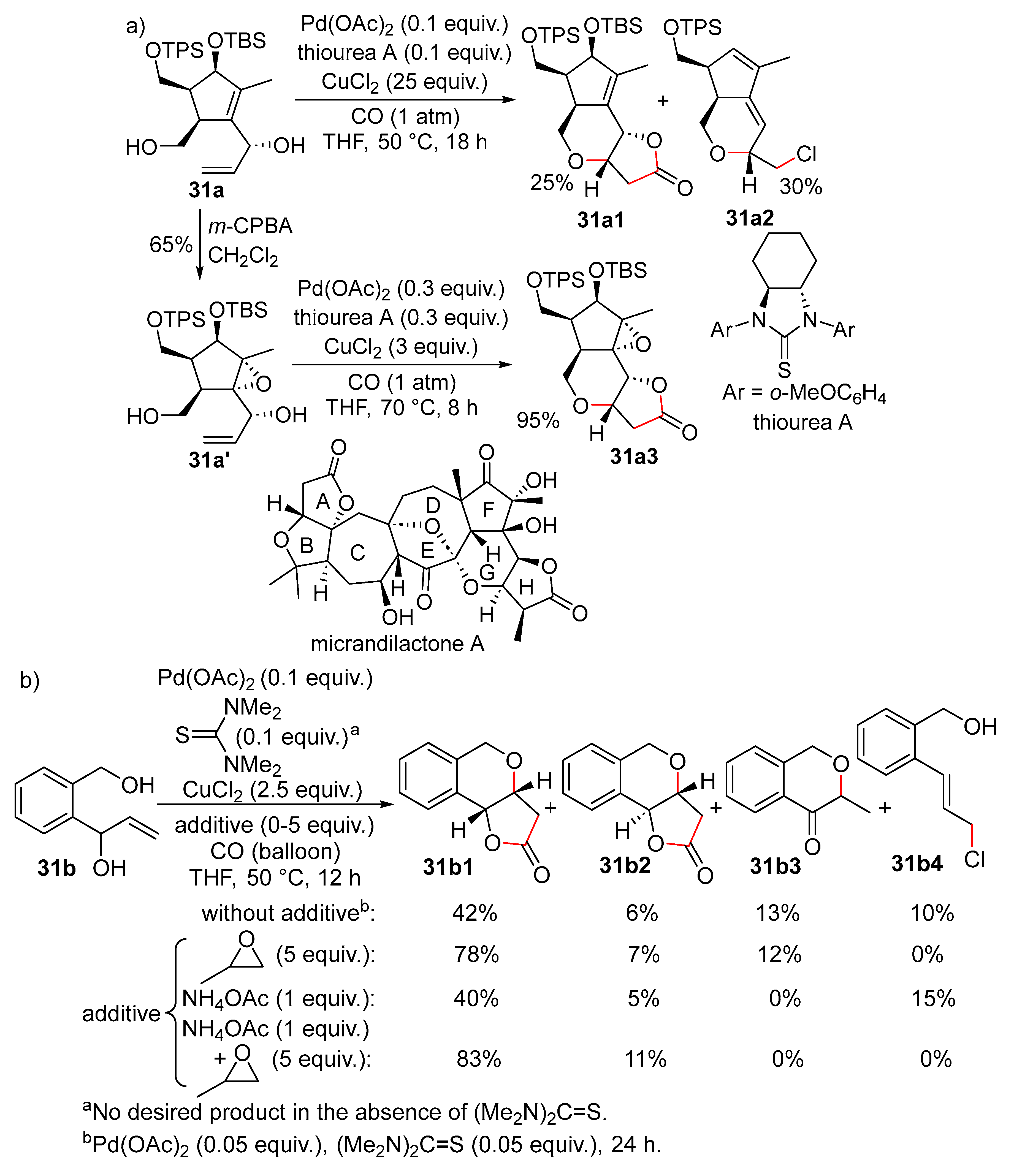
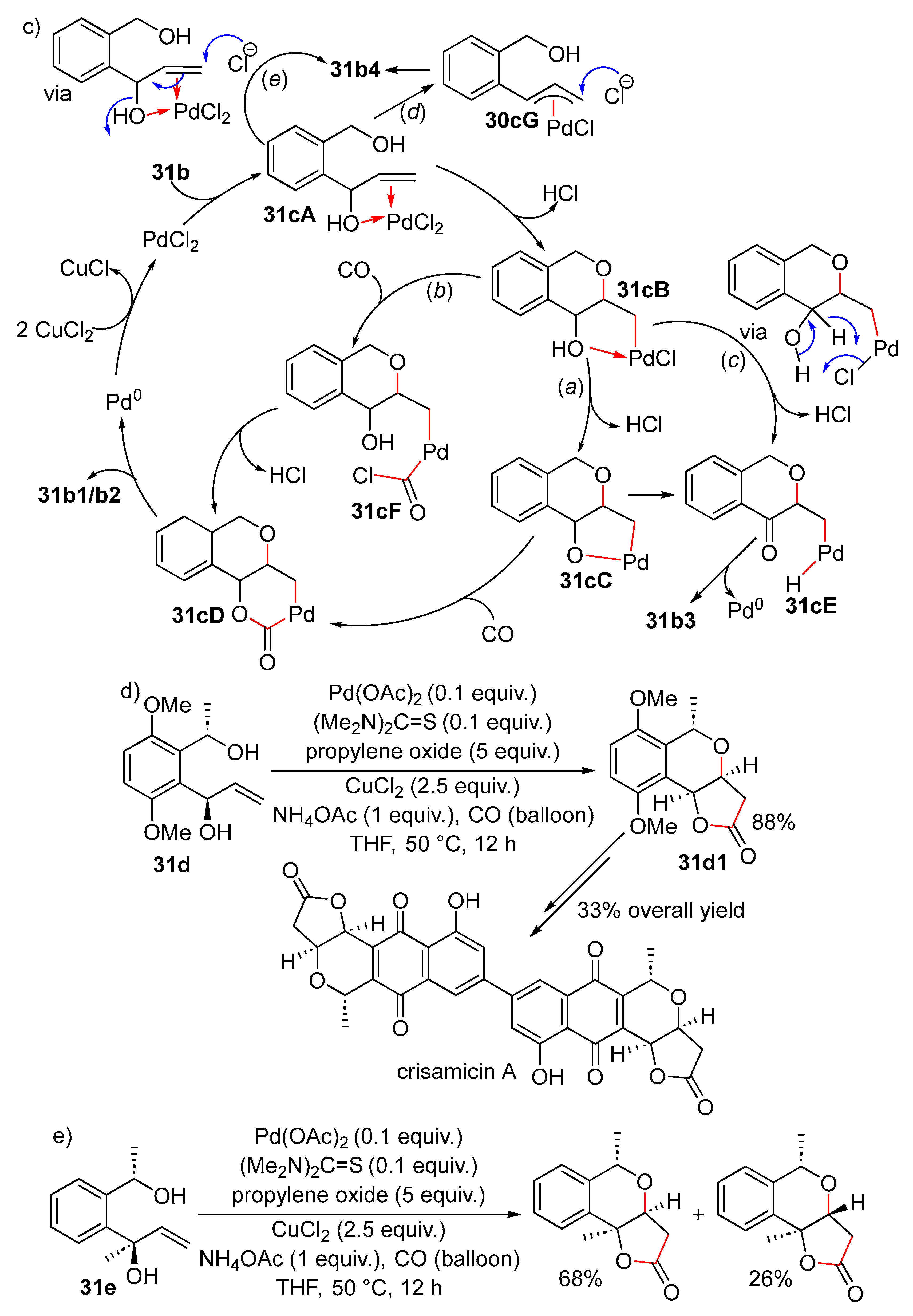
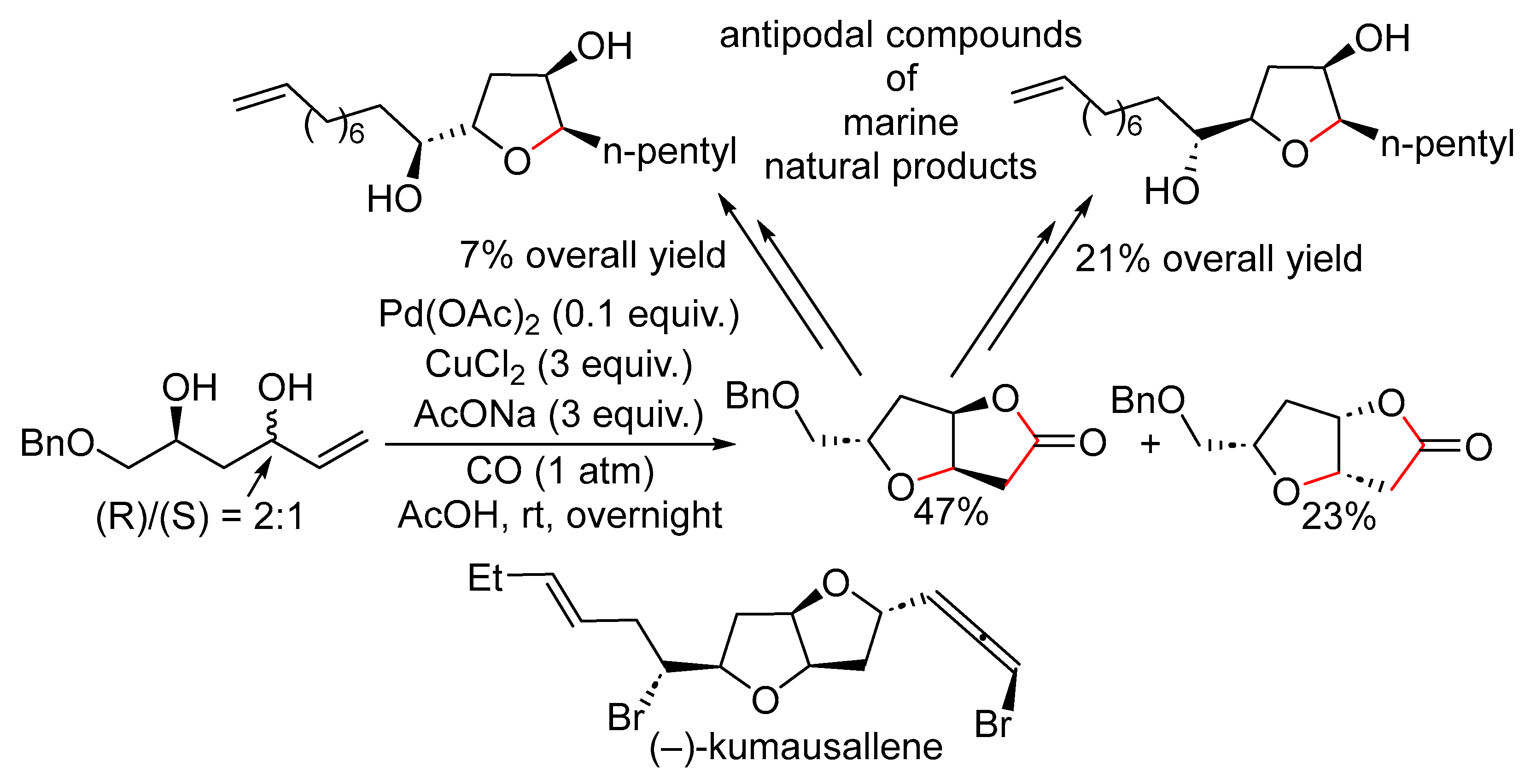
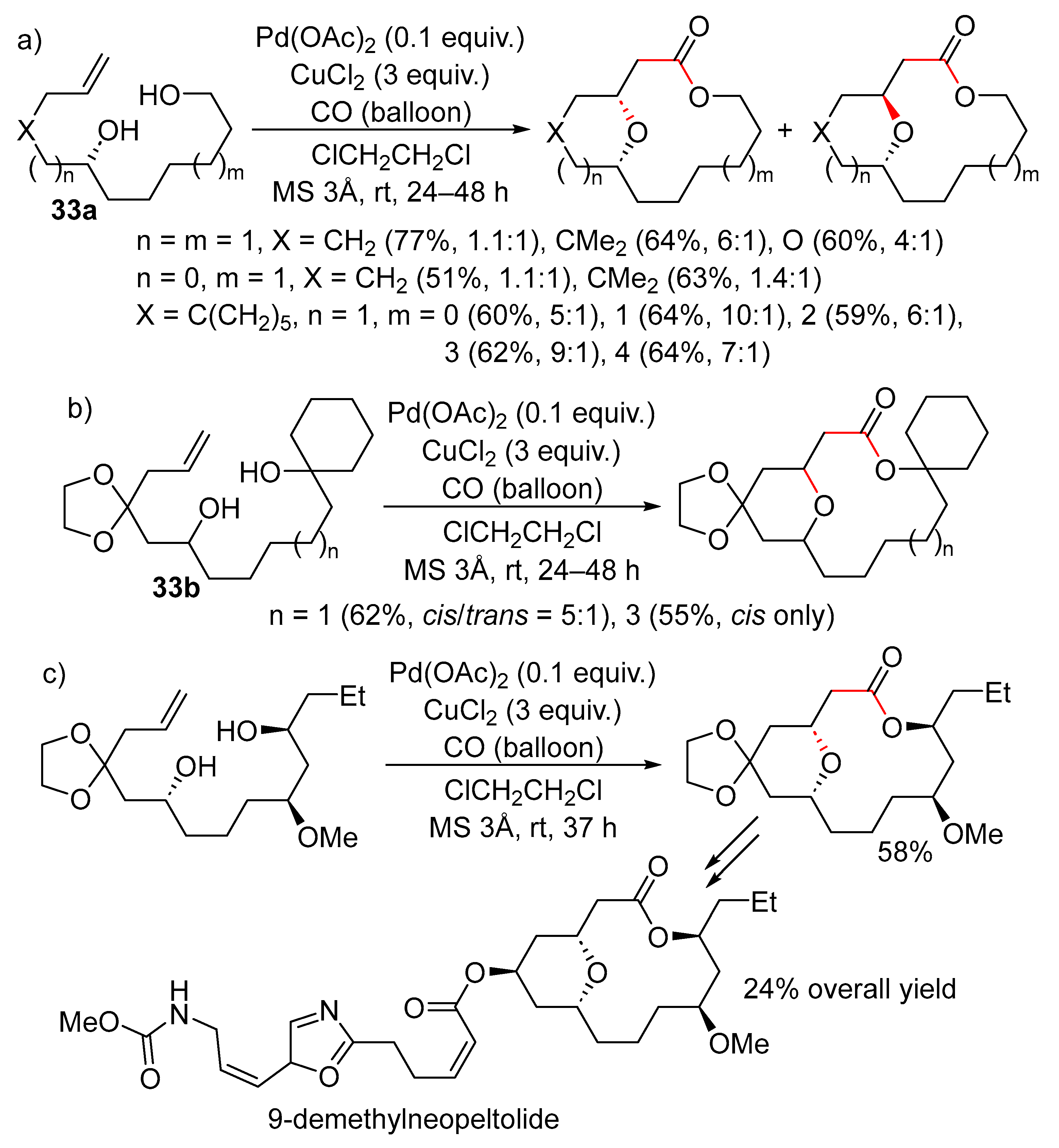
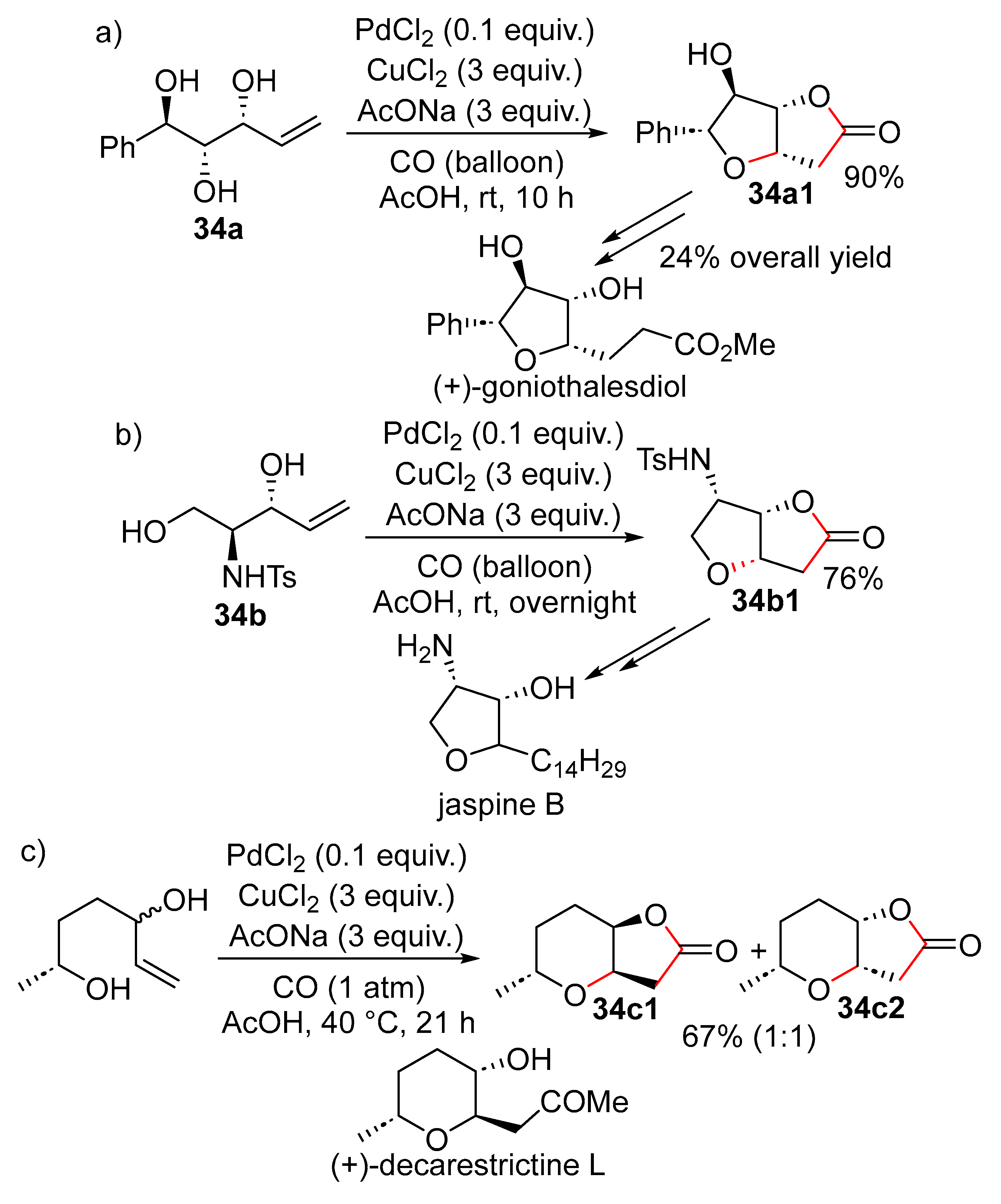
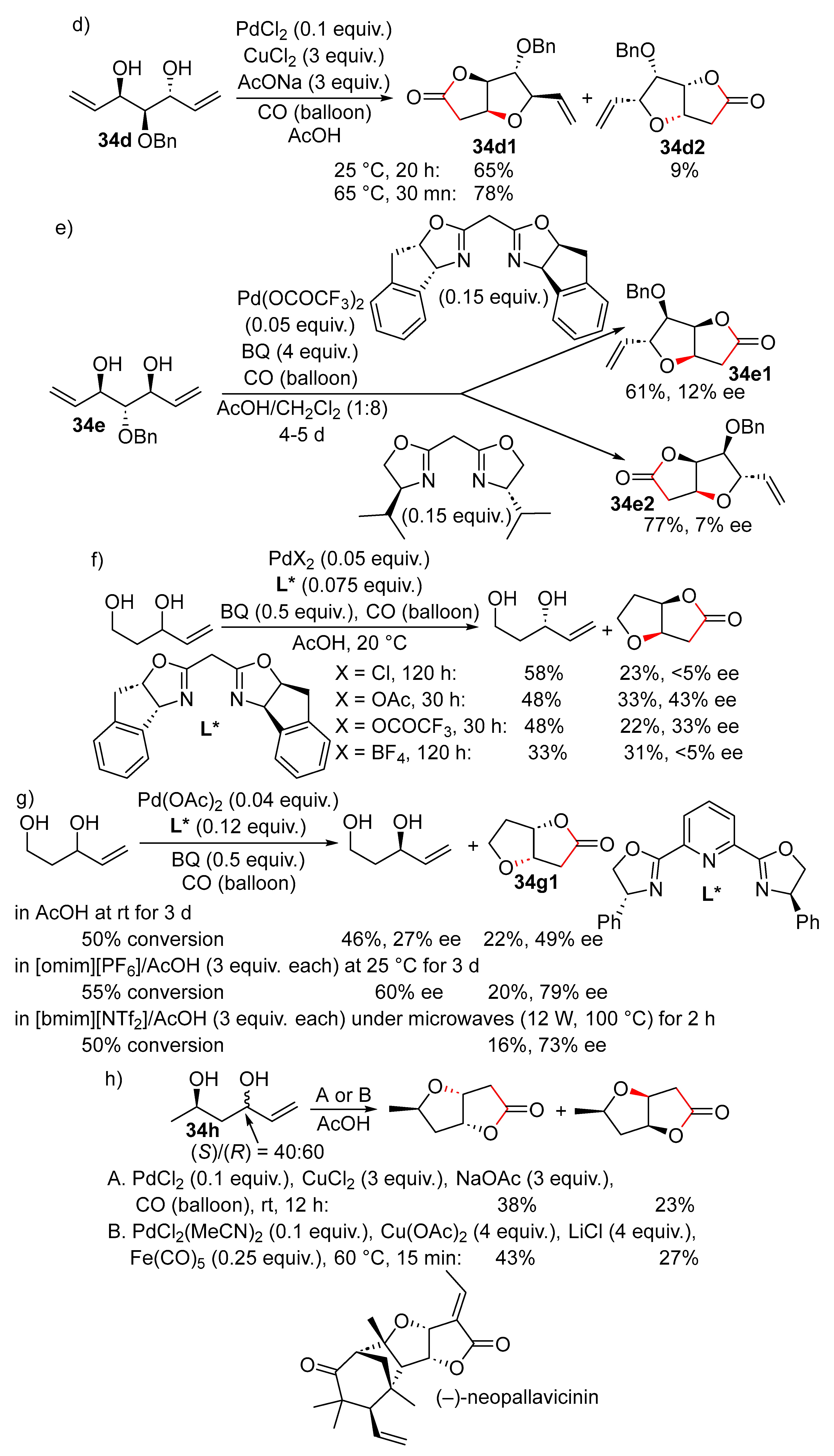
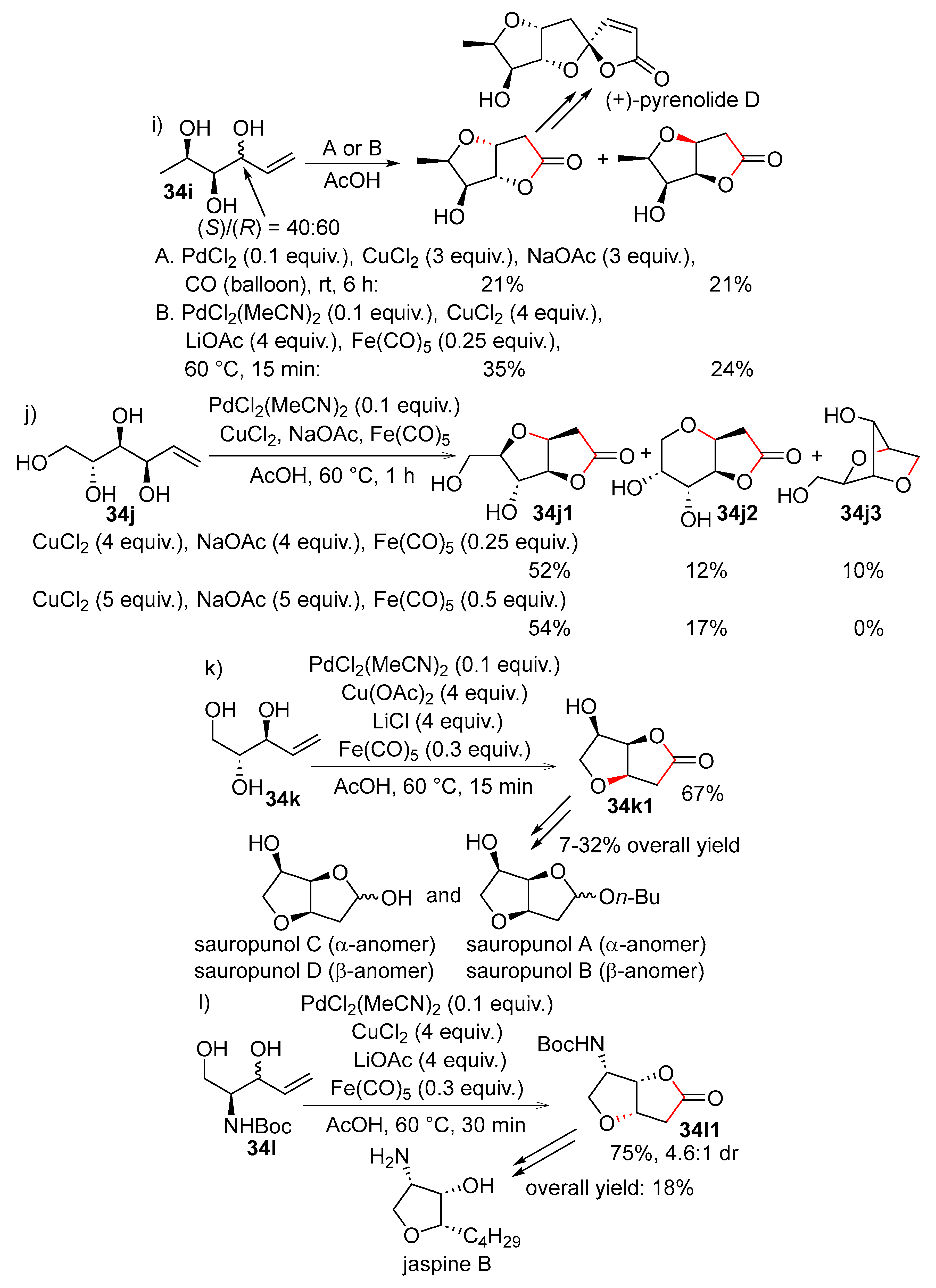
15. Cyclisation/Alkoxy- or Hydroxycarbonylation
16. Cyclisation/Alkoxycarbonylative Lactonisation
17. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Phillips, F.C. Researches upon the phenomena of oxidation and chemical properties of gases. Am. Chem. J. 1894, 16, 255–277. [Google Scholar]
- Phillips, F.C. Untersuchungen über die chemischen eigenschaften von gasen. I. Mitteilung. Erscheinungen bei der oxydation von wasserstoff und kohlenwasserstoffen. Z. Anorg. Chem. 1894, 6, 213–228. [Google Scholar] [CrossRef]
- Smidt, J.; Hafner, W.; Jira, R.; Sedlmeier, J.; Sieber, R.; Rüttinger, R.; Kojer, H. Katalytische umsetzungen von olefinen an platinmetall-verbindungen. Das consortium-verfahren zur herstellung von acetaldehyde. Angew. Chem. 1959, 71, 176–182. [Google Scholar] [CrossRef]
- Hafner, W.; Jira, R.; Sedlmeier, J.; Smidt, J. Über die reaktionen von olefinen mit wässrigen lösungen with palladiumsalzen. Chem. Ber. 1962, 95, 1575–1581. [Google Scholar] [CrossRef]
- Smidt, J.; Hafner, W.; Jira, R.; Sedlmeier, J.; Sieber, R.; Ruttinger, R.; Kojer, H. The oxidation of olefins with palladium chloride catalysts. Angew. Chem. Int. Ed. 1962, 1, 80–88. [Google Scholar] [CrossRef]
- Jira, R. Acetaldehyde from Ethylene—A Retrospective on the Discovery of the Wacker Process. Angew. Chem. Int. Ed. 2009, 48, 9034–9037. [Google Scholar] [CrossRef]
- Keith, J.A.; Henry, P.M. The mechanism of the Wacker reaction: A tale of two hydroxypalladations. Angew. Chem. Int. Ed. 2009, 48, 9038–9049. [Google Scholar] [CrossRef]
- Stirling, A.; Nair, N.N.; Lledós, A.; Ujaque, G. Challenges in modelling homogeneous catalysis: New answers from ab initio molecular dynamics to the controversy over the Wacker process. Chem. Soc. Rev. 2014, 43, 4940–4952. [Google Scholar] [CrossRef]
- Moiseev, I.I.; Vargaftik, M.N.; Syrkin, Y.K. Oxidation reactions of olefins. Dokl. Akad. Nauk SSSR 1960, 130, 820–823. [Google Scholar]
- Tsuji, J. Synthetic applications of the palladium-catalyzed oxidation of olefins to ketones. Synthesis 1984, 1984, 369–384. [Google Scholar] [CrossRef]
- Takacs, J.M.; Jiang, X.-T. The Wacker reaction and related alkene oxidation reaction. Curr. Org. Chem. 2003, 7, 369–396. [Google Scholar] [CrossRef]
- Cornell, C.N.; Sigman, M.S. Recent progress in Wacker oxidations: Moving toward molecular oxygen as the sole oxidant. Inorg. Chem. 2007, 46, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Jose, D.E.; Mathew, T.V. Advances in Wacker oxidation: From palladium to first-row transition metal catalysts. J. Organomet. Chem. 2025, 1032, 123625. [Google Scholar] [CrossRef]
- Muzart, J. Aldehydes from Pd-catalysed oxidation of terminal olefins. Tetrahedron 2007, 63, 7505–7521. [Google Scholar] [CrossRef]
- Muzart, J. Progress in the synthesis of aldehydes from Pd-catalyzed Wacker-type reactions of terminal olefins. Tetrahedron 2021, 87, 132024. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Jha, A.K.; Kumar, P. Recent advances in Wacker oxidation: From conventional to modern variants and applications. Catal. Sci. Technol. 2020, 10, 7448–7470. [Google Scholar] [CrossRef]
- Mhaldar, S.N.; Gaikwad, M.M.; Tilve, S.G. Wacker Oxidation: A Powerful Tool for Natural Product Synthesis. Asian J. Org. Chem. 2025, 14, e202500117. [Google Scholar] [CrossRef]
- Clement, W.H.; Selwitz, C.M. Improved procedures for converting higher α-olefins to methyl ketones with palladium chloride. J. Org. Chem. 1964, 29, 241–243. [Google Scholar] [CrossRef]
- McQuillin, F.J.; Parker, D.G. Complexing of terpenes with transition metals. Part IV. A comparison of the reactions of (+)-3,7-dimethylocta-1,6-diene with palladium(II) and with mercury(II). J. Chem. Soc. Perkin Trans. 1 1974, 1974, 809–815. [Google Scholar] [CrossRef]
- Tsuji, J.; Shimizu, I.; Yamamoto, K. Convenient general synthetic method for 1,4- and 1,5-diketones by palladium catalyzed oxidation of α-allyl and α-3-butenyl ketones. Tetrahedron Lett. 1976, 34, 2975–2976. [Google Scholar] [CrossRef]
- Tsuji, J.; Nagashima, H.; Nemoto, H. A general method for the preparation of methyl ketones from terminal olefins: 2-decanone. In Organic Syntheses; Semmelhack, M.F., Ed.; Wiley: Hoboken, NJ, USA, 1984; Volume 62, pp. 9–11. [Google Scholar]
- Yick, C.-Y.; Wong, H.N.C. Regiospecific substitution of the carbon–boron bond of tris(4-methylfuran-3-yl)boroxine: A model ring C→BC→ABC approach towards eudesmanolides. Tetrahedron 2001, 57, 6935–6940. [Google Scholar] [CrossRef]
- Thadani, A.N.; Rawal, V.H. Multifunctional palladium catalysis. 2. Tandem haloallylation followed by Wacker-Tsuji oxidation or Sonogashira cross-coupling. Org. Lett. 2002, 4, 4321–4323. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhou, H.; Yu, J.; Cook, J.M. An improved total synthesis of (+)-macroline and alstonerine as well as the formal total synthesis of (−)-talcarpine and (−)-anhydromacrosalhine-methine. J. Org. Chem. 2006, 71, 8884–8890. [Google Scholar] [CrossRef]
- Li, J.J. Wacker-Tsuji oxidation. In Name Reactions for Functional Group Transformations; Li, J.J., Corey, E.J., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 2007; pp. 309–326. [Google Scholar]
- Roussel, M.; Mimoun, H. Palladium-catalyzed oxidation of terminal olefins to methyl ketones by hydrogen peroxide. J. Org. Chem. 1980, 45, 5387–5390. [Google Scholar] [CrossRef]
- Nishimura, T.; Kakiuchi, N.; Onoue, T.; Ohe, K.; Uemura, S. Palladium(II)-catalyzed oxidation of terminal alkenes to methyl ketones using molecular oxygen. J. Chem. Soc. Perkin Trans. 1 2000, 2000, 1915–1918. [Google Scholar] [CrossRef]
- Muzart, J. Palladium-catalysed reactions of alcohols. Part C: Formation of ether linkages. Tetrahedron 2005, 61, 5955–6008. [Google Scholar] [CrossRef]
- Zeni, G.; Larock, R.C. Synthesis of heterocycles via palladium π-olefin and π-alkyne chemistry. Chem. Rev. 2004, 104, 2285–2309. [Google Scholar] [CrossRef]
- Sharma, G.V.M.; Krishna, P.R. Carbohydrates: From ‘chirons’ to new glycosubstances. Curr. Org. Chem. 2004, 8, 1187–1209. [Google Scholar] [CrossRef]
- Doháňošová, J.; Gracza, T. Asymmetric palladium-catalysed intramolecular Wacker-type cyclisations of unsaturated alcohols and amino alcohols. Molecules 2013, 18, 6173–6192. [Google Scholar] [CrossRef]
- Beccalli, E.M.; Broggini, G.; Martinelli, M.; Sottocornola, S. C-C, C-O, C-N bond formation on sp2 carbon by Pd(II)-catalyzed reactions involving oxidant agents. Chem. Rev. 2007, 107, 5318–5365. [Google Scholar] [CrossRef]
- Butt, N.A.; Zhang, W. Synthesis of heterocycles via palladium-catalyzed Wacker-type oxidative cyclization reactions of hydroxy- and amino-alkenes. Top. Heterocycl. Chem. 2013, 32, 77–108. [Google Scholar]
- Hosokawa, T.; Yamanaka, T.; Murahashi, S.-I. Palladium(II)-catalysed asymmetric acetalization of alkenes. J. Chem. Soc. Chem. Commun. 1993, 1993, 117–119. [Google Scholar] [CrossRef]
- Hosokawa, T.; Yamanaka, T.; Itotani, M.; Murahashi, S.-I. Palladium(II)-catalyzed asymmetric acetalization of alkenes. J. Org. Chem. 1995, 60, 6159–6167. [Google Scholar] [CrossRef]
- Henry, P.M. Palladium Catalyzed Oxidation of Hydrocarbons; Reidel: Dordrecht, The Netherlands, 1980; pp. 41–84. [Google Scholar]
- ten Brink, G.-J.; Arends, I.W.C.E.; Papadogianakis, G.; Sheldon, R.A. Catalytic conversions in water: Part 13. Aerobic oxidation of olefins to methyl ketones catalysed by a water-soluble palladium complex—Mechanistic investigations. Appl. Catal. A 2000, 194–195, 435–442. [Google Scholar] [CrossRef]
- Nelson, D.J.; Li, R.; Brammer, C. Correlation of relative rates of PdCl2 oxidation of functionalized acyclic alkenes versus alkene ionization potentials, HOMOs, and LUMOs. J. Am. Chem. Soc. 2001, 123, 1564–1568. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamasaki, K.; Mimura, M.; Uozumi, Y. Deuterium-labeling studies establishing stereochemistry at the oxypalladation step in Wacker-type oxidative cyclization of an o-allylphenol. J. Am. Chem. Soc. 2004, 126, 3036–3037. [Google Scholar] [CrossRef]
- Sakakibara, T.; Tanaka, Y.; Yamasaki, S.-I. Palladium-promoted aromatic annelation with acetylene dicarboxylates. Chem. Lett. 1986, 15, 797–800. [Google Scholar] [CrossRef]
- Wu, G.; Rheingold, A.L.; Geib, S.J.; Heck, R.F. Palladium-catalyzed annelation of aryl iodides with diphenylacetylene. Organometallics 1987, 6, 1941–1946. [Google Scholar] [CrossRef]
- Wang, W.; Wang, F.; Shi, M. Bis(NHC)-palladium(II) complex-catalyzed dioxygenation of alkenes. Organometallics 2010, 29, 928–933. [Google Scholar] [CrossRef]
- Takenaka, K.; Dhagea, Y.D.; Sasai, H. Enantioselective Pd(II)–Pd(IV) catalysis utilizing a SPRIX ligand: Efficient construction of chiral 3-oxy-tetrahydrofurans. Chem. Commun. 2013, 49, 11224–11226. [Google Scholar] [CrossRef]
- Giofrè, S.; Molteni, L.; Nava, D.; Lo Presti, L.; Beccalli, E.M. Enantio- and regioselective palladium(II)-catalyzed dioxygenation of (aza-)alkenols. Angew. Chem. Int. Ed. 2021, 60, 21723–21727. [Google Scholar] [CrossRef]
- Muzart, J. Pd0- and PdII-catalyzed oxaheterocyclization of substrates having both an allylic leaving group and a hydroxylated tether. J. Mol. Catal. A Chem. 2010, 319, 1–29. [Google Scholar] [CrossRef]
- Trend, R.M.; Ramtohul, Y.K.; Stoltz, B.M. Oxidative cyclizations in a nonpolar solvent using molecular oxygen and studies on the stereochemistry of oxypalladation. J. Am. Chem. Soc. 2005, 127, 17778–17788. [Google Scholar] [CrossRef]
- Ebner, D.C.; Novak, Z.; Stoltz, B.M. Oxidative kinetic resolution-Claisen rearrangement sequence to enantioenriched arylcycloalkenes. Synlett 2006, 2006, 3533–3539. [Google Scholar] [CrossRef]
- Jin, L.; Nemoto, T.; Nakamura, H.; Hamada, Y. Pd-catalyzed asymmetric allylic alkylation of 2-substituted cycloalkenyl carbonates using a chiral diaminophosphine oxide: (S,RP)-Ph-DIAPHOX. Tetrahedron Asymmetry 2008, 19, 1106–1113. [Google Scholar] [CrossRef]
- Kuramochi, A.; Usuda, H.; Yamatsugu, K.; Kanai, M.; Shibasaki, M. Total synthesis of (±)-garsubellin A. J. Am. Chem. Soc. 2005, 127, 14200–14201. [Google Scholar] [CrossRef]
- Kawai, N.; Hande, S.M.; Uenishi, J. Stereoselective synthesis of (−)-diospongins A and B and their stereoisomers at C-5. Tetrahedron 2007, 63, 9049–9056. [Google Scholar] [CrossRef]
- Parreira, L.A.; Gonçalves, D.C.; Menini, L.; Gusevskaya, E.V. Aerobic oxidation of naturally occurring -bisabolol catalyzed by palladium(II) salts as sole catalysts. Appl. Catal. A 2016, 524, 126–133. [Google Scholar] [CrossRef]
- Govender, S.; Mmutlane, E.M.; van Otterlo, W.A.L.; de Koning, C.B. Bidirectional racemic synthesis of the biologically active quinone cardinalin 3. Org. Biomol. Chem. 2007, 5, 2433–2440. [Google Scholar] [CrossRef]
- Tsuji, J.; Nagashima, H.; Hori, K. A new preparative method for 1, 3-dicarbonyl compounds by the regioselective oxidation of α, β-unsaturated carbonyl compounds, catalyzed by PdCl2 using hydroperoxides as the reoxidant of Pd0. Chem. Lett. 1980, 9, 257–260. [Google Scholar] [CrossRef]
- Liao, X.; Zhou, H.; Wearing, X.Z.; Ma, J.; Cook, J.M. The first regiospecific, enantiospecific total synthesis of 6-oxoalstophylline and an improved total synthesis of alstonerine and alstophylline as well as the bisindole alkaloid macralstonine. Org. Lett. 2005, 7, 3501–3504. [Google Scholar] [CrossRef] [PubMed]
- Cort, A.D. A simple and convenient method for the cleavage of silylethers. Synth. Commun. 1990, 20, 757–760. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Pollart, D.; Monforte, J.; Kotsuki, H. Pd(II)-catalyzed acetal/ketal hydrolysis/exchange reactions. Tetrahedron Lett. 1985, 26, 705–708. [Google Scholar] [CrossRef]
- Cormier, J.F. Removal of TBDMS protecting groups from carbohydrates using catalytic transfer hydrogenation. Tetrahedron Lett. 1991, 32, 187–188. [Google Scholar] [CrossRef]
- Takenaka, K.; Nakatsuka, S.; Tsujihara, T.; Koranne, P.S.; Sasai, H. Divergent synthesis of chiral spiro (isoxazole–isoxazoline) hybrid ligands. Tetrahedron Asymmetry 2008, 19, 2492–2496. [Google Scholar] [CrossRef]
- Takenaka, K.; Nagano, T.; Takizawa, S.; Sasai, H. Asymmetric synthesis of chiral spiro bis(isoxazoline) and spiro (isoxazole–isoxazoline) ligands. Tetrahedron Asymmetry 2010, 21, 379–381. [Google Scholar] [CrossRef]
- Broggini, G.; Beccalli, E.M.; Borsini, E.; Fasana, A.; Zecchi, G. Intramolecular oxidative Pd(II)-catalyzed alkoxylation of 3-aza-5-alkenols with O2 as sole oxidant: Mild conditions for the synthesis of 1,4-oxazine derivatives. Synlett 2011, 2011, 227–230. [Google Scholar] [CrossRef]
- ten Brink, G.-J.; Arends, I.W.C.E.; Papadogianakis, G.; Sheldon, R.A. Catalytic conversions in water. Part 10. Aerobic oxidation of terminal olefins to methyl ketones catalysed by water soluble palladium complexes. Chem. Commun. 1998, 1998, 2359–2360. [Google Scholar] [CrossRef]
- Mitsudome, T.; Umetani, T.; Nosaka, N.; Mori, K.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Convenient and efficient Pd-catalyzed regioselective oxyfunctionalization of terminal olefins by using molecular oxygen as sole reoxidant. Angew. Chem. Int. Ed. 2006, 45, 481–485. [Google Scholar] [CrossRef]
- Cornell, C.N.; Sigman, M.S. Discovery of a practical direct O2-coupled Wacker Oxidation with Pd[(-)-sparteine]Cl2. Org. Lett. 2006, 8, 4117–4120. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.L. A concise stereospecific synthesis of repinotan (BAY × 3702). Tetrahedron Lett. 2003, 44, 8563–8565. [Google Scholar] [CrossRef]
- Thiery, E.; Chevrin, C.; Le Bras, J.; Hakarat, D.; Muzart, J. Mechanism insights into the palladiumII-catalyzed hydroalkoxylation of 2-allylphenols. J. Org. Chem. 2007, 72, 1859–1862. [Google Scholar] [CrossRef]
- Fan, J.; Wan, C.; Wang, Q.; Gao, L.; Zheng, X.; Wang, Z. Palladium catalyzed isomerization of alkenes: A pronounced influence of an o-phenol hydroxyl group. Org. Biomol. Chem. 2009, 7, 3168–3172. [Google Scholar] [CrossRef]
- Nishiwaki, N.; Kamimura, R.; Shono, K.; Kawakami, T.; Nakayama, K.; Nishino, K.; Nakayama, T.; Takahashi, K.; Nakamura, A.; Hosokawa, T. Efficient double bond migration of allylbenzenes catalyzed by Pd(OAc)2–HFIP system with unique substituent effect. Tetrahedron Lett. 2010, 51, 3590–3592. [Google Scholar] [CrossRef]
- Landis, C.R.; Morales, C.M.; Stahl, S.S. Insights into the spin-forbidden reaction between L2Pd0 and molecular oxygen. J. Am. Chem. Soc. 2004, 126, 16302–16303. [Google Scholar] [CrossRef]
- Gligorich, K.M.; Sigman, M.S. Recent advancements and challenges of palladiumII-catalyzed oxidation reactions with molecular oxygen as the sole oxidant. Chem. Commun. 2009, 2009, 3854–3867. [Google Scholar] [CrossRef]
- Scheuermann, M.L.; Goldberg, K.I. Reactions of Pd and Pt complexes with molecular oxygen. Chem. Eur. J. 2014, 20, 14556–14568. [Google Scholar] [CrossRef]
- Gligorich, K.M.; Sigman, M.S. Mechanistic questions about the reaction of molecular oxygen with palladium in oxidase catalysis. Angew. Chem. Int. Ed. 2006, 118, 6612–6615. [Google Scholar] [CrossRef] [PubMed]
- Muzart, J. Molecular oxygen to regenerate PdII active species. Chem. Asian J. 2006, 1, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Keith, J.M.; Goddard, W.A., III; Oxgaard, J. Pd-Mediated activation of molecular oxygen: Pd(0) versus direct insertion. J. Am. Chem. Soc. 2007, 129, 10361–10369. [Google Scholar] [CrossRef]
- Konnick, M.M.; Stahl, S.S. Reaction of molecular oxygen with a PdII-hydride to produce a PdII-hydroperoxide: Experimental evidence for an HX-reductive-elimination pathway. J. Am. Chem. Soc. 2008, 130, 5753–5762. [Google Scholar] [CrossRef]
- Muzart, J.; Pète, J.-P. Dehydrogenation of cyclohexanones catalyzed by palladiumII trifluoroacetate. J. Mol. Catal. 1982, 15, 373–376. [Google Scholar] [CrossRef]
- Keith, J.M.; Muller, R.P.; Kemp, R.A.; Goldberg, K.I.; Goddard, W.A., III; Oxgaard, J. Mechanism of direct molecular oxygen insertion in a palladium(II)-hydride bond. Inorg. Chem. 2006, 45, 9631–9633. [Google Scholar] [CrossRef]
- Chowdhury, S.; Rivalta, I.; Russo, N.; Sicilia, E. Theoretical investigation of the mechanism of acid-catalyzed oxygenation of a Pd(II)-hydride to produce a Pd(II)-hydroperoxide. J. Chem. Theory Comput. 2008, 4, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.J.; Villarreal, J.A. Novel palladium-catalyzed oxidative intramolecular cyclization of β-citronellol with H2O2: A green and selective process to synthesize oxocine. Catal. Lett. 2017, 147, 1646–1653. [Google Scholar] [CrossRef]
- Reiter, M.; Turner, H.; Mills-Webb, R.; Gouverneur, V. Palladium-catalyzed oxidative cyclizations: Synthesis of dihydropyranones and furanones. J. Org. Chem. 2005, 70, 8478–8485. [Google Scholar] [CrossRef]
- Masal, D.P.; Choudhury, R.; Singh, A.; Reddy, S. Ready access to densely substituted furans using Tsuji-Wacker-type cyclization. J. Org. Chem. 2022, 87, 556–568. [Google Scholar] [CrossRef]
- Chevrin, C.; Le Bras, J.; Hénin, F.; Muzart, J. One-pot catalytic synthesis of 2-(1,2-dihydroxypropyl)-phenol derivatives from 2-allylphenols in aqueous media. Synthesis 2005, 2005, 2615–2618. [Google Scholar] [CrossRef]
- Schultz, M.J.; Sigman, M.S. Palladium(II)-catalyzed aerobic dialkoxylation of styrenes: A profound influence of an o-phenol. J. Am. Chem. Soc. 2006, 128, 1460–1461. [Google Scholar] [CrossRef]
- Zhang, Y.; Sigman, M.S. Palladium(II)-catalyzed enantioselective aerobic dialkoxylation of 2-propenyl phenols: A pronounced effect of copper additives on enantioselectivity. J. Am. Chem. Soc. 2007, 129, 3076–3077. [Google Scholar] [CrossRef]
- Jensen, K.H.; Pathak, T.P.; Zhang, Y.; Sigman, M.S. Palladium-catalyzed enantioselective addition of two distinct nucleophiles across alkenes capable of quinone methide formation. J. Am. Chem. Soc. 2009, 131, 17074–17075. [Google Scholar] [CrossRef]
- Pathak, T.P.; Gligorich, K.M.; Welm, B.E.; Sigman, M.S. Synthesis and preliminary biological studies of 3-substituted indoles accessed by a palladium-catalyzed enantioselective alkene difunctionalization reaction. J. Am. Chem. Soc. 2010, 132, 7870–7871. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.H.; Webb, J.D.; Sigman, M.S. Advancing the mechanistic understanding of an enantioselective palladium-catalyzed alkene difunctionalization reaction. J. Am. Chem. Soc. 2010, 132, 17471–17482. [Google Scholar] [CrossRef]
- Shiina, I.; Kawakita, Y.; Ibuka, R.; Yokoyama, K.; Tamai, Y. Total synthesis of buergerinin F via effective construction of the asymmetric quaternary carbons using an enantioselective aldol reaction. Chem. Commun. 2005, 2005, 4062–4064. [Google Scholar] [CrossRef] [PubMed]
- Paterson, I.; Razzak, M.; Anderson, E.A. Total synthesis of (−)-saliniketals A and B. Org. Lett. 2008, 10, 3295–3298. [Google Scholar] [CrossRef]
- Yadav, J.S.; Hossain, S.S.; Madhu, M.; Mohapatra, D.K. Formal total synthesis of (−)-saliniketals. J. Org. Chem. 2009, 74, 8822–8825. [Google Scholar] [CrossRef]
- Williams, P.G.; Asolkar, R.N.; Kondratyuk, T.; Pezzuto, J.M.; Jensen, P.R.; Fenical, W. Saliniketals A and B, bicyclic polyketides from the marine actinomycete Salinispora arenicola. J. Nat. Prod. 2007, 70, 83–88. [Google Scholar] [CrossRef]
- Tadross, P.M.; Bugga, P.; Stoltz, B.M. A rapid and convergent synthesis of the integrastatin core. Org. Biomol. Chem. 2011, 9, 5354–5357. [Google Scholar] [CrossRef]
- Singh, S.B.; Zink, D.L.; Quamina, D.S.; Pelaez, F.; Teran, A.; Felock, P.; Hazuda, D.J. Integrastatins: Structure and HIV-1 integrase inhibitory activities of two novel racemic tetracyclic aromatic heterocycles produced by two fungal species. Tetrahedron Lett. 2002, 43, 2351–2354. [Google Scholar] [CrossRef]
- Balija, A.M.; Stowers, K.J.; Schultz, M.J.; Sigman, M.S. Pd(II)-catalyzed conversion of styrene derivatives to acetals: Impact of (−)-sparteine on regioselectivity. Org. Lett. 2006, 8, 1121–1124. [Google Scholar] [CrossRef]
- Roll, D.M.; Scheuer, P.J.; Matsumoto, G.K.; Clardy, J. Halenaquinone, a pentacyclic polyketide from a marine sponge. J. Am. Chem. Soc. 1983, 105, 6177–6178. [Google Scholar] [CrossRef]
- Goswami, S.; Harada, K.; El-Mansy, M.F.; Lingampally, R.; Carter, R.G. Enantioselective synthesis of (−)-halenaquinone. Angew. Chem. Int. Ed. 2018, 57, 9117–9121. [Google Scholar] [CrossRef]
- Muzart, J. Palladium-catalysed oxidation of primary and secondary alcohols. Tetrahedron 2003, 59, 5789–5816. [Google Scholar] [CrossRef]
- Muzart, J. Oxidation adjacent to oxygen of alcohols catalyzed by palladium/dimethyl sulfoxide. In Comprehensive Organic Synthesis, 2nd ed.; Molander, G.A., Knochel, P., Eds.; Elsevier: Oxford, UK, 2014; Volume 7, pp. 295–301. [Google Scholar]
- Zheng, M.; Chen, P.; Huang, L.; Wu, W.; Jiang, H. Nucleo-palladation-triggering alkene functionalization: A route to γ-lactones. Org. Lett. 2017, 19, 5756–5759. [Google Scholar] [CrossRef]
- Gazzola, S.; Beccalli, E.M.; Borelli, T.; Castellano, C.; Diamante, D.; Broggini, G. Selective 7-endo-cyclization of 3-aza-5-alkenols through oxidative Pd(II)-catalyzed olefin oxyarylation. Synlett 2018, 29, 503–508. [Google Scholar]
- Broggini, G. (Università dell’Insubria, Como, Italy). Personal communication, 2025.
- Muzart, J. N,N-Dimethylformamide: Much more than a solvent. Tetrahedron 2009, 65, 8313–8323. [Google Scholar] [CrossRef]
- Le Bras, J.; Muzart, J. N,N-Dimethylformamide and N,N-dimethylacetamide as carbon, hydrogen, nitrogen and/or oxygen sources. In Solvents as Reagents in Organic Synthesis, Reactions and Applications; Wu, X.-F., Ed.; Wiley-VCH: Weinheim, Germany, 2017; pp. 199–314. [Google Scholar]
- Le Bras, J.; Muzart, J. Recent uses of N,N-dimethylformamide and N,N-dimethylacetamide as reagents. Molecules 2018, 23, 1939. [Google Scholar] [CrossRef] [PubMed]
- Broggini, G.; Barbera, V.; Beccalli, E.M.; Borsini, E.; Galli, S.; Lanza, G.; Zecchi, G. Palladium(II)/copper halide/solvent combination for selective intramolecular domino reactions of indolecarboxylic acid allylamides: An unprecedented arylation/esterification sequence. Adv. Synth. Catal. 2012, 354, 159–170. [Google Scholar] [CrossRef]
- Szolcsányi, P.; Gracza, T. PdCl2/CuCl2-catalysed chlorocyclisation of sugar-derived aminoalkenitols in the synthesis of new iminohexitols. Tetrahedron 2006, 62, 8498–8502. [Google Scholar] [CrossRef]
- Palík, M.; Kožíšek, J.; Koóš, P.; Gracza, T. Palladium-catalysed cyclisation of alkenols: Synthesis of oxaheterocycles as core intermediates of natural compounds. Beilstein J. Org. Chem. 2014, 10, 2077–2086. [Google Scholar] [CrossRef]
- Babjak, M.; Remeň, L.; Karlubíková, O.; Gracza, T. Synthesis of tetrahydrofurans through a novel pseudo-meso-trick. Synlett 2005, 2005, 1609–1611. [Google Scholar] [CrossRef]
- Babjak, M.; Remeň, L.; Szolcsányi, P.; Zálupský, P.; Mikloš, D.; Gracza, T. Novel bicyclisation of unsaturated polyols in PdCl2-CuCl2-AcOH catalytic system. J. Organomet. Chem. 2006, 691, 928–940. [Google Scholar] [CrossRef]
- Palík, M.; Karlubíková, O.; Lásiková, A.; Kožíšek, J.; Gracza, T. Total synthesis of (+)-varitriol from dimethyl L-tartrate. Eur. J. Org. Chem. 2009, 2009, 709–715. [Google Scholar] [CrossRef]
- Malmstrøm, J.; Christophersen, C.; Barrero, A.F.; Oltra, J.E.; Justicia, J.; Rosales, A. Bioactive metabolites from a marine-derived strain of the fungus Em11ericella variecolor. J. Nat. Prod. 2002, 65, 364–367. [Google Scholar] [CrossRef]
- Palík, M.; Karlubíková, O.; Lackovičová, D.; Lásiková, A.; Gracza, T. Formal synthesis of (+)-varitriol. Application of Pd(II)/Cu(II)-catalysed bicyclisation of unsaturated polyols. Tetrahedron 2010, 66, 5244–5249. [Google Scholar] [CrossRef]
- Brooks, J.L.; Xu, L.; Wiest, O.; Tan, D.S. Diastereoselective synthesis of highly substituted tetrahydrofurans by Pd-catalyzed tandem oxidative cyclization-redox relay reactions controlled by intramolecular hydrogen bonding. J. Org. Chem. 2017, 82, 57–75. [Google Scholar] [CrossRef]
- Gal, J. Problems of Stereochemical Nomenclature and Terminology. 1. The Homochiral Controversy. Its Nature and Origins, and a Proposed Solution. Enantiomer 1998, 3, 263–273. [Google Scholar]
- Gal, J. On the meaning and use of homochiral. J. Chromatogr. A 1998, 829, 417–418. [Google Scholar] [CrossRef]
- Uenishi, J.; Ohmi, M.; Ueda, A. PdII-catalyzed stereospecific formation of tetrahydro- and 3,6-dihydro [2H]pyran rings: 1,3-chirality transfer by intramolecular oxypalladation reaction. Tetrahedron Asymmetry 2005, 16, 1299–1303. [Google Scholar] [CrossRef]
- Uenishi, J.; Ohmi, M. Total synthesis of (−)-laulimalide; Pd-catalyzed stereospecific ring construction of substituted 3,6-dihydro [2H]pyran. Angew. Chem. Int. Ed. 2005, 44, 2756–2760. [Google Scholar] [CrossRef] [PubMed]
- Kawi, N.; Lagrange, J.-M.; Ohmi, M.; Uenishi, J. Palladium-catalyzed stereospecific synthesis of 2,6-disubstituted tetrahydropyrans: 1,3-chirality transfer by an intramolecular oxypalladation reaction. J. Org. Chem. 2006, 71, 4530–4537. [Google Scholar] [CrossRef]
- Kawai, N.; Lagrange, J.-M.; Uenishi, J. Stereochemistry and construction of tetrasubstituted chiral carbon centers in intramolecular Pd-catalyzed 1,3-chirality transfer reactions. Eur. J. Org. Chem. 2007, 2007, 2808–2814. [Google Scholar] [CrossRef]
- Uenishi, J.; Vikhe, Y.S.; Kawai, N. Stereochemistry and mechanistic study of intramolecular PdII-catalyzed oxypalladation and 1,3-chirality-transfer reactions. Chem. Asian J. 2008, 3, 473–484. [Google Scholar] [CrossRef]
- Hande, S.M.; Uenishi, J. Total synthesis of aspergillide B and structural discrepancy of aspergillide A. Tetrahedron Lett. 2009, 50, 189–192. [Google Scholar] [CrossRef]
- Uenishi, J.; Vikhe, Y.S. A short access to chiral non-racemic oxa- and azaheterocycles by cross-metathesis and Pd-catalyzed cyclization sequence. Heterocycles 2010, 80, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Ida, A.; Kitao, K.; Hoshiya, N.; Uenishi, J. Hydroxy group directed stereochemistry in oxypalladation of chiral allylic alcohol. Tetrahedron Lett. 2015, 56, 1956–1959. [Google Scholar] [CrossRef]
- Murata, Y.; Uenishi, J. Stereochemistry of PdII-catalyzed THF ring formation of ε-hydroxy allylic alcohols and synthesis of 2,3,5-trisubstituted and 2,3,4,5-tetrasubstituted tetrahydrofurans. J. Org. Chem. 2016, 81, 7471–7485. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Sasaki, M.; Nakagishi, T.; Ueda, T.; Hoshiya, N.; Uenishi, J. Construction of iterative tetrahydrofuran ring units and total synthesis of (+)-goniocin. Org. Lett. 2016, 18, 2248–2251. [Google Scholar] [CrossRef]
- Ghebreghiorgis, T.; Kirk, B.H.; Aponick, A.; Ess, D.H. Multiple mechanisms in Pd(II)-catalyzed SN2′ reactions of allylic alcohols. J. Org. Chem. 2013, 78, 7664–7673. [Google Scholar] [CrossRef]
- Zawisza, A.; Sinou, D. An unexpected palladium-catalyzed cyclization of bis-hydroxy allylic alcohols to dioxabicyclo [2.2.2]octanes. Tetrahedron Lett. 2006, 47, 3271–3274. [Google Scholar] [CrossRef]
- Olszewska, B.; Kryczka, B.; Zawisza, A. Synthesis of 2,5-substituted tetrahydrofurans by Pd(II)-catalyzed cyclization. Lett. Org. Chem. 2012, 9, 563–567. [Google Scholar] [CrossRef]
- Borah, M.; Borthakur, U.; Saikia, A.K. Diastereoselective synthesis of substituted morpholines from N-tethered alkenols: Total synthesis of (±)-chelonin A. J. Org. Chem. 2017, 82, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Ida, A.; Hoshiya, N.; Uenishi, J. Investigation of Pd(II)-catalyzed cyclization of chiral θ-hydroxy-α,β,γ,δ-unsaturated dienol. Heterocycles 2015, 90, 1082–1093. [Google Scholar]
- Yasumoto, T. The chemistry and biological function of natural marine toxins. Chem. Rec. 2001, 1, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Nishida, K.; Togawa, T.; Yamagami, M.; Miyazawa, M.; Hirai, Y. Stereoselective synthesis of the KJ ring system of yessotoxin by Pd(II)-catalyzed cyclization. Tetrahedron Lett. 2016, 57, 4379–4381. [Google Scholar] [CrossRef]
- Miyazawa, M.; Yano, Y.; Hayashi, C.; Okuno, M.; Hara, K.; Hirai, Y.; Yokoyama, H. Efficient synthesis of seven-membered cyclic ethers using Pd(II) catalyst. Heterocycles 2017, 94, 1885–1894. [Google Scholar] [CrossRef]
- Yokoyama, H.; Nakayama, S.; Murase, M.; Miyazawa, M.; Yamaguchi, S.; Hirai, Y. Pd(II)-catalyzed cyclization to ether and its application to the synthesis of the trans-fused polyether core. Heterocycles 2009, 77, 211–215. [Google Scholar] [CrossRef]
- Yokoyama, H.; Kusumoto, Y.; Sumiyoshi, K.; Miyazawa, M.; Hirai, Y. Synthetic studies of yessotoxin: Iterative synthesis of the AB ring system via Pd(II)-catalyzed cyclization of alcohol. Heterocycles 2014, 89, 353–358. [Google Scholar] [CrossRef]
- Sugimoto, M.; Nosho, S.; Hikosaka, G.; Hattori, Y.; Umezawa, K.; Kawamura, A.; Makabe, H. Synthesis of (+)-muconin via diastereoselective oxypalladation. J. Org. Chem. 2021, 86, 4859–4866. [Google Scholar] [CrossRef]
- Furuhata, S.; Hattori, Y.; Okajima, M.; Konno, H.; Abe, M.; Miyoshi, H.; Goto, T.; Makabe, H. Synthesis of pyranicin and its deoxygenated analogues and their inhibitory action with bovine heart mitochondrial complex I. Tetrahedron 2008, 64, 7695–7703. [Google Scholar] [CrossRef]
- Borrero, N.V.; Aponick, A. Total synthesis of acortatarin A using a Pd(II)-catalyzed spiroketalization strategy. J. Org. Chem. 2012, 77, 8410–8416. [Google Scholar] [CrossRef] [PubMed]
- Awasaguchi, K.; Miyazawa, M.; Uoya, I.; Inoue, K.; Nakamura, K.; Yokoyama, H.; Kakuda, H.; Hirai, Y. Synthesis of spiro C-arylglycoriboside via Pd(II)-catalyzed spirocyclization. Synlett 2010, 2010, 2392–2396. [Google Scholar]
- Koranne, P.S.; Tsujihara, T.; Arai, M.A.; Bajracharya, G.B.; Suzuki, T.; Onitsuka, K.; Sasai, H. Design and synthesis of chiral hybrid spiro (isoxazole–isoxazoline) ligands. Tetrahedron Asymmetry 2007, 18, 919–923. [Google Scholar] [CrossRef]
- Lankri, D.; Mostinski, Y.; Tsvelikhovsky, D. Palladium-catalyzed cascade assembly of tricyclic spiroethers from diene-alcohol precursors. J. Org. Chem. 2017, 82, 9452–9463. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Epa, W.R. Catalytic tandem oxy-palladation and vinylation. Tetrahedron Lett. 1993, 34, 7205–7208. [Google Scholar] [CrossRef]
- Lásiková, A.; Doháňošová, J.; Hlavínová, L.; Toffano, M.; Vo-Thanh, G.; Kožíšek, J.; Gracza, T. Domino reaction: Pd(II)-catalyzed cyclization of unsaturated polyols and cross-coupling. Tetrahedron Asymmetry 2012, 23, 818–827. [Google Scholar] [CrossRef]
- Abbineni, C.; Sasmal, P.K.; Mukkanti, K.; Iqbal, J. Synthesis of the C1-C12 fragment of amphidinolide T. Tetrahedron Lett. 2007, 48, 4259–4262. [Google Scholar] [CrossRef]
- Hewitt, J.F.M.; Williams, L.; Aggarwal, P.; Smith, C.D.; France, D.J. Palladium-catalyzed heteroallylation of unactivated alkenes—Synthesis of citalopram. Chem. Sci. 2013, 4, 3538–3543. [Google Scholar] [CrossRef]
- Keller, M.B. Citalopram therapy for depression: A review of 10 years of European experience and data from U.S. clinical trials. J. Clin. Psychiatry 2000, 61, 896–908. [Google Scholar] [CrossRef]
- Zhu, R.; Buchwald, S.L. Combined oxypalladation/C-H functionalization: Palladium(II)-catalyzed intramolecular oxidative oxyarylation of hydroxyalkenes. Angew. Chem. Int. Ed. 2012, 51, 1926–1929. [Google Scholar] [CrossRef]
- Ferreira, E.M.; Stoltz, B.M. Catalytic C-H bond functionalization with palladium(II): aerobic oxidative annulations of indoles. J. Am. Chem. Soc. 2003, 125, 9578–9579. [Google Scholar] [CrossRef]
- Lafrance, M.; Rowley, C.N.; Woo, T.K.; Fagnou, K. Catalytic intermolecular direct arylation of perfluorobenzenes. J. Am. Chem. Soc. 2006, 128, 8754–8756. [Google Scholar] [CrossRef]
- Le Bras, J.; Muzart, J. Intermolecular dehydrogenative Heck reactions. Chem. Rev. 2011, 111, 1170–1214. [Google Scholar] [CrossRef]
- Matsuura, B.S.; Condie, A.G.; Buff, R.C.; Karahalis, G.J.; Stephenson, C.R.J. Intercepting Wacker intermediates with arenes: C–H functionalization and dearomatization. Org. Lett. 2011, 13, 6320–6323. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, P.R.; Browder, C.C.; Hong, J.; Lincoln, C.M.; Nagornyy, P.A.; Robarge, L.A.; Wardrop, D.J.; White, J.D. Total synthesis of polycavernoside A, a lethal toxin of the red alga Polycavernosa tsudai. J. Org. Chem. 2005, 70, 5449–5460. [Google Scholar] [CrossRef] [PubMed]
- White, J.D.; Kranemann, C.L.; Kuntiyong, P. Intramolecular palladium(II)-mediated alkoxy carbonylation as a route to functionalized tetrahydropyrans. Synthesis of the C9-C32 segment of phorboxazole A. Org. Lett. 2001, 3, 4003–4006. [Google Scholar] [CrossRef]
- White, J.D.; Kuntiyong, P.; Lee, T.H. Total synthesis of phorboxazole A. 1. Preparation of four subunits. Org. Lett. 2006, 8, 6039–6042. [Google Scholar] [CrossRef]
- White, J.D.; Lee, T.H.; Kuntiyong, P. Total synthesis of phorboxazole A. 2. Assembly of subunits and completion of the synthesis. Org. Lett. 2006, 8, 6043–6046. [Google Scholar] [CrossRef] [PubMed]
- Searle, P.A.; Molinski, T.F. Phorboxazoles A and B: Potent cytostatic macrolides from marine sponge Phorbas sp. J. Am. Chem. Soc. 1995, 117, 8126–8131. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, X.; Jing, P.; Zhao, G.; Zheng, J.; Zhao, C.; She, X. Total synthesis of cyanolide A. Org. Biomol. Chem. 2011, 9, 984–986. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kim, H.S. Total synthesis of cyanolide A and confirmation of its absolute configuration. Org. Lett. 2010, 12, 2880–2883. [Google Scholar] [CrossRef]
- Erickson, K.L.; Gustafson, K.R.; Pannell, L.K.; Beutler, J.A.; Boyd, M.R. New dimeric macrolide glycosides from the marine sponge Myriastra clavosa. J. Nat. Prod. 2002, 65, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Karlubíková, O.; Babjak, M.; Gracza, T. Tetrahydropyran synthesis by palladium(II)-catalysed hydroxycarbonylation of hexenols: Synthesis of (±)-diospongin A and (+)-civet cat compound. Tetrahedron 2011, 67, 4980–4987. [Google Scholar] [CrossRef]
- Yin, J.; Kouda, K.; Yasuhiro, T.; Tran, Q.L.; Miyahara, T.; Chen, Y.; Kadota, S. New diarylheptanoids from the rhizomes of Dioscorea spongiosa and their antiosteoporotic activity. Planta Med. 2004, 70, 54–58. [Google Scholar] [PubMed]
- Hegedus, L.S.; Allen, G.F.; Olsen, D.J. Palladium-assisted cyclization-insertion reactions. Synthesis of functionalized heterocycles. J. Am. Chem. Soc. 1980, 102, 3583–3587. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Bodurow, C. Intramolecular alkoxypalladation/carbonylation of alkenes. J. Am. Chem. Soc. 1984, 106, 1496–1498. [Google Scholar] [CrossRef]
- Ambrosini, L.M.; Cernak, T.A.; Lambert, T.H. Development of oxidative formylation and ketonylation reactions. Synthesis 2010, 2010, 870–881. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Bodurow, C.; Baum, M. Direct synthesis of pyran-lactones related to naphthoquinoneantibiotics. Tetrahedron Lett. 1984, 25, 3171–3174. [Google Scholar] [CrossRef]
- Tamaru, Y.; Kobayashi, T.; Kawamura, S.; Ochiai, H.; Hojo, M.; Yoshida, Z. Palladium catalyzed oxycarbonylation of 4-penten-1,3-diols: Efficient stereoselective synthesis of cis 3-hydroxytetrahydrofuran 2-acetic acid lactones. Tetrahedron Lett. 1985, 26, 3207–3210. [Google Scholar] [CrossRef]
- Gracza, T. Intramolecular oxycarbonylation in steroselective synthesis. In Stereoselective Synthesis of Drugs and Natural Products, 1st ed.; Andrushko, V., Andrushko, N., Eds.; Wiley: Oxford, UK, 2013; pp. 421–440. [Google Scholar]
- Semmelhack, M.F.; Shanmugam, P. Development of an approach to the synthesis of the plakortones. Tetrahedron Lett. 2000, 41, 3567–3571. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Bean, M.F.; Carte, B.K.; Westley, J.W.; Johnson, R.K.; Lahouratate, P. The plakortones, novel bicyclic lactones from the sponge Plakortis halichondrioides: Activators of cardiac SR-Ca2+-pumping ATPase. Tetrahedron 1996, 52, 377–394. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Hooley, R.J.; Kraml, C.M. Synthesis of plakortone B and analogs. Org. Lett. 2006, 8, 5203–5206. [Google Scholar] [CrossRef]
- Hayes, P.Y.; Kitching, W. Total synthesis and absolute stereochemistry of plakortone D. J. Am. Chem. Soc. 2002, 124, 9718–9719. [Google Scholar] [CrossRef]
- Hayes, P.Y.; Chow, S.; Rahm, F.; Bernhardt, P.V.; De Voss, J.J.; Kitching, W. Synthesis of the sponge-derived plakortone series of bioactive compounds. J. Org. Chem. 2010, 75, 6489–6501. [Google Scholar] [CrossRef]
- Li, R.; Zhao, Q.; Li, S.; Han, Q.; Sun, H.; Lu, Y.; Zhang, L.; Zheng, Q. Micrandilactone A: A novel triterpene from Schisandra micrantha. Org. Lett. 2003, 5, 1023–1026, Erratum in Org. Lett. 2006, 8, 801. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Dai, M.; Luo, T.; Deng, L.; Chen, J.; Yang, Z. A highly efficient synthesis of the FGH ring of micrandilactone A. Application of thioureas as ligands in the Co-catalyzed Pauson−Khand reaction and Pd-catalyzed carbonylative annulation. Org. Lett. 2005, 7, 885–888. [Google Scholar] [CrossRef]
- Dai, M.; Liang, B.; Wang, C.; Chen, J.; Yang, Z. Synthesis of a novel C2- symmetric thiourea and its application in the Pd-catalyzed cross-coupling reactions with arenediazonium salts under aerobic conditions. Org. Lett. 2004, 6, 221–224. [Google Scholar] [CrossRef]
- Dai, M.; Wang, C.; Dong, G.; Xiang, J.; Luo, Y.; Liang, B.; Chen, J.; Yang, Z. Development of thiourea-based ligands for the palladium-catalyzed bis(methoxycarbonylation) of terminal olefins. Eur. J. Org. Chem. 2003, 2003, 4346–4348. [Google Scholar] [CrossRef]
- Dai, M.; Liang, B.; Wang, C.; You, Z.; Xiang, J.; Dong, G.; Chen, J.; Yang, Z. A novel thiourea ligand applied in the Pd-catalyzed Heck, Suzuki and Suzuki carbonylative reactions. Adv. Synth. Catal. 2004, 346, 1669–1673. [Google Scholar]
- Nelson, R.A.; Pope, J.A., Jr.; Luedemann, G.M.; McDaniel, L.E.; Schaffner, C.P. Crisamicin A, a new antibiotic from Micromonospora. I. Taxonomy of the producing strain, fermentation, isolation, physico-chemical characterization and antimicrobial. J. Antibiot. 1986, 39, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, Y.; Tang, Y.; Dai, M.; Wang, G.; Wang, Z.; Yang, Z. Total synthesis of crisamicin A. Org. Lett. 2008, 10, 3017–3020. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, Y.; Jiao, Z.; Wu, N.; Wang, D.Z.; Yang, Z. Diversity-oriented synthesis of fused pyran γ-lactones via an efficient Pd-thiourea-catalyzed alkoxycarbonylative annulation. Org. Lett. 2008, 10, 5163–5166. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, C.L.; McErlean, C.S.P. Total synthesis of C19 lipid diols containing a 2,5-disubstituted-3-oxygenated tetrahydrofuran. Org. Biomol. Chem. 2011, 9, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, C.L.; McErlean, C.S.P. An expedient synthesis of 2,5-disubstituted-3-oxygenated tetrahydrofurans. Tetrahedron Lett. 2009, 50, 6318–6320. [Google Scholar] [CrossRef]
- Lee, E.; Yoo, S.-K.; Choo, H.; Song, H.Y. Radical cyclization of β-alkoxyacrylates: A formal synthesis of (−)-kumausallene. Tetrahedron Lett. 1998, 39, 317–318. [Google Scholar] [CrossRef]
- Evans, P.A.; Murthy, V.S.; Roseman, J.D.; Rheingold, A.L. Enantioselective total synthesis of the nonisoprenoid sesquiterpene (−)-kumausallene. Angew. Chem. Int. Ed. 1999, 38, 3175–3177. [Google Scholar] [CrossRef]
- Bai, Y.; Davis, D.C.; Dai, M. Synthesis of tetrahydropyran/tetrahydrofuran-containing macrolides by palladium-catalyzed alkoxycarbonylative macrolactonizations. Angew. Chem. Int. Ed. 2014, 53, 6519–6522. [Google Scholar] [CrossRef]
- Gracza, T.; Hasenöhrl, T.; Stahl, U.; Jäger, V. Synthesis of 3,5-anhydro-2-deoxy-1,4-glyconolactones by palladium(II)-catalyzed, regioselective oxycarbonylation of C5- and C6-enitols. ω-Homologation of aldoses to produce intermediates for C-glycoside/C-nucleoside synthesis. Synthesis 1991, 1991, 1108–1118. [Google Scholar] [CrossRef]
- Babjak, M.; Kapitán, P.; Gracza, T. The first total synthesis of goniothalesdiol. Tetrahedron Lett. 2002, 43, 6983–6985. [Google Scholar] [CrossRef]
- Babjak, M.; Kapitán, P.; Gracza, T. Synthesis of (+)-goniothalesdiol and (+)-7-epi-goniothalesdiol. Tetrahedron 2005, 61, 2471–2479. [Google Scholar] [CrossRef]
- Bhaket, P.; Morris, K.; Stauffer, C.S.; Datta, A. Total synthesis of cytotoxic anhydrophytosphingosine pachastrissamine (jaspine B). Org. Lett. 2005, 7, 875–876. [Google Scholar] [CrossRef]
- Caletková, O.; Ďurišová, D.; Gracza, T.; Prónayová, N. Aminohydroxylation of divinylcarbinol and its application to the synthesis of bicyclic hydroxypyrrolidine and aminotetrahydrofuran building blocks. Chem. Pap. 2013, 67, 66–75. [Google Scholar] [CrossRef]
- Cintulová, D.; Slahúčková, M.; Paštrnák, J.; Prónayová, N.; Szolcsányi, P. Preparation of cis-fused tetrahydropyranyl lactones via palladium-catalysed cyclocarbonylation of enediols. Synthesis 2017, 49, 755–762. [Google Scholar]
- Kapitán, P.; Gracza, T. Stereocontrolled oxycarbonylation of 4-benzyloxy-hepta-1,6-diene-3,5-diols promoted by chiral palladium(II) complexes. Tetrahedron Asymmetry 2008, 19, 38–44. [Google Scholar] [CrossRef]
- Kapitán, P.; Gracza, T. Asymmetric intramolecular Pd(II)-catalysed oxycarbonylation of alkene-1,3-diols. Arkivoc 2008, 8, 8–17. [Google Scholar] [CrossRef]
- Doháňošová, J.; Lásiková, A.; Toffano, M.; Gracza, T.; Vo-Thanh, G. Kinetic resolution of pent-4-ene-1,3-diol by Pd(II)-catalysed oxycarbonylation in ionic liquids. New J. Chem. 2012, 36, 1744–1750. [Google Scholar] [CrossRef]
- Markovič, M.; Ďuranová, M.; Koóš, P.; Szolcsányi, P.; Gracza, T. Synthesis of bis-tetrahydrofuran subunit of (−)-neopallavicinin. Tetrahedron 2013, 69, 4185–4189. [Google Scholar] [CrossRef]
- Babjak, M.; Markovič, M.; Kandríková, B.; Gracza, T. Asymmetric formal synthesis of (+)-pyrenolide D. Synthesis 2014, 46, 817–821. [Google Scholar]
- Zhang, C.; Liu, J.; Du, Y. A concise total synthesis of (+)-pyrenolide D. Tetrahedron Lett. 2013, 54, 3278–3280. [Google Scholar] [CrossRef]
- Babjak, M.; Markovič, M.; Kandríková, B.; Gracza, T. Homogeneous cyclocarbonylation of alkenols with iron pentacarbonyl. Synthesis 2014, 46, 809–816. [Google Scholar] [CrossRef]
- Markoviča, M.; Koóša, P.; Gracza, T. A short asymmetric synthesis of sauropunols A–D. Synthesis 2017, 49, 2939–2942. [Google Scholar]
- Lopatka, P.; Gavenda, M.; Markovič, M.; Koóš, P.; Gracza, T. Flow Pd(II)-catalyzed carbonylative cyclisation in the total synthesis of jaspine B. Catalysts 2021, 11, 1513. [Google Scholar] [CrossRef]
- Lopatka, P.; Markovič, M.; Koóš, P.; Ley, S.V.; Gracza, T. Continuous Pd-catalyzed carbonylative cyclization using iron pentacarbonyl as a CO source. J. Org. Chem. 2019, 84, 14394–14406. [Google Scholar] [CrossRef]
- Markoviča, M.; Lopatka, P.; Gracza, T.; Koóš, P. Recent applications of continuous flow in homogeneous palladium catalysis. Synthesis 2020, 52, 3511–3529. [Google Scholar] [CrossRef]
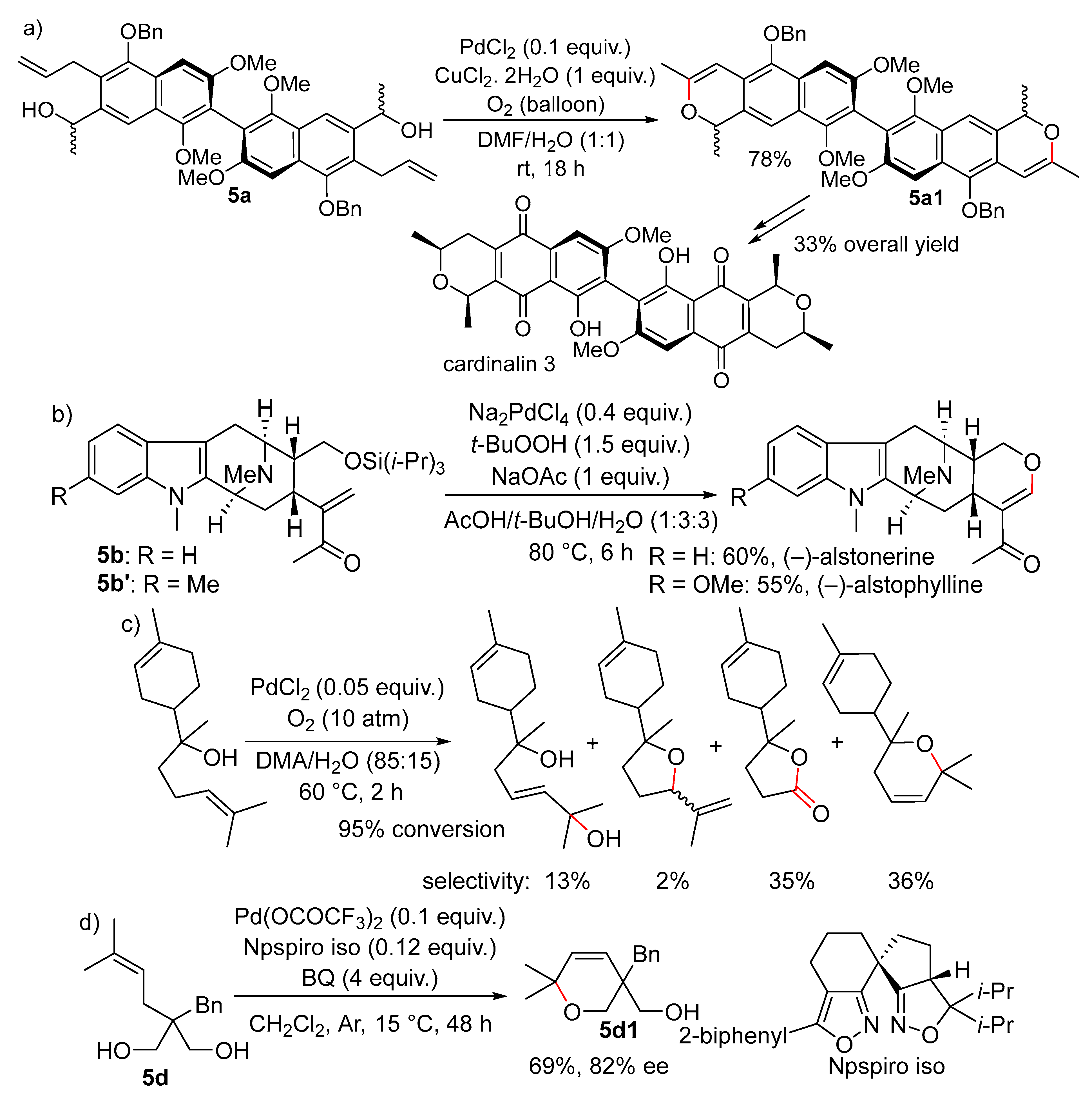
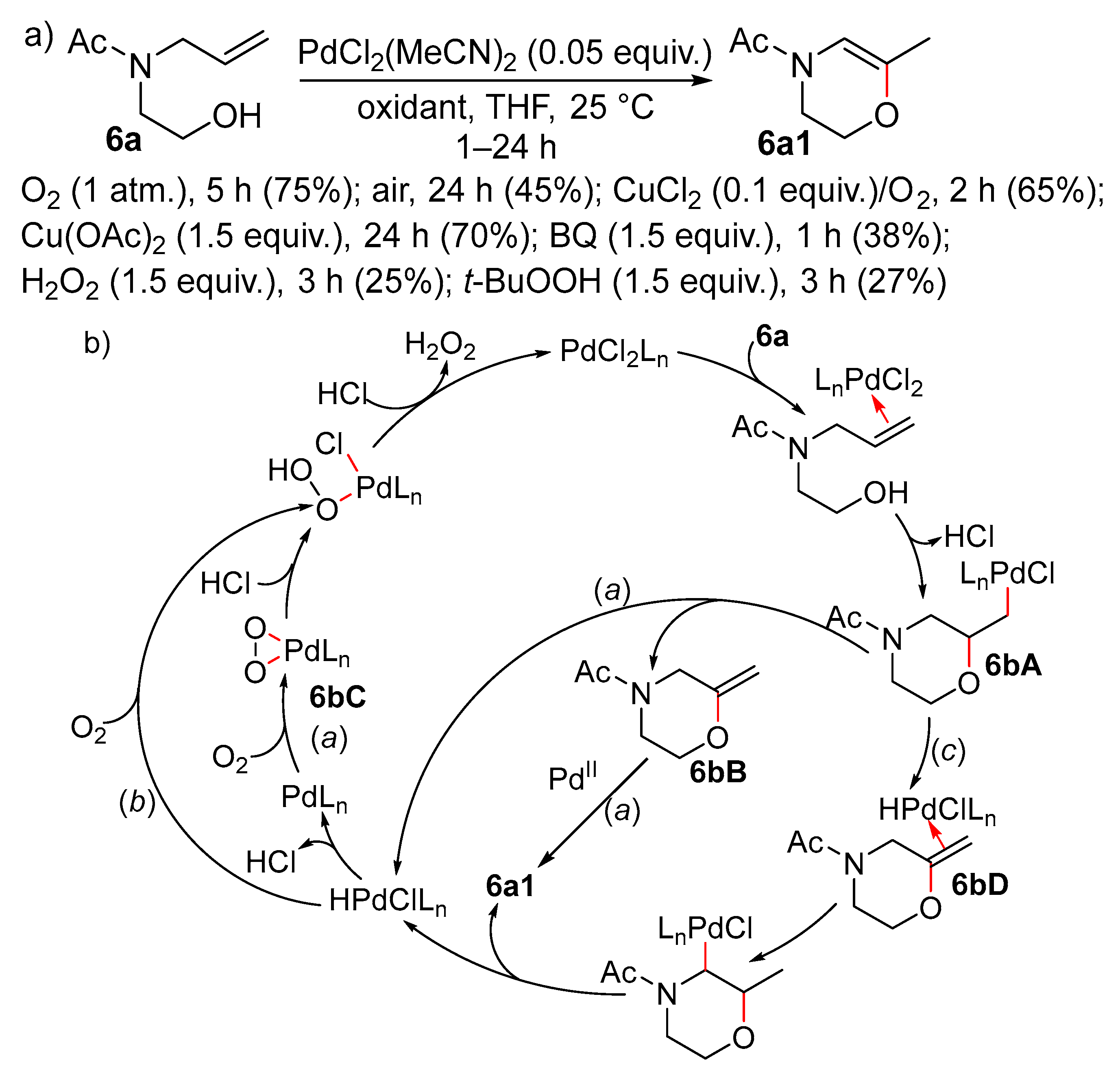


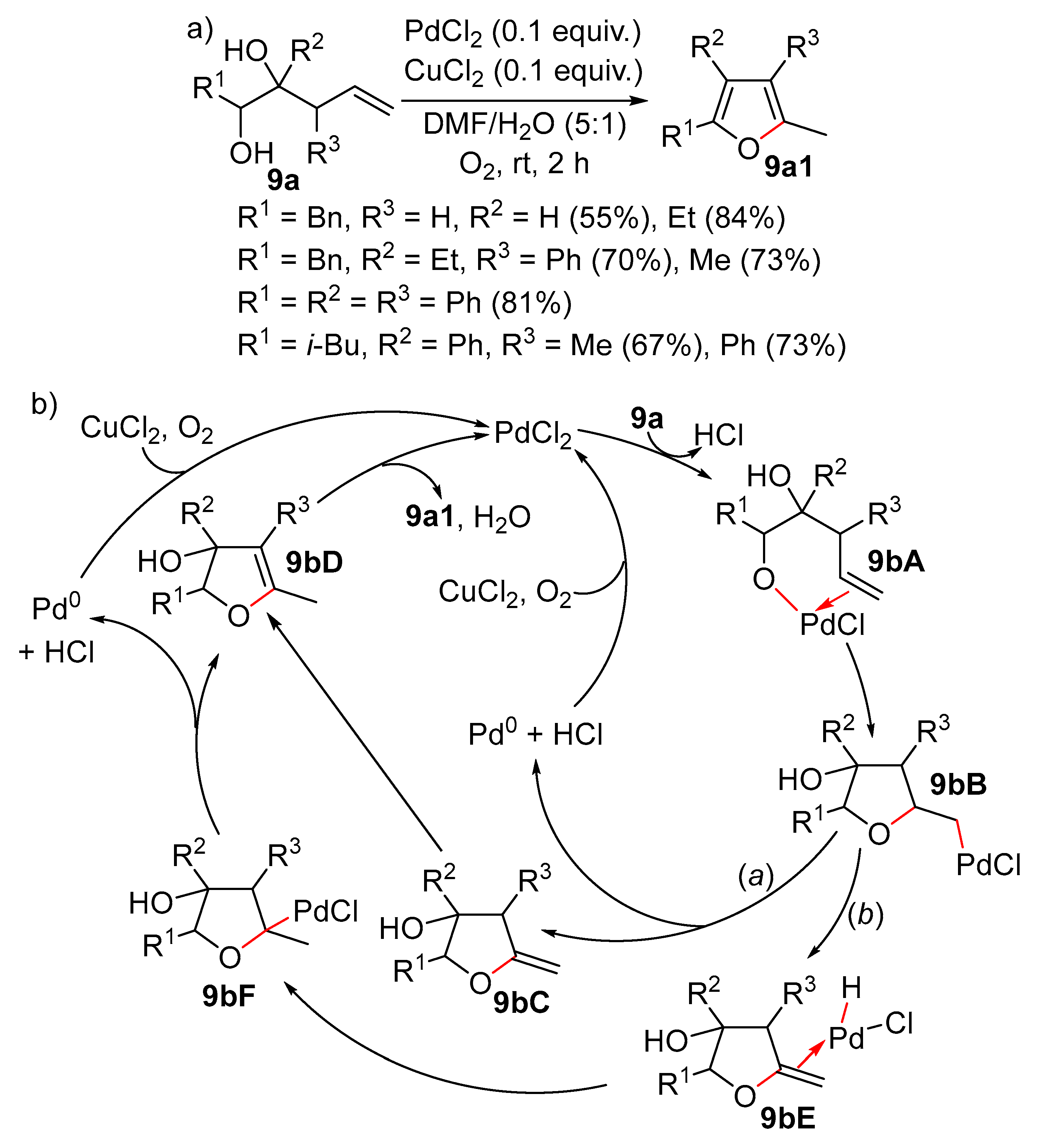
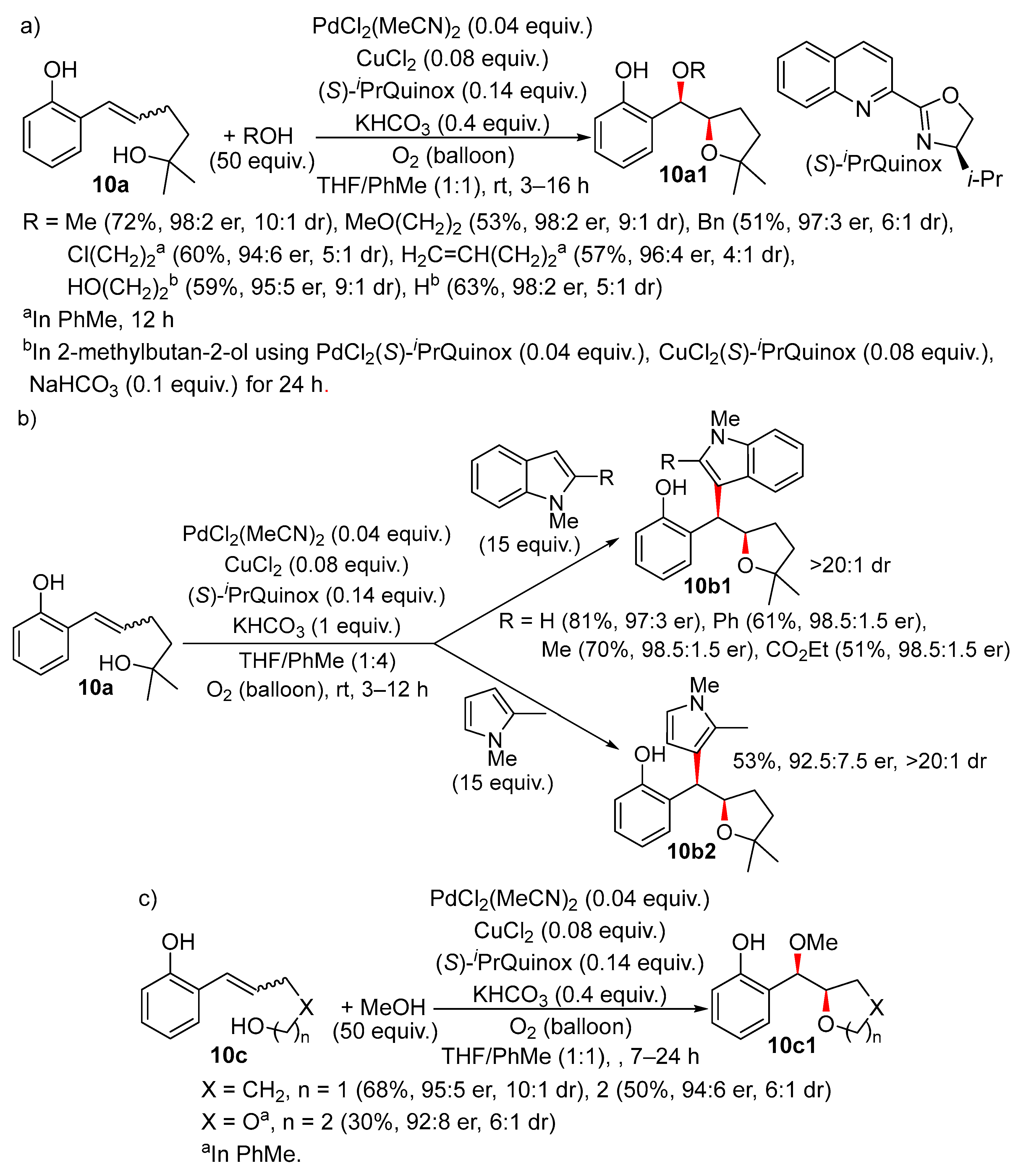










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzart, J. PdII Catalysis: Recent Advances in the Intramolecular Wacker-Type Reaction of Alkenols and Related Domino Reactions. Catalysts 2025, 15, 845. https://doi.org/10.3390/catal15090845
Muzart J. PdII Catalysis: Recent Advances in the Intramolecular Wacker-Type Reaction of Alkenols and Related Domino Reactions. Catalysts. 2025; 15(9):845. https://doi.org/10.3390/catal15090845
Chicago/Turabian StyleMuzart, Jacques. 2025. "PdII Catalysis: Recent Advances in the Intramolecular Wacker-Type Reaction of Alkenols and Related Domino Reactions" Catalysts 15, no. 9: 845. https://doi.org/10.3390/catal15090845
APA StyleMuzart, J. (2025). PdII Catalysis: Recent Advances in the Intramolecular Wacker-Type Reaction of Alkenols and Related Domino Reactions. Catalysts, 15(9), 845. https://doi.org/10.3390/catal15090845








