Abstract
The management of solid waste and the supply of energy are two of the most important environmental problems of our time. Projections indicate that by 2050, the global demand for electrical energy is expected to increase by 35% and the amount of solid waste generated to increase by 45%. In this context, polymeric waste materials such as biomass and plastics can be valorised through thermochemical processes for the generation of energy. Gasification, which converts carbonaceous materials into syngas, tar, and char, is one of the most promising recycling technologies. The composition and relative quantities of the products are influenced by the process configuration, operating parameters, and the type of fuel used. Tar removal is facilitated by adding specific catalysts to the process. The co-processing of biomass and plastics in the gasification process, called co-gasification, improves the gas yield and reduces solid residues. This review evaluates catalytic and non-catalytic co-gasification of biomass waste and non-biodegradable plastics, with a focus on syngas production and its energy potential.
1. Introduction
Humanity’s development leads to economic growth, urbanisation, and industrialisation. This development requires more energy and hence more waste generation [1]. No human activity is exempt from generating some amount of waste that will require subsequent disposal. The waste generated can be classified as hazardous and non-hazardous. Then, as municipal and industrial waste, as shown in Figure 1 [2]. Both subcategories include carbon-containing waste such as plastics and biomass. Managing both urban and industrial waste disposal requires investments in the billions of dollars. By 2030, industrial waste management alone will represent an expenditure of $1.08 billion [3], of which approximately 14% corresponds to agricultural waste [4]. Plastic waste, on the other hand, is incinerated, recycled, or simply disposed of. By 2015, nearly 5,000 Mt of plastic waste had accumulated in the environment. Geyer et al. [5] estimate that by 2050, this amount will rise to 12,000 Mt of plastic waste.
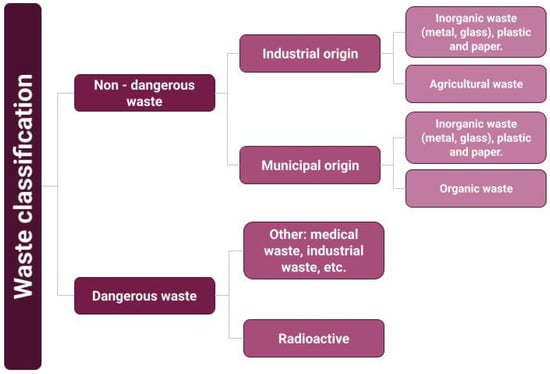
Figure 1.
Waste classification [2].
According to the European Renewable Energy Directive [6], biomass is understood as the biodegradable fraction of products, waste, and residues of biological origin from agriculture, including plant and animal substances, from forestry and related industries, including fisheries and aquaculture, as well as the biodegradable fraction of waste, including industrial and municipal waste of biological origin. Agricultural waste is produced by agricultural and livestock operations. It refers to crop residues (leaves, corn stalks, rice straw, wheat straw, barley straw, seed pods, and other plant remnants from harvesting), livestock waste (wastewater, manure), and by-products from food processing industries (molasses, bagasse from sugar industries, rice husk, fruit and vegetable skins and pomace, deoiled cakes, starch residues, eggshells, poultry, and meat industry waste) [7]. Of these categories, crop residues and some by-products from the food processing industry (bagasse, husks, etc.) are lignocellulosic biomass. The most used material in bioenergy production is wood, whether it be in the form of pellets, wood chips, briquettes, sawdust, or firewood [6].
Plastics, for their part, can be classified according to their functionality, structure, molecular bonds, the origin of the raw materials, or their biodegradability [8]. For this review, it is interesting to consider the classification of different types of plastic according to their final disposal. According to Nayanathara Thathsarani Pilapitiya and Amila Sandaruwan Ratnayake [8], plastics can be biodegradable and decompose in the environment, integrating into the biological cycle, and can be recycled or not, depending on how easy this process is for the material. Figure 2 groups the most common types of plastics according to this classification.

Figure 2.
Plastics classification according to its final dispose [8].
The biodegradable plastics represent only ~1% of global production and require industrial composting. While frequently recycled plastics dominate mechanical recycling, PVC, PS, and mixed plastics often end up in incineration or landfills due to their difficulty in being recycled [8].
Table 1 compares the heating values and ash content of some lignocellulosic biomasses and some of the most common non-biodegradable plastics. The LHV values of biomass are below 20 MJ/kg, while plastics have higher values, exceeding 30 MJ/kg. Furthermore, biomass contains significant percentages of ash compared to plastics.

Table 1.
LHV and ash content of lignocellulosic biomasses and common non-biodegradable plastics.
Biomass/plastic blends could produce a solid fuel with higher volumetric heating values than lignocellulosic biomass [13]. However, the environmental impact of plastics—particularly their embodied energy and CO2 footprint—remains a concern. Embodied energy (shown conceptually in Figure 3) represents the total energy consumed across a material’s lifecycle, including extraction, manufacturing, transportation, and disposal [14]. For plastics, this ranges from 80 to 120 MJ per kg (or 2–10 kg CO2 per kg), primarily due to fossil-fuel-dependent production [15]. In contrast, wood’s embodied energy is tenfold lower, as its lifecycle relies on photosynthetic carbon uptake and minimal processing energy (Figure 3).
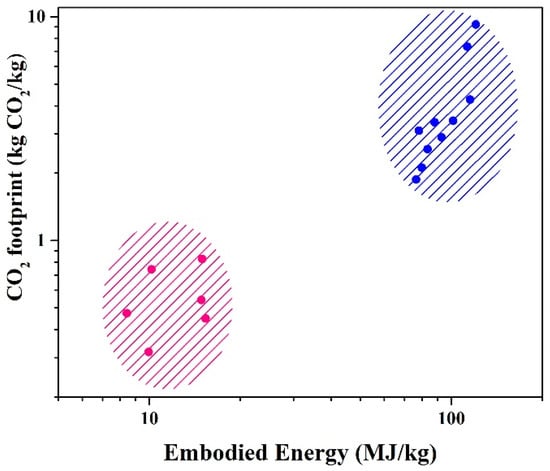
Figure 3.
CO2 footprint and embodied energy for materials like wood (pink) and plastics (blue) [15].
U.S. Energy Information Administration’s International Energy Outlook 2023 [16] projected that the global energy demand and CO2 emissions will increase through 2050, assuming no major policy changes. Although zero-carbon technologies like renewables and nuclear are expected to supply most of the new energy demand, they will not be sufficient to reduce overall CO2 emissions under current laws and regulations. Repurposing waste plastics as co-feedstocks in biomass gasification offers a partial solution, recovering embodied energy by converting plastics into syngas/H2, offsetting virgin fossil fuel use and reducing net carbon footprint by diverting plastics from landfills.
Despite their high environmental cost, plastics can be repurposed as an energy source when combined with biomass. This offers a partial recovery of their embodied energy and could reduce their overall carbon footprint. This approach diverts plastic waste from landfills and supports cleaner energy production.
According to the IEA [17], biomass gasification is an economically viable and environmentally beneficial method of producing climate-positive hydrogen. As negative carbon emissions are essential for meeting climate goals, biomass gasification is one of the few technologies capable of producing hydrogen with net-negative emissions. In this context, incorporating waste plastics into gasification or co-processing strategies could complement existing biomass feedstock, contributing to waste valorisation and supporting low-carbon energy systems.
This review applied a selection criterion: (1) inclusion of experimental studies comparing catalytic and non-catalytic co-gasification of well-characterised biomass–plastic mixtures, (2) requirement of individual feedstock performance data (biomass and plastics separately) to enable synergy evaluation, and (3) restriction to peer-reviewed publications (2011–2024). These criteria ensured a targeted examination of mixture-specific synergistic effects on syngas production, distinct from broader gasification process studies.
2. Gasification Fundamentals
2.1. Basics of Gasification: Reactions, Products (Syngas, Tar, and Char), and Energy Potential
Gasification is a thermochemical process of partial oxidation of carbonaceous material, which can convert the raw material into a mixture of gases and condensable that can be used for energy generation. If gasification occurs under certain conditions and the products are purified, the so-called “synthesis gas” or syngas (CO and H2) is obtained. This process occurs at temperatures ranging from 600 °C to 1000 °C and can include different gasifying agents (oxygen, air, and steam). The process of gasification involves several subprocesses, including drying, pyrolysis, reforming, cracking, oxidation, and reduction [18]. There are different types of reactors, and the relationships between the feed, the gasifying agent (oxidant), and the operating temperature determine the H2/CO ratio. The reaction system is complex and generates several products such as carbonaceous solids (char and ashes), liquids (oils and tars), and gases (CO, CO2, CH4, H2, C2H2, etc.) [19,20,21,22].
The drying process begins at low temperatures and continues up to 100 °C, where any existing moisture converts to vapour and there is no decomposition of the carbonaceous material. During the next process, pyrolysis, thermal degradation begins (cracking, free radical formation, and depolymerisation), which leading the release of volatiles. This stage has temperatures between 200 and 700 °C, volatile compounds (H2, CO2, tar, CO, water vapour, and light hydrocarbons) are released, and char, tars, oils, and ash are produced. During the oxidation process, the gasifying agent used (which will be present in substoichiometric quantities during gasification) reacts with the char to produce oxidised carbon species, preferably CO. Finally, reduction and reforming reactions will occur [22]. The system of reactions involved in the process is extremely complex and will vary according to the raw material used, but the main reactions are highlighted [22,23,24] as follows:
- Boudouard reaction
- Water—gas or steam
- Hydrogasification
- Oxidation reactions
- Shift reaction
- Methanation reaction
- Steam reforming reaction
The process can be carried out autothermally (where the heat required for the overall process is obtained from the exothermic reactions) or allothermally (the required heat is supplied externally) [22].
Several types of reactors are traditionally used in coal or biomass gasification, the most common being fixed-bed, fluidised-bed, and entrained-flow reactors. However, plastics have particular properties that must be considered when adding to reaction systems (high volatile material content, high viscosity, sticking, and high tar production). Therefore, a co-gasification system must consider [22]
- Good heat transfer to ensure rapid depolymerisation.
- Good sticking and high viscosity management.
- A residence time (or the incorporation of a catalytic system) that ensures the transformation of tars into gaseous products of interest.
Fixed-bed reactors are of little interest for the co-gasification of biomass and plastics, used on a small scale or in laboratories. Fluidised-bed reactors (particularly bubbling fluidised-bed and conical fluidised-bed reactors) offer advantages, such as good gas–solid contact, high heat and mass transfer, and improved control over sticking processes [22]. The suitability of a reactor configuration is determined by its ability to overcome the fundamental challenges of heterogeneous feedstock, such as agglomeration and clogging. The key operational characteristics, advantages, and central challenges of the main reactor types for co-gasification are systematically compared in Table 2.

Table 2.
Comparison of reactor types for plastic–biomass co-gasification: key advantages, and challenges [25,26,27,28].
Gasification has potential for biogenic valorisation and waste management. However, its commercial use has faced barriers, including technical and economic issues. Recent failures of European projects like Guessing and GobiGas highlight the struggle to compete with cheaper fossil-based alternatives [29]. Nonetheless, gasification remains a viable option in regions with limited access to fossil fuels or a supportive policy environment, and can sustainably meet energy demands.
2.2. Feedstock Characteristics
- Biomass waste: Types (agricultural, forestry) and properties (moisture, ash content).
Once the carbonaceous nature of the effluents analysed in this publication has been established, their main physicochemical characteristics can be described.
The organic matter found in municipal waste has significant lignocellulosic components (wood and paper) [13]. Similarly, agricultural and wood industry waste is mostly discarded lignocellulosic biomass [30]. Lignocellulosic biomass is composed of three major components: cellulose, hemicellulose, and lignin, which are structural components of plants and support their cell walls.
Cellulose is a high-grade polymer of the monomer cellobiose (a disaccharide of D-glucose with β-1,4 glycosidic linkages). Cellulose fibres are held together by hydrogen bonds and Van der Waals forces, making cellulose highly stable. The hydrogen bonds formed with the large number of OH groups present in the structure give it a strong structure, crystalline or amorphous, depending on the region. The reactivity of cellulose is greatest in its structural amorphous regions, while its crystalline regions determine its chemical stability and mechanical strength (the structural strength of leaves, stems, roots, etc.). This stability also makes it insoluble in most solvents [31].
Hemicellulose is a long-chain, heterogeneous, branched polymer of C5 and C6 sugars. It is the second most abundant lignocellulosic biopolymer, and due to its amorphous nature, its reactivity is greater than that of cellulose, and it is easily hydrolysed. Hydrolysis is carried out by enzymes, acids, or bases, even in dilute form. The hemicellulose chains are linked to the cellulose by hydrogen bonds, thus creating a highly rigid matrix composed of cellulose, hemicellulose, and finally lignin, with which it is covalently linked [31].
Finally, the third most abundant compound is lignin, an aromatic polymer whose monomers are phenylpropanoids linked by alkyl-aryl, alkyl-alkyl, and aryl-aryl ether bonds. Lignin is hydrophobic, highly complex, and amorphous. It is the “glue” that binds cellulose to hemicellulose, acting as a cement and forming a complex structure of three-dimensional networks in cell walls. Thus, lignin enables the creation of a structure of strong mechanical resistance and is insoluble in water. The cross-linking between lignin and polysaccharides results in the vascular tissue that conducts water in plants. This polymer is responsible for the protection of cellulose and hemicellulose, resisting external agents (pathogens, enzymes, oxidizing agents, microorganisms, etc.) [31].
The thermal stability of these components is ordered according to hemicellulose < cellulose < lignin [31]. The content of these compounds varies according to the nature of the biomass, as is shown in Table 3.

Table 3.
Lignocellulosic distribution in woody, grassy, and agro-industrial biomass [32].
The order of decreasing lignin content of different species is as follows: softwood > hardwood > grass. In addition, biomass contains other non-structural (organic and inorganic) compounds, including proteins, fats, waxes, sugars, resins, sugar polymers, pectins, etc. These components are responsible for characteristics such as colour, aroma, and flavour. Among the inorganic compounds are elements such as N, K, Ca, Mg, P, and S, which are the compounds with the greatest presence after C, H, and O [31,33]. Other compounds have a minor presence but also form part of important functions: Cl, Fe, Mn, Zn, B, CU, Ni, Mo, Si, Na, Al, Se, Cr, Ti, and Cd [31,33]. These compounds form part of what, after combustion, will be called ash [31].
- Non-biodegradable plastics and their challenges (chlorine content and melting behaviour).
The most common plastics used for all human activities are HDPE, LDPE, PP, PET, PS, PVC, and others, such as rubber [8]. The decomposition of these polymers can lead to the formation of compounds derived from their respective monomers, such as ethylene, propylene, styrene, terephthalic acid and ethylene glycol, aromatics, olefins, chlorinated compounds, and sulphur organic compounds from vulcanised rubber [34,35,36].
This decomposition process, called depolymerisation, is one of the multiple stages that occur in the global gasification process, which starts with pyrolysis. Generally, depolymerisation begins with the formation of free radicals that then trigger a series of reactions, among which random scission (RS), unzipping (UZ), and backbiting (BB) can be highlighted [10,34,37].
The pathway of depolymerisation through random scission (RS) implies intermolecular hydrogen transfers, followed by mid-chain Beta-scission to give light intermediaries. The backbiting (BB) reaction path, on the other hand, is related to intramolecular hydrogen transfers that then also lead to mid-chain Beta-scissions. Finally, the unzipping (UZ) refers to end-chain Beta-scissions [10,34]. These reactions can lead to the formation of recombination olefins [10].
The combination of all these mechanisms will break the polymeric chains, releasing hydrogen and generating free radicals that will continue reacting. At temperatures greater than 400 °C, other alpha-scission reactions will take place and become dominant, which can produce heavier compounds [10]. The RS and BB mechanisms are common in PE and PP, while in plastics such as PS, the main depolymerisation mechanisms are via RS and UZ [10,37].
3. Co-Gasification Mechanisms
3.1. Synergistic Effects: How Plastics Enhance H2/CO in Syngas (e.g., Plastics Act as Hydrogen Donors)
To understand how materials behave thermally, their decomposition can be studied using thermogravimetry, and mass loss curves can be observed in inert environments (usually N2 or Ar). Figure 4 shows a compilation of thermogravimetry curves during the pyrolysis of cellulose, hemicellulose, lignin, and some plastics, with N2 atmosphere.
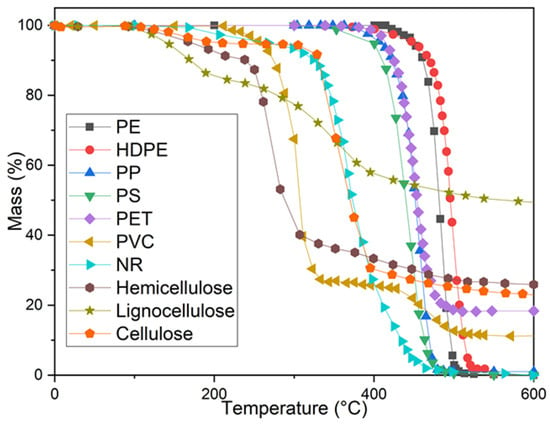
Figure 4.
Thermogravimetry curves for pyrolysis of cellulose, hemicellulose, lignin, and plastics. N2 atmosphere [37,38,39,40,41].
When analysing the pyrolysis of lignocellulosic biomass and plastics by thermogravimetry analysis (TGA), it can be observed that the loss of mass in biomass samples begins at lower temperatures, probably mostly due to the release of moisture, and that mass is then rapidly lost until the mass stabilises [38,42,43].
Hemicellulose begins to degrade at relatively low temperatures, starting with dehydration and then with glycosidic bond-breaking reactions, which require less energy (activation energy ~117.67 kJ/mol), rapidly releasing volatiles [44]. Cellulose, on the other hand, decomposes rapidly over short temperature ranges (less than 50 °C), with dehydrogenation, decarboxylation, and hydrogen bond-breaking reactions, releasing volatiles and leaving carbonaceous residues [44].
The most stable compound, lignin, reports having free radical concentrations in pyrolysis even at temperatures just above 200 °C, temperatures at which it begins to decompose and generate light compounds such as CO2, OH, and CO groups from carbonyl groups, as well as tars. The temperature increase generates stable and unstable free radicals that favour lignin depolymerisation. Free radical formation increases directly with temperature to values close to 475 °C, the value from which free radical formation decreases, probably because the free radical formation process swings with the process of destruction of lignin [45]. At temperatures greater than 500 °C, aromatic rings and hydrogen formation [46] are observed. TGA of the pyrolysis process shows how the formation of light compounds from 200 °C occurs similarly in all hemicellulose, cellulose, and lignin structures, being more abundant at temperatures between 400 and 500 °C, where the concentration of free radicals is greater. Lignin is the compound with the lowest mass loss throughout the analysis, which agrees with its thermal stability [38]. Even at high temperatures, under pyrolysis conditions, the lignin, cellulose, and hemicellulose structures generate a carbonaceous residue that is difficult to degrade thermally [38,42,43].
TGA of the pyrolysis of different plastics shows that the mass loss of the plastics starts from 200 to 250 °C (for NR [39]), or 350 to 400 °C (for PP [40], PS [37]), and continues until the total degradation of the polymers in the cases of PE, PP, PS, and NR [37,38,39,40,41,47], but remaining residues are observed in PVC and PET [37,38], even at temperatures above 750 °C. As can be seen in Figure 3, most plastics decompose completely at 500 °C; however, PET and PVC leave a remnant char. In the case of virgin PP, some analysis shows a small coal remnant, close to 1% [37,48].
In previous sections, various plastic depolymerisation mechanisms were described. When PE begins to decompose, free radicals are formed through scission (RS) mechanisms. As a material containing carbons with very similar chemical environments, under certain conditions, C-C bond fragmentation occurs rapidly, impartially, and simultaneously, giving rise to a wide variety of volatile compounds and leaving no carbonaceous residues [48]. The methyl side chains in PP split preferentially from the polymer chains, generating highly reactive radicals and rapidly triggering the scission of the main chain into light hydrocarbons. This also explains why a small proportion of char formation can be observed in the case of PP, because radical intermediates have a short lifespan, which prevents the formation of carbonaceous cross-linked intermediates [48].
Figure 5 shows the proximate and elemental analyses of lignocellulosic material and common plastics. Unlike biomass, plastics have low ash contents, so the residue obtained during pyrolysis cannot be attributed to ash. Thermal degradation of PET occurs rapidly between 400 and 500 °C [49], generating volatile residues and a non-volatile residue consisting of a char composed almost entirely of a network of interconnected aromatic rings [49,50].
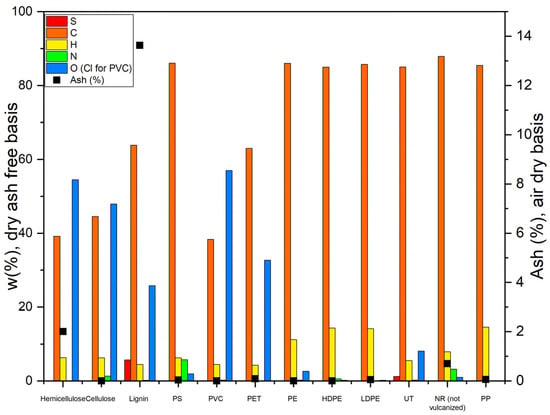
Figure 5.
Ash content and ultimate analyses for some common plastics and pure hemicellulose, cellulose, and lignin [38,51,52,53,54].
For its part, PVC exhibits a first stage of thermal decomposition corresponding to the release of HCl [48,55,56]. HCl generation occurs via two pathways. The intramolecular pathway occurs when the H-Cl pair is obtained from the PVC chain itself and generates two free radicals that rapidly self-annihilate through the formation of C=C bonds, forming polyenes derived from the PVC backbone. The species formed by this mechanism combine to form aromatic char precursors and ultimately char itself. This pathway for the formation of cross-linked species appears to be similar to that observed in the decomposition of PP, so it is inferred that it is not responsible for the elevated char formation in PVC (compared to that observed in PP), probably because the radicals formed by this pathway are short-lived [48,55]. Therefore, the elevated char formation in PVC can be explained by the second pathway for the release of HCl during the first stage of decomposition, which is the intermolecular pathway. When HCl is formed by this pathway (where Cl and H belong to different chains), it facilitates the formation of cross-links throughout. The cross-linked networks are important precursors of char (second stage of decomposition) generated by PVC [48,55].
Meng et al. [38] analysed the gasification of biomass and plastics with CO2 as a gasifying agent by thermogravimetry tests and found that gasification with CO2 reduces the carbonaceous residue of biomass, probably due to the Boudouard reaction (1); however, the residue generated by plastics (PVC and PET) does not present the same reactivity with this gasifying agent, nor does it show any influence on their decomposition temperatures. However, other authors [57] observed differences in PVC gasification with CO2, but do not attribute the decrease in char to the Boudouard reaction (1); instead, they mention that the CO2 interrupts the formation reactions of aromatics that are produced from the elimination of the Cl as HCl during the PVC depolymerisation [48,55].
Chin et al. [42] also showed by TGA that gasification with an air/steam mixture improves the decomposition of biomass char, probably because steam generates better carbon porosity [58] compared to a pyrolysis process under the same conditions in an Ar atmosphere. However, gasification of HDPE with an air/steam mixture does not show significant improvements in terms of decomposition rate, to the pyrolysis process [42]. Also, the gasifying agent seems to have a greater influence on the carbonaceous residue formed by lignocellulosic structures (oxygenated organic compounds and ash with a high inorganic content) [58] than on that residue generated by char-forming plastics like PVC or PET [38]. However, the activation energies in the gasification and pyrolysis of biomass and plastics mixtures show lower values than the reactions of plastics alone [42].
Pinto et al. [59] experimentally explored the co-gasification of rice residue and PE mixtures using different gasifying agents (air, oxygen, steam, CO2, or their mixtures). The results show that using steam combined with oxygen avoids the dilution of the syngas by N2, which increases the calorific value of the gas by around 42%, making this configuration the most technically efficient. Producing pure oxygen is expensive, but the option of air enriched with up to 40% oxygen also significantly improves the calorific value compared to gasification with air alone. Furthermore, the use of CO2 as a gasifying agent favours the Boudouard reaction (1), which reduces tar formation by almost 45% and increases the gas yield by approximately 70%. However, both steam and CO2 require additional heat due to their participation in endothermic reactions, so the authors suggest that a suitable mixture of CO2 + O2 + steam could represent the most balanced alternative from an operational and economic standpoint. For different biomass varieties, the study confirms that the inclusion of PE increases the energy content of the mixture, but also increases tar production when air is used as the gasifier, a production significantly mitigated when using steam or CO2, thanks to reforming reactions. This observation indicates that the mixture’s response to different gasifying agents depends on the biomass composition and that the effectiveness of thermal techniques varies depending on the presence of plastic.
Abdullahi et al. [44] studied the synergy between lignocellulosic biomass compounds and some plastics (PET and PVC) during the pyrolysis process by TGA. They found that there appears to be a synergistic effect that increases volatile production. They attribute this effect to a contribution of hydrogen atoms from PVC or PET to the biomass molecules. Furthermore, there is a decrease in the activation energy of thermal degradation reactions when mixtures are used, compared to individual materials. As observed by Abdullahi et al. [44], the free radicals generated by both materials promote degradation. PET appears to generate a greater amount of aromatics, while PVC gives rise to chlorinated organic species.
It is recommended that plastic utilisation, such as PVC [57] and rubber that contain sulphur [60], be avoided on account of their chemical compositions and the emissions that they generate during thermochemical processes. Moreover, plastics such as PET have a viable recycling route available [8], and these materials should be reused. It has been demonstrated that other types of plastic, including PP and LDPE, can be utilised as binders in biomass torrefaction [61]. Alternatively, these plastics can be blended with other materials for use in other thermochemical processes. In the context of thermochemical processes, the composition of biomass/plastic blends should be optimised to enhance the quality of the resulting products.
3.2. Plastics and Biomass Blends: Physicochemical Advantages of Co-Feeding
Later, the improvement of the quantity and quality of the gas obtained during gasification through biomass/plastic blends will be analysed. However, this section will discuss the improvements obtained by simply blending the materials in terms of their physicochemical properties.
As can be seen in Figure 5, plastics have a higher carbon and hydrogen content compared to biomass materials, especially polyolefins like PE, while biomass has a higher oxygen content and an elevated moisture content.
Beyond fundamental composition, the physical-chemical properties of the feedstock, such as moisture content, particle size, and heterogeneity, critically influence gasification efficiency and syngas quality. Consequently, pretreatment is often an essential prerequisite step [62]. For high-moisture biomass, methods like drying are necessary to reduce its energy penalty and increase the reactor’s thermal efficiency [63]. Co-processing with inherently low-moisture fuels, such as plastics, presents an effective strategy to dilute the overall moisture content of the feedstock blend. Furthermore, pretreatment extends beyond moisture control to include size reduction and palletisation, which mitigate handling issues like bridging and segregation, and thermal treatments like torrefaction [64], which increase the energy density and improve the grindability of biomass. These steps are vital for ensuring consistent feedstock flow and optimising the co-gasification process.
Furthermore, a mixture of biomass material and plastics such as PE, PP, and PS would act as carbon boosters in the generated gas, thus improving gas efficiency, and biomass adds part of the oxygen required for the process.
Biomass, for its part, contains significant levels of inorganic compounds [31], which are part of the residual ash. It has been observed that some of this ash can melt into slag at high temperatures, accumulate on the reactor walls, and combine with solid slag. If not removed, it can form agglomerations [65]. Since high ash content causes slagging, biomass with low ash content should be used for syngas production. Combining biomass with plastics can dilute these inorganic compounds and prevent the formation of agglomerates. However, the used plastic should have low ash content, and the gasification temperature must not rise too high, which can lead to the growth of mineral agglomerates with high aluminium content [66]. Otherwise, plastic blends should be avoided due to their ash slagging and fouling tendency [66].
Figure 5 shows that plastics decompose rapidly within narrow temperature ranges (~100 °C) [37,40,41,47]. Biomass components, on the other hand, initially release moisture and then decompose over a much wider temperature range than plastics [38]. A combined feed can decrease the global moisture and parallelly thermally balance energy requirements, where the endothermic reactions of biomass decomposition obtain the necessary energy from the exothermic decomposition of plastics. During their decomposition, some plastics generate undesirable compounds such as HCl [48,55,56,66], sulphur derivatives, and toxins generated by the volatilisation of some structures such as PS [13]. If the plastics also contain additives, these additives may contain heavy metals [13]. In this case, it is advisable to dilute the plastic content with biomass to reduce the problems generated by these pollutants. But managing the dilution effects and contaminant issues is crucial by carefully selecting materials and optimizing process conditions.
Other authors list more reasons for co-gasifying biomass and plastics: it helps with the seasonal shortage of some biomass varieties; it allows the gasification of plastics in downdraft reactors, due to how biomass acts as a bed allowing the feeding of plastics that would otherwise agglomerate; some authors mention the operational problems of gasifying only plastics (heat transfer problems, sticking, high viscosity, cooking, etc.) [67]; Robinson et al. [68] analysed these improvements with biomass/PET pellets; in addition, palletisation can solve the problems of reactor feed, related to the difference of density between plastics and biomass, due to the close contact between materials, which may cause some interaction that prevents coke formation, according to Robinson et al. [68]; co-gasification eliminates the need for separation of materials, which is advantageous when waste is difficult to separate [67].
Furthermore, the gasification process is also influenced by many operational parameters, including temperature, heating rate, reaction time, reactor type, and catalyst [24]. This article analyses the behaviour of mixtures of biomass materials with different plastics under different gasification scenarios, to determine the potential advantages of co-gasification over the exclusive gasification of one or the other material.
3.3. Exploration of Co-Gasification Studies
Biomass/plastic blends’ co-gasification has attracted growing research interest due to its potential to enhance syngas quality. Numerous studies have examined this technique, yielding valuable insights into process efficiency, tar reduction, and hydrogen production. The following section provides an overview of selected studies focused on catalytic co-gasification for syngas generation.
3.3.1. Biomass/Plastic Blends Co-Gasification, Non-Catalytic
Recent studies have demonstrated growing interest in non-catalytic co-gasification, primarily due to its potential to enhance energy efficiency. The addition of plastics to biomass during gasification has been shown to increase hydrogen yield and overall gas production. Moreover, the energy content of the resulting gas, expressed as LHV, also improves. Table 4 provides an introductory overview of synergistic effects observed in non-catalytic co-gasification of biomass/plastic blends, illustrating key trends in hydrogen yield and syngas energy content (LHV). As representative examples, the data highlight how plastic addition generally enhances gasification performance, though outcomes depend strongly on plastic type. Polyolefins (LDPE, HDPE, PE, PP, and PS) consistently improve both H2 production and LHV. For instance, a 25% HDPE blend increases H2 yield from 15.5% to 19.5% and LHV from 16.0 to 19.6 MJ/kg in wood gasification [55]. At higher composition, 50% of PE raises H2 by ~4.4% and nearly doubles LHV (10.7 → 18.9 MJ/kg) with sawdust [69]. PS and PP show notable synergy even at low blends (20%), increasing H2 by ~6–8% [70]. In contrast, heteroatom-containing plastics exhibit limitations, PET reduces both H2 (8.1% → 5.4%) and LHV (5.0 → 4.2 MJ/kg) [68], likely due to oxygen-driven tar formation and PVC shows marginal LHV gains (11.9 → 12.6 MJ/kg) despite higher H2 [71], suggesting chlorine inhibits energy recovery. These examples underscore that polyolefins are prime candidates for co-gasification, while PET/PVC require tailored approaches. These findings highlight the beneficial effect of plastic addition on the energy performance of biomass gasification.

Table 4.
Synergistic effects from non-catalytic blends.
As shown before, there is a tendency to produce more hydrogen, progressively, with higher plastic content in the feedstock. This is primarily attributed to the higher hydrogen-to-carbon (H/C) molar ratio of plastics compared to biomass. As the proportion of plastics increases, the depolymerisation pathway (RS, BB, and UZ; section 3) will break the polymeric chains, releasing hydrogen and generating free radicals that will continue reacting. As well, in the presence of biomass still present, stable and unstable OH radicals are generated that favour the decompositions [70]. This combination enhances the decomposition of oxygenated compounds (biomass) and hydrocarbons, and H and OH radicals are released and act as hydrogen donor species, promoting the decomposition of compounds, while also shifting endothermic reactions towards the formation of CO and H2. As outlined in the report by Liu et al. [75], endothermic reactions, such as steam reforming (13–14), the Boudouard reaction (1), and the water–gas reaction (2), become predominant at temperatures above 800 °C.
It is a general tendency for the H2/CO ratio in the gas composition to increase with the percentage of plastic in the mixture (see Figure 6). In all the cases analysed, an increase in H2 content is observed, although the evolution of the H2/CO ratio does not always follow a linear trend: this can remain constant or increase, depending on the percentage of plastic.
While many studies report increased hydrogen yield with higher plastic content, this trend shows important exceptions. Most studies observe that polyolefin plastics (PE, PP, and PS) enhance hydrogen production when blended with biomass. Lopez et al. [76] demonstrated that increasing PE content by 25 to 50% in wood blends raised H2/CO ratio by 50% in a conical spouted bed reactor, attributing this to the high hydrogen content of plastic–biomass (>2) and abundant hydrogen radicals. Zhu et al. [69] found that PP additions up to 50% increased the H2/CO ratio by more than 50% in a fluidised-bed reactor at 850 °C. However, several studies report deviations from this general trend. Wang et al. [77] demonstrated rubber’s positive effects in wood/rubber co-gasification, as higher rubber content increased H2 yield and H2/CO ratio. This stems from rubber’s ash-rich composition, where inorganics enhance char porosity and reactivity in the Boudouard reaction. The reduced biomass fraction also decreases oxygenated compounds. Studies [78,79] confirm such inorganic-rich chars develop superior properties—higher surface area, porosity, and active site accessibility—improving gasification performance.
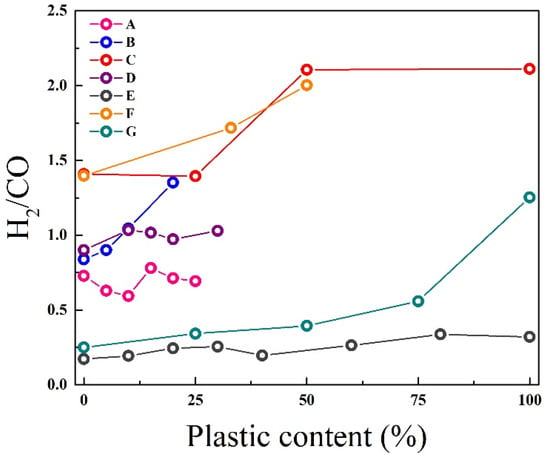
Figure 6.
Variation of H2/CO ratio with plastic content in feedstock. (A) LDPE [66]; (B) PP [71]; (C) HDPE [76]; (D) PE [74]; (E) PS [80]; (F) PE [69]; (G) tyre [77].
Fluctuant effect emerges in Yu et al. [74], as PE bends in an entrained-flow gasification reactor at 1000 °C increased H2 content, but also the concentration of CO increased. This difference is attributable to different gasification temperatures, which promote endothermic reaction. Related to process-dependent effects, the hydrogen enhancement mechanism varies significantly by reactor type and conditions. In autothermal systems, Fazil et al. [66] identified a critical threshold around 15% plastic content, below which CO production dominates, and above which H2 formation accelerates due to temperature increases (700→900 °C). Déparrois et al. [80] found paper/PS co-gasification yields less H2 and CO than mono-gasification, with PS > 30% causing negative synergy, and gas production fell below individual processes. PS’s char-free decomposition reduces carbon availability for CO2 reactions, decreasing CO yield versus paper alone. While PS volatiles can form CO, blend interactions (competition and dilution) further inhibit production. Mishra [81] confirms higher plastic ratios reduce char, limiting reactive carbon. CO2 gasification systems display different patterns, with some studies reporting nearly constant H2/CO ratios across blend compositions [80].
Negative synergy effects emerge at high plastic concentrations (>30%) for certain blends. PET blends consistently show reduced hydrogen yields compared to biomass alone (Table 4). Robinson et al. [68] observed 33% lower H2 production from 50% PET/wood blends compared to wood mono-gasification, due to oxygen interference in reforming reactions. PVC exhibits complex behaviour, and while some studies report modest H2 increases (12%), others show no improvement or even reduction, particularly in systems operating below 850 °C where chlorine promotes char formation [71].
The variability in hydrogen yield responses stems from competing factors, positively as high H/C ratio, hydrogen radical supply, and endothermic reaction promotion, and negatively as heteroatom interference (O, Cl), and reduced carbon accessibility. This analysis suggests that while the general trend of increasing hydrogen with plastic content holds for many systems, careful consideration of plastic type, blend ratio, and process conditions is essential for accurate prediction of gasification performance.
Figure 7 illustrates the synergistic effects observed in the biomass/plastic blends co-gasification. It presents as carbon conversion efficiency (CCE) expressed as the fraction of atomic carbon in the cumulative product gas yield relative to the solid feedstock. The inclusion of plastics in biomass gasification generally enhances CCE. In most cases, the carbon conversion achieved by co-gasification exceeds the expected values based on the weighted average of individual gasification of biomass and plastics alone, represented by the line between the gasification of biomass and plastic separately.
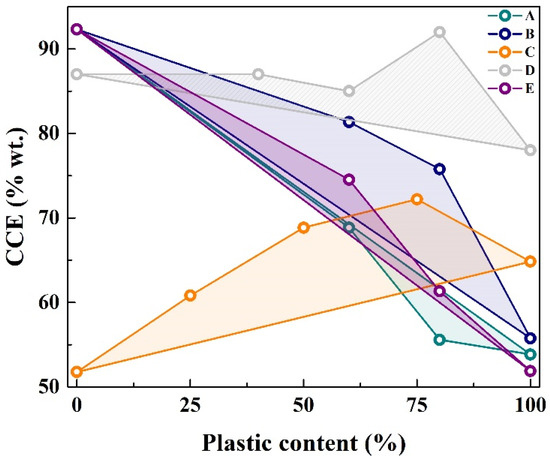
Figure 7.
Variation of carbon conversion efficiency (CCE) with plastic content in feedstock. (A) PP [82]; (B) BPC [82]; (C) [23]; (D) [83]; (E) PET [82].
However, the degree and nature of synergy, whether positive or negative, critically depend on several factors: the specific type of plastic used [84], since some plastics like PVC release corrosive substances such as HCl; the biomass-to-plastic ratio (low plastic content can reduce gas production or reactivity); the gasification conditions; and the ash content and behaviour of the biomass, which can lead to issues such as fouling or slagging.
Ahmed, Nipattummakul, and Gupta [85] investigated steam co-gasification of wood chips with plastics such as PP, PET, PE, and BPC. Their findings consistently demonstrated that the co-gasification process yielded more syngas than the theoretical sum of the yields from each material individually. This enhancement was attributed to hydrogen-rich free radicals released from the plastics, which facilitate secondary reactions involving the volatiles released by biomass, enhancing gas quality. Furthermore, the presence of oxygen in biomass increases oxidation potential, while methyl and benzyl radicals from plastics interact differently with both the gas and solid phases. A complementary study by Burra and Gupta [82] observed positive synergy in carbon conversion efficiency when blending biomass with PET and BPC, but not with PP. This difference became more evident when analysing tar and gaseous hydrocarbon yields (CH4, C2H6, C2H4, and C2H2), which were lower during co-gasification with PP compared to their mono-gasification. Research has indicated that the behaviour is linked to the chemical structure of the plastics. PP is composed primarily of methyl radicals, which have been shown to promote thermal cracking. In contrast, PET contains more stable benzyl linkages that are less reactive under typical residence times. This limits steam reforming and favours only cracking reactions.
To summarise, non-catalytic co-gasification of biomass with plastics shows great promise in improving gas quality, hydrogen yield, and carbon conversion efficiency. While synergistic effects are generally beneficial, certain plastics or specific process parameters may result in reduced performance or operational challenges. Therefore, a deeper understanding of the complex interactions between feedstock composition, reactor conditions, and product distribution is crucial for optimising co-gasification processes. Future research should focus on elucidating these mechanisms and developing strategies to mitigate negative effects, ultimately enhancing the viability of co-gasification as a sustainable waste-to-energy technology.
3.3.2. Biomass/Plastic Blends Co-Gasification, Catalytic
While the co-gasification of biomass and plastics has clear advantages, such as increased syngas yield and calorific value, there are several technical challenges to be addressed, particularly related to tar formation and process efficiency.
Furthermore, while biomass gasification tends to generate substantial amounts of char, plastics contribute predominantly to tar formation (Table 5) [74,82,86,87]. While the incorporation of plastics into biomass feedstocks has been shown to enhance the calorific value of the resulting gas, it has also been observed to increase tar-related concerns, which can negatively impact overall gasification efficiency [88].

Table 5.
Tar yield from various plastic feedstocks.
These tar-related challenges are multifaceted, impacting not only the gasifier’s operation but also the entire downstream process chain (Table 6). The following table summarises the primary technical challenges associated with tar formation and their specific consequences for process efficiency. In the case of certain plastics, such as PVC, the release of corrosive gases like HCl further complicates the process [57]. Furthermore, materials such as PP tend to melt within the gasifier, which complicates temperature control and reactor operation.

Table 6.
Technical challenges and efficiency impacts of tar in gasification.
Tar management strategies can be broadly categorised into primary and secondary methods. Primary methods focus on mitigating tar formation within the gasifier itself by controlling process parameters [89]; this includes operating at high temperatures (>800–900 °C) [90], employing elevated equivalence ratios to promote partial oxidation [91,92], and designing reactors that ensure long residence times and excellent mixing (e.g., fluidised beds). Secondary methods, applied to the raw syngas downstream, include thermal cracking at very high temperatures (1100–1300 °C), which is highly effective but energetically costly, and physical removal techniques such as wet scrubbing (which transfers the tar problem to a wastewater stream), cyclones, and barrier filtration (e.g., ceramic filters) which can efficiently capture tar. While non-catalytic approaches avoid the issues of catalyst cost, sensitivity, and deactivation, they often do so at the expense of process efficiency, through high energy consumption, or by creating secondary waste streams that require further treatment.
The choice between primary and secondary catalytic methods involves a direct trade-off between simplicity and control. Primary catalytic gasification, where the catalyst is mixed directly with the feedstocks, benefits from a simpler reactor design and lower capital costs but offers less control over the process and faster catalyst deactivation. Conversely, the secondary method, which utilises a separate downstream catalytic reactor, is more complex and costly but enables precise control over reaction conditions. This enhanced control facilitates better management of catalyst lifetime and allows for tailoring the product distribution, typically enhancing gas quality [93].
The composition of tar is highly dependent on the feedstock’s chemical structure. Consequently, the tar produced from the gasification of biomass, which is rich in oxygenated compounds, differs from that derived from plastics. Plastic-derived tars are primarily composed of linear hydrocarbons (e.g., from PP and PE), aromatics (e.g., from PS), and oxygenated compounds (e.g., from PET) [10,94].
To address these drawbacks, catalytic approaches, particularly the use of primary or secondary catalysts, have been widely investigated. The following section will focus on recent advances in catalytic co-gasification for improving process performance and syngas composition.
Catalysts play two essential roles in the gasification process: they enhance fuel conversion efficiency by reducing tar formation and help tailor the gas composition towards desired products such as H2, CO, or CH4. Table 7 provides an overview of the main catalytic reactions involved in gasification, along with the catalysts commonly used to promote each reaction.

Table 7.
Main catalysts and associated reactions in the gasification process.
In co-gasification studies, the catalysts employed are largely the same as those used in the gasification of individual feedstocks. Research in this area has focused primarily on well-established catalysts, particularly alkali metals (Na and K), alkaline earth metals (Ca and Mg), and transition metals such as Ni, Fe, and Co. These are typically supported on high surface area materials such as alumina, zeolites, or activated carbon. Potassium stands out for its high reactivity, especially in soluble forms like K2O, while sodium, though generally less active, has shown good performance under certain conditions, particularly as NaOH or Na2CO3 [103]. Research indicates that the co-gasification of biomass and plastics in the presence of catalysts can yield synergistic effects. For instance, the gasification of soda lignin with polyethylene (PE) has been demonstrated to enhance process efficiency, with the sodium present in the biomass naturally acting as a catalyst [104]. In a similar vein, Yu, Yang and Chen [74] demonstrated that Na2CO3 promotes the formation of H2 and CO2 via steam reforming reactions. Furthermore, the inherent presence of alkali and alkaline earth metals in biomass serves as a catalytic promoter, increasing the reactivity of biomass-derived carbon. When these metals are removed through acid washing, stronger interactions with plastics are observed, leading to higher hydrocarbon yields and enhanced catalytic reforming activity [55].
Catalytic mechanisms in gasification generally involve three primary steps: adsorption of the gasifying agent onto the catalyst surface, reaction at the carbon–catalyst interface, and diffusion of reactive species. It should be noted that highly active catalysts, such as potassium, have been shown to promote micropore formation within the carbon matrix, thereby enhancing carbon conversion. The “nanoworm” model illustrates how such catalysts penetrate the carbon structure during gasification [103].
Among the catalysts studied, CaO is particularly noteworthy for its dual functionality: it facilitates CO2 adsorption and water–gas shift (8) reaction equilibrium toward greater H2 production [105]. The combined use of Ni and CaO has shown very promising results in terms of enhancing H2 yields. More broadly, the use of alkaline earth metal oxides as catalysts has been shown to improve the conversion of intermediate hydrocarbons (HCs) to CO2, while also capturing CO2. This has been found to enhance the H/C ratio of the resulting syngas [106].
Conversely, the incorporation of Fe onto a CaO support has been shown to improve the co-gasification performance of pine sawdust blended with PE [75]. The Fe2O3/CaO catalyst exhibited significant enhancements during reuse experiments. Although a slight decrease in cold gas efficiency (CGE) was observed, the system maintained consistent reactivity for syngas production, with a notably high H2/CO ratio. The formation of a new active phase, Ca2Fe2O5, played a key role in promoting critical reactions such as the water–gas shift (8) and Boudouard (1) reactions, which also contributed to a substantial reduction in tar formation. After five gasification cycles, both Ca2Fe2O5 and CaO remained stable, highlighting the catalyst’s durability and reusability.
It is also important to note that naturally occurring minerals such as dolomite and olivine, which are rich in Ca and Mg, are widely used as low-cost catalysts for tar reduction due to their effectiveness. Dolomite typically contains lower concentrations of CaO and MgO compared to olivine, which in turn affects its tar cracking efficiency. The use of steam as a gasifying agent has been identified as a promising strategy to enhance dolomite’s catalytic performance [107]. Olivine is mainly composed of MgO and SiO2; however, under gasification conditions, the presence of inert elements such as Si and Al can negatively impact its efficiency due to the formation of aluminosilicate compounds [103].
Yu, Yang, and Chen [74] evaluated the efficiency of dolomite and Na2CO3 catalysts in the co-gasification of rice straw/PE blends at an 80:20 ratio. Dolomite exhibited superior performance in terms of CGE and LHV, whereas Na2CO3 excelled in reducing tar content by nearly 50%, although a decrease in LHV accompanied this. These results highlight the trade-off between maximizing gas quality and minimizing tar formation.
Several experimental studies have investigated nickel-based catalysts in co-gasification, testing various biomass–plastic combinations. For example, Alvarez et al. [70] studied Ni/Al2O3 catalysts applied to mixtures of wood and plastics including PE, PET, PS, and PP. The catalyst significantly increased hydrogen production in 80:20 biomass/PE and biomass/PP blends, with improvements of 45% and 58%, respectively. Additionally, tar content was notably reduced by 4% to 10%. However, plastics such as PS and PET yielded lower gas production due to the formation of aromatic tar and corrosive compounds.
Kumagai et al. [108] developed a Ni-Mg-Al-Ca catalyst for the co-gasification of pine wood and PP at a 75:25 ratio. The catalyst exhibited outstanding performance, achieving a 54% increase in H2 yield alongside a 30% reduction in tar content. The in situ CO2 capture by CaO and the spinel-structured MgAl2O4 support effectively prevented Ni sintering, enhancing catalyst stability.
Xu et al. [109] investigated the co-gasification of biomass waste and PE using a Ni/γ- Al2O3 catalyst. As consistently emphasised, blending with PE optimises syngas production. Furthermore, Xu et al. [109] observed that increasing PE content influences the type of coke deposited; at a 50% blend, the formation of high-quality carbon nanotubes (CNTs) predominates over amorphous carbon, which slows catalyst deactivation and helps maintain its activity. However, exceeding 50% PE leads to the accumulation of heavy hydrocarbons that hinder decomposition, thereby reducing H2 and total gas yields and negatively impacting catalyst performance. The addition of steam as a gasifying agent is a well-established method to mitigate amorphous carbon formation. Consequently, the biomass-to-plastic ratio must be carefully optimised to balance catalytic efficiency and system durability.
Zhu et al. [69] highlighted the catalytic efficacy of zeolites, particularly ZSM-5 and Na-Y, due to their porous structures and acidic sites that facilitate cracking reactions and promote H2 production. Both ZSM-5 and Na-Y significantly enhanced H2 yields—more than doubling production—while reducing tar levels by 48–58%. Notably, Na-Y exhibited the highest CGE, attributed to its high adsorption capacity and accessible active sites, making it the most effective catalyst under air gasification conditions. The presence of steam tends to moderate or align yields with those from biomass gasification alone, diminishing the catalyst’s impact on reaction parameters. In summary, catalytic effectiveness follows this order: Na-Y > ZSM-5 > olivine > sand, underscoring the superior performance of zeolites in co-gasification.
Overall, incorporating catalysts into the co-gasification of biomass–plastic mixtures substantially improves syngas quality and reduces tar formation. Nevertheless, significant gaps remain in designing catalysts tailored specifically for these complex feedstock blends. Catalysts based on alkali metals, nickel, and supports such as zeolites or CaO have demonstrated notable efficiency. However, their performance depends on factors like the biomass/plastic ratio, gasifying agent, and operating conditions. Future research should prioritise catalyst stability, reusability, contaminant resistance, and validation under realistic operational settings.
4. Conclusions
Carbonaceous waste from biomass and non-biodegradable plastics is abundant in all types of human activity. Although the nature of the decomposition of these materials has been analysed, the synergy of decomposition in catalytic and non-catalytic gasification systems appears to be limited to the formation of free radicals by plastic materials. However, due to the multiple combination possibilities, further research is still needed regarding the thermodynamic complexity of the system. The addition of catalysts improves the quality of the obtained gas, especially regarding cracking reactions, but they are susceptible to multiple combinations of co-gasification mixtures. Extensive research is still needed regarding the process conditions that optimise the gasification process if it is intended to be used for processing feedstocks without a prior waste separation process.
Author Contributions
Conceptualization, F.N. and J.M.B.; methodology, M.B. and L.D.; validation, C.R.V.; formal analysis, M.B. and L.D.; investigation, F.N., M.B. and L.D.; writing—original draft preparation, M.B. and L.D.; writing—review and editing, M.B. and L.D.; supervision, J.M.B. and C.R.V.; project administration, J.M.B. and C.R.V.; funding acquisition, J.M.B. and C.R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors gratefully acknowledge the support of the National Council for Scientific and Technological Research (CONICET), the National Agency for Scientific and Technological Promotion (ANPCyT), the National University of the Litoral (UNL), and the Santa Fe Agency for Science, Technology, and Innovation (AsaCTeI).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| PLA | Polylactic Acid |
| PHA | Polyhydroxyalkanoates |
| PET | polyethylene terephthalate |
| PETE | Polyethylene terephthalate |
| PP | polypropylene |
| HDPE | High-Density Polyethylene |
| LDPE | Low-Density Polyethylene |
| PVC | Polyvinyl Chloride |
| PS | Polystyrene plastic |
| LHV | Lower Heating Value |
| PE | polyethylene |
| IEA | International Energy Agency |
| NR | natural rubber |
| UT | used tires |
References
- Kaur, A.; Bharti, R.; Sharma, R. Municipal solid waste as a source of energy. Mater. Today Proc. 2023, 81, 904–915. [Google Scholar] [CrossRef]
- 1.1. Definition and Classification of Waste—MOOC: Auditing Waste Management, University of Tartu. Available online: https://sisu.ut.ee/waste/11-definition-and-classification-waste/ (accessed on 27 May 2025).
- Industrial Waste Management Market Size & Share to 2023–2030, KBV Research. Available online: https://www.kbvresearch.com/industrial-waste-management-market/ (accessed on 27 May 2025).
- Industrial Waste Management Market Revenue & Growth [2032], Fortune. Available online: https://www.fortunebusinessinsights.com/industry-reports/industrial-waste-management-market-100116 (accessed on 27 May 2025).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Biomass, Energy. Available online: https://energy.ec.europa.eu/topics/renewable-energy/bioenergy/biomass_en (accessed on 28 May 2025).
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef]
- Pilapitiya, P.G.C.N.T.; Ratnayake, A.S. The world of plastic waste: A review. Clean. Mater. 2024, 11, 100220. [Google Scholar] [CrossRef]
- Chidepatil, A.; Cárdenas, J.F.M.; Sankaran, K. Circular Economy of Plastics: Wishful Thinking or A Way Forward? J. Inst. Eng. (India) Ser. C 2021, 103, 647–653. [Google Scholar] [CrossRef]
- Bashir, M.A.; Ji, T.; Weidman, J.; Soong, Y.; Gray, M.L.; Shi, F.; Wang, P. Plastic waste gasification for low-carbon hydrogen production: A comprehensive review. Energy Adv. 2025, 4, 330–363. [Google Scholar] [CrossRef]
- Ioelovich, M. Recent findings and the energetic potential of plant biomass as a renewable source of biofuels-A review. BioResources 2014, 10, 1879–1914. [Google Scholar] [CrossRef]
- Alhulaybi, Z.; Dubdub, I. Comprehensive Kinetic Study of PET Pyrolysis Using TGA. Polymers 2023, 15, 3010. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Hall, P. Densification of Biomass and Waste Plastic Blends as a Solid Fuel: Hazards, Advantages, and Perspectives. Front. Energy Res. 2020, 8, 58. [Google Scholar] [CrossRef]
- Leary, M. 25-Materials selection and substitution using aluminium alloys. In Fundamentals of Aluminium Metallurgy; Woodhead Publishing: Sawston, UK, 2011; pp. 784–827. [Google Scholar] [CrossRef]
- Barcelo, L.; Kline, J.; Walenta, G.; Gartner, E. Cement and carbon emissions. Mater. Struct. 2013, 47, 1055–1065. [Google Scholar] [CrossRef]
- EIA. Available online: https://www.eia.gov/pressroom/releases/press542.php (accessed on 7 July 2025).
- Lundgren, J.; Vreugdenhil, B.; Ganjkhanlou, Y.; Baldwin, R. Biomass Gasification for Hydrogen Production. Available online: https://www.ieabioenergy.com/wp-content/uploads/2025/03/IEA-Bioenergy_T33_Bio-H2_Final_v2.pdf (accessed on 9 June 2025).
- Garcia, L.; Cordoba, M.; Dosso, L.; Nardi, F.; Vera, C.; Quiroga, M.; Busto, M.; Badano, J. Catalytic Gasification and Reforming of Residual Biomass in a Bench Scale System with Low Cost Catalysts. ChemPlusChem 2023, 88, e202300376. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, Q.; Wang, Y.; Guo, P.; Wang, Z.; Liu, H.; Akbari, A. Investigation of Biomass Gasification Potential in Syngas Production: Characteristics of Dried Biomass Gasification Using Steam as the Gasification Agent. Energy Fuels 2019, 34, 1033–1040. [Google Scholar] [CrossRef]
- Lv, P.M.; Xiong, Z.H.; Chang, J.; Wu, C.Z.; Chen, Y.; Zhu, J.X. An experimental study on biomass air–steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, Y.-L.; Zhao, X.-Y.; Cao, J.-P. Biomass thermochemical conversion: A review on tar elimination from biomass catalytic gasification. J. Energy Inst. 2020, 93, 1083–1098. [Google Scholar] [CrossRef]
- Shah, H.H.; Amin, M.; Iqbal, A.; Nadeem, I.; Kalin, M.; Soomar, A.M.; Galal, A.M. A review on gasification and pyrolysis of waste plastics. Front. Chem. 2023, 10, 960894. [Google Scholar] [CrossRef]
- Aentung, T.; Patcharavorachot, Y.; Wu, W. Co-Gasification of Plastic Waste Blended with Biomass: Process Modeling and Multi-Objective Optimization. Processes 2024, 12, 1906. [Google Scholar] [CrossRef]
- Reed, T.B. Principles and Technology of Biomass Gasification. In Advances in Solar Energy: An Annual Review of Research and Development Volume 2; Böer, K.W., Duffie, J.A., Eds.; Springer Nature: Boston, MA, USA, 1985; pp. 125–174. [Google Scholar] [CrossRef]
- Bartels, M.; Lin, W.; Nijenhuis, J.; Kapteijn, F.; Van Ommen, J.R. Agglomeration in fluidized beds at high temperatures: Mechanisms, detection and prevention. Prog. Energy Combust. Sci. 2008, 34, 633–666. [Google Scholar] [CrossRef]
- Donskoy, I. Particle Agglomeration of Biomass and Plastic Waste during Their Thermochemical Fixed-Bed Conversion. Energies 2023, 16, 4589. [Google Scholar] [CrossRef]
- Tremel, A.; Becherer, D.; Fendt, S.; Gaderer, M.; Spliethoff, H. Performance of entrained flow and fluidised bed biomass gasifiers on different scales. Energy Convers. Manag. 2013, 69, 95–106. [Google Scholar] [CrossRef]
- Chanthakett, A.; Arif, M.T.; Khan, M.M.K.; Oo, A.M.T. Performance assessment of gasification reactors for sustainable management of municipal solid waste. J. Environ. Manag. 2021, 291, 112661. [Google Scholar] [CrossRef]
- Hrbek, J. Gasification Developments in Europe and the USA. 2021. Available online: https://www.ieabioenergy.com/wp-content/uploads/2021/03/Hrbek-Gasification-developments-in-Europe-USA.pdf (accessed on 2 May 2025).
- Mujtaba, M.; Fraceto, L.F.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; De Medeiros, G.A.; Pereira, A.D.E.S.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Chandraratne, M.R.; Daful, A.G. Hydrothermal Conversion of Lignocellulosic Biomass to Hydrochar: Production, Characterization, and Applications. In From Biomass to Biobased Products; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Santana, H.E.P.; Jesus, M.; Santos, J.; Rodrigues, A.C.; Pires, P.; Ruzene, D.S.; Silva, I.P.; Silva, D.P. Lignocellulosic Biomass Gasification: Perspectives, Challenges, and Methods for Tar Elimination. Sustainability 2025, 17, 1888. [Google Scholar] [CrossRef]
- Lucas, Y. The Role of Plants in Controlling Rates and Products of Weathering: Importance of Biological Pumping. Annu. Rev. Earth Planet. Sci. 2001, 29, 135–163. [Google Scholar] [CrossRef]
- Levine, S.E.; Broadbelt, L.J. Detailed mechanistic modeling of high-density polyethylene pyrolysis: Low molecular weight product evolution. Polym. Degrad. Stab. 2009, 94, 810–822. [Google Scholar] [CrossRef]
- Hasan, M.M.; Haque, R.; Jahirul, M.I.; Rasul, M.G. Pyrolysis of plastic waste for sustainable energy Recovery: Technological advancements and environmental impacts. Energy Convers. Manag. 2025, 326, 119511. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology, NIST. Available online: https://www.nist.gov/ (accessed on 2 June 2025).
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. Thermal degradation of waste plastics under non-sweeping atmosphere: Part 1: Effect of temperature, product optimization, and degradation mechanism. J. Environ. Manag. 2019, 239, 395–406. [Google Scholar] [CrossRef]
- Meng, A.; Chen, S.; Long, Y.; Zhou, H.; Zhang, Y.; Li, Q. Pyrolysis and gasification of typical components in wastes with macro-TGA. Waste Manag. 2015, 46, 247–256. [Google Scholar] [CrossRef]
- Parvathi, K.; Ramesan, M.T. Structure, properties, and antibacterial behavior of nickel oxide reinforced natural rubber nanocomposites for flexible electronic applications. J. Appl. Polym. Sci. 2022, 139, e53120. [Google Scholar] [CrossRef]
- Ng, H.M.; Saidi, N.M.; Omar, F.S.; Ramesh, K.; Ramesh, S.; Bashir, S. Thermogravimetric Analysis of Polymers. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1–29. [Google Scholar] [CrossRef]
- Park, S.S.; Seo, D.K.; Lee, S.H.; Yu, T.-U.; Hwang, J. Study on pyrolysis characteristics of refuse plastic fuel using lab-scale tube furnace and thermogravimetric analysis reactor. J. Anal. Appl. Pyrolysis 2012, 97, 29–38. [Google Scholar] [CrossRef]
- Chin, B.L.F.; Yusup, S.; Al Shoaibi, A.; Kannan, P.; Srinivasakannan, C.; Sulaiman, S.A. Comparative studies on catalytic and non-catalytic co-gasification of rubber seed shell and high density polyethylene mixtures. J. Clean. Prod. 2014, 70, 303–314. [Google Scholar] [CrossRef]
- Fernandez, A.; Soria, J.; Rodriguez, R.; Baeyens, J.; Mazza, G. Macro-TGA steam-assisted gasification of lignocellulosic wastes. J. Environ. Manag. 2019, 233, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Shagali, A.A.; Hu, S.; Li, H.; He, L.; Han, H.; Chi, H.; Qing, H.; Xu, J.; Jiang, L.; Wang, Y.; et al. Synergistic interactions and co-pyrolysis characteristics of lignocellulosic biomass components and plastic using a fast heating concentrating photothermal TGA system. Renew. Energy 2023, 215, 118936. [Google Scholar] [CrossRef]
- Avni, E.; Suib, S.L. Free Radical Formation in Lignin During Pyrolysis. Holzforschung 1985, 39, 33–40. [Google Scholar] [CrossRef]
- Klapiszewski, U.; Szalaty, T.J.; Jesionowski, T. Depolymerization and Activation of Lignin: Current State of Knowledge and Perspectives. In Lignin—Trends and Applications; IntechOpen: London, UK, 2018; pp. 1–27. [Google Scholar] [CrossRef]
- Dubdub, I.; Al-Yaari, M. Pyrolysis of Mixed Plastic Waste: I. Kinetic Study. Materials 2020, 13, 4912. [Google Scholar] [CrossRef]
- Ye, L.; Li, T.; Hong, L. Understanding enhanced char formation in the thermal decomposition of PVC resin: Role of intermolecular chlorine loss. Mater. Today Commun. 2021, 26, 102186. [Google Scholar] [CrossRef]
- Das, P. Pyrolysis study of a waste plastic mixture through different kinetic models using isothermal and nonisothermal mechanism. RSC Adv. 2024, 14, 25599–25618. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. The thermal degradation of PET and analogous polyesters measured by thermal analysis–Fourier transform infrared spectroscopy. Polymer 2002, 43, 1835–1847. [Google Scholar] [CrossRef]
- Parekh, D.B.; Rotliwala, Y.C.; Parikh, P.A. Synergetic pyrolysis of high density polyethylene and Jatropha and Karanj cakes: A thermogravimetric study. J. Renew. Sustain. Energy 2009, 1, 033107. [Google Scholar] [CrossRef]
- Chaudhary, A.; Lakhani, J.; Dalsaniya, P.; Chaudhary, P.; Trada, A.; Shah, N.K.; Upadhyay, D.S. Slow pyrolysis of low-density Poly-Ethylene (LDPE): A batch experiment and thermodynamic analysis. Energy 2022, 263, 125810. [Google Scholar] [CrossRef]
- Osayi, J.I.; Iyuke, S.; Daramola, M.O.; Osifo, P.; Van Der Walt, I.J.; Ogbeide, S.E. Evaluation of pyrolytic oil from used tires and natural rubber (Hevea brasiliensis). Chem. Eng. Commun. 2018, 205, 805–821. [Google Scholar] [CrossRef]
- Wu, F.; Ben, H.; Yang, Y.; Jia, H.; Wang, R.; Han, G. Effects of Different Conditions on Co-Pyrolysis Behavior of Corn Stover and Polypropylene. Polymers 2020, 12, 973. [Google Scholar] [CrossRef]
- Li, J.; Burra, K.R.G.; Wang, Z.; Liu, X.; Gupta, A.K. Co-gasification of high-density polyethylene and pretreated pine wood. Appl. Energy 2021, 285, 116472. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Man, P. Kinetic Analysis of Thermal Decomposition of Polyvinyl Chloride at Various Oxygen Concentrations. Fire 2023, 6, 404. [Google Scholar] [CrossRef]
- Lee, T.; Lee, J.; Ok, Y.S.; Oh, J.-I.; Lee, S.-R.; Rinklebe, J.; Kwon, E.E. Utilizing CO2 to suppress the generation of harmful chemicals from thermal degradation of polyvinyl chloride. J. Clean. Prod. 2017, 162, 1465–1471. [Google Scholar] [CrossRef]
- Felix, C.B.; Chen, W.-H.; Ubando, A.T.; Park, Y.-K.; Lin, K.-Y.A.; Pugazhendhi, A.; Nguyen, T.-B.; Dong, C.-D. A comprehensive review of thermogravimetric analysis in lignocellulosic and algal biomass gasification. Chem. Eng. J. 2022, 445, 136730. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.; Miranda, M.; Neves, D.; Varela, F.; Santos, J. Effect of gasification agent on co-gasification of rice production wastes mixtures. Fuel 2016, 180, 407–416. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, M.; Jones, I.; Okoye, C.O.; Zhang, Z.; Gao, J.; Zhang, D. Transformation and fate of sulphur during steam gasification of a spent tyre pyrolysis char. Fuel 2022, 321, 124091. [Google Scholar] [CrossRef]
- Emadi, B.; Iroba, K.L.; Tabil, L.G. Effect of polymer plastic binder on mechanical, storage and combustion characteristics of torrefied and pelletized herbaceous biomass. Appl. Energy 2017, 198, 312–319. [Google Scholar] [CrossRef]
- Mishra, R.; Ong, H.C.; Lin, C.-W. Progress on co-processing of biomass and plastic waste for hydrogen production. Energy Convers. Manag. 2023, 284, 116983. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, L.; Guan, J.; Xiong, Q.; Zhang, S.; Jin, X. A Review on Biomass Gasification: Effect of Main Parameters on Char Generation and Reaction. Energy Fuels 2020, 34, 13438–13455. [Google Scholar] [CrossRef]
- Chen, W.-H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Fazil, A.; Kumar, S.; Mahajani, S.M. Downdraft co-gasification of high ash biomass and plastics. Energy 2022, 243, 123055. [Google Scholar] [CrossRef]
- Wang, B.; Gupta, R.; Bei, L.; Wan, Q.; Sun, L. A review on gasification of municipal solid waste (MSW): Syngas production, tar formation, mineral transformation and industrial challenges. Int. J. Hydrogen Energy 2023, 48, 26676–26706. [Google Scholar] [CrossRef]
- Block, C.; Ephraim, A.; Weiss-Hortala, E.; Minh, D.P.; Nzihou, A.; Vandecasteele, C. Co-pyrogasification of Plastics and Biomass, a Review. Waste Biomass Valorization 2018, 10, 483–509. [Google Scholar] [CrossRef]
- Robinson, T.; Bronson, B.; Gogolek, P.; Mehrani, P. Comparison of the air–blown bubbling fluidized bed gasification of wood and wood–PET pellets. Fuel 2016, 178, 263–271. [Google Scholar] [CrossRef]
- Zhu, H.L.; Zhang, Y.S.; Materazzi, M.; Aranda, G.; Brett, D.J.L.; Shearing, P.R.; Manos, G. Co-gasification of beech-wood and polyethylene in a fluidized-bed reactor. Fuel Process. Technol. 2019, 190, 29–37. [Google Scholar] [CrossRef]
- Alvarez, J.; Kumagai, S.; Wu, C.; Yoshioka, T.; Bilbao, J.; Olazar, M.; Williams, P.T. Hydrogen production from biomass and plastic mixtures by pyrolysis-gasification. Int. J. Hydrogen Energy 2014, 39, 10883–10891. [Google Scholar] [CrossRef]
- Baloch, H.A.; Yang, T.; Li, R.; Nizamuddin, S.; Kai, X.; Bhutto, A.W. Parametric study of co-gasification of ternary blends of rice straw, polyethylene and polyvinylchloride. Clean Technol. Environ. Policy 2016, 18, 1031–1042. [Google Scholar] [CrossRef]
- Basha, M.H.; Sulaiman, S.A.; Uemura, Y. Co-gasification of palm kernel shell and polystyrene plastic: Effect of different operating conditions. J. Energy Inst. 2020, 93, 1045–1052. [Google Scholar] [CrossRef]
- García-Bacaicoa, P.; Mastral, J.F.; Ceamanos, J.; Berrueco, C.; Serrano, S. Gasification of biomass/high density polyethylene mixtures in a downdraft gasifier. Bioresour. Technol. 2008, 99, 5485–5491. [Google Scholar] [CrossRef]
- Yu, H.; Yang, X.; Jiang, L.; Chen, D. Experimental Study on Co-gasification Characteristics of Biomass and Plastic Wastes. BioResources 2014, 9, 5615–5626. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.; Hu, C.; Peng, B.; Liu, C.; Li, Z.; Wu, K.; Zhang, H.; Xiao, R. High H2/CO ratio syngas production from chemical looping co-gasification of biomass and polyethylene with CaO/Fe2O3 oxygen carrier. Energy Convers. Manag. 2019, 199, 111951. [Google Scholar] [CrossRef]
- Lopez, G.; Erkiaga, A.; Amutio, M.; Bilbao, J.; Olazar, M. Effect of polyethylene co-feeding in the steam gasification of biomass in a conical spouted bed reactor. Fuel 2015, 153, 393–401. [Google Scholar] [CrossRef]
- Wang, Z.; Burra, K.G.; Lei, T.; Gupta, A.K. Co-gasification characteristics of waste tire and pine bark mixtures in CO2 atmosphere. Fuel 2019, 257, 116025. [Google Scholar] [CrossRef]
- Duman, G.; Uddin, A.; Yanik, J. The effect of char properties on gasification reactivity. Fuel Process. Technol. 2014, 118, 75–81. [Google Scholar] [CrossRef]
- Hongthong, S.; Sangsida, W.; Wongcharee, S.; Chanthakhot, A.; Aungthitipan, P.; Suwannahong, K.; Kreetachat, T.; Rioyo, J. Enhanced Biochar Production via Co-Pyrolysis of Biomass Residual with Plastic Waste after Recycling Process. Int. J. Chem. Eng. 2024, 1, 1176275. [Google Scholar] [CrossRef]
- Déparrois, N.; Singh, P.; Burra, K.G.; Gupta, A.K. Syngas production from co-pyrolysis and co-gasification of polystyrene and paper with CO2. Appl. Energy 2019, 246, 1–10. [Google Scholar] [CrossRef]
- Mishra, R.K. Co-pyrolysis of low-value wood sawdust and non-recyclable plastics into char: Effect of plastic loading on char yield and its properties. RSC Sustain. 2025, 3, 1774–1787. [Google Scholar] [CrossRef]
- Burra, K.G.; Gupta, A.K. Synergistic effects in steam gasification of combined biomass and plastic waste mixtures. Appl. Energy 2018, 211, 230–236. [Google Scholar] [CrossRef]
- Parrillo, F.; Ardolino, F.; Boccia, C.; Calì, G.; Marotto, D.; Pettinau, A.; Arena, U. Co-gasification of plastics waste and biomass in a pilot scale fluidized bed reactor. Energy 2023, 273, 127220. [Google Scholar] [CrossRef]
- Xu, D.; Xiong, Y.; Zhang, S.; Su, Y. The synergistic mechanism between coke depositions and gas for H2 production from co-pyrolysis of biomass and plastic wastes via char supported catalyst. Waste Manag. 2021, 121, 23–32. [Google Scholar] [CrossRef]
- Ahmed, I.I.; Nipattummakul, N.; Gupta, A.K. Characteristics of syngas from co-gasification of polyethylene and woodchips. Appl. Energy 2011, 88, 165–174. [Google Scholar] [CrossRef]
- Buentello-Montoya, D.A.; Duarte-Ruiz, C.A.; Maldonado-Escalante, J.F. Co-gasification of waste PET, PP and biomass for energy recovery: A thermodynamic model to assess the produced syngas quality. Energy 2022, 266, 126510. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Haciosmanoglu, M.; Akbulut, U.; Dincer, I. A review on converting plastic wastes into clean hydrogen via gasification for better sustainability. Int. J. Energy Res. 2021, 46, 4001–4032. [Google Scholar] [CrossRef]
- Wilk, V.; Hofbauer, H. Co-gasification of Plastics and Biomass in a Dual Fluidized-Bed Steam Gasifier: Possible Interactions of Fuels. Energy Fuels 2013, 27, 3261–3273. [Google Scholar] [CrossRef]
- Cortazar, M.; Santamaria, L.; Lopez, G.; Alvarez, J.; Zhang, L.; Wang, R.; Bi, X.; Olazar, M. A comprehensive review of primary strategies for tar removal in biomass gasification. Energy Convers. Manag. 2022, 276, 116496. [Google Scholar] [CrossRef]
- Cortazar, M.; Alvarez, J.; Lopez, G.; Amutio, M.; Santamaria, L.; Bilbao, J.; Olazar, M. Role of temperature on gasification performance and tar composition in a fountain enhanced conical spouted bed reactor. Energy Convers. Manag. 2018, 171, 1589–1597. [Google Scholar] [CrossRef]
- Upadhyay, D.S.; Sakhiya, A.K.; Panchal, K.; Patel, A.H.; Patel, R.N. Effect of equivalence ratio on the performance of the downdraft gasifier – An experimental and modelling approach. Energy 2019, 168, 833–846. [Google Scholar] [CrossRef]
- Basu, P. Chapter 6–Design of Biomass Gasifiers. In Biomass Gasification and Pyrolysis; Academic Press: Cambridge, MA, USA, 2010; pp. 167–228. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Wu, R.; Zeng, X.; Gao, S.; Xu, G. Fundamentals of Catalytic Tar Removal over in Situ and ex Situ Chars in Two-Stage Gasification of Coal. Energy Fuels 2013, 28, 58–66. [Google Scholar] [CrossRef]
- Tang, W.; Cao, J.; He, Z.; Jiang, W.; Wang, Z.; Zhao, X. Recent Progress of Catalysts for Reforming of Biomass Tar/Tar Models at Low Temperatures-A Short Review. ChemCatChem 2023, 15, e202300581. [Google Scholar] [CrossRef]
- Keller, M.; Sharma, A. Reverse Boudouard reforming produces CO directly suitable for the production of methanol from CO2 and CH4. Chem. Eng. J. 2022, 431, 134127. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, S.; Wang, Y.; Zhang, Y.; Zhu, Y.; Zong, B.; Hu, J.; Williams, P.; Wu, C. The role of reverse Boudouard reaction during integrated CO2 capture and utilisation via dry reforming of methane. Chem. Eng. J. 2024, 491, 151668. [Google Scholar] [CrossRef]
- Shahbaz, M.; Yusup, S.; Inayat, A.; Patrick, D.O.; Ammar, M. The influence of catalysts in biomass steam gasification and catalytic potential of coal bottom ash in biomass steam gasification: A review. Renew. Sustain. Energy Rev. 2017, 73, 468–476. [Google Scholar] [CrossRef]
- Ding, X.; Jiao, W.; Liu, Y.; Wang, Z.; Fang, Y. Methane production from high-pressure catalytic steam hydrogasification of sawdust char on K-modified transition metal composite catalysts. Fuel 2024, 363, 131000. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.; Byun, S.W.; Shin, H.; Ju, Y.; Min, H.; Kim, Y.J.; Heo, I.; Hazlett, M.J.; Kim, M.; et al. Impact of Pd:Pt ratio of Pd/Pt bimetallic catalyst on CH4 oxidation. Appl. Catal. B Environ. 2022, 316, 121623. [Google Scholar] [CrossRef]
- Yan, H.; Qin, X.-T.; Yin, Y.; Teng, Y.-F.; Jin, Z.; Jia, C.-J. Promoted Cu-Fe3O4 catalysts for low-temperature water gas shift reaction: Optimization of Cu content. Appl. Catal. B Environ. 2018, 226, 182–193. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Wang, Y.; Zhang, J.; Xu, D.; Gong, Y. Gasification of two and three-components mixture in supercritical water: Influence of NaOH and initial reactants of acetic acid and phenol. Int. J. Hydrogen Energy 2012, 37, 2278–2286. [Google Scholar] [CrossRef]
- Ngo, S.I.; García-Bordejé, E. Catalytic CO2 Methanation Reactors and Processes. Catalysts 2023, 13, 1422. [Google Scholar] [CrossRef]
- Arnold, R.A.; Hill, J.M. Catalysts for gasification: A review. Sustain. Energy Fuels 2019, 3, 656–672. [Google Scholar] [CrossRef]
- Cao, C.; Bian, C.; Wang, G.; Bai, B.; Xie, Y.; Jin, H. Co-gasification of plastic wastes and soda lignin in supercritical water. Chem. Eng. J. 2020, 388, 124277. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, M.; Gao, N.; Duan, Y.; Li, J. Experimental study on pyrolysis/gasification of biomass and plastics for H2 production under new dual-support catalyst. Chem. Eng. J. 2020, 396, 125260. [Google Scholar] [CrossRef]
- Niu, Y.-H.; Chi, Z.-Y.; Li, M.; Du, J.-Z.; Han, F.-T. Advancements in biomass gasification and catalytic tar-cracking technologies. Mater. Rep. Energy 2024, 4, 100295. [Google Scholar] [CrossRef]
- Islam, W. A review of dolomite catalyst for biomass gasification tar removal. Fuel 2020, 267, 117095. [Google Scholar] [CrossRef]
- Kumagai, S.; Alvarez, J.; Blanco, P.H.; Wu, C.; Yoshioka, T.; Olazar, M.; Williams, P.T. Novel Ni–Mg–Al–Ca catalyst for enhanced hydrogen production for the pyrolysis–gasification of a biomass/plastic mixture. J. Anal. Appl. Pyrolysis 2015, 113, 15–21. [Google Scholar] [CrossRef]
- Xu, D.; Xiong, Y.; Ye, J.; Su, Y.; Dong, Q.; Zhang, S. Performances of syngas production and deposited coke regulation during co-gasification of biomass and plastic wastes over Ni/-Al2O3 catalyst: Role of biomass to plastic ratio in feedstock. Chem. Eng. J. 2020, 392, 123728. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).