Synthesis of New Phenoxide-Modified Half-Titanocene Catalysts for Ethylene Polymerization
Abstract
1. Introduction
2. Results and Discussion
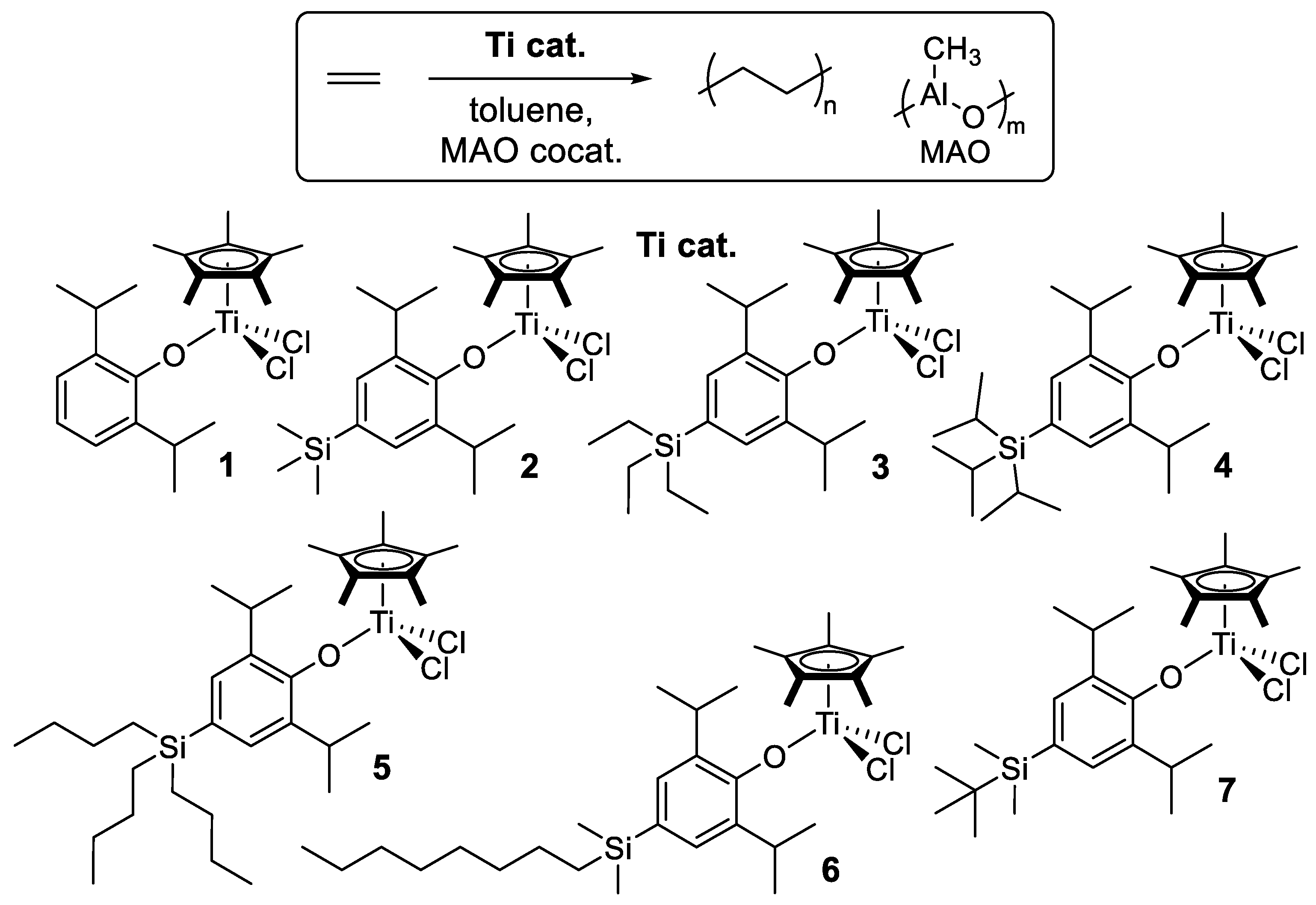
2.1. Synthesis of Half-Titanocenes Containing Different Trialkylsilyl Para-Phenoxy Substituents, Cp*TiCl2(O-2,6-iPr2-4-R-C6H2) (5–7)
2.2. Ethylene Polymerization by Cp*TiCl2(O-2,6-iPr2-4-R-C6H2) (1–7)
2.3. Ethylene Copolymerization with 1-Dodecene by Cp*TiCl2(O-2,6-iPr2-4-R-C6H2) (4–7)
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaminsky, W.; Engehausen, R.; Kopf, J. A Tailor-made metallocene for the copolymerization of ethene with bulky cycloalkenes. Angew. Chem. Int. Ed. Engl. 1995, 34, 2273–2275. [Google Scholar] [CrossRef]
- Kaminsky, W. New polymers by metallocene catalysis. Macromol. Chem. Phys. 1996, 197, 3907–3945. [Google Scholar] [CrossRef]
- Kaminsky, W.; Arndt, M. Metallocenes for polymer catalysis. In Polymer Synthesis/Polymer Catalysis; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1997; Volume 127, pp. 143–187. [Google Scholar] [CrossRef]
- Suhm, J.; Heinemann, J.; Wörner, C.; Müller, P.; Stricker, F.; Kressler, J.; Okuda, J.; Mülhaupt, R. Novel polyolefin materials via catalysis and reactive processing. Macromol. Symp. 1998, 129, 1–28. [Google Scholar] [CrossRef]
- McKnight, A.L.; Waymouth, R.M. Group 4 ansa-cyclopentadienyl-amido catalysts for olefin polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef]
- Gibson, V.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar] [CrossRef]
- Nomura, K.; Liu, J.; Padmanabhan, S.; Kitiyanan, B. Nonbridged half-metallocenes containing anionic ancillary donor ligands: New promising candidates as catalysts for precise olefin polymerization. J. Mol. Catal. A Chem. 2007, 267, 1–29. [Google Scholar] [CrossRef]
- Nomura, K. Half-titanocenes containing anionic ancillary donor ligands as promising new catalysts for precise olefin polymerization. Dalton Trans. 2009, 38, 8811–8823. [Google Scholar] [CrossRef]
- Nomura, K.; Liu, J. Half-titanocenes for precise olefin polymerization: Effects of ligand substituents and some mechanistic aspects. Dalton Trans. 2011, 40, 7666–7682. [Google Scholar] [CrossRef]
- Redshaw, C.; Tang, Y. Tridentate ligands and beyond in group IV metal α-olefin homo-/co-polymerization catalysis. Chem. Soc. Rev. 2012, 41, 4484–4510. [Google Scholar] [CrossRef]
- Baier, M.C.; Zuideveld, M.A.; Mecking, S. Post-metallocenes in the industrial production of polyolefins. Angew. Chem. Int. Ed. 2014, 53, 9722–9744. [Google Scholar] [CrossRef]
- van Doremaele, G.; van Duin, M.; Valla, M.; Berthoud, A. On the development of titanium κ1-amidinate complexes, commercialized as Keltan ACETM technology, enabling the production of an unprecedented large variety of EPDM polymer structures. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2877–2891. [Google Scholar] [CrossRef]
- Yuan, S.-F.; Yan, Y.; Solan, G.A.; Ma, Y.; Sun, W.-H. Recent advancements in N-ligated group 4 molecular catalysts for the (co)polymerization of ethylene. Coord. Chem. Rev. 2020, 411, 213254. [Google Scholar] [CrossRef]
- Organometallic Reactions and Polymerization; Osakada, K., Ed.; The Lecture Notes in Chemistry 85; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Handbook of Transition Metal Polymerization Catalysts, 2nd ed.; Hoff, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Nomura, K.; Kitphaitun, S. Catalysis for a Sustainable Environment: Reactions, Processes and Applied Technologies; Pombeiro, A.J.L., Sutradhar, M., Alegria, E.C.B.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2024; pp. 323–338. [Google Scholar]
- Kitphaitun, S.; Yan, Q.; Nomura, K. The effect of SiMe3 and SiEt3 para-substituents for high activity and introduction of a hydroxy group in ethylene copolymerization catalyzed by phenoxide-modified half-titanocenes. Angew. Chem. Int. Ed. 2020, 59, 23072–23076. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Nomura, K. Ethylene copolymerization with limonene and β-pinene: New bio-based polyolefins prepared by coordination polymerization. Macromolecules 2021, 54, 4693–4703. [Google Scholar] [CrossRef]
- Kitphaitun, S.; Chaimongkolkunasin, S.; Manit, J.; Makino, R.; Kadota, J.; Hirano, H.; Nomura, K. Ethylene/myrcene copolymers as new bio-based elastomers prepared by coordination polymerization using titanium catalysts. Macromolecules 2021, 54, 10049–10058. [Google Scholar] [CrossRef]
- Kawatsu, M.; Fujioka, T.; Losio, S.; Tritto, I.; Nomura, K. (Trialkylsilyl-cyclo-pentadienyl)titanium(IV) dichloride complexes containing ketimide ligands, Cp′TiCl2(N = CtBu2) (Cp′ = Me3SiC5H4, Et3SiC5H4), as efficient catalysts for ethylene copolymerisation with norbornene and tetracyclododecene. Catal. Sci. Technol. 2025, 15, 2757–2765. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, M.; Zou, C.; Chen, C.L. Direct synthesis of polar-functionalized polyolefin elastomers. Angew. Chem. 2025, 137, e202423814. [Google Scholar] [CrossRef]
- Losio, S.; Boggioni, L.; Vignali, A.; Bertini, F.; Nishiyama, A.; Nomura, K.; Tritto, I. Poly(propene-co-norbornene)s with high molar masses and tunable norbornene contents and properties, obtained in high yields using ketimide-modified half-titanocene catalysts. Polym. Chem. 2025, 16, 3709–3719. [Google Scholar] [CrossRef]
- Stephan, D.W.; Stewart, J.C.; Guérin, F.; Spence, R.E.v.H.; Xu, W.; Harrison, D.G. Phosphinimides as a Steric Equivalent to Cyclopentadienyl: An Approach to Ethylene Polymerization Catalyst Design. Organometallics 1999, 18, 1116–1118. [Google Scholar] [CrossRef]
- Stephan, D.W.; Stewart, J.C.; Guérin, F.; Courtenay, S.; Kickham, J.; Hollink, E.; Beddie, C.; Hoskin, A.; Graham, T.; Wei, P.; et al. An Approach to Catalyst Design: Cyclopentadienyl-titanium phosphinimide complexes in ethylene polymerization. Organometallics 2003, 22, 1937–1947. [Google Scholar] [CrossRef]
- Stephan, D.W. The road to early-transition-metal phosphinimide olefin polymerization catalysts. Organometallics 2005, 24, 2548–2560. [Google Scholar] [CrossRef]
- Kretschmer, W.P.; Dijkhuis, C.; Meetsma, A.; Hessen, B.; Teuben, J.H. A highly efficient titanium-based olefin polymerisation catalyst with a monoanionic iminoimidazolidide π-donor ancillary ligand. Chem. Commun. 2002, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Tamm, M.; Randoll, S.; Herdtweck, E.; Kleigrewe, N.; Kehr, G.; Erker, G.; Rieger, B. Imidazolin-2-iminato titanium complexes: Synthesis, structure and use in ethylenepolymerization catalysis. Dalton Trans. 2006, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Ijpeij, E.G.; Coussens, B.; Zuideveld, M.A.; van Doremaele, G.H.J.; Mountford, P.; Lutz, M.; Spek, A.L. Synthesis, solid state and DFT structure and olefin polymerization capability of a unique base-free dimeric methyl titanium dication. Chem. Commun. 2010, 46, 3339–3341. [Google Scholar] [CrossRef]
- Jiang, Y.; Shimoyama, D.; Gao, J.; Nomura, K. Synthesis of ethylene copolymers with 2-allylphenol by using half-titanocene catalysts containing SiEt3-, SiiPr3-substituted phenoxide ligands, Cp*TiCl2(O-2,6-iPr2-4-SiR3-C6H2) (R = Et, iPr). Catal. Sci. Technol. 2023, 14, 3800–3806. [Google Scholar] [CrossRef]
- Nomura, K.; Oya, K.; Imanishi, Y. Ethylene/α-olefin copolymerization by various nonbridged (cyclopentadienyl)(aryloxy)titanium(IV) complexes—MAO catalyst system. J. Mol. Catal. A Chem. 2001, 174, 127–140. [Google Scholar] [CrossRef]
- Randall, J.C. A review of high resolution liquid 13carbon nuclear magnetic resonance characterizations of ethylene based polymers. J. Macromol. Sci. Part C 1989, 29, 201–317. [Google Scholar] [CrossRef]
- Kissin, Y.V. Isospecific Polymerization of Olefin with Heterogeneous Ziegler-Natta Catalysts; Springer: New York, NY, USA, 1985. [Google Scholar]
- Sahgal, A.; La, H.M.; Hayduk, W. Solubility of ethylene in several polar and non-polar solvents. Can. J. Chem. Eng. 1978, 56, 354–357. [Google Scholar] [CrossRef]
- Heiland, K.; Kaminsky, W. Comparison of zirconocene and hafnocene catalysts for the polymerization of ethylene and 1-butene. Makromol. Chem. 1992, 193, 601–610. [Google Scholar] [CrossRef]
- Suhm, J.; Schneider, M.J.; Mülhaupt, R. Temperature dependence of copolymerization parameters in ethene/1-octene copolymerization using homogeneous rac-Me2Si(2-MeBenz[e]Ind)2ZrCl2/MAO catalyst. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 735–740. [Google Scholar] [CrossRef]
- Kakinuki, K.; Fujiki, M.; Nomura, K. Copolymerization of ethylene with α-olefins containing various substituents catalyzed by half-titanocenes: Factors affecting the monomer reactivities. Macromolecules 2009, 42, 4585–4595. [Google Scholar] [CrossRef]
- Kitphaitun, S.; Yan, Q.; Nomura, K. Effect of para-substituents in ethylene copolymerizations with 1-decene, 1-dodecene, and with 2-methyl-1-pentene using phenoxide modified half-titanocenes-MAO catalyst systems. ChemistryOpen 2021, 10, 867–876. [Google Scholar] [CrossRef]
- Nomura, K.; Naga, N.; Miki, M.; Yanagi, K. Olefin polymerization by (cyclopentadienyl)(aryloxy)titanium(IV) complexes-cocatalyst systems. Macromolecules 1998, 31, 7588–7597. [Google Scholar] [CrossRef]
- Kitphaitun, S.; Fujimoto, T.; Ochi, Y.; Nomura, K. Effect of borate cocatalysts toward activity and comonomer incorporation in ethylene copolymerization by half-titanocene catalysts in methylcyclohexane. ACS Org. Inorg. Au 2022, 2, 386–391. [Google Scholar] [CrossRef]

| Run | Cat. | Temp /°C | Yield /mg | Activity /kg-PE/mol-Ti·h | Mn 2 ×10−6 | Mw/Mn 2 |
|---|---|---|---|---|---|---|
| 1 | 1 | 25 | 124 | 49,600 | 1.06 | 3.18 |
| 2 | 1 | 50 | 120 | 48,000 | 0.67 | 2.41 |
| 3 | 2 | 25 | 136 | 54,400 | ||
| 4 | 2 | 50 | 147 | 58,800 | ||
| 5 | 3 | 25 | 139 | 55,600 | 1.64 | 3.84 |
| 6 | 3 | 50 | 161 | 64,400 | 0.55 | 2.96 |
| 7 | 4 | 25 | 141 | 56,400 | 1.17 | 2.67 |
| 8 | 4 | 50 | 127 | 50,800 | 0.43 | 2.31 |
| 9 | 5 | 25 | 164 | 65,600 | 0.88 | 2.84 |
| 10 | 5 | 25 | 170 | 68,000 | 1.21 | 2.78 |
| 11 | 5 | 50 | 157 | 62,800 | 0.53 | 2.44 |
| 12 | 6 | 25 | 154 | 61,600 | 0.78 | 2.89 |
| 13 | 6 | 25 | 157 | 62,800 | 0.67 | 2.49 |
| 14 | 6 | 50 | 179 | 71,600 | ||
| 15 | 7 | 25 | 147 | 58,800 | 1.06 | 2.43 |
| 16 | 7 | 50 | 157 | 62,800 | 0.44 | 2.72 |
| Run | Cat. /μmol | Temp /C | MAO /mol | Yield /g | Activity /g-PE/mol-Ti·h | Mn 2 ×10−6 | Mw/ Mn 2 |
|---|---|---|---|---|---|---|---|
| 17 | 4 (0.005) | 25 | 2.0 | 40 | 48,000 | 1.23 | 2.98 |
| 18 | 4 (0.005) | 25 | 3.0 | 60 | 72,000 | ||
| 19 | 4 (0.005) | 25 | 4.0 | 73 | 87,600 | 0.65 | 2.18 |
| 20 | 5 (0.005) | 25 | 2.0 | 80 | 96,000 | 0.65 | 2.81 |
| 21 | 5 (0.005) | 25 | 3.0 | 108 | 129,600 | ||
| 22 | 5 (0.005) | 25 | 4.0 | 120 | 144,000 | 0.55 | 2.20 |
| 23 | 5 (0.015) | 50 | 3.0 | 157 | 628,000 | 0.53 | 2.44 |
| 24 | 5 (0.015) | 50 | 4.0 | 161 | 644,000 | 0.37 | 1.95 |
| 25 | 5 (0.015) | 50 | 5.0 | 175 | 700,000 | 0.29 | 1.75 |
| 26 | 5 (0.015) | 50 | 6.0 | 161 | 644,000 | 0.24 | 2.28 |
| 27 | 5 (0.015) | 80 | 2.0 | 107 | 428,000 | 0.28 | 2.85 |
| 28 | 5 (0.015) | 80 | 3.0 | 124 | 496,000 | 0.27 | 1.87 |
| 29 | 5 (0.015) | 80 | 4.0 | 132 | 528,000 | 0.23 | 2.14 |
| 30 | 5 (0.015) | 80 | 5.0 | 151 | 604,000 | 0.17 | 2.05 |
| 31 | 5 (0.015) | 80 | 6.0 | 144 | 576,000 | 0.15 | 2.22 |
| Run | Cat. | Temp /°C | MAO /mmol | Yield /mg | Activity 2 /kg-Polymer/mol-Ti·h | Mn 3 ×10−5 | Mw/ Mn 3 | Cont. 4 /mol% |
|---|---|---|---|---|---|---|---|---|
| 32 | 1 | 25 | 2.0 | 16 | 160,000 | 2.54 | 1.90 | |
| 33 | 1 | 50 | 2.0 | 89 | 890,000 | |||
| 34 | 2 | 25 | 2.0 | 85 | 850,000 | 1.50 | 1.54 | |
| 35 | 2 | 50 | 2.0 | 105 | 1,050,000 | |||
| 36 | 3 | 25 | 2.0 | 109 | 1,090,000 | 1.67 | 1.62 | |
| 37 | 3 | 50 | 2.0 | 155 | 1,550,000 | |||
| 38 | 4 | 25 | 2.0 | 207 | 2,070,000 | 1.95 | 1.86 | 19.8 |
| 39 | 4 | 50 | 2.0 | 60 | 600,000 | |||
| 40 | 4 | 50 | 3.5 | 72 | 720,000 | 1.59 | 2.03 | 25.2 |
| 41 | 5 | 25 | 2.0 | 340 | 3,400,000 | 2.29 | 1.72 | 18.9 |
| 42 5 | 5 | 25 | 2.0 | 108 | 4,320,000 | |||
| 43 | 5 | 50 | 2.0 | 298 | 2,980,000 | |||
| 44 | 5 | 50 | 3.5 | 350 | 3,500,000 | 1.46 | 2.07 | 25.9 |
| 45 | 5 | 50 | 3.5 | 355 | 3,550,000 | |||
| 46 | 6 | 25 | 2.0 | 310 | 3,100,000 | 1.99 | 1.84 | 19.8 |
| 47 | 6 | 50 | 2.0 | 40 | 400,000 | |||
| 48 | 6 | 50 | 2.5 | 75 | 750,000 | |||
| 49 | 6 | 50 | 3.0 | 309 | 3,090,000 | |||
| 50 | 6 | 50 | 3.5 | 328 | 3,280,000 | 1.31 | 2.01 | 21.8 |
| 51 | 6 | 50 | 4.0 | 225 | 2,250,000 | |||
| 52 | 7 | 25 | 2.0 | 156 | 1,560,000 | 1.96 | 1.84 | 18.3 |
| 53 | 7 | 50 | 2.0 | 90 | 900,000 | |||
| 54 | 7 | 50 | 3.5 | 120 | 1,200,000 | 1.68 | 2.07 | 23.2 |
| Run | Cat. | Temp. | DD 2 | Triad Sequence Distribution 3/% | Dyads 4/% | rE 5 | rD 5 | rE·rD 6 | rE·rD 7 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| /°C | /mol% | EEE | EED + DEE | DED | EDE | DDE + EDD | DDD | EE | ED + DE | DD | ||||||
| 38 | 4 | 25 | 19.8 | 46.4 | 27.5 | 6.2 | 15.3 | 3.6 | 0.9 | 60.1 | 37.1 | 2.7 | 3.34 | 0.14 | 0.48 | 0.48 |
| 40 | 4 | 50 | 25.2 | 48.1 | 20.6 | 6.0 | 19.6 | 5.6 | trace | 58.5 | 38.7 | 2.8 | 4.05 | 0.11 | 0.44 | 0.44 |
| 41 | 5 | 25 | 18.9 | 52.5 | 25.3 | 3.3 | 15.4 | 2.8 | 0.7 | 65.1 | 32.8 | 2.1 | 4.09 | 0.12 | 0.50 | 0.50 |
| 44 | 5 | 50 | 25.9 | 42.9 | 26.7 | 4.4 | 20.4 | 3.6 | 1.9 | 56.3 | 40.0 | 3.7 | 3.77 | 0.14 | 0.51 | 0.51 |
| 46 | 6 | 25 | 19.7 | 47.3 | 28.7 | 4.3 | 16.3 | 1.1 | 2.3 | 61.6 | 35.5 | 2.9 | 3.57 | 0.16 | 0.56 | 0.57 |
| 50 | 6 | 50 | 21.8 | 46.7 | 27.7 | 3.7 | 17.3 | 4.0 | 0.5 | 60.6 | 36.9 | 2.5 | 4.40 | 0.10 | 0.44 | 0.45 |
| 52 | 7 | 25 | 18.3 | 54.5 | 23.9 | 3.2 | 15.4 | 2.4 | 0.5 | 66.5 | 31.7 | 1.7 | 4.32 | 0.11 | 0.46 | 0.46 |
| 54 | 7 | 50 | 23.2 | 48.2 | 22.9 | 5.6 | 17.3 | 5.4 | 0.5 | 59.7 | 37.1 | 3.2 | 4.31 | 0.13 | 0.55 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Sun, W.-H.; Nomura, K. Synthesis of New Phenoxide-Modified Half-Titanocene Catalysts for Ethylene Polymerization. Catalysts 2025, 15, 840. https://doi.org/10.3390/catal15090840
Gao J, Sun W-H, Nomura K. Synthesis of New Phenoxide-Modified Half-Titanocene Catalysts for Ethylene Polymerization. Catalysts. 2025; 15(9):840. https://doi.org/10.3390/catal15090840
Chicago/Turabian StyleGao, Jiahao, Wen-Hua Sun, and Kotohiro Nomura. 2025. "Synthesis of New Phenoxide-Modified Half-Titanocene Catalysts for Ethylene Polymerization" Catalysts 15, no. 9: 840. https://doi.org/10.3390/catal15090840
APA StyleGao, J., Sun, W.-H., & Nomura, K. (2025). Synthesis of New Phenoxide-Modified Half-Titanocene Catalysts for Ethylene Polymerization. Catalysts, 15(9), 840. https://doi.org/10.3390/catal15090840









