1. Introduction
Alkenes are a vital component of the petrochemical industry, serving as fundamental building blocks for an array of chemical products. The utility of alkenes stems from their facile functionalization, which gives rise to a number of valued products [
1,
2,
3,
4]. Alkenes of various lengths are traditionally made by the oligomerization of ethylene derived from coal or petroleum [
5,
6,
7,
8,
9]. The non-renewable fossil fuel origin (e.g., coal or crude oil) of the majority of bulk scale olefins presents an environmental and resource issue [
10,
11]. This is further compounded by the energy requirements of the processes involved (e.g., cracking).
Fats, oils, and fatty acids appear as interesting potential precursors for sourcing commodity alkenes from renewable sources, given the fact that they contain largely unfunctionalized hydrocarbon chains of various lengths [
10,
11,
12]. Furthermore, significant amounts of fat-based waste materials (e.g., waste cooking oil) are being produced by human activities [
13]. These waste materials could present an even more sustainable source to produce olefins than pristine oils and fats produced directly for this purpose. This is further augmented by the fact that improper disposal and storage of waste fats and oils can present danger for health and the environment [
13].
While the direct conversion of fats and oils to olefins has received limited attention, the interesting possibility of obtaining alkenes from fatty acids via the dehydrodecarboxylation reaction was investigated using both chemical and biological approaches. Over the last 60 years, several approaches for converting fatty acids into olefins have been developed. These mainly utilize homogeneous transition metal complexes (Pd, Rh, Ir, Fe, Ru, and Ni). Palladium-catalyzed reactions have received most of the interest so far. Tsuji and co-workers first reported such reaction in 1968 when they presented the decarbonylation of aliphatic acyl chlorides to olefins by a Pd catalyst [
14]. The reaction proceeds at 200 °C and provides a mixture of positional double bond isomers.
While other precious metals (e.g., Rh, Ru) have been used for this reaction as well, it is important to note that the reaction can also be catalyzed by more common first row transition metals. Several examples of alkene formation from carboxylic acids catalyzed by Ni(COD)
2 (COD = 1,5-cyclooctadiene) have been reported [
15,
16]. Similarly, the possibility to carry out this reaction with Fe-based catalysts has been demonstrated as well [
17]. Unfortunately, high temperatures were required in both cases.
Biocatalytic approaches to the conversion of carboxylic acids to alkenes have been developed as well [
11,
12,
18]. The first report of decarboxylation of long chain aliphatic fatty acids to alpha-olefins was reported by Rude and coworkers in 2011 [
19]. An interesting enzymatic system capable of the conversion of carboxylic acids to alkenes is the P450 enzyme OleT
JE, which catalyzes the decarboxylation of various fatty acids.
Photocatalysis, which has seen a renaissance in recent years due to the development of photoredox processes, presents another possible approach to the conversion of carboxylic acids to alkenes [
20]. This approach has been developed only recently, with one of the first examples being published by Tlahuext-Aca and coworkers in 2018 [
21].
The examples described in the previous sections focus on the conversion of carboxylic (fatty) acids into olefins. The possibility of directly converting triglycerides into olefins is of interest as well. Interestingly, Nguyen and coworkers have reported a method combining these two steps into a single process in 2019 [
5]. Their approach utilizes lipase-based hydrolysis of triglycerides into fatty acids and glycerol and a photocatalytic dehydrodecarboxylation step utilizing acridine- and cobalt-based catalytic system. Various reports on the use of chemo-enzymatic cascades exist in the literature [
22,
23,
24,
25]. Furthermore, Cui and coworkers published a report describing a biophotocatalytic cascade used for acylation of N-heterocycles in 2022 [
26]. However, the report by Nguyen and coworkers was the first describing this cascade to produce alkenes from bio-based oils.
Fats and oils constitute significant portion of waste generated by human activity [
27]. Thus, these materials are available on a large and meaningful scale. However, the quality of these materials, for example the presence of significant amounts of free fatty acids alongside triglycerides, complicates their valorization [
27,
28]. One of the most dominant fat waste materials is used cooking oil, which is produced on an annual scale reaching 190 million metric tons [
13,
29]. Several approaches to the valorization of used cooking oil are already being investigated and implemented. The main among these is its conversion to biodiesel, with conversions to urethanes, lubricants, or bioplastics being other less developed options [
13]. Direct conversion of used cooking oil to olefins has received minimal attention so far. Therefore, in this work, we focused on further developing the above-mentioned method of converting triglycerides to olefins by applying it to used cooking oil, a common fat-based waste [
5]. In addition, we explored the possibility of replacing dichloromethane used in the original procedure with a more environmentally friendly solvent [
30]. Finally, we also explored the possibility of reusing a part of the catalytic system to improve the effectivity of the reaction.
3. Discussion
We have undertaken this study to test the possibility of expanding the utility of a previously reported procedure for the conversion of fats and oils into olefins [
5]. The work has pursued two main ideas. Firstly, we aimed to investigate the possibility of carrying out this transformation with waste material rather than clean oil. The reason for this was the fact that fat-based waste materials are being produced on a significant scale, which makes their conversion into new useful chemicals desirable from the point of sustainability. We specifically chose used cooking oil as it is one of the most abundant fat-based wastes [
13]. Secondly, we tried to determine whether it would be possible to replace dichloromethane, which was used as the main organic solvent in the original report, with an eco-friendlier solvent [
30].
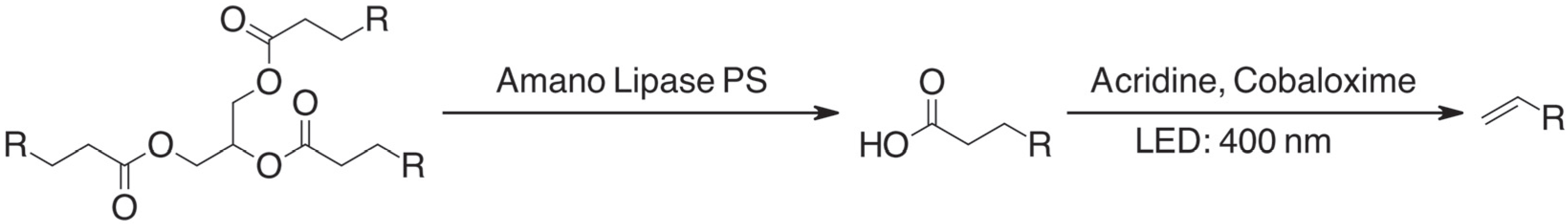
As mentioned above (
Scheme 1), the conversion of oils and fats in the explored reaction proceeds in two distinct steps. The first step is the enzymatic hydrolysis of triglycerides into glycerol and fatty acids, while the second step is the photocatalytic dehydrodecarboxylation of the fatty acids into the olefin products catalyzed by acridine and cobaloxime. The first step is a typical enzymatic hydrolysis step, which is mechanistically unremarkable. On the other hand, the photocatalytic dehydrodecarboxylation is less common. Therefore, we will briefly discuss its mechanism. A simplified diagram of the mechanism, which is based on the findings of Nguyen and coworkers, can be found in
Scheme S1 [
5]. The mechanism contains distinct acridine and cobaloxime catalytic cycles. The reaction starts in the acridine catalytic cycle with the formation of a hydrogen-bonded complex of acridine (I) and the carboxylic (fatty) acid (II). This complex (III) then undergoes light-promoted decomposition in which the acridine abstracts the shared hydrogen atom and forms a radical species (IV). The carboxylic acid is initially turned into carboxylic acid radical, which spontaneously decomposes into carbon dioxide (V) and an alkyl radical (VI). The hydrogen atom is subsequently abstracted from the alkyl radical by a Co
II species (VII) within the cobaloxime catalytic cycle. This results in the formation of the alkene product and (VIII) a Co
III hydride intermediate (IX). The acridine catalytic cycle can be closed via different pathways, which can either involve a carbocation (X) or a dihydro acridine species (XI). These steps in the acridine regeneration pathways are interconnected with the steps closing the cobaloxime (XII) catalytic cycle and result in the formation of molecular hydrogen (XIII).
Initial attempts at performing the conversion of waste cooking oil, which were carried out with a slight modification of the published procedure while still using dichloromethane as solvent, demonstrated the possibility to convert waste cooking oil into the desired olefin products.
Subsequently, we tested the possibility of replacing dichloromethane with an eco-friendlier solvent. Two candidate solvents, tert-butyl methyl ether and 2-methyl tetrahydrofuran, were chosen based on suggestions from the literature. These experiments have shown that the reaction could be carried out using tert-butyl methyl ether but not 2-methyl tetrahydrofuran as no product formation was observed when the latter solvent was used. Gratifyingly, better product yield was observed using tert-butyl methyl ether (41.2% (±2.6%)) than when dichloromethane was used (36.1% (±6.1%)).
The observation that the reaction mixtures, when using either dichloromethane or
tert-butyl methyl ether, separate into two phases suggested the possibility of recycling of the catalytic system, which was then explored. The initial focus was on recycling of the enzyme catalyst, which remains in the aqueous portion of the reaction mixture, which is not processed during the isolation and purification of the products. The initial recycling experiments were performed using both dichloromethane and
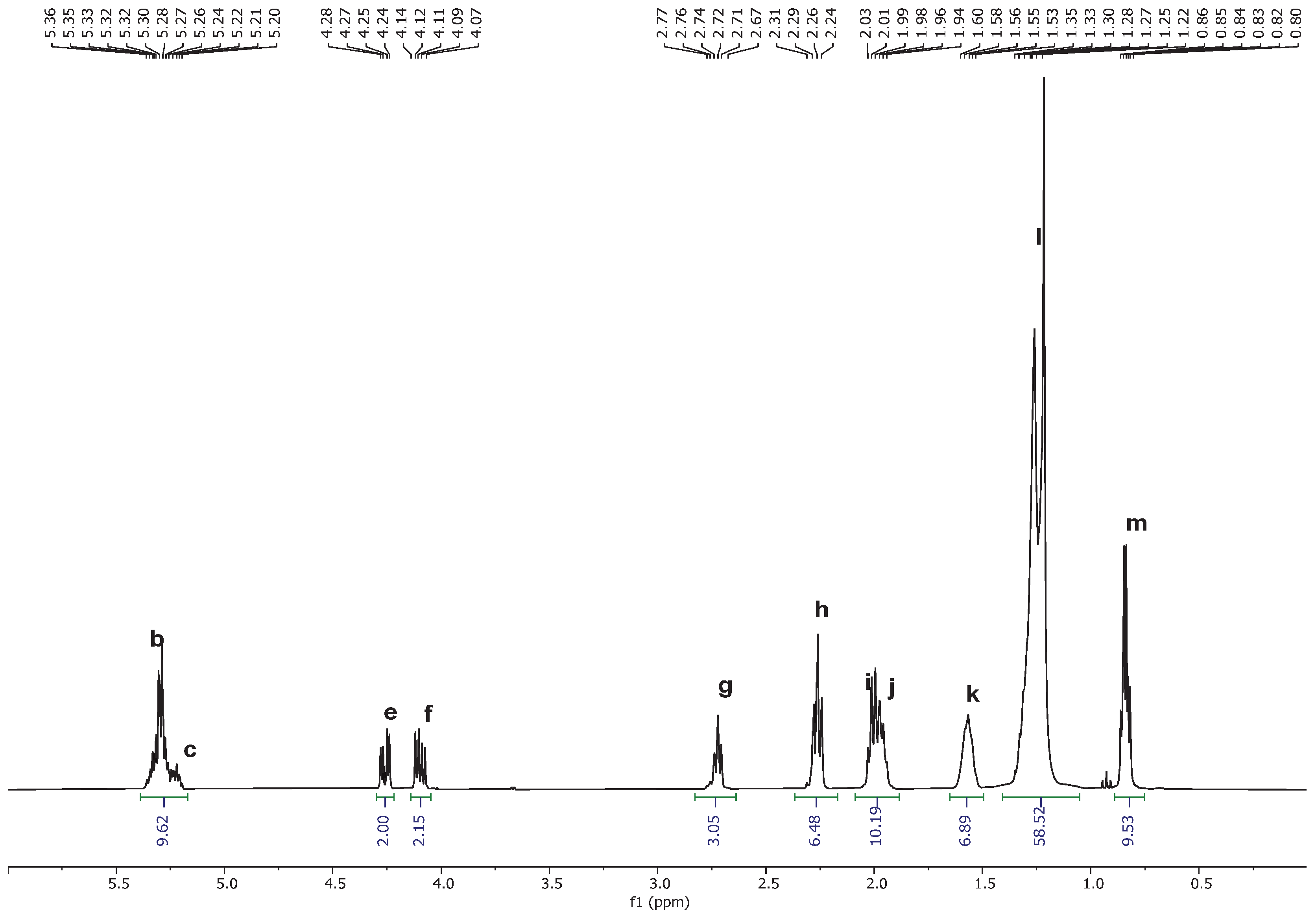
tert-butyl methyl ether as solvents. The results have shown that when dichloromethane is used as the principal organic solvent, substantial decrease in the performance of the reaction is already observed in the first repeat of the reaction. Investigation of the reaction mixture by
1H NMR (
Figure S4) suggests that this is due to the loss of the activity of the enzyme. On the other hand, reaction performance is markedly better in the first repeat of the reaction with recycled enzyme when
tert-butyl methyl ether is used as a solvent, albeit with some activity lost with respect to the initial run of the reaction. These results further support the idea that
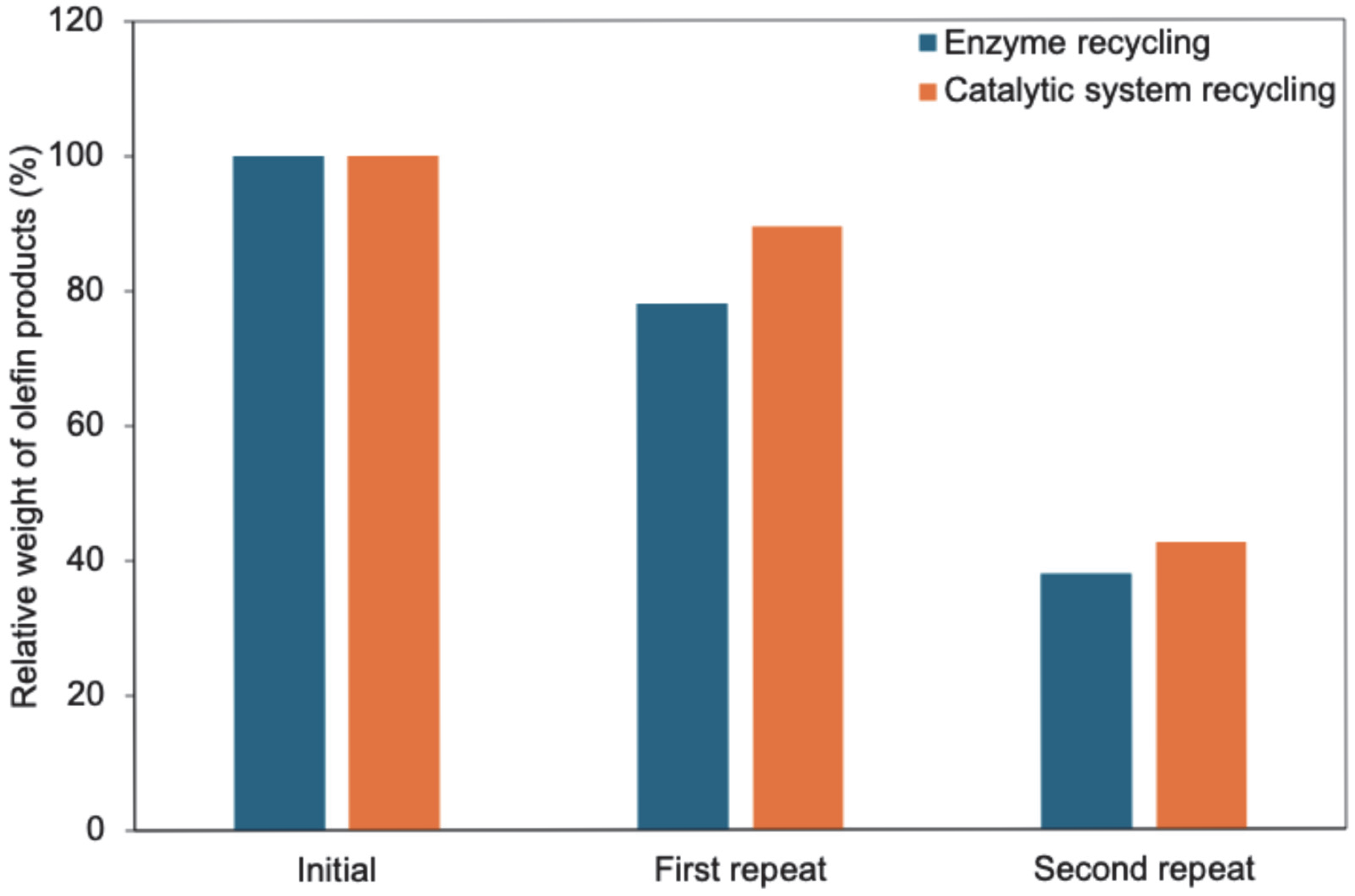
tert-butyl methyl ether can be used as a replacement solvent for dichloromentane. To investigate further the possibility of recycling of the catalytic system, we decided to also attempt to recycle the photocatalysts used in the reaction, which we believed could be recovered during the column chromatography purification of the olefin products. A comparison between recycling the enzyme catalyst and the whole catalytic system was performed in this experiment. A modest drop in activity was observed in both cases during the first reaction repeat. This was, unfortunately, followed by a steeper activity decline in the second reaction repeat. This was attributed to loss of enzyme activity in the case of enzyme recycling only, while a combination of both enzyme activity decreases and degradation of effectivity of the photocatalysts was observed in the case of recycling of the complete catalytic system. These results indicate that there is potential for recycling the catalytic system. However, further investigation is needed to achieve better outcomes.
As mentioned in previous sections, one of our aims was to find a greener alternative to dichloromethane as a solvent in this reaction. The importance of this goal is clearly demonstrated by the recent expansion of bans on dichloromethane use instituted by the EPA in the USA [
33]. Furthermore, the drive towards dichloromethane replacement is also in line with principle five (safer solvents and auxiliaries) of the twelve principles of green chemistry [
34]. Of the two alternative solvents chosen in this work, 2-methyltetrahydrofuran and
tert-butyl methyl ether, the former was a better alternative as it can be produced from cellulose [
35]. Unfortunately, this solvent proved to be unsuitable for the reaction.
Tert-butyl methyl ether still presents a better alternative to dichloromethane. This is demonstrated by the fact that in the CHEM21 solvent guide its environmental score of
Tert-butyl methyl ether is five, with dichloromethane being worse in this aspect with a score of seven [
36]. The fact that
tert-butyl methyl ether represents a better solvent choice is also demonstrated by the facts that the company Pfizer considers it a usable alternative to dichloromethane or that its use in industrial settings in Germany does not carry a special administrative burden [
30,
37]. However, the results of this work demonstrate that the replacement of dichloromethane with
tert-butyl methyl ether has an additional benefit from the point of view of sustainability. The improved performance in terms of recyclability, even if now limited to a single recycling step, does make the reaction in
tert-butyl methyl ether more efficient. This can be expressed in improvement in the process mass intensity (PMI) parameter as defined in the literature [
34]. Thus, in the case of a single recycling of the enzyme catalyst, the PMI value for the reaction improves from 184 when carried out in dichloromethane, to 72, when carried out in
tert-butyl methyl ether. Finally, the results obtained in this work with
tert-butyl methyl ether as a solvent raise the possibility that more environmentally friendly ether solvents, such as cyclopentyl methyl ether or
tert-butyl ethyl ether, could be used in this reaction [
35,
36].
Subsequently, we carried out the reaction on a scale that was three times that of the previous experiments. The desired product was observed. However, the yield was decreased (23.4% (±1.8%)) in comparison to the previous reactions carried out on a smaller scale. Therefore, we investigated the reaction progress using 1H-NMR, which indicated that the scaled-up reaction will require an extended reaction time, which resulted in improvement of the yield (30.6% (±3.8%)), albeit to values still lower than those observed in the smaller scale experiments. These experiments have shown that the enzymatic hydrolysis of the starting triglycerides runs to completion within the first 48 h of the reaction, while the photocatalytic conversion of the intermediate fatty acids proceeds at a slower rate and does not reach completion even after 240 h. These experiments demonstrated that it is the photochemical conversion of the intermediate fatty acids into olefins that is the bottleneck of this reaction. The discrepancy between the yields of the small scale and scaled-up reactions, which results from issues in the second photochemical step, might be due to the fact that the same reactor (20 mL glass vial) has been used for both scales of the reaction. This might be of importance as the solvent volumes were scaled with the same factor as the reagents. Thus, the heights of the individual reaction phases within the reactor are different. Furthermore, as identical stirrers and stirring rates were used for both scales of the experiment, the extent of intermixing of the reaction phases for the two scales of the reaction is likely different. This might affect factors such as mass transfer between the phases or even light propagation within the reactor, which in turn could result in the observed negative impact on the yields in the scaled-up reaction. Thus, there is a need to further improve the photocatalytic step of the reaction in the future, where one of the avenues should be the optimization of the photoreactor.
Finally, to further demonstrate the utility of this process we carried out the scaled-up reaction with waste pork fat as an alternative starting material. The desired olefin products were observed in this reaction as well (24.0% (±2.9%)). The products from the scaled-up conversion of both used cooking oil and waste pork products were investigated using GC-MS to provide insights into the identity of the major olefin products in the mixture. This analysis has shown that the product from used cooking oil mainly contains (8Z, 11Z)-1,8,11-heptadecatriene, (8Z)-1,8-heptadecadiene, and 1-pentadecene. On the other hand, the product from waste pork oil was shown to mainly contain 1-pentadecene, 1-heptadecene, and (8Z, 11Z)-1,8,11-heptadecatriene. These key olefin products are in line with the expectations based on the fatty acid compositions of the starting waste materials.
4. Materials and Methods
4.1. Material and Chemicals
Acridine and 2-methyltetrahydrofuran were obtained from Thermo Fisher Scientific Incorporated, Waltham, MA, USA. Acetonitrile, dichloromethane, di-Potassium hydrogen phosphate trihydrate potassium dihydrogen phosphate ACS-ISO, and tert-Butyl methyl ether were sourced from CARLO ERBA Reagents (DASIT Group, Cornaredo, Italy). Amano lipase PS from Burkholderia cepacia and Chloro(pyridine)bis(dimethylglyoximato)cobalt (III) were procured from Sigma-Aldrich Corporation, Burlington, USA. Chloroform-D was acquired from Cambridge Isotopes Laboratories Incorporated, Tewksbury, USA. Ethyl acetate and Hexane were supplied by RCI Labscan Limited, Bangkok, Thailand. Silica gel was received from Supelco Incorporated, Bellefonte, USA. The waste oil materials were obtained from domestic use. 1H-NMR spectra were acquired on a Bruker Avance-400 spectrometer (Bruker, Billerica, MA, USA) sing CDCl3 as a solvent. Used cooking oil, a mixture of soybean oil, sunflower oil, and palm oil, was obtained from domestic consumption. Waste pork fat was obtained from domestic frying of pork belly.
4.2. GC-MS Analysis
The composition of the product olefin mixture was analyzed using an Agilent 8890GC/7000DGC/MS Triple Quad instrument (Agilent Technologies, Santa Clara, CA, USA) equipped with the HP-5ms column (30 m × 250 μm × 0.25 μm). The samples were injected at 230 °C. The carrier gas (He) flowrate was 1 mL/min. The initial oven temperature was 50 °C for 1 min, then increased to 100 °C at 15 °C/min, then increased to 210 °C at 10 °C/min and held for 1 min, then increased to 310 °C at 5 °C and held for 8 min. Samples were analyzed in full-scan mode (m/z 100–500).
4.3. Initial Procedure for Biophotocatalytic Conversion of Used Cooking Oil
Used cooking oil (90 mg), acridine (12 mg), and chloro(pyridine)bis(dimethylglyoximato)cobalt (III) (12 mg), were mixed with dichloromethane (1.5 mL) and acetonitrile (0.15 mL) in a clear glass vial. Subsequently, Amano lipase PS from Burkholderia cepacia (200 mg) was dissolved in phosphate buffer (1 mL, pH 7.0) and added to the reaction mixture. The reaction mixture was stirred vigorously for 60 h under the irradiation of two UV lights (LED Light, 395 nm, 50 W, Dreamcast light, Samut Prakan, Thailand). Subsequently, the organic and aqueous phase of the reaction mixture were separated, and the aqueous phase was extracted with dichloromethane (2 × 2 mL). The combined organic fractions were evaporated to dryness. Purified olefin products were obtained after column purification on silica using hexane as the mobile phase. The crude and purified products were analyzed by 1H-NMR in chloroform-d.
4.4. Procedure for Biophotocatalytic Conversion of Used Cooking Oil with Extended Reaction Time
Used cooking oil (90 mg), acridine (12 mg), and chloro(pyridine)bis(dimethylglyoximato)cobalt (III) (12 mg), were mixed with dichloromethane (1.5 mL) and acetonitrile (0.15 mL) in a clear glass vial. Subsequently, Amano lipase PS from Burkholderia cepacia (200 mg) was dissolved in phosphate buffer (1 mL, pH 7.0) and added to the reaction mixture. The reaction mixture was stirred vigorously for 144 h under the irradiation of two UV lights (LED Light, 395 nm, 50 W, Dreamcast light, Samut Prakan, Thailand). Subsequently, the organic and aqueous phase of the reaction mixture were separated, and the aqueous phase was extracted with dichloromethane (2 × 2 mL). The combined organic fractions were evaporated to dryness. Purified olefin products were obtained after column purification on silica using hexane as the mobile phase. The crude and purified products were analyzed by 1H-NMR in chloroform-d.
4.5. Procedure for Selection of Alternative Solvent for Biophotocatalytic Conversion of Used Cooking Oil
Used cooking oil (90 mg), acridine (12 mg), and chloro(pyridine)bis(dimethylglyoximato)cobalt (III) (12 mg), were mixed with 2-methyltetrahydrofuran (or tert-butyl methyl ether) (1.5 mL) and acetonitrile (0.15 mL) in a clear glass vial. Subsequently, Amano lipase PS from Burkholderia cepacia (200 mg) was dissolved in phosphate buffer (1 mL, pH 7.0) and added to the reaction mixture. The reaction mixture was stirred vigorously for 144 h under the irradiation of two UV lights (LED Light, 395 nm, 50 W, Dreamcast light, Samut Prakan, Thailand). Subsequently, the organic and aqueous phase of the reaction mixture were separated, and the aqueous phase was extracted with the solvent originally used in the reaction mixture (2 × 2 mL). The combined organic fractions were evaporated to dryness. Purified olefin products were obtained after column purification on silica using hexane as the mobile phase. The crude and purified products were analyzed by 1H-NMR in chloroform-d.
4.6. Procedure for Enzyme Recycling in Biophotocatalytic Conversion of Used Cooking
Used cooking oil (90 mg), acridine (12 mg), and chloro(pyridine)bis(dimethylglyoximato)cobalt (III) (12 mg), were mixed with dichloromethane (or tert-butyl methyl ether) (1.5 mL) and acetonitrile (0.15 mL) in a clear glass vial. Subsequently, Amano lipase PS from Burkholderia cepacia (200 mg) was dissolved in phosphate buffer (1 mL, pH 7.0) and added to the reaction mixture. The reaction mixture was stirred vigorously for 144 h under the irradiation of two UV lights (LED Light, 395 nm, 50 W, Dreamcast light, Samut Prakan, Thailand). Subsequently, the organic and aqueous phase of the reaction mixture were separated, and the aqueous phase was extracted with the organic solvent originally used for the reaction (2 × 2 mL). The combined organic fractions were evaporated to dryness. Purified olefin products were obtained after column purification on silica using hexane as the mobile phase. A repeat of the reaction was set up using the recovered aqueous phase from the first run of the reaction to which used cooking oil (90 mg), acridine (12 mg), chloro(pyridine)bis(dimethylglyoximato)cobalt (III) (12 mg), dichloromethane (or tert-butyl methyl ether) (1.5 mL), and acetonitrile (0.15 mL) were added. The repeat reaction was conducted, worked up, and analyzed in a manner identical to the initial run. The crude and purified products were analyzed by 1H-NMR in chloroform-d.
4.7. Procedure for Enzyme Recycling in Biophotocatalytic Conversion of Used Cooking Oil
Used cooking oil (90 mg), acridine (12 mg), and chloro(pyridine)bis(dimethylglyoximato)cobalt (III) (12 mg), were mixed or tert-butyl methyl ether (1.5 mL) and acetonitrile (0.15 mL) in a clear glass vial. Subsequently, Amano lipase PS from Burkholderia cepacia (200 mg) was dissolved in phosphate buffer (1 mL, pH 7.0) and added to the reaction mixture. The reaction mixture was stirred vigorously for 144 h under the irradiation of two UV lights (LED Light, 395 nm, 50 W, Dreamcast light, Samut Prakan, Thailand). Subsequently, the organic and aqueous phase of the reaction mixture were separated, and the aqueous phase was extracted with the organic solvent originally used for the reaction (2 × 2 mL). The combined organic fractions were evaporated to dryness. Purified olefin products were obtained after column purification on silica using hexane as the mobile phase. The other components of the crude reaction mixture were obtained from the column using a mixture of ethyl acetate (70% v/v) and methanol (30% v/v). A repeat of the reaction was set up using the recovered aqueous phase from the first run of the reaction, which were added to a solution of the materials obtained from the column using the polar mobile phase dissolved in tert-butyl methyl ether (1.5 mL), and acetonitrile (0.15 mL). The repeat reaction was conducted, worked up, and analyzed in a manner identical to the initial run. The crude and purified products were analyzed by 1H-NMR in chloroform-d.
4.8. Procedure for Scaled Up Biophotocatalytic Conversion of Used Cooking Oil
Used cooking oil (270 mg), acridine (36 mg), and chloro(pyridine)bis(dimethylglyoximato)cobalt (III) (36 mg), were mixed with tert-butyl methyl ether (4.5 mL) and acetonitrile (0.45 mL) in a clear glass vial. Subsequently, Amano lipase PS from Burkholderia cepacia (600 mg) was dissolved in phosphate buffer (3 mL, pH 7.0) and added to the reaction mixture. The reaction mixture was stirred vigorously for 144 h under the irradiation of two UV lights (LED Light, 395 nm, 50 W, Dreamcast light, Samut Prakan, Thailand). Subsequently, the organic and aqueous phase of the reaction mixture were separated, and the aqueous phase was extracted with tert-butyl methyl ether (2 × 5 mL). The combined organic fractions were evaporated to dryness. Purified olefin products were obtained after column purification on silica using hexane as the mobile phase. The crude and purified products were analyzed by 1H-NMR in chloroform-d.
4.9. Procedure for Monitoring Reaction Progress of Scaled Up Biophotocatalytic Conversion of Fatty Acid Waste Materials
Used cooking oil (270 mg), acridine (36 mg), and chloro(pyridine)bis(dimethylglyoximato)cobalt (III) (36 mg), were mixed with
tert-butyl methyl ether (4.5 mL) and acetonitrile (0.45 mL) in a clear glass vial. Subsequently, Amano lipase PS from
Burkholderia cepacia (600 mg) was dissolved in phosphate buffer (3 mL, pH 7.0) and added to the reaction mixture. The reaction mixture was stirred vigorously under the irradiation of two UV lights (LED Light, 395 nm, 50 W, Dreamcast light, Samut Prakan, Thailand). Samples (1 mL) of the organic portion of the reaction mixture were collected every 48 h. The samples were evaporated to dryness, analyzed by
1H-NMR in chloroform-d. The percentage of reaction progress was calculated using Equation (1).
where
,
,
and
are the integral of the signal at 5.80 ppm in the reaction at a given time
t, the integral of the signal at 5.35 ppm at a given reaction time
t, the integral of the signal at 5.80 ppm in the product, and the integral of the signal at 5.35 ppm in the product, respectively.
Subsequently, the material used for the analysis was recovered, dissolved in tert-butyl methyl ether (1 mL), and returned to the reaction mixture.
Subsequently, the organic and aqueous phase of the reaction mixture were separated, and the aqueous phase was extracted with tert-butyl methyl ether (2 × 5 mL). The combined organic fractions were evaporated to dryness. Purified olefin products were obtained after column purification on silica using hexane as the mobile phase. The crude and purified products were analyzed by 1H-NMR in chloroform-d.
4.10. Procedure for Scaled Up Biophotocatalytic Conversion of Waste Pork Fat
Waste pork fat (270 mg), acridine (36 mg), and chloro(pyridine)bis(dimethylglyoximato)cobalt (III) (36 mg), were mixed with tert-butyl methyl ether (4.5 mL) and acetonitrile (0.45 mL) in a clear glass vial. Subsequently, Amano lipase PS from Burkholderia cepacia (600 mg) was dissolved in phosphate buffer (3 mL, pH 7.0) and added to the reaction mixture. The reaction mixture was stirred vigorously for 240 h under the irradiation of two UV lights (LED Light, 395 nm, 50 W, Dreamcast light, Samut Prakan, Thailand). Subsequently, the organic and aqueous phase of the reaction mixture were separated, and the aqueous phase was extracted with tert-butyl methyl ether (2 × 5 mL). The combined organic fractions were evaporated to dryness. Purified olefin products were obtained after column purification on silica using hexane as the mobile phase. The purified products were analyzed by 1H-NMR in chloroform-d.