Abstract
In this work, a theoretical study of the isoprene polymerization process was carried out using a cis-stereospecific neodymium Ziegler–Natta catalyst. The results obtained allowed us to conclude that the main reasons for the formation of highly stereoregular cis-1,4-polyisoprene are the lower activation energy of the monomer addition process in the cis-conformation (~50 kJ/mol) and the low probability of anti-syn-isomerization of the terminal unit of the growing macrochain. It was shown that for asymmetric isoprene, the most probable is the formation of macromolecules in which the monomer units are added according to the “tail-to-head” type.
1. Introduction
Industrial production of synthetic rubbers (SR) is of great importance, since without these materials the development of the automobile industry, tire production and many branches of modern industry is impossible. In 2022, global SR production reached 14.9 million tons [1], while the share of synthetic isoprene rubbers was almost 5% of the market [2].
A milestone for the global polymer industry was the creation of methods for obtaining stereoregular synthetic rubbers using Ziegler–Natta catalysts based on titanium compounds [3,4,5,6,7]. Such polyisoprenes already contained more than 90% cis-1,4-links, and the physical and mechanical properties of rubbers based on them approached the indicators of products made of natural rubber. However, the developed technologies had their own problems, some of which remain unresolved to this day. First of all, these include the occurrence of side processes that lead to the formation of toxic oligomers, branched macromolecules and insoluble gel fraction in the commercial product. Also, the residual content of titanium compounds in rubber—a metal of variable valence—leads to accelerated aging of rubber and products based on it. Increasing requirements on the performance characteristics of tires and rubber products are accompanied by intensified research aimed at obtaining SR with improved indicators. Over the past three decades, technologies for obtaining synthetic polyisoprenes using lanthanide, primarily neodymium-containing catalytic systems, have been actively developing. This is due to the possibility of solving most of the problems voiced: eliminating the formation of oligomers, forming a linear structure of macromolecules, obtaining not only highly stereo-, but also regio-regular polymers. An additional advantage of catalysts based on neodymium compounds is its stable oxidation state and the non-participation of the remains of the catalytic complex in rubber in oxidation–reduction reactions that contribute to the aging of the polymer [8,9,10,11,12].
The development of new effective catalysts is possible only with a deep understanding of the structure of active centers and all stages of the polymerization process. Despite the large number of published works, research in this area remains relevant today [13,14,15]. Modern methods of quantum chemistry based on calculations of structural and thermodynamic parameters allow a more detailed study of the polymerization process. The results we have obtained are consistent with the known experimental data and also deepen our understanding of the processes occurring on these catalysts. Correlating the calculated results with experimentally obtained data makes it possible to predict the activity and stereospecificity of new catalytic systems with a greater degree of reliability.
In the previous work [16], we studied the process of cis-1,4-stereospecific polymerization of 1,3-butadiene. The obtained results allowed us to clarify the mechanism of the 1,3-butadiene polymerization by the neodymium-based Ziegler–Natta system. The aim of the present work is to investigate in detail the elementary stages of the polymerization process of the asymmetric monomer—isoprene—in order to explain the reasons for both the high cis-stereospecificity and the outstanding regioselectivity of the neodymium-based Ziegler–Natta system.
2. Results and Discussion
The structure of the active center (AS) in the neodymium Ziegler–Natta catalyst used in this study is shown in Figure 1. The method for selecting a fragment for calculation within the framework of QM1 is completely analogous to our previous works and is described in detail in [16].
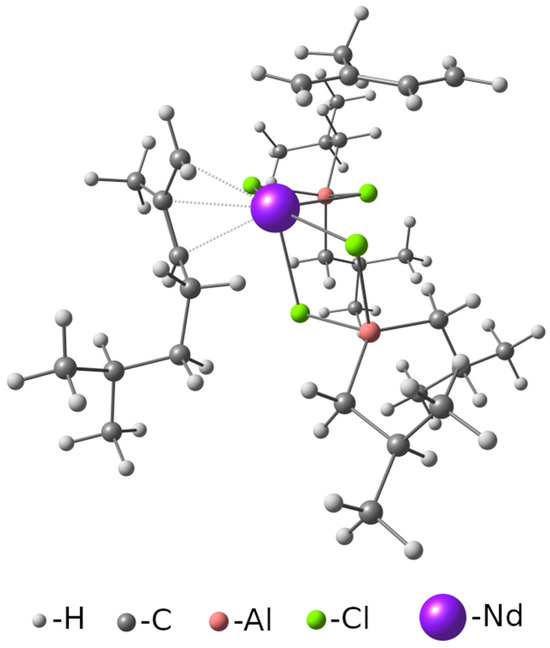
Figure 1.
Optimized structure of the active site of the cis-stereospecific neodymium Ziegler–Natta catalyst in which isoprene is bound to the coordinating site in the trans configuration.
2.1. Coordination Stage
It is known that conjugated dienes can exist in cis and trans conformations. In order to determine the relative stability of the monomer conformations in the volume of the reaction mixture, we will consider the activation energies of the trans–cis transition of isoprene:
where the transition state is associated with the rotation of the CH2 group around the CH–CH bond. For isoprene, the Gibbs free energy of activation of the forward reaction is ΔG≠→ = 26.2 kJ/mol, of the reverse reaction ΔG≠← = 14.2 kJ/mol, and for the entire process ΔG0298 = 12.0 kJ/mol. It is evident that for isoprene in the free state, the trans form is more stable, which is generally characteristic of dienes [17].
isoprene-trans (T) = transition state = isoprene-cis (C)
Previously in [16] we studied in detail the process of the 1,3-butadiene polymerization. The main difference between isoprene and 1,3-butadiene is that it is asymmetrical with respect to the CH-CH bond. In this regard, during the coordination binding of 1,3-butadiene with AS, there are two forms of complexes, which are determined by the cis- or trans-conformation of the monomer. For asymmetric isoprene, four combinations are possible, which are determined by both the conformation and the orientation of the monomer. While there are generally accepted rules for designating cis- and trans-isomers, designating orientations causes some complexity. For this reason, we will begin designating orientations by considering structures where the diene has already been built into the growing polymer chain. The orientation when the methyl group in the terminal link of the macromolecule is closer to the active center we will designate as CA and TA, and when it is closer to the growing polymer chain—CP and TP (Figure 2). We will designate the orientation of isoprene on the active center accordingly.
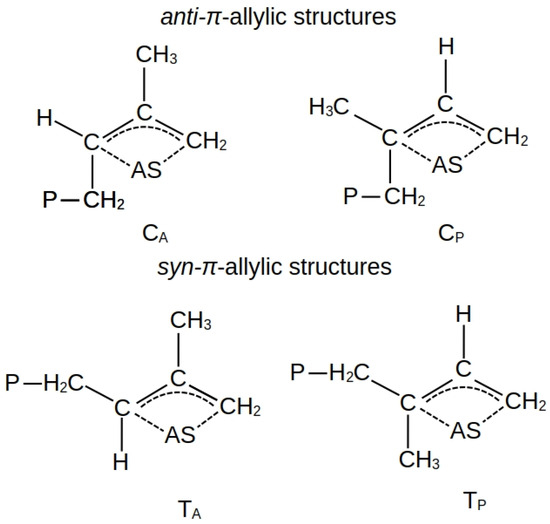
Figure 2.
Structures and proposed designs of the end link of a growing polymer chain with the introduction of isoprene, where P is i-C4H9 or the growing polymer chain.
Figure 3 and Table 1 show the scheme and energy parameters of the processes of orientation and trans–cis transformation of the isoprene molecule at the active center. Here it should be immediately noted that the obvious fact is that as a result of the elementary act of insertion of the monomer from the TA orientation, the terminal link of the growing polymer chain can be formed only in the CA orientation, and from the TP – CP.
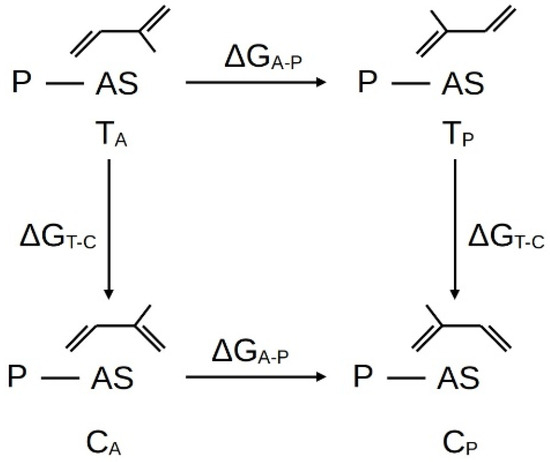
Figure 3.
Scheme of the trans–cis transition and reorientation of the isoprene interacting with the active center, where: P—i-C4H9, ΔGT-C—Gibbs free energy of the trans–cis reaction of diene transformation, ΔGA-P—Gibbs free energy of the diene reorientation reaction.

Table 1.
Gibbs free energy (kJ/mol) of the activation and reaction for trans–cis transition and of the reorientation of the isoprene interacting with the active center.
Table 1 shows that the most stable orientations for isoprene are TA and CP. In this case, the process of isoprene polymerization, unlike 1,3-butadiene, is supplemented by one more stage and can be described by the following elementary acts: coordination; reorientation; trans–cis isomerization; insertion (Figure 4).

Figure 4.
Energy diagram describing the incorporation of isoprene into the polymer chain, where: ΔGA-P is the reorientation Gibbs free energy, ΔG≠T-C is the activation energy of the trans–cis conversion of the isoprene, ΔGT-C is the Gibbs free energy of the trans–cis conversion of the isoprene, ΔG≠in is the activation energy of the incorporation of the isoprene into the polymer chain, ΔG≠tot is the total activation energy of the incorporation of the isoprene into the polymer chain, ΔGR is the Gibbs free energy of the process of incorporating the isoprene into the polymer chain.
As can be seen from Table 1, the energy of trans–cis transition in the TA and TP monomer orientations differs significantly. The same effect is observed at subsequent stages of the polymerization process studied in this work (see Tables 3–5 below). The reasons for these differences are clearly shown in Figure 3 and consist in the fact that during the trans–cis transition in TA both the -CH2 and -CH3 groups rotate, and in the case of TP only the -CH2 group. This is confirmed in particular by the fact that the activation energy of the trans–cis transition for the TP orientation of monomer practically coincides with that for the monomer in the volume of the reaction mixture, which was given above. If in both cases the -CH2 group rotates, then in the case of TA both end -CH2 groups of the monomer will be directed in the direction opposite to the growing polymer, and to begin the process of monomer incorporation into the polymer chain additional rotation of the monomer will be required, which will lead to additional energy costs.
2.2. Initiation Stage
Next, the activation parameters of the initiating stage of the isoprene polymerization process—the introduction of the first monomer molecule were analyzed. The activation parameters of this process, obtained according to the scheme shown in Figure 4, are presented in Table 2.

Table 2.
Results of calculations of energy and activation parameters (kJ/mol) of the initiating stage of the polymerization process.
The results obtained show that the processes of monomer insertion in the CA and TP orientations are characterized by minimal activation energies. However, in our opinion, the most probable is the formation of an anti-π-allyl structure of the terminal unit of the growing polymer chain from the monomer in the CA form, since, as shown above, the coordination of isoprene at the active center is more favorable in the TA form, and the transition of TA to TP is less probable than the transition of TA to CA (ΔGA-P = 8.3 kJ/mol, ΔG1 = ΔG(TA − CA) = 6.9 kJ/mol). Therefore, the most probable at the initiating stage should be considered the formation of an anti-π-allyl structure, in which the methyl group of the isoprene unit is closer to the active center (CA orientation). However, we began our investigation of the regular stage of polymerization by studying the interaction of the monomer with all possible orientations of the end units that can be formed at the initiating stage.
2.3. Polymer Chain Growth Stage
Based on the fact that 4 different structures of the polymer end link can be formed at the initiation stage, we decided to check the possibility of adding the next monomer molecule to each of these structures. Therefore, when studying the next stage, we considered the elementary acts corresponding to the addition of the second diene molecule to the growing polymer chain in all possible orientations. Taking into account the scheme (Figure 3) and the structures (Figure 2), it is clear that 16 different products can be formed for isoprene. The results of calculating the energy and kinetic parameters of the polymer chain growth stage for the second isoprene molecule according to the proposed scheme are presented in Table 3.

Table 3.
Results of calculations of energy and activation parameters (kJ/mol) of the growth stage of the polymerization process for the second diene molecule.
Taking into account the scheme (Figure 3) and the structures (Figure 2), it is clear that 16 different products can be formed for isoprene. The results of calculating the energy and kinetic parameters of the polymer chain growth stage for the second isoprene molecule according to the proposed scheme are presented in Table 3.
As can be seen from the Table 3, the Gibbs free energy of CA-CP and TA-TP reorientation of isoprene has a small value and is from 1.2 to 11.4 kJ/mol, i.e. the monomer can freely "rotate" near the active center. The activation energy of this process was not estimated since its mechanism remains unclear to this day and has no fundamental influence on understanding the entire process. The activation energy of the trans–cis transition of the isoprene to AS averages 27.8 kJ/mol and is close to that in the bulk of the reaction mixture—26.2 kJ/mol. The Gibbs energy of the trans–cis transition for most cases is in the range from 4 to 15 kJ/mol; against this background, the cases stand out when the terminal unit of polyisoprene has the least probable TP form, where ΔGT-C has small negative values.
The probability of the process proceeding according to one or another variant is characterized by the total activation energy. As can be seen from the Table 3, regardless of the structure of the terminal link of the growing polymer chain, the most advantageous is the addition of a new cis-link to it, and in the CA form. The total activation energy for the cis–cis addition is about 40 kJ/mol, while for the trans–cis addition this value is significantly higher.
The stability of the reaction product is affected by the ΔG value of the entire process. It is evident that the nature of this value for isoprene differs from the data we obtained earlier for the 1,4-butadiene [18]. Despite the fact that in both cases the addition of the cis-unit is more favorable, for the 1,4-butadiene the formation of the syn-π-allyl structure of the terminal unit is energetically more favorable. This indicates a high probability of the anti-syn-isomerization with subsequent formation of trans-units in the polymer. In contrast to butadiene, for isoprene the formation of the anti-π-allyl structure of the terminal unit is more favorable, which leads to a significantly lower probability of anti-syn-isomerization. In our opinion, an additional factor hindering this process is the presence of a methyl group in isoprene, which creates additional steric effects. The obtained data reveal the reason for the experimental results, which indicate an increase in the content of trans-units in polybutadiene with a slowdown in the polymerization process and the virtual absence of trans-structures in polyisoprenes synthesized in the presence of cis-regulating lanthanide catalysts [19].
According to the data of the Table 3, the obvious advantage of the formation of anti-π-allyl structures is evident. Also, based on the fact that to analyze all variants of the process of addition of the third isoprene molecule to the growing polymer chain, it would be necessary to perform calculations for 64 reactions, we decided to limit ourselves to considering the most probable products that can be obtained at the previous stage (see the selection in Table 3). The results obtained for the third isoprene molecule are presented in the Table 4.

Table 4.
Results of calculations of energy and activation parameters (kJ/mol) of the growth stage of the polymerization process for the third isoprene molecule.
The results presented in the Table 4 show that the further process of isoprene polymerization in the presence of the studied AS is accompanied by the formation of a cis-polymer. The main regularities discovered for the elementary acts of the process of adding a second molecule to the polymer chain (Table 3) are also characteristic of the subsequent stage. An interesting result is the formation of an anti-π-allyl structure of the terminal link of the growing macromolecule regardless of the type of complex entering into polymerization. This allows us to assume that the formation of "defective" links in the polymer chain will be of a single nature, and their distribution along the macromolecule will be statistical.
An important and new fact obtained in this work is the addition of the monomer in the CA form established during the polymerization of isoprene. This is the main reason for the regioselectivity of the studied neodymium-based Ziegler–Natta system and means that isoprene polymerizes in the so-called “tail to head” sequence. Therefore, it would be more correct to call the resulting product not cis-1,4-polyisoprene, but cis-4,1-polyisoprene.
Calculations were then performed for the formed cis-sequences upon introduction of the fourth diene molecule into the polymer chain. In this case, the number of monomer units in the resulting product exceeds the size of the Kuhn segment, which for the cis-1,4-polyisoprene is 2–3 monomer units and allows extrapolating the obtained results to real objects, the polymer chain of which reaches a length of several thousand monomer units. The obtained results (Table 5) indicate that for the process of isoprene polymerization using the cis-stereospecific neodymium Ziegler–Natta catalyst, the formation of regular cis-sequences is due to lower activation energies of the process of the monomer addition in the cis conformation. In this case, ΔG≠tot stabilizes at a level of 50 kJ/mol, which corresponds to experimental data obtained for the polymerization processes of conjugated dienes [8,13].

Table 5.
Results of calculations of energy and activation parameters (kJ/mol) of the growth stage of the polymerization process for the fourth diene molecule.
3. Computational Details
The technique used has proven itself well in similar studies and allows obtaining not only qualitative but also close to quantitative results [16,18,20]. For quantum chemical calculations, we used the Orca 5.0.3 software package [21,22]. The calculations were carried out within the framework of the ONIOM technique [23], in which the hybrid density functional PBE0 [24] in combination with the second-generation valence-split triple-zeta atomic basis set def2-TZVP of Ahlrichs et al. [25] was used as a high-level method (QM1). The semi-empirical method of the density functional theory XTB1 of Grimme et al. [26,27] was used as a low-level method (QM2). A feature of the systems under study is that weak dispersion interactions play an important role in them; accounting for dispersion interactions was carried out within the framework of the semi-empirical model D4 of Grimme et al. [28].
Optimization of geometric parameters was performed taking into account the influence of the solvent (hexane) in the continuum model ALPB—the method of analytical linearization of the Poisson–Boltzmann equation [29]. All calculations were performed with full optimization of geometric parameters without any symmetry restrictions. Since the Nd(III) ion has three unpaired electrons and the total multiplicity of all the complexes under study is 4 (confirmed by a series of additional calculations), the spin-unrestricted method was used for all calculations.
The calculations of vibrational spectra performed upon completion of the geometry optimization and did not contain imaginary modes. This means that the structures found correspond to minima on the total potential energy surface. These calculations were also used to estimate the thermal corrections needed to calculate the total Gibbs free energy of particles (at 298.15 K and 1 atm).
The search for transition states was carried out according to the standard procedure. All found transition states had one imaginary mode, which indicated the presence of a first-order saddle point. To establish the correspondence of the found saddle point to the transition state of the reaction under consideration, the calculation of "descents" was carried out according to the procedure of following the internal reaction coordinate IRC
4. Conclusions
The process of isoprene polymerization using a cis-stereospecific neodymium Ziegler–Natta catalyst can be described by the following elementary acts of interaction between AS and the monomer: coordination; reorientation; trans–cis isomerization; insertion.
It is shown that the formation of a regular cis-sequence of 1,4-polyisoprene under the studied conditions is due to a lower activation energy of the process of monomer addition in the cis-configuration, regardless of the structure of the upcoming terminal unit and a low probability of its anti-syn-isomerization. Based on the results of the study, the average activation energy of the polymerization process should be taken as ~50 kJ/mol.
It was established that the main reason for the regioselectivity of the studied polymerization system is the formation of a tail-to-head sequence, caused by favorable energy and activation parameters for the formation of the terminal link of the growing polymer chain in the form of an anti-π-allyl complex, in which the methyl group of the isoprene link is closer to the active center (CA orientation).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15090810/s1. The structures of all compounds discussed in this work are provided in xyz format in the Supplementary Materials file. The filenames of xyz files correspond to the names in Table 2, Table 3 and Table 4.
Author Contributions
Conceptualization, I.G.A. and I.M.D.; methodology, A.N.M. and A.M.K.; software, A.N.M.; validation, A.N.M., I.G.A., A.M.K. and I.M.D.; formal analysis, A.N.M. and I.G.A.; investigation, A.N.M. and I.G.A.; resources, A.M.K.; data curation, A.N.M. and I.G.A.; writing—original draft preparation, A.N.M. and I.G.A.; writing—review and editing, A.M.K. and I.M.D.; visualization, A.N.M.; supervision, I.M.D.; project administration, I.G.A. and I.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out at the expense of a grant provided in 2024 by the Academy of Sciences of the Republic of Tatarstan for the implementation of fundamental and applied scientific work in scientific and educational organizations, enterprises and organizations of the real sector of the economy of the Republic of Tatarstan. Grant 27/2024-ФИП.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Malaysian Rubber Council. Available online: https://www.myrubbercouncil.com/industry/world_production.php (accessed on 8 June 2023).
- Makhiyanov, N.; Akhmetov, I.G.; Vagizov, A.M. Microstructure of pol-yisoprenes synthesized with titanium- and neodymium-containing catalytic systems. Polym. Sci. Ser. A. 2012, 54, 942–949. [Google Scholar] [CrossRef]
- Natta, C. Stereospecific polymerizations. J. Polym. Sci. 1960, 48, 219–239. [Google Scholar] [CrossRef]
- Natta, G.; Porri, L.; Carbonaro, A.; Stoppa, G. Polymerization of conjugated diolefins by homogeneous aluminum alkyl-titaniumalkoxide catalyst systems. I. Cis-1,4 isotactic poly (1,3-pentadiene). Die Makromol. Chem. Macromol. Chem. Phys. 1964, 77, 114–125. [Google Scholar] [CrossRef]
- Natta, G.; Porri, L.; Carbonaro, A. Polymerization of conjugated diolefins by homogeneous aluminum alkyl-titanium alkoxide catalyst systems. II. 1,2-polybutadiene and 3, 4-polyisoprene. Die Makromol. Chem. Macromol. Chem. Phys. 1964, 77, 126–138. [Google Scholar] [CrossRef]
- Tinyakova, E.N.; Dolgoplosk, B.A.; Zhuravleva, T.G.; Kovalevskaya, R.N.; Kuren’Gina, T.N. Synthesis of cis and trans polymers of dienes on oxide catalysts and investigation of their structure and properties. J. Polym. Sci. 1961, 52, 159–167. [Google Scholar] [CrossRef]
- Cooper, W.; Vaughan, G. Recent developments in the polymerization of conjugated dienes. Prog. Polym. Sci. 1967, 1, 91–160. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Yeh, H.C. Polymerization of butadiene and isoprene with lanthanide catalysts; characterization and properties of homopolymers and co-polymers. Rubber Chem. Technol. 1985, 58, 117–145. [Google Scholar] [CrossRef]
- Fischbach, A.; Anwander, R. Neodymium Based Ziegler Catalysts Fundamental Chemistry; Nuyken, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 204, pp. 155–281. [Google Scholar] [CrossRef]
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-based Ziegler/Natta catalysts and their application in diene polymerization. Neodymium Based Ziegler Catal.–Fundam. Chem. 2006, 204, 1–154. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, D.; Wang, B.; Liu, B.; Yang, Y. Polymerization of 1,3-conjugated dienes with rare-earth metal precursors. Mol. Catal. Rare-Earth Elem. 2010, 137, 49–108. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Hu, Y.; Zhang, X. Lanthanide complexes mediated co-ordinative chain transfer polymerization of conjugated dienes. Sci. China Technol. Sci. 2018, 61, 1286–1294. [Google Scholar] [CrossRef]
- Kalinin, A.V.; Novikova, E.S.; Agibalova, L.V.; Saprykina, N.N.; Zuev, V.V. Isoprene polymerization using heterogeneous neodymium catalysts sup-ported by a polysiloxane covered nanodiamonds. Fuller. Nanotub. Carbon Nanostructures 2024, 32, 684–689. [Google Scholar] [CrossRef]
- Kang, D.; Ma, R.; Hu, H.; Zhou, Y.; Mao, G.; Xin, S. Novel High-Efficiency Single-Site Rare Earth (RE) Catalyst System for Isoprene Polymerization. Polymers 2025, 17, 1219. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Tian, L.; Zheng, W.; Wang, F.; Zhang, X. Stereo polymerblocking of dienes in switching stereoselectivity by addition of MMAO cocatalyst. J. Macromol. Sci. Part A 2025, 62, 118–125. [Google Scholar] [CrossRef]
- Masliy, A.N.; Akhmetov, I.G.; Kuznetsov, A.M.; Davletbaeva, I.M. DFT and ONIOM Simulation of 1, 3-Butadiene Polymerization Catalyzed by Neodymium-Based Ziegler–Natta System. Polymers 2023, 15, 1166. [Google Scholar] [CrossRef]
- Anno, T. Out-of-Plane Vibrations of a Conjugated Hydrocarbon: trans-Butadiene. J. Chem. Phys. 1958, 28, 944–949. [Google Scholar] [CrossRef]
- Masliy, A.N.; Akhmetov, I.G.; Kuznetsov, A.M.; Davletbaeva, I.M. Effect of DFT Methods and Dispersion Correction Models in ONIOM Methodology on the Activation Energy of Butadiene Polymerization on a Neodymium-Based Ziegler–Natta Catalyst. Int. J. Quantum Chem. 2024, 124, e27462. [Google Scholar] [CrossRef]
- Marina, N.G.; Monakov, Y.B.; Sabirov, Z.M.; Tolstikov, G.A. Lanthanide compounds—Catalysts of stereospecific polymerization of diene monomers. Review. Polym. Sci. USSR 1991, 33, 387–417. [Google Scholar] [CrossRef]
- Masliy, A.N.; Akhmetov, I.G.; Kuznetsov, A.M.; Davletbaeva, I.M. Theoretical Study of the Halogen Concentration Effect on the 1, 3-Butadiene Polymerization Catalyzed by the Neodymium-Based Ziegler–Natta System. Reactions 2024, 5, 753–764. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Dapprich, S.; Komáromi, I.; Byun, K.S.; Morokuma, K.; Frisch, M.J. A new ONIOM implementation in Gaussian98. Part I. The calculation of energy-gies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Struct. THEOCHEM 1999, 461, 1–21. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and as-sessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for all spd-block elements (Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]
- Caldeweyher, E.; Ehlert, S.; Hansen, A.; Neugebauer, H.; Spicher, S.; Bannwarth, C.; Grimme, S. A generally applicable atomic-charge dependent London dispersion correction. J. Chem. Phys. 2019, 150, 15. [Google Scholar] [CrossRef]
- Ehlert, S.; Stahn, M.; Spicher, S.; Grimme, S. Robust and efficient im-plicit solvation model for fast semiempirical methods. J. Chem. Theory Comput. 2021, 17, 4250–4261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).