Catalyst, Reactor, and Purification Technology in Methanol Steam Reforming for Hydrogen Production: A Review
Abstract
1. Introduction
2. Catalyst
2.1. Copper-Based Catalysts
2.2. The Influence of Synthesis Methods on the Performance of Copper-Based Catalysts
2.3. Noble Metal Catalysts
3. Reactor
3.1. Conventional Packed-Bed Reactor
3.2. Wall-Coated Microreactor
3.3. Application of Porous Catalyst Supports
4. Purification Technologies
4.1. Pressure Swing Adsorption
4.2. Separation Membranes
4.3. CO-Selective Oxidation
4.4. CO-Selective Methanation
5. Technical Application Prospects and Challenges
5.1. Application of MSR Technology
5.2. Challenges of MSR Technology
6. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Hu, H.; Li, P.; Shen, W. Preharvest Application of Hydrogen-Rich Water Not Only Affects Daylily Bud Yield but Also Contributes to the Alleviation of Bud Browning. Sci. Hortic. 2021, 287, 110267. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Chen, L.; Yarley, O.P.N.; Zhou, C. Facile Preparation of Sugarcane Bagasse-Derived Carbon Supported MoS2 Nanosheets for Hydrogen Evolution Reaction. Ind. Crops Prod. 2021, 172, 114064. [Google Scholar] [CrossRef]
- Liang, J.; Li, H.; Chen, L.; Ren, M.; Fakayode, O.A.; Han, J.; Zhou, C. Efficient Hydrogen Evolution Reaction Performance Using Lignin-Assisted Chestnut Shell Carbon-Loaded Molybdenum Disulfide. Ind. Crops Prod. 2023, 193, 116214. [Google Scholar] [CrossRef]
- Pan, S.; Zabed, H.M.; Wei, Y.; Qi, X. Technoeconomic and Environmental Perspectives of Biofuel Production from Sugarcane Bagasse: Current Status, Challenges and Future Outlook. Ind. Crops Prod. 2022, 188, 115684. [Google Scholar] [CrossRef]
- Yadav, D.; Lu, X.; Ma, B.-C.; Jing, D. Advancements in Microreactor Technology for Hydrogen Production via Steam Reforming: A Comprehensive Review of Numerical Studies. J. Power Sources 2024, 596, 234090. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Deng, J.; Zhang, J.; Han, B.; Wang, Y.; Li, Z.; Yu, H.; Cai, W.; Deng, Z. Embedded Ni Catalysts in Ni-O-Ce Solid Solution for Stable Hydrogen Production from Ethanol Steam Reforming Reaction. Fuel Process. Technol. 2019, 193, 94–101. [Google Scholar] [CrossRef]
- Ha, J.W.; Kundu, A.; Jang, J.H. Poly-Dimethylsiloxane (PDMS) Based Micro-Reactors for Steam Reforming of Methanol. Fuel Process. Technol. 2010, 91, 1725–1730. [Google Scholar] [CrossRef]
- Ploner, K.; Delir Kheyrollahi Nezhad, P.; Watschinger, M.; Schlicker, L.; Bekheet, M.F.; Gurlo, A.; Gili, A.; Doran, A.; Schwarz, S.; Stöger-Pollach, M.; et al. Steering the Methanol Steam Reforming Performance of Cu/ZrO2 Catalysts by Modification of the Cu-ZrO2 Interface Dimensions Resulting from Cu Loading Variation. Appl. Catal. Gen. 2021, 623, 118279. [Google Scholar] [CrossRef]
- Zheng, L.; Ambrosetti, M.; Zaio, F.; Beretta, A.; Groppi, G.; Tronconi, E. Direct Electrification of Rh/Al2O3 Washcoated SiSiC Foams for Methane Steam Reforming: An Experimental and Modelling Study. Int. J. Hydrogen Energy 2023, 48, 14681–14696. [Google Scholar] [CrossRef]
- Ambrosetti, M.; Bonincontro, D.; Balzarotti, R.; Beretta, A.; Groppi, G.; Tronconi, E. H2 Production by Methane Steam Reforming over Rh/Al2O3 Catalyst Packed in Cu Foams: A Strategy for the Kinetic Investigation in Concentrated Conditions. Catal. Today 2022, 387, 107–118. [Google Scholar] [CrossRef]
- Li, Y.-N.; Ma, R.; He, L.-N.; Diao, Z.-F. Homogeneous Hydrogenation of Carbon Dioxide to Methanol. Catal. Sci. Technol. 2014, 4, 1498–1512. [Google Scholar] [CrossRef]
- Ganesh, I. Conversion of Carbon Dioxide into Methanol—A Potential Liquid Fuel: Fundamental Challenges and Opportunities (a Review). Renew. Sustain. Energy Rev. 2014, 31, 221–257. [Google Scholar] [CrossRef]
- Guan, D.; Wang, F.; Zhang, X.; Dou, W.; Sun, Y. Comprehensive Study on Catalytic Coating Tubular Reactor with Electromagnetic Induction Heating for Hydrogen Production through Methanol Steam Reforming. Int. J. Hydrogen Energy 2024, 50, 1–17. [Google Scholar] [CrossRef]
- Sun, Z.; Aziz, M. Comparative Thermodynamic and Techno-Economic Assessment of Green Methanol Production from Biomass through Direct Chemical Looping Processes. J. Clean. Prod. 2021, 321, 129023. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Hsieh, T.-L.; Guo, M.; Qin, L.; Chung, C.; Fan, L.-S.; Tong, A. A Novel Chemical Looping Partial Oxidation Process for Thermochemical Conversion of Biomass to Syngas. Appl. Energy 2018, 222, 119–131. [Google Scholar] [CrossRef]
- Kathe, M.; Sandvik, P.; Fryer, C.; Kong, F.; Zhang, Y.; Grigonis, G.; Fan, L.-S. Coal Refining Chemical Looping Systems with CO2 as a Co-Feedstock for Chemical Syntheses. Energy Fuels 2018, 32, 1139–1154. [Google Scholar] [CrossRef]
- Luo, M.; Yi, Y.; Wang, S.; Wang, Z.; Du, M.; Pan, J.; Wang, Q. Review of Hydrogen Production Using Chemical-Looping Technology. Renew. Sustain. Energy Rev. 2018, 81, 3186–3214. [Google Scholar] [CrossRef]
- Nurdiawati, A.; Zaini, I.N.; Aziz, M. Dual-Stage Chemical Looping of Microalgae for Methanol Production with Negative-Carbon Emission. Energy Procedia 2019, 158, 842–847. [Google Scholar] [CrossRef]
- Gao, X.; Wen, Y.; Tan, R.; Huang, H.; Kawi, S. A Review of Catalyst Modifications for a Highly Active and Stable Hydrogen Production from Methane. Int. J. Hydrogen Energy 2023, 48, 6204–6232. [Google Scholar] [CrossRef]
- Ribeirinha, P.; Alves, I.; Vázquez, F.V.; Schuller, G.; Boaventura, M.; Mendes, A. Heat Integration of Methanol Steam Reformer with a High-Temperature Polymeric Electrolyte Membrane Fuel Cell. Energy 2017, 120, 468–477. [Google Scholar] [CrossRef]
- Kim, G.J.; Kim, M.S.; Byun, J.-Y.; Hong, S.C. Effects of Ru Addition to Pd/Al2O3 Catalysts on Methanol Steam Reforming Reaction: A Mechanistic Study. Appl. Catal. Gen. 2019, 572, 115–123. [Google Scholar] [CrossRef]
- Jiangyi, H.; Fan, W. Design and Testing of a Small Orchard Tractor Driven by a Power Battery. Eng. Agríc. 2023, 43, e20220195. [Google Scholar] [CrossRef]
- Yu, Y.; Hao, S.; Guo, S.; Tang, Z.; Chen, S. Motor Torque Distribution Strategy for Different Tillage Modes of Agricultural Electric Tractors. Agriculture 2022, 12, 1373. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, Y.; Wang, D.; Cai, Y.; Lai, L. Energy Saving Performance of Agricultural Tractor Equipped with Mechanic-Electronic-Hydraulic Powertrain System. Agriculture 2022, 12, 436. [Google Scholar] [CrossRef]
- Zhu, Z.; Zeng, L.; Chen, L.; Zou, R.; Cai, Y. Fuzzy Adaptive Energy Management Strategy for a Hybrid Agricultural Tractor Equipped with HMCVT. Agriculture 2022, 12, 1986. [Google Scholar] [CrossRef]
- Chen, W.-H.; Su, Y.-Q.; Lin, B.-J.; Kuo, J.-K.; Kuo, P.-C. Hydrogen Production from Partial Oxidation and Autothermal Reforming of Methanol from a Cold Start in Sprays. Fuel 2021, 287, 119638. [Google Scholar] [CrossRef]
- López-Rodríguez, L.; Araiza, D.G.; Arcos, D.G.; Gómez-Cortés, A.; Díaz, G. Bimetallic Cu-Pt Catalysts over Nanoshaped Ceria for Hydrogen Production via Methanol Decomposition. Catal. Today 2022, 394–396, 486–498. [Google Scholar] [CrossRef]
- Garcia, G.; Arriola, E.; Chen, W.-H.; De Luna, M.D. A Comprehensive Review of Hydrogen Production from Methanol Thermochemical Conversion for Sustainability. Energy 2021, 217, 119384. [Google Scholar] [CrossRef]
- Cai, F.; Guo, Y.; Ibrahim, J.J.; Zhang, J.; Sun, Y. A Highly Active and Stable Pd/MoC Catalyst for Hydrogen Production from Methanol Decomposition. Appl. Catal. B Environ. 2021, 299, 120648. [Google Scholar] [CrossRef]
- Chih, Y.-K.; Su, Y.-Q.; Chen, W.-H.; Lin, B.-J.; Kuo, J.-K.; You, S.; Lin, H.-P. Optimization for Hydrogen Production from Methanol Partial Oxidation over Ni–Cu/Al2O3 Catalyst under Sprays. Int. J. Hydrogen Energy 2022, 47, 40559–40572. [Google Scholar] [CrossRef]
- Mei, D.; Qiu, X.; Liu, H.; Wu, Q.; Yu, S.; Xu, L.; Zuo, T.; Wang, Y. Progress on Methanol Reforming Technologies for Highly Efficient Hydrogen Production and Applications. Int. J. Hydrogen Energy 2022, 47, 35757–35777. [Google Scholar] [CrossRef]
- Mohammed Abbas, A.H.; Cheralathan, K.K.; Porpatham, E.; Arumugam, S.K. Hydrogen Generation Using Methanol Steam Reforming—Catalysts, Reactors, and Thermo-Chemical Recuperation. Renew. Sustain. Energy Rev. 2024, 191, 114147. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for Methanol Steam Reforming—A Review. Appl. Catal. B Environ. 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Li, G.; Gu, C.; Zhu, W.; Wang, X.; Yuan, X.; Cui, Z.; Wang, H.; Gao, Z. Hydrogen Production from Methanol Decomposition Using Cu-Al Spinel Catalysts. J. Clean. Prod. 2018, 183, 415–423. [Google Scholar] [CrossRef]
- Liu, F.; Xiao, Y.; Sun, X.; Qin, G.; Song, X.; Liu, Y. Synergistic Catalysis over Hollow CeO2-CaO-ZrO2 Nanostructure for Polycarbonate Methanolysis with Methanol. Chem. Eng. J. 2019, 369, 205–214. [Google Scholar] [CrossRef]
- Baraj, E.; Ciahotný, K.; Hlinčík, T. The Water Gas Shift Reaction: Catalysts and Reaction Mechanism. Fuel 2021, 288, 119817. [Google Scholar] [CrossRef]
- Pal, D.B.; Chand, R.; Upadhyay, S.N.; Mishra, P.K. Performance of Water Gas Shift Reaction Catalysts: A Review. Renew. Sustain. Energy Rev. 2018, 93, 549–565. [Google Scholar] [CrossRef]
- Bepari, S.; Kuila, D. Steam Reforming of Methanol, Ethanol and Glycerol over Nickel-Based Catalysts-A Review. Int. J. Hydrogen Energy 2020, 45, 18090–18113. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Effect of Dopants on the Performance of CuO–CeO2 Catalysts in Methanol Steam Reforming. Appl. Catal. B Environ. 2007, 69, 226–234. [Google Scholar] [CrossRef]
- Huang, G.; Liaw, B.-J.; Jhang, C.-J.; Chen, Y.-Z. Steam Reforming of Methanol over CuO/ZnO/CeO2/ZrO2/Al2O3 Catalysts. Appl. Catal. Gen. 2009, 358, 7–12. [Google Scholar] [CrossRef]
- Jones, S.D.; Hagelin-Weaver, H.E. Steam Reforming of Methanol over CeO2- and ZrO2-Promoted Cu-ZnO Catalysts Supported on Nanoparticle Al2O3. Appl. Catal. B Environ. 2009, 90, 195–204. [Google Scholar] [CrossRef]
- Yang, S.-C.; Su, W.-N.; Lin, S.D.; Rick, J.; Hwang, B.-J. Preparation of Highly Dispersed Catalytic Cu from Rod-like CuO–CeO2 Mixed Metal Oxides: Suitable for Applications in High Performance Methanol Steam Reforming. Catal. Sci. Technol. 2012, 2, 807. [Google Scholar] [CrossRef]
- Yang, L.; Lin, G.-D.; Zhang, H.-B. Highly Efficient Pd–ZnO Catalyst Doubly Promoted by CNTs and Sc2O3 for Methanol Steam Reforming. Appl. Catal. Gen. 2013, 455, 137–144. [Google Scholar] [CrossRef]
- Haghofer, A.; Föttinger, K.; Girgsdies, F.; Teschner, D.; Knop-Gericke, A.; Schlögl, R.; Rupprechter, G. In Situ Study of the Formation and Stability of Supported Pd2Ga Methanol Steam Reforming Catalysts. J. Catal. 2012, 286, 13–21. [Google Scholar] [CrossRef]
- Yi, N.; Si, R.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Steam Reforming of Methanol over Ceria and Gold-Ceria Nanoshapes. Appl. Catal. B Environ. 2010, 95, 87–92. [Google Scholar] [CrossRef]
- Halevi, B.; Peterson, E.J.; Roy, A.; DeLariva, A.; Jeroro, E.; Gao, F.; Wang, Y.; Vohs, J.M.; Kiefer, B.; Kunkes, E.; et al. Catalytic Reactivity of Face Centered Cubic PdZnα for the Steam Reforming of Methanol. J. Catal. 2012, 291, 44–54. [Google Scholar] [CrossRef]
- Hassan, A. Controversial Mechanisms of Methanol Steam Reforming: A Review. Int. J. Hydrogen Energy 2024, 93, 1487–1501. [Google Scholar] [CrossRef]
- Jiang, W.; Ma, X.; Zhang, D.; Li, Z.; Fu, P. Highly Efficient Catalysts for Hydrogen Generation through Methanol Steam Reforming: A Critical Analysis of Modification Strategies, Deactivation, Mechanisms and Kinetics. J. Ind. Eng. Chem. 2024, 130, 54–72. [Google Scholar] [CrossRef]
- Achomo, M.A.; Kumar, A.; Peela, N.R.; Muthukumar, P. Hydrogen Production from Steam Reforming of Methanol: A Comprehensive Review on Thermodynamics, Catalysts, Reactors, and Kinetic Studies. Int. J. Hydrogen Energy 2024, 58, 1640–1672. [Google Scholar] [CrossRef]
- Wang, G.; Wang, F.; Li, L.; Zhang, G. Experiment of Catalyst Activity Distribution Effect on Methanol Steam Reforming Performance in the Packed Bed Plate-Type Reactor. Energy 2013, 51, 267–272. [Google Scholar] [CrossRef]
- Karim, A.; Bravo, J.; Gorm, D.; Conant, T.; Datye, A. Comparison of Wall-Coated and Packed-Bed Reactors for Steam Reforming of Methanol. Catal. Today 2005, 110, 86–91. [Google Scholar] [CrossRef]
- Kundu, A.; Park, J.M.; Ahn, J.E.; Park, S.S.; Shul, Y.G.; Han, H.S. Micro-Channel Reactor for Steam Reforming of Methanol. Fuel 2007, 86, 1331–1336. [Google Scholar] [CrossRef]
- Chein, R.-Y.; Chen, Y.-C.; Lin, Y.-S.; Chung, J.N. Experimental Study on the Hydrogen Production of Integrated Methanol-Steam Reforming Reactors for PEM Fuel Cells. Int. J. Therm. Sci. 2011, 50, 1253–1262. [Google Scholar] [CrossRef]
- Qian, M.; Mei, D.; Yi, Z.; Feng, Y.; Chen, Z. Fluid Flow and Heat Transfer Performance in a Micro-Reactor with Non-Uniform Micro-Pin-Fin Arrays for Hydrogen Production at Low Reynolds Number. Int. J. Hydrogen Energy 2017, 42, 553–561. [Google Scholar] [CrossRef]
- Kang, J.; Song, Y.; Kim, T.; Kim, S. Recent Trends in the Development of Reactor Systems for Hydrogen Production via Methanol Steam Reforming. Int. J. Hydrogen Energy 2022, 47, 3587–3610. [Google Scholar] [CrossRef]
- Yang, W.-W.; Ma, X.; Tang, X.-Y.; Dou, P.-Y.; Yang, Y.-J.; He, Y.-L. Review on Developments of Catalytic System for Methanol Steam Reforming from the Perspective of Energy-Mass Conversion. Fuel 2023, 345, 128234. [Google Scholar] [CrossRef]
- Han, L.; Li, J.; Zhao, D.; Xi, Y.; Gu, X.; Wang, N. Effect Analysis on Energy Conversion Enhancement and NOx Emission Reduction of Ammonia/Hydrogen Fuelled Wavy Micro-Combustor for Micro-Thermophotovoltaic Application. Fuel 2021, 289, 119755. [Google Scholar] [CrossRef]
- Lopes, F.V.S.; Grande, C.A.; Rodrigues, A.E. Activated Carbon for Hydrogen Purification by Pressure Swing Adsorption: Multicomponent Breakthrough Curves and PSA Performance. Chem. Eng. Sci. 2011, 66, 303–317. [Google Scholar] [CrossRef]
- Majlan, E.H.; Wan Daud, W.R.; Iyuke, S.E.; Mohamad, A.B.; Kadhum, A.A.H.; Mohammad, A.W.; Takriff, M.S.; Bahaman, N. Hydrogen Purification Using Compact Pressure Swing Adsorption System for Fuel Cell. Int. J. Hydrogen Energy 2009, 34, 2771–2777. [Google Scholar] [CrossRef]
- J2719; Information Report on the Development of a Hydrogen Quality Guideline for Fuel Cell Vehicles, SAE, J2719. Sae International: Warrendale, PA, USA, 2008.
- ISO 14687-2:2012; Hydrogen Fuel–Product Specification–Part 2: Proton Exchange Membrane (PEM) Fuel Cell Applications for Road Vehicles. ISO: Geneva, Switzerland, 2012.
- Basile, A.; Tosti, S.; Capannelli, G.; Vitulli, G.; Iulianelli, A.; Gallucci, F.; Drioli, E. Co-Current and Counter-Current Modes for Methanol Steam Reforming Membrane Reactor: Experimental Study. Catal. Today 2006, 118, 237–245. [Google Scholar] [CrossRef]
- Zhihua, L.; Xue, Z.; Xiaowei, H.; Xiaobo, Z.; Jiyong, S.; Yiwei, X.; Xuetao, H.; Yue, S.; Xiaodong, Z. Hypha-Templated Synthesis of Carbon/ZnO Microfiber for Dopamine Sensing in Pork. Food Chem. 2021, 335, 127646. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, X.; Ma, S.; Li, L.; You, T. Quantification of Zearalenone in Mildewing Cereal Crops Using an Innovative Photoelectrochemical Aptamer Sensing Strategy Based on ZnO-NGQDs Composites. Food Chem. 2020, 322, 126778. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Mehedi Hassan, M.; Zhu, J.; Ali, S.; Ahmad, W.; Wang, J.; Lv, C.; Chen, Q.; Li, H. Quantification of Deltamethrin Residues in Wheat by Ag@ZnO NFs-Based Surface-Enhanced Raman Spectroscopy Coupling Chemometric Models. Food Chem. 2021, 337, 127652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mei, S.; Manivel, P.; Ma, H.; Chen, X. Zinc Oxide Nanoparticles Synthesized Using Coffee Leaf Extract Assisted with Ultrasound as Nanocarriers for Mangiferin. Curr. Res. Food Sci. 2022, 5, 868–877. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Li, Y.; Li, Y.; Li, Z.; Zhang, W.; Zou, X.; Shi, J.; Huang, X.; Liu, C.; et al. Rapid Detection of Cadmium Ions in Meat by a Multi-Walled Carbon Nanotubes Enhanced Metal-Organic Framework Modified Electrochemical Sensor. Food Chem. 2021, 357, 129762. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Li, L.; Wang, K.; Liu, W.; Hu, B.; Cao, D.; Jiang, F.; Li, J.; Liu, K. Research on Cu-Based and Pt-Based Catalysts for Hydrogen Production through Methanol Steam Reforming. Acta Phys.-Chim. Sin. 2025, 41, 100049. [Google Scholar] [CrossRef]
- Toyir, J.; Ramírez De La Piscina, P.; Homs, N. Ga-Promoted Copper-Based Catalysts Highly Selective for Methanol Steam Reforming to Hydrogen; Relation with the Hydrogenation of CO2 to Methanol. Int. J. Hydrogen Energy 2015, 40, 11261–11266. [Google Scholar] [CrossRef]
- Liu, X.; Toyir, J.; Ramírez De La Piscina, P.; Homs, N. Hydrogen Production from Methanol Steam Reforming over Al2O3—And ZrO2 -Modified CuO ZnO Ga2O3 Catalysts. Int. J. Hydrogen Energy 2017, 42, 13704–13711. [Google Scholar] [CrossRef]
- Matsumura, Y. Development of Durable Copper Catalyst for Hydrogen Production by High Temperature Methanol Steam Reforming. Int. J. Hydrogen Energy 2013, 38, 13950–13960. [Google Scholar] [CrossRef]
- Cao, L.; Lu, M.; Li, G.; Zhang, S. Hydrogen Production from Methanol Steam Reforming Catalyzed by Fe Modified Cu Supported on Attapulgite Clay. React. Kinet. Mech. Catal. 2019, 126, 137–152. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Zhilyaeva, N.A.; Ermilova, M.M.; Orekhova, N.V.; Yaroslavtsev, A.B. Influence of the Support Structure and Composition of Ni–Cu-Based Catalysts on Hydrogen Production by Methanol Steam Reforming. Int. J. Hydrogen Energy 2015, 40, 9677–9684. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, G.; Liu, H.; Shu, Q.; Zhang, Q. Improved Charge Transfer and Morphology on Ti-Modified Cu/γ-Al2O3/Al Catalyst Enhance the Activity for Methanol Steam Reforming. Int. J. Hydrogen Energy 2022, 47, 18294–18304. [Google Scholar] [CrossRef]
- Pu, Y.-C.; Li, S.-R.; Yan, S.; Huang, X.; Wang, D.; Ye, Y.-Y.; Liu, Y.-Q. An Improved Cu/ZnO Catalyst Promoted by Sc2O3 for Hydrogen Production from Methanol Reforming. Fuel 2019, 241, 607–615. [Google Scholar] [CrossRef]
- Matsumura, Y. Durable Cu Composite Catalyst for Hydrogen Production by High Temperature Methanol Steam Reforming. J. Power Sources 2014, 272, 961–969. [Google Scholar] [CrossRef]
- Lu, J.; Li, X.; He, S.; Han, C.; Wan, G.; Lei, Y.; Chen, R.; Liu, P.; Chen, K.; Zhang, L.; et al. Hydrogen Production via Methanol Steam Reforming over Ni-Based Catalysts: Influences of Lanthanum (La) Addition and Supports. Int. J. Hydrogen Energy 2017, 42, 3647–3657. [Google Scholar] [CrossRef]
- Lei, Y.; Luo, Y.; Li, X.; Lu, J.; Mei, Z.; Peng, W.; Chen, R.; Chen, K.; Chen, D.; He, D. The Role of Samarium on Cu/Al2O3 Catalyst in the Methanol Steam Reforming for Hydrogen Production. Catal. Today 2018, 307, 162–168. [Google Scholar] [CrossRef]
- Yang, S.; He, J.; Zhang, N.; Sui, X.; Zhang, L.; Yang, Z. Effect of Rare-Earth Element Modification on the Performance of Cu/ZnAl Catalysts Derived from Hydrotalcite Precursor in Methanol Steam Reforming. J. Fuel Chem. Technol. 2018, 46, 179–188. [Google Scholar] [CrossRef]
- Bossola, F.; Roongcharoen, T.; Coduri, M.; Evangelisti, C.; Somodi, F.; Sementa, L.; Fortunelli, A.; Dal Santo, V. Discovering Indium as Hydrogen Production Booster for a Cu/SiO2 Catalyst in Steam Reforming of Methanol. Appl. Catal. B Environ. 2021, 297, 120398. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Abbandanak, M.H. Production of Hydrogen via Methanol Steam Reforming over Mesoporous CeO2–Cu/KIT-6 Nanocatalyst: Effects of Polar Aprotic Tetrahydrofuran Solvent and ZrO2 Promoter on Catalytic Performance. Int. J. Hydrogen Energy 2022, 47, 16362–16374. [Google Scholar] [CrossRef]
- Mohtashami, Y.; Taghizadeh, M. Performance of the ZrO2 Promoted CuZnO Catalyst Supported on Acetic Acid-Treated MCM-41 in Methanol Steam Reforming. Int. J. Hydrogen Energy 2019, 44, 5725–5738. [Google Scholar] [CrossRef]
- Sanches, S.G.; Flores, J.H.; Da Silva, M.I.P. Cu/ZnO and Cu/ZnO/ZrO2 Catalysts Used for Methanol Steam Reforming. Mol. Catal. 2018, 454, 55–62. [Google Scholar] [CrossRef]
- Seyedi, A.M.; Haghighi, M.; Rahemi, N. Significant Influence of Cutting-Edge Plasma Technology on Catalytic Properties and Performance of CuO-ZnO-Al2O3-ZrO2 Nanocatalyst Used in Methanol Steam Reforming for Fuel Cell Grade Hydrogen Production. Ceram. Int. 2017, 43, 6201–6213. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, M.; Wang, F.; Qiu, X. Efficient Cu/CeO2 Composites for Hydrogen Production from Photothermal Methanol Steam Reforming: The Utility of Synergism of Photo and Thermal Catalysis. Fuel 2023, 331, 125748. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, F.; Liu, Y.; Zhang, L.; Chen, Y.; Wang, H.; Tian, Y.; Zhang, C.; Liu, D. Morphology Effect of Ceria on the Performance of CuO/CeO2 Catalysts for Hydrogen Production by Methanol Steam Reforming. Int. J. Hydrogen Energy 2019, 44, 7252–7261. [Google Scholar] [CrossRef]
- Minaei, S.; Haghighi, M.; Jodeiri, N.; Ajamein, H.; Abdollahifar, M. Urea-Nitrates Combustion Preparation of CeO2 -Promoted CuO/ZnO/Al2O3 Nanocatalyst for Fuel Cell Grade Hydrogen Production via Methanol Steam Reforming. Adv. Powder Technol. 2017, 28, 842–853. [Google Scholar] [CrossRef]
- Linga Reddy, E.; Lee, H.C.; Kim, D.H. Steam Reforming of Methanol over Structured Catalysts Prepared by Electroless Deposition of Cu and Zn on Anodically Oxidized Alumina. Int. J. Hydrogen Energy 2015, 40, 2509–2517. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Wang, Q.; Yang, T.; Zhang, Q.; Zhang, L. Fast Start-up Structured CuFeMg/Al2O3 Catalyst Applied in Microreactor for Efficient Hydrogen Production in Methanol Steam Reforming. Chem. Eng. J. 2021, 426, 130644. [Google Scholar] [CrossRef]
- Tong, W.; West, A.; Cheung, K.; Yu, K.-M.; Tsang, S.C.E. Dramatic Effects of Gallium Promotion on Methanol Steam Reforming Cu–ZnO Catalyst for Hydrogen Production: Formation of 5 Å Copper Clusters from Cu–ZnGaOx. ACS Catal. 2013, 3, 1231–1244. [Google Scholar] [CrossRef]
- Tahay, P.; Khani, Y.; Jabari, M.; Bahadoran, F.; Safari, N. Highly Porous Monolith/TiO2 Supported Cu, Cu-Ni, Ru, and Pt Catalysts in Methanol Steam Reforming Process for H 2 Generation. Appl. Catal. Gen. 2018, 554, 44–53. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhou, W.; Lan, G.; Sun, X.; Wang, X.; Jiang, C.; Li, Y. High-Performance Cu/ZnO/Al2O3 Catalysts for Methanol Steam Reforming with Enhanced Cu-ZnO Synergy Effect via Magnesium Assisted Strategy. J. Energy Chem. 2021, 63, 550–557. [Google Scholar] [CrossRef]
- Tong, W.; Cheung, K.; West, A.; Yu, K.-M.; Tsang, S.C.E. Direct Methanol Steam Reforming to Hydrogen over CuZnGaOx Catalysts without CO Post-Treatment: Mechanistic Considerations. Phys. Chem. Chem. Phys. 2013, 15, 7240. [Google Scholar] [CrossRef]
- Khani, Y.; Bahadoran, F.; Safari, N.; Soltanali, S.; Taheri, S.A. Hydrogen Production from Steam Reforming of Methanol over Cu-Based Catalysts: The Behavior of ZnxLaxAl1-xO4 and ZnO/La2O3/Al2O3 Lined on Cordierite Monolith Reactors. Int. J. Hydrogen Energy 2019, 44, 11824–11837. [Google Scholar] [CrossRef]
- Khani, Y.; Safari, N.; Kamyar, N.; Bahadoran, F.; Torabi, M. High H2 Selectivity with Low Coke Formation for Methanol Steam Reforming over Cu/Y1.5Ce0.84Ru0.04O4 Catalyst in a Microchannel Plate Reactor. Int. J. Hydrogen Energy 2022, 47, 971–983. [Google Scholar] [CrossRef]
- Phongboonchoo, Y.; Thouchprasitchai, N.; Pongstabodee, S. Hydrogen Production with a Low Carbon Monoxide Content via Methanol Steam Reforming over Cu x Ce y Mg z /Al2O3 Catalysts: Optimization and Stability. Int. J. Hydrogen Energy 2017, 42, 12220–12235. [Google Scholar] [CrossRef]
- Tajrishi, O.Z.; Taghizadeh, M.; Kiadehi, A.D. Methanol Steam Reforming in a Microchannel Reactor by Zn-, Ce- and Zr- Modified Mesoporous Cu/SBA-15 Nanocatalyst. Int. J. Hydrogen Energy 2018, 43, 14103–14120. [Google Scholar] [CrossRef]
- Mrad, M.; Hammoud, D.; Gennequin, C.; Aboukaïs, A.; Abi-Aad, E. A Comparative Study on the Effect of Zn Addition to Cu/Ce and Cu/Ce–Al Catalysts in the Steam Reforming of Methanol. Appl. Catal. Gen. 2014, 471, 84–90. [Google Scholar] [CrossRef]
- He, J.; Yang, Z.; Zhang, L.; Li, Y.; Pan, L. Cu Supported on ZnAl-LDHs Precursor Prepared by in-Situ Synthesis Method on γ-Al2O3 as Catalytic Material with High Catalytic Activity for Methanol Steam Reforming. Int. J. Hydrogen Energy 2017, 42, 9930–9937. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, Y.; Fu, P.; Zheng, Q.; Fan, Q.; Wei, P.; Zheng, L. Achieving Strong Thermal Stability in Catalytic Reforming of Methanol over In-Situ Self-Activated Nano Cu2O/ZnO Catalyst with Dual-Sites of Cu Species. J. Environ. Chem. Eng. 2022, 10, 107676. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Yang, T.; Zhang, Q.; Zhang, L. In-Situ Self-Assembled Cu2O/ZnO Core-Shell Catalysts Synergistically Enhance the Durability of Methanol Steam Reforming. Appl. Catal. Gen. 2021, 616, 118072. [Google Scholar] [CrossRef]
- Park, J.E.; Yim, S.-D.; Kim, C.S.; Park, E.D. Steam Reforming of Methanol over Cu/ZnO/ZrO2/Al2O3 Catalyst. Int. J. Hydrogen Energy 2014, 39, 11517–11527. [Google Scholar] [CrossRef]
- Chen, M.; Sun, G.; Wang, Y.; Liang, D.; Li, C.; Wang, J.; Liu, Q. Steam Reforming of Methanol for Hydrogen Production over Attapulgite-Based Zeolite-Supported Cu-Zr Catalyst. Fuel 2022, 314, 122733. [Google Scholar] [CrossRef]
- Patel, S.; Pant, K.K. Activity and Stability Enhancement of Copper–Alumina Catalysts Using Cerium and Zinc Promoters for the Selective Production of Hydrogen via Steam Reforming of Methanol. J. Power Sources 2006, 159, 139–143. [Google Scholar] [CrossRef]
- Yu, X.; Tu, S.-T.; Wang, Z.; Qi, Y. Development of a Microchannel Reactor Concerning Steam Reforming of Methanol. Chem. Eng. J. 2006, 116, 123–132. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, L.; Ni, C.; Sun, T.; Zhao, S.; Wang, S.; Wang, A.; Hu, Y. CeO2–ZrO2-Promoted CuO/ZnO Catalyst for Methanol Steam Reforming. Int. J. Hydrogen Energy 2013, 38, 4397–4406. [Google Scholar] [CrossRef]
- Bagherzadeh, S.B.; Haghighi, M.; Rahemi, N. Novel Oxalate Gel Coprecipitation Synthesis of ZrO2-CeO2-Promoted CuO-ZnO-Al2O3 Nanocatalyst for Fuel Cell-Grade Hydrogen Production from Methanol: Influence of Ceria-Zirconia Loading. Energy Convers. Manag. 2017, 134, 88–102. [Google Scholar] [CrossRef]
- Jin, S.; Li, D.; Wang, Z.; Wang, Y.; Sun, L.; Zhu, M. Dynamics of the Cu/CeO2 Catalyst during Methanol Steam Reforming. Catal. Sci. Technol. 2022, 12, 7003–7009. [Google Scholar] [CrossRef]
- Liang, F.; Yu, Y.; Zhou, W.; Xu, X.; Zhu, Z. Highly Defective CeO2 as a Promoter for Efficient and Stable Water Oxidation. J. Mater. Chem. A 2015, 3, 634–640. [Google Scholar] [CrossRef]
- Roh, H.-S.; Jun, K.-W.; Dong, W.-S.; Park, S.-E.; Baek, Y.-S. Highly Stable Ni Catalyst Supported on Ce–ZrO2 for Oxy-Steam Reforming of Methane. Catal. Lett. 2001, 74, 31–36. [Google Scholar] [CrossRef]
- Ali, S.S.; Moawad, M.S.; Hussein, M.A.; Azab, M.; Abdelkarim, E.A.; Badr, A.; Sun, J.; Khalil, M. Efficacy of Metal Oxide Nanoparticles as Novel Antimicrobial Agents against Multi-Drug and Multi-Virulent Staphylococcus Aureus Isolates from Retail Raw Chicken Meat and Giblets. Int. J. Food Microbiol. 2021, 344, 109116. [Google Scholar] [CrossRef] [PubMed]
- Pohar, A.; Hočevar, S.; Likozar, B.; Levec, J. Synthesis and Characterization of Gallium-Promoted Copper–Ceria Catalyst and Its Application for Methanol Steam Reforming in a Packed Bed Reactor. Catal. Today 2015, 256, 358–364. [Google Scholar] [CrossRef]
- Kim, S.; Kang, M. Hydrogen Production from Methanol Steam Reforming over Cu–Ti–P Oxide Catalysts. J. Ind. Eng. Chem. 2012, 18, 969–978. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Akhoundzadeh, H.; Rezayan, A.; Sadeghian, M. Excellent Catalytic Performance of 3D-Mesoporous KIT-6 Supported Cu and Ce Nanoparticles in Methanol Steam Reforming. Int. J. Hydrogen Energy 2018, 43, 10926–10937. [Google Scholar] [CrossRef]
- He, W.; Liu, Q.; Yu, H.; Si, X.; Zhang, J. Efficient Synthesis of Octacosanol Linoleate Catalyzed by Ionic Liquid and Its Structure Characterization. J. Am. Oil Chem. Soc. 2016, 93, 509–517. [Google Scholar] [CrossRef]
- Ali, S.; Chen, X.; Ajmal Shah, M.; Ali, M.; Zareef, M.; Arslan, M.; Ahmad, S.; Jiao, T.; Li, H.; Chen, Q. The Avenue of Fruit Wastes to Worth for Synthesis of Silver and Gold Nanoparticles and Their Antimicrobial Application against Foodborne Pathogens: A Review. Food Chem. 2021, 359, 129912. [Google Scholar] [CrossRef]
- Sadalage, P.S.; Dar, M.A.; Bhor, R.D.; Bhalerao, B.M.; Kamble, P.N.; Paiva-Santos, A.C.; Nimbalkar, M.S.; Sonawane, K.D.; Pai, K.; Patil, P.S.; et al. Optimization of Biogenic Synthesis of Biocompatible Platinum Nanoparticles with Catalytic, Enzyme Mimetic and Antioxidant Activities. Food Biosci. 2022, 50, 102024. [Google Scholar] [CrossRef]
- Hwang, B.-Y.; Sakthinathan, S.; Chiu, T.-W. Production of Hydrogen from Steam Reforming of Methanol Carried out by Self-Combusted CuCr1-xFexO2 (x = 0–1) Nanopowders Catalyst. Int. J. Hydrogen Energy 2019, 44, 2848–2856. [Google Scholar] [CrossRef]
- Chiu, T.-W.; Yu, B.-S.; Wang, Y.-R.; Chen, K.-T.; Lin, Y.-T. Synthesis of Nanosized CuCrO2 Porous Powders via a Self-Combustion Glycine Nitrate Process. J. Alloys Compd. 2011, 509, 2933–2935. [Google Scholar] [CrossRef]
- Maiti, S.; Llorca, J.; Dominguez, M.; Colussi, S.; Trovarelli, A.; Priolkar, K.R.; Aquilanti, G.; Gayen, A. Combustion Synthesized Copper-Ion Substituted FeAl2O4 (Cu0.1Fe0.9Al2O4): A Superior Catalyst for Methanol Steam Reforming Compared to Its Impregnated Analogue. J. Power Sources 2016, 304, 319–331. [Google Scholar] [CrossRef]
- Ajamein, H.; Haghighi, M. On the Microwave Enhanced Combustion Synthesis of CuO–ZnO–Al2O3 Nanocatalyst Used in Methanol Steam Reforming for Fuel Cell Grade Hydrogen Production: Effect of Microwave Irradiation and Fuel Ratio. Energy Convers. Manag. 2016, 118, 231–242. [Google Scholar] [CrossRef]
- Xu, T.; Zou, J.; Tao, W.; Zhang, S.; Cui, L.; Zeng, F.; Wang, D.; Cai, W. Co-Nanocasting Synthesis of Cu Based Composite Oxide and Its Promoted Catalytic Activity for Methanol Steam Reforming. Fuel 2016, 183, 238–244. [Google Scholar] [CrossRef]
- Bagherzadeh, S.B.; Haghighi, M. Plasma-Enhanced Comparative Hydrothermal and Coprecipitation Preparation of CuO/ZnO/Al2O3 Nanocatalyst Used in Hydrogen Production via Methanol Steam Reforming. Energy Convers. Manag. 2017, 142, 452–465. [Google Scholar] [CrossRef]
- Ahmadi, F.; Haghighi, M.; Ajamein, H. Sonochemically Coprecipitation Synthesis of CuO/ZnO/ZrO2/Al2O3 Nanocatalyst for Fuel Cell Grade Hydrogen Production via Steam Methanol Reforming. J. Mol. Catal. Chem. 2016, 421, 196–208. [Google Scholar] [CrossRef]
- Fasanya, O.O.; Atta, A.Y.; Myint, M.T.Z.; Dutta, J.; Jibril, B.Y. Effects of Synthesis Methods on Performance of CuZn/MCM-41 Catalysts in Methanol Steam Reforming. Int. J. Hydrogen Energy 2021, 46, 3539–3553. [Google Scholar] [CrossRef]
- Ajamein, H.; Haghighi, M. Influence of Ambient Gas on Microwave-Assisted Combustion Synthesis of CuO–ZnO–Al2O3 Nanocatalyst Used in Fuel Cell Grade Hydrogen Production via Methanol Steam Reforming. Ceram. Int. 2016, 42, 17978–17989. [Google Scholar] [CrossRef]
- Ajamein, H.; Haghighi, M.; Alaei, S. The Role of Various Fuels on Microwave-Enhanced Combustion Synthesis of CuO/ZnO/Al2O3 Nanocatalyst Used in Hydrogen Production via Methanol Steam Reforming. Energy Convers. Manag. 2017, 137, 61–73. [Google Scholar] [CrossRef]
- Ajamein, H.; Haghighi, M.; Shokrani, R.; Abdollahifar, M. On the Solution Combustion Synthesis of Copper Based Nanocatalysts for Steam Methanol Reforming: Effect of Precursor, Ultrasound Irradiation and Urea/Nitrate Ratio. J. Mol. Catal. Chem. 2016, 421, 222–234. [Google Scholar] [CrossRef]
- Huang, R.-J.; Sakthinathan, S.; Chiu, T.-W.; Dong, C. Hydrothermal Synthesis of High Surface Area CuCrO2 for H2 Production by Methanol Steam Reforming. RSC Adv. 2021, 11, 12607–12613. [Google Scholar] [CrossRef] [PubMed]
- Lindström, B. Hydrogen Generation by Steam Reforming of Methanol over Copper-Based Catalysts for Fuel Cell Applications. Int. J. Hydrogen Energy 2001, 26, 923–933. [Google Scholar] [CrossRef]
- Lindström, B.; Pettersson, L.J.; Govind Menon, P. Activity and Characterization of Cu/Zn, Cu/Cr and Cu/Zr on γ-Alumina for Methanol Reforming for Fuel Cell Vehicles. Appl. Catal. Gen. 2002, 234, 111–125. [Google Scholar] [CrossRef]
- Patel, S.; Pant, K.K. Influence of Preparation Method on Performance of Cu(Zn)(Zr)-Alumina Catalysts for the Hydrogen Production via Steam Reforming of Methanol. J. Porous Mater. 2006, 13, 373–378. [Google Scholar] [CrossRef]
- Yao, C.; Wang, L.; Liu, Y.; Wu, G.; Cao, Y.; Dai, W.; He, H.; Fan, K. Effect of Preparation Method on the Hydrogen Production from Methanol Steam Reforming over Binary Cu/ZrO Catalysts. Appl. Catal. Gen. 2006, 297, 151–158. [Google Scholar] [CrossRef]
- Shen, J. Influence of Preparation Method on Performance of Cu/Zn-Based Catalysts for Low-Temperature Steam Reforming and Oxidative Steam Reforming of Methanol for H2 Production for Fuel Cells. Catal. Today 2002, 77, 89–98. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Wang, S.-F.; Tsai, A.-P.; Kameoka, S. Reduction Behaviors and Catalytic Properties for Methanol Steam Reforming of Cu-Based Spinel Compounds CuX2O4 (X = Fe, Mn, Al, La). Ceram. Int. 2014, 40, 4541–4551. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Wu, G.; Mao, D.; Lu, G. Influence of the Component Interaction over Cu/ZrO2 Catalysts Induced with Fractionated Precipitation Method on the Catalytic Performance for Methanol Steam Reforming. RSC Adv. 2016, 6, 30176–30183. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, L.; Ni, C.; Sun, T.; Wang, S.; Hu, Y.; Wang, A.; Zhao, S. Effects of Precipitation Aging Time on the Performance of CuO/ZnO/CeO2-ZrO2 for Methanol Steam Reforming. J. Fuel Chem. Technol. 2013, 41, 883–888. [Google Scholar] [CrossRef]
- Talkhoncheh, S.K.; Haghighi, M.; Minaei, S.; Ajamein, H.; Abdollahifar, M. Synthesis of CuO/ZnO/Al2O3/ZrO2/CeO2 Nanocatalysts via Homogeneous Precipitation and Combustion Methods Used in Methanol Steam Reforming for Fuel Cell Grade Hydrogen Production. Rsc Adv. 2016, 6, 57199–57209. [Google Scholar] [CrossRef]
- Sanches, S.G.; Huertas Flores, J.; Da Silva, M.I.P. Influence of Aging Time on the Microstructural Characteristics of a Cu/ZnO-Based Catalyst Prepared by Homogeneous Precipitation for Use in Methanol Steam Reforming. React. Kinet. Mech. Catal. 2017, 121, 473–485. [Google Scholar] [CrossRef]
- Shahsavar, H.; Taghizadeh, M.; Kiadehi, A.D. Effects of Catalyst Preparation Route and Promoters (Ce and Zr) on Catalytic Activity of CuZn/CNTs Catalysts for Hydrogen Production from Methanol Steam Reforming. Int. J. Hydrogen Energy 2021, 46, 8906–8921. [Google Scholar] [CrossRef]
- Ploner, K.; Nezhad, P.D.K.; Gili, A.; Kamutzki, F.; Gurlo, A.; Doran, A.; Cao, P.; Heggen, M.; Köwitsch, N.; Armbrüster, M. The Sol–Gel Autocombustion as a Route towards Highly CO2-Selective, Active and Long-Term Stable Cu/ZrO2 Methanol Steam Reforming Catalysts. Mater. Chem. Front. 2021, 5, 5093–5105. [Google Scholar] [CrossRef]
- Han, J.; Cai, Y.; Wang, L.; Mao, Y.; Ni, L.; Wang, Y. A High Efficiency Method Combining Metal Chelate Ionic Liquid-Based Aqueous Two-Phase Flotation with Two-Step Precipitation Process for Bromelain Purification. Food Chem. 2020, 309, 125749. [Google Scholar] [CrossRef]
- Shishido, T.; Yamamoto, Y.; Morioka, H.; Takaki, K.; Takehira, K. Active Cu/ZnO and Cu/ZnO/Al2O3 Catalysts Prepared by Homogeneous Precipitation Method in Steam Reforming of Methanol. Appl. Catal. Gen. 2004, 263, 249–253. [Google Scholar] [CrossRef]
- Bagherzadeh, S.B.; Haghighi, M. Texture-Evolution of Copper-Based Nanocatalyst via Hybrid Glow Discharge Plasma-Oxalate-Precipitation Method for Hydrogen Production from CH3OH/H2O Mixture. Appl. Catal. Gen. 2020, 591, 117402. [Google Scholar] [CrossRef]
- Föttinger, K.; Rupprechter, G. In Situ Spectroscopy of Complex Surface Reactions on Supported Pd–Zn, Pd–Ga, and Pd(Pt)–Cu Nanoparticles. Acc. Chem. Res. 2014, 47, 3071–3079. [Google Scholar] [CrossRef] [PubMed]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol Steam Reforming for Hydrogen Production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Iwasa, N.; Takezawa, N. New Supported Pd and Pt Alloy Catalysts for Steam Reforming and Dehydrogenation of Methanol. Top. Catal. 2003, 22, 215–224. [Google Scholar] [CrossRef]
- Tolmacsov, P.; Gazsi, A.; Solymosi, F. Decomposition and Reforming of Methanol on Pt Metals Supported by Carbon Norit. Appl. Catal. Gen. 2009, 362, 58–61. [Google Scholar] [CrossRef]
- Qi, C.; Amphlett, J.C.; Peppley, B.A. K (Na)-Promoted Ni, Al Layered Double Hydroxide Catalysts for the Steam Reforming of Methanol. J. Power Sources 2007, 171, 842–849. [Google Scholar] [CrossRef]
- Qi, C.; Amphlett, J.; Peppley, B. Hydrogen Production by Methanol Reforming on NiAl Layered Double Hydroxide Derived Catalyst: Effect of the Pretreatment of the Catalyst. Int. J. Hydrogen Energy 2007, 32, 5098–5102. [Google Scholar] [CrossRef]
- Qi, C.; Amphlett, J.C.; Peppley, B.A. Product Composition as a Function of Temperature over NiAl-Layered Double Hydroxide Derived Catalysts in Steam Reforming of Methanol. Appl. Catal. Gen. 2006, 302, 237–243. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Paxinou, A.; Słowik, G.; Neophytides, S.; Avgouropoulos, G. Steam Reforming of Methanol over Nanostructured Pt/TiO2 and Pt/CeO2 Catalysts for Fuel Cell Applications. Catalysts 2018, 8, 544. [Google Scholar] [CrossRef]
- Han, X.; Song, L.; Xu, H.; Ouyang, S. Light-Driven Low-Temperature Syngas Production from CH3OH and H2O over a Pt@SrTiO3 Photothermal Catalyst. Catal. Sci. Technol. 2018, 8, 2515–2518. [Google Scholar] [CrossRef]
- Cai, F.; Ibrahim, J.J.; Fu, Y.; Kong, W.; Zhang, J.; Sun, Y. Low-Temperature Hydrogen Production from Methanol Steam Reforming on Zn-Modified Pt/MoC Catalysts. Appl. Catal. B Environ. 2020, 264, 118500. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, S.; Liu, X.; Luo, H.; Huang, C.; Zhou, H.; Wu, Z.; Li, J.; Wang, H.; Sun, Y. Maximizing the Synergistic Effect between Pt0 and Ptδ+ in a Confined Pt-Based Catalyst for Durable Hydrogen Production. Appl. Catal. B Environ. 2022, 316, 121669. [Google Scholar] [CrossRef]
- Claudio-Piedras, A.; Ramírez-Zamora, R.M.; Alcántar-Vázquez, B.C.; Gutiérrez-Martínez, A.; Mondragón-Galicia, G.; Morales-Anzures, F.; Pérez-Hernández, R. One Dimensional Pt/CeO2-NR Catalysts for Hydrogen Production by Steam Reforming of Methanol: Effect of Pt Precursor. Catal. Today 2021, 360, 55–62. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, G.; Geng, J.; Jing, D.; Hong, X.; Guo, L. A High-Performance PdZn Alloy Catalyst Obtained from Metal-Organic Framework for Methanol Steam Reforming Hydrogen Production. Int. J. Hydrogen Energy 2019, 44, 24387–24397. [Google Scholar] [CrossRef]
- Heggen, M.; Penner, S.; Friedrich, M.; Dunin-Borkowski, R.E.; Armbrüster, M. Formation of ZnO Patches on ZnPd/ZnO during Methanol Steam Reforming: A Strong Metal–Support Interaction Effect? J. Phys. Chem. C 2016, 120, 10460–10465. [Google Scholar] [CrossRef]
- Iwasa, N.; Kudo, S.; Takahashi, H.; Masuda, S.; Takezawa, N. Highly Selective Supported Pd Catalysts for Steam Reforming of Methanol. Catal. Lett. 1993, 19, 211–216. [Google Scholar] [CrossRef]
- Iwasa, N.; Mayanagi, T.; Ogawa, N.; Sakata, K.; Takezawa, N. New Catalytic Functions of Pd–Zn, Pd–Ga, Pd–In, Pt–Zn, Pt–Ga and Pt–In Alloys in the Conversions of Methanol. Catal. Lett. 1998, 54, 119–123. [Google Scholar] [CrossRef]
- Iwasa, N.; Masuda, S.; Takezawa, N. Steam Reforming of Methanol over Ni, Co, Pd and Pt Supported on ZnO. React. Kinet. Catal. Lett. 1995, 55, 349–353. [Google Scholar] [CrossRef]
- Iwasa, N.; Mayanagi, T.; Nomura, W.; Arai, M.; Takezawa, N. Effect of Zn Addition to Supported Pd Catalysts in the Steam Reforming of Methanol. Appl. Catal. Gen. 2003, 248, 153–160. [Google Scholar] [CrossRef]
- Cai, F.; Lu, P.; Ibrahim, J.J.; Fu, Y.; Zhang, J.; Sun, Y. Investigation of the Role of Nb on Pd−Zr−Zn Catalyst in Methanol Steam Reforming for Hydrogen Production. Int. J. Hydrogen Energy 2019, 44, 11717–11733. [Google Scholar] [CrossRef]
- Kosinski, M.R.; Vizcaíno, A.J.; Gómez-Sainero, L.M.; Carrero, A.; Baker, R.T. Methanol Reforming by Nanostructured Pd/Sm-Doped Ceria Catalysts. Appl. Catal. B Environ. 2021, 286, 119935. [Google Scholar] [CrossRef]
- Wang, C.; Ouyang, M.; Li, M.; Lee, S.; Flytzani-Stephanopoulos, M. Low-Coordinated Pd Catalysts Supported on Zn1Zr1Ox Composite Oxides for Selective Methanol Steam Reforming. Appl. Catal. Gen. 2019, 580, 81–92. [Google Scholar] [CrossRef]
- Barrios, C.E.; Bosco, M.V.; Baltanás, M.A.; Bonivardi, A.L. Hydrogen Production by Methanol Steam Reforming: Catalytic Performance of Supported-Pd on Zinc–Cerium Oxides’ Nanocomposites. Appl. Catal. B Environ. 2015, 179, 262–275. [Google Scholar] [CrossRef]
- Yan, P.; Tian, P.; Cai, C.; Zhou, S.; Yu, X.; Zhao, S.; Tu, S.-T.; Deng, C.; Sun, Y. Antioxidative and Stable PdZn/ZnO/Al2O3 Catalyst Coatings Concerning Methanol Steam Reforming for Fuel Cell-Powered Vehicles. Appl. Energy 2020, 268, 115043. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Lin, J.; Qiao, B.; Yang, X.; Wang, A.; Wang, X. Reactivity of Methanol Steam Reforming on ZnPd Intermetallic Catalyst: Understanding from Microcalorimetric and FT-IR Studies. J. Phys. Chem. C 2018, 122, 12395–12403. [Google Scholar] [CrossRef]
- Azenha, C.; Lagarteira, T.; Mateos-Pedrero, C.; Mendes, A. Production of Hydrogen from Methanol Steam Reforming Using CuPd/ZrO2 Catalysts—Influence of the Catalytic Surface on Methanol Conversion and CO Selectivity. Int. J. Hydrogen Energy 2021, 46, 17490–17499. [Google Scholar] [CrossRef]
- Pojanavaraphan, C.; Luengnaruemitchai, A.; Gulari, E. Hydrogen Production by Oxidative Steam Reforming of Methanol over Au/CeO2 Catalysts. Chem. Eng. J. 2012, 192, 105–113. [Google Scholar] [CrossRef]
- Liu, D.; Men, Y.; Wang, J.; Kolb, G.; Liu, X.; Wang, Y.; Sun, Q. Highly Active and Durable Pt/In2O3/Al2O3 Catalysts in Methanol Steam Reforming. Int. J. Hydrogen Energy 2016, 41, 21990–21999. [Google Scholar] [CrossRef]
- Azenha, C.S.R.; Mateos-Pedrero, C.; Queirós, S.; Concepción, P.; Mendes, A. Innovative ZrO2-Supported CuPd Catalysts for the Selective Production of Hydrogen from Methanol Steam Reforming. Appl. Catal. B Environ. 2017, 203, 400–407. [Google Scholar] [CrossRef]
- Monyanon, S.; Luengnaruemitchai, A.; Pongstabodee, S. Optimization of Methanol Steam Reforming over a Au/CuO–CeO2 Catalyst by Statistically Designed Experiments. Fuel Process. Technol. 2012, 96, 160–168. [Google Scholar] [CrossRef]
- Pojanavaraphan, C.; Luengnaruemitchai, A.; Gulari, E. Effect of Catalyst Preparation on Au/Ce1−xZrxO2 and Au–Cu/Ce1−xZrxO2 for Steam Reforming of Methanol. Int. J. Hydrogen Energy 2013, 38, 1348–1362. [Google Scholar] [CrossRef]
- Iwasa, N.; Masuda, S.; Ogawa, N.; Takezawa, N. Steam Reforming of Methanol over Pd/ZnO: Effect of the Formation of PdZn Alloys upon the Reaction. Appl. Catal. Gen. 1995, 125, 145–157. [Google Scholar] [CrossRef]
- Karim, A.; Conant, T.; Datye, A. The Role of PdZn Alloy Formation and Particle Size on the Selectivity for Steam Reforming of Methanol. J. Catal. 2006, 243, 420–427. [Google Scholar] [CrossRef]
- Danwittayakul, S.; Dutta, J. Zinc Oxide Nanorods Based Catalysts for Hydrogen Production by Steam Reforming of Methanol. Int. J. Hydrogen Energy 2012, 37, 5518–5526. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Dagle, V.L.; Halevi, B.; Datye, A.K.; Wang, Y. Influence of ZnO Facets on Pd/ZnO Catalysts for Methanol Steam Reforming. ACS Catal. 2014, 4, 2379–2386. [Google Scholar] [CrossRef]
- Liu, S.; Takahashi, K.; Ayabe, M. Hydrogen Production by Oxidative Methanol Reforming on Pd/ZnO Catalyst: Effects of Pd Loading. Catal. Today 2003, 87, 247–253. [Google Scholar] [CrossRef]
- Pfeifer, P.; Schubert, K.; Liauw, M.A.; Emig, G. PdZn Catalysts Prepared by Washcoating Microstructured Reactors. Appl. Catal. Gen. 2004, 270, 165–175. [Google Scholar] [CrossRef]
- Ranganathan, E.S.; Bej, S.K.; Thompson, L.T. Methanol Steam Reforming over Pd/ZnO and Pd/CeO2 Catalysts. Appl. Catal. Gen. 2005, 289, 153–162. [Google Scholar] [CrossRef]
- Lorenz, H.; Penner, S.; Jochum, W.; Rameshan, C.; Klötzer, B. Pd/Ga2O3 Methanol Steam Reforming Catalysts: Part II. Catalytic Selectivity. Appl. Catal. Gen. 2009, 358, 203–210. [Google Scholar] [CrossRef]
- Men, Y.; Kolb, G.; Zapf, R.; O’Connell, M.; Ziogas, A. Methanol Steam Reforming over Bimetallic Pd–In/Al2O3 Catalysts in a Microstructured Reactor. Appl. Catal. Gen. 2010, 380, 15–20. [Google Scholar] [CrossRef]
- Matsumura, Y. Enhancement in Activity of Pd–Zn Catalyst for Methanol Steam Reforming by Coprecipitation on Zirconia Support. Appl. Catal. Gen. 2013, 468, 350–358. [Google Scholar] [CrossRef]
- Mayr, L.; Lorenz, H.; Armbrüster, M.; Villaseca, S.A.; Luo, Y.; Cardoso, R.; Burkhardt, U.; Zemlyanov, D.; Haevecker, M.; Blume, R.; et al. The Catalytic Properties of Thin Film Pd-Rich GaPd2 in Methanol Steam Reforming. J. Catal. 2014, 309, 231–240. [Google Scholar] [CrossRef]
- Liu, L.; Lin, Y.; Hu, Y.; Lin, Z.; Lin, S.; Du, M.; Zhang, L.; Zhang, X.; Lin, J.; Zhang, Z.; et al. ZnAl2O4 Spinel-Supported PdZnβ Catalyst with Parts per Million Pd for Methanol Steam Reforming. ACS Catal. 2022, 12, 2714–2721. [Google Scholar] [CrossRef]
- Nehe, P.; Reddy, V.M.; Kumar, S. Investigations on a New Internally-Heated Tubular Packed-Bed Methanol–Steam Reformer. Int. J. Hydrogen Energy 2015, 40, 5715–5725. [Google Scholar] [CrossRef]
- Karim, A.; Bravo, J.; Datye, A. Nonisothermality in Packed Bed Reactors for Steam Reforming of Methanol. Appl. Catal. Gen. 2005, 282, 101–109. [Google Scholar] [CrossRef]
- Wang, F.; Wang, G. Performance and Cold Spot Effect of Methanol Steam Reforming for Hydrogen Production in Micro-Reactor. Int. J. Hydrogen Energy 2016, 41, 16835–16841. [Google Scholar] [CrossRef]
- Vidal Vázquez, F.; Simell, P.; Pennanen, J.; Lehtonen, J. Reactor Design and Catalysts Testing for Hydrogen Production by Methanol Steam Reforming for Fuel Cells Applications. Int. J. Hydrogen Energy 2016, 41, 924–935. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, C.; Yu, H.; Wu, H.; Jin, F.; Xiao, F.; Liao, Z. Enhancement of Methanol Steam Reforming in a Tubular Fixed-Bed Reactor with Simultaneous Heating inside and Outside. Energy 2022, 254, 124330. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, M.; Ying, W.; Fang, D. Two-Dimensional Modeling of a Plant-Scale Fixed-Bed Reactor for Hydrogen Production from Methanol Steam Reforming. Int. J. Hydrogen Energy 2016, 41, 16932–16943. [Google Scholar] [CrossRef]
- Perng, S.-W.; Horng, R.-F.; Ku, H.-W. Effects of Reaction Chamber Geometry on the Performance and Heat/Mass Transport Phenomenon for a Cylindrical Methanol Steam Reformer. Appl. Energy 2013, 103, 317–327. [Google Scholar] [CrossRef]
- Li, X.; You, B.; Shum, H.C.; Chen, C.-H. Future Foods: Design, Fabrication and Production through Microfluidics. Biomaterials 2022, 287, 121631. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wang, S.; Cheng, L.; Ma, H.; Gao, X.; Brennan, C.S.; Yan, J.-K. Micro-Nano-Bubble Technology and Its Applications in Food Industry: A Critical Review. Food Rev. Int. 2023, 39, 4213–4235. [Google Scholar] [CrossRef]
- Zhang, R.; Cheng, Z.; Liang, Y.; Hu, X.; Shen, T.; Li, Y.; Han, Z.; Zhang, X.; Zou, X. A Novel Strategy for Accelerating Pumpable Ice Slurry Production with Ozone Micro–Nano Bubbles and Extending the Shelf Life of Larimichthys Polyactis. Foods 2023, 12, 2206. [Google Scholar] [CrossRef]
- Zheng, Z.; He, Y.; He, Y.; Zhan, J.; Shi, C.; Xu, Y.; Wang, X.; Wang, J.; Zhang, C. Micro-Nano Bubble Water Subsurface Drip Irrigation Affects Strawberry Yield and Quality by Modulation of Microbial Communities. Agric. Water Manag. 2025, 307, 109228. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, S.; Darko, R.O. Characteristics of Water and Droplet Size Distribution from Fluidic Sprinklers. Irrig. Drain. 2016, 65, 522–529. [Google Scholar] [CrossRef]
- Burns, M.A.; Johnson, B.N.; Brahmasandra, S.N.; Handique, K.; Webster, J.R.; Krishnan, M.; Sammarco, T.S.; Man, P.M.; Jones, D.; Heldsinger, D.; et al. An Integrated Nanoliter DNA Analysis Device. Science 1998, 282, 484–487. [Google Scholar] [CrossRef]
- Cassotta, M.; Forbes-Hernández, T.Y.; Calderón Iglesias, R.; Ruiz, R.; Elexpuru Zabaleta, M.; Giampieri, F.; Battino, M. Links between Nutrition, Infectious Diseases, and Microbiota: Emerging Technologies and Opportunities for Human-Focused Research. Nutrients 2020, 12, 1827. [Google Scholar] [CrossRef]
- Jiang, L.; Hassan, M.M.; Ali, S.; Li, H.; Sheng, R.; Chen, Q. Evolving Trends in SERS-Based Techniques for Food Quality and Safety: A Review. Trends Food Sci. Technol. 2021, 112, 225–240. [Google Scholar] [CrossRef]
- Losey, M.W.; Schmidt, M.A.; Jensen, K.F. Microfabricated Multiphase Packed-Bed Reactors: Characterization of Mass Transfer and Reactions. Ind. Eng. Chem. Res. 2001, 40, 2555–2562. [Google Scholar] [CrossRef]
- Wei, T. All-in-One Design Integrates Microfluidic Cooling into Electronic Chips. Nature 2020, 585, 188–189. [Google Scholar] [CrossRef]
- Zhang, R.; Cheng, Z.; Ding, F.; Hua, L.; Fang, Y.; Han, Z.; Shi, J.; Zou, X.; Xiao, J. Improvements in Chitosan-Based Slurry Ice Production and Its Application in Precooling and Storage of Pampus Argenteus. Food Chem. 2022, 393, 133266. [Google Scholar] [CrossRef]
- Sammarco, T.S.; Burns, M.A. Thermocapillary Pumping of Discrete Drops in Microfabricated Analysis Devices. AIChE J. 1999, 45, 350–366. [Google Scholar] [CrossRef]
- Liao, J.; Luo, X.; Wang, P.; Zhou, Z.; O’Donnell, C.C.; Zang, Y.; Hewitt, A.J. Analysis of the Influence of Different Parameters on Droplet Characteristics and Droplet Size Classification Categories for Air Induction Nozzle. Agronomy 2020, 10, 256. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Karimi, M. Biomedical Applications of Microfluidic Devices; Academic Press: London, UK, 2021; ISBN 978-0-12-818792-0. [Google Scholar]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic Mixer for Microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef]
- Fredrickson, C.K.; Fan, Z.H. Macro-to-Micro Interfaces for Microfluidic Devices. Lab. Chip 2004, 4, 526. [Google Scholar] [CrossRef]
- Ganguli, A.; Bhatt, V. Hydrogen Production Using Advanced Reactors by Steam Methane Reforming: A Review. Front. Therm. Eng. 2023, 3, 1143987. [Google Scholar] [CrossRef]
- Dong, Z.; Wen, Z.; Zhao, F.; Kuhn, S.; Noël, T. Scale-up of Micro- and Milli-Reactors: An Overview of Strategies, Design Principles and Applications. Chem. Eng. Sci. X 2021, 10, 100097. [Google Scholar] [CrossRef]
- Mei, D.; Qian, M.; Liu, B.; Jin, B.; Yao, Z.; Chen, Z. A Micro-Reactor with Micro-Pin-Fin Arrays for Hydrogen Production via Methanol Steam Reforming. J. Power Sources 2012, 205, 367–376. [Google Scholar] [CrossRef]
- Pattekar, A.V.; Kothare, M.V. A Microreactor for Hydrogen Production in Micro Fuel Cell Applications. J. Microelectromechanical Syst. 2004, 13, 7–18. [Google Scholar] [CrossRef]
- Pan, L.; Wang, S. Methanol Steam Reforming in a Compact Plate-Fin Reformer for Fuel-Cell Systems. Int. J. Hydrogen Energy 2005, 30, 973–979. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Q.; Mei, D.; Wang, Y. A Methanol Fuel Processing System with Methanol Steam Reforming and CO Selective Methanation Modules for PEMFC Application. Int. J. Energy Res. 2021, 45, 6163–6173. [Google Scholar] [CrossRef]
- Iulianelli, A.; Ribeirinha, P.; Mendes, A.; Basile, A. Methanol Steam Reforming for Hydrogen Generation via Conventional and Membrane Reactors: A Review. Renew. Sustain. Energy Rev. 2014, 29, 355–368. [Google Scholar] [CrossRef]
- Lee, M.; Greif, R.; Grigoropoulos, C.P.; Park, H.G.; Hsu, F.K. Transport in Packed-Bed and Wall-Coated Steam-Methanol Reformers. J. Power Sources 2007, 166, 194–201. [Google Scholar] [CrossRef]
- Sanz, O.; Velasco, I.; Pérez-Miqueo, I.; Poyato, R.; Odriozola, J.A.; Montes, M. Intensification of Hydrogen Production by Methanol Steam Reforming. Int. J. Hydrogen Energy 2016, 41, 5250–5259. [Google Scholar] [CrossRef]
- Du, X.; Shen, Y.; Yang, L.; Shi, Y.; Yang, Y. Experiments on Hydrogen Production from Methanol Steam Reforming in the Microchannel Reactor. Int. J. Hydrogen Energy 2012, 37, 12271–12280. [Google Scholar] [CrossRef]
- Pan, M.; Wu, Q.; Jiang, L.; Zeng, D. Effect of Microchannel Structure on the Reaction Performance of Methanol Steam Reforming. Appl. Energy 2015, 154, 416–427. [Google Scholar] [CrossRef]
- Mei, D.; Liang, L.; Qian, M.; Feng, Y. A Performance Study of Methanol Steam Reforming in an A-Type Microchannel Reactor. Int. J. Hydrogen Energy 2014, 39, 17690–17701. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, F.; Huang, X. Mass Transfer and Reaction in Methanol Steam Reforming Reactor with Fractal Tree-like Microchannel Network. Int. J. Heat Mass Transf. 2015, 87, 279–283. [Google Scholar] [CrossRef]
- Zhuang, X.; Xia, X.; Xu, X.; Li, L. Experimental Investigation on Hydrogen Production by Methanol Steam Reforming in a Novel Multichannel Micro Packed Bed Reformer. Int. J. Hydrogen Energy 2020, 45, 11024–11034. [Google Scholar] [CrossRef]
- Zhuang, X.; Xu, X.; Li, L.; Deng, D. Numerical Investigation of a Multichannel Reactor for Syngas Production by Methanol Steam Reforming at Various Operating Conditions. Int. J. Hydrogen Energy 2020, 45, 14790–14805. [Google Scholar] [CrossRef]
- Hao, Y.; Du, X.; Yang, L.; Shen, Y.; Yang, Y. Numerical Simulation of Configuration and Catalyst-Layer Effects on Micro-Channel Steam Reforming of Methanol. Int. J. Hydrogen Energy 2011, 36, 15611–15621. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, R.; Toan, S.; Xu, R.; Zhou, F.; Sun, Z.; Sun, Z. Microchannel Structure Design for Hydrogen Supply from Methanol Steam Reforming. Chem. Eng. J. 2022, 429, 132286. [Google Scholar] [CrossRef]
- Zeng, D.; Pan, M.; Wang, L.; Tang, Y. Fabrication and Characteristics of Cube-Post Microreactors for Methanol Steam Reforming. Appl. Energy 2012, 91, 208–213. [Google Scholar] [CrossRef]
- Mei, D.; Qian, M.; Yao, Z.; Liu, B.; Lou, X.; Chen, Z. Effects of Structural Parameters on the Performance of a Micro-Reactor with Micro-Pin-Fin Arrays (MPFAR) for Hydrogen Production. Int. J. Hydrogen Energy 2012, 37, 17817–17827. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Jang, J.-Y.; Cheng, C.-H. Fractal Channel Design in a Micro Methanol Steam Reformer. Int. J. Hydrogen Energy 2014, 39, 1998–2007. [Google Scholar] [CrossRef]
- Yao, F.; Chen, Y.; Peterson, G.P. Hydrogen Production by Methanol Steam Reforming in a Disc Microreactor with Tree-Shaped Flow Architectures. Int. J. Heat Mass Transf. 2013, 64, 418–425. [Google Scholar] [CrossRef]
- Perng, S.-W.; Wu, H.-W. Effect of Depth and Diameter of Cylindrical Cavity in a Plate-Type Methanol Steam Reformer on Estimated Net Power of PEMFC. Energy Convers. Manag. 2018, 177, 190–209. [Google Scholar] [CrossRef]
- Chu, X.; Zeng, X.; Zheng, T.; Zhuang, W.; Yang, Y.; Zhou, W.; Hong, Y. Structural Design and Performance Research of Methanol Steam Reforming Microchannel for Hydrogen Production Based on Mixing Effect. Int. J. Hydrogen Energy 2020, 45, 20859–20874. [Google Scholar] [CrossRef]
- Ma, G.; Mao, H.; Bu, Q.; Han, L.; Shabbir, A.; Gao, F. Effect of Compound Biochar Substrate on the Root Growth of Cucumber Plug Seedlings. Agronomy 2020, 10, 1080. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, J.; Yan, H.; Akhlaq, M.; Ni, Y.; Xue, R.; Li, J. Effects of Different Irrigation Amounts and Biochar Application on Soil Physical and Mechanical Properties in the Short Term. Irrig. Drain. 2024, 73, 866–881. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Han, I.; Wang, P.; Mei, Q.; Huang, Y. Effects of Different Straw Biochars on Soil Organic Carbon, Nitrogen, Available Phosphorus, and Enzyme Activity in Paddy Soil. Sci. Rep. 2020, 10, 8837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, X.; Yan, H.; Ullah, I.; Zuo, Z.; Li, L.; Yu, J. Effects of Irrigation Quantity and Biochar on Soil Physical Properties, Growth Characteristics, Yield and Quality of Greenhouse Tomato. Agric. Water Manag. 2020, 241, 106263. [Google Scholar] [CrossRef]
- Fan, X.; Peng, L.; Wang, X.; Han, S.; Yang, L.; Wang, H.; Hao, C. Efficient Capture of Lead Ion and Methylene Blue by Functionalized Biomass Carbon-Based Adsorbent for Wastewater Treatment. Ind. Crops Prod. 2022, 183, 114966. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, Z.; Zhang, T.; Chen, Q.; Qiu, F.; Peng, Y.; Tang, S. Fabrication of Functional Biomass Carbon Aerogels Derived from Sisal Fibers for Application in Selenium Extraction. Food Bioprod. Process. 2018, 111, 93–103. [Google Scholar] [CrossRef]
- Jing, Z.; Ding, J.; Zhang, T.; Yang, D.; Qiu, F.; Chen, Q.; Xu, J. Flexible, Versatility and Superhydrophobic Biomass Carbon Aerogels Derived from Corn Bracts for Efficient Oil/Water Separation. Food Bioprod. Process. 2019, 115, 134–142. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, B.; Chen, Q.; Peng, X.; Yang, D.; Qiu, F. Layered Double Hydroxide Functionalized Biomass Carbon Fiber for Highly Efficient and Recyclable Fluoride Adsorption. Appl. Biol. Chem. 2019, 62, 12. [Google Scholar] [CrossRef]
- Xu, Y.; Kutsanedzie, F.Y.H.; Hassan, M.; Zhu, J.; Ahmad, W.; Li, H.; Chen, Q. Mesoporous Silica Supported Orderly-Spaced Gold Nanoparticles SERS-Based Sensor for Pesticides Detection in Food. Food Chem. 2020, 315, 126300. [Google Scholar] [CrossRef]
- Shao, S.; Sun, T.; Li, X.; Wang, Y.; Ma, L.; Liu, Z.; Wu, S. Preparation of Heavy Bio-Oil-Based Porous Carbon by Pyrolysis Gas Activation and Its Performance in the Aldol Condensation for Aviation Fuel as Catalyst Carrier. Ind. Crops Prod. 2024, 218, 118963. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, W. Sponge Effect of Aerated Concrete on Phosphorus Adsorption-Desorption from Agricultural Drainage Water in Rainfall. Soil Water Res. 2020, 15, 220–227. [Google Scholar] [CrossRef]
- Bu, Q.; Chen, K.; Morgan, H.M.; Liang, J.; Zhang, X.; Yan, L.; Mao, H. Thermal Behavior and Kinetic Study of the Effects of Zinc-Modified Biochar Catalyst on Lignin and Low-Density Polyethylene (LDPE) Co-Pyrolysis. Trans. ASABE 2018, 61, 1783–1793. [Google Scholar] [CrossRef]
- Banhart, J. Manufacturing Routes for Metallic Foams. Jom 2000, 52, 22–27. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Dhakad, S.K. Preparation of Metal Foam by Different Methods: A Review. Mater. Today Proc. 2020, 26, 1784–1790. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, W.; Lin, Y.; Chen, L.; Chu, X.; Zheng, T.; Wan, S.; Lin, J. Novel Copper Foam with Ordered Hole Arrays as Catalyst Support for Methanol Steam Reforming Microreactor. Appl. Energy 2019, 246, 24–37. [Google Scholar] [CrossRef]
- Zhou, W.; Ke, Y.; Pei, P.; Yu, W.; Chu, X.; Li, S.; Yang, K. Hydrogen Production from Cylindrical Methanol Steam Reforming Microreactor with Porous Cu-Al Fiber Sintered Felt. Int. J. Hydrogen Energy 2018, 43, 3643–3654. [Google Scholar] [CrossRef]
- Li, J.-R.; Yu, C.-L.; Xu, Z.-J.; Wang, Q.-H.; Zhou, W.; Zheng, T.-Q. Preparing a Novel Gradient Porous Metal Fiber Sintered Felt with Better Manufacturability for Hydrogen Production via Methanol Steam Reforming. Int. J. Hydrogen Energy 2019, 44, 23983–23995. [Google Scholar] [CrossRef]
- Pan, M.; Wei, X.; Tang, Y. Factors Influencing Methanol Steam Reforming inside the Oriented Linear Copper Fiber Sintered Felt. Int. J. Hydrogen Energy 2012, 37, 11157–11166. [Google Scholar] [CrossRef]
- Pan, M.; Tang, Y.; Wei, X.; Jiang, X. Oriented Linear Cutting Fiber Sintered Felt as an Innovative Catalyst Support for Methanol Steam Reforming. Int. J. Hydrogen Energy 2011, 36, 7066–7073. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Q.; Qiu, Q.; Tang, Y.; Tu, J.; Hui, K.S.; Hui, K.N. Heat and Mass Transfer Characterization of Porous Copper Fiber Sintered Felt as Catalyst Support for Methanol Steam Reforming. Fuel 2015, 145, 136–142. [Google Scholar] [CrossRef]
- Ke, Y.; Zhou, W.; Chu, X.; Yuan, D.; Wan, S.; Yu, W.; Liu, Y. Porous Copper Fiber Sintered Felts with Surface Microchannels for Methanol Steam Reforming Microreactor for Hydrogen Production. Int. J. Hydrogen Energy 2019, 44, 5755–5765. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Yang, S.; Zhou, W.; Li, J.-R.; Xu, Z.-J.; Ke, Y.-Z.; Yu, W.; Hu, G.-H. Optimizing the Porosity Configuration of Porous Copper Fiber Sintered Felt for Methanol Steam Reforming Micro-Reactor Based on Flow Distribution. Appl. Energy 2018, 216, 243–261. [Google Scholar] [CrossRef]
- Liao, M.; Qin, H.; Guo, W.; Gao, P.; Xiao, H. Porous Reticular CuO/ZnO/CeO2/ZrO2 Catalyst Derived from Polyacrylic Acid Hydrogel System on Al2O3 Foam Ceramic Support for Methanol Steam Reforming Microreactor. Ceram. Int. 2021, 47, 33667–33677. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Mei, D.; Wu, Q.; Zhou, H. A Novel Thermally Autonomous Methanol Steam Reforming Microreactor Using SiC Honeycomb Ceramic as Catalyst Support for Hydrogen Production. Int. J. Hydrogen Energy 2021, 46, 25878–25892. [Google Scholar] [CrossRef]
- Moreno, A.M.; Wilhite, B.A. Autothermal Hydrogen Generation from Methanol in a Ceramic Microchannel Network. J. Power Sources 2010, 195, 1964–1970. [Google Scholar] [CrossRef]
- Liao, M.; Guo, C.; Guo, W.; Hu, T.; Qin, H.; Gao, P.; Xiao, H. Hydrogen Production in Microreactor Using Porous SiC Ceramic with a Pore-in-Pore Hierarchical Structure as Catalyst Support. Int. J. Hydrogen Energy 2020, 45, 20922–20932. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Z.; Cen, S.; Liang, N.; Shi, J.; Huang, X.; Zou, X. Preparation of Pangasius Hypophthalmus Protein-Stabilized Pickering Emulsions and 3D Printing Application. J. Food Eng. 2023, 341, 111333. [Google Scholar] [CrossRef]
- Guo, Z.; Arslan, M.; Li, Z.; Cen, S.; Shi, J.; Huang, X.; Xiao, J.; Zou, X. Application of Protein in Extrusion-Based 3D Food Printing: Current Status and Prospectus. Foods 2022, 11, 1902. [Google Scholar] [CrossRef]
- Pan, J.; Chen, X.; Zhu, Y.; Xu, B.; Li, C.; Khin, M.N.; Cui, H.; Lin, L. Design and Development of Dual-Extruder Food 3D Printer Based on Selective Compliance Assembly Robot Arm and Printing of Various Inks. J. Food Eng. 2024, 370, 111973. [Google Scholar] [CrossRef]
- Lei, H.-Y.; Li, J.-R.; Wang, Q.-H.; Xu, Z.-J.; Zhou, W.; Yu, C.-L.; Zheng, T.-Q. Feasibility of Preparing Additive Manufactured Porous Stainless Steel Felts with Mathematical Micro Pore Structure as Novel Catalyst Support for Hydrogen Production via Methanol Steam Reforming. Int. J. Hydrogen Energy 2019, 44, 24782–24791. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Fan, Y.; Zhou, W. Fabrication of Porous Metal by Selective Laser Melting as Catalyst Support for Hydrogen Production Microreactor. Int. J. Hydrogen Energy 2020, 45, 10–22. [Google Scholar] [CrossRef]
- Zheng, T.; Zhou, W.; Geng, D.; Li, Y.; Liu, Y.; Zhang, C. Methanol Steam Reforming Microreactor with Novel 3D-Printed Porous Stainless Steel Support as Catalyst Support. Int. J. Hydrogen Energy 2020, 45, 14006–14016. [Google Scholar] [CrossRef]
- Li, X.; Rezaei, F.; Rownaghi, A.A. 3D-Printed Zeolite Monoliths with Hierarchical Porosity for Selective Methanol to Light Olefin Reaction. React. Chem. Eng. 2018, 3, 733–746. [Google Scholar] [CrossRef]
- Tubío, C.R.; Azuaje, J.; Escalante, L.; Coelho, A.; Guitián, F.; Sotelo, E.; Gil, A. 3D Printing of a Heterogeneous Copper-Based Catalyst. J. Catal. 2016, 334, 110–115. [Google Scholar] [CrossRef]
- Mei, D.; Feng, Y.; Qian, M.; Chen, Z. An Innovative Micro-Channel Catalyst Support with a Micro-Porous Surface for Hydrogen Production via Methanol Steam Reforming. Int. J. Hydrogen Energy 2016, 41, 2268–2277. [Google Scholar] [CrossRef]
- Gyak, K.-W.; Vishwakarma, N.K.; Hwang, Y.-H.; Kim, J.; Yun, H.; Kim, D.-P. 3D-Printed Monolithic SiCN Ceramic Microreactors from a Photocurable Preceramic Resin for the High Temperature Ammonia Cracking Process. React. Chem. Eng. 2019, 4, 1393–1399. [Google Scholar] [CrossRef]
- Roman-Manso, B.; Muth, J.; Gibson, L.J.; Ruettinger, W.; Lewis, J.A. Hierarchically Porous Ceramics via Direct Writing of Binary Colloidal Gel Foams. ACS Appl. Mater. Interfaces 2021, 13, 8976–8984. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, X.; Xiong, H.; Zhou, K.; Zhang, D. Optimized Preceramic Polymer for 3D Structured Ceramics via Fused Deposition Modeling. J. Eur. Ceram. Soc. 2021, 41, 5066–5074. [Google Scholar] [CrossRef]
- Schmidt, J.; Colombo, P. Digital Light Processing of Ceramic Components from Polysiloxanes. J. Eur. Ceram. Soc. 2018, 38, 57–66. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Mei, D.; Yu, S. Direct Ink Writing of 3D SiC Scaffold as Catalyst Support for Thermally Autonomous Methanol Steam Reforming Microreactor. Renew. Energy 2022, 187, 923–932. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Xue, F.; Huang, Y.; Zhou, K.; Zhang, D. 3D Printing of SiC Ceramic: Direct Ink Writing with a Solution of Preceramic Polymers. J. Eur. Ceram. Soc. 2018, 38, 5294–5300. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, W.; Leng, J.; Liu, X.; Xu, H.; Ding, H.; Zhou, J.; Cui, L. Review and Research Prospects on Additive Manufacturing Technology for Agricultural Manufacturing. Agriculture 2024, 14, 1207. [Google Scholar] [CrossRef]
- Avril, A.; Hornung, C.H.; Urban, A.; Fraser, D.; Horne, M.; Veder, J.-P.; Tsanaktsidis, J.; Rodopoulos, T.; Henry, C.; Gunasegaram, D.R. Continuous Flow Hydrogenations Using Novel Catalytic Static Mixers inside a Tubular Reactor. React. Chem. Eng. 2017, 2, 180–188. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.; et al. Adsorption Properties of Macroporous Adsorbent Resins for Separation of Anthocyanins from Mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Hou, B.; Hao, C.; Li, X.; Wu, J. Construction of a Lignosulfonate–Lysine Hydrogel for the Adsorption of Heavy Metal Ions. J. Agric. Food Chem. 2020, 68, 3050–3060. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Qiu, F.; Zhang, T.; Xu, J. Controllable Preparation of FeOOH/CuO@WBC Composite Based on Water Bamboo Cellulose Applied for Enhanced Arsenic Removal. Food Bioprod. Process. 2020, 123, 177–187. [Google Scholar] [CrossRef]
- Han, J.; Wang, L.; Wang, L.; Li, C.; Mao, Y.; Wang, Y. Fabrication of a Core-Shell-Shell Magnetic Polymeric Microsphere with Excellent Performance for Separation and Purification of Bromelain. Food Chem. 2019, 283, 1–10. [Google Scholar] [CrossRef]
- Chao, Y.; Pang, J.; Bai, Y.; Wu, P.; Luo, J.; He, J.; Jin, Y.; Li, X.; Xiong, J.; Li, H.; et al. Graphene-like BN@SiO2 Nanocomposites as Efficient Sorbents for Solid-Phase Extraction of Rhodamine B and Rhodamine 6G from Food Samples. Food Chem. 2020, 320, 126666. [Google Scholar] [CrossRef]
- Luo, P.; Han, J.; Li, Y.; Wang, Y.; Wang, L.; Ni, L. Preparation of Dendritic Polymer-based Magnetic Carrier for Application of Bromelain Separation and Purification. J. Food Biochem. 2019, 43, e12976. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, X.; Yu, F.; Quan, Y. Preparation of Dummy Molecularly Imprinted Polymers Based on Dextran-Modified Magnetic Nanoparticles Fe3O4 for the Selective Detection of Acrylamide in Potato Chips. Food Chem. 2020, 317, 126431. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, L.; Wang, Y.; Cai, Y.; Mao, Y.; Ni, L.; Xie, X. Preparation of Temperature-Sensitive Magnetic Microspheres for Separation and Purification of Bromelain. Food Bioprod. Process. 2019, 114, 253–262. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, C.; Bao, Q.; Zheng, J.; Deng, D.; Duan, Y.; Shen, L. The Physicochemical Characterization, Equilibrium, and Kinetics of Heavy Metal Ions Adsorption from Aqueous Solution by Arrowhead Plant (Sagittaria trifolia L.) Stalk. J. Food Biochem. 2018, 42, e12448. [Google Scholar] [CrossRef]
- Xiao, J.; Li, C.; Fang, L.; Böwer, P.; Wark, M.; Bénard, P.; Chahine, R. Machine Learning–Based Optimization for Hydrogen Purification Performance of Layered Bed Pressure Swing Adsorption. Int. J. Energy Res. 2020, 44, 4475–4492. [Google Scholar] [CrossRef]
- Casas, N.; Schell, J.; Pini, R.; Mazzotti, M. Fixed Bed Adsorption of CO2/H2 Mixtures on Activated Carbon: Experiments and Modeling. Adsorption 2012, 18, 143–161. [Google Scholar] [CrossRef]
- Mondal, M.; Datta, A. Energy Transfer in Hydrogen Separation from Syngas Using Pressure Swing Adsorption (PSA) Process: A Thermodynamic Model: Hydrogen Separation Using PSA. Int. J. Energy Res. 2017, 41, 448–458. [Google Scholar] [CrossRef]
- Yang, J.; Lee, C.-H.; Chang, J.-W. Separation of Hydrogen Mixtures by a Two-Bed Pressure Swing Adsorption Process Using Zeolite 5A. Ind. Eng. Chem. Res. 1997, 36, 2789–2798. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, J.-N.; Cho, S.-H.; Kim, J.-D.; Yang, R.T. Adsorber Dynamics and Optimal Design of Layered Beds for Multicomponent Gas Adsorption. Chem. Eng. Sci. 1998, 53, 3951–3963. [Google Scholar] [CrossRef]
- Jang, S.-C.; Yang, S.-I.; Oh, S.-G.; Choi, D.-K. Adsorption Dynamics and Effects of Carbon to Zeolite Ratio of Layered Beds for Multicomponent Gas Adsorption. Korean J. Chem. Eng. 2011, 28, 583–590. [Google Scholar] [CrossRef]
- Férey, G.; Serre, C.; Devic, T.; Maurin, G.; Jobic, H.; Llewellyn, P.L.; De Weireld, G.; Vimont, A.; Daturi, M.; Chang, J.-S. Why Hybrid Porous Solids Capture Greenhouse Gases? Chem. Soc. Rev. 2011, 40, 550–562. [Google Scholar] [CrossRef]
- Silva, B.; Solomon, I.; Ribeiro, A.M.; Lee, U.-H.; Hwang, Y.K.; Chang, J.-S.; Loureiro, J.M.; Rodrigues, A.E. H2 Purification by Pressure Swing Adsorption Using CuBTC. Sep. Purif. Technol. 2013, 118, 744–756. [Google Scholar] [CrossRef]
- Golmakani, A.; Nabavi, S.A.; Manović, V. Effect of Impurities on Ultra-Pure Hydrogen Production by Pressure Vacuum Swing Adsorption. J. Ind. Eng. Chem. 2020, 82, 278–289. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Chen, X.; Xia, Q.; Li, Z. Adsorption of CO2 on Zeolite 13X and Activated Carbon with Higher Surface Area. Sep. Sci. Technol. 2010, 45, 710–719. [Google Scholar] [CrossRef]
- Dantas, T.L.P.; Luna, F.M.T.; Silva, I.J.; De Azevedo, D.C.S.; Grande, C.A.; Rodrigues, A.E.; Moreira, R.F.P.M. Carbon Dioxide–Nitrogen Separation through Adsorption on Activated Carbon in a Fixed Bed. Chem. Eng. J. 2011, 169, 11–19. [Google Scholar] [CrossRef]
- Brea, P.; Delgado, J.A.; Águeda, V.I.; Uguina, M.A. Comparison between MOF UTSA-16 and BPL Activated Carbon in Hydrogen Purification by PSA. Chem. Eng. J. 2019, 355, 279–289. [Google Scholar] [CrossRef]
- Abdeljaoued, A.; Relvas, F.; Mendes, A.; Chahbani, M.H. Simulation and Experimental Results of a PSA Process for Production of Hydrogen Used in Fuel Cells. J. Environ. Chem. Eng. 2018, 6, 338–355. [Google Scholar] [CrossRef]
- Shi, W.; Yang, H.; Shen, Y.; Fu, Q.; Zhang, D.; Fu, B. Two-Stage PSA/VSA to Produce H2 with CO2 Capture via Steam Methane Reforming (SMR). Int. J. Hydrogen Energy 2018, 43, 19057–19074. [Google Scholar] [CrossRef]
- Jamali, S.; Mofarahi, M.; Rodrigues, A.E. Investigation of a Novel Combination of Adsorbents for Hydrogen Purification Using Cu-BTC and Conventional Adsorbents in Pressure Swing Adsorption. Adsorption 2018, 24, 481–498. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Grande, C.A.; Lopes, F.V.S.; Loureiro, J.M.; Rodrigues, A.E. A Parametric Study of Layered Bed PSA for Hydrogen Purification. Chem. Eng. Sci. 2008, 63, 5258–5273. [Google Scholar] [CrossRef]
- Cen, P.; Yang, R.T. Bulk Gas Separation by Pressure Swing Adsorption. Ind. Eng. Chem. Fundam. 1986, 25, 758–767. [Google Scholar] [CrossRef]
- You, Y.-W.; Lee, D.-G.; Yoon, K.-Y.; Moon, D.-K.; Kim, S.M.; Lee, C.-H. H2 PSA Purifier for CO Removal from Hydrogen Mixtures. Int. J. Hydrogen Energy 2012, 37, 18175–18186. [Google Scholar] [CrossRef]
- Ahn, S.; You, Y.-W.; Lee, D.-G.; Kim, K.-H.; Oh, M.; Lee, C.-H. Layered Two- and Four-Bed PSA Processes for H2 Recovery from Coal Gas. Chem. Eng. Sci. 2012, 68, 413–423. [Google Scholar] [CrossRef]
- Nikolic, D.; Giovanoglou, A.; Georgiadis, M.C.; Kikkinides, E.S. Modelling and Simulation of Multi-Bed Pressure Swing Adsorption Processes. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2007; Volume 24, pp. 159–164. ISBN 978-0-444-53157-5. [Google Scholar] [CrossRef]
- Xiao, J.; Peng, Y.; Bénard, P.; Chahine, R. Thermal Effects on Breakthrough Curves of Pressure Swing Adsorption for Hydrogen Purification. Int. J. Hydrogen Energy 2016, 41, 8236–8245. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Cho, S. Performance Analysis of Four-bed H2 PSA Process Using Layered Beds. AIChE J. 2000, 46, 790–802. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Lee, D.; Ahn, H.; Kim, M.; Lee, C. Heat-exchange Pressure Swing Adsorption Process for Hydrogen Separation. AIChE J. 2008, 54, 2054–2064. [Google Scholar] [CrossRef]
- Huang, Q.; Malekian, A.; Eić, M. Optimization of PSA Process for Producing Enriched Hydrogen from Plasma Reactor Gas. Sep. Purif. Technol. 2008, 62, 22–31. [Google Scholar] [CrossRef]
- Lee, S.; Lim, H. Utilization of CO2 Arising from Methane Steam Reforming Reaction: Use of CO2 Membrane and Heterotic Reactors. J. Ind. Eng. Chem. 2020, 91, 201–212. [Google Scholar] [CrossRef]
- Giaconia, A.; Iaquaniello, G.; Morico, B.; Salladini, A.; Palo, E. Techno-Economic Assessment of Solar Steam Reforming of Methane in a Membrane Reactor Using Molten Salts as Heat Transfer Fluid. Int. J. Hydrogen Energy 2021, 46, 35172–35188. [Google Scholar] [CrossRef]
- Sá, S.; Sousa, J.M.; Mendes, A. Steam Reforming of Methanol over a CuO/ZnO/Al2O3 Catalyst Part II: A Carbon Membrane Reactor. Chem. Eng. Sci. 2011, 66, 5523–5530. [Google Scholar] [CrossRef]
- Saidi, M. Performance Assessment and Evaluation of Catalytic Membrane Reactor for Pure Hydrogen Production via Steam Reforming of Methanol. Int. J. Hydrogen Energy 2017, 42, 16170–16185. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, S.; Ganguly, S.; Patwardhan, A.V. Steam Reforming of Methane and Methanol in Simulated Macro & Micro-Scale Membrane Reactors: Selective Separation of Hydrogen for Optimum Conversion. J. Nat. Gas Sci. Eng. 2014, 18, 286–295. [Google Scholar] [CrossRef]
- Chicano, J.; Dion, C.T.; Pasaogullari, U.; Valla, J.A. Simulation of 12-Bed Vacuum Pressure-Swing Adsorption for Hydrogen Separation from Methanol-Steam Reforming off-Gas. Int. J. Hydrogen Energy 2021, 46, 28626–28640. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, A.; Upadhyay, R.K. Performance Comparison of Methanol Steam Reforming Integrated to Pd-Ag Membrane: Membrane Reformer vs. Membrane Separator. Sep. Purif. Technol. 2017, 183, 194–203. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Liu, H.; Liu, G.; He, Y. Numerical Studies of Sorption-Enhanced Glycerol Steam Reforming in a Fluidized Bed Membrane Reactor at Low Temperature. Int. J. Hydrogen Energy 2020, 45, 8346–8356. [Google Scholar] [CrossRef]
- Cifuentes, A.; Soler, L.; Torres, R.; Llorca, J. Methanol Steam Reforming over PdZn/ZnAl2O4/Al2O3 in a Catalytic Membrane Reactor: An Experimental and Modelling Study. Int. J. Hydrogen Energy 2022, 47, 11574–11588. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Harasi, J.N.; Amiri, T.Y.; Basile, A.; Iulianelli, A. Methanol Steam Reforming for Hydrogen Generation: A Comparative Modeling Study between Silica and Pd-Based Membrane Reactors by CFD Method. Fuel Process. Technol. 2020, 199, 106273. [Google Scholar] [CrossRef]
- Amiri, T.Y.; Ghasemzageh, K.; Iulianelli, A. Membrane Reactors for Sustainable Hydrogen Production through Steam Reforming of Hydrocarbons: A Review. Chem. Eng. Process.-Process Intensif. 2020, 157, 108148. [Google Scholar] [CrossRef]
- Li, C.; He, Z.; Ban, X.; Li, N.; Chen, C.; Zhan, Z. Membrane-Based Catalytic Partial Oxidation of Ethanol Coupled with Steam Reforming for Solid Oxide Fuel Cells. J. Membr. Sci. 2021, 622, 119032. [Google Scholar] [CrossRef]
- Sheu, W.-J.; Hsu, Z.-W.; Chen, W.-H.; Chen, Y.-C. Investigation of Steam Methane Reforming in a Pd–Ru Membrane Reactor with a Counter-Current Configuration. Int. J. Hydrogen Energy 2024, 52, 938–952. [Google Scholar] [CrossRef]
- Chen, T.; Butt, F.S.; Zhang, M.; Wei, X.; Lewis, A.; Radacsi, N.; Semiao, A.J.C.; Han, J.; Huang, Y. Ultra-Permeable Zeolitic Imidazolate Frameworks-Intercalated Graphene Oxide Membranes for Unprecedented Ultrafast Molecular Separation. Chem. Eng. J. 2021, 419, 129507. [Google Scholar] [CrossRef]
- Khorramdel, H.; Omidvar, M.; Tajaddini, M.; Huang, Y.; Saeb, M.R.; Seidi, F.; Xiao, H. Surface Engineering of Graphene Oxide Membranes for Selective Separation of Perfluorooctanoic Acids. J. Membr. Sci. 2022, 664, 121047. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhang, C.; Dang, W.; Xue, L.; Liu, H.; Cheng, H.; Yan, X. Facile and Selective Separation of Anthraquinones by Alizarin-Modified Iron Oxide Magnetic Nanoparticles. J. Chromatogr. A 2023, 1702, 464088. [Google Scholar] [CrossRef] [PubMed]
- Khanipour, M.; Mirvakili, A.; Bakhtyari, A.; Farniaei, M.; Rahimpour, M.R. A Membrane-Assisted Hydrogen and Carbon Oxides Separation from Flare Gas and Recovery to a Commercial Methanol Reactor. Int. J. Hydrogen Energy 2020, 45, 7386–7400. [Google Scholar] [CrossRef]
- Shen, Q.; Cai, Z.; Zhang, X.; Chen, G.; Yang, G.; Li, S. Novel Spinel Oxide Catalysts CuFexAl2−xO4 with High H2 Selectivity with Low CO Generation in Methanol Steam Reforming. J. Alloys Compd. 2023, 951, 169878. [Google Scholar] [CrossRef]
- Ling, M.; Zhao, G.; Chen, W.; Wang, M.; Xue, Q.; Lu, Y. Microfibrous Structured Catalytic Packings for Miniature Methanol Fuel Processor: Methanol Steam Reforming and CO Preferential Oxidation. Int. J. Hydrogen Energy 2011, 36, 12833–12842. [Google Scholar] [CrossRef]
- Iulianelli, A.; Longo, T.; Basile, A. Methanol Steam Reforming in a Dense Pd–Ag Membrane Reactor: The Pressure and WHSV Effects on CO-Free H2 Production. J. Membr. Sci. 2008, 323, 235–240. [Google Scholar] [CrossRef]
- Xu, X.; Lan, T.; Zhao, G.; Nie, Q.; Jiang, F.; Lu, Y. Interface-Hydroxyl Enabling Methanol Steam Reforming toward CO-Free Hydrogen Production over Inverse ZrO2/Cu Catalyst. Appl. Catal. B Environ. 2023, 334, 122839. [Google Scholar] [CrossRef]
- Geng, J.; Guo, Q.; Pan, J.; Chi, B.; Pu, J. Enhanced Stability of Co-Reforming Diesel and Methanol into Hydrogen-Enriched Gases for Solid Oxide Fuel Cell Application. J. Power Sources 2023, 564, 232830. [Google Scholar] [CrossRef]
- Ritzkopf, I.; Vukojević, S.; Weidenthaler, C.; Grunwaldt, J.-D.; Schüth, F. Decreased CO Production in Methanol Steam Reforming over Cu/ZrO2 Catalysts Prepared by the Microemulsion Technique. Appl. Catal. Gen. 2006, 302, 215–223. [Google Scholar] [CrossRef]
- Perng, S.-W.; Horng, R.-F.; Ku, H.-W. Numerical Predictions of Design and Operating Parameters of Reformer on the Fuel Conversion and CO Production for the Steam Reforming of Methanol. Int. J. Hydrogen Energy 2013, 38, 840–852. [Google Scholar] [CrossRef]
- He, B.; Yu, Y.; Gong, X.; Liu, S.; Tian, H.; Leng, E. Mechanism of Acid-Catalyzed Pyrolysis of Levoglucosan: Formation of Anhydro-Disaccharides. Fuel 2023, 345, 128242. [Google Scholar] [CrossRef]
- Gallucci, F.; Basile, A. Co-Current and Counter-Current Modes for Methanol Steam Reforming Membrane Reactor. Int. J. Hydrogen Energy 2006, 31, 2243–2249. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Neyman, K.M.; Gordienko, A.B.; Rösch, N. Surface Structure and Stability of PdZn and PtZn Alloys: Density-Functional Slab Model Studies. Phys. Rev. B 2003, 68, 075417. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Song, Y.; Min, Z.; Mourant, D.; Li, T.; Gunawan, R.; Li, C.-Z. Catalytic Steam Reforming of Cellulose-Derived Compounds Using a Char-Supported Iron Catalyst. Fuel Process. Technol. 2013, 116, 234–240. [Google Scholar] [CrossRef]
- Wei, D.; Peng, Q.; Wang, H.; Tian, X.; Xiao, H.; Liu, H.; Fu, G. Experimental Investigation of Blended H2/CH4 Combustion in Combustors with Block for Micro-Thermophotovoltaic. Fuel 2024, 357, 129869. [Google Scholar] [CrossRef]
- Masoudi Soltani, S.; Lahiri, A.; Bahzad, H.; Clough, P.; Gorbounov, M.; Yan, Y. Sorption-Enhanced Steam Methane Reforming for Combined CO2 Capture and Hydrogen Production: A State-of-the-Art Review. Carbon Capture Sci. Technol. 2021, 1, 100003. [Google Scholar] [CrossRef]
- Cormos, C.-C.; Cormos, A.-M.; Petrescu, L.; Dragan, S. Techno-Economic Assessment of Decarbonized Biogas Catalytic Reforming for Flexible Hydrogen and Power Production. Appl. Therm. Eng. 2022, 207, 118218. [Google Scholar] [CrossRef]
- Wu, S.-L.; Wey, M.-Y. Synthesis of Ni@Al2O3 Nanocomposite with Superior Activity and Stability for Hydrogen Production from Plastic-Derived Syngas by CO2-Sorption-Enhanced Reforming. Int. J. Hydrogen Energy 2021, 46, 39728–39735. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Pasha, A.A.; Khan, H.W.; Imteyaz, B.; Irshad, K. Sustainable and Energy Efficient Hydrogen Production via Glycerol Reforming Techniques: A Review. Int. J. Hydrogen Energy 2022, 47, 41397–41420. [Google Scholar] [CrossRef]
- Kontchouo, F.M.B.; Zhang, X.; Shao, Y.; Gao, G.; Zhang, S.; Wang, Z.; Hu, X. Steam Reforming of Guaiacol and N-Hexanol for Production of Hydrogen: Effects of Aromatic and Aliphatic Structures on Properties of the Coke. Mol. Catal. 2022, 528, 112498. [Google Scholar] [CrossRef]
- Park, M.-J.; Kim, H.-M.; Lee, Y.-H.; Jeon, K.-W.; Jeong, D.-W. Optimization of a Renewable Hydrogen Production System from Food Waste: A Combination of Anaerobic Digestion and Biogas Reforming. Waste Manag. 2022, 144, 272–284. [Google Scholar] [CrossRef]
- Akande, O.; Lee, B. Plasma Steam Methane Reforming (PSMR) Using a Microwave Torch for Commercial-Scale Distributed Hydrogen Production. Int. J. Hydrogen Energy 2022, 47, 2874–2884. [Google Scholar] [CrossRef]
- Wang, P.; Xie, H.; Zhang, J.; Jia, L.; Yu, Z.; Li, R. Optimization of Two Bio-Oil Steam Reforming Processes for Hydrogen Production Based on Thermodynamic Analysis. Int. J. Hydrogen Energy 2022, 47, 9853–9863. [Google Scholar] [CrossRef]
- Mao, X.; Li, W.; Yuan, Y.; Yang, L. Numerical Analysis of Methanol Steam Reforming Reactor Heated by Catalytic Combustion for Hydrogen Production. Int. J. Hydrogen Energy 2022, 47, 14469–14482. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, X.; Xue, Z.; Wang, X.; Shen, Y.; Zhu, J.; Wu, Y.; Meng, B.; Meng, X.; Yang, N. NaCl Induced Active Hcp Co Nanosheet for Hydrogen Production and Formaldehyde Abatement by Formaldehyde Steam Reforming. Chem. Eng. J. 2022, 433, 134600. [Google Scholar] [CrossRef]
- Janošovský, J.; Boháčiková, V.; Kraviarová, D.; Variny, M. Multi-Criteria Decision Analysis of Steam Reforming for Hydrogen Production. Energy Convers. Manag. 2022, 263, 115722. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Zheng, B.; Sun, P.; Hu, J.; Qiu, C.; Hu, W. Local Percolation of Non-Spherical Particles in Moving Bed Waste Heat Recovery Unit for Hydrogen Production by Methanol Steam Reforming. Int. J. Hydrogen Energy 2023, 48, 11463–11475. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Chen, J.; Yin, C.; Liu, X. Design, Fabrication and Performance Evaluation of an Integrated Reformed Methanol Fuel Cell for Portable Use. J. Power Sources 2018, 389, 37–49. [Google Scholar] [CrossRef]
- Chiu, W.-C.; Hou, S.-S.; Chen, C.-Y.; Lai, W.-H.; Horng, R.-F. Hydrogen-Rich Gas with Low-Level CO Produced with Autothermal Methanol Reforming Providing a Real-Time Supply Used to Drive a kW-Scale PEMFC System. Energy 2022, 239, 122267. [Google Scholar] [CrossRef]
- González-Espasandín, Ó.; Leo, T.J.; Raso, M.A.; Navarro, E. Direct Methanol Fuel Cell (DMFC) and H2 Proton Exchange Membrane Fuel (PEMFC/H2) Cell Performance under Atmospheric Flight Conditions of Unmanned Aerial Vehicles. Renew. Energy 2019, 130, 762–773. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, D.; Li, W.; Wang, Z.; Huang, Y.; You, Y.; Becker, S. Current Technologies and Challenges of Applying Fuel Cell Hybrid Propulsion Systems in Unmanned Aerial Vehicles. Prog. Aerosp. Sci. 2020, 116, 100620. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Z.; Yue, B.; Sun, Z.; Sun, Z. Chemical Looping Induced CH3OH–H2-PEMFC Scheme for Fuel Cell Vehicle: Parameter Optimization and Feasibility Analysis. J. Power Sources 2020, 479, 228790. [Google Scholar] [CrossRef]
- Wu, W.; Pai, C.-T.; Viswanathan, K.; Chang, J.-S. Comparative Life Cycle Assessment and Economic Analysis of Methanol/Hydrogen Production Processes for Fuel Cell Vehicles. J. Clean. Prod. 2021, 300, 126959. [Google Scholar] [CrossRef]
- Wu, W.; Liew, K.B.; Abbas, S.; Raza, M.A.; Hwang, J.J.; Yang, S.-B.; Li, Z. Exergy-Based Modular Design of an on-Board MeOH-to-H2 Processor for Fuel Cell Vehicles. Int. J. Hydrogen Energy 2020, 45, 19880–19890. [Google Scholar] [CrossRef]
- Krummrich, S.; Llabrés, J. Methanol Reformer—The next Milestone for Fuel Cell Powered Submarines. Int. J. Hydrogen Energy 2015, 40, 5482–5486. [Google Scholar] [CrossRef]
- Jung, S.-K.; Cha, W.-S.; Park, Y.-I.; Kim, S.-H.; Choi, J. Conceptual Design Development of a Fuel-Reforming System for Fuel Cells in Underwater Vehicles. Energies 2020, 13, 2000. [Google Scholar] [CrossRef]
- Ji, H.; Lee, J.; Choi, E.; Cho, J. Underwater Vehicle Hydrogen Production from Methanol Steam Reforming Using Hydrogen Peroxide. Int. J. Hydrogen Energy 2021, 46, 30310–30319. [Google Scholar] [CrossRef]
- Li, H.; Chen, L.; Zhang, Z. A Study on the Utilization Rate and Influencing Factors of Small Agricultural Machinery: Evidence from 10 Hilly and Mountainous Provinces in China. Agriculture 2022, 13, 51. [Google Scholar] [CrossRef]
- Liu, J.; Xia, C.; Jiang, D.; Sun, Y. Development and Testing of the Power Transmission System of a Crawler Electric Tractor for Greenhouses. Appl. Eng. Agric. 2020, 36, 797–805. [Google Scholar] [CrossRef]
- Wang, B.; Du, X.; Wang, Y.; Mao, H. Multi-Machine Collaboration Realization Conditions and Precise and Efficient Production Mode of Intelligent Agricultural Machinery. Int. J. Agric. Biol. Eng. 2024, 17, 27–36. [Google Scholar] [CrossRef]
- Sun, L.; Liu, M.; Wang, Z.; Wang, C.; Luo, F. Research on Load Spectrum Reconstruction Method of Exhaust System Mounting Bracket of a Hybrid Tractor Based on MOPSO-Wavelet Decomposition Technique. Agriculture 2023, 13, 1919. [Google Scholar] [CrossRef]
- Dong, G.; Chen, B.; Liu, B.; Hounjet, L.J.; Cao, Y.; Stoyanov, S.R.; Yang, M.; Zhang, B. Advanced Oxidation Processes in Microreactors for Water and Wastewater Treatment: Development, Challenges, and Opportunities. Water Res. 2022, 211, 118047. [Google Scholar] [CrossRef]
- Baydir, E.; Aras, Ö. Methanol Steam Reforming in a Microchannel Reactor Coated with Spray Pyrolysis Method for Durable Cu/ZnO Nanocatalyst. J. Anal. Appl. Pyrolysis 2021, 158, 105278. [Google Scholar] [CrossRef]
- Qing, W.; Liu, F.; Yao, H.; Sun, S.; Chen, C.; Zhang, W. Functional Catalytic Membrane Development: A Review of Catalyst Coating Techniques. Adv. Colloid Interface Sci. 2020, 282, 102207. [Google Scholar] [CrossRef]
- Yuan, W.; Zhou, B.; Tang, Y.; Zhang, Z.; Deng, J. Effects of Environmental Factors on Corrosion Behaviors of Metal-Fiber Porous Components in a Simulated Direct Methanol Fuel Cell Environment. Int. J. Miner. Metall. Mater. 2014, 21, 913–918. [Google Scholar] [CrossRef]
- Chen, J.; Li, T. Design Issues of Thermally Integrated Methanol Reforming Systems for the Production of Hydrogen: Effects of Channel Dimensions and Catalyst Properties. Energy Fuels 2019, 33, 12026–12040. [Google Scholar] [CrossRef]
- Shah, K.; Besser, R. Understanding Thermal Integration Issues and Heat Loss Pathways in a Planar Microscale Fuel Processor: Demonstration of an Integrated Silicon Microreactor-Based Methanol Steam Reformer. Chem. Eng. J. 2008, 135, S46–S56. [Google Scholar] [CrossRef]
- Pardo, E.G.; Blanco-Linares, J.; Velázquez, D.; Serradilla, F. Optimization of a Steam Reforming Plant Modeled with Artificial Neural Networks. Electronics 2020, 9, 1923. [Google Scholar] [CrossRef]
- Jäger, M.O.; Ranawat, Y.S.; Canova, F.F.; Morooka, E.V.; Foster, A.S. Efficient Machine-Learning-Aided Screening of Hydrogen Adsorption on Bimetallic Nanoclusters. ACS Comb. Sci. 2020, 22, 768–781. [Google Scholar] [CrossRef]
- Saber, M.; Commenge, J.M.; Falk, L. Microreactor Numbering-up in Multi-Scale Networks for Industrial-Scale Applications: Impact of Flow Maldistribution on the Reactor Performances. Chem. Eng. Sci. 2010, 65, 372–379. [Google Scholar] [CrossRef]
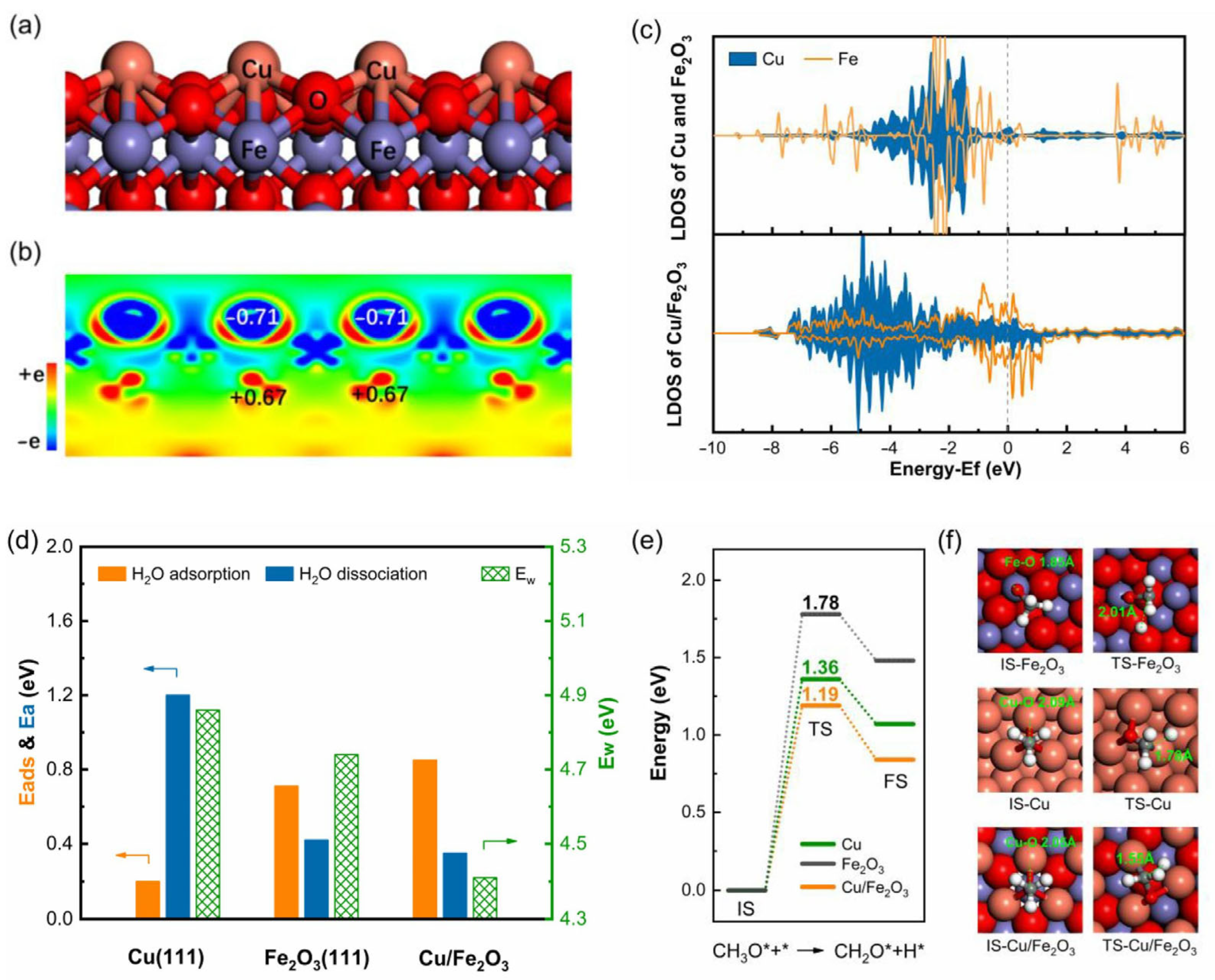

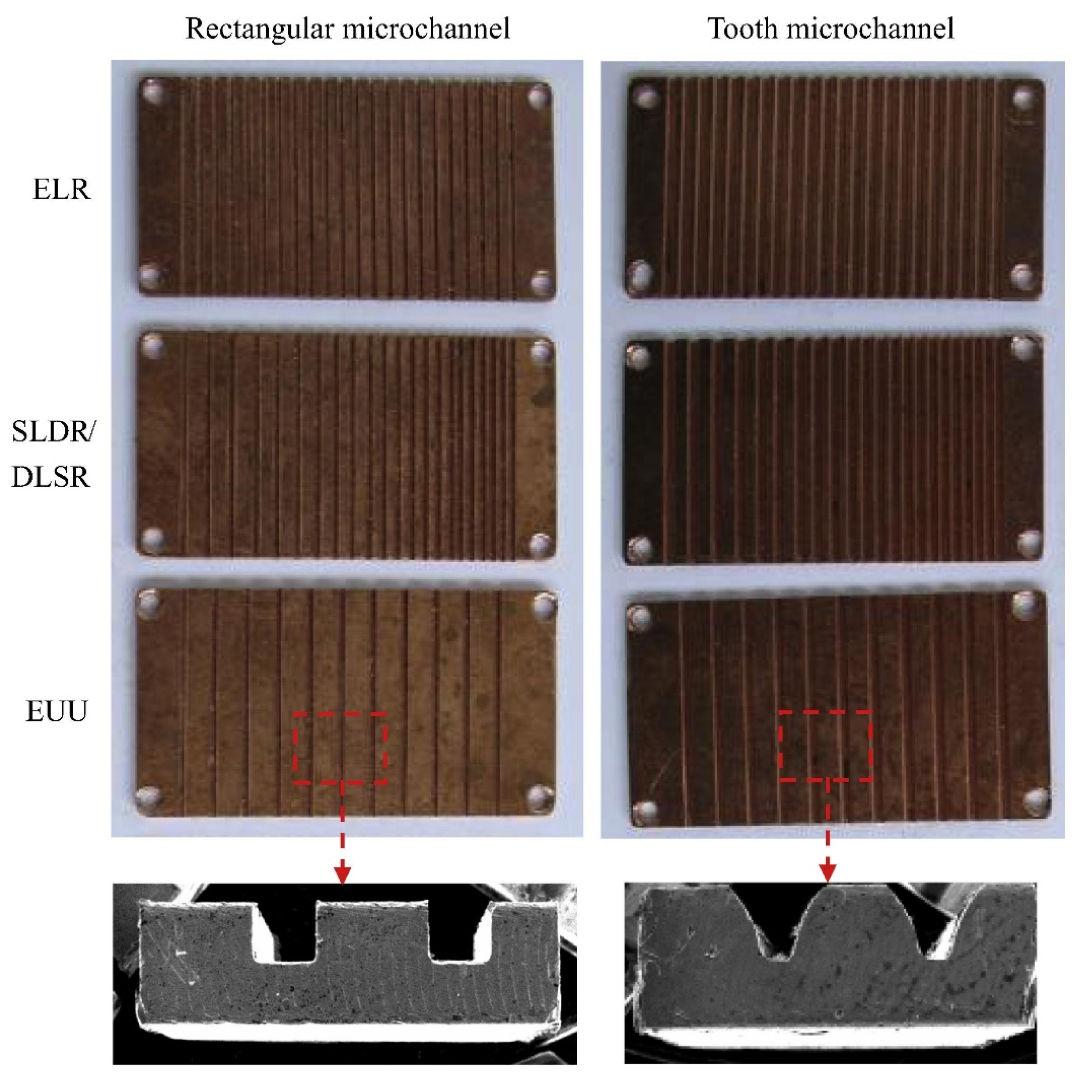

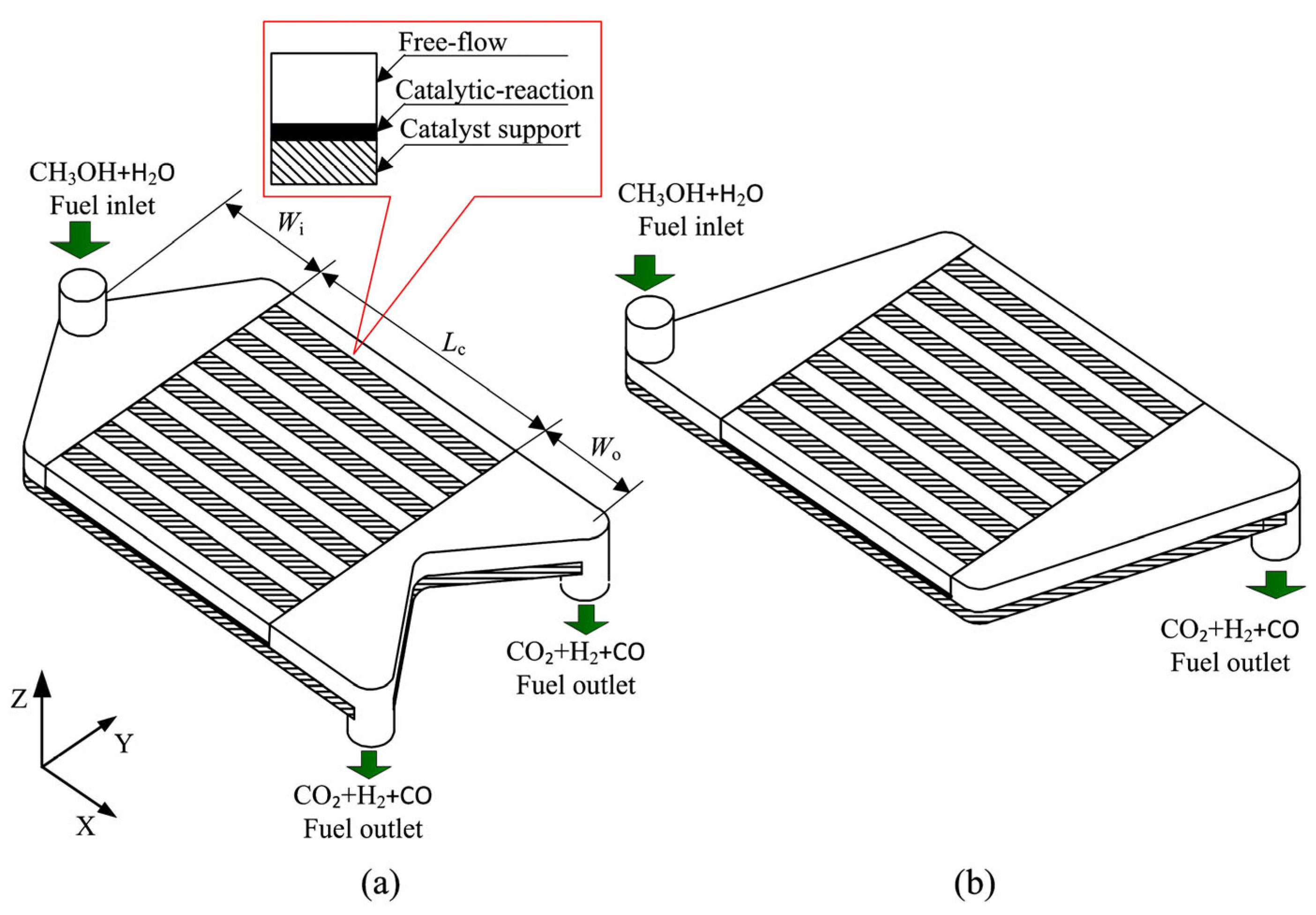
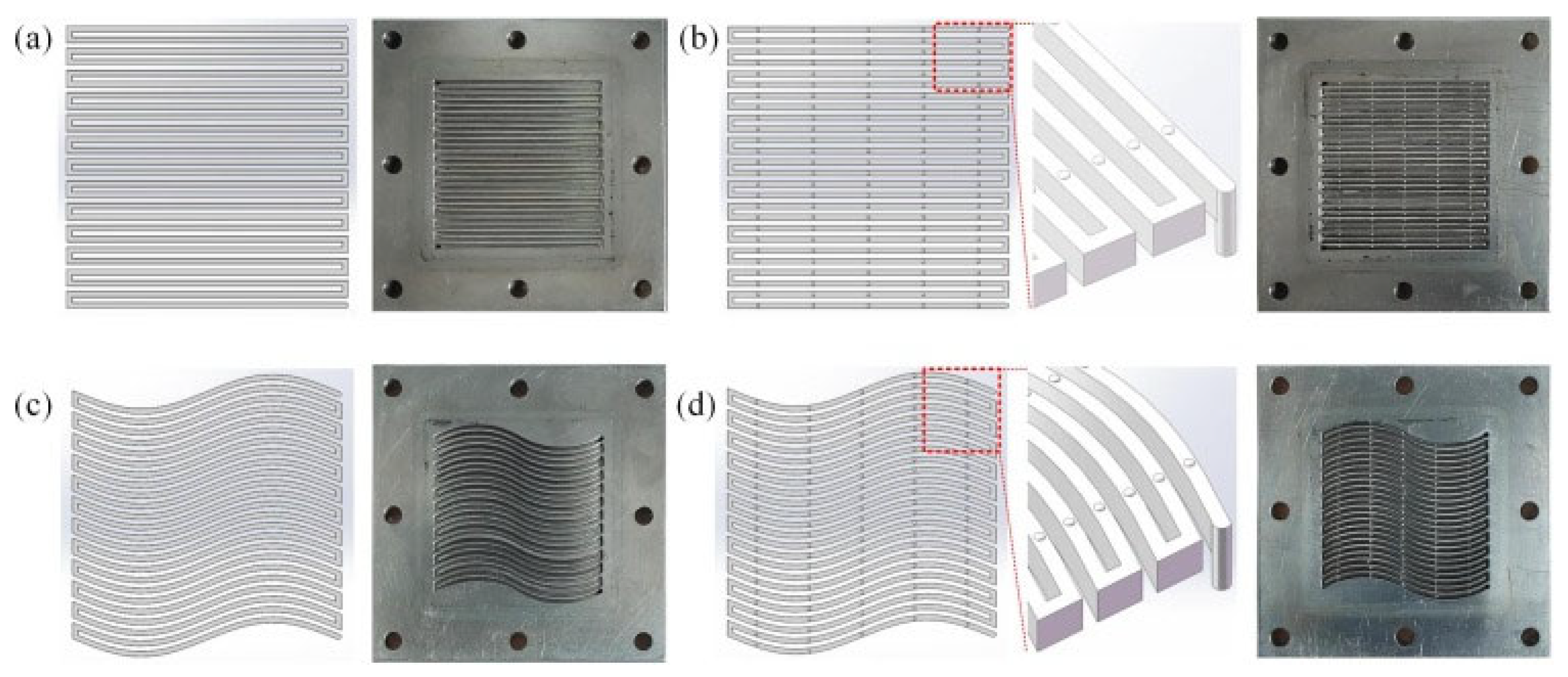
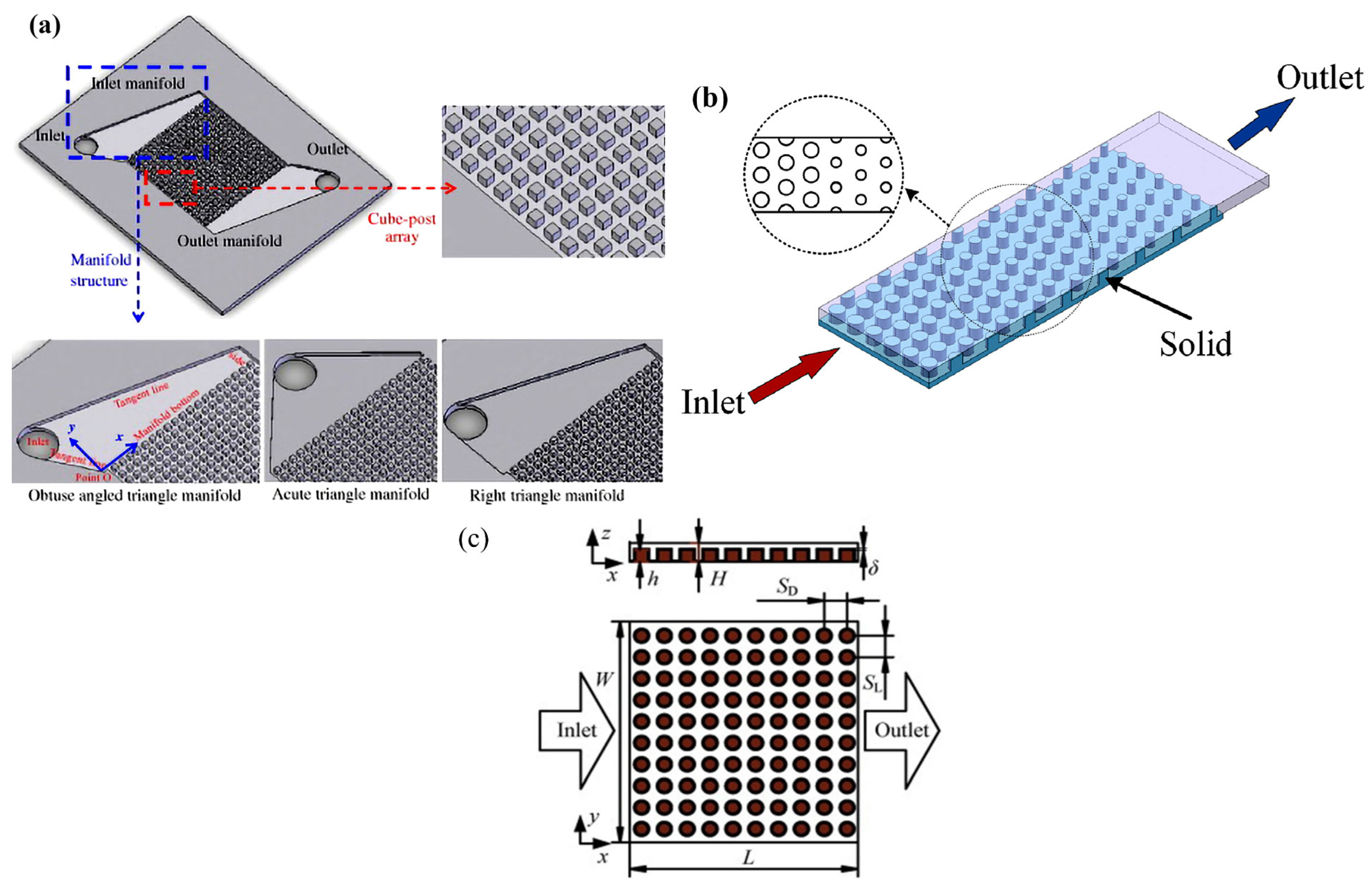
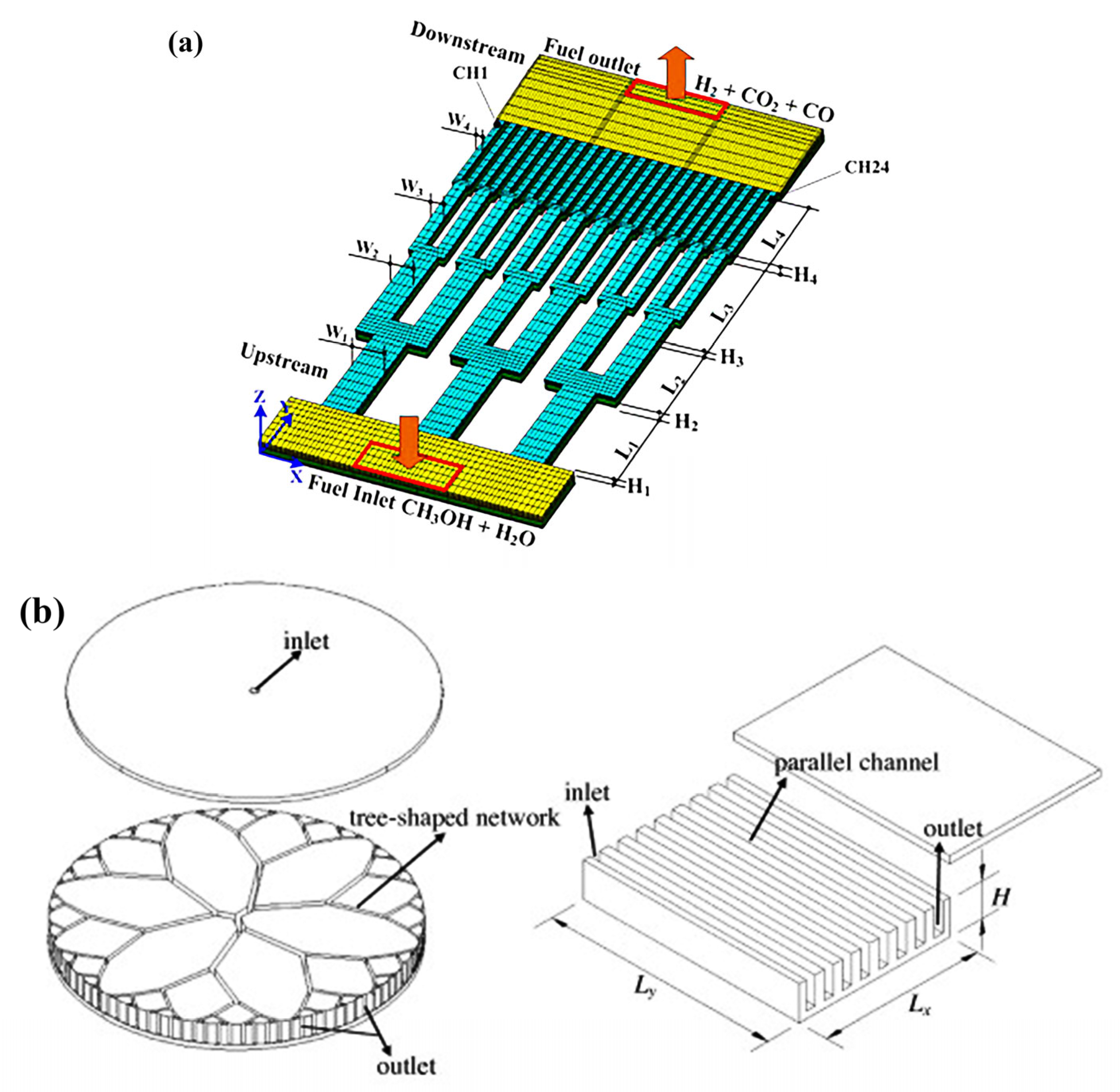
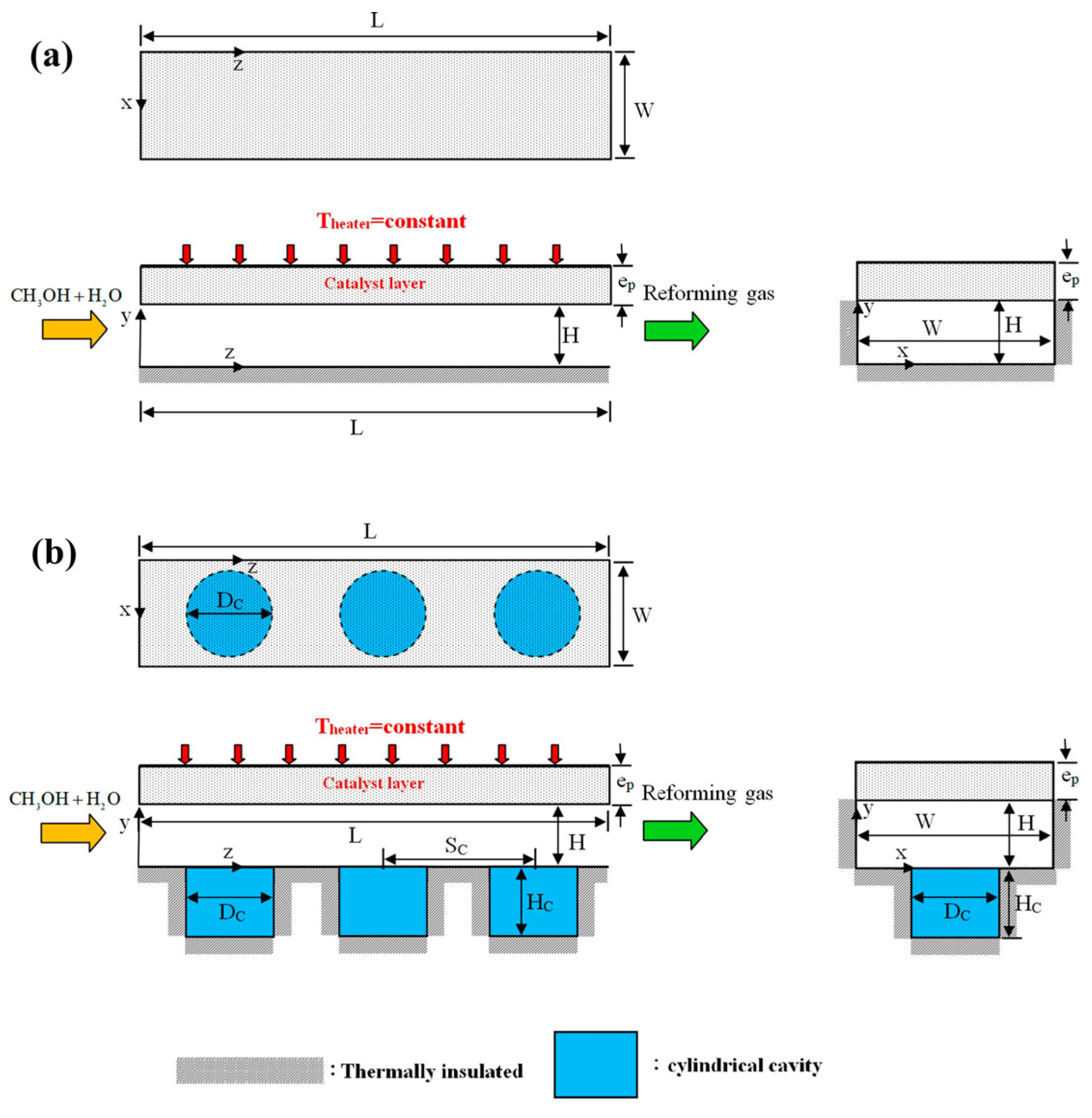
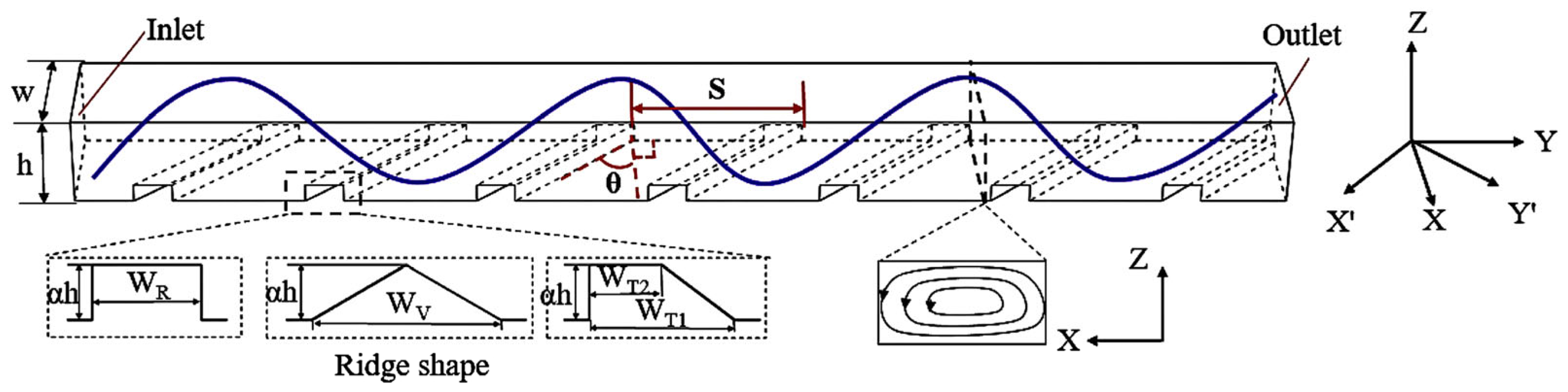
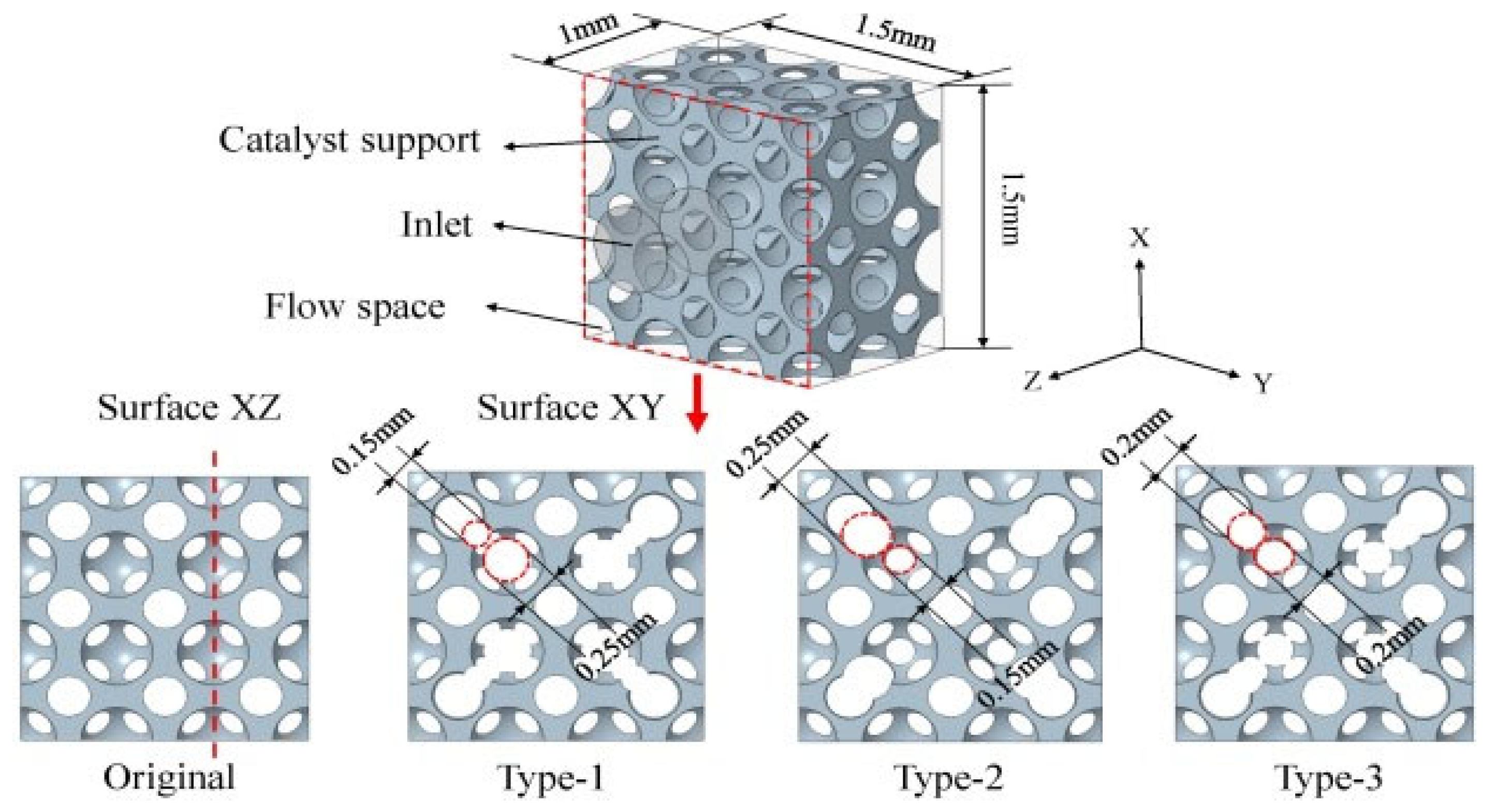
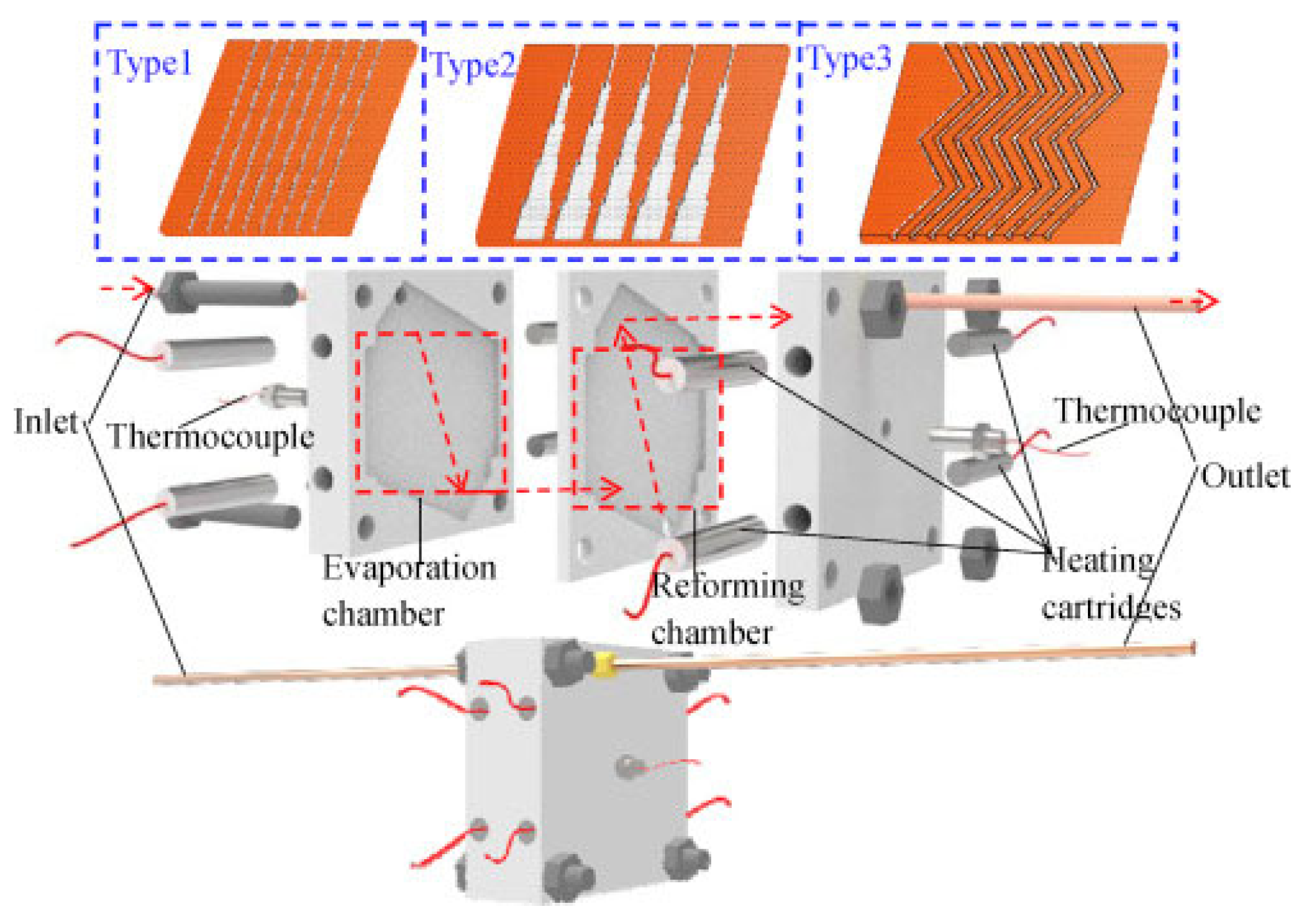


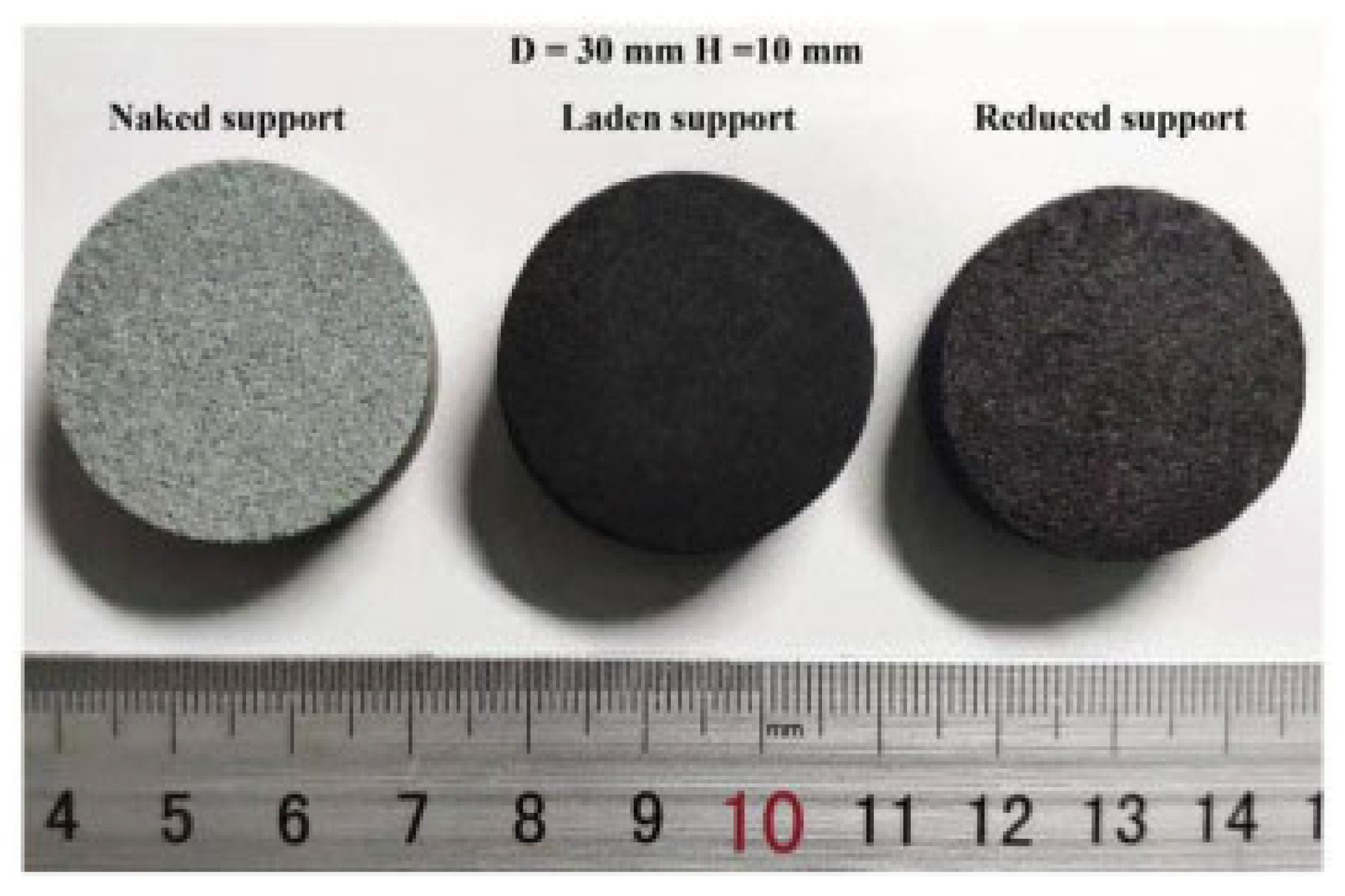
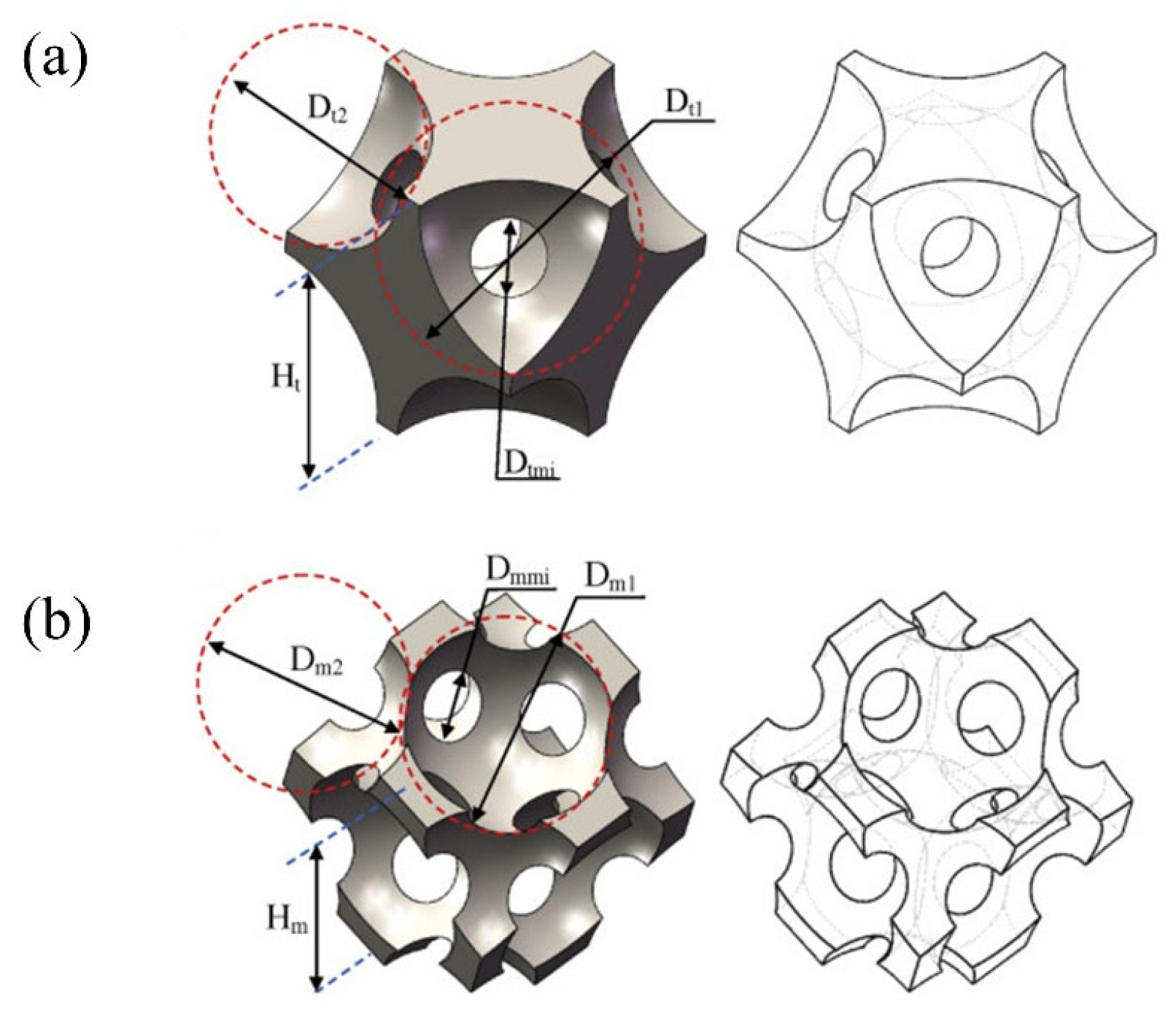
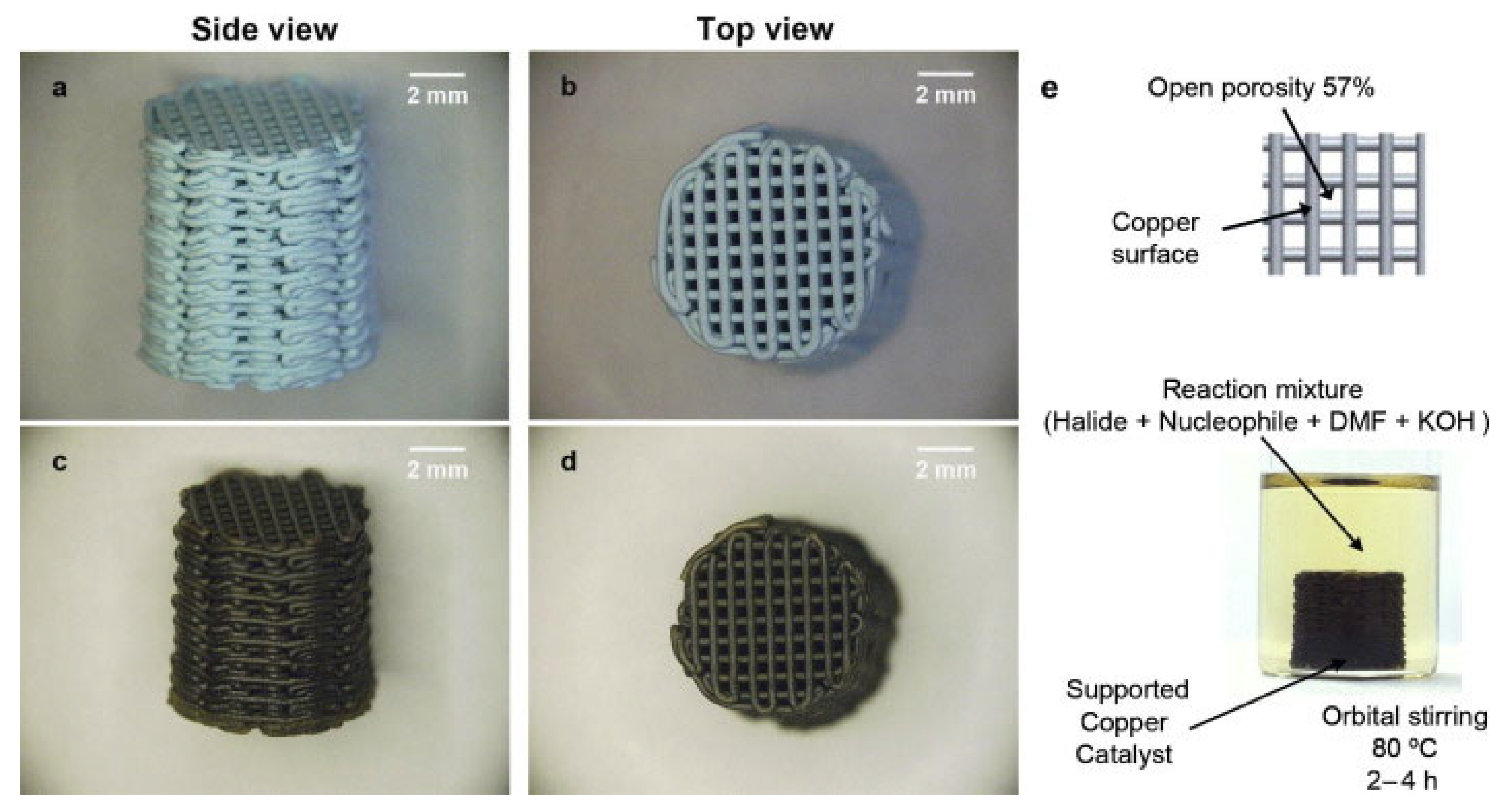
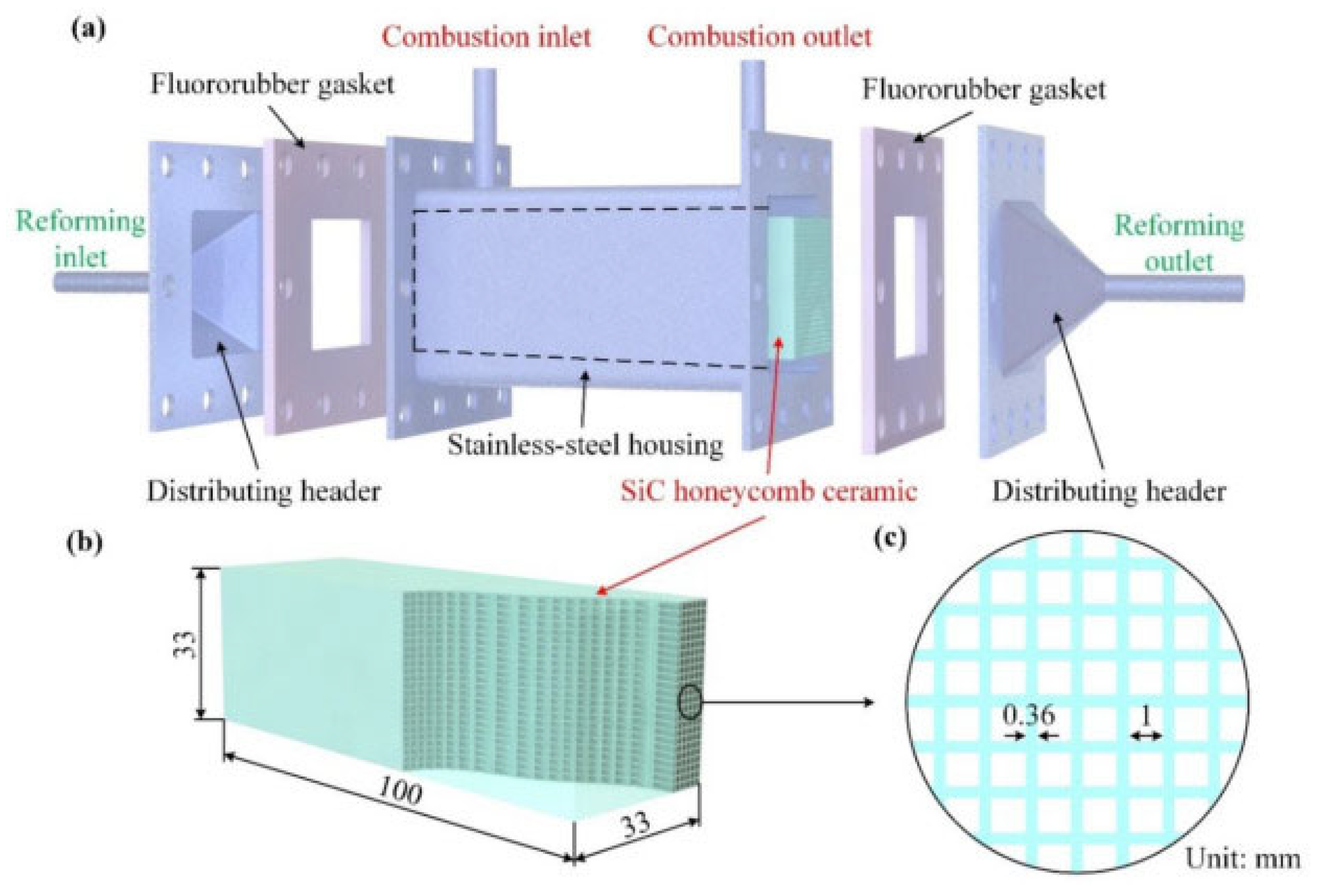
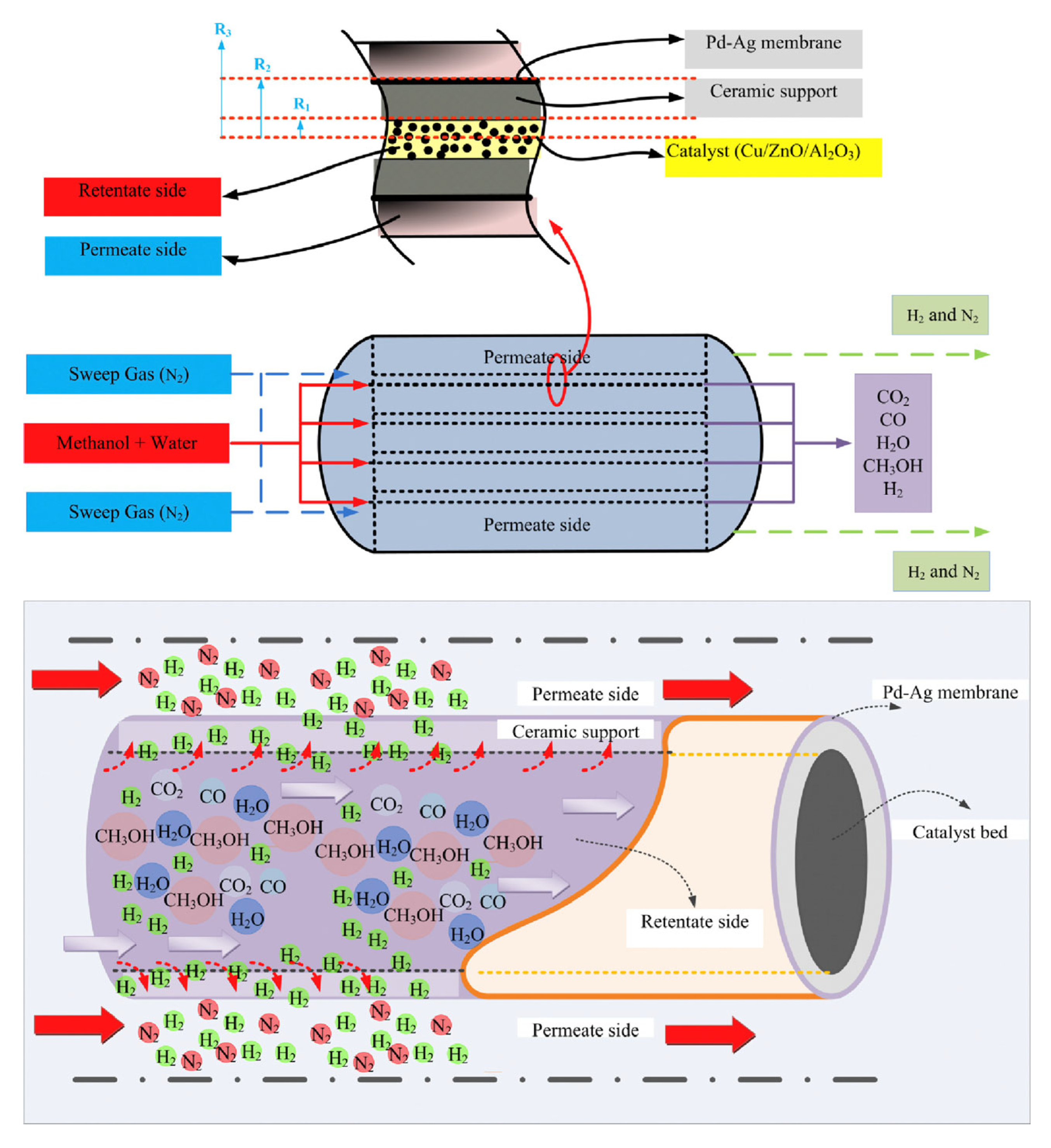
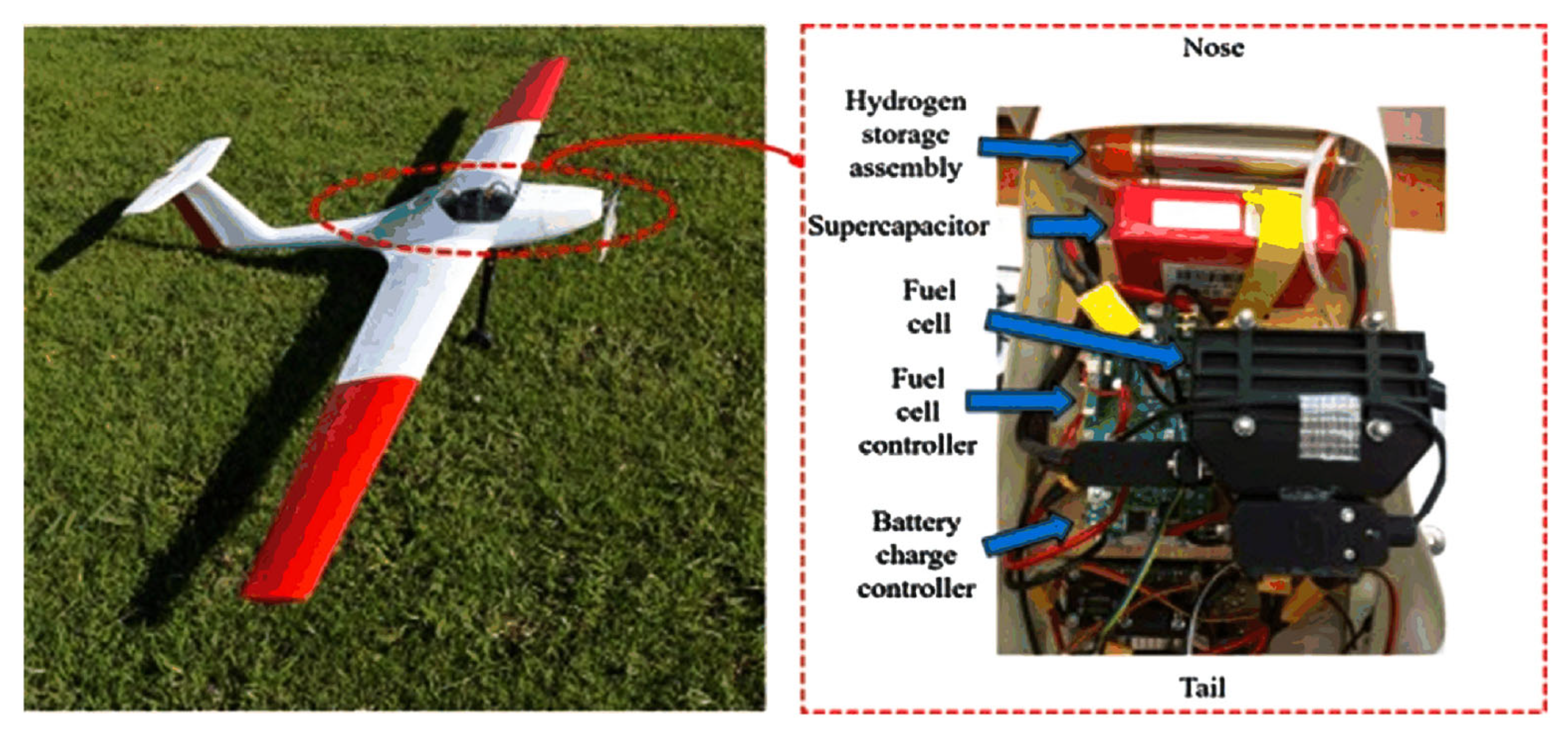
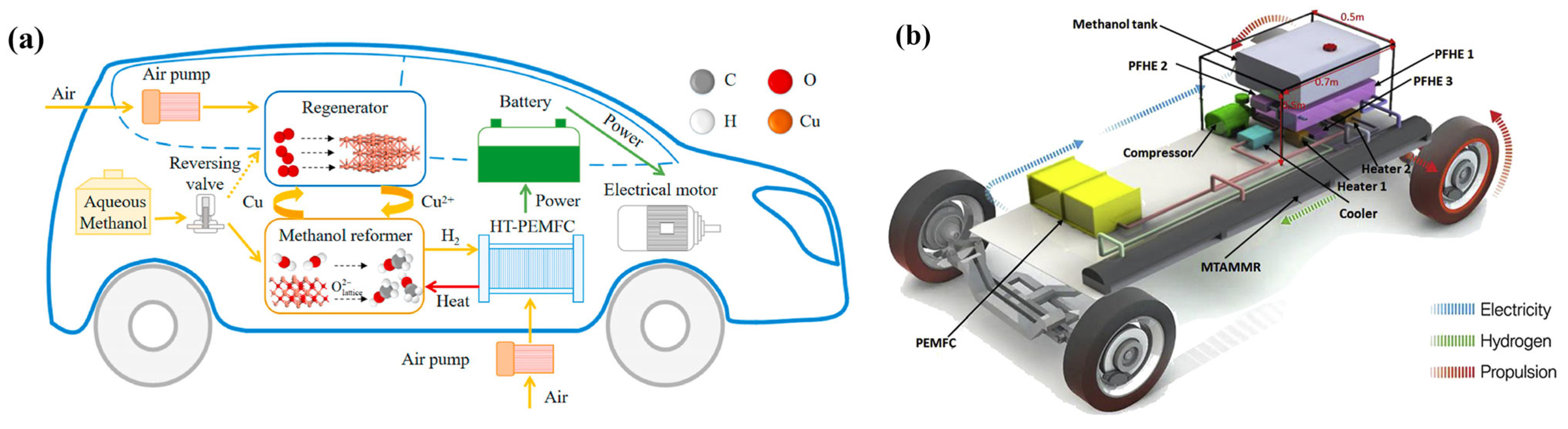
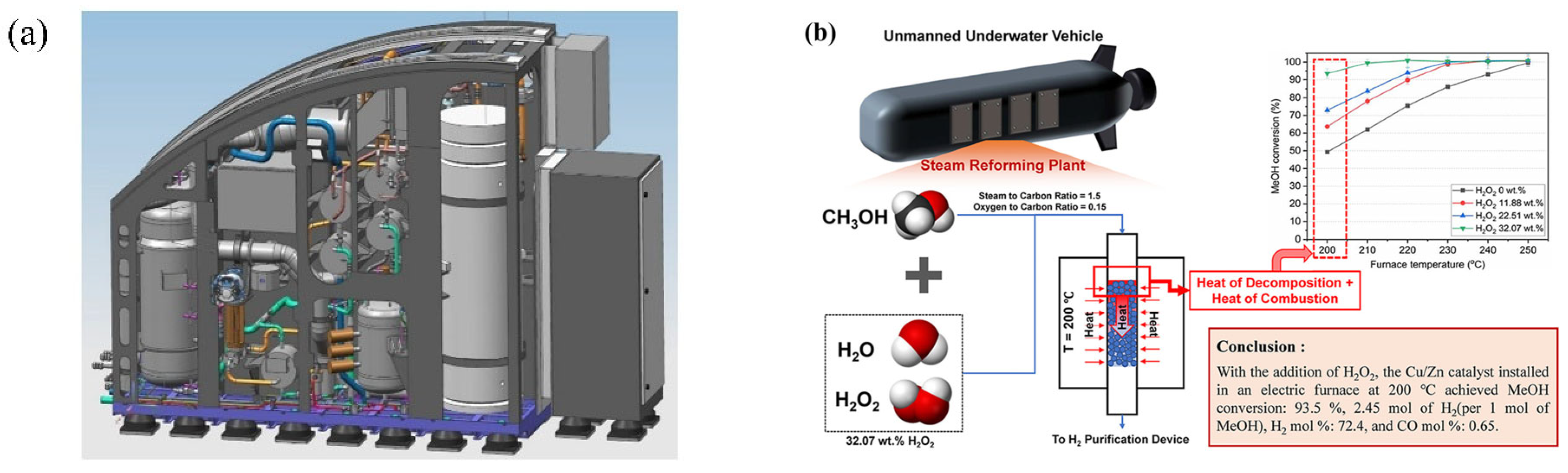
| Method | Advantages | Disadvantages |
|---|---|---|
| Methanol steam reforming | • Mild reaction conditions • High conversion rate • High hydrogen content • Low CO generation | • Expensive • Requires external heat supply |
| Methanol decomposition | • H2 can be produced directly from methanol • No extra reactants needed | • Highly selective towards CO • High CO concentrations must be removed for fuel cell use • Requires external energy source |
| Partial oxidation of methanol | • Exothermic • No extra heating needed | • Low H2 yield • High CO content • Requires pure oxygen supply |
| Oxidative steam reforming of methanol | • Thermally neutral process • No external heating is required | • High reaction temperature • Requires pure oxygen supply |
| Catalyst | T (°C) | Methanol Conversion (%) | H2 Selectivity (%) | Activity | CO Selectivity (%) | Refs. |
|---|---|---|---|---|---|---|
| Cu-ZnO | 300 | 40 | - | 0 | [76] | |
| Cu/Se2O3-ZnO | 300 | 90 | - | 0 | [76] | |
| Cu-Ga/ZnO | 320 | 96 | - | - | [70] | |
| Cu/Zr | 260 | 20 | - | - | [81] | |
| Cu/ZrSi | 260 | 73 | - | - | [81] | |
| CuZn3GaZr | 275 | 75 | - | 312 mlg−1min−1 | 0.3 | [71] |
| ZrO2-CeO2-Cu/KIT-6 | 300 | 96 | 99.8 | - | 0.7 | [82] |
| Cu-ZnO-ZrO2/MCM-41 | 300 | 97.8 | 99 | - | 0.4 | [83] |
| Cu/ZnO/ZrO2 | 250 | 88.6 | 75 | 806.4 molmol−1h−1 | - | [84] |
| Cu-ZnO-Al2O3-ZrO2 | 240 | 100 | 75 | - | - | [85] |
| Cu/CeO2 | 250 | 95.5 | - | - | - | [86] |
| CuO/CeO2 | 260 | 100 | - | - | 2.4 | [87] |
| CuO/ZnO/CeO2/Al2O3 | 260 | 98 | 65 | - | - | [88] |
| Cu-Zn/Y-Al2O2/Al | 350 | 74 | - | - | - | [89] |
| CuFeMg/Al2SO3 | 250 | 100 | - | 2.3 | [90] | |
| Cu/ZnO/Sc2O3 | 300 | 87 | - | 0.2 | [76] | |
| Cu/SiO2 | 300 | 94.67 | - | - | 0.36 | [41] |
| Cu-In/SiO2 | 300 | 96.1 | - | - | 0.07 | [41] |
| CuZnOx | 150 | 18.8 | - | - | - | [91] |
| CuZnGaOx | 150 | 22.5 | - | - | - | [91] |
| Cu/TiO2 | 300 | 90.2 | - | - | 7.6 | [92] |
| CuNi/TiO2 | 300 | 92.6 | - | - | 9.6 | [92] |
| CuZnAl | 200 | 56 | - | - | - | [93] |
| Cu/Zn/GaOx | 195 | 98 | - | 0.2 | [94] | |
| Ce/Cu/ZnAl | 240 | 92 | - | - | 0.9 | [80] |
| CuZnAl-Mg | 200 | 68 | - | - | [93] | |
| CuZnGaZr | 275 | 75 | - | - | [71] | |
| Cu/Zn1.11La1.26Al0.5O4.27 | 300 | 96 | 86 | - | - | [95] |
| Cu/Y1.6Ce0.76Ru0.03O4 | 300 | 99.5 | 98.7 | - | - | [96] |
| Cu0.5Ce0.25Mg0.05/Al | 250 | 100 | 46.5 | - | 0.15 | [97] |
| Cu/SBA-15 | 300 | 91 | - | - | 2.8 | [98] |
| Cu/ZnO/SBA-15 | 300 | 92.1 | - | - | 1.7 | [98] |
| Cu/ZnO/CeO2/SBA-15 | 300 | 93 | - | - | 1.1 | [98] |
| Cu/ZnO/ZrO2/SBA-15 | 300 | 96.8 | - | - | 2.1 | [98] |
| Cu/ZnO/CeO2/ZrO2/SBA15 | 300 | 95.2 | - | - | 1.4 | [98] |
| 5Cu10Ce | 350 | 99.7 | 75.1 | 5.3 molmin−1g−1 | 0 | [99] |
| 5Zn5Cu10Ce | 90.9 | 75 | - | 4.2 molmin−1g−1 | 0 | [99] |
| Cu/ZnAl-LDHs/γ-Al2O3 | 300 | 100 | - | 1 | [100] |
| Catalyst | T (°C) | Methanol Conversion (%) | CO Selectivity (%) | Reference | |
|---|---|---|---|---|---|
| Pd/ZnO | 300 | 98 | - | 13.7 | [158] |
| Pd/Al2O3 | 350 | 99 | - | 3 | [159] |
| Pd/La2O3 | 200 | - | 248 | - | [160] |
| Pd/Nd2O3 | 200 | - | 300 | - | [160] |
| Pd/Nb2O5 | 200 | - | 124 | - | [160] |
| Pd/In2O3 | 220 | 28.3 | - | 4.5 | [161] |
| Pd/Ga2O3 | 220 | 21.2 | - | 5.4 | [161] |
| Pd/SiO2 | 220 | 15.7 | - | 100 | [161] |
| Pd/MgO | 220 | 41.0 | - | 93 | [161] |
| Pd/ZrO2 | 220 | 64.3 | - | 81.6 | [161] |
| Pd/ZnO | 220 | 56.3 | - | - | [162] |
| Pd/In2O3 | 220 | 54.2 | - | 0.8 | [148] |
| Pd/Ga2O3 | 220 | 21.2 | - | 5.4 | [148] |
| Pd/Al2O3 | 220 | 58.9 | - | 69.6 | [148] |
| Pd/MgO | 220 | 41.0 | - | 93.4 | [148] |
| Pd/CeO2 | 220 | 62.4 | - | 77.3 | [148] |
| Pd/A.C. | 220 | - | - | 100 | [148] |
| Pd/ZnO/Al2O3 | 250 | 100 | - | - | [163] |
| Pd–Zr | 350 | 61 | ≈170 | 77 | [164] |
| Pd–Zr–Zn | 300 | 92 | ≈307 | ≈38 | [164] |
| Pd–Zn | 300 | 87 | ≈307 | ≈24 | [164] |
| Pd/Sm–Ce | 400 | 97.4 | - | - | [165] |
| Pd/Zn1Zr1Ox | 330 | 46 | 100 | 0 | [166] |
| Pd/ZnO/CeO2 | 250 | 100 | - | 45 | [167] |
| PdZn/ZnO/Al2O3 | 250 | 0.998 | - | - | [168] |
| PdZnSc–CNTs | 275 | 21.4 | - | 10 | [44] |
| Pd–Ru/Al2O3 | 300 | 88 | - | - | [22] |
| Ru-Pd/Al2O3 | 350 | 100 | - | 12.5 | [159] |
| Zn-Pd/ZnO | 300 | 98 | - | 5 | [169] |
| CuPd/ZnO2 | 220 | ≈65 | ≈193 | 5 | [170] |
| 1Nb–Pd–Zr–Zn | 300 | 81.5 | ≈300 | ≈7 | [164] |
| Au/CeO2 | 300 | 100 | - | - | [171] |
| Pt/In2O3/Al2O3 | 350 | 99 | - | 5 | [172] |
| CuPd/ZrO2 | 240 | 87 | - | - | [173] |
| Au/CuO–CeO2 | 300 | 100 | - | - | [174] |
| Au–Cu/Ce0.75Zr0.25O2 | 350 | 96 | - | 78 | [175] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Ma, T.; Ding, R.; Liu, W.; Sun, D. Catalyst, Reactor, and Purification Technology in Methanol Steam Reforming for Hydrogen Production: A Review. Catalysts 2025, 15, 802. https://doi.org/10.3390/catal15090802
Wang R, Ma T, Ding R, Liu W, Sun D. Catalyst, Reactor, and Purification Technology in Methanol Steam Reforming for Hydrogen Production: A Review. Catalysts. 2025; 15(9):802. https://doi.org/10.3390/catal15090802
Chicago/Turabian StyleWang, Ruochen, Te Ma, Renkai Ding, Wei Liu, and Dong Sun. 2025. "Catalyst, Reactor, and Purification Technology in Methanol Steam Reforming for Hydrogen Production: A Review" Catalysts 15, no. 9: 802. https://doi.org/10.3390/catal15090802
APA StyleWang, R., Ma, T., Ding, R., Liu, W., & Sun, D. (2025). Catalyst, Reactor, and Purification Technology in Methanol Steam Reforming for Hydrogen Production: A Review. Catalysts, 15(9), 802. https://doi.org/10.3390/catal15090802








