Abstract
The synthesis of heterocyclic compounds has gained significant attention in organic chemistry due to their diverse pharmacological properties. However, traditional synthetic approaches often involve hazardous chemicals, high energy consumption, and tedious workup procedures, leading to environmental concerns and low yields. In response, green chemistry strategies have emerged, emphasizing safer and more sustainable alternatives. Among these, magnetic nanoparticle (MNP)-based catalysts have shown remarkable promise in facilitating one-pot multicomponent reactions (MCRs), offering enhanced catalytic efficiency, ease of recovery, and reusability. This article provides a comprehensive overview of multicomponent reactions (MCRs) for the construction of a wide range of heterocyclic scaffolds—including chromenes, pyrazoles, phenazines, triazoles, tetrazoles, xanthenes, furans, indoles, imidazoles, pyridines, pyrimidines, oxazoles, and acridine derivatives—catalyzed by magnetic nanoparticles under sustainable and environmentally benign conditions. This review highlights recent advances (2018–2024) in the development and application of modified magnetic nanoparticles for green multicomponent synthesis. Emphasis is placed on their structural features, catalytic roles, and benefits in eco-friendly organic transformations.
1. Introduction
Nanoparticles (NPs) have attracted considerable interest in organic synthesis owing to their distinct physicochemical characteristics that set them apart from bulk materials. With sizes typically between 1 and 100 nm, NPs possess a high surface-to-volume ratio, which enhances their catalytic efficiency and makes them ideal supports for immobilizing homogeneous catalysts [1,2,3,4,5]. Among these nanomaterials, magnetic nanoparticles (MNPs) are especially appealing for catalytic applications due to their simple recovery using an external magnetic field, cost-effectiveness, high surface reactivity, and ease of use [6,7,8].
Despite their advantages, MNPs face certain limitations. They tend to aggregate due to anisotropic dipolar attractions, which impairs their dispersibility and catalytic efficiency [9]. Additionally, in acidic environments, MNPs are prone to degradation, resulting in the loss of their magnetic properties. These challenges can be overcome by coating MNPs with chemically stable and sustainable layers, creating core–shell nanostructures that enhance both stability and functionality [10,11].
In the context of green chemistry, catalysts play a central role in minimizing environmental impact. Ideal green catalysts must possess properties such as high activity, selectivity, low production cost, operational stability, and excellent recyclability [12]. Conventional catalysts fall into two categories: homogeneous and heterogeneous. Homogeneous catalysts offer high activity and selectivity, and their mechanisms can be studied in detail, allowing for precise optimization. However, their separation from reaction media is challenging, often leading to metal contamination—an issue particularly critical in the pharmaceutical industry [13,14,15].
On the other hand, heterogeneous catalysts are more environmentally benign due to their reusability and ease of separation, although they sometimes exhibit lower catalytic activity due to reduced surface interaction with substrates [16,17,18,19]. To address this trade-off, nano catalysts—especially MNP-based systems—offer a hybrid solution, combining the benefits of both catalyst types. The immobilization of homogeneous metal complexes onto MNPs enhances catalytic efficiency while allowing magnetic separation for reuse [20,21,22,23,24,25,26].
Multicomponent reactions (MCRs), which involve the combination of three or more reactants in a single reaction vessel to yield complex products, have emerged as an essential tool in synthetic chemistry [27]. MCRs offer several advantages: they are atom-economical, time-efficient, energy-saving, and environmentally friendly. These one-pot reactions reduce the need for multiple purification steps and minimize waste generation, aligning perfectly with the principles of green chemistry [28,29,30,31]. MCRs have found widespread applications in fields ranging from pharmaceutical development and drug discovery to materials science and chemical biology [32,33]. An ideal MCR allows all reagents, catalysts, and solvents to interact under unified conditions, streamlining synthesis and increasing overall efficiency [34,35]. The one-pot nature of these transformations enables the construction of complex molecular architectures with high yields, excellent regioselectivity, and product purity, making them especially attractive for the synthesis of bioactive compounds.
The growing field of green chemistry provides a framework for developing sustainable synthetic methodologies. As outlined by Anastas and Warner’s 12 principles of green chemistry, the focus is on reducing hazardous substances, improving energy efficiency, and using renewable feedstocks [36,37,38,39,40]. Several innovative green methods have been developed in recent years, including aqueous-phase organic synthesis [41], nanoparticle-catalyzed protocols [42], microwave-assisted techniques [43], and ultrasound-assisted reactions [44,45].
The integration of magnetic nano catalysts with multicomponent reactions is poised to emerge as a promising strategic research direction, offering an optimal platform for advancing sustainable approaches in green synthetic chemistry [46,47,48,49]. Between 2018 and 2024, a wide range of magnetic nano catalysts has been explored for use in MCRs. This review provides a comprehensive overview of recent advances in this area, emphasizing the design, functionalization, and catalytic applications of MNPs in green, one-pot multicomponent synthesis.
2. Magnetic Nanoparticle-Catalysed One-Pot Multicomponent Reactions
In this review, we present a comprehensive overview of various types of magnetic nano catalysts employed in one-pot multicomponent reactions (MCRs) for the synthesis of biologically active derivatives, highlighting the key advantages and green chemistry benefits of the reported methodologies.
2.1. Preparation of Pyrazole and Pyrano Pyrazole Derivatives
Pyrazoles represent a prominent class of heterocyclic compounds that hold significant potential in the development of novel pharmaceutical agents. Extensive research has revealed the presence of pyrazole moieties in numerous established drugs across diverse pharmacological categories, underscoring their versatility and medicinal relevance [50]. In recent years, pyrazole chemistry has garnered growing attention owing to the extensive array of biological activities revealed by its derivatives. These include antidiabetic [51], antibacterial [52], antioxidant [53], anticancer [54], antiviral [55], and antituberculosis [56] properties.
In 2020, Amirnejat co-workers. Ref. [57] developed a superparamagnetic nano catalyst, Fe3O4@alginate-supported L-arginine, representing a robust inorganic–organic hybrid system with heterogeneous and recyclable properties. The catalytic performance of this material was assessed in a one-pot cyclocondensation reaction involving malononitrile (1), aromatic aldehydes (2), and phenyl hydrazine (3) to synthesize annulated pyrazole derivatives (4). The reaction proceeded efficiently under mild conditions, delivering excellent yields between 90% and 97%, as illustrated in Scheme 1. The catalyst was recovered after the first run, washed with ethanol and acetone, dried, and reused for up to seven consecutive cycles without significant loss of activity.
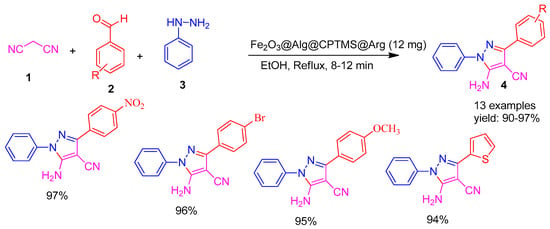
Scheme 1.
Synthesis of annulated pyrazole derivatives using Fe2O3@Alg@CPTMS@Arg catalyst.
In 2020, Nikpassand and co-workers [58] reported the synthesis of silica-coated NiFe2O4 nanoparticles functionalized with amino glucose, yielding a magnetically separable and reusable nano catalyst. This catalyst demonstrated excellent efficiency in a multicomponent reaction involving pyrazole carbaldehydes (5), semicarbazide (6), and alcohols (7), leading to the formation of novel 3-pyrazolyl-4H-1,2,4-triazole derivatives (8) with excellent yields ranging from 89% to 95% (Scheme 2). After the reaction, the catalyst was recovered, washed with warm ethanol, dried at 80 °C, and reused under identical conditions. NiFe2O4@SiO2-nPr@aminoglucose demonstrated excellent reusability, retaining its activity over six consecutive cycles without significant loss. The protocol aligns well with green chemistry principles and offers notable advantages such as high yields, magnetic recovery, catalyst recyclability, environmental benignity, and reduced waste generation.
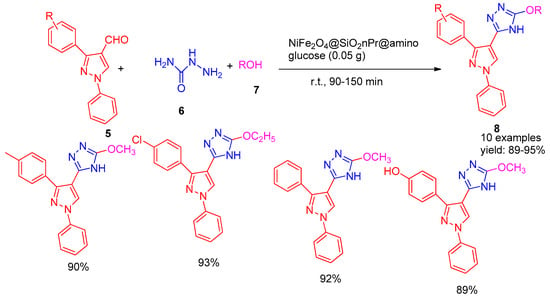
Scheme 2.
Synthesis of 3-pyrazolyl-4H-1,2,4-triazole derivatives using NiFe2O4@SiO2nPr@amino glucose catalyst.
In 2024, Ramezaninejad and co-workers [59] introduced a novel magnetic heterogeneous nano catalyst synthesized by co-precipitating tris (hydroxymethyl)aminomethane with MgFe2O4 nanoparticles. The catalytic performance of this nanomaterial was demonstrated in the one-pot multicomponent synthesis of pyrazolo[3,4-d]pyrimidine derivatives (13) via the reaction of barbituric acid (10), aromatic aldehydes (9), hydrazine hydrate (12), and ethyl acetoacetate (11), using water as a green solvent at room temperature (Scheme 3). The protocol afforded excellent yields ranging from 80% to 98%, under mild and environmentally friendly conditions. The reusability was evaluated and the catalyst maintained consistent performance over five cycles with no significant loss in activity. A key advantage of this methodology is the magnetic separability of the catalyst, allowing for easy recovery and reuse.
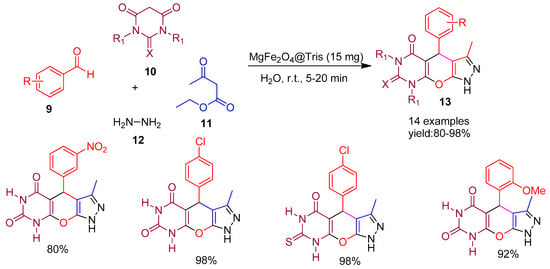
Scheme 3.
Synthesis of pyrazolo pyrimidine derivatives by MgFe2O4@Tris catalyst.
Ghasemzadeh and co-workers [60] (2019) developed Fe3O4@L-arginine nanocomposite as an efficient heterogeneous catalyst for the solvent-free, room-temperature multicomponent synthesis of spirooxindole derivatives (18) (Scheme 4). The reaction involved isatins (14), β-keto esters (15), hydrazine (16), and ethyl cyanoacetate or malononitrile (17) in a one-pot fashion. The catalyst demonstrated excellent reusability, retaining its activity over at least five cycles. This green and operationally simple method offers a sustainable alternative to traditional multi-step syntheses, highlighting the advantages of solid-supported nano catalysis.
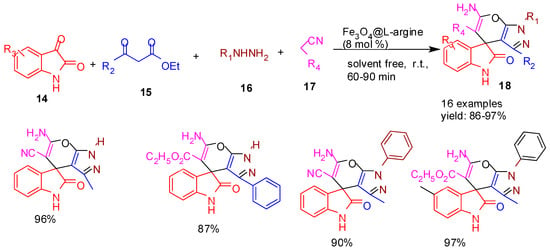
Scheme 4.
Synthesis of indoline−pyrano−pyrazole derivatives using Fe3O4@L−arginine catalyst.
In 2020, Hosseini Mohtasham [61] and co-workers synthesized a high surface area nanomesoporous silica using horsetail (Equisetum arvense), a medicinal plant, as a natural silica source. This bio-derived silica served as a platform for the development of a highly efficient magnetically retrievable solid acid catalyst, Fe3O4@SiO2−EP−NH−HPA, by immobilizing H3PW12O40 (a heteropoly acid) onto aminated epibromohydrin-functionalized Fe3O4@SiO2 nanoparticles. This catalyst exhibited excellent performance in the one-pot synthesis of pyrano−pyrazole derivatives (23) by reacting hydrazine hydrate (19), ethyl acetoacetate (20), various benzaldehyde (21) and malononitrile (22) in aqueous media at room temperature, affording excellent yields ranging from 89% to 99%, as illustrated in Scheme 5.
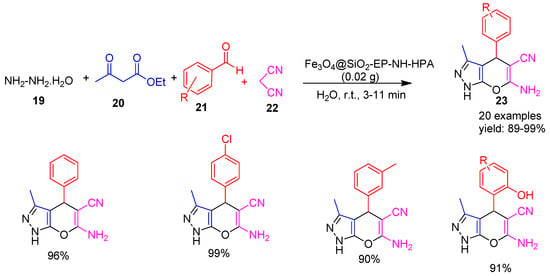
Scheme 5.
Synthesis of pyrano−pyrazole derivatives using Fe3O4@SiO2−EP−NH−HPA catalyst.
2.2. Preparation of Pyrano-Phenazine Derivatives
Phenazines constitute a broad class of nitrogen-containing heterocyclic compounds [62] and are recognized as key structural elements in various bacterial species [63,64]. Both naturally occurring and synthetically derived phenazine compounds have been extensively studied for their diverse biological properties. These include antimalarial [62], trypanocidal [65], fungicidal [66], antiplatelet [67], and antiphrastic activities [62]. Moreover, phenazines are known for their strong DNA intercalating capabilities and have demonstrated significant antitumor potential, particularly against leukemia and various solid tumors [68].
In 2020, Taheri and co-workers [69] reported the synthesis of a sulfonic acid-functionalized magnetic nano catalyst, Fe3O4@TiO2-SO3H, and evaluated its catalytic performance in the one-pot multicomponent synthesis of pyrazolo[4′,3′:5,6]pyrano[2,3−c]phenazin−15−yl)methanone derivatives (28). The reaction involved 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (24), benzene−1,2−diamine (25), 2−hydroxynaphthalene−1,4−dione (26), and aryl glyoxal (27) under microwave irradiation, affording the desired products in high yields ranging from 80% to 91%, as illustrated in Scheme 6. Furthermore, the antibacterial properties of the synthesized compounds were evaluated, with most exhibiting promising activity against Gram-positive Staphylococcus aureus, highlighting their potential as bioactive agents.
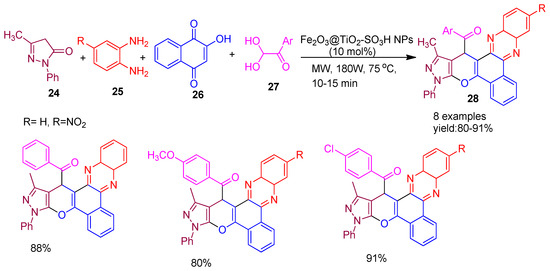
Scheme 6.
Synthesis of pyrazolo-pyrano-phenazine derivatives using Fe3O4@TiO2-SO3H catalyst.
In 2018, Esmaeilpour and co-workers [11] developed an efficient, environmentally benign, and cost-effective method for the synthesis of poly-substituted benzo[a]pyrano[2,3-c]phenazine derivatives (33) via a four-component reaction involving aldehydes (29), diamines (30), malononitrile (31), and 2−hydroxynaphthalene−1,4−dione (32), catalysed by a Fe3O4-based magnetic nano catalyst under reflux conditions (Scheme 7). The reaction progressed efficiently, reaching completion within 20–60 min and yielding the desired products in excellent yields of 81–95%. The reusability studies demonstrated that the catalyst could be effectively reused for five consecutive cycles without any significant loss in catalytic activity. The use of this recyclable nano catalyst was associated with a short reaction time and high efficiency.
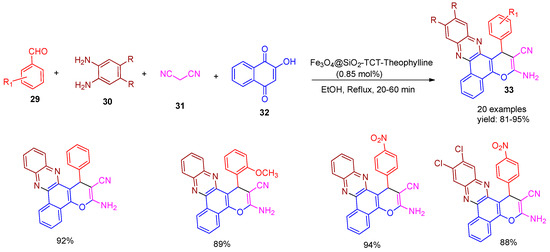
Scheme 7.
Synthesis of pyrano-phenazine derivatives catalysed by Fe3O4@SiO2-TCT-Theophylline.
2.3. Preparation of Pyran Derivatives
Pyran-based compounds are pharmacologically active heterocycles known for their diverse therapeutic applications. They have been reported to exhibit antitubercular [64], antifungal [70], antidiabetic [71], antibacterial [72], calcium channel antagonistic [73], anticancer [74], and anti-HIV activities [75]. Notably, a novel pyran-based phosphodiesterase 9A inhibitor has shown promising efficacy in the treatment of neurodegenerative disorders [76], in addition to demonstrating insulin-sensitizing properties [77].
Ahankar and co-workers [78] (2020) reported a green synthesis of Ni0.5Cu0.5 Fe2O4 MNPS magnetic nanoparticles (MNPs) using Arabic gum as a natural, non-toxic template and stabilizing agent. These MNPs served as efficient nano catalysts for a one-pot, multi-component synthesis of tetrahydropyran derivatives (37) (Scheme 8) via the reaction of dimedone (34), aldehydes (35), and malononitrile (36) under solvent-free, microwave-assisted conditions. The method afforded excellent product yields ranging from 82% to 97%.
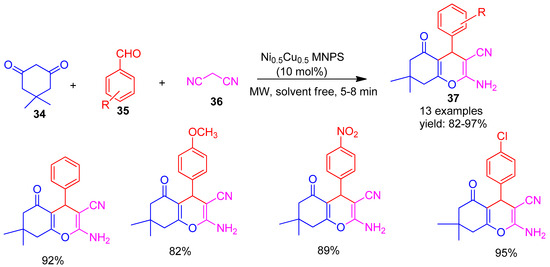
Scheme 8.
Synthesis of tetrahydro pyran using Ni0.5Cu0.5Fe2O4 catalyst.
In 2018, Maleki and co-workers [79] developed and characterized a magnetic bio-nanocomposite based on chitosan, which was subsequently utilized as an efficient heterogeneous catalyst for the one-pot multicomponent synthesis of various heterocyclic compounds. The nano catalyst facilitated the synthesis of 2-amino-4H-pyrans (46) via the reaction of aryl aldehydes (38), malononitrile (41), and ethyl acetoacetate (43); 2-amino-4H-chromene derivatives (45) through the reaction of dimedone (39), aryl aldehydes (38), and malononitrile (41); and polyhydroquinoline derivatives (42 & 44) using aryl aldehydes (38), malononitrile (41), ammonium acetate (40) and ethyl acetoacetate (43) or dimedone (39), all under mild conditions at room temperature in ethanol as a green solvent (Scheme 9). This methodology offers several advantages, including the use of a non-toxic, biodegradable catalyst, easy magnetic separation, recyclability up to seven runs, and high yields making it a promising green synthetic protocol for heterocyclic scaffolds.
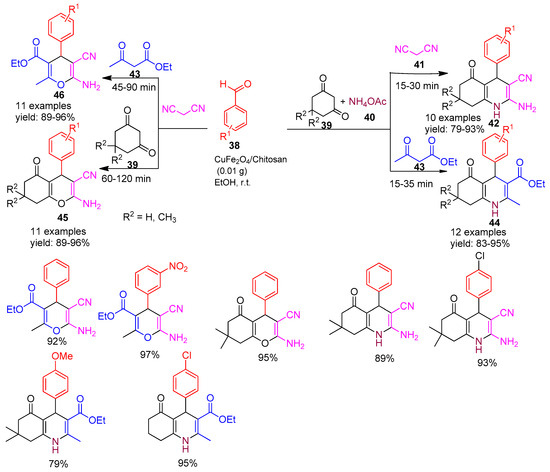
Scheme 9.
Synthesis of pyran, chromene and polyhydro quinoline derivatives.
In 2019, Aghajani and co-workers [80] developed Fe3O4 nanoparticles functionalized with a molybdenum Schiff base complex, named MoO25CML@Fe3O4@SiO2, and utilized them as an efficient nanocatalyst for the solvent-free synthesis of 2-amino-4H-benzo[h]chromene derivatives (50). This one-pot condensation reaction of benzaldehyde (47), 1-naphthol (48), and malononitrile (49) produced the target compounds in excellent yields ranging from 81% to 98%, as depicted in Scheme 10. Furthermore, the catalyst demonstrated good reusability, maintaining significant catalytic activity over at least seven consecutive reaction cycles with minimal loss of performance.
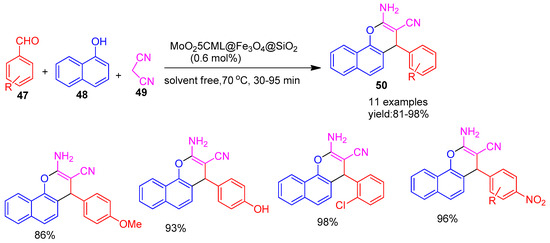
Scheme 10.
Synthesis of 2-amino-4H-benzo[h]chromene derivatives using MoO25CML@Fe3O4@SiO2 catalyst.
Nikpassand and co-workers [81], in 2020, synthesized silica-coated Fe3O4 nanoparticles functionalized with tannic acid (Fe3O4@SiO2@Tannic acid), adhering to green chemistry principles. These magnetically separable nanocatalysts were employed in a solvent-free and environmentally benign synthesis of 1,3-oxazine-2-thione derivatives (54) through a multicomponent reaction involving thiourea (51), aldehydes (52), and β-naphthol (53), carried out by manual grinding using a mortar and pestle. After the reaction, the mixture was dissolved in hot ethanol, and the catalyst was magnetically separated. The method afforded excellent product yields ranging from 87% to 94% (Scheme 11), highlighting its efficiency, eco-friendliness, and potential for sustainable organic synthesis.
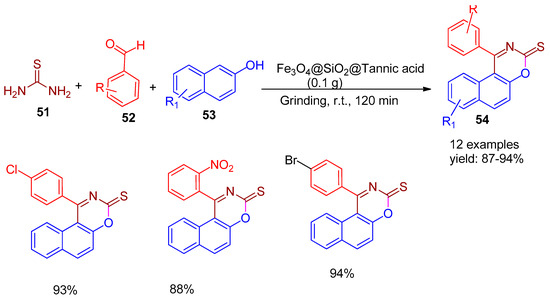
Scheme 11.
Synthesis of 1,3-oxazine-2-thione derivatives using Fe3O4@SiO2@Tannic acid catalyst.
2.4. Preparation of Furan Derivatives
Furans are significant structural motifs frequently present in numerous natural products and pharmaceutical agents [82]. A wide array of furan-containing compounds has exhibited diverse biological activities, such as antitumor [83], antioxidant [84], anticancer [85], antifungal [86], antiplasmodial [87], and anti-inflammatory [88] as well as anti-HIV and estrogenic properties [89].
In 2020, Shirzaei and co-workers [90] reported the synthesis of functionalized furan derivatives (58) via a one-pot multicomponent reaction using equimolar amounts of dialkyl acetylene dicarboxylic acid (55), aromatic aldehydes (56), and amines (57), catalysed by a reusable nano catalyst, Fe3O4@SiO2(CH2)3-thiocarbohydrazide–SO3H (Scheme 12). The reaction was performed at room temperature in ethanol, a green solvent, with reaction times ranging from 19 to 51 min. The catalyst’s reusability was evaluated using magnetic separation, it was washed, dried, and reused for seven consecutive runs, showing no significant loss of activity compared to the fresh catalyst. The protocol yielded excellent product yields in the range of 89–97%. Key advantages of this method include mild reaction conditions, the use of non-toxic ethanol as a solvent, and the facile magnetic separation and recyclability.
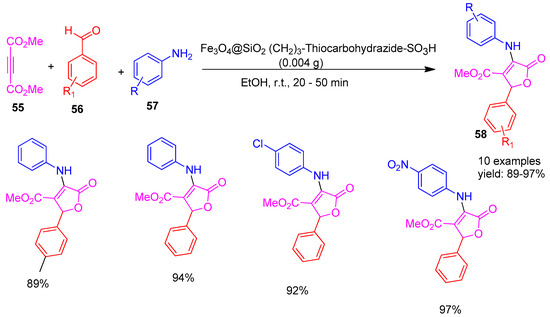
Scheme 12.
Synthesis of furan derivatives catalysed by Fe3O4@SiO2(CH2)3-Thiocarbohydrazide-SO3H.
Ahankar and co-workers [91] reported the use of silica-coated iron oxide magnetic nanoparticles functionalized with tetramethylguanidine (Fe3O4-TMG) as a green, efficient, and recyclable catalyst for the three-component, one-pot synthesis of furanone derivatives (62) (Scheme 13). The reaction involved aniline (61), dialkyl acetylenedicarboxylate (59), and aromatic aldehydes (60), carried out in ethanol at 40 °C for 10 to 13 h. Fe3O4-TMG was reused for five cycles with consistent yields (91–92%), showing excellent stability and catalytic performance. This method stands out for its high efficiency, excellent product yields ranging from 85% to 92%, environmentally friendly reaction conditions, and easy magnetic separation and reuse of the nano catalyst.
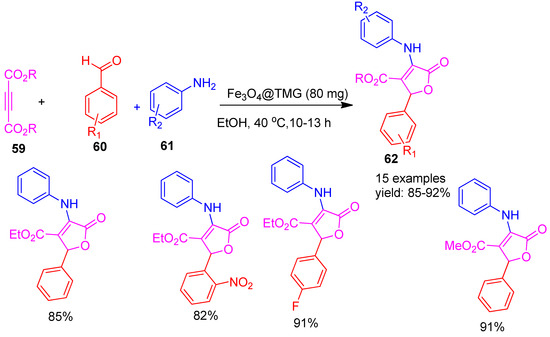
Scheme 13.
Synthesis of furanone derivatives by Fe3O4-TMG catalyst.
2.5. Preparation of Xanthene Derivatives
Xanthenes are a class of oxygen-containing heterocyclic compounds that are commonly found in natural products, synthetic bioactive molecules, and fluorescent dyes [92]. The xanthene core structure imparts a wide range of physicochemical and pharmacological properties. Compounds bearing this scaffold have demonstrated diverse biological activities, including antiviral [93], analgesic [94], antibacterial [95], anti-inflammatory [96], and anticancer [97].
In 2018, Arzehgar and co-workers [98] developed silver-functionalized hydroxyapatite-coated magnetic γ-Fe2O3 nanoparticles (γ-Fe2O3@HAp–Ag) and employed them as a magnetically recoverable, environmentally friendly, and reusable catalyst. Their catalytic efficiency was demonstrated in the solvent-free synthesis of aryl-dibenzo-xanthenes (65) through the condensation of substituted benzaldehydes (64) with β-naphthol (63) at 60 °C (Scheme 14). The reaction without the catalyst yielded no product. Furthermore, the magnetically separable MNP catalyst was reused for seven cycles with no significant loss in its initial catalytic activity.
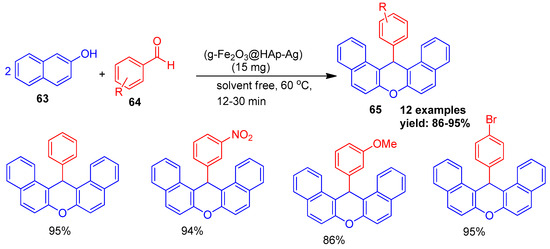
Scheme 14.
Synthesis of xanthene derivatives using γFe2O3@HAp-Ag catalyst.
In 2019, Sonei and co-workers [99] developed a magnetically reusable nano catalyst by immobilizing Cu(II) onto Fe3O4@APTMS-DFX nanoparticles. This catalyst was effectively utilized for the solvent-free synthesis of substituted 8,9,10,12-tetrahydrobenzoxanthen-11-one derivatives (69) via a three-component reaction involving 2-naphthol (67), dimedone (68), and aromatic aldehydes (66) at 120 °C, achieving excellent yields ranging from 80% to 97% (Scheme 15). Further, the reaction was carried out under solvent-free conditions at 120 °C without any catalyst, yielding only 14% after 24 h. The catalyst was successfully recycled for six runs without appreciable copper leaching from its surface. The method offers several advantages, including short reaction times, high product yields, straightforward work-up, and simple purification through recrystallization.
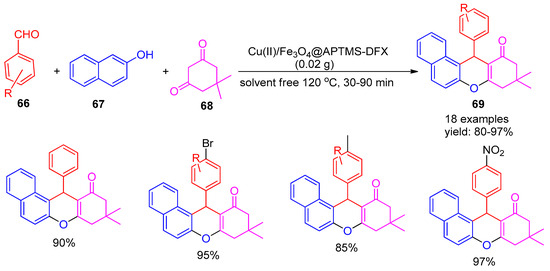
Scheme 15.
Synthesis of tetrahydro benzoxanthen-11-one derivatives using Cu (II)/Fe3O4@APTMS-DFX catalyst.
2.6. Preparation of Imidazole Derivatives
Nitrogen-containing aromatic heterocyclic compounds, particularly imidazoles, have attracted considerable attention in both academic and industrial research due to their wide spectrum of biological and pharmacological activities [100]. Imidazole scaffolds play a crucial role in the design and synthesis of biologically active molecules [101], contributing to the development of therapeutic agents with anticancer [102], anticoagulant [103], anti-inflammatory [104], antitubercular [105], antimicrobial [106], antimalarial [107], and antioxidant [108] properties.
In 2021, Sakhdari and co-workers [109] reported the synthesis and application of magnetic nanoparticle-supported sulfonic acid (γ-Fe2O3–SO3H) as an efficient and reusable catalyst for the preparation of imidazole derivatives (Scheme 16). The catalyst facilitated the formation of 1,2,4,5-tetrasubstituted imidazoles (75) via a one-pot four-component reaction involving ammonium acetate (72), aldehydes (71), benzil (70), and amine (74) derivatives, as well as 2,4,5-trisubstituted imidazoles (73) through a three-component reaction using benzil (70), aldehydes (71), and ammonium acetate (72). The reactions proceeded rapidly, with tetrasubstituted imidazoles forming in 30–40 min and trisubstituted imidazoles in 40–70 min. The method afforded excellent yields, ranging from 94–98% for tetrasubstituted and 92–98% for trisubstituted imidazole derivatives, highlighting the catalyst’s high efficiency and selectivity.
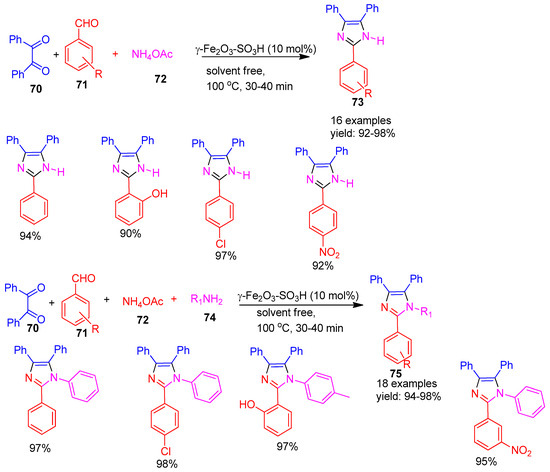
Scheme 16.
Synthesis of tri and tetra-substituted-imidazole derivatives by γ-Fe2O3−SO3H catalyst.
In 2020, Khalifeh and co-workers [110] developed a novel core–shell structured, green, and recyclable nano catalyst, Fe3O4@SiO2−EPIM, synthesized by modifying Fe3O4@SiO2 with 1-methylimidazole and epichlorohydrin. The catalytic activity of this material was evaluated in the one-pot synthesis of trisubstituted imidazoles (79) using a condensation reaction between aldehydes (76), benzil (78), and ammonium acetate (77) in polyethylene glycol (PEG−200) as a green solvent. The reaction proceeded efficiently, affording the desired imidazole derivatives in moderate to excellent yields ranging from 57% to 98%, as shown in Scheme 17. After each reaction, the catalyst was separated, washed, dried, and reused under identical conditions. It retained high activity over six runs, confirming excellent stability. The catalyst demonstrated environmentally friendly features, including ease of recovery, reusability, and high efficiency under mild reaction conditions.
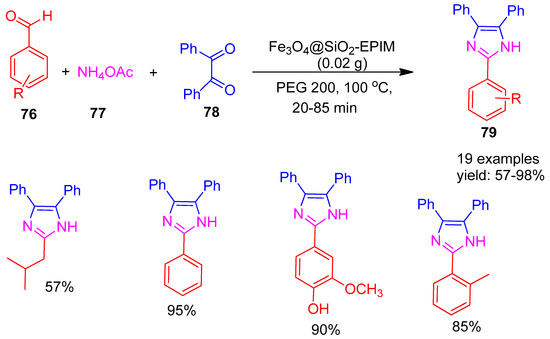
Scheme 17.
Synthesis of trisubstituted imidazole derivatives using Fe3O4@SiO2−EPIM catalyst.
2.7. Preparation of and Indole Derivatives
The indole core, a well-established pharmacophore in medicinal chemistry, is a versatile heterocyclic scaffold known for its broad range of biological activities [111]. Indole-containing compounds have demonstrated diverse pharmacological properties, including anticancer, antifungal, anti-HIV, anti-inflammatory, antiviral, antitubercular, and antimicrobial activities [112]. Additionally, they exhibit antihypertensive [111], antidiabetic [113], and photochemotherapeutic effects [114], making the indole nucleus a valuable structural motif in the development of new therapeutic agents.
In 2018, Esmaeilpour and co-workers [11] reported a green, single-step multicomponent synthesis of spirooxindole derivatives (83) using ethyl cyanoacetate or malononitrile (82), 5-amino-1,3-dimethyluracil (80), and isatin (81), catalyzed by a Fe3O4@SiO2-TCT-theophylline nanomagnetic catalyst under reflux conditions in water as a green solvent (Scheme 18). The protocol successfully yielded twelve spirooxindole derivatives with excellent yields ranging from 90% to 95%, within a reaction time of 6 to 12 h. After the reaction, the mixture was cooled, and the catalyst was magnetically separated, washed with hot ethanol, dried, and reused. It maintained its activity over five consecutive cycles without significant loss.
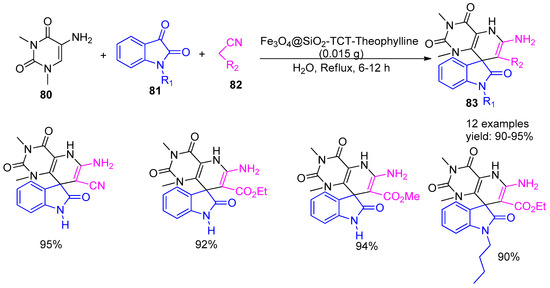
Scheme 18.
Synthesis of spiroxoindole derivatives catalysed by Fe3O4@SiO2-TCT-Theophylline.
Safari and co-workers [115] (2019) reported the synthesis of indeno[1,2-b]indole derivatives (87) using montmorillonite (MMT)-supported Fe3O4 magnetic nanoparticles (MMT@Fe3O4) as a heterogeneous catalyst (Scheme 19). The reaction involved aromatic amines (84), 1,3-dicarbonyl compounds such as 1,3-cyclohexadione or dimedone (85), and ninhydrin (86), carried out in water at 70 °C with stirring for 20–40 min. After the reaction, the catalyst was magnetically separated, washed, air-dried, and reused with fresh substrates for six runs without significant loss of activity.
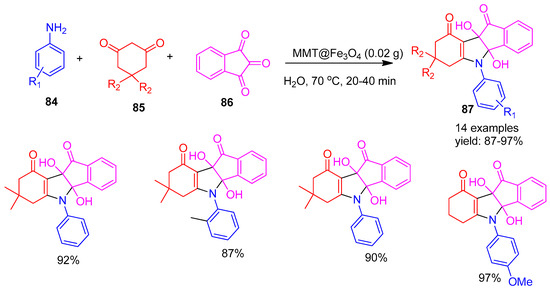
Scheme 19.
Synthesis of indeno-indole derivatives using MMT@Fe3O4 catalyst.
Nongthombam and co-workers [116] (2023) developed a novel acidic ionic liquid supported on ferrite nanoparticles, designated as Fe3O4@PCmIm-HSO4, and employed it as an efficient heterogeneous nano catalyst for the synthesis of bis (indolyl) alkane derivatives (90) (Scheme 20). The reaction involved indole or 2-methylindole (88) and aliphatic or aromatic carbonyl compounds (89), carried out in an ethanol–water mixture at room temperature, yielding products in the range of 83% to 93%. Upon completion, the catalyst was magnetically separated, washed with water and diethyl ether, dried at room temperature, and reused. It retained its catalytic activity for at least six cycles without significant loss. This method offers a green and sustainable approach, and easy magnetic recovery.
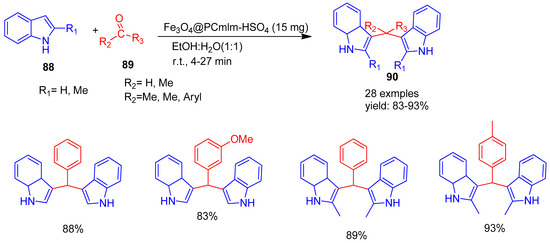
Scheme 20.
Synthesis of bis (indolyl) alkane derivatives using Fe3O4@PCmIm-HSO4 catalyst.
2.8. Preparation of Pyridine Derivatives
Pyridine derivatives are a particularly interesting type of heterocycles that can be found in both synthetic and natural molecules. The pyridine ring system is frequently used in responsive pharmacophores, sustainable agrochemicals, cosmetics, and biomimetic applications in addition to being present in bioactive compounds [117]. A wide range of biological and pharmacological activities are also displayed by pyridine-containing heterocyclic compounds, such as antimicrobial [118], antiviral [119], antioxidant [120], antidiabetic [121], anticancer [122], antimalarial [123], and anti-inflammatory [124].
Maleki and co-workers [125] (2018) developed a novel nanomagnetic organocatalyst, Fe3O4@SiO2@propyltriethoxysilane@L-proline (LPSF) and evaluated its catalytic efficiency in the solvent-free synthesis of 2,4,6-triarylpyridine derivatives (94). The reaction was carried out via a three-component condensation of acetophenones (91), various benzaldehydes (92), and ammonium acetate (93) under stirring at 60 °C, affording good to excellent yields (75–94%) as illustrated in Scheme 21. To assess the recyclability of the catalyst, it was magnetically separated, washed with ethanol and water, dried, and reused. It maintained high activity over seven cycles without significant loss in product yield.
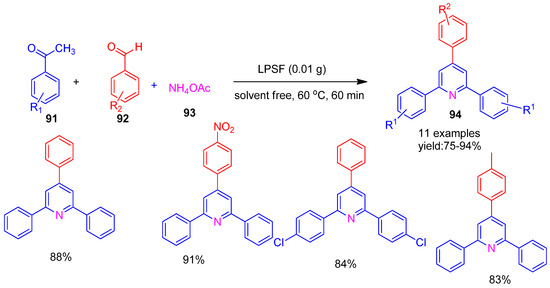
Scheme 21.
Synthesis of triaryl pyridine derivatives using LPSF catalyst.
Thrilokraj and co-workers [126] (2024) reported a green and cost-effective biogenic synthesis of Fe3O4 nanoparticles using Bacopa monnieri (Brahmi) extract. The catalytic efficiency of the biosynthesized Fe3O4 NPs was evaluated in a solvent-free multicomponent reaction involving benzaldehyde (95), malononitrile (96), acetophenone (97), and ammonium acetate (98) for the synthesis of 2-amino-3-cyanopyridine derivatives (99). The reaction proceeded smoothly under mild conditions, affording the desired products in excellent yields ranging from 79% to 91%, as illustrated in Scheme 22. The reaction without the catalyst resulted in a poor yield. This approach highlights the potential of plant-mediated magnetic nanoparticles as eco-friendly and efficient catalysts for green organic transformations.
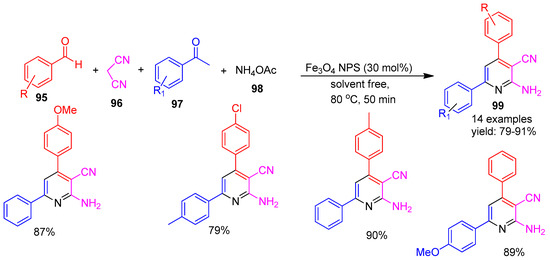
Scheme 22.
Synthesis of functionalized pyridine derivatives using Fe3O4 catalyst.
Maleki [127] and co-workers (2020) reported a novel and environmentally friendly method for synthesizing polyfunctionalized pyridines (103) using a magnetic Fe2O3@Fe3O4@Co3O4 nanocomposite as a catalyst. The reaction was carried out under solvent-free conditions via a multicomponent coupling of malononitrile (101), various aldehydes (100), and ammonium acetate (102), affording products in excellent yields ranging from 78% to 97%, as shown in Scheme 23. Furthermore, the same reaction using Co3O4 yielded the product in low amounts after 2 h, while Fe2O3 and Fe3O4 gave only trace yields. The catalyst is magnetically separable and reusable for up to four runs without loss of activity. This method offers high yields, operational simplicity, a straightforward workup, broad applicability, and minimal environmental impact owing to its solvent-free conditions.
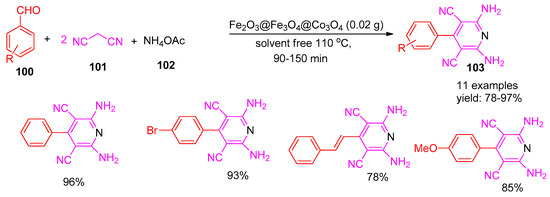
Scheme 23.
Synthesis of functionalized pyridine derivatives using Fe2O3@Fe3O4@Co3O4 catalyst.
In 2019, Rakhtshah [128] and co-workers developed a novel manganese Schiff base complex supported on chitosan-coated iron oxide magnetic nanoparticles (Fe3O4@CSBMn). This magnetically recoverable nano catalyst was applied in a multicomponent reaction involving trimethylsilyl cyanide (105), aldehydes (106), and 2-aminopyridine (104) for the synthesis of 3-iminoaryl-imidazo-pyridine derivatives (107), as depicted in Scheme 24. Furthermore, using silica-sulfuric acid as the catalyst gave a low yield even after 3 days. The protocol yielded the desired products in high yields (85–97%) within short reaction times (10–35 min). Although the catalyst exhibited good reusability, recycling studies revealed a noticeable decline in catalytic activity after six cycles.
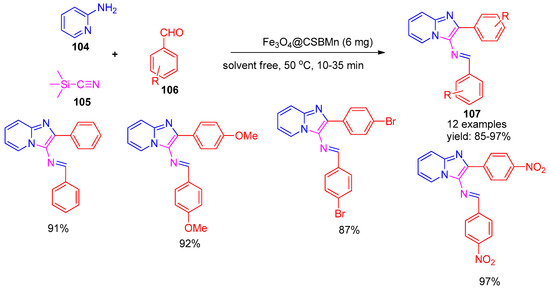
Scheme 24.
Synthesis of imidazo-pyridine derivatives using Fe3O4@CSBMn catalyst.
In 2020, Ahadi and co-workers [129] designed and synthesized a heterogeneous magnetic nano catalyst, MNP@BSAT@Cu(OAc)2, by functionalizing manganese ferrite (MnFe2O4) nanoparticles with a Schiff base encapsulated in a silica shell, followed by coordination with copper acetate. The catalytic efficiency of the nanocomposite was evaluated in a one-pot multicomponent reaction involving dimedone (108), benzaldehyde (111), ethyl acetoacetate (110), and ammonium acetate (109) for the synthesis of 1,4-dihydropyridine derivatives (112). The method afforded excellent yields ranging from 80% to 97%, as shown in Scheme 25. The reaction was also performed in the absence of a catalyst and low yield of product was obtained in a prolonged reaction time. Post-reaction, the catalyst was separated using a magnet, washed with ethanol, dried, and reused. It remained effective for five cycles without significant activity loss.
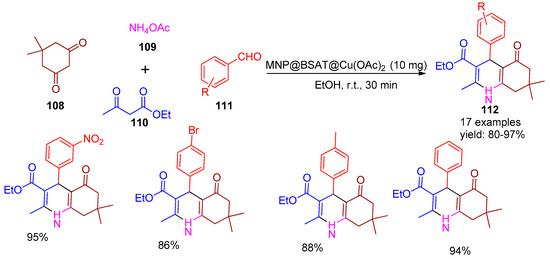
Scheme 25.
Synthesis of 1,4-dihydropyridines derivatives using MNP@BSAT@Cu(OAc)2 catalyst.
In 2021, Maleki and co-workers [130] developed a convenient and eco-friendly nano catalyst by supporting glutathione on magnetic Fe3O4 nanoparticles. This catalyst was successfully applied in the synthesis of a wide range of 1,4-dihydropyridine derivatives (116 & 119) via a multicomponent reaction involving benzaldehyde (113), ammonium acetate (114), ethyl acetoacetate (115), and dimedone (117) under solvent-free conditions at 110 °C (Scheme 26). Furthermore, the nano-FGT catalyst was employed for the solvent-free synthesis of acridines (118) via the reaction of various aldehydes (113), ammonium acetate (114), and dimedone (117), affording yields of 81–92%. Furthermore, control experiments confirmed that nano-FGT outperformed unfunctionalized nanoparticles and glutathione alone, which yielded significantly less product. After the reaction, nano-FGT was magnetically separated, dried at 50 °C, and reused three times in the same reaction, consistently yielding similar results in each cycle.
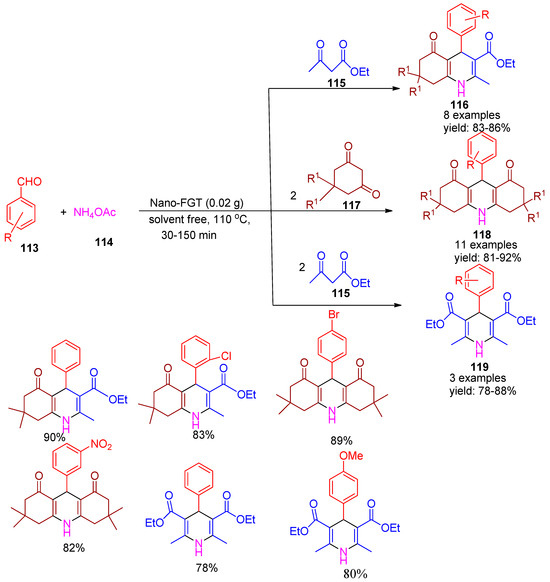
Scheme 26.
Synthesis of 1,4-dihydropyridine derivatives using Fe3O4 supported glutathione catalyst.
Bodaghifard [131] (2020) introduced silica-coated Fe3O4 nanoparticles functionalized with bis-sulfamic acid (MNPs-TBSA), forming a unique inorganic-organic core–shell magnetic nanostructure, as an efficient and reusable heterogeneous acidic catalyst. The catalytic potential of this nanomaterial was demonstrated in the green synthesis of pharmaceutically relevant polyhydroquinoline derivatives (124) via a multicomponent condensation of ethyl acetoacetate (120), dimedone (121), various benzaldehydes (122), and ammonium acetate (123) in ethanol at 70 °C (Scheme 27). Furthermore, the same reaction without a catalyst yielded only ~17% after 90 min. This protocol provided high yields (89–95%) and offered several advantages, including mild reaction conditions, simple work-up, exceptional product purity, short reaction times, and catalyst recyclability.
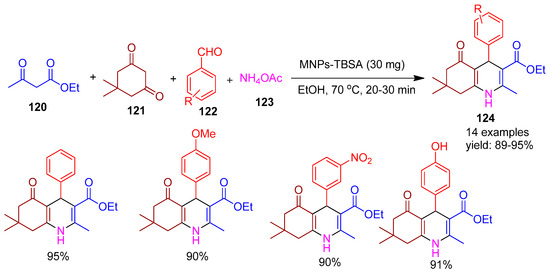
Scheme 27.
Synthesis of polyhydro quinoline derivatives using MNPs-TBSA catalyst.
In 2019, Bodaghifard [132] reported the development of a retrievable heterogeneous catalyst based on 4-aminoquinaldine embedded on silica-coated nano-Fe3O4 particles (MNPs-AQ). The catalytic performance of MNPs-AQ was evaluated in the eco-friendly, one-pot multicomponent synthesis of substituted 1,4-dihydropyridine derivatives (129) via the reaction of ethyl acetoacetate (127), malononitrile (125), various benzaldehydes (128), and ammonium acetate (126) under reflux in a H2O/EtOH mixture, as shown in Scheme 28. Further, performing the same reaction without a catalyst in refluxing ethanol gave only 23% yield after 90 min. This method offered several advantages over conventional approaches, including high yields (83–97%), exceptional product purity, shorter reaction times, and environmental compatibility, along with easy magnetic recovery and reusability of the catalyst.
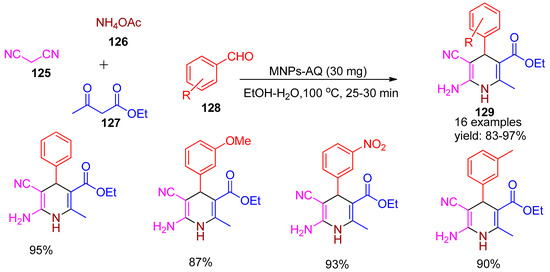
Scheme 28.
Synthesis of 1,4-dihydropyridine derivatives using MNPs-AQ catalyst.
2.9. Preparation of Pyrimidine and Acridine Derivatives
Heterocyclic compounds are of significant biological interest due to their diverse chemical and physical properties [133]. Among them, pyrimidine derivatives have garnered considerable attention for their wide range of pharmacological activities, including antimicrobial [134] analgesic [135], anticancer [136], antioxidant [137], anti-inflammatory [138], and anti-HIV properties [139]. Although several synthetic routes for pyrimidines have been established for decades, the development of more economical, efficient, and environmentally benign methods remains an area of substantial importance [140,141].
In 2021, Rostami [142] co-workers. developed a reusable heterogeneous magnetic nano catalyst, CoFe2O4@SiO2-PA-CC-guanidine-SA, for green organic synthesis. The catalyst was applied in the one-pot synthesis of pyrido[2,3-d:5,6-d’]dipyrimidine derivatives (133) via a multicomponent reaction of benzaldehyde (130), thiobarbituric acid or barbituric acid (132), and ammonium acetate (131) in water at room temperature. The process afforded high product yields ranging from 86% to 95%, as shown in Scheme 29. The use of water as a solvent, ambient conditions, and the magnetic recyclability of the catalyst underscore the method’s environmental and operational advantages.
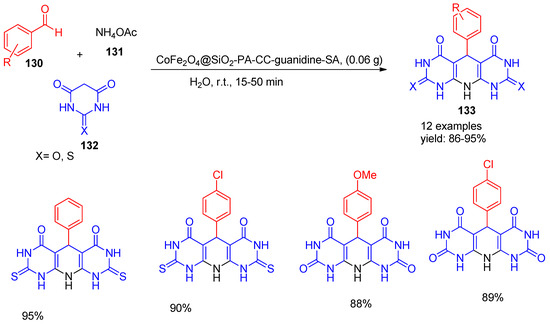
Scheme 29.
Synthesis of pyrimidine derivatives using CoFe2O4@SiO2-PA-CC-guanidine-SA catalyst.
In 2023, Sayahi and co-workers [143] synthesized a novel and eco-friendly magnetic nano catalyst, SPION@CS-IL, by modifying chitosan-functionalized ionic liquids with iron oxide nanoparticles. This heterogeneous, reusable catalyst was effectively employed for the synthesis of pyrido[2,3-d]pyrimidine derivatives (137) via a three-component, one-pot reaction involving 4-hydroxycoumarin (134), thiobarbituric acid (135), and various aldehydes (136), conducted in ethanol solvent using ultrasonic-assisted method to produce 84–92% yield (Scheme 30). This methodology offers numerous advantages, including short reaction times, low catalyst loading, absence of toxic reagents, and recyclability of the catalyst for up to five cycles without significant loss of activity.
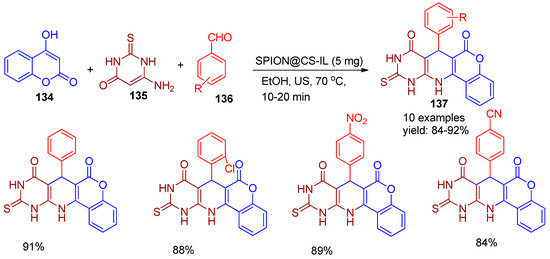
Scheme 30.
Synthesis of pyrimidine derivatives by SPION@CS-IL catalyst.
In 2021, Karimi and co-workers [144] reported the use of magnetic nanoparticles functionalized with 3-(propylthio)propane-1-sulfonic acid as an efficient heterogeneous catalyst for the solvent-free synthesis of various tetrazole-fused heterocycles (Scheme 31). The catalyst demonstrated excellent activity in the one-pot synthesis of dihydro-tetrazolo[1,5-a]pyrimidine derivatives (141) via the reaction of aldehydes (138), acetophenone (140), and 5-aminotetrazole (139), carried out under stirring at 70 °C. Additionally, it was applied to the synthesis of tetrahydro-tetrazolo[5,1-b]quinazolinone derivatives (145) from dimedone (144), aryl aldehydes (138), and 5-aminotetrazole (139), and to the formation of dihydro-tetrazolo[1,5-a]pyrimidine-6-carboxylate (143) derivatives using 5-aminotetrazole (139), aryl aldehydes (138), and ethyl acetoacetate (142). All reactions proceeded efficiently within 15–30 min, affording high yields. Further, under identical conditions without a catalyst, only trace yield was obtained after 60 min. Key advantages of this protocol include solvent-free conditions, short reaction times, excellent yields, and magnetic recoverability of the catalyst, making it a standout method in the field of sustainable synthesis.
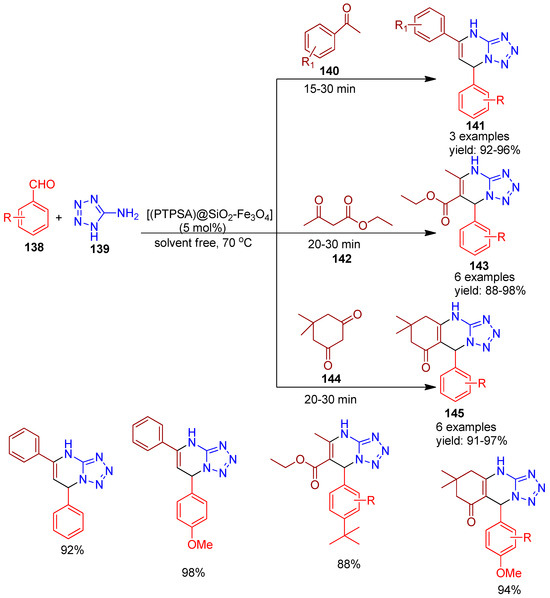
Scheme 31.
Synthesis of dihydro-tetrazolo- pyrimidine derivatives using [(PTPSA)@SiO2-Fe3O4] catalyst.
In 2021, Wenxin and co-workers [145] successfully synthesized SnCl2-grafted magnetic nanoparticles (SnCl2@MNPs) via a co-precipitation method, where the core–shell structure was achieved through alkali-catalyzed hydrolysis of tetraethyl orthosilicate. The catalytic efficiency of SnCl2@MNPs was evaluated through the Biginelli reaction, a one-pot, three-component synthesis of 3,4-dihydropyrimidinones (149) (Scheme 32). This transformation involved the condensation of thiourea or urea (147), aromatic or heterocyclic aldehydes (148), and α,β-dicarbonyl compounds (146), affording the desired products in excellent yields ranging from 82% to 96%.
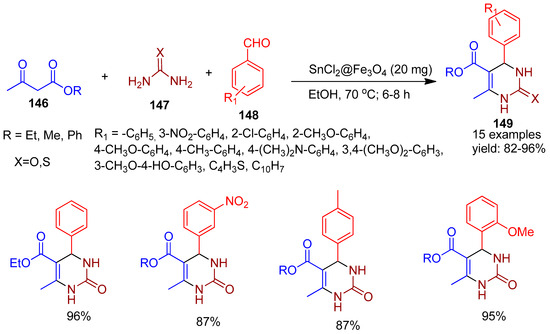
Scheme 32.
Synthesis of 3,4-dihydropyrimidinones derivatives using SnCl2@Fe3O4 catalyst.
In 2020, Alishahi and co-workers [146] developed a novel acidic nicotine-based ionic liquid immobilized on magnetic nanoparticles, denoted as [NicTC]HSO4@MNPs. The catalytic performance of this material was evaluated in a multicomponent reaction involving β-ketoesters (152), 2-aminobenzothiazole (150), and various benzaldehydes (151) for the synthesis of mono- and pyrimido-benzothiazole derivatives (153). The reaction proceeded efficiently, yielding the target compounds in 69–83%, as illustrated in Scheme 33. Further, under identical conditions without a catalyst, no product was obtained even after 7 h. This approach offers several advantages, including short reaction times, high product yields, simple work-up procedures, elimination of hazardous organic solvents, and excellent catalyst recyclability.
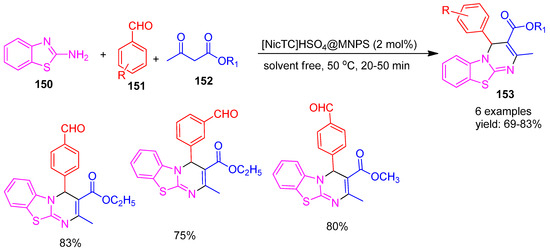
Scheme 33.
Synthesis of pyrimido benzothiazole derivatives using [NicTC]HSO4@MNP catalyst.
Verma and co-workers [147], in 2020, developed a green biocatalyst based on starch-functionalized magnetite nanoparticles (s-Fe3O4) for use under ultrasonic irradiation conditions. This eco-friendly catalyst was employed in a multicomponent reaction involving benzaldehyde (154), malononitrile (155), and 2-aminobenzimidazole (156) in water as a green solvent to synthesize imidazole-pyrimidine derivatives (157). The method yielded excellent results, with product yields ranging from 91% to 98% (Scheme 34). Under the same reaction conditions, ultrasound irradiation of the reaction mixture without a catalyst resulted in no product formation. The use of ultrasonic irradiation significantly enhanced the reaction rate and efficiency, making this protocol both environmentally sustainable and operationally simple.
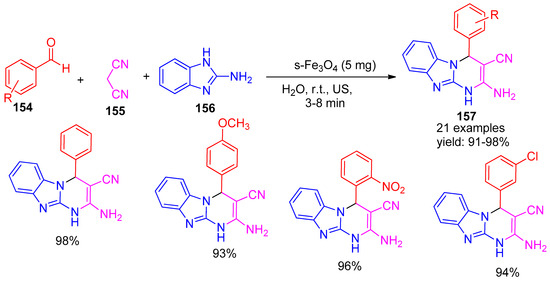
Scheme 34.
Synthesis of pyrimidine derivatives using s-Fe3O4 catalyst.
Saberikhah [148] and co-workers synthesized a series of pyrido[2,3-d] pyrimidine derivatives (161 & 163) using an organic-inorganic hybrid magnetic nano catalyst, Fe3O4@TiO2@NH2@PMo12O40. The catalyst was employed in a three-component reaction involving malononitrile (160), 6-amino-2-(thio or alkylthio) pyrimidinone (158 & 162), and various aryl aldehydes (159) in water at 80 °C, affording excellent yields (92–98%) within short reaction times (5–15 min), as illustrated in Scheme 35. Further, under the same reaction conditions without a catalyst, no product was obtained even after 30 h. Notably, the catalyst could be efficiently separated using an external magnet and reused for up to eight consecutive cycles without significant loss of activity.
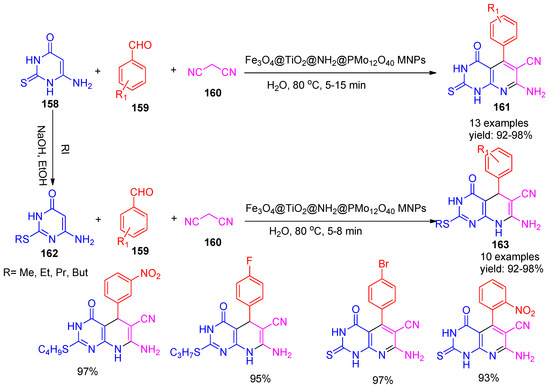
Scheme 35.
Synthesis of pyrimidine derivatives using Fe3O4@TiO2@NH2@PMo12O40 catalyst.
In 2022, Taheri Hatkehlouei [149] and co-workers developed sulfonated magnetic nanoparticles (Fe3O4@C@OSO3H) and employed them as efficient catalysts for solvent-free Biginelli reactions involving β-dicarbonyl compounds (164 & 166), aromatic aldehydes (162), and urea or thiourea (163). This protocol led to the synthesis of biologically active 3,4,5,6-tetrahydropyrimidinone/thione and 3,4-dihydropyrimidinone/thione derivatives (165 & 167) with excellent yields ranging from 80% to 97%, as illustrated in Scheme 36. Furthermore, under identical conditions without the catalyst, only trace amounts of the product were observed, with most of the starting materials remaining unreacted even after prolonged reaction time. Additionally, the nanoparticles were easily separated magnetically and Fe3O4@C@OSO3H was efficiently recycled for seven runs with minimal loss of catalytic activity. The method features several advantages, including a simple and eco-friendly work-up, high product yields, short reaction times, and easy magnetic recovery of the catalyst for potential reuse.
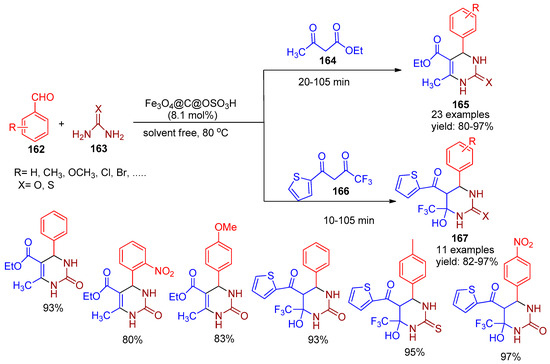
Scheme 36.
Synthesis of tetra/dihydro pyrimidinone/thione derivatives using Fe3O4@C@OSO3H catalyst.
Ghafuri and co-workers [150] (2021) reported the use of Fe3O4@Polyaniline-SO3H as a reusable, heterogeneous nano catalyst for the rapid and efficient green synthesis of acridine dione derivatives (171) (Scheme 37). The reaction was performed under reflux conditions in ethanol using ammonium acetate (170), dimedone (169), and aromatic aldehydes (168), with a remarkably short reaction time of 10–15 min and excellent product yields ranging from 90% to 98%. The nano catalyst can be easily retrieved from the reaction mixture using a magnet and reused for six cycles without any noticeable decline in its catalytic performance. Key advantages of this methodology include excellent yields, ease of product isolation, and a short reaction time.
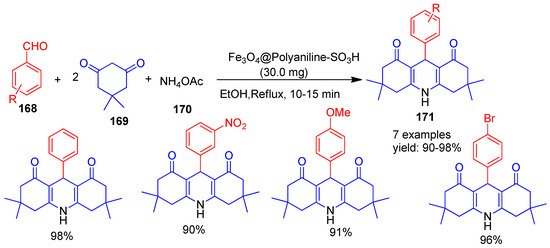
Scheme 37.
Synthesis of acridine derivatives by Fe3O4@Polyaniline-SO3H catalyst.
In 2021, Mousavi and co-workers [151] developed magnetically recoverable graphene-based nanoparticles (MSrGO NCs) as an efficient catalyst for the synthesis of acridine derivatives (175 & 177). The reaction was carried out under solvent-free conditions using aromatic amines (174) or ammonium acetate (176), dimedone (172), and aromatic aldehydes (173), yielding the desired products with high efficiency (Scheme 38). The reaction carried out without any catalyst at room temperature showed no progress even after 48 h. Furthermore, the MSrGO NCs demonstrated excellent reusability, maintaining their catalytic efficiency over seven consecutive cycles without significant loss of activity. These protocols offer notable advantages such as operational simplicity, low catalyst loading, better control over reaction conditions, shorter reaction times, and high product yields.
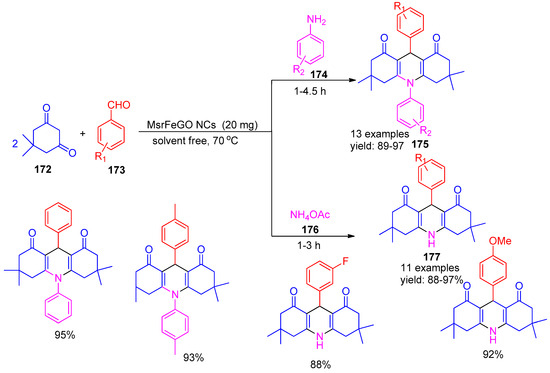
Scheme 38.
Synthesis of acridine derivatives using MSrGO NCs catalyst.
2.10. Preparation of Azole and Propargylamine Derivatives
“Azole” is a general term referring to five-membered heterocyclic rings—such as triazole, tetrazole, pentazole, oxazole, thiazole, and isoxazole-that contain at least one nitrogen atom and other heteroatoms [152,153,154]. Numerous azole-based sulfonamide inhibitors have been developed, particularly triazole-based antifungal agents like fluconazole, voriconazole, posaconazole, itraconazole, and isavuconazole, as well as imidazole-based drugs including ketoconazole, miconazole, and clotrimazole [153,154]. These triazole antifungal agents are employed for the treatment and prevention of both superficial and systemic fungal infections [155].
In 2021, Mirshafiee [156] and co-workers reported the synthesis and characterization of magnetic nanoparticles modified with copper iodide supported on 3-thionicotinyl-urea (MNPs@ThNU-CuI). The structure and composition of the catalyst were confirmed using various analytical techniques. Its catalytic performance was evaluated through a one-pot multicomponent reaction involving sodium azide (180), aryl halides (178), and terminal alkynes (179) to synthesize 1,2,3-triazole derivatives (181). The reaction was conducted in a deep eutectic solvent (DES) composed of PEG and choline chloride, which served as an environmentally friendly and recyclable medium. After each cycle, the catalyst was magnetically separated, washed, vacuum-dried at 70 °C, and reused. MNPs@ThNU-CuI maintained its activity over five cycles with no significant loss. This method afforded triazoles in good to excellent yields (69–94%), as shown in Scheme 39.
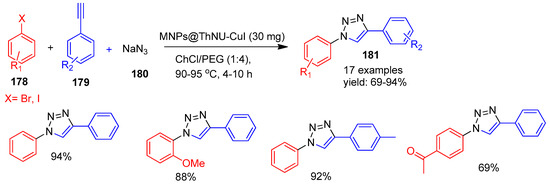
Scheme 39.
Synthesis of triazole derivatives using MNPs@ThNU-CuI catalyst.
Eisavi and co-workers [35] (2021) introduced a MgFe2O4/Cu nanocomposite as a simple and efficient nano catalyst for the regioselective synthesis of β-thiol-1,4-disubstituted-1,2,3-triazole derivatives (185) (Scheme 40). The reaction was carried out via a three-component protocol involving thiirane (182), alkynes (183), and sodium azide (184) in water under magnetic stirring at 60 °C for 2–4 h to produce triazole derivatives from 80 to 95% yield. Both the nano catalyst and the synthesized triazole derivatives were reported as novel, representing a completely new synthetic approach. The nanoparticles were easily recovered using a magnet, washed with ethyl acetate and water, dried, and reused for six cycles without significant loss of activity. This methodology offers several notable advantages, short reaction times, high yields, use of a green solvent (water), and recyclability of the catalyst.
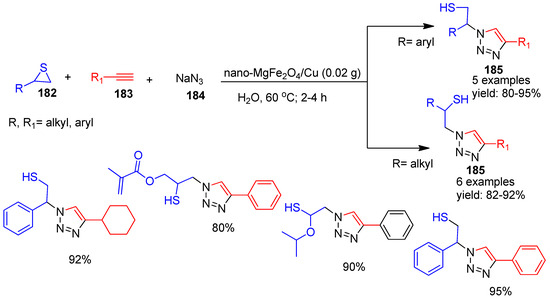
Scheme 40.
Synthesis of triazole derivatives by MgFe2O4/Cu nanocomposite catalyst.
In 2019, Soleimani-Amiri [157] and co-workers reported a green synthesis of magnetic iron oxide nanoparticles (Fe3O4-MNPs) using orange peel water extract as a natural reducing agent for ferric chloride. These eco-friendly nanoparticles were employed as catalysts in the room-temperature synthesis of dihydro-2H-cyclopenta[d][1,3] oxazole derivatives (189) via a multicomponent reaction involving dialkyl acetylene dicarboxylates (187), α-haloketones (188), and 1,3-oxazole-2(3H)-thione derivatives (186). The protocol provided excellent yields ranging from 83% to 97%, as illustrated in Scheme 41. Further, these reactions gave low yields and produced a mixture of products in the absence of a catalyst. For catalyst reusability, it was magnetically separated from the reaction mixture, washed with ethyl acetate, air-dried, and reused under identical conditions without further purification. The catalyst maintained its activity over five cycles with no significant reduction in product yield. Furthermore, the antioxidant activity of the synthesized compounds was evaluated using the DPPH assay, revealing that several of them exhibited significant radical scavenging properties.
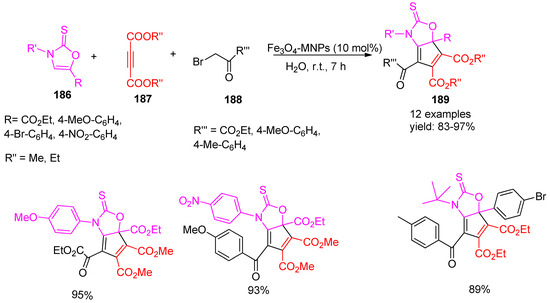
Scheme 41.
Synthesis of oxazole derivatives using Fe3O4-MNPs catalyst.
In 2020, Ahmadi and co-workers [158] developed a copper-supported magnetic nano catalyst, Fe3O4@MCM-41-SB-Cu, and explored its catalytic effectiveness in the synthesis of 5-substituted-1H-tetrazole derivatives (193) via a [3+2] cycloaddition reaction. The protocol involved the reaction of hydroxylamine hydrochloride (191), sodium azide (192), and aldehydes (190) at 120 °C, yielding the desired products in 72–91% as illustrated in Scheme 42. Catalyst reusability was studied under optimized conditions, and the catalyst was magnetically separated, washed with ethanol and water, dried at 60 °C, and reused. The immobilized catalyst retained its efficiency and selectivity over five cycles without significant loss of activity.
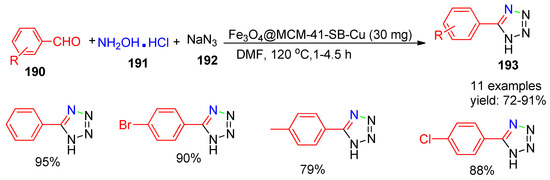
Scheme 42.
Synthesis of tetrazole derivatives using Fe3O4@MCM-41-SB-Cu catalyst.
2.11. Preparation of Propargylamine Derivatives
Propargylamines are highly valuable and versatile intermediates extensively employed in the synthesis of nitrogen-containing bioactive molecules, including agrochemicals, β-lactams, peptides, and isosteres [159]. Several traditional approaches have been developed for their synthesis, which generally involve the direct reaction of amines with propargyl halides, phosphates, or triflates, as well as the nucleophilic addition of metal acetylides to imines. These reactions often require strong bases such as hydroxides, alkoxides, organometallic reagents like butyllithium, or lithium diisopropylamide (LDA) [160,161].
In 2021, Ghasemi [162] and co-workers developed a novel magnetic nanocomposite catalyst, AgNPs/Fe3O4@chitosan/PVA, by functionalizing chitosan polymer chains with polyvinyl alcohol (PVA) and incorporating silver nanoparticles (AgNPs) along with magnetic Fe3O4. The catalytic performance of this nanocomposite was evaluated in multicomponent A3-coupling and click reactions involving piperidine (194), benzaldehyde (195), and phenylacetylene (196). The reactions efficiently yielded propargylamine derivatives (197) in excellent yields ranging from 80% to 98%, as illustrated in Scheme 43. The nano catalyst was magnetically separated, washed with ethanol (5 mL), vacuum-dried overnight, and reused for six consecutive cycles without significant loss of activity.
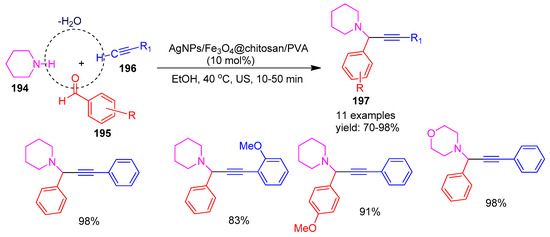
Scheme 43.
Synthesis of propargylamine derivatives using AgNPs/Fe3O4@chitosan/PVA catalyst.
In 2023, Hasan and co-workers [163] developed an efficient and magnetically recoverable catalyst based on Fe3O4-chitosan functionalized with a Cu(II) Schiff base complex. The catalyst exhibited excellent activity under microwave irradiation for the A3-coupling reaction of amines (199), alkynes (200), and aldehydes (198), affording propargylamine derivatives (201) in good to excellent yields ranging from 65% to 97%, as shown in Scheme 44. The catalyst exhibited excellent recyclability, retaining 95% efficiency after six cycles.
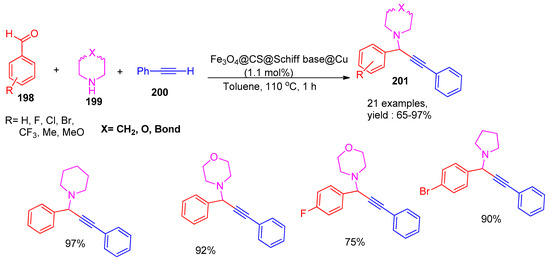
Scheme 44.
Synthesis of propargyl amine derivatives using Fe3O4@CS@Schiff base@Cu catalyst.
In 2024, Nasrin Moeini-Eghbali and co-workers [164] developed a novel and highly efficient magnetic nanocatalyst by immobilizing nickel (Ni) nanoparticles on the surface of magnetic titanium dioxide (Fe3O4/TiO2), which had been surface-modified using epibromohydrin and N-(2-aminoethyl) piperazine as linkers. The catalytic activity of this material was evaluated in a solvent-free A3 coupling reaction involving various aldehydes (202), terminal alkynes (203), and amines (204), leading to the formation of propargylamine derivatives (205) in good yields (Scheme 45). Notably, the catalyst demonstrated excellent recyclability and could be reused effectively for at least six consecutive cycles without significant loss in activity.
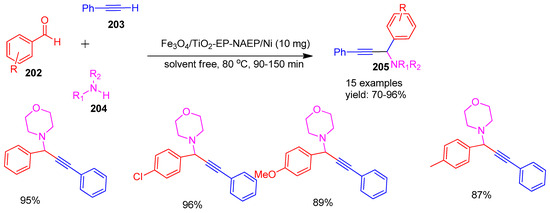
Scheme 45.
Synthesis of propargyl amine derivatives using Fe3O4/TiO2-EP-NAEP/Ni catalyst.
3. Conclusions
This review highlights recent advancements in the green synthesis of heterocyclic compounds through one-pot multicomponent reactions (MCRs) catalysed by magnetic nanoparticles (MNPs). MNPs have emerged as highly versatile and efficient catalysts, finding applications across diverse fields such as biomedicine, agriculture, environmental remediation, catalysis, and biosensing. In this review, we have comprehensively summarized the recent advancements in the functionalization of magnetic nanoparticles (MNPs), with a particular focus on their catalytic applications in the synthesis of heterocyclic compounds.
The studies reviewed, spanning from 2018 to 2024, demonstrate the growing importance of MNPs in facilitating efficient, sustainable, and high-yielding one-pot transformations. Compared to traditional homogeneous catalysis, MNP-based systems offer notable advantages, including ease of catalyst recovery, reusability, operational simplicity, and compatibility with green solvents or solvent-free conditions. Overall, the integration of magnetic nano catalysts into one-pot MCRs offers a powerful and eco-friendly strategy for the construction of structurally diverse heterocycles, especially fused and polyfunctional derivatives, underscoring the potential of MNPs in the advancement of green and sustainable synthetic methodologies.
Author Contributions
Conceptualization, methodology, software, writing—original draft, supervision, V.K.; draft writing, software, M.E.S.A.Z.; draft writing, editing, H.B.N. and H.S.; investigation, writing—original draft, S.-K.C., L.S.W. and K.P.; writing—original draft preparation, H.S.; writing—review and editing, K.P.; writing—original draft preparation supervision, S.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
All the authors express their sincere gratitude to CVR College of Engineering, Hyderabad, India, for its encouragement and provision of scientific resources that facilitated the completion of this project. The authors also acknowledge the valuable research support received from INTI International University, Malaysia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tasnim, A.; Roy, A.; Akash, S.R.; Ali, H.; Habib, M.R.; Barasarathi, J.; Muthukumaran, M.; Sayyed, R.Z.; Yeasmin, T. Hibiscus sabdariffa L. petal biomass: A green source of nanoparticles of multifarious potential. Open Agric. 2024, 9, 20220332. [Google Scholar] [CrossRef]
- Leong, L.M.; Ong, G.H.; Loh, K.E. Green synthesis of Chrysanthemum morifolium silver nanoparticles and evaluation of its antibacterial activity. Malays. Appl. Biol. 2024, 53, 1–6. [Google Scholar] [CrossRef]
- Safaei, M.; Imani, M.M.; Sharifi, R.; Mobarakeh, M.S.; Mozaffari, H.R.; Hashim, M.; Wong, L.S.; Nhlapo, A.; Rezaei, R. Optimization of green synthesis of nickel nanoparticles by Halomonas elongata as antifungal agent. Asian J. Green Chem. 2024, 8, 779–793. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, G.; Bi, X.; Chen, X. Facile assembly of a hierarchical core@shell Fe3O4@CuMgAl-LDH magnetic nanocatalyst for the hydroxylation of phenol. J. Mater. Chem. A 2013, 1, 5934–5942. [Google Scholar] [CrossRef]
- Lim, C.W.; Lee, I.S. Magnetically recyclable nanocatalyst systems for the organic reactions. Nano Today 2010, 5, 412–434. [Google Scholar] [CrossRef]
- Minakata, S.; Komatsu, M. Organic reactions on silica in water. Chem. Rev. 2009, 109, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, H.; Iranpoor, N.; Gholinejad, M.; Akbari, S.; Jeddi, N. Palladium nanoparticles supported on agarose-functionalized magnetic nanoparticles of Fe3O4 as a recyclable catalyst for C–C bond formation via Suzuki–Miyaura, Heck–Mizoroki and Sonogashira–Hagihara coupling reactions. RSC Adv. 2014, 4, 17060. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Luque, R.; Fihri, A.; Zhu, H.; Bouhrara, M.; Basset, J.M. Magnetically recoverable nanocatalysts. Chem. Rev. 2011, 111, 3036–3075. [Google Scholar] [CrossRef] [PubMed]
- Sardarian, A.R.; Eslahi, H.; Esmaeilpour, M. Copper(II) complex supported on Fe3O4@SiO2 coated by polyvinyl alcohol as reusable nanocatalyst in N-arylation of amines and N(H)-heterocycles and green synthesis of 1H-tetrazoles. ChemistrySelect 2018, 3, 1499–1511. [Google Scholar] [CrossRef]
- Yi, D.K.; Lee, S.S.; Ying, J.Y. Synthesis and applications of magnetic nanocomposite catalysts. Chem. Mater. 2006, 18, 2459–2461. [Google Scholar] [CrossRef]
- Esmaeilpour, M.; Sardarian, A.R.; Firouzabadi, H. Theophylline supported on modified silica-coated magnetite nanoparticles as a novel, efficient, reusable catalyst in green one-pot synthesis of spirooxindoles and phenazines. ChemistrySelect 2018, 3, 9236–9248. [Google Scholar] [CrossRef]
- Kalidindi, S.B.; Jagirdar, B.R. Nanocatalysis and prospects of green chemistry. ChemSusChem 2012, 5, 65–75. [Google Scholar] [CrossRef]
- Wang, J.; Lee, S.A.; Jang, H.W.; Shokouhimehr, M. Emerging two-dimensional-based nanostructured catalysts: Applications in sustainable organic transformations. Langmuir 2022, 38, 9064–9072. [Google Scholar] [CrossRef]
- Zhang, K.; Kim, J.; Kirlikovali, K.O.; Wang, J.; Lee, T.H.; Kim, S.Y.; Varma, R.S.; Jang, H.W.; Farha, O.K.; Shokouhimehr, M. Magnetically recyclable nanocomposites via lanthanide-based MOFs grown on natural sea sponge: Screening hydrogenation of nitrophenol to amino phenol. Mol. Catal. 2022, 528, 112459. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Motahharifar, N.; Sajjadi, M.; Naserimanesh, A.; Shokouhimehr, M. Functionalization of chitosan by grafting Cu(II)-5-amino-1H-tetrazole complex as a magnetically recyclable catalyst for CN coupling reaction. Inorg. Chem. Commun. 2022, 136, 109135. [Google Scholar] [CrossRef]
- Alamgholiloo, H.; Pesyan, N.N.; Mohammadi, R.; Rostamnia, S.; Shokouhimehr, M. Synergistic advanced oxidation process for the fast degradation of ciprofloxacin antibiotics using a GO/CuMOF-magnetic ternary nanocomposite. J. Environ. Chem. Eng. 2021, 9, 105486. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Hong, K.; Lee, T.H.; Moon, C.W.; Hong, S.P.; Zhang, K.; Suh, J.M.; Choi, K.S.; Varma, R.S.; Jang, H.W. Magnetically retrievable nanocomposite adorned with Pd nano catalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
- Wang, J.; Cheon, W.S.; Lee, J.Y.; Yan, W.; Jung, S.; Jang, H.W.; Shokouhimehr, M. Magnetic boron nitride adorned with Pd nanoparticles: An efficient catalyst for the reduction of nitroarenes in aqueous media. Dalton Trans. 2023, 52, 3567–3574. [Google Scholar] [CrossRef]
- Astruc, D. Nanoparticles and Catalysis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Somorjai, G.A.; Frei, H.; Park, J.Y. Advancing the frontiers in nanocatalysis, biointerfaces, and renewable energy conversion by innovations of surface techniques. J. Am. Chem. Soc. 2009, 131, 16589–16605. [Google Scholar] [CrossRef]
- Veisi, H.; Pirhayati, M.; Mohammadi, P.; Tamoradi, T.; Hemmati, S.; Karmakar, B. Recent advances in the application of magnetic nano catalysts in multicomponent reactions. RSC Adv. 2023, 13, 20530–20556. [Google Scholar] [CrossRef]
- Shylesh, S.; Schweizer, J.; Demeshko, S.; Schünemann, V.; Ernst, S.; Thiel, W.R. Nanoparticle supported, magnetically recoverable oxodiperoxo molybdenum complexes: Efficient catalysts for selective epoxidation reactions. Adv. Synth. Catal. 2009, 351, 1789–1795. [Google Scholar] [CrossRef]
- Sharma, R.K.; Dutta, S.; Sharma, S.; Zboril, R.; Varma, R.S.; Gawande, M.B. Fe3O4 (iron oxide)-supported nano catalysts: Synthesis, characterization and applications in coupling reactions. Green Chem. 2016, 18, 3184–3209. [Google Scholar] [CrossRef]
- Zeng, T.; Yang, L.; Hudson, R.; Song, G.; Moores, A.R.; Li, C.J. Fe3O4 nanoparticle-supported copper(I) pybox catalyst: Magnetically recoverable catalyst for enantioselective direct-addition of terminal alkynes to imines. Org. Lett. 2011, 13, 442–445. [Google Scholar] [CrossRef]
- Sun, J.; Yu, G.; Liu, L.; Li, Z.; Kan, Q.; Huo, Q.; Guan, J. Core–shell structured Fe3O4@SiO2 supported cobalt(II) or copper(II) acetylacetonate complexes: Magnetically recoverable nano catalysts for aerobic epoxidation of styrene. Catal. Sci. Technol. 2014, 4, 1246–1252. [Google Scholar] [CrossRef]
- Aghajani, M.; Monadi, N. Cu(II) Schiff Base Complex Supported on Fe3O4 Nanoparticles as an Efficient Nanocatalyst for the Selective Aerobic Oxidation of Alcohols. Appl. Organomet. Chem. 2018, 32, e4433. [Google Scholar] [CrossRef]
- Saranya, S.; Rohit, K.R.; Radhika, S.; Anilkumar, G. Palladium-Catalyzed Multicomponent Reactions: An Overview. Org. Biomol. Chem. 2019, 17, 8048–8061. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.M.; Rodrigues, M.O.; Neto, B.A. Mechanistic Knowledge and Noncovalent Interactions as the Key Features for Enantioselective Catalysed Multicomponent Reactions: A Critical Review. Org. Biomol. Chem. 2019, 17, 7260–7269. [Google Scholar] [CrossRef]
- Das, K.K.; Manna, S.; Panda, S. Transition Metal Catalyzed Asymmetric Multicomponent Reactions of Unsaturated Compounds Using Organoboron Reagents. Chem. Commun. 2021, 57, 441–459. [Google Scholar] [CrossRef]
- Biesen, L.; Müller, T.J.J. Multicomponent and One-Pot Syntheses of Quinoxalines. Adv. Synth. Catal. 2021, 363, 980–1006. [Google Scholar] [CrossRef]
- Insuasty, D.; Castillo, J.; Becerra, D.; Rojas, H.; Abonia, R. Synthesis of Biologically Active Molecules through Multicomponent Reactions. Molecules 2020, 25, 505. [Google Scholar] [CrossRef]
- Mohlala, R.L.; Rashamuse, T.J.; Coyanis, E.M. Highlighting Multicomponent Reactions as an Efficient and Facile Alternative Route in the Chemical Synthesis of Organic-Based Molecules: A Tremendous Growth in the Past 5 Years. Front. Chem. 2024, 12, 1469677. [Google Scholar] [CrossRef]
- Wang, Z.; Dömling, A. Multicomponent Reactions in Medicinal Chemistry. In Multicomponent Reactions Towards Heterocycles: Concepts and Applications; Wiley-VCH: Weinheim, Germany, 2022; pp. 91–137. [Google Scholar] [CrossRef]
- Allochio Filho, J.F.; Lemos, B.C.; de Souza, A.S.; Pinheiro, S.; Greco, S.J. Multicomponent Mannich Reactions: General Aspects, Methodologies and Applications. Tetrahedron 2017, 73, 6977–7004. [Google Scholar] [CrossRef]
- Eisavi, R.; Naseri, K. Preparation, Characterization and Application of MgFe2O4/Cu Nanocomposite as a New Magnetic Catalyst for One-Pot Regioselective Synthesis of β-Thiol-1,4-Disubstituted-1,2,3-Triazoles. RSC Adv. 2021, 11, 13061–13076. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J. Organic Reactions in Aqueous Media with a Focus on Carbon–Carbon Bond Formations: A Decade Update. Chem. Rev. 2005, 105, 3095–3166. [Google Scholar] [CrossRef]
- Potewar, T.M.; Ingale, S.A.; Srinivasan, K.V. Catalyst-Free Efficient Synthesis of 2-Aminothiazoles in Water at Ambient Temperature. Tetrahedron 2008, 64, 5019–5022. [Google Scholar] [CrossRef]
- Horváth, I.T. Introduction: Sustainable Chemistry. Chem. Rev. 2018, 118, 369–371. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Anastas, P.T.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Kappe, O.C. Microwave Dielectric Heating in Synthetic Organic Chemistry. Chem. Rev. 2008, 108, 1127–1150. [Google Scholar] [CrossRef]
- Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef]
- Chanda, A.; Fokin, V.V. Organic Synthesis “on Water”. Chem. Rev. 2009, 109, 725–748. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, K.; Kumar, K.M.; Sivakumar, A. Synthesis, Characterization and Anticancer Molecular Docking Studies of Phenothiazine Derivatives—A Green Chemical Approach. ChemistrySelect 2023, 8, e202302613. [Google Scholar] [CrossRef]
- Venkatesan, K.; Basha, N.H.; Jagadish, T.; Reddy, P.V.; Shaik, H.; Pasupathi, M. Ultrasound Assisted Synthesis of Phenothiazine Based Chalcone Derivatives, Their Antibacterial Studies and Molecular Docking against COVID-19 Virus Spike Protein Inhibitor. Russ. J. Bioorg. Chem. 2024, 50, 1133–1140. [Google Scholar] [CrossRef]
- Chen, M.N.; Mo, L.P.; Cui, Z.S.; Zhang, Z.H. Magnetic Nanocatalysts: Synthesis and Application in Multicomponent Reactions. Curr. Opin. Green Sustain. Chem. 2019, 15, 27–37. [Google Scholar] [CrossRef]
- Nandi, S.; Jamatia, R.; Sarkar, R.; Sarkar, F.K.; Alam, S.; Pal, A.K. One-Pot Multicomponent Reaction: A Highly Versatile Strategy for the Construction of Valuable Nitrogen-Containing Heterocycles. ChemistrySelect 2022, 7, e202201901. [Google Scholar] [CrossRef]
- Das, D. Multicomponent Reactions in Organic Synthesis Using Copper-Based Nanocatalysts. ChemistrySelect 2016, 1, 1959–1980. [Google Scholar] [CrossRef]
- Yadav, M.; Dutta, M.; Tanwar, P.; Jain, R.; Srivastava, A.; Sharma, R.K. Microwave-Assisted C–C, C–O, C–N, C–S Bond Formation and Multicomponent Reactions Using Magnetic Retrievable Nanocatalysts. Curr. Microw. Chem. 2021, 8, 96–116. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.H.; Faouzi, M.E.A. Overview of Recent Developments of Pyrazole Derivatives as an Anticancer Agent in Different Cell Line. Bioorg. Chem. 2020, 97, 103470. [Google Scholar] [CrossRef] [PubMed]
- Karrouchi, K.; Brandán, S.A.; Sert, Y.; El-Marzouqi, H.; Radi, S.; Ferbinteanu, M.; Faouzi, M.E.A.; Garcia, Y.; Ansar, M.H. Synthesis, X-ray Structure, Vibrational Spectroscopy, DFT, Biological Evaluation and Molecular Docking Studies of (E)-N′-(4-(Dimethylamino)benzylidene)-5-methyl-1H-pyrazole-3-carbohydrazide. J. Mol. Struct. 2020, 1219, 128541. [Google Scholar] [CrossRef]
- Vashisht, K.; Sethi, P.; Ramasamy, S.K.; Bansal, A.; Dar, M.O.; Singh, M.; Alkhanjaf, A.A.A.; Ibrahim, A.A.; Umar, A.; Kumar, R.; et al. Synthesis, Characterization, and Antibacterial Activity of Novel Pyrazole Derivatives. J. Mol. Struct. 2025, 1332, 141706. [Google Scholar] [CrossRef]
- Karrouchi, K.; Fettach, S.; Radi, S.; Yousfi, E.B.; Taoufik, J.; Mabkhot, Y.N.; Alterary, S.; Faouzi, M.E.; Ansar, M. Synthesis, Characterization, Free-Radical Scavenging Capacity and Antioxidant Activity of Novel Series of Hydrazone, 1,3,4-Oxadiazole and 1,2,4-Triazole Derived from 3,5-Dimethyl-1H-pyrazole. Lett. Drug Des. Discov. 2019, 16, 712–720. [Google Scholar] [CrossRef]
- Chaudhary, M.; Kumar, N.; Baldi, A.; Chandra, R.; Babu, M.A.; Madan, J. Chloro and Bromo-Pyrazole Curcumin Knoevenagel Condensates Augmented Anticancer Activity against Human Cervical Cancer Cells: Design, Synthesis, In Silico Docking and In Vitro Cytotoxicity Analysis. J. Biomol. Struct. Dyn. 2020, 38, 200–218. [Google Scholar] [CrossRef]
- Abu-Melha, S.; Edrees, M.M.; Riyadh, S.M.; Abdelaziz, M.R.; Elfiky, A.A.; Gomha, S.M. Clean Grinding Technique: A Facile Synthesis and In Silico Antiviral Activity of Hydrazones, Pyrazoles, and Pyrazines Bearing Thiazole Moiety against SARS-CoV-2 Main Protease (Mpro). Molecules 2020, 25, 4565. [Google Scholar] [CrossRef] [PubMed]
- Pogaku, V.; Krishna, V.S.; Sriram, D.; Rangan, K.; Basavoju, S. Ultrasonication-Ionic Liquid Synergy for the Synthesis of New Potent Anti-Tuberculosis 1,2,4-Triazol-1-yl-Pyrazole Based Spirooxindolopyrrolizidines. Bioorg. Med. Chem. Lett. 2019, 29, 1682–1687. [Google Scholar] [CrossRef] [PubMed]
- Amirnejat, S.; Nosrati, A.; Javanshir, S. Superparamagnetic Fe3O4@Alginate Supported L-Arginine as a Powerful Hybrid Inorganic–Organic Nanocatalyst for the One-Pot Synthesis of Pyrazole Derivatives. Appl. Organomet. Chem. 2020, 34, e5888. [Google Scholar] [CrossRef]
- Nikpassand, M.; Farshami, M.J. One-Pot Synthesis of Novel 3-Pyrazolyl-4H-1,2,4-Triazoles Using Aminoglucose-Functionalized Silica-Coated NiFe2O4 Nanoparticles as a Magnetically Separable Catalyst. J. Clust. Sci. 2021, 32, 975–982. [Google Scholar] [CrossRef]
- Ramezaninejad, Z.; Shiri, L. MgFe2O4@Tris Magnetic Nanoparticles: An Effective and Powerful Catalyst for One-Pot Synthesis of Pyrazolopyranopyrimidine and Tetrahydro Dipyrazolopyridine Derivatives. RSC Adv. 2024, 14, 6006–6015. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.A.; Mirhosseini-Eshkevari, B.; Abdollahi-Basir, M.H. Green Synthesis of Spiro[indoline-3,4′-pyrano[2,3-c]pyrazoles] Using Fe3O4@L-Arginine as a Robust and Reusable Catalyst. BMC Chem. 2019, 13, 119. [Google Scholar] [CrossRef]
- Hosseini Mohtasham, N.; Gholizadeh, M. Nano Silica Extracted from Horsetail Plant as a Natural Silica Support for the Synthesis of H3PW12O40 Immobilized on Aminated Magnetic Nanoparticles (Fe3O4@SiO2-EP-NH-HPA): A Novel and Efficient Heterogeneous Nano Catalyst for the Green One-Pot Synthesis of Pyrano[2,3-c]pyrazole Derivatives. Res. Chem. Intermed. 2020, 46, 3037–3066. [Google Scholar] [CrossRef]
- Laursen, J.B.; Nielsen, J. Phenazine Natural Products: Biosynthesis, Synthetic Analogues, and Biological Activity. Chem. Rev. 2004, 104, 1663–1686. [Google Scholar] [CrossRef]
- Bonsignore, L.; Loy, G.; Secci, D.; Calignano, A. Synthesis and Pharmacological Activity of 2-Oxo-(2H)-1-benzopyran-3-carboxamide Derivatives. Eur. J. Med. Chem. 1993, 28, 517–520. [Google Scholar] [CrossRef]
- Ferreira, S.B.; de Carvalho da Silva, F.; Bezerra, F.A.; Lourenço, M.C.; Kaiser, C.R.; Pinto, A.C.; Ferreira, V.F. Synthesis of α- and β-Pyran Naphthoquinones as a New Class of Antitubercular Agents. Arch. Pharm. 2010, 343, 81–90. [Google Scholar] [CrossRef]
- Gamage, S.A.; Spicer, J.A.; Rewcastle, G.W.; Milton, J.; Sohal, S.; Dangerfield, W.; Mistry, P.; Vicker, N.; Charlton, P.A.; Denny, W.A. Structure−Activity Relationships for Pyrido-, Imidazo-, Pyrazolo-, Pyrazino-, and Pyrrolophenazinecarboxamides as Topoisomerase-Targeted Anticancer Agents. J. Med. Chem. 2002, 45, 740–743. [Google Scholar] [CrossRef]
- Ligon, J.M.; Hill, D.S.; Hammer, P.E.; Torkewitz, N.R.; Hofmann, D.; Kempf, H.J.; van Pée, K.H. Natural Products with Antifungal Activity from Pseudomonas Biocontrol Bacteria. Pest Manag. Sci. 2000, 56, 688–695. [Google Scholar] [CrossRef]
- Muller, M.; Sorrell, T.C. Inhibition of the Human Platelet Cyclooxygenase Response by the Naturally Occurring Phenazine Derivative, 1-Hydroxyphenazine. Prostaglandins 1995, 50, 301–311. [Google Scholar] [CrossRef]
- Gao, J.; Chen, M.; Tong, X.; Zhu, H.; Yan, H.; Liu, D.; Li, W.; Qi, S.; Xiao, D.; Wang, Y.; et al. Synthesis, Antitumor Activity, and Structure–Activity Relationship of Some Benzo[a]pyrano[2,3-c]phenazine Derivatives. Comb. Chem. High Throughput Screen. 2015, 18, 960–974. [Google Scholar] [CrossRef]
- Taheri, M.; Mohebat, R. Synthesis of One-Pot Pyrazolo[4′,3′:5,6]pyrano[2,3-c]phenazin-15-yl) Methanone Derivatives via a Multi-Component Using Fe3O4@TiO2–SO3H as a Recoverable Magnetic Catalyst under Microwave Irradiation. Green Chem. Lett. Rev. 2020, 13, 165–178. [Google Scholar] [CrossRef]
- Safari, F.; Hosseini, H.; Bayat, M.; Ranjbar, A. Synthesis and Evaluation of Antimicrobial Activity, Cytotoxic and Pro-Apoptotic Effects of Novel Spiro-4H-Pyran Derivatives. RSC Adv. 2019, 9, 24843–24851. [Google Scholar] [CrossRef]
- Venkatesan, K.; Rao, T.S.; Sridhar, V. Ultrasound Assisted Synthesis of Pyran Derivatives Catalysed by Uranyl Nitrate and Their Molecular Docking against Glycogen Synthase Kinase-3 Beta Receptor. Russ. J. Bioorg. Chem. 2024, 50, 2580–2588. [Google Scholar] [CrossRef]
- Bedair, A.H.; Emam, H.A.; El-Hady, N.A.; Ahmed, K.A.; El-Agrody, A.M. Synthesis and Antimicrobial Activities of Novel Naphtho[2, -b]pyran, Pyrano[2,3-d]pyrimidine and Pyrano[3,2-e][1,2,4]triazolo[2,3-c]pyrimidine Derivatives. Il Farmaco 2001, 56, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Shahrisa, A.; Zirak, M.; Mehdipour, A.R. Synthesis and Calcium Channel Antagonist Activity of New Symmetrical and Asymmetrical 4-[2-Chloro-2-(4-Chloro-6-methyl-2-oxo-2H-pyran-3-yl)vinyl]-Substituted 1,4-Dihydropyridines. Chem. Heterocycl. Compd. 2011, 46, 1354–1363. [Google Scholar] [CrossRef]
- da Rocha, D.R.; de Souza, A.C.; Resende, J.A.; Santos, W.C.; dos Santos, E.A.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V.; Montenegro, R.C.; Ferreira, V.F. Synthesis of New 9-Hydroxy-α- and 7-Hydroxy-β-Pyran Naphthoquinones and Cytotoxicity against Cancer Cell Lines. Org. Biomol. Chem. 2011, 9, 4315–4322. [Google Scholar] [CrossRef]
- Sirous, H.; Chemi, G.; Gemma, S.; Butini, S.; Debyser, Z.; Christ, F.; Saghaie, L.; Brogi, S.; Fassihi, A.; Campiani, G.; et al. Identification of Novel 3-Hydroxy-Pyran-4-one Derivatives as Potent HIV-1 Integrase Inhibitors Using In Silico Structure-Based Combinatorial Library Design Approach. Front. Chem. 2019, 7, 574. [Google Scholar] [CrossRef]
- Verhoest, P.R.; Fonseca, K.R.; Hou, X.; Proulx-LaFrance, C.; Corman, M.; Helal, C.J.; Claffey, M.M.; Tuttle, J.B.; Coffman, K.J.; Liu, S.; et al. Design and Discovery of 6-[(3S,4S)-4-Methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl]-1-(tetrahydro-2H-pyran-4-yl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (PF-04447943), a Selective Brain Penetrant PDE9A Inhibitor for the Treatment of Cognitive Disorders. J. Med. Chem. 2012, 55, 9045–9054. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.S.; Jaiswal, N.; Sharma, A.; Fatima, S.; Sharma, R.; Rahuja, N.; Srivastava, A.K.; Bajpai, V.; Kumar, B.; Tripathi, R.P. A Convenient Synthesis of Novel Pyranosyl Homo-C-Nucleosides and Their Antidiabetic Activities. Carbohydr. Res. 2011, 346, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Ahankar, H.; Fardood, S.T.; Ramazani, A. One-Pot Three-Component Synthesis of Tetrahydrobenzo[b]pyrans in the Presence of Ni0.5Cu0.5Fe2O4 Magnetic Nanoparticles under Microwave Irradiation in Solvent-Free Conditions. Iran. J. Catal. 2020, 10, 3561. [Google Scholar]
- Maleki, A.; Ghassemi, M.; Firouzi-Haji, R. Green Multicomponent Synthesis of Four Different Classes of Six-Membered N-Containing and O-Containing Heterocycles Catalyzed by an Efficient Chitosan-Based Magnetic Bionano Composite. Pure Appl. Chem. 2018, 90, 387–394. [Google Scholar] [CrossRef]
- Aghajani, M.; Monadi, N. A One-Pot Green Synthesis of 2-Amino-4H-benzo[h]chromenes Catalyzed by a Dioxomolybdenum Schiff Base Complex Supported on Magnetic Nanoparticles as an Efficient and Recyclable Nanocatalyst. J. Chin. Chem. Soc. 2019, 66, 775–784. [Google Scholar] [CrossRef]
- Nikpassand, M.; Kasmaei, S.A. Tannic Acid-Functionalized Silica-Coated Fe3O4 Nanoparticles as a Novel and Magnetically Separable Catalyst for Green Synthesis of Aryl Naphtho[1,3]oxazine-2-thiones. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4917–4926. [Google Scholar] [CrossRef]
- Shill, M.C.; Das, A.K.; Itou, T.; Karmakar, S.; Mukherjee, P.K.; Mizuguchi, H.; Kashiwada, Y.; Fukui, H.; Nemoto, H. The Isolation and Synthesis of a Novel Benzofuran Compound from Tephrosia purpurea, and the Synthesis of Several Related Derivatives, Which Suppress Histamine H1 Receptor Gene Expression. Bioorg. Med. Chem. 2015, 23, 6869–6874. [Google Scholar] [CrossRef]
- Kucuksayan, E.; Ozben, T. Hybrid Compounds as Multitarget Directed Anticancer Agents. Curr. Top. Med. Chem. 2017, 17, 907–918. [Google Scholar] [CrossRef]
- Tang, H.J.; Zhang, X.W.; Yang, L.; Li, W.; Li, J.H.; Wang, J.X.; Chen, J. Synthesis and Evaluation of Xanthine Oxidase Inhibitory and Antioxidant Activities of 2-Arylbenzo[b]furan Derivatives Based on Salvianolic Acid C. Eur. J. Med. Chem. 2016, 124, 637–648. [Google Scholar] [CrossRef]
- Zaher, A.F.; Abuel-Maaty, S.M.; El-Nassan, H.B.; Amer, S.A.; Abdelghany, T.M. Synthesis, Antitumor Screening and Cell Cycle Analysis of Novel Benzothieno[3,2-b]pyran Derivatives. J. Enzyme Inhib. Med. Chem. 2016, 31, 145–153. [Google Scholar] [CrossRef]
- Hiremathad, A.; Patil, M.R.; Chand, K.; Santos, M.A.; Keri, R.S. Benzofuran: An Emerging Scaffold for Antimicrobial Agents. RSC Adv. 2015, 5, 96809–96828. [Google Scholar] [CrossRef]
- Bowyer, P.W.; Tate, E.W.; Leatherbarrow, R.J.; Holder, A.A.; Smith, D.F.; Brown, K.A. N-Myristoyltransferase: A Prospective Drug Target for Protozoan Parasites. ChemMedChem 2008, 3, 402–408. [Google Scholar] [CrossRef]
- Hwang, J.W.; Choi, D.H.; Jeon, J.H.; Kim, J.K.; Jun, J.G. Facile Preparation of 2-Arylbenzo[b]furan Molecules and Their Anti-Inflammatory Effects. Bull. Korean Chem. Soc. 2010, 31, 965–970. [Google Scholar] [CrossRef]
- Halabalaki, M.; Alexi, X.; Aligiannis, N.; Alexis, M.N.; Skaltsounis, A.L. Ebenfurans IV−VIII from Onobrychis ebenoides: Evidence That C-Prenylation Is the Key Determinant of the Cytotoxicity of 3-Formyl-2-arylbenzofurans. J. Nat. Prod. 2008, 71, 1934–1937. [Google Scholar] [CrossRef] [PubMed]
- Shirzaei, M.; Mollashahi, E.; Maghsoodlou, M.T.; Lashkari, M. Novel Synthesis of Silica-Coated Magnetic Nanoparticles Based on Acidic Ionic Liquid, as a Highly Efficient Catalyst for Three-Component System Leads to Furans Derivatives. J. Saudi Chem. Soc. 2020, 24, 216–222. [Google Scholar] [CrossRef]
- Ahankar, H.; Ramazani, A.; Fattahi, N.; Ślepokura, K.; Lis, T.; Asiabi, P.A.; Kinzhybalo, V.; Hanifehpour, Y.; Joo, S.W. Tetramethylguanidine-Functionalized Silica-Coated Iron Oxide Magnetic Nanoparticles Catalyzed One-Pot Three-Component Synthesis of Furanone Derivatives. J. Chem. Sci. 2018, 130, 1–13. [Google Scholar] [CrossRef]
- Khurana, J.M.; Magoo, D.; Aggarwal, K.; Aggarwal, N.; Kumar, R.; Srivastava, C. Synthesis of Novel 12-Aryl-8,9,10,12-Tetrahydrobenzo[a]xanthene-11-thiones and Evaluation of Their Biocidal Effects. Eur. J. Med. Chem. 2012, 58, 470–477. [Google Scholar] [CrossRef]
- El-Sabbagh, O.I.; El-Sadek, M.E.; El-Kalyoubi, S.; Ismail, I. Synthesis, DNA Binding and Antiviral Activity of New Uracil, Xanthine, and Pteridine Derivatives. Arch. Pharm. 2007, 340, 26–31. [Google Scholar] [CrossRef]
- Zygmunt, M.; Sapa, J.; Drabczyńska, A.; Karcz, T.; Müller, C.; Köse, M.; Latacz, G.; Schabikowski, J.; Bednarski, M.; Kieć-Kononowicz, K. Synthesis and Analgesic Activity of Annelated Xanthine Derivatives in Experimental Models in Rodents. Arch. Pharm. 2015, 348, 704–714. [Google Scholar] [CrossRef]
- Dianov, V.M.; Bulgakov, A.K. Synthesis and Antimicrobial Activity of 3-Methyl-Substituted 6,8-Dimethylthiazolo-[2,3-f]xanthines. Pharm. Chem. J. 2006, 40, 551–553. [Google Scholar] [CrossRef]
- Hafez, H.N.; Hegab, M.I.; Ahmed-Farag, I.S.; El-Gazzar, A.B.A.A. Facile Regioselective Synthesis of Novel Spiro-Thioxanthene and Spiro-Xanthene-9′,2-[1,3,4]Thiadiazole Derivatives as Potential Analgesic and Anti-Inflammatory Agents. Bioorg. Med. Chem. Lett. 2008, 18, 4538–4543. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S. Recent Developments in the Synthesis and Anti-Cancer Activity of Acridine and Xanthine-Based Molecules. Phys. Sci. Rev. 2023, 8, 2405–2439. [Google Scholar] [CrossRef]
- Arzehgar, Z.; Aydi, A.; Mirzaei Heydari, M. Silver Functionalized on Hydroxyapatite-Core-Shell Magnetic γ-Fe2O3: An Environmentally and Readily Recyclable Nanocatalyst for the One-Pot Synthesis of 14H-Dibenzo[a,j]xanthenes Derivatives. Asian J. Green Chem. 2018, 2, 281–298. [Google Scholar] [CrossRef]
- Sonei, S.; Gholizadeh, M.; Taghavi, F. Cu(II) Anchored on Modified Magnetic Nanoparticles: As a Green and Efficient Recyclable Nano Catalyst for One Pot Synthesis of 12-Aryl-8,9,10,12-Tetrahydrobenzo[a]xanthene-11-One. Polycycl. Aromat. Compd. 2020, 40, 1127–1142. [Google Scholar] [CrossRef]
- Lee, C.F.; Holownia, A.; Bennett, J.M.; Elkins, J.M.; Denis, J.D.S.; Adachi, S.; Yudin, A.K. Oxalyl Boronates Enable Modular Synthesis of Bioactive Imidazoles. Angew. Chem. Int. Ed. 2017, 56, 6264–6267. [Google Scholar] [CrossRef]
- Gaba, M.; Mohan, C. Development of Drugs Based on Imidazole and Benzimidazole Bioactive Heterocycles: Recent Advances and Future Directions. Med. Chem. Res. 2016, 25, 173–210. [Google Scholar] [CrossRef]
- Zhang, M.; Ding, Y.; Qin, H.X.; Xu, Z.G.; Lan, H.T.; Yang, D.L.; Yi, C. One-Pot Synthesis of Substituted Pyrrole–Imidazole Derivatives with Anticancer Activity. Mol. Divers. 2020, 24, 1177–1184. [Google Scholar] [CrossRef]
- Gurevich, K.G.; Urakov, A.L.; Basantsev, A.V.; Samorodov, A.V.; Danilin, A.A.; Purygin, P.P.; Klenova, N.A.; Bashirov, I.I.; Bashirova, L.I.; Golovanov, A.A.; et al. Synthesis of New N-Mono- and N,N-Dialkylated Imidazole Derivatives and Their Antiplatelet and Anticoagulation Activity. Pharm. Chem. J. 2021, 55, 119–122. [Google Scholar] [CrossRef]
- Nascimento, M.V.P.; Munhoz, A.C.; Theindl, L.C.; Mohr, E.T.B.; Saleh, N.; Parisotto, E.B.; Rossa, T.A.; Zamoner, A.; Creczynski-Pasa, T.B.; Filippin-Monteiro, F.B.; et al. A Novel Tetrasubstituted Imidazole as a Prototype for the Development of Anti-Inflammatory Drugs. Inflammation 2018, 41, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Parwani, D.; Bhattacharya, S.; Rathore, A.; Mallick, C.; Asati, V.; Agarwal, S.; Rajoriya, V.; Das, R.; Kashaw, S.K. Current Insights into the Chemistry and Antitubercular Potential of Benzimidazole and Imidazole Derivatives. Mini-Rev. Med. Chem. 2021, 21, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Verma, B.K.; Kapoor, S.; Kumar, U.; Pandey, S.; Arya, P. Synthesis of New Imidazole Derivatives as Effective Antimicrobial Agents. Indian J. Pharm. Biol. Res. 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Roy, D.; Anas, M.; Manhas, A.; Saha, S.; Kumar, N.; Panda, G. Synthesis, Biological Evaluation, Structure−Activity Relationship Studies of Quinoline-Imidazole Derivatives as Potent Antimalarial Agents. Bioorg. Chem. 2022, 121, 105671. [Google Scholar] [CrossRef] [PubMed]
- Gunaseeli, P.I.; Ruban, Y.J.V.; Venkatesan, K. Microwave-Assisted Synthesis and Biological Studies of Phenothiazine-Based Imidazole Derivatives. Russ. J. Gen. Chem. 2025, 95, 1068–1074. [Google Scholar] [CrossRef]
- Sakhdari, M.; Amoozadeh, A.; Kolvari, E. Magnetic Nanoparticle-Supported Sulfonic Acid as a Green Catalyst for the One-Pot Synthesis of 2,4,5-Trisubstituted Imidazoles and 1,2,4,5-Tetrasubstituted Imidazoles under Solvent-Free Conditions. Heterocycl. Commun. 2021, 27, 71–78. [Google Scholar] [CrossRef]
- Khalifeh, R.; Naseri, V.; Rajabzadeh, M. Synthesis of Imidazolium-Based Ionic Liquid on Modified Magnetic Nanoparticles for Application in One-Pot Synthesis of Trisubstituted Imidazoles. ChemistrySelect 2020, 5, 11453–11462. [Google Scholar] [CrossRef]
- Abele, E.; Abele, R.; Dzenitis, O.; Lukevics, E. Indole and Isatin Oximes: Synthesis, Reactions, and Biological Activity. Chem. Heterocycl. Compd. 2003, 39, 3–35. [Google Scholar] [CrossRef]
- Al-Hiari, Y.; Qaisi, A.; El-Abadelah, M. Synthesis and Antibacterial Activity of Some Substituted 3-(Aryl)- and 3-(Heteroaryl)indoles. Monatsh. Chem. 2006, 137, 243–248. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wu, H.S.; Tang, L.; Feng, C.R.; Yu, J.H.; Li, Y.; Yang, Y.S.; Yang, B.; He, Q.J. The Potential Insulin Sensitizing and Glucose Lowering Effects of a Novel Indole Derivative in Vitro and in Vivo. Pharmacol. Res. 2007, 56, 335–343. [Google Scholar] [CrossRef]
- Barraja, P.; Sciabica, L.; Diana, P.; Lauria, A.; Montalbano, A.; Almerico, A.M.; Dattolo, G.; Cirrincione, G.; Disarò, S.; Basso, G.; et al. Synthesis and Photochemotherapeutic Activity of Thiopyrano[2,3-e]indol-2-ones. Bioorg. Med. Chem. Lett. 2005, 15, 2291–2294. [Google Scholar] [CrossRef]
- Safari, J.; Hosseini Nasab, N. Fe3O4 Magnetic Nanoparticles in the Layers of Montmorillonite as a Valuable Heterogeneous Nano Catalyst for the One-Pot Synthesis of Indeno[1,2-b]indolone Derivatives in Aqueous Media. Res. Chem. Intermed. 2019, 45, 1025–1038. [Google Scholar] [CrossRef]
- Nongthombam, G.S.; Kathing, C.; Nongrum, R.; Kharmawlong, G.K.; Nongkhlaw, R. Fe3O4 Supported Acidic Ionic Liquid: An Efficient and Recyclable Magnetic Nanoparticles Catalyst for One-Pot Synthesis of Bis(indolyl)methanes. Indian J. Chem. 2023, 62, 16–23. [Google Scholar] [CrossRef]
- Min, L.; Pan, B.; Gu, Y. Synthesis of Quinoline-Fused 1-Benzazepines through a Mannich-Type Reaction of a C,N-Bisnucleophile Generated from 2-Aminobenzaldehyde and 2-Methylindole. Org. Lett. 2016, 18, 364–367. [Google Scholar] [CrossRef]
- Carreño, A.; Zúñiga, C.; Páez-Hernández, D.; Gacitúa, M.; Polanco, R.; Otero, C.; Arratia-Pérez, R.; Fuentes, J.A. Study of the Structure–Bioactivity Relationship of Three New Pyridine Schiff Bases: Synthesis, Spectral Characterization, DFT Calculations and Biological Assays. New J. Chem. 2018, 42, 8851–8863. [Google Scholar] [CrossRef]
- Chen, Z.; Li, P.; Hu, D.; Dong, L.; Pan, J.; Luo, L.; Zhang, W.; Xue, W.; Jin, L.; Song, B. Synthesis, Antiviral Activity, and 3D-QSAR Study of Novel Chalcone Derivatives Containing Malonate and Pyridine Moieties. Arab. J. Chem. 2019, 12, 2685–2696. [Google Scholar] [CrossRef]
- Worachartcheewan, A.; Prachayasittikul, S.; Pingaew, R.; Nantasenamat, C.; Tantimongcolwat, T.; Ruchirawat, S.; Prachayasittikul, V. Antioxidant, Cytotoxicity, and QSAR Study of 1-Adamantylthio Derivatives of 3-Picoline and Phenylpyridines. Med. Chem. Res. 2012, 21, 3514–3522. [Google Scholar] [CrossRef]
- Riaz, S.; Khan, I.U.; Bajda, M.; Ashraf, M.; Shaukat, A.; Rehman, T.U.; Mutahir, S.; Hussain, S.; Mustafa, G.; Yar, M. Pyridine Sulfonamide as a Small Key Organic Molecule for the Potential Treatment of Type-II Diabetes Mellitus and Alzheimer’s Disease: In Vitro Studies against Yeast α-Glucosidase, Acetylcholinesterase and Butyrylcholinesterase. Bioorg. Chem. 2015, 63, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Masaret, G.S. Convenient Synthesis and Anticancer Evaluation of Novel Pyrazolyl-Thiophene, Thieno[3,2-b]pyridine, Pyrazolo[3,4-d]thieno[3,2-b]pyridine and Pyrano[2,3-d]thieno[3,2-b]pyridine Derivatives. J. Heterocycl. Chem. 2021, 58, 1344–1358. [Google Scholar] [CrossRef]
- Elkanzi, N.A.; Bakr, R.B.; Ghoneim, A.A. Design, Synthesis, Molecular Modeling Study, and Antimicrobial Activity of Some Novel Pyrano[2,3-b]pyridine and Pyrrolo[2,3-b]pyrano[2,3-d]pyridine Derivatives. J. Heterocycl. Chem. 2019, 56, 406–416. [Google Scholar] [CrossRef]
- Ali, E.M.; Abdel-Maksoud, M.S.; Hassan, R.M.; Mersal, K.I.; Ammar, U.M.; Se-In, C.; He-Soo, H.; Kim, H.K.; Lee, A.; Lee, K.T.; et al. Design, Synthesis and Anti-Inflammatory Activity of Imidazol-5-yl Pyridine Derivatives as p38α/MAPK14 Inhibitor. Bioorg. Med. Chem. 2021, 31, 115969. [Google Scholar] [CrossRef]
- Maleki, A.; Firouzi-Haji, R. L-Proline Functionalized Magnetic Nanoparticles: A Novel Magnetically Reusable Nano Catalyst for One-Pot Synthesis of 2,4,6-Triarylpyridines. Sci. Rep. 2018, 8, 17303. [Google Scholar] [CrossRef]
- Thrilokraj, R.; Ghosh, A.; Limaye, A.S.; Małecki, J.G.; Budagumpi, S.; Deokar, R.C.; Dateer, R.B. Fe3O4NPs: A Heterogeneous and Reusable Magnetic Nano Catalyst for Base and Solvent Free One-Pot Multicomponent Synthesis of Pyridine Derivatives and Their Photophysical Study. Mol. Catal. 2024, 557, 113978. [Google Scholar] [CrossRef]
- Maleki, B.; Natheghi, H.; Tayebee, R.; Alinezhad, H.; Amiri, A.; Hossieni, S.A.; Nouri, S.M.M. Synthesis and Characterization of Nanorod Magnetic Co–Fe Mixed Oxides and Its Catalytic Behavior Towards One-Pot Synthesis of Polysubstituted Pyridine Derivatives. Polycycl. Aromat. Compd. 2020, 40, 633–643. [Google Scholar] [CrossRef]
- Rakhtshah, J.; Yaghoobi, F. Catalytic Application of New Manganese Schiff-Base Complex Immobilized on Chitosan-Coated Magnetic Nanoparticles for One-Pot Synthesis of 3-Iminoaryl-imidazo[1,2-a]pyridines. Int. J. Biol. Macromol. 2019, 139, 904–916. [Google Scholar] [CrossRef]
- Ahadi, N.; Mobinikhaledi, A.; Bodaghifard, M.A. One-Pot Synthesis of 1,4-Dihydropyridines and N-Arylquinolines in the Presence of Copper Complex Stabilized on MnFe2O4 (MFO) as a Novel Organic–Inorganic Hybrid Material and Magnetically Retrievable Catalyst. Appl. Organomet. Chem. 2020, 34, e5822. [Google Scholar] [CrossRef]
- Maleki, B.; Atharifar, H.; Reiser, O.; Sabbaghzadeh, R. Glutathione-Coated Magnetic Nanoparticles for One-Pot Synthesis of 1,4-Dihydropyridine Derivatives. Polycycl. Aromat. Compd. 2021, 41, 721–734. [Google Scholar] [CrossRef]
- Bodaghifard, M.A. Organic Base Grafted on Magnetic Nanoparticles as a Recoverable Catalyst for the Green Synthesis of Hydropyridine Rings. J. Iran. Chem. Soc. 2020, 17, 483–492. [Google Scholar] [CrossRef]
- Bodaghifard, M.A. Bis Sulfamic Acid Functionalized Magnetic Nanoparticles as a Retrievable Nano Catalyst for the Green Synthesis of Polyhydroquinolines and Tetrahydrobenzopyrans. J. Nanostruct. 2019, 9, 29–40. [Google Scholar] [CrossRef]
- Brown, R.C. Recent Developments in Solid-Phase Organic Synthesis. J. Chem. Soc., Perkin Trans. 1 1998, 19, 3293–3320. [Google Scholar] [CrossRef]
- Pershin, G.N.; Shcherbakova, L.I.; Zykova, T.N.; Sokolova, V.N. Antibacterial Activity of Pyrimidine and Pyrrolo-(3,2-d)-Pyrimidine Derivatives. Farmakol. Toksikol. 1972, 35, 466–471. [Google Scholar]
- Amr, A.E.G.E.; Sayed, H.H.; Abdulla, M.M. Synthesis and Reactions of Some New Substituted Pyridine and Pyrimidine Derivatives as Analgesic, Anticonvulsant and Antiparkinsonian Agents. Arch. Pharm. 2005, 338, 433–440. [Google Scholar] [CrossRef]
- Keshk, R.M.; Salama, Z.A.; Elsaedany, S.K. Synthesis, Antimicrobial, Anti-inflammatory, Antioxidant and Cytotoxicity of New Pyrimidine and Pyrimidopyrimidine Derivatives. Sci. Rep. 2025, 15, 9328. [Google Scholar] [CrossRef]
- Kostova, I.; Atanasov, P.Y. Antioxidant Properties of Pyrimidine and Uracil Derivatives. Curr. Org. Chem. 2017, 21, 2096–2108. [Google Scholar] [CrossRef]
- Sondhi, S.M.; Singh, N.; Johar, M.; Kumar, A. Synthesis, Anti-inflammatory and Analgesic Activities Evaluation of Some Mono, Bi and Tricyclic Pyrimidine Derivatives. Bioorg. Med. Chem. 2005, 13, 6158–6166. [Google Scholar] [CrossRef]
- Huang, B.; Kang, D.; Tian, Y.; Daelemans, D.; De Clercq, E.; Pannecouque, C.; Zhan, P.; Liu, X. Design, Synthesis, and Biological Evaluation of Piperidinyl-Substituted [1,2,4]Triazolo[1,5-a]Pyrimidine Derivatives as Potential Anti-HIV-1 Agents with Reduced Cytotoxicity. Chem. Biol. Drug Des. 2021, 97, 67–76. [Google Scholar] [CrossRef]
- Spivey, A.C.; Srikaran, R.; Diaper, C.M.; Turner, D.J. Traceless Solid Phase Synthesis of 2-Substituted Pyrimidines Using an ‘Off-the-Shelf’ Chlorogermane-Functionalised Resin. Org. Biomol. Chem. 2003, 1, 1638–1640. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Soloducho, J.; Belyakov, S. Bis-4-Halophenyl-Pyrimidines and –1,2,4,5-Tetrazines. Arkivoc 2000, 1, 37–42. [Google Scholar] [CrossRef]
- Rostami, H.; Shiri, L. One-Pot Synthesis of Pyrido[2,3-d:5,6-d′]Dipyrimidines Using CoFe2O4@SiO2-PA-CC-Guanidine-SA Magnetic Nanoparticles in Water. Appl. Organomet. Chem. 2021, 35, e6293. [Google Scholar] [CrossRef]
- Sayahi, M.H.; Sepahdar, A.; Bazrafkan, F.; Dehghani, F.; Mahdavi, M.; Bahadorikhalili, S. Ionic Liquid Modified SPION@Chitosan as a Novel and Reusable Superparamagnetic Catalyst for Green One-Pot Synthesis of Pyrido[2,3-d]Pyrimidine-Dione Derivatives in Water. Catalysts 2023, 13, 290. [Google Scholar] [CrossRef]
- Karimi, F.; Tighsazzadeh, B.; Asadi, B.; Mohammadpoor-Baltork, I.; Layeghi, M.; Mirkhani, V.; Tangestaninejad, S.; Moghadam, M. 3-(Propylthio)Propane-1-Sulfonic Acid Immobilized on Functionalized Magnetic Nanoparticles as an Efficient Catalyst for One-Pot Synthesis of Dihydrotetrazolo[1,5-a]Pyrimidine and Tetrahydrotetrazolo[5,1-b]Quinazolinone Derivatives. RSC Adv. 2022, 12, 22180–22187. [Google Scholar] [CrossRef] [PubMed]
- Wenxin, Z.; Mengjun, H.; Shengnan, L.; Yujing, L.; Zhongqiu, L.; An’guo, Y. One-Pot Synthesis of 3,4-Dihydropyrimidine-2-One Derivatives via Biginelli Reactions Catalyzed by SnCl2@MNPs. Chin. J. Org. Chem. 2021, 41, 2743–2749. [Google Scholar] [CrossRef]
- Alishahi, N.; Nasr-Esfahani, M.; Mohammadpoor-Baltork, I.; Tangestaninejad, S.; Mirkhani, V.; Moghadam, M. Nicotine-Based Ionic Liquid Supported on Magnetic Nanoparticles: An Efficient and Recyclable Catalyst for Selective One-Pot Synthesis of Mono- and Bis-4H-Pyrimido[2,1-b]Benzothiazoles. Appl. Organomet. Chem. 2020, 34, e5681. [Google Scholar] [CrossRef]
- Verma, P.; Pal, S.; Chauhan, S.; Mishra, A.; Sinha, I.; Singh, S.; Srivastava, V. Starch Functionalized Magnetite Nanoparticles: A Green, Biocatalyst for One-Pot Multicomponent Synthesis of Imidazopyrimidine Derivatives in Aqueous Medium under Ultrasound Irradiation. J. Mol. Struct. 2020, 1203, 127410. [Google Scholar] [CrossRef]
- Saberikhah, E.; Mamaghani, M.; Mahmoodi, N.O.; Fallah Shojaei, A. Magnetic Fe3O4@TiO2@NH2@PMo12O40 Nanoparticles: A Recyclable and Efficient Catalyst for Convergent One-Pot Synthesis of Pyrido[2,3-d]Pyrimidine Derivatives. Polycycl. Aromat. Compd. 2021, 42, 297–315. [Google Scholar] [CrossRef]
- Taheri Hatkehlouei, S.F.; Mirza, B.; Soleimani-Amiri, S. Solvent-Free One-Pot Synthesis of Diverse Dihydropyrimidinones/Tetrahydropyrimidinones Using Biginelli Reaction Catalyzed by Fe3O4@C@OSO3H. Polycycl. Aromat. Compd. 2022, 42, 1341–1357. [Google Scholar] [CrossRef]
- Ghafuri, H.; Moradi, S.; Ghanbari, N.; Dogari, H.; Ghafori, M. Efficient and Green Synthesis of Acridinedione Derivatives Using Highly Fe3O4@Polyaniline-SO3H as Efficient Heterogeneous Catalyst. Chem. Proc. 2021, 8, 23. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Rashidi Nodeh, H.; Foroumadi, A. Magnetically Recoverable Graphene-Based Nanoparticles for the One-Pot Synthesis of Acridine Derivatives under Solvent-Free Conditions. Polycycl. Aromat. Compd. 2021, 41, 746–760. [Google Scholar] [CrossRef]
- Singh, A.; Singh, K.; Sharma, A.; Kaur, K.; Chadha, R.; Bedi, P.M.S. Recent Advances in Antifungal Drug Development Targeting Lanosterol 14α-Demethylase (CYP51): A Comprehensive Review with Structural and Molecular Insights. Chem. Biol. Drug Des. 2023, 102, 606–639. [Google Scholar] [CrossRef]
- Monk, B.C.; Keniya, M.V. Roles for Structural Biology in the Discovery of Drugs and Agrochemicals Targeting Sterol 14α-Demethylases. J. Fungi 2021, 7, 67. [Google Scholar] [CrossRef]
- Imran, M.; Bawadekji, A.; Alotaibi, N. Synthesis and Evaluation of Antimicrobial Properties of Some Azole Derivatives. Trop. J. Pharm. Res. 2020, 19, 377–382. [Google Scholar] [CrossRef]
- Maertens, J.A. History of the Development of Azole Derivatives. Clin. Microbiol. Infect. 2004, 10, 1–10. [Google Scholar] [CrossRef]
- Mirshafiee, S.; Salamatmanesh, A.; Heydari, A. A Sustainable Approach for Efficient One-Pot Synthesis of 1-Aryl-1,2,3-Triazoles Using Copper Iodide Supported on 3-Thionicotinyl-Urea-Modified Magnetic Nanoparticles in DES. Appl. Organomet. Chem. 2021, 35, e6255. [Google Scholar] [CrossRef]
- Soleimani-Amiri, S.; Shafaei, F.; Varasteh Moradi, A.; Gholami-Orimi, F.; Rostami, Z. A Novel Synthesis and Antioxidant Evaluation of Functionalized [1,3]Oxazoles Using Fe3O4-Magnetic Nanoparticles. J. Heterocycl. Chem. 2019, 56, 2744–2752. [Google Scholar] [CrossRef]
- Naslhajian, H.; Amini, M.; Farnia, S.M.F.; Janczak, J. Synthesis and Characterization of a New Polyoxovanadate for the One-Pot Three-Component (A3) Coupling of Aldehydes, Amines and Alkynes. Mol. Catal. 2020, 483, 110769. [Google Scholar] [CrossRef]
- Mirabedini, M.; Motamedi, E.; Kassaee, M.Z. Magnetic CuO Nanoparticles Supported on Graphene Oxide as an Efficient Catalyst for A3-Coupling Synthesis of Propargylamines. Chin. Chem. Lett. 2015, 26, 1085–1090. [Google Scholar] [CrossRef]
- Kaiba, A.; Geesi, M.H.; Guionneau, P.; Aljohani, T.A.; Bih, L.; Bih, H.; Kassou, S. Synthesis, Structural and Raman Spectroscopic in Organic–Inorganic Halide Perovskites Based on β-Alanine. J. Mol. Struct. 2020, 1204, 127380. [Google Scholar] [CrossRef]
- Ghasemi, K.; Darroudi, M.; Rahimi, M.; Rouh, H.; Gupta, A.R.; Cheng, C.; Amini, A. Magnetic AgNPs/Fe3O4@Chitosan/PVA Nanocatalyst for Fast One-Pot Green Synthesis of Propargylamine and Triazole Derivatives. New J. Chem. 2021, 45, 16119–16130. [Google Scholar] [CrossRef]
- Ahmadi, A.; Sedaghat, T.; Motamedi, H.; Azadi, R. Anchoring of Cu(II)-Schiff Base Complex on Magnetic Mesoporous Silica Nanoparticles: Catalytic Efficacy in One-Pot Synthesis of 5-Substituted-1H-Tetrazoles, Antibacterial Activity Evaluation and Immobilization of α-Amylase. Appl. Organomet. Chem. 2020, 34, e5572. [Google Scholar] [CrossRef]
- Hasan, K.; Joseph, R.G.; Patole, S.P.; Al-Qawasmeh, R.A. Development of Magnetic Fe3O4-Chitosan Immobilized Cu(II) Schiff Base Catalyst: An Efficient and Reusable Catalyst for Microwave Assisted One-Pot Synthesis of Propargylamines via A3 Coupling. Catal. Commun. 2023, 174, 106588. [Google Scholar] [CrossRef]
- Moeini-Eghbali, N.; Eshghi, H. Immobilized Nickel Nanoparticles on Modified Magnetic Titanium Dioxide: A Proficient and Eco-Friendly Nanocatalyst for the Green A3-Coupling Synthesis of Propargylamines. J. Mol. Struct. 2024, 1305, 137727. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).