Fabrication of VOx/AFCC-PG Catalyst from Waste Support for Hg0 Removal in Flue Gas
Abstract
1. Introduction
2. Results and Discussion
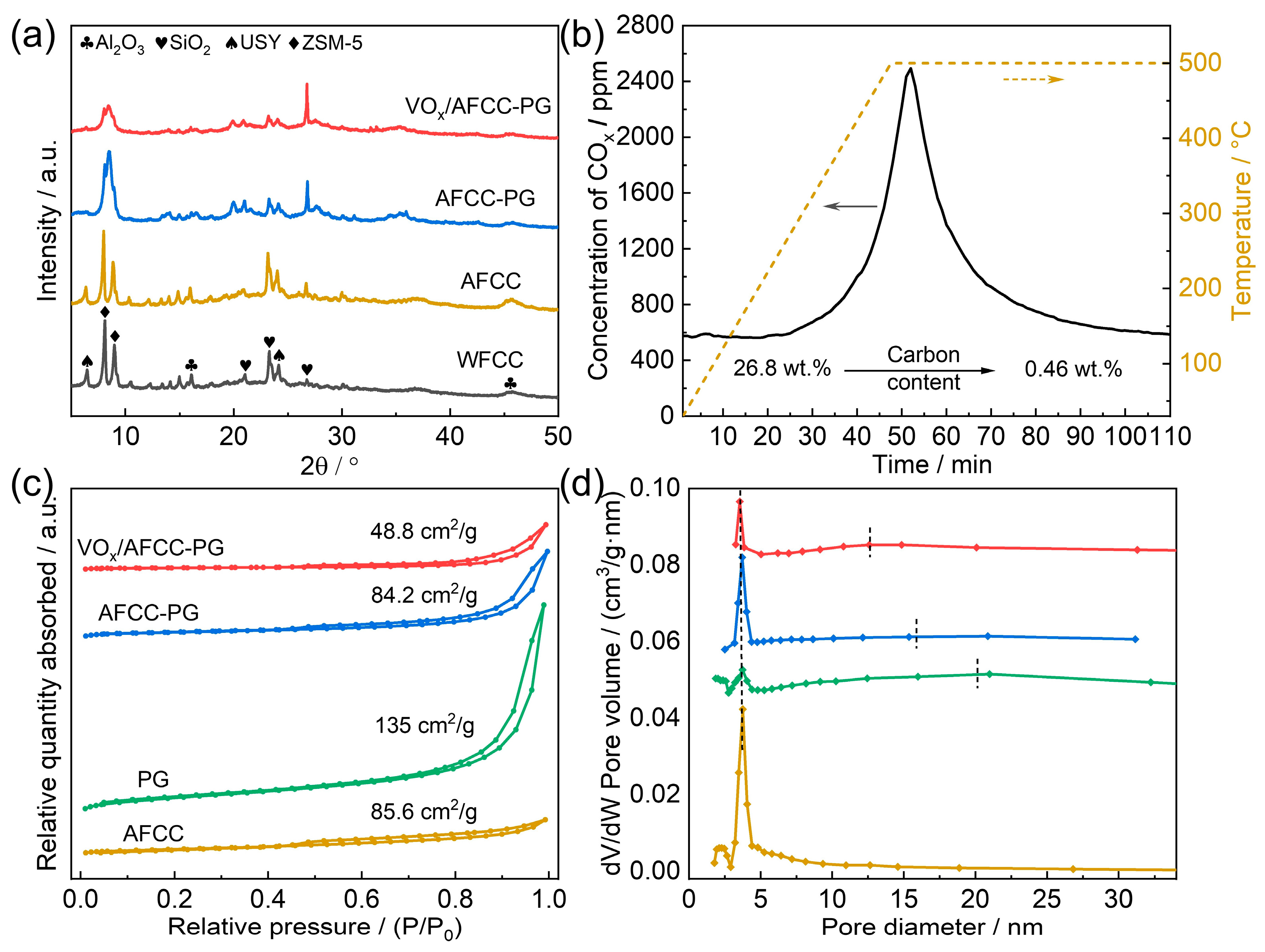
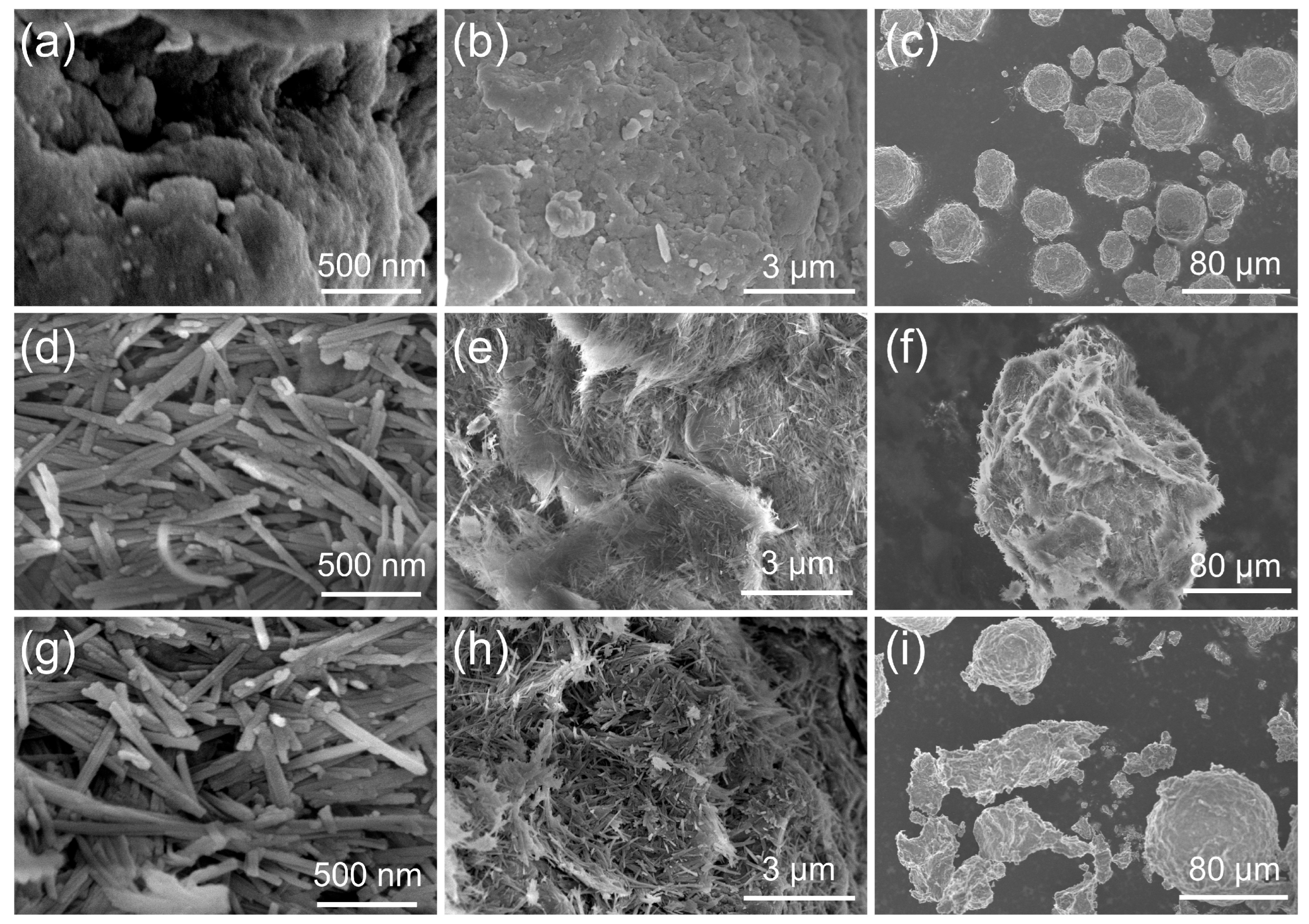
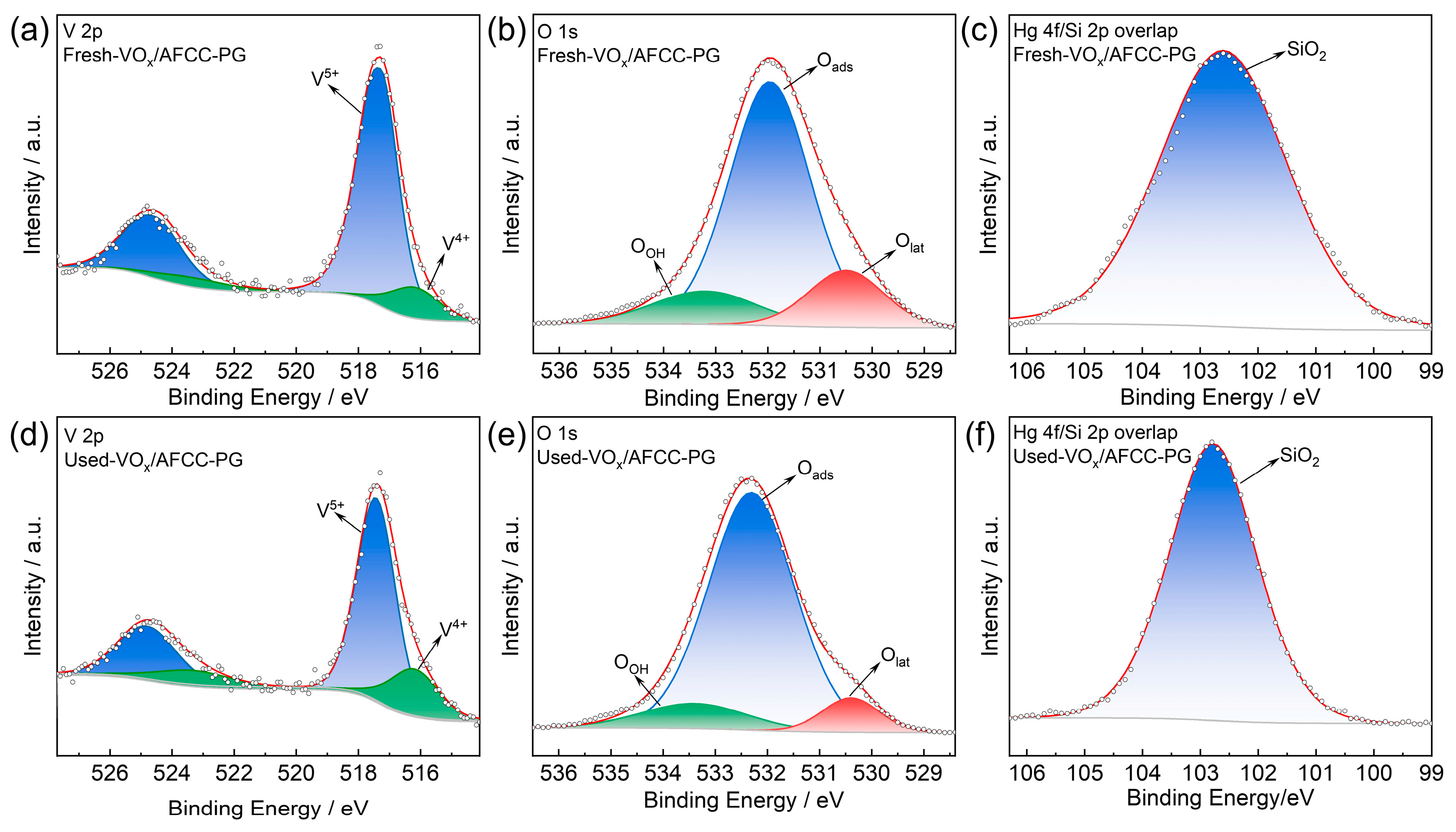
2.1. Catalyst Characterization
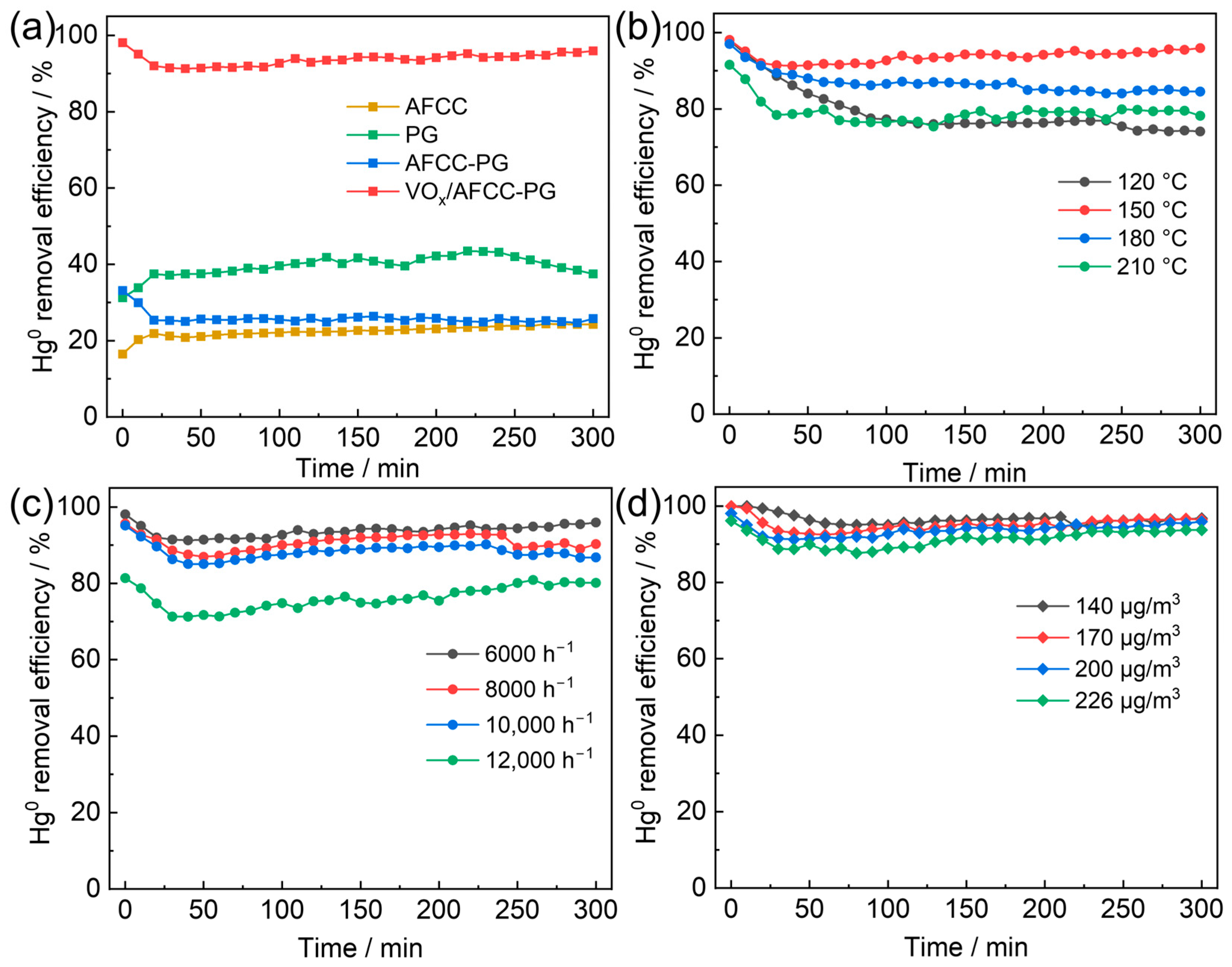
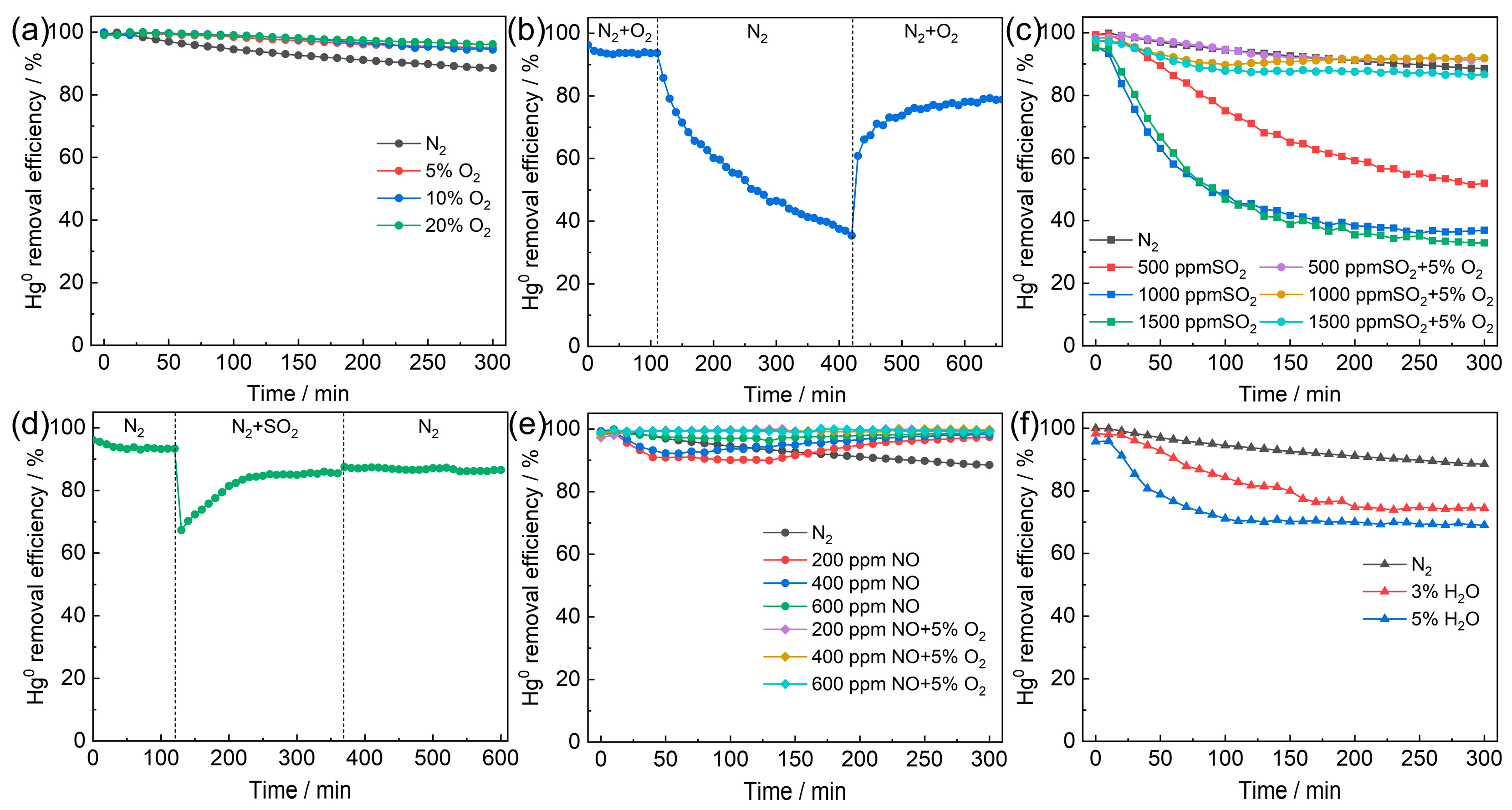
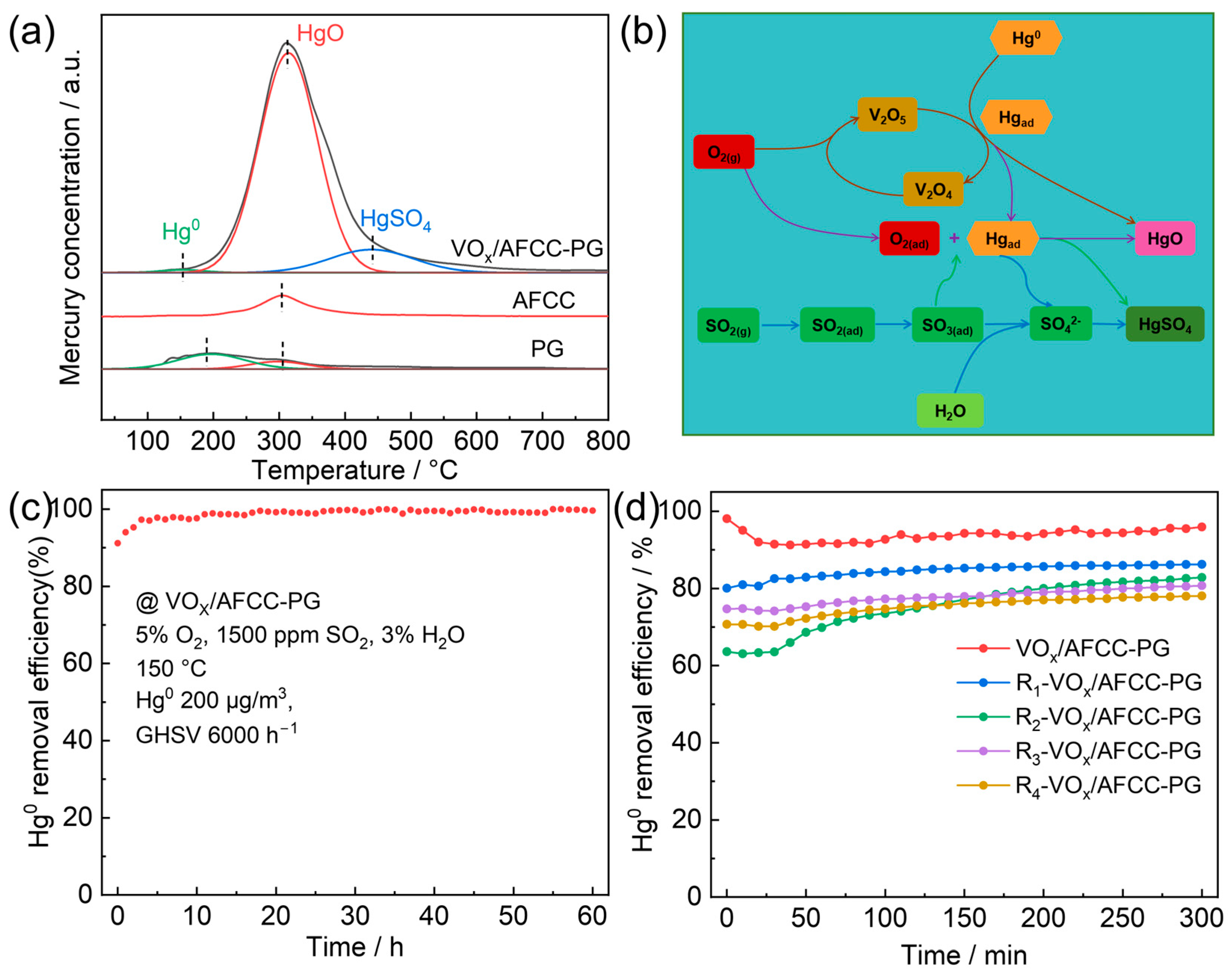
2.2. Catalytic Activity
2.2.1. Effect of Reaction Conditions on Catalytic Activity
2.2.2. Effect of Flue Gas Components on Catalytic Activity
2.3. Catalytic Mechanism and Application Potential
3. Materials and Methods
3.1. Synthesis of Catalysts
3.2. Activity Measurement
3.3. Materials Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.Y.; Kang, H.H.; Zhao, L.J.; Guo, J.M.; Zhang, Y.; Xie, C.; Dong, X.Y.; Kang, S.C.; Liu, X.H. Climate and industrial pollution determine the seasonal and spatial mercury variations in the China’s Weihe River. Sci. Total Environ. 2024, 912, 186555. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.Q.; Zheng, W.; Leng, L.J.; Yang, J.P.; Qu, W.Q.; Li, H.L. A review on monolithic remediators for mercury pollution control in industrial flue gas and effluents. Sep. Purif. Technol. 2025, 363, 131917. [Google Scholar] [CrossRef]
- Liu, D.Y.; Wang, C.R.; Fan, Y.P.; Liu, Q.Q.; Wang, X.D.; Xu, K.L.; Jin, J.; Ma, J.J.; Ma, J.C. Mercury transformation and removal in chemical looping combustion of coal: A review. Fuel 2023, 347, 128440. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Zhang, H.; Jin, Y.L.; Wang, Y.D.; Yang, X.D.; Hou, R.J.; Hou, D.Y. Assessing mercury pollution at a primary ore site with both ancient and industrial mining and smelting activities. Environ. Pollut. 2023, 336, 122413. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.C.; Chen, Z.K.; Liu, Y.X.; Wang, R.X.; Yan, K.; Xu, Z.F.; Li, J.; Liu, Z.L.; Li, J.Y.; Liu, H. Engineering sulfur-rich MoS2 adsorbent with abundant unsaturated coordination sulfur sites for gaseous mercury capture from high-concentration SO2 smelting flue gas. Chem. Eng. J. 2024, 483, 149122. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, C.Z.; Liu, F.H.; Zheng, R.J.; Xiao, R.; Liu, X.L.; Zhu, T.Y.; Chang, H.Z. Simultaneous catalytic oxidation of Hg0 and NO over CoxCuy metal oxides catalyst. Catal. Today 2024, 439, 114801. [Google Scholar] [CrossRef]
- Cui, S.P.; Liao, Y.J.; Gao, Z.Y.; Fu, D. Dielectric barrier discharge coupling catalytic oxidation for efficient conversion of Hg0 from flue gas. Fuel 2021, 287, 119521. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.; Li, S.; Zhang, W.; Du, X.; Huang, L.; Zhu, Y.; Zhai, Y.; Synergetic, G. Superior performance and resistance to SO2 and H2O over CoOx-modified MnOx/biomass activated carbons for simultaneous Hg0 and NO removal. Chem. Eng. J. 2019, 371, 781–795. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, D.; Zhang, A.; Zhang, Z.; Liu, Z.; Hou, L. CrOx-MnOx-TiO2 adsorbent with high resistance to SO2 poisoning for Hg0 removal at low temperature. J. Ind. Eng. Chem. 2017, 55, 119–127. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Zhang, A.; Jing, Z.; Li, Y.; Wang, F.; Wang, Y. Analysis of mercury removal and sulfur resistance of CrOx-MnOx-SnOx-TiO2 catalysts. Process Saf. Environ. Prot. 2024, 188, 1536–1549. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Zhang, A.; Zhang, Z.; Li, Y.; Wang, F.; Wang, Q. Ruthenium-modified catalyst Ru-CeO2 significantly enhances mercury removal from sulfur-containing coal flue gas. Process Saf. Environ. Prot. 2024, 189, 840–855. [Google Scholar] [CrossRef]
- Wei, X.; Du, R.; Zhao, R.; Xu, Y.; Wang, H.; Jiang, B.; Fang, G.; Wang, J. Functionalized MnOx/Montmorillonite for High-efficiency Hg0 Removal in Simulated Flue Gas: Synergistic Hydrophobic and Polar Modifications. Catal. Lett. 2025, 155, 255. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Z.; Lu, H.; Liu, Z.; Xiang, J.; Zhou, C.; Xing, W.; Sun, L. Effect of Promotion with Ru Addition on the Activity and SO2 Resistance of MnOx-TiO2 Adsorbent for Hg0 Removal. Ind. Eng. Chem. Res. 2015, 54, 2930–2939. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Zhang, B.; Zhao, Y.; Chen, X.; Shen, F. Experimental and theoretical studies of mercury oxidation over CeO2-WO3/TiO2 catalysts in coal-fired flue gas. Chem. Eng. J. 2017, 317, 758–765. [Google Scholar] [CrossRef]
- Li, J.; Xu, H.; Huang, Z.; Hong, Q.; Qiu, Y.; Yan, N.; Qu, Z. Strengthen the Affinity of Element Mercury on the Carbon-Based Material by Adjusting the Coordination Environment of Single-Site Manganese. Environ. Sci. Technol. 2021, 55, 14126–14135. [Google Scholar] [CrossRef]
- Singh, A.; Srivastava, V.C.; Janowska, I. Utilization of carbon-black industry waste to synthesize electrode material for supercapacitors. Energy Storage 2024, 6, e677. [Google Scholar] [CrossRef]
- İlknur, A.; Münevver, S. Preparation of TiO2-polystyrene photocatalyst from waste material and its usability for removal of various pollutants. Appl. Catal. B Environ. 2014, 144, 694–701. [Google Scholar]
- Kaur, J.; Kathuria, J.; Babu, J.N.; Arora, M. Waste plastic bottles an alternate material for synthesis of metal organic frameworks (MOFs) with potential applications. Next Sustain. 2025, 5, 100068. [Google Scholar] [CrossRef]
- Wang, H.S.; Ren, Y.J.; Deng, S.; Huang, J.Y.; Guo, F.Y.; Tian, G. Removal of Hg0 from simulated coal-fired flue gas by using activated spent FCC catalysts. J. Fuel Chem. Technol. 2020, 48, 1466–1475. [Google Scholar] [CrossRef]
- Salahudeen, N. Effect of ZSM5 in the catalytic activity of a fluid catalytic cracking catalyst. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 173–181. [Google Scholar] [CrossRef]
- Roncolatto, R.E.; Cardoso, M.J.B.; Cerqueira, H.S.; Lam, Y.L.; Schmal, M. XPS Study of Spent FCC Catalyst Regenerated under Different Conditions. Ind. Eng. Chem. Res. 2007, 46, 1148–1152. [Google Scholar] [CrossRef]
- Tashima, M.M.; Soriano, L.; Akasaki, J.L.; Castaldelli, V.N.; Monzó, J.M.; Payá, J.; Borrachero, M.V. Spent FCC catalyst for preparing alkali-activated binders: An opportunity for a high-degree valorization. Key Eng. Mater. 2014, 600, 709–716. [Google Scholar] [CrossRef]
- Mathieu, Y.; Corma, A.; Echard, M.L.; Bories, M. Single and combined Fluidized Catalytic Cracking (FCC) catalyst deactivation by iron and calcium metal–organic contaminants. Appl. Catal. A Gen. 2014, 469, 451–465. [Google Scholar] [CrossRef]
- Su, B.; Shi, L.; Meng, X.; Wang, X.; Liu, N. From waste to best: Excellent desulfurization performance of spent FCC catalyst. J. Sulfur Chem. 2018, 40, 75–87. [Google Scholar] [CrossRef]
- Nazarova, G.; Elena, I.; Emiliya, O.; Alexandra, D.; Irena, P.; Mariya, P. Modeling of the catalytic cracking: Catalyst deactivation by coke and heavy metals. Fuel Process. Technol. 2020, 200, 106318. [Google Scholar] [CrossRef]
- Álvarez-Hernández, D.; Ivanova, S.; Domínguez, M.I.; Blanes, J.M.M.; Centeno, M.Á. V2O5/TiO2 Catalyst for Catalytic Glucose Oxidation to Formic Acid in Batch Reactor: Vanadium Species Nature and Reaction Conditions Optimization. Top. Catal. 2025, 68, 49–58. [Google Scholar] [CrossRef]
- Liu, H.; Chang, L.; Liu, W.; Xiong, Z.; Zhao, Y.; Zhang, J. Advances in mercury removal from coal-fired flue gas by mineral adsorbents. Chem. Eng. J. 2020, 379, 122263. [Google Scholar] [CrossRef]
- Ma, Y.P.; Mu, B.L.; Yuan, D.L.; Zhang, H.Z.; Xu, H.M. Design of MnO2/CeO2-MnO2 hierarchical binary oxides for elemental mercury removal from coal-fired flue gas. J. Hazard. Mater. 2020, 333, 186–193. [Google Scholar] [CrossRef]
- Wu, L.; Shang, Z.; Zhu, H.; Li, Z.; Luo, G.; Yao, H. Gas-Phase Mercury Removal by Modified Activated Carbons Treated with Ar-O2 Non-Thermal Plasma under Different O2 Concentrations. Int. J. Chem. React. Eng. 2018, 17, 20180093. [Google Scholar] [CrossRef]
- Chen, Y.; Lisbona, P.; Perez, V.; Guo, X. Performance of MnCl2 doped magnetic iron-carbon sorbent on mercury removal from flue gas: The effect of O2 and SO2. Fuel 2021, 285, 119064. [Google Scholar] [CrossRef]
- Shi, J.; Chen, J.; Wang, J.; Yin, R.; Wang, B.; Xiong, S.; Wang, Z.; Liu, H.; Li, J. Vanadium-density-dependent reactivity for simultaneous removal of NOx and Hg0 over V2O5/TiO2 catalyst. Fuel 2023, 332, 126189. [Google Scholar] [CrossRef]
- Park, J.H.; Nguyen, T.P.T.; Kim, M.H.; Hong, Y. Effect of Vanadium Oxide Loading for the Simultaneous NO Reduction and Hg0 Oxidation over a V2O5-WO3/TiO2 Catalyst. Ind. Eng. Chem. Res. 2023, 62, 10–21. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Y.; Yan, N.; Wu, D.; He, H.; Xie, J.; Qu, Z.; Jia, J. Remarkable effect of the incorporation of titanium on the catalytic activity and SO2 poisoning resistance of magnetic Mn-Fe spinel for elemental mercury capture. Appl. Catal. B Environ. 2011, 101, 698–708. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Zhang, Y.; Wang, J.; Sun, B.; Pan, W.P. A review on adsorbent/catalyst application for mercury removal in flue gas: Effect of sulphur oxides (SO2, SO3). J. Clean. Prod. 2020, 276, 124220. [Google Scholar] [CrossRef]
- Li, H.; Peng, X.; An, M.; Zhang, J.; Cao, Y.; Liu, W. Negative effect of SO2 on mercury removal over catalyst/sorbent from coal-fired flue gas and its coping strategies: A review. Chem. Eng. J. 2023, 455, 140751. [Google Scholar] [CrossRef]
- Shi, Q.; Lu, L.; Kang, D.; Shen, B.; Bian, Y.; Zhang, X.; Lu, H.H.; Li, S. Rational modulation of redox equilibrium over Mn modified Ce-Sn catalyst for low-temperature elimination of NO and Hg0. Fuel 2024, 368, 131486. [Google Scholar] [CrossRef]
- Marchon, B.; Carrazza, J.; Heinemann, H.; Somorjai, G.A. TPD and XPS studies of O2, CO2, and H2O adsorption on clean polycrystalline graphite. Carbon 1988, 26, 507–514. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Q.Y.; Liu, Z.Y.; Huang, Z.G.; Guo, Y.X.; Yang, J.L. Roles of lattice oxygen in V2O5 and activated coke in SO2 removal over coke-supported V2O5 catalysts. Appl. Catal. B Environ. 2008, 82, 114–119. [Google Scholar] [CrossRef]
- Lietti, L. Reactivity of V2O5WO3TiO2 de-NOx catalysts by transient methods. Appl. Catal. B Environ. 1996, 10, 281–297. [Google Scholar] [CrossRef]
- Zhang, X.; Han, X.; Wei, Y.; Wang, X.; Zhang, N.; Bao, J.; He, G. Single-atom Co-N-C catalyst for efficient Hg0 oxidation at low temperature. Chem. Eng. J. 2022, 428, 132660. [Google Scholar] [CrossRef]
- Yang, W.; Liu, X.; Chen, X.; Cao, Y.; Cui, S.; Jiao, L.; Wu, C.; Chen, C.; Fu, D.; Gates, I.D. A Sulfur-Tolerant MOF-Based Single-Atom Fe Catalyst for Efficient Oxidation of NO and Hg0. Adv. Mater. 2022, 34, 1242–1264. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Wang, Z.; Yu, Y. Reaction mechanism of elemental mercury oxidation to HgSO4 during SO2/SO3 conversion over V2O5/TiO2 catalyst. Proc. Combust. Inst. 2021, 38, 4317–4325. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Hsi, H.-C.; Lin, H.-P.; Chang, T.-C. Effects of properties of manganese oxide-impregnated catalysts and flue gas condition on multipollutant control of Hg0 and NO. J. Hazard. Mater. 2015, 291, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xu, Y.; Zhang, B.; Luo, G.; Sun, P.; Zou, R.; Yao, H. Elemental mercury adsorption and regeneration performance of sorbents FeMnOx enhanced via non-thermal plasma. Chem. Eng. J. 2017, 309, 503–512. [Google Scholar] [CrossRef]
- Yang, B.; Li, Z.; Huang, Q.; Chen, M.; Xu, L.; Shen, Y.; Zhu, S. Synergetic removal of elemental mercury and NO over TiCe0.25Sn0.25Ox catalysts from flue gas: Performance and mechanism study. Chem. Eng. J. 2019, 360, 990–1002. [Google Scholar] [CrossRef]
- Li, H.; Wu, C.-Y.; Li, Y.; Li, L.; Zhao, Y.; Zhang, J. Impact of SO2 on elemental mercury oxidation over CeO2–TiO2 catalyst. Chem. Eng. J. 2013, 219, 319–326. [Google Scholar] [CrossRef]
- Li, Y.; Murphy, P.D.; Wu, C.Y.; Powers, K.W.; Bonzongo, J.C. Development of silica/vanadia/titania catalysts for removal of elemental mercury from coal-combustion flue gas. Environ. Sci. Technol. 2008, 42, 5304–5309. [Google Scholar] [CrossRef]
- Li, J.; Yan, N.; Qu, Z.; Qiao, S.; Yang, S.; Guo, Y.; Liu, P.; Jia, J. Catalytic oxidation of elemental mercury over the modified catalyst Mn/α-Al2O3 at lower temperatures. Environ. Sci. Technol. 2010, 44, 426–431. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.-Q.; Qiao, F.; Zhu, X.; Wang, T.; Pan, W.-P. Engineering single-atom Pd sites in ZIF-derived porous Co3O4 for enhanced elementary mercury removal. Sep. Purif. Technol. 2023, 309, 123050. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Zhou, Y.; Li, J.; Hu, S.; Huang, W.; Qu, Z. Interface modulation of Mn-N4-C with optimized oxygen-containing functional groups for highly efficient mercury adsorption. J. Hazard. Mater. 2024, 461, 132498. [Google Scholar] [CrossRef]
- Shen, L.; Xiong, Z.; Du, Z.; Wang, H.; Li, W.; Zhang, Z.; Wu, H.; Zhou, C.; Zhou, Y.; Tang, G.; et al. Unveiling the electron-acceptor effect in selenium-doped Cu-N-C catalyst with atomically dispersed active sites for boosting Hg0 oxidation. Sep. Purif. Technol. 2024, 334, 126078. [Google Scholar] [CrossRef]
- Zhao, L.; Li, C.; Zhang, J.; Zhang, X.; Zhan, F.; Ma, J.; Xie, Y.; Zeng, G. Promotional effect of CeO2 modified support on V2O5-WO3/TiO2 catalyst for elemental mercury oxidation in simulated coal-fired flue gas. Fuel 2015, 153, 361–369. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, J.; Li, B.; Xu, H.; Liu, D. Removal of elemental mercury from simulated flue gas by ZSM-5 modified with Mn-Fe mixed oxides. Chem. Eng. J. 2019, 375, 121946. [Google Scholar] [CrossRef]
- Guo, Z.; Jing, G.; Tolba, S.A.; Yuan, C.-s.; Li, Y.-H.; Zhang, X.; Huang, Z.; Zhao, H.; Wu, X.; Shen, H.; et al. Design and construction of an O-Au-O coordination environment in Au single atom-doped Ti4+ defected TiO2 for an enhanced oxidative ability of lattice oxygen for Hg0 oxidation. Chem. Eng. J. 2023, 451, 138895. [Google Scholar] [CrossRef]
- Xu, W.; Tong, L.; Qi, H.; Zhou, X.; Wang, J.; Zhu, T. Effect of flue gas components on Hg0 oxidation over Fe/HZSM-5 Catalyst. Ind. Eng. Chem. Res. 2014, 54, 146–152. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Wang, Z.; Zhang, J.; Wang, L.; Zheng, C. Integrated removal of NO and mercury from coal combustion flue gas using manganese oxides supported on TiO2. J. Environ. Sci. 2017, 53, 141–150. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Wang, X.; Hu, J. Activity of CuCl2-modified cobalt catalyst supported on Ti-Ce composite for simultaneous catalytic oxidation of Hg0 and NO in a simulated pre-sco process. Chem. Eng. J. 2017, 316, 1103–1113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Du, R.; Zhao, R.; Li, W.; Jiang, C.; Wang, J. Fabrication of VOx/AFCC-PG Catalyst from Waste Support for Hg0 Removal in Flue Gas. Catalysts 2025, 15, 799. https://doi.org/10.3390/catal15090799
Wei X, Du R, Zhao R, Li W, Jiang C, Wang J. Fabrication of VOx/AFCC-PG Catalyst from Waste Support for Hg0 Removal in Flue Gas. Catalysts. 2025; 15(9):799. https://doi.org/10.3390/catal15090799
Chicago/Turabian StyleWei, Xuhui, Ruoyang Du, Rushan Zhao, Wenzhi Li, Caihong Jiang, and Junwei Wang. 2025. "Fabrication of VOx/AFCC-PG Catalyst from Waste Support for Hg0 Removal in Flue Gas" Catalysts 15, no. 9: 799. https://doi.org/10.3390/catal15090799
APA StyleWei, X., Du, R., Zhao, R., Li, W., Jiang, C., & Wang, J. (2025). Fabrication of VOx/AFCC-PG Catalyst from Waste Support for Hg0 Removal in Flue Gas. Catalysts, 15(9), 799. https://doi.org/10.3390/catal15090799






