Flow Field Structure Optimization and Inlet Parameters in Tubular Photocatalytic Reactors: A CFD-Based Study
Abstract
1. Introduction
2. Results and Discussion
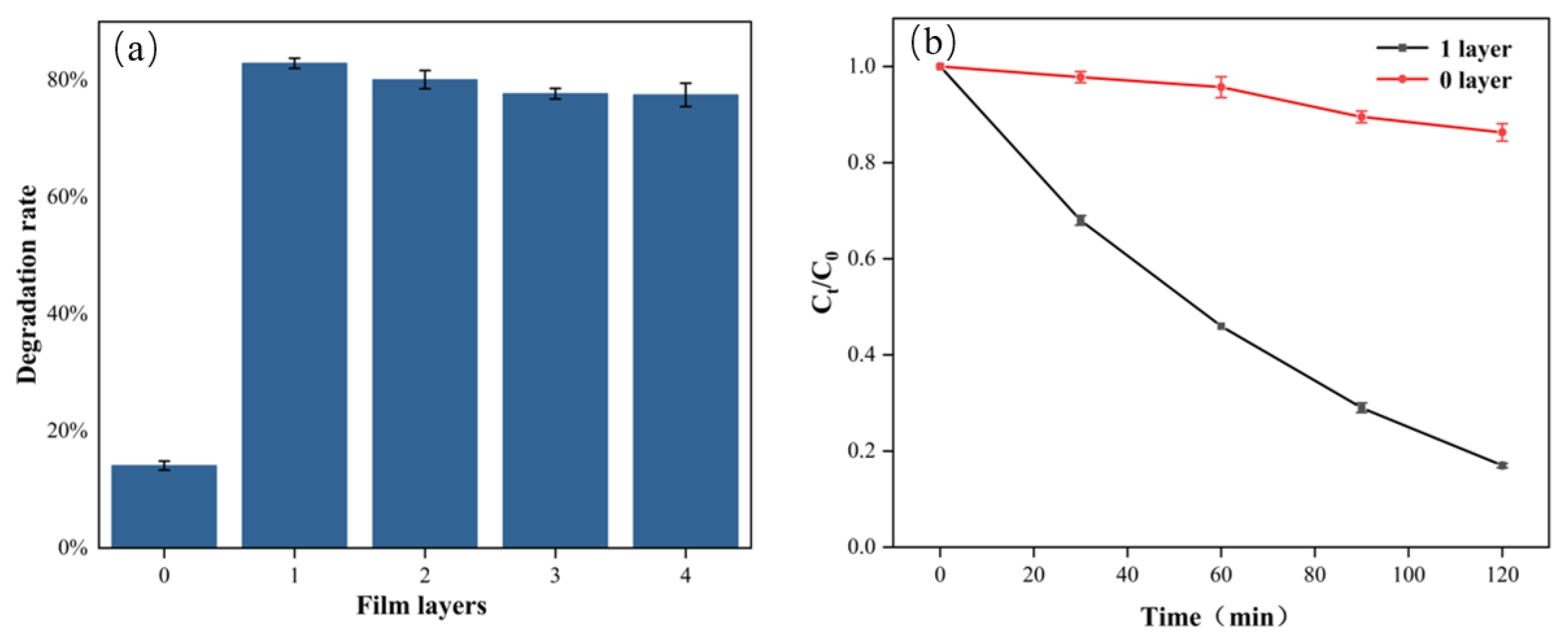
2.1. Effect of the Number of TiO2 Film Layers on Photocatalytic Efficiency
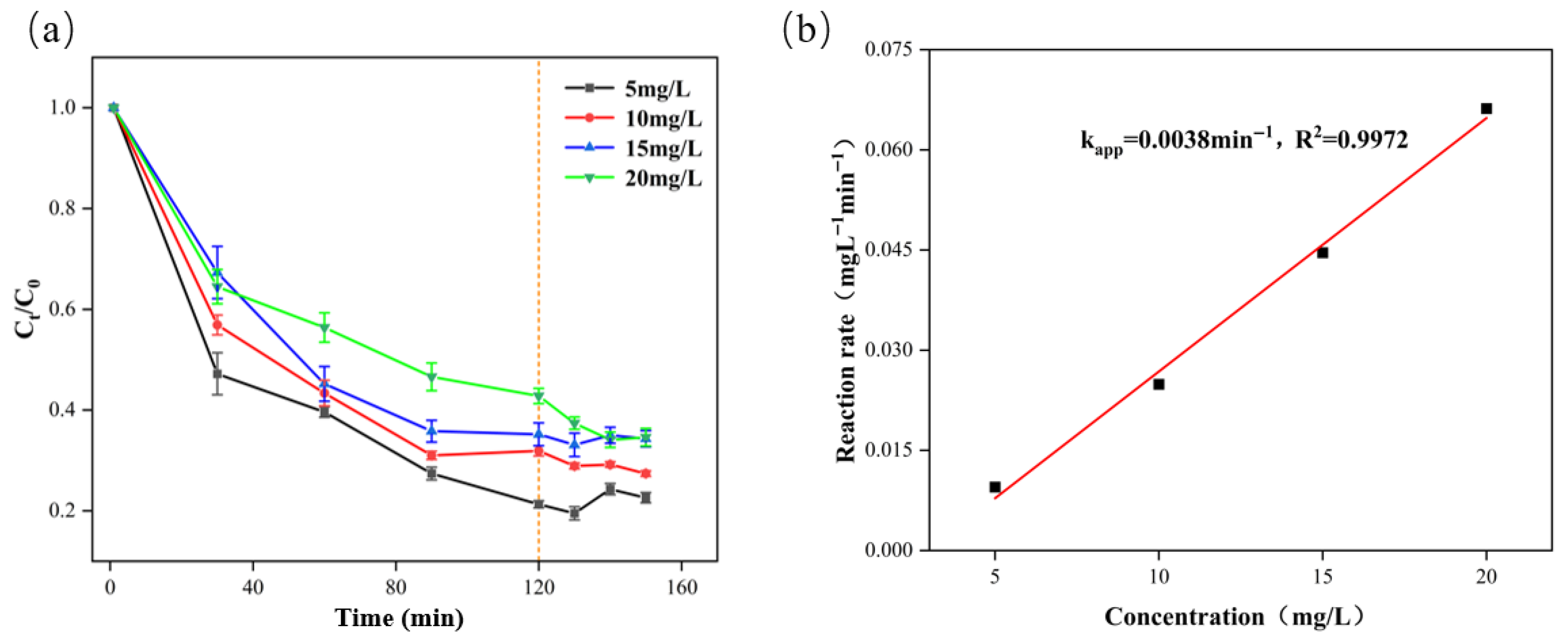
2.2. Degradation Kinetics Analysis
2.3. Analysis of Model Validity
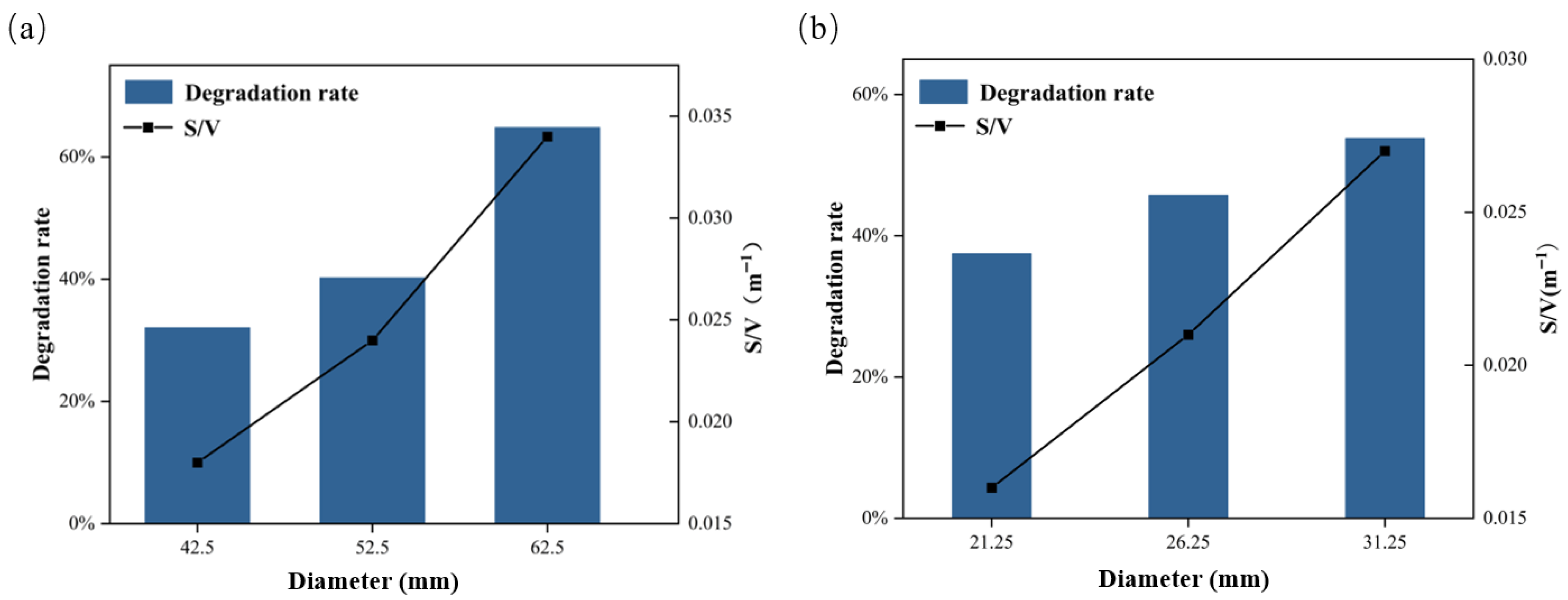
2.4. Effect of Film Surface Area on Photocatalytic Efficiency
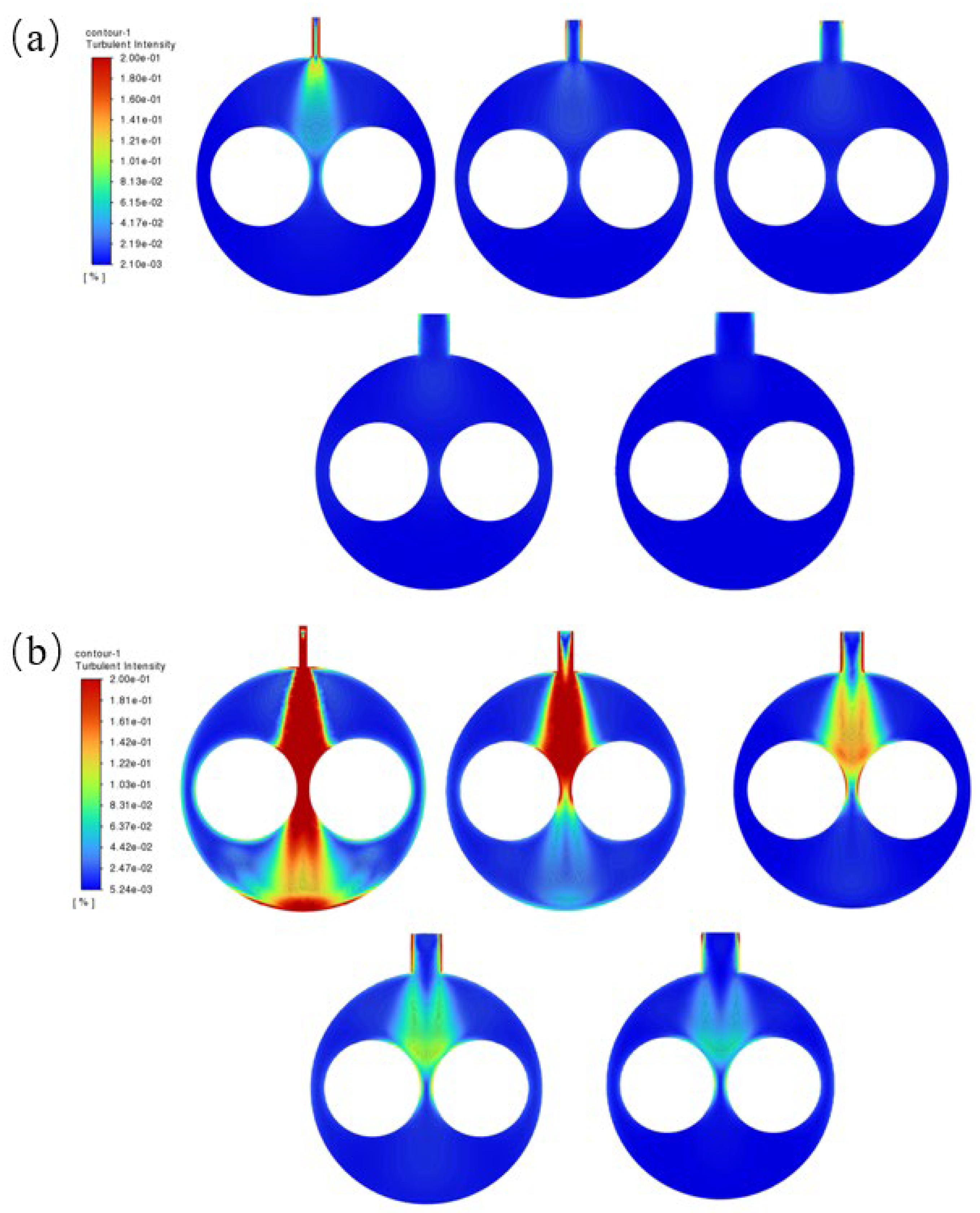
2.5. Effect of Flow Field Configuration on Photocatalytic Efficiency
2.6. Effect of S/V Value on Photocatalytic Efficiency
2.7. Effect of Inlet Parameters on Photocatalytic Efficiency
3. Materials and Methods
3.1. Materials
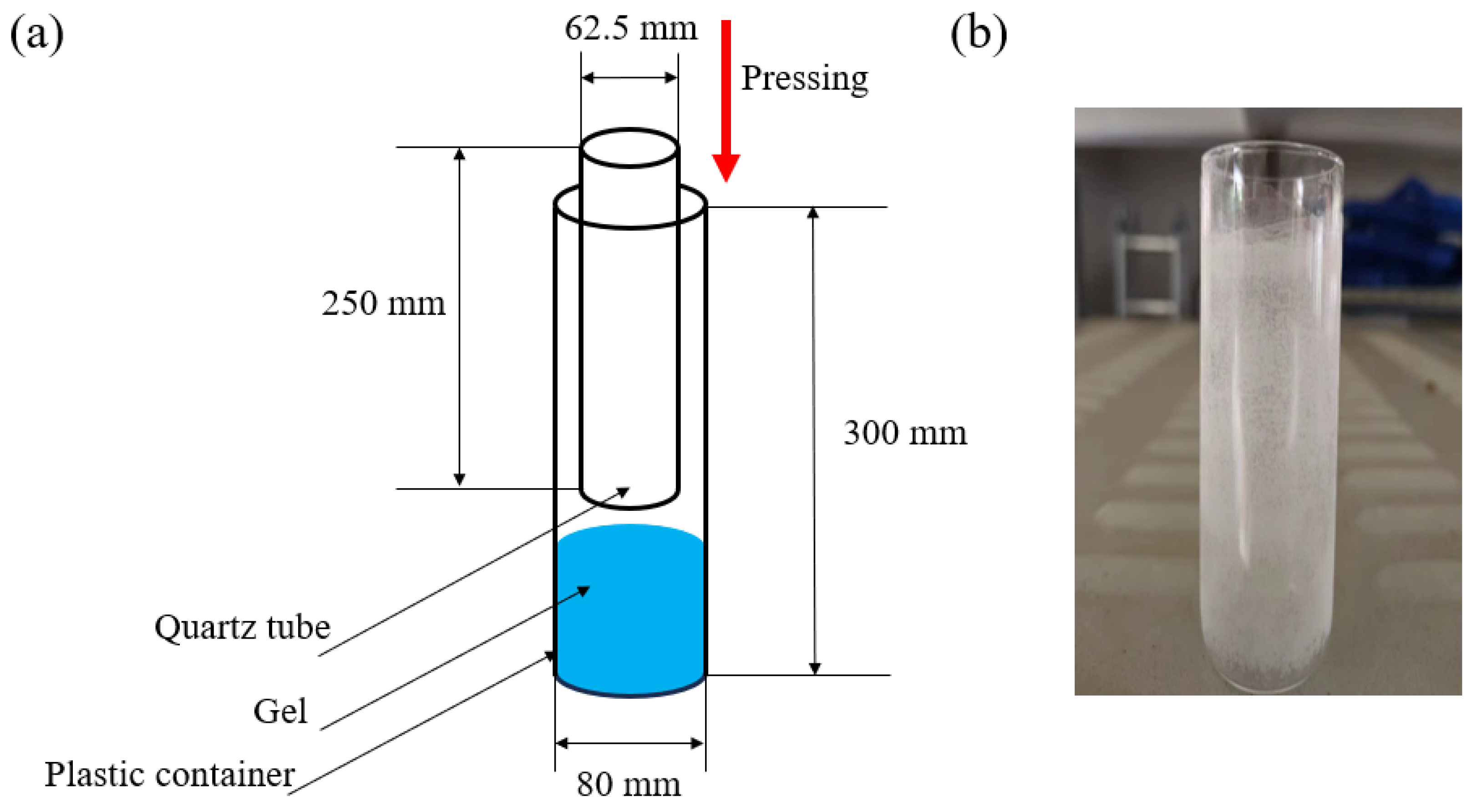
3.2. Catalyst Film Preparation
3.3. Photocatalytic Activity Testing
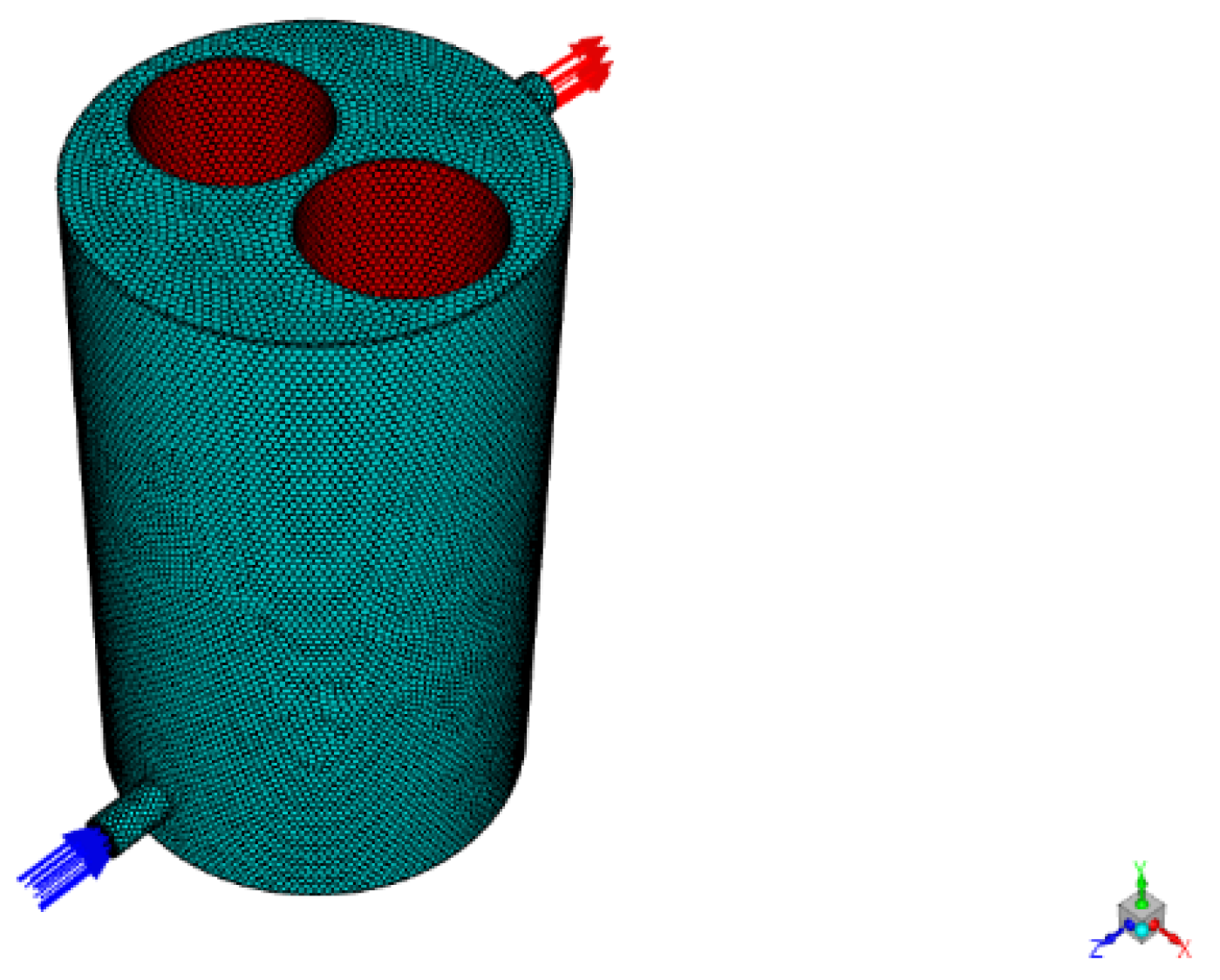
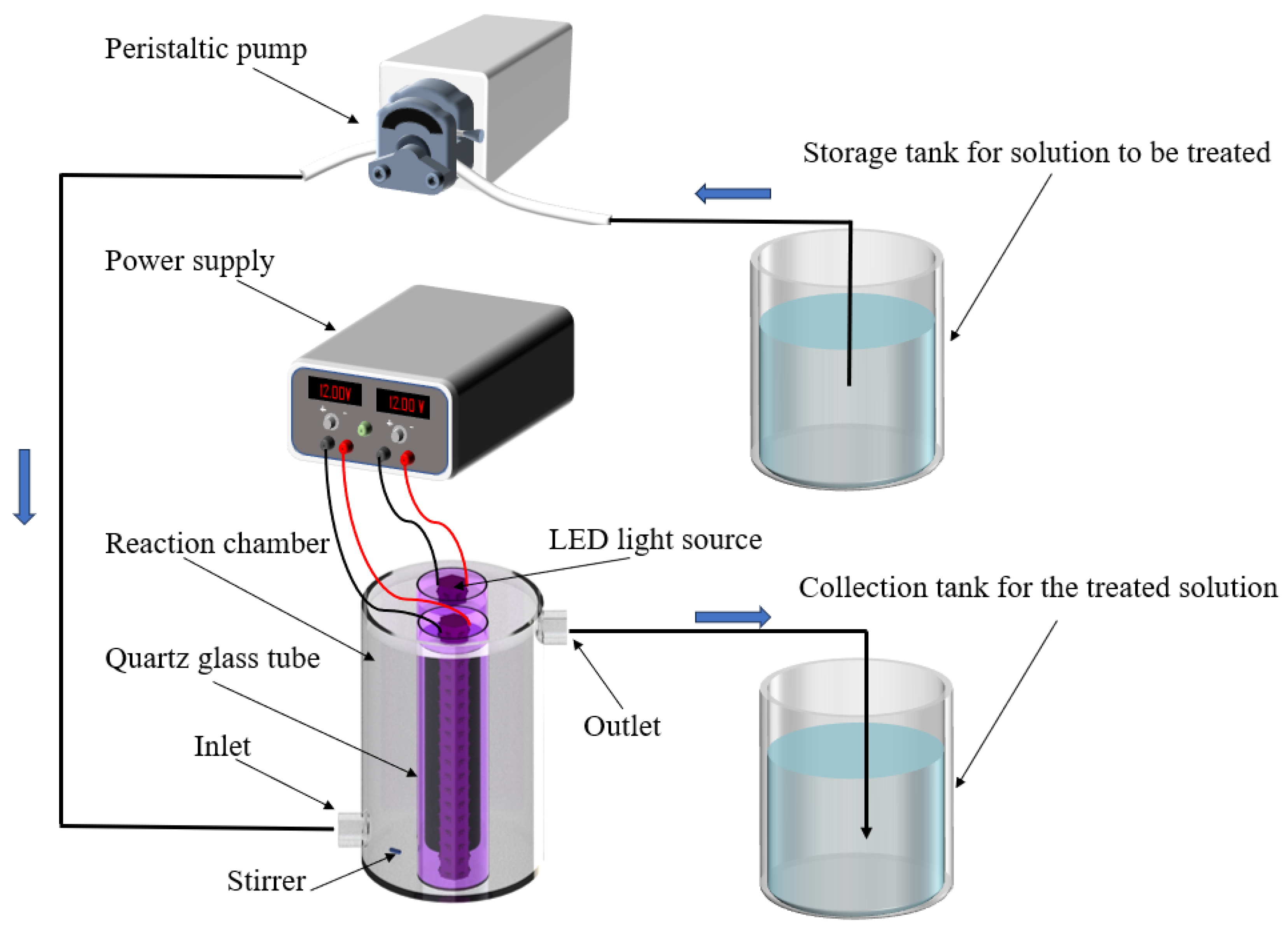
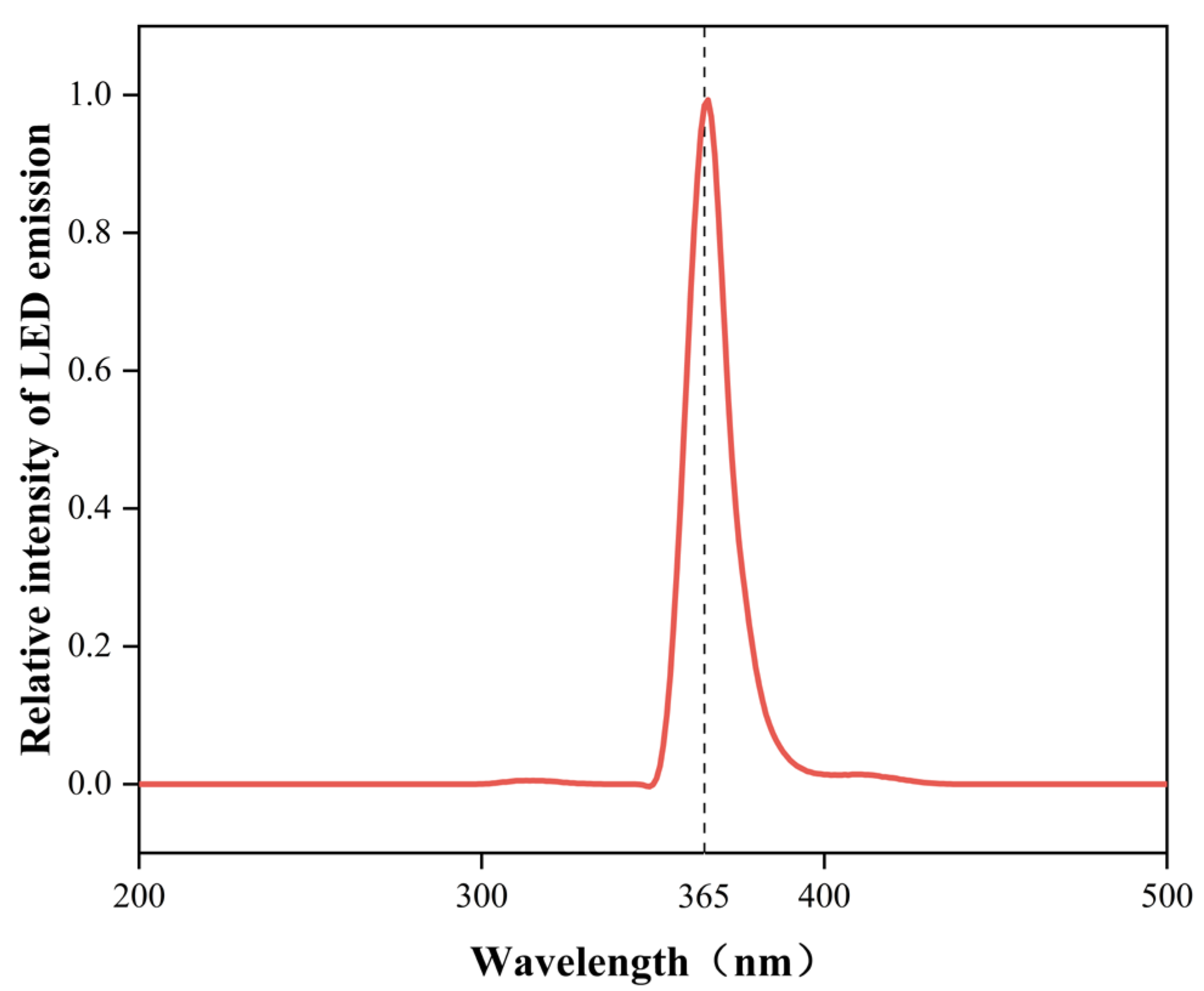
3.3.1. Photocatalytic Reactor
3.3.2. Photocatalytic Degradation Experiments
3.4. Basic Governing Equations of Fluid Dynamics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shen, Y.; Wang, Y.; Shan, P.; Xu, R.; Sun, X.; Hou, J.; Guo, F.; Li, C.; Shi, W. Boosted photo-self-fenton degradation activity by fe-doped carbon dots as dual-function active sites for in-situ H2O2 generation and activation. Sep. Purif. Technol. 2025, 353, 128529. [Google Scholar] [CrossRef]
- Qin, H.; He, Y.; Xu, P.; Zhu, Y.; Wang, H.; Wang, Z.; Zhao, Y.; Xie, H.; Tian, Q.; Wang, C.; et al. Carbon-doped CuFe2O4 with C--O--M channels for enhanced Fenton-like degradation of tetracycline hydrochloride: From construction to mechanism. Green Energy Environ. 2024, 9, 732–747. [Google Scholar] [CrossRef]
- Deng, H.; Wu, Y.; Li, L.; Jiang, X.; Wang, P.; Fang, K.; Li, J.; Hao, D.; Zhu, H.; Wang, Q.; et al. Synergistic mechanisms for efficient and safe antibiotic removal: Effective adsorption and photocatalytic degradation using aerogels. Sep. Purif. Technol. 2025, 354, 129455. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, J.; Shi, J.; Deng, H. Bi2Fe4O9/rGO nanocomposite with visible light photocatalytic performance for tetracycline degradation. Environ. Res. 2024, 249, 118361. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Ye, Q.; Qu, C.; Meng, F.; Wang, L.; Li, Y. Enhanced hydrogen evolution and tetracycline degradation by a Z-scheme g-C3N4 nanosheets loaded CeO2 photocatalyst under visible light irradiation. J. Environ. Chem. Eng. 2024, 12, 112563. [Google Scholar] [CrossRef]
- Wang, X.; Lin, S.; Cui, N.; Qi, K.; Liu, S.; Khan, I. Synthesis of ZnWO4/NiWO4 photocatalysts and their application in tetracycline hydrochloride degradation and antibacterial activities. J. Taiwan Inst. Chem. Eng. 2024, 157, 105408. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; Rao, R.; Chen, J.; Han, X.; Jiang, S.; Yang, Y.; Wang, Y.; Wang, L. Highly efficient visible-light-driven photocatalytic degradation of gaseous toluene by rutile-anatase TiO2@MIL-101 composite with two heterojunctions. J. Environ. Sci. 2023, 134, 21–33. [Google Scholar] [CrossRef]
- Khan, H. Sol-Gel Synthesis of TiO2 from TiOSO4 (Part 2): Kinetics and Photocatalytic Efficiency of Methylene Blue Degradation Under UV Irradiation. Catalysts 2025, 15, 64. [Google Scholar] [CrossRef]
- Haque, F.; Blanchard, A.; Laipply, B.; Dong, X. Visible-Light-Activated TiO2-Based Photocatalysts for the Inactivation of Pathogenic Bacteria. Catalysts 2024, 14, 855. [Google Scholar] [CrossRef]
- Lu, Z.; Peng, J.; Song, M.; Liu, Y.; Liu, X.; Huo, P.; Dong, H.; Yuan, S.; Ma, Z.; Han, S. Improved recyclability and selectivity of environment-friendly mfa-based heterojunction imprinted photocatalyst for secondary pollution free tetracycline orientation degradation. Chem. Eng. J. 2019, 360, 1262–1276. [Google Scholar] [CrossRef]
- Pérez, T.A.; Gerónimo, E.S.; Torres, J.G.; Del Angel Montes, G.A.; Vázquez, I.R.; García, A.C.; Uribe, A.C.; Pavon, A.A.; Pérez, J.C. Photocatalytic Oxidation of Pesticides with TiO2-CeO2 Thin Films Using Sunlight. Catalysts 2025, 15, 46. [Google Scholar] [CrossRef]
- Morante, N.; De Guglielmo, L.; Oliva, N.; Monzillo, K.; Femia, N.; Di Capua, G.; Vaiano, V.; Sannino, D. Influence of UV-A Light Modulation on Phenol Mineralization by TiO2 Photocatalytic Process Coadjuvated with H2O2. Catalysts 2024, 14, 544. [Google Scholar] [CrossRef]
- Wu, C.; Maggay, I.V.; Chiang, C.; Chen, W.; Chang, Y.; Hu, C.; Venault, A. Removal of tetracycline by a photocatalytic membrane reactor with MIL-53(Fe)/PVDF mixed-matrix membrane. Chem. Eng. J. 2023, 451, 138990. [Google Scholar] [CrossRef]
- Lian, Z.; Wu, T.; Zhang, X.; Cai, S.; Xiong, Y.; Yang, R. Synergistic degradation of tetracycline from Mo2C/MoOx films mediated peroxymonosulfate activation and visible-light triggered photocatalysis. Chem. Eng. J. 2023, 469, 143774. [Google Scholar] [CrossRef]
- Xu, C.; Ye, S.; Cui, X.; Song, X.; Xie, X. Modelling photocatalytic detoxification of aflatoxin B1 in peanut oil on TiO2 layer in a closed-loop reactor. Biosyst. Eng. 2019, 180, 87–95. [Google Scholar] [CrossRef]
- Das, S.; Mahalingam, H. Novel immobilized ternary photocatalytic polymer film based airlift reactor for efficient degradation of complex phthalocyanine dye wastewater. J. Hazard. Mater. 2020, 383, 121219. [Google Scholar] [CrossRef]
- Deng, B.; Jiang, Y.; Gao, L.; Zhao, B. CFD modeling of ethylene degradation in gas-phase photocatalytic reactors. Environ. Sci. Pollut. Res. 2023, 30, 24132–24142. [Google Scholar] [CrossRef]
- Heris, S.Z.; Etemadi, M.; Mousavi, S.B.; Mohammadpourfard, M.; Ramavandi, B. Preparation and characterizations of TiO2/ZnO nanohybrid and its application in photocatalytic degradation of tetracycline in wastewater. J. Photochem. Photobiol. A Chem. 2023, 443, 114893. [Google Scholar]
- Lira, J.O.B.; Riella, H.G.; Padoin, N.; Soares, C. CFD + DoE optimization of a flat plate photocatalytic reactor applied to NOx abatement. Chem. Eng. Process.-Process Intensif. 2020, 154, 107998. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Sattar, M.A.; Jahirul, M.I. Phenol degradation of waste and stormwater on a flat plate photocatalytic reactor with TiO2 on glass slide: An experimental and modelling investigation. J. Water Process Eng. 2022, 47, 102769. [Google Scholar] [CrossRef]
- Khataee, A.; Farshchi, M.E.; Fathinia, M.; Aghdasinia, H. Photocatalytic ozonation process for degradation of an anthelmintic drug using ceramic coated TiO2 NPs: CFD simulation coupling with kinetic mechanisms. Process Saf. Environ. Prot. 2020, 141, 37–48. [Google Scholar] [CrossRef]
- Tong, K.; Yang, L.; Du, X.; Yang, Y. Review of modeling and simulation strategies for unstructured packing bed photoreactors with CFD method. Renew. Sustain. Energy Rev. 2020, 131, 109986. [Google Scholar] [CrossRef]
- Alarcón, A.; Busqué, R.; Andreu, T.; Guilera, J. Design of a Multi-Tubular Catalytic Reactor Assisted by CFD Based on Free-Convection Heat-Management for Decentralised Synthetic Methane Production. Catalysts 2022, 12, 1053. [Google Scholar] [CrossRef]
- Phuan, Y.W.; Ismail, H.M.; Garcia-Segura, S.; Chong, M.N. Design and CFD modelling of the anodic chamber of a continuous photofuelcell reactor for water treatment. Process Saf. Environ. Prot. 2017, 111, 449–461. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Keulemans, M.; van Walsem, J.; Tytgat, T.; Lenaerts, S.; Denys, S. CFD modeling of transient adsorption/desorption behavior in a gas phase photocatalytic fiber reactor. Chem. Eng. J. 2016, 292, 42–50. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Lenaerts, S.; Denys, S. Analytic versus CFD approach for kinetic modeling of gas phase photocatalysis. Chem. Eng. J. 2015, 262, 1–8. [Google Scholar] [CrossRef]
- Matiazzo, T.; Vilar, V.J.P.; Riella, H.G.; Padoin, N.; Soares, C. CFD and radiation field modeling of the NETmix milli-photocatalytic reactor for n-decane oxidation at gas phase: Effect of LEDs number and arrangement. Chem. Eng. J. 2022, 444, 136577. [Google Scholar] [CrossRef]
- van Walsem, J.; Verbruggen, S.W.; Modde, B.; Lenaerts, S.; Denys, S. CFD investigation of a multi-tube photocatalytic reactor in non-steady-state conditions. Chem. Eng. J. 2016, 304, 808–816. [Google Scholar] [CrossRef]
- Luo, M.; Zeng, F.; Jeong, T.; Wu, G.; Guan, Q. CFD Modeling of UV/H2O2 Process in Internal Airlift Circulating Photoreactor. Water 2020, 12, 3237. [Google Scholar] [CrossRef]
- Duran, J.E.; Mohseni, M.; Taghipour, F. Design improvement of immobilized photocatalytic reactors using a CFD-Taguchi combined method. Ind. Eng. Chem. Res. 2011, 50, 824–831. [Google Scholar] [CrossRef]
- Tedesco, G.C.; Moraes, P.B. Innovative design of a continuous flow photoelectrochemical reactor: Hydraulic design, CFD simulation and prototyping. J. Environ. Chem. Eng. 2021, 9, 105917. [Google Scholar] [CrossRef]
- Einaga, H.; Tokura, J.; Teraoka, Y.; Ito, K. Kinetic analysis of TiO2-catalyzed heterogeneous photocatalytic oxidation of ethylene using computational fluid dynamics. Chem. Eng. J. 2015, 263, 325–335. [Google Scholar] [CrossRef]
- Wang, X.; Tan, X.; Yu, T. Kinetic study of ozone photocatalytic decomposition using a thin film of TiO2 coated on a glass plate and the CFD modeling approach. Ind. Eng. Chem. Res. 2014, 53, 7902–7909. [Google Scholar] [CrossRef]
- Tong, K.; Yang, L.; Du, X. Modelling of TiO2-based packing bed photocatalytic reactor with Raschig rings for phenol degradation by coupled CFD and DEM. Chem. Eng. J. 2020, 400, 125988. [Google Scholar] [CrossRef]
- Balestrin, E.; Valle, R.D.C.S.; de Souza, S.M.A.G.; Valle, J.A.B.; Da Silva, A. Performance analysis of photocatalytic reactor with immobilized catalyst for emerging pollutants water treatment using CFD simulation and optimization method. Chem. Eng. Process.-Process Intensif. 2024, 205, 110016. [Google Scholar] [CrossRef]
- Pandey, P.; Mohanan, S.; Surenjan, A. Photocatalytic degradation of metformin on a rectangular baffled reactor: CFD modeling and validation investigation. Chem. Eng. Process.-Process Intensif. 2024, 202, 109833. [Google Scholar] [CrossRef]
- de OB Lira, J.; Padoin, N.; Vilar, V.J.P.; Soares, C. Photocatalytic NOx abatement: Mathematical modeling, CFD validation and reactor analysis. J. Hazard. Mater. 2019, 372, 145–153. [Google Scholar] [CrossRef]
- Zhu, K.; Ma, L.; Duan, J.; Fang, Z.; Yang, Z. Photocatalytic Degradation of Tetracycline Hydrochloride Using TiO2/CdS on Nickel Foam Under Visible Light and RSM–BBD Optimization. Catalysts 2025, 15, 113. [Google Scholar] [CrossRef]
- Li, C.; Lu, Z.; Ao, X.; Sun, W.; Huang, X. Degradation kinetics and removal efficiencies of pharmaceuticals by photocatalytic ceramic membranes using ultraviolet light-emitting diodes. Chem. Eng. J. 2022, 427, 130828. [Google Scholar] [CrossRef]
- Ma, L.; Duan, J.; Ji, B.; Liu, Y.; Li, C.; Li, C.; Zhao, W.; Yang, Z. Ligand-metal charge transfer mechanism enhances TiO2/Bi2WO6/rGO nanomaterials photocatalytic efficient degradation of norfloxacin under visible light. J. Alloys Compd. 2021, 869, 158679. [Google Scholar] [CrossRef]
- Muñoz, V.; Casado, C.; Suárez, S.; Sánchez, B.; Marugán, J. Photocatalytic NOx removal: Rigorous kinetic modelling and ISO standard reactor simulation. Catal. Today 2019, 326, 82–93. [Google Scholar] [CrossRef]
- Borrás-Jiménez, D.; Silva-López, W.; Nieto-Londoño, C. Towards the configuration of a photoelectrocatalytic reactor: Part 2—Selecting photoreactor flow configuration and operating variables by a numerical approach. Nanomaterials 2022, 12, 3030. [Google Scholar] [CrossRef] [PubMed]
- Rasul, M.G.; Ahmed, S.; Sattar, M.A.; Jahirul, M.I. Hydrodynamic performance assessment of photocatalytic reactor with baffles and roughness in the flow path: A modelling approach with experimental validation. Heliyon 2023, 9, e19623. [Google Scholar] [CrossRef] [PubMed]
- Kaisare, N.S.; Di Sarli, V. The Effect of Catalyst Placement on the Stability of a U-Bend Catalytic Heat-Recirculating Micro-Combustor: A Numerical Investigation. Catalysts 2021, 11, 1560. [Google Scholar] [CrossRef]
- Garretón, G.; Maxwell, L.; Cornejo, I. Transition of the flow regime inside of monolith microchannel reactors fed with highly turbulent flow. Catalysts 2023, 13, 938. [Google Scholar] [CrossRef]
- Whyte, H.E.; Subrenat, A.; Raillard, C.; Héquet, V. Understanding the influence of media geometry on the degradation of acrylonitrile: Experimental and CFD analysis. Chem. Eng. Sci. 2019, 209, 115217. [Google Scholar] [CrossRef]
- De Brito Lira, J.O.; Riella, H.G.; Padoin, N.; Soares, C. An Overview of Photoreactors and Computational Modeling for the Intensification of Photocatalytic Processes in the Gas-Phase: State-of-Art. J. Environ. Chem. Eng. 2021, 9, 105068. [Google Scholar] [CrossRef]
- Lira, J.O.B.; Riella, H.G.; Padoin, N.; Soares, C. Computational fluid dynamics (CFD), artificial neural network (ANN) and genetic algorithm (GA) as a hybrid method for the analysis and optimization of micro-photocatalytic reactors: NOx abatement as a case study. Chem. Eng. J. 2022, 431, 133771. [Google Scholar] [CrossRef]
- Gholami, D.; Shahbazi, S.; Mosleh, S.; Ghoorchian, A.; Hajati, S.; Dashtian, K.; Yasin, G. In situ growth of CuFeS2/CuS bridged heterojunction catalyst with mixed redox-couple cations for excellent photocatalytic degradation of organophosphate insecticide: CFD and DFT modeling. Chem. Eng. J. 2023, 461, 141950. [Google Scholar] [CrossRef]
- Gao, J.; Dong, P.; Tan, J.; Zhang, L.; Wang, C. Optimal design of novel honeycomb photocatalytic reactors for numerical analysis of formaldehyde degradation by CFD modeling. Res. Chem. Intermed. 2023, 49, 1683–1700. [Google Scholar] [CrossRef]






| Inlet Velocity (m/s) | Number of Meshes | Outlet Velocity (m/s) |
|---|---|---|
| 1 | 157,116 | 0.9683 |
| 1 | 363,343 | 0.9679 |
| 1 | 584,693 | 0.9675 |
| Configuration | S/V (m−1) | Degradation Rate (%) |
|---|---|---|
| a | 0.073 | 53.45 |
| b | 0.034 | 64.83 |
| c | 0.034 | 36.1 |
| d | 0.028 | 44.2 |
| e | 0.028 | 49.77 |
| f | 0.028 | 32.15 |
| g | 0.028 | 54.29 |
| h | 0.0268 | 44.69 |
| i | 0.0268 | 46.5 |
| j | 0.0268 | 53.76 |
| k | 0.0258 | 51.46 |
| l | 0.0258 | 49.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Ma, L.; Duan, J.; Zhu, K.; Zhang, X.; Yang, Z. Flow Field Structure Optimization and Inlet Parameters in Tubular Photocatalytic Reactors: A CFD-Based Study. Catalysts 2025, 15, 798. https://doi.org/10.3390/catal15090798
Fang Z, Ma L, Duan J, Zhu K, Zhang X, Yang Z. Flow Field Structure Optimization and Inlet Parameters in Tubular Photocatalytic Reactors: A CFD-Based Study. Catalysts. 2025; 15(9):798. https://doi.org/10.3390/catal15090798
Chicago/Turabian StyleFang, Zhiyong, Lizhe Ma, Jieli Duan, Kefu Zhu, Xiangshu Zhang, and Zhou Yang. 2025. "Flow Field Structure Optimization and Inlet Parameters in Tubular Photocatalytic Reactors: A CFD-Based Study" Catalysts 15, no. 9: 798. https://doi.org/10.3390/catal15090798
APA StyleFang, Z., Ma, L., Duan, J., Zhu, K., Zhang, X., & Yang, Z. (2025). Flow Field Structure Optimization and Inlet Parameters in Tubular Photocatalytic Reactors: A CFD-Based Study. Catalysts, 15(9), 798. https://doi.org/10.3390/catal15090798








