Boosting the Bifunctional Catalytic Activity of La0.85Y0.15Ni0.7Fe0.3O3 Perovskite Air Electrode with Facile Hybrid Strategy of Metallic Oxide for Rechargeable Zn–Air Batteries
Abstract
1. Introduction
2. Results and Discussion
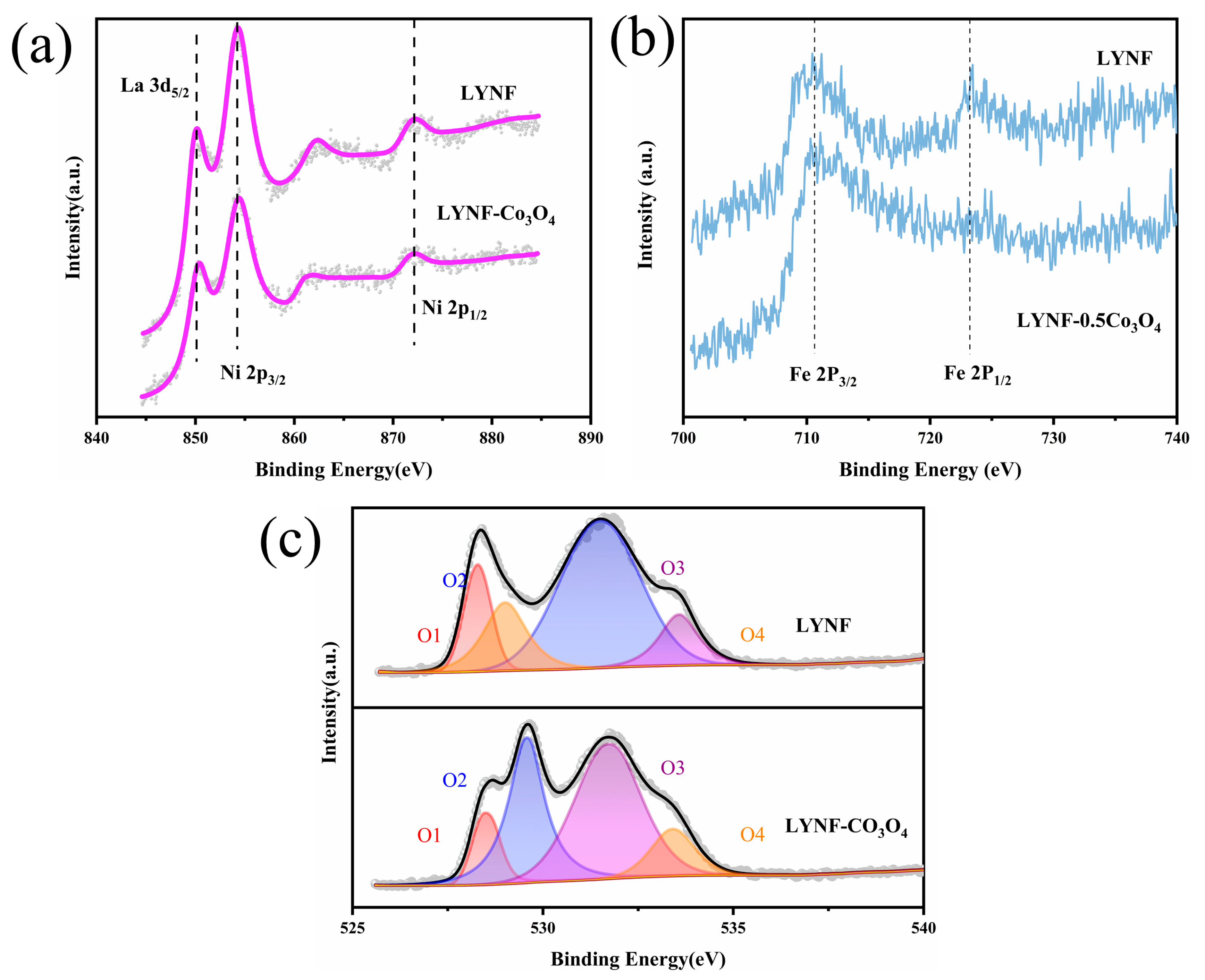
2.1. Material Structure and Analysis
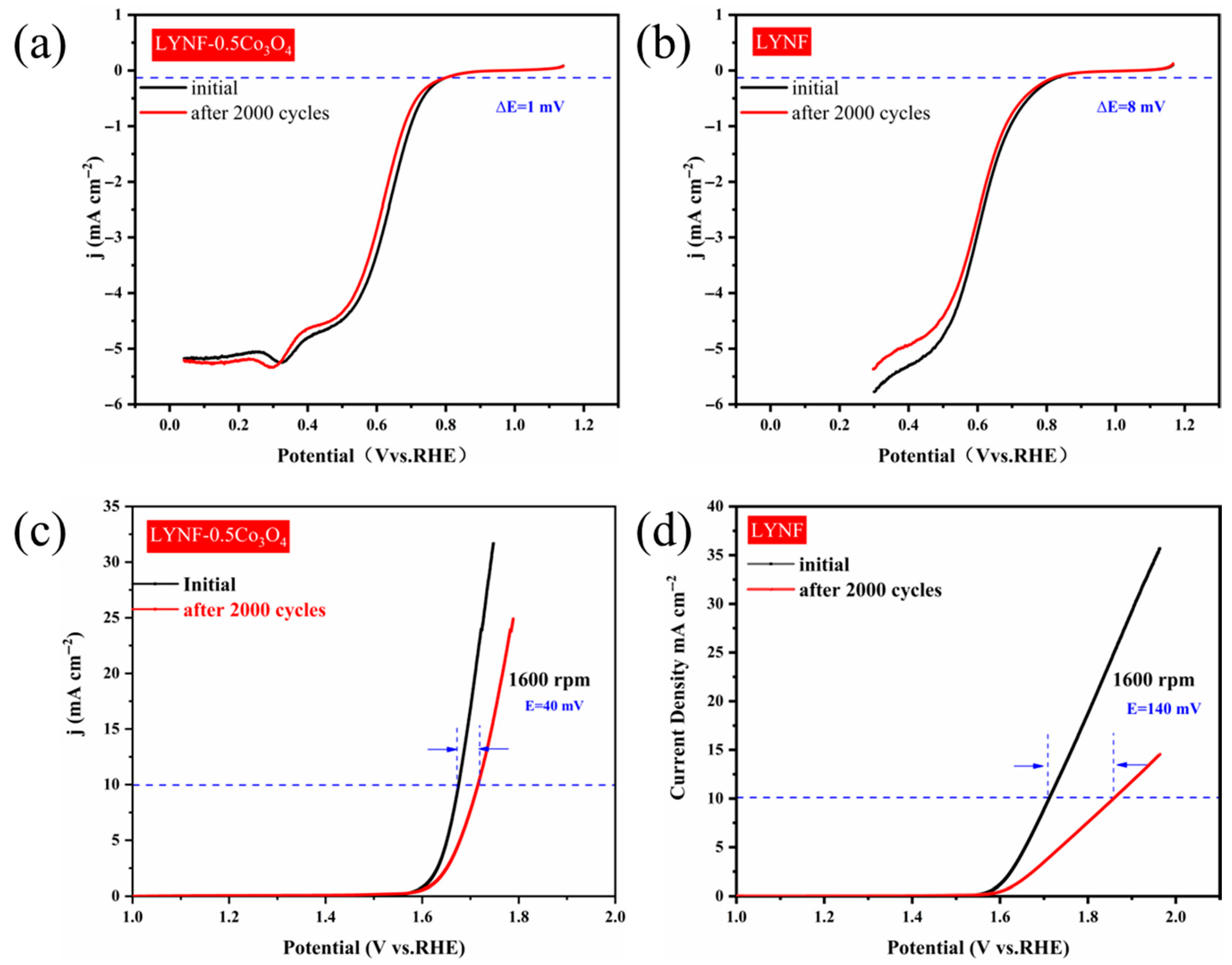
2.2. Electrochemical Performance
2.3. Zn–Air Battery Performance
3. Experimental Section
3.1. Synthesis of Samples
3.2. Characterization
3.3. Electrochemical Test
4. Challenges and Limitations
4.1. Contributions of LYNF-xCo3O4
4.2. Limitations of LYNF-xCo3O4
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhoyate, S.D.; Kim, J.; de Souza, F.M.; Lin, J.; Lee, E.; Kumar, A.; Gupta, R.K. Science and engineering for non-noble-metal-based electrocatalysts to boost their ORR performance: A critical review. Coord. Chem. Rev. 2023, 474, 214854. [Google Scholar] [CrossRef]
- Wang, J.; Gao, S.; Hu, S. Zinc-air battery-H2O2 generation system: Current progress, key challenges, optimization strategies and future developments. Chin. Chem. Lett. 2025, 111000, in press. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, J.; Chen, Y.; Liu, X.; Zhang, M.; Yang, M.; Liu, M.; Wu, J.; Li, S.; Huo, F. Leather waste as precursor to prepare bifunctional catalyst for alkaline and neutral zinc-air batteries. Chin. Chem. Lett. 2023, 34, 107756. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, Q.; Huang, Z.; Xiao, Y.; Wan, Y.; Li, S.; Lee, C.-S. Water-Splitting Based and Related Therapeutic Effects: Evolving Concepts, Progress, and Perspectives. Small 2020, 16, 2004551. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, T.; Miao, S.; Cho, Y. Emerging Energy Harvesting Technology for Electro/Photo-Catalytic Water Splitting Application. Catalysts 2021, 11, 142. [Google Scholar] [CrossRef]
- Akram, S.; Hussain, S.; Arif, M.; Ali, M.H.; Tariq, M.; Rauf, A.; Munawar, K.S.; Alkahtani, H.M.; Alhaj Zen, A.; Ali Shah, S.A. Psidium guajava mediated green synthesized cobalt oxide nanoparticles dispersed on reduced graphene oxide for electrocatalytic water splitting. RSC Adv. 2025, 15, 13786–13798. [Google Scholar] [CrossRef]
- Chen, R.; Hung, S.-F.; Zhou, D.; Gao, J.; Yang, C.; Tao, H.; Yang, H.B.; Zhang, L.; Zhang, L.; Xiong, Q.; et al. Layered Structure Causes Bulk NiFe Layered Double Hydroxide Unstable in Alkaline Oxygen Evolution Reaction. Adv. Mater. 2019, 31, e1903909. [Google Scholar] [CrossRef]
- Tan, D.; Xiong, H.; Zhang, T.; Fan, X.; Wang, J.; Xu, F. Recent progress in noble-metal-free electrocatalysts for alkaline oxygen evolution reaction. Front. Chem. 2022, 10, 1071274. [Google Scholar] [CrossRef]
- Hou, A.; Ouyang, R. Origin of the Different Trends of Experimental Activity on Perovskite Catalysts between OER and ORR. ACS Appl. Energy Mater. 2025, 8, 3481–3490. [Google Scholar] [CrossRef]
- Ren, X.; Zhai, Y.; Yang, N.; Wang, B.; Liu, S. Lattice Oxygen Redox Dynamics in Zeolite-Encapsulated CsPbBr3 Perovskite OER Electrocatalysts. Adv. Sci. 2025, 12, e2412679. [Google Scholar] [CrossRef]
- Du, X.; Ai, H.; Chen, M.; Liu, D.; Chen, S.; Wang, X.; Lo, K.H.; Pan, H. PLD-fabricated perovskite oxide nanofilm as efficient electrocatalyst with highly enhanced water oxidation performance. Appl. Catal. B Environ. 2020, 272, 119046. [Google Scholar] [CrossRef]
- Li, S.-F.; Zheng, J.; Hu, L.; Ma, Y.; Yan, D. Facile surface defect engineering on perovskite oxides for enhanced OER performance. Dalton Trans. 2023, 52, 4207–4213. [Google Scholar] [CrossRef]
- Pavlovska, O.B.; Vasylechko, L.O.; Lutsyuk, I.V.; Koval, N.M.; Zhydachevskii, Y.A.; Pieniazek, A. Structure Peculiarities of Micro- and Nanocrystalline Perovskite Ferrites La1−xSmxFeO3. Nanoscale Res. Lett. 2017, 12, 153. [Google Scholar] [CrossRef]
- Guo, B.-R.; Chen, M.-X.; Li, S.-W.; Gao, R.-H.; Sang, B.-H.; Ren, X.-Q.; Liu, Z.; Cao, X.; Liu, J.; Ding, Y.-N.; et al. Construction of iron oxyhydroxide/nickel sulfate hydroxide hybrid electrocatalyst for efficient oxygen evolution. Rare Met. 2024, 43, 6394–6404. [Google Scholar] [CrossRef]
- Qiao, Y.; Pan, Y.; Fan, W.; Long, G.; Zhang, F. Polyoxometalate-incorporated NiFe-based oxyhydroxides for enhanced oxygen evolution reaction in alkaline media. Chem. Commun. 2024, 60, 11287–11290. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Islam, M.R.; Islam, M.R. Improved Electrochemical Performance of Co3O4 Incorporated MnO2 Nanowires for Energy Storage Applications. Arab. J. Sci. Eng. 2025, 50, 583–596. [Google Scholar] [CrossRef]
- Ahmed, S.; Lee, M.-C.; Rim, H.-R.; Lee, H.-K.; Shim, J.; Park, G. Highly porous Co3O4 and Ni-deposited Co3O4 nanoflowers as bifunctional catalysts for oxygen reduction and evolution reactions. Inorg. Chem. Commun. 2020, 113, 107799. [Google Scholar] [CrossRef]
- Quan, W.; Chen, T.; Sun, S.; Sun, C.; Wu, C. Facile hybrid strategy of SrCo0.5Fe0.3Mo0.2O3-dCo3O4 heterostructure for efficient oxygen evolution reaction. J. Alloys Compd. 2022, 917, 165508. [Google Scholar] [CrossRef]
- Zhang, C.; Antonietti, M.; Fellinger, T.-P. Blood Ties: Co3O4 Decorated Blood Derived Carbon as a Superior Bifunctional Electrocatalyst. Adv. Funct. Mater. 2014, 24, 7655–7665. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, H.; Qian, B.; Wang, S.; Zheng, Y.; Ge, L.; Chen, H. Y and Fe co-doped LaNiO3 perovskite as a novel bifunctional electrocatalyst for rechargeable zinc-air batteries. Int. J. Hydrogen Energy 2023, 48, 8082–8092. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, W.; Chen, Y.; Xu, Z.; Cai, D.; Xu, M.; Tong, R. Cobalt-containing ZIF-derived catalysts for Zn-air batteries. Mater. Chem. Front. 2024, 8, 2394–2419. [Google Scholar] [CrossRef]
- Shang, Z.; Zhang, H.; Liang, J.; You, Z.; Wang, R.; Wan, L.; Lei, D.; Li, Z. Highly adhesive hydrogel electrolytes driven by adenosine monophosphate and Fe3+ for high-voltage asymmetric flexible zinc-air batteries. Energy Storage Mater. 2024, 71, 103599. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Li, T.; Tian, X.; Zhang, K.; Miao, Y.; Xia, C.; Cai, L.; Hui, B.; Chen, C. Defective wood-based chainmail electrocatalysts boost performances of seawater-medium Zn-air batteries. J. Energy Chem. 2025, 102, 134–143. [Google Scholar] [CrossRef]
- Liu, T.; Dong, X.; Tang, B.; Zhao, R.; Xu, J.; Li, H.; Gao, S.; Fang, Y.; Chao, D.; Zhou, Z. Engineering electrolyte additives for stable zinc-based aqueous batteries: Insights and prospects. J. Energy Chem. 2024, 98, 311–326. [Google Scholar] [CrossRef]
- Harisha, B.S.; Akkinepally, B.; Shim, J.; Lim, J. NiO.Co3O4@TiO2: Multimetallic nanocomposite electrode for supercapacitors: Experimental evaluation and simulation insights. Chem. Eng. J. 2025, 508, 160207. [Google Scholar] [CrossRef]
- Yu, H.; Qin, S.; Qi, X.; Wang, K.; Li, Y.; Du, Y.; Feng, X.; Shan, W.; Xiong, Y. P123-assisted synthesis of Co3O4 catalyst for efficiently catalyzing N2O decomposition under simulated real tail-gases. Mol. Catal. 2024, 565, 114378. [Google Scholar] [CrossRef]
- Zheng, F.; Lu, J.; Liu, C.; Zheng, H.; Xu, Q. Synergistic engineering of oxygen vacancies, cation vacancies, and surface acidity in MOFs-derived Co-Mn spinel oxides via acid etching: A pathway to enhanced toluene oxidation performance. J. Hazard. Mater. 2025, 492, 138239. [Google Scholar] [CrossRef]
- Que, L.; Lu, L.; Xu, Y.; Xu, X.; Zhu, M.; Pan, J.; Cao, J.; Wang, J.; Zheng, Y.; Li, C. The Ni2+-LaNiO3/CdS hollow core-shell heterojunction towards enhanced visible light overall water splitting H2 evolution via HER/OER synergism of Ni2+/Ov. Chem. Eng. J. 2023, 469, 143902. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, C.-R.; Ding, T.-Y.; Gu, J.; Yan, J.-W.; Cheng, J.; Zhang, K.H.L. Direct observation of the dynamic reconstructed active phase of perovskite LaNiO3 for the oxygen-evolution reaction. Chem. Sci. 2023, 14, 5906–5911. [Google Scholar] [CrossRef]
- Zhu, M.; Sheng, Z.; Gao, J.; Li, Y.; Zhang, C.; Zhu, X. Synthesis of porous LaNiO3 thin films by chemical solution deposition for enhanced oxygen evolution reaction. Dalton Trans. 2023, 52, 9903–9907. [Google Scholar] [CrossRef]
- Kuchipudi, A.; Madhu, R.; Arunmuthukumar, P.; Sundarravalli, S.; Sreedhar, G.; Kundu, S. Decoration of Au Nanoparticles over LaFeO3: A High Performance Electrocatalyst for Total Water Splitting. Inorg. Chem. 2023, 62, 14448–14458. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Liu, M.; Qi, J.; Hu, L.; Feng, M.; Lu, W. The modulated oxygen evolution reaction performance of LaFeO3 with abundant electronic structures via a design of stoichiometry offset. Phys. Chem. Chem. Phys. 2023, 25, 11725–11731. [Google Scholar] [CrossRef]
- Yu, J.; Yang, G.; Li, Z.; Zhu, W.; Jiang, S.; Chen, D.; Shao, Z.; Ni, M. Boosting the lattice oxygen reactivity of perovskite electrocatalyst via less Ru substitution. Int. J. Hydrogen Energy 2024, 84, 650–657. [Google Scholar] [CrossRef]
- Inoue, Y.; Miyahara, Y.; Miyazaki, K.; Lee, C.; Sakamoto, R.; Abe, T. Synergistic Interplay between Fe-Based Perovskite Oxides and Co in Electrolyte for Efficient Oxygen Evolution Reaction. Chemsuschem 2025, 18, e202401982. [Google Scholar] [CrossRef]
- Chen, J.; Huang, J.; Wang, H.; Feng, W.; Luo, T.; Hu, Y.; Yuan, C.; Cao, L.; Jie, Y.; Kajiyoshi, K.; et al. Phase-mediated cobalt phosphide with unique core-shell architecture serving as efficient and bifunctional electrocatalyst for hydrogen evolution and oxygen reduction reaction. Chin. Chem. Lett. 2022, 33, 3752–3756. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, K.; Yang, B. Architecting double-shelled hollow carbon nanocages embedded bimetallic sites as bifunctional oxygen electrocatalyst for zinc-air batteries. Chin. Chem. Lett. 2025, 36, 110538. [Google Scholar] [CrossRef]
- Huang, K.; Hu, J.; Cao, J.; Wei, X.; Liu, S.; Dai, Q.; Shen, F.; Zhang, X.; Zhao, X.; Peng, Y.; et al. Biomass-templated strategy to synthesize Fe2P/Co2P heterojunction bifunctional electrocatalyst for high performance flexible zinc-air batteries. Sci. China Chem. 2025, 68, 3056–3063. [Google Scholar] [CrossRef]
- Saha, P.; Shah, S.S.; Ali, M.; Shaikh, M.N.; Aziz, M.A.; Saleh Ahammad, A.J. Cobalt Oxide-Based Electrocatalysts with Bifunctionality for High-Performing Rechargeable Zinc-Air Batteries. Chem. Rec. 2024, 24, e202300216. [Google Scholar] [CrossRef]
- Liang, Q.; Brocks, G.; Bieberle-Hutter, A. Oxygen evolution reaction (OER) mechanism under alkaline and acidic conditions. J. Phys. Energy 2021, 3, 026001. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, W.; Zhang, F.-M.; Yang, Z.-D.; Zhang, G.; Zeng, X.C. COF-C4N Nanosheets with uniformly anchored single metal sites for electrocatalytic OER: From theoretical screening to target synthesis. Appl. Catal. B Environ. Energy 2023, 325, 122366. [Google Scholar] [CrossRef]
- Jiang, T.; Jiang, H.; Wang, W.; Mu, H.; Zhang, Y.; Li, B. Atomically Dispersed High-Active Site Density Copper Electrocatalyst for the Reduction of Oxygen. Materials 2024, 17, 5030. [Google Scholar] [CrossRef]
- Yoo, D.; Park, S.; Oh, S.; Kim, M.P.; Park, K. In Situ Analysis of Binder Degradation during Catalyst-Accelerated Stress Test of Polymer Electrolyte Membrane Fuel Cells. Materials 2024, 17, 4425. [Google Scholar] [CrossRef]
- Han, Y.; Jin, K.; Zhao, T.; Yu, Y. Recent advances in solid-state zinc-air and zinc-ion batteries. Chin. Chem. Lett. 2025, 111387, in press. [Google Scholar] [CrossRef]
- Zhang, D.; Song, Y.; Du, Z.; Wang, L.; Li, Y.; Goodenough, J.B. Active LaNi1−xFexO3 bifunctional catalysts for air cathodes in alkaline media. J. Mater. Chem. A 2015, 3, 9421–9426. [Google Scholar] [CrossRef]
- Gong, C.; Zhao, L.; Li, S.; Wang, H.; Gong, Y.; Wang, R.; He, B. Atomic layered deposition iron oxide on perovskite LaNiO3 as an efficient and robust bi-functional catalyst for lithium oxygen batteries. Electrochim. Acta 2018, 281, 338–347. [Google Scholar] [CrossRef]
- Chandrappa, S.G.; Moni, P.; Chen, D.; Karkera, G.; Prakasha, K.R.; Caruso, R.A.; Prakash, A.S. The influence of ruthenium substitution in LaCoO3 towards bi-functional electrocatalytic activity for rechargeable Zn-air batteries. J. Mater. Chem. A 2020, 8, 20612–20620. [Google Scholar] [CrossRef]
- Zheng, Q.L.; Zhang, Y.D.; Wang, C.C.; Zhang, C.H.; Guo, Y.M. CoO Enhanced Oxygen Evolution Kinetics of LaMnO3 Perovskite As a Potential Cathode for Rechargeable Zn-Air Batteries. Energy Fuels 2022, 36, 1091–1099. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, X.; Zhuang, G.; Bai, J.; Yan, J.; Zheng, Y. Boosting the Bifunctional Catalytic Activity of La0.85Y0.15Ni0.7Fe0.3O3 Perovskite Air Electrode with Facile Hybrid Strategy of Metallic Oxide for Rechargeable Zn–Air Batteries. Catalysts 2025, 15, 785. https://doi.org/10.3390/catal15080785
Yi X, Zhuang G, Bai J, Yan J, Zheng Y. Boosting the Bifunctional Catalytic Activity of La0.85Y0.15Ni0.7Fe0.3O3 Perovskite Air Electrode with Facile Hybrid Strategy of Metallic Oxide for Rechargeable Zn–Air Batteries. Catalysts. 2025; 15(8):785. https://doi.org/10.3390/catal15080785
Chicago/Turabian StyleYi, Xiankai, Guangwei Zhuang, Junhua Bai, Jiaxing Yan, and Yifeng Zheng. 2025. "Boosting the Bifunctional Catalytic Activity of La0.85Y0.15Ni0.7Fe0.3O3 Perovskite Air Electrode with Facile Hybrid Strategy of Metallic Oxide for Rechargeable Zn–Air Batteries" Catalysts 15, no. 8: 785. https://doi.org/10.3390/catal15080785
APA StyleYi, X., Zhuang, G., Bai, J., Yan, J., & Zheng, Y. (2025). Boosting the Bifunctional Catalytic Activity of La0.85Y0.15Ni0.7Fe0.3O3 Perovskite Air Electrode with Facile Hybrid Strategy of Metallic Oxide for Rechargeable Zn–Air Batteries. Catalysts, 15(8), 785. https://doi.org/10.3390/catal15080785






