Synergistic Catalysis for Algae Control: Integrating Sonocavitation and Chemical Catalysis
Abstract
1. Introduction
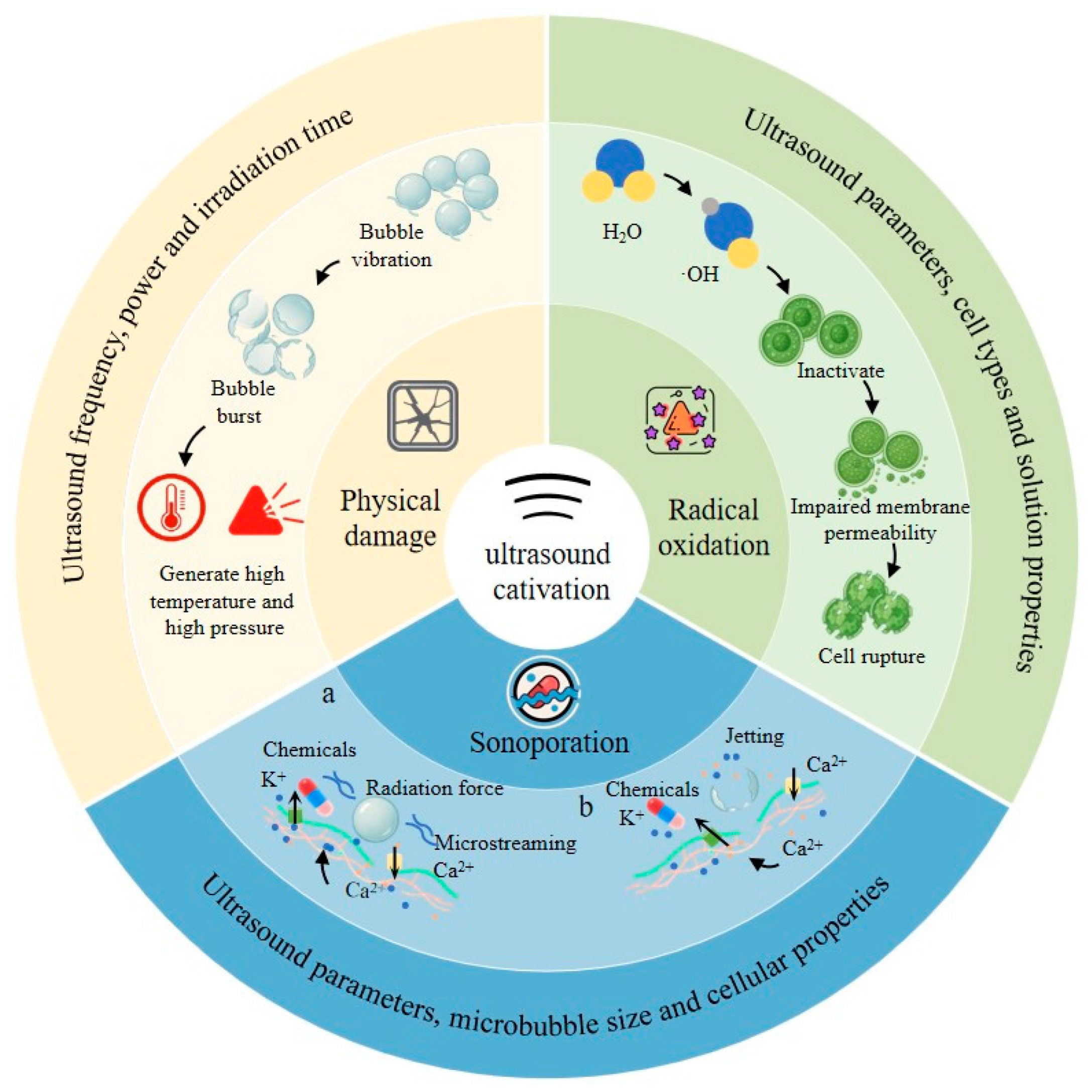
2. Research Progress in Ultrasonic Bioeffects
2.1. Physical Damage
2.2. Free Radical Oxidation
2.3. Sonoporation
3. Algae Removal by Ultrasound Synergized with Catalysts
3.1. Carbon-Based Catalyst Systems
3.2. Iron-Based Catalyst Systems
3.3. TiO2-Based Catalytic Systems
4. Ultrasound-Assisted Oxidant Catalysis for Algae Removal
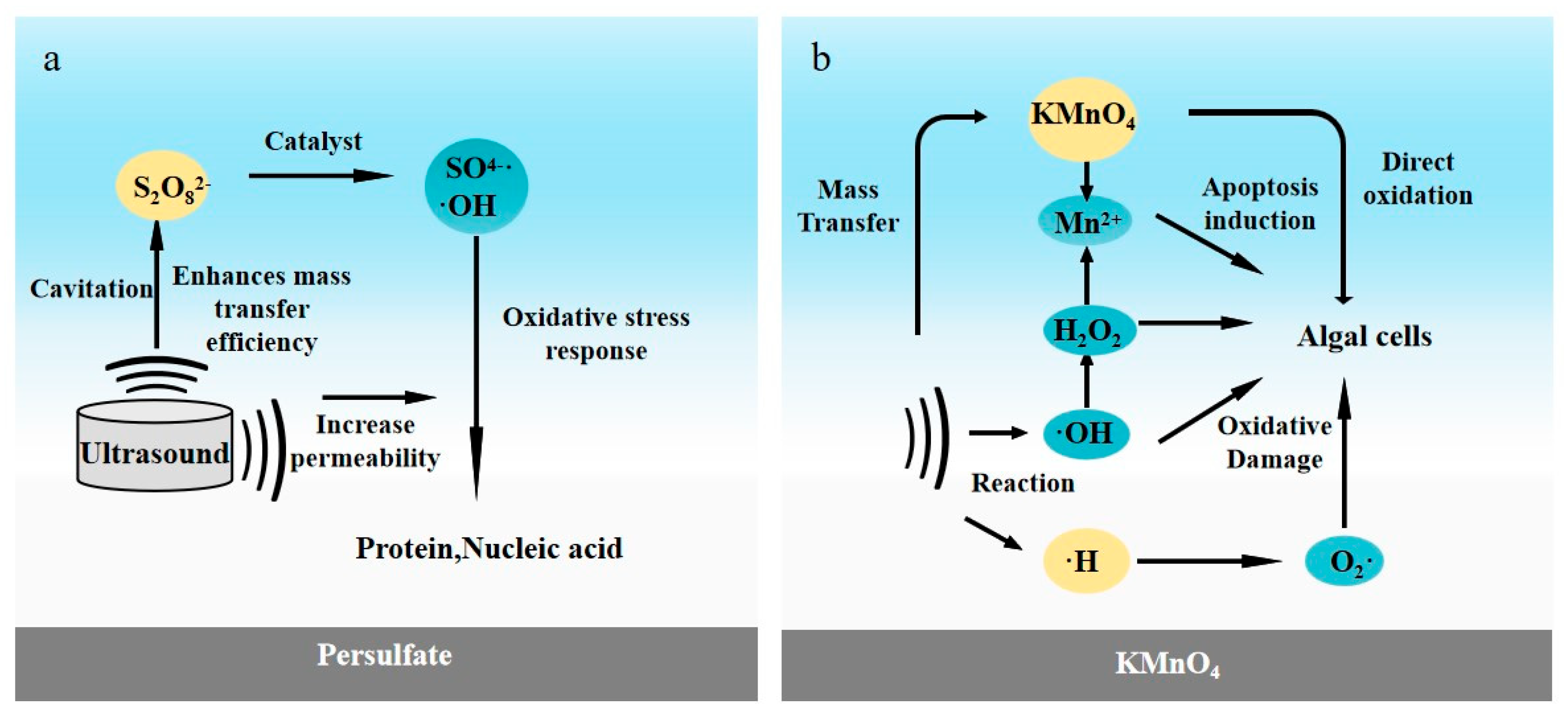
4.1. Potassium Permanganate
4.2. Persulfate
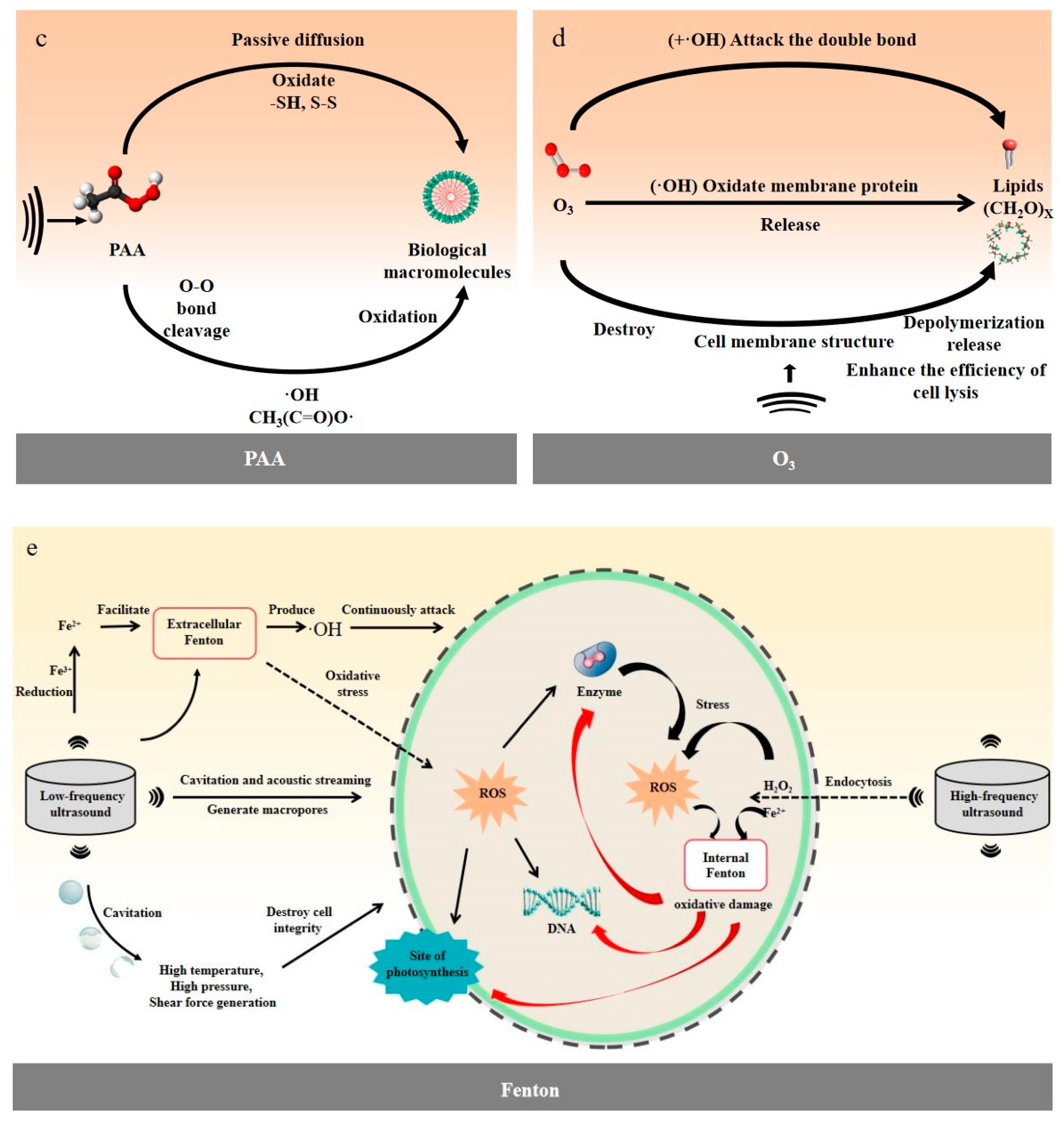
4.3. Ozone
4.4. Peroxyacetic Acid
4.5. Hydrogen Peroxide and Fenton’s Reagent
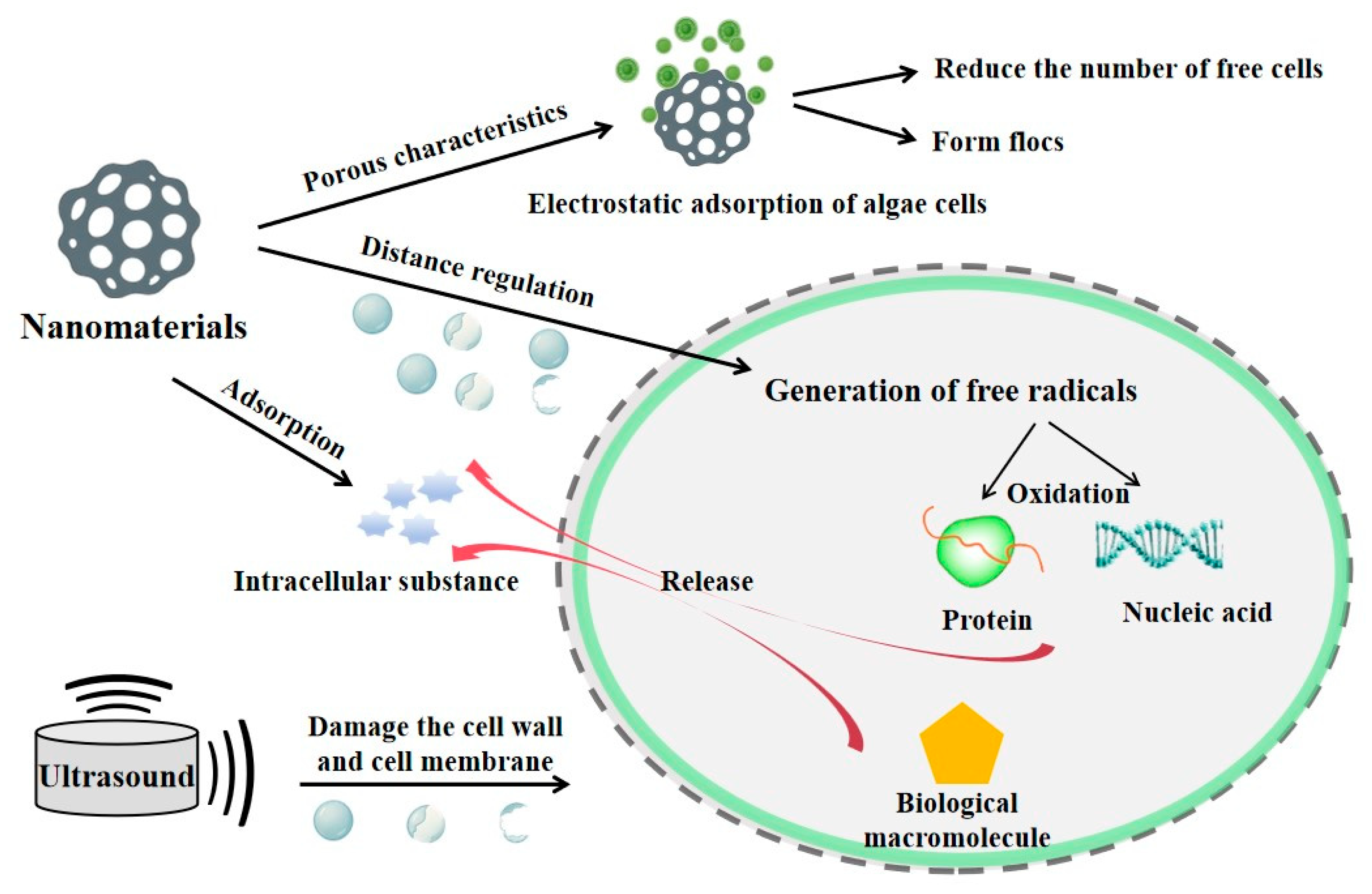
5. Ultrasound-Assisted Nanomaterial Catalysis for Algae Removal
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- Wu, M.R.; Zhang, C.; Xie, X.Q.; Feng, H.J.; Ho, G.W.; Xu, Y.F. Sustainable microalgae extraction for proactive water bloom prevention. Nat. Water 2024, 2, 172–182. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Ren, B.X.; Weitzel, K.A.; Duan, X.D.; Nadagouda, M.N.; Dionysiou, D.D. A comprehensive review on algae removal and control by coagulation-based processes: Mechanism, material, and application. Sep. Purif. Technol. 2022, 293, 121106. [Google Scholar] [CrossRef]
- Xie, P.C.; Chen, Y.Q.; Ma, J.; Zhang, X.; Zou, J.; Wang, Z.P. A mini review of preoxidation to improve coagulation. Chemosphere 2016, 155, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.L.; Hua, L.C.; Hung, S.K.; Huang, C. algae removal from cyanobacteria-rich waters by preoxidation-assisted coagulation-flotation: Effect of algogenic organic matter release on algae removal and trihalomethane formation. J. Environ. Sci. 2018, 63, 147–155. [Google Scholar] [CrossRef]
- Qi, J.; Ma, B.W.; Miao, S.Y.; Liu, R.P.; Hu, C.Z.; Qu, J.H. Pre-oxidation enhanced cyanobacteria removal in drinking water treatment: A review. J. Environ. Sci. 2021, 110, 160–168. [Google Scholar] [CrossRef]
- Wang, J.L.; Tang, J.T. Fe-based Fenton-like catalysts for water treatment: Preparation, characterization and modification. Chemosphere 2021, 276, 130177. [Google Scholar] [CrossRef]
- Bao, J.F.; Guo, S.S.; Fan, D.D.; Cheng, J.L.; Zhang, Y.; Pang, X. Sonoactivated nanomaterials: A potent armament for wastewater treatment. Ultrason. Sonochem. 2023, 99, 106569. [Google Scholar] [CrossRef]
- Dan, H.B.; Han, S.L.; Gao, Y.; Gao, B.Y.; Yue, Q.Y. Sono-enhanced heterogeneous Fenton catalysis: Magnetic halloysite nanotube synthesis and accelerated free radical generation. Environ. Sci. Pollut. Res. 2023, 30, 90799–90813. [Google Scholar] [CrossRef]
- Kozmus, G.; Zevnik, J.; Hočevar, M.; Dular, M.; Petkovšek, M. Characterization of cavitation under ultrasonic horn tip—Proposition of an acoustic cavitation parameter. Ultrason. Sonochem. 2022, 89, 106159. [Google Scholar] [CrossRef]
- Feng, H.R.; Wang, J.A.; Wang, L.; Jin, J.M.; Wu, S.W.; Zhou, C.C. Study on a novel omnidirectional ultrasonic cavitation removal system for Microcystis aeruginosa. Ultrason. Sonochem. 2022, 86, 106008. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Liu, Y.; Umar, A.; Ma, H.; Wang, H. Ultrasonic cavitation: Tackling organic pollutants in wastewater. Chemosphere 2024, 350, 141024. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.R.; Li, H.Z.; Wei, X.M.; Wang, D.H.; Liu, Y.T.; Li, L. The effect of low frequency ultrasonic treatment on the release of extracellular organic matter of Microcystis aeruginosa. Chem. Eng. J. 2020, 383, 123141. [Google Scholar] [CrossRef]
- Huang, Y.R.; Ding, S.K.; Li, L.; Liao, Q.Y.; Chu, W.H.; Li, H.Z. Ultrasound-enhanced coagulation for Microcystis aeruginosa removal and disinfection by-product control during subsequent chlorination. Water Res. 2021, 201, 117334. [Google Scholar] [CrossRef]
- Huang, Y.R.; Li, L.; Luan, X.M.; Wei, X.M.; Li, H.Z.; Gao, N.Y.; Yao, J.J. Ultrasound-enhanced coagulation for cyanobacterial removal: Effects of ultrasound frequency and energy density on coagulation performance, leakage of intracellular organic matters and toxicity. Water Res. 2021, 201, 117348. [Google Scholar] [CrossRef]
- Kong, Y.; Peng, Y.Z.; Zhang, Z.; Zhang, M.; Zhou, Y.H.; Duan, Z. Removal of Microcystis aeruginosa by ultrasound: Inactivation mechanism and release of algae organic matter. Ultrason. Sonochem. 2019, 56, 447–457. [Google Scholar] [CrossRef]
- Li, Y.T.; Shi, X.D.; Zhang, Z.; Peng, Y.Z. Enhanced coagulation by high-frequency ultrasound in Microcystis aeruginosa-laden water: Strategies and mechanisms. Ultrason. Sonochem. 2019, 55, 232–242. [Google Scholar] [CrossRef]
- Maršálek, B.; Zezulka, Š.; Maršálková, E.; Pochylý, F.; Rudolf, P. Synergistic effects of trace concentrations of hydrogen peroxide used in a novel hydrodynamic cavitation device allows for selective removal of cyanobacteria. Chem. Eng. J. 2020, 382, 122383. [Google Scholar] [CrossRef]
- Wu, X.G.; Liu, J.L.; Zhu, J.J. Sono-Fenton hybrid process on the inactivation of Microcystis aeruginosa: Extracellular and intracellular oxidation. Ultrason. Sonochem. 2019, 53, 68–76. [Google Scholar] [CrossRef]
- Zhu, T.T.; Liu, B. Mechanism study on the effect of peracetic acid (PAA), UV/PAA and ultrasonic/PAA oxidation on ultrafiltration performance during algae-laden water treatment. Water Res. 2022, 220, 118705. [Google Scholar] [CrossRef]
- Wang, A.; Xu, H.; Chen, C.G.; Chen, L.; Lin, T.; Ma, J.; Ding, M.M. Critical review on advances and perspectives of ultrasound assisted membrane technologies for water purification. Chem. Eng. J. 2024, 482, 148873. [Google Scholar] [CrossRef]
- Pandur, Ž.; Zevnik, J.; Podbevšek, D.; Stojković, B.; Stopar, D.; Dular, M. Water treatment by cavitation: Understanding it at a single bubble-bacterial cell level. Water Res. 2023, 236, 119956. [Google Scholar] [CrossRef] [PubMed]
- Pandur, Ž.; Dular, M.; Kostanjšek, R.; Stopar, D. Bacterial cell wall material properties determine E. coli resistance to sonolysis. Ultrason. Sonochem. 2022, 83, 105919. [Google Scholar] [CrossRef] [PubMed]
- Petkovšek, M.; Hočevar, M.; Dular, M. Visualization and measurements of shock waves in cavitating flow. Exp. Therm. Fluid Sci. 2020, 119, 110215. [Google Scholar] [CrossRef]
- Liao, X.; Li, J.; Suo, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Multiple action sites of ultrasound on Escherichia coli and Staphylococcus aureus. Food Sci. Hum. Wellness 2018, 7, 102–109. [Google Scholar] [CrossRef]
- Wu, X.G.; Shen, T.T.; Liu, X.Y.; Zhang, G.M.; Qian, X.Q.; Yang, W.L. Unveiling the mechanisms of ultrasonic radiation-induced free radical stress on algae communities: Insights into growth inhibition, photosynthetic disruption, and antioxidant defense responses. Ultrason. Sonochem. 2025, 115, 107297. [Google Scholar] [CrossRef]
- Popova, S.; Tsenter, I.; Garkusheva, N.; Beck, S.E.; Matafonova, G.; Batoev, V. Evaluating (sono)-photo-Fenton-like processes with high-frequency ultrasound and UVA LEDs for degradation of organic micropollutants and inactivation of bacteria separately and simultaneously. J. Environ. Chem. Eng. 2021, 9, 105249. [Google Scholar] [CrossRef]
- Wijesiri, N.H. Development of Nanoparticle-Based Hybrid Sensitizers for Therapeutic Applications. Ph.D. Thesis, University of Cincinnati, Cincinnati, OH, USA, 2020. [Google Scholar]
- Nöpel, J.A.; Ayela, F. Experimental evidences of radicals production by hydrodynamic cavitation: A short review. Comptes Rendus Chim. 2023, 26, 157–166. [Google Scholar] [CrossRef]
- Babiak, W.; Krzeminska, I. Extracellular Polymeric Substances (EPS) as microalgae bioproducts: A review of factors affecting EPS synthesis and application in flocculation processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Goltermann, L.; Shahryari, S.; Rybtke, M.; Nielsen, T.T. Microbial Primer: The catalytic biofilm matrix. Microbiology 2024, 170, 001497. [Google Scholar] [CrossRef]
- Tu, J.; Yu, A.C.H. Ultrasound-Mediated Drug Delivery: Sonoporation Mechanisms, Biophysics, and Critical Factors. Bme Front. 2022, 2022, 9807347. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.R.; Guo, K.H.; Kang, X.W.; Zhang, J.S.; Li, C.H.; Fang, J.Y. Complete removal of organoarsenic by the UV/Permanganate process via HO• Oxidation and in situ-formed manganese dioxide adsorption. ACS EST Eng. 2021, 1, 794–803. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Ye, J.X.; Li, Z.Y.; Chen, H.J.; Gao, Y. Recent progress in sono-photodynamic cancer therapy: From developed new sensitizers to nanotechnology-based efficacy-enhancing strategies. Acta Pharm. Sin. B 2021, 11, 2197–2219. [Google Scholar] [CrossRef] [PubMed]
- Maciulevicius, M.; Tamosiunas, M.; Navickaite, D.; Satkauskas, S.; Venslauskas, M.S. Free- and liposomal- doxorubicin delivery via microbubble inertial cavitation. J. Drug Deliv. Sci. Technol. 2022, 72, 103386. [Google Scholar] [CrossRef]
- Wu, X.G.; Babu, A.G.; Kim, B.L.; Kim, J.O.; Shin, J.H.; Kim, D.P. Ultrasound-mediated intracellular delivery of fluorescent dyes and DNA into microalgal cells. Algal Res.-Biomass Biofuels Bioprod. 2016, 15, 210–216. [Google Scholar] [CrossRef]
- Shi, C.C.; Fang, W.J.; Ma, M.R.; Xu, W.; Ye, J.J. Changes in Extracellular Microcystins (MCs) Accompanying Algae/Cyanobacteria Removal during Three Representative Algae/Cyanobacteria Inactivation Processes and an MC Diffusion Model in Still Water. Water 2023, 15, 3591. [Google Scholar] [CrossRef]
- Peng, Y.Z.; Xiao, X.; Ren, B.Z.; Zhang, Z.; Luo, J.; Yang, X.Z.; Zhu, G.C. Biological activity and molecular mechanism of inactivation of Microcystis aeruginosa by ultrasound irradiation. J. Hazard. Mater. 2024, 468, 133742. [Google Scholar] [CrossRef]
- Li, J.P.; Long, H.; Song, C.; Wu, W.; Yeabah, T.O.; Qiu, Y.J. Study on the removal of algae from lake water and its attendant water quality changes using ultrasound. Desalination Water Treat. 2014, 52, 4762–4771. [Google Scholar] [CrossRef]
- Fan, G.D.; Liu, D.M.; Zhu, G.C.; Lin, Q.; Chen, L.R. Influence factors in kinetics during removal of harmful algae by ultrasonic irradiation process. Desalination Water Treat. 2014, 52, 7317–7322. [Google Scholar] [CrossRef]
- Wei, P.Y.; Tang, M.X.; Wang, Y.; Hu, B.W.; Qu, X.L.; Wang, Y.F.; Gao, G.D. Low-frequency ultrasound assisted contact-electro-catalysis for efficient inactivation of Microcystis aeruginosa. J. Hazard. Mater. 2024, 478, 135537. [Google Scholar] [CrossRef]
- Ninomiya, K.; Ogino, C.; Kawabata, S.; Kitamura, K.; Maki, T.; Hasegawa, H.; Shimizu, N. Ultrsaonic inactivation of Microcystis aeruginosa in the presence of TiO2 particles. J. Biosci. Bioenfineering 2013, 116, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.D.; Zhang, L.; Lin, X.; Cao, X.F.; Li, H.; Luo, J.; Zou, T.Y.; Hong, Z.L.; Xu, K.Q. Fabrication of heterostructured T-BaTiO3/Ag3PO4 forefficient piezophotocatalytic inactivation of M. aeruginosa under xisible light with ultrasound. Sep. Purif. Technol. 2024, 338, 126522. [Google Scholar] [CrossRef]
- Wu, X.G.; Xu, G.F.; Wang, J.J. Ultrasound-assisted coagulation for Microcystis aeruginosa removal using Fe3O4-loaded carbon nanotubes. Rsc Adv. 2020, 10, 13525–13531. [Google Scholar] [CrossRef] [PubMed]
- Shahryari, T.; Alizadeh, V.; Kazemzadeh, P.; Jadoun, S.; Chauhan, N.P.S.; Sargazi, G. A controllable procedure for removing Navicula algae from drinking water using an ultrasonic-assisted electrospun method for highly efficient synthesis of Co-MOF/PVA polymeric network. Appl. Phys. A-Mater. Sci. Process. 2022, 128, 396. [Google Scholar] [CrossRef]
- Wu, X.G.; Yang, S.; Li, W.S.; Wang, J.J.; Dular, M.; Tan, X. Improving Microcystis aeruginosa removal efficiency through enhanced sonosensitivity of nitrogen-doped nanodiamonds. Ultrason. Sonochem. 2024, 109, 106993. [Google Scholar] [CrossRef]
- Wang, X.X.; Wang, Y.; Zhao, C.; Zhu, Y.H.; Sun, Z.H.; Fan, H.J.S.; Hu, X.B.; Zheng, H.L. Ciprofloxacin removal by ultrasound-enhanced carbon nanotubes/permanganate process: In situ generation of free reactive manganese species via electron transfer. Water Res. 2021, 202, 117393. [Google Scholar] [CrossRef]
- Egbedina, A.O.; Bolade, O.P.; Ewuzie, U.; Lime, E.C. Emerging trends in the application of carbon-based materials: A review. J. Environ. Chem. Eng. 2022, 10, 107260. [Google Scholar] [CrossRef]
- Hao, L.M.; Zhang, J.K.; Liu, J.; Min, Y.T.; Chen, C.G. Applications of carbon-based materials in activated peroxymonosulfate for the degradation of organic pollutants: A review. Chem. Rec. 2023, 23, e202300203. [Google Scholar] [CrossRef]
- Rao, N.; Singh, R.; Bashambu, L. Carbon-based nanomaterials: Synthesis and prospective applications. Mater. Today Proc. 2021, 44, 608–614. [Google Scholar] [CrossRef]
- Liu, S.Y.; Lai, C.; Li, B.S.; Liu, X.G.; Zhou, X.R.; Zhang, C.; Qin, L.; Li, L.; Zhang, M.M.; Yi, H.; et al. Heteroatom doping in metal-free carbonaceous materials for the enhancement of persulfate activation. Chem. Eng. J. 2022, 427, 131655. [Google Scholar] [CrossRef]
- Liu, Q.R.; Tian, H.; Dai, Z.H.; Sun, H.G.; Liu, J.; Ao, Z.M.; Wang, S.B.; Han, C.; Liu, S.M. Nitrogen-doped Carbon Nanospheres-Modified Graphitic Carbon Nitride with Outstanding Photocatalytic Activity. Nano-Micro Lett. 2020, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xian, G.; Li, X.M.; Zhang, P.Y.; Zhang, G.G.; Zhu, J. Iron Based Catalysts Used in Water Treatment Assisted by Ultrasound: A Mini Review. Front. Chem. 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Lai, J.H.; Li, R.C.; Fang, X.; Zhang, D.F.; Tsiakaras, P.; Wang, Y. Enhanced Ultrasonic-Assisted Heterogeneous Fenton Degradation of Organic Pollutants over a New Copper Magnetite (Cu-Fe3O4/Cu/C) Nanohybrid Catalyst. Ind. Eng. Chem. Res. 2020, 59, 12431–12440. [Google Scholar] [CrossRef]
- Zhong, Q.; Xue, Y.; Qi, Z.H.; Sun, Y.; Wu, L.L.; Sun, D.Y.; Xu, C.M.; Ri, K.; Yang, S.G.; Zhu, J.D.; et al. FeSeS@C cage-in-cage superlattices for peroxymonosulfate activation: Surface acidity regulates Fe spin state. Appl. Catal. B Environ. Energy 2025, 360, 124539. [Google Scholar] [CrossRef]
- Li, Y.Q.; Qu, C.; Ye, Q.; Meng, F.W.; Yang, D.C.; Wang, L.Y. Enhanced tetracycline degradation by novel Mn-FeOOH/CNNS photocatalysts in a visible-light-driven photocatalysis coupled peroxydisulfate system. Environ. Res. 2024, 257, 119293. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, L.S.; Gao, W.Z.; Chen, F.; Wu, X.M.; Wang, K.Z.; He, Y.L.; Zhang, P.P.; Yang, G.H.; Tsubaki, N. Multi-Promoters Regulated Iron Catalyst with Well-Matching Reverse Water-Gas Shift and Chain Propagation for Boosting CO2 Hydrogenation. J. CO2 Util. 2021, 52, 101700. [Google Scholar] [CrossRef]
- Moussa, S.O.; Panchakarla, L.S.; Ho, M.Q.; El-Shall, M.S. Graphene-Supported, Iron-Based Nanoparticles for Catalytic Production of Liquid Hydrocarbons from Synthesis Gas: The Role of the Graphene Support in Comparison with Carbon Nanotubes. ACS Catal. 2014, 4, 535–545. [Google Scholar] [CrossRef]
- Wu, X.G.; Xu, G.F.; Zhu, J.J. Sonochemical synthesis of Fe3O4/carbon nanotubes using low frequency ultrasonic devices and their performance for heterogeneous sono-persulfate process on inactivation of Microcystis aeruginosa. Ultrason. Sonochem. 2019, 58, 104634. [Google Scholar] [CrossRef]
- Chang, C.W.; Huo, X.C.; Lin, T.F. Exposure of Microcystis aeruginosa to hydrogen peroxide and titanium dioxide under visible light conditions: Modeling the impact of hydrogen peroxide and hydroxyl radical on cell rupture and microcystin degradation. Water Res. 2018, 141, 217–226. [Google Scholar] [CrossRef]
- Sendra, M.; Moreno-Garrido, I.; Yeste, M.P.; Gatica, J.M.; Blasco, J. Toxicity of TiO(2), in nanoparticle or bulk form to freshwater and marine microalgae under visible light and UV-A radiation. Environ. Pollut. 2017, 227, 39–48. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, H.; Lu, J.F. Inactivation of algae by visible-light-driven modified photocatalysts: A review. Sci. Total Environ. 2023, 858, 159640. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Song, X.L.; He, J.L.; Zhang, M.; Zhang, S.W.; Liu, J.L. A new perspective for surface modifying TiO2 to effectively enhance its visible light photocatalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133116. [Google Scholar] [CrossRef]
- Mapukata, S.; Ntsendwana, B.; Mokhena, T.; Sikhwivhilu, L. Advances on sonophotocatalysis as a water and wastewater treatment technique: Efficiency, challenges and process optimisation. Front. Chem. 2023, 11, 1252191. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mohamed, A.A. Advances in ultrasound-assisted synthesis of photocatalysts and sonophotocatalytic processes: A review. iScience 2024, 27, 108583. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, J.; Zhan, J.; Luo, J.; Lin, J.; Qu, F.; Du, B.; Tang, D.; Xie, B.; Yan, Z. Recyclable self-floating A-GUN-coated foam as effective visible-light-driven photocatalyst for inactivation of Microcystis aeruginosa. J. Hazard. Mater. 2021, 419, 126407. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, Y.T.; Li, W.S.; Wu, X.G. Enhancement of KMnO4 treatment on cyanobacteria laden-water via 1000 kHz ultrasound at a moderate intensity. Ultrason. Sonochem. 2023, 98, 106502. [Google Scholar] [CrossRef]
- Keris-Sen, U.D.; Sen, U.; Gurol, M.D. Combined effect of ozone and ultrasound on disruption of microalgae cells. Sep. Sci. Technol. 2019, 54, 1853–1861. [Google Scholar] [CrossRef]
- González-Balderas, R.M.; Velásquez-Orta, S.B.; Ledesma, M.T.O. Biorefinery process intensification by ultrasound and ozone for phosphorus and biocompounds recovery from microalgae. Chem. Eng. Process.-Process Intensif. 2020, 153, 107951. [Google Scholar] [CrossRef]
- Qi, J.; Lan, H.C.; Miao, S.Y.; Xu, Q.; Liu, R.P.; Liu, H.J.; Qu, J.H. KMnO4-Fe(II) pretreatment to enhance Microcystis aeruginosa removal by aluminum coagulation: Does it work after long distance transportation? Water Res. 2016, 88, 127–134. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Ling, J.F.; Li, L.; Guan, X.H. The effectiveness of bisulfite-activated permanganate technology to enhance the coagulation efficiency of Microcystis aeruginosa. Chin. Chem. Lett. 2020, 31, 1545–1549. [Google Scholar] [CrossRef]
- Pandya, K.; Singh, T.S.A.; Kodgire, P.; Simon, S. Combined ultrasound cavitation and persulfate for the treatment of pharmaceutical wastewater. Water Sci. Technol. 2022, 86, 2157–2174. [Google Scholar] [CrossRef] [PubMed]
- Tsenter, I.; Kobunova, E.; Matafonova, G.; Batoev, V. Synergistic Piezo-Catalytic Inactivation of Bacteria by Dual-Frequency Ultrasound (120+1700 kHz) Using Persulfate and ZnO Nano- and Microparticles. Water 2023, 15, 2937. [Google Scholar] [CrossRef]
- Yang, L.; Xue, J.M.; He, L.Y.; Wu, L.; Ma, Y.F.; Chen, H.; Li, H.; Peng, P.; Zhang, Z.L. Review on ultrasound assisted persulfate degradation of organic contaminants in wastewater: Influences, mechanisms and prospective. Chem. Eng. J. 2019, 378, 122146. [Google Scholar] [CrossRef]
- Qiu, Y.T.; Luo, Y.L.; Zhang, T.X.; Du, X.; Wang, Z.H.; Liu, F.; Liang, H. Comparison between permanganate pre-oxidation and persulfate/iron(II) enhanced coagulation as pretreatment for ceramic membrane ultrafiltration of surface water contaminated with manganese and algae. Environ. Res. 2021, 196, 110942. [Google Scholar] [CrossRef]
- Shokoohi, R.; Rahmani, A.; Asgari, G.; Ashrafi, M.; Ghahramani, E. The effect of the combined system of hydrodynamic cavitation, ozone, and hydrogen peroxide on chlorophyll a and organic substances removal in the raw water. Sci. Rep. 2023, 13, 10102. [Google Scholar] [CrossRef]
- Wu, X.G.; Joyce, E.M.; Mason, T.J. Evaluation of the mechanisms of the effect of ultrasound on Microcystis aeruginosa at different ultrasonic frequencies. Water Res. 2012, 46, 2851–2858. [Google Scholar] [CrossRef]
- Rokhina, E.V.; Makarova, K.; Lahtinen, M.; Golovina, E.A.; Van As, H.; Virkutyte, J. Ultrasound-assisted MnO2 catalyzed homolysis of peracetic acid for phenol degradation: The assessment of process chemistry and kinetics. Chem. Eng. J. 2013, 221, 476–486. [Google Scholar] [CrossRef]
- Cao, L.S.; Wang, J.W.; Wang, Z.P.; Cheng, Y.J.; Dai, J.Y.; Ma, J.; Chen, Y.Q.; Liu, Z.Z.; Xie, P.C. Comparison of peracetic acid and sodium hypochlorite enhanced Fe(II) coagulation on algae-laden water treatment. J. Hazard. Mater. 2023, 445, 130571. [Google Scholar] [CrossRef]
- Cao, L.S.; Wang, J.W.; Wang, Z.P.; Yu, S.W.; Cheng, Y.J.; Ma, J.; Xie, P.C. Inactivation of Microcystis aeruginosa by peracetic acid combined with ultraviolet: Performance and characteristics. Water Res. 2022, 208, 117847. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Q.N.; Al-Dhabi, N.A.; Zhang, C.F.; Tang, W.W.; Deng, L.; Mao, X.; Chang, H.Q. Mechanistic insight into peracetic acid-enhanced coagulation for algae-laden water treatment. J. Environ. Chem. Eng. 2024, 12, 112041. [Google Scholar] [CrossRef]
- Huang, Y.R.; Li, L.; Wei, X.M.; Li, H.Z.; Zeng, J.Y.; Kuang, R. An investigation of mechanisms for the enhanced coagulation removal of Microcystis aeruginosa by low-frequency ultrasound under different ultrasound energy densities. Ultrason. Sonochem. 2020, 69, 105278. [Google Scholar] [CrossRef]
- Zhang, Q.; Xue, H.H.; Zhang, H.J.; Chen, Y.Q.; Liu, Z.J.; Fan, Z.; Guo, X.S.; Wu, X.G.; Zhang, D.; Tu, J. Enhanced thrombolytic effect induced by acoustic cavitation generated from nitrogen-doped annealed nanodiamond particles. Ultrason. Sonochem. 2023, 99, 106563. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Liang, H.Z.; Tang, C.; Cheng, Y.; Cheng, L. Exploration of ultrasound-sensitive biomaterials in cancer theranostics. Adv. Funct. Mater. 2024, 34, 13454. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.Y.; Xue, H.H.; Zhang, H.J.; Yuan, Z.Y.; Shen, Y.C.; Guo, X.S.; Fan, Z.; Wu, X.G.; Zhang, D.; et al. Intensified and controllable vaporization of phase-changeable nanodroplets induced by simultaneous exposure of laser and ultrasound. Ultrason. Sonochem. 2023, 94, 106312. [Google Scholar] [CrossRef] [PubMed]
- Postema, M.; Carlson, C.S.; Anderton, N.; Hu, X.Y.; Yamasaku, M.; Petit, L.; Massera, J.; Kudo, N. High-speed photography of gas release from bioactive glass. Jpn. J. Appl. Phys. 2024, 63, 028001. [Google Scholar] [CrossRef]
- McLaughlan, J.R.; Cowell, D.M.J.; Freear, S. Gold nanoparticle nucleated cavitation for enhanced high intensity focused ultrasound therapy. Phys. Med. Biol. 2018, 63, 015004. [Google Scholar] [CrossRef]
- Chen, Y.R.; Zheng, H.R.; Truong, V.N.T.; Xie, G.Y.; Liu, Q.X. Selective aggregation by ultrasonic standing waves through gas nuclei on the particle surface. Ultrason. Sonochem. 2020, 63, 104924. [Google Scholar] [CrossRef]
- Keller, S.B.; LuTheryn, G.; Gray, M.D.; Lyons, B.; Cleveland, R.O.; Stride, E.; Coussios, C.C. Quantitative evaluation of anti-biofilm cavitation activity seeded from microbubbles or protein cavitation nuclei by passive acoustic mapping. Phys. Med. Biol. 2024, 69, 215008. [Google Scholar] [CrossRef]
- Ohl, S.W.; Reese, H.; Ohl, C.D. Cavitation bubble collapse near a rigid wall with an oil layer. Int. J. Multiph. Flow 2024, 174, 104761. [Google Scholar] [CrossRef]
- Reese, H.; Ohl, S.W.; Ohl, C.D. Cavitation bubble induced wall shear stress on an elastic boundary. Phys. Fluids 2023, 35, 076122. [Google Scholar] [CrossRef]
- Abbondanza, D.; Gallo, M.; Casciola, C.M. Cavitation over solid surfaces: Microbubble collapse, shock waves, and elastic response. Meccanica 2023, 58, 1109–1119. [Google Scholar] [CrossRef]
- Ding, Q.M.; Li, X.M.; Cui, Y.Y.; Yang, S.G.; Li, L.F. Dynamic response of the elastic boundary near a single cavitation bubble. Int. J. Multiph. Flow 2024, 177, 104884. [Google Scholar] [CrossRef]
- Xiong, X.; Teschner, T.R.; Moulitsas, I.; Józsa, T.I. Critical assessment of the lattice Boltzmann method for cavitation modelling based on single bubble dynamics. Discov. Appl. Sci. 2024, 6, 241. [Google Scholar] [CrossRef]
- Abaszadeh, M.; Safavinejad, A.; Amiri, H.; Delouei, A.A. A direct-forcing IB-LBM implementation for thermal radiation in irregular geometries. J. Therm. Anal. Calorim. 2022, 147, 11169–11181. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, Z.; Peng, Y.Z. Multi-objective optimization of ultrasonic algae removal technology by using response surface method and non-dominated sorting genetic algorithm-II. Ecotoxicol. Environ. Saf. 2022, 230, 113151. [Google Scholar] [CrossRef]
- Zheng, T.W.; Hou, D.B.; Wu, N.J.; Wang, M.X.; Luo, N.; Luo, H.L.; Leng, W.P.; Li, P.Z.; Wei, W.X. Synergistic effect and removal mechanism of trichloroethylene (TCE) by nano scale zero-valent iron (nZVI) supported on biological calcium carbonate (CaCO3). J. Environ. Chem. Eng. 2023, 11, 111573. [Google Scholar] [CrossRef]
| Catalysts | Dosage | Algae | Density | Frequency | Time | Removal Rate (%) | Reference |
|---|---|---|---|---|---|---|---|
| FeSO4 | 1 mg/L | Microcystis aeruginosa | 4.19 × 106 cells/mL | 20 kHz, | 5 min | 89.26% | [21] |
| Fe3O4/CNTs | 20 mg/L | Microcystis aeruginosa | 1.8 × 106 cells/mL | 40 kHz | 20 s | 94.40% | [46] |
| Co-MOF/PVA | 0.5 mg | Navicula | Using appropriate growth concentration | 20 kHz | 137.2 s | 99.96% | [47] |
| Nitrogen-doped nanodiamonds (N-NDs) | 20 mg/L | Microcystis aeruginosa | 1.5 × 106 cells/L | 800 kHz | 5 min | Over 90% | [48] |
| Polytetrafluoroethylene (PTFE) film | 5 × 5 cm PTFE film | Microcystis aeruginosa | 1.76 × 106 cells/mL | 40 kHz | 5 h | 60.19% | [43] |
| Fe3O4/multi-walled carbon nanotubes (Fe3O4/MWCNTs) | 5 mg/L | Microcystis aeruginosa | 2.0 × 106 cells/mL | 20 kHz (probe), 40 kHz (bath, for catalyst preparation), 600 kHz (synergistic treatment) | 30 min | 90.58% | [21] |
| TiO2 | 0.5 g/mL | Microcystis aeruginosa | 5 × 106 cells/mL | 36 kHz | 15 min | 87% | [44] |
| TiO2/biochar (TiO2/BC) | 50 mg/L | Microcystis aeruginosa | 1.3 × 107 cells/mL | 600 kHz | 90 s | 92% | [49] |
| T-BaTiO3/Ag3PO4 (T-BTO/AP-50) | 50 mg/L | Microcystis aeruginosa | - | 28 kHz | 4 h | 96.10% | [45] |
| Oxidant | Concentration | Free Radicals | Species | Density | Time (min) | Frequency | Intensity | Device | Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| PS | 20 mg/L | SO4−·, ·OH | Microcystis aeruginosa | 2.0 × 106 cells/mL | 30 | 0.3 W/mL | 20 kHz probe, 40 kHz cleaning bath, 600 kHz device | 90.58 | [63] | |
| Peracetic acid | 5–20 mg/L | ·OH, ·CH3CO2, ·CH3CO3 | Microcystis aeruginosa | 2.2 × 106 cells/mL | 20 | 20 kHz | A 20 kHz ultrasonic generator | Reduction of membrane fouling resistance by 76.26 | [22] | |
| Potassium permanganate (KMnO4) | 5–30 mg/L | ·OH, O2− | Microcystis aeruginosa | 2.0 × 106 cells/mL | 10 | 1000 kHz | 0.12 W/mL, 0.39 W/mL | 1000 kHz ultrasonic generator | [70] | |
| O3 | 0.27 g O3/g | ·OH | Cenedesmus sp., Chloroccum sp. | 0.5 g/L | 30 kHz | 50 W | Ultrasonic processor equipped with an 8 cm long and 7 mm diameter ultrasonic probe | [71] | ||
| O3 | 27 mg O3/L | ·OH | Scenedesmus obliquus | 42 kHz | 100 W | 2.81 L ultrasonic bath | [72] | |||
| H2O2, Fe2+ | H2O2: 1 mg/L; Fe2+: 1 mg/L | ·OH | Microcystis aeruginosa | 4.19 × 106 cells/mL | 5 | 20 kHz | 0.42 W/mL | Probe | 89.26 | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wu, X.; Ashokkumar, M. Synergistic Catalysis for Algae Control: Integrating Sonocavitation and Chemical Catalysis. Catalysts 2025, 15, 784. https://doi.org/10.3390/catal15080784
Zhang Y, Wu X, Ashokkumar M. Synergistic Catalysis for Algae Control: Integrating Sonocavitation and Chemical Catalysis. Catalysts. 2025; 15(8):784. https://doi.org/10.3390/catal15080784
Chicago/Turabian StyleZhang, Yunxi, Xiaoge Wu, and Muthupandian Ashokkumar. 2025. "Synergistic Catalysis for Algae Control: Integrating Sonocavitation and Chemical Catalysis" Catalysts 15, no. 8: 784. https://doi.org/10.3390/catal15080784
APA StyleZhang, Y., Wu, X., & Ashokkumar, M. (2025). Synergistic Catalysis for Algae Control: Integrating Sonocavitation and Chemical Catalysis. Catalysts, 15(8), 784. https://doi.org/10.3390/catal15080784








