Interfacial Electron Transfer in Strategically Engineered Pt3Rh/C Ultrafine Alloy Nanoparticle Catalysts Facilitates Exceptional Performance in Li-O2 Batteries
Abstract
1. Introduction
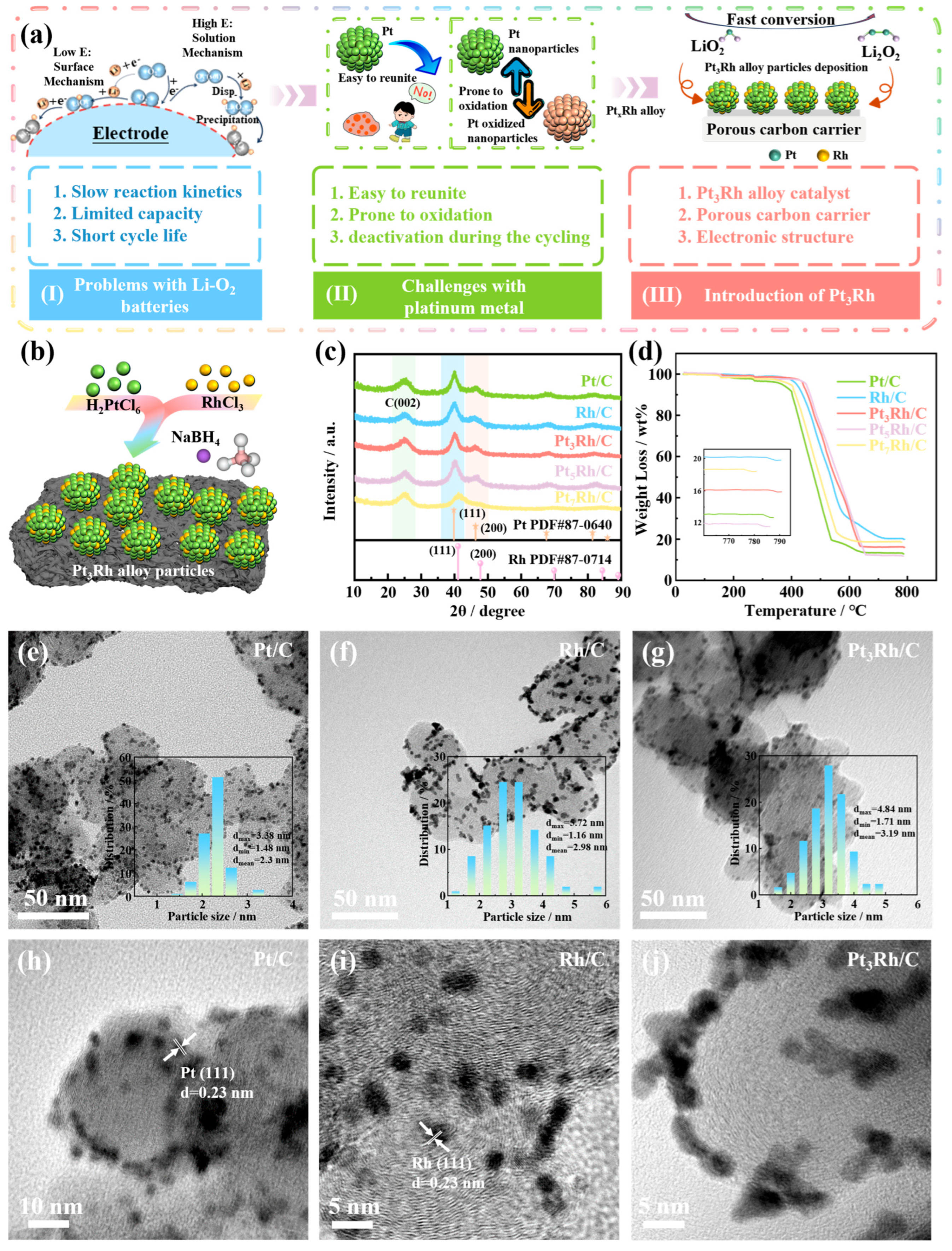
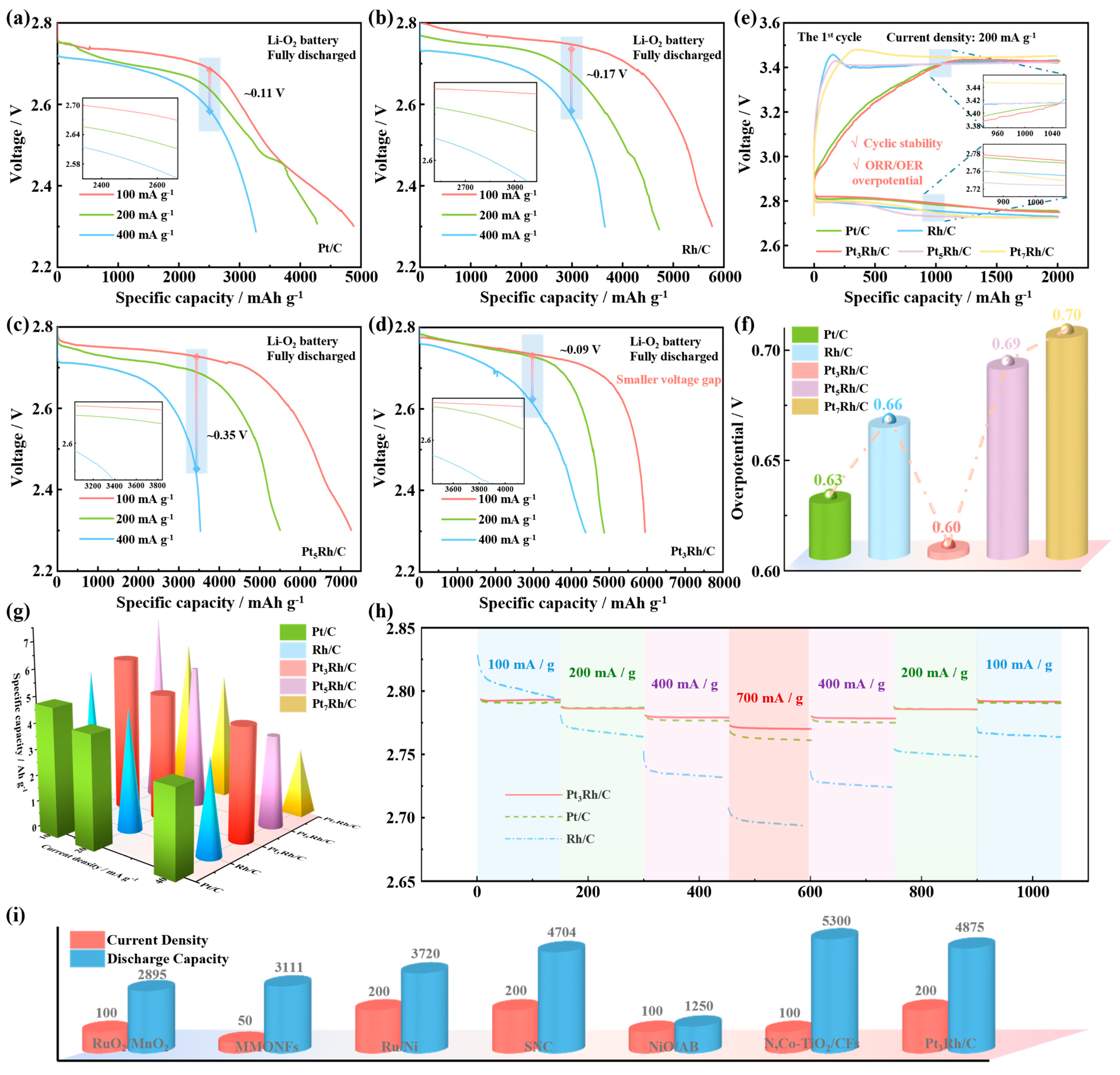
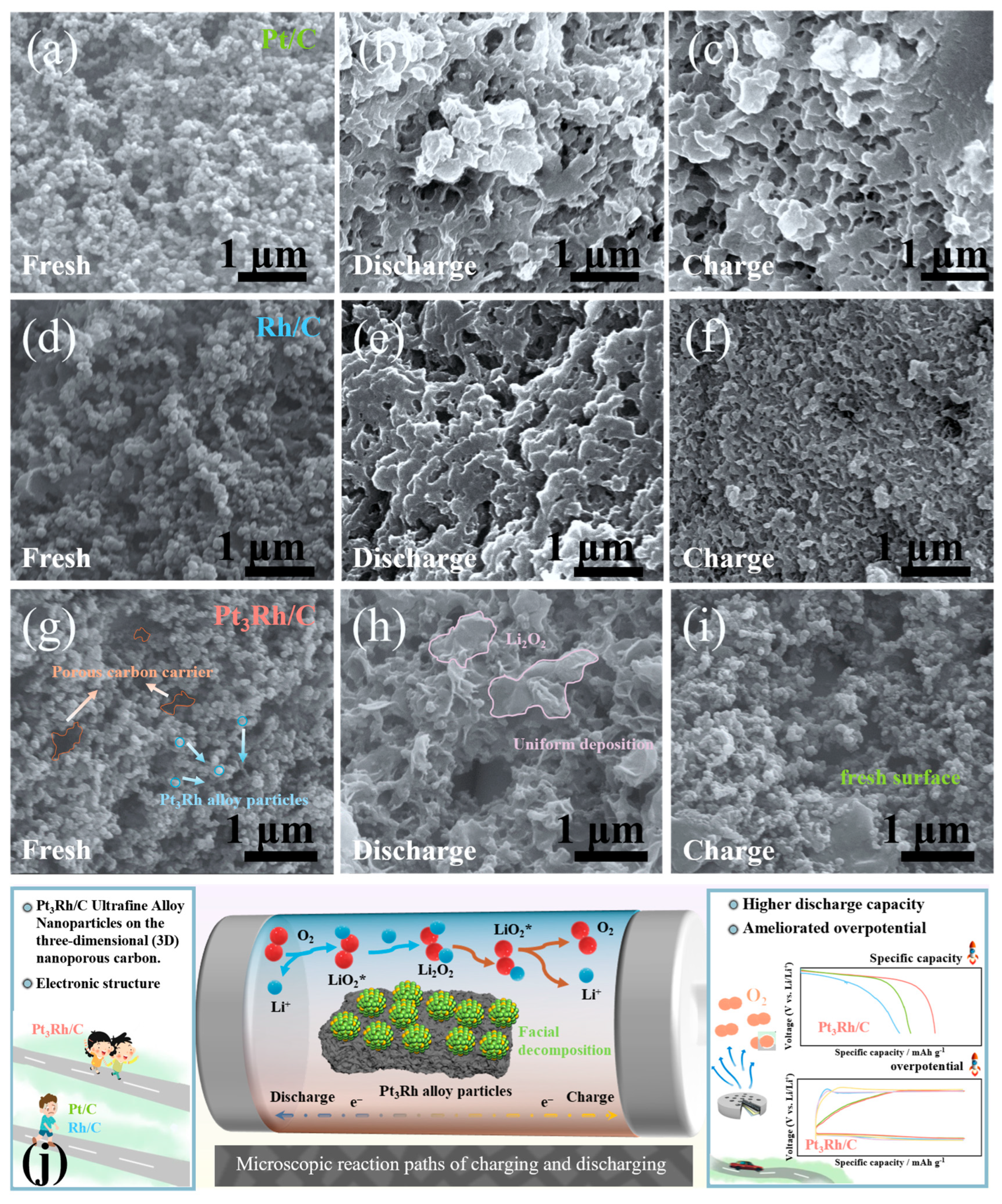
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Electrocatalysts’ Synthesis
3.3. Characterization Techniques
3.3.1. Structural and Chemical Analysis
3.3.2. Li-O2 Cell Assembly and Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Black, R.; Adams, B.; Nazar, L.F. Non-Aqueous and Hybrid Li-O2 Batteries. Adv. Energy Mater. 2012, 2, 801–815. [Google Scholar] [CrossRef]
- Du, J.; Han, G.; Zhang, W.; Li, L.; Yan, Y.; Shi, Y.; Zhang, X.; Geng, L.; Wang, Z.; Xiong, Y.; et al. CoIn Dual-Atom Catalyst for Hydrogen Peroxide Production via Oxygen Reduction Reaction in Acid. Nat. Commun. 2023, 14, 4766. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Temprano, I.; Lei, J.; Tang, L.; Li, J.; Grey, C.P.; Liu, T. Recent Progress in Developing a LiOH-Based Reversible Nonaqueous Lithium-Air Battery. Adv. Mater. 2022, 35, 2201384. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Li, L. Hybrid and Aqueous Lithium-Air Batteries. Adv. Energy Mater. 2014, 5, 1401302. [Google Scholar] [CrossRef]
- Wang, P.; Ren, Y.; Wang, R.; Zhang, P.; Ding, M.; Li, C.; Zhao, D.; Qian, Z.; Zhang, Z.; Zhang, L.; et al. Atomically Dispersed Cobalt Catalyst Anchored on Nitrogen-Doped Carbon Nanosheets for Lithium-Oxygen Batteries. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Wang, J.; McKee, W.C.; Xu, Y.; Peng, Z. Potential-Dependent Generation of O2− and LiO2 and Their Critical Roles in O2 Reduction to Li2O2 in Aprotic Li-O2 Batteries. J. Phys. Chem. C 2016, 120, 3690–3698. [Google Scholar] [CrossRef]
- Choi, R.; Jung, J.; Kim, G.; Song, K.; Kim, Y.-I.; Jung, S.C.; Han, Y.-K.; Song, H.; Kang, Y.-M. Ultra-Low Overpotential and High Rate Capability in Li-O2 Batteries through Surface Atom Arrangement of PdCu Nanocatalysts. Energy Environ. Sci. 2014, 7, 1362–1368. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Y.; Gu, Q.; Gu, Q.; Yin, K.; Yin, K.; Li, Y.; Li, Y.; Tao, L.; Tao, L.; et al. Engineering eg Orbital Occupancy of Pt with Au Alloying Enables Reversible Li-O2 Batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202201416. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, K.; Gu, Q.; Tao, L.; Li, Y.; Tan, H.; Zhou, J.; Zhang, W.; Li, H.; Guo, S. Lewis-Acidic PtIr Multipods Enable High-Performance Li-O2 Batteries. Angew. Chem. Int. Ed. Engl. 2021, 60, 26592–26598. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Q.; Qian, J.; Wang, Y.; Mu, Y.; Zhang, Z.; Li, Z.; Zhao, T.; Liu, B.; Zeng, L. Pt-Pyrrole Complex-Assisted Synthesis of Carbon-Supported Pt Intermetallics for Oxygen Reduction in Proton Exchange Membrane Fuel Cells. ACS Catal. 2024, 14, 6992–7000. [Google Scholar] [CrossRef]
- Li, L.; Hua, M.; Li, J.; Zhang, P.; Nie, Y.; Wang, P.; Lin, X.; Zhang, Z.; Wang, R.; Ge, X.; et al. Tuning Dual Catalytic Active Sites of Pt Single Atoms Paired with High-Entropy Alloy Nanoparticles for Advanced Li-O2 Batteries. ACS Nano 2025, 19, 4391–4402. [Google Scholar] [CrossRef]
- Tong, Z.; Lv, C.; Zhou, Y.; Zhang, P.; Xiang, C.; Li, Z.; Wang, Z.; Liu, Z.; Li, J.; Sun, S. Highly Dispersed Ru-Co Nanoparticles Interfaced With Nitrogen-Doped Carbon Polyhedron for High Efficiency Reversible Li-O2 Battery. Small 2022, 18, 2204836. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Huang, Y.; Li, Y.; Zhang, T.; Wu, Z. Regulating Surface Electron Structure of PtNi Nanoalloy via Boron Doping for High-current-density Li-O2 Batteries with Low Overpotential and Long-life Cyclability. SmartMat 2022, 5, e1150. [Google Scholar] [CrossRef]
- An, Z.; Li, H.; Zhang, X.; Xu, X.; Xia, Z.; Yu, S.; Chu, W.; Wang, S.; Sun, G. Structural Evolution of a PtRh Nanodendrite Electrocatalyst and Its Ultrahigh Durability toward Oxygen Reduction Reaction. ACS Catal. 2022, 12, 3302–3308. [Google Scholar] [CrossRef]
- Du, W.; Zhang, W.; Zhu, C.; Guo, W.; He, M.; Zhao, H.; Chen, R. Platinum Group Metals-Based Intermetallic Compounds: Syntheses and Application in Electrocatalysis. Co-ord. Chem. Rev. 2025, 530, 216473. [Google Scholar] [CrossRef]
- Li, F.; Yao, C.; Jeon, J.-P.; Han, G.-F.; Shin, T.J.; Han, A.; Fu, Z.; Lu, Y.; Baek, J.-B. Rhodium and Carbon Sites with Strong d–p Orbital Interaction for Efficient Bifunctional Catalysis. ACS Nano 2023, 17, 24282–24289. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-X.; Yu, Z.-X. Arene Reduction by Rh/Pd or Rh/Pt under 1 Atm Hydrogen Gas and Room Temperature. Org. Lett. 2024, 26, 3458–3462. [Google Scholar] [CrossRef]
- Su, L.; Wu, H.; Zhang, S.; Cui, C.; Zhou, S.; Pang, H. Insight Into Intermediate Behaviors and Design Strategies of Platinum Group Metal-Based Alkaline Hydrogen Oxidation Catalysts. Adv. Mater. 2024, 37, 2414628. [Google Scholar] [CrossRef]
- Moriyama, T.; Muratsugu, S.; Sato, M.; Higuchi, K.; Takagi, Y.; Tada, M. Pt2Gd Alloy Nanoparticles from Organometallic Pt and Gd Complexes and Hollow Mesoporous Carbon Spheres: Enhanced Oxygen Reduction Reaction Activity and Durability. J. Am. Chem. Soc. 2024, 147, 1262–1270. [Google Scholar] [CrossRef]
- Li, X.; Duan, X.; Zhang, S.; Wang, C.; Hua, K.; Wang, Z.; Wu, Y.; Li, J.; Liu, J. Strategies for Achieving Ultra–Long ORR DurabilityRh Activates Interatomic Interactions in Alloys. Angew. Chem. Int. Ed. Engl. 2024, 63, e202400549. [Google Scholar] [CrossRef]
- Zhao, Y.; Meng, K.; Luo, T.; Chen, M.; Niu, S.; Lin, C.; Xing, X.; Yang, Q.; Kong, X.; Zhang, D.; et al. Electronic Structure Engineering of RuCo Nanoalloys Supported on Nanoporous Carbon for Li-O2 Batteries. J. Power Sources 2024, 597, 234130. [Google Scholar] [CrossRef]
- Guan, S.; Li, X.; Zhao, Y.; Han, G.; Lou, S.; Zhu, Y. Single-Atom Tailored Transition Metal Oxide Enhances d-p Hybridization in Catalytic Conversion for Lithium-Oxygen Batteries. Chem. Eng. J. 2024, 488, 151064. [Google Scholar] [CrossRef]
- Lee, K.B.; Jo, S.; Zhang, L.; Kim, M.; Sohn, J.I. Hierarchically Interconnected 3D Catalyst Structure of Porous Multi-Metal Oxide Nanofibers for High-Performance Li-O2 Batteries. Small Methods 2024, 8, 2301728. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Zhang, T.; Wang, Y.; Li, F.; Jian, Z.; Yu, H.; Zhou, H. Nanoporous Ru as a Carbon-and Binder-Free Cathode for Li-O2 Batteries. ChemSusChem 2015, 8, 1429–1434. [Google Scholar] [CrossRef]
- Sun, B.; Zheng, W.; Kang, C.; Xie, B.; Qian, Z.; Wang, Y.; Ye, S.; Lou, S.; Kong, F.; Mei, B.; et al. Tailoring the p-Band Center of NS Pair for Accelerating High-Performance Lithium-Oxygen Battery. Small 2023, 19, 2207461. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.-L.; Xu, S.-M.; Xu, C.-Y.; Zhang, Q.; Wang, H.-H.; Zhang, Z.; Chen, X.; Dong, S.-Y.; Liu, Y.-S.; Xu, Z.-X.; et al. Free-Standing N, Co-Codoped TiO2 Nanoparticles for LiO2-Based Li-O2 Batteries. J. Mater. Chem. A 2019, 7, 23046–23054. [Google Scholar] [CrossRef]
- Cai, S.; Zheng, M.S.; Lin, X.; Lei, M.; Yuan, R.; Dong, Q.F. A Synergistic Catalytic Mechanism for Oxygen Evolution Reaction in Aprotic Li-O2 Battery. ACS Catal. 2018, 8, 7983–7990. [Google Scholar] [CrossRef]
- Tong, S. Mesoporous NiO with a Single-Crystalline Structure Utilized as a Noble Metal-Free Catalyst for Non-Aqueous Li-O2 Batteries. J. Mater. Chem. A 2015, 3, 16177–16182. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Guan, S.; Gao, Y.; Gong, S.; Han, G.; Gao, P.; Lou, S.; Zhu, Y.; Li, X. Atomically Dispersed Ir Lewis Acid Sites on (111)-Oriented CeO2 Enable Enhanced Reaction Kinetics for Li-O2 Batteries. Chem. Eng. J. 2024, 500, 156972. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Gao, Y.; Li, X. Interfacial Electron Transfer in Strategically Engineered Pt3Rh/C Ultrafine Alloy Nanoparticle Catalysts Facilitates Exceptional Performance in Li-O2 Batteries. Catalysts 2025, 15, 777. https://doi.org/10.3390/catal15080777
Xu X, Gao Y, Li X. Interfacial Electron Transfer in Strategically Engineered Pt3Rh/C Ultrafine Alloy Nanoparticle Catalysts Facilitates Exceptional Performance in Li-O2 Batteries. Catalysts. 2025; 15(8):777. https://doi.org/10.3390/catal15080777
Chicago/Turabian StyleXu, Xing, Yinkun Gao, and Xudong Li. 2025. "Interfacial Electron Transfer in Strategically Engineered Pt3Rh/C Ultrafine Alloy Nanoparticle Catalysts Facilitates Exceptional Performance in Li-O2 Batteries" Catalysts 15, no. 8: 777. https://doi.org/10.3390/catal15080777
APA StyleXu, X., Gao, Y., & Li, X. (2025). Interfacial Electron Transfer in Strategically Engineered Pt3Rh/C Ultrafine Alloy Nanoparticle Catalysts Facilitates Exceptional Performance in Li-O2 Batteries. Catalysts, 15(8), 777. https://doi.org/10.3390/catal15080777




