Advancements in Non-Precious Metal Catalysts for High-Temperature Proton-Exchange Membrane Fuel Cells: A Comprehensive Review
Abstract
1. Introduction
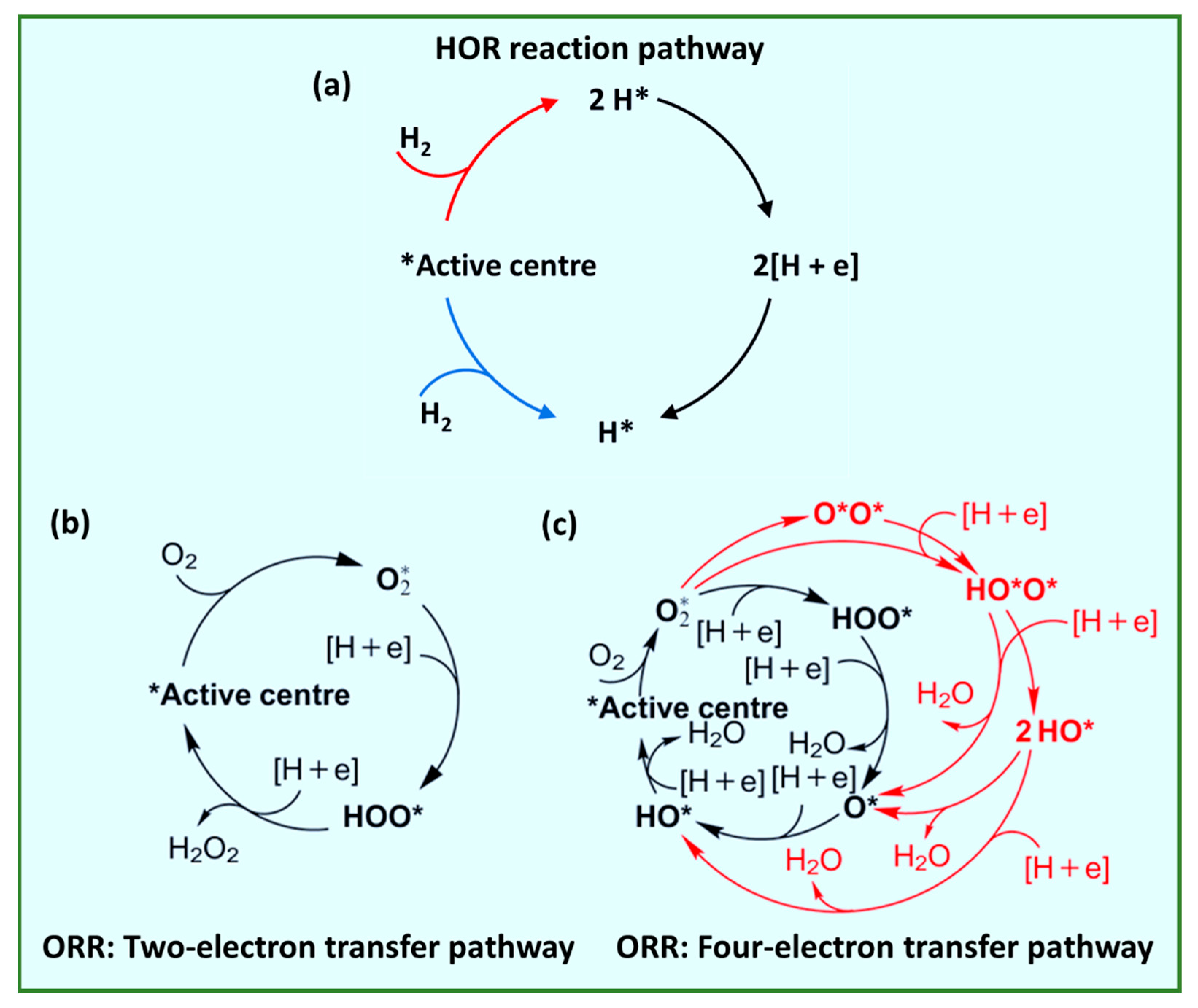
1.1. Working Principles and Electrochemistry of HT-PEMFCs
Two-electron transfer: O2 + 2H+ + 2e− → H2O2
1.2. Problem Associates with PGM Catalyst in HT-PEMFCs
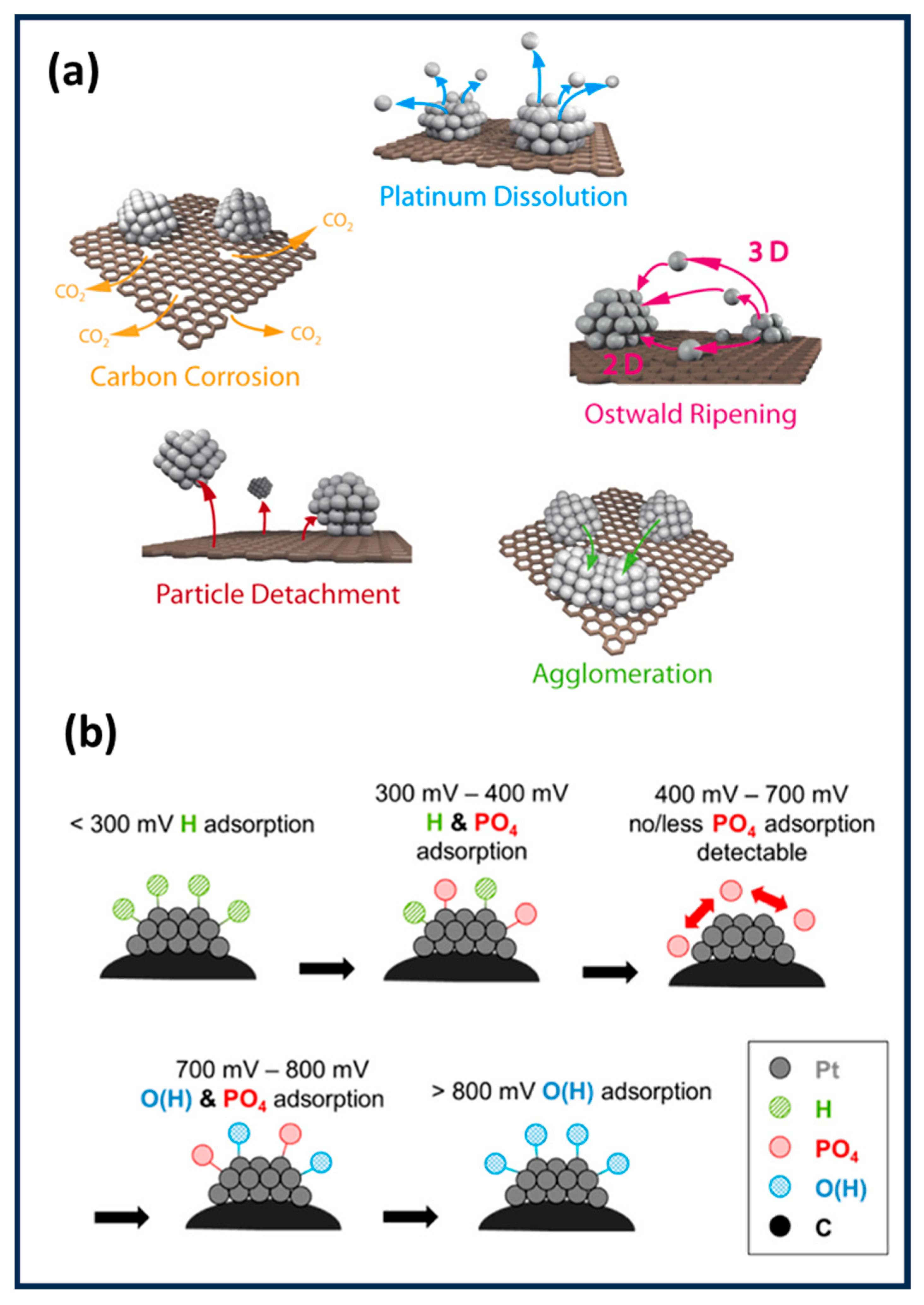
2. Types of Non-Precious Metal (NPM) Catalysts
2.1. Transition Metal Catalysts
2.2. Metal–Nitrogen–Carbon (M-N-C) Catalysts
2.3. P-Doped Metal–Nitrogen–Carbon (M-N-C) Catalysts
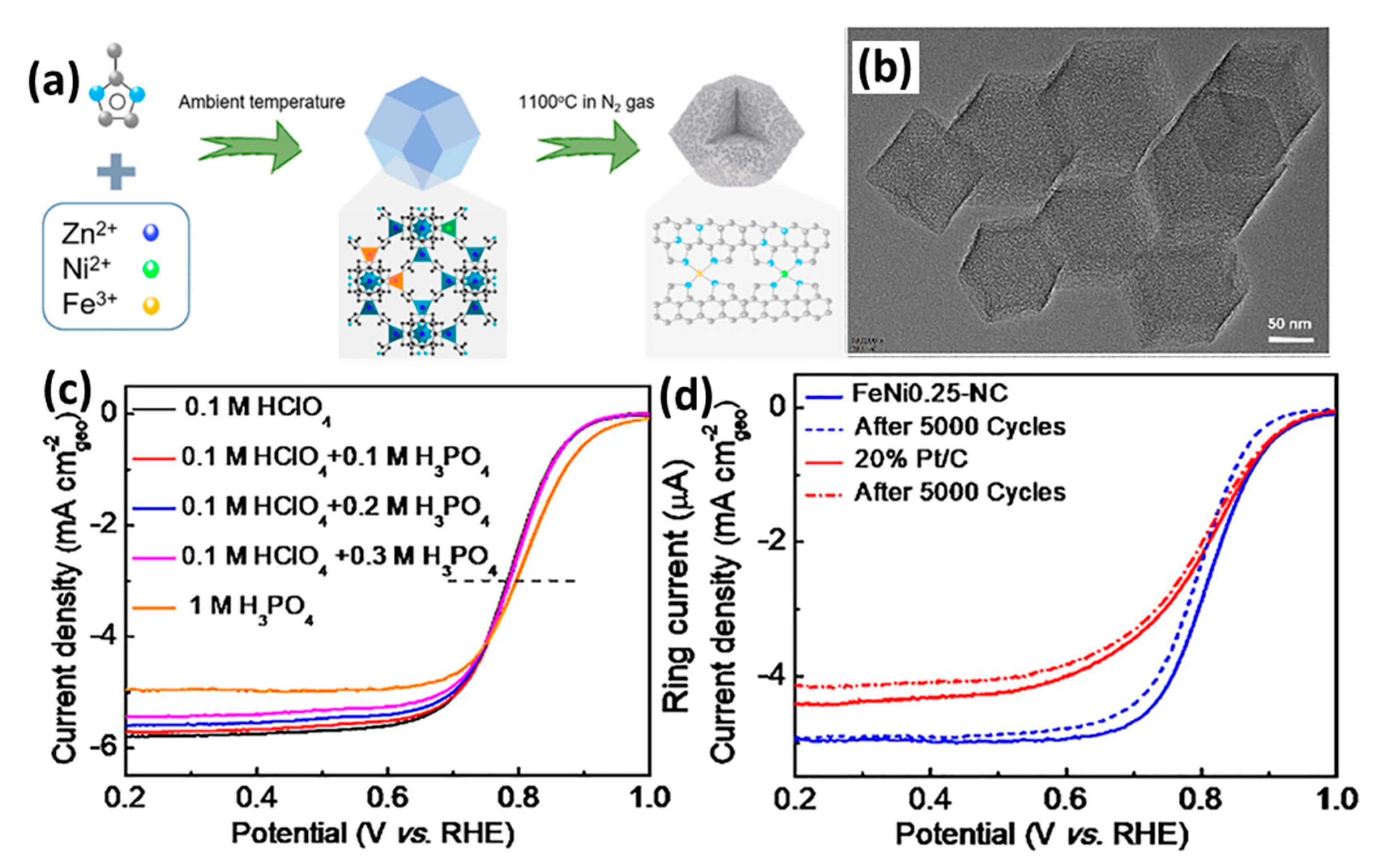
2.4. Multi-Metallic M-N-C Systems
2.5. Advanced Carbon Supports
2.6. Metal-Free Heteroatom-Doped Carbon Catalysts
3. Challenges and Future Perspectives
3.1. Current Challenges in NPM Catalyst Development for HT-PEMFCs
- (1)
- Limitation between Activity and Stability: An ongoing and essential problem in the fabrication of M-N-C catalysts is the contrary interaction between the initial activity and stability. The high-temperature pyrolysis process is necessary to synthesize the highly active and graphitic M-Nx sites, often due to carbon support being more sensitive to electrochemical corrosion [135]. On the other hand, the synthesis conditions provide more durable catalysts, but amorphous carbon support might not provide sufficient active sites [130,136]. One of the main objectives of the field is to overcome this limitation in order to produce a catalyst that is both extremely active and extremely durable.
- (2)
- Mass Transport and Electrode Engineering: As mentioned earlier, thick catalyst layers are required to obtain sufficient performance due to the lower volumetric activity of NPMs in comparison to Pt [137]. This leads to a series of mass transit-related engineering issues. A high thickness and porous layer will severely hinder the transport of oxygen toward the active sites and the outflow of water vapor, resulting in concentration losses and reduction in the power density of fuel cells, particularly under high load [92]. Additionally, regulating PA ionomer diffusion within this thick coating layer is essential, as too little acid leads to inefficient proton conductivity and poor active sites, while a surplus amount of acid (flooding) blocks the pores and stops the diffusion of gas [137,138]. This is an electrode-level technical challenge and equally significant as the synthesis of catalyst materials [139].
- (3)
- Understanding and Mitigating Degradation in PA: Fe-N-C catalysts have been admired because of their ability to withstand phosphate poisoning, which involves reversibly blocking active sites, although they are not impervious to the general harshness of the HT-PEMFC environment [89]. The corrosive properties of concentrated PA, high electrode potential, and high temperature (160–200 °C) can all speed up basic degradation processes such as carbon support corrosion and dissolution of the active metal centers [140]. The mechanism of degradation is a complicated, interconnected phenomenon in which the PA electrolyte, the Polybenzimidazole (PBI) membrane, and the catalyst all interact with one another and degrade over time [141]. Developing an extensive understanding of these interconnected system-level failure mechanisms requires designing an extremely long-lasting MEA.
- (4)
- Scalability and Standardization: There is an enormous gap between laboratory-scale innovations and industrial-scale production. A lot of sophisticated synthesis techniques, which produce high-performance catalysts, such as multi-step templating or Chemical Vapor Deposition (CVD), are complicated and might not be commercially scalable [137]. For commercial transformation, developing more straightforward and reliable synthesis methods is essential. At the same time, the absence of widely accepted, standardized processes for MEA development and long-term testing makes it very challenging to consistently evaluate performance data from various research groups and determine which catalyst candidates show the greatest promise for future development [137].
3.2. Innovative Approaches and Future Research Directions
- Urgent Need for Standardized Protocols:
- Proposed Framework for Future Benchmarking:
- (i)
- Standard operating window: Temperature = 160–180 °C, pressure = 1 atm, and RH = 0–20%, using doped PBI-type membranes.
- (ii)
- Electrode architecture: Standardized catalyst loading (e.g., 1.0 ± 0.2 mg/cm2), gas diffusion layer, and ink composition.
- (iii)
- Electrochemical protocols: Report initial activity via polarization curves and ORR onset potential, and assess stability via 100 h chronoamperometry or accelerated stress tests (ASTs).
- (iv)
- Post-mortem analysis: Encourage the use of XPS, XAS, and HR-TEM to understand degradation mechanisms.
- (v)
- Data reporting format: Include both gravimetric and areal performance metrics (mA/mg, mA/cm2), membrane resistance, and cell voltage at defined current densities.
4. Conclusions and Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Roser, M. CO2 emissions. Our World Data 2020. Available online: https://ourworldindata.org/co2-emissions (accessed on 10 August 2025).
- IEA. Global Energy Review 2025; IEA: Paris, France, 2025. [Google Scholar]
- Yan, H.; Acquah, S.J.; Zhang, C.; Wang, G.; Huang, S.; Zhang, H.; Zhao, B.; Wu, H. Energy partitioning of greenhouse cucumber based on the application of Penman-Monteith and Bulk Transfer models. Agric. Water Manage. 2019, 217, 201–211. [Google Scholar] [CrossRef]
- Khan, K.Y.; Tang, Y.; Cheng, P.; Song, Y.; Li, X.; Lou, J.; Iqbal, B.; Zhao, X.; Hameed, R.; Li, G.; et al. Effects of degradable and non-degradable microplastics and oxytetracycline co-exposure on soil N2O and CO2 emissions. Appl. Soil Ecol. 2024, 197, 105331. [Google Scholar] [CrossRef]
- Jiangyi, H.; Fan, W. Design and testing of a small orchard tractor driven by a power battery. Eng. Agríc. 2023, 43, e20220195. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, P.K. Advances in the high performance polymer electrolyte membranes for fuel cells. Chem. Soc. Rev. 2012, 41, 2382–2394. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zeng, L.; Chen, L.; Zou, R.; Cai, Y. Fuzzy Adaptive Energy Management Strategy for a Hybrid Agricultural Tractor Equipped with HMCVT. Agriculture 2022, 12, 1986. [Google Scholar] [CrossRef]
- Zhu, Z.; Chai, X.; Xu, L.; Quan, L.; Yuan, C.; Tian, S. Design and performance of a distributed electric drive system for a series hybrid electric combine harvester. Biosystems Eng. 2023, 236, 160–174. [Google Scholar] [CrossRef]
- Sheng, B.; Pan, T.; Luo, Y.; Jermsittiparsert, K. System Identification of the PEMFCs based on Balanced Manta-Ray Foraging Optimization algorithm. Energy Reports 2020, 6, 2887–2896. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.-F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar] [CrossRef]
- Moorthy, S.; Sivasubramanian, G.; Kannaiyan, D.; Deivanayagam, P. Neoteric advancements in polybenzimidazole based polymer electrolytes for high-temperature proton exchange membrane fuel cells-A versatile review. Int. J. Hydrogen Energy 2023, 48, 28103–28118. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, Z.; Lu, Y.; He, Z.; Shao, Z.; Yu, J. Optimization of porous layer structure of high-temperature proton exchange membrane fuel cell based on deep learning and Monte Carlo method. Int. J. Hydrogen Energy 2024, 50, 1004–1019. [Google Scholar] [CrossRef]
- Wieser, C. Novel polymer electrolyte membranes for automotive applications–requirements and benefits. Fuel Cells 2004, 4, 245–250. [Google Scholar] [CrossRef]
- Ding, X.; Ahmad, W.; Wu, J.; Rong, Y.; Ouyang, Q.; Chen, Q. Bipyridine-mediated fluorescence charge transfer process based on copper ion grafted upconversion nanoparticle platform for ciprofloxacin sensing in aquatic products. Food Chem. 2023, 404, 134761. [Google Scholar] [CrossRef]
- Hu, D.; Liu, J.; Yi, F.; Yang, Q.; Zhou, J. Enhancing heat dissipation to improve efficiency of two-stage electric air compressor for fuel cell vehicle. Energy Convers. Manag. 2022, 251, 115007. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from microalgae: Technologies, challenges and opportunities. Renew. Sustain. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Chen, D.; Zou, Y.; Shi, W.; Serbin, S.; You, H. Proton exchange membrane fuel cells using new cathode field designs of multi-inlet shunt intake design. Int. J. Energy Res. 2021, 45, 9948–9960. [Google Scholar] [CrossRef]
- Sun, L.; Zheng, B.; Liu, W. Constructing high-throughput and highly adsorptive lithium–sulfur battery separator coatings based on three-dimensional hexagonal star-shaped MOF derivatives. J. Colloid. Interface Sci. 2025, 679, 197–205. [Google Scholar] [CrossRef]
- Jiao, K.; Alaefour, I.E.; Li, X. Three-dimensional non-isothermal modeling of carbon monoxide poisoning in high temperature proton exchange membrane fuel cells with phosphoric acid doped polybenzimidazole membranes. Fuel 2011, 90, 568–582. [Google Scholar] [CrossRef]
- Lopes, P.P.; Freitas, K.S.; Ticianelli, E.A. CO tolerance of PEMFC anodes: Mechanisms and electrode designs. Electrocatalysis 2010, 1, 200–212. [Google Scholar] [CrossRef]
- Nachiappan, N.; Kalaignan, G.P.; Sasikumar, G. Influence of methanol impurity in hydrogen on PEMFC performance. Ionics 2013, 19, 517–522. [Google Scholar] [CrossRef]
- Simon Araya, S.; Liso, V.; Cui, X.; Li, N.; Zhu, J.; Sahlin, S.L.; Jensen, S.H.; Nielsen, M.P.; Kær, S.K. A review of the methanol economy: The fuel cell route. Energies 2020, 13, 596. [Google Scholar] [CrossRef]
- Profatilova, I.; Jacques, P.-A.; Escribano, S. Evaluation of parameters accelerating the aging of PEMFCs operating under reformate containing carbon monoxide. J. Electrochem. Soc. 2018, 165, F3251. [Google Scholar] [CrossRef]
- Dias, V.; Pochet, M.; Contino, F.; Jeanmart, H. Energy and economic costs of chemical storage. Front. Mech. Eng. 2020, 6, 21. [Google Scholar] [CrossRef]
- Authayanun, S.; Im-Orb, K.; Arpornwichanop, A. A review of the development of high temperature proton exchange membrane fuel cells. Chin. J. Catal. 2015, 36, 473–483. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Bethune, K.; Rubio, M.A.; Rocheleau, R. Study of low concentration CO poisoning of Pt anode in a proton exchange membrane fuel cell using spatial electrochemical impedance spectroscopy. J. Power Sources 2014, 269, 344–362. [Google Scholar] [CrossRef]
- St-Pierre, J. PEMFC contaminant tolerance limit—CO in H2. Electrochim. Acta 2010, 55, 4208–4211. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Q.; Mei, D.; Wang, Y. Development of highly efficient methanol steam reforming system for hydrogen production and supply for a low temperature proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2020, 45, 25317–25327. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, Z.; Zhao, S.; Liu, J.; Gao, R.; Jiang, P. Characterization of volatile organic compounds of different pigmented rice after puffing based on gas chromatography-ion migration spectrometry and chemometrics. Food Res. Int. 2023, 169, 112879. [Google Scholar] [CrossRef]
- Subianto, S. Recent advances in polybenzimidazole/phosphoric acid membranes for high-temperature fuel cells. Polym. Int. 2014, 63, 1134–1144. [Google Scholar] [CrossRef]
- Chandan, A.; Hattenberger, M.; El-Kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)–A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Shabani, B.; Hafttananian, M.; Khamani, S.; Ramiar, A.; Ranjbar, A.A. Poisoning of proton exchange membrane fuel cells by contaminants and impurities: Review of mechanisms, effects, and mitigation strategies. J. Power Sources 2019, 427, 21–48. [Google Scholar] [CrossRef]
- Oono, Y.; Fukuda, T.; Sounai, A.; Hori, M. Influence of operating temperature on cell performance and endurance of high temperature proton exchange membrane fuel cells. J. Power Sources 2010, 195, 1007–1014. [Google Scholar] [CrossRef]
- Tse, E.C.M.; Gewirth, A.A. Effect of temperature and pressure on the kinetics of the oxygen reduction reaction. J. Phys. Chem. A 2015, 119, 1246–1255. [Google Scholar] [CrossRef]
- Zhang, J.; Aili, D.; Lu, S.; Li, Q.; Jiang, S.P. Advancement toward polymer electrolyte membrane fuel cells at elevated temperatures. Research 2020, 2020, 9089405. [Google Scholar] [CrossRef]
- Belgacem, N.; Pauchet, J.; Prat, M. On the current distribution at the channel–rib scale in polymer-electrolyte fuel cells. Int. J. Hydrog. Energy 2018, 43, 5112–5123. [Google Scholar] [CrossRef]
- Lv, R.; Huang, X.; Aheto, J.H.; Dai, C.; Tian, X. Research on reaction mechanism of colorimetric sensor array with lead and its application for determination of lead content of fish. J. Food Process Eng. 2019, 42, e13075. [Google Scholar] [CrossRef]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrog. Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Herdem, M.S.; Sinaki, M.Y.; Farhad, S.; Hamdullahpur, F. An overview of the methanol reforming process: Comparison of fuels, catalysts, reformers, and systems. Int. J. Energy Res. 2019, 43, 5076–5105. [Google Scholar] [CrossRef]
- Li, N.; Cui, X.; Zhu, J.; Zhou, M.; Liso, V.; Cinti, G.; Sahlin, S.L.; Araya, S.S. A review of reformed methanol-high temperature proton exchange membrane fuel cell systems. Renew. Sustain. Energy Rev. 2023, 182, 113395. [Google Scholar] [CrossRef]
- Xia, X.; Ren, M.; He, W.-S.; Jia, C.; Zhang, X. The preparation of phytosteryl succinyl sucrose esters and improvement of their water solubility and emulsifying properties. Food Chem. 2022, 373, 131501. [Google Scholar] [CrossRef]
- Mei, S.; Yang, C.; Song, X.; Wang, T.; Wang, Y.; Geng, Y.; Wang, J.; Su, S. Analysis of the effect of sterilization and storage on the quality of Pinus yunnanensis pollen based on untargeted metabonomics. J. Food Process. Preserv. 2021, 45, e16033. [Google Scholar] [CrossRef]
- Bayat, M.; Özalp, M. Effects of leak current density and doping level on energetic, exergetic and ecological performance of a high-temperature PEM fuel cell. Int. J. Hydrog. Energy 2023, 48, 23212–23229. [Google Scholar] [CrossRef]
- Xu, H.; Song, Y.; Kunz, H.R.; Fenton, J.M. Effect of elevated temperature and reduced relative humidity on ORR kinetics for PEM fuel cells. J. Electrochem. Soc. 2005, 152, A1828. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Wang, X.; Guo, Y.; Ni, C.; Wu, J.; Hao, C. High performance hybrid supercapacitors assembled with multi-cavity nickel cobalt sulfide hollow microspheres as cathode and porous typha-derived carbon as anode. Ind. Crops Prod. 2022, 189, 115863. [Google Scholar] [CrossRef]
- Oshchepkov, A.G.; Bonnefont, A.; Parmon, V.N.; Savinova, E.R. On the effect of temperature and surface oxidation on the kinetics of hydrogen electrode reactions on nickel in alkaline media. Electrochim. Acta 2018, 269, 111–118. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, J.; Sun, W.; Pan, H. Non-platinum group metal electrocatalysts toward efficient hydrogen oxidation reaction. Adv. Funct. Mater. 2021, 31, 2010633. [Google Scholar] [CrossRef]
- Liang, J.; Li, H.; Chen, L.; Ren, M.; Fakayode, O.A.; Han, J.; Zhou, C. Efficient hydrogen evolution reaction performance using lignin-assisted chestnut shell carbon-loaded molybdenum disulfide. Ind. Crops Prod. 2023, 193, 116214. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, J.; Chen, Y.; Huang, J. Modelling electrocatalytic reactions with a concerted treatment of multistep electron transfer kinetics and local reaction conditions. J. Phys. Condens. Matter 2021, 33, 504002. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhong, Y.; Chen, R.; Ling, G.; Wang, X.; Shen, Y.; Hao, C. Cu-Ag-C@Ni3S4 with core shell structure and rose derived carbon electrode materials: An environmentally friendly supercapacitor with high energy and power density. Ind. Crops Prod. 2024, 222, 119676. [Google Scholar] [CrossRef]
- Kuzmin, A.V.; Shainyan, B.A. Mechanisms of catalytic electrochemical reactions of oxygen reduction (ORR) and carbon dioxide reduction (CO2RR). Russ. Chem. Rev. 2023, 92, 5085. [Google Scholar] [CrossRef]
- Yang, Y.; Peltier, C.R.; Zeng, R.; Schimmenti, R.; Li, Q.; Huang, X.; Yan, Z.; Potsi, G.; Selhorst, R.; Lu, X. Electrocatalysis in alkaline media and alkaline membrane-based energy technologies. Chem. Rev. 2022, 122, 6117–6321. [Google Scholar] [CrossRef]
- Yang, F.; Liao, L.-w.; Li, M.-f.; Mei, D.; Chen, Y.-x. Kinetics study on O2 adsorption OHad desorption at Pt(111), its implication to oxygen reduction reaction kinetics. Chin. J. Chem. Phys. 2014, 27, 479–484. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Chen, L.; Yarley, O.P.N.; Zhou, C. Facile preparation of sugarcane bagasse-derived carbon supported MoS2 nanosheets for hydrogen evolution reaction. Ind. Crops Prod. 2021, 172, 114064. [Google Scholar] [CrossRef]
- Unnikrishnan, A.; Rajalakshmi, N.; Janardhanan, V.M. Kinetics of electrochemical charge transfer in HT-PEM fuel cells. Electrochim. Acta 2019, 293, 128–140. [Google Scholar] [CrossRef]
- Hooshyari, K.; Amini Horri, B.; Abdoli, H.; Fallah Vostakola, M.; Kakavand, P.; Salarizadeh, P. A review of recent developments and advanced applications of high-temperature polymer electrolyte membranes for PEM fuel cells. Energies 2021, 14, 5440. [Google Scholar] [CrossRef]
- Kerr, R.; García, H.R.; Rastedt, M.; Wagner, P.; Alfaro, S.M.; Romero, M.T.; Terkelsen, C.; Steenberg, T.; Hjuler, H.A. Lifetime and degradation of high temperature PEM membrane electrode assemblies. Int. J. Hydrogen Energy 2015, 40, 16860–16866. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, X.; Zhang, Y.; Xu, G. Remarkably durable platinum cluster supported on multi-walled carbon nanotubes with high performance in an anhydrous polymer electrolyte fuel cell. RSC Adv. 2016, 6, 108158–108163. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Yousfi-Steiner, N.; Moçotéguy, P.; Candusso, D.; Hissel, D. A review on polymer electrolyte membrane fuel cell catalyst degradation and starvation issues: Causes, consequences and diagnostic for mitigation. J. Power Sources 2009, 194, 130–145. [Google Scholar] [CrossRef]
- Honji, A.; Mori, T.; Tamura, K.; Hishinuma, Y. Agglomeration of platinum particles supported on carbon in phosphoric acid. J. Electrochem. Soc. 1988, 135, 355. [Google Scholar] [CrossRef]
- Tseung, A.C.C.; Dhara, S.C. Loss of surface area by platinum and supported platinum black electrocatalyst. Electrochim. Acta 1975, 20, 681–683. [Google Scholar] [CrossRef]
- Watanabe, M.; Tsurumi, K.; Mizukami, T.; Nakamura, T.; Stonehart, P. Activity and stability of ordered and disordered Co-Pt alloys for phosphoric acid fuel cells. J. Electrochem. Soc. 1994, 141, 2659. [Google Scholar] [CrossRef]
- Berejnov, V.; Martin, Z.; West, M.; Kundu, S.; Bessarabov, D.; Stumper, J.; Susac, D.; Hitchcock, A.P. Probing platinum degradation in polymer electrolyte membrane fuel cells by synchrotron X-ray microscopy. Phys. Chem. Chem. Phys. 2012, 14, 4835–4843. [Google Scholar] [CrossRef]
- Ascarelli, P.; Contini, V.; Giorgi, R. Formation process of nanocrystalline materials from x-ray diffraction profile analysis: Application to platinum catalysts. J. Appl. Phys. 2002, 91, 4556–4561. [Google Scholar] [CrossRef]
- Zhang, J. PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Yoda, T.; Uchida, H.; Watanabe, M. Effects of operating potential and temperature on degradation of electrocatalyst layer for PEFCs. Electrochim. Acta 2007, 52, 5997–6005. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Han, J.; Nguyen, X.L.; Yu, S.; Goo, Y.-M.; Le, D.D. Review of the durability of polymer electrolyte membrane fuel cell in long-term operation: Main influencing parameters and testing protocols. Energies 2021, 14, 4048. [Google Scholar] [CrossRef]
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent developments in catalyst-related PEM fuel cell durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Kaserer, S.; Caldwell, K.M.; Ramaker, D.E.; Roth, C. Analyzing the influence of H3PO4 as catalyst poison in high temperature PEM fuel cells using in-operando X-ray absorption spectroscopy. J. Phys. Chem. C 2013, 117, 6210–6217. [Google Scholar] [CrossRef]
- He, Q.; Yang, X.; Chen, W.; Mukerjee, S.; Koel, B.; Chen, S. Influence of phosphate anion adsorption on the kinetics of oxygen electroreduction on low index Pt (hkl) single crystals. Phys. Chem. Chem. Phys. 2010, 12, 12544–12555. [Google Scholar] [CrossRef]
- Caldwell, K.M.; Kaserer, S.; Roth, C.; Ramaker, D.E. Following Adsorbate Coverage on Anodes of High-Temperature Polymer Electrolyte Membrane Fuel Cells in the Presence of CO and H2O by using In Operando X-ray Absorption Spectroscopy. ChemElectroChem 2015, 2, 1502–1509. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Liu, P.; Zhao, K.; Ye, S.; Liang, G. Rapid, ultrasensitive and non-enzyme electrochemiluminescence detection of hydrogen peroxide in food based on the ssDNA/g-C3N4 nanosheets hybrid. Food Chem. 2021, 357, 129753. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Peng, W.; Ji, Y.; Wei, J.; Che, J.; Huang, Y.; Huang, W.; Yang, W.; Xu, W. A self-powered photoelectrochemical aptasensor using 3D-carbon nitride and carbon-based metal-organic frameworks for high-sensitivity detection of tetracycline in milk and water. J. Food Sci. 2024, 89, 8022–8035. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, P.; Zhang, T.; Zhu, Y.; Qiu, F. Fabrication of recyclable magnetic double-base aerogel with waste bioresource bagasse as the source of fiber for the enhanced removal of chromium ions from aqueous solution. Food Bioprod. Process. 2020, 119, 257–267. [Google Scholar] [CrossRef]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Witte, J.; Bongard, H.J.; Topalov, A.A.; Baldizzone, C.; Mezzavilla, S.; Schüth, F.; Mayrhofer, K.J.J. Design criteria for stable Pt/C fuel cell catalysts. Beilstein J. Nanotech. 2014, 5, 44–67. [Google Scholar] [CrossRef]
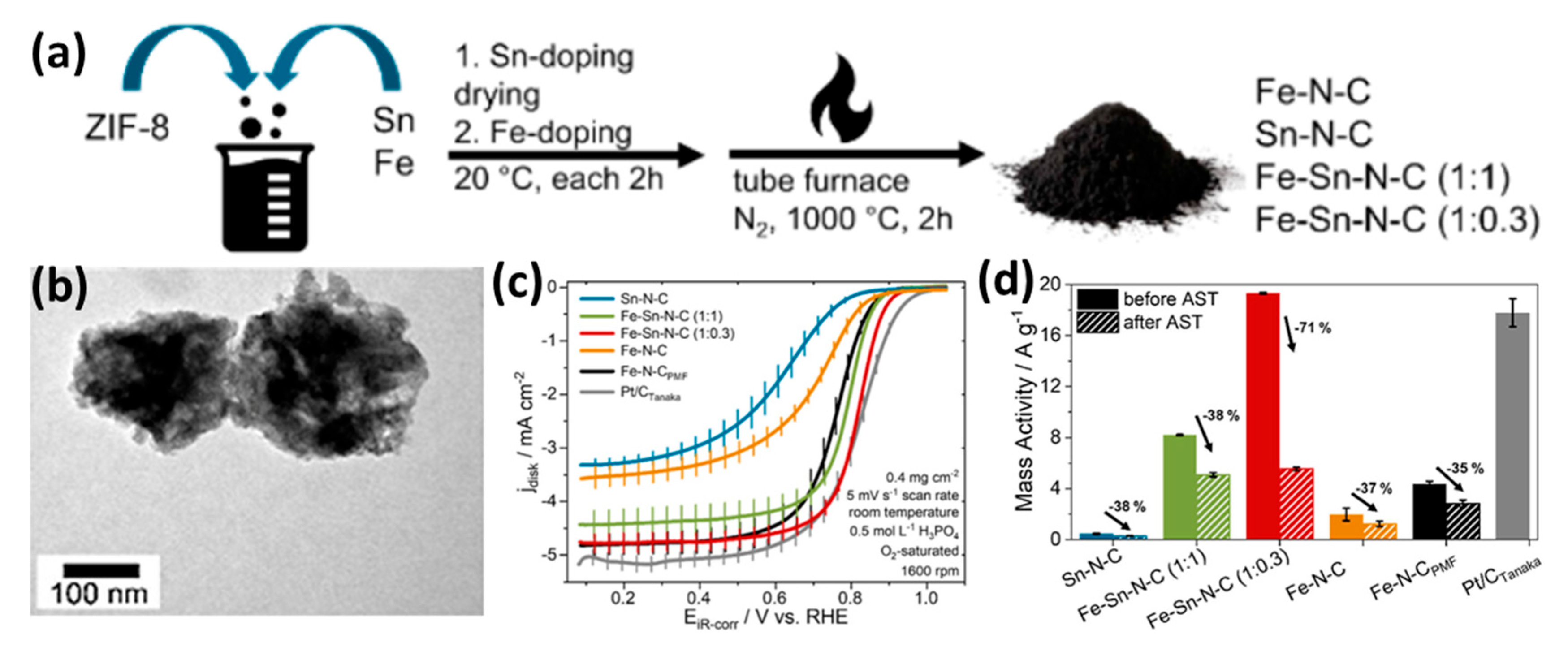
- Buschermöhle, J.G.; Müller-Hülstede, J.; Schmies, H.; Schonvogel, D.; Zierdt, T.; Lucka, R.; Renz, F.; Wagner, P.; Wark, M. Fe–Sn–N–C Catalysts: Advancing Oxygen Reduction Reaction Performance. ACS Catal. 2025, 15, 4477–4488. [Google Scholar] [CrossRef]
- Zucconi, A.; Hack, J.; Stocker, R.; Suter, T.A.M.; Rettie, A.J.E.; Brett, D.J.L. Challenges and opportunities for characterisation of high-temperature polymer electrolyte membrane fuel cells: A review. J. Mater. Chem. A 2024, 12, 8014–8064. [Google Scholar] [CrossRef]
- Li, Q.; Wu, G.; Cullen, D.A.; More, K.L.; Mack, N.H.; Chung, H.T.; Zelenay, P. Phosphate-tolerant oxygen reduction catalysts. ACS Catal. 2014, 4, 3193–3200. [Google Scholar] [CrossRef]
- Wang, J.; Ahmad, W.; Mehedi Hassan, M.; Zareef, M.; Viswadevarayalu, A.; Arslan, M.; Li, H.; Chen, Q. Landing microextraction sediment phase onto surface enhanced Raman scattering to enhance sensitivity and selectivity for chromium speciation in food and environmental samples. Food Chem. 2020, 323, 126812. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Yosri, N.; Chen, Q.; Elseedi, H.R.; Zou, X.; Yang, H. Detection of Heavy Metals in Food and Agricultural Products by Surface-enhanced Raman Spectroscopy. Food Rev. Int. 2023, 39, 1440–1461. [Google Scholar] [CrossRef]
- Hu, Y.; Jensen, J.O.; Zhang, W.; Martin, S.; Chenitz, R.; Pan, C.; Xing, W.; Bjerrum, N.J.; Li, Q. Fe3C-based oxygen reduction catalysts: Synthesis, hollow spherical structures and applications in fuel cells. J. Mater. Chem. A 2015, 3, 1752–1760. [Google Scholar] [CrossRef]
- Mamtani, K.; Jain, D.; Zemlyanov, D.; Celik, G.; Luthman, J.; Renkes, G.; Co, A.C.; Ozkan, U.S. Probing the oxygen reduction reaction active sites over nitrogen-doped carbon nanostructures (CN x) in acidic media using phosphate anion. ACS Catal. 2016, 6, 7249–7259. [Google Scholar] [CrossRef]
- Holst-Olesen, K.; Reda, M.; Hansen, H.A.; Vegge, T.; Arenz, M. Enhanced oxygen reduction activity by selective anion adsorption on non-precious-metal catalysts. ACS Catal. 2018, 8, 7104–7112. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, A.; Ahmad, W.; Adade, S.Y.-S.S.; Chen, Q.; Wei, W.; Chen, X.; Wei, J.; Jiao, T.; Chen, Q. A three-channel biosensor based on stimuli-responsive catalytic activity of the Fe3O4@Cu for on-site detection of tetrodotoxin in fish. Food Chem. 2024, 460, 140566. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Wang, C.; Tian, X.; Chang, X.; Ren, Y.; Yu, S. Applications of surface functionalized Fe3O4 NPs-based detection methods in food safety. Food Chem. 2021, 342, 128343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, X.; Yu, F.; Quan, Y. Preparation of dummy molecularly imprinted polymers based on dextran-modified magnetic nanoparticles Fe3O4 for the selective detection of acrylamide in potato chips. Food Chem. 2020, 317, 126431. [Google Scholar] [CrossRef]
- Ma, S.; Pan Lg You, T.; Wang, K. g-C3N4/Fe3O4 Nanocomposites as Adsorbents Analyzed by UPLC-MS/MS for Highly Sensitive Simultaneous Determination of 27 Mycotoxins in Maize: Aiming at Increasing Purification Efficiency and Reducing Time. J. Agric. Food. Chem. 2021, 69, 4874–4882. [Google Scholar] [CrossRef]
- Strickland, K.; Pavlicek, R.; Miner, E.; Jia, Q.; Zoller, I.; Ghoshal, S.; Liang, W.; Mukerjee, S. Anion resistant oxygen reduction electrocatalyst in phosphoric acid fuel cell. ACS Catal. 2018, 8, 3833–3843. [Google Scholar] [CrossRef]
- Razmjooei, F.; Yu, J.-H.; Lee, H.-Y.; Lee, B.-J.; Singh, K.P.; Kang, T.-H.; Kim, H.-J.; Yu, J.-S. Single-atom iron-based electrocatalysts for high-temperature polymer electrolyte membrane fuel cell: Organometallic precursor and pore texture tailoring. ACS Appl. Energy Mater. 2020, 3, 11164–11176. [Google Scholar] [CrossRef]
- Jain, D.; Gustin, V.; Basu, D.; Gunduz, S.; Deka, D.J.; Co, A.C.; Ozkan, U.S. Phosphate tolerance of nitrogen-coordinated-iron-carbon (FeNC) catalysts for oxygen reduction reaction: A size-related hindrance effect. J. Catal. 2020, 390, 150–160. [Google Scholar] [CrossRef]
- Müller-Hülstede, J.; Zierdt, T.; Schmies, H.; Schonvogel, D.; Meyer, Q.; Zhao, C.; Wagner, P.; Wark, M. Implementation of different Fe–N–C catalysts in high temperature proton exchange membrane fuel cells–effect of catalyst and catalyst layer on performance. J. Power Sources 2022, 537, 231529. [Google Scholar] [CrossRef]
- Yang, X.; Huang, Z.; Du, L.; Li, Q.; Ye, S. Active Sites and Stability Study of Fe/N/C Catalyst in PEMFCs: A Decade of Stunning Progress and the Remaining Challenges. ACS Catal. 2024, 14, 15471–15488. [Google Scholar] [CrossRef]
- Hu, Y.; Jensen, J.O.; Pan, C.; Cleemann, L.N.; Shypunov, I.; Li, Q. Immunity of the Fe-NC catalysts to electrolyte adsorption: Phosphate but not perchloric anions. Appl. Catal. B 2018, 234, 357–364. [Google Scholar] [CrossRef]
- Gokhale, R.; Asset, T.; Qian, G.; Serov, A.; Artyushkova, K.; Benicewicz, B.C.; Atanassov, P. Implementing PGM-free electrocatalysts in high-temperature polymer electrolyte membrane fuel cells. Electrochem. Commun. 2018, 93, 91–94. [Google Scholar] [CrossRef]
- Müller-Hülstede, J.; Schmies, H.; Schonvogel, D.; Meyer, Q.; Nie, Y.; Zhao, C.; Wagner, P.; Wark, M. What determines the stability of Fe-NC catalysts in HT-PEMFCs? Int. J. Hydrog. Energy 2024, 50, 921–930. [Google Scholar] [CrossRef]
- Byeon, A.; Lee, K.J.; Lee, M.J.; Lee, J.S.; Lee, I.H.; Park, H.Y.; Lee, S.Y.; Yoo, S.J.; Jang, J.H.; Kim, H.J. Effect of Catalyst Pore Size on the Performance of Non-Precious Fe/N/C-Based Electrocatalysts for High-Temperature Polymer Electrolyte Membrane Fuel Cells. ChemElectroChem 2018, 5, 1805–1810. [Google Scholar] [CrossRef]
- Bevilacqua, N.; Asset, T.; Schmid, M.A.; Markötter, H.; Manke, I.; Atanassov, P.; Zeis, R. Impact of catalyst layer morphology on the operation of high temperature PEM fuel cells. J. Power Sources Adv. 2021, 7, 100042. [Google Scholar] [CrossRef]
- Li, Y.; Luo, S.; Sun, L.; Kong, D.; Sheng, J.; Wang, K.; Dong, C. A Green, Simple, and Rapid Detection for Amaranth in Candy Samples Based on the Fluorescence Quenching of Nitrogen-Doped Graphene Quantum Dots. Food Anal. Methods 2019, 12, 1658–1665. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Li, Q.; Shi, Y.; Zhai, X.; Xu, Y.; Li, Z.; Huang, X.; Wang, X.; Shi, J.; et al. Copper nanoclusters @ nitrogen-doped carbon quantum dots-based ratiometric fluorescence probe for lead (II) ions detection in porphyra. Food Chem. 2020, 320, 126623. [Google Scholar] [CrossRef] [PubMed]
- Najam, T.; Shah, S.S.A.; Ding, W.; Wei, Z. Role of P-doping in antipoisoning: Efficient MOF-derived 3D hierarchical architectures for the oxygen reduction reaction. J. Phys. Chem. C 2019, 123, 16796–16803. [Google Scholar] [CrossRef]
- Yang, W.; Liu, C.; Zhang, B.; Wu, C.; Cao, Y.; Huang, W.; Xu, W. Construction of a Nitrogen-Doped Carbon Quantum Dot Fluorescent Molecularly Imprinted Sensor for Ultra-Sensitive Detection of Sulfadiazine in Pork Samples. Food Anal. Methods 2024, 17, 1689–1701. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Li, Y.; Shi, J.; Huang, X.; Li, Z.; Zhang, J.; Li, W.; Xu, Y.; Zou, X. Easy-to-Use Visual Sensing System for Milk Freshness, Sensitized with Acidity-Responsive N-Doped Carbon Quantum Dots. Foods 2022, 11, 1855. [Google Scholar] [CrossRef]
- Jin, X.; Li, Y.; Sun, H.; Gao, X.; Li, J.; Lü, Z.; Liu, W.; Sun, X. Phosphorus induced activity-enhancement of Fe-NC catalysts for high temperature polymer electrolyte membrane fuel cells. Nano Res. 2023, 16, 6531–6536. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, H.; Wang, Y.; Luo, E.; Li, K.; Gao, L.; Wang, Y.; Wu, Z.; Jin, Z.; Ge, J. Bridge bonded oxygen ligands between approximated FeN4 sites confer catalysts with high ORR performance. Angew. Chem. Int. Ed. 2020, 132, 14027–14032. [Google Scholar] [CrossRef]
- Jing, Y.; Cheng, Y.; Wang, L.; Liu, Y.; Yu, B.; Yang, C. MOF-derived Co, Fe, and Ni co-doped N-enriched hollow carbon as efficient electrocatalyst for oxygen reduction reaction. Chem. Eng. J. 2020, 397, 125539. [Google Scholar] [CrossRef]
- Brea, C.; Hu, G. Dual-Atom Catalysts for the Oxygen Reduction Reaction: Unraveling Atomic Structures under Reaction Conditions. J. Am. Chem. Soc. 2025, 147, 19210–19216. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Z.; Liu, W.; Chang, C.; Tang, H.; Li, Z.; Chen, W.; Jia, C.; Yao, T.; Wei, S. Design of N-coordinated dual-metal sites: A stable and active Pt-free catalyst for acidic oxygen reduction reaction. J. Am. Chem. Soc. 2017, 139, 17281–17284. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Luo, G.; Li, Z.; Zhao, C.; Zhang, H.; Zhu, M.; Xu, Q.; Wang, X.; Zhao, C. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energy Environ. Sci. 2018, 11, 3375–3379. [Google Scholar] [CrossRef]
- Xiao, M.; Chen, Y.; Zhu, J.; Zhang, H.; Zhao, X.; Gao, L.; Wang, X.; Zhao, J.; Ge, J.; Jiang, Z. Climbing the apex of the ORR volcano plot via binuclear site construction: Electronic and geometric engineering. J. Am. Chem. Soc. 2019, 141, 17763–17770. [Google Scholar] [CrossRef]
- Kattel, S.; Wang, G. A density functional theory study of oxygen reduction reaction on Me–N4 (Me = Fe, Co, or Ni) clusters between graphitic pores. J. Mater. Chem. A 2013, 1, 10790–10797. [Google Scholar] [CrossRef]
- Liu, J.; Fan, C.; Liu, G.; Jiang, L. MOF-derived dual metal (Fe, Ni)–nitrogen–doped carbon for synergistically enhanced oxygen reduction reaction. Appl. Surf. Sci. 2021, 538, 148017. [Google Scholar] [CrossRef]
- Ameena, S.; Rajesh, N.; Anjum, S.M.; Khadri, H.; Riazunnisa, K.; Mohammed, A.; Kari, Z.A. Antioxidant, Antibacterial, and Anti-diabetic Activity of Green Synthesized Copper Nanoparticles of Cocculus hirsutus (Menispermaceae). Appl. Biochem. Biotechnol. 2022, 194, 4424–4438. [Google Scholar] [CrossRef]
- Qin, C.; Guo, W.; Liu, Y.; Liu, Z.; Qiu, J.; Peng, J. A Novel Electrochemical Sensor Based on Graphene Oxide Decorated with Silver Nanoparticles–Molecular Imprinted Polymers for Determination of Sunset Yellow in Soft Drinks. Food Anal. Methods 2017, 10, 2293–2301. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, Z.; Zhang, T.; Chen, Q.; Qiu, F.; Peng, Y.; Tang, S. Fabrication of functional biomass carbon aerogels derived from sisal fibers for application in selenium extraction. Food Bioprod. Process. 2018, 111, 93–103. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, Y.; Wang, J.; Gan, L.; He, M.; Zhang, T.; Li, P.; Qiu, F. Preparation and application of Mg–Al composite oxide/coconut shell carbon fiber for effective removal of phosphorus from domestic sewage. Food Bioprod. Process. 2021, 126, 293–304. [Google Scholar] [CrossRef]
- Shen, L.Q.; Zhou, X.; Zhang, C.X.; Yin, H.B.; Wang, A.L.; Wang, C.T. Functional characterization of bimetallic CuPdx nanoparticles in hydrothermal conversion of glycerol to lactic acid. J. Food Biochem. 2019, 43, e12931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, B.; Chen, Q.; Peng, X.; Yang, D.; Qiu, F. Layered double hydroxide functionalized biomass carbon fiber for highly efficient and recyclable fluoride adsorption. Appl. Biol. Chem. 2019, 62, 12. [Google Scholar] [CrossRef]
- Bu, Q.; Chen, K.; Morgan, H.M.; Liang, J.; Zhang, X.; Yan, L.; Mao, H. Thermal Behavior and Kinetic Study of the Effects of Zinc-Modified Biochar Catalyst on Lignin and Low-Density Polyethylene (LDPE) Co-Pyrolysis. Trans. ASABE 2018, 61, 1783–1793. [Google Scholar] [CrossRef]
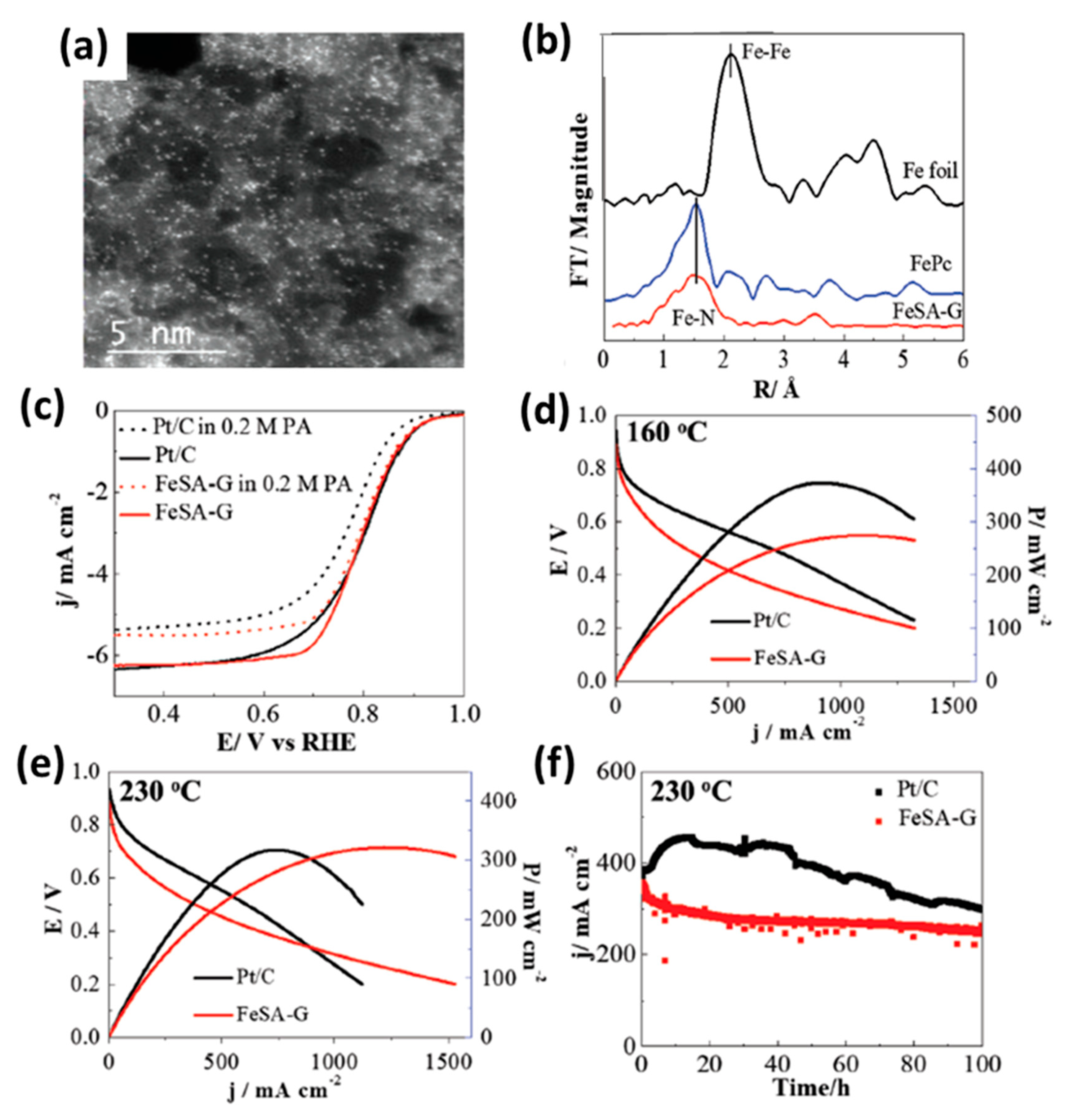
- Cheng, Y.; He, S.; Lu, S.; Veder, J.P.; Johannessen, B.; Thomsen, L.; Saunders, M.; Becker, T.; De Marco, R.; Li, Q.; et al. Iron single atoms on graphene as nonprecious metal catalysts for high-temperature polymer electrolyte membrane fuel cells. Adv. Sci. 2019, 6, 1802066. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Wu, X.; Tang, C.; Yang, S.-z.; Su, P.; Thomsen, L.; Zhao, F.; Lu, S.; Liu, J. A template-free method to synthesis high density iron single atoms anchored on carbon nanotubes for high temperature polymer electrolyte membrane fuel cells. Nano Energy 2021, 80, 105534. [Google Scholar] [CrossRef]
- Wang, M.; Lu, S.; Wu, X.; Veder, J.-P.; Johannessen, B.; Thomsen, L.; Zhang, J.; Yang, S.-z.; Jiang, S.P. First demonstration of phosphate enhanced atomically dispersed bimetallic FeCu catalysts as Pt-free cathodes for high temperature phosphoric acid doped polybenzimidazole fuel cells. Appl. Catal. B 2021, 284, 119717. [Google Scholar]
- Chenitz, R.; Kramm, U.I.; Lefèvre, M.; Glibin, V.; Zhang, G.; Sun, S.; Dodelet, J.-P. A specific demetalation of Fe–N4 catalytic sites in the micropores of NC_Ar + NH3 is at the origin of the initial activity loss of the highly active Fe/N/C catalyst used for the reduction of oxygen in PEM fuel cells. Energy Environ. Sci. 2018, 11, 365–382. [Google Scholar] [CrossRef]
- Zhang, T.; Yuan, D.; Guo, Q.; Qiu, F.; Yang, D.; Ou, Z. Preparation of a renewable biomass carbon aerogel reinforced with sisal for oil spillage clean-up: Inspired by green leaves to green Tofu. Food Bioprod. Process. 2019, 114, 154–162. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Fang, Z.; Shi, G.; Lou, L.; Ren, K.; Cai, Q. Italian ryegrass–rice rotation system for biomass production and cadmium removal from contaminated paddy fields. J. Soils Sediments 2020, 20, 874–882. [Google Scholar] [CrossRef]
- Choi, C.H.; Baldizzone, C.; Grote, J.P.; Schuppert, A.K.; Jaouen, F.; Mayrhofer, K.J.J. Stability of Fe-N-C catalysts in acidic medium studied by operando spectroscopy. Angew. Chem. Int. Ed. 2015, 54, 12753–12757. [Google Scholar] [CrossRef]
- Kinumoto, T.; Inaba, M.; Nakayama, Y.; Ogata, K.; Umebayashi, R.; Tasaka, A.; Iriyama, Y.; Abe, T.; Ogumi, Z. Durability of perfluorinated ionomer membrane against hydrogen peroxide. J. Power Sources 2006, 158, 1222–1228. [Google Scholar] [CrossRef]
- Zhang, Q.; Mamtani, K.; Jain, D.; Ozkan, U.; Asthagiri, A. CO poisoning effects on FeNC and CN x ORR catalysts: A combined experimental–computational study. J. Phys. Chem. C 2016, 120, 15173–15184. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, L. Nitrogen, phosphorus, and fluorine tri-doped graphene as a multifunctional catalyst for self-powered electrochemical water splitting. Angew. Chem. Int. Ed. 2016, 128, 13490–13494. [Google Scholar] [CrossRef]
- Wang, D.-W.; Su, D. Heterogeneous nanocarbon materials for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 576–591. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Qiu, F.; Zhang, T.; Xu, J. Controllable preparation of FeOOH/CuO@WBC composite based on water bamboo cellulose applied for enhanced arsenic removal. Food Bioprod. Process. 2020, 123, 177–187. [Google Scholar] [CrossRef]
- Shao, Z.; Xing, C.; Xue, M.; Fang, Y.; Li, P. Selective removal of Pb(II) from yellow rice wine using magnetic carbon-based adsorbent. J. Sci. Food Agric. 2023, 103, 6929–6939. [Google Scholar] [CrossRef] [PubMed]
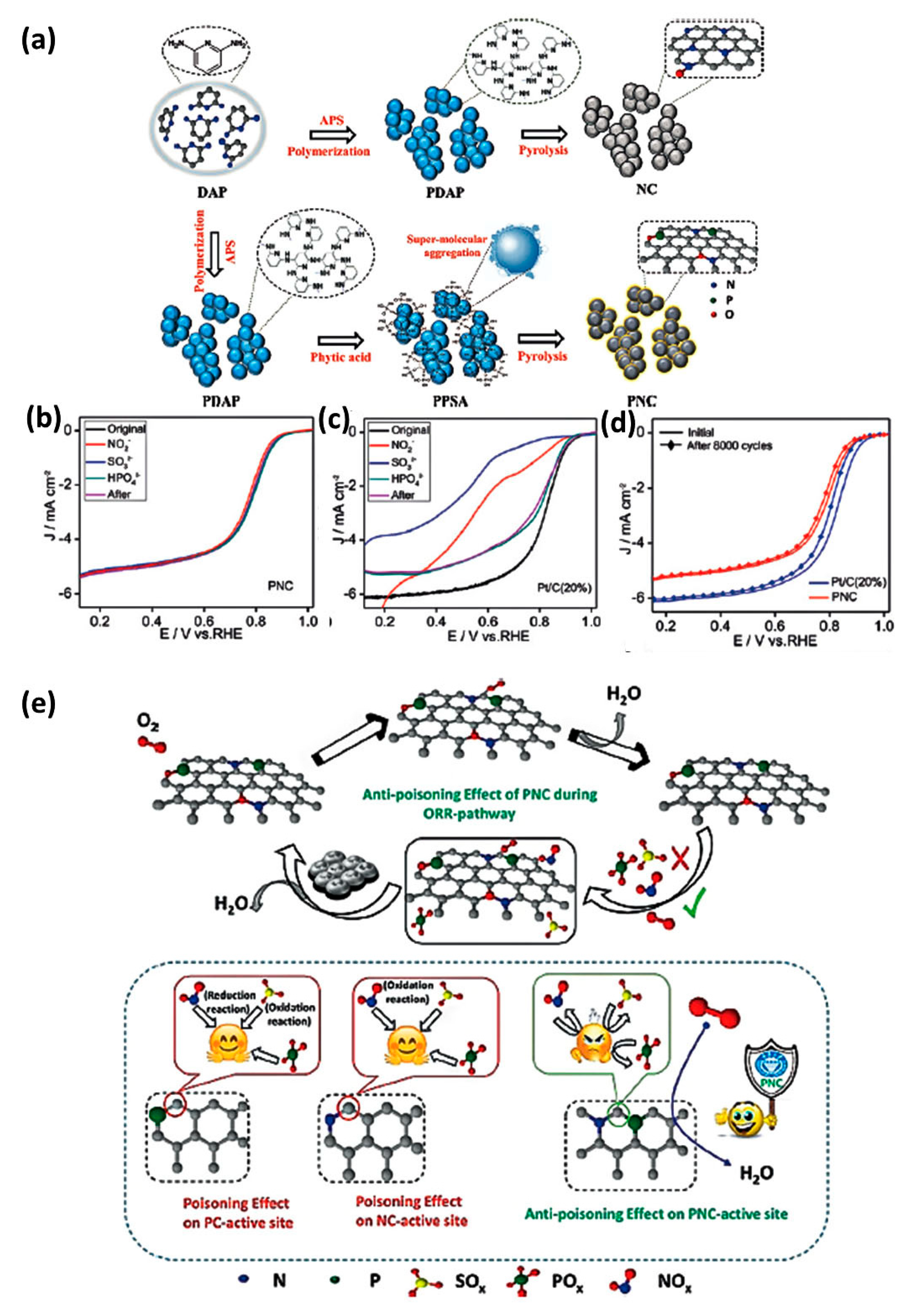
- Najam, T.; Shah, S.S.A.; Ding, W.; Jiang, J.; Jia, L.; Yao, W.; Li, L.; Wei, Z. An efficient anti-poisoning catalyst against SOx, NOx, and POx: P, N-doped carbon for oxygen reduction in acidic media. Angew. Chem. Int. Ed. 2018, 130, 15321–15326. [Google Scholar] [CrossRef]
- Mooste, M.; Müller-Hülstede, J.; Schonvogel, D.; Zierdt, T.; Buschermöhle, J.; Fuhrmann, K.; Wilhelm, M.; Wagner, P.; Friedrich, K.A. Binary transition metal and ZIF-8 functionalised polymer-derived ceramic catalysts for high temperature PEM fuel cell cathode. Electrochim. Acta 2025, 514, 145620. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Mohamed, T.A.; Essa, A.F.; Abd-El Gawad, A.M.; Alqahtani, A.S.; Shahat, A.A.; Yoneyama, T.; Farrag, A.R.H.; Noji, M.; El-Seedi, H.R.; et al. Recent Advances in Kaempferia Phytochemistry and Biological Activity: A Comprehensive Review. Nutrients 2019, 11, 2396. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Dubau, L.; Jaouen, F.; Maillard, F. Review on the degradation mechanisms of metal-NC catalysts for the oxygen reduction reaction in acid electrolyte: Current understanding and mitigation approaches. Chem. Rev. 2023, 123, 9265–9326. [Google Scholar] [CrossRef]
- Zierdt, T.; Müller-Hülstede, J.; Schmies, H.; Schonvogel, D.; Wagner, P.; Friedrich, K.A. Effect of polytetrafluorethylene content in Fe-N-C-based catalyst layers of gas diffusion electrodes for HT-PEM fuel cell applications. ChemElectroChem 2024, 11, e202300583. [Google Scholar] [CrossRef]
- Han, J.; Feng, H.; Wu, J.; Li, Y.; Zhou, Y.; Wang, L.; Luo, P.; Wang, Y. Construction of Multienzyme Co-immobilized Hybrid Nanoflowers for an Efficient Conversion of Cellulose into Glucose in a Cascade Reaction. J. Agric. Food. Chem. 2021, 69, 7910–7921. [Google Scholar] [CrossRef] [PubMed]
- Martinaiou, I.; Daletou, M.K. Enhancing Electrode Efficiency in Proton Exchange Membrane Fuel Cells with PGM-Free Catalysts: A Mini Review. Energies 2024, 17, 3443. [Google Scholar] [CrossRef]
- Chowdury, M.S.K.; Park, Y.; Park, S.B.; Park, Y.-i. Degradation Mechanisms Long-Term durability Challenges mitigation methods for proton exchange membranes membrane electrode assemblies with Pt/Celectrocatalysts in Low-Temperature High-Temperature fuel Cells: Acomprehensive review. J. Electroanal. Chem. 2024, 975, 118712. [Google Scholar] [CrossRef]
- Lim, K.H.; Matanovic, I.; Maurya, S.; Kim, Y.; De Castro, E.S.; Jang, J.-H.; Park, H.; Kim, Y.S. High temperature polymer electrolyte membrane fuel cells with high phosphoric acid retention. ACS Energy Lett. 2022, 8, 529–536. [Google Scholar] [CrossRef]
- Tellez-Cruz, M.M.; Escorihuela, J.; Solorza-Feria, O.; Compañ, V. Proton exchange membrane fuel cells (PEMFCs): Advances and challenges. Polymers 2021, 13, 3064. [Google Scholar] [CrossRef]
- Hossen, M.M.; Artyushkova, K.; Atanassov, P.; Serov, A. Synthesis and characterization of high performing Fe-N-C catalyst for oxygen reduction reaction (ORR) in Alkaline Exchange Membrane Fuel Cells. J. Power Sources 2018, 375, 214–221. [Google Scholar] [CrossRef]

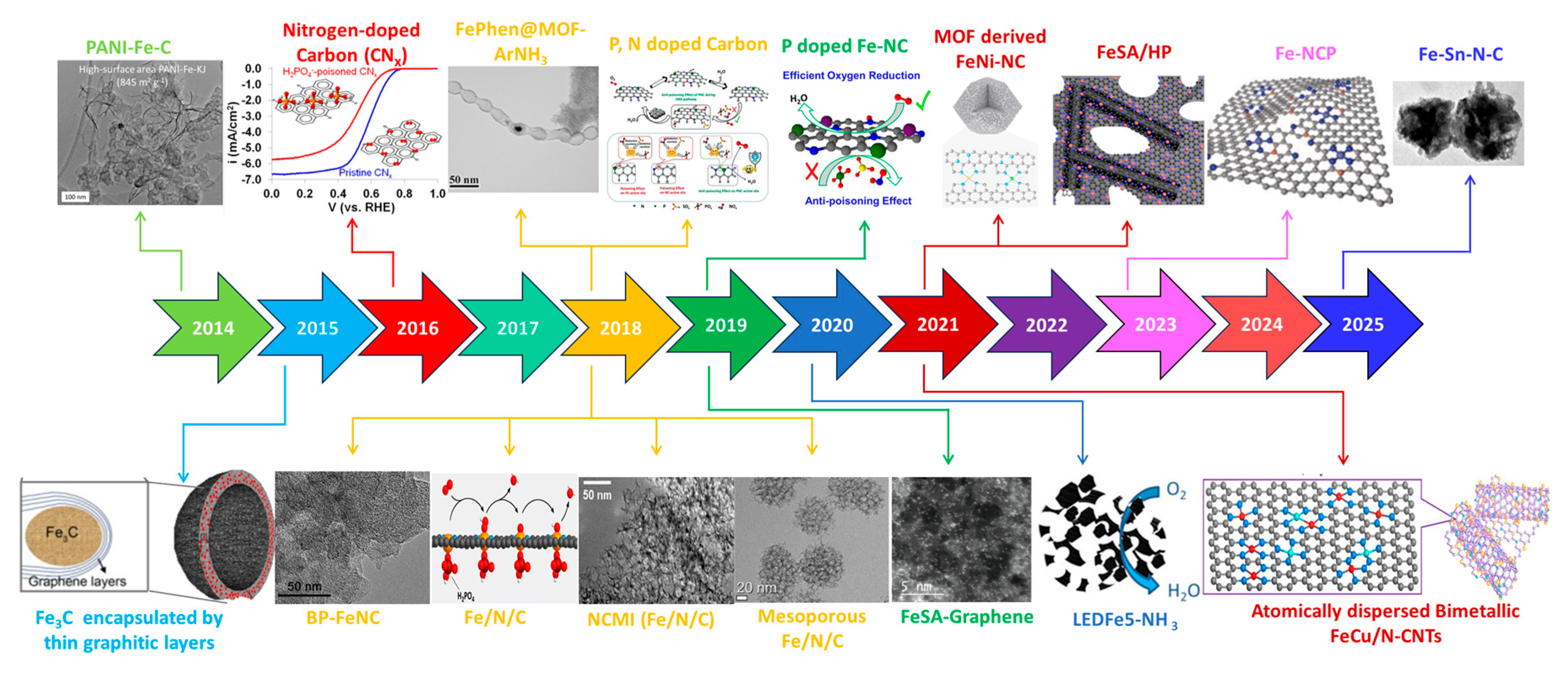
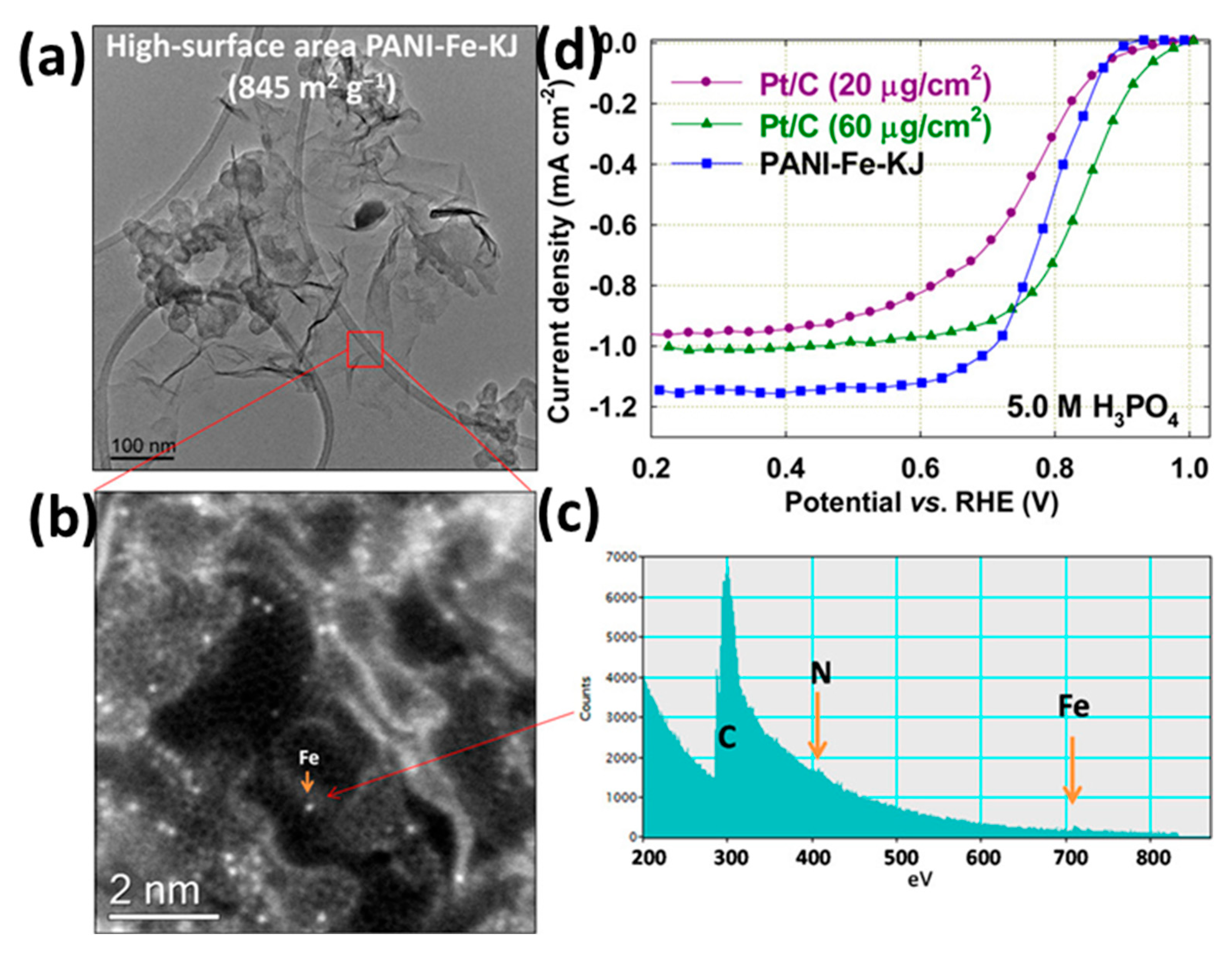
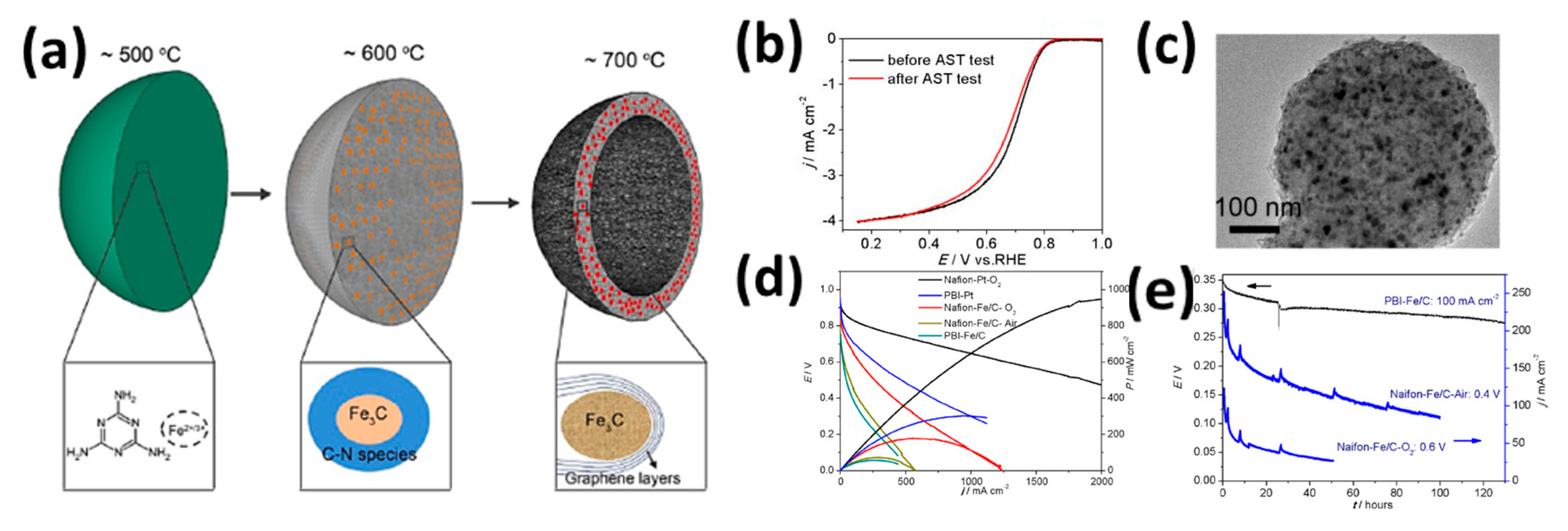
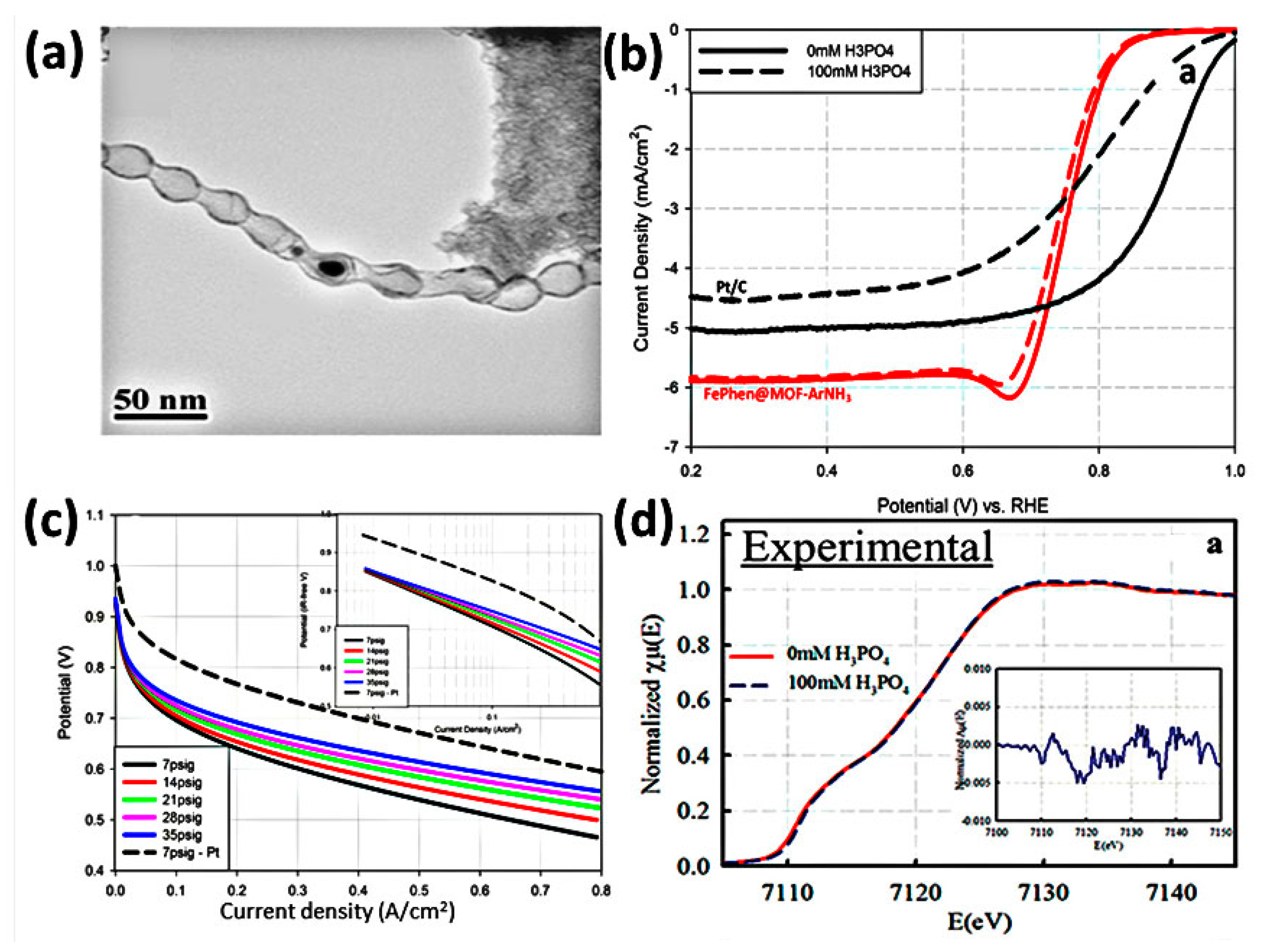
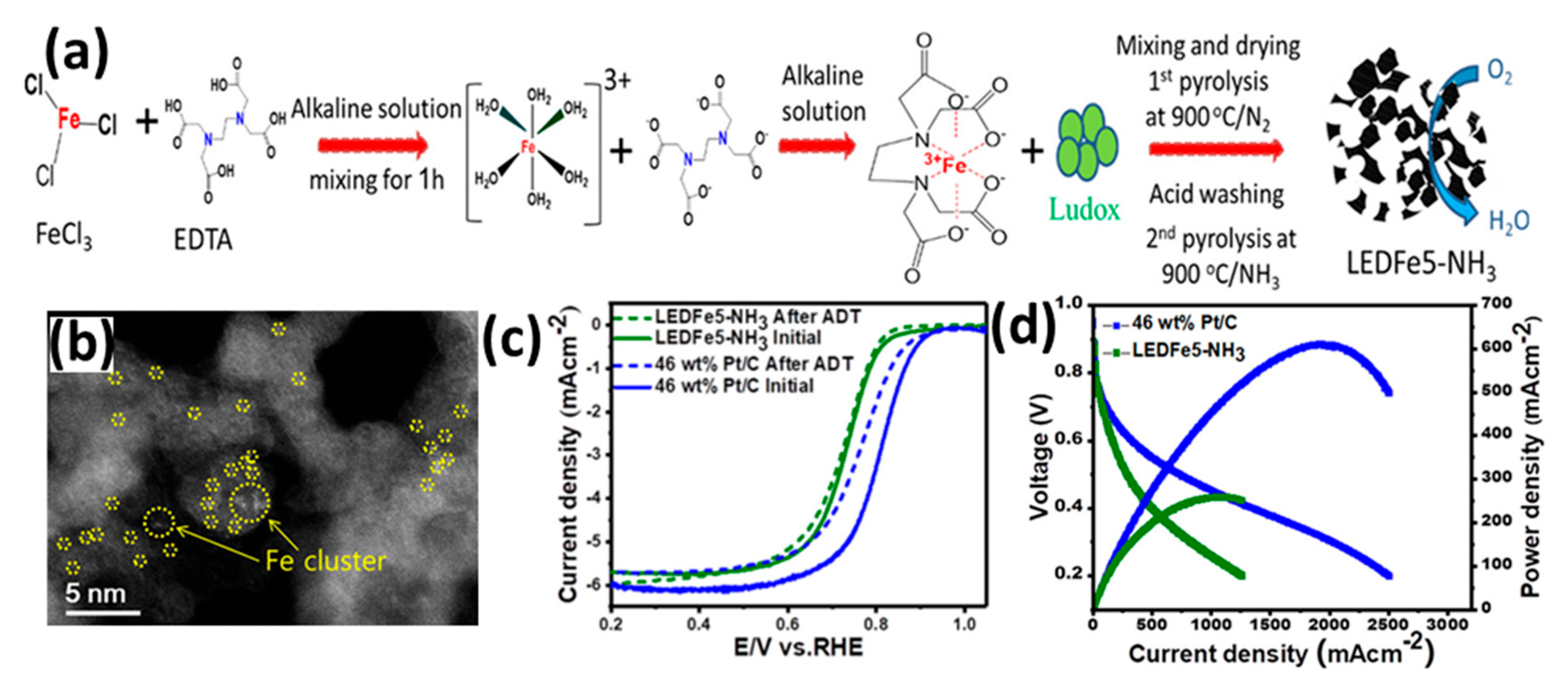
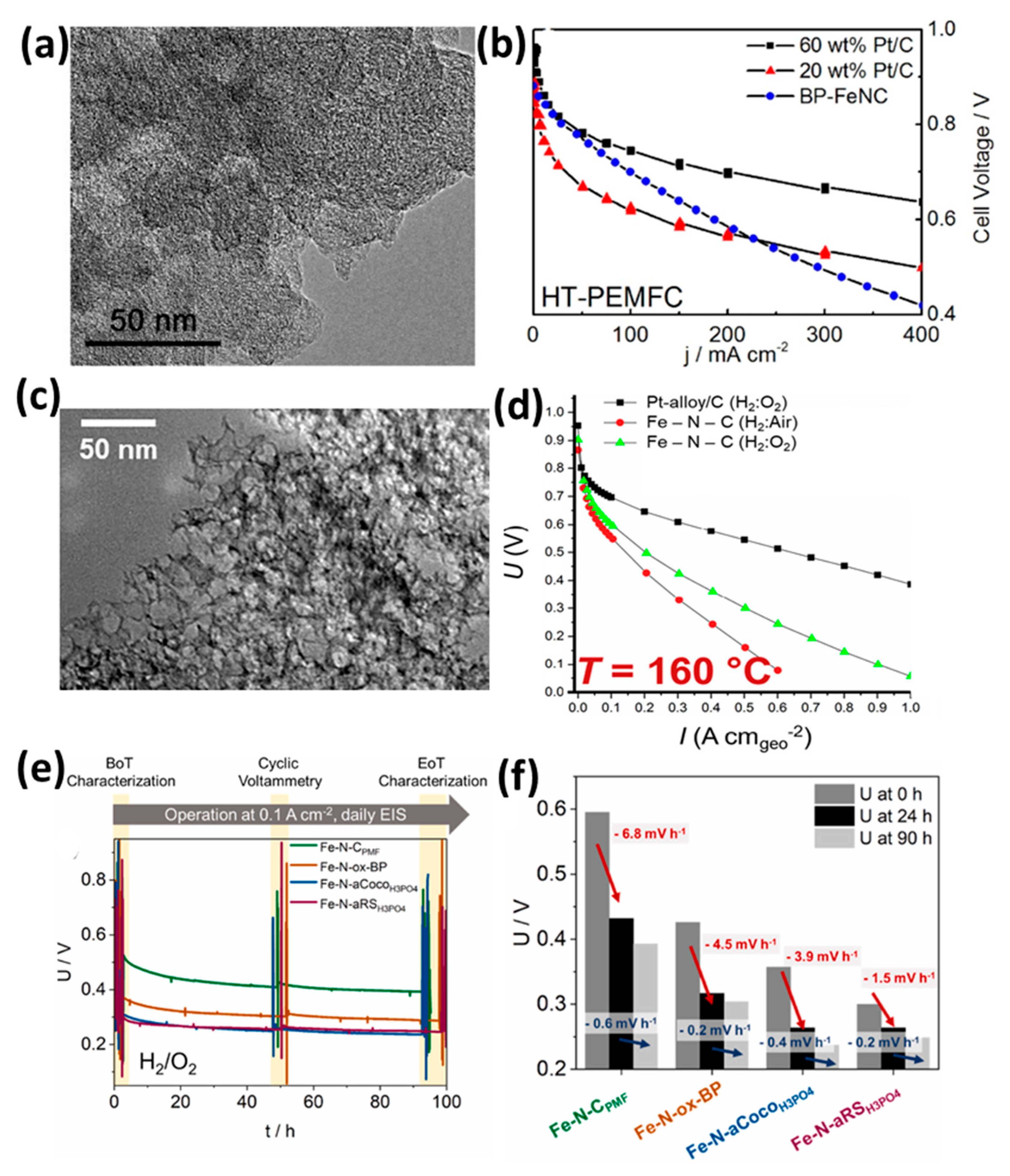
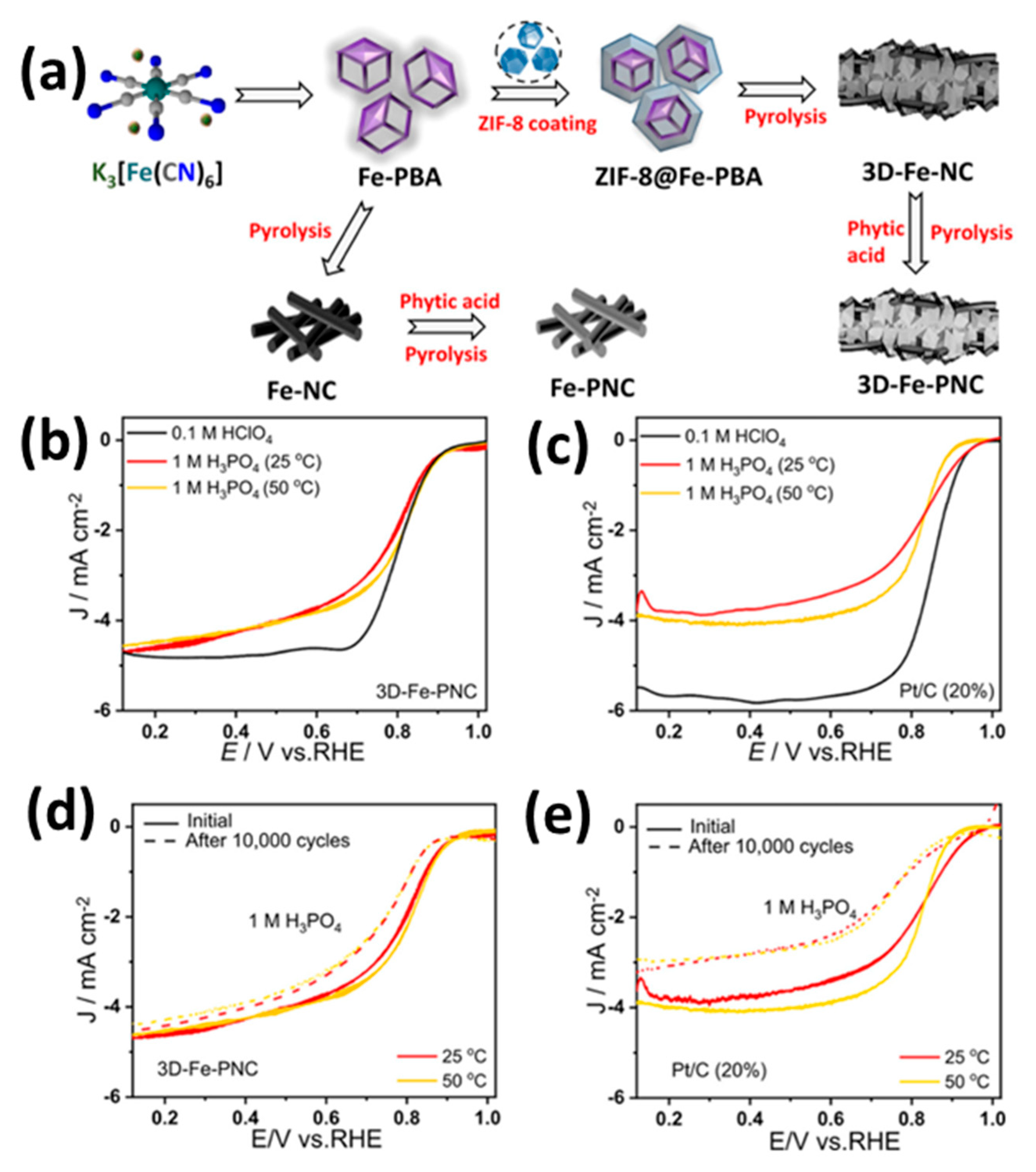
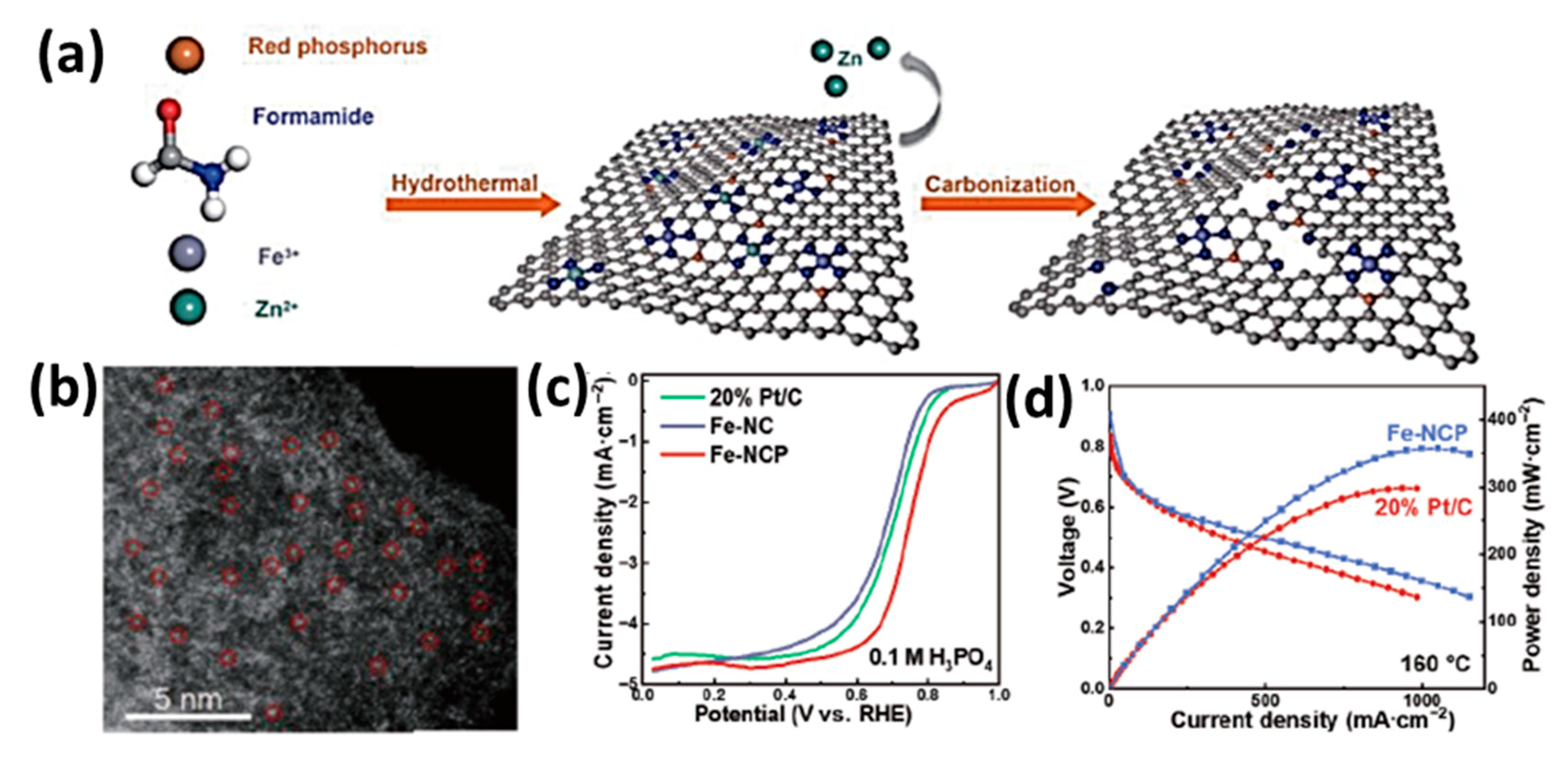

| Feature | LT-PEMFC | HT-PEMFC |
|---|---|---|
| Operating Temperature | 50–100 °C | 120–200 °C |
| Electrolyte | Perfluorosulfonic acid (PFSA) polymer (e.g., Nafion) | Phosphoric acid (PA)-doped polymer (e.g., Polybenzimidazole, PBI) |
| Proton Conduction Mechanism | Vehicular (via H2O molecules) | Grotthuss (hopping via PA network) |
| Water Management | Requires external humidification; prone to flooding/dehydration | No external humidification needed; simplified water removal (vapor phase) |
| Thermal Management | Requires large cooling system due to small ΔT | Simplified with smaller cooling system due to large ΔT; high-quality waste heat |
| Fuel Impurity Tolerance (CO) | Very low (<10 ppm) | Very high (1–3%) |
| Typical Cathode Catalyst | Pt/C, Pt-alloy/C | Pt/C, Pt-alloy/C |
| Typical Cathode Pt Loading | 0.1–0.4 mg cm−2 | >1.0 mg cm−2 |
| Key Advantages | Fast start-up and high power density | Fuel flexibility, simplified BoP, CHP potential, and enhanced kinetics |
| Key Challenges | Water management, CO poisoning, and high-purity H2 requirement | Catalyst poisoning by phosphate, long-term durability, and acid management |
| Catalyst Type | Cathode Loading (mg cm−2) | Anode Catalyst | Membrane | Temp. (°C) | Test Conditions | Peak Power Density (mW cm−2) | Performance Metric | Durability/Degradation | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Fe/C-700 | 6.3 | Pt/C | PA-doped PBI membrane | 120–180 | H2/O2, 2.0 bar | 60 @ 160 °C | 0.44 A cm−2 @ 0.8 V | 130 h | [82] |
| BP-Fe-N-C | 7.8 | Pt/C | PA-doped PBI membrane | 160 | H2/O2, 20 mL min−1 | 700 | 0.57 A cm−2 @ 0.6 V | 34% loss @ 0.6 V after 400 h | [94] |
| FePhen@MOF-ArNH3 | 2 | Commercial Advent A1100W Pt GDE | PBI membrane | 200 | H2/O2 | 137 | 0.55 A cm−2 @ 0.6 V | - | [89] |
| Fe-N-C | 2 | Pt/C | PBI membrane | 160 | H2/O2, 500/400 sccm | - | 0.65 V @ 0.2 A cm−2 | 250 h | [95] |
| Fe-N-C | 21 | Pt/C | PA-doped BASF membrane | 150 | H2/O2, 200/600 mL min−1 | 20 | 20 mA cm−2 @ 0.6 V | - | [97] |
| FeSA-G | 0.3FeSA | Pt/C | SiO2 nanoparticle-doped PA/PBI membrane | 230 | H2/O2 | 325 | 353 mA cm−2 @ 0.5 V | 16% loss @ 0.5 V after 100 h | [120] |
| LEDFe5-NH3 | 3.8 | Pt/C | PA-doped PBI membrane | 150 | H2/O2, 1.5 bar | 260 | 1260 mA cm−2 @ 0.2 V and 78 mA cm−2 @ 0.7 V | - | [90] |
| FeCu/N-CNTs | 4 (0.12FeCu) | Pt/C | SiO2 nanoparticle-doped PA/PBI membrane | 230 | H2/O2, 100 mL min−1 | 302 | 320 mA cm−2 @ 0.5 V | No loss @ 0.5 V after 100 h | [122] |
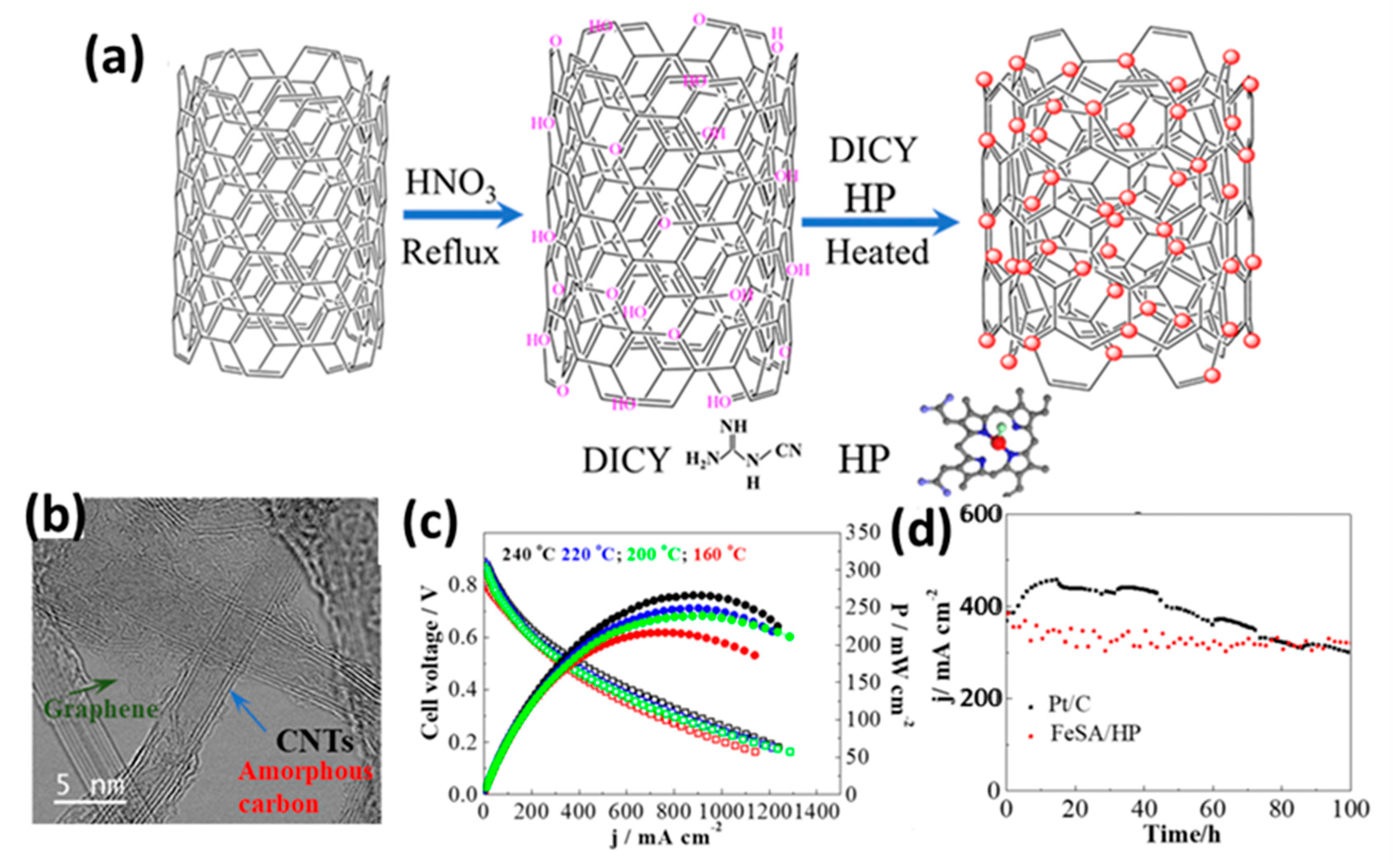
| FeSA/HP | 4 (0.13Fe) | Pt/C | SiO2 nanoparticle-doped PA/PBI membrane | 240 | Anhydrous H2/O2, 150/100 sccm | 266 | 365 mA cm−2 @ 0.5 V | 12% loss @ 0.5 V after 100 h | [121] |
| Fe-NCP | 2.5 | Pt/C | PA-doped PBI membrane | 160 | H2/O2 | 357 | - | - | [104] |
| CoFe-N-SiOCa | 3 | Pt/C | PA-doped PBI membrane | 160 | H2/O2, 1.5/9.5 | 34 | - | - | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, N.; Ravichandran, B.; Emayavaramban, I.; Liu, H.; Su, H. Advancements in Non-Precious Metal Catalysts for High-Temperature Proton-Exchange Membrane Fuel Cells: A Comprehensive Review. Catalysts 2025, 15, 775. https://doi.org/10.3390/catal15080775
Narayanan N, Ravichandran B, Emayavaramban I, Liu H, Su H. Advancements in Non-Precious Metal Catalysts for High-Temperature Proton-Exchange Membrane Fuel Cells: A Comprehensive Review. Catalysts. 2025; 15(8):775. https://doi.org/10.3390/catal15080775
Chicago/Turabian StyleNarayanan, Naresh, Balamurali Ravichandran, Indubala Emayavaramban, Huiyuan Liu, and Huaneng Su. 2025. "Advancements in Non-Precious Metal Catalysts for High-Temperature Proton-Exchange Membrane Fuel Cells: A Comprehensive Review" Catalysts 15, no. 8: 775. https://doi.org/10.3390/catal15080775
APA StyleNarayanan, N., Ravichandran, B., Emayavaramban, I., Liu, H., & Su, H. (2025). Advancements in Non-Precious Metal Catalysts for High-Temperature Proton-Exchange Membrane Fuel Cells: A Comprehensive Review. Catalysts, 15(8), 775. https://doi.org/10.3390/catal15080775









