Synergistic Effects of UV Radiation and H2O2 on Chloramphenicol Degradation by REE-Based Catalysts
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of REE Oxides
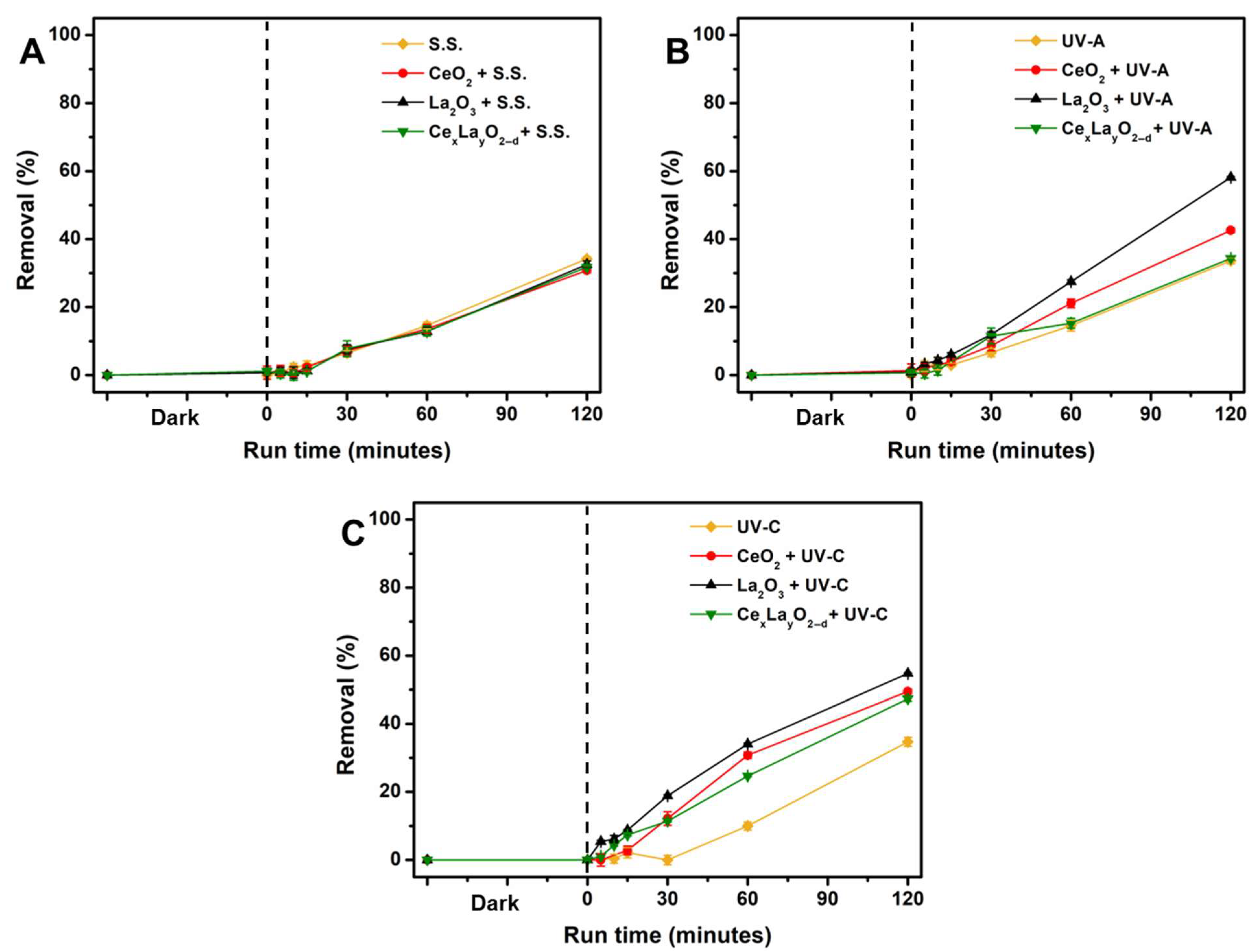
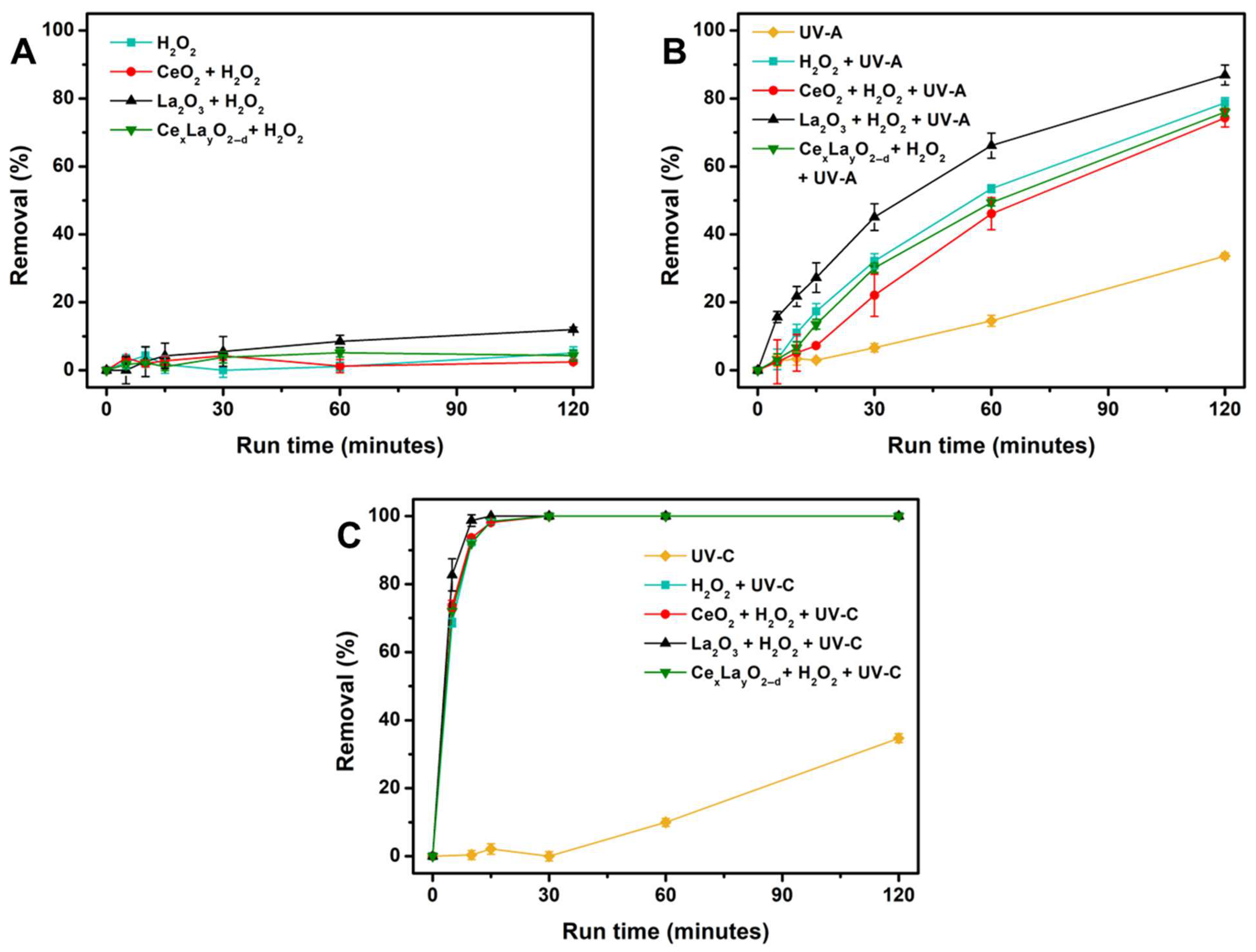
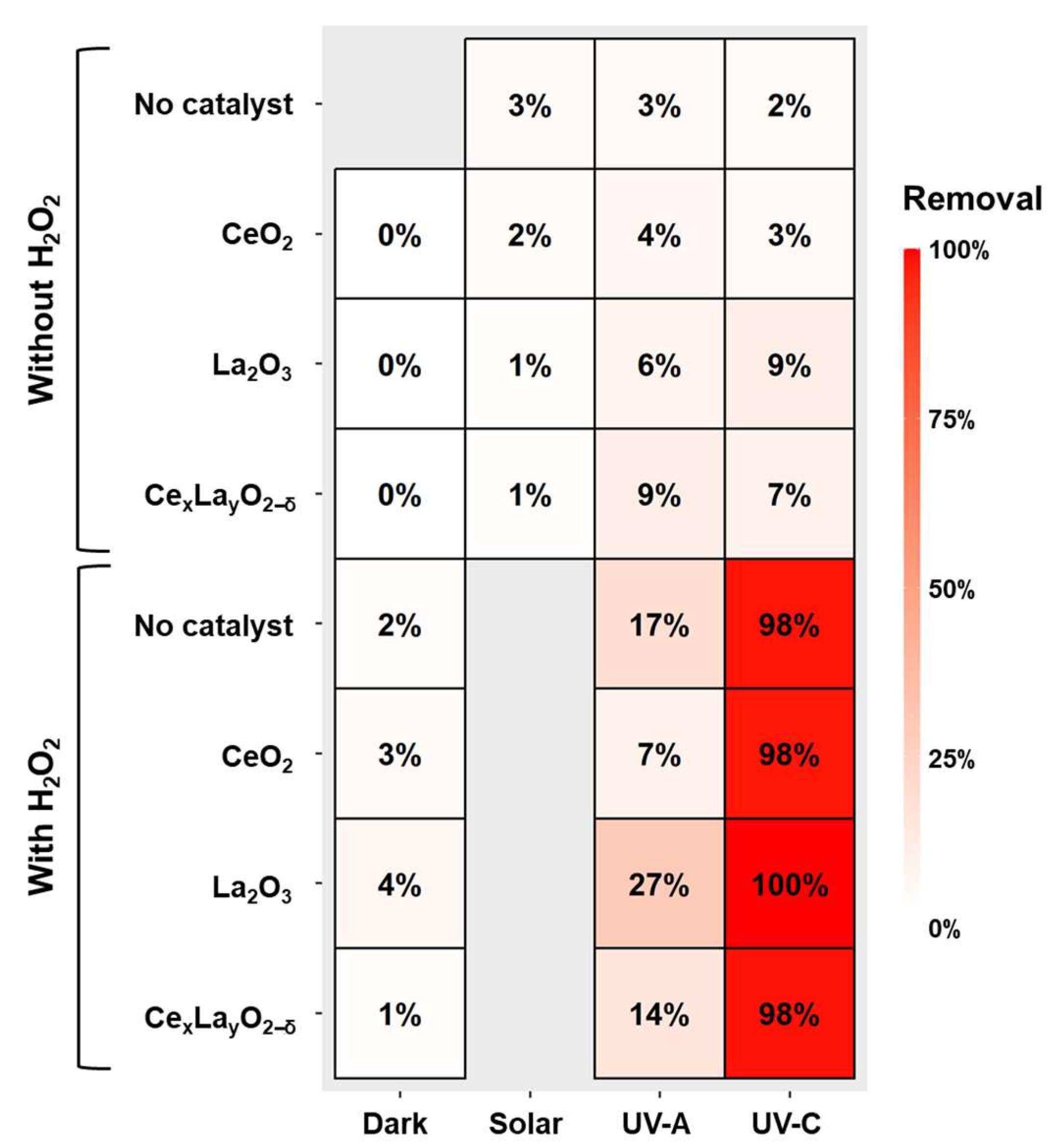
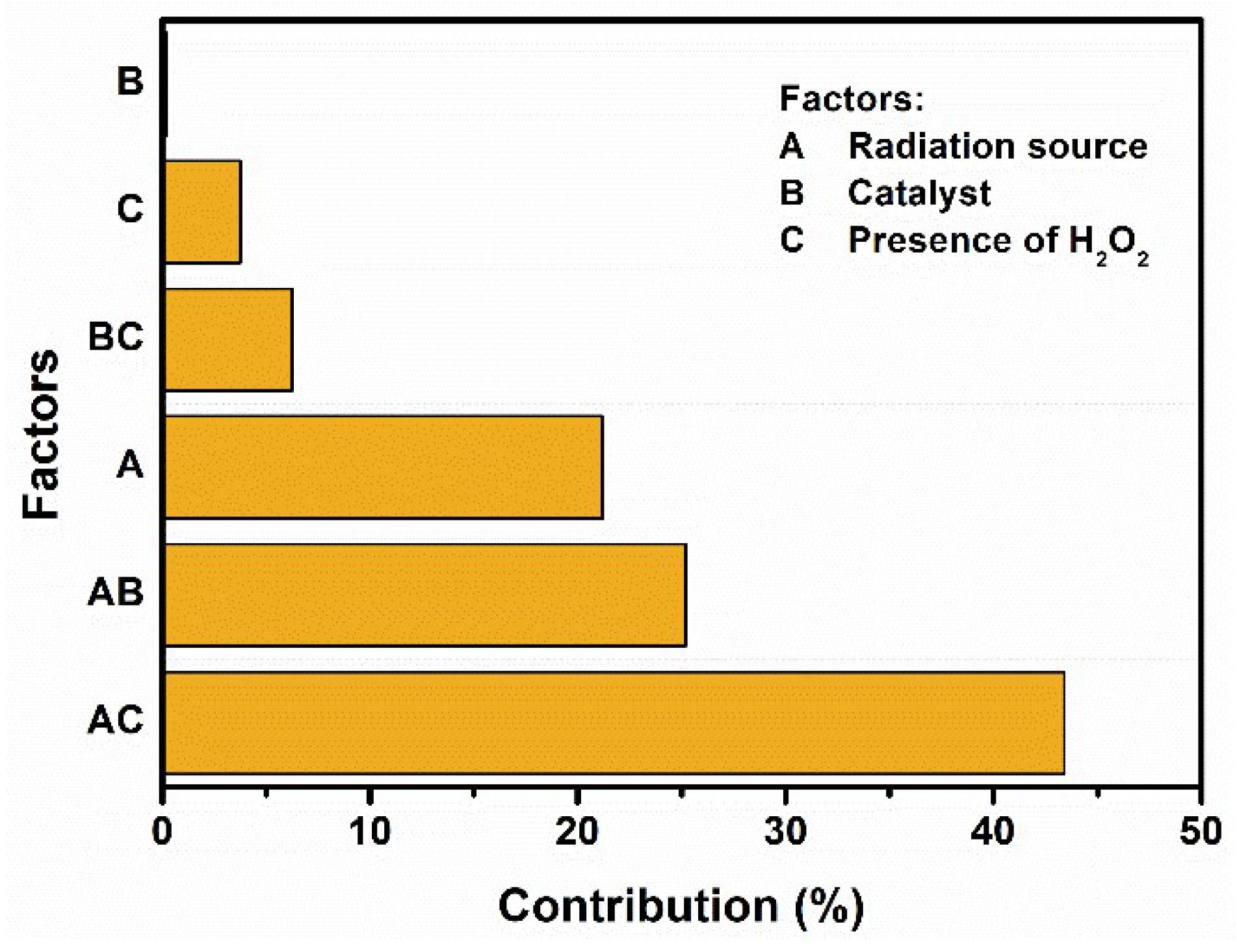
2.2. Photolysis and Photocatalytic Degradation of CAP
3. Materials and Methods
3.1. Synthesis of RRE Oxides
3.2. Degradation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Shen, X.; Jiang, W.; Xi, Y.; Li, S. Comprehensive review of emerging contaminants: Detection technologies, environmental impact, and management strategies. Ecotoxicol. Environ. Saf. 2024, 278, 116420. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, C.; Geladakis, G.; Kommata, V.; Batargias, C.; Lagoumintzis, G. Insights in pharmaceutical pollution: The prospective role of eDNA metabarcoding. Toxics 2023, 11, 903. [Google Scholar] [CrossRef]
- Ehalt Macedo, H.; Lehner, B.; Nicell, J.A.; Khan, U.; Klein, E.Y. Antibiotics in the global river system arising from human consumption. PNAS Nexus 2025, 4, pgaf096. [Google Scholar] [CrossRef]
- Barrocas, B.T.; Fernandes, S.M.; Alcobia, T.; Lourenço, M.C.; Oliveira, M.C.; Marques, A.C. Optimization of TiO2 loaded sol-gel derived MICROSCAFS® for enhanced minocycline removal from water and real wastewater. J. Sol-Gel Sci. Technol. 2025, 1–16. [Google Scholar] [CrossRef]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jonidi Jafari, A.; Rezaei Kalantary, R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of emerging concern: A review of new approach in AOP technologies. Environ. Monit. Assess. 2017, 189, 414. [Google Scholar] [CrossRef]
- Yadav, D.; Rangabhashiyam, S.; Verma, P.; Singh, P.; Devi, P.; Kumar, P.; Mustansar Hussain, C.; Gaurav, G.K.; Kumar, K.S. Environmental and health impacts of contaminants of emerging concerns: Recent treatment challenges and approaches. Chemosphere 2021, 272, 129492. [Google Scholar] [CrossRef]
- Saviano, L.; Brouziotis, A.A.; Padilla Suarez, E.G.; Siciliano, A.; Spampinato, M.; Guida, M.; Trifuoggi, M.; Del Bianco, D.; Carotenuto, M.; Romano Spica, V.; et al. Catalytic activity of rare earth elements (REEs) in advanced oxidation processes of wastewater pollutants: A review. Molecules 2023, 28, 6185. [Google Scholar] [CrossRef]
- Li, Q.; Song, L.; Liang, Z.; Sun, M.; Wu, T.; Huang, B.; Luo, F.; Du, Y.; Yan, C.H. A review on CeO2-based electrocatalyst and photocatalyst in energy conversion. Adv. Energy Sustain. Res. 2021, 2, 2000063. [Google Scholar] [CrossRef]
- Khan, A.A.; Partho, A.T.; Arnab, M.H.; Khyam, M.A.; Kumar, N.; Tahir, M. Recent advances in Lanthanum-based photocatalysts with engineering aspects for photocatalytic hydrogen production: A critical review. Mater. Sci. Semicond. Process. 2024, 184, 108809. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Han, D.H.; Lee, J.; Cho, M.H. Defect-induced band gap narrowed CeO2 nanostructures for visible light activities. Ind. Eng. Chem. Res. 2014, 53, 9754–9763. [Google Scholar] [CrossRef]
- Pandey, A.; Jain, G.; Vyas, D.; Irusta, S.; Sharma, S. Nonreducible, Basic La2O3 to Reducible, Acidic La2–xSbxO3 with Significant Oxygen Storage Capacity, Lower Band Gap, and Effect on the Catalytic Activity. J. Phys. Chem. C 2017, 121, 481–489. [Google Scholar] [CrossRef]
- Rehman, Y.; Morlando, A.; Chaki Borras, M.; Sluyter, R.; Wang, X.; Huang, X.F.; Konstantinov, K. Defect-rich La2O3 nanoparticles with antioxidant activity for human keratinocytes. ACS Appl. Nano Mater. 2021, 4, 6345–6356. [Google Scholar] [CrossRef]
- Hu, J.; Wu, B.; Chen, L.; Song, C.; Yang, H.; Long, F.; Sun, J.; Chi, R.A. Influences of CeO2 morphology on enhanced performance of electro-Fenton for wastewater treatment. J. Rare Earths 2022, 40, 1870–1877. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Sayyar, Z. Rare-Earth-Based Materials for Heterogeneous Photocatalysis. In Concepts of Semiconductor Photocatalysis; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Alobi, N.O.; Ita, B.I.; Odey, M.T.; Nyong, B.E. The activity of γ–Al2O3 and La2O3 in peroxide decomposition. J. Ind. Technol. 2016, 1, 69–72. [Google Scholar]
- Singh, K.; Kumar, K.; Srivastava, S.; Chowdhury, A. Effect of rare-earth doping in CeO2 matrix: Correlations with structure, catalytic and visible light photocatalytic properties. Ceram. Int. 2017, 43, 17041–17047. [Google Scholar] [CrossRef]
- Cardito, A.; Albarano, L.; Sacco, O.; Vaiano, V.; Lettieri, M.; Libralato, G.; Lofrano, G.; Carotenuto, M. Removal and toxicity effects of chloramphenicol and acid orange solutions using zero-valent iron nanoparticles. J. Water Process Eng. 2025, 69, 106868. [Google Scholar] [CrossRef]
- Nguyen, L.M.; Nguyen, N.T.T.; Nguyen, T.T.T.; Nguyen, T.T.; Nguyen, D.T.C.; Tran, T.V. Occurrence, toxicity and adsorptive removal of the chloramphenicol antibiotic in water: A review. Environ. Chem. Lett. 2022, 20, 1929–1963. [Google Scholar] [CrossRef]
- Iannaco, M.C.; Mancuso, A.; Mottola, S.; Pipolo, A.; Vaiano, V.; De Marco, I. Visible-Light-Driven Degradation of Chloramphenicol Using CeO2 Nanoparticles Prepared by a Supercritical CO2 Route: A Proof of Concept. Nanomaterials 2025, 15, 102. [Google Scholar] [CrossRef]
- Razali, N.A.; Conte, M.; McGregor, J. The role of impurities in the La2O3 catalysed carboxylation of crude glycerol. Catal. Lett. 2019, 149, 1403–1414. [Google Scholar] [CrossRef]
- Jayakumar, G.; Irudayaraj, A.A.; Raj, A.D. Particle size effect on the properties of cerium oxide (CeO2) nanoparticles synthesized by hydrothermal method. Mech. Mater. Sci. Eng. J. 2017, 9. [Google Scholar] [CrossRef]
- Singh, A.; Palakollu, V.; Pandey, A.; Kanvah, S.; Sharma, S. Green synthesis of 1, 4-benzodiazepines over La2O3 and La(OH)3 catalysts: Possibility of Langmuir–Hinshelwood adsorption. RSC Adv. 2016, 6, 103455–103462. [Google Scholar] [CrossRef]
- Chahal, S.; Singh, S.; Kumar, A.; Kumar, P. Oxygen-deficient lanthanum doped cerium oxide nanoparticles for potential applications in spintronics and photocatalysis. Vacuum 2020, 177, 109395. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Zarinkamar, M.; Firoozabadi, T.P. Synthesis of Cerium Oxide (CeO2) nanoparticles using simple CO-precipitation method. Rev. Mex. De Física 2016, 62, 496–499. [Google Scholar]
- Saviano, L.; Mancuso, A.; Cardito, A.; Sacco, O.; Vaiano, V.; Carotenuto, M.; Libralato, G.; Lofrano, G. Photocatalytic Degradation of Levofloxacin and Inactivation of Enterococci Levofloxacin-Resistant Bacteria Using Pure Rare-Earth Oxides. Separations 2024, 11, 272. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, K.; Jiang, L.; Hou, J.; Yu, X.; Feng, M.; Ye, C. Removal of chloramphenicol antibiotics in natural and engineered water systems: Review of reaction mechanisms and product toxicity. Sci. Total Environ. 2022, 850, 158059. [Google Scholar] [CrossRef]
- Belikov, Y.A.; Snytnikova, O.A.; Sheven, D.G.; Fedunov, R.G.; Grivin, V.P.; Pozdnyakov, I.P. Laser flash photolysis and quantum chemical studies of UV degradation of pharmaceutical drug chloramphenicol: Short-lived intermediates, quantum yields and mechanism of photolysis. Chemosphere 2024, 351, 141211. [Google Scholar] [CrossRef]
- da Rocha, O.R.S.; Pinheiro, R.B.; Duarte, M.M.B.; Dantas, R.F.; Ferreira, A.P.; Benachour, M.; da Silva, V.L. Degradation of the antibiotic chloramphenicol using photolysis and advanced oxidation process with UVC and solar radiation. Desalin. Water Treat. 2013, 51, 7269–7275. [Google Scholar] [CrossRef]
- Trovó, A.G.; de Paiva, V.A.; Machado, A.E.; de Oliveira, C.A.; Santos, R.O. Degradation of the antibiotic chloramphenicol by photo-Fenton process at lab-scale and solar pilot plant: Kinetic, toxicity and inactivation assessment. Sol. Energy 2013, 97, 596–604. [Google Scholar] [CrossRef]
- Lofrano, G.; Libralato, G.; Adinolfi, R.; Siciliano, A.; Iannece, P.; Guida, M.; Volpi Ghirardini, A.; Carotenuto, M. Photocatalytic degradation of the antibiotic chloramphenicol and effluent toxicity effects. Ecotoxicol. Environ. Saf. 2016, 123, 65–71. [Google Scholar] [CrossRef]
- Tan, C.; Fu, D.; Gao, N.; Qin, Q.; Xu, Y.; Xiang, H. Kinetic degradation of chloramphenicol in water by UV/persulfate system. J. Photochem. Photobiol. A Chem. 2017, 332, 406–412. [Google Scholar] [CrossRef]
- Nie, M.; Yan, C.; Xiong, X.; Wen, X.; Yang, X.; Dong, W. Degradation of chloramphenicol using a combination system of simulated solar light, Fe2+ and persulfate. Chem. Eng. J. 2018, 348, 455–463. [Google Scholar] [CrossRef]
- Marson, E.O.; Paniagua, C.E.; Costa-Serge, N.M.; Sousa, R.M.; Silva, G.D.; Becker, R.W.; Sirtori, C.; Starling, M.C.V.M.; Carvalho, S.R.; Trovó, A.G. Chemical and toxicological evaluation along with unprecedented transformation products during photolysis and heterogeneous photocatalysis of chloramphenicol in different aqueous matrices. Environ. Sci. Pollut. Res. 2021, 28, 23582–23594. [Google Scholar] [CrossRef]
- Qu, X.; Wu, H.; Zhang, T.; Liu, Q.; Wang, M.; Yateh, M.; Tang, Y. Degradation of chloramphenicol using UV-LED based advanced oxidation processes: Kinetics, mechanisms, and enhanced formation of disinfection by-products. Water 2021, 13, 3035. [Google Scholar] [CrossRef]
- Zuorro, A.; Fidaleo, M.; Fidaleo, M.; Lavecchia, R. Degradation and antibiotic activity reduction of chloramphenicol in aqueous solution by UV/H2O2 process. J. Environ. Manag. 2014, 133, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Moreno Andrés, J.; Tierno-Galán, M.; Romero Martínez, L.; Acevedo Merino, A.; Nebot Sanz, E. Inactivation of the waterborne marine pathogen Vibrio alginolyticus by photo-chemical processes driven by UV-A, UV-B, or UV-C LED combined with H2O2 or HSO5. Water Res. 2023, 232, 119686. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, R.; Chowdhury, A. Synthesis of La-doped ceria nanoparticles: Impact of lanthanum depletion. J. Mater. Sci. 2016, 51, 4134–4141. [Google Scholar] [CrossRef]
- Akhtar, A.; Akram, K.; Aslam, Z.; Ihsanullah, I.; Baig, N.; Bello, M.M. Photocatalytic degradation of p-nitrophenol in wastewater by heterogeneous cobalt supported ZnO nanoparticles: Modeling and optimization using response surface methodology. Environ. Prog. Sustain. Energy 2023, 42, e13984. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Y.; Liu, Y.; Luo, C.; Wu, D. Enhanced degradation of chloramphenicol at alkaline conditions by S (-II) assisted heterogeneous Fenton-like reactions using pyrite. Chemosphere 2017, 188, 557–566. [Google Scholar] [CrossRef]
- Chatzitakis, A.; Berberidou, C.; Paspaltsis, I.; Kyriakou, G.; Sklaviadis, T.; Poulios, I. Photocatalytic degradation and drug activity reduction of chloramphenicol. Water Res. 2008, 42, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Palma, T.L.; Vieira, B.; Nunes, J.; Lourenço, J.P.; Monteiro, O.C.; Costa, M.C. Photodegradation of chloramphenicol and paracetamol using PbS/TiO2 nanocomposites produced by green synthesis. J. Iran. Chem. Soc. 2020, 17, 2013–2031. [Google Scholar] [CrossRef]
- Balamurugan, K.S.; Rohini, V.; Minnam Reddy, V.R.; Kim, W.K.; Afzal, M. Effective photocatalytic degradation of antibiotic chloramphenicol and anionic direct violet 51 dye using g-C3N4 embedded NiO nanocomposite. Ionics 2024, 30, 4245–4255. [Google Scholar] [CrossRef]
- Xu, Q.; Song, Z.; Ji, S.; Xu, G.; Shi, W.; Shen, L. The photocatalytic degradation of chloramphenicol with electrospun Bi2O2CO3-poly (ethylene oxide) nanofibers: The synthesis of crosslinked polymer, degradation kinetics, mechanism and cytotoxicity. RSC Adv. 2019, 9, 29917–29926. [Google Scholar] [CrossRef] [PubMed]
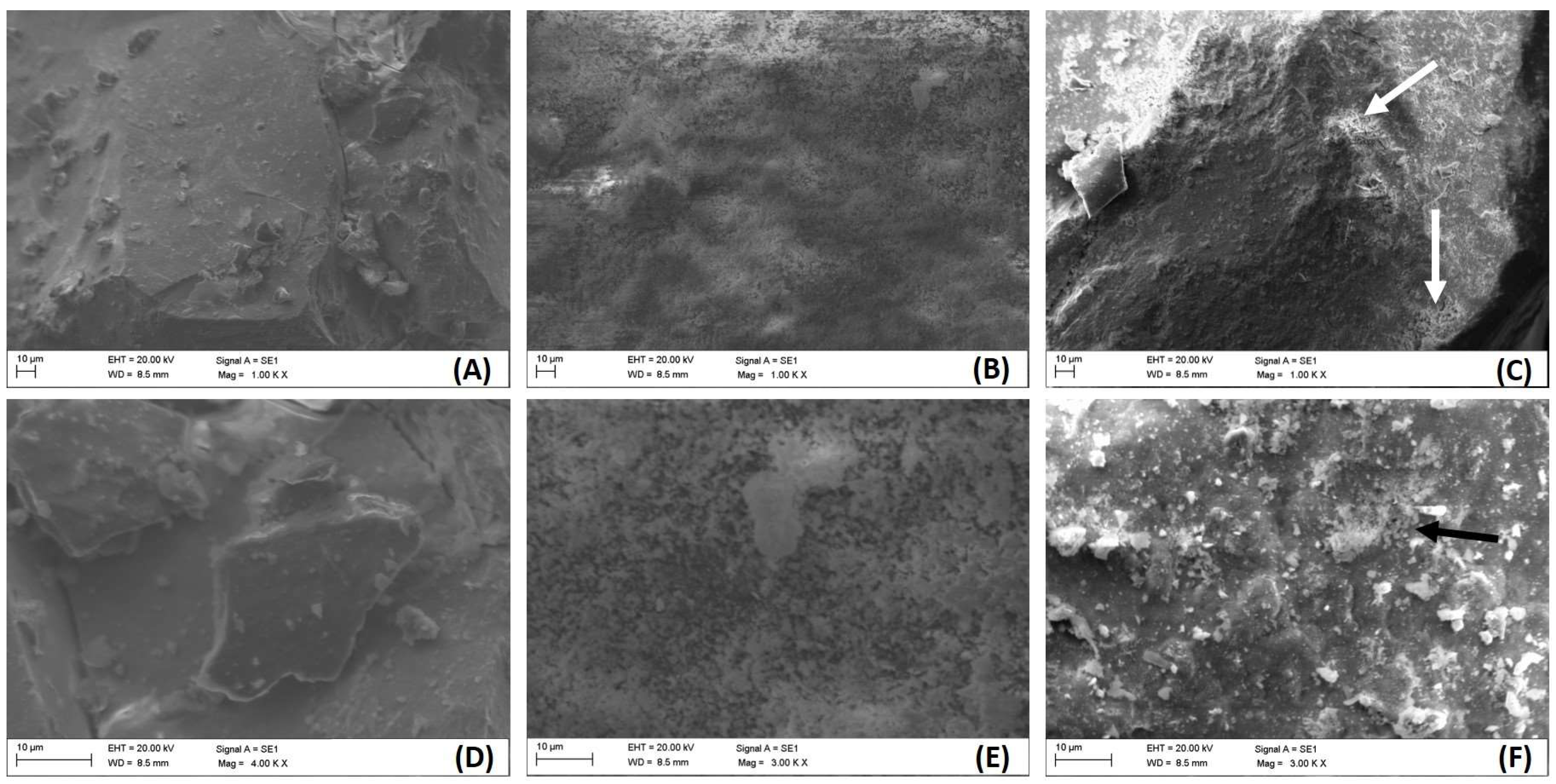
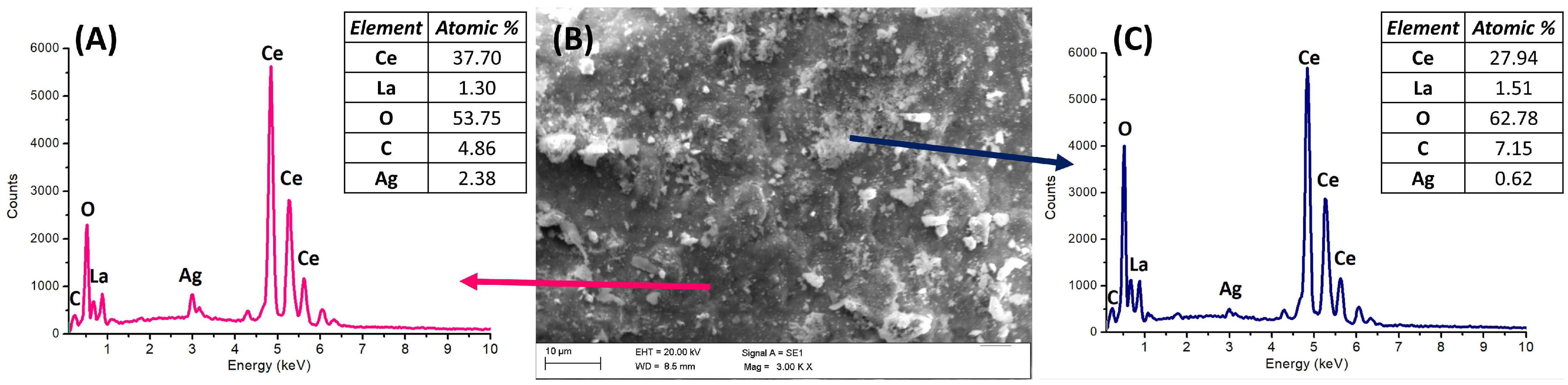
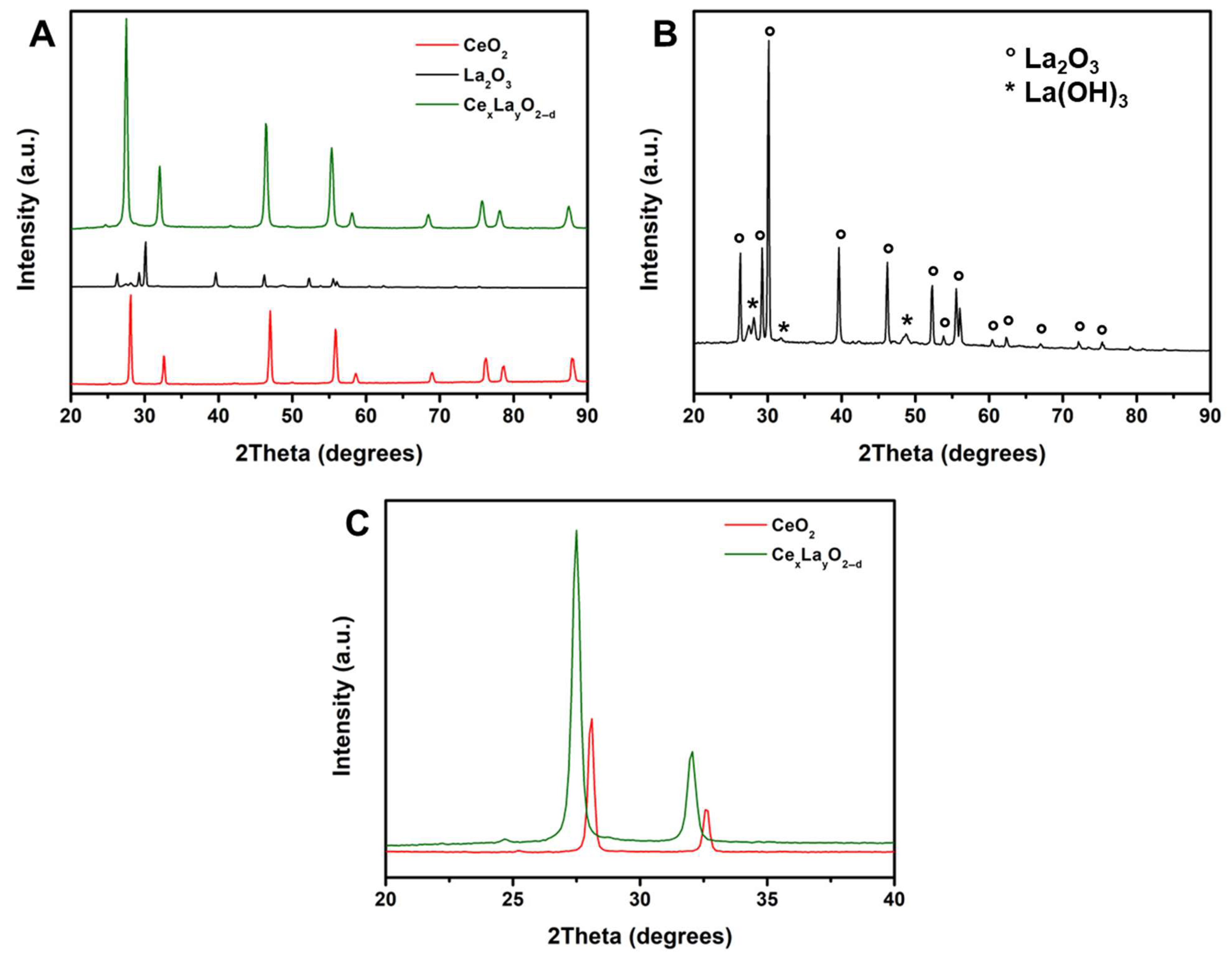
| Ce | O | La | C | Ag | |
|---|---|---|---|---|---|
| CeO2 | 39 ± 11 | 53 ± 11 | --- | 6 ± 2 | 2.3 ± 0.5 |
| La2O3 | --- | 57 ± 4 | 28 ± 6 | 14 ± 6 | 1.6 ± 0.3 |
| CexLayO2−δ | 37 ± 7 | 53 ± 8 | 1.4 ± 0.2 | 7 ± 2 | 2.1 ± 0.9 |
| Initial CAP Concentration (mg/L) | Radiation | Time (min) | Removal | Reference |
|---|---|---|---|---|
| 20 | Solar | 720 | 21.8% | [29] |
| UV-C | 83.3% | [29] | ||
| 200 | Solar | 60 | 34% | [30] |
| 25 | UV-C | 120 | 40% | [31] |
| 9.69 | UV-C | 120 | 20.3% | [32] |
| 16.16 | Solar | 100 | 21.9% | [33] |
| 0.969 | UV-A | 30 | 20% | [34] |
| 5 | UV | 60 | 40% | [35] |
| 1 | Solar simulator | 120 | 34% | This study |
| UV-A | 34% | |||
| UV-C | 35% |
| Process | kobs (min−1) | R2 |
|---|---|---|
| Solar | 0.0032 | 0.98 |
| UV-A | 0.0032 | 0.98 |
| UV-C | 0.0030 | 0.89 |
| Solar/CeO2 | 0.0029 | 0.99 |
| Solar/La2O3 | 0.0030 | 0.96 |
| Solar/CexLayO2−δ | 0.0030 | 0.97 |
| UV-A/CeO2 | 0.0044 | 0.99 |
| UV-A/La2O3 | 0.0067 | 0.98 |
| UV-A/CexLayO2−δ | 0.0034 | 0.98 |
| UV-C/CeO2 | 0.0057 | 0.99 |
| UV-CLa2O3 | 0.0067 | 1.00 |
| UV-C/CexLayO2−δ | 0.0052 | 0.99 |
| UV-A/H2O2 | 0.1280 | 1.00 |
| UV-A/H2O2/CeO2 | 0.1090 | 0.99 |
| UV-A/H2O2/La2O3 | 0.0174 | 1.00 |
| UV-A/H2O2/CexLayO2−δ | 0.0117 | 1.00 |
| UV-C/H2O2 | 0.2669 | 1.00 |
| UV-C/H2O2/CeO2 | 0.2683 | 1.00 |
| UV-C/H2O2/La2O3 | 0.4188 | 0.99 |
| UV-C/H2O2/CexLayO2−δ | 0.2700 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardito, A.; Lettieri, M.; Saviano, L.; Sacco, O.; Lofrano, G.; Vaiano, V.; Libralato, G.; Guida, M.; Carotenuto, M. Synergistic Effects of UV Radiation and H2O2 on Chloramphenicol Degradation by REE-Based Catalysts. Catalysts 2025, 15, 776. https://doi.org/10.3390/catal15080776
Cardito A, Lettieri M, Saviano L, Sacco O, Lofrano G, Vaiano V, Libralato G, Guida M, Carotenuto M. Synergistic Effects of UV Radiation and H2O2 on Chloramphenicol Degradation by REE-Based Catalysts. Catalysts. 2025; 15(8):776. https://doi.org/10.3390/catal15080776
Chicago/Turabian StyleCardito, Alice, Mariateresa Lettieri, Lorenzo Saviano, Olga Sacco, Giusy Lofrano, Vincenzo Vaiano, Giovanni Libralato, Marco Guida, and Maurizio Carotenuto. 2025. "Synergistic Effects of UV Radiation and H2O2 on Chloramphenicol Degradation by REE-Based Catalysts" Catalysts 15, no. 8: 776. https://doi.org/10.3390/catal15080776
APA StyleCardito, A., Lettieri, M., Saviano, L., Sacco, O., Lofrano, G., Vaiano, V., Libralato, G., Guida, M., & Carotenuto, M. (2025). Synergistic Effects of UV Radiation and H2O2 on Chloramphenicol Degradation by REE-Based Catalysts. Catalysts, 15(8), 776. https://doi.org/10.3390/catal15080776











