Improvement of Cleaner Composting Production by Manganese Dioxide Nanozyme with Streptomyces rochei ZY-2: From the Humus Formation to Greenhouse Gas Emissions
Abstract
1. Introduction
2. Results
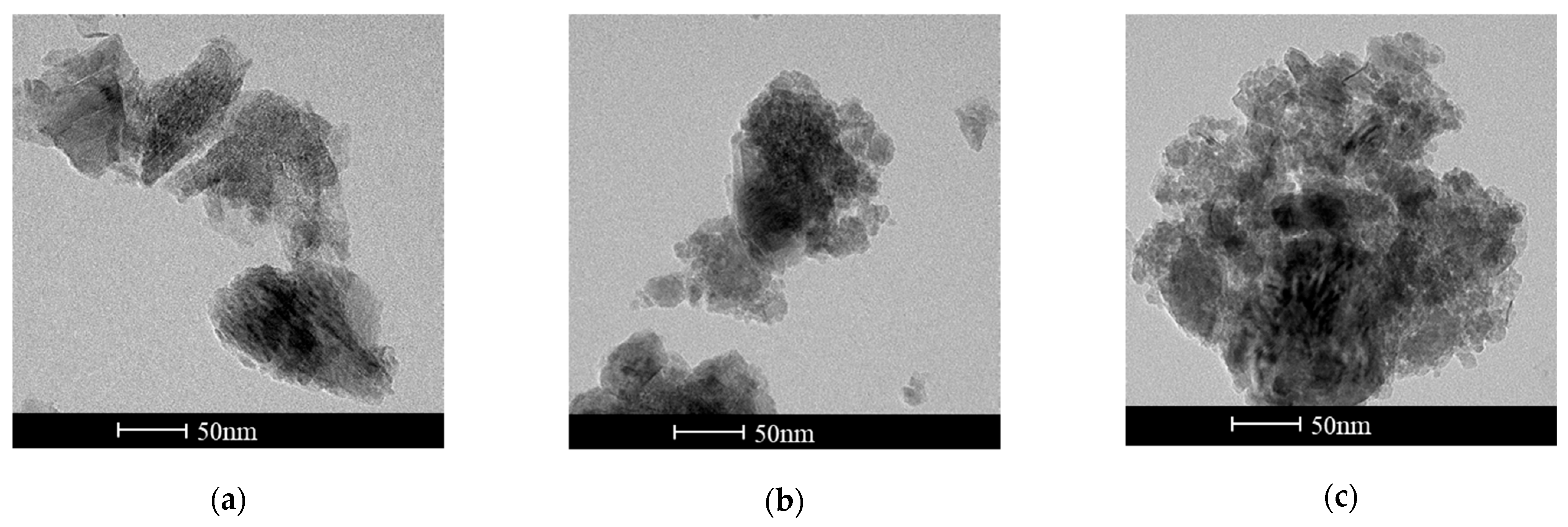
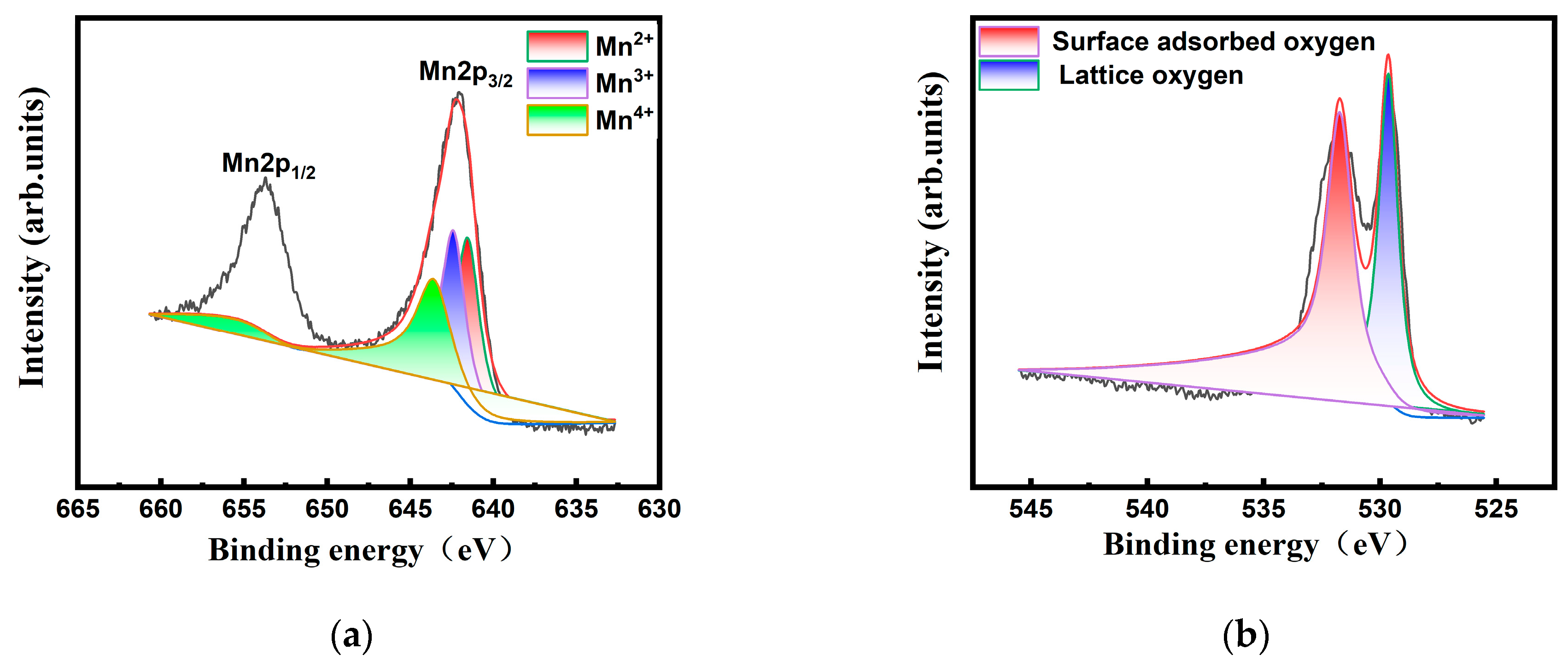
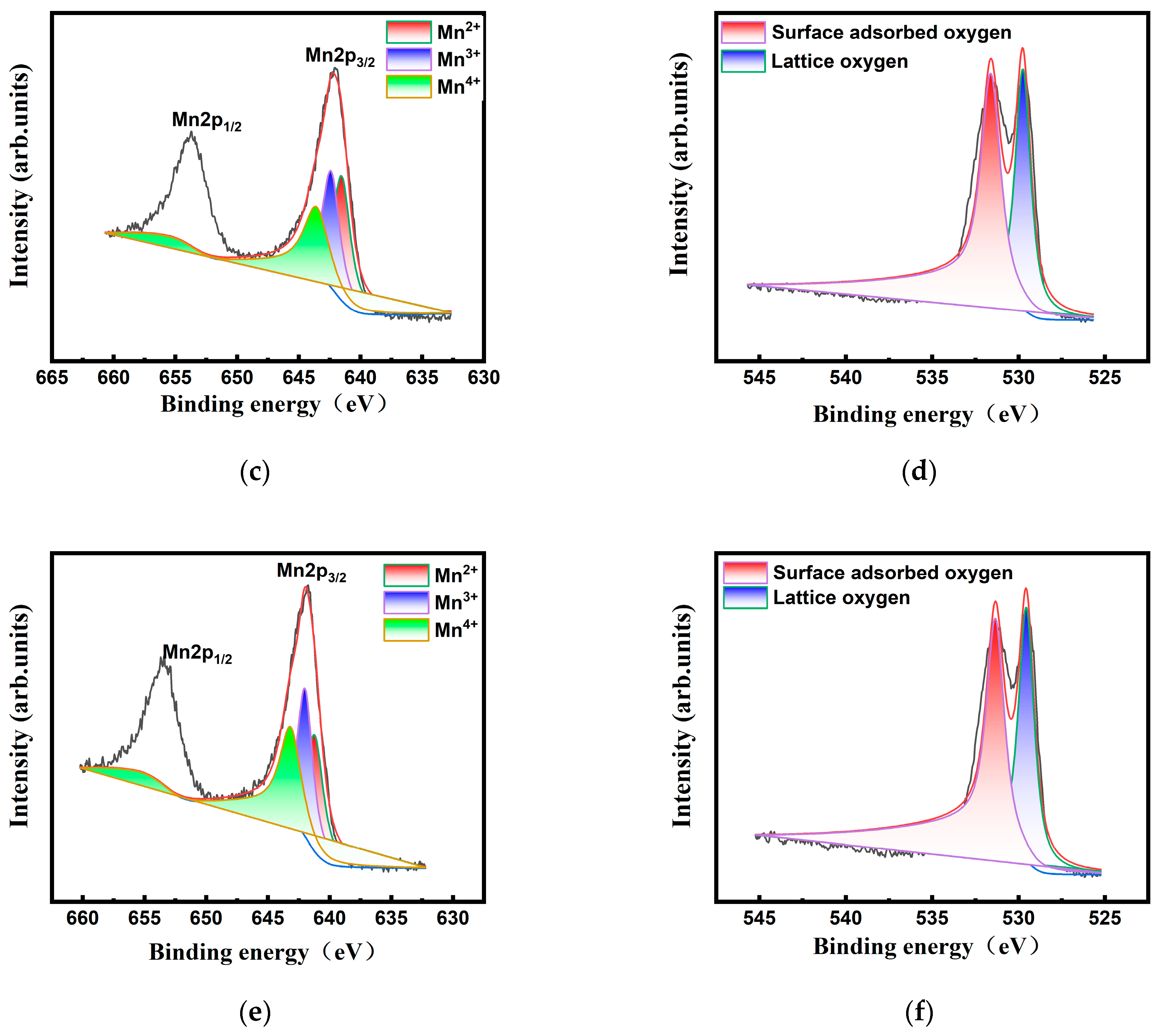
2.1. Characterizations of MnO2 Nanoenzyme
2.2. Physical and Chemical Properties, Humification, and Gas Abatement
2.2.1. Changes in Physical and Chemical Properties During Composting
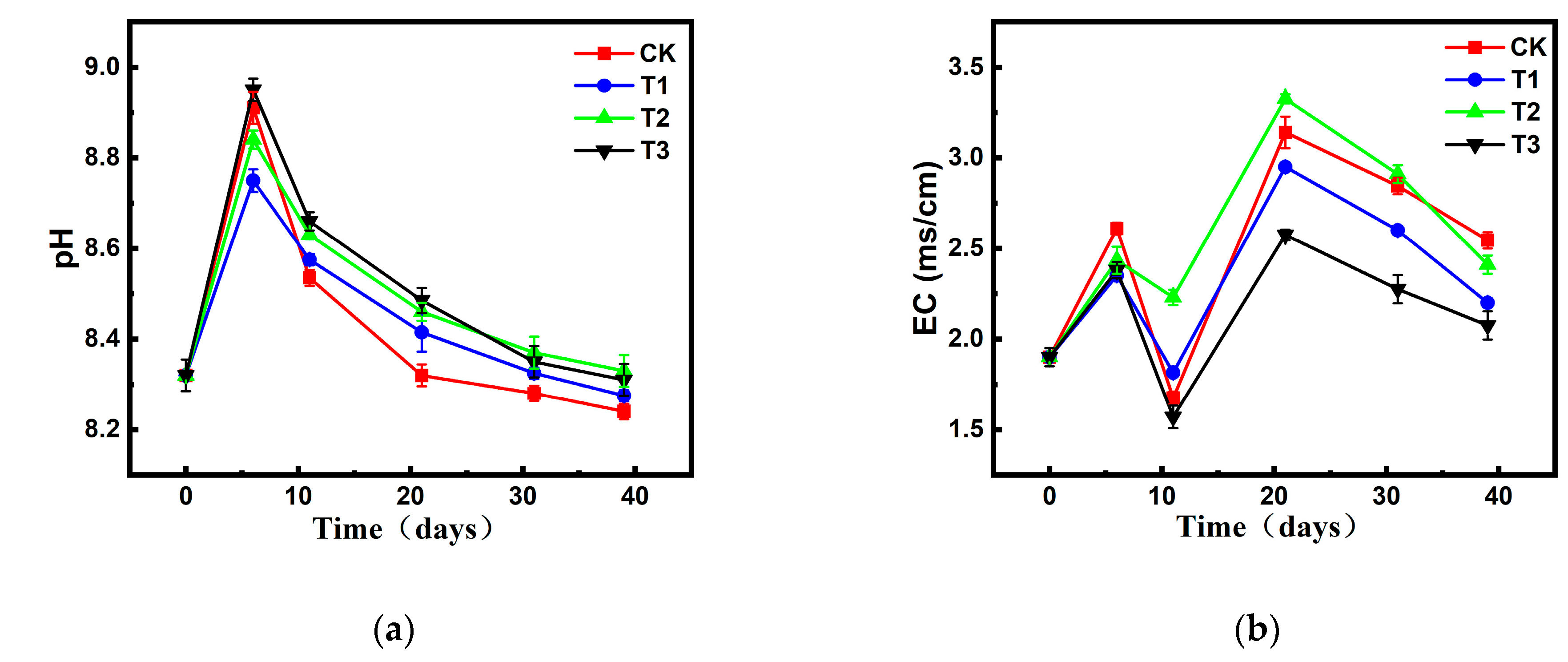
Changes in pH and EC During Composting
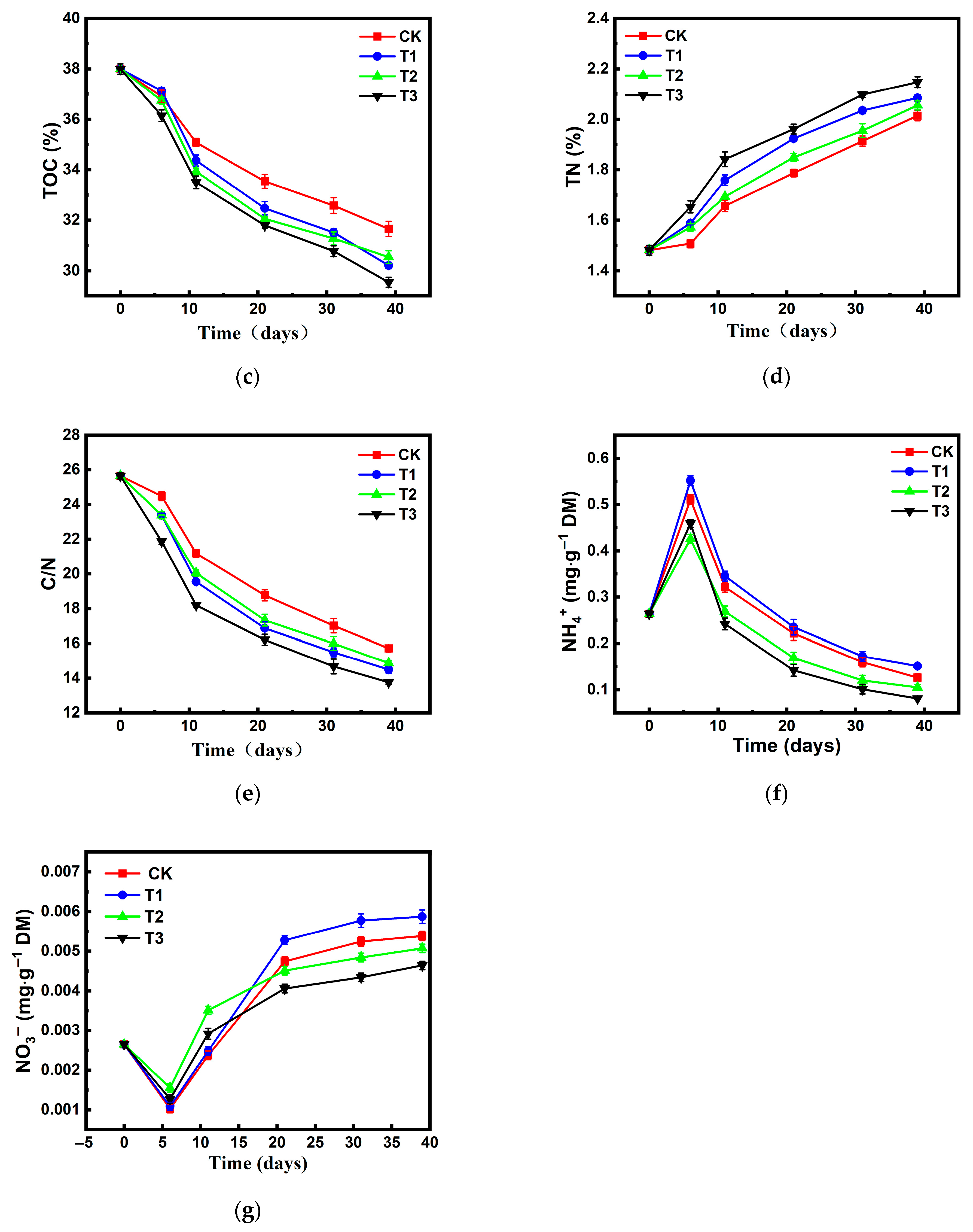
Changes in Carbon and Nitrogen Transformation During Composting
Changes in and During Composting
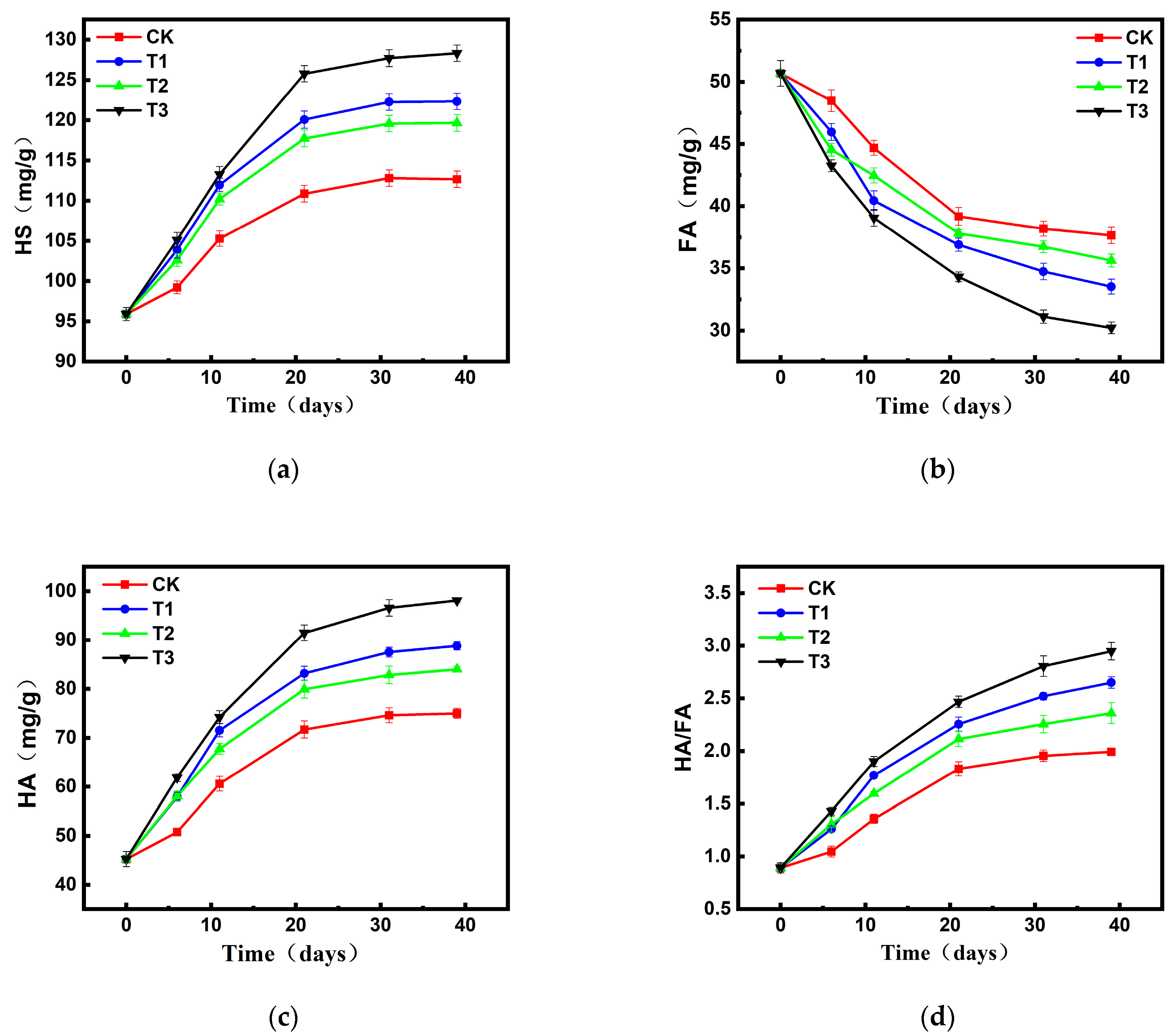
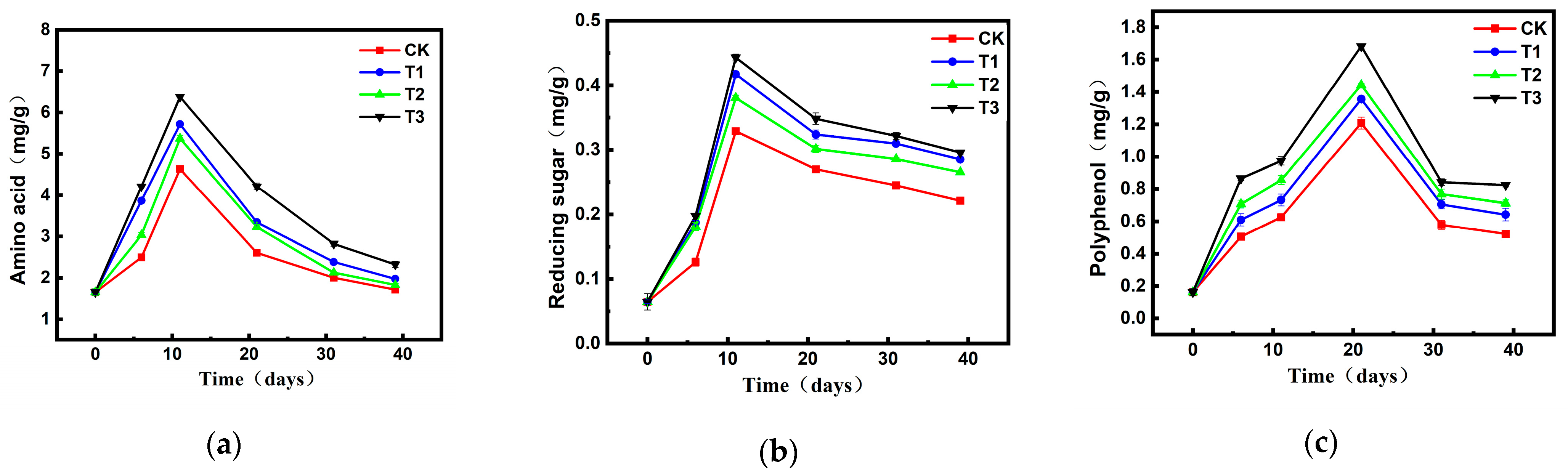
2.2.2. Changes in Humus Composition and Content During Composting
2.2.3. Greenhouse Gas Emissions
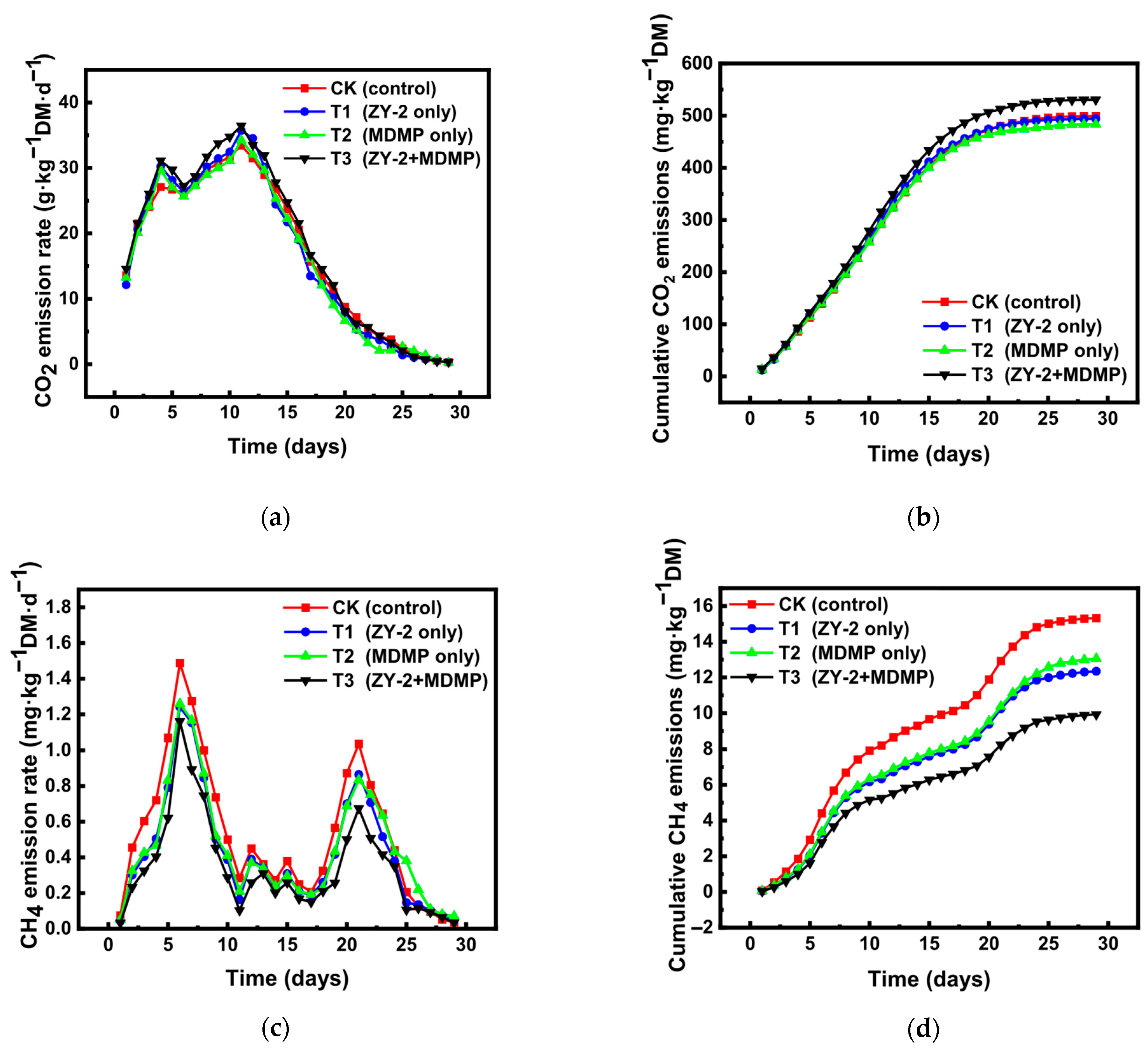
Changes in CO2 Emissions During Composting
Changes in CH4 Emissions During Composting
Changes in Nitrous Oxide Emissions During Composting
Ammonia Emission Analysis
2.3. Research on the Composting Mechanism
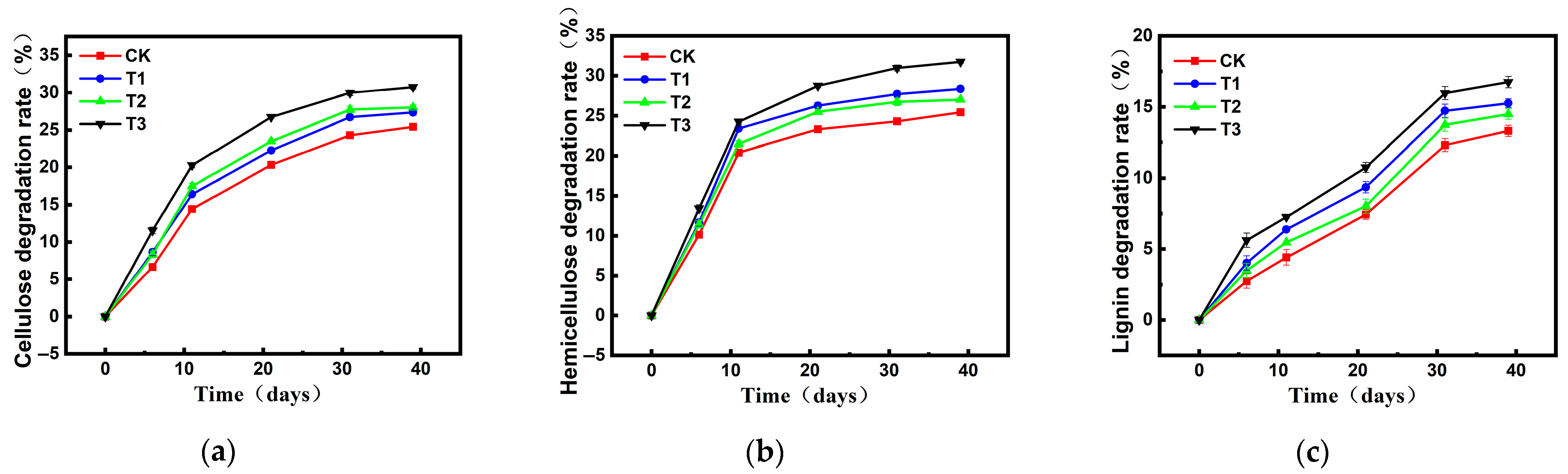
2.3.1. Changes in Cellulose, Hemicellulose, and Lignin During Composting
2.3.2. Changes in Humus Precursors During Composting
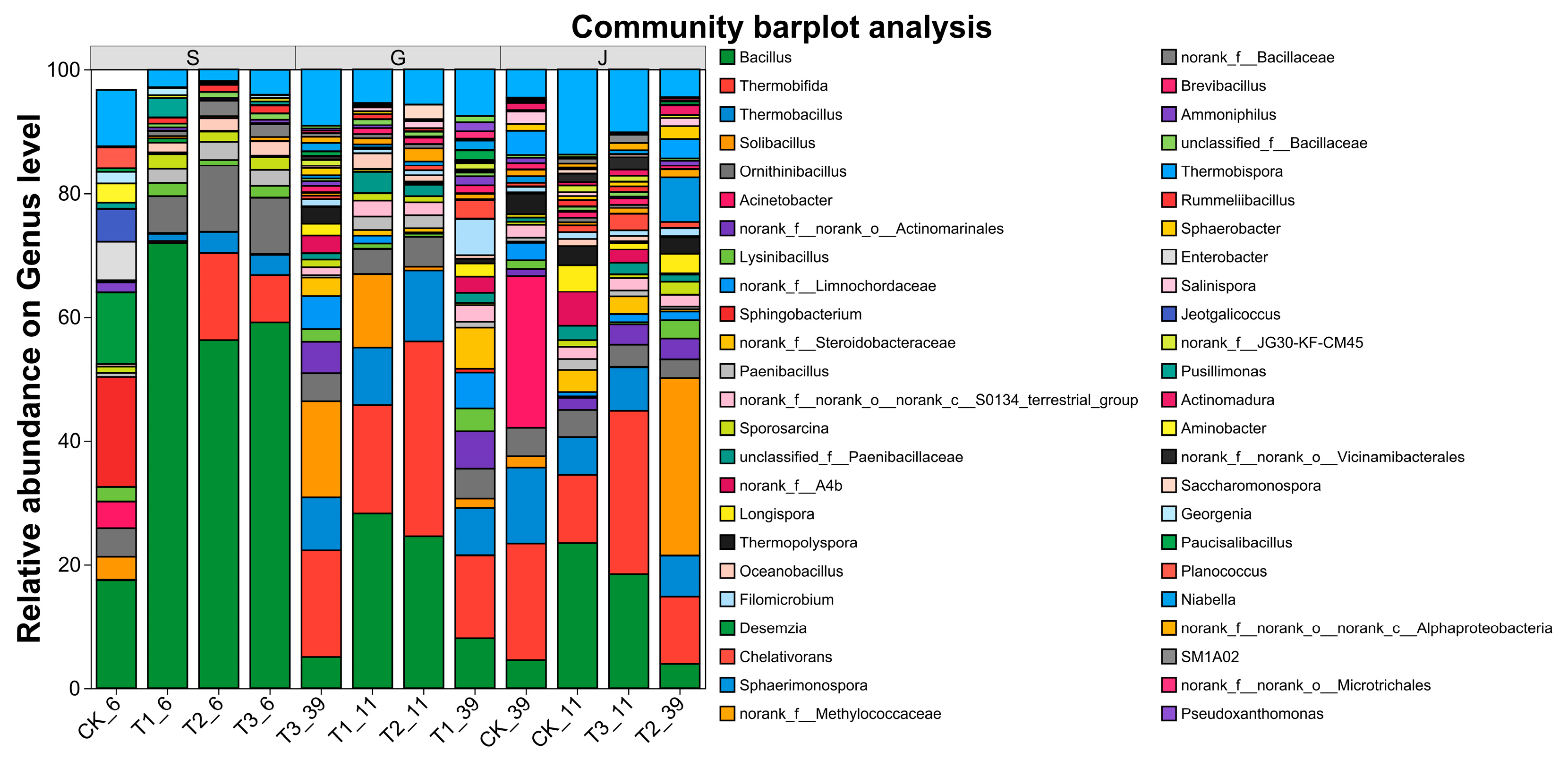
2.3.3. Microbial Community Composition and Succession Analysis
Bacterial Community Diversity and Richness Analysis
Analysis of Bacterial Community Composition
3. Materials and Methods
3.1. Raw Material Preparation and Composting Experiment
3.2. Determination of Physicochemical Properties
3.3. Greenhouse Gas Emissions
3.4. High-Throughput Sequencing
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDMP | manganese dioxide mineral powder |
| Vo | oxygen vacancies |
| HA | humic acid |
| FA | fulvic acid |
| GHG | significant greenhouse gas |
References
- Khan, H.M.; Iqbal, T.; Yasin, S.; Ali, C.H.; Abbas, M.M.; Jamil, M.A.; Hussain, A.; Soudagar, M.E.M.; Rahman, M.M. Application of Agricultural Waste as Heterogeneous Catalysts for Biodiesel Production. Catalysts 2021, 11, 1215. [Google Scholar] [CrossRef]
- Briassoulis, D.; Babou, E.; Hiskakis, M.; Scarascia, G.; Picuno, P.; Guarde, D.; Dejean, C. Review, mapping and analysis of the agricultural plastic waste generation and consolidation in Europe. Waste Manag. Res. 2013, 31, 1262–1278. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, H.; Wei, Y. The Practical Significance and Countermeasures of Agricultural Waste Resources Utilization. In Proceedings of the International Symposium on Agriculture, Food and Biotechnology (ISAFB), Dubai, United Arab Emirates, 9–10 August 2019. [Google Scholar]
- Cheng, K.H.; Huang, M.C.; Lu, M.F.; Chou, Y.J.; Lin, J.J.M. Assessment of Degree of Maturity of Compost Produced by Different Kitchen Waste Composting Methods. In Proceedings of the 3rd International Conference on Advances in Materials Manufacturing (ICAMMP 2012), Beihai, China, 22–23 December 2013. [Google Scholar]
- Yang, F.; Li, Y.; Han, Y.; Qian, W.; Li, G.; Luo, W. Performance of mature compost to control gaseous emissions in kitchen waste composting. Sci. Total Environ. 2019, 657, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Guo, J. Effect of matured compost as a bulking agent and inoculating agent on Composting of Municipal Solid Waste. In Proceedings of the 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–16 June 2009. [Google Scholar]
- Zanella, A.; Ponge, J.F.; Jabiol, B.; Sartori, G.; Kolb, E.; Le Bayon, R.C.; Gobat, J.M.; Aubert, M.; De Waal, R.; Van Delft, B.; et al. Humusica 1, article 5: Terrestrial humus systems and forms—Keys of classification of humus systems and forms. Appl. Soil Ecol. 2018, 122, 75–86. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Z.; Zhu, Z.; Zhao, Y.; Jia, L.; Lv, P. Humus formation driven by ammonia-oxidizing bacteria during mixed materials composting. Bioresour. Technol. 2020, 311, 123500. [Google Scholar] [CrossRef]
- Wu, J.; Yao, W.; Zhao, L.; Zhao, Y.; Qi, H.; Zhang, R.; Song, C.; Wei, Z. Estimatingthe synergistic formation of humus by abiotic and biotic pathways during composting. J. Clean. Prod. 2022, 363, 132470. [Google Scholar] [CrossRef]
- Yu, C.; Lu, Q.; Fu, C.; Jiang, Z.; Huang, J.; Jiang, F.; Wei, Z. Exploring the internal driving mechanism underlying bacterial community-induced organic component conversion and humus formation during rice straw composting with tricarboxylic acid cycle regulator addition. Bioresour. Technol. 2022, 365, 128149. [Google Scholar] [CrossRef]
- Chen, X.; Qin, X.; Wang, A.; Li, Q. Alkali-activated persulfate mediated the depolymerization and oxygenation of lignin to synthesize humus-like substances for agricultural applications. Biomass Convers. Biorefin. 2025, 15, 8553–8565. [Google Scholar] [CrossRef]
- Li, J.; Wu, S.; Zheng, J.; Sun, X.; Hu, C. Combining citrus waste-derived function microbes with biochar promotes humus formation by enhancing lignocellulose degradation in citrus waste compost. Chemosphere 2024, 368, 143754. [Google Scholar] [CrossRef]
- Dai, J.; Patti, A.F.; Styles, G.N.; Nanayakkara, S.; Spiccia, L.; Arena, F.; Italiano, C.; Saito, K. Lignin oxidation by MnO2 under the irradiation of blue light. Green Chem. 2019, 21, 2005–2014. [Google Scholar] [CrossRef]
- Zhai, R.; Hu, J.; Chen, X.; Xu, Z.; Wen, Z.; Jin, M. Facile synthesis of manganese oxide modified lignin nanocomposites from lignocellulosic biorefinery wastes for dye removal. Bioresour. Technol. 2020, 315, 123846. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Akiyama, T.; Yokoyama, T.; Matsumoto, Y. Differences in the Mechanisms of MnO2 Oxidation between Lignin Model Compounds with the p-Hydroxyphenyl, Guaiacyl, and Syringyl Nuclei. J. Agric. Food Chem. 2020, 68, 6819–6825. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Ma, Y.; Li, D.; Jiang, M.; Yu, C. Hydrothermal Synthesis of MnO2 Microspheres and Their Degradation of Toluene. Acs Omega 2023, 8, 49150–49157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Feng, J.; Ji, Y.; Li, Y. First-Principles Prediction of Novel Metastable MnO2 for the Oxygen Evolution Reaction. J. Phys. Chem. C 2025, 129, 5260–5267. [Google Scholar] [CrossRef]
- Zheng, X.; Han, B.; Wu, S.; Li, T.; Mohamed, A.F.; Liu, S. Optimized preparation of delta-manganese oxide for energetic zinc-manganese oxide batteries. J. Power Sources 2025, 630, 236114. [Google Scholar] [CrossRef]
- Culotta, V.C.; Daly, M.J. Manganese Complexes: Diverse Metabolic Routes to Oxidative Stress Resistance in Prokaryotes and Yeast. Antioxid. Redox Signal. 2013, 19, 933–944. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Su, G.; Zheng, M.; Huang, C.; Wang, M.; Ma, C.; Wei, D. The Regular/Persistent Free Radicals and Associated Reaction Mechanism for the Degradation of 1,2,4-Trichlorobenzene over Different MnO2 Polymorphs. Environ. Sci. Technol. 2018, 52, 13351–13360. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Jiao, L.; Yan, H.; Gu, W.; Zhu, C. Research Progress of Carbon-based Nanozymes for Biosensing. Chin. J. Anal. Chem. 2021, 49, 907–921. [Google Scholar]
- Gao, L.; Chen, L.; Zhang, R.; Yan, X. Nanozymes: Next-generation artificial enzymes. Sci. Sin. Chim. 2022, 52, 1649–1663. [Google Scholar] [CrossRef]
- Feng, M.; Li, X.; Zhang, X.; Huang, Y. Recent advances in the development and analytical applications of oxidase-like nanozymes. TrAC Trends Anal. Chem. 2023, 166, 117220. [Google Scholar] [CrossRef]
- Ye, Y.; Zou, J.; Wu, W.; Wang, Z.; Wen, S.; Liang, Z.; Liu, S.; Lin, Y.; Chen, X.; Luo, T.; et al. Advanced nanozymes possess peroxidase-like catalytic activities in biomedical and antibacterial fields: Review and progress. Nanoscale 2024, 16, 3324–3346. [Google Scholar] [CrossRef]
- Ndayiragije, S.; Zhang, Y.; Zhou, Y.; Song, Z.; Wang, N.; Majima, T.; Zhu, L. Mechanochemically tailoring oxygen vacancies of MnO2 for efficient degradation of tetrabromobisphenol A with peroxymonosulfate. Appl. Catal. B Environ. Energy 2022, 307, 121168. [Google Scholar] [CrossRef]
- Chai, H.; Zhang, Z.; Zhou, Y.; Zhu, L.; Lv, H.; Wang, N. Roles of intrinsic Mn3+ sites and lattice oxygen in mechanochemical debromination and mineralization of decabromodiphenyl ether with manganese dioxide. Chemosphere 2018, 207, 41–49. [Google Scholar] [CrossRef]
- Qiu, K.; Lu, Y.; Zhang, D.; Cheng, J.; Yan, H.; Xu, J.; Liu, X.; Kim, J.K.; Luo, Y. Mesoporous, hierarchical core/shell structured ZnCo2O4/MnO2 nanocone forests for high-performance supercapacitors. Nano Energy 2015, 11, 687–696. [Google Scholar] [CrossRef]
- Cheng, Z.; Lu, G.; Qi, Y.; Huang, J.; Yan, G.; Fang, L.; Avdeev, M.; Yang, T.; Jiang, P. Structure and photoluminescence of Mn2+/4+-activated doubly ordered spinel Mg4(Ga/Al)SbO8: Site-selective Al3+-to-Ga3+ substitution enabling Mn4+ accumulation, excellent anti-thermal quenching of Mn2+ green-emission, and optical thermometry. Inorg. Chem. Front. 2024, 11, 1923–1936. [Google Scholar] [CrossRef]
- Hardie, A.G.; Dynes, J.J.; Kozak, L.M.; Huang, P.M. The role of glucose in abiotic humification pathways as catalyzed by birnessite. J. Mol. Catal. A Chem. 2009, 308, 114–126. [Google Scholar] [CrossRef]
- Ewert, J.; Glück, C.; Strasdeit, H.; Fischer, L.; Stressler, T. Influence of the metal ion on the enzyme activity and kinetics of PepA from Lactobacillus delbrueckii. Enzym. Microb. Technol. 2018, 110, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Kucharzyk, K.H.; Pawlik, A.; Staszczak, M.; Paszczynski, A.J. Fungal laccase, manganese peroxidase and lignin peroxidase: Gene expression and regulation. Enzym. Microb. Technol. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, A.; Du, Z.; Wu, J.; Chen, X.; Zhang, X.; Zhao, Y.; Wei, Z.; Xie, X.; Li, Y.; et al. δ-MnO2 changed the structure of humic-like acid during co-composting of chicken manure and rice straw. Waste Manag. 2021, 128, 16–24. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Meng, H.; Li, L.; Cui, H.; Wei, Z.; Yang, T.; Dang, Q. Revealing the Inner Dynamics of Fulvic Acid from Different Compost-Amended Soils through Microbial and Chemical Analyses. J. Agric. Food Chem. 2020, 68, 3722–3728. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, R.; Jia, L.; Wang, L.; Pan, C.; Zhang, R.; Wei, Z. Microbial inoculants reshape structural distribution of complex components of humic acid based on spectroscopy during straw waste composting. Bioresour. Technol. 2022, 359, 127472. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, D.; Wei, D.; Zhao, Y.; Wu, J.; Xie, X.; Zhang, R.; Wei, Z. Improved lignocellulose-degrading performance during straw composting from diverse sources with actinomycetes inoculation by regulating the key enzyme activities. Bioresour. Technol. 2019, 271, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, B.; Zhang, D.; Chu, S.; Zhi, Y.; Hayat, K.; Wang, J.; Chen, X.; Hui, N.; Zhou, P. Streptomyces griseorubens JSD-1 promotes rice straw composting efficiency in industrial-scale fermenter: Evaluation of change in physicochemical properties and microbial community. Bioresour. Technol. 2021, 321, 124465. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, C.; Feng, Z.; Zhang, R. Ultralong α-MnO2 Nanowires Capable of Catalytically Degrading Methylene Blue at Low Temperature. Catal. Lett. 2018, 148, 2822–2829. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, H.; Liu, Z.; Peng, Z.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Greatly enhanced oxidative activity of δ-MnO2 to degrade organic pollutants driven by dominantly exposed {−111} facets. J. Hazard. Mater. 2021, 413, 125285. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Zhang, Z.; Wei, Y.; Wang, H.; Lu, Q.; Li, Y.; Wei, Z. Effect of thermo-tolerant actinomycetes inoculation on cellulose degradation and the formation of humic substances during composting. Waste Manag. 2017, 68, 64–73. [Google Scholar] [CrossRef]
- Kelzenberg, S.; Eisenreich, N.; Knapp, S.; Koleczko, A.; Schuppler, H.; Fietzek, H. Chemical Kinetics of the Oxidation of Manganese and of the Decomposition of MnO2 by XRD and TG Measurements. Propell. Explos. Pyrot. 2019, 44, 714–724. [Google Scholar] [CrossRef]
- Sivakumar, S.; Prabu, L.N. Synthesis and Characterization of α-MnO2 nanoparticles for Supercapacitor application. Mater. Today Proc. 2021, 47, 52–55. [Google Scholar] [CrossRef]
- Yan, W.; Liu, D.; Tan, D.; Yuan, P.; Chen, M. FTIR spectroscopy study of the structure changes of palygorskite under heating. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2012, 97, 1052–1057. [Google Scholar] [CrossRef]
- Wang, Y.; Ai, X.; Lu, S.; Xing, T.; Qi, N.; Chen, G. Fabrication of a type of silk/PEDOT conductive fibers for wearable sensor. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126909. [Google Scholar] [CrossRef]
- Chen, Z.; Xing, R.; Yang, X.; Zhao, Z.; Liao, H.; Zhou, S. Enhanced in situ Pb(II) passivation by biotransformation into chloropyromorphite during sludge composting. J. Hazard. Mater. 2021, 408, 124973. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Xia, J.; Chen, Y. Effect of microbial inoculation on physicochemical properties and bacterial community structure of citrus peel composting. Bioresour. Technol. 2019, 291, 121843. [Google Scholar] [CrossRef]
- Rogers, J.R.; Bennett, P.C. Mineral stimulation of subsurface microorganisms: Release of limiting nutrients from silicates. Chem. Geol. 2004, 203, 91–108. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, W.; Xu, L. Adsorption of copper and cadmium by Cu- and Cd-resistant bacteria and their composites with soil colloids and kaolinite. Geomicrobiol. J. 2005, 22, 227–236. [Google Scholar] [CrossRef]
- Medina, J.; Monreal, C.M.; Orellana, L.; Calabi-Floody, M.; González, M.E.; Meier, S.; Borie, F.; Cornejo, P. Influence of saprophytic fungi and inorganic additives on enzyme activities and chemical properties of the biodegradation process of wheat straw for the production of organo-mineral amendments. J. Environ. Manag. 2020, 255, 109922. [Google Scholar] [CrossRef]



| CO2 accrue | CH4 accrue | N2O accrue | NH3 accrue | C loss | N loss | GWP | |
|---|---|---|---|---|---|---|---|
| CK | 422,276.23 | 16.56 | 29.31 | 118.81 | 422.29 | 0.15 | 8.23 |
| T1 | 424,028.93 | 15.37 | 27.98 | 114.63 | 424.04 | 0.14 | 7.84 |
| T2 | 429,212.12 | 13.60 | 25.31 | 93.56 | 429.22 | 0.12 | 7.09 |
| T3 | 427,921.41 | 14.51 | 26.57 | 111.52 1 | 427.93 | 0.14 | 7.45 2 |
| ACE | Chao1 | Shannon | Simpson | ||
|---|---|---|---|---|---|
| Temperature raising period | CK | 216.02 | 259.50 | 2.64 | 0.097 |
| T1 | 319.13 | 334.23 | 3.39 | 0.064 | |
| T2 | 276.25 | 301.02 | 3.14 | 0.074 | |
| T3 | 377.76 | 390.93 | 3.80 | 0.049 | |
| Megathermal period | CK | 202.28 | 234.60 | 2.17 | 0.100 |
| T1 | 361.61 | 356.55 | 3.23 | 0.082 | |
| T2 | 326.40 | 313.87 | 3.55 | 0.068 | |
| T3 | 431.91 | 396.28 | 4.13 | 0.037 | |
| Maturation stage | CK | 272.05 | 325.07 | 2.89 | 0.063 |
| T1 | 412.65 | 456.26 | 3.79 | 0.035 | |
| T2 | 381.19 | 399.25 | 3.96 | 0.023 | |
| T3 | 464.45 | 491.68 | 4.76 | 0.017 |
| Group | Code | Treatment | Additive Dosage |
|---|---|---|---|
| CK | CK | No additives | — |
| ZY-2 | T1 | Streptomyces rochei ZY-2 | 5% (v/w) compost dry mass |
| MDMP | T2 | MnO2 nanozyme (ball-milled 2 h) | 0.05% (w/w) compost dry mass |
| ZY-2+MDMP | T3 | ZY-2 + MnO2 nanozyme | 5% (v/w) ZY-2 + 0.05% (w/w) MnO2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Lin, L.; Zhang, J.; Sun, E.; Yong, C.; Chen, L.; Huang, H.; Jin, H.; Qu, P. Improvement of Cleaner Composting Production by Manganese Dioxide Nanozyme with Streptomyces rochei ZY-2: From the Humus Formation to Greenhouse Gas Emissions. Catalysts 2025, 15, 774. https://doi.org/10.3390/catal15080774
Liu G, Lin L, Zhang J, Sun E, Yong C, Chen L, Huang H, Jin H, Qu P. Improvement of Cleaner Composting Production by Manganese Dioxide Nanozyme with Streptomyces rochei ZY-2: From the Humus Formation to Greenhouse Gas Emissions. Catalysts. 2025; 15(8):774. https://doi.org/10.3390/catal15080774
Chicago/Turabian StyleLiu, Guoxiang, Lili Lin, Jing Zhang, Enhui Sun, Cheng Yong, Ling Chen, Hongying Huang, Hongmei Jin, and Ping Qu. 2025. "Improvement of Cleaner Composting Production by Manganese Dioxide Nanozyme with Streptomyces rochei ZY-2: From the Humus Formation to Greenhouse Gas Emissions" Catalysts 15, no. 8: 774. https://doi.org/10.3390/catal15080774
APA StyleLiu, G., Lin, L., Zhang, J., Sun, E., Yong, C., Chen, L., Huang, H., Jin, H., & Qu, P. (2025). Improvement of Cleaner Composting Production by Manganese Dioxide Nanozyme with Streptomyces rochei ZY-2: From the Humus Formation to Greenhouse Gas Emissions. Catalysts, 15(8), 774. https://doi.org/10.3390/catal15080774







