Improved Electrochemical Performance Using Transition Metal Doped ZnNi/Carbon Nanotubes as Conductive Additive in Li/CFx Battery
Abstract
1. Introduction
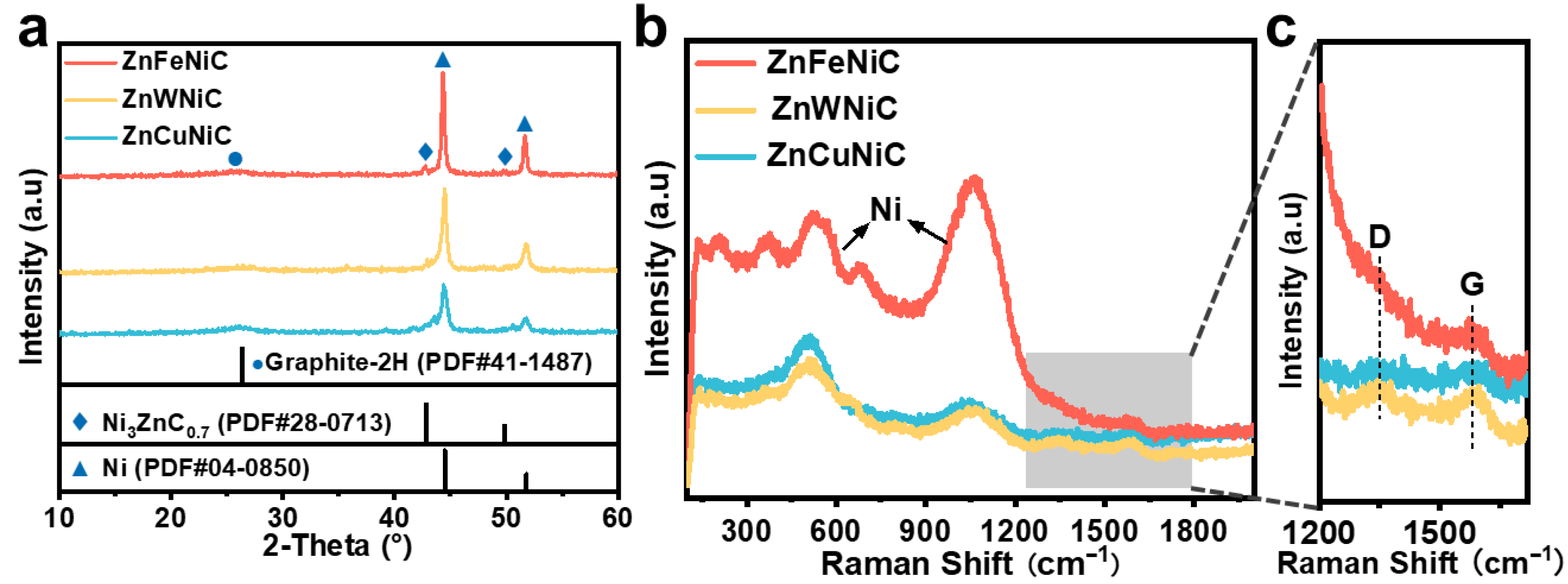
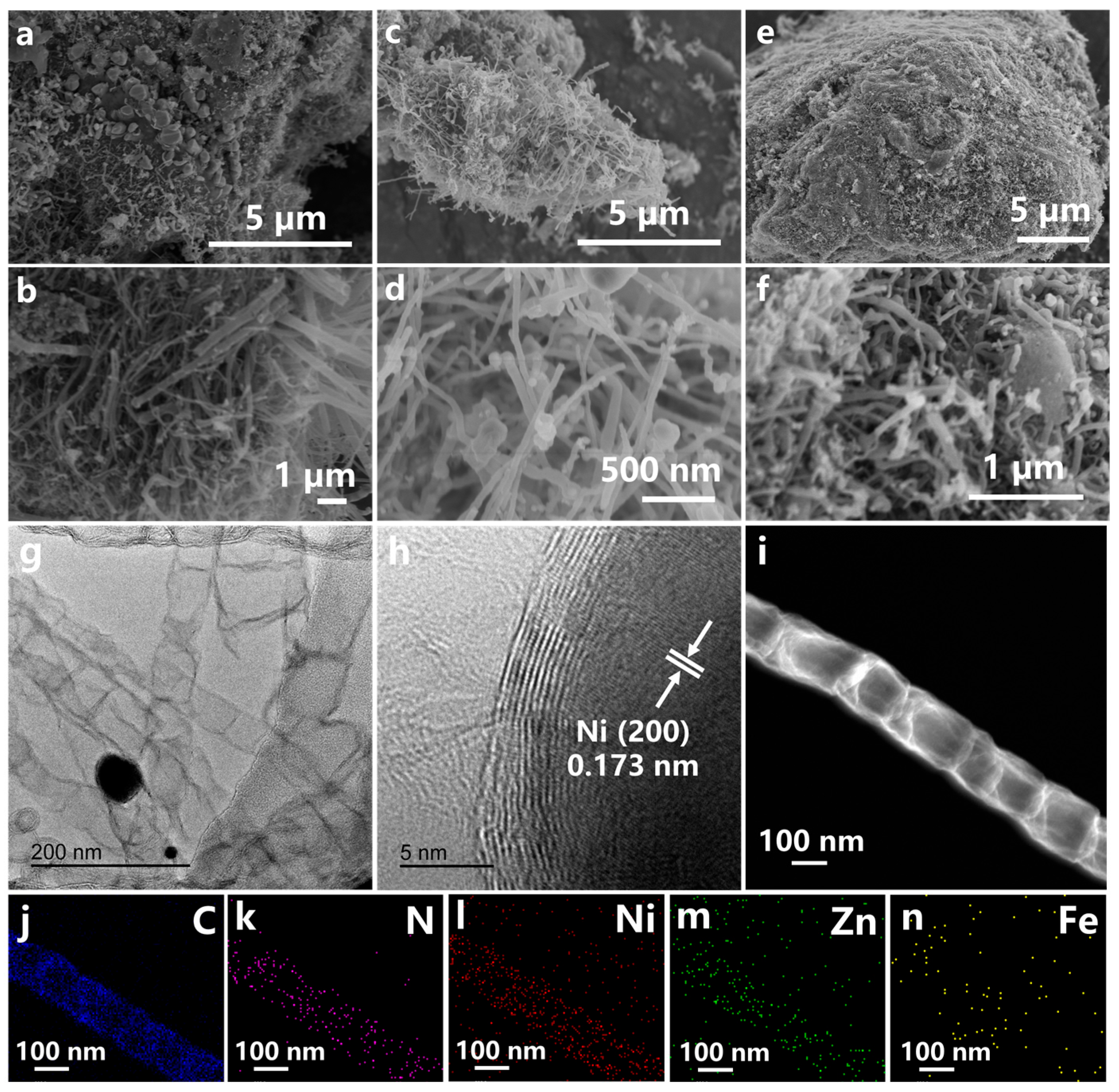
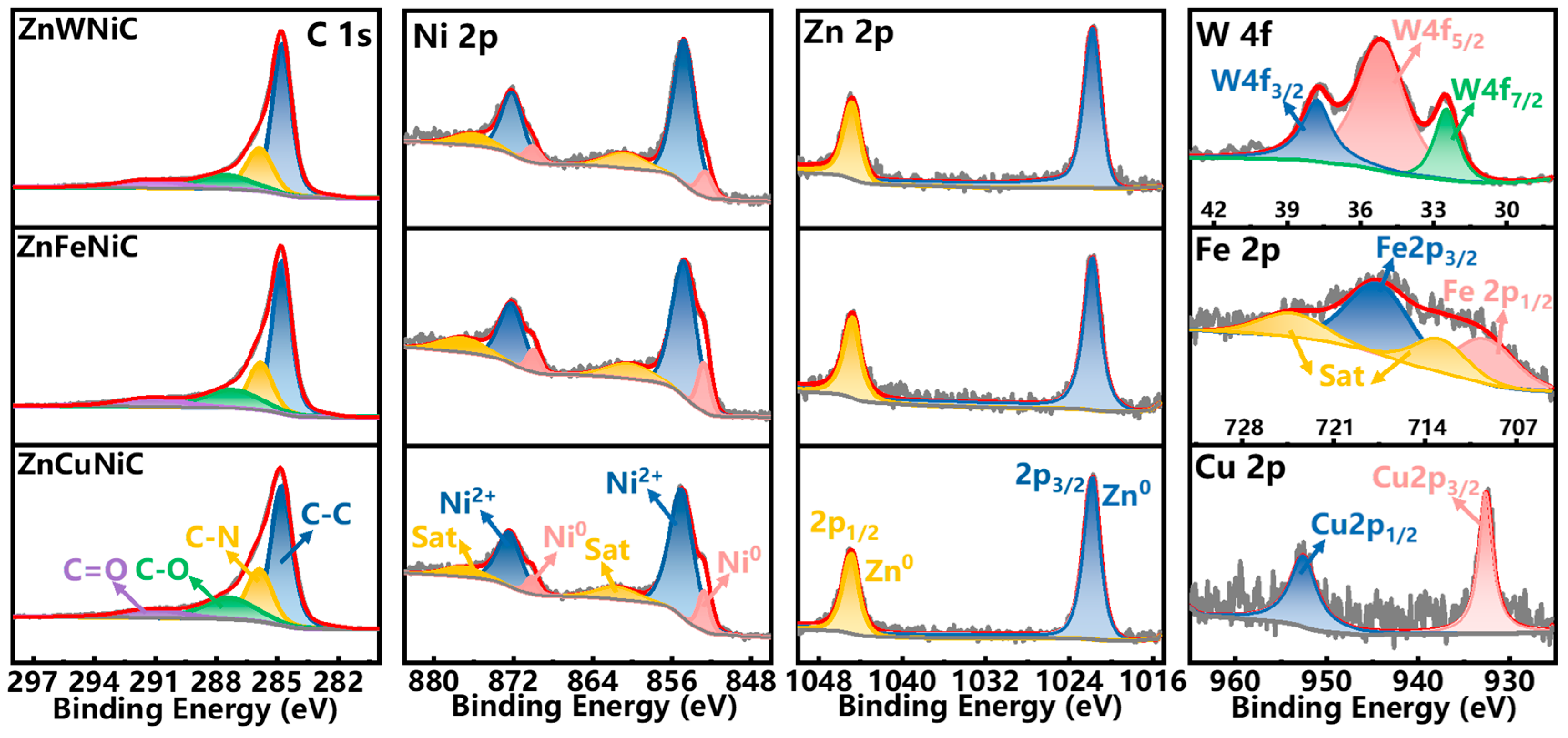
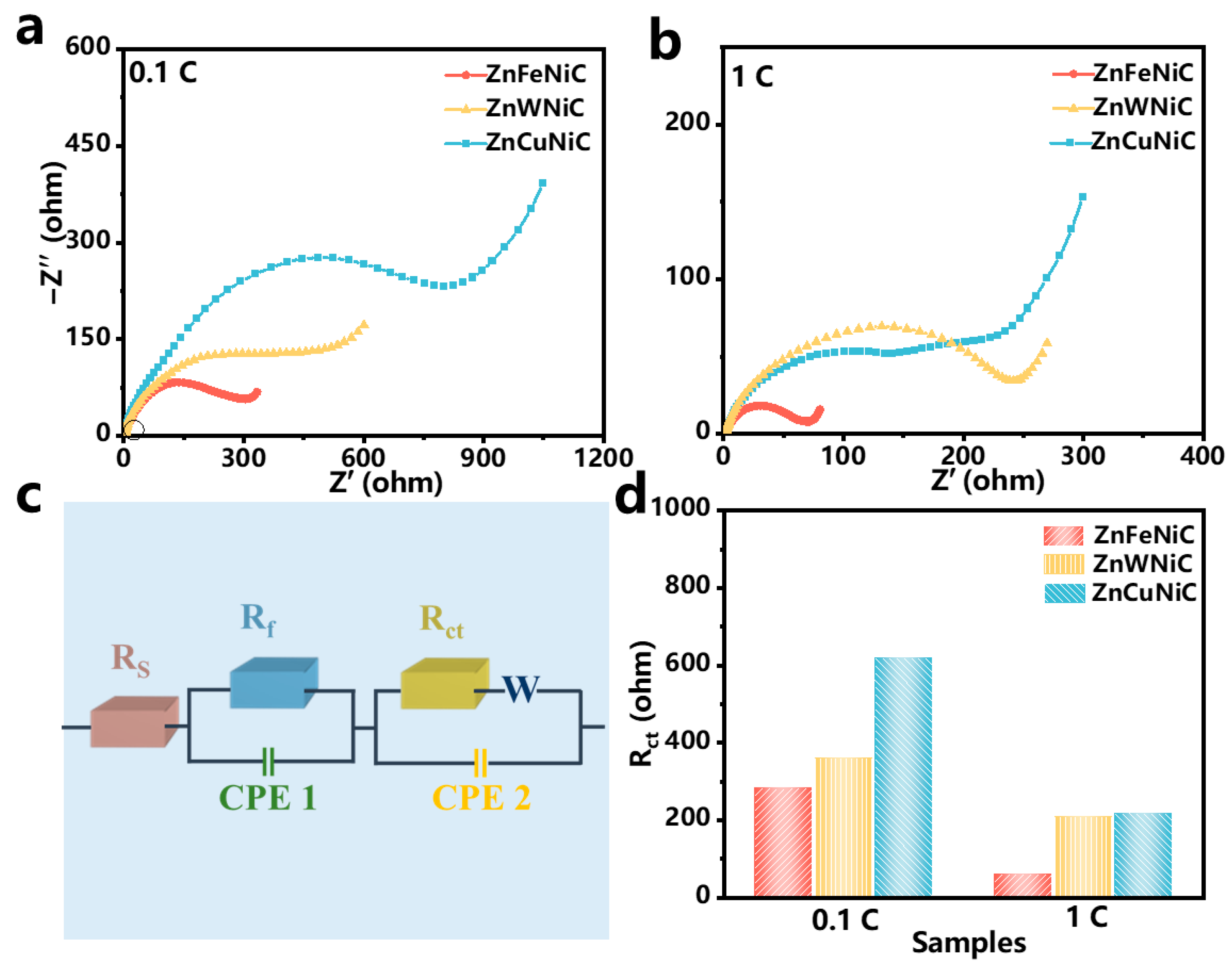
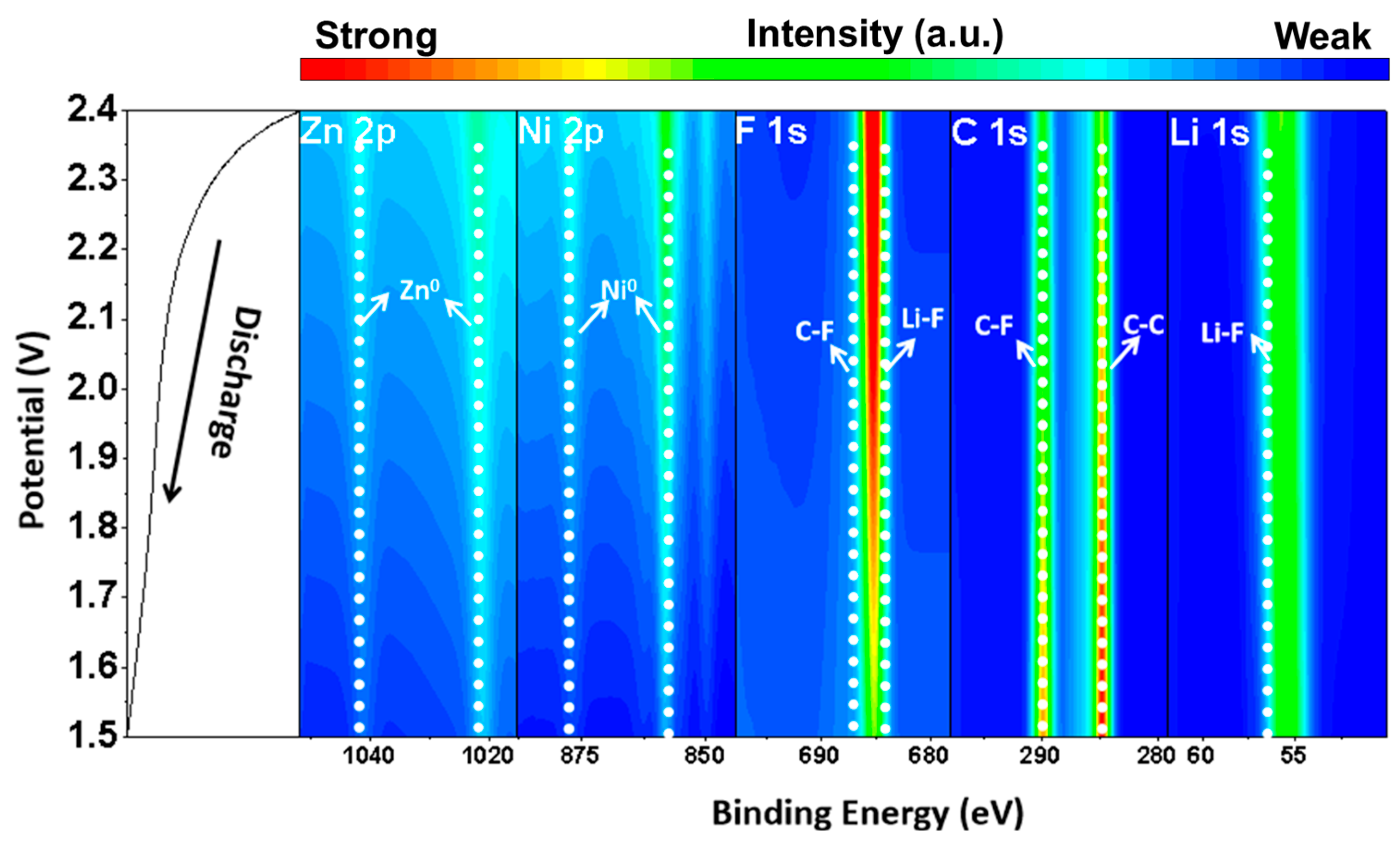
2. Results and Discussion
3. Experimental Section
3.1. Preparation of ZnFeNiC/ZnWNiC/ZnCuNiC
3.2. Materials Characterization
3.3. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, P.; Wang, B.; Wu, Z.; Niu, X.; Ouyang, C.; Li, H.; Wang, L. Fluorinated soft carbon as an ultra-high energy density potassium-ion battery cathode enabled by a ternary phase KxFC. J. Energy Chem. 2023, 77, 38–44. [Google Scholar] [CrossRef]
- Gao, M.T.; Cai, D.M.; Luo, S.F.; Yang, Y.H.; Xie, Y.; Zhu, L.C.; Yuan, Z.Z. Research progress in fluorinated carbon sources and the discharge mechanism for Li/CFx primary batteries. J. Mater. Chem. A 2023, 11, 16519–16538. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Jiang, J.M.; Feng, Q.L.; Zhou, F.; Qiu, X.; Xu, C.C.; Qiu, L.B.; Ju, Z.C.; Cui, Y.H.; Zhuang, Q.C. Transforming Li/CFx primary batteries with a novel additive: Engineering solvation structure regulation and electrode/electrolyte interface modification enables superior discharge performance in wide temperatures. J. Energy Storage 2025, 123, 116764. [Google Scholar] [CrossRef]
- Chen, G.; Duan, Y.; Zhao, H.; Feng, H.; Zeng, B.; Zang, Z.; Ravivarma, M.; Fan, H.; Gao, F.; Wu, J.; et al. Unveiling Essentials and Prospects of Electrolytes for Li/CFx Primary Battery: A Review. Adv. Funct. Mater. 2025, 2503144. [Google Scholar] [CrossRef]
- Meng, J.S.; Xiao, Z.T.; Zhu, L.J.; Zhang, X.; Hong, X.F.; Jia, Y.F.; Liu, F.; Pang, Q.Q. Fluorinated electrode materials for high-energy batteries. Matter 2023, 6, 1685–1716. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, Y.; Ali, R.; Ma, J.; Hou, J.; Yang, X.; Jian, X. Air plasma-induced carbon fluoride enabling active C-F bonds for double-high energy/power densities of Li/CFx primary battery. J. Alloys Compd. 2022, 905, 164151. [Google Scholar] [CrossRef]
- Hu, Y.H.; Kong, L.C.; Li, W.Y.; Sun, L.D.; Peng, C.; Qin, M.M.; Zhao, Z.Y.; Li, Y.; Feng, W. Fluorinated microporous carbon spheres for Li/CFx batteries with high volumetric energy density. Compos. Commun. 2023, 40, 101607. [Google Scholar] [CrossRef]
- Chen, G.B.; Cao, F.; Li, Z.X.; Fu, J.A.; Wu, B.S.; Liu, Y.F.; Jian, X. Helical fluorinated carbon nanotubes/iron(III) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery. Nanotechnol. Rev. 2023, 12, 20230108. [Google Scholar] [CrossRef]
- Qin, G.L.; Liu, Y.F.; He, J.C.; Wang, N.K.; Wang, J.W.; Jian, X. Porous Hollow Carbon Alkali-Activated Nanoonions As a Conductive Additive for High-Rate Lithium Primary Batteries. ACS Appl. Energy Mater. 2024, 7, 7936–7944. [Google Scholar] [CrossRef]
- Chen, G.B.; Fan, Y.; Shoaib, M.; Yang, X.X.; Liu, Y.F.; Jian, X. Constructing the charge channel with CFx using a well-dispersed carbon nanotube arrays to boost high-rate primary battery. J. Alloys Compd. 2024, 994, 174483. [Google Scholar] [CrossRef]
- Qiao, J.J.; Han, Y.Y.; Feng, L.Y.; Li, Y.T.; Ding, J.N.; Xu, F.; Lin, B.C. Composite of Double Transition Metals (Fe, Ni) and N-Doped Carbon Nanotubes as Cathode Catalysts for Zinc-Air Batteries. ACS Appl. Nano Mater. 2023, 6, 22897–22906. [Google Scholar] [CrossRef]
- Jia, Z.; Shang, J.Y.; Xue, K.J.; Yang, X.; Wang, S.M.; Xu, C.H.; Wang, Q.G. N-doped Nanocarbon Inserted NiCo-LDH Nanoplates on NF with High OER/ORR Performances for Zinc-Air Battery. ChemCatChem 2023, 15, e202201469. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, Z.H.; Zhang, S.Y.; Guan, X.Z.; Xu, W.C.; Liang, Y.Q.; Jiang, H.; Li, Z.Y.; Wu, S.L.; Cui, Z.D.; et al. Asymmetric-Charge-Distributed Co-Mn Diatomic Catalyst Enables Efficient Oxygen Reduction Reaction. Adv. Funct. Mater. 2025, 2504260. [Google Scholar] [CrossRef]
- Zheng, X.J.; Cao, X.C.; Sun, Z.H.; Zeng, K.; Yan, J.; Strasser, P.; Chen, X.; Sun, S.H.; Yang, R.Z. Indiscrete metal/metal-N-C synergic active sites for efficient and durable oxygen electrocatalysis toward advanced Zn-air batteries. Appl. Catal. B-Environ. 2020, 272, 118967. [Google Scholar] [CrossRef]
- Ma, J.; Xing, S.Y.; Wang, Y.B.; Yang, J.H.; Yu, F. Kinetic-Thermodynamic Promotion Engineering toward High-Density Hierarchical and Zn-Doping Activity-Enhancing ZnNiO@CF for High-Capacity Desalination. Nano-Micro Lett. 2024, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Liu, G.P.; Fu, A.; Xiao, Y.K.; Sun, J.P.; Zhang, Z.R.; Yang, Y. Electrolyte Strategy Enables High-Rate Lithium Carbon Fluoride (Li/CFx) Primary Batteries in All-Climate Environments. Adv. Funct. Mater. 2025, 35, 2413423. [Google Scholar] [CrossRef]
- Luan, T.Y.; Zhao, J.M.; Gao, T.C.; Wang, F.D.; Xu, H.C.; Song, Z.Q.; Luo, L.X.; Gong, S.; Liu, B. Efficient Design of PtFeCoNiX Ordered High-Entropy Alloys as Multifunctional High-Performance Electrocatalysts. Adv. Funct. Mater. 2025, 2506851. [Google Scholar] [CrossRef]
- Zhang, J.X.; Pei, A.; Yang, H.Y.; Zhou, W.W.; Feng, Z.Z.; Tian, H.; Zhao, Y.; Chen, G.X.; Peng, J. Copper-Optimized Active Sites in Cobalt Oxide Nanocubes for Selective Electrooxidation of 5-Hydroxymethylfurfural. ACS Catal. 2025, 15, 4596–4604. [Google Scholar] [CrossRef]
- Cui, P.; Li, G.-T.; Zhang, P.-P.; Wan, T.; Li, M.-Q.; Chen, X.-L.; Zhou, Y.; Guo, R.-Q.; Su, M.-R.; Liu, Y.-J.; et al. Arranging cation mixing and charge compensation of TiNb2O7 with W6+ doping for high lithium storage performance. Rare Met. 2023, 42, 3364–3377. [Google Scholar] [CrossRef]
- Cai, H.M.; Dou, B.; Xue, L.F.; Cheng, B.; Zhao, Y.M.; Wan, D.; Xue, Y.F. Engineering Ti-Cr-Mo-based alloys for hydrogen storage: Fe doping as a strategy for improved reversibility and stability. Int. J. Hydrogen Energy 2025, 128, 499–510. [Google Scholar] [CrossRef]
- Bala, N.; Singh, H.K.; Verma, S.; Rath, S. Magnetic-order induced effects in nanocrystalline NiO probed by Raman spectroscopy. Phys. Rev. B 2020, 102, 024423. [Google Scholar] [CrossRef]
- Fukushima, T.; Tsuchimoto, K.; Oyamada, N.; Sato, D.; Minamimoto, H.; Murakoshi, K. Raman Spectroscopic Observation of Electrolyte-Dependent Oxygen Evolution Reaction Intermediates in Nickel-Based Electrodes. J. Phys. Chem. C 2024, 128, 20156–20164. [Google Scholar] [CrossRef]
- Faid, A.Y.; Barnett, A.O.; Seland, F.; Sunde, S. Ni/NiO nanosheets for alkaline hydrogen evolution reaction: In situ electrochemical-Raman study. Electrochim. Acta 2020, 361, 137040. [Google Scholar] [CrossRef]
- Uleviciene, V.; Balciunaite, A.; Upskuviene, D.; Plavniece, A.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Niaura, G.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Synthesis of Nitrogen-Doped Biomass-Based Activated-Carbon-Supported Nickel Nanoparticles for Hydrazine Oxidation. Catalysts 2025, 15, 400. [Google Scholar] [CrossRef]
- Hua, R.; Li, H.; Zheng, J.; Wang, R.; Ma, Q.; Zhou, T.; Zhang, L.; Kang, H.; Zhang, C.; Zheng, Y. Spatially confined construction of heterostructured SnSe/SnTe nanodots in porous carbon fibers with high-level N-doping for superior sodium storage. J. Power Sources 2023, 554, 232333. [Google Scholar] [CrossRef]
- Bae, S.H.; Karthikeyan, K.; Lee, Y.S.; Oh, I.K. Microwave self-assembly of 3D graphene-carbon nanotube-nickel nanostructure for high capacity anode material in lithium ion battery. Carbon 2013, 64, 527–536. [Google Scholar] [CrossRef]
- Chatla, A.; Abu-Rub, F.; Prakash, A.V.; Ibrahim, G.; Elbashir, N.O. Highly stable and coke-resistant Zn-modified Ni-Mg-Al hydrotalcite derived catalyst for dry reforming of methane: Synergistic effect of Ni and Zn. Fuel 2022, 308, 122042. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Z.; Xu, L.; Wang, J.; Fan, L.; Kong, F.; Shi, Q.; Piao, Y.; Diao, G.; Chen, M. Consecutive hybrid mechanism boosting Na+ storage performance of dual-confined SnSe2 in N, Se-doping double-walled hollow carbon spheres. J. Energy Chem. 2022, 74, 8–17. [Google Scholar] [CrossRef]
- Hu, X.; Yang, X.; Liu, Y.; Qiu, M.; Tian, Z.; Guo, Y.; Yuan, J.; Ding, Y.; Zhan, H.; Wen, Z. Confined replacement synthesis of SnSe nanoplates in N-doped hollow carbon nanocages for high-performance sodium-ion batteries. Inorg. Chem. Front. 2023, 10, 793–803. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Wang, Q.; Gu, S.; Wang, L.; Yu, J.; Zhou, G.; Xu, L. SnSe2/NiSe2@N-Doped Carbon Yolk-Shell Heterostructure Construction and Selenium Vacancies Engineering for Ultrastable Sodium-Ion Storage. Adv. Energy Mater. 2023, 13, 2302901. [Google Scholar] [CrossRef]
- Luo, C.W.; Zhang, K.; Zeng, H.Y.; Yan, W.; Lv, S.B.; Wu, G.Z. Engineering ZnNi-LDH with improved wettability by N-direct-doping for high-performance supercapacitor. J. Alloys Compd. 2025, 1010, 177406. [Google Scholar] [CrossRef]
- Li, J.Y.; Qian, C.; Hu, Y.F.; Huang, J.F.; Chen, G.J.; Cao, L.Y.; Wang, F.M.; Kajiyoshi, K.; Zhao, Y.; Liu, Y.J.; et al. Tetrahedral Bonding Structure (Ni3-Se) Induced by Lattice-Distortion of Ni to Achieve High Catalytic Activity in Na-Se Battery. Small 2023, 19, 2302100. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Wang, Z.; Chen, L.; Gao, C.; Xu, B.; Jia, Z.; Wu, G. Synthesis of 3D flower-like ZnO/ZnCo2O4 composites with the heterogeneous interface for excellent electromagnetic wave absorption properties. J. Colloid Interface Sci. 2021, 586, 479–490. [Google Scholar] [CrossRef]
- Liu, P.; Han, J.; Zhu, K.; Dong, Z.; Jiao, L. Heterostructure SnSe2/ZnSe@PDA Nanobox for Stable and Highly Efficient Sodium-Ion Storage. Adv. Energy Mater. 2020, 10, 2000741. [Google Scholar] [CrossRef]
- Wei, S.Y.; Huang, J.F.; Wang, Y.T.; Huang, Q.Q.; Bai, S.Z.; Kajiyoshi, K.; Liu, Y.J.; Li, Z.J.; Cao, L.Y.; Li, J.Y. Embedding WS2 sheets parallel to SnS sheets for high performance in K-ion batteries. Chem. Eng. J. 2024, 484, 149455. [Google Scholar] [CrossRef]
- Ma, M.; Cao, L.Y.; Li, J.Y.; Yao, K.; Huang, J.F.; Qi, H.; Chen, S.Y. Tailoring mulberry-like Fe2O3 architecture assembled by quantum dots on rGO to enable high pseudocapacitance and controllable solid electrolyte interphase. Chem. Eng. J. 2020, 388, 124119. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Q.; Han, Q.; Zhao, L.; Liu, Y.; Wang, Y.; Li, Z.; Dong, C.; Sun, X.; Yang, J.; et al. Design of High-Capacity MoS3 Decorated Nitrogen Doped Carbon Coated Cu2S Electrode Structures with Dual Heterogenous Interfaces for Outstanding Sodium-Ion Storage. Small 2023, 19, 2303742. [Google Scholar] [CrossRef]
- Kong, L.C.; Li, Y.; Peng, C.; Sun, L.D.; Wang, K.; Liu, Y.; Feng, W. Defective nano-structure regulating C-F bond for lithium/fluorinated carbon batteries with dual high-performance. Nano Energy 2022, 104, 107905. [Google Scholar] [CrossRef]
- Li, L.; Zhu, L.; Pan, Y.; Lei, W.X.; Ma, Z.S.; Li, Z.Z.; Cheng, J.J.; Zhou, J. Integrated Polyaniline-coated CFx Cathode Materials with Enhanced Electrochemical Capabilities for Li/CFx Primary Battery. Int. J. Electrochem. Sci. 2016, 11, 6838–6847. [Google Scholar] [CrossRef]
- Yin, X.D.; Li, Y.; Feng, Y.Y.; Feng, W. Polythiophene/graphite fluoride composites cathode for high power and energy densities lithium primary batteries. Synth. Met. 2016, 220, 560–566. [Google Scholar] [CrossRef]
- Moon, C.E.; Nguyen, A.G.; Yang, J.S.; Nazir, A.; Verma, R.; Park, C.J. Confining SnSe particles in nitrogen-doped carbon nanofibers: A free-standing, binder-free anode for potassium-ion batteries. Carbon 2024, 218, 118741. [Google Scholar] [CrossRef]
- Hu, J.X.; Xie, Y.Y.; Zhou, X.L.; Zhang, Z.A. Engineering Hollow Porous Carbon-Sphere-Confined MoS2 with Expanded (002) Planes for Boosting Potassium-Ion Storage. ACS Appl. Mater. Interfaces 2020, 12, 1232–1240. [Google Scholar] [CrossRef]
- He, Z.M.; Wang, H.Y.; Liang, M.M.; Ma, H.; Zhang, C.H.; Zhao, Y.Z.; Qu, Y.; Miao, Z.C. Controlled synthesis of spindle-like CoNi2S4 as electrode material for aqueous energy storage application. Int. J. Hydrogen Energy 2024, 49, 81–89. [Google Scholar] [CrossRef]
- Wang, H.Y.; Liang, M.M.; Yang, P.F.; Miao, Z.C. Moss-like Ti3C2Tx based Fe/H2V3O8 composite as bifunctional electrode material for ammonium ion and zinc ion storage. Chem. Eng. J. 2024, 495, 153530. [Google Scholar] [CrossRef]
- Li, L.Y.; Wu, R.Z.; Ma, H.C.; Cheng, B.B.; Rao, S.Q.; Lin, S.; Xu, C.B.; Li, L.; Ding, Y.; Mai, L.Q. Toward the High-Performance Lithium Primary Batteries by Chemically Modified Fluorinate Carbon with d-MnO2. Small 2023, 19, 2300762. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Li, J.; Zheng, Y.; Dong, X.; Zhao, Y.; He, Z.; Li, M.; Lin, L.; He, D.; Miao, Z.; et al. Improved Electrochemical Performance Using Transition Metal Doped ZnNi/Carbon Nanotubes as Conductive Additive in Li/CFx Battery. Catalysts 2025, 15, 758. https://doi.org/10.3390/catal15080758
Wang F, Li J, Zheng Y, Dong X, Zhao Y, He Z, Li M, Lin L, He D, Miao Z, et al. Improved Electrochemical Performance Using Transition Metal Doped ZnNi/Carbon Nanotubes as Conductive Additive in Li/CFx Battery. Catalysts. 2025; 15(8):758. https://doi.org/10.3390/catal15080758
Chicago/Turabian StyleWang, Fangmin, Jiayin Li, Yuxin Zheng, Xue Dong, Yuzhen Zhao, Zemin He, Manni Li, Lei Lin, Danyang He, Zongcheng Miao, and et al. 2025. "Improved Electrochemical Performance Using Transition Metal Doped ZnNi/Carbon Nanotubes as Conductive Additive in Li/CFx Battery" Catalysts 15, no. 8: 758. https://doi.org/10.3390/catal15080758
APA StyleWang, F., Li, J., Zheng, Y., Dong, X., Zhao, Y., He, Z., Li, M., Lin, L., He, D., Miao, Z., Zhang, H., Tan, H., & Huang, J. (2025). Improved Electrochemical Performance Using Transition Metal Doped ZnNi/Carbon Nanotubes as Conductive Additive in Li/CFx Battery. Catalysts, 15(8), 758. https://doi.org/10.3390/catal15080758







