Anchor Biochar from Potato Peels with Magnetite Nanoparticles for Solar Photocatalytic Treatment of Oily Wastewater Effluent
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphological and Structural Characterization of POT400-M
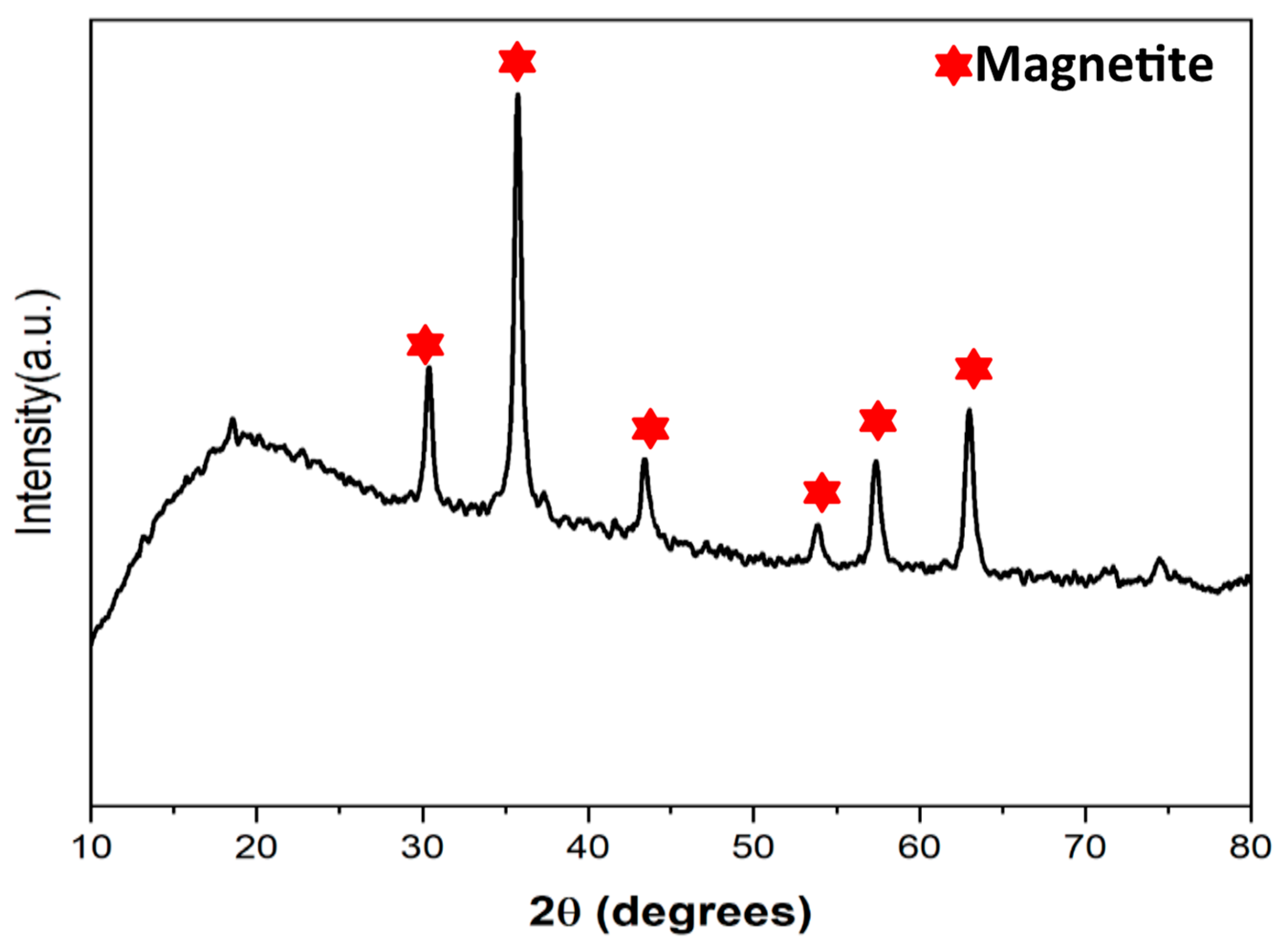
2.1.1. XRD
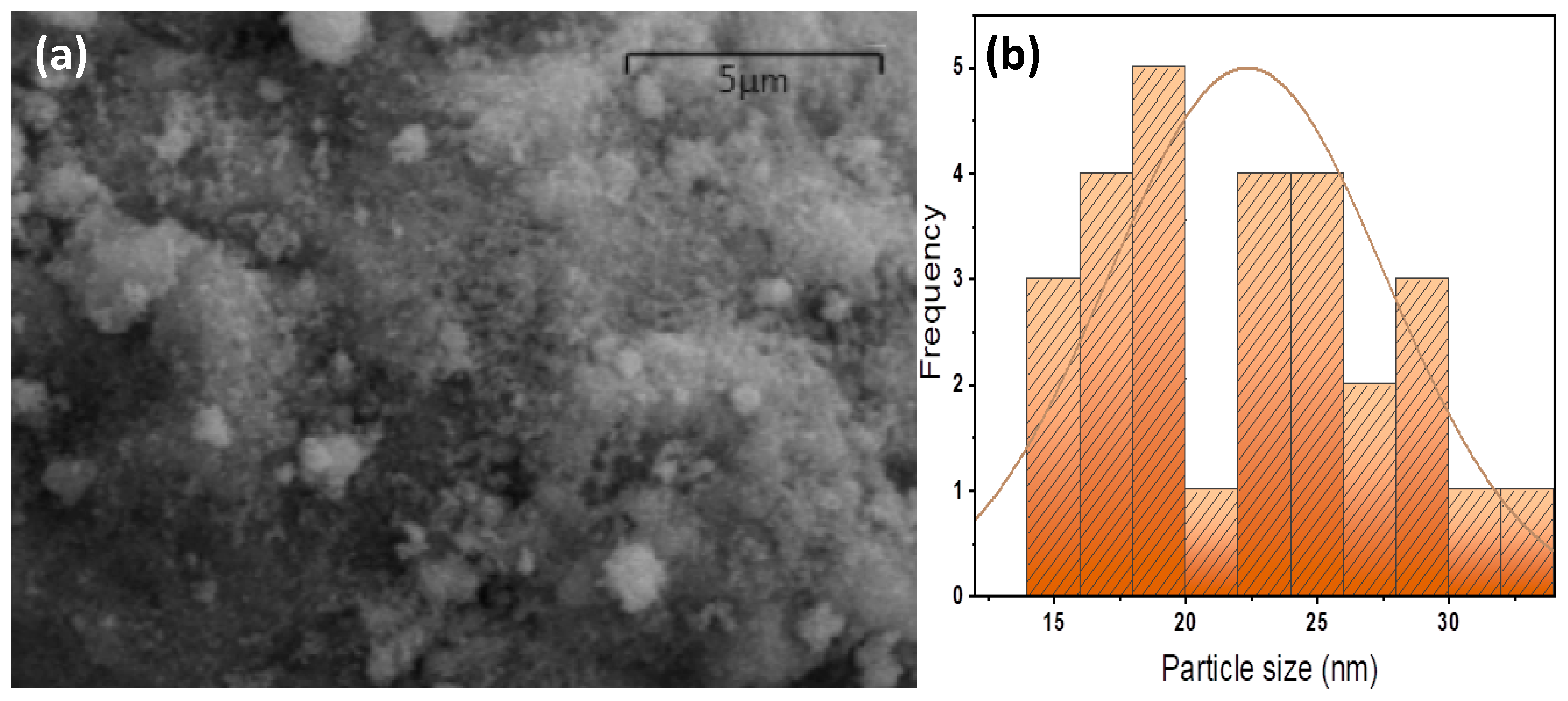
2.1.2. SEM Morphology and Particle Size Distribution
2.1.3. EDX Analysis
2.2. Oil Spill Oxidation
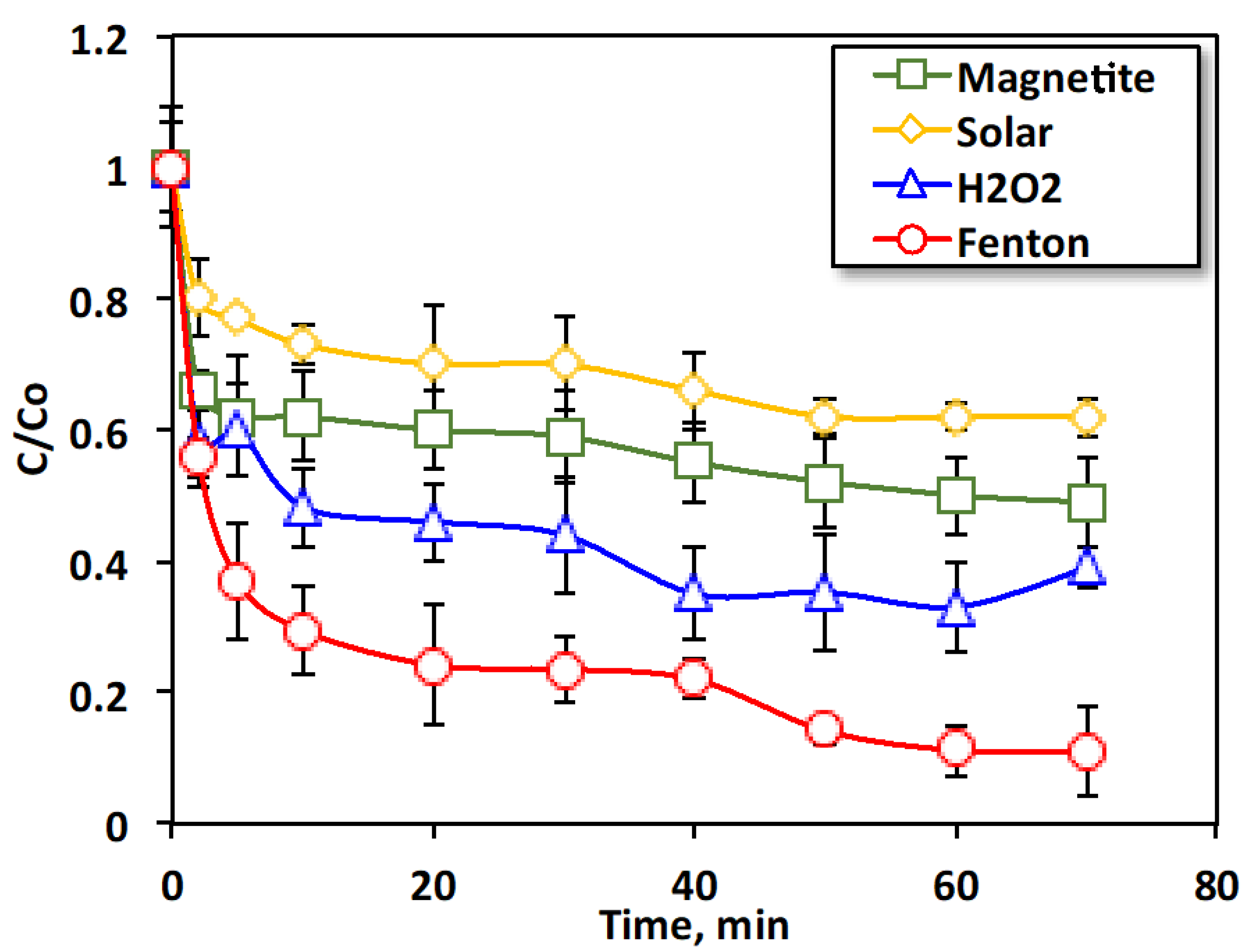
2.2.1. Effect of Oxidation Time on Different Oxidation Systems
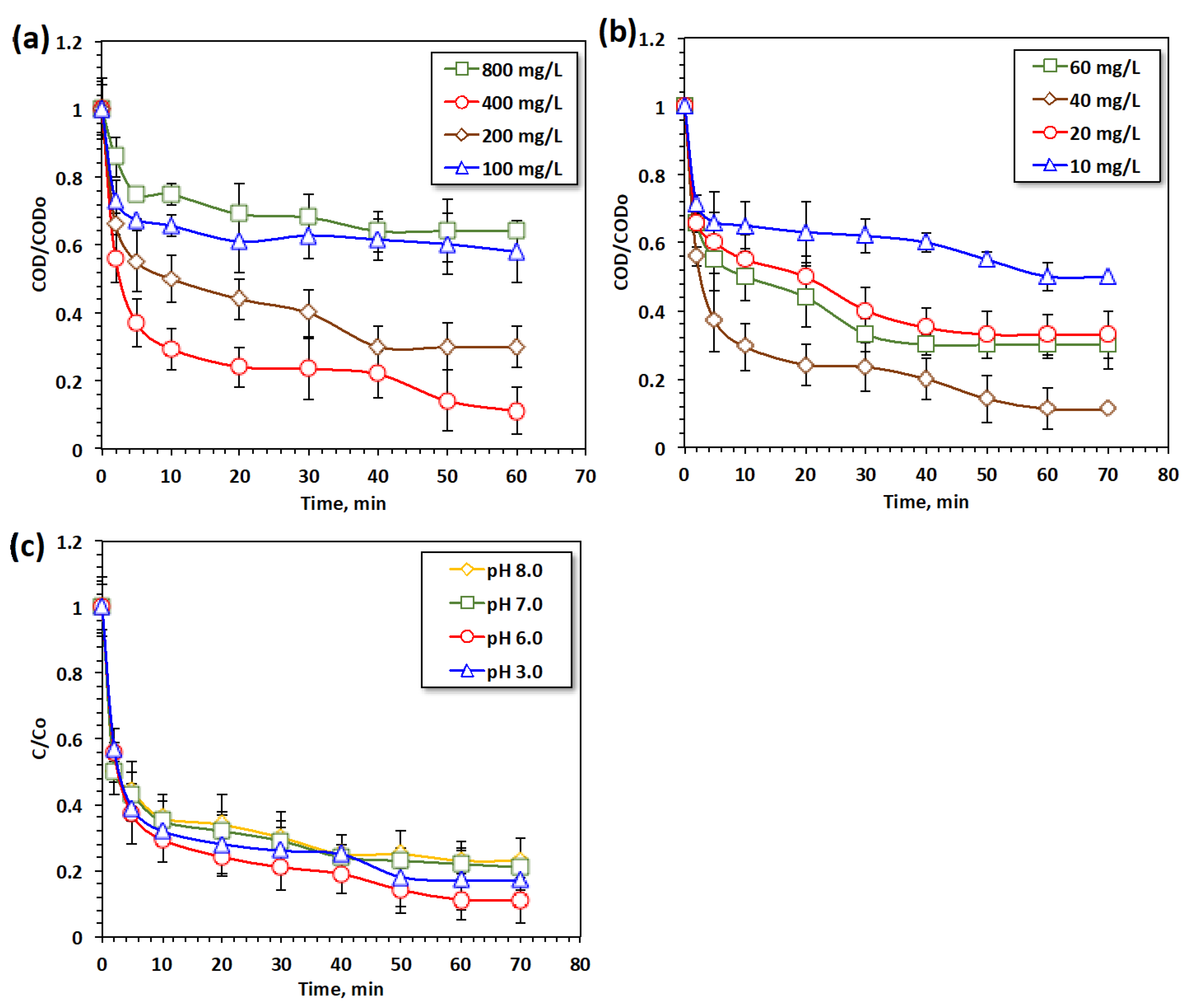
2.2.2. Effectiveness of Modified Fenton-like System’s Multiple Parameters (POT400-M Catalyst, Hydrogen Peroxide, and pH Value)
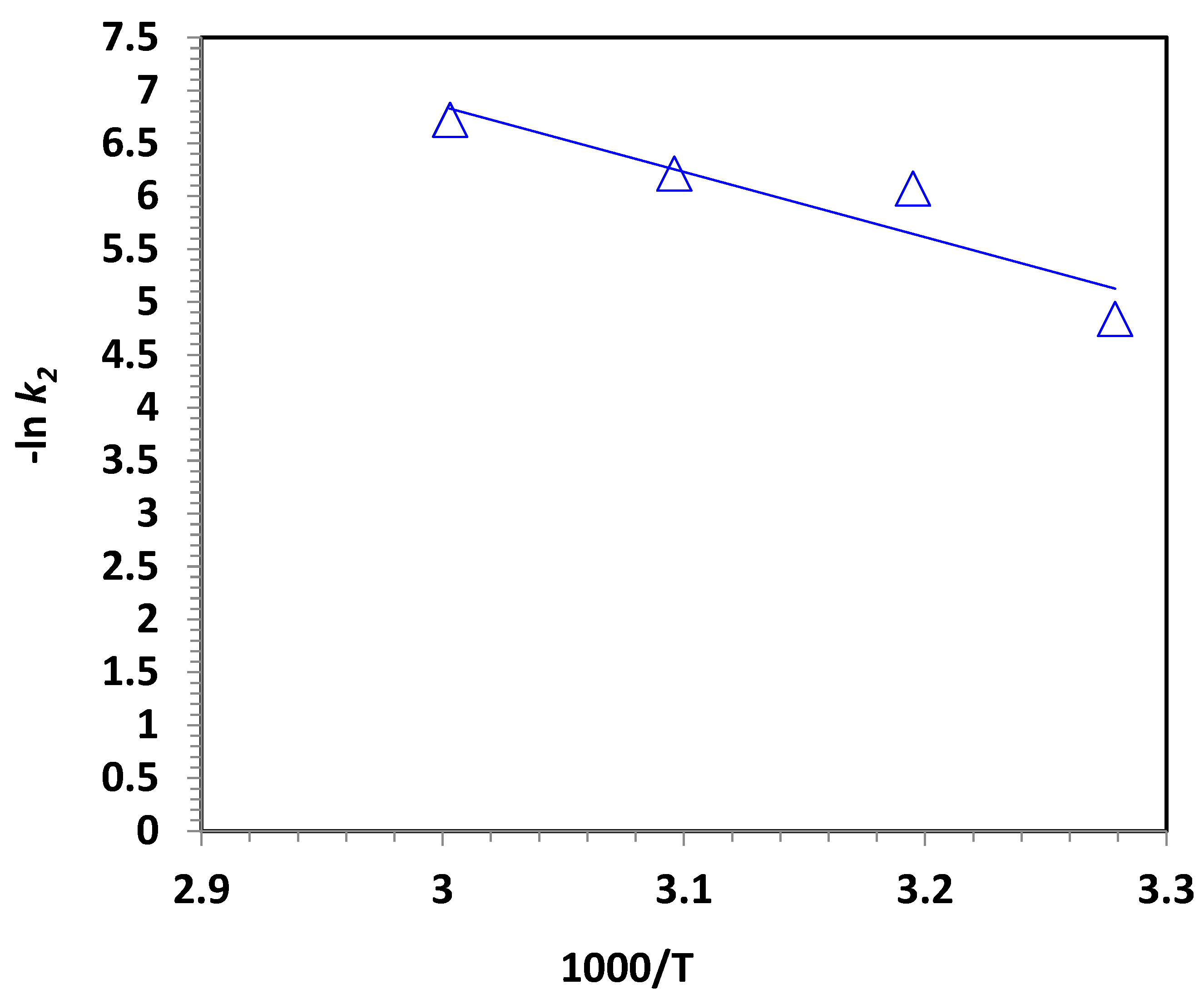
2.2.3. Effect of Temperature on Oxidation Kinetics and Thermodynamics
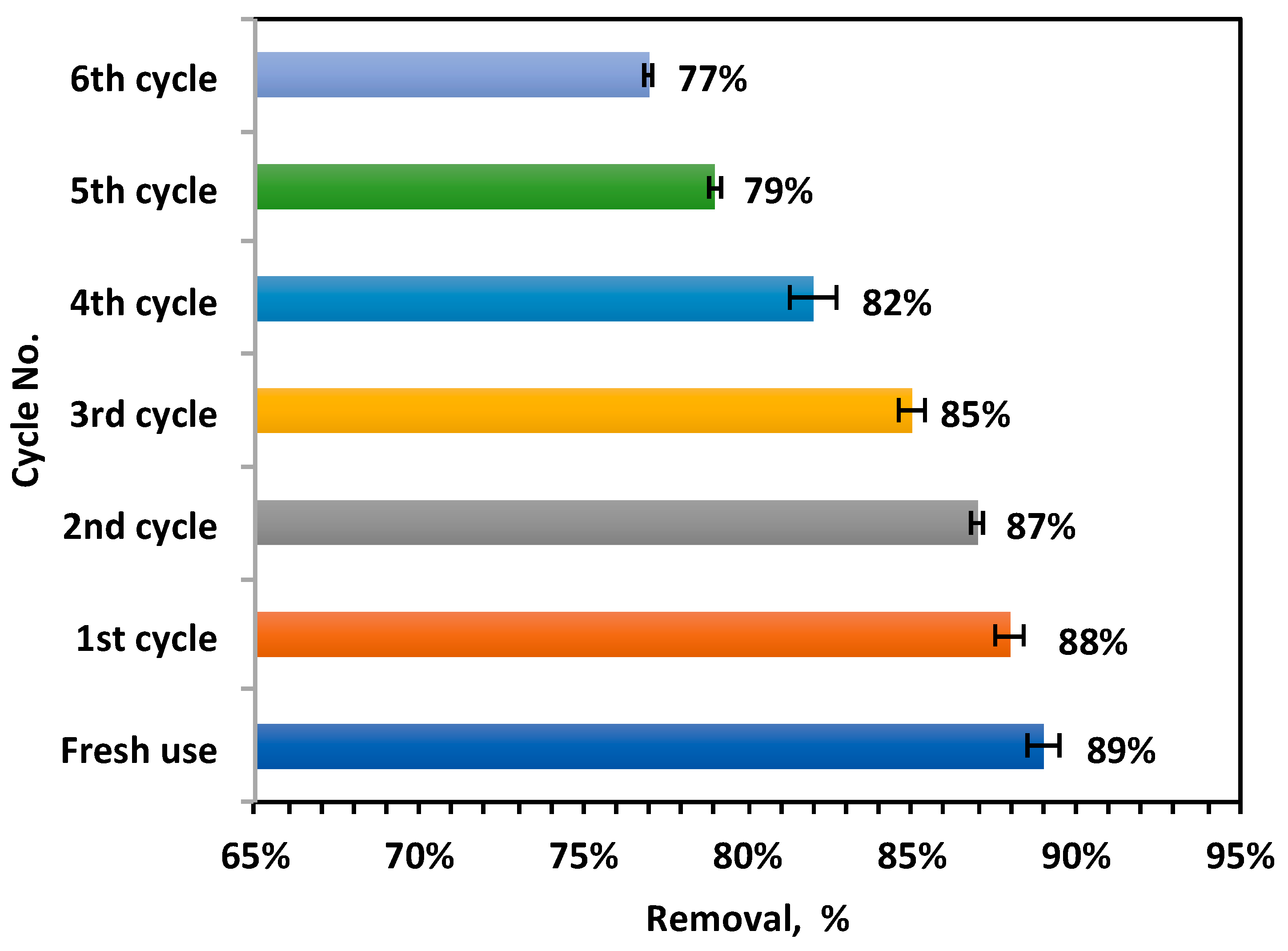
2.2.4. Recyclability and Stability
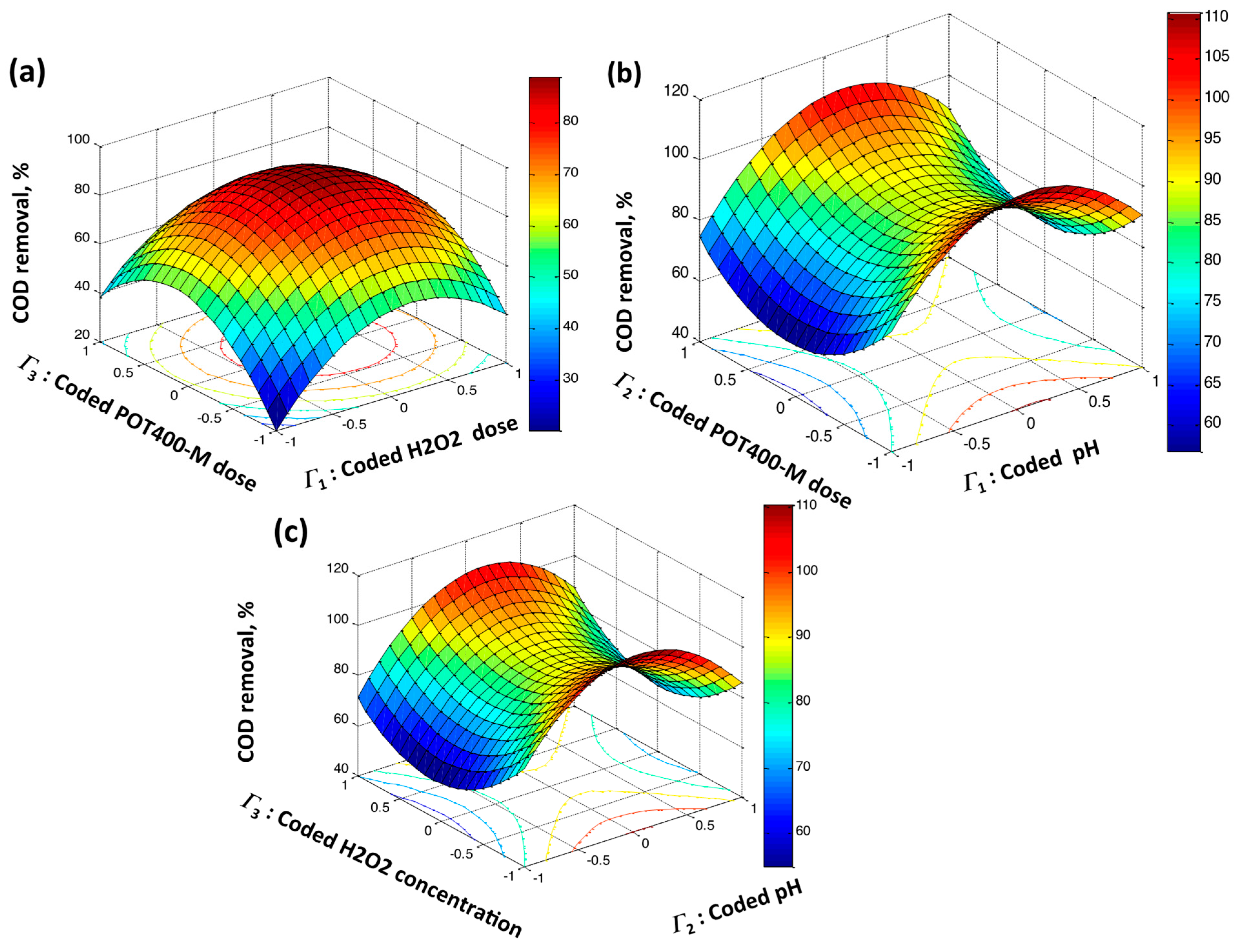
2.2.5. Numerical Optimization and Experimental Design
2.2.6. Comparative Analysis
3. Materials and Methods
3.1. Field Sampling and Tested Oil
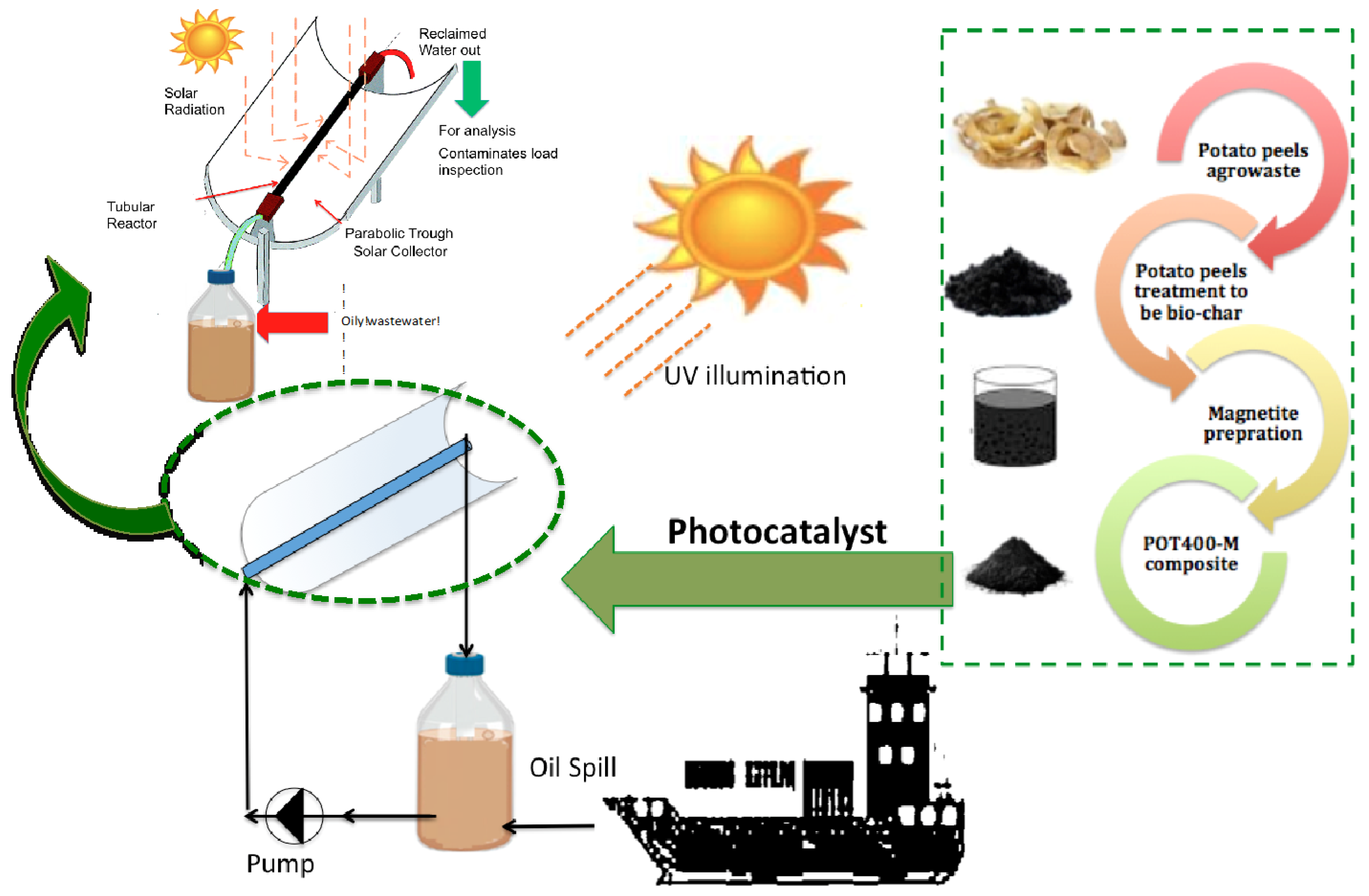
3.2. Magnetic Bio-Char Preparation
3.3. Characterization of the Material
3.4. Solar Photochemical Reactor Configuration
3.5. Methodology and Photocatalytic Determination
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ifelebuegu, A.O.; Lale, E.E.; Mbanaso, F.U.; Theophilus, S.C. Facile Fabrication of Recyclable, Superhydrophobic, and Oleophilic Sorbent from Waste Cigarette Filters for the Sequestration of Oil Pollutants from an Aqueous Environment. Processes 2018, 6, 140. [Google Scholar] [CrossRef]
- Tony, M.A.; Nabwey, H.A. Recent advances in solar still technology for solar water desalination. Appl. Water Sci. 2024, 14, 147. [Google Scholar] [CrossRef]
- Xu, E.G.; Richardot, W.H.; Li, S.; Buruaem, L.; Wei, H.-H.; Dodder, N.G.; Schick, S.F.; Novotny, T.; Schlenk, D.; Gersberg, R.M.; et al. Assessing Toxicity and in Vitro Bioactivity of Smoked Cigarette Leachate Using Cell-Based Assays and Chemical Analysis. Chem. Res. Toxicol. 2019, 32, 1670–1679. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, M.; Li, Y.; Cheng, F.; Liu, Y.; Gao, M.; Liu, G.; Hu, L.; Liang, Y. Effectiveness of discarded cigarette butts derived carbonaceous adsorbent for heavy metals removal from water. Microchem. J. 2021, 168, 106474. [Google Scholar] [CrossRef]
- Bi, R.; Pang, S.K.; Yung, K.C.; Yin, L.K. Comprehensive study of used cigarette filters-derived porous activated carbon for Supercapacitors: From biomass waste to sustainable energy source. J. Electroanal. Chem. 2022, 925, 116915. [Google Scholar] [CrossRef]
- Almojjly, A.; Johnson, D.; Oatley-Radcliffe, D.; Hilal, N. Removal of oil from oil-water emulsion by hybrid coagulation/sand filter as pre-treatment. J. Water Process Eng. 2018, 26, 17–27. [Google Scholar] [CrossRef]
- Guo, S.; Dan, Z.; Duan, N.; Chen, G.; Gao, W.; Zhao, W. Zn (II), Pb (II), and Cd (II) adsorption from aqueous solution by magnetic silica gel: Preparation, characterization, and adsorption. Environ. Sci. Pollut. Res. 2018, 25, 30938–30948. [Google Scholar] [CrossRef]
- Raut-Jadhav, S.; Pinjari, D.V.; Saini, D.R.; Sonawane, S.H.; Pandit, A.B. Intensification of degradation of methomyl (carbamate group pesticide) by using the combination of ultrasonic cavitation and process intensifying additives. Ultrason. Sonochem. 2016, 31, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Nan, Z. Formation of octahedron-shaped ZnFe2O4/SiO2 with yolk–shell structure. J. Phys. Chem. Solids 2020, 141, 109410. [Google Scholar] [CrossRef]
- Pourali, P.; Behzad, M.; Arfaeinia, H.; Ahmadfazeli, A.; Afshin, S.; Poureshgh, Y.; Rashtbari, Y. Removal of acid blue 113 from aqueous solutions using low-cost adsorbent: Adsorption isotherms, thermodynamics, kinetics and regeneration studies. Sep. Sci. Technol. 2021, 56, 3079–3091. [Google Scholar] [CrossRef]
- Phan, T.T.N.; Nikoloski, A.N.; Bahri, P.A.; Li, D. Facile fabrication of perovskite-incorporated hierarchically mesoporous/macroporous silica for efficient photoassisted-Fenton degradation of dye. Appl. Surf. Sci. 2019, 491, 488–496. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Huynh, K.A.; Padungthon, S.; Pranudta, A.; Amonpattaratkit, P.; Tran, L.B.; Phan, P.T.; Nguyen, N.H. Synthesis of natural flowerlike iron-alum oxide with special interaction of Fe-Si-Al oxides as an effective catalyst for heterogeneous Fenton process. J. Environ. Chem. Eng. 2021, 9, 105732. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Z.; Shen, S.; Zhong, W.; Cao, S. Advances in designing heterojunction photocatalytic materials. Chin. J. Catal. 2021, 42, 710–730. [Google Scholar] [CrossRef]
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. A Review of Direct Z-Scheme Photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Li, F.; Zhu, G.; Jiang, J.; Yang, L.; Deng, F.; Arramel; Li, X. A review of updated S-scheme heterojunction photocatalysts. J. Mater. Sci. Technol. 2024, 177, 142–180. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Al-Zaqri, N.; EI marghany, A.; Alghamdi, A.A.; Alorabi, A.Q.; Baghdadi, N.; AL-Shehri, H.S.; Wahab, R.; Ahmad, N. Synthesis of nanocauliflower ZnO photocatalyst by potato waste and its photocatalytic efficiency against dye. J. Mater. Sci. Mater. Electron. 2020, 31, 11538–11547. [Google Scholar] [CrossRef]
- Sun, H.; Xu, R.; Jia, X.; Liu, Z.; Chen, H.; Lu, T. Recent advances in the photocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran. Biomass Convers. Biorefinery 2023, 14, 26707–26724. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, Q.; Zhang, H.; Wang, N.; Chang, S.; Wang, H.; Pang, H.; Chen, H. Treatment of real benzene dye intermediates wastewater by the Fenton method: Characteristics and multi-response optimization. RSC Adv. 2018, 8, 80–90. [Google Scholar] [CrossRef]
- Bolobajev, J.; Katte, E.; Viisimaa, M.; Go, A.; Trapido, M.; Tenno, T.; Dulova, N. Reuse of ferric sludge as an iron source for the Fenton-based process in wastewater treatment. Chem. Eng. J. 2014, 255, 8–13. [Google Scholar] [CrossRef]
- Pintor, A.M.; Vilar, V.J.; Boaventura, R.A. Decontamination of cork wastewaters by solar-photo-Fenton process using cork bleaching wastewater as H2O2 source. Sol. Energy 2011, 85, 579–587. [Google Scholar] [CrossRef]
- Magnago, R.F.; Berselli, D.; Medeiros, P. Treatment of wastewater from car wash by fenton and photo-fenton oxidative processes. J. Eng. Sci. Technol. 2018, 13, 838–850. [Google Scholar]
- Choi, H.; Cloud, R.M. Natural Sorbents in Oil Spill Cleanup. Environ. Sci. Technol. 1992, 26, 772–776. [Google Scholar] [CrossRef]
- El-Sayed, M.A.; El-Samni, T.M. Physical and Chemical Properties of Rice Straw Ash and Its Effect on the Cement Paste produced from different cement types materials and methods. J. King Saud. Univ. Eng. Sci. 2006, 19, 21–30. [Google Scholar] [CrossRef]
- Tayeb, A.M.; Farouq, R.; Mohamed, O.; Tony, M.A. Oil spill clean-up using combined sorbents: A comparative investigation and design aspects. Int. J. Environ. Anal. Chem. 2019, 100, 311–325. [Google Scholar] [CrossRef]
- APHA; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; Port City Press: Baltimore, MD, USA, 2005. [Google Scholar]
- Primo, O.; Rueda, A.; Rivero, M.J.; Ortiz, I. An Integrated Process, Fenton Reaction−Ultrafiltration, for the Treatment of Landfill Leachate: Pilot Plant Operation and Analysis. Ind. Eng. Chem. Res. 2008, 47, 946–952. [Google Scholar] [CrossRef]
- Jabbar, H.A.; Alatabe, M.J.A. Treatment oilfield produced water using coagulation/flocculation process (case study: Alahdab Oilfield). Pollution 2021, 7, 787–797. [Google Scholar]
- Mico, M.M.; Chourdaki, S.J.; Bacardit, J.; Sans, C. Degradation of p-nitrophenol using CuO/Al2O3 as a Fenton-like catalyst under microwave irradiation. Ozone Sci. Eng. 2010, 32, 259–264. [Google Scholar]
- Mirshahghassemi, S.; Aminzadeh, B.; Torabian, A. Optimizing electrocoagulation and electro-Fenton process for treating car wash wastewater. Environ. Health Eng. Manag. J. 2017, 4, 37–43. [Google Scholar] [CrossRef]
- Poorsargol, M.; Razmara, Z.; Amiri, M.M. The role of hydroxyl and carboxyl functional groups in adsorption of copper by carbon nanotube and hybrid graphene–carbon nanotube: Insights from molecular dynamic simulation. Adsorption 2020, 26, 397–405. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, Y.; Liu, P.; Men, Y.-L.; Pan, Y.-X. Boosting the adsorption and removal of dye from water by COOH-functionalized carbon nanotubes. Green Chem. Eng. 2023, 4, 88–98. [Google Scholar] [CrossRef]
- Nam, S.W.; Choi, D.J.; Kim, S.K.; Her, N.; Zoh, K.D. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J. Hazard. Mater. 2014, 270, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Ying, D.; Liang, X.; Zhu, M.; Lin, X.; Xu, Q.; Cai, Z.; Xu, X.; Zhang, L. A novel cationic polyelectrolyte microsphere for ultrafast and ultra-efficient removal of heavy metal ions and dyes. Chem. Eng. J. 2021, 410, 128404. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Tan, J.; Jiang, M.; Fu, S.; Zhang, T.; Wang, X. In situ synthesis of novel peroxo-functionalized Ti3C2Tx adsorbent for aqueous pollutants removal: Role of oxygen-containing terminal groups. Chemosphere 2022, 286, 131801. [Google Scholar] [CrossRef]
- Li, N.; Bai, R. Development of chitosan-based granular adsorbents for enhanced and selective adsorption performance in heavy metal removal. Water Sci. Technol. 2006, 54, 103–113. [Google Scholar] [CrossRef]
- Shaban, A.M.; Kamal, M.M.; Kenawy, N.; El Manakhly, H. Role of interior structure of agro and non-agro materials for industrial wastewater treatment. J. Appl. Sci. Res. 2009, 5, 978–985. [Google Scholar]
- Luna, J.M.; Rufino, R.D.; Jara, A.M.A.T.; Brasileiro, P.P.F.; Sarubbo, L.A. Environmental applications of the biosurfactant produced by Candida sphaerica cultivated in low-cost substrates. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 413–418. [Google Scholar] [CrossRef]
- Liu, X.; Ge, L.; Li, W.; Wang, X.; Li, F. Layered double hydroxide functionalized textile for effective oil/water separation and selective oil adsorption. ACS Appl. Mater. Interfaces 2015, 7, 791–800. [Google Scholar] [CrossRef]
- Yue, C.; Liu, J.; Zhang, H.; Dai, L.; Wei, B.; Chang, Q. Increasing the hydrophobicity of filter medium particles for oily water treatment using coupling agents. Heliyon 2018, 4, e00809. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gawad, H.S. Oil and Grease Removal from Industrial Wastewater Using New Utility Approach. Adv. Environ. Chem. 2014, 2014, 6. [Google Scholar] [CrossRef]
- Udonne, J.D. Chemical treatment of emulsion problem in crude oil production. J. Pet. Gas Eng. 2012, 7, 135–141. [Google Scholar]
- Hussain, F.A.; Zamora, J.; Ferrer, I.M.; Kinyuac, M.; Velázqueza, J.M. Adsorption of Crude Oil from Crude Oil-Water Emulsion by Mesoporous Hafnium Oxide Ceramics. Environ. Sci. Water Res. Technol. 2020, 6, 2035–2042. [Google Scholar] [CrossRef]
- Wei, B.; Liu, J.; Wang, G.; Chang, Q. Filtration of oil from oily wastewater via hydrophobic modified quartz sand filter medium. J. Water Reuse Desalinat. 2018, 8, 544–552. [Google Scholar] [CrossRef]
- Roushenas, P.; Yusop, Z.; Majidnia, Z.; Nasrollahpour, R. Photocatalytic degradation of spilled oil in sea water using maghemite nanoparticles. Desalinat. Water Treat. 2016, 57, 5837–5841. [Google Scholar] [CrossRef]
- Sadikina, A.N.; Mohd nawawia, M.G.; Othmana, N.; Rasit Alib, R.; Umi, A.A. Removal of Oily Wastewater Using Chitosan-filled Filter Media. J. Teknol. 2015, 74, 111–115. [Google Scholar] [CrossRef][Green Version]
- Nour, M.M.; Tony, M.A.; Nabwey, H.A.; Shaaban, S.M. Experimental Design and Numerical Optimization of Photochemical Oxidation Removal of Tetracycline from Water Using Fe3O4-Supported Fruit Waste Activated Carbon. Catalysts 2025, 15, 351. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1989. [Google Scholar]
- Zhang, J.; Xu, H.; Guo, J.; Chen, T.; Liu, H. Superhydrophobic Polypyrrole-Coated Cigarette Filters for Effective Oil/Water Separation. Appl. Sci. 2020, 10, 1985. [Google Scholar] [CrossRef]
- Nabwey, H.A.; Tony, M.A. Dewatered Sludge Decorated with Nanoparticles for Alum Sludge Conditioning towards the Concept of “End-of-Waste”. Nanomaterials 2023, 13, 2903. [Google Scholar] [CrossRef] [PubMed]
- Thabet, R.H.; Fouad, M.K.; El Sherbiny, S.A.; Tony, M.A. Zero-waste approach: Assessment of aluminum-based waste as a photocatalyst for industrial wastewater treatment ecology. Int. J. Environ. Res. 2022, 16, 36. [Google Scholar] [CrossRef]
- Doyan, A.; Leong, C.L.; Bilad, M.R.; Kurnia, K.A.; Susilawati, S.; Prayogi, S.; Narkkun, T.; Faungnawakij, K. Cigarette Butt Waste as Material for Phase Inverted Membrane Fabrication Used for Oil/Water Emulsion Separation. Polymers 2021, 13, 1907. [Google Scholar] [CrossRef]
- Ou, J.; Wan, B.; Wang, F.; Xue, M.; Wu, H.; Li, W. Superhydrophobic fibers from cigarette filters for oil spill cleanup. RSC Adv. 2016, 6, 44469. [Google Scholar] [CrossRef]
- Tony, M.A. Recent frontiers in solar energy storage via nanoparticles enhanced phase change materials: Succinct review on basics, applications and their environmental aspects. Energy Storage 2021, 3, e238. [Google Scholar] [CrossRef]
- Tony, M.A.; Eltabey, M.M. End-of-life waste criteria: Synthesis and utilization of Mn-Zn ferrite nanoparticles as a super paramagnetic photocatalyst for synergistic wastewater remediation. Appl. Water Sci. 2022, 12, 21. [Google Scholar] [CrossRef]
- Nascimento, T.A.; Dutra, F.V.A.; Pires, B.C.; Tarley, C.R.T.; Manoa, V.; Borges, K.B. Preparation and characterization of a composite based on polyaniline, polypyrrole and cigarette filters: Adsorption studies and kinetics of phenylbutazone in aqueous media. RSC Adv. 2016, 6, 64450. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Y.; Fang, C.; Shi, J.; Chen, J.; Han, H. Novel and multifunctional adsorbent fabricated by Zeolitic imidazolate framworks-8 and waste cigarette filters for wastewater treatment: Effective adsorption and photocatalysis. J. Solid State Chem. 2021, 299, 122190. [Google Scholar] [CrossRef]
- Nabwey, H.A.; Tony, M.A. Distinct pathway of multiferroic silver-decorated zinc ferrite nanocatalyst performance for Acinate insecticide oxidation. Sci. Rep. 2024, 14, 27078. [Google Scholar] [CrossRef]
- Li, L.; Jia, C.; Zhu, X.; Zhang, S. Utilization of cigarette butt waste as functional carbon precursor for supercapacitors and adsorbents. J. Clean. Prod. 2020, 256, 120326. [Google Scholar] [CrossRef]
- Abu-Danso, E.; Bagheri, A.; Bhatnagar, A. Facile functionalization of cellulose from discarded cigarette butts for the removal of diclofenac from water. Carbohydr. Polym. 2019, 219, 46–55. [Google Scholar] [CrossRef]
- Nabwey, H.A.; Abdelkreem, M.; Tony, M.A.; Al Hoseny, N.F. Smart win-win waste management: Superhydrophobic filter using valorized cellulose acetate from discarded cigarette butts for cleaning-up marine oil spill at Hurghada Red Sea shore in Egypt. Frontiers 2024, 11, 1270026. [Google Scholar] [CrossRef]
- Hassan, E.A.; Tony, M.A.; Nabwey, H.A.; Awad, M.M. Potential of the Biomass Waste Originating from Saccharum officinarum as a Fenton Precursor for the Efficient Oxidation of Azo Dye from an Aqueous Stream. Processes 2023, 11, 1394. [Google Scholar] [CrossRef]
- Gamaralalage, D.; Sawai, O.; Nunoura, T. Reusing the generated sludge as Fe source in Fenton process for treating crepe rubber wastewater. J. Mater. Cycles Waste Manag. 2019, 21, 248–257. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, J.; Zhou, J.; Lei, J. Study on adsorption of methylene blue by a novel composite material of TiO2 and alum sludge. RSC Adv. 2018, 8, 32799–32807. [Google Scholar] [CrossRef] [PubMed]
- Mulinari, J.; da Silva Júnior, A.H.; de Oliveira, C.R.S.; Júnior, F.W.R. Valorization and treatment of oily wastewaters from agro-industries using lipases: An overview. In Science, Technology and Innovation: From the Field to the Meal; FAO: Rome, Italy, 2020. [Google Scholar]
- Pan, J.R.; Huang, C.; Lin, S. Reuse of fresh water sludge in cement making. Water Sci. Technol. 2004, 50, 183–188. [Google Scholar] [CrossRef] [PubMed]


| Kinetic Model | Linear Equation | Parameters | Values | |||
|---|---|---|---|---|---|---|
| T(K) | ||||||
| 305 | 313 | 323 | 333 | |||
| Zero-order | K (min−1) | 0.19 | 0.13 | 0.14 | 0.21 | |
| t0.5 | 53.47 | 74.73 | 73.20 | 43.29 | ||
| R2 | 0.55 | 0.59 | 0.66 | 0.73 | ||
| First-order | K (min−1) | 0.075 | 0.033 | 0.031 | 0.027 | |
| t0.5 | 9.21 | 20.74 | 22.07 | 25.66 | ||
| R2 | 0.93 | 0.92 | 0.92 | 0.91 | ||
| Second-order | K (Lgmg−1min−1) | 0.0079 | 0.0023 | 0.002 | 0.0012 | |
| t0.5 | 6.32 | 21.73 | 25.01 | 41.67 | ||
| R2 | 0.99 | 0.92 | 0.94 | 0.92 | ||
| Temperature, K | Ln k2 | Ea, kJ/mol | ΔG′, kJ/mol | ΔH′, kJ/mol | ΔS′, J/mol |
|---|---|---|---|---|---|
| 305 | −4.84 | −51.34 | 87.03 | −53.88 | −462.00 |
| 313 | −6.07 | 92.59 | −53.94 | −468.17 | |
| 323 | −6.21 | 96.01 | −54.03 | −464.51 | |
| 333 | −6.72 | 100.48 | −54.11 | −464.24 |
| Parameters | Symbols | Levels | |||
|---|---|---|---|---|---|
| Natural | Coded | −1 | 0 | 1 | |
| POT400-M (mg/L) | τ1 | Γ1 | 20 | 40 | 60 |
| Hydrogen peroxide (mg/L) | τ | Γ2 | 200 | 400 | 600 |
| pH | τ3 | Γ3 | 5 | 6 | 7 |
| Run No. | Codified Variables | Natural Variables | ||||
|---|---|---|---|---|---|---|
| Γ1 | Γ2 | Γ3 | τ1 | τ2 | τ3 | |
| 1 | −1 | −1 | 0 | 20 | 200 | 6 |
| 2 | −1 | 1 | 0 | 20 | 600 | 6 |
| 3 | 1 | −1 | 0 | 60 | 200 | 6 |
| 4 | 1 | 1 | 0 | 60 | 600 | 6 |
| 5 | 0 | −1 | −1 | 40 | 200 | 5 |
| 6 | 0 | −1 | 1 | 40 | 200 | 7 |
| 7 | 0 | 1 | −1 | 40 | 600 | 5.0 |
| 8 | 0 | 1 | 1 | 40 | 600 | 7.0 |
| 9 | −1 | 0 | −1 | 20 | 400 | 5 |
| 10 | 1 | 0 | −1 | 60 | 400 | 5 |
| 11 | −1 | 0 | 1 | 20 | 400 | 7 |
| 12 | 1 | 0 | 1 | 60 | 400 | 7 |
| 13 | 0 | 0 | 0 | 40 | 400 | 6 |
| 14 | 0 | 0 | 0 | 40 | 400 | 6 |
| 15 | 0 | 0 | 0 | 40 | 400 | 6 |
| Source | Degrees of Freedom (DF) | Sum of Squares (SS) | Mean Squares (MS) | Fisher (F-Value) | Probability (p-Value) |
|---|---|---|---|---|---|
| Model | 9 | 7624.333 | 847.1481 | 13.16811 | 0.005544 |
| Linear | 3 | 579 | 579 | 9 | 0.753014 |
| Square | 3 | 2338.564 | 2338.564 | 36.35074 | 0.001806 |
| Interaction | 3 | 4377.82 | 4377.82 | 68.049031 | 0.566131 |
| Error | 5 | 321.6667 | 64.33333 | ||
| Total | 14 | 7946 |
| Technique | Oily Wastewater Type | Removal (%) | Ref. |
|---|---|---|---|
| POT400-M/ H2O2 | Oil spill in sea | 93 | Current work |
| Coagulation/sand filter | Vegetable oil | 88 | [39] |
| Candida biological treatment | Motor oil | 40 | [35] |
| Sand filter/DN-101/KH-570 | Gasoline oil | 87 | [41] |
| Magnetite NP/UV/H2O2 | Oil spill in sea | 90 | [42] |
| Sand filter media/ TiO2 | Gasoline engine oil | 36 | [37] |
| Cigarette filters | Diesel oil spill | 99 | [60] |
| Adsorption (mesoporous ceramics) | Crude oil | 99 | [40] |
| Filtration (chitosan packing) | Palm oil | 29 | [43] |
| Textile/ LDH | Diesel oil | 97 | [37] |
| Biological/ adsorption (enzyme/zeolite) | Oil/detergent | 72 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nour, M.M.; Nabwey, H.A.; Tony, M.A. Anchor Biochar from Potato Peels with Magnetite Nanoparticles for Solar Photocatalytic Treatment of Oily Wastewater Effluent. Catalysts 2025, 15, 731. https://doi.org/10.3390/catal15080731
Nour MM, Nabwey HA, Tony MA. Anchor Biochar from Potato Peels with Magnetite Nanoparticles for Solar Photocatalytic Treatment of Oily Wastewater Effluent. Catalysts. 2025; 15(8):731. https://doi.org/10.3390/catal15080731
Chicago/Turabian StyleNour, Manasik M., Hossam A. Nabwey, and Maha A. Tony. 2025. "Anchor Biochar from Potato Peels with Magnetite Nanoparticles for Solar Photocatalytic Treatment of Oily Wastewater Effluent" Catalysts 15, no. 8: 731. https://doi.org/10.3390/catal15080731
APA StyleNour, M. M., Nabwey, H. A., & Tony, M. A. (2025). Anchor Biochar from Potato Peels with Magnetite Nanoparticles for Solar Photocatalytic Treatment of Oily Wastewater Effluent. Catalysts, 15(8), 731. https://doi.org/10.3390/catal15080731








