Recent Progress of 2D Pt-Group Metallic Electrocatalysts for Energy-Conversion Applications
Abstract
1. Introduction
2. Synthesis of 2D Pt-Group Metallic Electrocatalysts
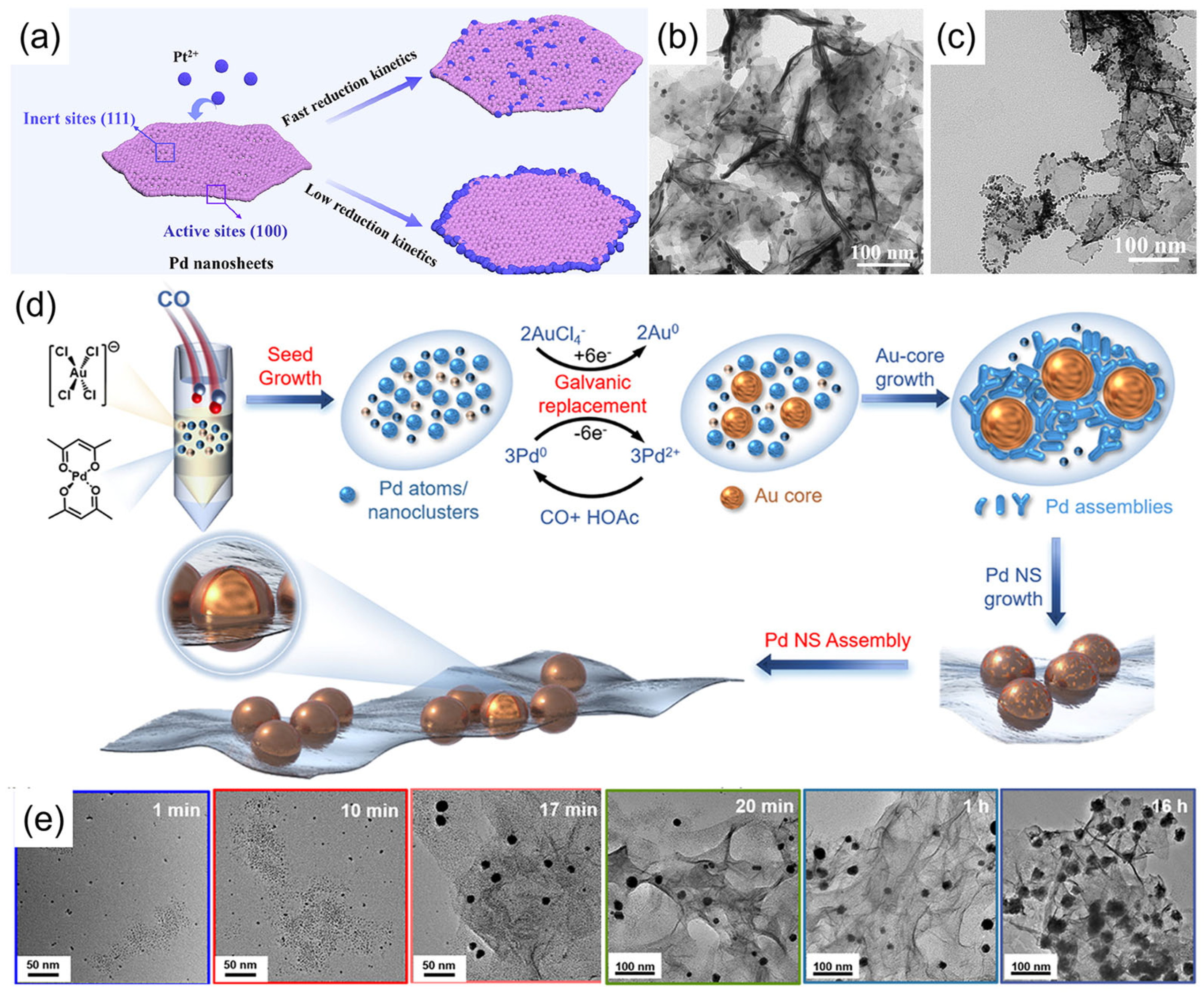
2.1. CO-Confined Growth Methods
2.2. Self-Assembly Methods
2.3. Template Methods
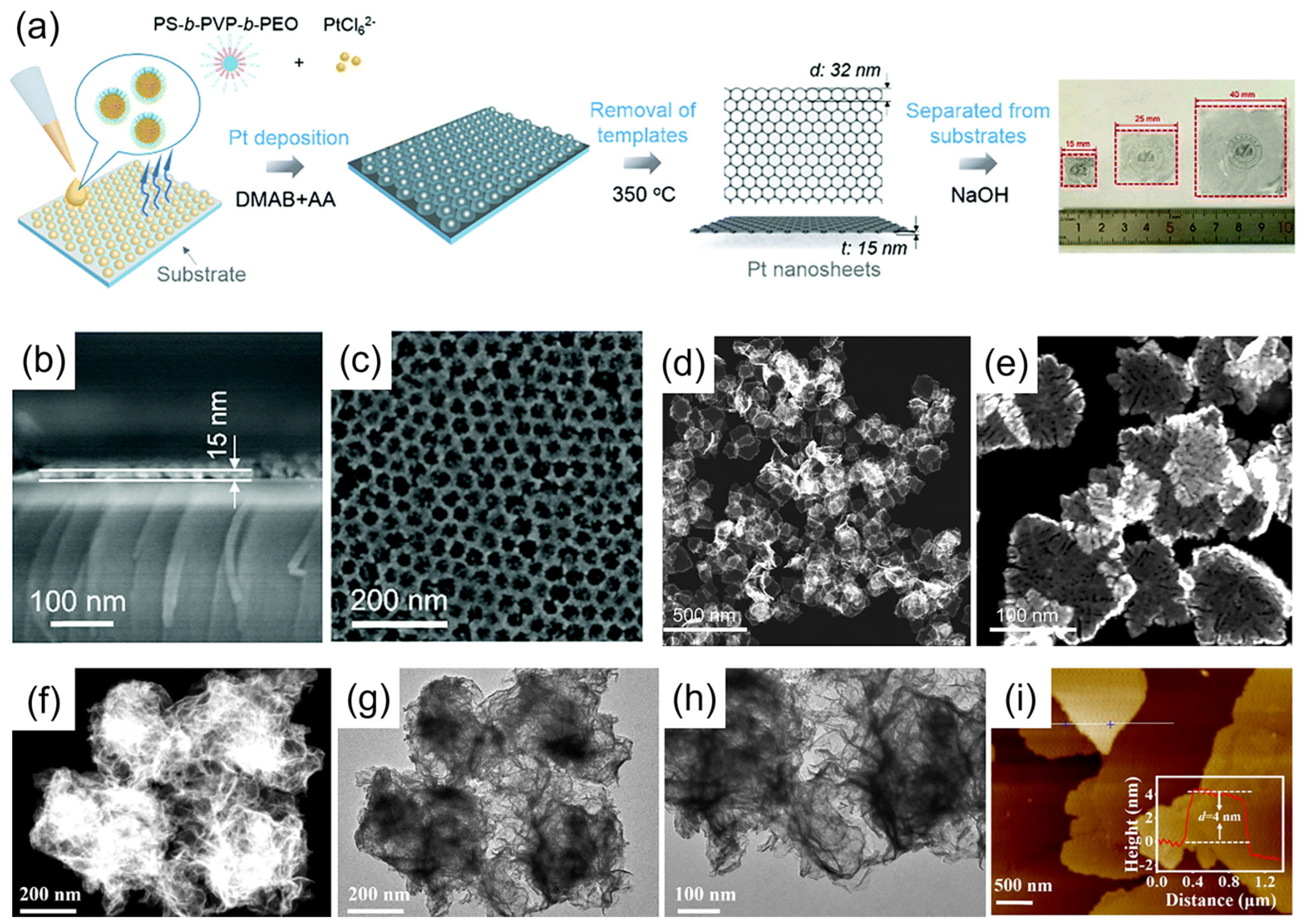
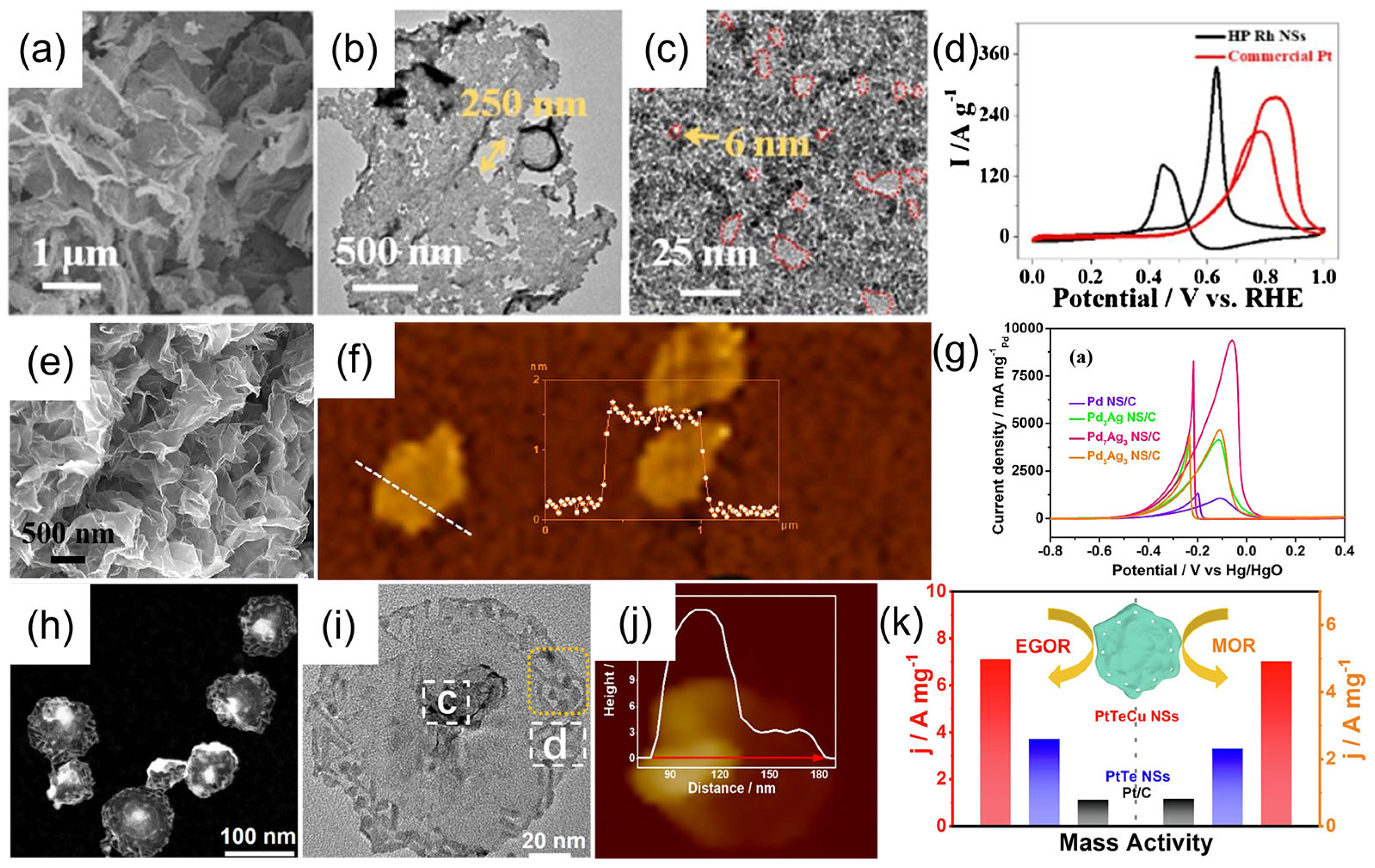
2.4. In Situ Growth Methods
2.5. Other Synthesis Methods
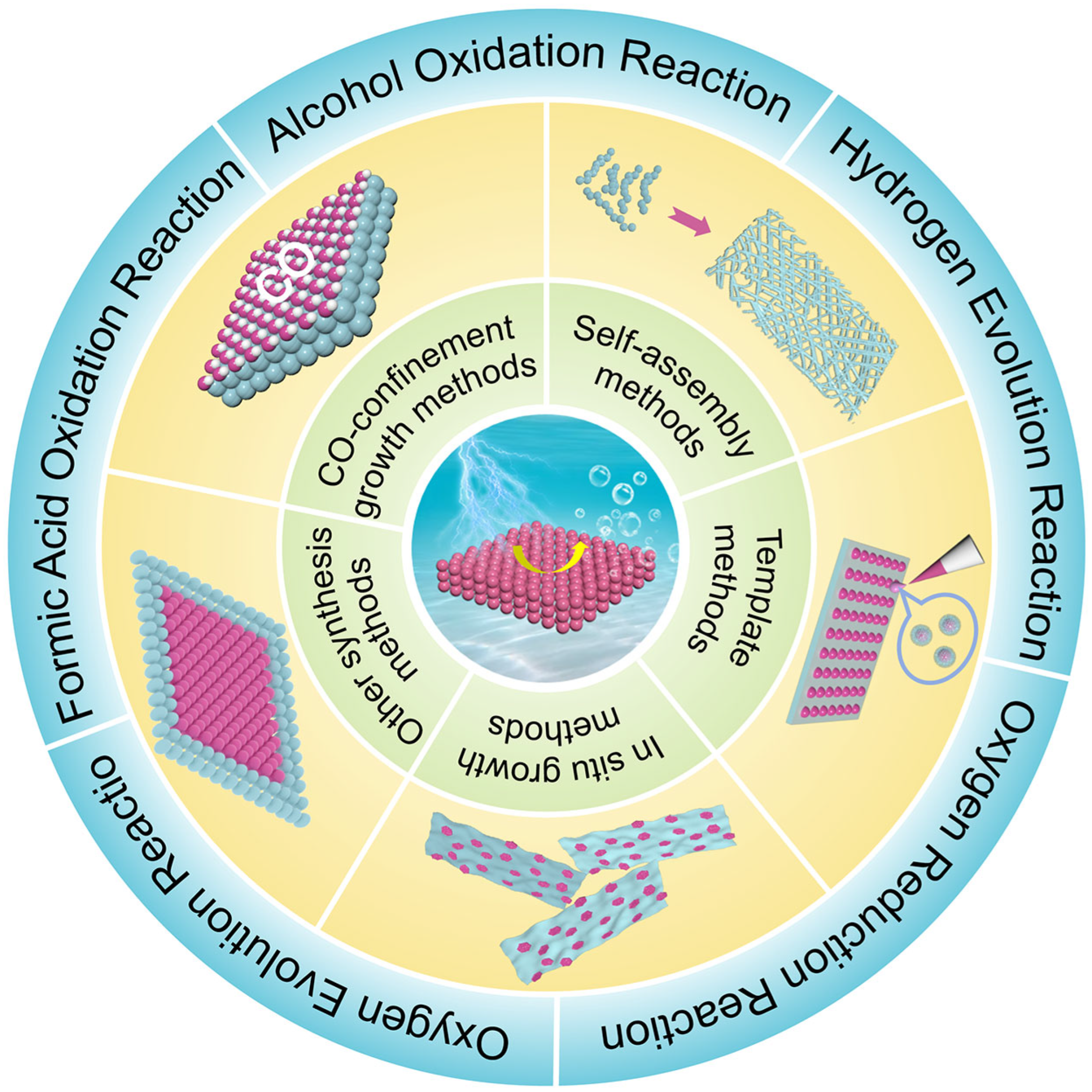
3. Applications of 2D Pt-Group Metallic Electrocatalysts for Energy Conversion
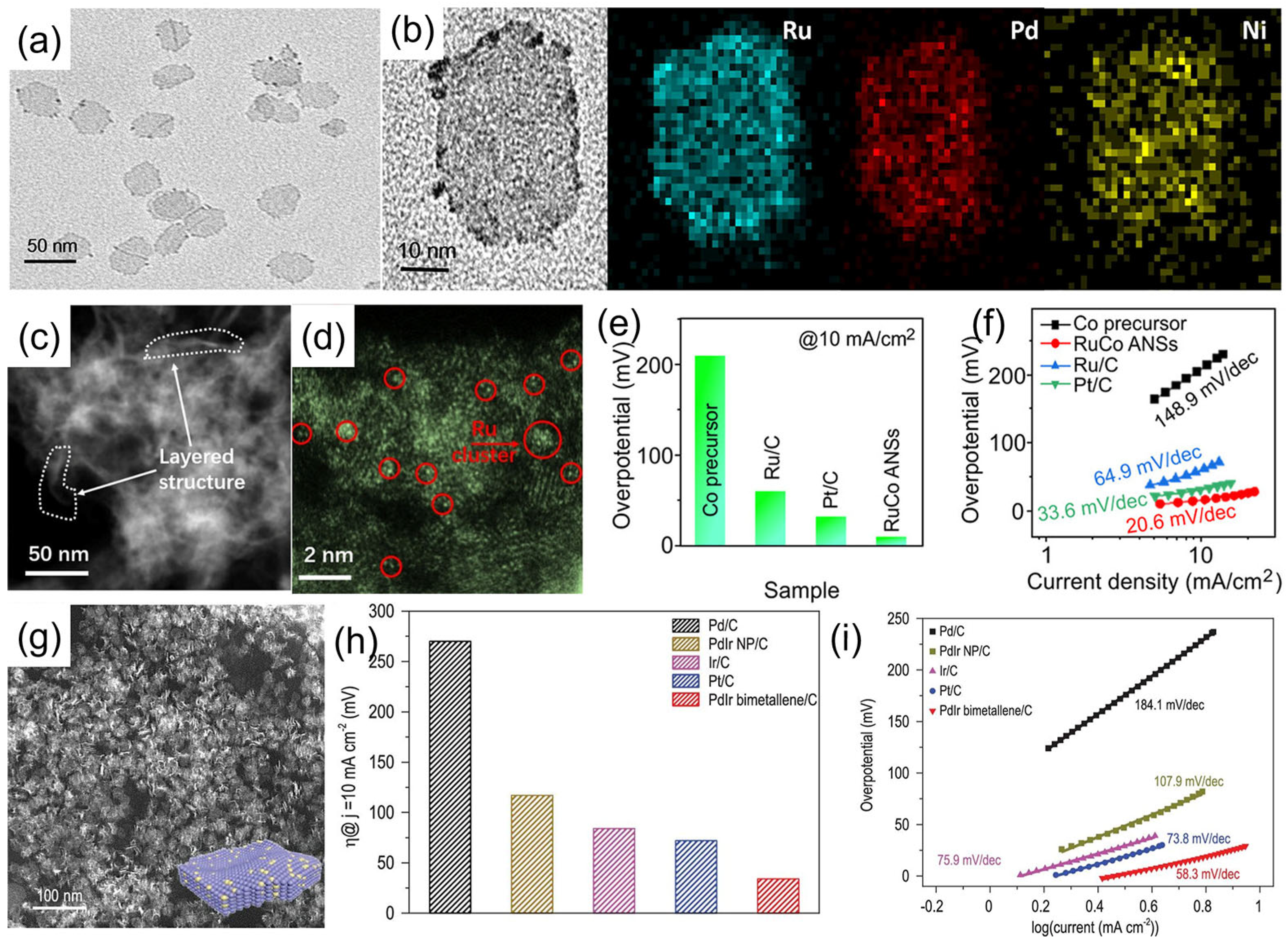
3.1. Hydrogen Evolution Reaction
3.2. Oxygen Evolution Reaction
3.3. Oxygen Reduction Reaction

3.4. Formic Acid Oxidation Reaction
3.5. Alcohol Oxidation Reactions
4. Conclusions, Challenges, and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 0D | zero-dimensional |
| 1D | one-dimensional |
| 2D | two-dimensional |
| 3D | three-dimensional |
| TMDs | transition metal disulfides |
| h-BN | hexagonal boron nitride |
| MOFs | metal-organic frameworks |
| COFs | covalent organic frameworks |
| HER | hydrogen evolution reaction |
| OER | oxygen evolution reaction |
| ORR | oxygen reduction reaction |
| FAOR | formic acid oxidation reaction |
| AOR | alcohol oxidation reaction |
| CO | carbon monoxide |
| LDHs | layered double hydroxides |
| RGO | reduced graphene oxide |
| DFT | density functional theory |
| PEMWE | polymer electrolyte membrane water electrolyzer |
| PEMFCs | proton exchange membrane fuel cells |
| ADT | accelerated durability testing |
| RRDE | rotating ring disk electrode |
| DEMS | differential electrochemical mass spectrometry |
| ECSA | electrochemical active surface area |
| CV | cyclic voltammetry |
| LSV | linear sweep voltammetry |
| MA | mass activity |
| DFAFCs | direct formic acid fuel cells |
| EN | ethylenediamine |
| DAFCs | direct alcohol fuel cells |
| EGOR | ethylene glycol oxidation reaction |
| MOR | methanol oxidation reaction |
References
- He, Q.; Sheng, B.; Zhu, K.; Zhou, Y.; Qiao, S.; Wang, Z.; Song, L. Phase Engineering and Synchrotron-Based Study on Two-Dimensional Energy Nanomaterials. Chem. Rev. 2023, 123, 10750–10807. [Google Scholar] [CrossRef]
- Johnson, N.; Liebreich, M.; Kammen, D.M.; Ekins, P.; McKenna, R.; Staffell, I. Realistic Roles for Hydrogen in the Future Energy Transition. Nat. Rev. Clean Technol. 2025, 1, 351–371. [Google Scholar] [CrossRef]
- Qiu, H.; Wen, S.; Fu, Q.; Zhao, X. Oxygen Reduction Reactions of Catalysts with Asymmetric Atomic Structures: Mechanisms, Applications, and Challenges. Catalysts 2025, 15, 615. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Shao, Z.; Jung, W. Advanced Electrocatalysts with Unusual Active Sites for Electrochemical Water Splitting. InfoMat 2023, 6, e12494. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Hamzat, A.K.; Whidborne, J.; Kuang, B.; Jenkins, K.W. Integration of Renewable Energy Sources in Tandem with Electrolysis: A Technology Review for Green Hydrogen Production. Int. J. Hydrogen Energy 2025, 107, 218–240. [Google Scholar] [CrossRef]
- He, Q.; Zhou, Y.; Shou, H.; Wang, X.; Zhang, P.; Xu, W.; Qiao, S.; Wu, C.; Liu, H.; Liu, D.; et al. Synergic Reaction Kinetics over Adjacent Ruthenium Sites for Superb Hydrogen Generation in Alkaline Media. Adv. Mat. 2022, 34, 2110604. [Google Scholar] [CrossRef]
- Li, S.; Shu, J.; Ma, S.; Yang, H.; Jin, J.; Zhang, X.; Jin, R. Engineering Three-Dimensional Nitrogen-Doped Carbon Black Embedding Nitrogen-doped Graphene Anchoring Ultrafine Surface-Clean Pd Nanoparticles as Efficient Ethanol Oxidation Electrocatalyst. Appl. Catal. B Environ. 2021, 280, 119464. [Google Scholar] [CrossRef]
- Yang, C.; He, H.; Jiang, Q.; Liu, X.; Shah, S.P.; Huang, H.; Li, W. Pd Nanocrystals Grown on MXene and Reduced Graphene Oxide CO-Constructed Three-Dimensional Nanoarchitectures for Efficient Formic Acid Oxidation Reaction. Int. J. Hydrogen Energy 2021, 46, 589–598. [Google Scholar] [CrossRef]
- Xu, C.; He, H.; Zhu, Y.; Wang, Y.; Hao, L.; Huang, H. Mutual-Coupling 0D/1D/2D Heterostructure of Cobalt Selenide/Carbon Nanotube/MXene for Enhanced Electrocatalytic Hydrogen Evolution. Int. J. Hydrogen Energy 2025, 100, 1038–1045. [Google Scholar] [CrossRef]
- Wang, J.; Kong, H.; Zhang, J.; Hao, Y.; Shao, Z.; Ciucci, F. Carbon-Based Electrocatalysts for Sustainable Energy Applications. Prog. Mater. Sci. 2021, 116, 100717. [Google Scholar] [CrossRef]
- Yao, J.; Huang, W.; Fang, W.; Kuang, M.; Jia, N.; Ren, H.; Liu, D.; Lv, C.; Liu, C.; Xu, J.; et al. Promoting Electrocatalytic Hydrogen Evolution Reaction and Oxygen Evolution Reaction by Fields: Effects of Electric Field, Magnetic Field, Strain, and Light. Small Methods 2020, 4, 200494. [Google Scholar] [CrossRef]
- Yue, L.; Jiang, Q.; Ma, L.; Li, Y.; Yang, L.; Zhang, J.; He, H.; Huang, H. Confined Palladium Nanocrystals within Covalent Organic Framework-Intercalated MXene Nanoarchitectures toward Highly Efficient Methanol Electrooxidation. Chem. Mater. 2025, 37, 2125–2135. [Google Scholar] [CrossRef]
- Wu, X.; Yan, Q.; Wang, H.; Wu, D.; Zhou, H.; Li, H.; Yang, S.; Ma, T.; Zhang, H. Heterostructured Catalytic Materials as Advanced Electrocatalysts: Classification, Synthesis, Characterization, and Application. Adv. Funct. Mater. 2024, 34, 2404535. [Google Scholar] [CrossRef]
- Li, S.; Ma, S.; Zhang, Y.; Zhao, L.; Yang, H.; Jin, R. Metal-Organic Interface Engineering for Coupling Palladium Nanocrystals over Functionalized Graphene as an Advanced Electrocatalyst of Methanol and Ethanol Oxidation. J. Colloid Interface Sci. 2021, 588, 384–392. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Chen, Z.; Zhang, Y.; He, H.; Huang, H. Palladium Nanocrystals Immobilized on Boron and Nitrogen Codoped Mesoporous Carbon Spheres as Efficient Methanol Oxidation Electrocatalysts. Colloids Surf. A 2025, 705, 135598. [Google Scholar] [CrossRef]
- Wang, H.; Yan, Z.; Cheng, F.; Chen, J. Advances in Noble Metal Electrocatalysts for Acidic Oxygen Evolution Reaction: Construction of Under-Coordinated Active Sites. Adv. Sci. 2024, 11, 2401652. [Google Scholar] [CrossRef]
- Gao, G.; Zhao, G.; Zhu, G.; Sun, B.; Sun, Z.; Li, S.; Lan, Y.-Q. Recent Advancements in Noble-Metal Electrocatalysts for Alkaline Hydrogen Evolution Reaction. Chin. Chem. Lett. 2025, 36, 109557. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, Q.; He, H.; Huang, H. Ultrasmall Rh-Decorated Porous Heterostructures Stereoassembled from MXene and Graphene for Efficient Methanol Electrooxidation. Int. J. Hydrogen Energy 2024, 92, 535–543. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Zhang, C.; Yang, L.; Jiang, Q.; Zhang, J.; He, H.; Huang, H. Mesoporous Hollow Carbon Sphere-Embedded MXene Architectures Decorated with Ultrafine Rh Nanocrystals toward Methanol Electrooxidation. Inorg. Chem. 2024, 63, 16888–16896. [Google Scholar] [CrossRef]
- Huang, C.; Wang, F.; Chen, X.; Li, J.; Shao, M.; Wei, Z. Innovative Strategies for Designing and Constructing Efficient Fuel Cell Electrocatalysts. Chem. Commun. 2025, 61, 2658–2683. [Google Scholar] [CrossRef]
- Shu, J.; Li, R.; Lian, Z.; Zhang, W.; Jin, R.; Yang, H.; Li, S. In-Situ Oxidation of Palladium–Iridium Nanoalloy Anchored on Nitrogen-Doped Graphene as an Efficient Catalyst for Methanol Electrooxidation. J. Colloid Interface Sci. 2022, 605, 44–53. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, Z.; He, H.; Ying, G.; Huang, H. Immobilizing Ultrafine PtRu Alloy Nanoparticles onto 3D Interconnected MXene-Graphene Frameworks for Highly Efficient Methanol Oxidation. Ceram. Int. 2024, 50, 16443–16451. [Google Scholar] [CrossRef]
- Huang, H.; Wei, Y.; Yang, Y.; Yan, M.; He, H.; Jiang, Q.; Yang, X.; Zhu, J. Controllable Synthesis of Grain Boundary-Enriched Pt Nanoworms Decorated on Graphitic Carbon Nanosheets for Ultrahigh Methanol Oxidation Catalytic Activity. J. Energy Chem. 2021, 57, 601–609. [Google Scholar] [CrossRef]
- Meng, W.; He, H.; Yang, L.; Jiang, Q.; Yuliarto, B.; Yamauchi, Y.; Xu, X.; Huang, H. 1D-2D Hybridization: Nanoarchitectonics for Grain Boundary-Rich Platinum Nanowires Coupled with MXene Nanosheets as Efficient Methanol Oxidation Electrocatalysts. Chem. Eng. J. 2022, 450, 137932. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, M.-M.; Du, W.-F.; Yin, C.-Y.; Zhang, J.; Yang, L.; Kang, Y.-Q.; He, H.-Y.; Huang, H.-J. Spatial Construction of Ultrasmall Pt-Decorated 3D Spinel Oxide-Modified N-Doped Graphene Nanoarchitectures as High-Efficiency Methanol Oxidation Electrocatalysts. Rare Met. 2023, 43, 186–197. [Google Scholar] [CrossRef]
- Hu, X.; Yang, B.; Ke, S.; Liu, Y.; Fang, M.; Huang, Z.; Min, X. Review and Perspectives of Carbon-Supported Platinum-Based Catalysts for Proton Exchange Membrane Fuel Cells. Energy Fuels 2023, 37, 11532–11566. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Chen, S.; Yan, H.; Wang, C.; Wu, C.; Haleem, Y.A.; Duan, S.; Lu, J.; Ge, B.; et al. Atomically Dispersed Platinum Supported on Curved Carbon Supports for Efficient Electrocatalytic Hydrogen Evolution. Nat. Energy 2019, 4, 512–518. [Google Scholar] [CrossRef]
- Wang, W.; Lv, F.; Lei, B.; Wan, S.; Luo, M.; Guo, S. Tuning Nanowires and Nanotubes for Efficient Fuel-Cell Electrocatalysis. Adv. Mater. 2016, 28, 10117–10141. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Q.; Meng, W.; Yang, C.; Jiang, Q.; Zhang, C.; He, H.; Ying, G. Grain Boundary-Enriched Wavy Pd Nanowires Intertwined with MXene Nanosheets toward Formic Acid and Methanol Electrooxidation. Inorg. Chem. 2025, 64, 7690–7697. [Google Scholar] [CrossRef]
- Shen, B.; Wei, Y.; Sun, P.; He, H.; Ying, G.; Huang, H. Immobilizing Ultrasmall Pt nanocrystals on 3D Interweaving BCN Nanosheet-Graphene Networks Enables Efficient Methanol Oxidation Reaction. Dalton Trans. 2023, 52, 13644–13652. [Google Scholar] [CrossRef]
- Zhu, Z.; Qin, J.; Yang, Q.; He, H.; Yang, L.; Huang, H.; Ying, G. Interconnected Pd Nanowire Networks Stereoassembled on Biomass-Derived Porous Carbon Skeletons as Bifunctional Electrocatalysts for Efficient Methanol and Formic Acid Oxidation. ACS Sustain. Chem. Eng. 2024, 12, 10615–10623. [Google Scholar] [CrossRef]
- Xiao, D.; Jiang, Q.; Xu, C.; Yang, C.; Yang, L.; He, H.; Huang, H. Interfacial Engineering of Worm-Shaped Palladium Nanocrystals Anchored on Polyelectrolyte-Modified MXene Nanosheets for Highly Efficient Methanol Oxidation. J. Colloid Interface Sci. 2022, 616, 781–790. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Zhang, Y.; Zhang, C.; Yang, L.; Jiang, Q.; He, H.; Ying, G.; Huang, H. Carbon Nanotube-Bridged MXene Nanoarchitectures Decorated with Ultrasmall Rh Nanoparticles for Efficient Methanol Oxidation. Mater. Today Energy 2024, 40, 101495. [Google Scholar] [CrossRef]
- Chen, T.; Kong, X.; Liu, Q. RuCu Nanorod Arrays Synergistically Promote Efficient Water-Splitting. Catalysts 2025, 15, 98. [Google Scholar] [CrossRef]
- Huang, H.; Guo, X.; Zhang, C.; Yang, L.; Jiang, Q.; He, H.; Amin, M.A.; Alshahrani, W.A.; Zhang, J.; Xu, X.; et al. Advancements in Noble Metal-Decorated Porous Carbon Nanoarchitectures: Key Catalysts for Direct Liquid Fuel Cells. ACS Nano 2024, 18, 10341–10373. [Google Scholar] [CrossRef]
- Chen, T.; Li, Y.; Li, L.; Zhao, Y.; Shi, S.; Jiang, R.; Ma, H. Cu Modified Pt Nanoflowers with Preferential (100) Surfaces for Selective Electroreduction of Nitrate. Catalysts 2019, 9, 536. [Google Scholar] [CrossRef]
- Yang, S.; Chen, Y.; Jiang, C. Strain Engineering of Two-Dimensional Materials: Methods, Properties, and Applications. InfoMat 2021, 3, 397–420. [Google Scholar] [CrossRef]
- Zhao, Y.; Niu, S.; Xi, B.; Du, Z.; Yu, T.; Wan, T.; Lei, C.; Lyu, S. Recent Developments in Two-Dimensional Carbon-Based Nanomaterials for Electrochemical Water Oxidation: A Mini Review. Catalysts 2024, 14, 221. [Google Scholar] [CrossRef]
- Wang, J.; Du, C.F.; Xue, Y.; Tan, X.; Kang, J.; Gao, Y.; Yu, H.; Yan, Q. MXenes as a Versatile Platform for Reactive Surface Modification and Superior Sodium-Ion Storages. Exploration 2021, 1, 20210024. [Google Scholar] [CrossRef]
- Liu, L.; Lian, H.; Deng, H.; Zhang, W. Mxene-Supported Ni–Co Bimetallic MOF 2D Lamellar Membrane for Enhanced Electrochemical Oxygen Reactions and Li–O2 Battery. Sci. Rep. 2025, 15, 13995. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Feng, J.; Qian, Y. Mxenes and their Derivatives for Advanced Aqueous Rechargeable Batteries. Mater. Today 2022, 52, 225–249. [Google Scholar] [CrossRef]
- Zhao, J.; Li, T.; Yue, Y.; Li, X.; Xie, Z.; Zhang, H.; Tian, X. Advancements in Employing Two-Dimensional Nanomaterials for Enhancing Skin Wound Healing: A Review of Current Practice. J. Nanobiotechnol. 2024, 22, 520. [Google Scholar] [CrossRef]
- Yang, C.; Huang, H.; He, H.; Yang, L.; Jiang, Q.; Li, W. Recent Advances in Mxene-Based Nanoarchitectures as Electrode Materials for Future Energy Generation and Conversion Applications. Coord. Chem. Rev. 2021, 435, 213806. [Google Scholar] [CrossRef]
- Xu, H.; Shang, H.; Wang, C.; Du, Y. Recent Progress of Ultrathin 2D Pd-Based Nanomaterials for Fuel Cell Electrocatalysis. Small 2021, 17, 2005092. [Google Scholar] [CrossRef]
- Tian, H.; Yu, Y.; Wang, Q.; Li, J.; Rao, P.; Li, R.; Du, Y.; Jia, C.; Luo, J.; Deng, P.; et al. Recent Advances in Two-Dimensional Pt Based Electrocatalysts for Methanol Oxidation Reaction. Int. J. Hydrogen Energy 2021, 46, 31202–31215. [Google Scholar] [CrossRef]
- Tian, J.; Song, Y.; Hao, X.; Wang, X.; Shen, Y.; Liu, P.; Wei, Z.; Liao, T.; Jiang, L.; Guo, J.; et al. Greatly Enhanced Oxygen Reduction Reaction in Anion Exchange Membrane Fuel Cell and Zn-Air Battery via Hole Inner Edge Reconstruction of 2D Pd Nanomesh. Adv. Mater. 2024, 37, 2412051. [Google Scholar] [CrossRef]
- Gu, X.; Wang, D.; Zhang, N.; Zhang, Y.; Ye, C.; Du, Y. Solvothermal Synthesis of PdCu Nanorings with High Catalytic Performance for Alcohol Electrooxidation. J. Colloid Interface Sci. 2025, 677, 750–757. [Google Scholar] [CrossRef]
- Gu, B.S.; Dutta, S.; Hong, Y.R.; Ngome Okello, O.F.; Im, H.; Ahn, S.; Choi, S.Y.; Woo Han, J.; Ryu, S.; Lee, I.S. Harmonious Heterointerfaces Formed on 2D-Pt Nanodendrites by Facet-Respective Stepwise Metal Deposition for Enhanced Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2023, 62, e202307816. [Google Scholar] [CrossRef]
- Zhu, E.; Yan, X.; Wang, S.; Xu, M.; Wang, C.; Liu, H.; Huang, J.; Xue, W.; Cai, J.; Heinz, H.; et al. Peptide-Assisted 2-D Assembly toward Free-Floating Ultrathin Platinum Nanoplates as Effective Electrocatalysts. Nano Lett. 2019, 19, 3730–3736. [Google Scholar] [CrossRef]
- Tian, J.; Rao, Y.; Shi, W.; Yang, J.; Ning, W.; Li, H.; Yao, Y.; Zhou, H.; Guo, S. Sabatier Relations in Electrocatalysts Based on High-entropy Alloys with Wide-distributed d-band Centers for Li-O2 Batteries. Angew. Chem. Int. Ed. 2023, 62, e202310894. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Z.; Xu, X. Dynamic Evolution of the Active Center Driven by Hemilabile Coordination in Cu/CeO2 Single-Atom Catalyst. Nat. Commun. 2023, 14, 2512. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.-X. Sabatier Principle of Metal-Support Interaction for Design of Ultrastable Metal Nanocatalysts. Science 2021, 374, 1360–1365. [Google Scholar] [CrossRef]
- Chen, Z.W.; Li, J.; Ou, P.; Huang, J.E.; Wen, Z.; Chen, L.; Yao, X.; Cai, G.; Yang, C.C.; Singh, C.V.; et al. Unusual Sabatier Principle on High Entropy Alloy Catalysts for Hydrogen Evolution Reactions. Nat. Commun. 2024, 15, 359. [Google Scholar] [CrossRef]
- Yang, C.; Wang, T.; Li, C.; He, H.; Liu, D.; Huang, H. PdMo Bimetallene Coupled with MXene Nanosheets as Efficient Bifunctional Electrocatalysts for Formic Acid and Methanol Oxidation Reactions. ACS Appl. Mater. Interfaces 2023, 15, 49195–49203. [Google Scholar] [CrossRef]
- Wei, J.; Song, Y.; Gan, M.; An, G.; Shen, Y.; Zhao, X.; Zhang, Y.; Liu, P.; Xu, B.; Guo, J. A Heterostructure Coupling of Ru Nanosheets and NiCo LDH for Hydrazine-Assisted Overall Seawater Splitting. Sustain. Mater. Technol. 2025, 44, e01413. [Google Scholar] [CrossRef]
- Cui, Z.; Bai, X. Ultrasonic-Assisted Synthesis of Two Dimensional Coral-Like Pd Nanosheets Supported on Reduced Graphene Oxide for Enhanced Electrocatalytic Performance. Ultrason. Sonochem. 2021, 70, 105309. [Google Scholar] [CrossRef]
- He, H.; Lan, Y.; Qin, J.; Jiang, Q.; Yang, L.; Zhang, J.; Huang, H. Three-Dimensional Porous Rhodium–Copper Alloy Nanoflowers Stereoassembled on Ti3C2Tx MXene as Highly-Efficient Methanol Oxidation Electrocatalysts. Inorg. Chem. Front. 2024, 11, 8564–8574. [Google Scholar] [CrossRef]
- Zhao, F.; Nie, S.; Wu, L.; Yuan, Q.; Wang, X. Porous, Ultrathin PtAgBiTe Nanosheets for Direct Hydrazine Hydrate Fuel Cell Devices. Adv. Mater. 2023, 35, 2303672. [Google Scholar] [CrossRef]
- Li, L.; Bu, L.; Huang, B.; Wang, P.; Shen, C.; Bai, S.; Chan, T.S.; Shao, Q.; Hu, Z.; Huang, X. Compensating Electronic Effect Enables Fast Site-to-Site Electron Transfer over Ultrathin RuMn Nanosheet Branches toward Highly Electroactive and Stable Water Splitting. Adv. Mater. 2021, 33, 2105308. [Google Scholar] [CrossRef]
- Qin, J.; Huang, H.; Xie, Y.; Pan, S.; Chen, Y.; Yang, L.; Jiang, Q.; He, H. MXene Supported Rhodium Nanocrystals for Efficient Electrocatalysts towards Methanol Oxidation. Ceram. Int. 2022, 48, 15327–15333. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, Q.; Liu, H.; Yang, L.; He, H.; Huang, H.; Li, W. Pt-on-Pd Bimetallic Nanodendrites Stereoassembled on MXene Nanosheets for Use as High-Efficiency Electrocatalysts toward the Methanol Oxidation Reaction. J. Mater. Chem. A 2021, 9, 15432–15440. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, Q.; Huang, H.; He, H.; Yang, L.; Li, W. Polyelectrolyte-Induced Stereoassembly of Grain Boundary-Enriched Platinum Nanoworms on Ti3C2Tx MXene Nanosheets for Efficient Methanol Oxidation. ACS Appl. Mater. Interfaces 2020, 12, 23822–23830. [Google Scholar] [CrossRef]
- Huang, H.; Wei, Y.; Shen, B.; Zhang, Y.; He, H.; Jiang, Q.; Yang, L.; Nanjundan, A.K.; Na, J.; Xu, X.; et al. Synthesis of Multiple-Twinned Pd Nanoparticles Anchored on Graphitic Carbon Nanosheets for Use as Highly-Active Multifunctional Electrocatalyst in Formic Acid and Methanol Oxidation Reactions. Adv. Mater. Interfaces 2020, 7, 2000142. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Q.; Ren, Q.; Yang, Q.; Zhao, D.; Feng, Y.; Chen, H.; Wang, Y. Facile Synthesis of Pd and PdPtNi Trimetallic Nanosheets as Enhanced Oxygen Reduction Electrocatalysts. Small 2021, 18, 2103665. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Dong, Z.; Zhang, N.; Zhang, Q.; Xie, C.; Wu, Z.; Xu, G.-R.; Wang, L. One-Step CO Assisted Synthesis of Hierarchical Porous PdRuCu Nanosheets as Advanced Bifunctional Catalysts for Hydrogen Evolution and Glycerol Oxidation. Int. J. Hydrogen Energy 2022, 47, 33319–33328. [Google Scholar] [CrossRef]
- Luo, M.; Zhao, Z.; Zhang, Y.; Sun, Y.; Xing, Y.; Lv, F.; Yang, Y.; Zhang, X.; Hwang, S.; Qin, Y.; et al. PdMo Bimetallene for Oxygen Reduction Catalysis. Nature 2019, 574, 81–85. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Ouyang, Y.; Wang, S.; Wu, D.; Jiang, M.; Wang, F.; Yuan, W.; Li, C.M. Perforated Pd Nanosheets with Crystalline/Amorphous Heterostructures as a Highly Active Robust Catalyst toward Formic Acid Oxidation. Small 2019, 15, 1904245. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, L.; Yang, P.; Hu, C.; Luo, Z.; Chang, X.; Zhao, Z.J.; Gong, J. Low-Coordinated Edge Sites on Ultrathin Palladium Nanosheets Boost Carbon Dioxide Electroreduction Performance. Angew. Chem. Int. Ed. 2018, 57, 11544–11548. [Google Scholar] [CrossRef]
- Cheng, Z.; Huang, B.; Pi, Y.; Li, L.; Shao, Q.; Huang, X. Partially Hydroxylated Ultrathin Iridium Nanosheets as Efficient Electrocatalysts for Water Splitting. Natl. Sci. Rev. 2020, 7, 1340–1348. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Mao, Q.; Yu, H.; Deng, K.; Xu, Y.; Li, X.; Wang, Z.; Wang, L. Tensile Strained PdNi Bimetallene for Energy-Efficient Hydrogen Production Integrated with Formate Oxidation. Chem. Eng. J. 2022, 450, 137995. [Google Scholar] [CrossRef]
- Huang, X.; Tang, S.; Mu, X.; Dai, Y.; Chen, G.; Zhou, Z.; Ruan, F.; Yang, Z.; Zheng, N. Freestanding Palladium Nanosheets with Plasmonic and Catalytic Properties. Nat. Nanotechnol. 2010, 6, 28–32. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, X.; Yang, W.; Jia, C.; Chen, X.; Ren, W.; Smith, S.C.; Zhao, C. Surface Reconstruction of Ultrathin Palladium Nanosheets During Electrocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2020, 59, 21493–21498. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, C.; Su, H.; Liang, J.; Lin, S.; Gu, L.; Wang, X.; Chen, M.; Zheng, N. Single-Crystalline Rhodium Nanosheets with Atomic Thickness. Adv. Sci. 2015, 2, 1500100. [Google Scholar] [CrossRef]
- Yang, X.; Ouyang, B.; Zhao, L.; Shen, Q.; Chen, G.; Sun, Y.; Li, C.; Xu, K. Ultrathin Rh Nanosheets with Rich Grain Boundaries for Efficient Hydrogen Oxidation Electrocatalysis. J. Am. Chem. Soc. 2023, 145, 27010–27021. [Google Scholar] [CrossRef]
- Ando, S.; Yamamoto, E.; Kobayashi, M.; Kumatani, A.; Osada, M. Facile Synthesis of Pd Nanosheets and Implications for Superior Catalytic Activity. ACS Nano 2023, 18, 461–469. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Zhang, Y.; Chen, X.; Ye, J.; Chen, J.; Lyu, Z.; Chen, X.; Kuang, Q.; Xie, S.; et al. Edge Enrichment of Ultrathin 2D PdPtCu Trimetallic Nanostructures Effectuates Top-Ranked Ethanol Electrooxidation. Nano Lett. 2020, 20, 5458–5464. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Ji, Y.; Liu, X.; Su, D.; Zhuang, Z.; Chang, Y.-C.; Pao, C.-W.; Shao, Q.; Hu, Z.; et al. Atomic-Thick Metastable Phase RhMo Nanosheets for Hydrogen Oxidation Catalysis. Nat. Commun. 2023, 14, 1761. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, P.; Zhang, H.; Deng, K.; Yu, H.; Xu, Y.; Li, X.; Wang, H.; Wang, L. PdCu Bimetallene for Enhanced Oxygen Reduction Electrocatalysis. Inorg. Chem. 2023, 62, 5622–5629. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Li, J.; Zhang, N.; Wu, Z.; Du, Y. Rapid Synthesis of PdCu Nanosheets with Enhanced Electrocatalytic Activity toward Polyalcohol Oxidation Reaction. Int. J. Hydrogen Energy 2024, 74, 193–200. [Google Scholar] [CrossRef]
- Chu, X.; Wang, X.; Wei, R.; Yan, M.; Wei, X.; Zhang, X.; Zhu, Z.; Wang, Y.; Wang, L.; Yin, S. Defect-Rich PdCo Bimetallene Constructed by a Self-Reduction Strategy for Enhanced Ethanol Oxidation Reaction. Mater. Today Phys. 2024, 43, 101416. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, K.; Wang, X.; Wang, C.; Li, Z.; Gao, F.; Du, Y. Rapid Synthesis of Palladium-Platinum-Nickel Ultrathin Porous Nanosheets with High Catalytic Performance for Alcohol Electrooxidation. J. Colloid Interface Sci. 2023, 650, 350–357. [Google Scholar] [CrossRef]
- Ao, Y.; Ling, X.; Zheng, J.; Han, M.; Xu, D. Effective PdPtMoCrCoNi Alloy Nanosheets Boost Electrocatalytic Activity and Stability for Ethylene Glycol Oxidation. Surf. Interfaces. 2024, 45, 103928. [Google Scholar] [CrossRef]
- Li, H.; Zeng, R.; Feng, X.; Wang, H.; Xu, W.; Lu, X.; Xie, Z.; Abruña, H.D. Oxidative Stability Matters: A Case Study of Palladium Hydride Nanosheets for Alkaline Fuel Cells. J. Am. Chem. Soc. 2022, 144, 8106–8114. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, J.; Wu, X.-R.; Chen, P.; Jin, P.-J.; Yao, H.-C.; Chen, Y. Atomically Ultrathin RhCo Alloy Nanosheet Aggregates for Efficient Water Electrolysis in Broad Ph Range. J. Mater. Chem. A 2019, 7, 16437–16446. [Google Scholar] [CrossRef]
- Gao, C.; Sun, H.; Du, J. Unusual Endotaxy Growth of Hexagonal Nanosheets by the Self-Assembly of a Homopolymer. Angew. Chem. Int. Ed. 2025, 64, 2420079. [Google Scholar] [CrossRef]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-Assembly as a Key Player for Materials Nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef]
- Cai, R.; Yang, D.; Lin, K.-T.; Lyu, Y.; Zhu, B.; He, Z.; Zhang, L.; Kitamura, Y.; Qiu, L.; Chen, X.; et al. Generalized Preparation of Two-Dimensional Quasi-nanosheets via Self-assembly of Nanoparticles. J. Am. Chem. Soc. 2019, 141, 1725–1734. [Google Scholar] [CrossRef]
- Saleem, F.; Xu, B.; Ni, B.; Liu, H.; Nosheen, F.; Li, H.; Wang, X. Atomically Thick Pt-Cu Nanosheets: Self-Assembled Sandwich and Nanoring-Like Structures. Adv. Mater. 2015, 27, 2013–2018. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Li, M.; Hu, J.; Liu, Y.; Sun, D.; Fu, G.; Tang, Y. Iminodiacetonitrile Induce-Synthesis of Two-Dimensional PdNi/Ni@Carbon Nanosheets with Uniform Dispersion and Strong Interface Bonding as an Effective Bifunctional Eletrocatalyst in Air-Cathode. Energy Storage Mater. 2021, 42, 118–128. [Google Scholar] [CrossRef]
- Saravani, H.; Farsadrooh, M.; Mollashahi, M.S.; Hajnajafi, M.; Douk, A.S. Two-Dimensional Engineering of Pd Nanosheets as Advanced Electrocatalysts toward Formic Acid Oxidation. Int. J. Hydrogen Energy 2020, 45, 21232–21240. [Google Scholar] [CrossRef]
- Zu, L.; Qian, X.; Zhao, S.; Liang, Q.; Chen, Y.E.; Liu, M.; Su, B.-J.; Wu, K.-H.; Qu, L.; Duan, L.; et al. Self-Assembly of Ir-Based Nanosheets with Ordered Interlayer Space for Enhanced Electrocatalytic Water Oxidation. J. Am. Chem. Soc. 2022, 144, 2208–2217. [Google Scholar] [CrossRef]
- Fu, L.; Liu, K.; Zhen, C.; Zhu, Y.; Lyu, Z.; Du, G.; Xie, S. Lamellar-Assembled PdNi Super-Nanosheets as Effective Oxygen Redox Dual-Electrocatalysts for Rechargeable Zn-Air Batteries. Nano Res. 2022, 16, 2163–2169. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, H.; Wu, P.; Zhang, F.; Wei, S.; Sun, D.; Xu, L.; Tang, Y. One-Pot Synthesis of Freestanding Porous Palladium Nanosheets as Highly Efficient Electrocatalysts for Formic Acid Oxidation. Adv. Funct. Mater. 2016, 27, 1603852. [Google Scholar] [CrossRef]
- Jiang, N.; Niu, X.; Liu, D.; Zhang, K.; Guo, Z.; Qin, Y.; Zhao, W.; Zhang, X.; Wang, Q. Constructing Pt/Hierarchical HY Bifunctional Catalysts for Selective Hydroisomerization of Phenanthrene to Alkyl-Adamantanes. Catalysts 2025, 15, 413. [Google Scholar] [CrossRef]
- Shin, H.J.; Ryoo, R.; Liu, Z.; Terasaki, O. Template Synthesis of Asymmetrically Mesostructured Platinum Networks. J. Am. Chem. Soc. 2001, 123, 1246–1247. [Google Scholar] [CrossRef]
- Burpo, F.J.; Nagelli, E.A.; Losch, A.R.; Bui, J.K.; Forcherio, G.T.; Baker, D.R.; McClure, J.P.; Bartolucci, S.F.; Chu, D.D. Salt-Templated Platinum-Copper Porous Macrobeams for Ethanol Oxidation. Catalysts 2019, 9, 662. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Wang, H.; Liu, B.; Zhao, N.; Zhong, C.; Hu, W. Designing Nanoporous Coral-Like Pt Nanowires Architecture for Methanol and Ammonia Oxidation Reactions. Adv. Funct. Mater. 2021, 32, 2110702. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Zhou, M.; Huang, Y.-F.; Ma, Y.-Y.; Yan, L.-Y.; Zhou, X.-W.; Ma, X.-Z.; Zhao, X.-L.; Chen, C.; Bai, J.; et al. Enhanced Ethanol Oxidation over Pd Nanoparticles Supported Porous Graphene-Doped MXene Using Polystyrene Particles as Sacrificial Templates. Rare Met. 2022, 41, 3170–3179. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, J.; Xiong, D.; Chen, X.; Liu, M.; Zhong, Z.; Malgras, V.; Bando, Y.; Yamauchi, Y.; et al. A Centimeter Scale Self-Standing Two-Dimensional Ultra-Thin Mesoporous Platinum Nanosheet. Mater. Horiz. 2020, 7, 489–494. [Google Scholar] [CrossRef]
- Xu, D.; Liu, X.; Lv, H.; Liu, Y.; Zhao, S.; Han, M.; Bao, J.; He, J.; Liu, B. Ultrathin Palladium Nanosheets with Selectively Controlled Surface Facets. Chem. Sci. 2018, 9, 4451–4455. [Google Scholar] [CrossRef]
- Yamamoto, E.; Suzuki, A.; Kobayashi, M.; Osada, M. Tailored Synthesis of Molecularly Thin Platinum Nanosheets Using Designed 2D Surfactant Solids. Nanoscale 2022, 14, 11561–11567. [Google Scholar] [CrossRef]
- Teng, Z.; Li, M.; Li, Z.; Liu, Z.; Fu, G.; Tang, Y. Facile Synthesis of Channel-Rich Ultrathin Palladium-Silver Nanosheets for Highly Efficient Formic Acid Electrooxidation. Mater. Today Energy 2021, 19, 100596. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Yang, W.; Yang, J.; Wang, S.; Zhang, H.; Cui, P.; Yin, S.; Wang, Y.; Qi, J. Binary Nonmetal Boron and Phosphorus CO-Doping into PdRh Nanosheets Boosts Electro-Upgrading Polyethylene Terephthalate. Chem. Eng. J. 2024, 490, 151696. [Google Scholar] [CrossRef]
- Jang, S.W.; Dutta, S.; Kumar, A.; Hong, Y.-R.; Kang, H.; Lee, S.; Ryu, S.; Choi, W.; Lee, I.S. Holey Pt Nanosheets on NiFe-Hydroxide Laminates: Synergistically Enhanced Electrocatalytic 2D Interface toward Hydrogen Evolution Reaction. ACS Nano 2020, 14, 10578–10588. [Google Scholar] [CrossRef]
- Gu, K.; Pan, X.; Wang, W.; Ma, J.; Sun, Y.; Yang, H.; Shen, H.; Huang, Z.; Liu, H. In Situ Growth of Pd Nanosheets on g-C3N4 Nanosheets with Well-Contacted Interface and Enhanced Catalytic Performance for 4-Nitrophenol Reduction. Small 2018, 14, 1801812. [Google Scholar] [CrossRef]
- Chen, M.-T.; Zhang, R.-L.; Feng, J.-J.; Mei, L.-P.; Jiao, Y.; Zhang, L.; Wang, A.-J. A Facile One-Pot Room-Temperature Growth of Self-Supported Ultrathin Rhodium-Iridium Nanosheets as High-Efficiency Electrocatalysts for Hydrogen Evolution Reaction. J. Colloid Interface Sci. 2022, 606, 1707–1714. [Google Scholar] [CrossRef]
- Bao, X.; Gong, Y.; Zheng, X.; Chen, J.; Mao, S.; Wang, Y. Highly Performed Platinum Nanosheets Synthesized under in Situ Reaction Conditions for Hydrogen Generation. J. Energy Chem. 2020, 51, 272–279. [Google Scholar] [CrossRef]
- Yang, S.; Qiu, P.; Yang, G. Graphene Induced Formation of Single Crystal Pt Nanosheets through 2-Dimensional Aggregation and Sintering of Nanoparticles on Molten Salt Medium. Carbon 2014, 77, 1123–1131. [Google Scholar] [CrossRef]
- Yang, C.; Wang, T.; Wang, T.; He, H.; Liu, D.; Huang, H. Ultrathin PdMo Bimetallene Immobilized on Graphene Nanosheets as an Efficient Bifunctional Electrocatalyst towards Formic Acid and Methanol Oxidation Reactions. Int. J. Hydrogen Energy 2024, 80, 1174–1181. [Google Scholar] [CrossRef]
- He, C.; Tao, J.; Shen, P.K. Solid Synthesis of Ultrathin Palladium and Its Alloys’ Nanosheets on RGO with High Catalytic Activity for Oxygen Reduction Reaction. ACS Catal. 2018, 8, 910–919. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Q.; Yang, X.; Zhang, C.; Zhang, J.; Yang, L.; He, H.; Ying, G.; Huang, H. Heterointerface Engineering of Rhombic Rh Nanosheets Confined on MXene for Efficient Methanol Oxidation. J. Energy Chem. 2024, 93, 419–428. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, D.; Zhu, Z.; Zhang, C.; Yang, L.; He, H.; You, J.; Jiang, Q.; Xu, X.; Yamauchi, Y. A 2D/2D Heterojunction of Ultrathin Pd Nanosheet/MXene towards Highly Efficient Methanol Oxidation Reaction: The Significance of 2D Material Nanoarchitectonics. Chem. Sci. 2023, 14, 9854–9862. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Y.; Yang, C.; Yang, L.; Zhang, C.; Jiang, Q.; He, H.; Ying, G.; Huang, H. Confining Rhodium Nanocrystals into Polyaniline Nanorod-Embedded MXene Nanoarchitectures Enables Efficient Methanol Electrooxidation. J. Colloid Interface Sci. 2025, 695, 137809. [Google Scholar] [CrossRef]
- Lan, Y.; He, H.; Liu, C.; Qin, J.; Luo, L.; Zhu, F.; Zhao, Y.; Zhang, J.; Yang, L.; Huang, H. Ultrasmall Pd Nanocrystals Confined into Co-Based Metal Organic Framework-Decorated MXene Nanoarchitectures for Efficient Methanol Electrooxidation. J. Power Sources 2024, 603, 234438. [Google Scholar] [CrossRef]
- Qin, J.; Huang, H.; Zhang, J.; Zhu, F.; Luo, L.; Zhang, C.; Yang, L.; Jiang, Q.; He, H. Stereoassembly of Ultrasmall Rh-Decorated Zeolite Imidazolate Framework–MXene Heterostructures for Boosted Methanol Oxidation Reaction. J. Mater. Chem. A 2023, 11, 2848–2856. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zeng, T.; Zheng, L.; Wang, Y.; Yuan, W.; Niu, M.; Guo, C.X.; Cao, D.; Li, C.M. Epitaxial Growth of Pt–Pd Bimetallic Heterostructures for the Oxygen Reduction Reaction. Adv. Powder Mater. 2023, 2, 100131. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Zhang, Y.; Hata, S.; Zhang, K.; Ye, C.; Shiraishi, Y.; Du, Y. Advanced Heterostructure of Pd Nanosheets@Pt Nanoparticles Boosts Methanol Electrooxidation. J. Energy Chem. 2023, 85, 430–438. [Google Scholar] [CrossRef]
- Ding, J.; Wang, F.; Pan, F.; Yu, P.; Gao, N.; Goldsmith, R.H.; Cai, S.; Yang, R.; He, J. Two-Dimensional Palladium Nanosheet Intercalated with Gold Nanoparticles for Plasmon-Enhanced Electrocatalysis. ACS Catal. 2021, 11, 13721–13732. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, S.; Shen, B.; He, H.; Huang, H. Confining Tungsten Disulfide Quantum Dots on MXene Nanosheets Enables Efficient Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2025, 8, 2747–2754. [Google Scholar] [CrossRef]
- Ma, C.; He, H.; Qin, J.; Luo, L.; Lan, Y.; Zhang, J.; Yang, L.; Jiang, Q.; Huang, H. The Marriage of Hydrazone-Linked Covalent Organic Frameworks and MXene Enables Efficient Electrocatalytic Hydrogen Evolution. Small Struct. 2023, 5, 2300279. [Google Scholar] [CrossRef]
- Ma, C.; He, H.; Qin, J.; Hao, L.; Jia, L.; Yang, L.; Huang, H. Combining MXene Nanosheets with Iron-Based Metal-Organic Frameworks for Enhanced Electrocatalytic Hydrogen Evolution Reaction. Mater. Today Chem. 2023, 30, 101531. [Google Scholar] [CrossRef]
- He, H.; Chen, Y.; Yang, C.; Yang, L.; Jiang, Q.; Huang, H. Constructing 3D Interweaved MXene/Graphitic Carbon Nitride Nanosheets/Graphene Nanoarchitectures for Promoted Electrocatalytic Hydrogen Evolution. J. Energy Chem. 2022, 67, 483–491. [Google Scholar] [CrossRef]
- Luo, L.; Meng, W.; Wang, G.; Qin, J.; He, H.; Huang, H. MnO2 Nanoflowers-Decorated MXene Nanosheets with Enhanced Supercapacitor Performance. J. Alloys Compd. 2023, 957, 170411. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, X.; Wei, W.; Wang, S.; Ni, B.-J. Iridium-Based Nanomaterials for Electrochemical Water Splitting. Nano Energy 2020, 78, 105270. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, Y.; Ma, T.; Yang, C.; Peng, Y.; Zhou, Z.; Zhou, M.; Li, S.; Wang, Y.; Cheng, C. Designing MOF Nanoarchitectures for Electrochemical Water Splitting. Adv. Mater. 2021, 33, 2006042. [Google Scholar] [CrossRef]
- Hao, L.; He, H.; Xu, C.; Zhang, M.; Feng, H.; Yang, L.; Jiang, Q.; Huang, H. Ultrafine Cobalt Selenide Nanowires Tangled with MXene Nanosheets as Highly Efficient Electrocatalysts toward the Hydrogen Evolution Reaction. Dalton Trans. 2022, 51, 7135–7141. [Google Scholar] [CrossRef]
- Zhou, F.; Zhou, Y.; Liu, G.-G.; Wang, C.-T.; Wang, J. Recent Advances in Nanostructured Electrocatalysts for Hydrogen Evolution Reaction. Rare Met. 2021, 40, 3375–3405. [Google Scholar] [CrossRef]
- Araújo, H.F.; Gómez, J.A.; Santos, D.M.F. Proton-Exchange Membrane Electrolysis for Green Hydrogen Production: Fundamentals, Cost Breakdown, and Strategies to Minimize Platinum-Group Metal Content in Hydrogen Evolution Reaction Electrocatalysts. Catalysts 2024, 14, 845. [Google Scholar] [CrossRef]
- Hua, W.; Sun, H.-H.; Xu, F.; Wang, J.-G. A Review and Perspective on Molybdenum-Based Electrocatalysts for Hydrogen Evolution Reaction. Rare Met. 2020, 39, 335–351. [Google Scholar] [CrossRef]
- Li, T.; Dong, Y.; Zhang, J.; Wang, L.; Duan, F.; Wang, D.; Zeng, H. Carbon Dots-Based Composites Electrocatalysts in Hydrogen Evolution Reaction and Oxygen Evolution Reaction: A Mini Review. Int. J. Hydrogen Energy 2024, 77, 359–372. [Google Scholar] [CrossRef]
- Fan, J.; Wu, J.; Cui, X.; Gu, L.; Zhang, Q.; Meng, F.; Lei, B.-H.; Singh, D.J.; Zheng, W. Hydrogen Stabilized RhPdH 2D Bimetallene Nanosheets for Efficient Alkaline Hydrogen Evolution. J. Am. Chem. Soc. 2020, 142, 3645–3651. [Google Scholar] [CrossRef]
- Lin, B.-L.; Chen, X.; Niu, B.-T.; Lin, Y.-T.; Chen, Y.-X.; Lin, X.-M. The Research Progress of Ruthenium-Based Catalysts for the Alkaline Hydrogen Evolution Reaction in Water Electrolysis. Catalysts 2024, 14, 671. [Google Scholar] [CrossRef]
- Shen, B.; Huang, H.; Jiang, Y.; Xue, Y.; He, H. 3D Interweaving MXene–Graphene Network–Confined Ni–Fe Layered Double Hydroxide Nanosheets for Enhanced Hydrogen Evolution. Electrochim. Acta 2022, 407, 139913. [Google Scholar] [CrossRef]
- Huang, H.; Xue, Y.; Xie, Y.; Yang, Y.; Yang, L.; He, H.; Jiang, Q.; Ying, G. MoS2 Quantum Dot-Decorated MXene Nanosheets as Efficient Hydrogen Evolution Electrocatalysts. Inorg. Chem. Front. 2022, 9, 1171–1178. [Google Scholar] [CrossRef]
- Xue, Y.; Xie, Y.; Xu, C.; He, H.; Jiang, Q.; Ying, G.; Huang, H. 0D/2D Heterojunction of Graphene Quantum Dots/MXene Nanosheets for Boosted Hydrogen Evolution Reaction. Surf. Interfaces 2022, 30, 101907. [Google Scholar] [CrossRef]
- Hansen, J.N.; Prats, H.; Toudahl, K.K.; Mørch Secher, N.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is There Anything Better than Pt for HER? ACS Energy Lett. 2021, 6, 1175–1180. [Google Scholar] [CrossRef]
- Guo, F.; Macdonald, T.J.; Sobrido, A.J.; Liu, L.; Feng, J.; He, G. Recent Advances in Ultralow-Pt-Loading Electrocatalysts for the Efficient Hydrogen Evolution. Adv. Sci. 2023, 10, e2301098. [Google Scholar] [CrossRef]
- Hao, L.; He, H.; Qin, J.; Ma, C.; Luo, L.; Yang, L.; Huang, H. MXene Nanosheets Induce Efficient Iron Selenide Active Sites to Boost the Electrocatalytic Hydrogen Evolution Reaction. Inorg. Chem. 2022, 61, 21087–21094. [Google Scholar] [CrossRef]
- Zhao, Y.; Cong, H.; Li, P.; Wu, D.; Chen, S.; Luo, W. Hexagonal RuSe2 Nanosheets for Highly Efficient Hydrogen Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2021, 60, 7013–7017. [Google Scholar] [CrossRef]
- Ma, C.; Chen, W.; Wu, Y.; Wang, W.; Xu, L.; Chen, C.; Zheng, L.; Wang, G.; Han, P.; Gu, P.; et al. Undercoordinated Two-Dimensional Pt Nanoring Stabilized by a Ring-on-Sheet Nanoheterostructure for Highly Efficient Alkaline Hydrogen Evolution Reaction. Nano Lett. 2025, 25, 3212–3220. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, H.; Huang, B.; Li, B.; Li, H.; Han, Y.; Wang, Z.; Wu, X.; Pan, Y.; Sun, Y.; et al. Advanced Ultrathin RuPdM (M = Ni, Co, Fe) Nanosheets Electrocatalyst Boosts Hydrogen Evolution. ACS Cent. Sci. 2019, 5, 1991–1997. [Google Scholar] [CrossRef]
- Cai, C.; Liu, K.; Zhu, Y.; Li, P.; Wang, Q.; Liu, B.; Chen, S.; Li, H.; Zhu, L.; Li, H.; et al. Optimizing Hydrogen Binding on Ru Sites with RuCo Alloy Nanosheets for Efficient Alkaline Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202113664. [Google Scholar] [CrossRef]
- Lv, F.; Huang, B.; Feng, J.; Zhang, W.; Wang, K.; Li, N.; Zhou, J.; Zhou, P.; Yang, W.; Du, Y.; et al. A Highly Efficient Atomically Thin Curved PdIr Bimetallene Electrocatalyst. Natl. Sci. Rev. 2021, 8, nwab019. [Google Scholar] [CrossRef]
- Jin, X.; Kwon, S.J.; Kim, M.G.; Kim, M.; Hwang, S.-J. Crucial Role of Metal Coordination Number in Optimizing Electrocatalyst Activity of Holey Large-Area 2D Ru Nanosheets. ACS Nano 2024, 18, 15194–15203. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Z.; Gao, W.; Maxson, T.; Raciti, D.; Giroux, M.; Pan, A.; Wang, C.; Greeley, J. Tunable Intrinsic Strain in Two-Dimensional Transition Metal Electrocatalysts. Science 2019, 363, 870–874. [Google Scholar] [CrossRef]
- Huo, J.; Dou, Y.; Wu, C.; Liu, H.; Dou, S.; Yuan, D. Defect Engineering of Metal-Based Atomically Thin Materials for Catalyzing Small-Molecule Conversion Reactions. Adv. Mater. 2025, 37, e2416483. [Google Scholar] [CrossRef]
- Li, Q.; Kudo, A.; Ma, J.; Kawashima, R.; Toyama, K.; Xu, W.; Gao, Z.; Liang, Y.; Jiang, H.; Li, Z.; et al. Tuning Electrocatalytic Activities of Dealloyed Nanoporous Catalysts by Macroscopic Strain Engineering. Nano Lett. 2024, 24, 5543–5549. [Google Scholar] [CrossRef]
- Zhao, C.; Shu, C.; Zheng, R.; Du, D.; Ren, L.; He, M.; Li, R.; Xu, H.; Wen, X.; Long, J. Adjusting the D-Band Center of Metallic Sites in NiFe-Based Bimetal-Organic Frameworks via Tensile Strain to Achieve High-Performance Oxygen Electrode Catalysts for Lithium-Oxygen Batteries. J. Colloid Interface Sci. 2022, 607, 1215–1225. [Google Scholar] [CrossRef]
- Xia, Z.; Guo, S. Strain Engineering of Metal-Based Nanomaterials for Energy Electrocatalysis. Chem. Soc. Rev. 2019, 48, 3265–3278. [Google Scholar] [CrossRef]
- Guan, C.; Yue, X.; Xiang, Q. The Role of Lattice Distortion in Catalysis: Functionality and Distinctions from Strain. Adv. Mater. 2025; ahead of print. [Google Scholar] [CrossRef]
- Hou, Z.; Cui, C.; Li, Y.; Gao, Y.; Zhu, D.; Gu, Y.; Pan, G.; Zhu, Y.; Zhang, T. Lattice-Strain Engineering for Heterogenous Electrocatalytic Oxygen Evolution Reaction. Adv. Mater. 2023, 35, e2209876. [Google Scholar] [CrossRef]
- Kari, J.; Olsen, J.P.; Jensen, K.; Badino, S.F.; Krogh, K.B.R.M.; Borch, K.; Westh, P. Sabatier Principle for Interfacial (Heterogeneous) Enzyme Catalysis. ACS Catal. 2018, 8, 11966–11972. [Google Scholar] [CrossRef]
- Yin, S.; Liu, S.; Wang, Z.; Xu, Y.; Li, X.; Wang, H.; Wang, L. Methanol-Assisted Energy-Saving Hydrogen Production over Defect-Rich Perforated PdIn Bimetallene. Chem. Eng. J. 2022, 435, 134711. [Google Scholar] [CrossRef]
- Ding, H.; Liu, H.; Chu, W.; Wu, C.; Xie, Y. Structural Transformation of Heterogeneous Materials for Electrocatalytic Oxygen Evolution Reaction. Chem. Rev. 2021, 121, 13174–13212. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Q.; Zhang, Y.; Li, J.; Liu, P.; Wang, N.; Gong, X. Engineering High-Entropy Alloy Nanosheets toward Efficient Electrocatalytic Water Oxidation. Fuel 2024, 358, 130011. [Google Scholar] [CrossRef]
- Lu, X.; Xue, H.; Gong, H.; Bai, M.; Tang, D.; Ma, R.; Sasaki, T. 2D Layered Double Hydroxide Nanosheets and their Derivatives toward Efficient Oxygen Evolution Reaction. Nano-Micro Lett. 2020, 12, 86. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the Oxygen Evolution Reaction: Recent Development and Future Perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Xie, X.; Du, L.; Yan, L.; Park, S.; Qiu, Y.; Sokolowski, J.; Wang, W.; Shao, Y. Oxygen Evolution Reaction in Alkaline Environment: Material Challenges and Solutions. Adv. Funct. Mater. 2022, 32, 2110036. [Google Scholar] [CrossRef]
- Mo, J.; Fu, N.; Mu, S.; Peng, J.; Liu, Y.; Zhang, G. Facile Synthesis of Metal/Carbide Hybrid toward Overall Water Splitting. Catalysts 2024, 14, 730. [Google Scholar] [CrossRef]
- Shen, B.; Feng, Y.; Wang, Y.; Sun, P.; Yang, L.; Jiang, Q.; He, H.; Huang, H. Holey MXene Nanosheets Intimately Coupled with Ultrathin Ni–Fe Layered Double Hydroxides for Boosted Hydrogen and Oxygen Evolution Reactions. Carbon 2023, 212, 118141. [Google Scholar] [CrossRef]
- He, L.; Tang, Q.; Fan, Q.; Zhuang, H.; Wang, S.; Pang, Y.; Liang, K. Vertically Ti3CN@NiFe LDH Nanoflakes as Self-Standing Catalysts for Enhanced Oxygen Evolution Reaction. Catalysts 2024, 14, 708. [Google Scholar] [CrossRef]
- Song, Y.; Chen, H.; Wang, X.; Weng, C.; Zou, K.; Wang, C.; Yuan, Y.; Ma, Y.; Yang, X.; Lin, W. Engineering Ir-based Catalysts for High Current Density Applications in Proton Exchange Membrane Water Electrolyzers. Energy Environ. Sci. 2025, 18, 130–154. [Google Scholar] [CrossRef]
- Gao, G.; Sun, Z.; Chen, X.; Zhu, G.; Sun, B.; Yamauchi, Y.; Liu, S. Recent Advances in Ru/Ir-Based Electrocatalysts for Acidic Oxygen Evolution Reaction. Appl. Catal. B Environ. 2024, 343, 123584. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhang, G.; Gu, Z.; Chen, H.; Wei, G.; Shen, S.; Cheng, J.; Zhang, J. Recent Progress in Balancing the Activity, Durability, and Low Ir Content for Ir-Based Oxygen Evolution Reaction Electrocatalysts in Acidic Media. Small 2024, 21, 2410407. [Google Scholar] [CrossRef]
- Thao, N.T.T.; Jang, J.U.; Nayak, A.K.; Han, H. Current Trends of Iridium-Based Catalysts for Oxygen Evolution Reaction in Acidic Water Electrolysis. Small Sci. 2024, 4, 2300109. [Google Scholar] [CrossRef]
- Yu, H.; Ke, J.; Shao, Q. Two Dimensional Ir-Based Catalysts for Acidic OER. Small 2023, 19, e2304307. [Google Scholar] [CrossRef]
- Chen, H.; Shi, L.; Sun, K.; Zhang, K.; Liu, Q.; Ge, J.; Liang, X.; Tian, B.; Huang, Y.; Shi, Z.; et al. Protonated Iridate Nanosheets with a Highly Active and Stable Layered Perovskite Framework for Acidic Oxygen Evolution. ACS Catal. 2022, 12, 8658–8666. [Google Scholar] [CrossRef]
- Jiang, B.; Guo, Y.; Kim, J.; Whitten, A.E.; Wood, K.; Kani, K.; Rowan, A.E.; Henzie, J.; Yamauchi, Y. Mesoporous Metallic Iridium Nanosheets. J. Am. Chem. Soc. 2018, 140, 12434–12441. [Google Scholar] [CrossRef]
- Chatterjee, S.; Peng, X.; Intikhab, S.; Zeng, G.; Kariuki, N.N.; Myers, D.J.; Danilovic, N.; Snyder, J. Nanoporous Iridium Nanosheets for Polymer Electrolyte Membrane Electrolysis. Adv. Energy Mater. 2021, 11, 2101438. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, C.; Li, D.; Shao, X.; Cao, L.; Yang, J. Designing of Hexagonal Nanosheets with Edge-Sharing [IrO6] Octahedral Crystals for Efficient and Stable Acidic Water Splitting. Adv. Funct. Mater. 2023, 34, 2313375. [Google Scholar] [CrossRef]
- Kong, X.; Xu, K.; Zhang, C.; Dai, J.; Norooz Oliaee, S.; Li, L.; Zeng, X.; Wu, C.; Peng, Z. Free-Standing Two-Dimensional Ru Nanosheets with High Activity toward Water Splitting. ACS Catal. 2016, 6, 1487–1492. [Google Scholar] [CrossRef]
- Chen, F.-Y.; Wu, Z.-Y.; Adler, Z.; Wang, H. Stability Challenges of Electrocatalytic Oxygen Evolution Reaction: From Mechanistic Understanding to Reactor Design. Joule 2021, 5, 1704–1731. [Google Scholar] [CrossRef]
- Minke, C.; Suermann, M.; Bensmann, B.; Hanke-Rauschenbach, R. Is Iridium Demand a Potential Bottleneck in the Realization of Large-Scale Pem Water Electrolysis? Int. J. Hydrogen Energy 2021, 46, 23581–23590. [Google Scholar] [CrossRef]
- Li, S.; Zhao, S.; Hu, F.; Li, L.; Ren, J.; Jiao, L.; Ramakrishna, S.; Peng, S. Exploring the Potential Ru-Based Catalysts for Commercial-Scale Polymer Electrolyte Membrane Water Electrolysis: A Systematic Review. Prog. Mater. Sci. 2024, 145, 101294. [Google Scholar] [CrossRef]
- Qin, R.; Chen, G.; Feng, X.; Weng, J.; Han, Y. Ru/Ir-Based Electrocatalysts for Oxygen Evolution Reaction in Acidic Conditions: From Mechanisms, Optimizations to Challenges. Adv. Sci. 2024, 11, e2309364. [Google Scholar] [CrossRef]
- Yuan, J.H.; Li, L.H.; Zhang, W.; Xue, K.H.; Wang, C.; Wang, J.; Miao, X.S.; Zeng, X.C. Pt5Se4 Monolayer: A Highly Efficient Electrocatalyst toward Hydrogen and Oxygen Electrode Reactions. ACS Appl. Mater. Interfaces 2020, 12, 13896–13903. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Y.; Gao, L.; Zeng, G.; Li, M.; Huang, H. Stabilizing Pt-Based Electrocatalysts for Oxygen Reduction Reaction: Fundamental Understanding and Design Strategies. Adv. Mater. 2021, 33, e2006494. [Google Scholar] [CrossRef]
- Tian, X.; Lu, X.F.; Xia, B.Y.; Lou, X.W. Advanced Electrocatalysts for the Oxygen Reduction Reaction in Energy Conversion Technologies. Joule 2020, 4, 45–68. [Google Scholar] [CrossRef]
- Chen, J.W.; Zhang, Z.; Yan, H.M.; Xia, G.J.; Cao, H.; Wang, Y.G. Pseudo-Adsorption and Long-Range Redox Coupling During Oxygen Reduction Reaction on Single Atom Electrocatalyst. Nat. Commun. 2022, 13, 1734. [Google Scholar] [CrossRef]
- Ao, X.; Wang, H.; Zhang, X.; Wang, C. Atomically Dispersed Metal-Nitrogen-Carbon Catalysts for Acidic Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2025, 17, 2844–2862. [Google Scholar] [CrossRef]
- Yu, J.; Su, C.; Shang, L.; Zhang, T. Single-Atom-Based Oxygen Reduction Reaction Catalysts for Proton Exchange Membrane Fuel Cells: Progress and Perspective. ACS Nano 2023, 17, 19514–19525. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Y.; Lu, Z.; Liu, C.; Xu, Y.; Chen, H.; Wang, Q.; Wu, D.; Dang, D.; Deng, Y.; et al. Oxygen-Coordinated Cr Single-Atom Catalyst for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. Angew. Chem. Int. Ed. 2025, 64, e202500500. [Google Scholar] [CrossRef]
- Pruchyathamkorn, J.; Yang, M.; Amin, H.M.A.; Batchelor-McAuley, C.; Compton, R.G. Imaging Electrode Heterogeneity Using Chemically Confined Fluorescence Electrochemical Microscopy. J. Phys. Chem. Lett. 2017, 8, 6124–6127. [Google Scholar] [CrossRef]
- Pan, L.; Merzdorf, T.; Campos-Roldàn, C.A.; Guo, A.; Lu, J.; Schmidt, J.; Heggen, M.; Klingenhof, M.; Wang, X.; Möhle, S.; et al. From Seeds to Cell: Improving PEMFC Performance and Durability by Seed-Mediation Synthesis for PtNiIr ORR Nanocatalysts. Adv. Sci. 2025; ahead of print. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Yu, J.; Wang, H.; Qin, Y.; Xing, L.; Du, L. Research Progress of Pt-Based Catalysts toward Cathodic Oxygen Reduction Reactions for Proton Exchange Membrane Fuel Cells. Catalysts 2024, 14, 569. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S. Current Status and Future Development of Catalyst Materials and Catalyst Layers for Proton Exchange Membrane Fuel Cells: An Industrial Perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]
- Sinniah, J.D.; Wong, W.Y.; Loh, K.S.; Yunus, R.M.; Timmiati, S.N. Perspectives on Carbon-Alternative Materials as Pt Catalyst Supports for a Durable Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. J. Power Sources 2022, 534, 231422. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Baltruschat, H.; Wittmaier, D.; Friedrich, K.A. A Highly Efficient Bifunctional Catalyst for Alkaline Air-Electrodes Based on a Ag and Co3O4 Hybrid: RRDE and Online DEMS Insights. Electrochim. Acta 2015, 151, 332–339. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Konigshoven, P.; Hegemann, M.; Baltruschat, H. Role of Lattice Oxygen in the Oxygen Evolution Reaction on Co3O4: Isotope Exchange Determined Using a Small-Volume Differential Electrochemical Mass Spectrometry Cell Design. Anal. Chem. 2019, 91, 12653–12660. [Google Scholar] [CrossRef]
- Liang, J.; Li, S.; Chen, Y.; Liu, X.; Wang, T.; Han, J.; Jiao, S.; Cao, R.; Li, Q. Ultrathin and Defect-Rich Intermetallic Pd2Sn Nanosheets for Efficient Oxygen Reduction Electrocatalysis. J. Mater. Chem. A 2020, 8, 15665–15669. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Z.; Ali, A.; Luo, Y.; Feng, H.; Luo, Y.; Tsiakaras, P.; Kang Shen, P. Shell-Thickness-Dependent Pd@Ptni Core–Shell Nanosheets for Efficient Oxygen Reduction Reaction. Chem. Eng. J. 2022, 427, 131565. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, S.; Cao, W.; Luo, J.; Gu, Y.; Liu, X.; Tan, P.; Wang, Z.; Pan, J. Microwave Heating-Assisted Synthesis of Ultrathin Platinum-Based Trimetallic Nanosheets as Highly Stable Catalysts towards Oxygen Reduction Reaction in Acidic Medium. J. Colloid Interface Sci. 2024, 675, 1108–1118. [Google Scholar] [CrossRef]
- Peng, X.; Lu, D.; Qin, Y.; Li, M.; Guo, Y.; Guo, S. Pt-on-Pd Dendritic Nanosheets with Enhanced Bifunctional Fuel Cell Catalytic Performance. ACS Appl. Mater. Interfaces 2020, 12, 30336–30342. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Guo, C.X.; Cao, H.; Wang, S.; Ouyang, Y.; Xu, B.; Guo, P.; Li, C.M. Highly Wrinkled Palladium Nanosheets as Advanced Electrocatalysts for the Oxygen Reduction Reaction in Acidic Medium. Chem. Eng. J. 2022, 431, 133237. [Google Scholar] [CrossRef]
- Chen, W.; Gao, W.; Tu, P.; Robert, T.; Ma, Y.; Shan, H.; Gu, X.; Shang, W.; Tao, P.; Song, C.; et al. Neighboring Pt Atom Sites in an Ultrathin FePt Nanosheet for the Efficient and Highly CO-Tolerant Oxygen Reduction Reaction. Nano Lett. 2018, 18, 5905–5912. [Google Scholar] [CrossRef]
- Li, Y.; Yao, M.S.; He, Y.; Du, S. Recent Advances of Electrocatalysts and Electrodes for Direct Formic Acid Fuel Cells: From Nano to Meter Scale Challenges. Nano-Micro Lett. 2025, 17, 148. [Google Scholar] [CrossRef]
- Al-Nayili, A.; Majdi, H.S.; Albayati, T.M.; Saady, N.M.C. Formic Acid Dehydrogenation Using Noble-Metal Nanoheterogeneous Catalysts: Towards Sustainable Hydrogen-Based Energy. Catalysts 2022, 12, 324. [Google Scholar] [CrossRef]
- Guo, X.; Yang, L.; Shen, B.; Wei, Y.; Yang, Y.; Yang, C.; Jiang, Q.; He, H.; Huang, H. Ultrafine Pd Nanocrystals Anchored onto Single-Walled Carbon Nanohorns: A Highly-Efficient Multifunctional Electrocatalyst with Ultra-Low Pd Loading for Formic Acid and Methanol Oxidation. Mater. Chem. Phys. 2020, 250, 123167. [Google Scholar] [CrossRef]
- Zheng, J.-H.; Zhang, J.; Li, G.; Zhang, J.-M.; Zhang, B.-W.; Jiang, Y.-X.; Sun, S.-G. Tuning Atomic Pt Site Surface on Ptau Alloy toward Electro-Oxidation of Formic Acid. Mater. Today Energy 2022, 27, 101028. [Google Scholar] [CrossRef]
- Hu, X.; Xiao, Z.; Wang, W.; Bu, L.; An, Z.; Liu, S.; Pao, C.W.; Zhan, C.; Hu, Z.; Yang, Z.; et al. Platinum-Lead-Bismuth/Platinum-Bismuth Core/Shell Nanoplate Achieves Complete Dehydrogenation Pathway for Direct Formic Acid Oxidation Catalysis. J. Am. Chem. Soc. 2023, 145, 15109–15117. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, M.; Huang, Y.; Zou, Y.; Liu, Z.; Tao, L.; Li, Y.; Dong, C.L.; Wang, S. In Situ Exfoliation and Pt Deposition of Antimonene for Formic Acid Oxidation via a Predominant Dehydrogenation Pathway. Research 2020, 2020, 5487237. [Google Scholar] [CrossRef]
- Hu, X.; An, Z.; Wang, W.; Lin, X.; Chan, T.S.; Zhan, C.; Hu, Z.; Yang, Z.; Huang, X.; Bu, L. Sub-Monolayer SbOx on PtPb/Pt Nanoplate Boosts Direct Formic Acid Oxidation Catalysis. J. Am. Chem. Soc. 2023, 145, 19274–19282. [Google Scholar] [CrossRef]
- Ge, Z.-X.; Miao, B.-Q.; Tian, X.-L.; He, B.; Chen, Y. Chemical Functionalization of Commercial Pt/C Electrocatalyst towards Formic Acid Electrooxidation. Chem. Eng. J. 2023, 476, 146529. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Kim, S.; Jang, J.-H.; Sohn, H.; Hong, S.J.; Kim, J.; Han, G.H.; Choe, S.; Sung, Y.-E.; et al. Atomic Pt clusters on Au dendrite for Formic Acid Oxidation. Chem. Eng. J. 2023, 451, 138664. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, B.; Song, H.; Zhou, S.; Ye, J.; Liao, H.-G.; Dong, L.; Huang, X.; Bu, L. Platinum–Tellurium Heterojunction Nanosheet Assemblies for Efficient Direct Formic Acid Electrooxidation Catalysis. ACS Nano 2024, 18, 10008–10018. [Google Scholar] [CrossRef]
- Liu, L.; Jin, L.; Xiao, Z.; Fang, N.; Lin, X.; Ji, Y.; Wang, Y.; Li, Y.; Huang, X.; Bu, L. Heterostructured Pt-PbS Nanobelt Achieves Remarkable Direct Formic Acid Oxidation Catalysis. Nano Lett. 2024, 24, 8162–8170. [Google Scholar] [CrossRef]
- Zhou, Y.-W.; Chen, Y.-F.; Qin, X.; Jiang, K.; Lin, W.-F.; Cai, W.-B. Boosting Electrocatalytic Oxidation of Formic Acid on SnO2-Decorated Pd Nanosheets. J. Catal. 2021, 399, 8–14. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, Z.; Chen, B.; Huang, Y.; Chen, J.; Lai, Z.; Chen, Y.; Sindoro, M.; Wang, A.L.; Cheng, H.; et al. Synthesis of Ultrathin PdCu Alloy Nanosheets Used as a Highly Efficient Electrocatalyst for Formic Acid Oxidation. Adv. Mater. 2017, 29, 1700768. [Google Scholar] [CrossRef]
- Wei, J.; Wu, F.; Sun, H.; Xia, S.; Sang, X.; Li, F.; Zhang, Z.; Han, S.; Niu, W. Modulate the Metallic Sb State on Ultrathin PdSb-Based Nanosheets for Efficient Formic Acid Electrooxidation. J. Colloid Interface Sci. 2023, 648, 473–480. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ebrahimifar, H.; Askari, M.B. Alcohol Electrooxidation on Three-Component NiO/La2O3/MWCNTs Catalyst for DAFC Application. Electrochem. Commun. 2025, 176, 107963. [Google Scholar] [CrossRef]
- Wu, F.; Zeng, L.; Zhu, L.; Wang, W.; Cao, C.; Yang, Z.; Shang, C.; Ye, H.; Guo, Z. Cooperative Pd@CoNi/rG Catalyst for Highly Efficient and Stable Electrocatalytic Methanol and Ethanol Oxidations. Chem. Eng. J. 2024, 502, 158010. [Google Scholar] [CrossRef]
- Guo, X.; Xiong, J.; Wang, Q.; Zhang, J.; He, H.; Huang, H. Ultrafine Rh Nanocrystals Grown onto a Boron and Nitrogen Codoped Carbon Support with a Horn-Shaped Structure for Highly Efficient Methanol Oxidation. Dalton Trans. 2022, 51, 16982–16989. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, J.; Yang, C.; Yang, Y.; Zhang, Y.; Yang, F.; Ma, R.; Yang, L.; He, H.; Huang, H. Pd Nanocrystals Anchored on 3D Hybrid Architectures Constructed from Nitrogen-Doped Graphene and Low-Defect Carbon Nanotube as High-Performance Multifunctional Electrocatalysts for Formic Acid and Methanol Oxidation. Mater. Today Energy 2020, 16, 100409. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; He, H.; Huang, H. Building 3D Interconnected MoS2 Nanosheet–Graphene Networks Decorated with Rh Nanoparticles for Boosted Methanol Oxidation Reaction. ACS Sustain. Chem. Eng. 2022, 10, 8940–8948. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Q.; Li, Y.; Chen, Y.; Yang, L.; He, H.; Xu, X.; Huang, H. Nanosized Rh Grown on Single-Walled Carbon Nanohorns for Efficient Methanol Oxidation Reaction. Rare Met. 2022, 41, 2108–2117. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Q.; He, H.; Huang, H. Boron and Nitrogen Codoped Carbon Nanohorn-Supported Pt Nanocrystals as Highly-Efficient Methanol Oxidation Electrocatalysts. J. Mater. Res. Technol. 2022, 19, 722–731. [Google Scholar] [CrossRef]
- Berretti, E.; Osmieri, L.; Baglio, V.; Miller, H.A.; Filippi, J.; Vizza, F.; Santamaria, M.; Specchia, S.; Santoro, C.; Lavacchi, A. Direct Alcohol Fuel Cells: A Comparative Review of Acidic and Alkaline Systems. Electrochem. Energy Rev. 2023, 6, 30. [Google Scholar] [CrossRef]
- Elsaid, K.; Abdelfatah, S.; Abdel Elabsir, A.M.; Hassiba, R.J.; Ghouri, Z.K.; Vechot, L. Direct Alcohol Fuel Cells: Assessment of the Fuel’s Safety and Health Aspects. Int. J. Hydrogen Energy 2021, 46, 30658–30668. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, T.; Jiang, X.; Chen, K.; Wu, Q.; Zhang, Y.; Shi, D.; Li, H. Advances in the Preparation Strategies and Structural Regulation for CeO2-Containing Electrocatalysts Applied to the Anodes of Direct Alcohol Fuel Cells: A Comprehensive Review. J. Mater. Chem. A 2025, 13, 20143–20175. [Google Scholar] [CrossRef]
- Mohammadi, T.; Hosseini, M.G.; Diaz-Coello, S.; Pastor, E.; Ahadzadeh, I. Monitoring of Ethanol Electrooxidation on Highly Efficient Conductive Runi Metal-Organic Framework by Mass Spectrometry. J. Power Sources 2024, 611, 234758. [Google Scholar] [CrossRef]
- Xue, Y.; Xiong, J.; Zhang, H.; He, H.; Huang, H. Ultrafine Rh Nanocrystals Immobilized on 3D Boron and Nitrogen Co-Doped Graphene–Carbon Nanotube Networks: High-Efficiency Electrocatalysts towards the Methanol Oxidation Reaction. Catal. Sci. Technol. 2022, 12, 6016–6023. [Google Scholar] [CrossRef]
- Qiang, L.; Wen, W.; Yan, Q.; Zhao, P.; Ma, J.; Liu, C.; Zhao, M.; He, Y.; Xiao, H.; Jia, J. Recent Advances in Constructing Efficient Electrocatalysts for Ethanol Oxidation Reaction. J. Alloys Compd. 2024, 1001, 175120. [Google Scholar] [CrossRef]
- Ipadeola, A.K.; Eid, K.; Lebechi, A.K.; Abdullah, A.M.; Ozoemena, K.I. Porous Multi-Metallic Pt-Based Nanostructures as Efficient Electrocatalysts for Ethanol Oxidation: A Mini-Review. Electrochem. Commun. 2022, 140, 107330. [Google Scholar] [CrossRef]
- Bai, S.; Xu, Y.; Cao, K.; Huang, X. Selective Ethanol Oxidation Reaction at the Rh-SnO2 Interface. Adv. Mater. 2021, 33, e2005767. [Google Scholar] [CrossRef]
- Liu, C.; Shen, Y.; Zhang, J.; Li, G.; Zheng, X.; Han, X.; Xu, L.; Zhu, S.; Chen, Y.; Deng, Y.; et al. Multiple Twin Boundary-Regulated Metastable Pd for Ethanol Oxidation Reaction. Adv. Energy Mater. 2022, 12, 2103505. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Liu, T.; Ma, X.; Feng, Y.; Xu, B.; Cai, W.; Li, Y.; Su, D.; Shao, Q.; et al. Rhombohedral Pd–Sb Nanoplates with Pd-Terminated Surface: An Efficient Bifunctional Fuel-Cell Catalyst. Adv. Mater. 2022, 34, 2202333. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Hu, M.; Wu, Z.; Du, Y. 2D Petal-Like Pdag Nanosheets Promote Efficient Electrocatalytic Oxidation of Ethanol and Methanol. Nanoscale 2024, 16, 14096–14100. [Google Scholar] [CrossRef]
- Lan, B.; Wang, Q.-L.; Ma, Z.-X.; Wu, Y.-J.; Jiang, X.-L.; Jia, W.-S.; Zhou, C.-X.; Yang, Y.-Y. Efficient Electrochemical Ethanol-To-CO2 Conversion at Rhodium and Bismuth Hydroxide Interfaces. Appl. Catal. B Environ. 2022, 300, 120728. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, H.; Xiong, J.; He, H.; Huang, H. Well-Dispersive Pt Nanocrystals Anchored onto 3D Boron and Nitrogen Double-Doped Reduced Graphene Oxide–Carbon Nanotube Frameworks as Efficient Electrocatalysts for Methanol Oxidation. J. Electroanal. Chem. 2022, 921, 116705. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Chen, S.; Xue, Q.; Li, F.-M.; Yao, H.-C.; Xu, L.; Chen, Y. Hierarchical Porous Rh Nanosheets for Methanol Oxidation Reaction. Appl. Catal. B Environ. 2020, 264, 118520. [Google Scholar] [CrossRef]
- Meng, X.; Ouyang, Y.; Wu, H.; Huang, H.; Wang, F.; Wang, S.; Jiang, M.; Zhang, L.Y. Hierarchical Defective Palladium-Silver Alloy Nanosheets for Ethanol Electrooxidation. J. Colloid Interface Sci. 2021, 586, 200–207. [Google Scholar] [CrossRef]
- Dong, K.; Dai, H.; Pu, H.; Zhang, T.; Wang, Y.; Deng, Y. Constructing Efficient Ternary PtTeCu Nano-Catalysts with 2D Ultrathin-Sheet Structures for Oxidation Reaction of Alcohols. Appl. Surf. Sci. 2023, 609, 155301. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wang, C.; Liu, D.; Yu, R.; Ye, C.; Du, Y. Activable Ru-PdRu Nanosheets with Heterogeneous Interface for High-Efficiency Alcohol Oxidation Reaction. J. Colloid Interface Sci. 2023, 647, 519–527. [Google Scholar] [CrossRef]
- Ji, L.; Che, H.; Qian, N.; Li, J.; Luo, S.; Li, X.; Wu, X.; Xu, Q.; Gong, X.; Cui, X.; et al. Unconventional s-p-d Hybridization in Modulating Frontier Orbitals of Carbonaceous Radicals on PdBi Nanosheets for Efficient Ethanol Electrooxidation. Appl. Catal. B Environ. 2023, 328, 122521. [Google Scholar] [CrossRef]
- Li, L.; Chu, M.; Song, R.; Liu, S.; Ren, G.; Xu, Y.; Wang, L.; Xu, Q.; Shao, Q.; Lu, J.; et al. CO Spillover on Ultrathin Bimetallic Rh/Rh-M Nanosheets. Chem Catal. 2022, 2, 1709–1719. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Wang, Y.; He, H.; Huang, H. Recent Progress of 2D Pt-Group Metallic Electrocatalysts for Energy-Conversion Applications. Catalysts 2025, 15, 716. https://doi.org/10.3390/catal15080716
Chen Z, Wang Y, He H, Huang H. Recent Progress of 2D Pt-Group Metallic Electrocatalysts for Energy-Conversion Applications. Catalysts. 2025; 15(8):716. https://doi.org/10.3390/catal15080716
Chicago/Turabian StyleChen, Ziyue, Yuerong Wang, Haiyan He, and Huajie Huang. 2025. "Recent Progress of 2D Pt-Group Metallic Electrocatalysts for Energy-Conversion Applications" Catalysts 15, no. 8: 716. https://doi.org/10.3390/catal15080716
APA StyleChen, Z., Wang, Y., He, H., & Huang, H. (2025). Recent Progress of 2D Pt-Group Metallic Electrocatalysts for Energy-Conversion Applications. Catalysts, 15(8), 716. https://doi.org/10.3390/catal15080716






