1. Introduction
Clean water is essential for human health, environmental preservation, and sustainable development. However, the presence of various pollutants poses a serious threat to the quality of surface and groundwater sources [
1]. Organic contaminants are particularly persistent, as they are difficult to degrade and can disrupt aquatic ecosystems. Conventional treatment methods, including physical, chemical, and biological approaches, often fall short due to inefficiency, high costs, or secondary pollution. As a result, developing advanced and sustainable water purification technologies has become a global priority. In this context, heterogeneous photocatalysis using semiconductor materials has emerged as an effective, environmentally friendly, and potentially low-cost strategy for the degradation and removal of a broad range of organic pollutants from water systems [
2].
Titanium dioxide (TiO
2) is a transition metal oxide widely studied for photocatalytic applications due to its semiconducting properties, good chemical stability, non-toxicity, and abundance [
3]. As a semiconductor, TiO
2 can generate electron-hole pairs when exposed to light with energy greater than its band gap. However, its relatively wide band gap (~3.2 eV) limits its absorption to the UV region of the spectrum. Since UV light accounts for only about 5% of the solar spectrum, this significantly restricts the practical use of TiO
2 in solar-driven photocatalytic applications. Therefore, TiO
2-based materials must be modified to enable visible light absorption and enhance photocatalytic performance [
4]. The efficiency of TiO
2 in photocatalysis strongly depends on its morphology, crystal structure, and chemical composition, which includes doping with metal and non-metal dopants and the presence of structural defects [
5]. Furthermore, the rapid recombination of photogenerated charge carriers significantly reduces its photocatalytic efficiency. These limitations have prompted research into doping strategies and heterostructure formation to enhance visible light response and improve charge separation [
6]. In particular, TiO
2-based heterojunctions with materials such as g-C
3N
4 [
7] and Cu
2O [
8] have demonstrated enhanced photocatalytic activity through improved charge separation and extended light absorption.
One promising approach to enhance the visible-light activity of TiO
2 involves coupling it with titanium nitride (TiN), a conductive and thermally stable material that exhibits plasmonic properties similar to those of noble metals such as gold and silver [
9]. These plasmonic characteristics enhance visible light absorption [
10] and can facilitate charge carrier separation at the TiN/TiO
2 interface, thereby reducing electron-hole recombination and improving overall photocatalytic efficiency [
11]. Additionally, the combination of TiN and TiO
2 offers the possibility of nitrogen incorporation into the TiO
2 lattice during post-deposition annealing, creating N-doped TiO
2 with improved photocatalytic performance. Research results summarized in the review by Chakraborty et al. [
12] indicate that nitrogen doping can significantly enhance the photocatalytic activity of TiO
2 in the visible region; however, the extent of this enhancement varies considerably depending on the synthesis method, nature of the pollutant, and testing conditions. Nitrogen can easily integrate into the TiO
2 structure due to its atomic size being comparable to oxygen, along with its low ionization energy and high stability [
13]. Producing N-doped TiO
2 by magnetron sputtering is usually performed by tuning the ratio between O
2 and N
2 reactive gases during the material growth [
14,
15,
16,
17,
18]. Other methods for producing N-doped TiO
2 thin films include electrochemical anodization of TiN [
19], nitrogen ion implantation [
20], plasma nitriding [
21], pulsed laser deposition [
22], among others.
Although literature suggests that deposition of noble metals increases photocatalytic activity [
23], it is possible that in some cases Au acts as a recombination center [
24]. The addition of gold is intended to improve visible-light photocatalysis through multiple mechanisms. Gold can form a Schottky junction at the metal–semiconductor interface, where its lower Fermi level enables it to trap conduction band electrons from TiO
2 [
25]. This facilitates charge separation by suppressing recombination and enhancing electron transfer to reducible species, such as dissolved oxygen or organic pollutants [
26]. Additionally, the plasmonic properties of gold nanoparticles can increase visible light absorption [
27], while their interface with TiO
2 can create localized surface and quantum size effects that influence charge carrier dynamics [
28]. However, literature reports show that the photocatalytic activity of Au/TiO
2 systems is highly sensitive to deposition method, particle size, and gold loading, as Cojocaru et al. [
29] demonstrated that reactive sputtering allows precise control of Au layer thickness and nanoparticle size, with photocatalytic activity for acetone oxidation strongly depending on both Au content and the underlying titania phase. Similarly, Jung et al. [
30] reported that gold-buffered TiO
2 thin films, prepared by magnetron sputtering, exhibited enhanced photocatalytic performance compared to pure anatase films due to improved charge separation at the Au interface. Based on these insights, our study aims to examine how gold modification affects TiN/TiO
2 bilayer systems, particularly in the context of plasmonic enhancement and interfacial charge dynamics.
A particular focus of this research is on the use of thin films as photocatalytic materials, due to their numerous advantages for real-world applications. Thin films deposited by physical methods such as reactive sputtering offer strong adhesion to substrates, precise control over film thickness, high homogeneity, excellent reproducibility, and the ability to be deposited on various substrates and over large areas [
31]. In addition, thin films can be reused multiple times without removal from the reaction medium, which presents a significant advantage over nanoparticle-based systems that are difficult to separate from solution and may contribute to secondary pollution [
32]. Although thin films generally possess a lower specific surface area and fewer active sites compared to nanoparticles, these limitations can be overcome through careful optimization of synthesis conditions. Controlling layer thickness, tailoring material structure, introducing dopants, and modifying surface and electronic properties can all contribute to improved photocatalytic performance and enhanced degradation rates of organic pollutants [
5].
This study extends our previous investigations into the photocatalytic properties of TiO
2-based thin films. Our earlier works explored the influence of nitrogen doping via annealing on TiO
2-N thin films [
17] and the effect of surface-formed gold structures on TiO
2 thin films deposited by DC sputtering [
33]. In the present work, we introduce a bilayer system in which sub-stoichiometric TiO
2 films are deposited directly onto TiN thin film substrates, enabling diffusion-driven nitrogen doping during annealing and creating an engineered heterostructure not previously explored in our studies. Comprehensive structural and optical analyses were performed to shed more light on the influence of N doping and N diffusion in TiO
2/TiN composites and its influence on the photocatalytic activity of these films. The presence of vacancies in the structure is expected to positively affect on nitrogen diffusion through the layer structure, and they can be a tool for tailoring the presence of nitrogen species at the surface. The consensus across experimental and theoretical studies indicates that nitrogen atomic concentrations between 1 at% and 3 at% optimize the visible-light photocatalytic activity of TiO
2 [
34]. Deviations from this range may lead to either insufficient bandgap narrowing or defect-dominated recombination. The photocatalytic properties were assessed by studying the degradation of methylene blue dye (MB). MB is a synthetic dye commonly used in various industries, making its presence in wastewater a significant environmental concern, particularly in developing countries. Due to its known toxicity, the development of effective removal methods has been widely studied [
35]. Another reason for selecting MB is its well-established degradation behavior under photocatalytic conditions, which allows for detailed kinetic studies. For example, Houas et al. [
36] investigated the degradation pathway and identified intermediate products, showing that hydroxyl radicals (•OH) attack the C–S
+=C functional group, while ring-opening requires the presence of heteroatoms such as S and N. This suggests that photocatalysts capable of degrading MB may also be effective against structurally similar pollutants, including sulfur heterocycles present in pharmaceuticals [
37] and agrochemicals [
38]. Furthermore, to enhance the photocatalytic degradation rate of MB, a thin film of gold (5 nm) was deposited as a buffer 3 nm below the TiO
2 surface, as described in our earlier paper [
33] on the annealed TiN/TiO
2 films, to demonstrate the synergistic effect of gold on different TiO
2/TiN bilayer structures.
2. Results
2.1. Structural and Morphological Characterization
In order to gather detailed information on the microstructure of the as-prepared samples, transmission electron microscopy (TEM) was employed. The results of this analysis are displayed as representative micrographs (
Figure 1) taken from the pure TiO
2 film and from the film containing N75 (see
Table 1 for information regarding sample naming) as an example. Low-magnification bright field micrographs of the selected samples are presented in
Figure 1a,d, revealing the general microstructure of the films. It can be noticed that both films exhibit similar morphology, characterized by the irregularly positioned grains, various in size and shape. The observed surface roughness may be the result of the strong effect of the FIB during the preparation of the sample. The thickness of the films was measured to be around 110 nm, measuring from the end of the Silicone substrate to the protective Pt layer. The presence of numerous voids characterizing the nanostructured morphology was observed. The voids are mostly situated directly on top of the underlying Si substrate. It can be noticed that the number of voids rises as the quantity of deposited TiN phase increases, indicating the influence of the film composition and/or preparation procedure on the sample morphology.
To achieve a closer inspection of the microstructure, images were acquired at higher magnification, for the highly ordered lattice planes to be distinctly observed. Interplanar spacing distances were measured, revealing the presence of TiO
2 phase, and/or TiN phases, depending on the observed sample.
Figure 1b,e represent enlarged parts of a few selected grains, clearly showing the crystalline nature of the deposited films induced by annealing. The
d-spacing value for an isolated grain from pure TiO
2 film (
Figure 1b) was measured to be 0.352 nm, corresponding well with the plane (101) for anatase TiO
2 (PDF card no. 21-1272). The estimated interplanar spacing for N75 (
Figure 1e) was 0.160 nm, which is in agreement with the reference value for the plane with Miller index (220) for TiN (PDF card no. 2-1159). Moreover, attained FFT images (presented as insets in
Figure 1b,e) show highly oriented bright spots, validating the existence of the ordered lattice stripes, which is an additional confirmation that the samples possess a crystalline structure.
To further investigate the crystalline nature of the deposited films, selected area electron diffraction analysis was performed, and the results are demonstrated in
Figure 1c,f. SAED images consist of numerous fragments of diverse diffraction rings, revealing the polycrystalline structure of the samples. Measuring the radii of the rings, interplanar spacing distances can be calculated for the various planes, thus verifying the presence of existing phases in the sample. For TiO
2 film (
Figure 1c),
d-spacing values were found to be 0.238, 0.196, 0.139, 0.125, 0.115, and 0.083 nm, which corresponds well with planes (112), (200), (211), (224), (305), and (415) for the TiO
2 anatase phase, respectively. Moreover, planes (202) and (018) were measured with the spacing of 0.210 and 0.162 nm, corresponding to the Ti
2O
3 phase (PDF card no. 10-0063), respectively. The formation of the Ti
2O
3 phase can be a consequence of oxygen vacancies that usually occur in TiO
2 structures. The outer ring fragments originate from the reflections of high index planes of the titania phases. Additionally, the first inner ring was measured to have a
d-spacing of 0.302 nm, which can be attributed to the Si phase (PDF card no. 01-075-0841) originating from the Si substrate. For the TiO
2/TiN film, the SAED image (
Figure 1f) exhibits a similar diffraction pattern to that observed for the pure TiO
2 film. Interplanar spacings were determined to have the values of 0.239, 0.197, 0.139, and 0.085 nm, which correspond well to the crystallographic planes with Miller indices (112), (200), (211), and (415) for TiO
2 anatase, respectively. Moreover, the Ti
2O
3 phase was also discovered in the form of plane (211), having an interplanar spacing distance of 0.205 nm. The formation of TiN was evidenced by the
d-spacing values of 0.217, 0.156, and 0.112 nm which can be ascribed to the planes (200), (220), and (400), respectively. Accomplished results clearly point to a polycrystalline structure of the as-deposited films, revealing firm evidence for the presence of different TiO
2 and TiN phases formed during the chosen preparation method.
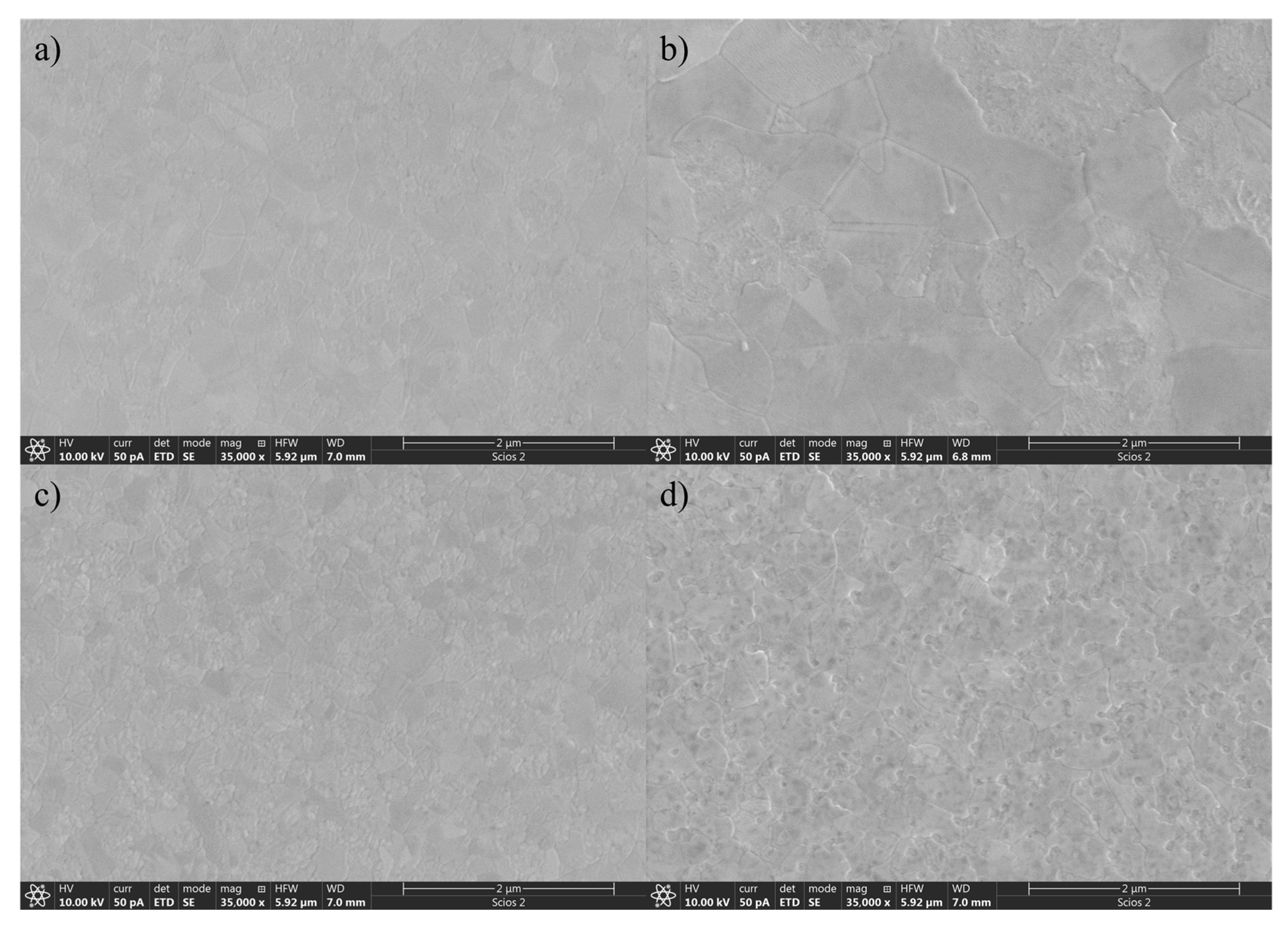
Morphological changes of the surface caused by annealing and N diffusion were examined using scanning electron microscopy (SEM). Following the sequential deposition of TiN and TiO
2 thin films by reactive sputtering without substrate heating, the samples were subjected to annealing in air at 600 °C. SEM images acquired after annealing are shown in
Figure 2 and reveal significant morphological differences between the samples as a function of the TiN/TiO
2 ratio. The surface of the pure TiO
2 film exhibits a uniform and compact morphology with well-defined faceted grains, characteristic of polycrystalline anatase. With increasing TiN content, the differences in grain size become visible. At 25% TiN, the grain size appears larger, indicating that the influence of N diffusion through the TiO
2 upper layer influences crystal growth. The 50% TiN sample shows morphological distortion with smaller or irregular grains. In the sample containing 75% TiN, the surface becomes highly rough and non-uniform.
Despite these morphological changes, the anatase phase is retained, implying that the oxygen-deficient TiO2 top layer transforms into anatase even at that temperature because of the influence of N incorporation into the TiO2 lattice. The presence of TiN during deposition and its transformation during annealing appear to affect the crystallization kinetics of TiO2, leading to notable changes in surface texture that could influence optical, electrical, or catalytic properties.
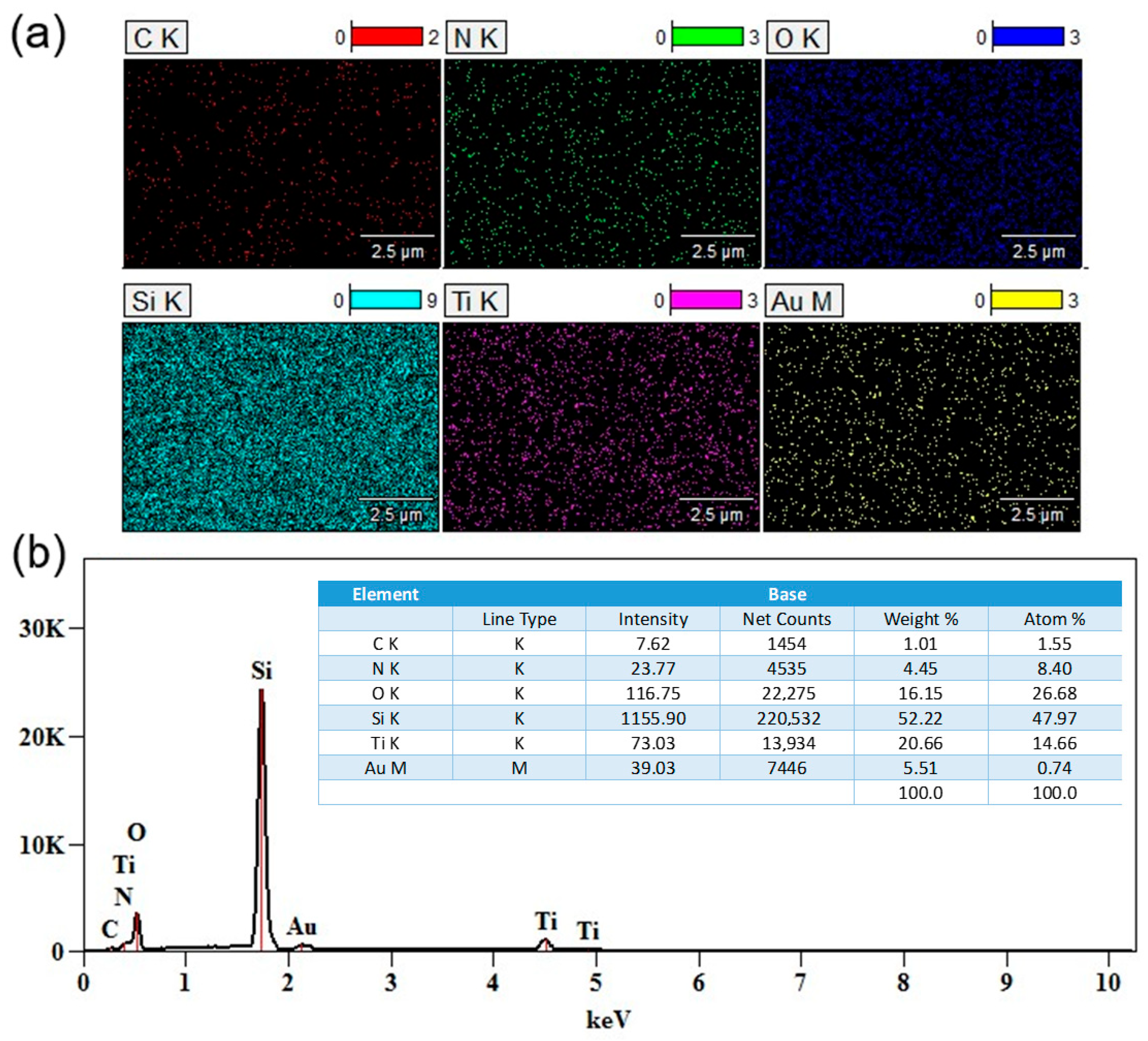
Energy-dispersive X-ray spectroscopy (EDS) mapping confirmed the presence and distribution of the main elements in the obtained TiO
2/TiN–Au bilayer systems.
Figure 3a shows the elemental mapping of Si, Ti, O, N, C, and Au, with different colors representing each element. The Au signal appears to be homogeneously distributed, which indicates the presence of Au on the subsurface in the form of an ultrathin film. A more detailed analysis of the elemental weight and atomic percentages is presented in the EDS spectrum shown in
Figure 3b, where Au incorporation is measured at approximately 0.74 at%.
2.2. Surface Chemical Composition
Understanding the chemical state of elements in thin films is essential for explaining both light absorption in the visible spectrum and photocatalytic activity. These properties in TiO
2 thin films depend on the introduced chemical species, particularly nitrogen atoms, and their corresponding states after doping. Additionally, the chemical state is strongly influenced by the preparation methods, which will be discussed in this section. The survey spectra of all the samples show the presence of Ti 2s, 2p, 3s, and 3p lines, O 1s and 2s lines, and N 1s from the samples, along with a C 1s signal attributed to organic contamination after deposition, as seen in
Figure 4. Additionally, on survey spectra for Au-buffered thin films, the presence of Au 4d and Au 4f lines was detected.
Table 1.
XPS composition of the surface for TiO2/TiN composites.
Table 1.
XPS composition of the surface for TiO2/TiN composites.
| Sample | TiO2/TiN [nm] | Ti 2p
[at%] | O 1s
[at%] | Ov
[%] | N 1s
[at%] | Nsub [%] | Nint [%] | Nch [%] |
|---|
| N0 | 0/100 | 26.5 | 69.9 | 15.4 | 3.6 | 16.7 | 14.1 | 69.2 |
| N25 | 25/100 | 27.1 | 70.1 | 9.2 | 2.8 | 31.7 | 23.6 | 44.7 |
| N50 | 50/50 | 26 | 72.5 | 8.8 | 1.5 | 16.7 | 14.5 | 68.8 |
| N75 | 75/25 | 24.8 | 74.4 | 8.7 | 0.8 | 14 | 22.2 | 63.8 |
From survey spectra, nitrogen incorporation of 3.6 at% was observed on the surface of the pure TiO
2 sample. This value decreased gradually to 0.77 at% as the TiN contribution increased in the samples (see
Table 1). We have previously described the phenomenon of nitrogen incorporation on the surface of TiO
2 during the annealing process in air, in our earlier work [
17], and the underlying mechanism is detailed by Guillen et al. [
18].
Table 1 presents the atomic percentages of Ti, N, and O derived from survey spectra. Additionally, percentages of oxygen vacancies and nitrogen incorporation sites, calculated from high-resolution spectra, are included. Oxygen vacancy concentrations were determined from the contribution areas in deconvoluted high-resolution O 1s spectra (
Figure S1), while nitrogen contributions were derived from high-resolution N 1s spectra (Figure 6). The observed concentration of oxygen atoms is higher than expected for stoichiometric TiO
2 layers. This is attributed to the presence of C-O and C=O bonds on the surface, originating from adventitious carbon (
Figure S2).
The high-resolution XPS spectra of the Ti 2p photoelectron line, shown in
Figure 5, were analyzed for all samples, revealing a Ti 2p doublet with binding energies of 464.2 ± 0.2 eV and 458.5 ± 0.2 eV. Orbital splitting of 5.7 eV and the position of the peaks corelate to Ti
4+ contribution [
39]. It is evident that nitrogen doping does not cause any significant shift in the binding energy of the Ti 2p peaks across the samples, which implies no formation of Ti-N bonds on the surface, for which concentration of N atoms on the surface is crucial.
A well-documented fact is that exposing TiOx films to air results in surface oxidation, forming a few nanometers thick TiO
2 layer. This phenomenon is supported by studies showing that titanium oxide films readily oxidize in ambient conditions, with TiO
2 forming as the stable surface phase due to oxygen interaction, as observed by surface analysis techniques such as XPS and AES [
40,
41]. To analyze the subsurface region (>5 nm) of the TiO
2 layer, the surface of the material was sputtered with 4 KeV Ar
+ ions for 20 s. Analysis showed a non-stoichiometric structure characterized by the presence of Ti
3+ species. Sputtering parameters during preparation of all TiO
2/TiN heterostructures were optimized for obtaining TiO
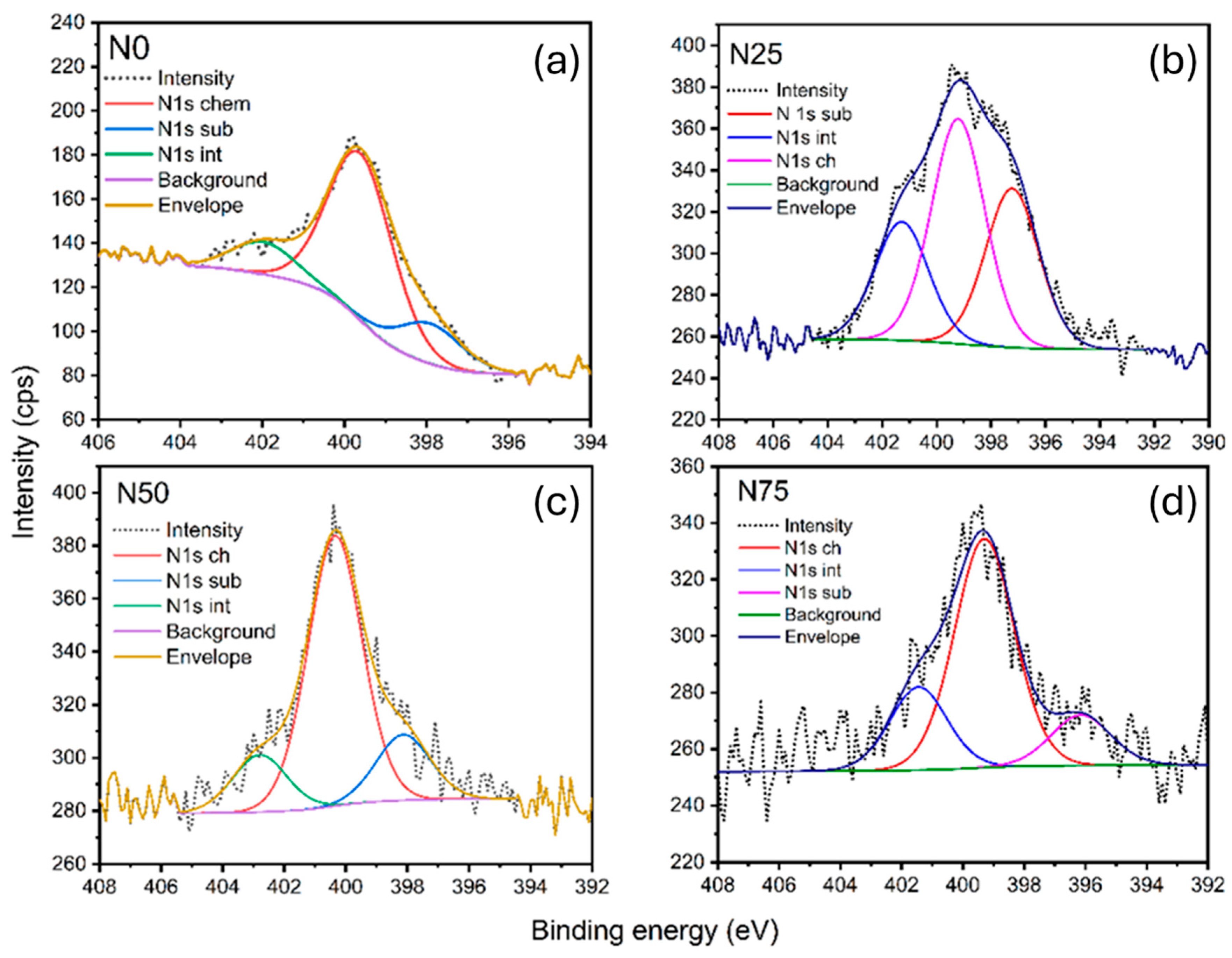
2−x films rich in oxygen vacancies to enhance nitrogen diffusion during subsequent annealing. Indeed, we have found with surface analysis that the presence of N1s bonds in annealed samples varied between 0.8 and 3.6 at%. Nitrogen was partially incorporated as substitutional in place of oxygen vacancies, while part of the nitrogen was found in interstitial positions, as can be seen in
Figure 6, where N1s high-resolution spectra for all heterostructures are presented. The concentration of substitutional nitrogen was found to be dependent on the ratio between TiN and TiO
2 layers. The most substitutional nitrogen was found in heterostructures with 25% TiN, while as the mass ratio of the TiN phase increased, the interstitial and chemiabsorbed nitrogen rate is larger. One can conclude that the smallest amount of the TiN phase was enough to fill oxygen vacancies by N diffusion in the TiO
2 lattice as substitutional nitrogen, while with further increases in the mass ratio of TiN, the interstitial and chemisorbed portions of nitrogen at the surface grow.
The inverse relationship between TiN content and surface nitrogen concentration occurs because higher TiN portions lead to more extensive thermal oxidation at 600 °C, which releases nitrogen as gas through newly formed pores and voids rather than allowing it to accumulate at the surface. The pure TiO2 sample shows the highest N1s concentration because it lacks the nitrogen-depleting oxidation mechanism present in bi-layer systems and instead retains nitrogen from ambient adsorption or initial contamination during the annealing process. These conclusions are in agreement with observed TEM and SEM analysis.
The high-resolution XPS spectra of O 1s for all samples reveal a peak that can be deconvoluted into three to four components, as shown in
Figure S1 in the Supplementary Materials. The most intense peak, located at 529.9 ± 0.1 eV, corresponds to oxygen in the TiO
2 lattice. The component at 531.2 ± 0.2 eV is attributed to oxygen vacancies and/or adsorbed hydroxyl (–OH) groups on the metal oxide surface. Analysis of the intensity of this peak demonstrates a significant decrease between pristine TiO
2 film and films with TiN as a substrate. This reduction may occur from nitrogen diffusion leading to a decrease in oxygen vacancies, either through substitutional doping of nitrogen atoms or by compensating electron-deficient vacancies with interstitial nitrogen. While N-doping is often suggested to induce oxygen vacancies [
42], there is also evidence that post-annealing nitridation of TiO
2 can reduce surface oxygen vacancies [
43]. In the case presented here, increasing the mass percentage of TiN in the composite material did not affect noticeable O
v concentrations, confirming once again that the lowest concentration of TiN, as a substrate, was enough to fill oxygen vacancy spots in the TiO
2 lattice and further increases of the TiN component affected only Nint and Nch concentrations and overall N presence on the surface. The remaining two components on O1s spectra above 532 eV are attributed to the presence of carbon contamination species and adsorbed water.
2.3. Optical Properties
The efficiency of TiO
2 thin films for photocatalytic applications strongly depends on their optical properties, as increased light absorption can lead to a higher generation of electron-hole pairs [
44]. UV-Vis spectroscopy was used to measure the transmission of TiO
2/TiN thin films with various contributions of TiN and TiO
2 after annealing. In samples N0, N25, N50, and N75, the percentage of TiN contribution increases, and the absorption edge shifts to a longer wavelength.
In
Figure 7a, the transmittance of the samples measured in the UV-Vis wavelength range (280–800 nm) is shown. The N25 and N75 samples exhibit high transmittance (65–85%), while the N50 sample shows a drop to 50–60% within the 450–600 nm range. The reduced transmittance of the N50 and N75 samples in the 450–600 nm range can be attributed to their higher optical absorption, likely due to a favorable density of defect states and more effective nitrogen incorporation and achieving a balance between interstitial and substitutional nitrogen species within the TiO
2 lattice and the formation of new sub-bandgap states [
45]. Outside this range, the transmittance of the N50 sample aligns with that of N25 and N75. These results are for annealed samples, and it is worth mentioning that they show an increase in optical transmittance compared to the non-annealed samples (~10–40%). However, since the non-annealed samples were not used in the further experiments, their data are not presented.
The bandgap energy (
) of a semiconductor represents the minimum energy needed to excite an electron from the valence band (VB) to the conduction band (CB). The bandgap plays a crucial role in determining the ability of a material to absorb light and initiate photocatalytic reactions. Doping TiO
2 with N atoms has been shown to affect the bandgap, which can also be modified by annealing N-doped TiO
2 thin films [
18]. The bandgap value is estimated from a Tauc plot using the relation:
Here,
represents the optical absorption coefficient, calculated using the equation
, where d denotes the thickness of the thin films, and T is the transmittance. The plot of
as a function of photon energy
is presented in
Figure 7b. The values of
were obtained by extrapolating the linear part of the graph down to the photon energy axis. From
Figure 7b, it can be seen that increasing the TiN content leads to a slight reduction in the band gap, from 3.40 eV for N25 to 3.26 eV for N75. Additionally, in the absorption edge region of all samples, two distinct slopes are observed, indicating the presence of additional energy levels within the forbidden band gap of the TiN/TiO
2 samples. These additional energy levels are located at 3.21 eV, 2.77 eV, and 2.57 eV for N25, N50, and N75, respectively.
To complement the optical analyses, we also observed the effect of the TiO
2/TiN ratio on the optical constants n and k, which affect overall optical properties. Detailed analyses and conclusions can be found in
Supplementary Materials.
2.4. Contact-Angle Measurements
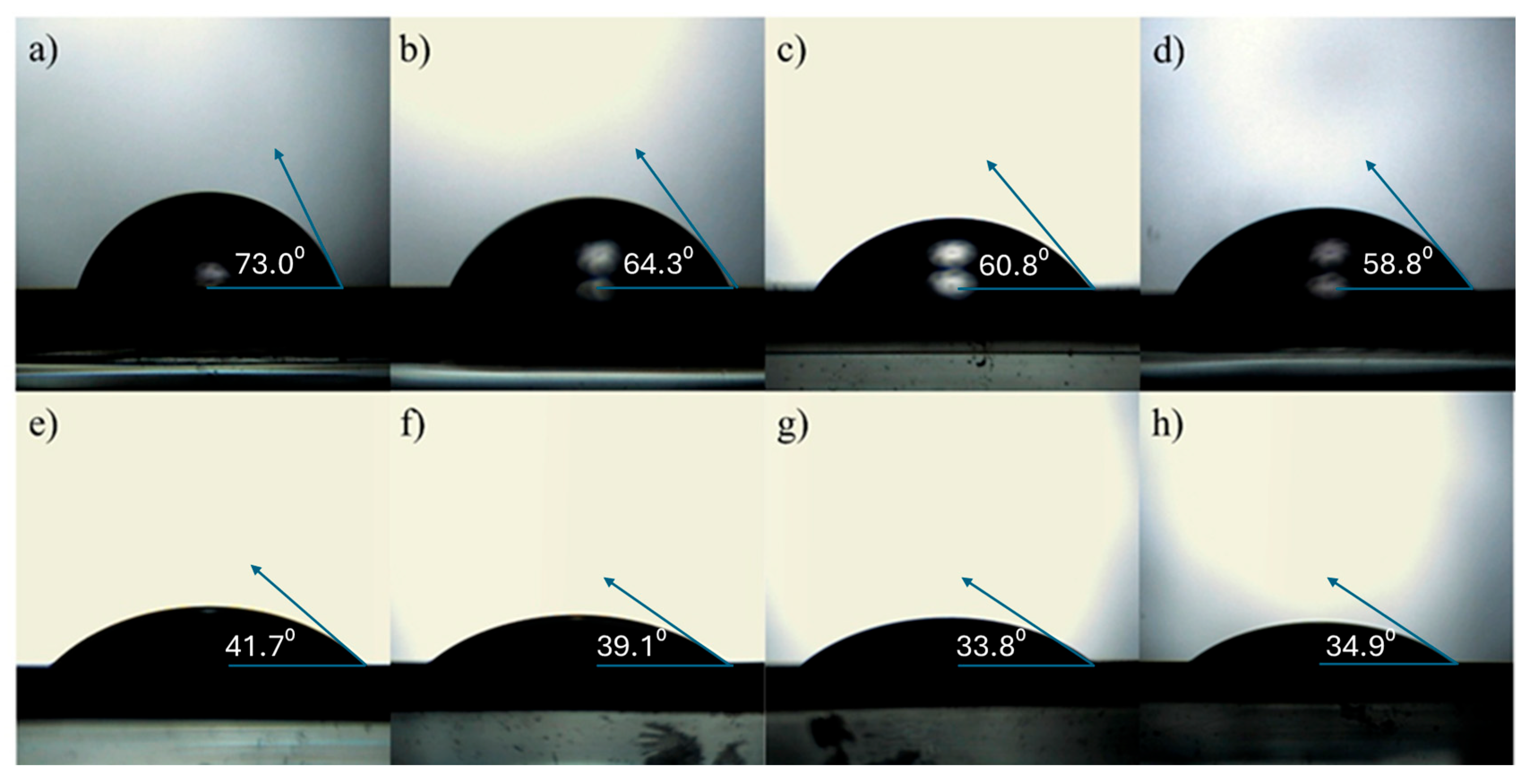
Surface free energy (SFE) can be defined as the excess energy of the surface compared to the bulk of the material. In a practical sense, it characterizes the interaction of the material’s surface with different liquids. Using both deionized water and diiodomethane as reference liquids, as shown in
Figure 8, for a–d for water and e–h for diiodomethane, for contact angle measurements, it was found that the water contact angle decreases for samples with higher TiN content.
This trend indicates an increase in surface hydrophilicity, which is favorable for photocatalytic applications. These findings are consistent with previous reports on N-doped TiO
2 surfaces [
46], where a strong correlation between photocatalytic activity and surface wettability has been established. A more hydrophilic surface can provide additional active sites for pollutant adsorption and facilitate photocatalytic degradation. Both the wettability and photocatalytic activity of TiO
2 films are known to be influenced by film morphology, chemical composition, and the presence of surface defects, all of which are dependent on the deposition and post-treatment conditions. The influence of surface nitrogen content on the SFE of the films is summarized in
Table 2.
The increase, from 6.3 for N0 to 12.3 for N75, in the polar component of the SFE for samples containing TiN indicates that the surface becomes more hydrophilic compared to unmodified TiO2. The dispersive component also slightly increased with increasing TiN content. This change suggests a higher presence of surface hydroxyl groups and/or nitrogen-containing species. Enhanced surface hydrophilicity is beneficial for photocatalytic activity, as it facilitates stronger interactions between water-dissolved pollutants (such as MB) and the surface while also promoting more efficient generation of reactive oxygen species (e.g., •OH).
2.5. Photocatalytic Measurements
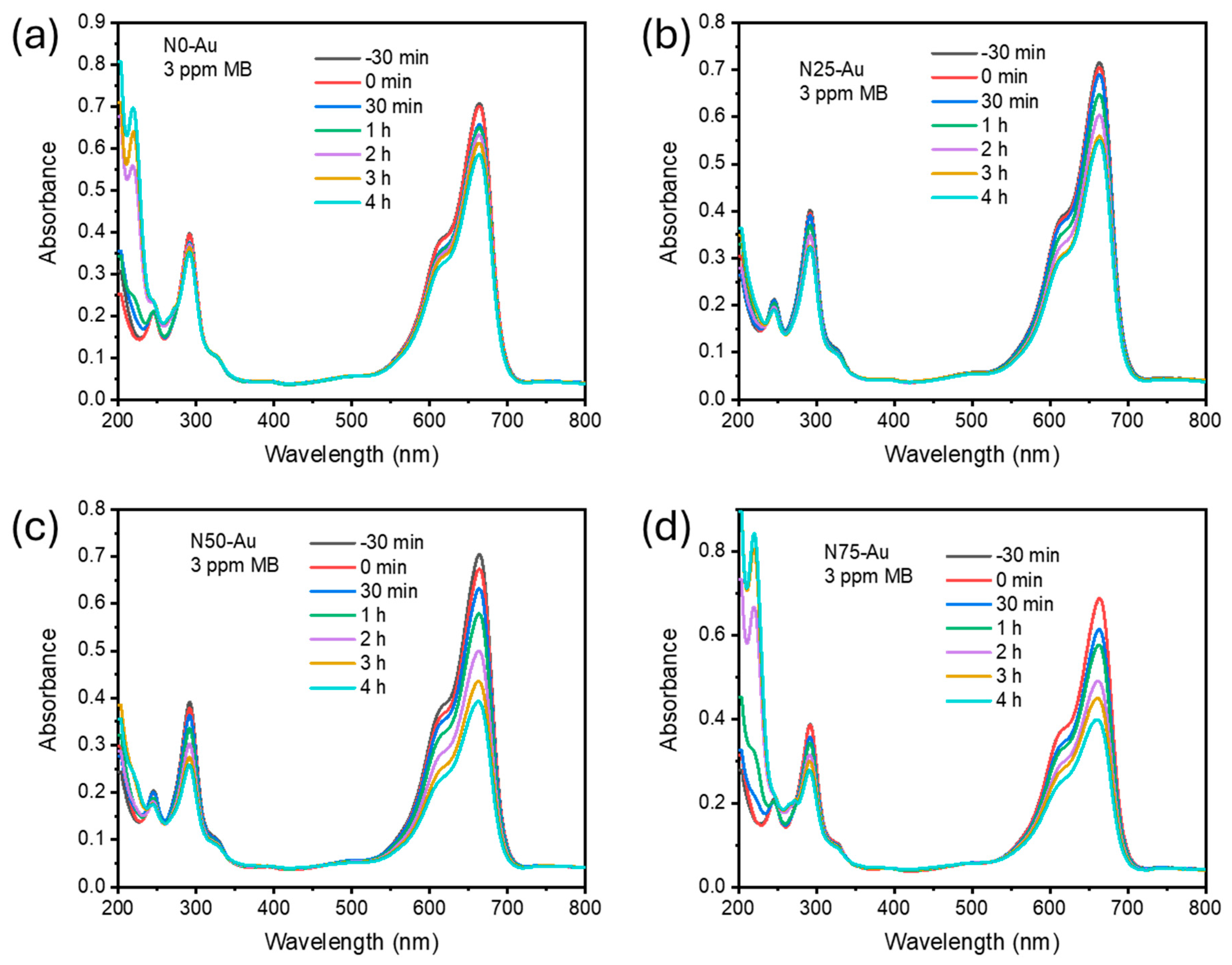
The photocatalytic activity (PA) of TiN/TiO
2 thin films was studied to assess the effect of nitrogen doping on the surface of TiO
2. The degradation of MB dye was monitored using UV-Vis spectroscopy by measuring the absorbance in the wavelength range of 200–800 nm. The first aliquot was collected immediately after immersing the film sample in the MB dye solution. A second aliquot was taken after 30 min in the dark to ensure adsorption-desorption equilibrium. Subsequent aliquots were collected at 0.5, 1, 2, 3, and 4 h during light exposure. The absorbance spectra of all TiN/TiO
2 samples are presented in
Figure 9. A characteristic absorbance peak of MB dye at 663 nm is observed, which decreases over time with illumination. Although the peak intensity diminishes with increasing exposure time, the characteristic shape of the peak remains unchanged. To compare the PA of different samples, the graph on the left side in Figure 11 presents the absorbance ratio (C/C
0) for N0, N25, N50, and N75 measured at 663 nm as a function of illumination time.
From the comparative kinetic curves in Figure 11a, it is clearly observed that all samples exhibit some degree of photocatalytic activity and that the incorporation of nitrogen (via TiN) into TiO2 thin films can enhance the PA by up to 30%. However, it is also evident that nitrogen incorporation does not necessarily guarantee improved performance. For instance, the N25 sample, which contains the lowest amount of TiN, showed no significant improvement in PA compared to pure TiO2. This is despite the presence of both substitutional and interstitial nitrogen species on the surface, as confirmed by XPS analysis.
In contrast, the N50 and N75 samples demonstrated enhanced photocatalytic performance, clearly surpassing that of pure TiO2. This suggests that nitrogen incorporation can lead to increased photocatalytic activity, but only when it is effectively integrated into the structure. The observed improvement in PA can be attributed to two main factors. Firstly, the presence of both substitutional and interstitial nitrogen in appropriate proportions can narrow the bandgap or introduce mid-gap states, thereby enhancing visible-light absorption. Secondly, the formation of a heterojunction between the conductive TiN and semiconducting TiO2 may facilitate more efficient separation of photogenerated charge carriers, thus reducing recombination losses.
Because of annealing after the deposition of TiN/TiO
2 heterostructures, nitrogen diffusion is induced, and a small amount of N is detected at the surface (as confirmed by XPS and SEM). Based on XPS analysis, nitrogen is present in interstitial and substitutional positions, along with chemisorbed N
2. These N species introduce additional energy states within the bandgap: substitutional N typically leads to shallow acceptor levels above the valence band, while interstitial N contributes to more localized mid-gap states [
42,
47]. As shown by Yang et al. [
34], such localized N 2p states can enhance visible-light absorption and photocatalytic activity at low doping levels. In our case, the thermally induced N diffusion likely contributes to bandgap narrowing and surface modification, which correlates with improved photoactivity under visible light. This supports the concept of an optimal N-doping concentration, beyond which further doping has limited benefits or even detrimental effects due to increased recombination.
Furthermore, Zeng et al. [
48] emphasized the importance of avoiding excess oxygen during synthesis in order to retain substitutional nitrogen and surface hydroxyl groups, both of which significantly enhance photocatalytic activity. Their findings also suggest that the photocatalytic activity of samples containing substitutional nitrogen is higher than that of those with predominantly interstitial nitrogen.
Interestingly, in our study, the highest photocatalytic activity was observed in the N50 sample, where the concentrations of interstitial and substitutional nitrogen are comparable to annealed TiO2 thin film (N0). This indicates that overall nitrogen concentration on the surface plays a key role in PA, while the place of their incorporation has secondary effects if the concentration of N atoms on the surface is in optimal ranges.
As shown in
Table 2, the polar component of the SFE rises from 6.3 mJ cm
−2 for N0 to 11.1 mJ cm
−2 for N50 and 12.3 mJ cm
−2 for N75, reflecting progressively greater hydrophilicity. Higher polar SFE implies a higher density of polar functional groups (–OH and nitrogen species) on the surface, which serve as active sites for MB adsorption. XPS results (
Table 1) reveal that the N50 sample combines a low oxygen-vacancy content (8.8%) with a nearly balanced ratio of substitutional (16.7%) and interstitial (14.5%) nitrogen. Our present work identifies this as optimal for bandgap narrowing and charge-separation efficiency. This synergy of surface wettability and beneficial N incorporation yields the highest MB degradation rates for N50 (Figure 11).
Although N75 has an even higher polar SFE, its much lower total surface N (0.8 at%) limits the number of truly active N-centers, so its photocatalytic rate to fall slightly below N50. Conversely, N25, while rich in surface N, exhibits lower polar SFE (10.1 mJ cm−2) and higher oxygen vacancies (9.2%), resulting in intermediate activity. N0, with the lowest polar SFE and no intentional N-doping, shows the poorest MB degradation.
Additionally, the formation of the TiO2/TiN heterostructure itself may contribute to the enhanced photocatalytic performance, suggesting that interfacial engineering combined with controlled nitrogen incorporation is essential for optimizing activity. Results of reproducibility tests, shown in Figure 11c, confirm that these materials are stable during illumination and photocatalytic degradation of MB.
Overall, the results indicate that both the chemical state of nitrogen and the structural configuration of the TiO2/TiN interface play crucial roles in determining photocatalytic efficiency. Optimal nitrogen doping and heterostructure design are therefore key to maximizing performance.
Functionalization of TiO2/TiN Thin Films with Gold
The effect of gold deposition on TiO
2/TiN thin films was examined in the second set of photocatalytic experiments, where all TiO
2/TiN heterostructures were further modified by depositing a thin gold layer (~5 nm), followed by an additional TiO
2 overlayer (~3 nm). The introduction of Au was intended to act as a co-catalyst to improve visible-light absorption and facilitate charge carrier separation. However, the photocatalytic behavior after this modification revealed notable differences among the samples. The absorption spectra of photocatalytic degradation for N0-Au, N25-Au, N50-Au, and N75-Au are presented in
Figure 10, with comparative kinetic curves presented in
Figure 11. Similar to the TiN/TiO
2 heterostructures, in the case of incorporation of Au, there are no additional peaks at lower wavelength values in the visible part of the MB absorption spectra that could be ascribed to de-ethylated forms of MB. So, we can conclude that in the systems presented here, degradation (not decolorization) of MB is the main process. Additional reproducibility measurements confirm the stability of our material, as demonstrated in
Figure 11c.
The pure TiO2 sample modified with gold (N0-Au) exhibited a decrease in photocatalytic activity compared to the unmodified N0. This suggests that in the absence of an effective heterojunction (i.e., no underlying TiN), the presence of Au may have introduced recombination centers or partially blocked active TiO2 sites, thus reducing the efficiency of the photocatalytic process. The thin TiO2 overlayer may also have been insufficient to maintain optimal photocatalytic activity in this configuration.
On the other hand, the N50-Au and N75-Au samples, which contain higher TiN content underneath the Au/TiO2 surface layers, demonstrated significantly improved photocatalytic activity. Their kinetic curves indicate very similar and enhanced performance, confirming a beneficial synergistic effect. In these cases, the TiO2/TiN heterojunction likely facilitates efficient electron transfer, while the Au layer further improves charge separation and may serve as an electron sink or plasmonic enhancer. The final TiO2 overlayer may stabilize surface reactions and preserve photocatalytic functionality.
These results indicate that the photocatalytic efficiency of TiO2-based heterostructures can be further enhanced by incorporating noble metal nanoparticles such as gold, but only in systems where the underlying structure supports efficient charge transfer. In particular, the combination of TiN (as a conductive scaffold), Au (as a plasmonic sensitizer), and TiO2 (as a photocatalytic surface) offers a promising architecture for visible-light-driven degradation of organic pollutants.
Understanding the mechanism behind this photocatalytic activity in TiO
2/TiN structures was part of one of our group’s previous works [
49]. In that work, we demonstrated through photoluminescence analysis that Au particles on the surface and near-surface regions significantly reduce electron-hole recombination in the TiN/TiO
2 systems. In
Figure S3, positions of calculated valence and conduction bands for the TiN/TiO
2 system with positions of MB highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), and possible charge transfer processes in the illuminated system are presented. In forementioned paper it was suggested that photogenerated electrons can engage in reduction processes, either directly reducing the dye or interacting with electron acceptors such as dissolved O
2, leading to the formation of superoxide radical anions (O
2−•). Concurrently, photogenerated holes can oxidize the organic molecule to generate R
+ or react with OH
− or H
2O to produce hydroxyl radicals (OH•) [
49].
Table 3 presents a comprehensive comparison of TiO
2-based and gold-enhanced TiO
2 photocatalysts, focusing on materials with similar gold content that demonstrate photocatalytic activity under UV and visible light illumination. Comparing different systems is challenging because photocatalytic activity depends on many factors, including dye concentration, light source and intensity, structural defects, gold content and morphology, illumination time, and others. Therefore, the data in this table should be interpreted with these variables in mind. The comparison includes both nanopowder and thin film formulations, evaluating their effectiveness in degrading organic pollutants through various synthesis methods such as hydrothermal synthesis, photoreduction, RF sputtering, magnetron sputtering, and sol-gel techniques. The analysis shows that nanopowders generally exhibit superior photocatalytic performance compared to thin films, with decomposition efficiencies reaching 75–80%, while thin films achieve 20–64%. The most efficient nanopowder systems include chitosan-assisted UV photoreduction and hydrothermal nanoshells, which achieve high degradation rates in relatively short times [
50,
51]. Among thin films, seven different material compositions are considered, including RF-sputtered TiO
2/WOx achieving 20% methylene blue decomposition in 9 h under visible light [
52], magnetron-sputtered TiO
2-Au with 5 nm nanoclusters reaching 40% decomposition in 6 h [
53], interfacial pyrolytic assembly with 5 nm nanoparticles with 64% decomposition in 3 h under visible light [
54], and various DC sputtering techniques yielding morphologies from buffered continuous films to nanoparticles, with decomposition rates ranging from 33% to 44.5% under solar simulation conditions [
33,
50,
55].
3. Materials and Methods
TiN/TiO
2 thin films were deposited using a DC reactive sputtering system (Balzers Sputtron II, Oerlikon Balzers Coating AG; Balzers, Liechtenstein). Four series of samples (denoted as N0, N25, N50, and N75 and detailed in
Table 1) were prepared in oxygen and nitrogen atmospheres during deposition, respectively. A high-purity titanium target (99.99%) was used for sputtering. Before starting the deposition, the base vacuum was maintained at 5 × 10
−6 Torr. The partial pressure of argon was 10
−3 Torr for all sample series, while the partial pressures of nitrogen and oxygen were kept constant at 3 × 10
−4 Torr and 6 × 10
−4 Torr, respectively. The deposition times of titanium in nitrogen and oxygen atmospheres were varied to obtain different thickness ratios of the TiN and TiO
2 layers: 0/100, 25/75, 50/50, and 75/25 nm for the N0, N25, N50, and N75 samples, respectively. The oxygen pressure during TiO
2 deposition was selected to be lower than that pressure required for stoichiometric TiO
2 formation. After deposition, all TiN and TiO
2 films had an approximate thickness of 100 nm. No substrate heating was applied during the deposition process. Following deposition, all samples were annealed at 600 °C in an air atmosphere to modify the film structure through nitrogen diffusion into the non-stoichiometric TiO
2 structure.
All thin film series were simultaneously deposited onto two types of substrates: microscope glass and Si wafers. The glass substrates were used for optical and photocatalytic measurements, as well as for determining the surface free energy of the thin films, while the Si substrates were used for structural characterization by SEM, TEM, and XPS analyses.
Microstructural analysis of as-deposited thin films was completed on an FEI Talos F200X microscope (Thermo Fisher Scientific, Waltham, MA, USA) equipped with an X-FEG source, operating at 200 kV. Conventional and high-resolution transmission electron microscopy (TEM and HRTEM) were carried out along with the selected area diffraction (SAED) method combined with Fast Fourier Transform (FFT) analysis. The samples were prepared in the form of an electron-transparent lamella, using focused ion beam (FIB) on an FEI SCIOS2 Dual beam microscope. Study of the micrographs and determination of the interplanar spacing of the characteristic crystalline planes were completed by using ImageJ software version 1.54g (National Institutes of Health (NIH), Bethesda, MD, USA).
The morphology and qualitative chemical composition of the samples were investigated using field emission scanning electron microscopy equipped with an energy-dispersive X-ray spectrometer (FESEM-EDS, FEI SCIOS 2 Dual Beam, Hillsboro, OR, USA). All measurements were conducted under high vacuum conditions at an acceleration voltage of 20 kV.
The chemical composition of the samples was analyzed using X-ray photoelectron spectroscopy (XPS) with a SPECS system equipped with an XP50M X-ray source (for Focus 500) and a PHOIBOS 100/150 analyzer (SPECS Gmbh, Berlin, Germany). For this study, an Al Kα source (1486.74 eV) at 12.5 kV and 32 mA was used. Survey spectra were recorded over the 0–800 eV binding energy range using a constant pass energy of 40 eV, a step size of 0.5 eV, and a dwell time of 0.2 s in fixed analyzer transmission (FAT) mode. High-resolution spectra for C 1s, N 1s, O 1s, and Ti 2p regions were acquired with a pass energy of 20 eV, a step size of 0.1 eV, and a dwell time of 2 s, also in FAT mode. Spectra were obtained at a pressure of 5 × 10−8 Pa. All the peak positions were referenced to C 1s at 284.8 eV.
UV-Vis measurements were performed using a UV-2600i spectrophotometer (Shimadzu, Kyoto, Japan). Transmittance spectra were recorded in the wavelength range of 200–800 nm at a scanning speed of 300 nm/min, using bare microscope glass as the baseline reference.
Wettability measurements were performed using a device equipped with a CCD camera and a blue LED lamp (Kingbright Electronic Co., Ltd., Taipei, Taiwan) for sample illumination. All samples were evaluated under identical conditions. SFE of our samples was calculated using the Owens–Wendt 2 liquids model [
56]. Deionized water (polar liquid) and diiodomethane (non-polar liquid) were used as reference liquids for contact angle measurements. Contact angles were determined by applying three droplets of each liquid to the sample surface. According to this model, the SFE (
γs) can be expressed as a sum of 2 components, dispersive
γsd (Lifshitz-Van der Waals interactions) and polar
γsp (hydrogen bonding):
γs =
γsp +
γsd. The adhesion between a liquid and a solid material is related to the contact angle between the droplet and the surface of a material,
θ, by the following equation:
), where
γL is the liquid surface energy,
is the dispersive component of the liquid surface energy, and
is the polar component of the liquid surface energy. The values of the components of surface energy for the test liquids are known from the literature, so that by measuring contact angles of two different liquids, two equations could be expressed, and values of
γsp and
γsd retrieved from them, as well as the value of
γs.
To evaluate the photocatalytic degradation efficiency of the synthesized thin films, 50 mL of 3 ppm methylene blue (MB) solution in distilled water was prepared; the solution pH was 6.2. Thin films with dimensions of 2.5 cm × 2.5 cm were immersed in this solution, which was then exposed to simulated solar light using an Osram Vitalux lamp, Osram GmbH, Munich, Germany (300 W, simulating sunlight). The light source, positioned 30 cm above the solution, emitted white light, including UVB radiation (280–315 nm, 3.0 W) and UVA radiation (315–400 nm, 13.6 W), with the remaining spectrum comprising visible light and infrared. The MB solution was kept at the constant temperature of 8 °C. Aliquots of 2 cm3 were collected at regular intervals, and the absorption spectra of the extracted samples were recorded.