Photocatalytic Degradation of Environmental Contaminants: Transformation Products and Effects on Photocatalytic Performance
Abstract
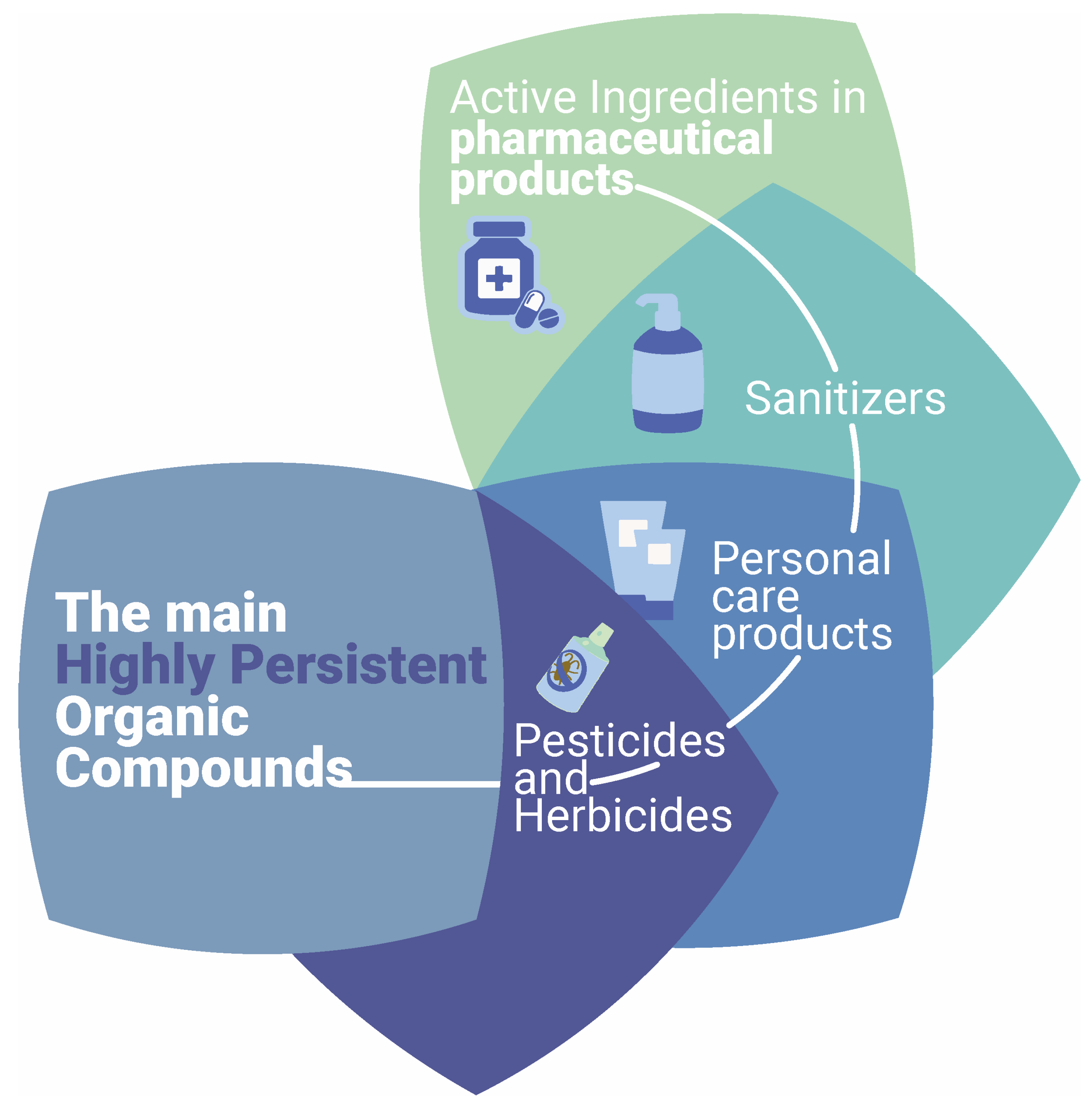
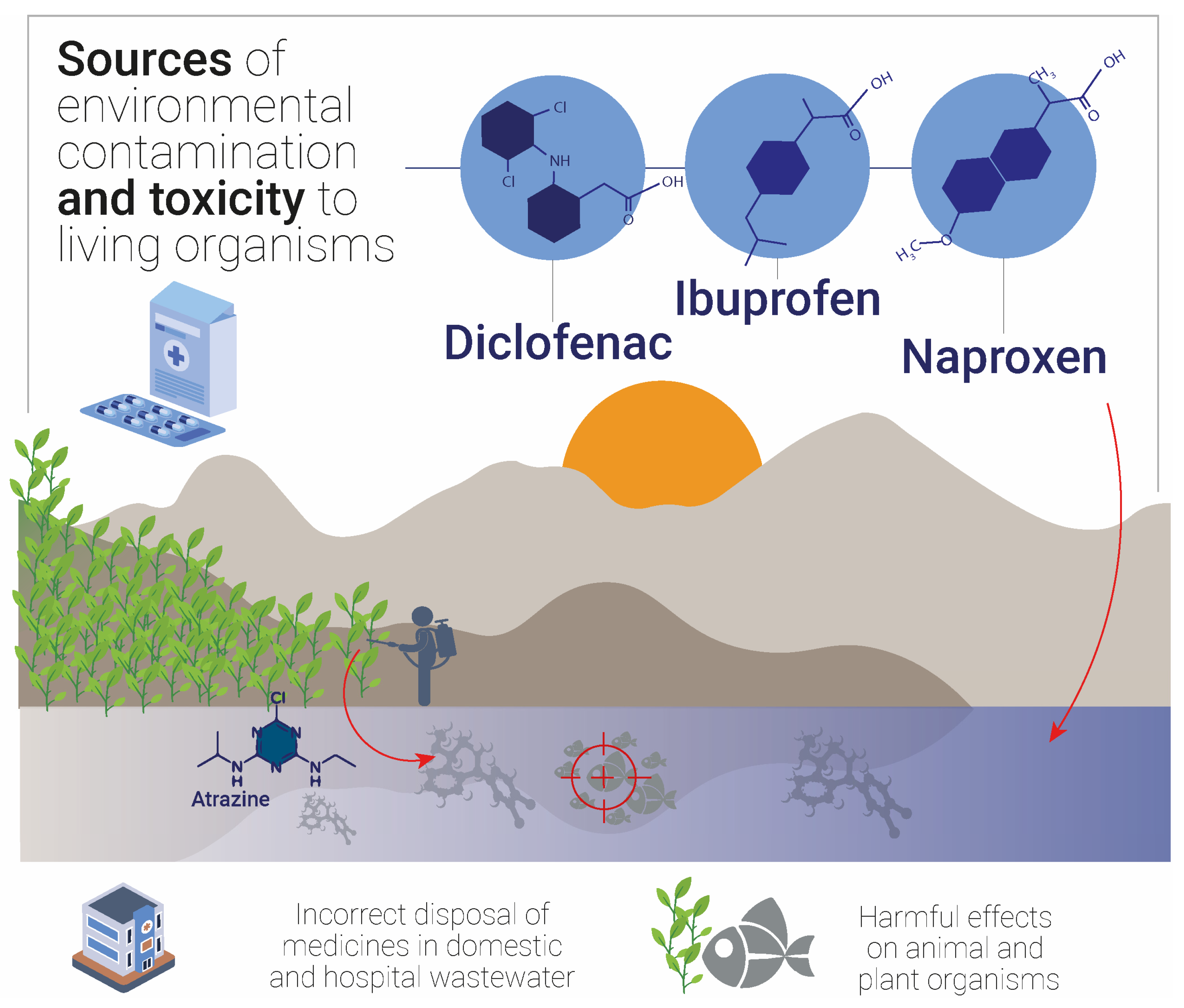
1. Introduction
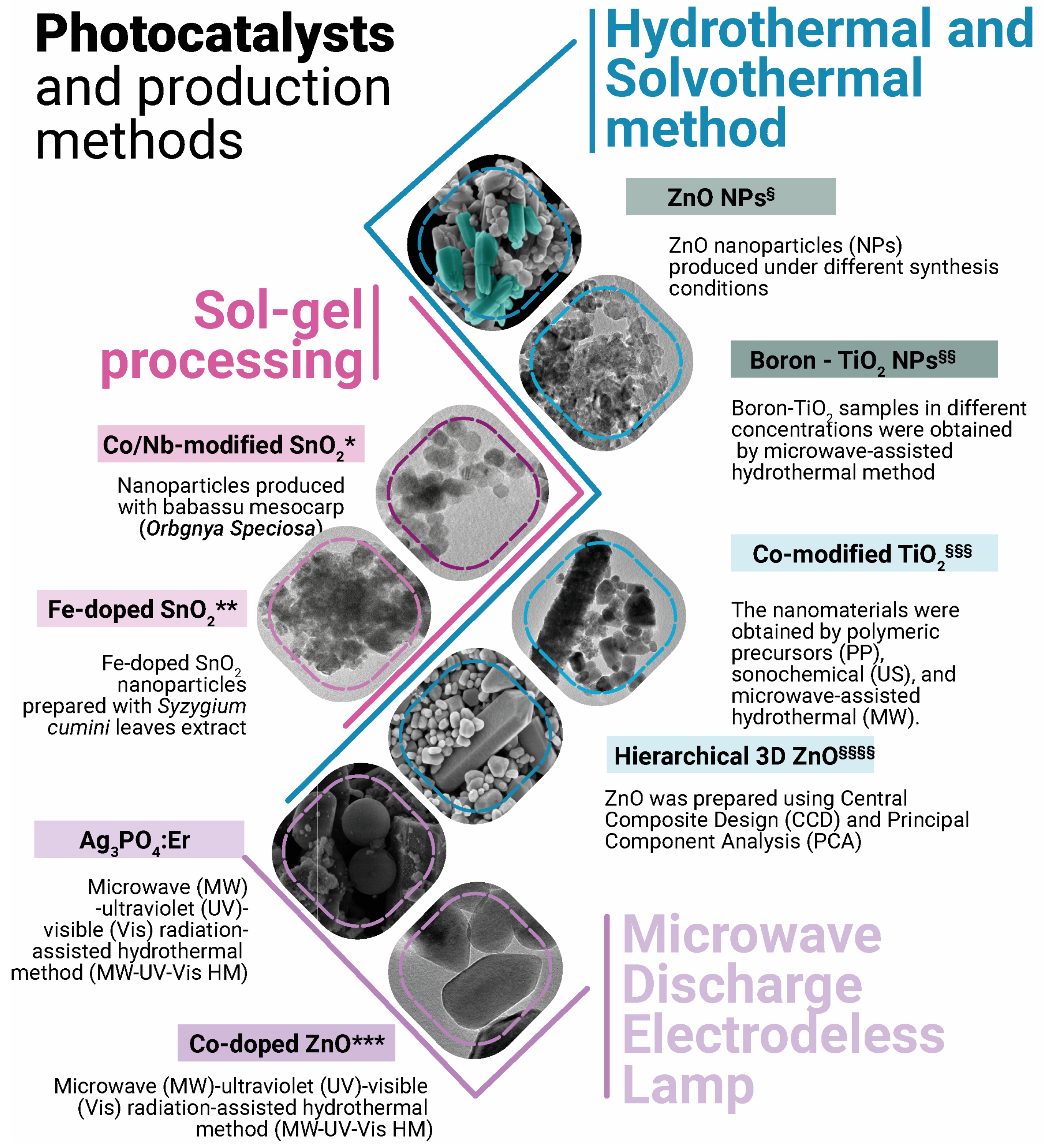
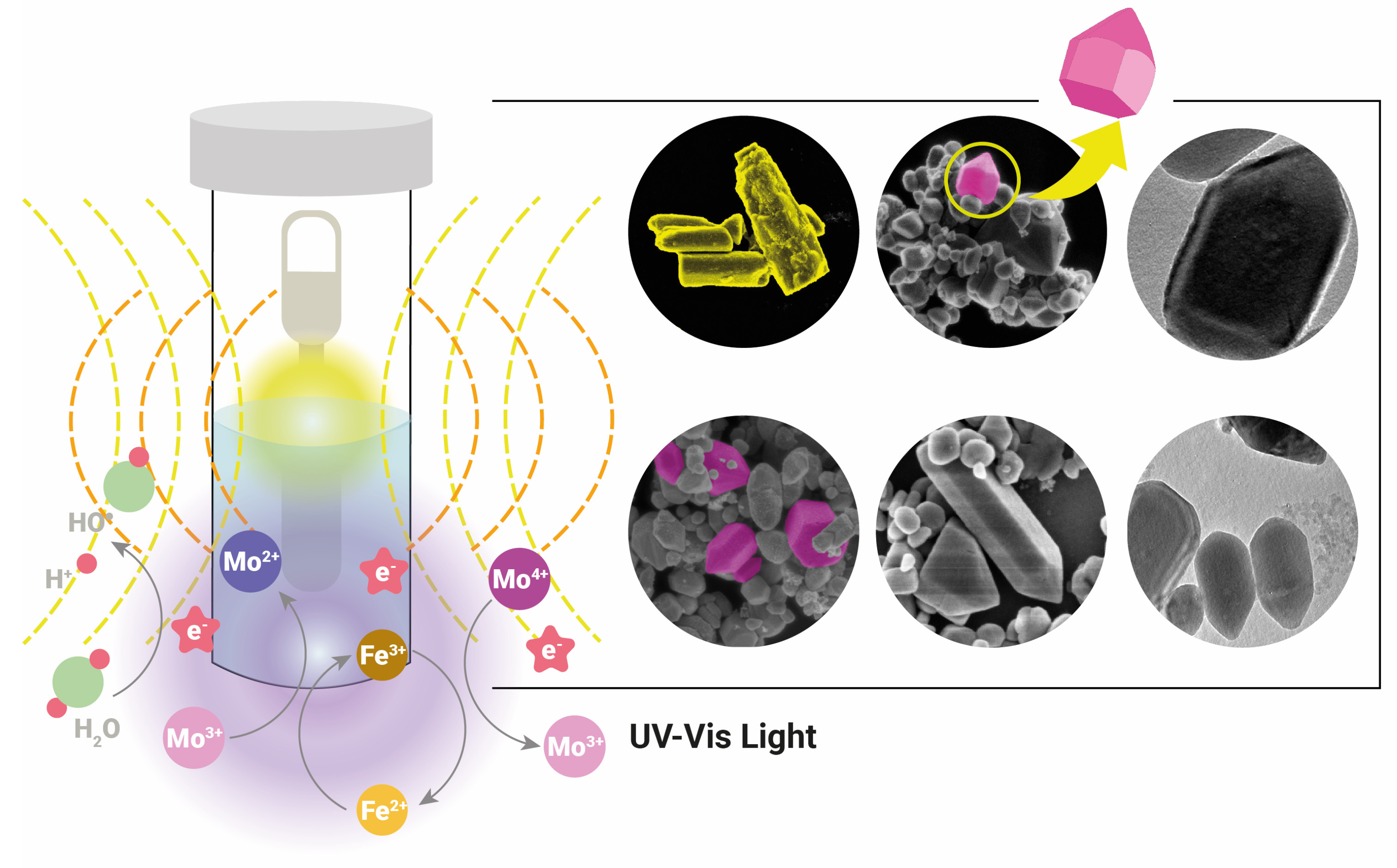
2. Photocatalysts, Synthesis, and Reaction Mechanisms
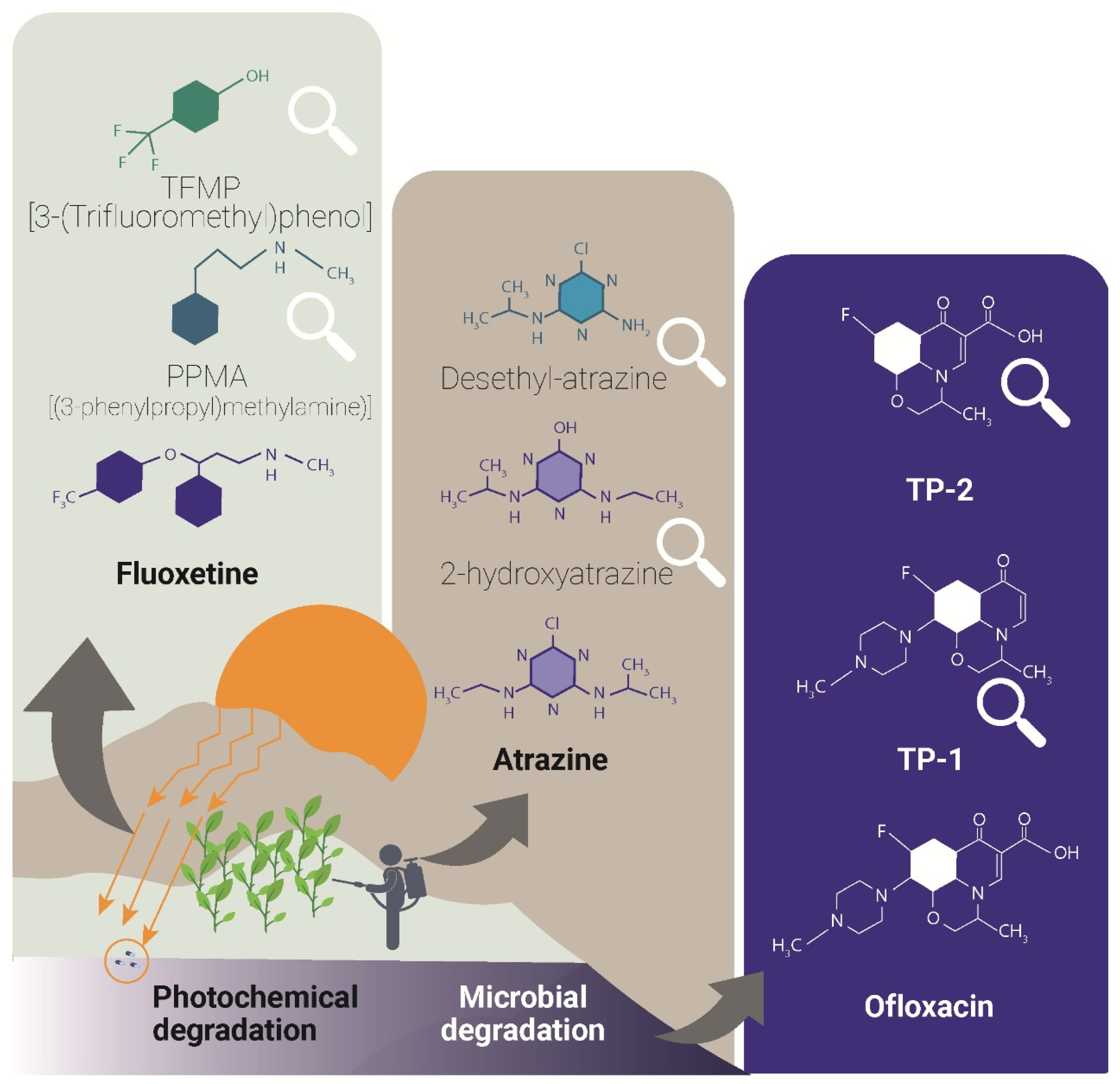
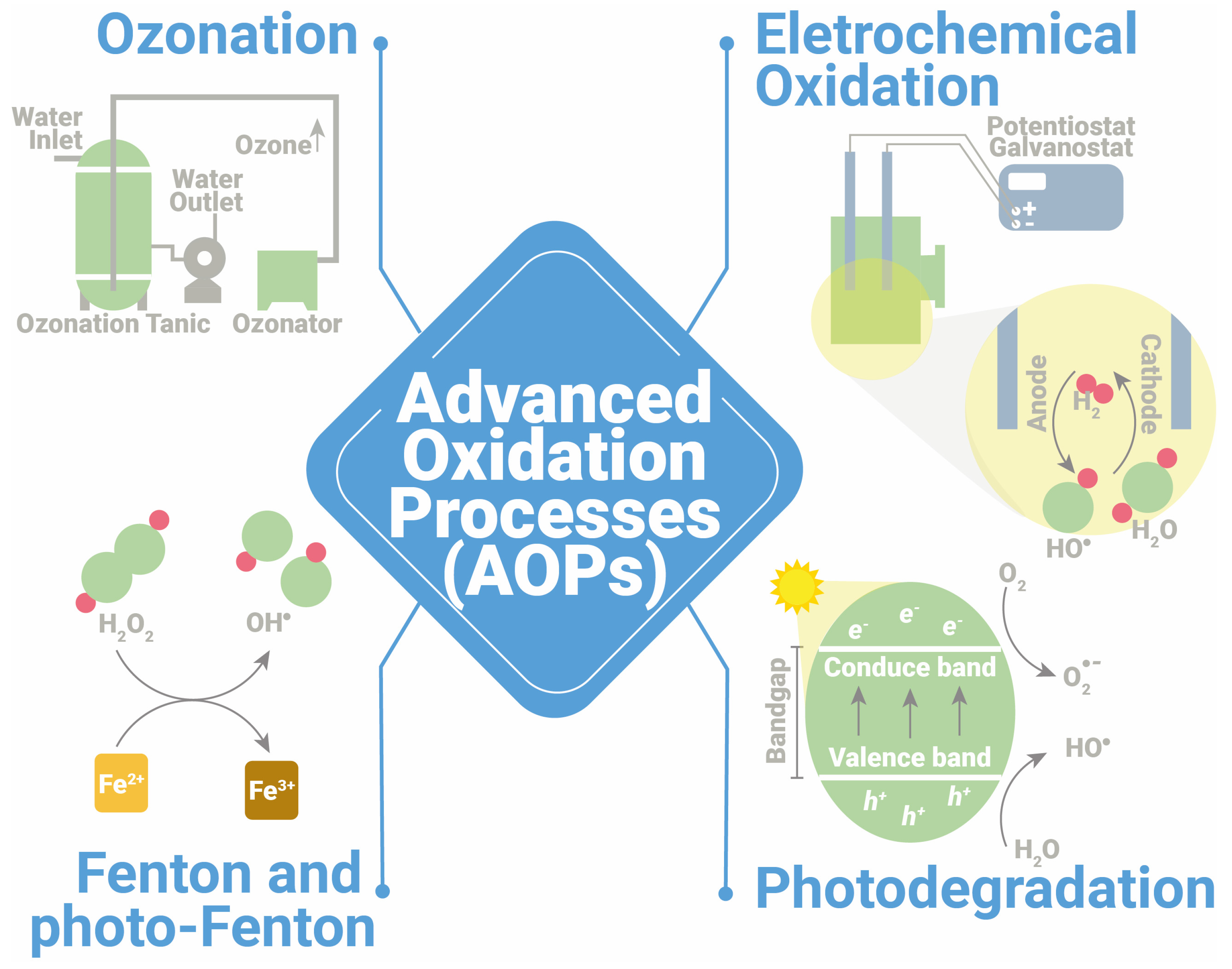
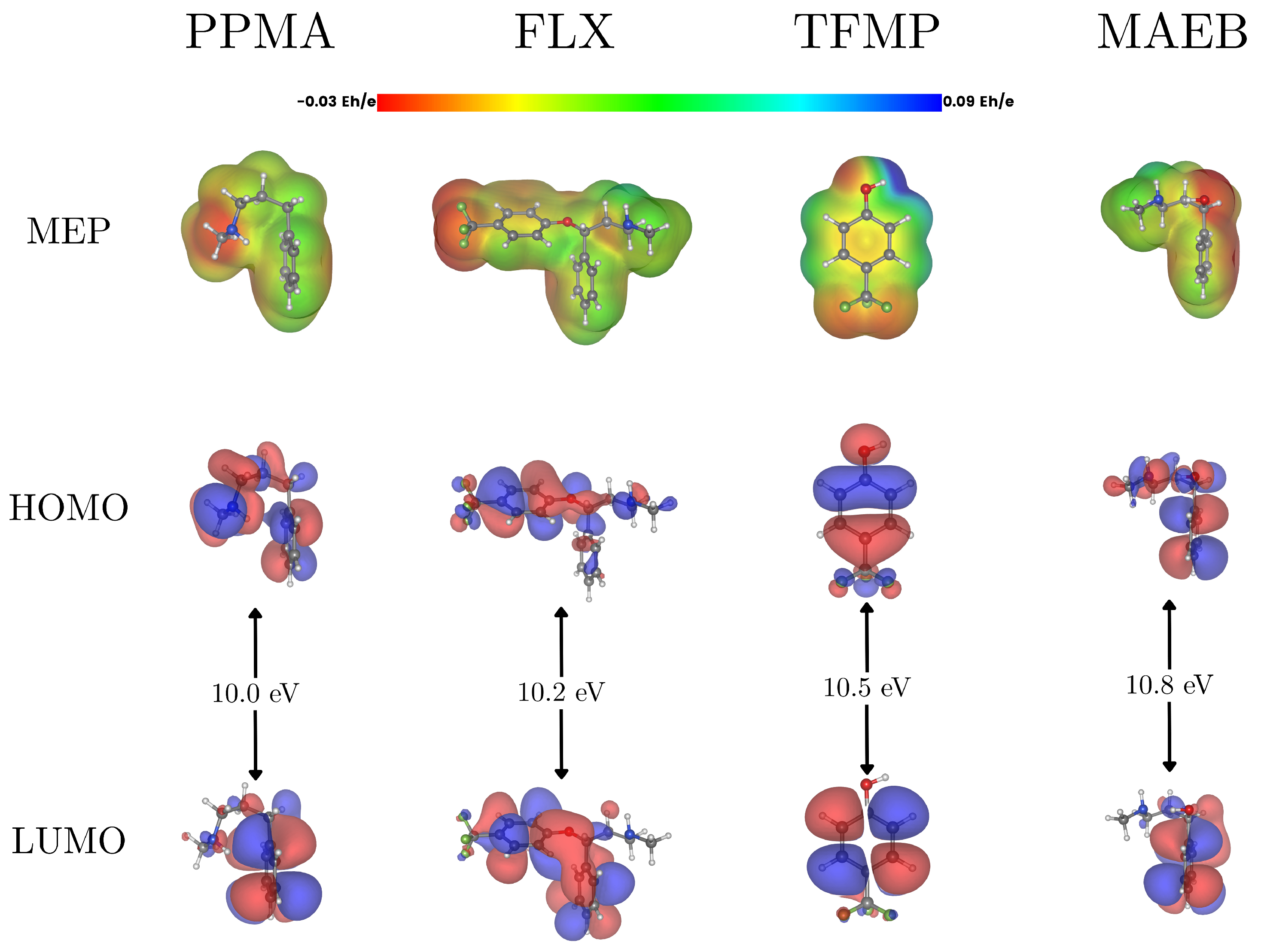
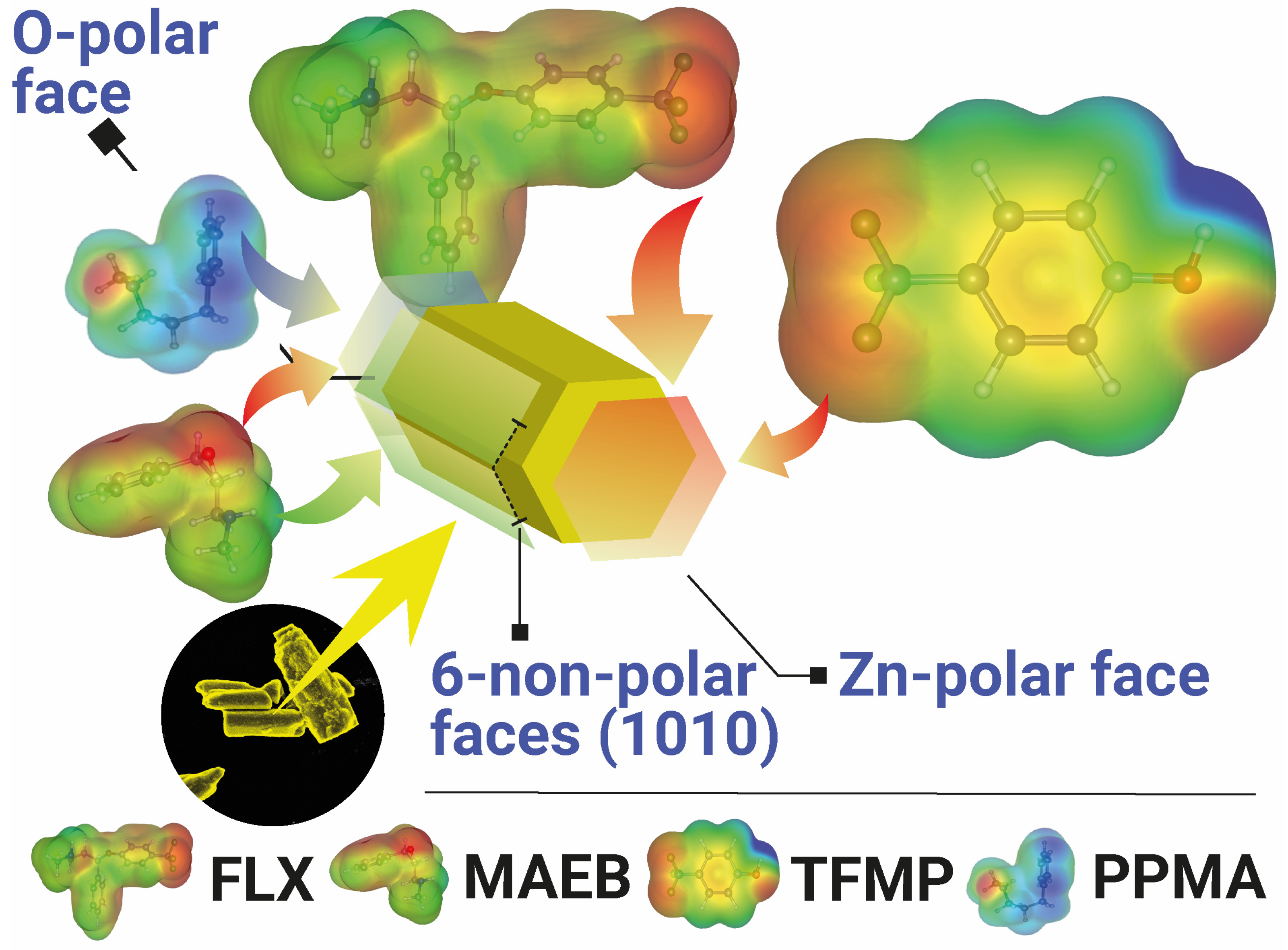
3. Photocatalytic Degradation: Performance and Degradation Mechanisms
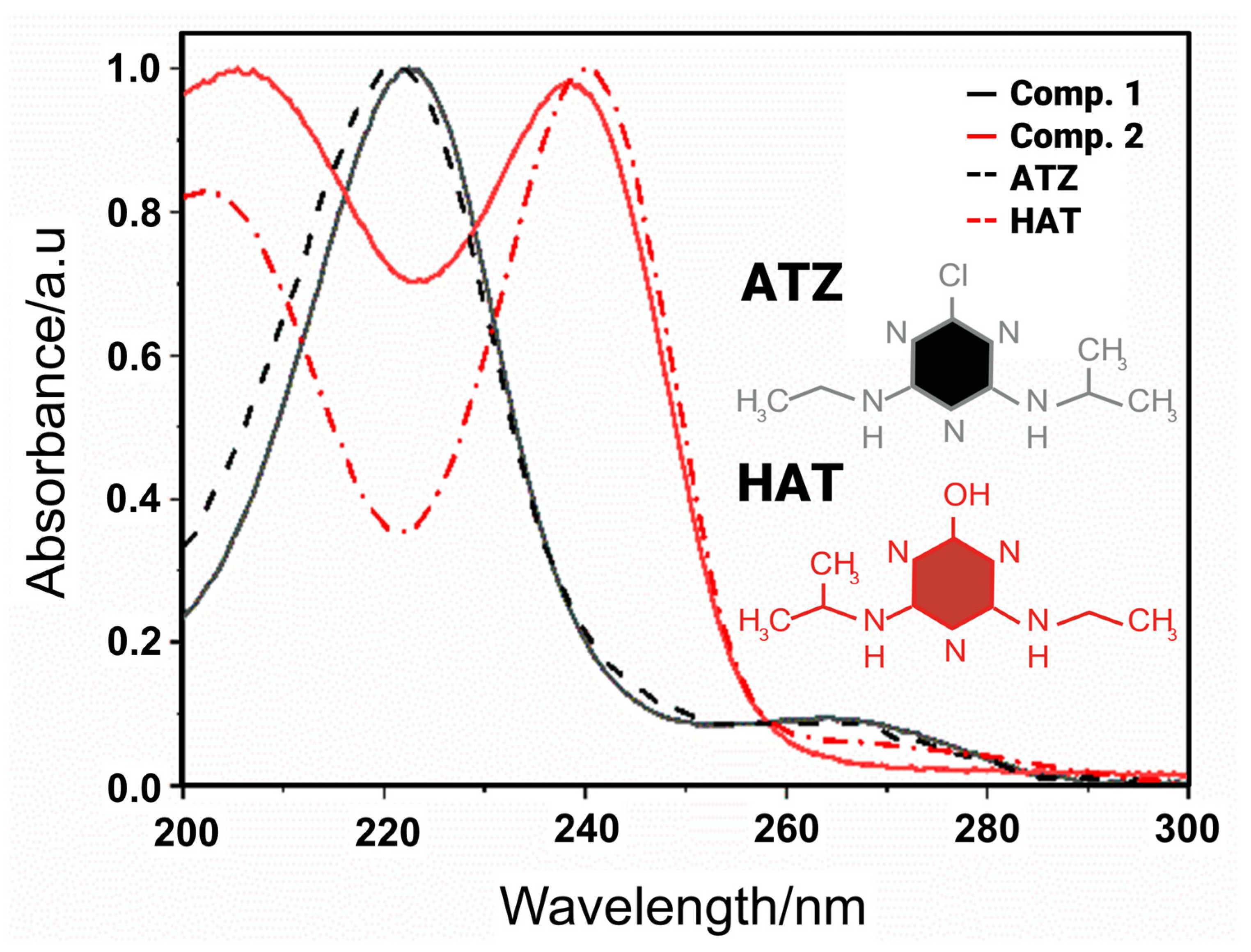
4. Higher-Order Calibration: An Alternative for Monitoring TP in Photodegradation Reactions
5. Challenges and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Sonne, C.; Bank, M.S.; Jenssen, B.M.; Cieseielski, T.M.; Rinklebe, J.; Lam, S.S.; Hansen, M.; Bossi, R.; Gustavson, K.; Dietz, R. PFAS Pollution Threatens Ecosystems Worldwide. Science (1979) 2023, 379, 887–888. [Google Scholar] [CrossRef] [PubMed]
- Sabri, N.A.A.; Razak, M.R.; Aris, A.Z. Fate of Microplastics and Emerging Contaminants: Mechanisms of Interactions, Bioaccumulation and Combined Toxicity to Aquatic Organisms. Mar. Pollut. Bull. 2025, 214, 117822. [Google Scholar] [CrossRef]
- Selak, A.; Reberski, J.L.; Klobučar, G. Assessing the Persistence, Mobility and Toxicity of Emerging Organic Contaminants in Croatian Karst Springs Used for Drinking Water Supply. Sci. Total Environ. 2023, 903, 166240. [Google Scholar] [CrossRef]
- Das, R.; Raj, D. Sources, Distribution, and Impacts of Emerging Contaminants—A Critical Review on Contamination of Landfill Leachate. J. Hazard. Mater. Adv. 2025, 17, 100602. [Google Scholar] [CrossRef]
- Saidulu, D.; Gupta, B.; Gupta, A.K.; Ghosal, P.S. A Review on Occurrences, Eco-Toxic Effects, and Remediation of Emerging Contaminants from Wastewater: Special Emphasis on Biological Treatment Based Hybrid Systems. J. Environ. Chem. Eng. 2021, 9, 105282. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, X.; Wang, X.; Li, Z.; Yang, L. Environmental Pollutant Exposure and Adverse Neurodevelopmental Outcomes: An Umbrella Review and Evidence Grading of Meta-Analyses. J. Hazard. Mater. 2025, 491, 137832. [Google Scholar] [CrossRef]
- Pang, L.; Li, M.; Dukureh, A.; Li, Y.; Ma, J.; Tang, Q.; Wu, W. Association between Prenatal Perfluorinated Compounds Exposure and Risk of Pregnancy Complications: A Meta-Analysis. Ecotoxicol. Environ. Saf. 2024, 272, 116017. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhu, T.; Sun, J.; Pan, X.; Li, J.; Luo, H. Emerging Organic Contaminants in Sewage Sludge: Current Status, Technological Challenges and Regulatory Perspectives. Sci. Total Environ. 2024, 955, 177234. [Google Scholar] [CrossRef]
- Puri, M.; Gandhi, K.; Kumar, M.S. Emerging Environmental Contaminants: A Global Perspective on Policies and Regulations. J. Environ. Manag. 2023, 332, 117344. [Google Scholar] [CrossRef]
- Corpa, C.; Balea, A.; Nieto, G.; Chernysh, Y.; Stejskalová, L.; Blanco, A.; Monte, M.C. Bibliometric Analysis of Emerging Contaminants and Cytostatic Compounds: Understanding the Current Challenges. J. Hazard. Mater. Adv. 2025, 17, 100538. [Google Scholar] [CrossRef]
- Meena, V.; Swami, D.; Chandel, A.; Joshi, N.; Prasher, S.O. Selected Emerging Contaminants in Water: Global Occurrence, Existing Treatment Technologies, Regulations and Associated Risk. J. Hazard. Mater. 2025, 483, 136541. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.A.; Santos, V.S.; Vizioli, B.C.; Ferreira, B.S.; Montagner, C.C. Pesticides in Rainwater: A Two-Year Occurrence Study in an Unexplored Environmental Compartment in Regions with Different Land Use in the State of São Paulo–Brazil. Chemosphere 2025, 372, 144093. [Google Scholar] [CrossRef]
- Devendrapandi, G.; Balu, R.; Ayyappan, K.; Ayyamperumal, R.; Alhammadi, S.; Lavanya, M.; Senthilkumar, R.; Karthika, P.C. Unearthing Earth’s Secrets: Exploring the Environmental Legacy of Contaminants in Soil, Water, and Sediments. Environ. Res. 2024, 249, 118246. [Google Scholar] [CrossRef]
- Jamal, E.; Reichelt-Brushett, A.; Gillmore, M.; Pearson, B.; Benkendorff, K. Pesticide Occurrence in a Subtropical Estuary, Australia: Complementary Sampling Methods. Environ. Pollut. 2024, 342, 123084. [Google Scholar] [CrossRef]
- Weng, B.; Zhang, M.; Lin, Y.; Yang, J.; Lv, J.; Han, N.; Xie, J.; Jia, H.; Su, B.-L.; Roeffaers, M.; et al. Photo-Assisted Technologies for Environmental Remediation. Nat. Rev. Clean Technol. 2025, 1, 201–215. [Google Scholar] [CrossRef]
- Ashina, C.; Pugazhenthiran, N.; Mangalaraja, R.V.; Sathishkumar, P. Review on Enhancing Solar Photocatalysis for Sustainable Degradation of Invisible Environmental Pollutants. Renew. Sustain. Energy Rev. 2025, 214, 115490. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, J.; Sun, P.; Liu, J.; Ji, Y.; Nakada, N.; Qiao, Z.; Tanaka, H.; Yang, Y. Nanomaterials for Treating Emerging Contaminants in Water by Adsorption and Photocatalysis: Systematic Review and Bibliometric Analysis. Sci. Total Environ. 2018, 627, 1253–1263. [Google Scholar] [CrossRef]
- Chen, Z.; Xia, D.; Liu, H.; Wang, R.; Huang, M.; Tang, T.; Lu, G. Tracing Contaminants of Emerging Concern and Their Transformations in the Whole Treatment Process of a Municipal Wastewater Treatment Plant Using Nontarget Screening and Molecular Networking Strategies. Water Res. 2024, 267, 122522. [Google Scholar] [CrossRef]
- Moreira, A.J.; Malafatti, J.O.D.; Giraldi, T.R.; Paris, E.C.; Pereira, E.C.; de Mendonça, V.R.; Mastelaro, V.R.; Freschi, G.P.G. Prozac® Photodegradation Mediated by Mn-Doped TiO2 Nanoparticles: Evaluation of by-Products and Mechanisms Proposal. J. Environ. Chem. Eng. 2020, 8, 104543. [Google Scholar] [CrossRef]
- Qian, Y.; Guan, L.; Ke, Y.; Wang, L.; Wang, X.; Yu, N.; Yu, Q.; Wei, S.; Geng, J. Unveiling Intricate Transformation Pathways of Emerging Contaminants During Wastewater Treatment Processes Through Simplified Network Analysis. Water Res. 2024, 253, 121299. [Google Scholar] [CrossRef] [PubMed]
- Malafatti, J.O.D.; Moreira, A.J.; Sciena, C.R.; Silva, T.E.M.; Freschi, G.P.G.; Pereira, E.C.; Paris, E.C. Prozac® Removal Promoted by HAP:Nb2O5 Nanoparticles System: By-Products, Mechanism, and Cytotoxicity Assessment. J. Environ. Chem. Eng. 2021, 9, 104820. [Google Scholar] [CrossRef]
- Moreira, A.J.; Coelho, D.; Dias, J.A.; Mascaro, L.H.; Freschi, G.P.G.; Mastelaro, V.R.; Pereira, E.C. Phase Control and Optimization of Photocatalytical Properties of Samarium Doped TiO2 Synthesized by Coupled Ultraviolet and Microwave Radiations. J. Alloys Compd. 2022, 905, 164217. [Google Scholar] [CrossRef]
- Moreira, A.J.; Dos Santos, B.R.M.; Dias, J.A.; Rabello, P.T.; Coelho, D.; Mascaro, L.H.; Freschi, G.P.G.; Gobato, Y.G.; Galeti, H.V.A.; Mastelaro, V.R.; et al. Photoactivity of Boron-or Nitrogen-Modified TiO2 for Organic Pollutants Degradation: Unveiling the Photocatalytic Mechanisms and by-Products. J. Environ. Chem. Eng. 2023, 11, 109207. [Google Scholar] [CrossRef]
- Alberto, D.; Couée, I.; Pateyron, S.; Sulmon, C.; Gouesbet, G. Low Doses of Triazine Xenobiotics Mobilize ABA and Cytokinin Regulations in a Stress- and Low-Energy-Dependent Manner. Plant Sci. 2018, 274, 8–22. [Google Scholar] [CrossRef]
- Fan, M.; Zhou, Y.; Luo, J.; Gao, Y.; Qiu, J.; Yang, X. Formation of Highly Toxic Halogenated Coupling Byproducts in UV/Chlorine Reaction of Phenols in Presence of Halides. Water Res. 2025, 284, 123981. [Google Scholar] [CrossRef]
- Sandré, F.; Duval, A.; Garrigue-Antar, L. From Pharmaceuticals to Toxic Threats: Unveiling the Impact of Furosemide and by-Products on Fish Model. Aquat. Toxicol. 2025, 286, 107455. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fu, C.; Zhang, G.; Chen, C.; Wang, X.; Wang, Y.; Liu, Y.; Xiang, Z. Disinfection By-Products of Metformin in the Environment: A Systematic Toxicity Evaluation on Gut-Liver-Brain Axis Homeostasis and Establishment of a Detection Method Based on NiFe-LDOs/N-BC Composite. Water Res. 2025, 272, 122895. [Google Scholar] [CrossRef]
- Chambial, P.; Thakur, N.; Kushawaha, J.; Kumar, R. Per- and Polyfluoroalkyl Substances in Environment and Potential Health Impacts: Sources, Remediation Treatment and Management, Policy Guidelines, Destructive Technologies, and Techno-Economic Analysis. Sci. Total Environ. 2025, 969, 178803. [Google Scholar] [CrossRef]
- Lendewig, M.; Marquez, R.; Franco, J.; Vera, R.E.; Vivas, K.A.; Forfora, N.; Venditti, R.A.; Gonzalez, R. PFAS Regulations and Economic Impact: A Review of U.S. Pulp & Paper and Textiles Industries. Chemosphere 2025, 377, 144301. [Google Scholar] [CrossRef]
- Mattoli, L.; Proietti, G.; Fodaroni, G.; Quintiero, C.M.; Burico, M.; Gianni, M.; Giovagnoni, E.; Mercati, V.; Santi, C. Suspect Screening Analysis to Improve Untargeted and Targeted UHPLC-QToF Approaches: The Biodegradability of a Proton Pump Inhibitor Medicine and a Natural Medical Device. Sci. Rep. 2024, 14, 51. [Google Scholar] [CrossRef]
- Li, Z.; Kaserzon, S.L.; Plassmann, M.M.; Sobek, A.; Gómez Ramos, M.J.; Radke, M. A Strategic Screening Approach to Identify Transformation Products of Organic Micropollutants Formed in Natural Waters. Environ. Sci. Process. Impacts 2017, 19, 488–498. [Google Scholar] [CrossRef]
- Wang, X.; Yu, N.; Jiao, Z.; Li, L.; Yu, H.; Wei, S. Machine Learning-Enhanced Molecular Network Reveals Global Exposure to Hundreds of Unknown PFAS. Sci. Adv. 2024, 10, 1039. [Google Scholar] [CrossRef]
- Anagnostopoulou, K.; Evgenidou, E.; Alampanos, V.; Lambropoulou, D.A. High-Resolution Mass Spectrometry Approaches for Screening Persistent and Mobile Organic Compounds in Wastewaters: Target Analysis, Suspect Analysis and Risk Assessment. Sci. Total Environ. 2025, 967, 178777. [Google Scholar] [CrossRef] [PubMed]
- Nováková, P.; Kodešová, R.; Fedorova, G.; Bořík, A.; Sadchenko, A.; Grabic, R. Identifying Organic Micropollutants’ Transformation Products from the Soil Dissipation Experiment by Non-Targeted High-Resolution Mass Spectrometry Approach: Can We Gain More than Transformation Product Identity? Environ. Pollut. 2024, 351, 124038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, J.; Qing, S.; Herath, T.M.; Zhao, H.; Klabklaydee, S.; Fu, Q.; Kwon, E.; Takeuchi, N.; Wang, D.; et al. Accurate Detection and High Throughput Profiling of Unknown PFAS Transformation Products for Elucidating Degradation Pathways. Water Res. 2025, 282, 123645. [Google Scholar] [CrossRef] [PubMed]
- Dudášová, S.; Wurz, J.; Berger, U.; Reemtsma, T.; Fu, Q.; Lechtenfeld, O.J. An Automated and High-Throughput Data Processing Workflow for PFAS Identification in Biota by Direct Infusion Ultra-High Resolution Mass Spectrometry. Anal. Bioanal. Chem. 2024, 416, 4833–4848. [Google Scholar] [CrossRef]
- Liberty, J.T.; Anil, A.; Ijimdiya, S.J.; Kwaji, M.J.; Ijimdiya, R.U. Harnessing the Potential of Nanostructured Materials for Sustainable Development. Nano-Struct. Nano-Objects 2024, 38, 101216. [Google Scholar] [CrossRef]
- Marques, G.N.; Moreira, A.J.; Nóbrega, E.T.D.; Braga, S.; Argentin, M.N.; da Cunha Camargo, I.L.B.; Azevedo, E.; Pereira, E.C.; Bernardi, M.I.B.; Mascaro, L.H. Selective Inhibitory Activity of Multidrug-Resistant Bacteria by Zinc Oxide Nanoparticles. J. Environ. Chem. Eng. 2024, 12, 111870. [Google Scholar] [CrossRef]
- Silva, T.E.M.; Moreira, A.J.; Nobrega, E.T.D.; Alencar, R.G.; Rabello, P.T.; Blaskievicz, S.F.; Marques, G.N.; Mascaro, L.H.; Paris, E.C.; Lemos, S.G.; et al. Hierarchical Structure of 3D ZnO Experimentally Designed to Achieve High Performance in the Sertraline Photocatalysis in Natural Waters. Chem. Eng. J. 2023, 475, 146235. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- de Oliveira, R.C.; Sousa, N.G.; da Silva, L.T.V.; do Nascimento, R.F.; Mascaro, L.H.; Casciano, P.N.S.; Oliveira, T.M.B.F.; de Lima-Neto, P.; Correia, A.N. The Role of FexCu(1−x) Electrodeposits from a Deep Eutectic Solvent in Promoting the Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2024, 93, 346–354. [Google Scholar] [CrossRef]
- Khalkhali, F.S.; Kowsari, E.; Ramakrishna, S.; Eqbalpour, M.; Gheibi, M.; Esmaili, H. A Review on the Photosensitizers Used for Enhancing the Photoelectrochemical Performance of Hydrogen Production with Emphasis on a Novel Toxicity Assessment Framework. Int. J. Hydrogen Energy 2024, 51, 990–1022. [Google Scholar] [CrossRef]
- Sivasubramanian, P.D.; Kumar, M.; Chen, C.L.; Kiran-kumar, V.S.; Samuel, M.S.; Chang, J.H. A Review of Metal Nanomaterials-Based Electrochemical Biosensors for Environmental Wastewater Monitoring and Their Remediation. Environ. Nanotechnol. Monit. Manag. 2024, 22, 101023. [Google Scholar] [CrossRef]
- Ramachandran, T.; Butt, H.; Zheng, L.; Rezeq, M. A Review of 2D Metal Boride-Derived Nanostructures: From Synthesis to Energy Storage and Conversion Applications. J. Energy Storage 2024, 99, 113425. [Google Scholar] [CrossRef]
- Yaghoubi, S.; Mousavi, S.M.; Babapoor, A.; Binazadeh, M.; Lai, C.W.; Althomali, R.H.; Rahman, M.M.; Chiang, W.H. Photocatalysts for Solar Energy Conversion: Recent Advances and Environmental Applications. Renew. Sustain. Energy Rev. 2024, 200, 114538. [Google Scholar] [CrossRef]
- Kumar, R.; Chaudhary, M.P.; Al-Ahmed, A.; Bhattacharyya, S.; von Gratowski, S.; Iqbal, J.; Inamuddin. Photo-to-Chemical Energy Transformation: Pioneering Photocatalysts, Surface and Interface Engineering. Mater. Res. Bull. 2024, 180, 113046. [Google Scholar] [CrossRef]
- Costa, M.B.; de Araújo, M.A.; Paiva, R.; Cruz, S.A.; Mascaro, L.H. Plasma Treatment of Electrodeposited Sb2Se3 Thin Films for Improvement of Solar-Driven Hydrogen Evolution Reaction. Chem. Eng. J. 2024, 485, 149526. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal Oxides Nanoparticles via Sol–Gel Method: A Review on Synthesis, Characterization and Applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal Processing of Materials: Past, Present and Future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Nobrega, E.T.D.; Araújo, K.C.D.; Moreira, A.J.; Oliveira, R.C.; da Silva, G.T.S.T.; Blaskievicz, S.F.; Soares, L.L.; Lemos, S.G.; Mascaro, L.H.; Pereira, E.C. Pure and Cobalt-Modified ZnO Nanostructures Prepared by a New Synthesis Route Applied to Environmental Remediation. J. Braz. Chem. Soc. 2024, 35, e-20240054. [Google Scholar] [CrossRef]
- Meng, L.Y.; Wang, B.; Ma, M.G.; Lin, K.L. The Progress of Microwave-Assisted Hydrothermal Method in the Synthesis of Functional Nanomaterials. Mater. Today Chem. 2016, 1, 63–83. [Google Scholar] [CrossRef]
- Lima, J.B.; Marques, G.N.; Ferreira, D.S.; Moreira, A.J.; Assis, M.; Longo, E.; Bernardi, M.I.B.; Rangel, J.H.G.; Azevedo, E.; Mascaro, L.H.; et al. Degradation of Emerging Contaminants Using Co/Nb-Modified SnO2 Nanoparticles and Produced with Babassu Mesocarp (Orbgnya Speciosa). J. Environ. Chem. Eng. 2025, 13, 115941. [Google Scholar] [CrossRef]
- da Silva Ribeiro, M.H.; Marques, G.N.; Moreira, A.J.; Oliveira, M.M.; Oliveira, R.C.; da Silva, R.T.; Krohling, A.C.; Macedo, W.A.A.; Bernardi, M.I.B.; Mascaro, L.H.; et al. Green-Assisted Synthesis of Highly Defective Nanostructured Fe-Doped SnO2: Magnetic and Photocatalytic Properties Evaluation. Acta Mater. 2024, 277, 120194. [Google Scholar] [CrossRef]
- Moreira, A.J.; Blaskievicz, S.F.; de Assis, M.; Marques, G.N.; Menezes, W.T.; Rabello, P.T.; Rabahi, C.; Gobato, Y.G.; Freschi, G.P.G.; Mascaro, L.H.; et al. Effect of Synthesis Methods of Co-Modified TiO2 in Its Photocatalytic and Bactericidal Activity. Res. Chem. Intermed. 2024, 50, 297–322. [Google Scholar] [CrossRef]
- Jasniewski, A.J.; Lee, C.C.; Ribbe, M.W.; Ribbe, M.W.; Hu, Y. Reactivity, Mechanism, and Assembly of the Alternative Nitrogenases. Chem. Rev. 2020, 120, 5107–5157. [Google Scholar] [CrossRef]
- Van Stappen, C.; Decamps, L.; Cutsail, G.E.; Bjornsson, R.; Henthorn, J.T.; Birrell, J.A.; Debeer, S. The Spectroscopy of Nitrogenases. Chem. Rev. 2020, 120, 5005–5081. [Google Scholar] [CrossRef]
- Yang, X.; Hu, J.; Wu, L.; Hou, H.; Liang, S.; Yang, J. Cooperation of Multiple Active Species Generated in Hydrogen Peroxide Activation by Iron Porphyrin for Phenolic Pollutants Degradation. Environ. Pollut. 2022, 313, 120097. [Google Scholar] [CrossRef]
- Lopes, O.F.; Paris, E.C.; Ribeiro, C. Synthesis of Nb2O5 Nanoparticles through the Oxidant Peroxide Method Applied to Organic Pollutant Photodegradation: A Mechanistic Study. Appl. Catal. B 2014, 144, 800–808. [Google Scholar] [CrossRef]
- Xu, B.J.; Wu, H.; Su, H.Z.; Lee, M.Y.; Zhang, Y.X.; Chen, Y.; Liu, M.; Wang, W.L.; Du, Y. A Novel Ultraviolet Light Source of Microwave Discharge Electrodeless Ultraviolet Lamp: Luminescence Mechanisms, Reactor Structures, and Environmental Applications. J. Clean. Prod. 2024, 436, 140706. [Google Scholar] [CrossRef]
- Xiang, J.L.; Wang, J.J.; Wu, Z.J.; Xu, B.J.; Du, H.S.; Chen, Y.; Liu, M.; Lee, M.Y.; Wang, W.L.; Du, Y. Efficient Wastewater Disinfection Using a Novel Microwave Discharge Electrodeless Ultraviolet System with Ozone at an Ultra-Low Dose. J. Hazard. Mater. 2024, 464, 133011. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.J.; Borges, A.C.; Gouvea, L.F.C.; MacLeod, T.C.O.; Freschi, G.P.G. The Process of Atrazine Degradation, Its Mechanism, and the Formation of Metabolites Using UV and UV/MW Photolysis. J. Photochem. Photobiol. A Chem. 2017, 347, 160–167. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhou, Y.Y.; Xiang, J.L.; Du, H.S.; Zhang, J.; Zheng, T.G.; Liu, M.; Ye, M.Q.; Chen, Z.; Du, Y. Disinfection of Wastewater by a Complete Equipment Based on a Novel Ultraviolet Light Source of Microwave Discharge Electrodeless Lamp: Characteristics of Bacteria Inactivation, Reactivation and Full-Scale Studies. Sci. Total Environ. 2024, 917, 170200. [Google Scholar] [CrossRef]
- Moreira, A.J.; Borges, A.C.; De Souza, B.B.; Barbosa, L.R.; De Mendonça, V.R.; Freschi, C.D.; Freschi, G.P.G. Microwave Discharge Electrodeless Mercury Lamp (Hg-MDEL): An Energetic, Mechanistic and Kinetic Approach to the Degradation of Prozac®. J. Environ. Chem. Eng. 2019, 7, 102916. [Google Scholar] [CrossRef]
- He, Y.; Yin, L.; Yuan, N.; Zhang, G. Adsorption and Activation, Active Site and Reaction Pathway of Photocatalytic CO2 Reduction: A Review. Chem. Eng. J. 2024, 481, 148754. [Google Scholar] [CrossRef]
- Zheng, J.; Tang, R.; Li, L.; Zhou, Z.; Li, W.; Gong, D.; Huang, Y.; Deng, Y. Recent Trends in Photocatalysts with Dual-Reaction Sites for Solar Driven Reactions: Fundamental Design Principles and Applications. Surf. Interfaces 2024, 49, 104395. [Google Scholar] [CrossRef]
- Klinbumrung, A.; Prachakiew, S.; Boonphan, S.; Boonruang, C.; Keereeta, Y. Diminishing Photoactivity in Microwave-Synthesized Vanadium-Doped TiO2: Resolving the Paradox of Defect-Mediated Charge Recombination. Micro Nanostructures 2025, 205, 208181. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Zhong, Y.; Ding, J. Novel Strategies for 2,8-Dichlorodibenzo-p-Dioxin Degradation Using Ternary Au-Modified Iron Doped TiO2 Catalysts under UV–Vis Light Illumination. Chemosphere 2022, 291, 132826. [Google Scholar] [CrossRef]
- Umair, M.; Ruiz-Aguirre, A.; Berruti, I.; Rodríguez, S.M.; Palmisano, L.; Loddo, V.; Bellardita, M. Biomass Derivatives Photoreforming in Pilot Plant Scale to Obtain H2 under Green Conditions by Using Ball Milling Cu2O-TiO2 P25 Photocatalysts. Chem. Eng. J. 2025, 504, 158585. [Google Scholar] [CrossRef]
- Ahmad, T.; Ansari, M.Z. Structural and Optical Characteristics of Sb Doped SnO2 Nanoparticles and Their Boosted Photocatalytic Activity Under Visible Light Irradiation. Ceram. Int. 2023, 49, 35740–35756. [Google Scholar] [CrossRef]
- Ramaripa, P.S.; Modibane, K.D.; Seleka, W.M.; Somo, T.R.; Makhado, E.; Makgopa, K.; Ogunbayo, T.B. Recent Applications of Analytical Techniques and Electrochemical Methods in Characterizations of the Titanium Dioxide Composites. Int. J. Electrochem. Sci. 2024, 19, 100444. [Google Scholar] [CrossRef]
- Bagus, P.S.; Freund, H.J. X-Ray Photoelectron Spectroscopy as a Useful Tool to Study Surfaces and Model Systems for Heterogeneous Catalysts: A Review and Perspective. Surf. Sci. 2024, 745, 122471. [Google Scholar] [CrossRef]
- Rocabruno-Valdés, C.I.; Ugalde-Saldivar, V.M.; Valencia-Garcia, K.; Hernández-Gordillo, A.; Rodil, S.E. Exploring the Electronic Structure of BiVO4 Thin Films Using Energy-Resolved Electrochemical Impedance Spectroscopy. Mater. Lett. 2024, 357, 135741. [Google Scholar] [CrossRef]
- Guo, Y.; Peng, B.; Liao, J.; Cao, W.; Liu, Y.; Nie, X.; Li, Z.; Ouyang, R. Recent Advances in the Role of Dissolved Organic Matter during Antibiotics Photodegradation in the Aquatic Environment. Sci. Total Environ. 2024, 916, 170101. [Google Scholar] [CrossRef]
- Duan, C.; Liu, F.; You, J.; Zhao, G.; Kong, M.; Hu, X.; Wang, Z.; Xu, H. Unraveling the Dual Roles of Dissolved Organic Matter on the Photodegradation of Aquatic Contaminants: Molecular Weight- and Type-Dependent Heterogeneities. J. Hazard. Mater. 2025, 485, 136879. [Google Scholar] [CrossRef] [PubMed]
- Gonfa, M.T.; Shen, S.; Chen, L.; Yin, S.F. Selective Hydroxylation of Benzene via Enhanced Generation and Utilization of Hydroxyl Radicals with CuZnSbO Photocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2025, 706, 135746. [Google Scholar] [CrossRef]
- Moreira, A.J.; Campos, L.O.; Maldi, C.P.; Dias, J.A.; Paris, E.C.; Giraldi, T.R.; Freschi, G.P.G. Photocatalytic Degradation of Prozac® Mediated by TiO2 Nanoparticles Obtained via Three Synthesis Methods: Sonochemical, Microwave Hydrothermal, and Polymeric Precursor. Environ. Sci. Pollut. Res. 2020, 27, 27032–27047. [Google Scholar] [CrossRef]
- Peng, X.; Zhou, M.; Yuan, Q.; Fan, M.; Zhu, J.; Gao, N.; Chen, Y. Degradation of Organic Pollutants in Water by Inactive and Chloride Salt-Activated Peroxymonosulfate (PMS): Performance, Kinetics, Mechanisms and Practical Applications. J. Ind. Eng. Chem. 2025; in press. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Action and Mechanism of Semiconductor Photocatalysis on Degradation of Organic Pollutants in Water Treatment: A Review. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100255. [Google Scholar] [CrossRef]
- Zango, Z.U.; Ibnaouf, K.H.; Garba, A.; Aldaghri, O.; Wadi, I.A.; Hosseini-Bandegharaei, A.; Baigenzhenov, O. Advances in Green Synthesis, Modification Strategies, and Photocatalytic Application of Metal Oxide Nanoparticles for Organic Pollutants Degradation: A Comprehensive and in-Depth Review. J. Mol. Liq. 2025, 428, 127497. [Google Scholar] [CrossRef]
- Chatzimpaloglou, A.; Christophoridis, C.; Nika, M.C.; Aalizadeh, R.; Fountoulakis, I.; Thomaidis, N.S.; Bais, A.F.; Fytianos, K. Degradation of Antineoplastic Drug Etoposide in Aqueous Environment by Photolysis and Photocatalysis. Identification of Photocatalytic Transformation Products and Toxicity Assessment. Chem. Eng. J. 2022, 431, 133969. [Google Scholar] [CrossRef]
- Lincho, J.; Mazierski, P.; Klimczuk, T.; Martins, R.C.; Gomes, J.; Zaleska-Medynska, A. TiO2 Nanotubes Modification by Photodeposition with Noble Metals: Characterization, Optimization, Photocatalytic Activity, and by-Products Analysis. J. Environ. Chem. Eng. 2024, 12, 112990. [Google Scholar] [CrossRef]
- Menacherry, S.P.M.; Aravind, U.K.; Aravindakumar, C.T. Critical Review on the Role of Mass Spectrometry in the AOP Based Degradation of Contaminants of Emerging Concern (CECs) in Water. J. Environ. Chem. Eng. 2022, 10, 108155. [Google Scholar] [CrossRef]
- Xu, J.; Xu, S.; Tang, S.; Wang, L.; Wan, K.; Wang, C. Efficient Photocatalytic Degradation of 2,4,6-Trichlorophenol by Z-Scheme α-MnO2/Bi2S3 Enriched with Double Vacancies under Visible Light: Mechanism and Degradation Pathway. Colloids Surf. A Physicochem. Eng. Asp. 2025, 719, 137059. [Google Scholar] [CrossRef]
- Wang, T.; Shi, H.; Kumar, A.; Zhang, D.; Wang, H.; Wang, S.; Zheng, J. Efficient Visible-Light Photocatalysis of Chloramphenicol Using Novel Engineered Biochar-Based Ti-Doped Bi2WO6 Composite: Mechanisms, Degradation Pathways, and Applications. Sep. Purif. Technol. 2024, 332, 125780. [Google Scholar] [CrossRef]
- Jiménez-Salcedo, M.; Monge, M.; Tena, M.T. The Photocatalytic Degradation of Sodium Diclofenac in Different Water Matrices Using G-C3N4 Nanosheets: A Study of the Intermediate by-Products and Mechanism. J. Environ. Chem. Eng. 2021, 9, 105827. [Google Scholar] [CrossRef]
- Li, X.; Liu, M.; Wu, N.; Sharma, V.K.; Qu, R. Enhanced Removal of Phenolic Compounds by Ferrate(VI): Unveiling the Bi(III)-Bi(V) Valence Cycle with in Situ Formed Bismuth Hydroxide as Catalyst. Water Res. 2024, 248, 120827. [Google Scholar] [CrossRef] [PubMed]
- Mohammed-Amine, E.; Kaltoum, B.; El Mountassir, E.M.; Abdelaziz, A.T.; Stephanie, R.; Stephanie, L.; Anne, P.; Pascal, W.W.C.; Alrashed, M.M.; Salah, R. Novel Sol-Gel Synthesis of TiO2/BiPO4 Composite for Enhanced Photocatalytic Degradation of Carbamazepine under UV and Visible Light: Kinetic, Identification of Photoproducts and Mechanistic Insights. J. Water Process Eng. 2025, 70, 107098. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Wu, N.; Zhu, C.; Qin, W.; Huang, D.; Zhou, D. Advanced Reduction Processes Initiated by Oxidative Radicals for Trichloroacetic Acid Degradation: Performance, Radical Generation, and Mechanism. Water Res. 2025, 268, 122587. [Google Scholar] [CrossRef]
- Chen, Q.; Xia, J.; Liu, F.; Fan, J.; Yan, P.; Sillanpää, M. Visible Light Photocatalytic Enhanced Heterogeneous Cobalt Catalyzed Peroxymonosulfate Synergistic Process to Degradation Atrazine: Efficiency, Influencing Factors, by-Products Removal and Mechanism. J. Environ. Sci. 2025; in press. [Google Scholar] [CrossRef]
- Becerra, L.C.I.F.; Malafatti, J.O.D.; Paris, E.C.; Joya, M.R.; Moreira, A.J.; Reis, R.Y.N.; Lima, J.B.; Vargas, C.A.P.; Páez, A.M.R. Photocatalytic Degradation of Fluoxetine Mediated by CuO/CuWO4 Nanostructures. J. Taiwan Inst. Chem. Eng. 2025, 174, 106215. [Google Scholar] [CrossRef]
- Giulietti, M.; Zuorro, A.; Lavecchia, R.; Baaloudj, O.; Garcia-Segura, S.; Brienza, M. Synergistic Effects of UV Irradiation and Hydrogen Peroxide in the Degradation of Chloramphenicol: Mechanism and Identification of Reaction Byproducts and Intermediates. J. Water Process Eng. 2025, 71, 107290. [Google Scholar] [CrossRef]
- Topolovec, B.; Jovanovic, O.; Puac, N.; Skoro, N.; Lumbaque, E.C.; Petrovic, M. Plasma Water Treatment for PFAS: Study of Degradation of Perfluorinated Substances and Their Byproducts by Using Cold Atmospheric Pressure Plasma Jet. J. Environ. Chem. Eng. 2024, 12, 112979. [Google Scholar] [CrossRef]
- Sun, X.; Xu, T.; Xian, T.; Yi, Z.; Liu, G.; Dai, J.; Yang, H. Insight on the Enhanced Piezo-Photocatalytic Mechanism of In2O3/BiFeO3 Heterojunctions for Degradation of Tetracycline Hydrochloride. Appl. Surf. Sci. 2023, 640, 158408. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, B.; Zhang, L.; Sun, Y.; Yang, X.; Zhang, L. 1D/2D CoNi-LDH/ZnIn2S4 S-Scheme Heterojunction for Effectively Tetracycline Degradation under Photocatalytic-Peroxymonosulfate Activation System: DFT Calculations and Mechanism Insights. Chem. Eng. J. 2023, 478, 147535. [Google Scholar] [CrossRef]
- Li, G.; Zeng, G.; Tang, N.; Lu, L.; Li, M.; Yu, J.; Long, W.; Hu, X.; Tan, X.; Tang, C. Floatable Expanded Perlite-Loaded Z-Scheme n-C3N5/Ag2CO3 Core-Shell Structure with C-Defects for Enhanced Adsorption and Photodegradation of Microcystin-LR: Insights into Performance and Mechanism. Appl. Catal. B Environ. Energy 2025, 361, 124614. [Google Scholar] [CrossRef]
- Deng, Z.; Zheng, X.; Guo, Y. Effective Degradation of Doxycycline Hydrochloride in Simulated and Real Water by S-Scheme Heterojunction 2D/1D Bi4O5I2/In2O3 Under Visible Light: DFT Calculation, Mechanism, Degradation Pathway and Toxicity Analysis. Appl. Surf. Sci. 2023, 641, 158407. [Google Scholar] [CrossRef]
- Yang, K.; Huang, Y.; Wang, T.; Li, Y.; Du, Y.; Ling, J.; Fan, Z.; Zhang, C.; Ma, C. In-Situ Anchoring of Co Single-Atom Synergistically with Cd Vacancy of Cadmium Sulfide for Boosting Asymmetric Charge Distribution and Photocatalytic Hydrogen Evolution. Adv. Mater. 2024, 36, 2409832. [Google Scholar] [CrossRef]
- Zhang, G.; Xia, S.; Li, H.; Du, H.; Li, T.; Cao, Y.; Meng, Y.; Wang, J.; Zhong, C.; Lau, W.M. Mediated Peroxydisulfate Activation at Oxygen Vacancy Sites: Synergistic Degradation of Norfloxacin by Radical Pathway and Non-Radical Pathway. Sep. Purif. Technol. 2025, 354, 129018. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, J.; He, X.; Xiong, B.; Li, X. Enhanced Photocatalytic Performance of B/P Doped g-C3N4 for Pollutant Degradation: First-Principles Calculation Study. Catal. Lett. 2025, 155, 99. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, F.; Wei, H.; Yu, W.; Yao, S. Novel Z-Scheme MgFe2O4/Bi2WO6 Heterojunction for Efficient Photocatalytic Degradation of Tetracycline Hydrochloride: Mechanistic Insight, Degradation Pathways and Density Functional Theory Calculations. J. Colloid Interface Sci. 2023, 652, 1282–1296. [Google Scholar] [CrossRef]
- Dai, X.; Gu, D.; Zhou, Q.; Zhang, X.; Zhang, C.; Sun, T.; Liu, X.; Song, D.; Gao, L.; Zheng, J.; et al. Interfacial Heterojunction-Engineered Magnetic Alpha-Fe2O3@NiFe LDH@biotemplate Catalyst for Heterogeneous Photocatalytic Activated Persulfate Removal of Organic Pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2023, 679, 132534. [Google Scholar] [CrossRef]
- Jaison, A.; Mohan, A.; Lee, Y.C. Machine Learning-Enhanced Photocatalysis for Environmental Sustainability: Integration and Applications. Mater. Sci. Eng. R Rep. 2024, 161, 100880. [Google Scholar] [CrossRef]
- Essenni, S.; Aflak, N.; Bahsis, L.; Agunaou, M. Templates Effects on the Morphological, Optical, and Photocatalytic Properties of ZnO Nanostructures Synthesized via Precipitation Method. Inorg. Chem. Commun. 2024, 170, 113437. [Google Scholar] [CrossRef]
- Yu, X.; Liu, M.; Xu, H.; Xu, J.; Yi, J. Improving Adsorption and Purification Performance of Bi2WO6/BiOCl by Oxygen Vacancies. Environ. Pollut. 2024, 363, 125296. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, F.L.P.; Silva, M.V.C.; Mendonça, A.L.F.; Araújo, C.S.; Sallum, L.O.; de Aguiar, A.S.N.; Lima, A.R.; Napolitano, H.B.; Calvete, M.J.F.; Dias, L.D. Photocatalytic Degradation of Ciprofloxacin: A Combined Experimental and Theoretical Study Using Curcumin and Hydrogen Peroxide. Separations 2024, 11, 260. [Google Scholar] [CrossRef]
- Gomzi, V.; Šapić, I.M.; Vidak, A. ReaxFF Force Field Development and Application for Toluene Adsorption on MnMOx (M = Cu, Fe, Ni) Catalysts. J. Phys. Chem. A 2021, 125, 10649–10656. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, R.Q.; Li, Z.; Shi, C.H.; Liu, E.Z.; Zheng, Z.; Zhou, B.; Ji, M.T.; Chen, H.Y. Enhancing Photocatalytic Degradation Efficiency via the Construction of Organic/Inorganic S-Scheme Supramolecular Hybrid Heterostructures. Appl. Surf. Sci. 2023, 641, 158481. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Liu, J.; Lei, Y.; Ma, W.; Song, X.; Zhou, W.; Liu, X.; Zhang, J.; Li, B.; et al. Fabricated S-Scheme CoS/CeO2 Heterojunction Photocatalyst Efficiently Activates Peroxymonosulfate for Polyethylene Terephthalate Plastic Degradation: Insight into Radicals and Electron Transfer. Chem. Eng. J. 2024, 502, 158124. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Wu, D. Highly Efficient CoAl-LDH/(110) Facet-Exposed BiOBr Catalysts: Promotional Effect of Z-Type Heterojunction and Oxygen Vacancies in Photocatalytic Ciprofloxacin Degradation. Mater. Sci. Semicond. Process. 2025, 186, 109105. [Google Scholar] [CrossRef]
- Moreira, A.J.; Pinheiro, B.S.; Araújo, A.F.; Freschi, G.P.G. Evaluation of Atrazine Degradation Applied to Different Energy Systems. Environ. Sci. Pollut. Res. 2016, 23, 18502–18511. [Google Scholar] [CrossRef]
- Moreira, A.J.; Lemos, S.G.; Coelho, D.; Mascaro, L.H.; Freschi, G.P.G.; Pereira, E.C. UV–Vis Spectrophotometry Coupled to Chemometric Analysis for the Performance Evaluation of Atrazine Photolysis and Photocatalysis. Environ. Sci. Pollut. Res. 2022, 29, 24010–24023. [Google Scholar] [CrossRef]
- Queral-Beltran, A.; Marín-García, M.; Lacorte, S.; Tauler, R. UV-Vis Absorption Spectrophotometry and LC-DAD-MS-ESI(+)-ESI(−) Coupled to Chemometrics Analysis of the Monitoring of Sulfamethoxazole Degradation by Chlorination, Photodegradation, and Chlorination/Photodegradation. Anal. Chim. Acta 2023, 1276, 341563. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, Y.Q.; Li, Y.; Xi, B.D.; Wei, Z.M.; Wang, X.L.; Zhao, Z.N.; Ding, J. Using UV-Vis Absorbance for Characterization of Maturity in Composting Process with Different Materials. Guang Pu Xue Yu Guang Pu Fen Xi/Spectrosc. Spectr. Anal. 2015, 35, 961–965. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Azcarate, S.M. How Chemometrics Revives the UV-Vis Spectroscopy Applications as an Analytical Sensor for Spectralprint (Nontargeted) Analysis. Chemosensors 2022, 11, 8. [Google Scholar] [CrossRef]
- Olivieri, A.C.; Escandar, G.M. Calibration Scenarios. In Practical Three-Way Calibration; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–9. [Google Scholar] [CrossRef]
- Araújo, K.C.; Barreto, M.C.; Siqueira, A.S.; Freitas, A.C.P.; Oliveira, L.G.; Bastos, M.E.P.A.; Rocha, M.E.P.; Silva, L.A.; Fragoso, W.D. Oil Spill in Northeastern Brazil: Application of Fluorescence Spectroscopy and PARAFAC in the Analysis of Oil-Related Compounds. Chemosphere 2021, 267, 129154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Guo, L.; Osburn, C.L. Fluorescence EEMs and PARAFAC Techniques in the Analysis of Petroleum Components in the Water Column. In Hydrocarbon and Lipid Microbiology Protocols; Springer: Berlin/Heidelberg, Germany, 2015; pp. 179–200. [Google Scholar] [CrossRef]
- Zhou, Z.; Guo, L.; Shiller, A.M.; Lohrenz, S.E.; Asper, V.L.; Osburn, C.L. Characterization of Oil Components from the Deepwater Horizon Oil Spill in the Gulf of Mexico Using Fluorescence EEM and PARAFAC Techniques. Mar. Chem. 2013, 148, 10–21. [Google Scholar] [CrossRef]
- Bianchi, T.S.; Osburn, C.; Shields, M.R.; Yvon-Lewis, S.; Young, J.; Guo, L.; Zhou, Z. Deepwater Horizon Oil in Gulf of Mexico Waters after 2 Years: Transformation into the Dissolved Organic Matter Pool. Environ. Sci. Technol. 2014, 48, 9288–9297. [Google Scholar] [CrossRef]
- Mendoza, W.G.; Riemer, D.D.; Zika, R.G. Application of Fluorescence and PARAFAC to Assess Vertical Distribution of Subsurface Hydrocarbons and Dispersant during the Deepwater Horizon Oil Spill. Environ. Sci. Process. Impacts 2013, 15, 1017–1030. [Google Scholar] [CrossRef]
- D’Sa, E.J.; Overton, E.B.; Lohrenz, S.E.; Maiti, K.; Turner, R.E.; Freeman, A. Changing Dynamics of Dissolved Organic Matter Fluorescence in the Northern Gulf of Mexico Following the Deepwater Horizon Oil Spill. Environ. Sci. Technol. 2016, 50, 4940–4950. [Google Scholar] [CrossRef]
- Beltrán, J.L.; Guiteras, J.; Ferrer, R. Three-Way Multivariate Calibration Procedures Applied to High-Performance Liquid Chromatography Coupled with Fast-Scanning Fluorescence Spectrometry Detection. Determination of Polycyclic Aromatic Hydrocarbons in Water Samples. Anal. Chem. 1998, 70, 1949–1955. [Google Scholar] [CrossRef]
- Ferrer, R.; Beltrán, J.L.; Guiteras, J. Multivariate Calibration Applied to Synchronous Fluorescence Spectrometry. Simultaneous Determination of Polycyclic Aromatic Hydrocarbons in Water Samples. Talanta 1998, 45, 1073–1080. [Google Scholar] [CrossRef]
- Jiji, R.D.; Cooper, G.A.; Booksh, K.S. Excitation-Emission Matrix Fluorescence Based Determination of Carbamate Pesticides and Polycyclic Aromatic Hydrocarbons. Anal. Chim. Acta 1999, 397, 61–72. [Google Scholar] [CrossRef]
- JiJi, R.D.; Nahorniak, M.L.; Fruitman, E.; Booksh, K.S. Application and Calibration of a Field-Portable Excitation-Emission Matrix Fluorometer for Analysis of Environmental Contaminants. In Pattern Recognition, Chemometrics, and Imaging for Optical Environmental Monitoring, Proceedings of the Photonics East ‘99, Boston, MA, USA, 19–22 September 1999; SPIE Publications: Bellingham, WA, USA, 1999; Volume 3854, pp. 73–82. [Google Scholar] [CrossRef]
- Booksh, K.S.; Muroski, A.R.; Myrick, M.L. Single-Measurement Excitation/Emission Matrix Spectrofluorometer for Determination of Hydrocarbons in Ocean Water. 2. Calibration and Quantitation of Naphthalene and Styrene. Anal. Chem. 1996, 68, 3539–3544. [Google Scholar] [CrossRef]
- Zhou, Z.; Guo, L. Evolution of the Optical Properties of Seawater Influenced by the Deepwater Horizon Oil Spill in the Gulf of Mexico. Environ. Res. Lett. 2012, 7, 025301. [Google Scholar] [CrossRef]
- Araújo, K.C.; Nóbrega, E.T.D.; Moreira, A.J.; Lemos, S.G.; Fragoso, W.D.; Pereira, E.C. Fast and Efficient Processes for Oxidation and Monitoring of Polycyclic Aromatic Hydrocarbons in Environmental Matrices. Catal. Commun. 2024, 187, 106834. [Google Scholar] [CrossRef]


| Degradation Process | Contaminant | Degradation (%)/Time (min) | TP (Amount Found) | Time of Maximum Formation (min) | Ref. |
|---|---|---|---|---|---|
| g-C3N4 nanosheets photocatalysis | Diclofenac | 96/120 | 4 | 15 [n = 4] * | [86] |
| Catalytic oxidation | 2-hydroxybenzophenone | 100/10 | 12 | 1.7 [n = 12] | [87] |
| TiO2/BiPO4 photocatalysis | Carbamazepine | 88/360 | 10 | 50 [n = 5] 110 [n = 3] 150 [n = 1] 240 [n = 1] | [88] |
| Catalytic oxidation | trichloroacetic acid | 100/60 | 2 | 20 [n = 1] 90 [n = 1] | [89] |
| Co2O4/TiO2+PMS | Atrazine | 100/50 | 3 | 20 [n = 1] 35 [n = 2] | [90] |
| HAP:Nb2O5 photocatalysis | Fluoxetine | 100/1 | 6 | 1 [n = 3] 2 [n = 2] 5 [n = 1] | [22] |
| Sm-doped TiO2 | Atrazine | 45/300 | 3 | 300 [n = 3] | [23] |
| Co3O4/TiO2+(PMS) | Atrazine | 100/20 | 3 | 20 [n = 1] 35 [n = 2] | [90] |
| CuO/CuWO4 nanostructures | Fluoxetine | 95/45 | 3 | 40 [n = 3] | [91] |
| UV-H2O2 | chloramphenicol | 100/40 | 4 | 10 [n = 2] 15 [n = 1] 25 [n = 1] | [92] |
| Plasma water treatment | PFAS | 100/5 | 4 | 3 [n = 1] 5 [n = 1] 10 [n = 2] | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, A.J.; Marques, G.N.; Araújo, K.C.d.; Moraes, A.S.d.; Mascaro, L.H.; Pereira, E.C. Photocatalytic Degradation of Environmental Contaminants: Transformation Products and Effects on Photocatalytic Performance. Catalysts 2025, 15, 643. https://doi.org/10.3390/catal15070643
Moreira AJ, Marques GN, Araújo KCd, Moraes ASd, Mascaro LH, Pereira EC. Photocatalytic Degradation of Environmental Contaminants: Transformation Products and Effects on Photocatalytic Performance. Catalysts. 2025; 15(7):643. https://doi.org/10.3390/catal15070643
Chicago/Turabian StyleMoreira, Ailton José, Gleison Neres Marques, Kelvin Costa de Araújo, Alex Silva de Moraes, Lucia Helena Mascaro, and Ernesto Chaves Pereira. 2025. "Photocatalytic Degradation of Environmental Contaminants: Transformation Products and Effects on Photocatalytic Performance" Catalysts 15, no. 7: 643. https://doi.org/10.3390/catal15070643
APA StyleMoreira, A. J., Marques, G. N., Araújo, K. C. d., Moraes, A. S. d., Mascaro, L. H., & Pereira, E. C. (2025). Photocatalytic Degradation of Environmental Contaminants: Transformation Products and Effects on Photocatalytic Performance. Catalysts, 15(7), 643. https://doi.org/10.3390/catal15070643







