Abstract
Constructing a heterojunction is considered one of the most effective strategies for enhancing photocatalytic activity. Herein, we employ Ta3N5 and tubular graphitic carbon nitride (TCN) to construct a Ta3N5/TCN van der Waals heterojunction via electrostatic self-assembly for enhanced photocatalytic H2 production. SEM and TEM results show that Ta3N5 particles (~300 nm in size) are successfully anchored onto the surface of TCN. The light absorption capability of the Ta3N5/TCN heterojunction is between those of Ta3N5 and TCN. The strong interaction between Ta3N5 and TCN with different energy structures (Fermi levels) by van der Waals force renders the formation of an interfacial electric field to drive the separation and transfer of photogenerated charge carriers in the Ta3N5/TCN heterojunction, as evidenced by the photoluminescence (PL) and photoelectrochemical (PEC) characterization results. Consequently, the optimal Ta3N5/TCN heterojunction exhibits a remarkable H2 production rate of 12.73 mmol g−1 h−1 under visible light irradiation, which is 3.3 and 16.8 times those of TCN and Ta3N5, respectively. Meanwhile, the cyclic experiment demonstrates excellent stability of the Ta3N5/TCN heterojunction upon photocatalytic reaction. Notably, the photocatalytic performance of 15-TaN/TCN outperforms the most previously reported CN-based and Ta3N5-based heterojunctions for H2 production. This work provides a new avenue for the rational design of CN-based van der Waals heterojunction photocatalysts with enhanced photocatalytic activity.
1. Introduction
Photocatalytic water splitting to generate hydrogen (H2) using semiconductor materials holds great promise for the conversion and storage of renewable solar energy [1,2,3,4]. Among various semiconductor materials, two-dimensional (2D) graphitic carbon nitride (CN) has emerged as one of the most promising photocatalysts for H2 production due to its appropriately aligned energy band structure; cost-effective, non-toxic nature; excellent photochemical stability over 15 h of continuous irradiation; and thermal stability up to 550 °C [5,6,7]. However, photocatalytic H2 production activity over bulk CN is limited, mainly due to its low specific surface area and rapid recombination of charge carriers [8,9,10]. Therefore, different strategies such as element doping, heterojunction construction, morphology design, and surface functionalization have been developed to modify CN for enhanced photocatalytic activity [11,12,13,14]. Among these approaches, fabrication of tubular CN (TCN) has emerged as a promising approach for enhanced light-scattering ability, increased specific surface area, and promoted ballistic charge carriers transfer, all of which are beneficial for hydrogen generation. In addition, compared to other 2D materials such as graphene, MXenes, and MBenes, TCN exhibits distinct advantages, including low cost, facile preparation, and an appropriate photoresponse range [15,16,17]. For instances, Wu et al. synthesized delaminated laminar TCN exhibiting a 32% higher photocatalytic degradation capacity for tetracycline hydrochloride than pristine CN [18]. Shan et al. prepared TCN, achieving a 4.4-fold higher photocatalytic H2 production rate than that of pristine CN [19]. Besides the morphology design strategy, constructing TCN-based heterostructures with other suitable components has been employed as an effective approach to further improve the photocatalytic H2 production activity of TCN. In the heterostructure, an interface electric field (IEF) can be generated to promote the separation and transfer of charge carries, thereby improving photocatalytic performance. For example, Chen et al. synthesized a novel 3D hierarchical hollow TCN/ZnIn2S4 nanosheets (HTCN/ZIS) type-II heterojunction photocatalyst via an in situ solvothermal treatment method. Under visible light illumination, the optimally proportioned HTCN/ZIS exhibited a remarkable photocatalytic H2 evolution rate of 20,738 μmol g−1 h−1, which was 2.5 times that of hollow TCN [20]. Huang et al. engineered a 1D/2D Schottky heterojunction (PTCN/TC) through the electrostatic self-assembly of phosphorus-doped TCN (PTCN) and delaminated 2D Ti3C2 MXene nanosheets. The 1D pathway of TCN, along with the IEF between Ti3C2 and PTCN, significantly facilitated the transfer and separation of photogenerated charge carriers, while the Schottky barrier simultaneously inhibited the recombination of charge carriers. The optimized PTCN/TC exhibited an H2 evolution rate of 565.0 μmol g−1 h−1 under simulated sunlight irradiation by using methanol as a sacrificial agent, which was 2.0 times that of PTCN [21]. Li et al. fabricated a double-shell microtube TCN/N-doped TiO2 heterostructure (TCN@TiO2). The TCN@TiO2 showed a high H2 production rate of 10.1 mmol g−1 h−1, which was four times that of TCN [22].
Tantalum nitride (Ta3N5), a typical n-type semiconductor, has attracted considerable attention due to its intriguing properties, such as a relatively narrow bandgap (~2.1 eV) and a theoretical solar-to-hydrogen conversion efficiency of 15.9% [23,24]. However, Ta3N5 is typically synthesized via high-temperature nitridation, during the process of which the catalyst is introduced into substantial defects such as nitrogen vacancies and low-valence Ta ions that may serve as recombination centers of photogenerated charge carriers, thereby limiting the photocatalytic H2 production activity [25]. Fortunately, Ta3N5 can be integrated with other 2D materials to promote the interfacial separation of photogenerated charge carriers. Zhan et al. prepared a Ta3N5/ZnIn2S4 1D/2D heterojunction, which exhibited an H2 production rate of 637.18 μmol g−1 h−1 under visible light irradiation, which was 88 times that of Ta3N5 [26]. In this context, constructing a Ta3N5/TCN heterojunction could be expected to exhibit significantly enhanced photocatalytic H2 production activity. To the best of our knowledge, however, studies on constructing a van der Waals heterojunction between Ta3N5 and TCN for photocatalytic applications have been rarely reported so far.
In this work, we designed and synthesized a Ta3N5/TCN (TaN/TCN) van der Waals heterojunction via an electrostatic self-assembly method for photocatalytic H2 production. Experimental results indicated that the strong coupling between Ta3N5 with TCN increased the separation efficiency of photogenerated charge carries via the IEF. As a result, the optimized sample exhibited excellent photocatalytic H2 production performance (12.73 mmol g−1 h−1), which was 9.3 and 16.8 times those of pristine CN and Ta3N5, respectively. This study provides deep insights into the charge separation/transfer mechanism, accounting for efficient photocatalytic H2 production over the developed TaN/TCN van der Waals heterojunction.
2. Results and Discussion
2.1. Characterization of Materials
The microstructure and morphology of the as-prepared photocatalysts were characterized by SEM and TEM. As shown in Figure 1a, the pristine CN exhibited an irregular agglomerated solid structure. After treatment with H3PO4, the obtained TCN displayed a tubular structure with a smooth surface (Figure 1b). Ta3N5 consisted of aggregated nanoparticles with an average size of ~1 μm (Figure 1c). In the electrostatically self-assembled 15-TaN/TCN heterostructure, the tubular structure of TCN was still maintained, and the Ta3N5 particles were attached to the surface of TCN (Figure 1d,e). The HR-TEM image of 15-TaN/TCN (Figure S1) revealed that Ta3N5 and TCN were in close contact, further indicating the successful formation of the heterojunction. Additionally, the particle size distribution histograms of CN, TCN, Ta3N5, and 15-TaN/TCN were obtained from corresponding SEM images (Figure S2). TEM-based energy-dispersive X-ray (EDX) elemental mapping of CN, TCN, and Ta3N5 revealed the homogeneous spatial distribution of the constituent elements (Figure S3). Furthermore, the high-angle annular dark field (HAADF) image (Figure 1f), EDX elemental mapping (Figure 1g–i), and corresponding elemental composition (Table S1) of 15-TaN/TCN confirmed the successful formation of the Ta3N5/TCN heterojunction. The Ta3N5 content in 15-TaN/TCN was precisely determined to be 15.55 wt% based on the inductively coupled plasma optical emission spectrometry (ICP–OES) data. The Zeta potentials of acidified TCN and Ta3N5 were measured to be 22.8 and −38.6 mV, respectively, indicating the feasibility of forming 15-TaN/TCN heterojunctions via electrostatic self-assembly (Table S2). SEM, TEM, HR-TEM, and Zeta potential collectively demonstrate that 15-TaN/TCN heterostructure was successfully constructed by the electrostatic self-assembly method.

Figure 1.
SEM images of (a) CN, (b) TCN, (c) Ta3N5, and (d) 15-TaN/TCN. (e) TEM and (f) HAADF image of 15-TaN/TCN. EDS mappings of (g) C, (h) N, and (i) Ta in 15-TaN/TCN.
The N2 adsorption–desorption isotherms of pristine CN, TCN, Ta3N5, and 15-TaN/TCN were measured to obtain their specific surface areas and pore characteristics (Figure S4). All catalysts exhibited type IV isotherms with type H3 hysteresis loops, indicating the existence of a mesoporous structure [27,28]. The specific surface areas of pristine CN and TCN were 14.8 and 37.0 m2 g−1, respectively. The larger specific surface area of the tubular TCN structure would favor the formation of heterojunctions. When TCN was combined with Ta3N5, the specific surface area of 15-TaN/TCN decreased to 28.0 m2 g−1, but it was still larger than that of Ta3N5 (13.5 m2 g−1). Nevertheless, 15-TaN/TCN exhibited a similar pore size distribution characteristic to that of TCN, indicating that Ta3N5 did not cover the pores on TCN.
The crystalline structures of the as-prepared TCN, Ta3N5, and x-TaN/TCN composites were analyzed by XRD. As shown in Figure 2a, two diffraction peaks at approximately 13.1° and 27.6° were observed in pristine CN, corresponding to the (100) and (002) planes, respectively. The former was associated with the periodic in-plane stacking of tri-s-triazine units, while the latter was related to the interlayer structural packing [29,30]. Notably, compared with the pristine CN, the two diffraction peaks of TCN at 13.1° and 27.6° became broader and weaker, which might be attributed to the size-dependent properties of the tubular structure [31]. The main peaks of Ta3N5 were observed at 24.5°, 31.5°, 35.1°, 36.1°, and 57.5°, corresponding to the (110), (023), (130), (113), and (223) lattice planes, respectively [32]. As for the x-TaN/TCN heterostructures, the XRD patterns displayed all the characteristic peaks of both TCN and Ta3N5, with no additional peaks detected. These results confirmed that the x-TaN/TCN composites were composed of TCN and Ta3N5, and no impurity phases were generated during the synthesis process. Additionally, the lattice constants of CN, TCN, and Ta3N5, as well as the crystallite sizes of CN, TCN, Ta3N5, and x-TaN/TCN, were calculated based on the XRD results (see Tables S3 and S4). The chemical structures of the x-TaN/TCN composites were analyzed by FT-IR spectroscopy (Figure 2b). For pristine CN, the characteristic peak at 810 cm−1 originated from the breathing mode of heptazine ring units, while the peak in the range of 1237–1647 cm−1 could be attributed to the stretching vibrations of aromatic N–C=N heterocycles [33,34]. The weak absorption in the range of 3000–3400 cm−1 was attributed to the stretching vibrations of –NHx groups and the surface adsorbed H2O (–OH) [35]. TCN exhibited similar characteristic peaks to pristine CN, indicating that H3PO4 treatment did not alter the fundamental framework. For Ta3N5, the peak at 870 cm−1 could be attributed to the stretching vibrations of the Ta–N bonds [36]. The characteristic peaks of both TCN and Ta3N5 were observed in the x-TaN/TCN heterostructures, confirming the successful combination of TCN with Ta3N5.
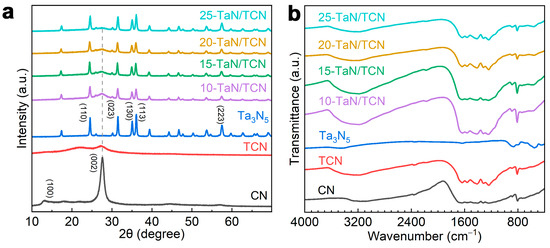
Figure 2.
(a) XRD patterns and (b) FT-IR spectra of CN, TCN, Ta3N5, and x-TaN/TCN.
The XPS measurements were performed to investigate the chemical composition and surface chemical states of the samples. The XPS survey spectrum confirmed that 15-TaN/TCN mainly consisted of C, N, O, and Ta elements (Figure S5), further indicating the effective recombination of TCN with Ta3N5. As shown in Figure 3a, four peaks were observed in the C 1s spectrum of TCN at 284.8, 286.1, 288.1, and 293.8 eV, which were attributed to the C–C/C=C bond of adventitious carbon, sp2-hybridized carbon in C–NHx, sp2-hybridized carbon in N–C=N, and the charging effect, respectively [37,38]. In the N 1s spectrum (Figure 3b), TCN showed four peaks at 398.6, 399.9, 401.1, and 404.4 eV, which could be assigned to sp2-hybridized nitrogen in C=N–C, nitrogen in N–Hx, sp3-hybridized nitrogen in N–C3, and the charging effect, respectively [39]. For Ta3N5, two peaks were obtained at 396.5 and 403.2 eV, which were attributed to Ta–N and Ta 4p3/2, respectively [40]. In the O 1s spectrum (Figure 3c), TCN displayed a peak at 532.0 eV, corresponding to surface adsorbed oxygen in –OH [41]. The O 1s spectrum of Ta3N5 could be deconvoluted into two peaks at 531.9 and 533.4 eV, which were attributed to surface lattice oxygen and adsorbed oxygen, respectively [40]. The Ta 4f spectrum of Ta3N5 could be deconvoluted into four peaks (Figure 3d). Among them, the two relatively strong peaks at 25.1 and 27.0 eV, with a fixed 4:3 area ratio, were attributed to 4f7/2 and 4f5/2 in N–Ta–N. The two relatively weak peaks at 26.0 and 27.9 eV, also with a fixed 4:3 area ratio, were assigned to 4f7/2 and 4f5/2 in O–Ta(V)–N [23]. The C 1s and N 1s spectra of 15-TaN/TCN were similar to those of TCN, but the N–C=N peak in the C 1s spectrum shifted to a more positive binding energy position. In addition, the Ta 4f spectrum of 15-TaN/TCN resembled that of Ta3N5, but an obvious negative shift of these peaks was observed. These results demonstrated that electrons were transferred from TCN to Ta3N5 in the 15-TaN/TCN heterojunction, leading to an increase of electron density in Ta3N5.
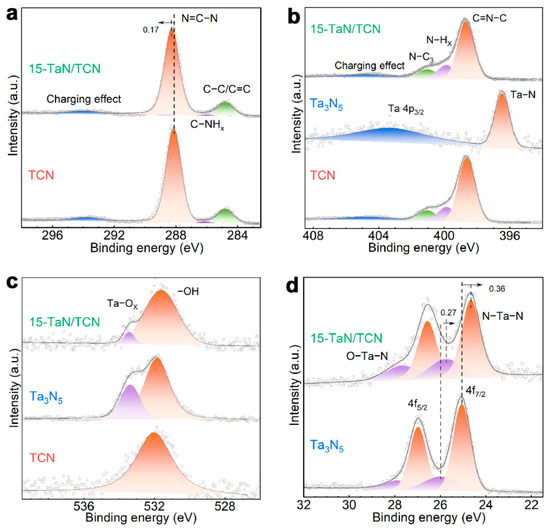
Figure 3.
(a) C 1s high-resolution XPS spectra of TCN and 15-TaN/TCN. (b) N 1s and (c) O 1s high-resolution XPS spectra of TCN, Ta3N5, and 15-TaN/TCN. (d) Ta 4f high-resolution XPS spectra of Ta3N5 and 15-TaN/TCN.
2.2. Photocatalytic H2 Evolution Performance
Photocatalytic hydrogen production was carried out using methanol as a hole scavenger under 300 W Xe lamp irradiation (λ ≥ 320 nm, see the light spectrum in Figure S6). Methanol could be oxidized by photogenerated holes to produce formaldehyde and protons, which were further utilized for H2 production [42]. As shown in Figure 4a, pristine CN and Ta3N5 exhibited poor performance, while TCN showed a much better performance than pristine CN. After coupling TCN with Ta3N5, the x-TaN/TCN heterostructure exhibited significantly enhanced photocatalytic H2 production activity. However, the activity gradually decreased when the Ta3N5 content exceeded 15%, possibly due to excessive Ta3N5 covering the active sites and blocking the incident light. Notably, 15-TaN/TCN showed the highest H2 evolution rate of 12.73 mmol g−1 h−1, which was 3.3, 9.3, and 16.8 times that of TCN (3.81 mmol g−1 h−1), pristine CN (1.37 mmol g−1 h−1), and Ta3N5 (0.76 mmol g−1 h−1), respectively. Furthermore, as a comparison, we prepared 15-Ta3N5 + TCN by combining Ta3N5 with non-acidified TCN. The H2 evolution rate of 15-Ta3N5 + TCN was much lower than that of TCN, indicating that non-acidified TCN and Ta3N5 did not form a heterojunction. Notably, the photocatalytic performance of 15-TaN/TCN outperformed the most previously reported CN-based and Ta3N5-based heterojunctions for H2 production (Table S5). Figure S7 presented the average H2 evolution rate with error bars of Ta3N5, TCN, and 15-TaN/TCN samples measured three times. The sample standard deviations were all within 0.35, indicating excellent reproducibility. Moreover, the BET surface-area-normalized H2 evolution rate of 15-TaN/TCN (455 μmol g−1 h−1 m−2) was 4.4 and 8.1 times higher than that of TCN (103 μmol g−1 h−1 m−2) and Ta3N5 (56 μmol g−1 h−1 m−2), respectively (Figure 4b), indicating that the enhanced photocatalytic activity of the 15-TaN/TCN system was primarily attributed to the successful construction of the heterojunction rather than differences in BET surface areas. Meanwhile, the effects of temperature and stirring time during the electrostatic self-assembly process on the photocatalytic activity of the 15-TaN/TCN heterojunction were investigated. As shown in Figure S8a, temperature had little impact on the photocatalytic activity of 15-TaN/TCN. The activity was stabilized after 24 h of stirring, indicating that Ta3N5 and TCN were sufficiently self-assembled (Figure S8b).
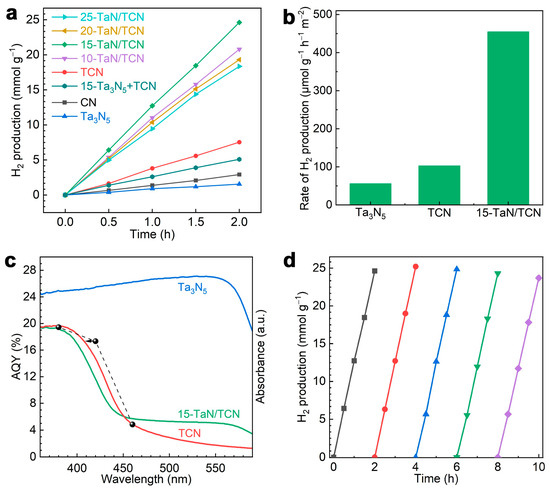
Figure 4.
(a) Time courses of photocatalytic H2 production of Ta3N5, CN, TCN, 15-Ta3N5 + TCN, and x-TaN/TCN under visible light irradiation. (b) BET surface area normalized H2 evolution rates over Ta3N5, TCN, and 15-TaN/TCN. (c) The wavelength-dependent AQY of Ta3N5, TCN, and 15-TaN/TCN. (d) The cyclic experiment of 15-TaN/TCN for photocatalytic H2 production.
The apparent quantum yields (AQYs) of 15-TaN/TCN at various wavelengths showed a good dependence on the light absorption spectrum of TCN (Figure S9), indicating that H2 production mainly occurred on TCN. The 15-TaN/TCN exhibited no H2 production activity in the dark. The above results demonstrated the light-driven nature of photocatalytic H2 production. Notably, the 15-TaN/TCN achieved an AQY of 17.33% at 420 nm, indicating efficient conversion of solar energy into H2 in the photocatalytic process. In addition, the photocatalytic activity of 15-TaN/TCN remained nearly unchanged after five consecutive cycles of 10 h total (Figure 4d), indicating excellent photocatalytic stability. Meanwhile, the XRD patterns and FTIR spectra of 15-TaN/TCN before and after the cycling experiment showed no significant difference (Figure S10), revealing the structural stability of the photocatalyst.
2.3. Mechanism of Photocatalytic H2 Production
The optical absorption property was an important factor that influenced the photocatalytic activity of a catalyst. Shown in Figure 5a are the UV–vis diffuse reflection spectra (DRS) of the as-prepared samples. Ta3N5 showed prominently higher light absorption capacity than pristine CN and TCN, which was consistent with their different colors (Figure S11). In addition, the absorption capability of the x-TaN/TCN composites was close to that of TCN due to the low content of Ta3N5. With increasing Ta3N5 content, the light absorption ability of x-TaN/TCN gradually increased. Furthermore, as shown in Figure 5b and Figure S12, the Tauc plots for CN, TCN, Ta3N5, and x-TaN/TCN were obtained using the Kubelka–Munk function ((Ahv)n = A(hν − Eg)) [43]. The bandgap values and morphological characteristics of samples are summarized in Table S6. Among these materials, the energy band gaps (Eg) of Ta3N5 and TCN were estimated to be 1.97 and 2.66 eV, respectively (Figure 5b). The Mott–Schottky plots of Ta3N5 and TCN exhibited positive slopes, suggesting the n-type semiconductor characteristics. Accordingly, the conduction band (CB) potentials (ECB) of Ta3N5 and TCN, which were approximately equal to their flat band potentials [44], were estimated to be −0.57 and −0.75 eV, respectively (Figure S12). According to the equation EVB = ECB + Eg (vs. SHE), the valence band (VB) potentials (EVB) of Ta3N5 and TCN were calculated to be 1.40 and 1.91 eV. Furthermore, XPS VB spectra revealed that the potential difference (ED) between the EVB and the Fermi levels (EF) (ED = EVB − EF) of Ta3N5 and TCN were 1.24 and 2.31 eV, respectively (Figure 5c) [45]. Thus, the EF values of Ta3N5 and TCN were estimated to be 0.16 and −0.4 eV, respectively.
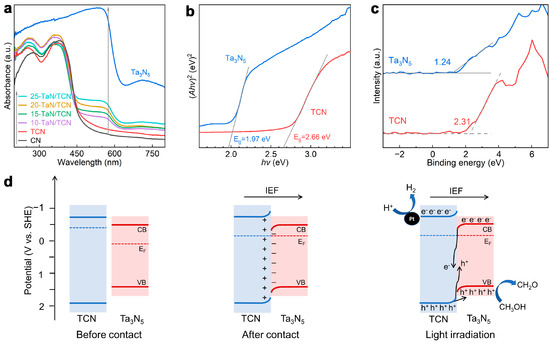
Figure 5.
(a) UV—vis diffuse reflectance spectra of CN, TCN, Ta3N5, and x-TaN/TCN. (b) Tauc plot of Ta3N5 and TCN. (c) XPS VB spectra of Ta3N5 and TCN. (d) Schematic diagram of the band structure, charge transfer, and photocatalytic H2 production mechanism of Ta3N5/TCN van der Waals heterojunction under light irradiation.
Based on the above results and discussion, we proposed the band structure, charge transfer, and photocatalytic H2 production mechanism over the Ta3N5/TCN van der Waals heterojunction. As shown in Figure 5d, the Fermi level (EF) of TCN is higher than that of Ta3N5. Upon the formation of the heterojunction, electrons spontaneously transfer from TCN to Ta3N5 and the eventual formation of an IEF directing from TCN to Ta3N5, being consistent with the XPS results. Subsequently, the CB and VB of TCN bend upward due to electrostatic repulsion by the IEF, while those of Ta3N5 bend downward [46]. Upon light irradiation, photogenerated electrons in the CB of Ta3N5 are driven by the IEF to transfer to the VB of TCN and combine with holes. Meanwhile, the remaining photogenerated holes in the VB of TCN are driven by the IEF to transfer to the VB of Ta3N5. Eventually, the accumulated photogenerated electrons in the CB of TCN migrate to the Pt nanoparticles cocatalyst for H2 production, while the accumulated photogenerated holes in the VB of Ta3N5 oxidize the sacrificial reagent methanol. Additionally, the TEM image of 3 wt% Pt-loaded 15-TaN/TCN (Figure S14) clearly showed that Pt nanoparticles were exclusively photodeposited on TCN nanotubes, with no observable nanoparticles on Ta3N5. This observation confirmed that H2PtCl6 was reduced by photogenerated electrons preferentially on TCN rather than on Ta3N5, which was consistent with the wavelength-dependent AQY results. The spatially selective deposition of Pt nanoparticles provided direct experimental evidence supporting the proposed charge transfer mechanism in the heterojunction.
The separation and transfer efficiency of photogenerated charge carriers were explored through steady-state photoluminescence (PL) and time-resolved photoluminescence (TRPL) decay spectra. The TCN exhibited a strong PL emission peak, indicating the rapid recombination of photogenerated electron and hole pairs (Figure 6a). The emission peak intensity of the 15-TaN/TCN van der Waals heterojunction was significantly reduced, indicating that the separation efficiency of electron and hole pairs was enhanced. Furthermore, the TRPL decay spectra provided more details of the charge carriers’ separation characteristics (Figure 6b). The average PL lifetimes (τa) of TCN and 15-TaN/TCN, derived from fitting the PL decay spectra by a triple-exponential model, were 4.85 and 3.93 ns, respectively. The shorter τa of 15-TaN/TCN suggested the existence of more efficient non-radiative charge transfer within the TaN/TCN heterojunction, which was beneficial to the enhanced photocatalytic H2 production.
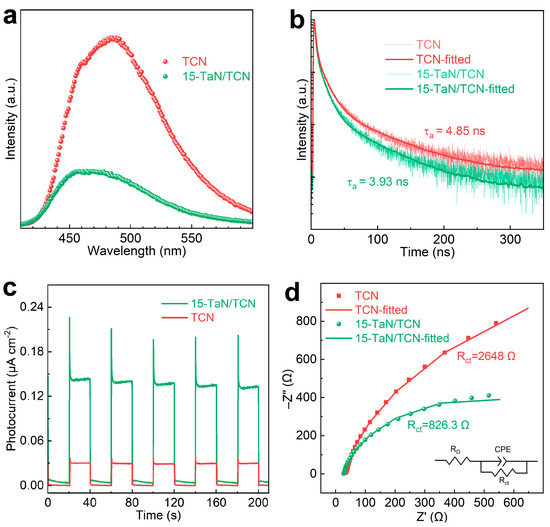
Figure 6.
(a) Steady-state PL spectra, (b) TRPL spectra, (c) transient photocurrent response spectra, and (d) EIS Nyquist plots of TCN and 15-TaN/TCN.
To further study the separation property of photogenerated electron and hole pairs in photocatalysts, the transient photocurrent response and electrochemical impedance spectroscopy (EIS) of TCN and 15-TaN/TCN were tested. As shown in Figure 6c, 15-TaN/TCN showed a higher photocurrent density, confirming that the TaN/TCN heterojunction effectively facilitated spatial separation of charge carriers. The radius of the EIS Nyquist plot reflected the charge transfer resistance, and a smaller radius indicated better efficient charge transfer. As determined by fitting the high-frequency region of EIS to the equivalent circuit diagram (Figure 6d), the charge transfer impedance from electrode to electrolyte was noticeably lower for 15-TaN/TCN (826.3 Ω) than for TCN (2648 Ω), indicating the smaller interface resistance of 15-TaN/TCN than TCN. Therefore, the PL, TRPL, photocurrent, and EIS results collectively indicated that the recombination of photogenerated charge carriers were effectively suppressed in the 15-TaN/TCN van der Waals heterojunction, thereby enhancing the photocatalytic H2 production activity significantly.
3. Materials and Methods
3.1. Materials Syntheses
Melamine (99.0%) and Ta2O5 (99.99%) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Phosphoric acid and hydrochloric acid were obtained from Tianjin Fengchuan Co., Ltd. (Tianjin, China). Methanol was obtained from Li’anlongbohua Co., Ltd. (Tianjin, China). All reagents were used without any further treatment.
Ta3N5 was synthesized by heating the commercial Ta2O5 under ammonia flow at 900 °C for 6 h, with a heating rate of 2.5 °C min−1. Typically, pristine CN was synthesized by thermolysis of melamine at 550 °C for 4 h with a heating rate of 2.5 °C min−1 in air. Tubular C3N4 (TCN) was fabricated by two steps. First, 1 g of melamine and 1.2 g of phosphoric acid were added to 60 mL deionized (DI) water and stirred for 0.5 h. The mixture was transferred into a Teflon autoclave and maintained at 180 °C for 10 h. Then, the obtained tube-like product was centrifugalized, washed using DI water, and dried at 60 °C overnight under vacuum conditions. After that, the dried product was calcined at 550 °C for 4 h under a flow of argon with ramping rate of 2.5 °C min−1. The resulting sample was noted as TCN.
Ta3N5/TCN van der Waals heterojunction/heterostructure was constructed by an electrostatic self-assembly method (Figure S15). First, the pre-synthesized TCN was added into an aqueous HCl solution (50 mL, 1 M) and sonicated for 1 h. Then, the mixture was stirred for 24 h. The resulting acid suspension was centrifuged and washed with deionized water until neutral. After drying at 60 °C overnight, a certain amount of acidified TCN was added into 50 mL DI water with the pre-synthesized Ta3N5 powder. The mixture was ultrasonicated for 1 h and stirred for 24 h. Finally, the sample was filtered, dried, and denoted as x-TaN/TCN (x = 10, 15, 20, 25; x was the mass of Ta3N5 in 100 mg of the total x-TaN/TCN heterostructures). Following the procedure for preparation of 15-TaN/TCN using acidified TCN, the physically mixed sample 15-Ta3N5 + TCN was prepared using unacidified TCN.
3.2. Characterizations
The morphology and composition were measured on a scanning electron microscope (SEM, S-4800, Hitachi, Tokyo, Japan) and a field-emission transmission electron microscope (TEM, Talos F200X, Thermo Fisher Scientific, Waltham, MA, USA) with energy-dispersive X-ray spectrometer (EDS). Brunauer–Emmett–Teller (BET) surface area, pore size, and porosity were conducted on a Quantachrome Autosorb-iQ instrument (Thermo Fisher Scientific, Waltham, MA, USA). The crystal structure of samples was obtained by X-ray powder diffraction (XRD) analysis using a D/max2500VB+/PC X-ray diffractometer (Rigaku, Tokyo, Japan) with monochromatic Cu Kα radiation (λ = 0.15418 nm). Fourier transform infrared spectroscopy (FT-IR) was tested by a Nicolet 6700 spectrometric analyzer (Thermo Fisher Scientific, Waltham, MA, USA). The ultraviolet–visible (UV–vis) absorption spectrum was evaluated by a UV-3600 ultraviolet–visible spectrophotometer (Shimadzu, Kyoto, Japan). The chemical states were analyzed by Escalab 250Xi X-ray photoelectron spectroscopy (Thermo Fisher Scientific, Waltham, MA, USA), and the C 1s peak was corrected to 284.8 eV. Photoluminescence (PL) and time-resolved PL (TRPL) were conducted using a HORIBA Fluorolog-3 spectrofluorometer (Horiba Scientific, Piscataway, NJ, USA) with an excitation wavelength of 380 nm at ambient temperature. The Ta element content in 15-TaN/TCN was quantified by inductively coupled plasma optical emission spectrometry (ICP-OES, 5110, Agilent, Santa Clara, CA, USA).
3.3. Photoelectrochemical (PEC) Measurements
The photocurrent, electrochemical impedance spectroscopy (EIS), and Mott–Schottky measurements were carried out on an electrochemical workstation (CHI760E, Chenhua, Shanghai, China) with a typical three-electrode configuration. Fluorine-doped tin oxide (FTO) glass (1 cm × 1 cm), Pt sheet, and Ag/AgCl electrode were used as the working electrode, counter electrode, and reference electrode, respectively. A 0.1 M Na2SO4 aqueous solution was employed as the electrolyte in photocurrent measurements, while a mixed aqueous solution containing 0.1 M KCl, 2.5 mM K3Fe(CN)6, and 2.5 mM K4Fe(CN)6 was used for the EIS and Mott–Schottky tests. During the photocurrent measurements, a 300 W Xe lamp was used as the light source, with alternating “light on” and “light off” conditions. The frequency range of EIS Nyquist plots were from 0.1 to 105 Hz. The Mott–Schottky curves were recorded at 800 and 900 Hz, respectively.
3.4. Photocatalytic Hydrogen Evolution Reaction (HER) Measurements
The photocatalytic activity was performed by a quartz glass reactor connected to a closed gas circulation system. A 300 W xenon lamp was used as light source. In a standard procedure, a 20 mg catalyst was uniformly dispersed by a magnetic stirrer in a 240 mL aqueous solution containing methanol (10 vol%) as a sacrificial agent and H2PtCl6·6H2O (3 wt% Pt loading) as the cocatalyst precursor. H2 yield was evaluated by a gas chromatograph (Shimadzu, GC-2014, Ar as the carrier gas) equipped with a thermal conductivity detector (TCD). The apparent quantum yield (AQY) for hydrogen evolution reaction (HER) involving one-step photoexcitation was conducted by a 300 W Xe lamp with a water filter and various band-pass filters of 380, 420, and 460 nm. The AQY was calculated according to the following equation:
where m(H2) and n(photons) represent the number of produced H2 molecules and incident photons, respectively.
AQY(%) = [2 × m(H2)]/n(photons)] × 100%
4. Conclusions
In summary, a Ta3N5/TCN van der Waals heterojunction photocatalyst was successfully constructed by electrostatic self-assembly method. Based on the energy band structure and XPS analysis, it was concluded that electrons transferred from TCN to Ta3N5, forming an IEF, which facilitated the separation and transfer of photogenerated electrons and holes as evidenced by the PL and PEC characterization results. Consequently, the TaN/TCN van der Waals heterojunction exhibited significantly improved photocatalytic H2 production activity. The optimal sample 15-TaN/TCN exhibited a high photocatalytic H2 production rate of 12.73 mmol g−1 h−1 under full-spectrum light irradiation, which was among the best of most previously reported CN-based and Ta3N5-based heterojunctions for H2 production, much better than those of pristine CN, TCN, and Ta3N5. This work demonstrates that constructing a CN-based van der Waals heterojunction is a viable approach to the development of efficient photocatalysts for H2 production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15070691/s1, Figure S1: HR-TEM image of the 15-TaN/TCN heterojunction; Figure S2: The particle size distribution histograms of (a) CN, (b) TCN, (c) Ta3N5, and (d) 15-TaN/TCN; Figure S3: TEM images of (a) CN, (b) TCN, and (c) Ta3N5. HAADF images of (d) CN, (h) TCN, and (l) Ta3N5. EDS mapping of (e) C, (f) N, and (g) O of CN; (i) C, (j) N, and (k) O of TCN; (m) N, (n) O, and (o) Ta of Ta3N5; Table S1: Atomic percentages of elements in TEM mapping; Table S2: The Zeta potentials of TCN, Ta3N5, and acidified TCN at pH = 7; Figure S4: (a) N2 sorption isotherms and (b) pore size (D) distribution curves obtained from the adsorption branches of the isotherms using the Barrett–Joyner–Halenda (BJH) method for CN, TCN, Ta3N5, and 15-TaN/TCN; Table S3: The crystal structure and lattice constants of CN, TCN, and Ta3N5; Table S4: The crystallite sizes of CN, TCN, Ta3N5, and x-TaN/TCN; Figure S5: Survey XPS spectra of TCN, Ta3N5, and 15-TaN/TCN; Figure S6: The light spectrum of the 300 W Xe lamp; Table S5: A comparison of hydrogen evolution activity between previous studies and this work; Figure S7: The H2 evolution rates of 15-TaN/TCN under different (a) temperatures and (b) stirring times; Figure S8: The H2 evolution rates of 15-TaN/TCN under different (a) temperatures and (b) stirring times; Figure S9: H2 production rate under (a) dark condition and (b) light irradiation of 15-TaN/TCN; Figure S10: (a) XRD patterns and (b) FTIR spectra of 15-TaN/TCN before and after cyclic experiments; Figure S11: Photographs of (a) pristine CN, (b) TCN, and (c) Ta3N5; Figure S12: Tauc plots of CN and x-TaN/TCN; Table S6: The bandgap and morphology information of CN, TCN, Ta3N5, and x-TaN/TCN; Figure S13: Mott–Schottky plots of (a) Ta3N5 and (b) TCN; Figure S14: TEM image of 3 wt% Pt-loaded 15-TaN/TCN; Figure S15: The construction process of the 15-TaN/TCN heterojunction. References [26,47,48,49,50,51,52,53,54,55,56,57] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, J.Y. (Junbo Yu), G.B. and D.W.; methodology and investigation, J.Y. (Junbo Yu), G.B., F.B., H.H. and D.W.; data curation, J.Y. (Junbo Yu); writing—original draft preparation, J.Y. (Junbo Yu) and G.B.; writing—review and editing, D.W.; supervision, D.W.; funding acquisition and resources, H.H., J.Y. (Jinhua Ye) and D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the National Natural Science Foundation of China (51572191).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jin, Z.; Yang, C.; Li, L.; Jiang, J. Rational Constructing 2D/3D p-n Heterojunctions to Modulate Hydrogen Evolution Efficient Pathways for Enhances Photocatalytic Hydrogen Production. J. Ind. Eng. Chem. 2025, 142, 449–462. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef]
- Rasool, M.A.; Sattar, R.; Anum, A.; Al-Hussain, S.A.; Ahmad, S.; Irfan, A.; Zaki, M.E. An Insight into Carbon Nanomaterial-Based Photocatalytic Water Splitting for Green Hydrogen Production. Catalysts 2023, 13, 66. [Google Scholar] [CrossRef]
- Mohsin, M.; Ishaq, T.; Bhatti, I.A.; Jilani, A.; Melaibari, A.A.; Abu-Hamdeh, N.H. Semiconductor Nanomaterial Photocatalysts for Water-Splitting Hydrogen Production: The Holy Grail of Converting Solar Energy to Fuel. Nanomaterials 2023, 13, 546. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yu, X.; Peng, L.; Luo, J.; Ning, X.; Fan, X.; Zhou, X.; Zhou, X. Pd (Ii) Coordination Molecule Modified G-C3N4 for Boosting Photocatalytic Hydrogen Production. J. Colloid Interface Sci. 2024, 671, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Gao, T.; Wang, Y.; Chen, Y.; Luo, W.; Wu, Y.; Xie, Y.; Wang, Y.; Zhang, Y. Engineering of Bimetallic Au–Pd Alloyed Particles on Nitrogen Defects Riched G-C3N4 for Efficient Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2024, 63, 1116–1127. [Google Scholar] [CrossRef]
- Palani, G.; Apsari, R.; Hanafiah, M.M.; Venkateswarlu, K.; Lakkaboyana, S.K.; Kannan, K.; Shivanna, A.T.; Idris, A.M.; Yadav, C.H. Metal-Doped Graphitic Carbon Nitride Nanomaterials for Photocatalytic Environmental Applications—A Review. Nanomaterials 2022, 12, 1754. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xiao, H.; Yang, J.; Luan, X.; Fang, D.; Yang, L.; Zi, J.; Lian, Z. Modulation of Electronic Density in Ultrathin G-C3N4 for Enhanced Photocatalytic Hydrogen Evolution through an Efficient Hydrogen Spillover Pathway. Appl. Catal. B Environ. 2024, 341, 123334. [Google Scholar] [CrossRef]
- Sewnet, A.; Alemayehu, E.; Abebe, M.; Mani, D.; Thomas, S.; Kalarikkal, N.; Lennartz, B. Single-Step Synthesis of Graphitic Carbon Nitride Nanomaterials by Directly Calcining the Mixture of Urea and Thiourea: Application for Rhodamine B (Rhb) Dye Degradation. Nanomaterials 2023, 13, 762. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Shan, P.; Shi, W.; Guo, F. Photothermal-Assisted Photocatalytic Degradation of Antibiotic by Black G-C3N4 Materials Derived from C/N Precursors and Tetrachlorofluorescein. Catalysts 2025, 15, 504. [Google Scholar] [CrossRef]
- Zhao, B.; Gong, W.; Liu, X.; Guo, H.; Yan, L.; Gao, A.; Lin, J. Ternary Synergism: Synthesis of S, C Co-Doped G-C3N4 Hexagonal Ultra-Thin Tubular Composite Photocatalyst for Efficient Visible-Light-Driven Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2024, 61, 1317–1329. [Google Scholar] [CrossRef]
- Meng, D.; Ruan, X.; Xu, M.; Jiao, D.; Fang, G.; Qiu, Y.; Zhang, Y.; Zhang, H.; Ravi, S.K.; Cui, X. An S-Scheme Artificial Photosynthetic System with H-TiO2/G-C3N4 Heterojunction Coupled with Mxene Boosts Solar H2 Evolution. J. Mater. Sci. Technol. 2025, 211, 22–29. [Google Scholar] [CrossRef]
- Ma, Z.; Jia, X.; Song, X.; Xie, Y.; Zhao, L.; Zhang, J. Efficient Photocatalytic Hydrogen Evolution by in Situ Construction of Nb4+ Charge-Carrier Channels in Hollow Porous Tubular C3N4 and Nb2O5 Z-Scheme Heterojunctions. Mater. Today Phys. 2024, 46, 101523. [Google Scholar] [CrossRef]
- Xu, X.; Huang, Z.; Tan, L.; Zhang, Z.; Chen, B.; Xia, X.; Cheng, G.; Chen, X. Surface Modification of G-C3N4 for Enhanced Photocatalytic Activity Via a Simple Illumination in Pure Water. Appl. Surf. Sci. 2024, 672, 160794. [Google Scholar] [CrossRef]
- Zheng, D.D.; Huang, C.J.; Wang, X.C. Post-Annealing Reinforced Hollow Carbon Nitride Nanospheres for Hydrogen Photosynthesis. Nanoscale 2015, 7, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Su, T.M.; Shao, Q.; Qin, Z.Z.; Guo, Z.H.; Wu, Z.L. Role of Interfaces in Two-Dimensional Photocatalyst for Water Splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Bury, D.; Jakubczak, M.; Purbayanto, M.A.K.; Rybak, M.; Birowska, M.; Wójcik, A.; Moszczynska, D.; Eisawi, K.; Prenger, K.; Presser, V.; et al. Wet-Chemical Etching and Delamination of MoAlB into Mbene and Its Outstanding Photocatalytic Performance. Adv. Funct. Mater. 2023, 33, 2308156. [Google Scholar] [CrossRef]
- Wu, T.; He, Q.; Liu, Z.; Shao, B.; Liang, Q.; Pan, Y.; Huang, J.; Peng, Z.; Liu, Y.; Zhao, C.; et al. Tube Wall Delamination Engineering Induces Photogenerated Carrier Separation to Achieve Photocatalytic Performance Improvement of Tubular G-C3N4. J. Hazard. Mater. 2022, 424, 127177. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Ge, G.; Zhao, Z. Fabrication of Tubular G-C3N4 with N-Defects and Extended Π-Conjugated System for Promoted Photocatalytic Hydrogen Production. ChemCatChem 2019, 11, 1534–1544. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, F.; Sun, H.; Shi, Y.; Shi, W. Well-Designed Three-Dimensional Hierarchical Hollow Tubular G-C3N4/ZnIn2S4 Nanosheets Heterostructure for Achieving Efficient Visible-Light Photocatalytic Hydrogen Evolution. J. Colloid Interface Sci. 2022, 607, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Li, C.; Zhang, X.; Wang, L.; Wang, W.; Meng, X. Self-Assembly Synthesis of Phosphorus-Doped Tubular G-C3N4/Ti3C2 Mxene Schottky Junction for Boosting Photocatalytic Hydrogen Evolution. Green Energy Environ. 2023, 8, 233–245. [Google Scholar] [CrossRef]
- Li, F.; Xiao, X.; Zhao, C.; Liu, J.; Li, Q.; Guo, C.; Tian, C.; Zhang, L.; Hu, J.; Jiang, B. TiO2-on-C3N4 Double-Shell Microtubes: In-Situ Fabricated Heterostructures toward Enhanced Photocatalytic Hydrogen Evolution. J. Colloid Interface Sci. 2020, 572, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Vequizo, J.J.M.; Hisatomi, T.; Rabeah, J.; Nakabayashi, M.; Wang, Z.; Xiao, Q.; Li, H.; Pan, Z.; Krause, M.; et al. Simultaneously Tuning the Defects and Surface Properties of Ta3N5 Nanoparticles by Mg–Zr Codoping for Significantly Accelerated Photocatalytic H2 Evolution. J. Am. Chem. Soc. 2021, 143, 10059–10064. [Google Scholar] [CrossRef] [PubMed]
- Dzade, N.Y. CO2 and H2O Coadsorption and Reaction on the Low-Index Surfaces of Tantalum Nitride: A First-Principles DFT-D3 Investigation. Catalysts 2020, 10, 1217. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, S.C.; Thaweesak, S.; Luo, B.; Wang, L.Z. Tantalum (Oxy)Nitride: Narrow Bandgap Photocatalysts for Solar Hydrogen Generation. Engineering 2017, 3, 365–378. [Google Scholar] [CrossRef]
- Zhan, X.Q.; Zheng, Y.P.; Li, B.; Fang, Z.; Yang, H.L.; Zhang, H.T.; Xu, L.Y.; Shao, G.; Hou, H.L.; Yang, W.Y. Rationally Designed Ta3N5/ZnIn2S4 1D/2D Heterojunctions for Boosting Visible-Light-Driven Hydrogen Evolution. Chem. Eng. J. 2022, 431, 134053. [Google Scholar] [CrossRef]
- Xiao, C.; Dong, G.; Yao, T.; Han, K.; Dong, T.; Zhou, T. The Visible Light Photocatalytic Performance of G-C3N4 Is Regulated by the Brønsted Acid Site on the Mullite Surface. Appl. Surf. Sci. 2024, 654, 159453. [Google Scholar] [CrossRef]
- Tang, S.; Yang, S.; Chen, Y.; Yang, Y.; Li, Z.; Zi, L.; Liu, Y.; Wang, Y.; Li, Z.; Fu, Z.; et al. Ionothermally Synthesized S-Scheme Isotype Heterojunction of Carbon Nitride with Significantly Enhanced Photocatalytic Performance for Hydrogen Evolution and Carbon Dioxide Reduction. Carbon 2023, 201, 815–828. [Google Scholar] [CrossRef]
- Negro, P.; Cesano, F.; Damin, A.; Brescia, R.; Scarano, D. Porous G-C3N4-Based Nanoarchitectures by Playing with Sustainable Precursors: Role of Urea/Melamine Ratio on the Structure/Properties Relationship. J. Alloys Compd. 2024, 1002, 175053. [Google Scholar] [CrossRef]
- Zažímal, F.; Atri, S.; Plašienka, D.; Vrána, L.; Stýskalík, A.; Vlk, A.; Čaplovičová, M.; Šob, M.; Monfort, O.; Homola, T. Fast Plasma Nanomodification of Graphitic Carbon Nitride by Amide and Carboxyl Groups for Enhanced Sulfamethoxazole Degradation in Wastewater: Detailed Experimental and DFT Study. J. Mater. Chem. A 2025, 13, 13909–13923. [Google Scholar] [CrossRef]
- Guo, S.; Deng, Z.; Li, M.; Jiang, B.; Tian, C.; Pan, Q.; Fu, H. Phosphorus-Doped Carbon Nitride Tubes with a Layered Micro-Nanostructure for Enhanced Visible-Light Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2016, 55, 1830–1834. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Ou, D.; Zheng, Y.; Li, B.; Xu, L.; Yang, H.; Yang, W.; Zhang, H.; Hou, H.; Yang, W. Boosted Photocatalytic Hydrogen Production over Two-Dimensional/Two-Dimensional Ta3N5/ReS2 Van Der Waals Heterojunctions. J. Colloid Interface Sci. 2023, 629, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, C.; Zheng, L.; Chen, F.; Qian, J.; Meng, X.; Chen, Z.; Zhong, S.; He, B. N3C-Defect-Tuned G-C3N4 Photocatalysts: Structural Optimization and Enhanced Tetracycline Degradation Performance. Nanomaterials 2025, 15, 466. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Peng, J.; Deng, F.; Li, X.; Zou, J.; Zhang, Y.; Luo, X. Preferential Degradation of Ofloxacin on All-Organic Molecularly Imprinted PDI/G-C3N4 Photocatalyst Via Specific Molecular Recognition. Sep. Purif. Technol. 2025, 353, 128499. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, C.; Tayyab, M.; Wei, Z.; Zheng, X.; Shangguan, W.; Zhang, S.; Chen, S.; Meng, S. Regulating Electron-Hole Pairs of G-C3N4 Efficiently Separated and Fully Utilized for Photosynthesis of H2O2 under Visible Light. Chem. Eng. J. 2025, 509, 161409. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, P.; Chen, Y.; Zhou, Z.; Yang, H.; Hong, Y.; Li, F.; Ni, L.; Yan, Y.; Gregory, D.H. Construction of Stable Ta3N5/G-C3N4 Metal/Non-Metal Nitride Hybrids with Enhanced Visible-Light Photocatalysis. Appl. Surf. Sci. 2017, 391, 392–403. [Google Scholar] [CrossRef]
- Alaya, Y.; Chouchene, B.; Medjahdi, G.; Balan, L.; Bouguila, N.; Schneider, R. Heterostructured S-TiO2/G-C3N4 Photocatalysts with High Visible Light Photocatalytic Activity. Catalysts 2024, 14, 226. [Google Scholar] [CrossRef]
- Mao, S.; Yao, G.; Liu, P.; Liu, C.; Wu, Y.; Ding, Z.; Ding, C.; Xia, M.; Wang, F. Construction of an Enhanced Built-in Electric Field in S-Doped G-C3N4/NiCo2O4 for Boosting Peroxymonosulfate Activation. Chem. Eng. J. 2023, 470, 144250. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Shi, W.X.; Zhuang, G.L.; Zhao, Q.P.; Ren, J.; Zhang, P.; Yin, H.Q.; Lu, T.B.; Zhang, Z.M. W Single-Atom Catalyst for CH4 Photooxidation in Water Vapor. Adv. Mater. 2022, 34, 2204448. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, T.; Dong, B.; Qi, Y.; Yuan, H.; Gao, J.; Yang, D.; Zhang, F. Flux-Assisted Synthesis of Prism-Like Octahedral Ta3N5 Single-Crystals with Controllable Facets for Promoted Photocatalytic H2 Evolution. Sol. RRL 2021, 5, 2000574. [Google Scholar] [CrossRef]
- Bharathkumar, S.; Mohan, S.; Alsaeedi, H.; Oh, T.H.; Vignesh, S.; Sundaramoorthy, A.; Valdes, H. Z-Scheme Driven Charge Transfer in G-C3N4/A-Fe2O3 Nanocomposites Enabling Photocatalytic Degradation of Crystal Violet and Chromium Reduction. Surf. Interfaces 2024, 54, 105299. [Google Scholar] [CrossRef]
- Liang, Y.-F.; Lu, J.-R.; Tian, S.-K.; Cui, W.-Q.; Liu, L. Pt Nanoclusters Modified Porous G-C3N4 Nanosheets to Significantly Enhance Hydrogen Production by Photocatalytic Water Reforming of Methanol. Chin. J. Chem. Eng. 2024, 66, 40–50. [Google Scholar] [CrossRef]
- Yu, C.; Yang, H.; Zhao, H.; Huang, X.; Liu, M.; Du, C.; Chen, R.; Feng, J.; Dong, S.; Sun, J.; et al. Simultaneous Hydrogen Production from Wastewater Degradation by Protonated Porous G-C3N4/BiVO4 Z-Scheme Composite Photocatalyst. Sep. Purif. Technol. 2024, 335, 126201. [Google Scholar] [CrossRef]
- Sun, Q.M.; Xu, J.J.; Tao, F.F.; Ye, W.; Zhou, C.; He, J.H.; Lu, J.M. Boosted Inner Surface Charge Transfer in Perovskite Nanodots@ Mesoporous Titania Frameworks for Efficient and Selective Photocatalytic CO2 Reduction to Methane. Angew. Chem. Int. Ed. 2022, 61, e202200872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, S.; Qin, H.; Zheng, Q.; Zhang, P.; Li, X.; Li, C.; Wang, T.; Li, N.; Zhang, S.; et al. Metal-Organic Framework-Derived Nitrogen-Doped Carbon-Coated Hollow Tubular In2O3/CdZnS Heterojunction for Efficient Photocatalytic Hydrogen Evolution. Sci. China Mater. 2023, 66, 1042–1052. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, M.; Li, H.; Wang, Z.; Wang, Y. Multi-Channel Charge Transfer in Self-Supporting B-G-C3Nx/Bi2S3/CdS Dual S-Scheme Heterojunction toward Enhanced Photothermal-Photocatalytic Performance. Nano Energy 2024, 120, 109164. [Google Scholar] [CrossRef]
- Zhan, X.Q.; Zhang, H.T.; Hou, H.L.; Gao, F.M.; Wang, L.; Ou, D.L.; Li, B.; Xu, L.Y.; Yang, W.Y. Rationally Designed Ta3N5/ZnO Core-Shell Nanofibers for Significantly Boosts Photocatalytic Hydrogen Production. Appl. Surf. Sci. 2023, 611, 155788. [Google Scholar] [CrossRef]
- Zhang, J.D.; Zhang, R.Y.; Jia, X.W.; Li, J.M.; Sun, M.L.; Zhang, S.K.; Guo, Z.F.; Jiao, X.Y.; Liu, X.C.; Jin, Z.S.; et al. Template-Free Synthesis of Honeycomb-Structured Ta3N5 Foam Nanoplates with Expanded Light Absorption, Abundant Active Sites and Fast Charges Transport for Visible-Light-Driven H2 Evolution. New J. Chem. 2025, 49, 8485–8493. [Google Scholar] [CrossRef]
- Zhou, H.R.; Ke, J.; Wu, H.; Liu, J.; Xu, D.S.; Zou, X.J. Manganese Tungstate/Graphitic Carbon Nitride S-Scheme Heterojunction for Boosting Hydrogen Evolution and Mechanism Exploration. Mater. Today Energy 2022, 23, 100918. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, W.W.; Wang, G.C.; Zhao, Z.K. Poly-(Imidazolium-Methylene) Chloride Mediated Self-Assembly Strategy to Modulate Electronic Structure of Carbon Nitride for Enhanced Visible-Light Photocatalytic Hydrogen Evolution. Chemcatchem 2023, 15, e202201620. [Google Scholar] [CrossRef]
- Shao, X.X.; Zhao, X.F.; Li, K.P.; Sun, M.H.; Ji, X.P.; Lu, H.D.; Liu, Y.P. Preparation of WP2/G-C3N4 Composite Photocatalysts and Their Enhanced Photocatalytic Performance. Ionics 2025, 31, 4725–4737. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Jia, X.F.; Ma, Z.Y.; Zhang, J.Y. 2D/1D Z-Scheme WO3/G-C3N4 Photocatalytic Heterojunction with Enhanced Photo-Induced Charge-Carriers Separation. J. Phys. D. Appl. Phys. 2022, 55, 434005. [Google Scholar] [CrossRef]
- Wang, B.C.; Huang, L.L.; Peng, T.; Wang, R.; Jin, J.; Wang, H.W.; He, B.B.; Gong, Y.S. Attapulgite-Intercalated G-C3N4/ZnIn2S4 3D Hierarchical Z-Scheme Heterojunction for Boosting Photocatalytic Hydrogen Production. J. Colloid Interf. Sci. 2024, 675, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.G.; Jia, R.M.; Wang, C.J.; Guan, W.; Wang, P.; Zhang, L.; Gan, Z.X.; Dong, L.F.; Yu, L.Y.; Sui, L.A. High Efficiency Photocatalytic Hydrogen Evolution by Black Phosphorus Quantum Dots Decorated 1D G-C3N4 Nanotubes. Int. J. Hydrogon Energ. 2024, 95, 766–772. [Google Scholar] [CrossRef]
- Ullah, I.; Lu, X.J.; Chen, S.; Li, J.H.; Habib, S.; Murtaza, G.; Tofaz, T.; Xu, A.W. Electron-Deficient Boron-Doped G-C3N4 as an Efficient and Robust Photocatalyst for Visible-Light Driven Hydrogen Evolution from Water Splitting. Adv. Sustain. Syst. 2024, 8, 2400103. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, D.; Deng, C.K.; Zhong, J.B.; Dou, L.; Huang, S.T. Rationally Construction of Dy2O3/G-C3N4 Heterojunctions with Largely Enhanced Photocatalytic Hydrogen Evolution Activity. Mater. Res. Bull. 2024, 179, 112971. [Google Scholar] [CrossRef]
- Zhang, Y.; Luan, J.D.; Li, P.P.; Jiang, L.D.; Yan, H.B.; Liu, W.A.; Yan, Z. Carbon Doping and Bridging Oxygen Benefit for G-C3N4 to Photocatalytic H2 Production from Methanol/Water Splitting: Experiments and Theoretical Calculations. Carbon 2024, 228, 119430. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).