Plasmon-Driven Catalytic Inhibition of pATP Oxidation as a Mechanism for Indirect Fe²⁺ Detection on a SERS-Active Platform
Abstract
1. Introduction
2. Results and Discussion
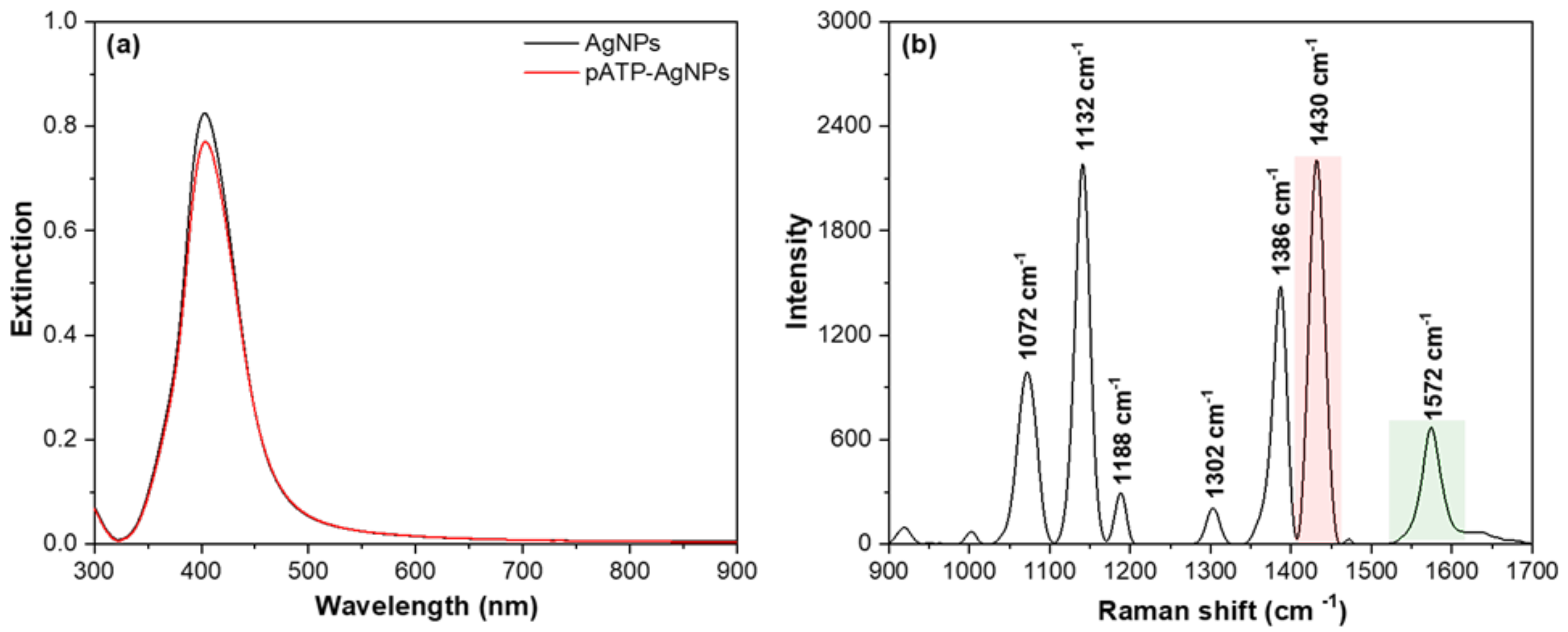
2.1. Synthesis of AgNPs and Functionalization with pATP
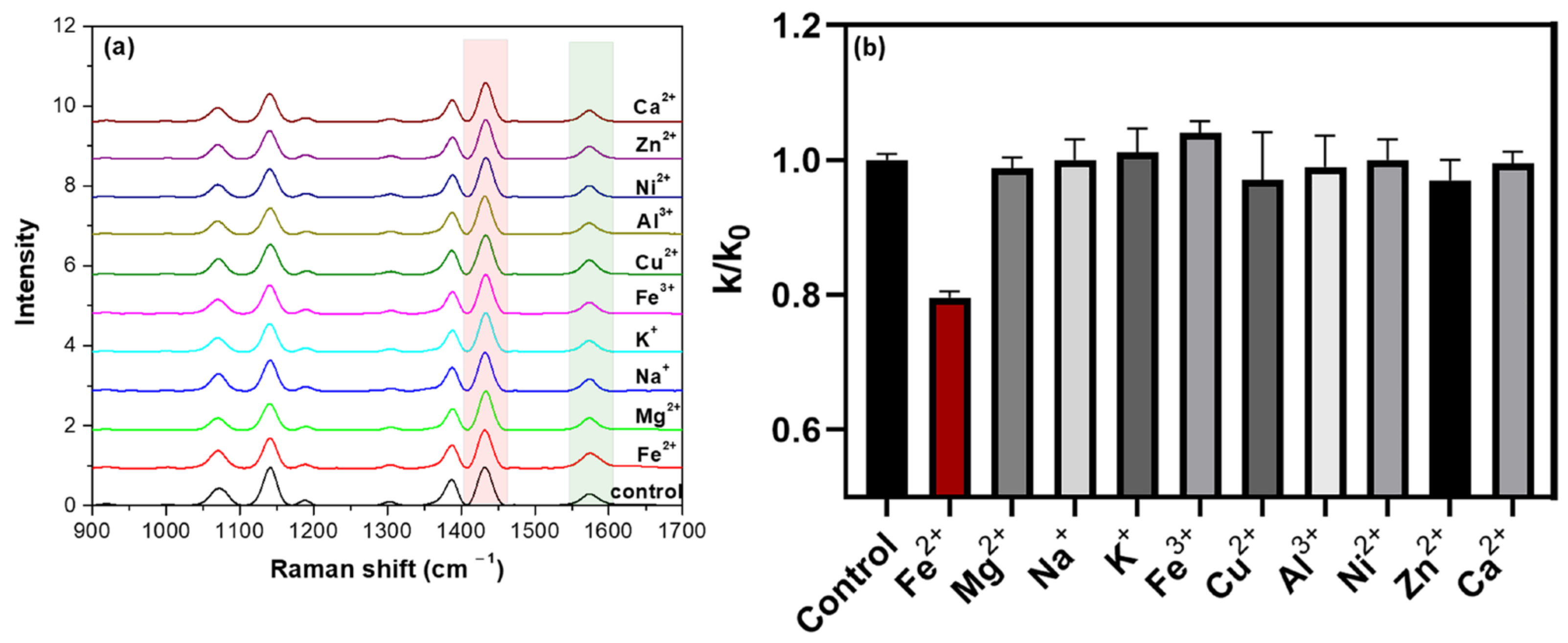
2.2. Detection of Fe2+ Using the Catalysis-Based Sensing Platform
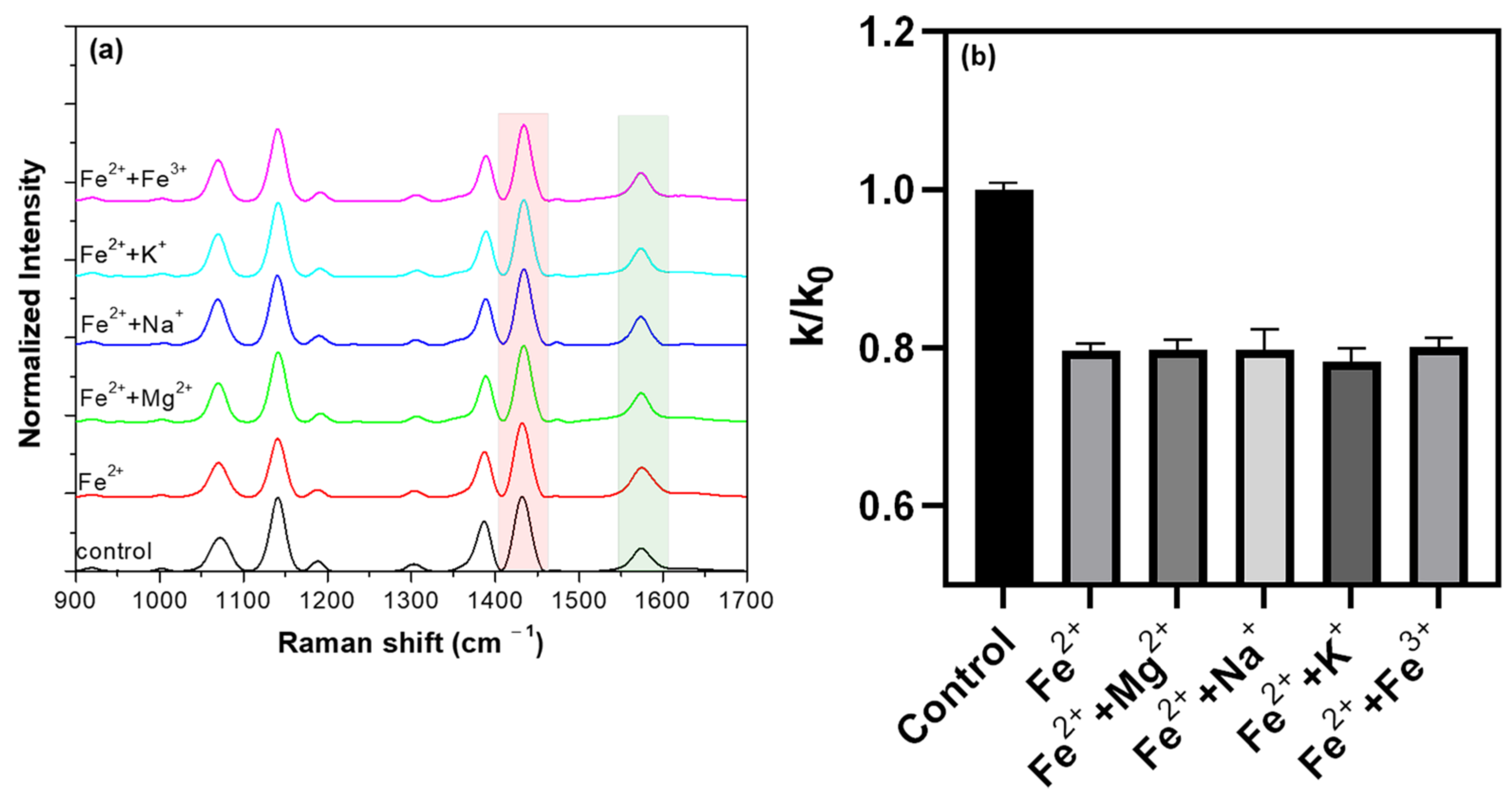
2.3. Competitivity and Real Water Samples Assays for the Detection of Fe2+
3. Materials and Methods
3.1. Materials
3.2. Synthesis of AgNPs
3.3. Functionalization of AgNPs with pATP
3.4. Sensitivity Assay
3.5. Selectivity Assay
3.6. Competitivity Assay
3.7. Real Sample Assay
3.8. Equipment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia, M.A. Surface Plasmons in Metallic Nanoparticles: Fundamentals and Applications. J. Phys. D Appl. Phys. 2011, 44, 283001. [Google Scholar] [CrossRef]
- Rossi, T.P.; Erhart, P.; Kuisma, M. Hot-Carrier Generation in Plasmonic Nanoparticles: The Importance of Atomic Structure. ACS Nano 2020, 14, 9963–9971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, C.; Zheng, H.; Xu, H. Plasmon-Driven Catalysis on Molecules and Nanomaterials. Acc. Chem. Res. 2019, 52, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, H.; Camden, J.P. Utilizing Light-Triggered Plasmon-Driven Catalysis Reactions as a Template for Molecular Delivery and Release. Chem. Sci. 2017, 8, 5902–5908. [Google Scholar] [CrossRef]
- Tamaki, K.; Verma, P.; Yoshii, T.; Shimojitosho, T.; Kuwahara, Y.; Mori, K.; Yamashita, H. Design of Au Nanorods-Based Plasmonic Catalyst in Combination with Nanohybrid Pd-rGO Layer for Boosting CO2 Hydrogenation to Formic Acid under Visible Light Irradiation. Catal. Today 2023, 411–412, 113795. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, P.; Yang, X.; Liang, W.; Sun, M. Surface Plasmon-Driven Photocatalysis in Ambient, Aqueous and High-Vacuum Monitored by SERS and TERS. J. Photochem. Photobiol. C: Photochem. Rev. 2016, 27, 100–112. [Google Scholar] [CrossRef]
- Verma, R.; Sharma, G.; Polshettiwar, V. The Paradox of Thermal vs. Non-Thermal Effects in Plasmonic Photocatalysis. Nat. Commun. 2024, 15, 7974. [Google Scholar] [CrossRef]
- Kim, Y.; Dumett Torres, D.; Jain, P.K. Activation Energies of Plasmonic Catalysts. Nano Lett. 2016, 16, 3399–3407. [Google Scholar] [CrossRef]
- Yao, X.; Ehtesabi, S.; Höppener, C.; Deckert-Gaudig, T.; Schneidewind, H.; Kupfer, S.; Gräfe, S.; Deckert, V. Mechanism of Plasmon-Induced Catalysis of Thiolates and the Impact of Reaction Conditions. J. Am. Chem. Soc. 2024, 146, 3031–3042. [Google Scholar] [CrossRef]
- Ren, X.; Cao, E.; Lin, W.; Song, Y.; Liang, W.; Wang, J. Recent Advances in Surface Plasmon-Driven Catalytic Reactions. RSC Adv. 2017, 7, 31189–31203. [Google Scholar] [CrossRef]
- Long, Y.; Liping, M.; Liu, Y.; Song, P.; Xia, L. Conversion of PATP to DMAB based on Ag+-induced catalytic oxidation. J. Raman Spect. 2020, 51, 838–843. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, B. pH Dependent Plasmon-Driven Surface-Catalysis Reactions of p,P′-Dimercaptoazobenzene Produced from Para-Aminothiophenol and 4-Nitrobenzenethiol. Sci. China Chem. 2012, 55, 2567–2572. [Google Scholar] [CrossRef]
- Canpean, V.; Iosin, M.; Astilean, S. Disentangling SERS Signals from Two Molecular Species: A New Evidence for the Production of p,P′-Dimercaptoazobenzene by Catalytic Coupling Reaction of p-Aminothiophenol on Metallic Nanostructures. Chem. Phys. Lett. 2010, 500, 277–282. [Google Scholar] [CrossRef]
- Sun, M.; Xu, H. A Novel Application of Plasmonics: Plasmon-Driven Surface-Catalyzed Reactions. Small 2012, 8, 2777–2786. [Google Scholar] [CrossRef]
- Gabudean, A.M.; Biro, D.; Astilean, S. Localized Surface Plasmon Resonance (LSPR) and Surface-Enhanced Raman Scattering (SERS) Studies of 4-Aminothiophenol Adsorption on Gold Nanorods. J. Mol. Struct. 2011, 993, 420–424. [Google Scholar] [CrossRef]
- Wang, J.L.; Ando, R.A.; Camargo, P.H.C. Investigating the Plasmon-Mediated Catalytic Activity of AgAu Nanoparticles as a Function of Composition: Are Two Metals Better than One? ACS Catal. 2014, 4, 3815–3819. [Google Scholar] [CrossRef]
- Zhong, H.; Chen, J.; Chen, J.; Tao, R.; Jiang, J.; Hu, Y.; Xu, J.; Zhang, T.; Liao, J. Plasmon Catalytic PATP Coupling Reaction on Ag-NPs/Graphite Studied via in Situ Electrochemical Surface-Enhanced Raman Spectroscopy. Phys. Chem. Chem. Phys. 2020, 22, 23482–23490. [Google Scholar] [CrossRef]
- Xu, J.-F.; Liu, G.-K. Laser-Induced Chemical Transformation of PATP Adsorbed on Ag Nanoparticles by Surface-Enhanced Raman Spectroscopy—A Study of the Effects from Surface Morphology of Substrate and Surface Coverage of PATP. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 873–877. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, D.; Zhao, Y.; Yang, Y.; Wu, S.; Wang, J.; Xia, L.; Song, P. Solvent-Controlled Plasmon-Assisted Surface Catalysis Reaction of 4-Aminothiophenol Dimerizing to p,p’-Dimercaptoazobenzene on Ag Nanoparticles. Heliyon 2019, 5, e01545. [Google Scholar] [CrossRef]
- Zhou, B.; Zhong, J.; Tang, X.; Liu, J.; Shen, J.; Wang, C.; Ou, W.; Wang, H.; Liu, L.; Pan, J.; et al. In Situ Surface-Enhanced Raman Spectroscopy Monitoring of Molecular Reorientation in Plasmon-Mediated Chemical Reactions. J. Catal. 2022, 413, 527–533. [Google Scholar] [CrossRef]
- Jiang, Y.; Cao, J.; Hu, S.; Cheng, T.; Wang, H.; Guo, X.; Ying, Y.; Liu, X.; Wang, F.; Wen, Y.; et al. Internal Standard Optimization Advances Sensitivity and Robustness of Ratiometric Detection Method. Analyst 2024, 149, 2806–2811. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, G.; Wang, Y.; Song, P.; Zhang, Y.; Xia, L. Surface Plasmon-Assisted Catalytic Reduction of p-Nitrothiophenol for the Detection of Fe2+ by Surface-Enhanced Raman Spectroscopy. Anal. Biochem. 2023, 680, 115314. [Google Scholar] [CrossRef] [PubMed]
- Beeram, R.; Vepa, K.R.; Soma, V.R. Recent Trends in SERS-Based Plasmonic Sensors for Disease Diagnostics, Biomolecules Detection, and Machine Learning Techniques. Biosensors 2023, 13, 328. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, Y.; Liu, Y.; Hu, X.; Chen, H. Current Strategies of Plasmonic Nanoparticles Assisted Surface-Enhanced Raman Scattering toward Biosensor Studies. Biosens. Bioelectron. 2023, 228, 115231. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.-H.; Oh, J.-W.; Nam, J.-M. Surface-Enhanced Raman Scattering-Based Detection of Hazardous Chemicals in Various Phases and Matrices with Plasmonic Nanostructures. Nanoscale 2019, 11, 20379–20391. [Google Scholar] [CrossRef]
- Hada, A.-M.; Zetes, M.; Focsan, M.; Astilean, S.; Craciun, A.-M. Photoluminescent Histidine-Stabilized Gold Nanoclusters as Efficient Sensors for Fast and Easy Visual Detection of Fe Ions in Water Using Paper-Based Portable Platform. IJMS 2022, 23, 12410. [Google Scholar] [CrossRef]
- Tremaine, P.R.; LeBlanc, J.C. The Solubility of Magnetite and the Hydrolysis and Oxidation of Fe2+ in Water to 300 °C. J. Solut. Chem. 1980, 9, 415–442. [Google Scholar] [CrossRef]
- Mundra, S.; Tits, J.; Wieland, E.; Angst, U.M. Aerobic and Anaerobic Oxidation of Ferrous Ions in Near-Neutral Solutions. Chemosphere 2023, 335, 138955. [Google Scholar] [CrossRef]
- World Health Organization. In Guidelines for Drinking-Water Quality: Fourth Edition Incorporating First Addendum, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0.
- Laschuk, N.O.; Ebralidze, I.I.; Quaranta, S.; Kerr, S.T.W.; Egan, J.G.; Gillis, S.; Gaspari, F.; Latini, A.; Zenkina, O.V. Rational Design of a Material for Rapid Colorimetric Fe2+ Detection. Mater. Des. 2016, 107, 18–25. [Google Scholar] [CrossRef]
- Yaman, M.; Kaya, G. Speciation of Iron (II) and (III) by Using Solvent Extraction and Flame Atomic Absorption Spectrometry. Anal. Chim. Acta 2005, 540, 77–81. [Google Scholar] [CrossRef]
- Neethipathi, D.K.; Beniwal, A.; Ganguly, P.; Bass, A.; Scott, M.; Dahiya, R. Electrochemical Detection of Fe2+ Ions in Water Using 2-Dimensional g-C3N4 Modified Glassy Carbon Electrode-Based Sensor. In Proceedings of the 2023 IEEE Applied Sensing Conference (APSCON), Bengaluru, India, 23–25 January 2023; pp. 1–3. [Google Scholar]
- Huang, Y.-F.; Wu, D.-Y.; Zhu, H.-P.; Zhao, L.-B.; Liu, G.-K.; Ren, B.; Tian, Z.-Q. Surface-Enhanced Raman Spectroscopic Study of p-Aminothiophenol. Phys. Chem. Chem. Phys. 2012, 14, 8485. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.M.P.S.; Nouws, H.P.A.; Delerue-Matos, C.; Martín-Yerga, D. Electrochemical Detection and Characterization of Nanoparticles: A Potential Tool for Environmental Purposes. Curr. Opin. Electrochem. 2020, 22, 58–64. [Google Scholar] [CrossRef]
- Li, H.; Xia, H.; Wang, D.; Tao, X. Simple Synthesis of Monodisperse, Quasi-Spherical, Citrate-Stabilized Silver Nanocrystals in Water. Langmuir 2013, 29, 5074–5079. [Google Scholar] [CrossRef] [PubMed]
- Desimoni, E.; Brunetti, B. About Estimating the Limit of Detection by the Signal to Noise Approach. Pharm. Anal. Acta 2015, 6, 4. [Google Scholar] [CrossRef]


| Added Ions | Added (mM) | Found (mM) | Recovered (%) |
|---|---|---|---|
| Fe2+ + Mg2+ | 0.2 | 0.198 ± 0.013 | 99.00 ± 6.50 |
| Fe2+ + Na+ | 0.202 ± 0.008 | 101.00 ± 4.00 | |
| Fe2+ + K+ | 0.199 ± 0.018 | 99.50 ± 9.00 | |
| Fe2+ + Fe3+ | 0.197 ± 0.011 | 98.50 ± 5.50 |
| Added (mM) | Found (mM) | Recovered (%) |
|---|---|---|
| 0 | Non-detactable | - |
| 0.5 | 0.497 ± 0.012 | 99.41 ± 2.40 |
| 1 | 0.966 ± 0.018 | 96.64 ± 1.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hada, A.-M.; Moruz, M.-M.; Holca, A.; Astilean, S.; Lamy de la Chapelle, M.; Focsan, M. Plasmon-Driven Catalytic Inhibition of pATP Oxidation as a Mechanism for Indirect Fe²⁺ Detection on a SERS-Active Platform. Catalysts 2025, 15, 667. https://doi.org/10.3390/catal15070667
Hada A-M, Moruz M-M, Holca A, Astilean S, Lamy de la Chapelle M, Focsan M. Plasmon-Driven Catalytic Inhibition of pATP Oxidation as a Mechanism for Indirect Fe²⁺ Detection on a SERS-Active Platform. Catalysts. 2025; 15(7):667. https://doi.org/10.3390/catal15070667
Chicago/Turabian StyleHada, Alexandru-Milentie, Mihail-Mihnea Moruz, Alexandru Holca, Simion Astilean, Marc Lamy de la Chapelle, and Monica Focsan. 2025. "Plasmon-Driven Catalytic Inhibition of pATP Oxidation as a Mechanism for Indirect Fe²⁺ Detection on a SERS-Active Platform" Catalysts 15, no. 7: 667. https://doi.org/10.3390/catal15070667
APA StyleHada, A.-M., Moruz, M.-M., Holca, A., Astilean, S., Lamy de la Chapelle, M., & Focsan, M. (2025). Plasmon-Driven Catalytic Inhibition of pATP Oxidation as a Mechanism for Indirect Fe²⁺ Detection on a SERS-Active Platform. Catalysts, 15(7), 667. https://doi.org/10.3390/catal15070667







