Mechanochemical and Transition-Metal-Catalyzed Reactions of Alkynes
Abstract
1. Introduction
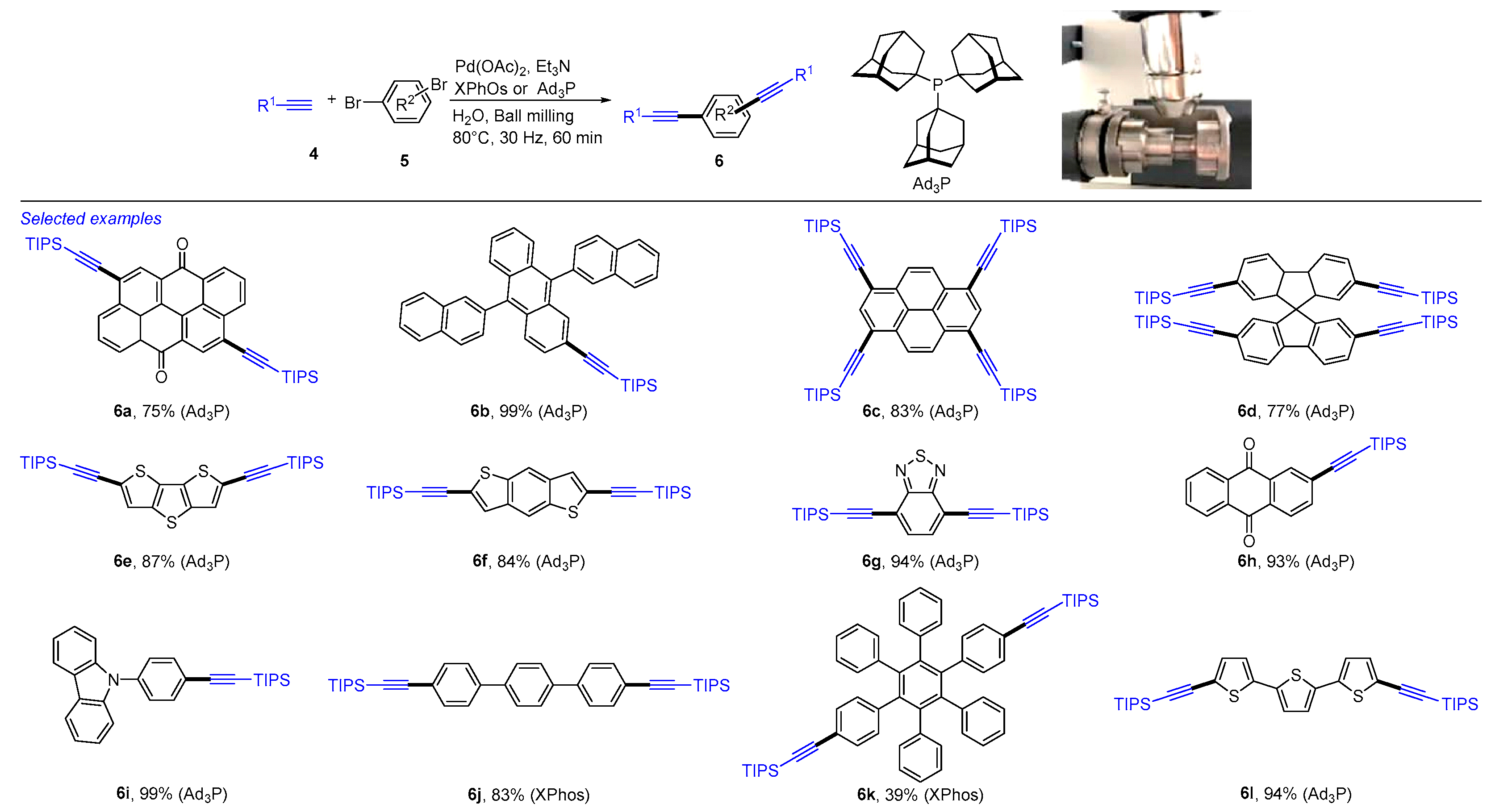
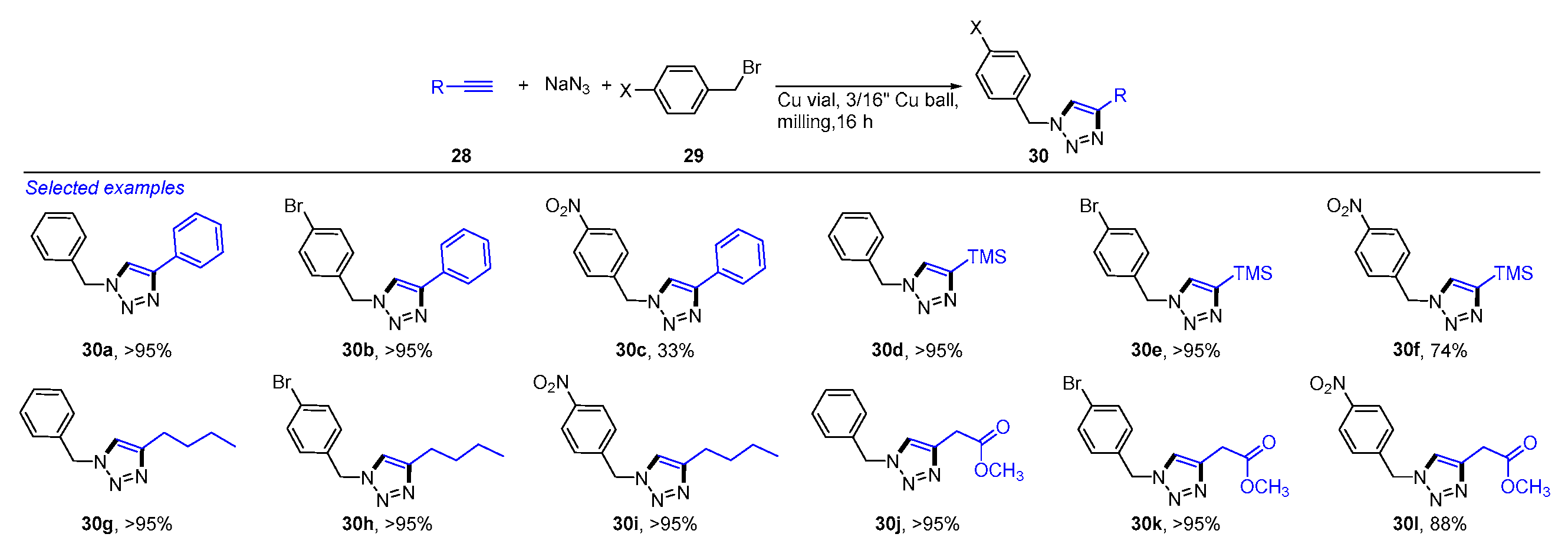
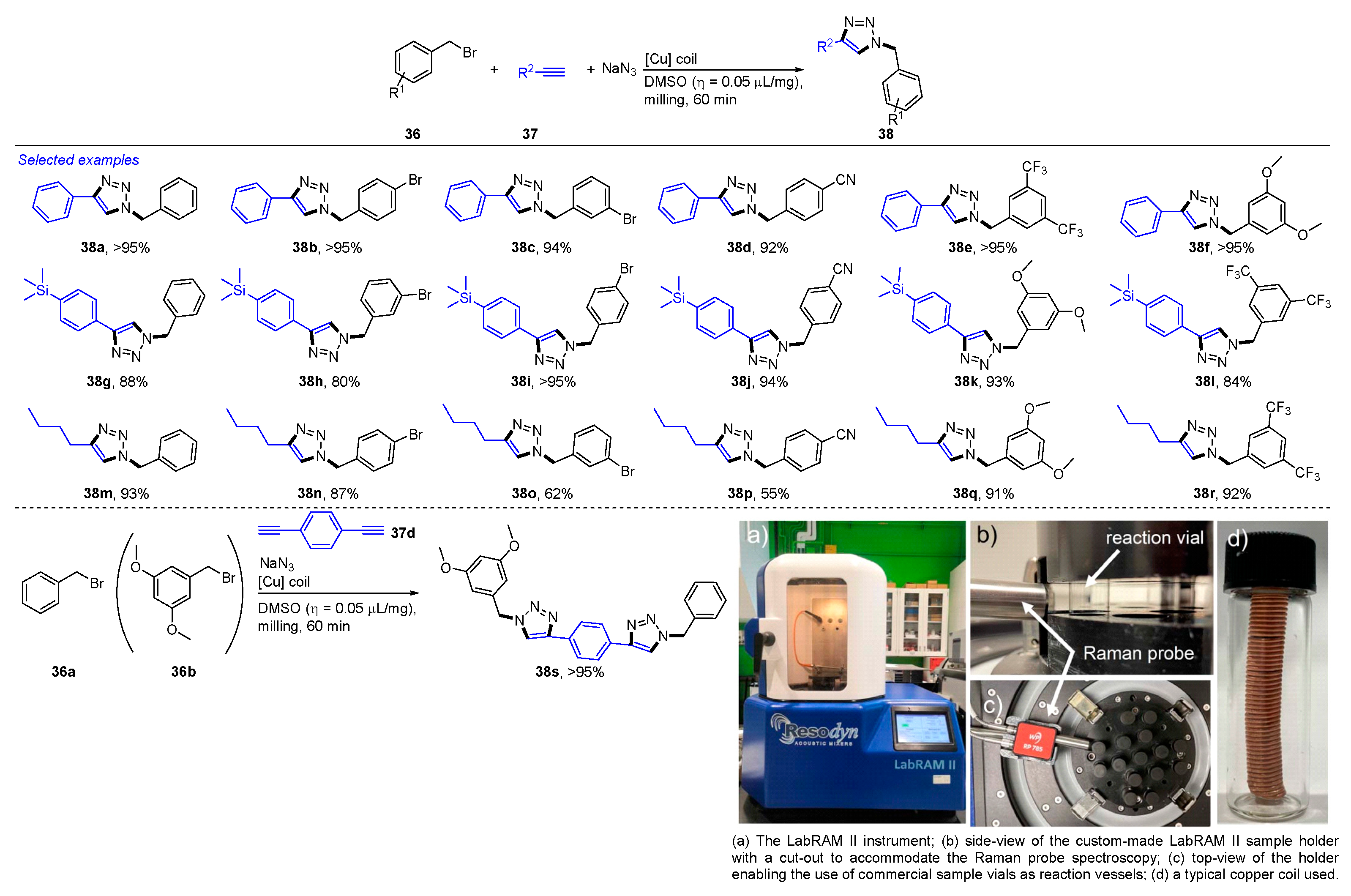
2. Mechanochemical and Transition-Metal-Catalyzed Coupling Reactions
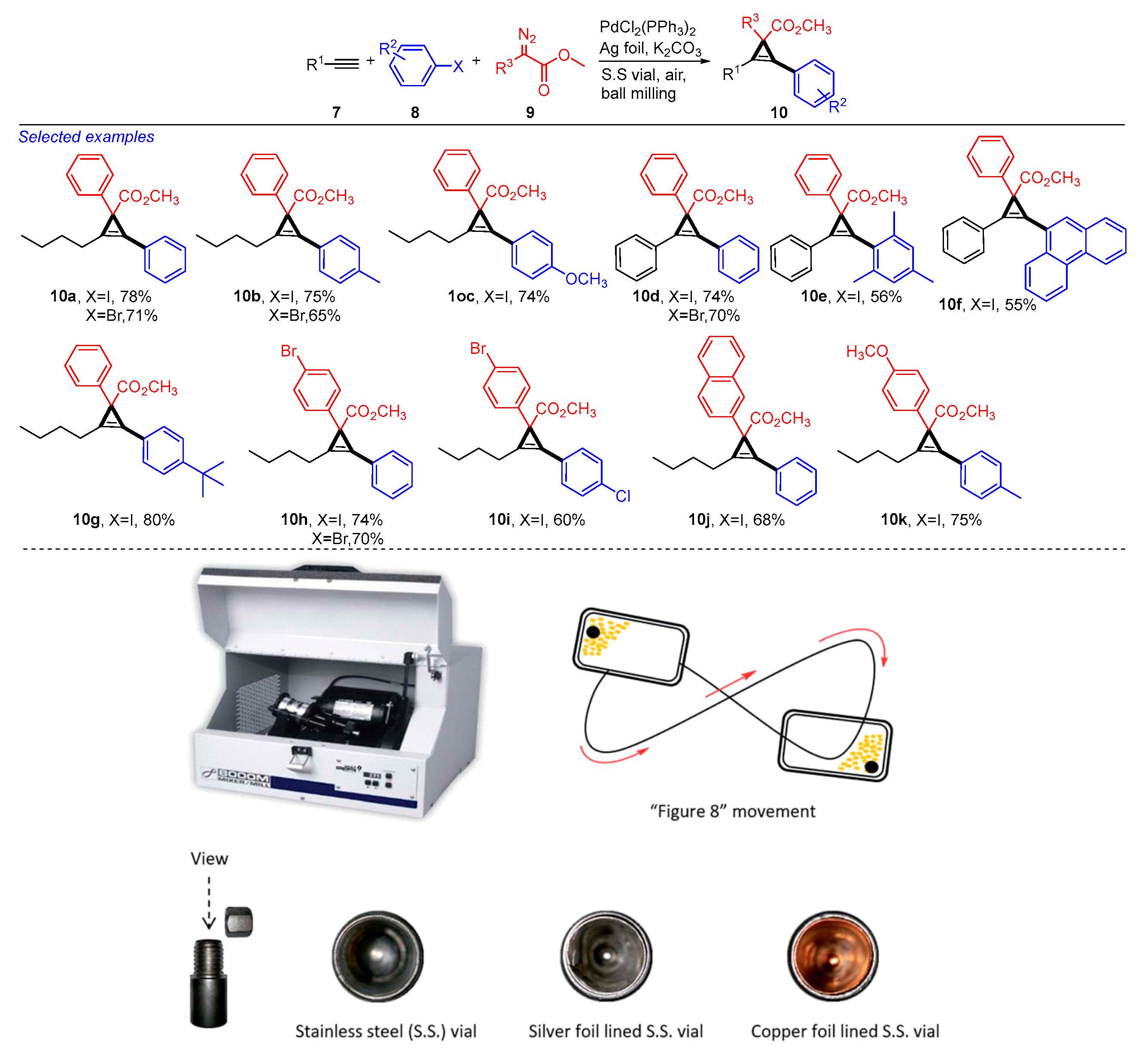
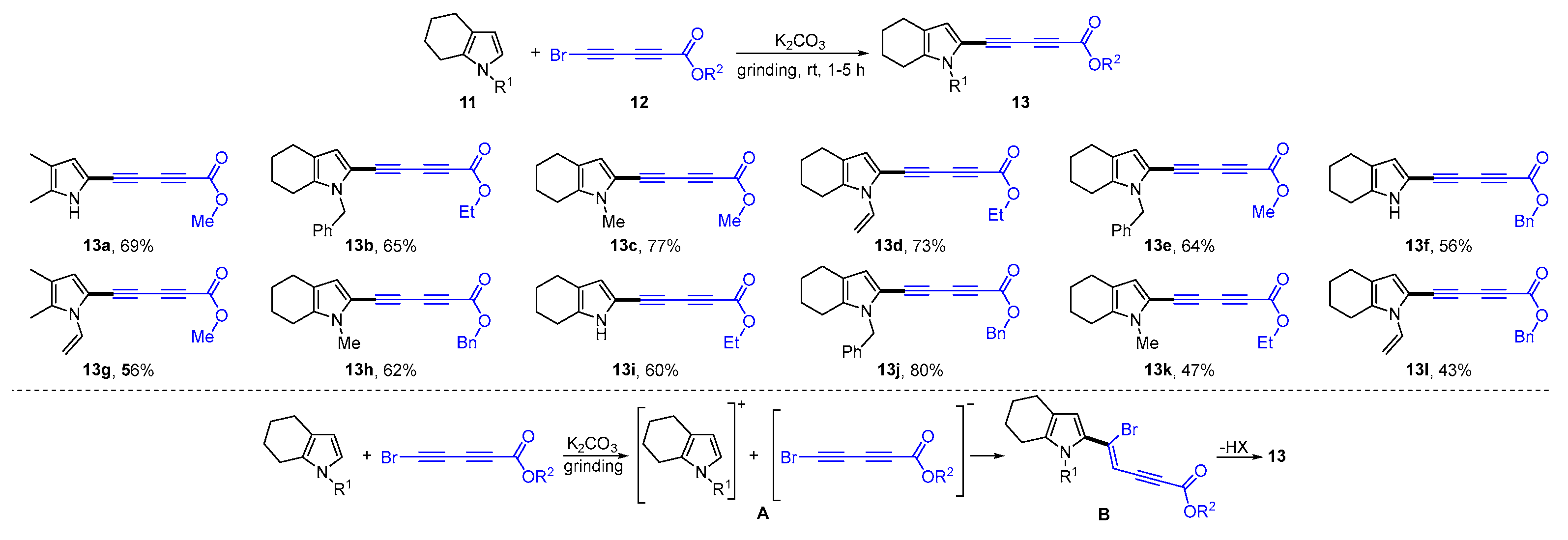
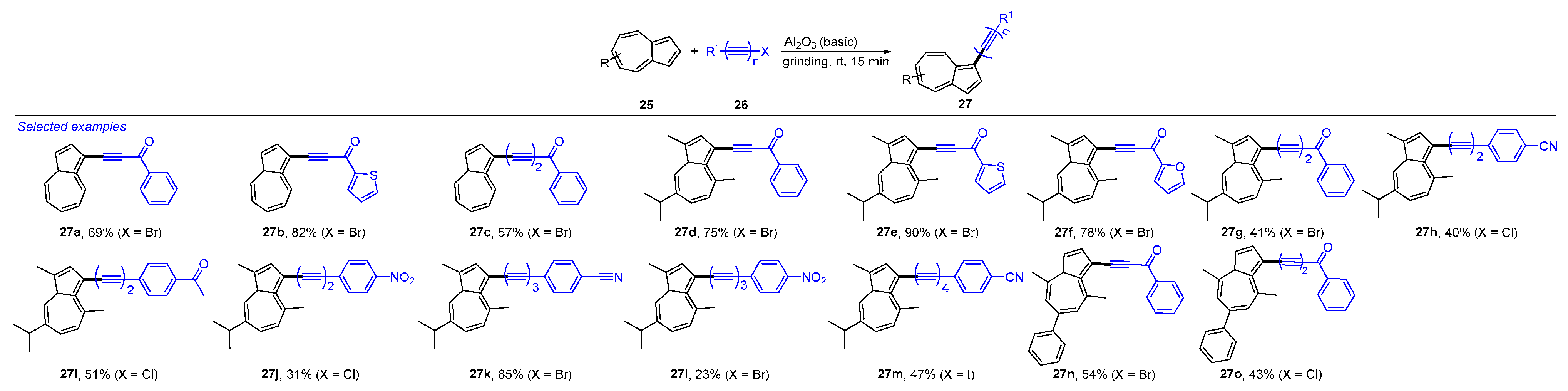
3. Mechanochemical and Transition-Metal-Catalyzed Cyclization
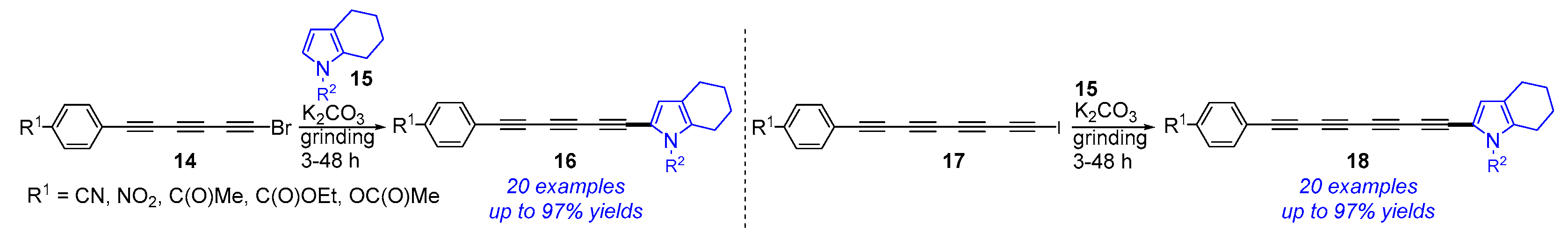
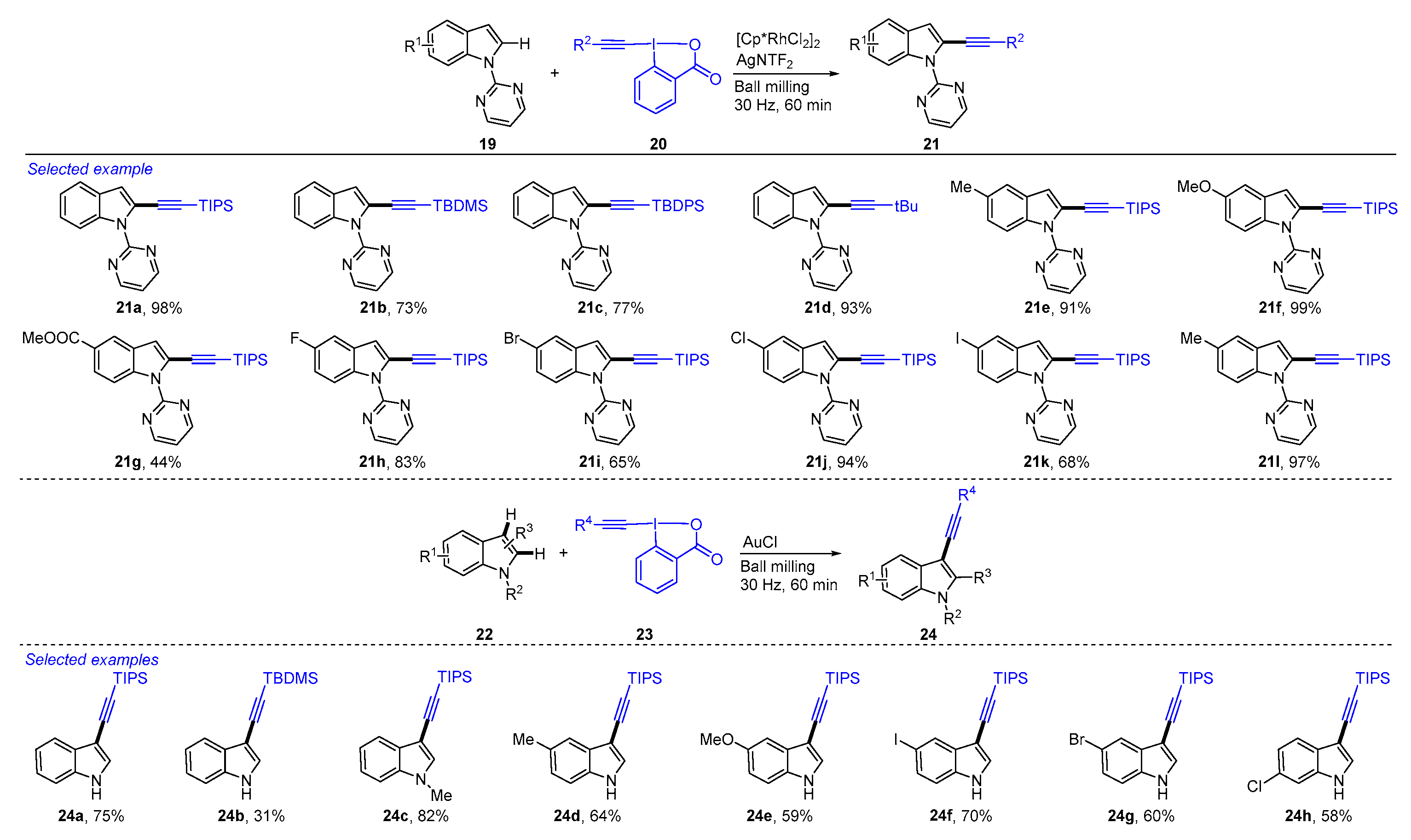
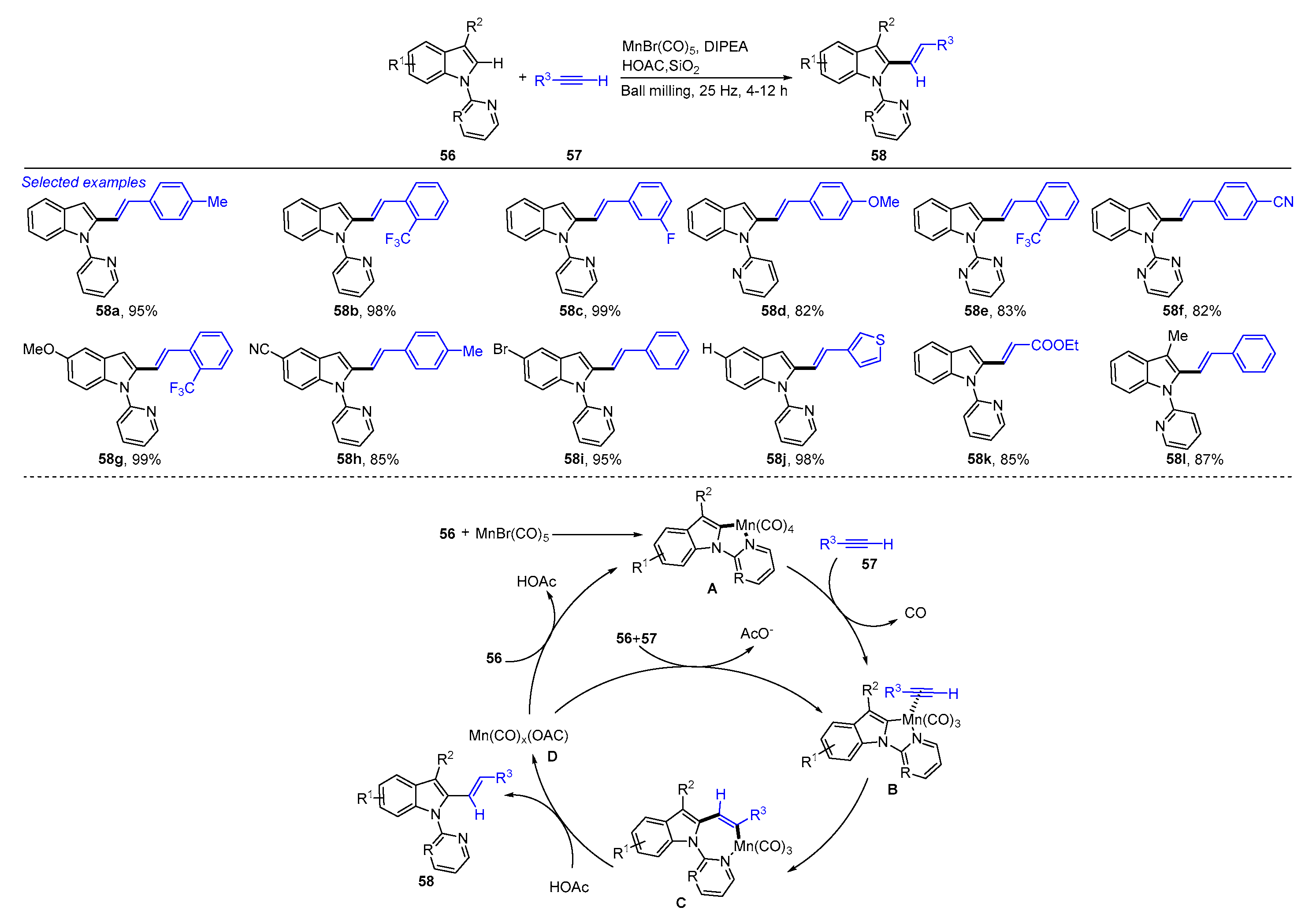
4. Mechanochemical and Transition-Metal-Catalyzed C−H Alkenylation
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yong, T.; Bati, G.; Garcia, F.; Stuparu, M.C. Mechanochemical transformation of planar polyarenes to curved fused-ring systems. Nat. Commun. 2021, 12, 5187. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Peng, H.; Wei, C.; Yuan, T.; Wojtas, L.; Shi, X. Gold-catalyzed oxidative coupling of alkynes toward the synthesis of cyclic conjugated diynes. Chemistry 2018, 4, 1983–1993. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, Y.-J.; Huang, C.; Xu, H.-C. Ruthenium-catalyzedelectrochemical dehydrogenative alkyne annulation. ACS Catal. 2018, 8, 3820–3824. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Cheng, Z.; Jiao, N.; Zhang, C. Selective nitrogen insertion into aryl alkanes. Nat. Commun. 2024, 15, 6016. [Google Scholar] [CrossRef] [PubMed]
- Parcero-Bouzas, S.; Correa, J.; Jimenez-Lopez, C.; Delgado Gonzalez, B.; Fernandez-Megia, E. Modular Synthesis of PEG-dendritic block copolymers by thermal azide-alkyne cycloaddition with internal alkynes and evaluation of their self-assembly for drug delivery applications. Biomacromolecules 2024, 25, 2780–2791. [Google Scholar] [CrossRef] [PubMed]
- Fehr, J.M.; Myrthil, N.; Garrison, A.L.; Price, T.W.; Lopez, S.A.; Jasti, R. Experimental and theoretical elucidation of SPAAC kinetics for strained alkyne-containing cycloparaphenylenes. Chem. Sci. 2023, 14, 2839–2848. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Yan, X.-Y.; Jiang, S.; Lu, Y.-N.; Tan, W.; Shi, F. Organocatalytic Nazarov-type cyclization of 3-alkynyl-2-indolylmethanols: Construction of axially chiral cyclopenta[b]indole scaffolds. Chem. Synth. 2023, 3, 6. [Google Scholar] [CrossRef]
- Wang, H.Q.; Wu, S.F.; Yang, J.R.; Zhang, Y.C.; Shi, F. Design and organocatalytic asymmetric synthesis of indolyl-pyrroloindoles bearing both axial and central chirality. J. Org. Chem. 2023, 88, 7684–7702. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhang, H. Advances in catalytic asymmetric reactions using 2-indolylmethanols as platform molecules. Chin. J. Org. Chem. 2022, 42, 3351–3372. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Z.-G.; Shi, F. Advances in catalytic enantioselective synthesis of chiral helicenes and helicenoids. Chem. Catal. 2022, 2, 3077–3111. [Google Scholar] [CrossRef]
- Zhang, H.H.; Shi, F. Organocatalytic atroposelective synthesis of indole derivatives bearing axial chirality: Strategies and applications. Acc. Chem. Res. 2022, 55, 2562–2580. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Zhang, S.; Yu, X.-Y.; Wang, Y.-H.; Wan, H.-L.; Zhang, S.; Tan, W.; Shi, F. Organocatalytic asymmetric synthesis of bioactive hexahydropyrrolo[2,3-b]indole-containing tetrasubstituted allenes bearing multiple chiral elements. Chemistry 2022, 1, 100007–100018. [Google Scholar] [CrossRef]
- Wang, J.Y.; Sun, M.; Yu, X.Y.; Zhang, Y.C.; Tan, W.; Shi, F. Atroposelective construction of axially chiral alkene-indole scaffolds via catalytic enantioselective addition reaction of 3-alkynyl-2-indolylmethanols. Chin. J. Chem. 2021, 39, 2163–2171. [Google Scholar] [CrossRef]
- Wang, C.S.; Li, T.Z.; Liu, S.J.; Zhang, Y.C.; Deng, S.; Jiao, Y.; Shi, F. Axially chiral aryl-alkene-indole framework: A nascent member of the atropisomeric family and its catalytic asymmetric construction. Chin. J. Chem. 2020, 38, 543–552. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, Y.; Okuda, Y.; Le, L.; Tang, Z.; Yin, S.F.; Qiu, R.; Orita, A. Process-divergent syntheses of 4- and 5-sulfur-functionalized 1,2,3-triazoles via copper-catalyzed azide-alkyne cycloadditions of 1phosphinyl-2-sulfanylethynes. J. Org. Chem. 2023, 88, 3089–3108. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Peng, L.; Qiu, R.; Orita, A. Recent progress of protecting groups for terminal alkynes. Chin. J. Org. Chem. 2020, 40, 3112–3119. [Google Scholar] [CrossRef]
- Peng, L.; Li, R.; Tang, Z.; Chen, J.; Yi, R.; Xu, X. Cesium-catalyzed highly regioselective synthesis of (Z)-vinylic selenosulfides via thioselenation of alkynes with unsymmetrical diorganoyl dichalcogenides. Tetrahedron 2017, 73, 3099–3105. [Google Scholar] [CrossRef]
- Peng, L.; Hu, Z.; Wang, H.; Wu, L.; Jiao, Y.; Tang, Z.; Xu, X. Direct cyanation, hydrocyanation, dicyanation and cyanofunctionalization of alkynes. RSC Adv. 2020, 10, 10232–10244. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Xu, L.; Zhou, M.; Yin, B.; Osuka, A.; Song, J. Expanded azaporphyrins consisting of multiple BODIPY units: Global aromaticity and high affinities towards alkali metal ions. Angew. Chem. Int. Ed. 2022, 61, e202206899. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Guo, M.; Wang, M.; Zhou, M.; Zhou, B.; Xu, L.; Rao, Y.; Yin, B.; Osuka, A.; Song, J. Efficient synthesis of multiply seven-membered-ring fused porphyrins by rhodium-catalyzed [5+2] annulation. Angew. Chem. Int. Ed. 2022, 61, e202209594. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Sagisaka, K.; Peng, L.; Watanabe, H.; Xu, F.; Pawlak, R.; Meyer, E.; Okuda, Y.; Orita, A.; Kawai, S. Head-to-tail oligomerization by silylene-tethered Sonogashira coupling on Ag(111). Angew. Chem. Int. Ed. 2021, 60, 19598–19603. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Krejci, O.; Foster, A.S.; Pawlak, R.; Xu, F.; Peng, L.; Orita, A.; Meyer, E. Diacetylene linked anthracene oligomers synthesized by one-shot homocoupling of trimethylsilyl on Cu(111). ACS Nano 2018, 12, 8791–8797. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Nishida, T.; Shinohara, K.; Peng, L.; Takezaki, M.; Kamada, T.; Akashi, H.; Nakamura, H.; Sugiyama, K.; Ohta, K.; et al. Trimethylsilyl group assisted stimuli response: Self-assembly of 1,3,6,8-tetrakis((trimethysilyl)ethynyl)pyrene. Organometallics 2017, 36, 556–563. [Google Scholar] [CrossRef]
- Zeng, C.; Yuan, Z.; Jiao, Y.; Peng, L.; Tang, Z.; Xu, X. Synthesis of alkynyl sulfides: Alkynyl trifluoromethyl sulfides and thiocyanates. Eur. J. Org. Chem. 2023, 26, e202300733. [Google Scholar] [CrossRef]
- Peng, L.; Yuan, Z.; Tang, Z.; Zeng, C.; Xu, X. Thioalkynes in ring forming reactions. Chem. Rec. 2023, 23, e202300242. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Chen, J.; Chen, Y.; Lu, H.; Okuda, Y.; Tang, Z.; Orita, A.; Qiu, R.; Yin, S.F. Sulfur-containing 1,2,3-triazoles: Synthesis and properties. Eur. J. Org. Chem. 2024, 27, e202301146. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green chemistry and resource efficiency: Towards a green economy. Green Chem. 2016, 18, 3180–3183. [Google Scholar] [CrossRef]
- Bikshapathi, R.; Parvathaneni, S.P.; Jayathirtha Rao, V. An atom-economical protocol for direct conversion of Baylis-Hillman alcohols to β-chloro aldehydes in water. Green Chem. 2017, 19, 4446–4450. [Google Scholar] [CrossRef]
- Markiewicz, M.; Lewandowski, P.; Spychalski, M.; Kukawka, R.; Feder-Kubis, J.; Beil, S.; Smiglak, M.; Stolte, S. New bifunctional ionic liquid-based plant systemic acquired resistance (SAR) inducers with an improved environmental hazard profile. Green Chem. 2021, 23, 5138–5149. [Google Scholar] [CrossRef]
- Tickner, J.A.; Simon, R.V.; Jacobs, M.; Pollard, L.D.; van Bergen, S.K. The nexus between alternatives assessment and green chemistry: Supporting the development and adoption of safer chemicals. Green Chem. Lett. Rev. 2020, 14, 23–44. [Google Scholar] [CrossRef]
- Pellis, A.; Byrne, F.P.; Sherwood, J.; Vastano, M.; Comerford, J.W.; Farmer, T.J. Safer bio-based solvents to replace toluene and tetrahydrofuran for the biocatalyzed synthesis of polyesters. Green Chem. 2019, 21, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.; Hecker, P.; Mann, M.; Ma, Q.; Gross, J.P.; Schwaiger, R.; Guillon, O.; Fattakhova-Rohlfing, D.; Finsterbusch, M. Reducing the environmental footprint of solid-electrolytes-a green synthesis route for LATP. Green Chem. 2024, 26, 2712–2720. [Google Scholar] [CrossRef]
- Schlögl, R. Chemical energy storage enables the transformation of fossil energy systems to sustainability. Green Chem. 2021, 23, 1584–1593. [Google Scholar] [CrossRef]
- Sun, L.; Xiao, G.; Qian, X.; An, X. Alkyne functionalized cellulose fibers: A versatile “clickable” platform for antibacterial materials. Carbohydr. Polym. 2019, 207, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, J.; Ai, Y.; Liu, Y.; Xiong, J.; Wang, H.; Qiao, Y.; Liu, W.; Tan, S.; Feng, S.; et al. A ppm level Rh-based composite as an ecofriendly catalyst for transfer hydrogenation of nitriles: Triple guarantee of selectivity for primary amines. Green Chem. 2019, 21, 1390–1395. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, J.; Jung, H.; Meyer, L.E.; Fioroni, G.M.; Stubbs, C.D.; Jeong, K.; McCormick, R.L.; St. John, P.C.; Kim, S. Designing green chemicals by predicting vaporization properties using explainable graph attention networks. Green Chem. 2024, 26, 10247–10264. [Google Scholar] [CrossRef]
- Stolte, S.; Bui, H.T.T.; Steudte, S.; Korinth, V.; Arning, J.; Białk-Bielińska, A.; Bottin-Weber, U.; Cokoja, M.; Hahlbrock, A.; Fetz, V.; et al. Preliminary toxicity and ecotoxicity assessment of methyltrioxorhenium and its derivatives. Green Chem. 2015, 17, 1136–1144. [Google Scholar] [CrossRef]
- Sneddon, H.F. Safety first. Green. Chem. 2016, 18, 5082–5085. [Google Scholar] [CrossRef]
- Tamiya, T.; Hsu, Y.-I.; Asoh, T.-A.; Uyama, H. Reinforcement of Microbial Thermoplastics by Grafting to Polystyrene with Propargyl-Terminated Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). ACS Appl. Polym. Mater. 2020, 2, 3948–3956. [Google Scholar] [CrossRef]
- Takacs, L. The historical development of mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Ji, D.; Mao, W.; Cui, Y.; Wang, Q.; Han, L.; Zhong, H.; Wei, Z.; Zhao, Y.; Nørgaard, K.; et al. Dry chemistry of ferrate(VI): A solvent-free mechanochemical way for versatile green oxidation. Angew. Chem. Int. Ed. 2018, 57, 10949–10953. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, G.-W. Mechanochemical solvent-free synthesis of indenones from aromatic carboxylic acids and alkynes. J. Org. Chem. 2021, 86, 14102–14112. [Google Scholar] [CrossRef] [PubMed]
- Fantozzi, N.; Volle, J.-N.; Porcheddu, A.; Virieux, D.; García, F.; Colacino, E. Green metrics in mechanochemistry. Chem. Soc. Rev. 2023, 52, 6680–6714. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T. Mechanochemical synthesis of metal oxide nanoparticles. Commun. Chem. 2021, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Rath, W.H.; Göstl, R.; Herrmann, A. Mechanochemical activation of DNAzyme by ultrasound. Adv. Sci. 2024, 11, 2306236. [Google Scholar] [CrossRef] [PubMed]
- Dalidovich, T.; Nallaparaju, J.V.; Shalima, T.; Aav, R.; Kananovich, D.G. Mechanochemical nucleophilic substitution of alcohols via isouronium intermediates. Chem. Sus. Chem. 2022, 15, e202102286. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Hu, A.; Gao, P.; Gao, Y.; Pang, Y.; Seo, T.; Jiang, J.; Maeda, S.; Takaya, H.; Kubota, K.; et al. Mechanochemical synthesis of magnesium-based carbon nucleophiles in air and their use in organic synthesis. Nat. Commun. 2021, 12, 6691. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, M.; Mkrtchyan, S.; Shkoor, M.; Lanka, S.; Budzák, Š.; Iliaš, M.; Skoršepa, M.; Iaroshenko, V.O. Mechanochemical conversion of aromatic amines to aryl frifluoromethyl ethers. J. Am. Chem. Soc. 2022, 144, 10438–10445. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; De Bo, G. Mechanochemical generation of aryne. Chem. Sci. 2024, 15, 13181–13184. [Google Scholar] [CrossRef] [PubMed]
- Hermann, G.N.; Jung, C.L.; Bolm, C. Mechanochemical indole synthesis by rhodium-catalysed oxidative coupling of acetanilides and alkynes under solventless conditions in a ball mill. Green Chem. 2017, 19, 2520–2523. [Google Scholar] [CrossRef]
- Das, D.; Bhosle, A.A.; Panjikar, P.C.; Chatterjee, A.; Banerjee, M. Mn(I)-Catalyzed mechanochemical C-H bond activation: C-2 selective alkenylation of indoles. ACS Sustain. Chem. Eng. 2020, 8, 19105–19116. [Google Scholar] [CrossRef]
- Cheng, H.; Hernández, J.G.; Bolm, C. Mechanochemical ruthenium-catalyzed hydroarylations of alkynes under ball-milling conditions. Org. Lett. 2017, 19, 6284–6287. [Google Scholar] [CrossRef] [PubMed]
- Baig, R.B.; Varma, R.S. Alternative energy input: Mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 2012, 41, 1559–1584. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xin, B.; Elsukova, A.; Eklund, P.; Solin, N. Mechanochemical formation of protein nanofibril: Graphene nanoplatelet hybrids and their thermoelectric properties. ACS Sustain. Chem. Eng. 2020, 8, 17368–17378. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wen, J.; Zhang, P.; Yu, B.; Chen, C.; Ma, T.; Lu, X.; Kim, S.H.; Qian, L. Nanomanufacturing of silicon surface with a single atomic layer precision via mechanochemical reactions. Nat. Commun. 2018, 9, 1542. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kouznetsova, T.B.; Boulatov, R.; Craig, S.L. Mechanical gating of a mechanochemical reaction cascade. Nat. Commun. 2016, 7, 13433. [Google Scholar] [CrossRef] [PubMed]
- Avdoshenko, S.M.; Makarov, D.E. Reaction coordinates and pathways of mechanochemical transformations. J. Phys. Chem. B 2016, 120, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.A. Review: Mechanochemistry of the kinesin-1 ATPase. Biopolymers 2016, 105, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Amrute, A.P.; Lodziana, Z.; Schreyer, H.; Weidenthaler, C.; Schuth, F. High-surface-area corundum by mechanochemically induced phase transformation of boehmite. Science 2019, 366, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.; Vugrin, L.; Miletic, G.; Kulcsar, M.J.; Ricci, P.C.; Halasz, I.; Delogu, F. Mechanochemical reactions from individual impacts to global transformation kinetics. Angew. Chem. Int. Ed. 2023, 62, e202308046. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.G.; Liu, S.; Guan, X.; Yoon, S.; Zhou, J.; Chen, W.Y.; Gaire, S.; Seylar, J.; Chen, H.; Wang, Z.; et al. Mechanochemically accessing a challenging-to-synthesize depolymerizable polymer. Nat. Commun. 2023, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef] [PubMed]
- Porcheddu, A.; Colacino, E.; De Luca, L.; Delogu, F. Metal-Mediated and Metal-Catalyzed Reactions Under Mechanochemical Conditions. ACS Catal. 2020, 10, 8344–8394. [Google Scholar] [CrossRef]
- Chen, X.; Shen, H.; Zhang, Z. Polymer mechanochemistry on reactive species generated from mechanochemical reactions. Chin. J. Chem. 2024, 42, 1418–1432. [Google Scholar] [CrossRef]
- Agrahari, A.K.; Bose, P.; Jaiswal, M.K.; Rajkhowa, S.; Singh, A.S.; Hotha, S.; Mishra, N.; Tiwari, V.K. Cu(I)-Catalyzed click chemistry in glycoscience and their diverse applications. Chem. Rev. 2021, 121, 7638–7956. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Zahoor, A.F.; Ahmad, S.; Noreen, R.; Khan, S.G.; Ahmad, H. Cross-coupling reactions towards the synthesis of natural products. Mol. Divers. 2021, 26, 647–689. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, I.; Mujahid, A.; Rasool, N.; Rizwan, K.; Malik, A.; Ahmad, G.; Shah, S.A.A.; Rashid, U.; Nasir, N.M. Palladium and copper catalyzed Sonogashira cross coupling an excellent methodology for C-C bond formation over 17 years: A review. Catalysts 2020, 10, 443. [Google Scholar] [CrossRef]
- Nair, P.P.; Philip, R.M.; Anilkumar, G. Nickel catalysts in Sonogashira coupling reactions. Org. Biomol. Chem. 2021, 19, 4228–4242. [Google Scholar] [CrossRef] [PubMed]
- Driowya, M.; Saber, A.; Marzag, H.; Demange, L.; Bougrin, K.; Benhida, R. Microwave-assisted syntheses of bioactive seven-membered, macro-sized heterocycles and their fused derivatives. Molecules 2016, 21, 1023. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Zarandi, M.A.; Zhou, X.; Jayawickramarajah, J. Synthesis and applications of porphyrin-biomacromolecule conjugates. Front. Chem. 2021, 9, 764137. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Kameda, M.; Hatakeyama, T.; Morisaki, Y. π-Stacked polymer consisting of a pseudo-meta-[2.2] paracyclophane Skeleton. Polymers 2018, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Sajjadi, M.; Suh, J.M.; Zhang, K.; Nasrollahzadeh, M.; Jang, H.W.; Varma, R.S.; Shokouhimehr, M. Palladium nanoparticles on assorted nanostructured supports: Applications for Suzuki, Heck, and Sonogashira cross-coupling reactions. ACS Appl. Nano Mater. 2020, 3, 2070–2103. [Google Scholar] [CrossRef]
- Haley, R.A.; Mack, J.; Guan, H. 2-In-1: Catalyst and reaction medium. Inorg. Chem. Front. 2017, 4, 52–55. [Google Scholar] [CrossRef]
- Pickhardt, W.; Siegfried, E.; Fabig, S.; Rappen, M.F.; Etter, M.; Wohlgemuth, M.; Gratz, S.; Borchardt, L. The Sonogashira coupling on palladium milling balls-a new reaction pathway in mechanochemistry. Angew. Chem. Int. Ed. 2023, 62, e202301490. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-B.; Wei, Y.; Shi, M. Recent developments of cyclopropene chemistry. Chem. Soc. Rev. 2011, 40, 5534–5563. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Feng, C.; Seo, T.; Kubota, K.; Ito, H. Efficient access to materials-oriented aromatic alkynes via the mechanochemical Sonogashira coupling of solid aryl halides with large polycyclic conjugated systems. Chem. Sci. 2022, 13, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Leslie, D.; Coleman, M.G.; Mack, J. Recyclable heterogeneous metal foil-catalyzed cyclopropenation of alkynes and diazoacetates under solvent-free mechanochemical reaction conditions. Chem. Sci. 2018, 9, 4650–4661. [Google Scholar] [CrossRef] [PubMed]
- Baird, M.S. Houben-Weyl: Methods of Organic Chemistry, 4th ed.; De Meijere, A., Ed.; Thieme: Stuttgart, Germany, 1996; Volume E17d, pp. 2695–2759. [Google Scholar]
- Ford, A.; Miel, H.; Ring, A.; Slattery, C.N.; Maguire, A.R.; McKervey, M.A. Modern Organic Synthesis with alpha-Diazocarbonyl Compounds. Chem. Rev. 2015, 115, 9981–10080. [Google Scholar] [CrossRef] [PubMed]
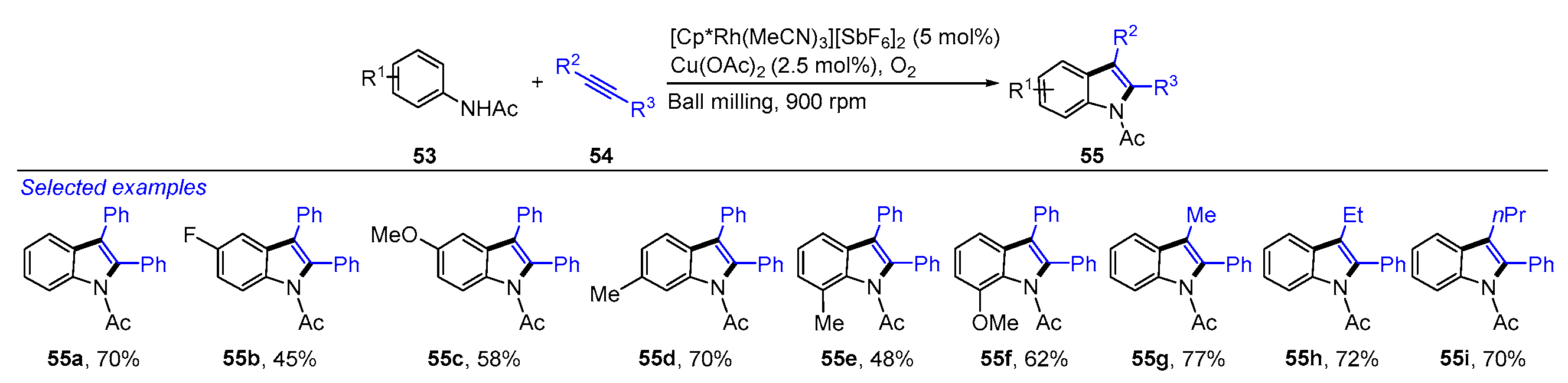
- Hermann, G.N.; Becker, P.; Bolm, C. Mechanochemical Rhodium(III)-catalyzed C-H bond functionalization of acetanilides under solventless conditions in a ball mill. Angew. Chem. Int. Ed. 2015, 54, 7414–7417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, P.; Cao, F.; Shi, L.; Zhang, N.; Xue, Z.; Luo, G. Mechanistic insights into rare-earth-catalysed C-H alkylation of sulfides: Sulfide facilitating alkene insertion and beyond. RSC Adv. 2022, 12, 13593–13599. [Google Scholar] [CrossRef] [PubMed]
- Tomilin, D.N.; Pigulski, B.; Gulia, N.; Arendt, A.; Sobenina, L.N.; Mikhaleva, A.b.I.; Szafert, S.; Trofimov, B.A. Direct synthesis of butadiynyl-substituted pyrroles under solvent- and transition metal-free conditions. RSC Adv. 2015, 5, 73241–73248. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Sobenina, L.N.; Stepanova, Z.V.; Vakul’skaya, T.I.; Kazheva, O.G.N.; Aleksandrov, G.G.; Dyachenko, O.A.; Mikhaleva, A.b.I. Reactions of 2-phenylpyrrole with bromobenzoylacetylene on metal oxides active surfaces. Tetrahedron 2008, 64, 5541–5544. [Google Scholar] [CrossRef]
- Pigulski, B.; Arendt, A.; Tomilin, D.N.; Sobenina, L.N.; Trofimov, B.A.; Szafert, S. Transition-metal free mechanochemical approach to polyyne substituted pyrroles. J. Org. Chem. 2016, 81, 9188–9198. [Google Scholar] [CrossRef] [PubMed]
- Hermann, G.N.; Unruh, M.T.; Jung, S.H.; Krings, M.; Bolm, C. Mechanochemical rhodium(III)- and gold(I)-catalyzed C-H bond alkynylations of indoles under solventless conditions in mixer mills. Angew. Chem. Int. Ed. 2018, 57, 10723–10727. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Loh, T.P. Rhodium-catalyzed C-H alkynylation of arenes at room temperature. Angew. Chem. Int. Ed. 2014, 53, 2722–2726. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.D.; Lied, F.; Glorius, F. Preparation of conjugated 1,3-enynes by Rh(III)-catalysed alkynylation of alkenes via C-H activation. Chem. Commun. 2014, 50, 4459–4461. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Patel, P.; Hwang, H.; Chang, S. Rhodium(III)-catalyzed C-C bond formation of quinoline N-oxides at the C-8 position under mild conditions. Org. Lett. 2014, 16, 4598–4601. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Pan, C.; Zhang, H.; Xu, P.; Cheng, Y.; Zhu, C. Rhodium-catalyzed direct C7 alkynylation of indolines. Adv. Synth. Catal. 2015, 357, 1149–1153. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhou, B.; Li, Y. Iridium(III)-catalyzed C-7 selective C-H alkynylation of indolines at room temperature. J. Org. Chem. 2015, 80, 1946–1951. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, P.; Kloeckner, U.; Nachtsheim, B.J. OH-Directed alkynylation of 2-vinylphenols with ethynyl benziodoxolones: A fast access to terminal 1,3-enynes. Angew. Chem. Int. Ed. 2015, 54, 4949–4952. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Hong, S. Rh(III) and Ru(II)-catalyzed site-selective C-H alkynylation of quinolones. Org Lett. 2015, 17, 1938–1941. [Google Scholar] [CrossRef] [PubMed]
- Boobalan, R.; Gandeepan, P.; Cheng, C.H. Ruthenium-catalyzed C-H alkynylation of aromatic amides with hypervalent iodine-alkyne reagents. Org. Lett. 2016, 18, 3314–3317. [Google Scholar] [CrossRef] [PubMed]
- Caspers, L.D.; Finkbeiner, P.; Nachtsheim, B.J. Direct electrophilic C-H alkynylation of inprotected 2-vinylanilines. Chem. Eur. J. 2017, 23, 2748–2752. [Google Scholar] [CrossRef] [PubMed]
- Jarszak-Tyl, A.; Pigulski, B.; Szafert, S. Solvent-free C-H alkynylation of azulenes. Org. Chem. Front. 2021, 8, 5674–5680. [Google Scholar] [CrossRef]
- Fabian, K.H.H.; Elwahy, A.H.M.; Hafner, K. Syntheses of mono-, di- and triethynylazulenes. Tetrahedron Let. 2000, 41, 2855–2858. [Google Scholar] [CrossRef]
- Martinez-Ferrero, E.; Boubekeur, K.; Ribot, F. Carboxylate-Containing Tin(IV) Isopropoxides: Synthesis and Characterization of [Sn(OiPr)2(O2CR)2]2 [R = (CH3)CCH2, C6H5, CH3]. Eur. J. Org. Chem. 2006, 2006, 802–807. [Google Scholar] [CrossRef]
- Shoji, T.; Araki, T.; Iida, N.; Kobayashi, Y.; Ohta, A.; Sekiguchi, R.; Ito, S.; Mori, S.; Okujima, T.; Yasunami, M. Molecular Transformation of 2-Methylazulenes: An Efficient and Practical Synthesis of 2-Formyl- and 2-Ethynylazulenes. Eur. J. Org. Chem. 2018, 2018, 1145–1157. [Google Scholar] [CrossRef]
- Avan, I.; Hall, C.D.; Katritzky, A.R. Peptidomimetics via modifications of amino acids and peptide bonds. Chem. Soc. Rev. 2014, 43, 3575–3594. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Messinis, A.M.; Li, J.; Warratz, S.; Ackermann, L. Chelation-Assisted Iron-Catalyzed C-H Activations: Scope and Mechanism. Acc. Chem. Res. 2024, 57, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Cattani, S.; Pandit, N.K.; Buccio, M.; Balestri, D.; Ackermann, L.; Cera, G. Iron-Catalyzed C-H Alkylation/Ring Opening with Vinylbenzofurans Enabled by Triazoles. Angew. Chem. Int. Ed. 2024, 63, e202404319. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Z. Development and applications of the copper-catalyzed azide-alkyne cycloaddition (CuAAC) as a bioorthogonal reaction. Molecules 2016, 21, 1393. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Fong, D.; Meichsner, E.; Adronov, A. A Survey of strain-promoted azide-alkyne cycloaddition in polymer chemistry. Chem. Eur. J. 2021, 27, 5057–5073. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Liu, Z.; Du, Z.; Zhang, L.; Ren, J.; Qu, X. A biocompatible heterogeneous MOF-Cu catalyst for in vivo drug synthesis in targeted subcellular organelles. Angew. Chem. Int. Ed. 2019, 58, 6987–6992. [Google Scholar] [CrossRef] [PubMed]
- Saal, A.; Friebe, C.; Schubert, U.S. Polymeric blatter’s radical via CuAAC and ROMP. Macromol. Chem. Phys. 2021, 222, 2100194. [Google Scholar] [CrossRef]
- Cook, T.L.; Walker, J.A.; Mack, J. Scratching the catalytic surface of mechanochemistry: A multi-component CuAAC reaction using a copper reaction vial. Green. Chem. 2013, 15, 617–619. [Google Scholar] [CrossRef]
- Kumar, R.J.; MacDonald, J.M.; Singh, T.B.; Waddington, L.J.; Holmes, A.B. Hierarchical self-assembly of semiconductor functionalized peptide alpha-helices and optoelectronic properties. J. Am. Chem. Soc. 2011, 133, 8564–8573. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, P.; Karmakar, I.; Mukherjee, D.; Bhowmick, A.; Brahmachari, G. Mechanochemical solvent-free one-pot synthesis of poly-functionalized 5-(arylselanyl)-1H-1,2,3-triazoles through a copper(I)-catalyzed click reaction. Chem. Eur. J. 2023, 29, e202302539. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sun, H.; Liu, Y.; Duan, Y.; Liu, J.; Yang, X.; Li, W.; Qin, S.; Xu, S.; Zhu, Z.; et al. Design, synthesis and biological evaluation of vinyl selenone derivatives as novel microtubule polymerization inhibitors. Eur. J. Med. Chem. 2020, 207, 112716. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xie, J.; Zhu, Z. 1,10-Phenanthroline: A versatile ligand to promote copper-catalyzed cascade reactions. Appl. Organomet. Chem. 2020, 34, e5926. [Google Scholar] [CrossRef]
- Li, X.; Yang, F.; Wu, Y.; Wu, Y. Copper-mediated oxidative decarboxylative coupling of arylpropiolic acids with dialkyl H-phosphonates in water. Org. Lett. 2014, 16, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Worrell, B.T.; Malik, J.A.; Fokin, V.V. Direct evidence of a dinuclear copper intermediate in Cu(I)-catalyzed azide-alkyne cycloadditions. Science 2013, 340, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Stefani, H.A.; Vasconcelos, S.N.S.; Manarin, F.; Leal, D.M.; Souza, F.B.; Madureira, L.S.; Zukerman-Schpector, J.; Eberlin, M.N.; Godoi, M.N.; de Souza Galaverna, R. Synthesis of 5-organotellanyl-1H-1,2,3-triazoles: Functionalization of the 5-position scaffold by the Sonogashira cross-coupling reaction. Eur. J. Org. Chem. 2013, 2013, 3780–3785. [Google Scholar] [CrossRef]
- Gooßen, L.J.; Rodríguez, N.; Manjolinho, F.; Lange, P.P. Synthesis of propiolic acids via copper-catalyzed insertion of carbon dioxide into the C-H bond of terminal alkynes. Adv. Synth. Catal. 2010, 352, 2913–2917. [Google Scholar] [CrossRef]
- Lennox, C.B.; Borchers, T.H.; Gonnet, L.; Barrett, C.J.; Koenig, S.G.; Nagapudi, K.; Friščić, T. Direct mechanocatalysis by resonant acoustic mixing (RAM). Chem. Sci. 2023, 14, 7475–7481. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, G.; McLeod, D.; Mohr, L.M.; Jorgensen, K.A. Organocatalytic enantioselective 1,3-dipolar [6+4] cycloadditions of tropone. Chem. Eur. J. 2020, 26, 15491–15496. [Google Scholar] [CrossRef] [PubMed]
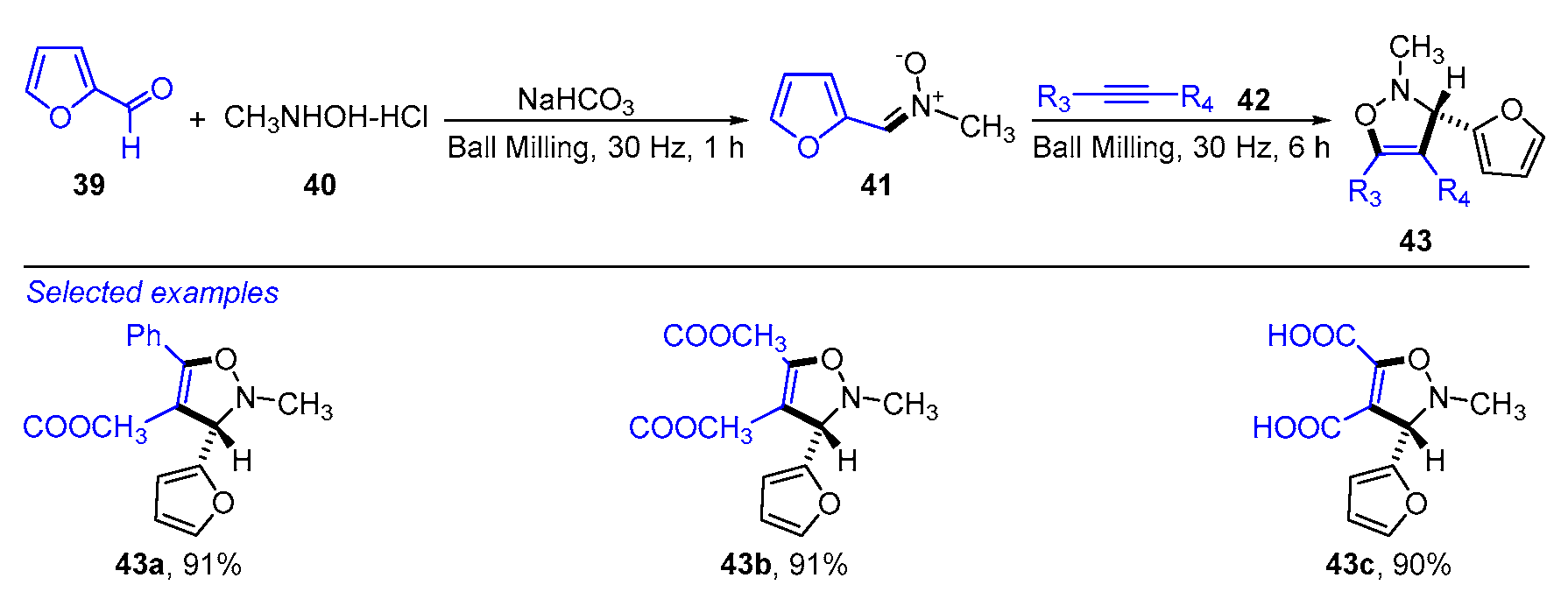
- Chakraborty, B. Solvent-free synthesis and 1,3-dipolar cycloaddition reactions of N-methyl-C-(2-furyl) nitrone in a ball mill and anticancer activities of the new cycloadducts. J. Heterocycl. Chem. 2019, 57, 477–485. [Google Scholar] [CrossRef]
- Hernandez, R.A.; Trakakis, I.; Do, J.L.; Cuccia, L.A.; Friščić, T.; Forgione, P. Mechanochemical desymmetrization of unbiased bis- and tris-alkynes to access 3,5-isoxazoles-alkyne adducts and unsymmetrical bis-3,5-isoxazoles. Eur. J. Org. Chem. 2023, 26, e202300374. [Google Scholar] [CrossRef]
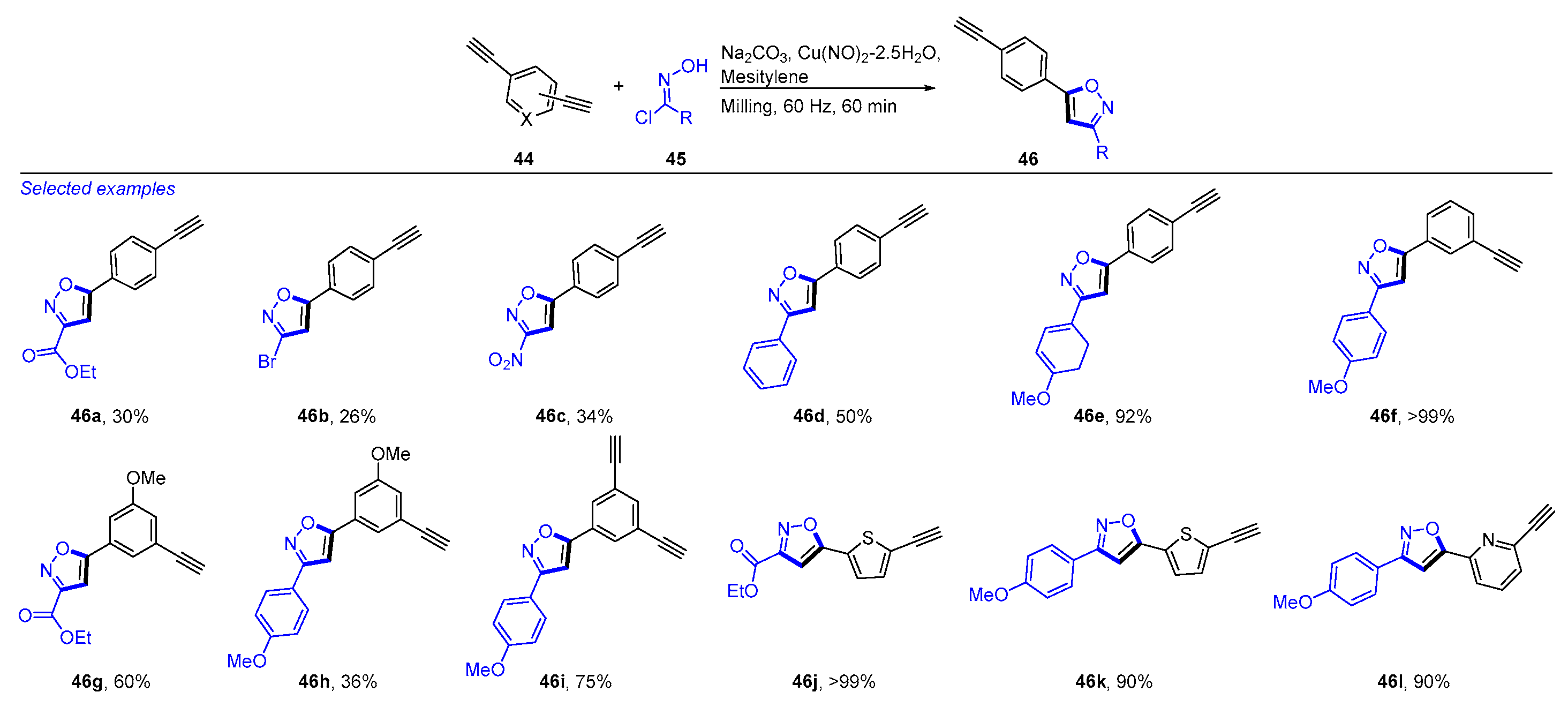
- Carloni, L.E.; Mohnani, S.; Bonifazi, D. Synthesis of 3,5-Disubstituted Isoxazoles through a 1,3-Dipolar Cycloaddition Reaction between Alkynes and Nitrile Oxides Generated from O-Silylated Hydroxamic Acids. Eur. J. Org. Chem. 2019, 2019, 7322–7334. [Google Scholar] [CrossRef]
- Montagnat, O.D.; Lessene, G.; Hughes, A.B. Synthesis of azide-alkyne fragments for “click” chemical applications. Part 2. Formation of oligomers from orthogonally protected chiral trialkylsilylhomopropargyl azides and homopropargyl alcohols. J. Org. Chem. 2010, 75, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.V.R.; Narendar, V.; Sampath Kumar, N.; Aparna, P.; Bhavani, A.K.D.; Solhi, H.; Le Guevel, R.; Roul, J.; Gautier, F.; Juin, P.; et al. Synthesis and biological studies of new piperidino-1,2,3-triazole hybrids with 3-aryl isoxazole side chains. Bioorg. Med. Chem. Lett. 2021, 52, 128390. [Google Scholar] [CrossRef] [PubMed]
- Fiandanese, V.; Maurantonio, S.; Punzi, A.; Rafaschieri, G.G. A general procedure for the synthesis of alkyl- and arylethynyl-1,2,3-triazole-fused dihydroisoquinolines. Org. Biomol. Chem. 2012, 10, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; David, A.L. Chemoselective Formation ofSuccessive Triazole Linkages in OnePot: “Click-Click” Chemistry. Org. Lett. 2006, 8, 4505–4507. [Google Scholar]
- Aizpurua, J.M.; Azcune, I.; Fratila, R.M.; Balentova, E.; Sagartzazu-Aizpurua, M.; Miranda, J.I. “Click” Synthesis of Nonsymmetrical Bis(1,2,3-triazoles). Org. Lett. 2010, 12, 1584–1587. [Google Scholar] [CrossRef] [PubMed]
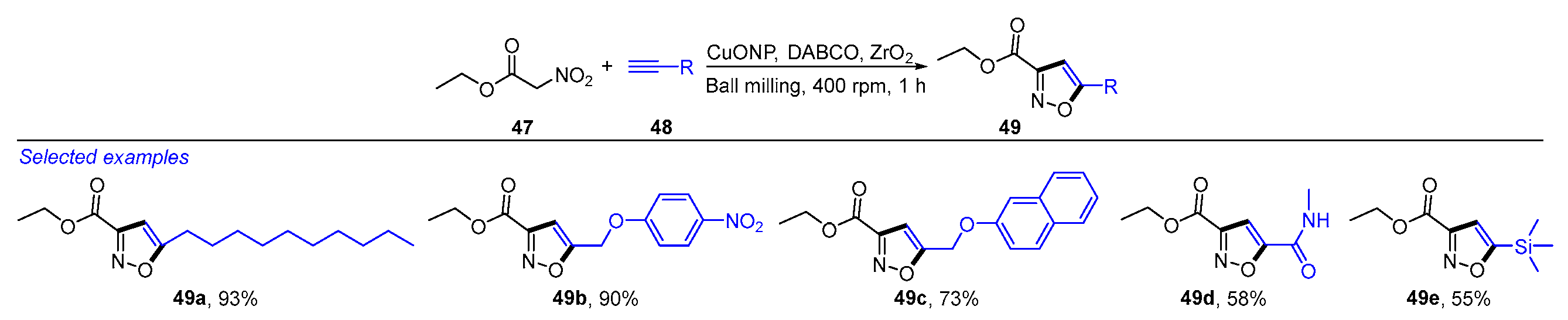
- Vadivelu, M.; Sampath, S.; Muthu, K.; Karthikeyan, K.; Praveen, C. Mechanochemistry enabled construction of isoxazole skeleton via CuO nanoparticles catalyzed intermolecular dehydrohalogenative annulation. Adv. Synth. Catal. 2021, 363, 4941–4952. [Google Scholar] [CrossRef]
- Hernandez, R.R.A.; Nabavi, N.; Patterson, S.J.; Forgione, P. Achieving regioselective control for mechanochemical reactions: A planetary ball-milling, Ru-catalyzed synthesis of 3,4- and 3,4,5-isoxazoles. Chem. Cat. Chem. 2024, 16, e202400406. [Google Scholar] [CrossRef]
- Grecian, S.; Fokin, V.V. Ruthenium-catalyzed cycloaddition of nitrile oxides and alkynes: Practical synthesis of isoxazoles. Angew. Chem. Int. Ed. 2008, 47, 8285–8287. [Google Scholar] [CrossRef] [PubMed]
- Oakdale, J.S.; Sit, R.K.; Fokin, V.V. Ruthenium-catalyzed cycloadditions of 1-haloalkynes with nitrile oxides and organic azides: Synthesis of 4-haloisoxazoles and 5-halotriazoles. Chem Eur. J. 2014, 20, 11101–11110. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Lygin, A.V. Cationic Ruthenium(II) Catalysts for Oxidative C-H/N-H Bond Functionalizations of Anilines with Removable Directing Group: Synthesis of Indoles in Water. Org. Lett. 2012, 14, 764. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ackermann, L. Nickel-catalyzed alkyne annulation by anilines: Versatile indole synthesis by C-H/N-H functionalization. Chem. Commun. 2013, 49, 6638–6640. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhong, X.; She, H.; Lei, Z.; Li, F. Manganese catalyzed C−H functionalization of indoles with alkynes to synthesize bis/ trisubstituted indolylalkenes and carbazoles: The acid is the key to control selectivity. Chem. Commun. 2015, 51, 7136–7139. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; Zou, Z.; Wang, T.; Liu, X.; Li, H.; Yuan, Z.; Zeng, C.; Xu, X.; Tang, Z.; Jiang, G. Mechanochemical and Transition-Metal-Catalyzed Reactions of Alkynes. Catalysts 2025, 15, 690. https://doi.org/10.3390/catal15070690
Peng L, Zou Z, Wang T, Liu X, Li H, Yuan Z, Zeng C, Xu X, Tang Z, Jiang G. Mechanochemical and Transition-Metal-Catalyzed Reactions of Alkynes. Catalysts. 2025; 15(7):690. https://doi.org/10.3390/catal15070690
Chicago/Turabian StylePeng, Lifen, Zhiling Zou, Ting Wang, Xirong Liu, Hui Li, Zhiwen Yuan, Chunling Zeng, Xinhua Xu, Zilong Tang, and Guofang Jiang. 2025. "Mechanochemical and Transition-Metal-Catalyzed Reactions of Alkynes" Catalysts 15, no. 7: 690. https://doi.org/10.3390/catal15070690
APA StylePeng, L., Zou, Z., Wang, T., Liu, X., Li, H., Yuan, Z., Zeng, C., Xu, X., Tang, Z., & Jiang, G. (2025). Mechanochemical and Transition-Metal-Catalyzed Reactions of Alkynes. Catalysts, 15(7), 690. https://doi.org/10.3390/catal15070690





