The Behavior of Divalent Metals in Double-Layered Hydroxides as a Fenton Bimetallic Catalyst for Dye Decoloration: Kinetics and Experimental Design
Abstract
1. Introduction
2. Results and Discussion
2.1. Material Characterization
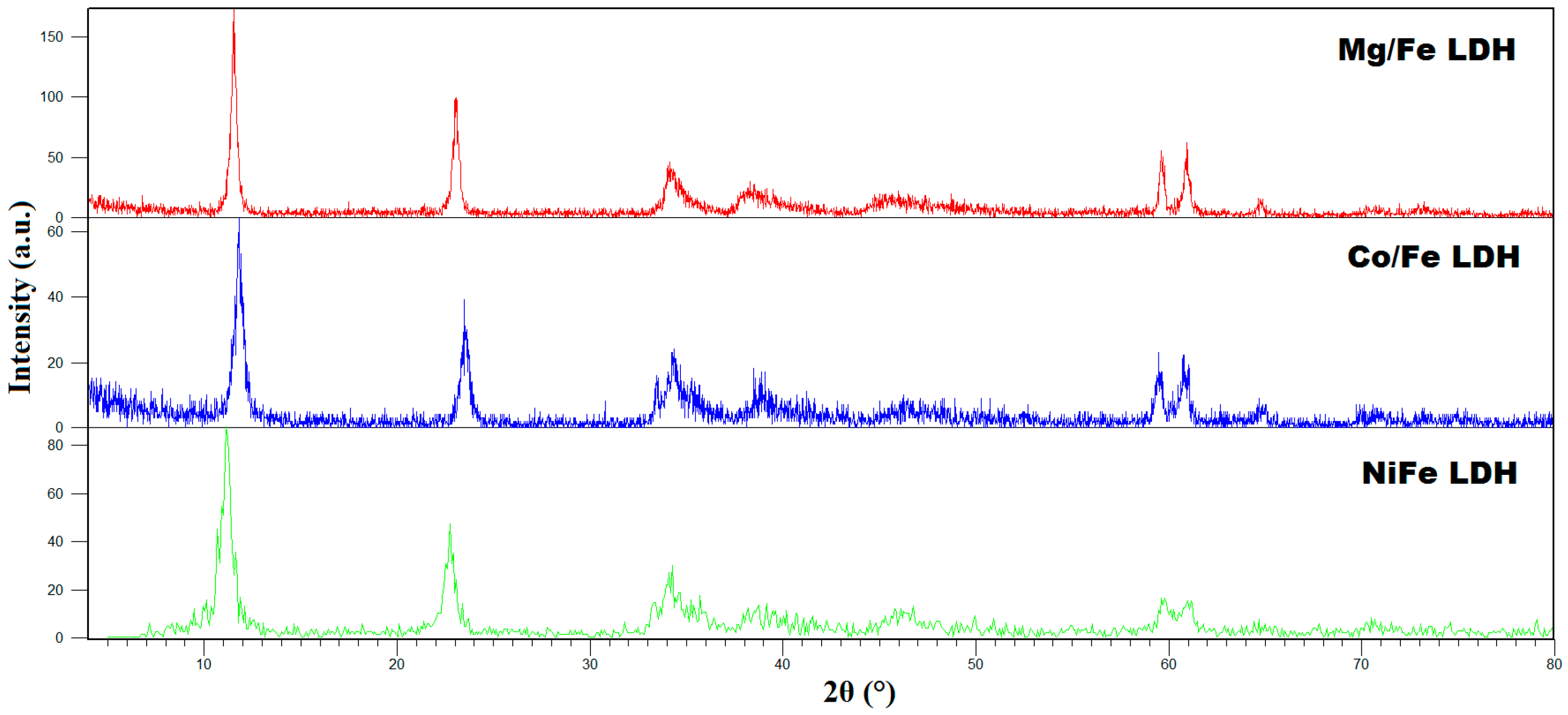
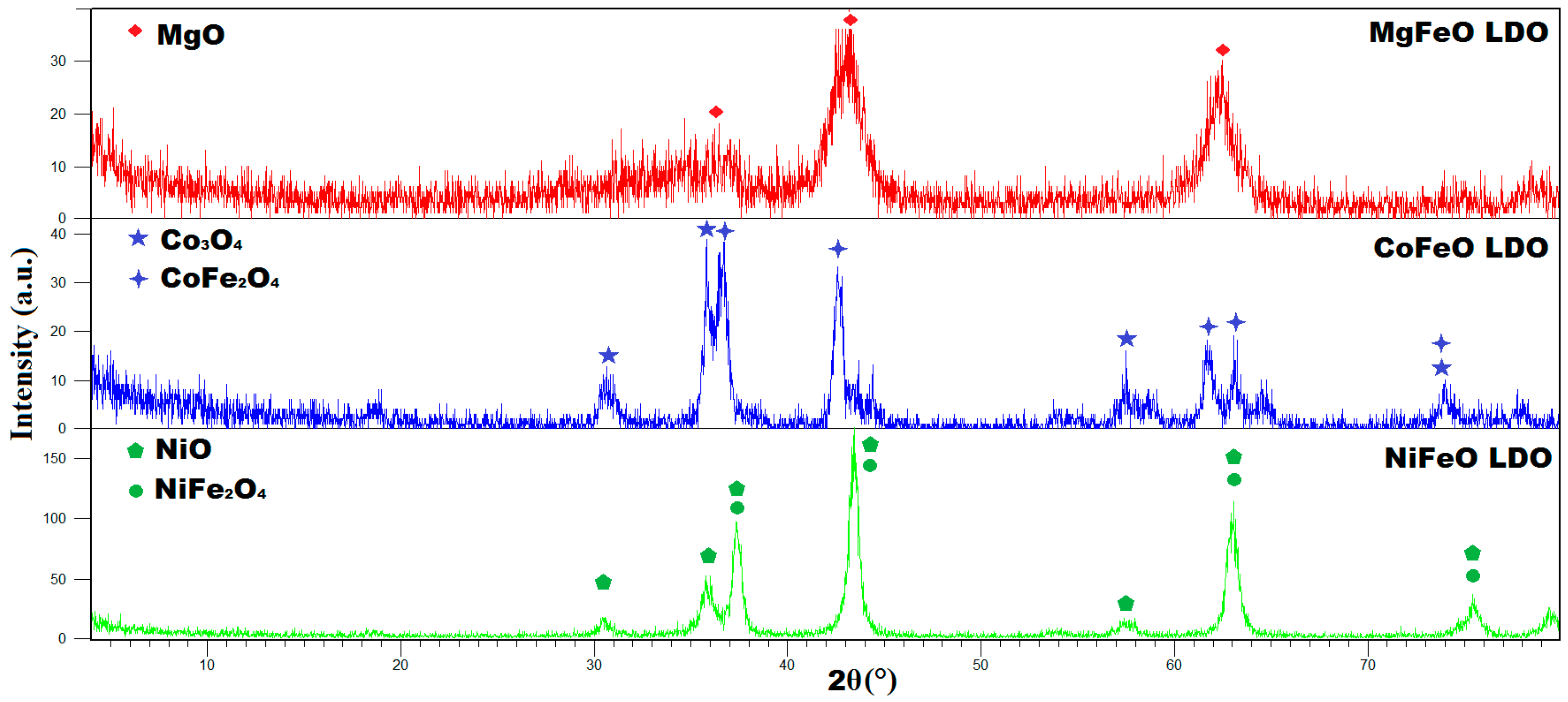
2.1.1. X-Ray Diffraction (XRD)
- D = crystallite size (nm);
- K = 0.9 Scherrer constant;
- λ = 0.15406 nm (Cu2α);
- Β = FWHM;
- θ = diffraction peak angle.
| Material | Average Crystallite Size [nm] |
|---|---|
| Mg/Fe | 48.47 |
| MgFeO | 59.70 |
| Co/Fe | 52.97 |
| CoFeO | 27.27 |
| Ni/Fe | 30.90 |
| NiFeO | 39.79 |
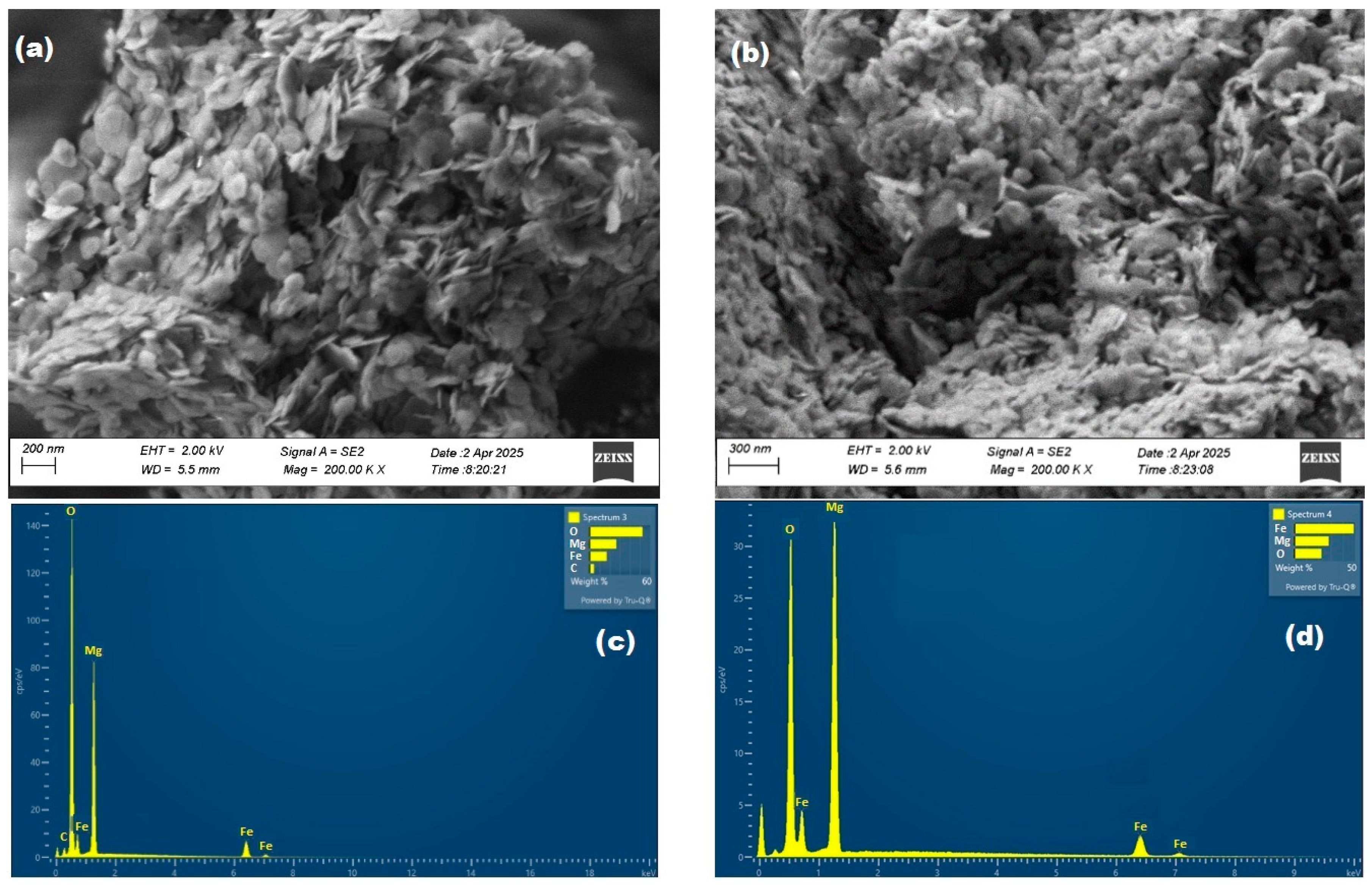
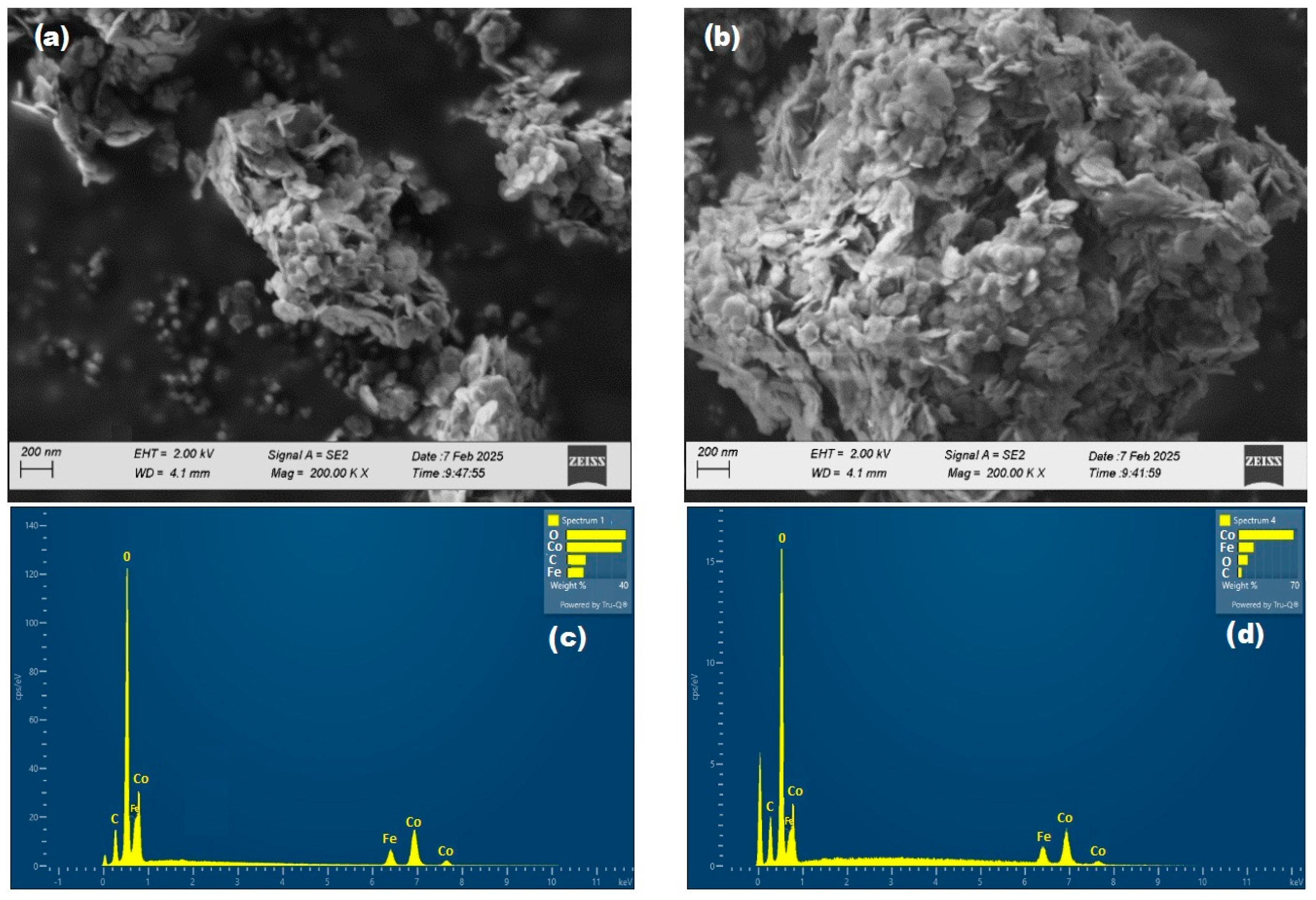
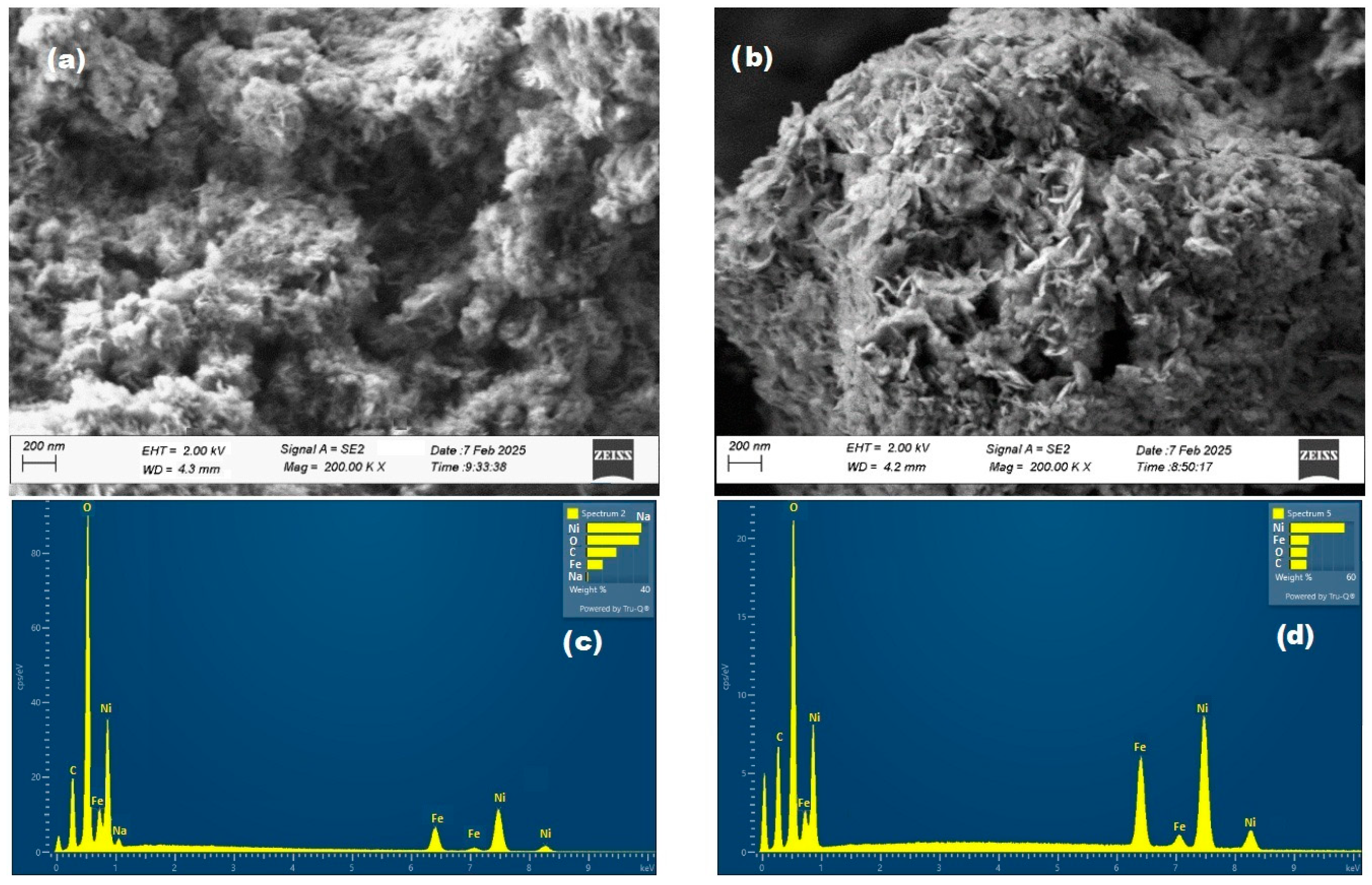
2.1.2. Morphological and Elemental Characterization (SEM-EDS)
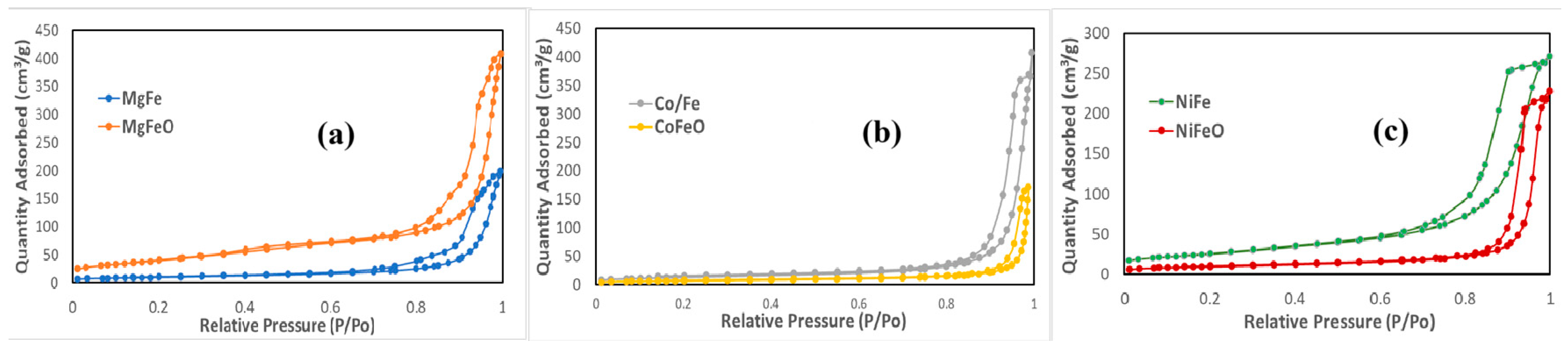
2.1.3. BET Surface Area and Textural Analysis of Catalyst Precursors
2.2. Decoloration of Crystal Violet and Methyl Blue Using LDH-Based Catalysts
- ;
- .
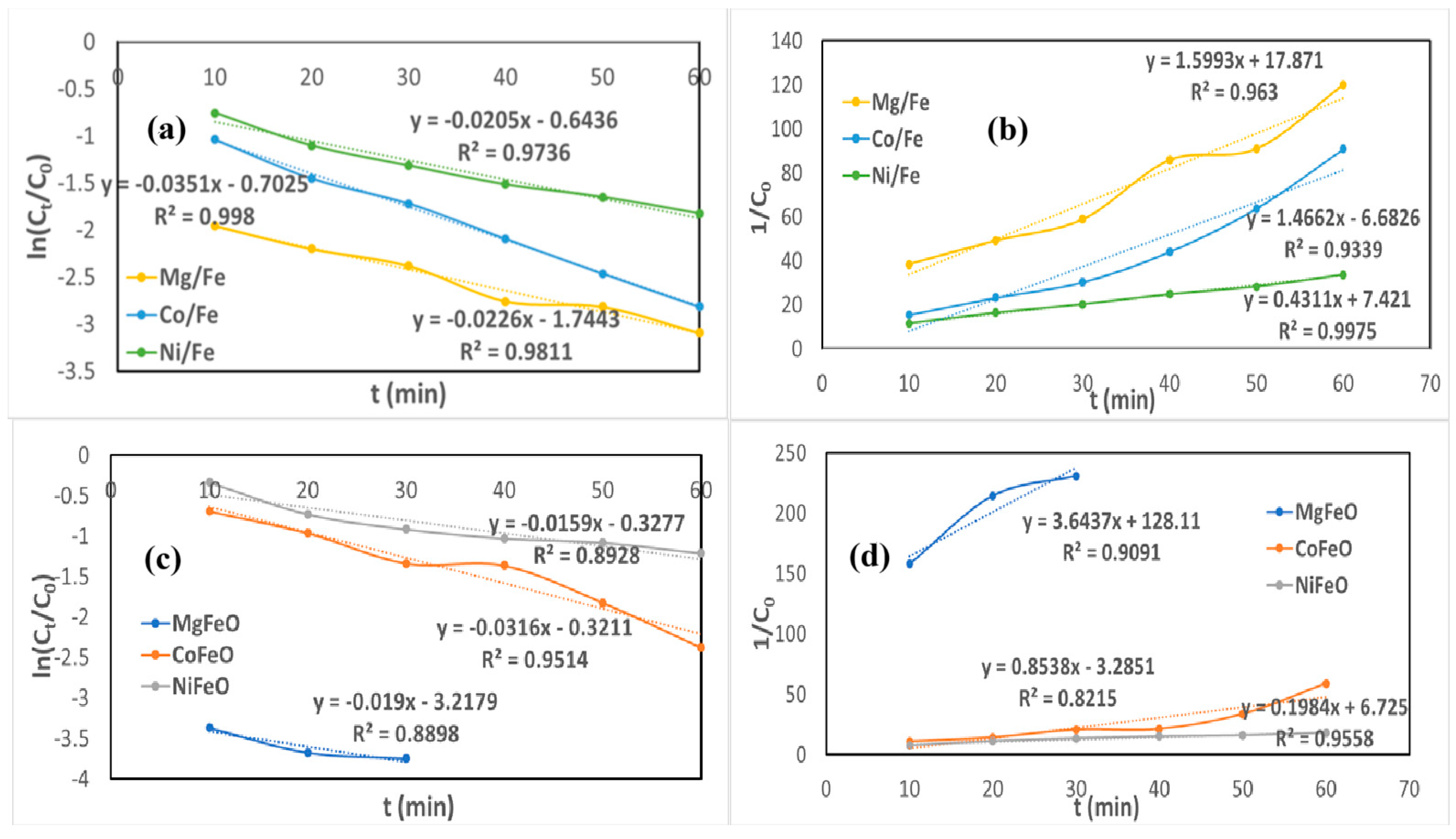
2.3. Decoloration Kinetics of Crystal Violet and Methyl Blue
2.4. Experimental Design Analysis
3. Materials and Methods
3.1. Material Synthesis
3.2. Characterization of Materials
3.3. Catalytic Evaluation
3.4. Experiment Design
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOP | Advanced Oxidation Process |
| BET | Brunauer-Emmett-Teller |
| CV | Crystal Violet |
| EDS | energy-dispersive spectroscopy |
| FWHM | Full Width at Half Maximum |
| IUPAC | International Union of Pure and Applied Chemistry |
| LDH | Layered Double Hydroxide |
| Rpm | revolutions per minute |
| MB | Methyl Blue |
| OER | oxygen evolution reaction |
| SEM | scanning electron microscopy |
| XRD | X-ray diffraction |
References
- Feng, J.B.; Li, Y.Y.; Zhang, Y.; Xu, Y.Y.; Cheng, X.W. Adsorptive Removal of Indomethacin and Diclofenac from Water by Polypyrrole Doped-GO/COF-300 Nanocomposites. Chem. Eng. J. 2022, 429, 132499. [Google Scholar] [CrossRef]
- Phan, K.H.; Le, L.T.; Ninh, T.T.N.; Tran, C.S.; Nguyen, T.T.; Nguyen, D.T.D.; Tra, V.T.; Tran, T.D.; Nguyen, T.B.; Mai, T.P.; et al. Decolorization and Degradation of Azo Dyes in Thermophilic Biological Wastewater Treatment Process: A Mini-Review. Case Stud. Chem. Environ. Eng. 2024, 10, 101018. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A Critical Review on Textile Wastewater Treatments: Possible Approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Anuse, D.D.; Patil, S.A.; Chorumale, A.A.; Kolekar, A.G.; Bote, P.P.; Walekar, L.S.; Pawar, S.P. Activated Carbon from Pencil Peel Waste for Effective Removal of Cationic Crystal Violet Dye from Aqueous Solutions. Results Chem. 2025, 13, 101949. [Google Scholar] [CrossRef]
- Alegbe, E.O.; Uthman, T.O. A Review of History, Properties, Classification, Applications and Challenges of Natural and Synthetic Dyes. Heliyon 2024, 10, e33646. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Chowdhury, S.; Das Saha, P. Adsorption of Crystal Violet from Aqueous Solution onto NaOH-Modified Rice Husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Nevondo, N.G.; Onwudiwe, D.C.; Onyango, M.S. Photocatalytic Degradation of Methyl Blue in Water Using Sawdust-Derived Cellulose Nanocrystals-Metal Oxide Nanocomposite. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2542–2552. [Google Scholar] [CrossRef]
- Mondello, L.; Pawliszyn, J.; Dugo, P. Comprehensive Sampling and Sample Preparation: Analytical Techniques for Scientists; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4, Extraction Techniques and Applications: Food and Beverage; ISBN 9780123813732. [Google Scholar]
- Zhu, W.; Chen, F.; Ye, L.; Wang, X.; Tang, Y.; Li, Y.; Song, Y. Pyrrhotite Promote Aerobic Granular Sludge Formation in Dye Wastewater: PH, Interfacial Free Energy, and Microbial Community Evolution. Bioresour. Technol. 2025, 419, 131922. [Google Scholar] [CrossRef] [PubMed]
- Oswaldo, L.C.E.; Refugio, R.V.; Deyanira, A.B.; Ricardo, L.M. Encapsulamiento de Colorantes Empleando Óxidos Dobles Laminares. Rev. Tend. Docencia Investig. Química 2024, 10, 512–518. [Google Scholar]
- Kumar, A.; Phor, L.; Bhargava, S.; Fatehmulla, A.; Singh, S.; Kumar, P.; Kumar, A.; Chahal, S. Controlled Synthesis of Nanosized Cd-CeO2 for Efficient PH Responsive Photocatalytic Degradation of CV Dye for Sustainable Wastewater Treatment. Mater. Sci. Eng. B 2025, 311, 117840. [Google Scholar] [CrossRef]
- Ismail, G.A.; Sakai, H. Review on Effect of Different Type of Dyes on Advanced Oxidation Processes (AOPs) for Textile Color Removal. Chemosphere 2022, 291, 132906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, X. Quantitative Analysis of Research Literature on Degradation of Pollutants by Fenton and Fenton-like AOPs Based on CiteSpace. Desalination Water Treat. 2024, 320, 100894. [Google Scholar] [CrossRef]
- Fu, W.; Yi, J.; Cheng, M.; Liu, Y.; Zhang, G.; Li, L.; Du, L.; Li, B.; Wang, G.; Yang, X. When Bimetallic Oxides and Their Complexes Meet Fenton-like Process. J. Hazard. Mater. 2022, 424, 127419. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Chen, H.; Hu, T.; Feng, J.; Xing, W.; Tang, L.; Tang, W. Recent Advances in the Applications of Encapsulated Transition-Metal Nanoparticles in Advanced Oxidation Processes for Degradation of Organic Pollutants: A Critical Review. Appl. Catal. B Environ. 2024, 342, 123401. [Google Scholar] [CrossRef]
- Lin, L.; Wang, J.; Zhao, Z.; Zhu, J.; Zhamaerding, A.; Feng, L.; Yang, D.; Meng, L.; He, C.; Wang, W.; et al. Multi-Dimensional Micro-Nano Scale Manganese Oxide Catalysts Induced Chemical-Based Advanced Oxidation Processes (AOPs) in Environmental Applications: A Critical Review. Chem. Eng. J. 2023, 474, 145600. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J. Fe-Based Fenton-like Catalysts for Water Treatment: Catalytic Mechanisms and Applications. J. Mol. Liq. 2021, 332, 115755. [Google Scholar] [CrossRef]
- Wang, H.; Shen, T.; Li, Y.; Wang, L.; Xiong, Y.; Wu, Y. Synthesis of γ-Cu2(OH)3Cl/LDH Composites as Fenton Catalysts to Mineralize Aniline: Successive Mineralization by Hydroxyl and Superoxide Radicals. New J. Chem. 2024, 48, 12740–12752. [Google Scholar] [CrossRef]
- Mkaddem, H.; Fdez-Sanromán, A.; Rosales, E.; Pazos, M.; Benamor, H.; Sanromán, M.A. Efficient Degradation of Organic Pollutants Using MnCuFe-LDH as a Photo-Fenton Catalyst. Chem. Eng. Sci. 2025, 311, 121611. [Google Scholar] [CrossRef]
- Keyikoğlu, R.; Khataee, A. Layered Double Hydroxides as Sustainable Catalysts in Electrocatalytic Processes for Water Treatment: Advances, Mechanisms, and Future Perspectives. J. Environ. Chem. Eng. 2025, 13, 115275. [Google Scholar] [CrossRef]
- Sarkar, S.; Upadhyay, C. Layered Double Hydroxides for Industrial Wastewater Remediation: A Review. Catal. Today 2025, 445, 115101. [Google Scholar] [CrossRef]
- Oswaldo, L.C.E.; Enrique, N.S.G.; Rita, V.R.M.; Erasmo, F.V.; Ricardo, L.M. Angeles Beltrán Deyanira. Remoción de Cu (II) En Efluentes Acuosos Utilizando Hidrotalcita Mg/Fe. Rev. Tend. Docencia Investig. Química 2020, 6, 164–169. [Google Scholar]
- Shin, J.; Rho, H.; Cho, Y.; Kwak, J.; Son, C.; Kim, S.; Ki, S.; Lee, Y.G.; Kim, H.J.; Lee, S.H.; et al. Decoration of Mg/Fe Layered Double Hydroxides on Coffee Waste Biochars for Enhanced Adsorption and Selectivity of Phosphate Ions: Mechanistic Studies. Desalination Water Treat. 2025, 321, 100990. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Zhou, J.; Zhang, L.; Shen, Y.; Wu, J.; Hao, C. Effective Removal of Congo Red and Hexavalent Chromium from Aqueous Solutions by Guar Gum/Sodium Alginate/Mg/Al-Layered Double Hydroxide Composite Microspheres. Int. J. Biol. Macromol. 2024, 293, 139385. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Sagar, F.B.; Rahman, M.L.; Rahman, F.; Rahman Nahid, M.M.; Sharmin, N. Efficient Adsorptive Removal of Ciprofloxacin from Wastewater Using Alginate Bearing Mg–Al Layered Double Hydroxide. J. Indian Chem. Soc. 2025, 102, 101519. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, Z.; Duan, X.; Ren, X.; Zhao, X. Enhancing Degradation of Sulfamethoxazole by Layered Double Hydroxide/Carbon Nanotubes Catalyst via Synergistic Effect of Photocatalysis/Persulfate Activation. Environ. Res. 2024, 261, 119647. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Wei, X.; Xin, C.; Yang, F. Synthesis of Flower-like NixFe1−x Layered Double Hydroxides with Enhanced Oxygen Evolution Catalysis. Int. J. Electrochem. Sci. 2024, 19, 100425. [Google Scholar] [CrossRef]
- Tian, K.; Zhang, J.; Liu, H.; Wang, R.; Zhang, Z. Mechanism of Carbonized Humic Acid and Magnesium Aluminum-Layered Double Hydroxide Promoting Biohydrogen Generation. Bioresour. Technol. 2024, 413, 131563. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhou, X.; Wang, F.; Ning, X.; Wen, Y.; Song, B.; Yang, C.; Wu, D.; Ke, X.; Peng, L. Insights into Memory Effect Mechanisms of Layered Double Hydroxides with Solid-State NMR Spectroscopy. Nat. Commun. 2022, 13, 6093. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wu, Z.; Bi, J.; Zhang, H.; Shahab, A. Calcined MgFe Layered Double Hydroxides for Magnetic Separation and Enhanced Adsorption Performance of Methyl Orange: Mechanism Based on the Memory Effect. J. Water Process. Eng. 2024, 63, 105418. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, D.; Chen, S.; Qiu, J.; Liu, X. Calcination Treatment of CoFe-Layered Double Hydroxide Enables Enhanced Chemodynamic and Photothermal Effects for Antibacterial and Antitumor Applications. Appl. Clay Sci. 2023, 239, 106949. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, X.; Xu, P.; Wang, H.; Liu, Y.; He, A. Elemental Mercury Removal from Flue Gas by CoFe2O4 Catalyzed Peroxymonosulfate. J. Hazard. Mater. 2018, 341, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, P.; Li, Y.; Wen, D.; Luo, J.; Wang, S.; Wu, F.; Fang, L.; Pang, Y. A Facile Synthesis of NiFe-Layered Double Hydroxide and Mixed Metal Oxide with Excellent Microwave Absorption Properties. Molecules 2021, 26, 5046. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Wang, L.; Xu, S.M.; Yang, X.; He, S.; Guan, S.; Waterhouse, G.I.N.; Zhou, S. Exploiting Co Defects in CoFe-Layered Double Hydroxide (CoFe-LDH) Derivatives for Highly Efficient Photothermal Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 54916–54926. [Google Scholar] [CrossRef] [PubMed]
- Milam, L.R.; Planalp, R.P. Bimetallic Fenton-like Catalysts in the Remediation of Dyes. Colorants 2023, 3, 1–16. [Google Scholar] [CrossRef]
- Tran, H.D.; Nguyen, D.Q.; Do, P.T.; Tran, U.N.P. Kinetics of Photocatalytic Degradation of Organic Compounds: A Mini-Review and New Approach. RSC Adv. 2023, 13, 16915–16925. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, C.; Yuan, Y.; Jin, Y.; Liu, Y.; Jiang, Z.; Li, X.; Dai, J.; Zhang, Y.; Siyal, A.A.; et al. Catalytic Degradation of Crystal Violet and Methyl Orange in Heterogeneous Fenton-like Processes. Chemosphere 2023, 344, 140406. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, A.A.; Ifebajo, A.O.; Gazi, M. Magnetic LDH-Based CoO–NiFe2O4 Catalyst with Enhanced Performance and Recyclability for Efficient Decolorization of Azo Dye via Fenton-like Reactions. Appl. Catal. B 2019, 243, 243–252. [Google Scholar] [CrossRef]
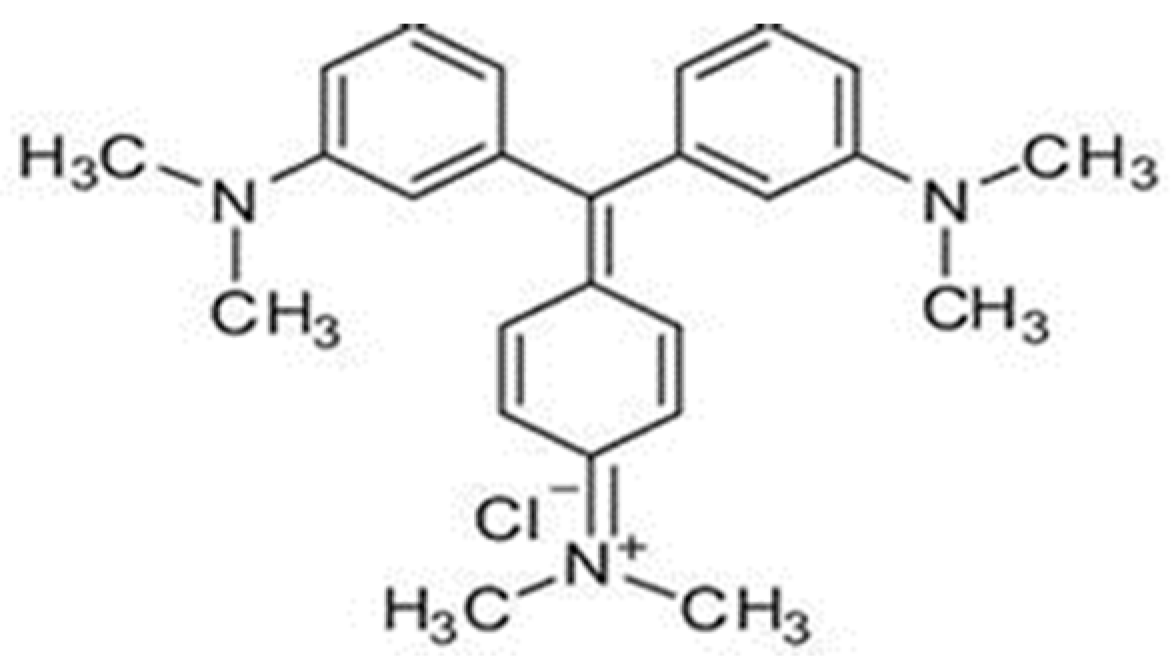
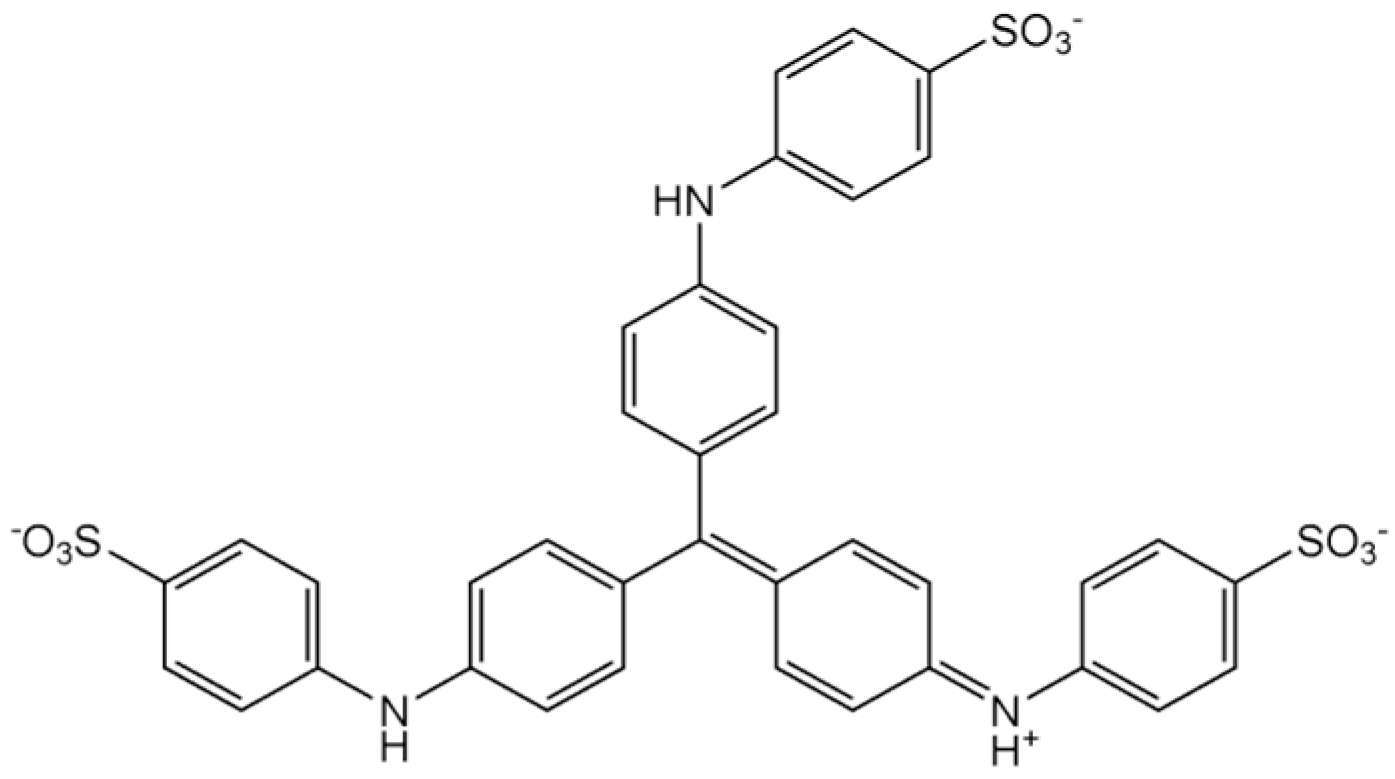
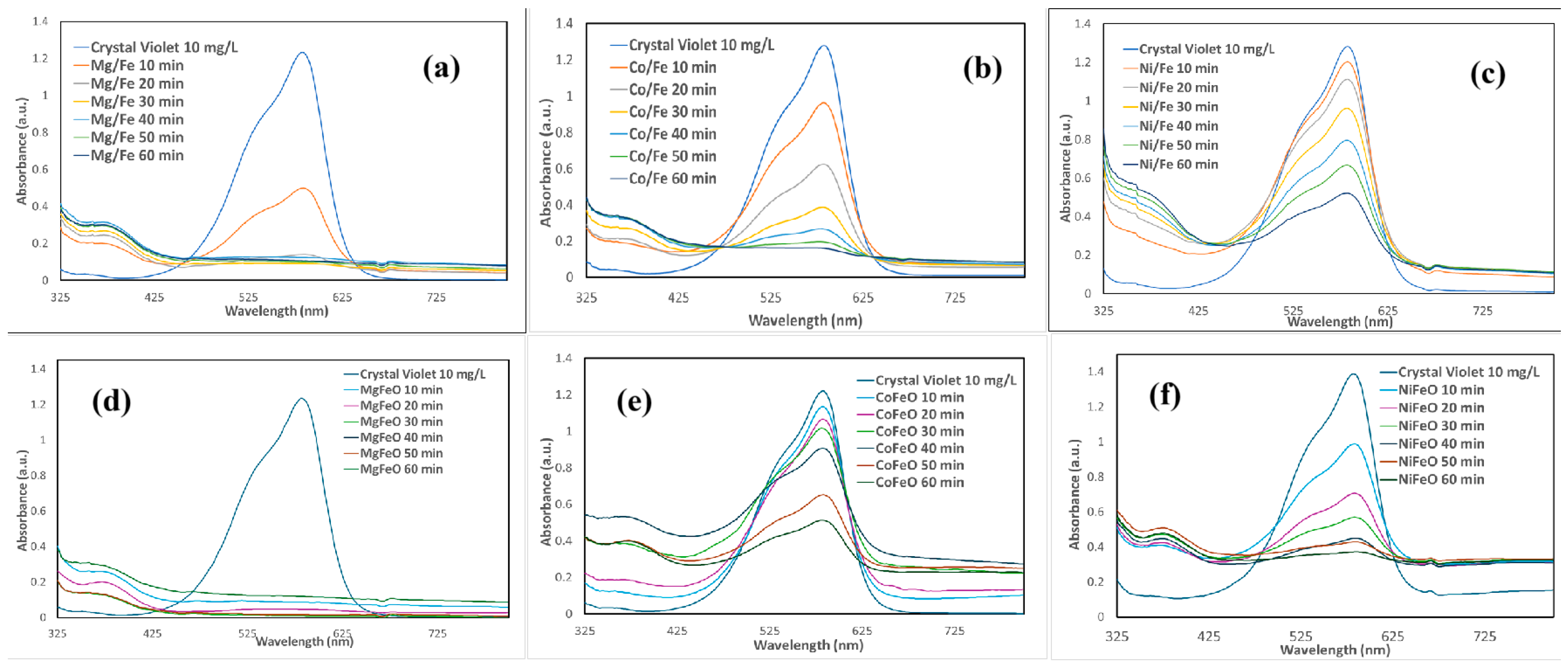
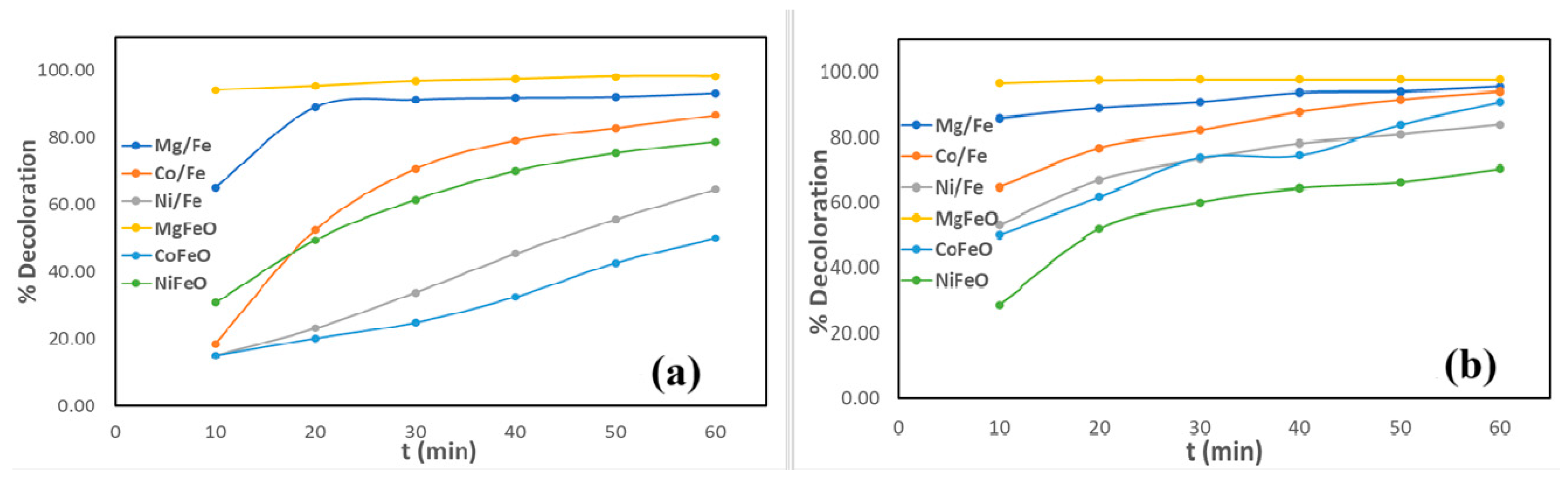
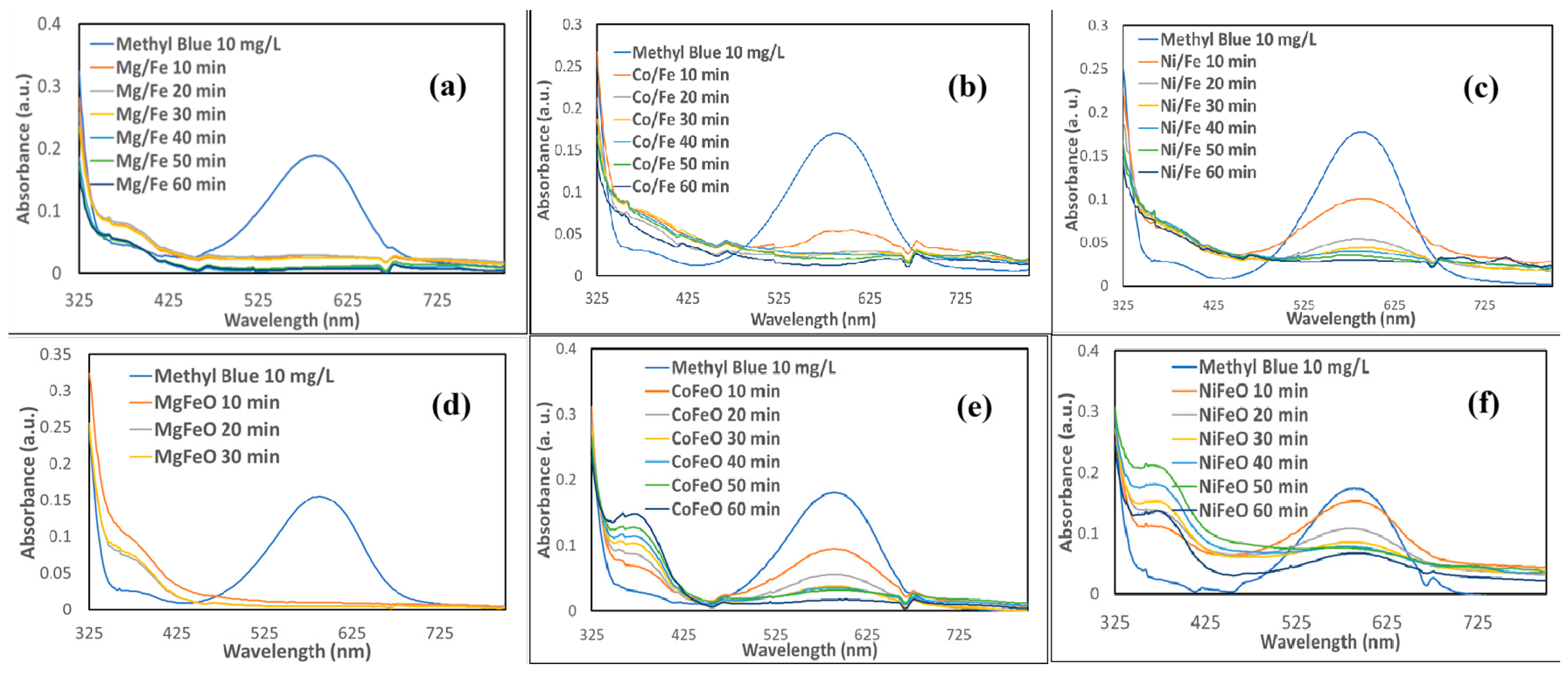
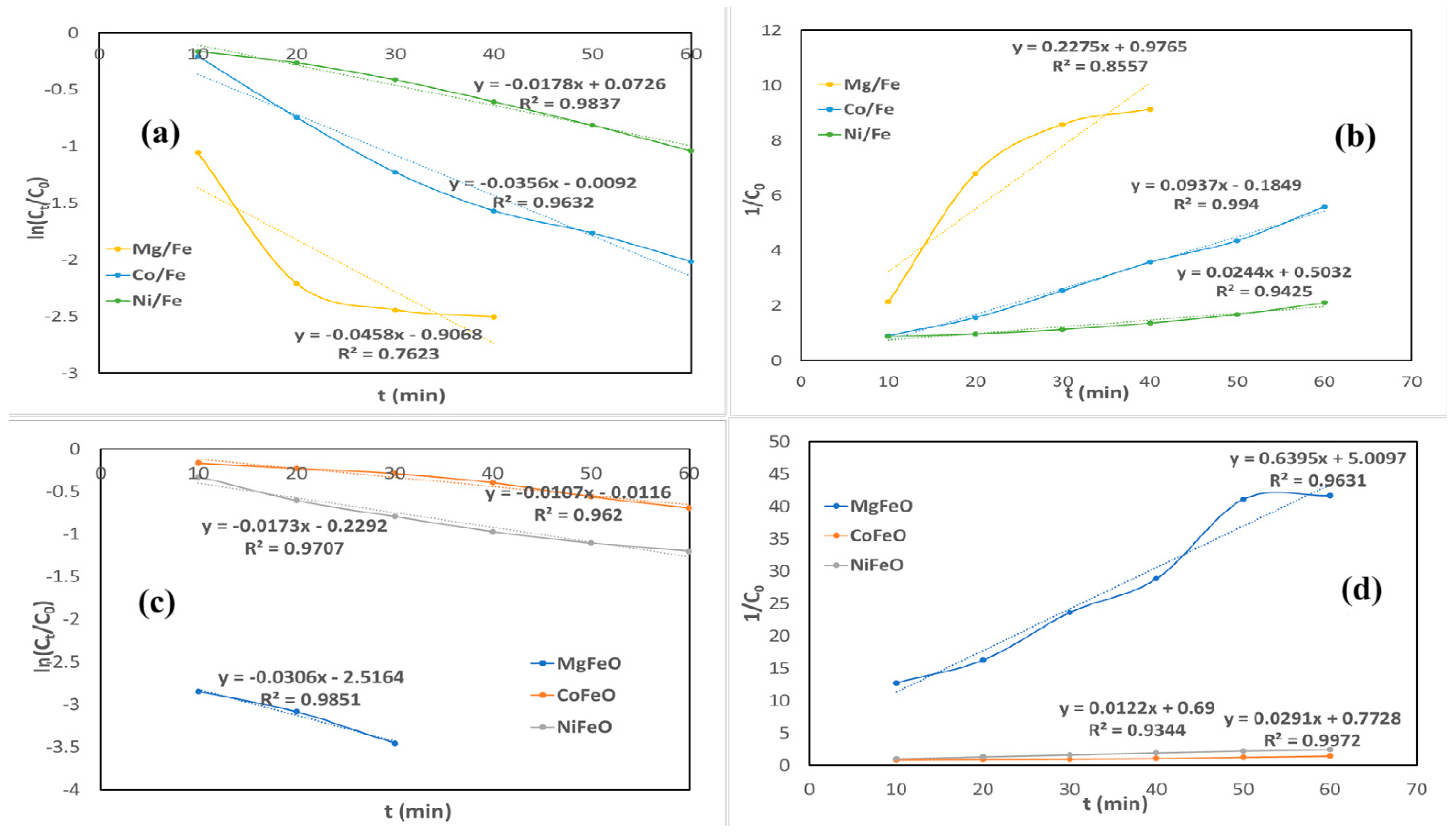
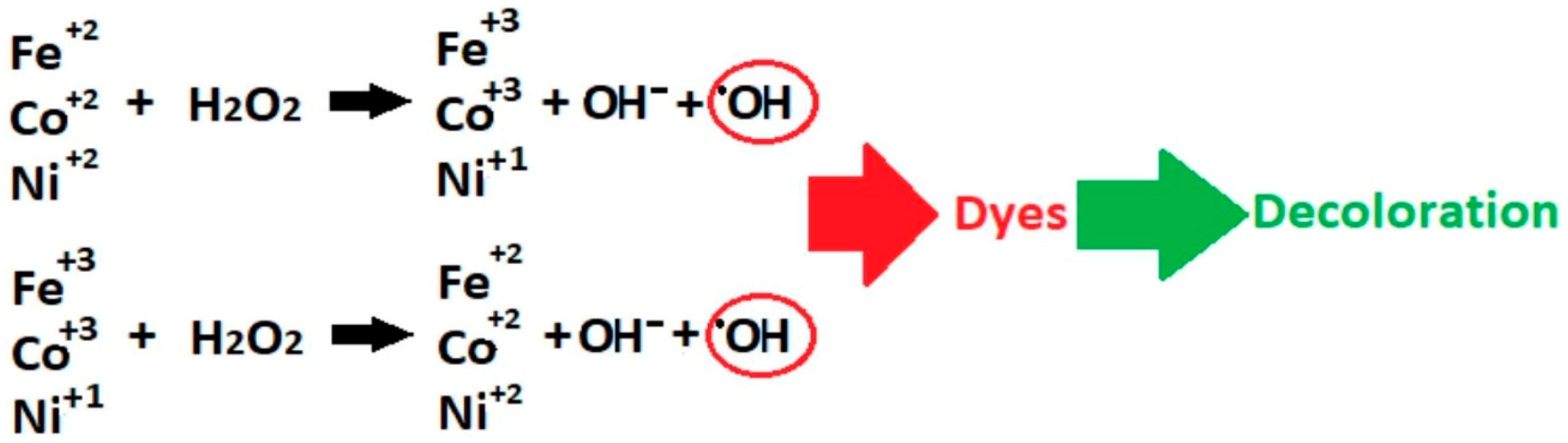
| Process | OH Radical Source | Optimal pH | Requires Light | H2O2 Generation | Operating Cost | Material/ Catalyst | Ref |
|---|---|---|---|---|---|---|---|
| Classical Fenton | H2O2 + Fe2+ | ~3 | No | External | Low | Fe3O4 (magnetite) | [17] |
| Fenton-like | H2O2 + transition metal | 3–6 | No | External | Low– medium | γ-Cu2(OH)3Cl/ Cu/Al-LDH | [18] |
| Photo- Fenton | H2O2 + Fe2+ + light | ~2.8–3.5 | Yes (UV/solar) | External | Medium | MnCuFe-LDH | [19] |
| Electro-Fenton | O2 (cathode) + Fe2+ | ~3 | No | In situ | Medium–high | CoFe LDH/carbon felt (CF) cathodes | [20] |
| Mg/Fe | Co/Fe | Ni/Fe | |
|---|---|---|---|
| Peak No. | Pos. [°2θ] | ||
| 1 | 11.54 | 11.82 | 11.62 |
| 2 | 23.06 | 23.55 | 23.17 |
| 3 | 34.19 | 34.36 | 34.66 |
| 4 | 38.39 | 38.93 | 39.13 |
| 5 | 45.56 | 46.57 | 46.93 |
| 6 | 59.63 | 59.52 | 60.16 |
| 7 | 60.94 | 60.85 | 61.40 |
| 8 | 64.77 | 64.80 | 65.26 |
| 9 | 73.22 | 70.17 | 71.16 |
| MgFeO | CoFeO | NiFeO | |
|---|---|---|---|
| Peak No. | Pos. [°2 θ] | ||
| 1 | 36.49 | 18.67 | 18.50 |
| 2 | 43.24 | 30.63 | 30.54 |
| 3 | 62.37 | 35.88 | 35.81 |
| 4 | 36.63 | 37.37 | |
| 5 | 42.68 | 43.46 | |
| 6 | 57.54 | 53.89 | |
| 7 | 61.76 | 57.55 | |
| 8 | 63.16 | 63.01 | |
| 9 | 64.56 | 75.47 | |
| 10 | 74.00 |
| Sample | %wt | |||||
|---|---|---|---|---|---|---|
| C | O | Mg | Co | Ni | Fe | |
| Mg/Fe | 4.28 | 52.44 | 16.84 | - | - | 16.84 |
| Mg/FeO | 4.81 | 38.04 | 22.38 | - | - | 34.77 |
| Co/Fe | 12.83 | 39.20 | - | 36.53 | - | 11.45 |
| Co/FeO | 9.26 | 23.56 | - | 53.02 | - | 16.03 |
| Ni/Fe | 7.77 | 43.49 | - | - | 36.29 | 12.45 |
| Ni/FeO | 4.74 | 22.21 | - | - | 55.81 | 17.25 |
| Material | BET Surface Area [m2/g] | Pore Volume [cm3/g] |
|---|---|---|
| Mg/Fe | 35.1 | 0.27 |
| MgFeO | 144 | 0.53 |
| Co/Fe | 47.6 | 0.51 |
| CoFeO | 22.8 | 0.17 |
| Ni/Fe | 89.4 | 0.41 |
| NiFeO | 33.6 | 0.34 |
| % Decoloration | ||||||
| Without Stirring | ||||||
| t [min] | Mg/Fe | Co/Fe | Ni/Fe | MgFeO | CoFeO | NiFeO |
| 10 | 8.43 | 6.87 | 1.31 | 61.24 | 6.49 | 27.84 |
| 20 | 31.75 | 16.01 | 5.45 | 94.93 | 10.45 | 45.42 |
| 30 | 58.28 | 30.97 | 12.16 | 95.55 | 17.96 | 54.80 |
| 40 | 77.95 | 41.83 | 18.69 | 95.72 | 26.57 | 62.11 |
| 50 | 87.91 | 53.51 | 25.45 | 95.77 | 32.61 | 66.82 |
| 60 | 92.50 | 62.24 | 31.64 | 95.90 | 39.20 | 69.90 |
| With Stirring (280 rpm) | ||||||
| t [min] | Mg/Fe | Co/Fe | Ni/Fe | MgFeO | CoFeO | NiFeO |
| 10 | 65.15 | 18.56 | 15.07 | 94.18 | 15.07 | 30.82 |
| 20 | 89.03 | 52.49 | 23.13 | 95.42 | 20.17 | 49.50 |
| 30 | 91.31 | 70.72 | 33.84 | 96.84 | 24.85 | 61.47 |
| 40 | 91.83 | 79.15 | 45.45 | 97.41 | 32.54 | 70.07 |
| 50 | 92.05 | 82.89 | 55.67 | 98.18 | 42.69 | 75.37 |
| 60 | 93.10 | 86.67 | 64.63 | 98.21 | 49.95 | 78.73 |
| Decoloration (%) | ||||||
| Without Stirring | ||||||
| t [min] | Mg/Fe | Co/Fe | Ni/Fe | MgFeO | CoFeO | NiFeO |
| 10 | 21.92 | 36.05 | 12.50 | 87.14 | 49.18 | 12.86 |
| 20 | 62.32 | 44.57 | 23.19 | 92.93 | 58.42 | 26.81 |
| 30 | 75.54 | 51.09 | 38.22 | 96.56 | 67.75 | 50.36 |
| 40 | 86.41 | 79.89 | 51.27 | 96.56 | 78.62 | 54.35 |
| 50 | 88.77 | 91.67 | 58.70 | 96.56 | 83.51 | 58.15 |
| 60 | 90.22 | 94.57 | 67.93 | 96.56 | 88.04 | 66.49 |
| With Stirring (280 rpm) | ||||||
| t [min] | Mg/Fe | Co/Fe | Ni/Fe | MgFeO | CoFeO | NiFeO |
| 10 | 85.87 | 64.67 | 53.08 | 96.56 | 50.00 | 28.44 |
| 20 | 88.95 | 76.63 | 66.85 | 97.46 | 61.78 | 51.81 |
| 30 | 90.76 | 82.07 | 73.19 | 97.64 | 73.73 | 59.96 |
| 40 | 93.66 | 87.68 | 78.08 | 97.64 | 74.46 | 64.31 |
| 50 | 94.02 | 91.49 | 80.80 | 97.64 | 83.88 | 66.12 |
| 60 | 95.47 | 94.02 | 83.88 | 97.64 | 90.76 | 70.29 |
| Kinetic Model | Equation | Lineal Adjusted |
|---|---|---|
| Pseudo-First Order | vs. t | |
| Pseudo-Second Order | vs. t |
| Crystal Violet | ||||
|---|---|---|---|---|
| PFO | PSO | |||
| Material | k1 | R2 | k2 | R2 |
| Mg/Fe | 0.0458 | 0.7623 | 0.2275 | 0.8557 |
| MgFeO | 0.0253 | 0.9678 | 0.6395 | 0.9631 |
| Co/Fe | 0.0356 | 0.9632 | 0.0973 | 0.9940 |
| CoFeO | 0.0173 | 0.9707 | 0.0291 | 0.9972 |
| Ni/Fe | 0.0178 | 0.9837 | 0.0244 | 0.9425 |
| NiFeO | 0.0107 | 0.9620 | 0.0122 | 0.9344 |
| Methyl Blue | ||||
| PFO | PSO | |||
| Material | k1 | R2 | k2 | R2 |
| Mg/Fe | 0.0226 | 0.9811 | 1.5993 | 0.9630 |
| MgFeO | 0.0190 | 0.8898 | 3.6437 | 0.9091 |
| Co/Fe | 0.0351 | 0.9980 | 1.4662 | 0.9339 |
| CoFeO | 0.0316 | 0.9514 | 0.8538 | 0.8215 |
| Ni/Fe | 0.0205 | 0.9736 | 0.4311 | 0.9975 |
| NiFeO | 0.0159 | 0.8928 | 0.1984 | 0.9558 |
| Factor | Level | Values (−1) | (+1) | (0) |
|---|---|---|---|---|
| Metal (II) | 3 | Mg | Co | Ni |
| Form | 2 | Hydroxide | Oxide | |
| Stirring (rpm) | 2 | 0 | 280 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva Cruz, E.O.; Negrete Godínez, D.; Angeles-Beltrán, D.; Rodríguez-Vázquez, R. The Behavior of Divalent Metals in Double-Layered Hydroxides as a Fenton Bimetallic Catalyst for Dye Decoloration: Kinetics and Experimental Design. Catalysts 2025, 15, 687. https://doi.org/10.3390/catal15070687
Leyva Cruz EO, Negrete Godínez D, Angeles-Beltrán D, Rodríguez-Vázquez R. The Behavior of Divalent Metals in Double-Layered Hydroxides as a Fenton Bimetallic Catalyst for Dye Decoloration: Kinetics and Experimental Design. Catalysts. 2025; 15(7):687. https://doi.org/10.3390/catal15070687
Chicago/Turabian StyleLeyva Cruz, Edgar Oswaldo, Diana Negrete Godínez, Deyanira Angeles-Beltrán, and Refugio Rodríguez-Vázquez. 2025. "The Behavior of Divalent Metals in Double-Layered Hydroxides as a Fenton Bimetallic Catalyst for Dye Decoloration: Kinetics and Experimental Design" Catalysts 15, no. 7: 687. https://doi.org/10.3390/catal15070687
APA StyleLeyva Cruz, E. O., Negrete Godínez, D., Angeles-Beltrán, D., & Rodríguez-Vázquez, R. (2025). The Behavior of Divalent Metals in Double-Layered Hydroxides as a Fenton Bimetallic Catalyst for Dye Decoloration: Kinetics and Experimental Design. Catalysts, 15(7), 687. https://doi.org/10.3390/catal15070687







