Recent Advances in Biocatalytic Dearomative Spirocyclization Reactions
Abstract
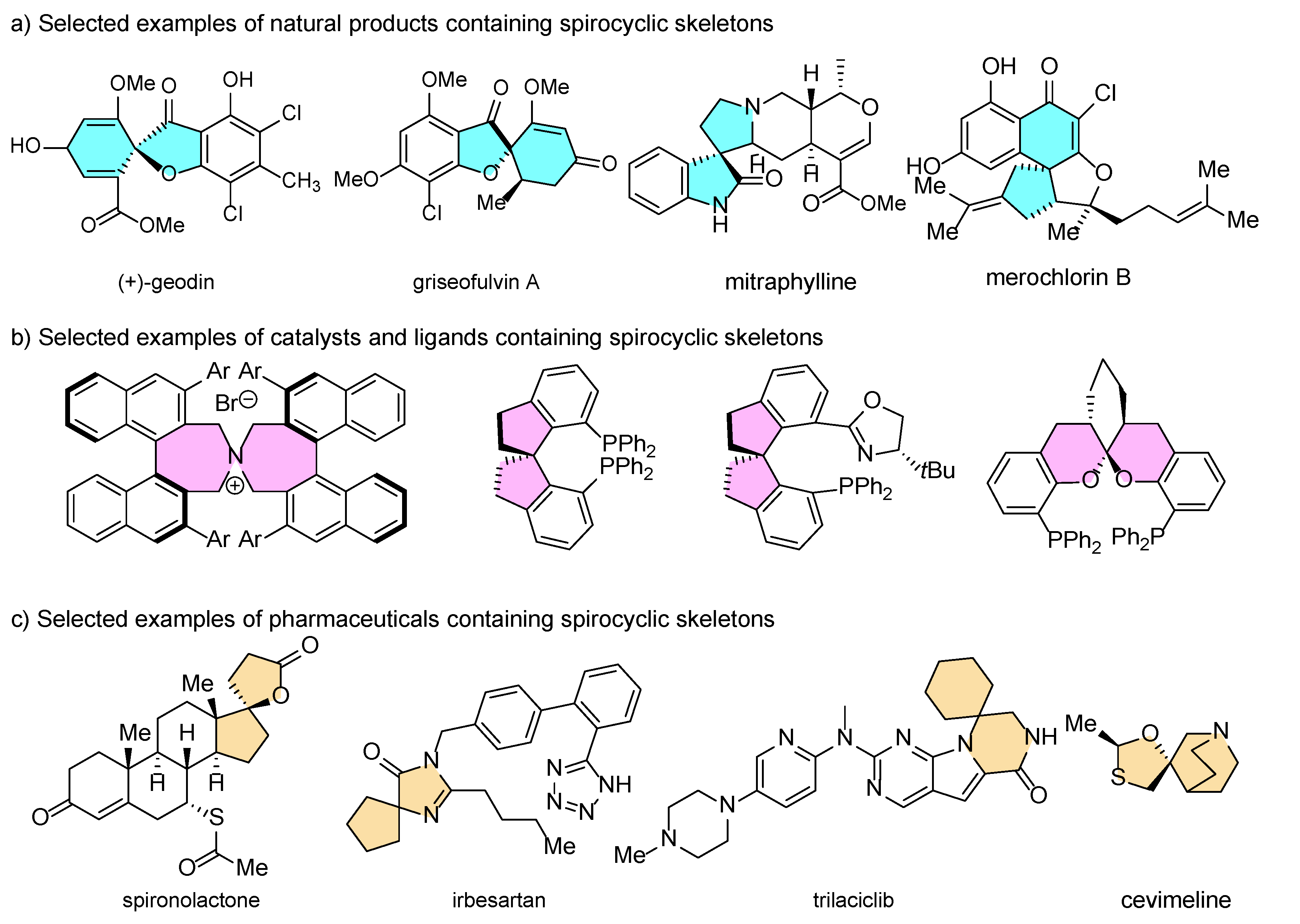
1. Introduction
2. Biocatalytic Dearomative Spirocyclization Reactions of All-Carbon Aromatic Rings
2.1. Dearomatization of Phenols
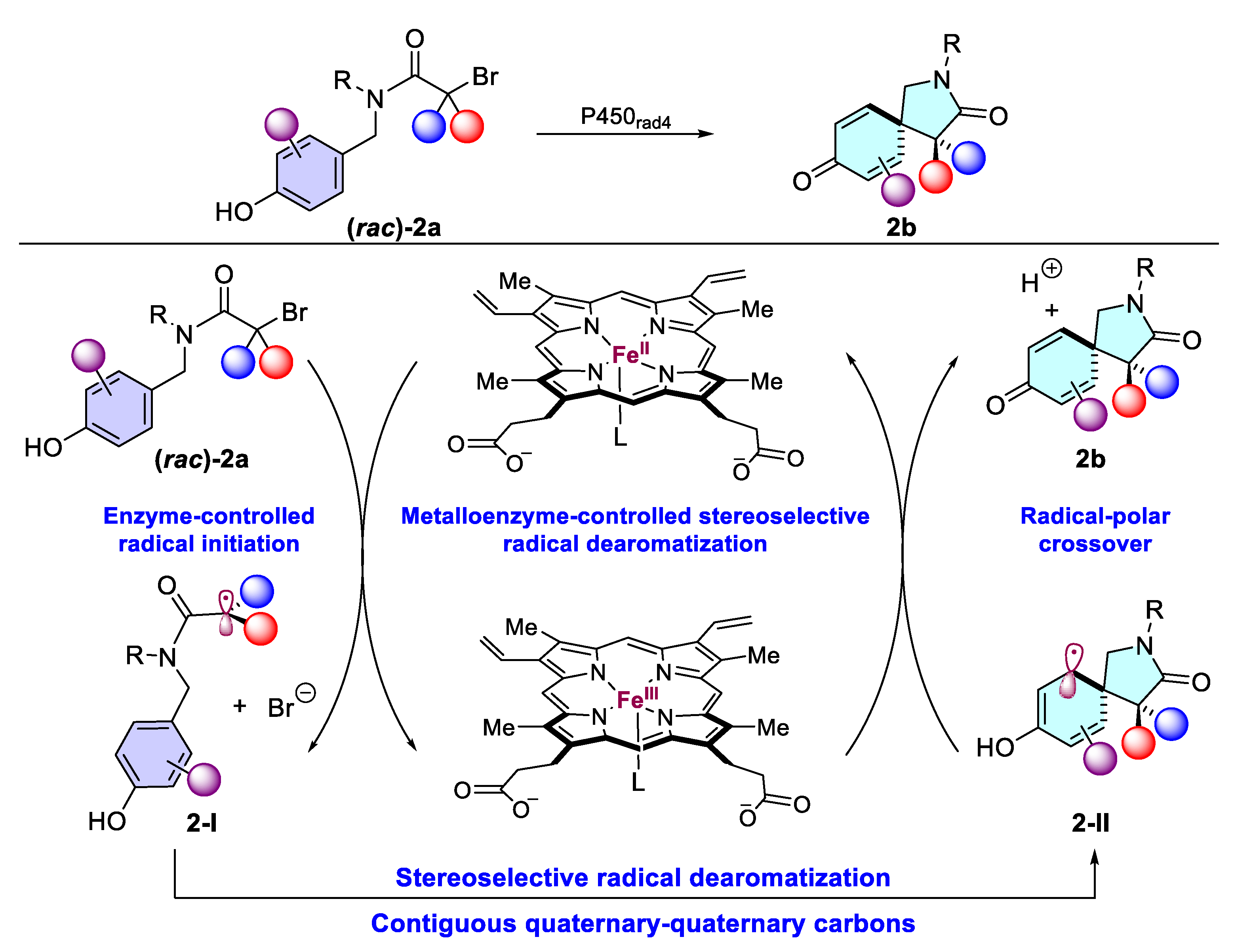
2.1.1. Dearomatization of Phenols via P450 Enzymes
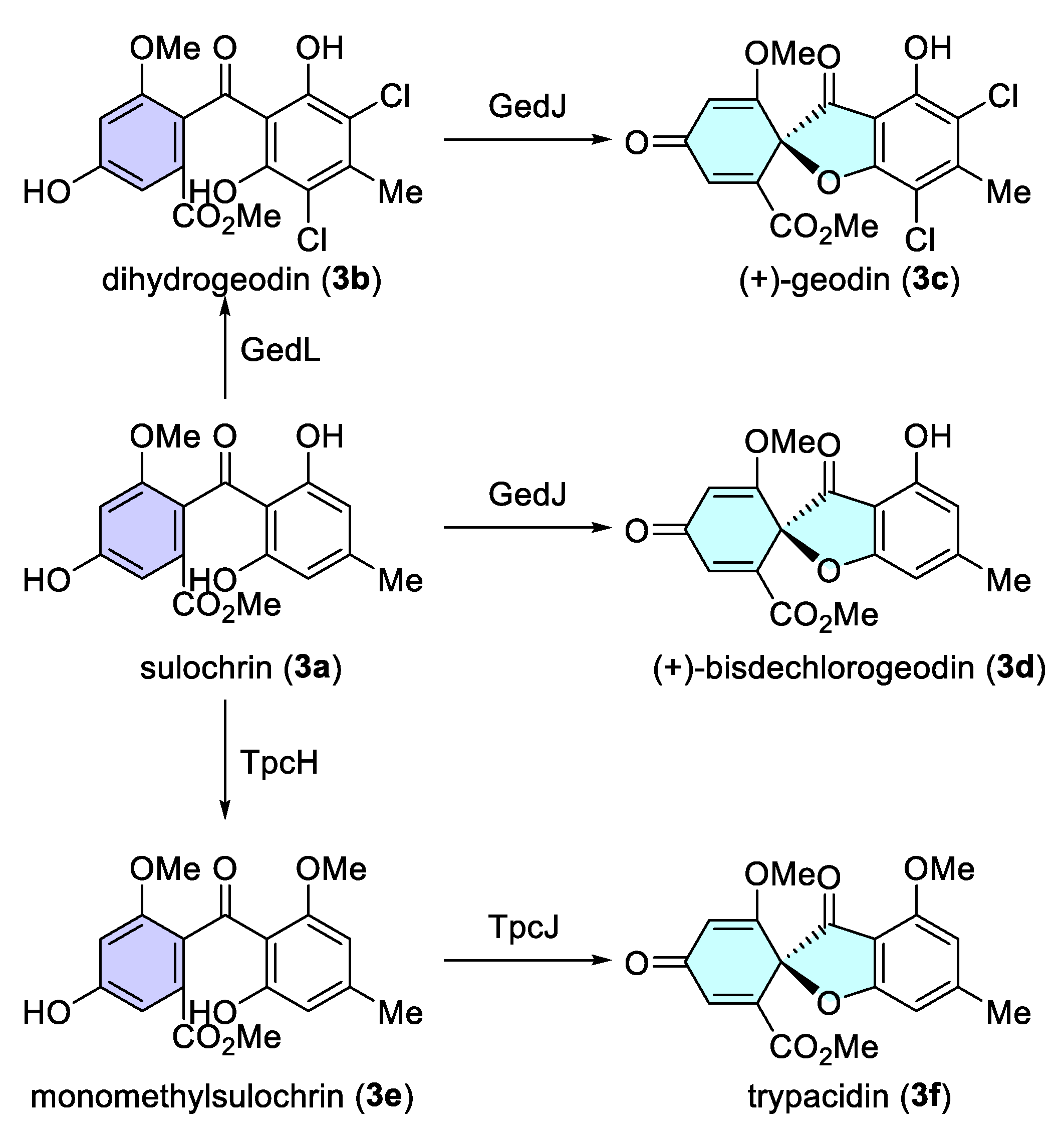
2.1.2. Dearomatization of Phenols via Copper-Dependent Oxidases
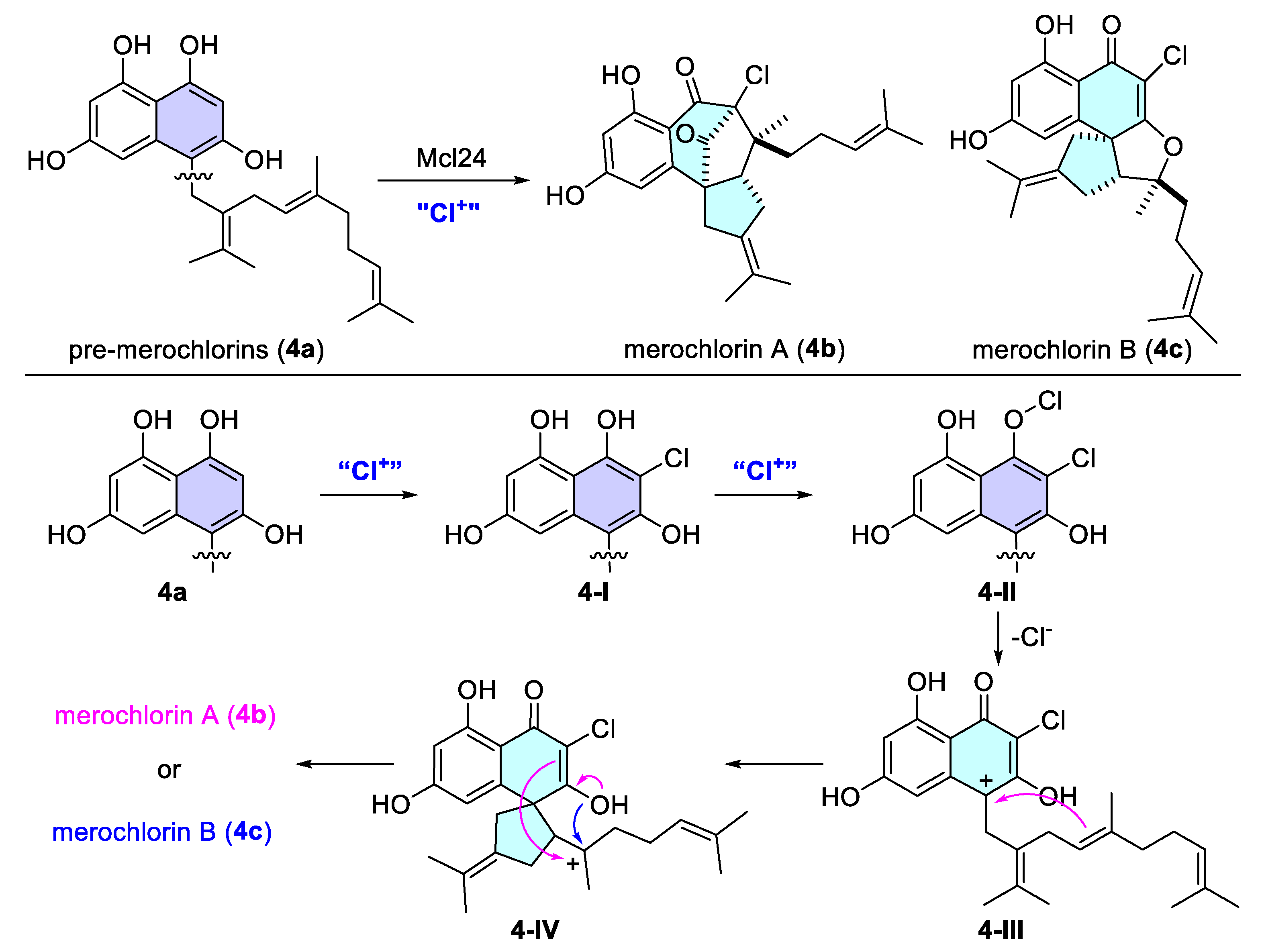
2.1.3. Dearomatization of Phenols via Vanadium-Dependent Chloroperoxidase
2.1.4. Dearomatization of Phenols via Methyltransferases
2.2. Dearomatization of Benzenes
3. Biocatalytic Dearomative Spirocyclization Reactions of Aza-Aromatic Rings
3.1. Dearomatization of Indoles
3.1.1. Dearomatization of Indoles via p450 Enzymes
3.1.2. Dearomatization of Indoles via Flavin-Dependent Oxygenases
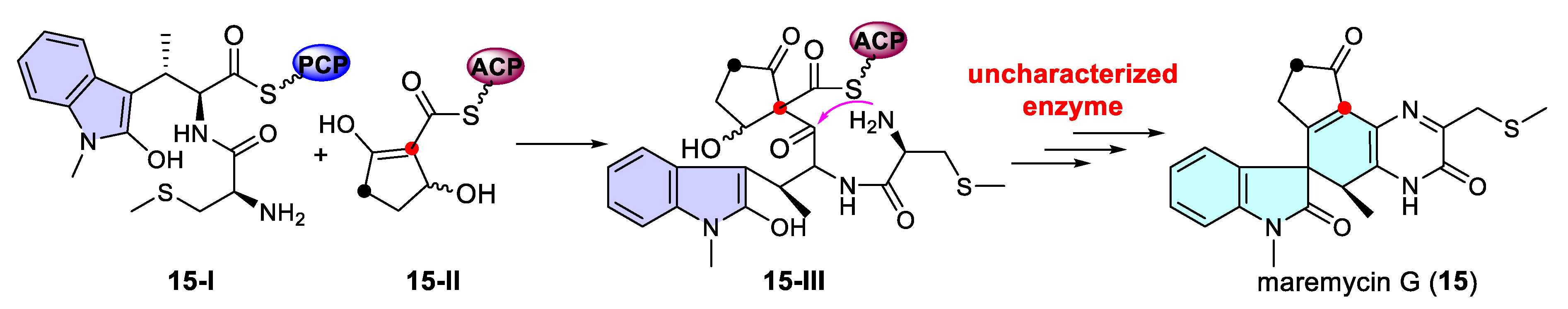
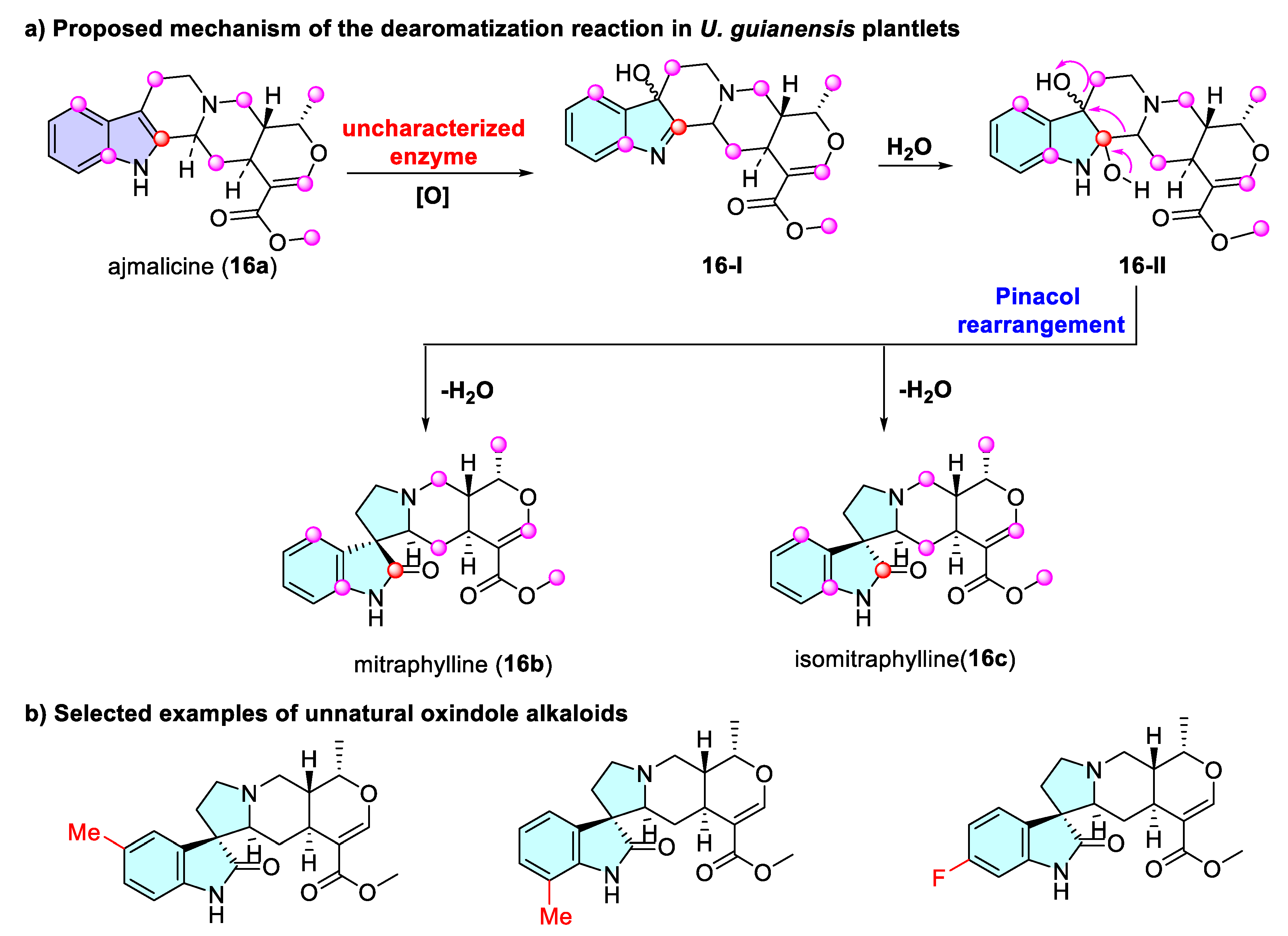
3.1.3. Dearomatization of Indoles via Uncharacterized Enzymes
3.2. Dearomatization of Pyrroles
3.2.1. Dearomatization of Pyrroles via P450
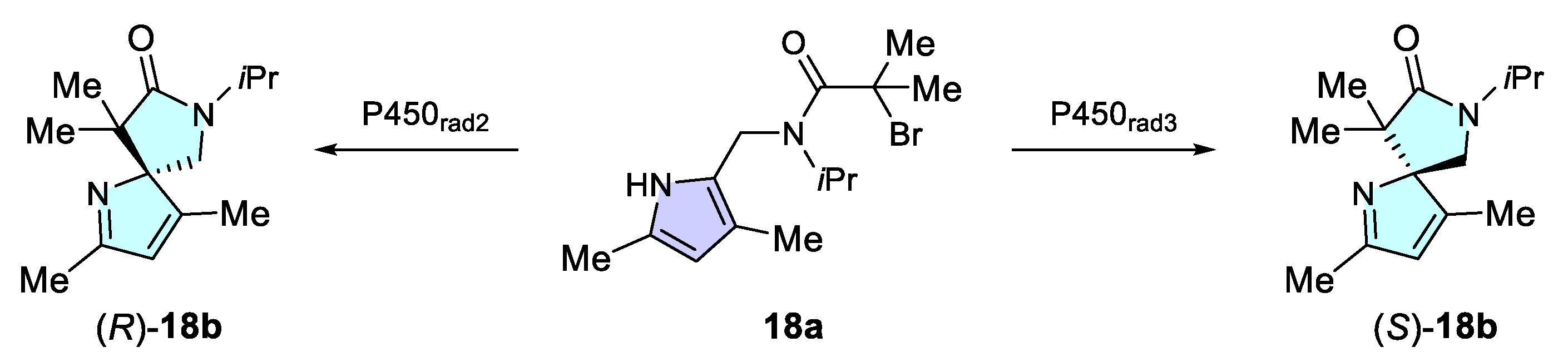
3.2.2. Dearomatization of Pyrroles via Uncharacterized Enzymes
4. Biocatalytic Dearomative Spirocyclization Reactions of Oxa-Aromatic Rings
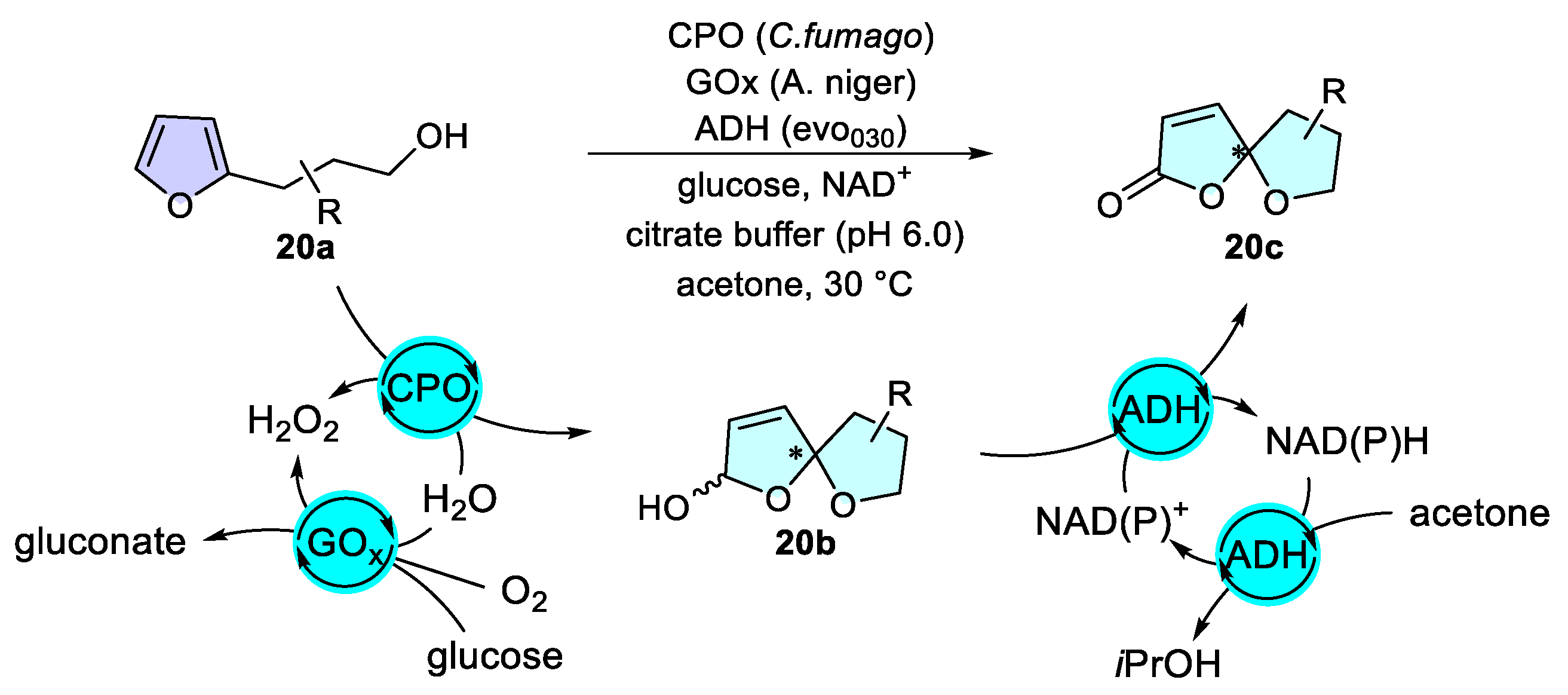
Dearomatization of Furans
5. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sperry, J.; Wilson, Z.E.; Rathwell, D.C.K.; Brimble, M.A. Isolation, biological activity and synthesis of benzannulated spiroketal natural products. Nat. Prod. Rep. 2010, 27, 1117. [Google Scholar] [CrossRef] [PubMed]
- Chupakhin, E.; Babich, O.; Prosekov, A.; Asyakina, L.; Krasavin, M. Spirocyclic Motifs in Natural Products. Molecules 2019, 24, 4165. [Google Scholar] [CrossRef] [PubMed]
- Ooi, T.; Kameda, M.; Maruoka, K. Design of N-Spiro C2-Symmetric Chiral Quaternary Ammonium Bromides as Novel Chiral Phase-Transfer Catalysts: Synthesis and Application to Practical Asymmetric Synthesis of α-Amino Acids. J. Am. Chem. Soc. 2003, 125, 5139–5151. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-H.; Zhou, Q. Magical Chiral Spiro Ligands. Acta Chim. Sinica 2014, 72, 778. [Google Scholar] [CrossRef]
- Rahman, A.; Lin, X. Development and application of chiral spirocyclic phosphoric acids in asymmetric catalysis. Org. Biomol. Chem. 2018, 16, 4753–4777. [Google Scholar] [CrossRef]
- Xu, C.; Hu, W. Recent Advances of ChiraI Spiro Ligands and Their Catalysts in Asymmetric Catalysis. Chem. J. Chin. Univ. 2020, 41, 2153. [Google Scholar]
- Wang, X.; Han, Z.; Wang, Z.; Ding, K. A Type of Structurally Adaptable Aromatic Spiroketal Based Chiral Diphosphine Ligands in Asymmetric Catalysis. Acc. Chem. Res. 2021, 54, 668–684. [Google Scholar] [CrossRef]
- Yang, F.; Xie, J.-H.; Zhou, Q.-L. Highly Efficient Asymmetric Hydrogenation Catalyzed by Iridium Complexes with Tridentate Chiral Spiro Aminophosphine Ligands. Acc. Chem. Res. 2023, 56, 332–349. [Google Scholar] [CrossRef]
- Xia, H.; Xie, K.; Zou, G. Advances in Spiropyrans/Spirooxazines and Applications Based on Fluorescence Resonance Energy Transfer (FRET) with Fluorescent Materials. Molecules 2017, 22, 2236. [Google Scholar] [CrossRef]
- Gangala, S.; Misra, R. Spiro-linked organic small molecules as hole-transport materials for perovskite solar cells. J. Mater. Chem. A 2018, 6, 18750–18765. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Wang, J.; Deng, Z.; Xu, Y.; Su, X.; Zhang, L.; Yang, S.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Spiro-materials with aggregation-induced emission. Matter 2024, 7, 3390–3421. [Google Scholar] [CrossRef]
- Zheng, Y.; Tice, C.M.; Singh, S.B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-M.; Ren-Yu, Q.; Yang, G.-F. An overview of spirooxindole as a promising scaffold for novel drug discovery. Expert Opin. Drug Dis. 2020, 15, 603–625. [Google Scholar] [CrossRef] [PubMed]
- Hiesinger, K.; Dar’in, D.; Proschak, E.; Krasavin, M. Spirocyclic Scaffolds in Medicinal Chemistry. J. Med. Chem. 2021, 64, 150–183. [Google Scholar] [CrossRef]
- Moshnenko, N.; Kazantsev, A.; Chupakhin, E.; Bakulina, O.; Dar’in, D. Synthetic Routes to Approved Drugs Containing a Spirocycle. Molecules 2023, 28, 4209. [Google Scholar] [CrossRef]
- Varela, M.T.; Dias, G.G.; de Oliveira, L.F.N.; de Oliveira, R.G.; Aguiar, F.D.; Nogueira, J.P.; Cruz, L.R.; Dias, L.C. Spirocyclic compounds as innovative tools in drug discovery for medicinal chemists. Eur. J. Med. Chem. 2025, 287, 117368. [Google Scholar] [CrossRef]
- Kumar, P.; Anuradha, S.; Rani, B.; Bellapukonda, S.; Indravath, R.S.; Ankita, D.; Srinivas, N.; Venkata Madhavi, Y. Spirocyclic compounds: Potential drug leads in the fight against Mycobacterium tuberculosis. Future Med. Chem. 2025, 17, 819–837. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Trapeznikova, O.g.A.; de Meijere, A.; Dzhemilev, U.M. Metal Complex Catalysis in the Synthesis of Spirocarbocycles. Chem. Rev. 2014, 114, 5775–5814. [Google Scholar] [CrossRef]
- James, M.J.; O’Brien, P.; Taylor, R.J.K.; Unsworth, W.P. Synthesis of Spirocyclic Indolenines. Chem. Eur. J. 2016, 22, 2856–2881. [Google Scholar] [CrossRef]
- Kotha, S.; Panguluri, N.R.; Ali, R. Design and Synthesis of Spirocycles. Eur. J. Org. Chem. 2017, 2017, 5316–5342. [Google Scholar] [CrossRef]
- Franz, A.K.; Hanhan, N.V.; Ball-Jones, N.R. Asymmetric Catalysis for the Synthesis of Spirocyclic Compounds. ACS Catal. 2013, 3, 540–553. [Google Scholar] [CrossRef]
- Ding, A.; Meazza, M.; Guo, H.; Yang, J.W.; Rios, R. New development in the enantioselective synthesis of spiro compounds. Chem. Soc. Rev. 2018, 47, 5946–5996. [Google Scholar] [CrossRef] [PubMed]
- Mei, G.-J.; Shi, F. Catalytic asymmetric synthesis of spirooxindoles: Recent developments. Chem. Commun. 2018, 54, 6607–6621. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.-W.; Yu, J.-S.; Chen, C.; Cao, Z.-Y.; Zhou, F.; Zhou, J. Catalytic Enantioselective Construction of Spiro Quaternary Carbon Stereocenters. ACS Catal. 2019, 9, 1820–1882. [Google Scholar] [CrossRef]
- Zheng, C.; You, S.-L. Exploring the Chemistry of Spiroindolenines by Mechanistically-Driven Reaction Development: Asymmetric Pictet–Spengler-type Reactions and Beyond. Acc. Chem. Res. 2020, 53, 974–987. [Google Scholar] [CrossRef]
- Boddy, A.J.; Bull, J.A. Stereoselective synthesis and applications of spirocyclic oxindoles. Org. Chem. Front. 2021, 8, 1026–1084. [Google Scholar] [CrossRef]
- Li, Q.; Pan, R.; Wang, M.; Yao, H.; Lin, A. Ligand-Controlled, Palladium-Catalyzed Asymmetric [4 + 4] and [2 + 4] Cycloadditions. Org. Lett. 2021, 23, 2292–2297. [Google Scholar] [CrossRef]
- Wang, Y.; Cobo, A.A.; Franz, A.K. Recent advances in organocatalytic asymmetric multicomponent cascade reactions for enantioselective synthesis of spirooxindoles. Org. Chem. Front. 2021, 8, 4315–4348. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Q.; Chen, L.; Yao, H.; Lin, A. Palladium-catalyzed asymmetric carbamoyl-carbonylation of alkenes. Sci. China Chem. 2021, 64, 1367–1371. [Google Scholar] [CrossRef]
- Wu, W.-T.; Zhang, L.; You, S.-L. Catalytic asymmetric dearomatization (CADA) reactions of phenol and aniline derivatives. Chem. Soc. Rev. 2016, 45, 1570–1580. [Google Scholar] [CrossRef]
- Sun, W.; Li, G.; Hong, L.; Wang, R. Asymmetric dearomatization of phenols. Org. Biomol. Chem. 2016, 14, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.-X.; Zheng, C.; You, S.-L. Transition-Metal-Catalyzed Asymmetric Allylic Dearomatization Reactions. Acc. Chem. Res. 2014, 47, 2558–2573. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.-X.; Jia, Y.-X. Aromatic π-Components for Enantioselective Heck Reactions and Heck/Anion-Capture Domino Sequences. Acc. Chem. Res. 2022, 55, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Claraz, A.; Masson, G. Asymmetric iodine catalysis-mediated enantioselective oxidative transformations. Org. Biomol. Chem. 2018, 16, 5386–5402. [Google Scholar] [CrossRef]
- Ali, S.; Israr, M. Asymmetric functionalization of benzenes via an organocatalytic hetero-Diels–Alder reaction. Chem. Commun. 2022, 58, 9851–9854. [Google Scholar] [CrossRef]
- Kamlar, M.; Urban, M.; Veselý, J. Enantioselective Synthesis of Spiro Heterocyclic Compounds Using a Combination of Organocatalysis and Transition-Metal Catalysis. Chem. Rec. 2023, 23, e202200284. [Google Scholar] [CrossRef]
- Patel, R.N. Biocatalysis: Synthesis of Key Intermediates for Development of Pharmaceuticals. ACS Catal. 2011, 1, 1056–1074. [Google Scholar] [CrossRef]
- Clouthier, C.M.; Pelletier, J.N. Expanding the organic toolbox: A guide to integrating biocatalysis in synthesis. Chem. Soc. Rev. 2012, 41, 1585–1605. [Google Scholar] [CrossRef]
- Nestl, B.M.; Hammer, S.C.; Nebel, B.A.; Hauer, B. New Generation of Biocatalysts for Organic Synthesis. Angew. Chem. Int. Ed. 2014, 53, 3070–3095. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Velikogne, S.; Hall, M.; Kroutil, W. Artificial Biocatalytic Linear Cascades for Preparation of Organic Molecules. Chem. Rev. 2018, 118, 270–348. [Google Scholar] [CrossRef]
- Xue, Y.-P.; Cao, C.-H.; Zheng, Y.-G. Enzymatic asymmetric synthesis of chiral amino acids. Chem. Soc. Rev. 2018, 47, 1516–1561. [Google Scholar] [CrossRef] [PubMed]
- Zwick, C.R.; Renata, H. Harnessing the biocatalytic potential of iron-and α-ketoglutarate-dependent dioxygenases in natural product total synthesis. Nat. Prod. Rep. 2020, 37, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.K.; Schrittwieser, J.H.; Kroutil, W. Power of Biocatalysis for Organic Synthesis. ACS Cent. Sci. 2021, 7, 55–71. [Google Scholar] [CrossRef]
- Watts, O.F.B.; Berreur, J.; Collins, B.S.L.; Clayden, J. Biocatalytic Enantioselective Synthesis of Atropisomers. Acc. Chem. Res. 2022, 55, 3362–3375. [Google Scholar] [CrossRef]
- Liu, C.; Miao, C.; Chen, X.; Zhang, Y.; Rao, Y.; Yuan, Z. Biocatalytic reactions, crystal structures and mechanisms of kynurenine formamidases. Tetrahedron Chem. 2024, 11, 100077. [Google Scholar] [CrossRef]
- O’Connell, A.; Barry, A.; Burke, A.J.; Hutton, A.E.; Bell, E.L.; Green, A.P.; O’Reilly, E. Biocatalysis: Landmark discoveries and applications in chemical synthesis. Chem. Soc. Rev. 2024, 53, 2828–2850. [Google Scholar] [CrossRef]
- Ji, P.; Park, J.; Gu, Y.; Clark, D.S.; Hartwig, J.F. Abiotic reduction of ketones with silanes catalysed by carbonic anhydrase through an enzymatic zinc hydride. Nat. Chem. 2021, 13, 312–318. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, X.; Zhuang, H.; Xie, Z.; Yu, L.; Fu, Z.; Sun, Y.; Wang, W.; Wu, R.; Ji, P. Stereoconvergent reduction of alkenes using a repurposed iron-based dioxygenase. Nat. Synth. 2025. [Google Scholar] [CrossRef]
- Li, Z.; Wan, Z.; Wang, W.; Chen, L.; Ji, P. Chemoenzymatic Sequential Catalysis with Carbonic Anhydrase for the Synthesis of Chiral Alcohols from Alkanes, Alkenes, and Alkynes. ACS Catal. 2024, 14, 8786–8793. [Google Scholar] [CrossRef]
- Bao, Y.; Li, Y.; Xie, Z.; Song, P.; Huang, J.; Zhang, X.; Ji, P. Designing γ-Carbonic Anhydrase as a Broad-Scope Metalloreductase with Ultrathermostability and Organic-Solvent Tolerance. ACS Catal. 2025, 15, 8036–8048. [Google Scholar] [CrossRef]
- Turner, N.; Gerlach, T. Biocatalytic Dearomatisation Reactions. Synthesis 2025, 57, 1102–1116. [Google Scholar] [CrossRef]
- Gai, K.; Fang, X.; Li, X.; Xu, J.; Wu, X.; Lin, A.; Yao, H. Synthesis of spiro [2.5]octa-4,7-dien-6-one with consecutive quaternary centers via 1,6-conjugate addition induced dearomatization of para-quinone methides. Chem. Commun. 2015, 51, 15831–15834. [Google Scholar] [CrossRef]
- Yuan, Z.; Fang, X.; Li, X.; Wu, J.; Yao, H.; Lin, A. 1,6-Conjugated Addition-Mediated [2+1] Annulation: Approach to Spiro[2.5]octa-4,7-dien-6-one. J. Org. Chem. 2015, 80, 11123–11130. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wei, W.; Lin, A.; Yao, H. Bifunctional Organo/Metal Cooperatively Catalyzed [3+2] Annulation of para-Quinone Methides with Vinylcyclopropanes: Approach to Spiro[4.5]deca-6,9-diene-8-ones. Org. Lett. 2016, 18, 3370–3373. [Google Scholar] [CrossRef]
- Zheng, J.; Li, P.; Gu, M.; Lin, A.; Yao, H. Synthesis of Spiropentadiene Pyrazolones by Rh(III)-Catalyzed Formal sp3 C–H Activation/Annulation. Org. Lett. 2017, 19, 2829–2832. [Google Scholar] [CrossRef]
- Yuan, Z.; Gai, K.; Wu, Y.; Wu, J.; Lin, A.; Yao, H. Tandem 1,6-addition/cyclization/vinylcyclopropane rearrangement at low temperature under metal-free conditions: An approach to spiro[4.5]cyclohexadienones. Chem. Commun. 2017, 53, 3485–3488. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, L.; Pan, R.; Yao, H.; Lin, A. Silver-Catalyzed Cascade 1,6-Addition/Cyclization of para-Quinone Methides with Propargyl Malonates: An Approach to Spiro[4.5]deca-6,9-dien-8-ones. J. Org. Chem. 2017, 82, 8743–8751. [Google Scholar] [CrossRef]
- Yuan, Z.; Pan, R.; Zhang, H.; Liu, L.; Lin, A.; Yao, H. Palladium-catalyzed Oxa-[4+2] Annulation of para-Quinone Methides. Adv. Synth. Catal. 2017, 359, 4244–4249. [Google Scholar] [CrossRef]
- Petersen, A.B.; Rønnest, M.H.; Larsen, T.O.; Clausen, M.H. The Chemistry of Griseofulvin. Chem. Rev. 2014, 114, 12088–12107. [Google Scholar] [CrossRef]
- Aris, P.; Wei, Y.; Mohamadzadeh, M.; Xia, X. Griseofulvin: An Updated Overview of Old and Current Knowledge. Molecules 2022, 27, 7034. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.-H.; Cacho, R.; Tang, Y. Identification of the Viridicatumtoxin and Griseofulvin Gene Clusters from Penicillium aethiopicum. Chem. Biol. 2010, 17, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Cacho, R.A.; Chooi, Y.-H.; Zhou, H.; Tang, Y. Complexity Generation in Fungal Polyketide Biosynthesis: A Spirocycle-Forming P450 in the Concise Pathway to the Antifungal Drug Griseofulvin. ACS Chem. Biol. 2013, 8, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Grandner, J.M.; Cacho, R.A.; Tang, Y.; Houk, K.N. Mechanism of the P450-Catalyzed Oxidative Cyclization in the Biosynthesis of Griseofulvin. ACS Catal. 2016, 6, 4506–4511. [Google Scholar] [CrossRef]
- Fu, W.; Fu, Y.; Zhao, Y.; Wang, H.; Liu, P.; Yang, Y. A metalloenzyme platform for catalytic asymmetric radical dearomatization. Nat. Chem. 2024, 16, 1999–2008. [Google Scholar] [CrossRef]
- Raistrick, H.; Smith, G. Studies in the biochemistry of micro-organisms: The metabolic products of Aspergillus terreus Thom. Part II. Two new chlorine-containing mould metabolic products, geodin and erdin. Biochem. J. 1936, 30, 1315–1322. [Google Scholar] [CrossRef]
- Rinderknecht, H.; Ward, J.L.; Bergel, F.; Morrison, A.L. Studies on antibiotics: 2. Bacteriological activity and possible mode of action of certain non-nitrogenous natural and synthetic antibiotics. Biochem. J. 1947, 41, 463–469. [Google Scholar] [CrossRef]
- Shinohara, C.; Chikanishi, T.; Nakashima, S.; Hashimoto, A.; Hamanaka, A.; Endo, A.; Hasumi, K. Enhancement of fibrinolytic activity of vascular endothelial cells by chaetoglobosin A, crinipellin B, geodin and triticone B. J. Antibiot. 2000, 53, 262–268. [Google Scholar] [CrossRef]
- Kozone, I.; Ueda, J.-y.; Takagi, M.; Shin-ya, K. JBIR-52, a new antimycin-like compound, from Streptomyces sp. ML55. J. Antibiot. 2009, 62, 593–595. [Google Scholar] [CrossRef]
- Nielsen, M.T.; Nielsen, J.B.; Anyaogu, D.C.; Holm, D.K.; Nielsen, K.F.; Larsen, T.O.; Mortensen, U.H. Heterologous reconstitution of the intact geodin gene cluster in Aspergillus nidulans through a simple and versatile PCR based approach. PLoS ONE 2013, 8, e72871. [Google Scholar] [CrossRef]
- Fujii, I.; Iijima, H.; Tsukita, S.; Ebizuka, Y.; Sankawa, U. Purification and Properties of Dihydrogeodin Oxidase from Aspergillus terreus. J. Biochem. 1987, 101, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.X.; Fujii, I.; Ebizuka, Y.; Gomi, K.; Sankawa, U. Molecular Cloning and Heterologous Expression of the Gene Encoding Dihydrogeodin Oxidase, a Multicopper Blue Enzyme from Aspergillus terreus. J. Biol. Chem. 1995, 270, 21495–21502. [Google Scholar] [CrossRef] [PubMed]
- De Mattos-Shipley, K.M.J.; Simpson, T.J. The ‘emodin family’ of fungal natural products–amalgamating a century of research with recent genomics-based advances. Nat. Prod. Rep. 2023, 40, 174–201. [Google Scholar] [CrossRef] [PubMed]
- Nordlöv, H.; Gatenbeck, S. Enzymatic synthesis of (+)-and (-)-bisdechlorogeodin with sulochrin oxidase from Penicillium frequentans and Oospora sulphurea-ochracea. Arch. Microbiol. 1982, 131, 208–211. [Google Scholar] [CrossRef]
- Scott, A.I. Oxidative coupling of phenolic compounds. Q. Rev. Chem. Soc. 1965, 19, 1–35. [Google Scholar] [CrossRef]
- Diethelm, S.; Teufel, R.; Kaysser, L.; Moore, B.S. A Multitasking Vanadium-Dependent Chloroperoxidase as an Inspiration for the Chemical Synthesis of the Merochlorins. Angew. Chem. Int. Ed. 2014, 53, 11023–11026. [Google Scholar] [CrossRef]
- Kaysser, L.; Bernhardt, P.; Nam, S.-J.; Loesgen, S.; Ruby, J.G.; Skewes-Cox, P.; Jensen, P.R.; Fenical, W.; Moore, B.S. Merochlorins A–D, Cyclic Meroterpenoid Antibiotics Biosynthesized in Divergent Pathways with Vanadium-Dependent Chloroperoxidases. J. Am. Chem. Soc. 2012, 134, 11988–11991. [Google Scholar] [CrossRef]
- Sakoulas, G.; Nam, S.-J.; Loesgen, S.; Fenical, W.; Jensen, P.R.; Nizet, V.; Hensler, M. Novel Bacterial Metabolite Merochlorin A Demonstrates in vitro Activity against Multi-Drug Resistant Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2012, 7, e29439. [Google Scholar] [CrossRef]
- Yeh, E.; Blasiak, L.C.; Koglin, A.; Drennan, C.L.; Walsh, C.T. Chlorination by a Long-Lived Intermediate in the Mechanism of Flavin-Dependent Halogenases. Biochemistry 2007, 46, 1284–1292. [Google Scholar] [CrossRef]
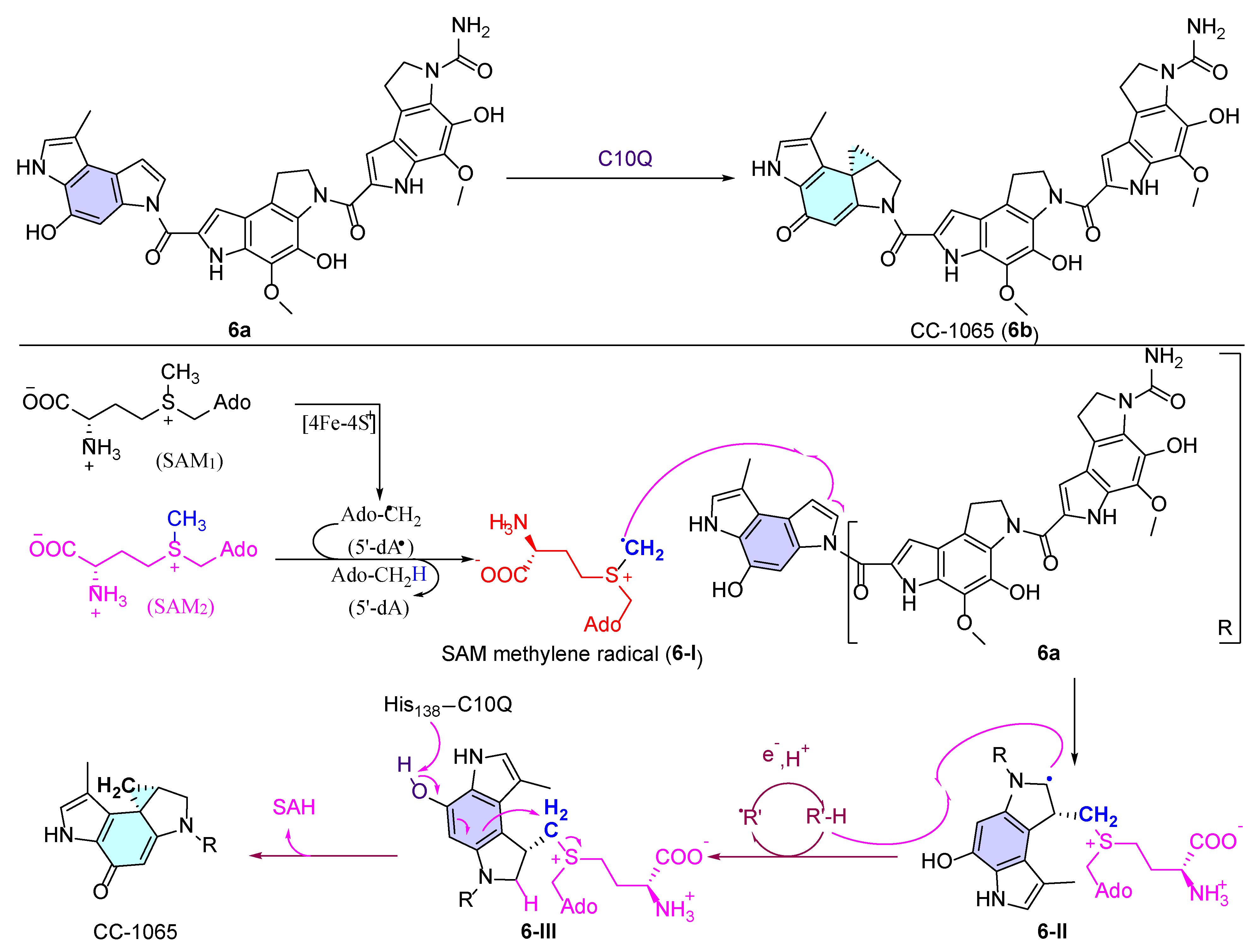
- Felber, J.G.; Thorn-Seshold, O. 40 Years of Duocarmycins: A Graphical Structure/Function Review of Their Chemical Evolution, from SAR to Prodrugs and ADCs. JACS Au 2022, 2, 2636–2644. [Google Scholar] [CrossRef]
- MacMillan, K.S.; Boger, D.L. Fundamental Relationships between Structure, Reactivity, and Biological Activity for the Duocarmycins and CC-1065. J. Med. Chem. 2009, 52, 5771–5780. [Google Scholar] [CrossRef] [PubMed]
- Tichenor, M.S.; Boger, D.L. Yatakemycin: Total synthesis, DNA alkylation, and biological properties. Nat. Prod. Rep. 2008, 25, 220–226. [Google Scholar] [CrossRef] [PubMed]
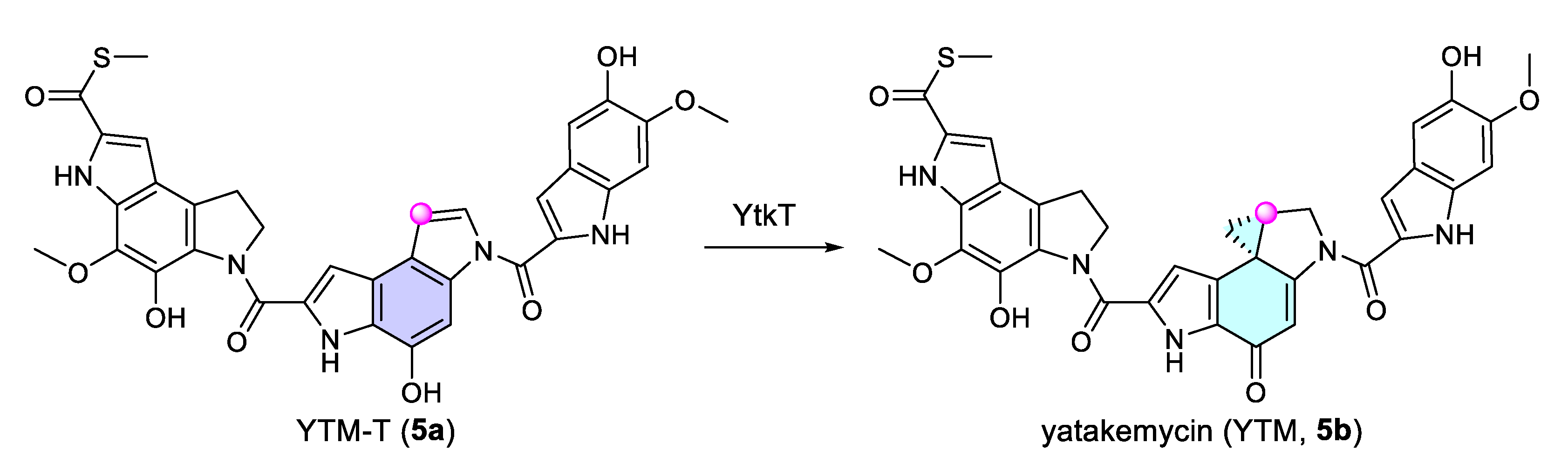
- Huang, W.; Xu, H.; Li, Y.; Zhang, F.; Chen, X.-Y.; He, Q.-L.; Igarashi, Y.; Tang, G.-L. Characterization of Yatakemycin Gene Cluster Revealing a Radical S-Adenosylmethionine Dependent Methyltransferase and Highlighting Spirocyclopropane Biosynthesis. J. Am. Chem. Soc. 2012, 134, 8831–8840. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.-B.; Wu, S.; Jian, X.-H.; Yuan, H.; Tang, G.-L. A radical S-adenosyl-L-methionine enzyme and a methyltransferase catalyze cyclopropane formation in natural product biosynthesis. Nat. Commun. 2018, 9, 2771. [Google Scholar] [CrossRef]
- Wertjes, W.C.; Southgate, E.H.; Sarlah, D. Recent advances in chemical dearomatization of nonactivated arenes. Chem. Soc. Rev. 2018, 47, 7996–8017. [Google Scholar] [CrossRef]
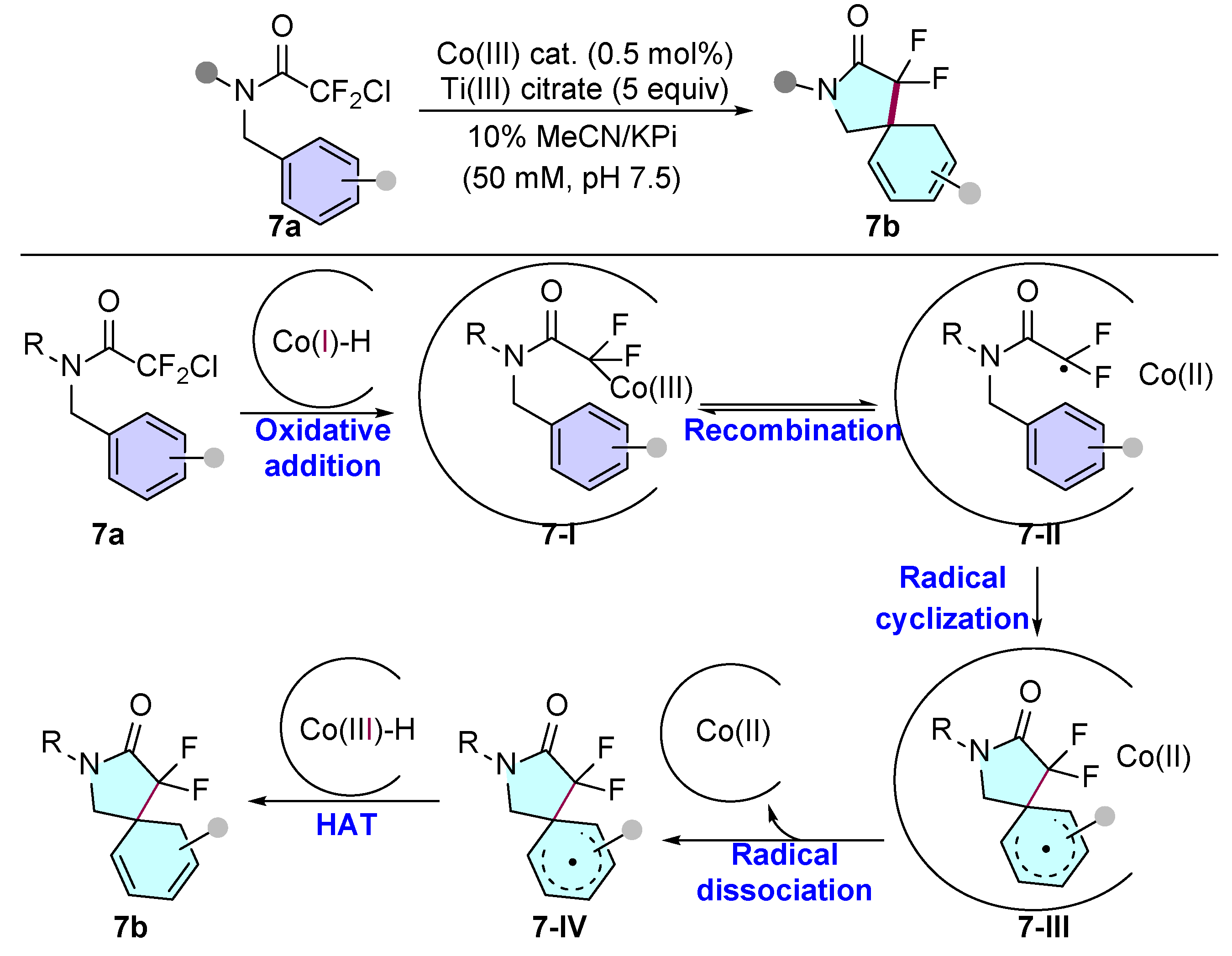
- Li, J.; Kumar, A.; Lewis, J.C. Non-native Intramolecular Radical Cyclization Catalyzed by a B12-Dependent Enzyme. Angew. Chem. Int. Ed. 2023, 62, e202312893. [Google Scholar] [CrossRef]
- Bandini, M.; Eichholzer, A. Catalytic Functionalization of Indoles in a New Dimension. Angew. Chem. Int. Ed. 2009, 48, 9608–9644. [Google Scholar] [CrossRef]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine Indole Alkaloids: Potential New Drug Leads for the Control of Depression and Anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef]
- Song, J.; Chen, D.-F.; Gong, L.-Z. Recent progress in organocatalytic asymmetric total syntheses of complex indole alkaloids. Natl. Sci. Rev. 2017, 4, 381–396. [Google Scholar] [CrossRef]
- Wan, Y.; Li, Y.; Yan, C.; Yan, M.; Tang, Z. Indole: A privileged scaffold for the design of anti-cancer agents. Eur. J. Med. Chem. 2019, 183, 111691. [Google Scholar] [CrossRef]
- Zheng, C.; You, S.-L. Catalytic asymmetric dearomatization (CADA) reaction-enabled total synthesis of indole-based natural products. Nat. Prod. Rep. 2019, 36, 1589–1605. [Google Scholar] [CrossRef] [PubMed]
- Sheng, F.-T.; Wang, J.-Y.; Tan, W.; Zhang, Y.-C.; Shi, F. Progresses in organocatalytic asymmetric dearomatization reactions of indole derivatives. Org. Chem. Front. 2020, 7, 3967–3998. [Google Scholar] [CrossRef]
- Buttard, F.; Guinchard, X. Spiroindoles as Intermediates/Products in Transition Metal-Catalyzed Dearomatization of Indoles. ACS Catal. 2023, 13, 9442–9475. [Google Scholar] [CrossRef]
- Das, S. Visible-Light-Induced Dearomative Annulation of Indoles toward Stereoselective Formation of Fused- and Spiro Indolines. ACS Omega 2024, 9, 36023–36042. [Google Scholar] [CrossRef] [PubMed]
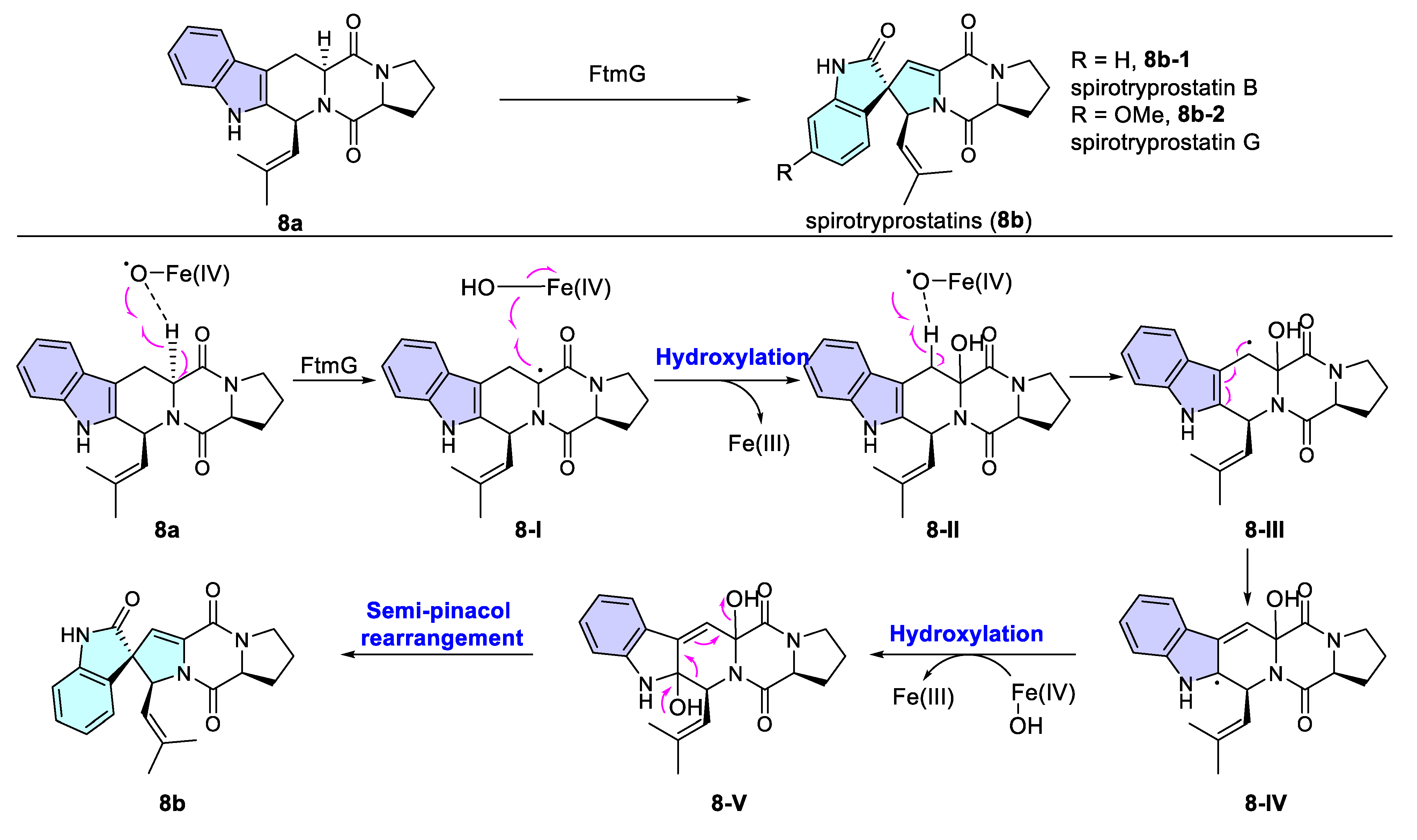
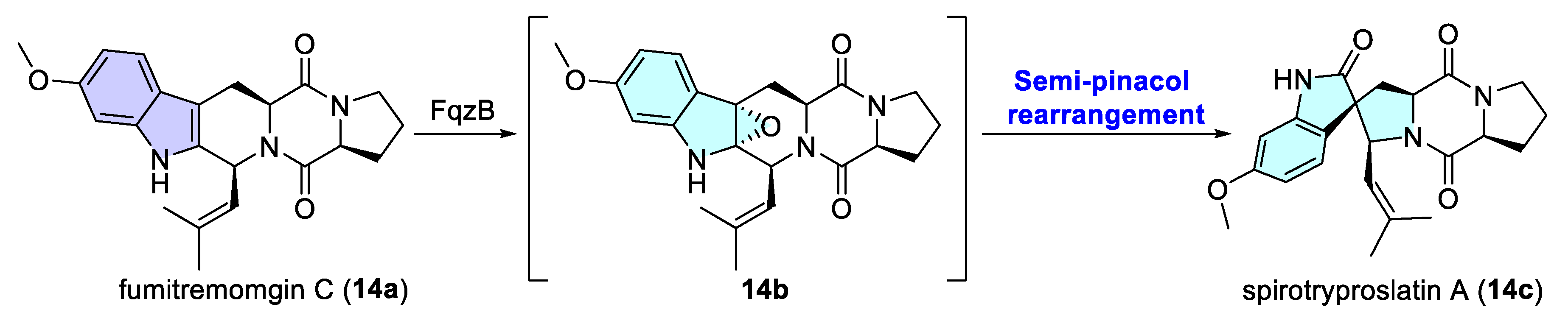
- Tsunematsu, Y.; Ishikawa, N.; Wakana, D.; Goda, Y.; Noguchi, H.; Moriya, H.; Hotta, K.; Watanabe, K. Distinct mechanisms for spiro-carbon formation reveal biosynthetic pathway crosstalk. Nat. Chem. Biol. 2013, 9, 818–825. [Google Scholar] [CrossRef]
- Cui, C.-B.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, spirotryprostatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 1996, 52, 12651–12666. [Google Scholar] [CrossRef]
- Cui, C.-B.; Kakeya, H.; Osada, H. Spirotryprostatin B, a novel mammalian cell cycle inhibitor produced by Aspergillus fumigatus. J. Antibiot. 1996, 49, 832–835. [Google Scholar] [CrossRef]
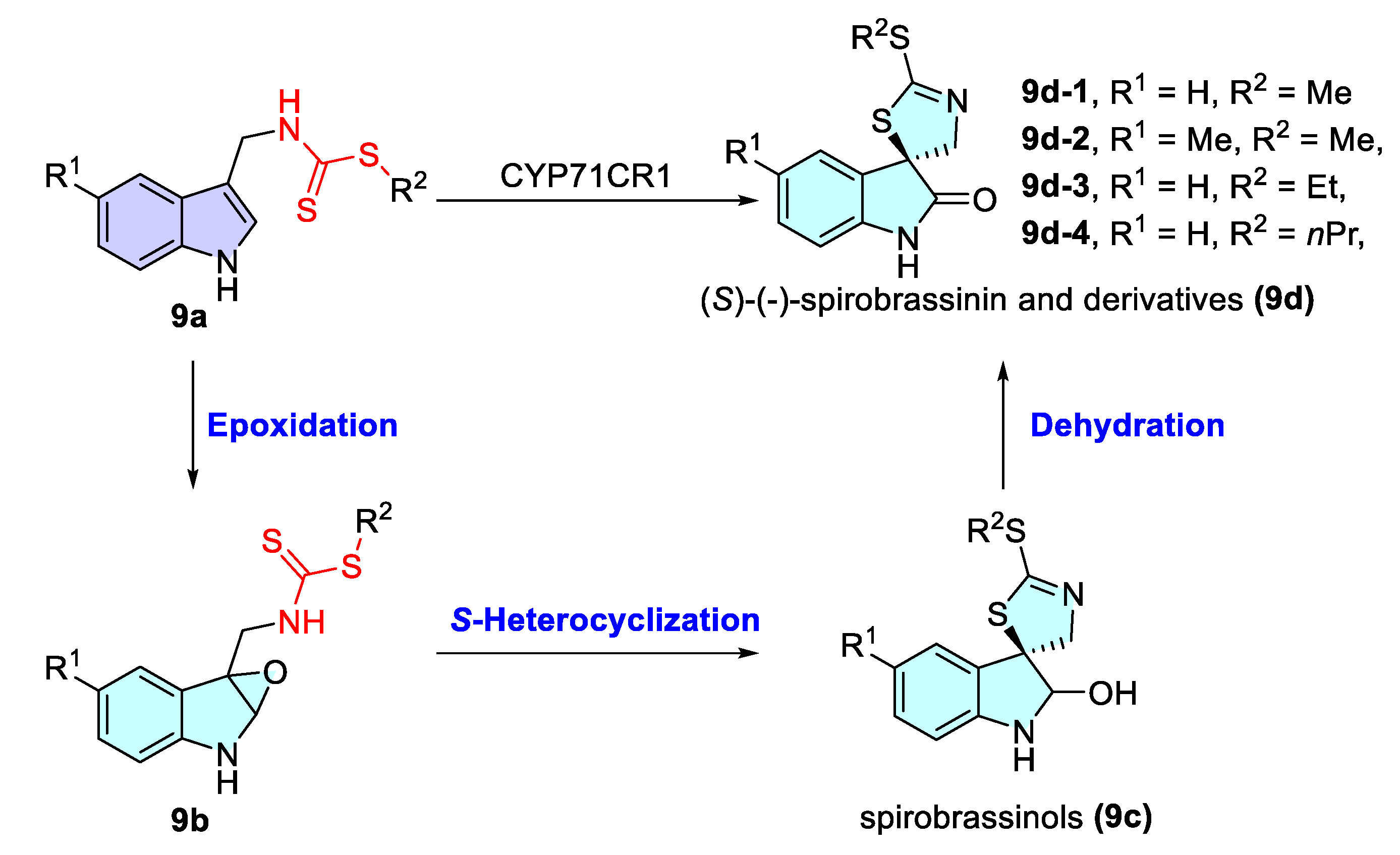
- Klein, A.P.; Sattely, E.S. Two cytochromes P450 catalyze S-heterocyclizations in cabbage phytoalexin biosynthesis. Nat. Chem. Biol. 2015, 11, 837–839. [Google Scholar] [CrossRef]
- Ryu, K.; Nakamura, S.; Nakashima, S.; Matsuda, H. One-pot enantioselective synthesis of (S)-spirobrassinin and non-natural (S)-methylspirobrassinin from amino acids using a turnip enzyme. J. Nat. Med. 2021, 75, 308–318. [Google Scholar] [CrossRef]
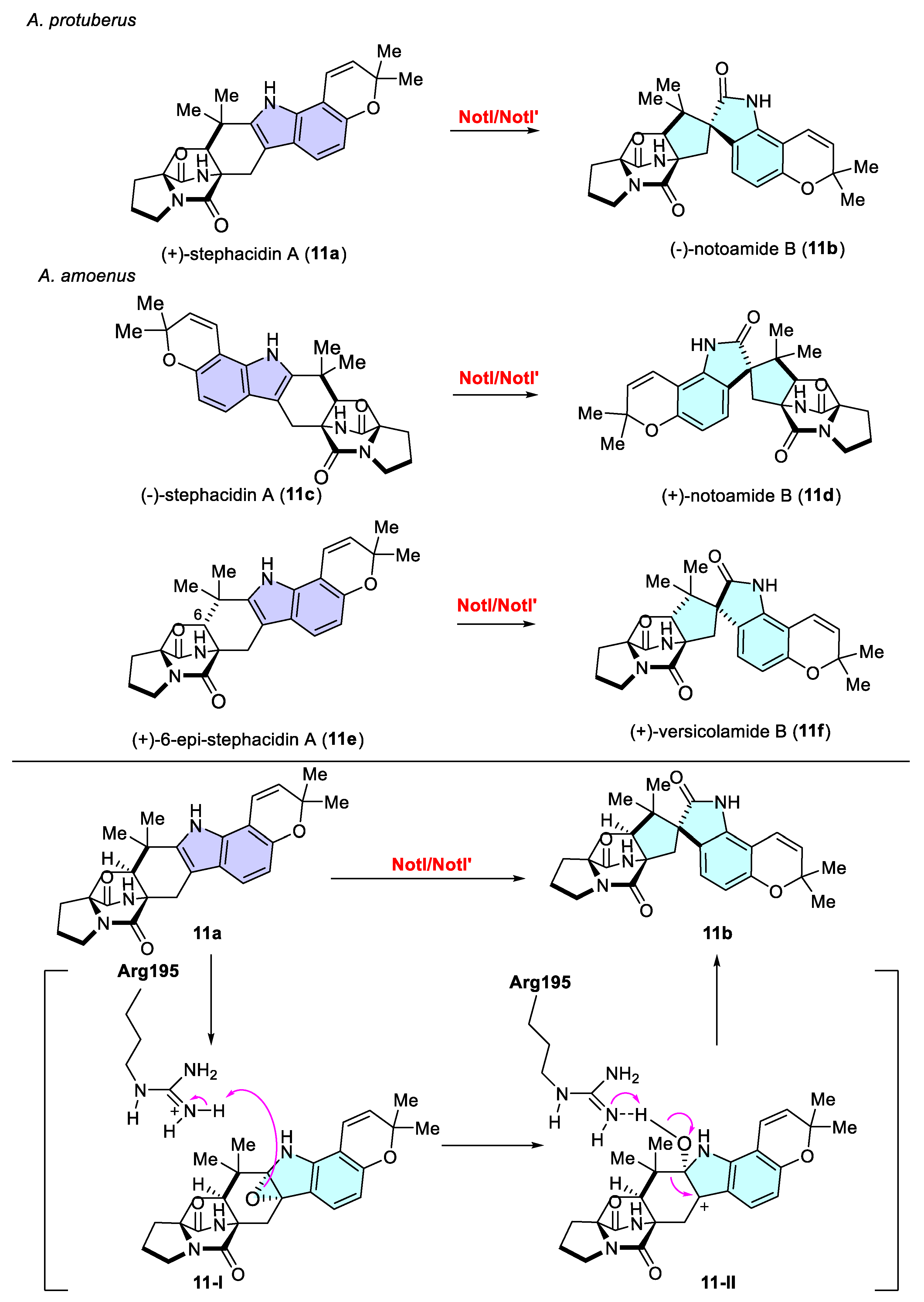
- Fraley, A.E.; Tran, H.T.; Kelly, S.P.; Newmister, S.A.; Tripathi, A.; Kato, H.; Tsukamoto, S.; Du, L.; Li, S.; Williams, R.M.; et al. Flavin-Dependent Monooxygenases NotI and NotI’ Mediate Spiro-Oxindole Formation in Biosynthesis of the Notoamides. ChemBioChem 2020, 21, 2449–2454. [Google Scholar] [CrossRef]
- Kato, H.; Yoshida, T.; Tokue, T.; Nojiri, Y.; Hirota, H.; Ohta, T.; Williams, R.M.; Tsukamoto, S. Notoamides A–D: Prenylated Indole Alkaloids Isolated from a Marine-Derived Fungus, Aspergillus sp. Angew. Chem. Int. Ed. 2007, 46, 2254–2256. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kato, H.; Greshock, T.J.; Hirota, H.; Ohta, T.; Williams, R.M. Isolation of Notoamide E, a Key Precursor in the Biosynthesis of Prenylated Indole Alkaloids in a Marine-Derived Fungus, Aspergillus sp. J. Am. Chem. Soc. 2009, 131, 3834–3835. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kato, H.; Samizo, M.; Nojiri, Y.; Onuki, H.; Hirota, H.; Ohta, T. Notoamides F−K, Prenylated Indole Alkaloids Isolated from a Marine-Derived Aspergillus sp. J. Nat. Prod. 2008, 71, 2064–2067. [Google Scholar] [CrossRef] [PubMed]
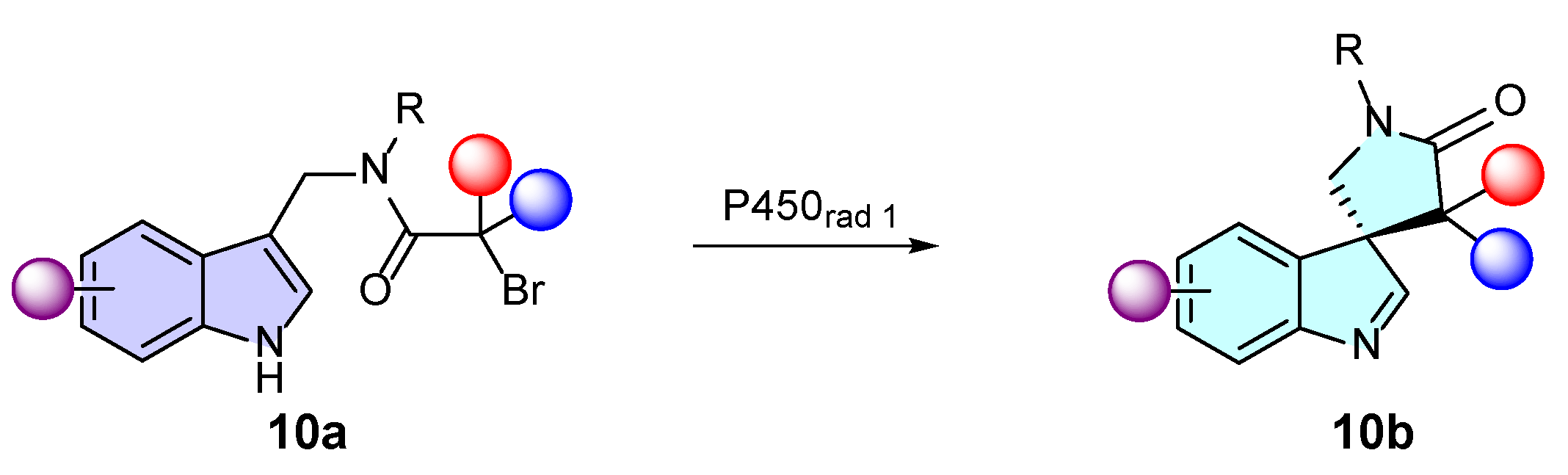
- Fraley, A.E.; Caddell Haatveit, K.; Ye, Y.; Kelly, S.P.; Newmister, S.A.; Yu, F.; Williams, R.M.; Smith, J.L.; Houk, K.N.; Sherman, D.H. Molecular Basis for Spirocycle Formation in the Paraherquamide Biosynthetic Pathway. J. Am. Chem. Soc. 2020, 142, 2244–2252. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, F.; Zhao, B.; Yang, J.; Ferrara, J.; Sankaran, B.; Venkataram Prasad, B.V.; Kundu, B.B.; Phillips, G.N.; Gao, Y.; et al. Structural basis of the stereoselective formation of the spirooxindole ring in the biosynthesis of citrinadins. Nat. Commun. 2021, 12, 4158. [Google Scholar] [CrossRef]
- Tsuda, M.; Kasai, Y.; Komatsu, K.; Sone, T.; Tanaka, M.; Mikami, Y.; Kobayashi, J.i. Citrinadin A, a Novel Pentacyclic Alkaloid from Marine-Derived Fungus Penicillium citrinum. Org. Lett. 2004, 6, 3087–3089. [Google Scholar] [CrossRef]
- Okamoto, M.; Yoshida, K.; Nishikawa, M.; Ando, T.; Iwami, M.; Kohsaka, M.; Aoki, H. FR-900452, a specific antagonist of platelet activating factor (PAF) produced by Streptomyces phaeofaciens I. Taxonomy, fermentation, isolation, and physico-chemical and biological characteristics. J. Antibiot. 1986, 39, 198–204. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, Y.; Huang, T.; Zou, Y.; Huang, T.; Hu, K.; Deng, Z.; Lin, S. Divergent biosynthesis of indole alkaloids FR900452 and spiro-maremycins. Org. Biomol. Chem. 2018, 16, 5446–5451. [Google Scholar] [CrossRef]
- Lopes, A.A.; Chioca, B.; Musquiari, B.; Crevelin, E.J.; França, S.d.C.; Fernandes da Silva, M.F.d.G.; Pereira, A.M.S. Unnatural spirocyclic oxindole alkaloids biosynthesis in Uncaria guianensis. Sci. Rep. 2019, 9, 11349. [Google Scholar] [CrossRef]
- Liang, X.; Huang, Z.-H.; Shen, W.-B.; Lu, X.-H.; Zhang, X.-X.; Ma, X.; Qi, S.-H. Prenylated indole diketopiperazine alkaloids as phosphatase inhibitors from the marine-derived fungus Talaromyces purpureogenus. Phytochemistry 2024, 223, 114119. [Google Scholar] [CrossRef]
- Cui, C.-B.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus II. Physico-chemical properties and structures. J. Antibiot. 1996, 49, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.; Gumber, D.; Abbot, V.; Dhiman, S.; Sharma, P. Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 2015, 5, 15233–15266. [Google Scholar] [CrossRef]
- O’Hagan, D. Pyrrole, pyrrolidine, pyridine, piperidine and tropane alkaloids. Nat. Prod. Rep. 2000, 17, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.K.; Rihak, K.J.; Bissember, A.C.; Smith, J.A. The Oxidation of Pyrrole. Chem. Asian J. 2016, 11, 155–167. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, X.; Zheng, C.; You, S.-L. Energy-Transfer-Enabled Dearomative Cycloaddition Reactions of Indoles/Pyrroles via Excited-State Aromatics. Acc. Chem. Res. 2022, 55, 2510–2525. [Google Scholar] [CrossRef]
- Choi, S.-M.; Kim, J.H. Recent Advances in Synthetic Methods for 2H-Pyrroles. Adv. Synth. Catal. 2024, 366, 2–17. [Google Scholar] [CrossRef]
- Tulinsky, A. The Structure of Isoquinocycline A. An X-Ray Crystallographic Determination. J. Am. Chem. Soc. 1964, 86, 5368–5369. [Google Scholar] [CrossRef]
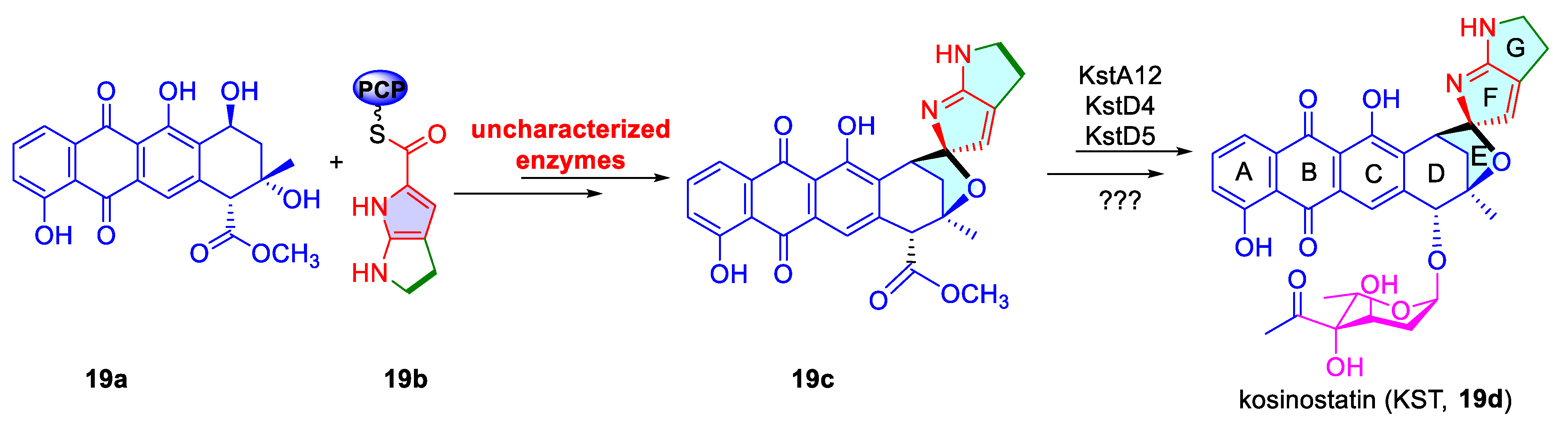
- Hu, Y.; Zhou, Q.; Zhang, Z.; Pan, H.-X.; Ilina, Y.; Metsä-Ketelä, M.; Igarashi, Y.; Tang, G.-L. Deciphering the Origin and Formation of Aminopyrrole Moiety in Kosinostatin Biosynthesis. Chin. J. Chem. 2021, 39, 3329–3333. [Google Scholar] [CrossRef]
- Zhang, Z.; Gong, Y.-K.; Zhou, Q.; Hu, Y.; Ma, H.-M.; Chen, Y.-S.; Igarashi, Y.; Pan, L.; Tang, G.-L. Hydroxyl regioisomerization of anthracycline catalyzed by a four-enzyme cascade. Proc. Natl. Acad. Sci. USA 2017, 114, 1554–1559. [Google Scholar] [CrossRef]
- Alizadeh, M.; Jalal, M.; Hamed, K.; Saber, A.; Kheirouri, S.; Pourteymour Fard Tabrizi, F.; Kamari, N. Recent updates on anti-inflammatory and antimicrobial effects of furan natural derivatives. J. Inflamm. Res. 2020, 13, 451–463. [Google Scholar] [CrossRef]
- Batool, Z.; Dan, X.; Xia, Z.; Xiaoxi, L.; Yuting, L.; Zhiyi, C.; Bing, L.; Li, L. A review on furan: Formation, analysis, occurrence, carcinogenicity, genotoxicity and reduction methods. Crit. Rev. Food Sci. Nutr. 2021, 61, 395–406. [Google Scholar] [CrossRef]
- Chatterjee, S.; Sahoo, R.; Nanda, S. Recent reports on the synthesis of γ-butenolide, γ-alkylidenebutenolide frameworks, and related natural products. Org. Biomol. Chem. 2021, 19, 7298–7332. [Google Scholar] [CrossRef] [PubMed]
- Ray Choudhury, A.; Mukherjee, S. Deconjugated butenolide: A versatile building block for asymmetric catalysis. Chem. Soc. Rev. 2020, 49, 6755–6788. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Rolfes, J.D.; Björklund, J.; Deska, J. Fully Biocatalytic Rearrangement of Furans to Spirolactones. ACS Catal. 2023, 13, 7256–7262. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhu, C.; Ji, L.; Liu, C.; Zhang, Y.; Rao, Y.; Yuan, Z. Recent Advances in Biocatalytic Dearomative Spirocyclization Reactions. Catalysts 2025, 15, 673. https://doi.org/10.3390/catal15070673
Chen X, Zhu C, Ji L, Liu C, Zhang Y, Rao Y, Yuan Z. Recent Advances in Biocatalytic Dearomative Spirocyclization Reactions. Catalysts. 2025; 15(7):673. https://doi.org/10.3390/catal15070673
Chicago/Turabian StyleChen, Xiaorui, Changtong Zhu, Luyun Ji, Changmei Liu, Yan Zhang, Yijian Rao, and Zhenbo Yuan. 2025. "Recent Advances in Biocatalytic Dearomative Spirocyclization Reactions" Catalysts 15, no. 7: 673. https://doi.org/10.3390/catal15070673
APA StyleChen, X., Zhu, C., Ji, L., Liu, C., Zhang, Y., Rao, Y., & Yuan, Z. (2025). Recent Advances in Biocatalytic Dearomative Spirocyclization Reactions. Catalysts, 15(7), 673. https://doi.org/10.3390/catal15070673







