Development of Ni-P-N-C/Nickel Foam for Efficient Hydrogen Production via Urea Electro-Oxidation
Abstract
1. Introduction
2. Results
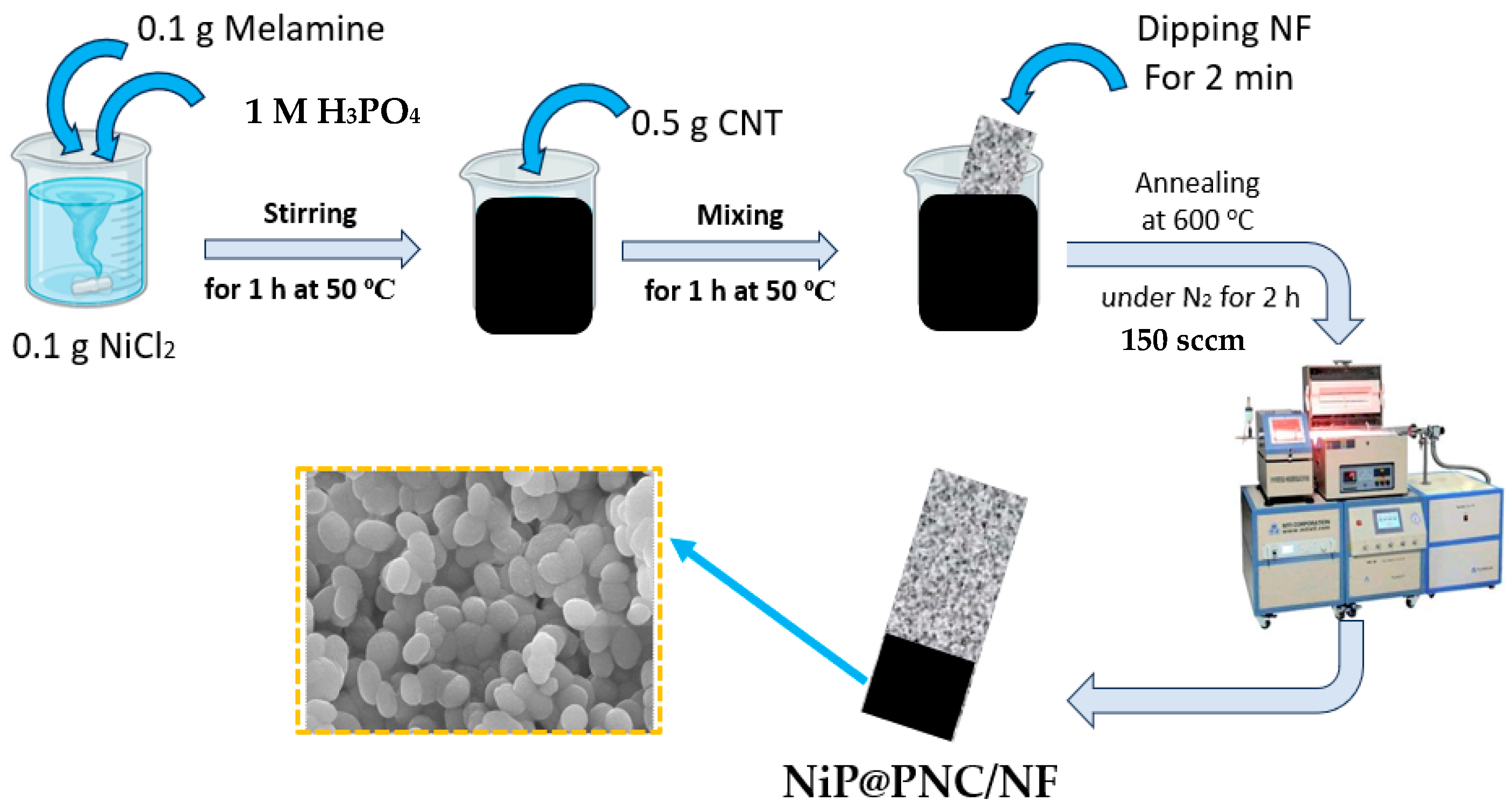
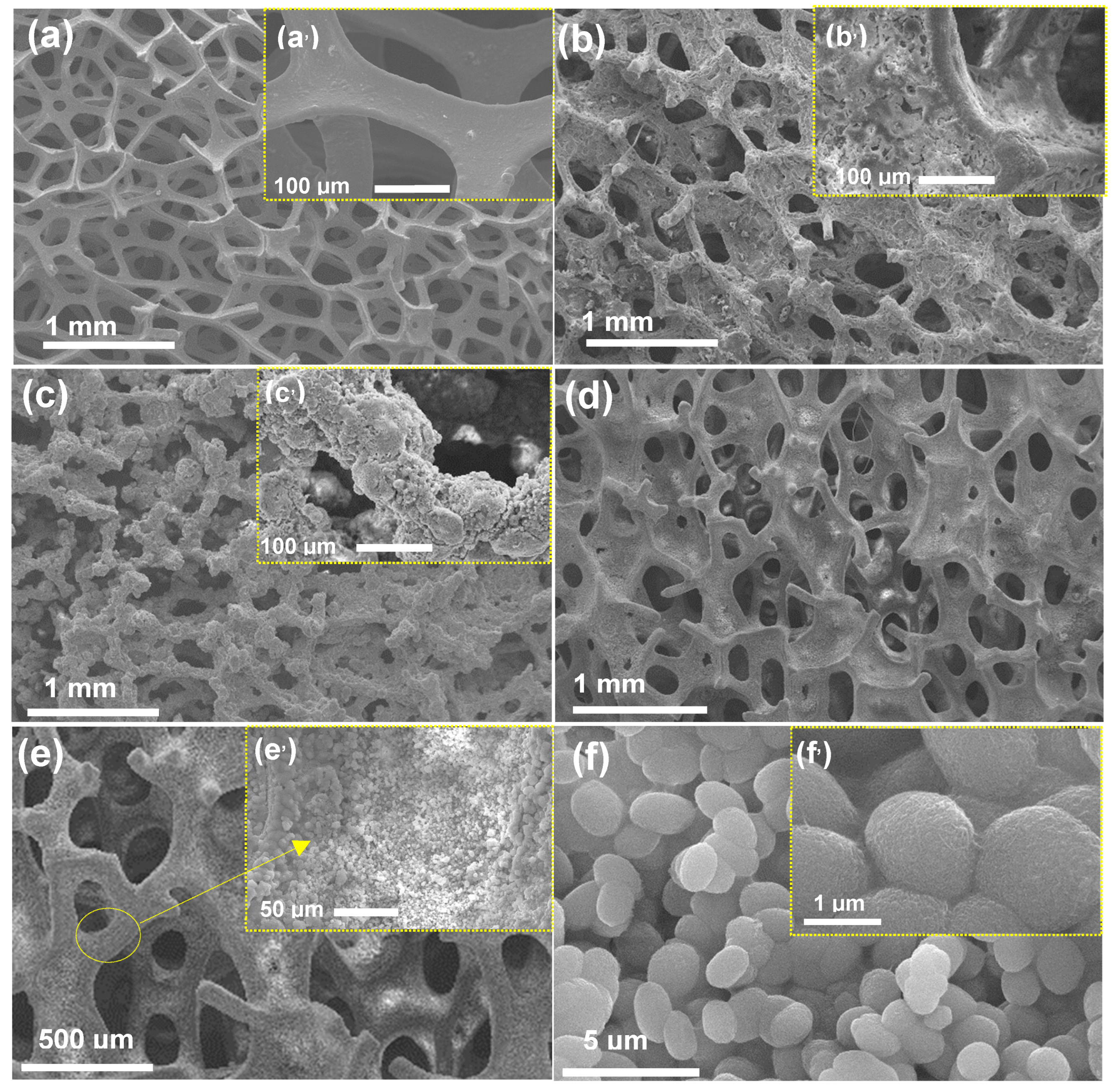
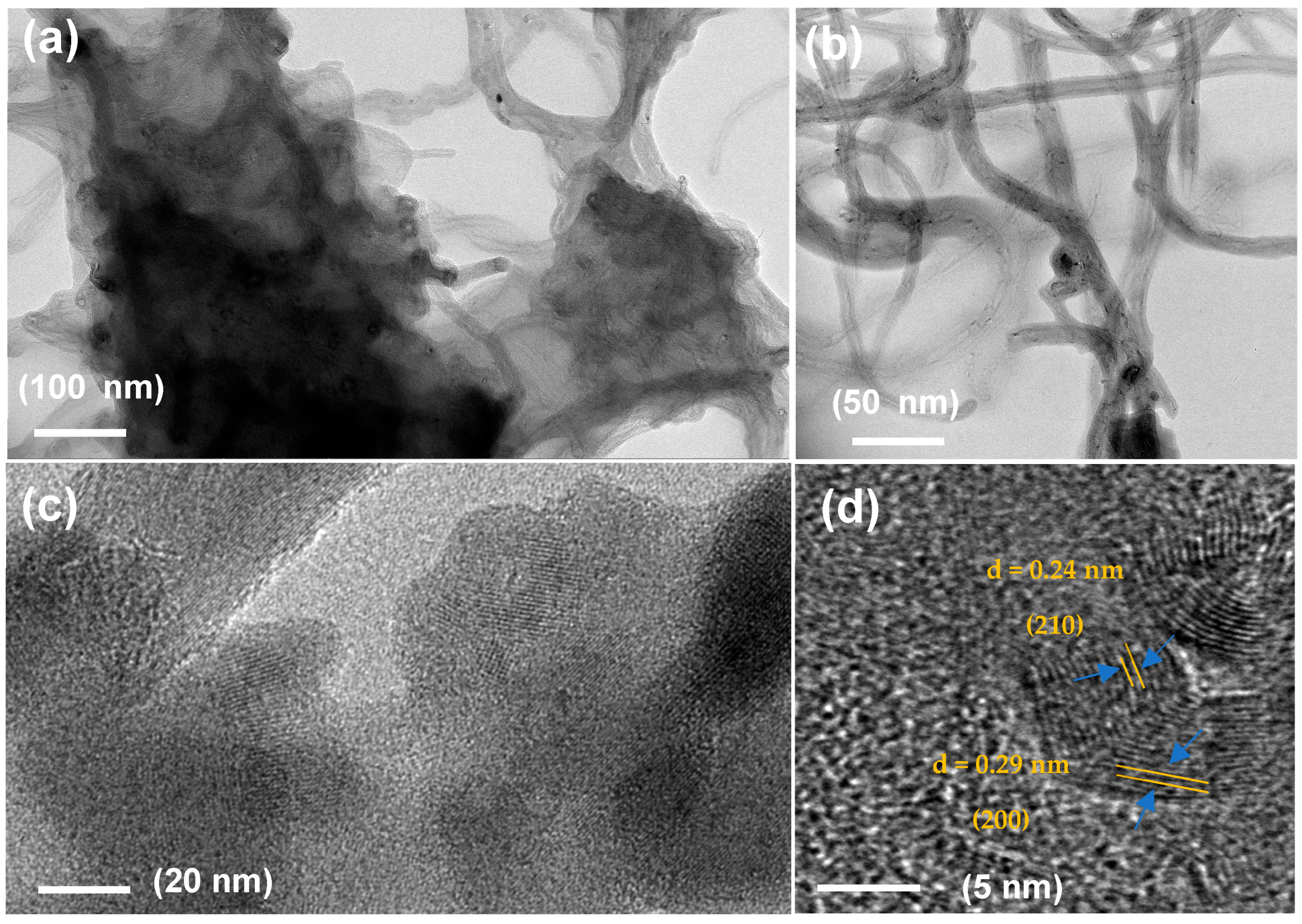
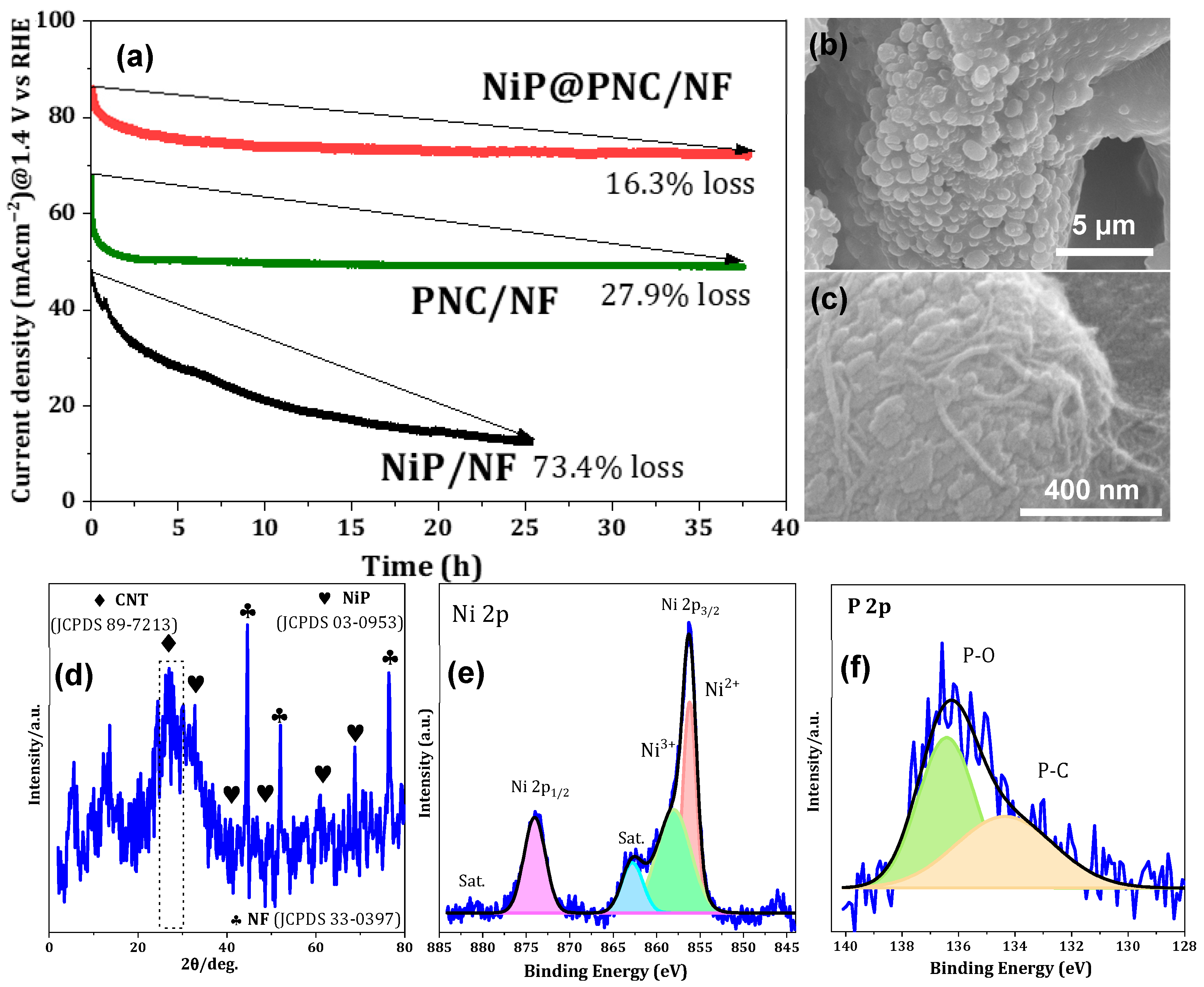
2.1. Morphological Features of NiP@PNC/NF
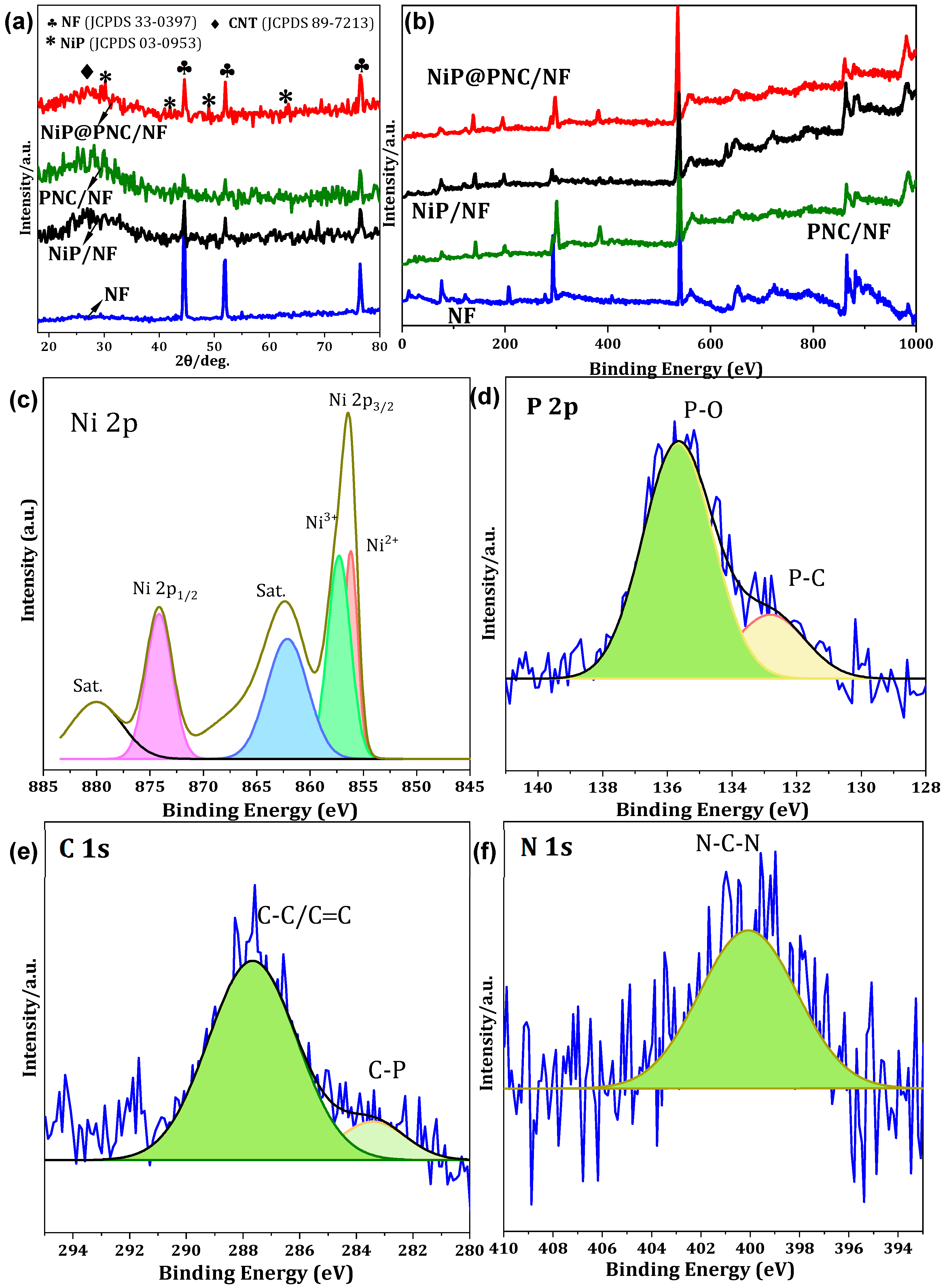
2.2. Crystalline and Chemical Composition Features of Electrodes
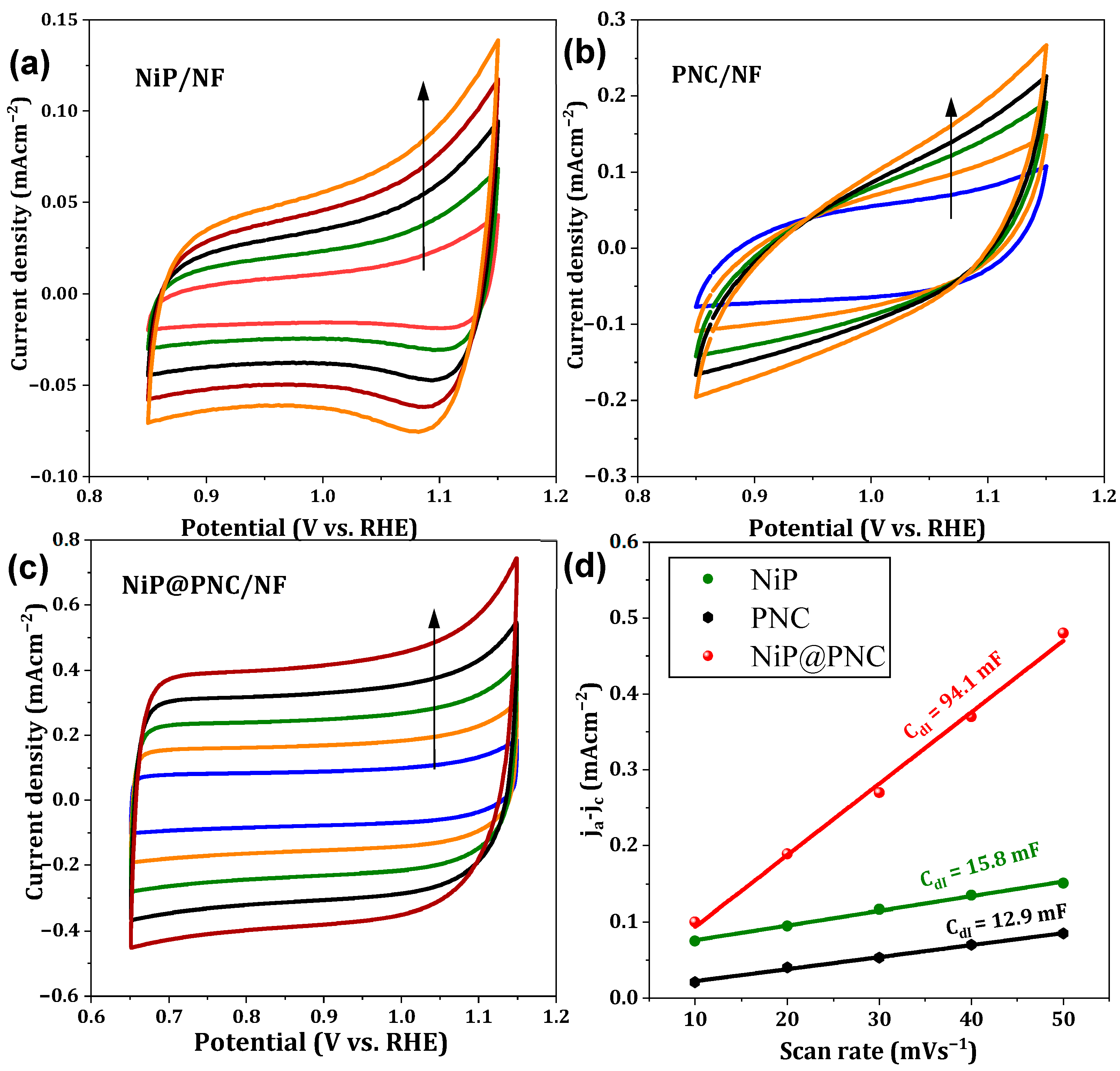
2.3. Electrochemical Properties of Catalysts
2.3.1. OER and UOR Characteristics
2.3.2. Long-Term Stability
3. Experimental Section
3.1. Materials
3.2. Fabrication of Nip/NF Electrode
3.3. Fabrication of PNC/NF Electrode
3.4. Fabrication of NiP@PNC/NF Electrode
3.5. Characterization
3.6. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Christopher, K.; Dimitrios, R. A Review on Exergy Comparison of Hydrogen Production Methods from Renewable Energy Sources. Energy Environ. Sci. 2012, 5, 6640–6651. [Google Scholar] [CrossRef]
- Dunn, S. Hydrogen Futures: Toward a Sustainable Energy System. Int. J. Hydrogen Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Alharthi, A.I.; Aladeemy, S.A.; Arunachalam, P. Engineering Oxygen Vacancy on Nickel-Doped Iron Oxide Nanorods as Efficient Bifunctional Electrocatalysts for Oxygen Evolution and Urea Oxidation Reaction. J. Taiwan Inst. Chem. Eng. 2024, 105928. [Google Scholar] [CrossRef]
- Al-Sulmi, W.A.; Ghanem, M.A.; Aladeemy, S.A.; Al-Mayouf, A.M.; Alotaibi, N.H. Chemical Deposition from a Liquid Crystal Template: A Highly Active Mesoporous Nickel Phosphate Electrocatalyst for Hydrogen Green Production via Urea. Arab. J. Chem. 2023, 16, 105266. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; Arunachalam, P.; Amer, M.S.; Al-Mayouf, A.M. Electrochemically Embedded Heterostructured Ni/NiS Anchored onto Carbon Paper as Bifunctional Electrocatalysts for Urea Oxidation and Hydrogen Evolution Reaction. RSC Adv. 2025, 15, 14–25. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; AlRijraji, T.R.; Amer, M.S.; Arunachalam, P.; Al-Mayouf, A.M. Electrooxidation of Ethylene Glycol Coupled with Hydrogen Production on Porous NiO/Ni@NF Nanosheet Electrocatalysts. Catal. Sci. Technol. 2025, 15, 2571–2583. [Google Scholar] [CrossRef]
- Arunachalam, P.; Shaddad, M.N.; Amer, M.S.; AL-Qadi, A. Enhanced Photoelectrochemical Water Splitting Coupled with Pharmaceutical Pollutants Degradation on Zr:BiVO4 Photoanodes by Synergetic Catalytic Activity of NiFeOOH Nanostructures. Alex. Eng. J. 2024, 99, 64–75. [Google Scholar] [CrossRef]
- Omar ben Gubaer, S.; Shaddad, M.N.; Arunachalam, P.; Amer, M.S.; Aladeemy, S.A.; Al-Mayouf, A.M. Enhanced Electrocatalytic Oxygen Redox Reactions of Iron Oxide Nanorod Films by Combining Oxygen Vacancy Formation and Cobalt Doping. RSC Adv. 2023, 13, 33242–33254. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Alanazi, A.A.; Aldawsari, A.M.; Alotaibi, M.A.; Aladeemy, S.A.; Abdulaziz, F.; Aljohani, Y.S. Multifunctional Nitrogen Doped Carbon Nanotube-Encapsulated Defect-Rich Cobalt-Iron Oxide Catalyst for Efficient Water Electrolysis and and Dye Removal from Water. J. Ind. Eng. Chem. 2025, in press. [CrossRef]
- Zhang, W.; Cui, L.; Liu, J. Recent Advances in Cobalt-Based Electrocatalysts for Hydrogen and Oxygen Evolution Reactions. J. Alloys Compd. 2020, 19, 821. [Google Scholar] [CrossRef]
- Babar, N.U.A.; Joya, Y.F.; Khalil, H.; Hussain, F.; Joya, K.S. Thin-Film Iron-Oxide Nanobeads as Bifunctional Electrocatalyst for High Activity Overall Water Splitting. Int. J. Hydrogen Energy 2021, 46, 7885–7902. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J. A Review on NiFe-Based Electrocatalysts for Efficient Alkaline Oxygen Evolution Reaction. J. Power Sources 2020, 448, 227375. [Google Scholar] [CrossRef]
- Wang, D.; Botte, G.G. In Situ X-Ray Diffraction Study of Urea Electrolysis on Nickel Catalysts. ECS Electrochem. Lett. 2014, 3, 29–32. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Direct Evidence of the Mechanism for the Electro-Oxidation of Urea on Ni(OH)2 Catalyst in Alkaline Medium. Electrochim. Acta 2013, 108, 660–665. [Google Scholar] [CrossRef]
- Daramola, D.A.; Singh, D.; Botte, G.G. Dissociation Rates of Urea in the Presence of NiOOH Catalyst: A DFT Analysis. J. Phys. Chem. A 2010, 114, 11513–11521. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, J.; Li, J.; Wu, Q. Urea Electrooxidation: Current Development and Understanding of Ni-Based Catalysts. ChemElectroChem 2020, 7, 3211–3228. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; Arunachalam, P.; Al-Mayouf, A.M.; Sudha, P.N.; Rekha, A.; Vidhya, A.; Hemapriya, J.; Latha, S.; Prasad, P.S.; Pavithra, S.; et al. Engineered CoS/Ni3S2 Heterointerface Catalysts Grown Directly on Carbon Paper as an Efficient Electrocatalyst for Urea Oxidation. Catalysts 2024, 14, 570. [Google Scholar] [CrossRef]
- Wu, T.H.; Hou, B.W. Superior Catalytic Activity of α-Ni(OH)2 for Urea Electrolysis. Catal. Sci. Technol. 2021, 11, 4294–4300. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Botte, G.G. Nickel and Cobalt Bimetallic Hydroxide Catalysts for Urea Electro-Oxidation. Electrochim. Acta 2012, 61, 25–30. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Alotaibi, M.A.; Alharthi, A.I.; Alanazi, A.A. Nickel Hexacyanoferrate on Nitrogen-Rich CNTs: An Efficient Bi-Functional Catalyst for Crystal Violet Removal and Electrochemical Urea Oxidation. Mater. Res. Bull. 2025, 185, 113300. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; Al-Mayouf, A.; Amer, M.; Alotaibi, N.; Weller, M.T.; Ghanem, M.A. Structure and Electrochemical Activity of Nickel Aluminium Fl Uoride Nanosheets during Urea Electro-Oxidation in an Alkaline Solution. RSC Adv. 2021, 11, 3190–3201. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; Al-Mayouf, A.M.; Shaddad, M.N.; Amer, M.S.; Almutairi, N.K.; Ghanem, M.A.; Alotaibi, N.H.; Arunachalam, P. Electrooxidation of Urea in Alkaline Solution Using Nickel Hydroxide Activated Carbon Paper Electrodeposited from DMSO Solution. Catalysts 2021, 11, 102. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, C.; Wang, Y.; Wang, K. Advanced Nickel-Based Catalysts for Urea Oxidation Reaction: Challenges and Developments. Catalysts 2022, 12, 337. [Google Scholar] [CrossRef]
- Singh, R.K.; Schechter, A. Electrochemical Investigation of Urea Oxidation Reaction on β Ni(OH)2 and Ni/Ni(OH)2. Electrochim. Acta 2018, 278, 405–411. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Jin, H.; Kim, B.; Lee, K. Nanostructured Materials on 3D Nickel Foam as Electrocatalysts for Water Splitting. Nanoscale 2017, 9, 12231–12247. [Google Scholar] [CrossRef]
- Hu, X.; Tian, X.; Lin, Y.-W.; Wang, Z. Nickel Foam and Stainless Steel Mesh as Electrocatalysts for Hydrogen Evolution Reaction, Oxygen Evolution Reaction and Overall Water Splitting in Alkaline Media. RSC Adv. 2019, 9, 31563–31571. [Google Scholar] [CrossRef]
- Kumar, V.; Ahlawat, D.S.; AarifUl Islam, S.; Singh, A. Ce Doping Induced Modifications in Structural, Electrical and Magnetic Behaviour of Hematite Nanoparticles. Mater. Sci. Eng. B 2021, 272, 115327. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Khalaf, M.M.; Mohamed, I.M.A. Advances in Phosphorus-Based Catalysts for Urea Electrooxidation: A Pathway to Sustainable Waste to Energy Conversion Through Electrocatalysis. Catalysts 2024, 14, 937. [Google Scholar] [CrossRef]
- Eom, S.; Jung, J.; Kim, D.H. One-Pot Synthesis of Nanostructured Ni@Ni(OH)2 and Co-Doped Ni@Ni(OH)2 via Chemical Reduction Method for Supercapacitor Applications. Materials. 2023, 16, 380. [Google Scholar] [CrossRef]
- Su, J.; Musgrave, C.B.; Song, Y.; Huang, L.; Liu, Y.; Li, G.; Xin, Y.; Xiong, P.; Li, M.M.-J.; Wu, H.; et al. Strain Enhances the Activity of Molecular Electrocatalysts via Carbon Nanotube Supports. Nat. Catal. 2023, 6, 818–828. [Google Scholar] [CrossRef]
- Aslam, M.M.A.; Kuo, H.W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Yao, C.; Xu, J.; Zhu, Y.; Zhang, R.; Shen, Y.; Xie, A. Porous CoP@N/P Co-Doped Carbon/CNTs Nanocubes: In-Situ Autocatalytic Synthesis and Excellent Performance as the Anode for Lithium-Ion Batteries. Appl. Surf. Sci. 2020, 513, 145777. [Google Scholar] [CrossRef]
- Scardamaglia, M.; Amati, M.; Llorente, B.; Mudimela, P.; Colomer, J.-F.F.; Ghijsen, J.; Ewels, C.; Snyders, R.; Gregoratti, L.; Bittencourt, C. Nitrogen Ion Casting on Vertically Aligned Carbon Nanotubes: Tip and Sidewall Chemical Modification. Carbon N. Y. 2014, 77, 319–328. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, S.-H.; Li, P.; Qian, L.; Li, P.; Yan, S. Highly Dispersed Fe-Doped CoP Nanoparticles Encapsulated with P, N Co-Doped Hollow Carbon Shell toward Robust Bifunctional Oxygen Electrocatalysis and Rechargeable Li–O2 Batteries. Int. J. Hydrogen Energy 2024, 53, 49–59. [Google Scholar] [CrossRef]
- Wu, T.-H.; Qiu, Z.-T.; Hsieh, C.-N. Obtaining Ni P Electrocatalyst in Minutes via Electroless Plating on Carbon Nanotubes Decorated Substrate for Alkaline Urea Electrolysis. Appl. Surf. Sci. 2024, 645, 158831. [Google Scholar] [CrossRef]
- Qiao, L.; Zhu, A.; Liu, D.; Feng, J.; Chen, Y.; Chen, M.; Zhou, P.; Yin, L.; Wu, R.; Ng, K.W.; et al. Crystalline Phosphides/Amorphous Oxides Composite for Energy-Saving Hydrogen Production Assisted by Efficient Urea Oxidation Reaction. Chem. Eng. J. 2023, 454, 140380. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, X.; Zhang, K.; Duan, D.; Zhou, X.; Wang, J.; Liu, S. Electrodeposition Synthesis of Co-MnP/MnO@NiP/NF Catalytic Materials and Investigation of Their Performance in Hydrogen Evolution Reaction and Urea Oxidation Reaction. Appl. Surf. Sci. 2025, 688, 162464. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Ullah, Z.; Nazar, M.F.; Younas, M.; Suliman, M. One Step Fabrication of Nanostructured Nickel Thin Films on Porous Nickel Foam for Drastic Electrocatalytic Oxygen Evolution. Int. J. Hydrogen Energy 2023, 48, 15784–15795. [Google Scholar] [CrossRef]
- Luo, K.-H.; Cheng, C.-K.; Lin, J.-Y.; Huang, C.-H.; Yeh, T.-K.; Hsieh, C.-K. Highly-Porous Hierarchically Microstructure of Graphene-Decorated Nickel Foam Supported Two-Dimensional Quadrilateral Shapes of Cobalt Sulfide Nanosheets as Efficient Electrode for Methanol Oxidation. Surf. Coatings Technol. 2020, 393, 125850. [Google Scholar] [CrossRef]
- Wang, H.; Zou, H.; Liu, Y.; Liu, Z.; Sun, W.; Lin, K.A.; Li, T.; Luo, S. Ni2P Nanocrystals Embedded Ni-MOF Nanosheets Supported on Nickel Foam as Bifunctional Electrocatalyst for Urea Electrolysis. Sci. Rep. 2021, 11, 21414. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Wang, X.; Ma, Y.; Li, Y.; Yang, X.; Li, Y. Multi-Electron Transfer Mechanism and Band Structures of CNTs in MOF-Derived CNTs/Co Hybrids for Enhanced Electromagnetic Wave Absorption Property. Mater. Today Commun. 2024, 39, 108936. [Google Scholar] [CrossRef]
- Morant, C.; Andrey, J.; Prieto, P.; Mendiola, D.; Sanz, J.M.; Elizalde, E. XPS Characterization of Nitrogen-Doped Carbon Nanotubes. Phys. Status Solidi Appl. Mater. Sci. 2006, 203, 1069–1075. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, T.; Ma, X.; Shen, Y.; Deng, Q.; Zhang, W.; Zhao, Y. Hydrogen Production from Urea Sewage on NiFe-Based Porous Electrocatalysts. ACS Sustain. Chem. Eng. 2020, 8, 11007–11015. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, R.; Sun, Z.; Lin, L.; Yang, H.; Li, H.; Nan, C.; Sun, G.; Ma, S. Morphology-Controlled Synthesis of Ni-MOFs with Highly Enhanced Electrocatalytic Performance for Urea Oxidation. Inorg. Chem. 2019, 58, 11449–11457. [Google Scholar] [CrossRef]
- He, M.; Feng, C.; Liao, T.; Hu, S.; Wu, H.; Sun, Z. Low-Cost Ni2P/Ni0.96S Heterostructured Bifunctional Electrocatalyst toward Highly Efficient Overall Urea-Water Electrolysis. ACS Appl. Mater. Interfaces 2020, 12, 2225–2233. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, F.; Zhang, H.; Huang, Y. Ultrafine NiFe Clusters Anchored on N-Doped Carbon as Bifunctional Electrocatalysts for Efficient Water and Urea Oxidation. Dalt. Trans. 2020, 49, 13962–13969. [Google Scholar] [CrossRef]
- Wen, X. NiFe-LDH/MWCNTs/NF Nanohybrids as a High-Performance Bifunctional Electrocatalyst for Overall Urea Electrolysis. Int. J. Hydrogen Energy 2020, 45, 14660–14668. [Google Scholar] [CrossRef]
- Rezaee, S.; Shahrokhian, S. 3D Ternary Ni: XCo2- XP/C Nanoflower/Nanourchin Arrays Grown on HCNs: A Highly Efficient Bi-Functional Electrocatalyst for Boosting Hydrogen Production via the Urea Electro-Oxidation Reaction. Nanoscale 2020, 12, 16123–16135. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, X.; Zhang, J.; Huang, Y. Hierarchical NiFe Hydroxide/Ni3N Nanosheet-on-Nanosheet Heterostructures for Bifunctional Oxygen Evolution and Urea Oxidation Reactions. ACS Sustain. Chem. Eng. 2021, 9, 12584–12590. [Google Scholar] [CrossRef]
- Lv, Z.; Li, Z.; Tan, X.; Li, Z.; Wang, R.; Wen, M.; Liu, X.; Wang, G.; Xie, G.; Jiang, L. One-Step Electrodeposited NiFeMo Hybrid Film for Efficient Hydrogen Production via Urea Electrolysis and Water Splitting. Appl. Surf. Sci. 2021, 552, 149514. [Google Scholar] [CrossRef]
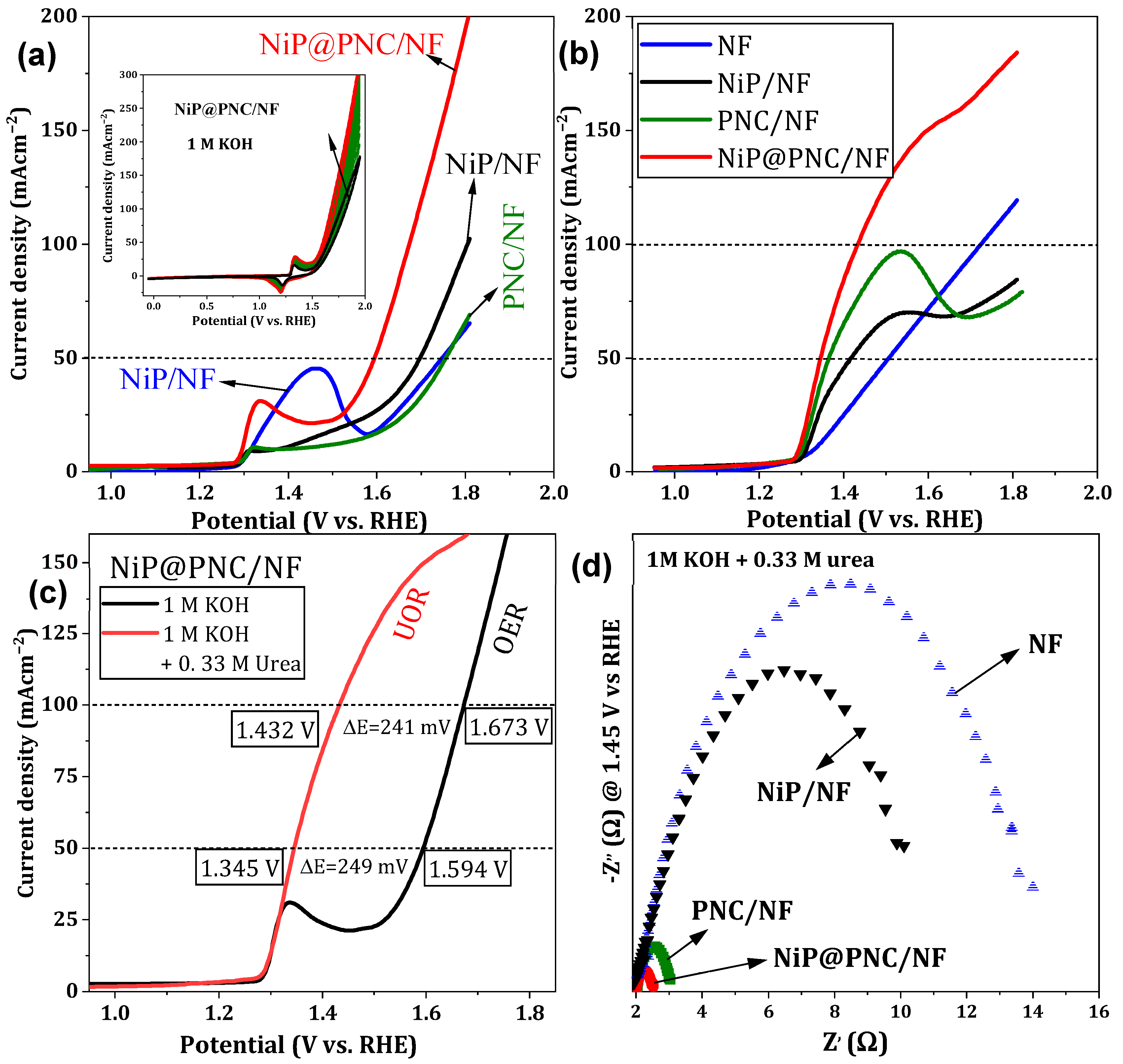
| Electrodes | OER | UOR | ||
|---|---|---|---|---|
| 50 mAcm−2 | 100 mAcm−2 | 50 mAcm−2 | 100 mAcm−2 | |
| NF | 1.695 V | 1.80 | 1.48 V | 1.72 V |
| NiP/NF | 1.63 V | - | 1.414 V | - |
| PNC/NF | 1.74 V | - | 1.365 V | - |
| NiP@PNC/NF | 1.594 V | 1.673 V | 1.345 V | 1.432 V |
| Anodic Materials | Urea (M) | Stability (h) | Potential (V vs. RHE) | J (mAcm−2) | Ref. |
|---|---|---|---|---|---|
| c-CoNiPx/a-P-MnOy | 0.33 | 300 | 1.33 | 50 | [36] |
| Co-MnP/MnO@NiP/NF | 0.33 | 60 | 1.41 | 50 | [37] |
| NP-Ni0.70Fe0.30, NP-Ni | 0.33 | 10 | 1.51 | 50 | [43] |
| Ni-MOF nanowires | 0.33 | 1 | 142 | 50 | [44] |
| Ni2P/Ni0.96S | 0.33 | 20 | 1.415 | 50 | [45] |
| NiFe/N-C | 0.33 | 12 | 137 | 50 | [46] |
| NiFe-LDH/MWCNTs/NF | 0.33 | 3 | 1.405 | 50 | [47] |
| Ni0.4Co1.6P/C@HCNs/GCE | 0.33 | 10 | 1.45 | 50 | [48] |
| NiFe(OH)x/Ni3N | 0.33 | 10 | 1.38 | 50 | [49] |
| NiFeMo | 0.33 | 25 | 1.44 | 50 | [50] |
| NiP@PNC/NF | 0.33 | ~35 | 1.34 | 50 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldawsari, A.M.; Shaddad, M.N.; Aladeemy, S.A. Development of Ni-P-N-C/Nickel Foam for Efficient Hydrogen Production via Urea Electro-Oxidation. Catalysts 2025, 15, 662. https://doi.org/10.3390/catal15070662
Aldawsari AM, Shaddad MN, Aladeemy SA. Development of Ni-P-N-C/Nickel Foam for Efficient Hydrogen Production via Urea Electro-Oxidation. Catalysts. 2025; 15(7):662. https://doi.org/10.3390/catal15070662
Chicago/Turabian StyleAldawsari, Abdullah M., Maged N. Shaddad, and Saba A. Aladeemy. 2025. "Development of Ni-P-N-C/Nickel Foam for Efficient Hydrogen Production via Urea Electro-Oxidation" Catalysts 15, no. 7: 662. https://doi.org/10.3390/catal15070662
APA StyleAldawsari, A. M., Shaddad, M. N., & Aladeemy, S. A. (2025). Development of Ni-P-N-C/Nickel Foam for Efficient Hydrogen Production via Urea Electro-Oxidation. Catalysts, 15(7), 662. https://doi.org/10.3390/catal15070662









