Challenges and Opportunities for g-C3N4-Based Heterostructures in the Photodegradation of Environmental Pollutants
Abstract
1. Introduction
2. Preparation Methods
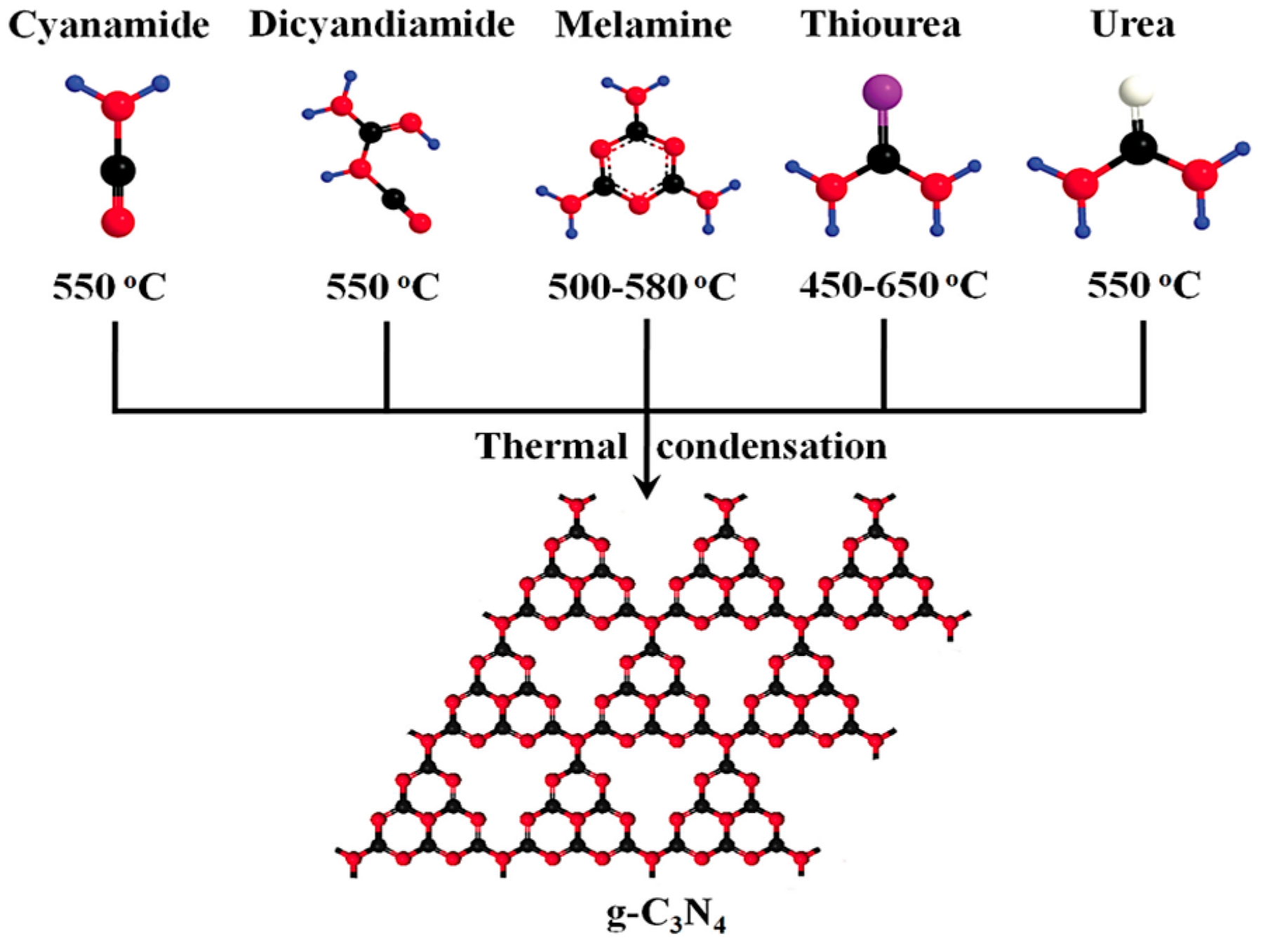
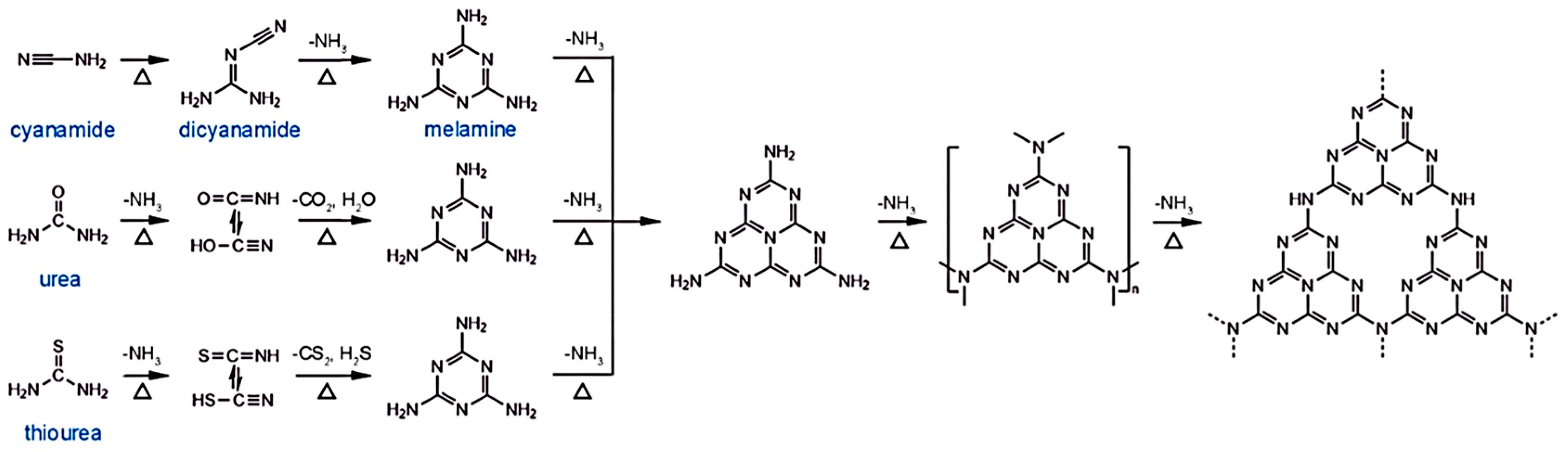
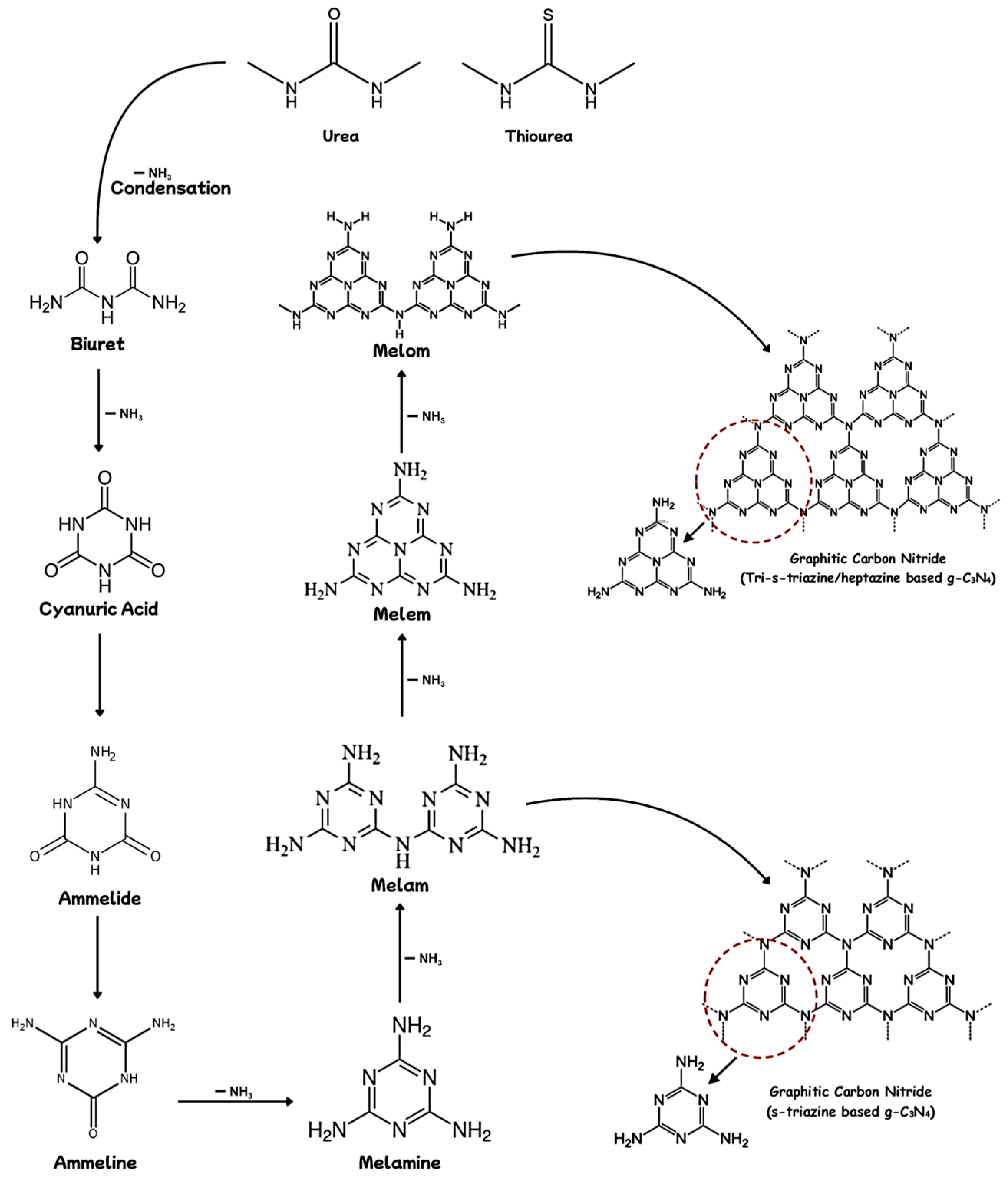
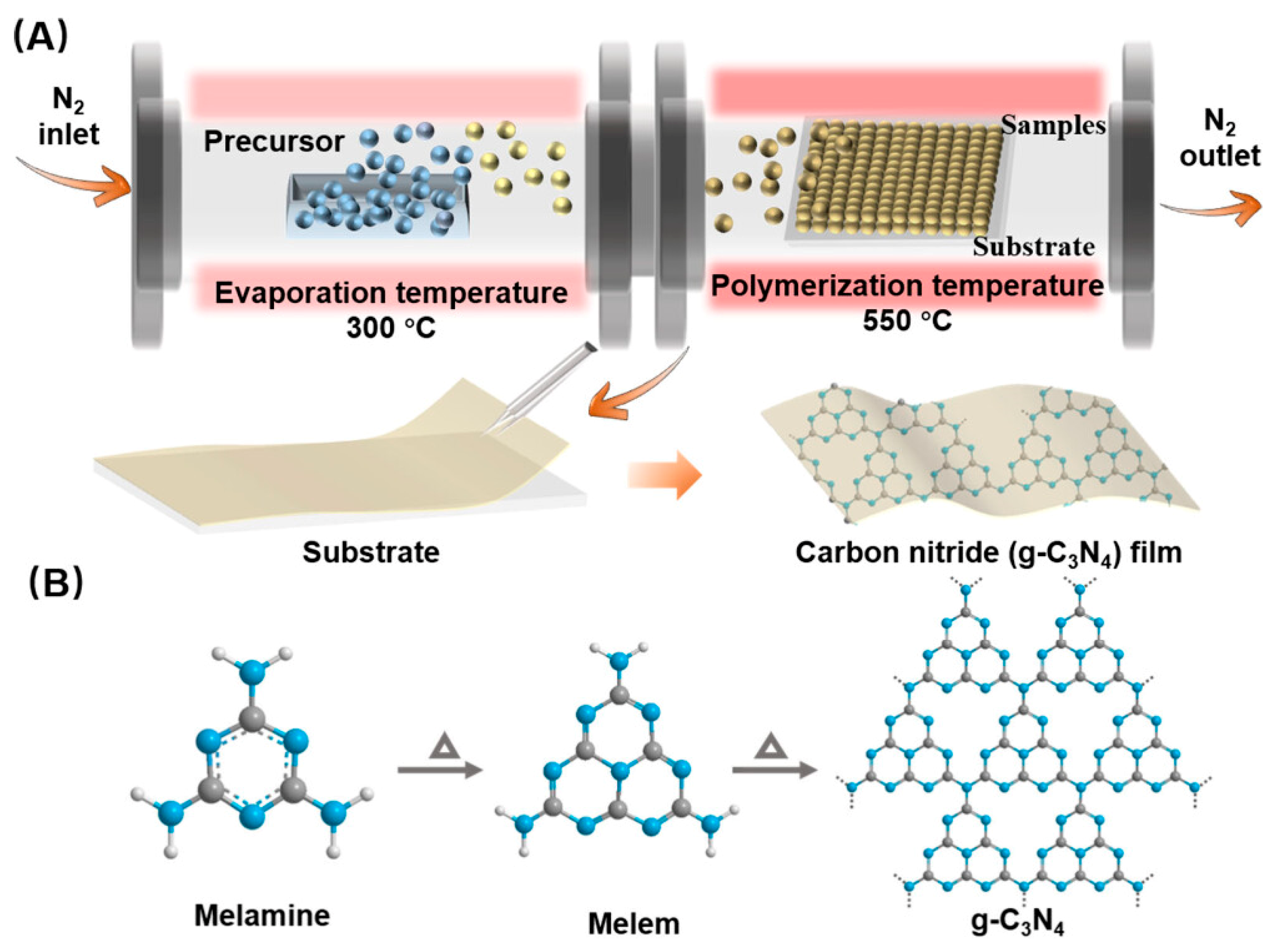
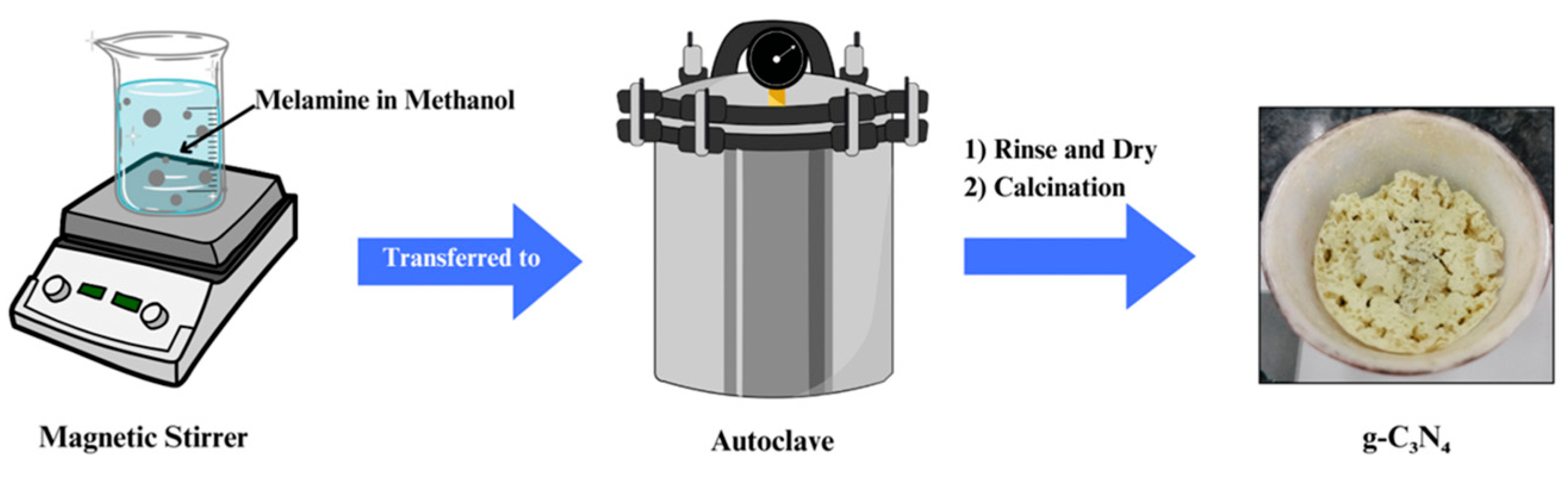
2.1. Synthesis Methods for g-C3N4
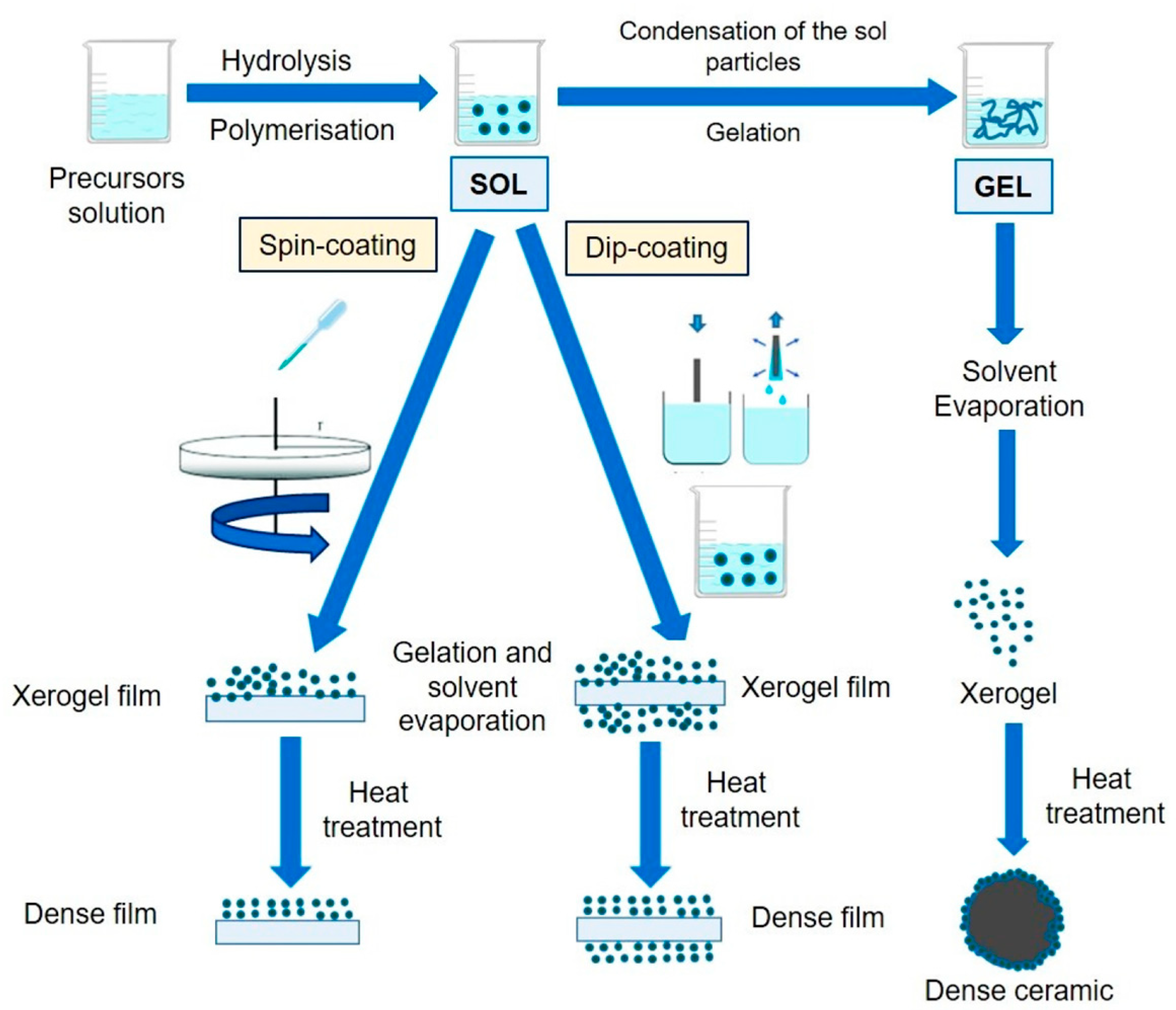
2.2. Preparation of g-C3N4-Based Heterostructures
- g-C3N4/TiO2: A widely studied type II heterojunction that leverages the robustness of TiO2 and extends its visible-light response thanks to g-C3N4 [28].
- g-C3N4/ZnO: A type II or S-scheme system (depending on doping) that combines ZnO, is high conductivity with g-C3N4 is visible-light absorption. It shows improved dye degradation (97% degradation of crystal violet by g-C3N4/ZnO in 150 min). ZnO must be stabilized against photocorrosion through coupling [32].
- g-C3N4/BiVO4: A prototype direct Z-scheme heterostructure, where g-C3N4 (strong reducer) couples with BiVO4 (strong oxidizer). It retains high redox-potential carriers and has demonstrated effective degradation of pesticides, dyes, and pharmaceuticals under visible light [70].
- g-C3N4/graphene (or rGO): A conductor-dielectric nanocomposite. Graphene does not generate carriers, but it enhances conductivity by acting as a bridge and adsorbent. A g-C3N4/graphene composite achieved 100% atrazine degradation in 5 h vs. 40% with g-C3N4 alone. It improves electron transfer speed and stability [31].
- g-C3N4/MXenes (Ti3C2): Similar to graphene, MXenes are 2D conductors that, when coupled with g-C3N4, enhance charge separation and provide adsorption sites for charged pollutants. Notable improvements have been observed in anionic dye degradation [71].
- g-C3N4/MOFs: Incorporating g-C3N4 into porous metal–organic frameworks combines photoactivity with high adsorption. For example, ZIF-67/MIL-100(Fe)@g-C3N4 showed accelerated PFOA removal by combining MOFs’ high PFAS adsorption affinity with g-C3N4 photocatalytic activation [72]. The challenge lies in achieving good photoelectric contact between organic and inorganic phases.
- g-C3N4/Noble metals: Although not a semiconductor-semiconductor heterojunction, loading g-C3N4 with Au, Ag, or Pt creates Schottky heterostructures. The metallic nanoparticles trap electrons, prolonging hole lifetimes in g-C3N4, which has been shown to improve dye degradation [28].
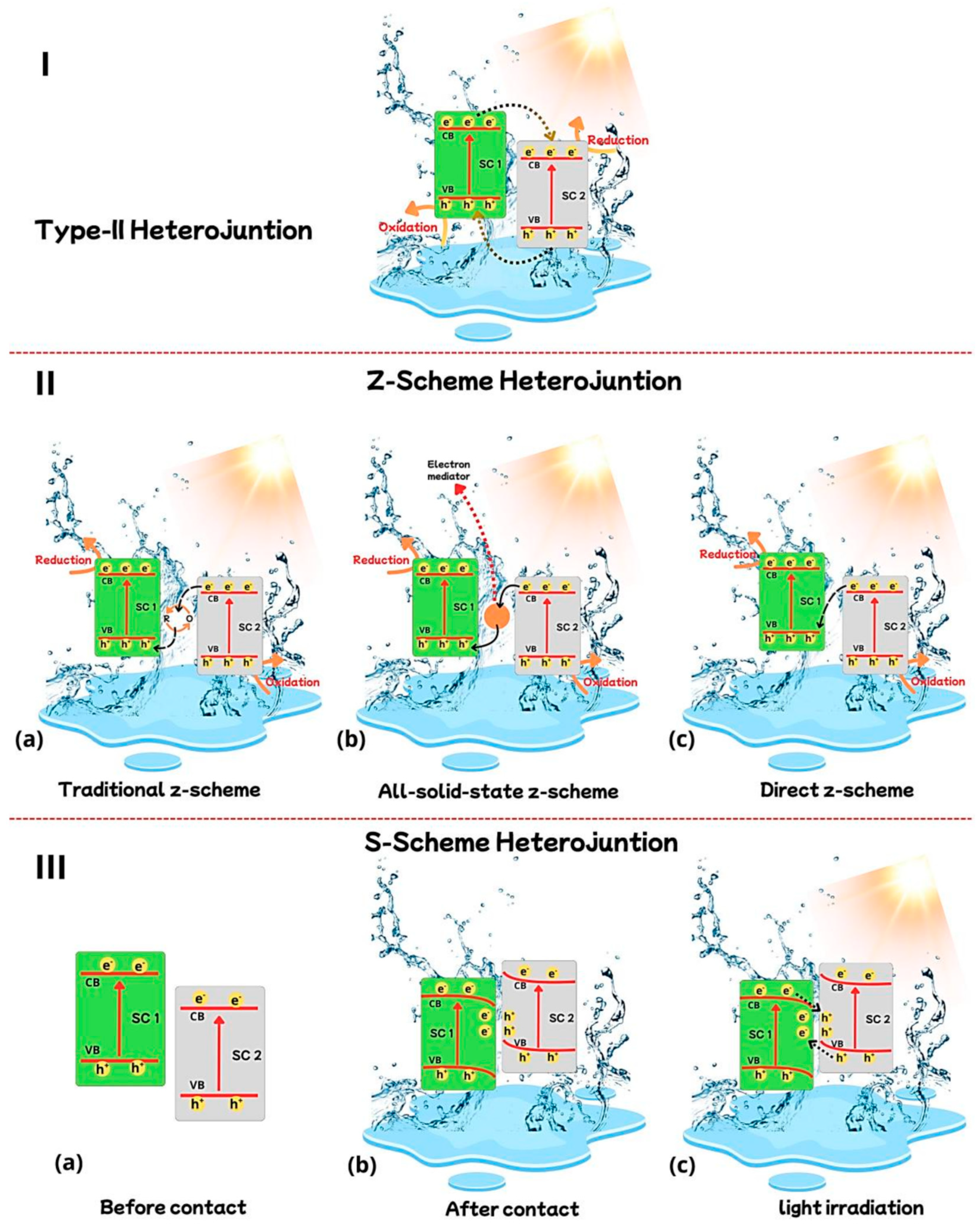
3. Charge Transfer Mechanism
3.1. g-C3N4 Type II Heterostructures
3.2. g-C3N4 Z-Scheme Heterostructures
3.3. g-C3N4 S-Scheme Heterostructures
4. Photodegradation of Environmental Pollutants
- ✓
- The effect of the synthesis method of g-C3N4-based heterostructures on the photocatalytic degradation performance of organic pollutants.
- ✓
- The effect of the morphology and design of g-C3N4-based heterostructures (nanosheets vs. bulk, mesoporosity, etc.) on the photocatalytic degradation performance of organic pollutants.
- ✓
- The effect of g-C3N4 doping in the coupled heterostructures and its influence on the photocatalytic degradation performance of organic pollutants.
- ✓
- The influence of the type of coupled semiconductor and the involved charge transfer mechanism (type II, Z, and S) on the photocatalytic degradation performance of organic pollutants.
4.1. g-C3N4 Organic Toxic Dyes
4.2. Pharmaceutical Pollutants
4.2.1. Antibiotics
4.2.2. Analgesics and Anti-Inflammatories
4.3. Pesticides
4.4. Per- and Polyfluoroalkyl Substances (PFAS)
5. Conclusions and Future Perspectives
- ✓
- Developing innovative and sustainable synthesis techniques that enable precise control over the morphology, composition, and interfacial structure of g-C3N4 heterostructures.
- ✓
- Deepening the fundamental understanding of interfacial charge transfer mechanisms through advanced techniques such as transient spectroscopy and computational modeling, facilitating the rational design of photocatalysts with optimized performance.
- ✓
- Exploring ternary and quaternary combinations with advanced materials (MXenes, MOFs, metallic nanoparticles) to further expand spectral absorption and enhance charge separation, promoting synergies that significantly increase photocatalytic efficiency.
- ✓
- Carrying out a comprehensive evaluation of economic and environmental feasibility for scaling these processes to an industrial level, including life cycle assessments (LCA) and pilot studies validating their performance under real operational conditions.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, N.A.; Khan, S.U.; Ahmed, S.; Farooqi, I.H.; Yousefi, M.; Mohammadi, A.A.; Changani, F. Recent Trends in Disposal and Treatment Technologies of Emerging-Pollutants—A Critical Review. TrAC Trends Anal. Chem. 2020, 122, 115744. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Pot, E.J.; Milakovic, M.; Chaumot, A.; Seidensticker, S.; Melling, M.; Supriatin, A.; Sherif, S. Pharmaceutical Pollution of the World’s Rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef]
- aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the Environment-Global Occurrences and Perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of Pesticide Pollution at the Global Scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Grunfeld, D.A.; Gilbert, D.; Hou, J.; Jones, A.M.; Lee, M.J.; Kibbey, T.C.G.; O’Carroll, D.M. Underestimated Burden of Per-and Polyfluoroalkyl Substances in Global Surface Waters and Groundwaters. Nat. Geosci. 2024, 17, 340–346. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; Dewitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An Overview of the Uses of Per-And Polyfluoroalkyl Substances (PFAS). Environ. Sci. Process Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions—A Review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Z.; Yao, B.; Zhou, Y. Occurrence, Bioaccumulation, Fate, and Risk Assessment of Emerging Pollutants in Aquatic Environments: A Review. Sci. Total Environ. 2024, 923, 171388. [Google Scholar] [CrossRef]
- Pereira, L.C.; de Souza, A.O.; Bernardes, M.F.F.; Pazin, M.; Tasso, M.J.; Pereira, P.H.; Dorta, D.J. A Perspective on the Potential Risks of Emerging Contaminants to Human and Environmental Health. Environ. Sci. Pollut. Res. 2015, 22, 13800–13823. [Google Scholar] [CrossRef]
- Gomes, A.R.; Justino, C.; Rocha-Santos, T.; Freitas, A.C.; Duarte, A.C.; Pereira, R. Review of the Ecotoxicological Effects of Emerging Contaminants to Soil Biota. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2017, 52, 992–1007. [Google Scholar] [CrossRef]
- Shehata, N.; Egirani, D.; Olabi, A.G.; Inayat, A.; Abdelkareem, M.A.; Chae, K.J.; Sayed, E.T. Membrane-Based Water and Wastewater Treatment Technologies: Issues, Current Trends, Challenges, and Role in Achieving Sustainable Development Goals, and Circular Economy. Chemosphere 2023, 320, 137993. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, R.; Chen, H.; Zhou, B.; Cui, Z.; Zhu, B. Advancements in Inorganic Membrane Filtration Coupled with Advanced Oxidation Processes for Wastewater Treatment. Molecules 2024, 29, 4267. [Google Scholar] [CrossRef]
- Annamalai, J.; Ummalyma, S.B.; Pandey, A. Recent Trends in the Microbial Degradation and Bioremediation of Emerging Pollutants in Wastewater Treatment System. In Development in Wastewater Treatment Research and Processes: Innovative Microbe-Based Applications for Removal of Chemicals and Metals in Wastewater Treatment Plants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 99–125. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment Technologies for Emerging Contaminants in Wastewater Treatment Plants: A Review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of Adsorption Process for Effective Removal of Emerging Contaminants from Water and Wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of Emerging Contaminants from Water and Wastewater by Modified Biochar: A Review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Zawadzki, P. Visible Light–Driven Advanced Oxidation Processes to Remove Emerging Contaminants from Water and Wastewater: A Review. Water Air Soil Pollut 2022, 233, 374. [Google Scholar] [CrossRef]
- Fast, S.A.; Gude, V.G.; Truax, D.D.; Martin, J.; Magbanua, B.S. A Critical Evaluation of Advanced Oxidation Processes for Emerging Contaminants Removal. Environ. Process. 2017, 4, 283–302. [Google Scholar] [CrossRef]
- Bracamontes-Ruelas, A.R.; Ordaz-Díaz, L.A.; Bailón-Salas, A.M.; Ríos-Saucedo, J.C.; Reyes-Vidal, Y.; Reynoso-Cuevas, L. Emerging Pollutants in Wastewater, Advanced Oxidation Processes as an Alternative Treatment and Perspectives. Processes 2022, 10, 1041. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment Technologies for Emerging Contaminants in Water: A Review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Hussien, M.S.A.; Bouzidi, A.; Abd-Rabboh, H.S.M.; Yahia, I.S.; Zahran, H.Y.; Abdel-Wahab, M.S.; Alharbi, W.; Awwad, N.S.; Ibrahim, M.A. Fabrication and Characterization of Highly Efficient As-Synthesized WO3/Graphitic-C3 N4 Nanocomposite for Photocatalytic Degradation of Organic Compounds. Materials 2022, 15, 2482. [Google Scholar] [CrossRef]
- Liu, Q.; Liang, D.; Xiong, J.; Cai, X.; Huang, Z.; Gan, T.; Hu, H.; Zhang, Y. Bi2WO6/Spontaneously Polarized Ceramic–g-C3N4 Heterojunction with Dynamic Cascade Electric Fields to Deplete Unilaterally Accumulated Charges and Break Carrier Shielding Effect for Boosting Piezo-Photocatalysis. J. Mater. Sci. Technol. 2025, 230, 93–105. [Google Scholar] [CrossRef]
- Bhanderi, D.; Lakhani, P.; Modi, C.K. Graphitic Carbon Nitride (g-C3N4) as an Emerging Photocatalyst for Sustainable Environmental Applications: A Comprehensive Review. RSC Sustain. 2023, 2, 265–287. [Google Scholar] [CrossRef]
- Basumatary, F.; Sarkar, A.; Mushahary, N.; Das, B.; Saikia, P.; Selvaraj, M.; Basumatary, S. Graphitic Carbon Nitride Composites as Advanced Versatile Materials for Adsorption and Photocatalytic Degradation of Emerging Pollutants from Wastewater. Process Saf. Environ. Prot. 2024, 191, 2416–2468. [Google Scholar] [CrossRef]
- Cecconet, D.; Sturini, M.; Malavasi, L.; Capodaglio, A.G. Graphitic Carbon Nitride as a Sustainable Photocatalyst Material for Pollutants Removal. State-of-the Art, Preliminary Tests and Application Perspectives. Materials 2021, 14, 7368. [Google Scholar] [CrossRef]
- Pham, T.H.; Viet, N.M.; Hoai, P.T.T.; Jung, S.H.; Kim, T.Y. Graphitic Carbon Nitride Metal-Free Photocatalyst for the Simultaneous Removal of Emerging Pharmaceutical Pollutants in Wastewater. Environ. Res. 2023, 231, 116246. [Google Scholar] [CrossRef]
- Pei, J.; Li, H.; Yu, D.; Zhang, D. g-C3N4-Based Heterojunction for Enhanced Photocatalytic Performance: A Review of Fabrications, Applications, and Perspectives. Catalysts 2024, 14, 825. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of Graphitic Carbon Nitride for Photocatalysis: A Review. Appl. Catal. B 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Suhag, M.H. Development of Advanced Technology for Photocatalytic Degradation of Organic Pollutants in Aqueous Solution with Modified g-C3N4 Composites. Ph.D Thesis, Mie University, Mie, Japan, September 2024. [Google Scholar]
- Altendji, K.; Hamoudi, S. Efficient Photocatalytic Degradation of Aqueous Atrazine over Graphene-Promoted g-C3N4 Nanosheets. Catalysts 2023, 13, 1265. [Google Scholar] [CrossRef]
- Manimozhi, R.; Mathankumar, M.; Prakash, A.P.G. Synthesis of g-C3N4/ZnO Heterostructure Photocatalyst for Enhanced Visible Degradation of Organic Dye. Optik 2021, 229, 165548. [Google Scholar] [CrossRef]
- Chen, M.; Jia, Y.; Li, H.; Wu, Z.; Huang, T.; Zhang, H. Enhanced Pyrocatalysis of the Pyroelectric BiFeO3/g-C3N4 Heterostructure for Dye Decomposition Driven by Cold-Hot Temperature Alternation. J. Adv. Ceram. 2021, 10, 338–346. [Google Scholar] [CrossRef]
- Rubab, R.; Mansoor, S.; Javed, M.; Hamza, A.; Bahadur, A.; Iqbal, S.; Mahmood, S.; Qamar, M.A.; Shoaib, M.; Alotaibi, K.M.; et al. Harnessing Solar Power for Enhanced Photocatalytic Degradation of Coloured Pollutants Using Novel Mg-Doped-ZnFe2O4/S@g-C3N4 Heterojunction: A Facile Hydrothermal Synthesis Approach. Luminescence 2024, 39, e4758. [Google Scholar] [CrossRef]
- Sert, B.; Bilici, Z.; Ocakoglu, K.; Dizge, N.; Rad, T.S.; Khataee, A. Preparation of S-Scheme g-C3N4/ZnO Heterojunction Composite for Highly Efficient Photocatalytic Destruction of Refractory Organic Pollutant. Catalysts 2023, 13, 485. [Google Scholar] [CrossRef]
- Singhal, M.; Jangir, S.; Upadhyay, S.; Rajawat, D.S. Advances in Graphitic Carbon Nitride: A Comprehensive Review of Synthesis, Properties, Applications and Recent Developments with Photoelectrochemical Water Splitting. Discov. Chem. 2024, 1, 64. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Chouhan, R.S.; Jerman, I.; Heath, D.; Bohm, S.; Gandhi, S.; Sadhu, V.; Baker, S.; Horvat, M. Emerging Tri-s-triazine-based Graphitic Carbon Nitride: A Potential Signal-transducing Nanostructured Material for Sensor Applications. Nano Sel. 2021, 2, 712–743. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, Y.; Fan, J.; Xue, Y.; Chang, H.; Masubuchi, Y.; Yin, S. Synthesis of Graphitic Carbon Nitride from Different Precursors by Fractional Thermal Polymerization Method and Their Visible Light Induced Photocatalytic Activities. J. Alloys Compd. 2018, 735, 1297–1305. [Google Scholar] [CrossRef]
- Saman, F.; Ling, C.H.S.; Ayub, A.; Rafeny, N.H.B.; Mahadi, A.H.; Subagyo, R.; Nugraha, R.E.; Prasetyoko, D.; Bahruji, H. Review on Synthesis and Modification of g-C3N4 for Photocatalytic H2 Production. Int. J. Hydrogen Energy 2024, 77, 1090–1116. [Google Scholar] [CrossRef]
- Jing, L.; Li, Z.; Chen, Z.; Li, R.; Hu, J. Engineering Polyheptazine and Polytriazine Imides for Photocatalysis. Angew. Chem. Int. Ed. 2024, 63, e202406398. [Google Scholar] [CrossRef]
- Pérez-Molina, Á.; Pastrana-Martínez, L.M.; Morales-Torres, S.; Maldonado-Hódar, F.J. Photodegradation of Cytostatic Drugs by g-C3N4: Synthesis, Properties and Performance Fitted by Selecting the Appropriate Precursor. Catal. Today 2023, 418, 114068. [Google Scholar] [CrossRef]
- Aoudi, B.; Kahkeci, J.; Boluk, Y.; El-Din, M.G. Enhanced G-C3N4 for Sustainable Solar Degradation of 1,3-Diphenylguanidine (DPG) in Wastewater: Investigating the Effects of Precursor, Temperature, and Potassium Doping. Chem. Eng. J. 2024, 487, 150665. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Hussain, M.I.; Zhou, W.; Chen, Y.; Wang, L.N. g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials 2022, 12, 121. [Google Scholar] [CrossRef]
- Wu, Q.; Jeong, T.; Kim, S.H.; Song, Y.J. Synthesis of Large Area Graphitic Carbon Nitride Nanosheet by Chemical Vapor Deposition. J. Alloys Compd. 2022, 900, 163310. [Google Scholar] [CrossRef]
- Chubenko, E.B.; Maximov, S.E.; Bui, C.D.; Pham, V.T.; Borisenko, V.E. Rapid Chemical Vapor Deposition of Graphitic Carbon Nitride Films. Materialia 2023, 28, 101724. [Google Scholar] [CrossRef]
- Yadav, R.M.; Kumar, R.; Aliyan, A.; Dobal, P.S.; Biradar, S.; Vajtai, R.; Singh, D.P.; Martí, A.A.; Ajayan, P.M. Facile Synthesis of Highly Fluorescent Free-Standing Films Comprising Graphitic Carbon Nitride (g-C3N4) Nanolayers. New J. Chem. 2020, 44, 2644–2651. [Google Scholar] [CrossRef]
- Chubenko, E.B.; Kovalchuk, N.G.; Komissarov, I.V.; Borisenko, V.E. Chemical Vapor Deposition of 2D Crystallized g-C3N4 Layered Films. J. Phys. Chem. C 2022, 126, 4710–4714. [Google Scholar] [CrossRef]
- Chen, L.; Feng, Q.; Giusto, P.; Luo, D.; Zhang, W.; Liu, J.; Antonietti, M.; Xiao, K. Controlled Growth of Ultrathin Graphitic Carbon Nitride Films by Chemical Vapor Deposition. ACS Mater. Lett. 2025, 7, 869–875. [Google Scholar] [CrossRef]
- Hu, C.C.; Wang, M.S.; Hung, W.Z. Influence of Solvothermal Synthesis on the Photocatalytic Degradation Activity of Carbon Nitride under Visible Light Irradiation. Chem. Eng. Sci. 2017, 167, 1–9. [Google Scholar] [CrossRef]
- Abdullahi, M.T.; Ali, M.; Farooq, W.; Khan, M.; Younas, M.; Tahir, M.N. Solvothermal Synthesis of Carbon Nitride (g-C3N4): Bandgap Engineering for Improved Photocatalytic Performance. Sustain. Energy Fuels 2025, 9, 1109–1119. [Google Scholar] [CrossRef]
- Yan, L.; Zhong, S.; Xiao, C.; Chen, Y. Optimization of Hydrothermal Pretreatment to Prepare g-C3N4 with Enhanced Catalyst Yield and Photocatalytic Activity for Aqueous Contaminant Removal. Mater. Today Sustain. 2023, 22, 100374. [Google Scholar] [CrossRef]
- Zhuang, Q.; Sun, L.; Ni, Y. One-Step Synthesis of Graphitic Carbon Nitride Nanosheets with the Help of Melamine and Its Application for Fluorescence Detection of Mercuric Ions. Talanta 2017, 164, 458–462. [Google Scholar] [CrossRef]
- Esposito, S. “Traditional” Sol-Gel Chemistry as a Powerful Tool for the Preparation of Supported Metal and Metal Oxide Catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef]
- De Matteis, V.; Cannavale, A.; Ayr, U. Titanium Dioxide in Chromogenic Devices: Synthesis, Toxicological Issues, and Fabrication Methods. Appl. Sci. 2020, 10, 8896. [Google Scholar] [CrossRef]
- Liu, X.; Chen, N.; Li, Y.; Deng, D.; Xing, X.; Wang, Y. A General Nonaqueous Sol-Gel Route to g-C3N4-Coupling Photocatalysts: The Case of Z-Scheme g-C3N4/TiO2 with Enhanced Photodegradation toward RhB under Visible-Light. Sci. Rep. 2016, 6, 39531. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor Heterojunction Photocatalysts: Design, Construction, and Photocatalytic Performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
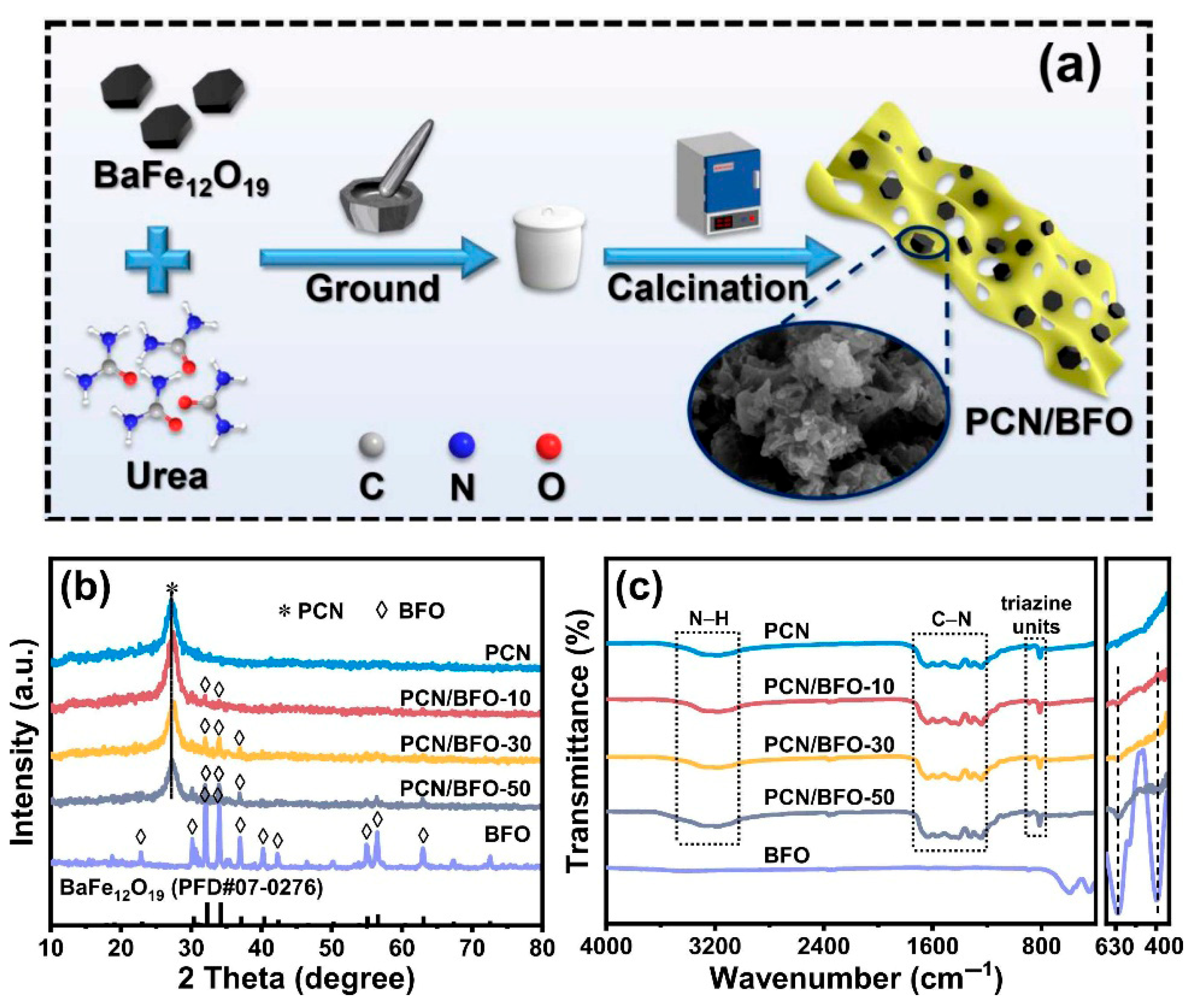
- Zhou, P.; Wang, Y.; Yan, X.; Gan, Y.; Xia, C.; Xu, Y.; Xie, M. Nitrogen-Defect-Modified g-C3N4/BaFe12O19 S-Scheme Heterojunction Photocatalyst with Enhanced Advanced Oxidation Technology Synergistic Photothermal Degradation Ability of Antibiotic: Insights into Performance, Electron Transfer Pathways and Toxicity. Appl. Catal. B 2024, 343, 123485. [Google Scholar] [CrossRef]
- Tan, Y.; Shu, Z.; Zhou, J.; Li, T.; Wang, W.; Zhao, Z. One-Step Synthesis of Nanostructured g-C3N4/TiO2 Composite for Highly Enhanced Visible-Light Photocatalytic H2 Evolution. Appl. Catal. B 2018, 230, 260–268. [Google Scholar] [CrossRef]
- Zou, Y.; Xie, Y.; Yu, S.; Chen, L.; Cui, W.; Dong, F.; Zhou, Y. SnO2 Quantum Dots Anchored on g-C3N4 for Enhanced Visible-Light Photocatalytic Removal of NO and Toxic NO2 Inhibition. Appl. Surf. Sci. 2019, 496, 143630. [Google Scholar] [CrossRef]
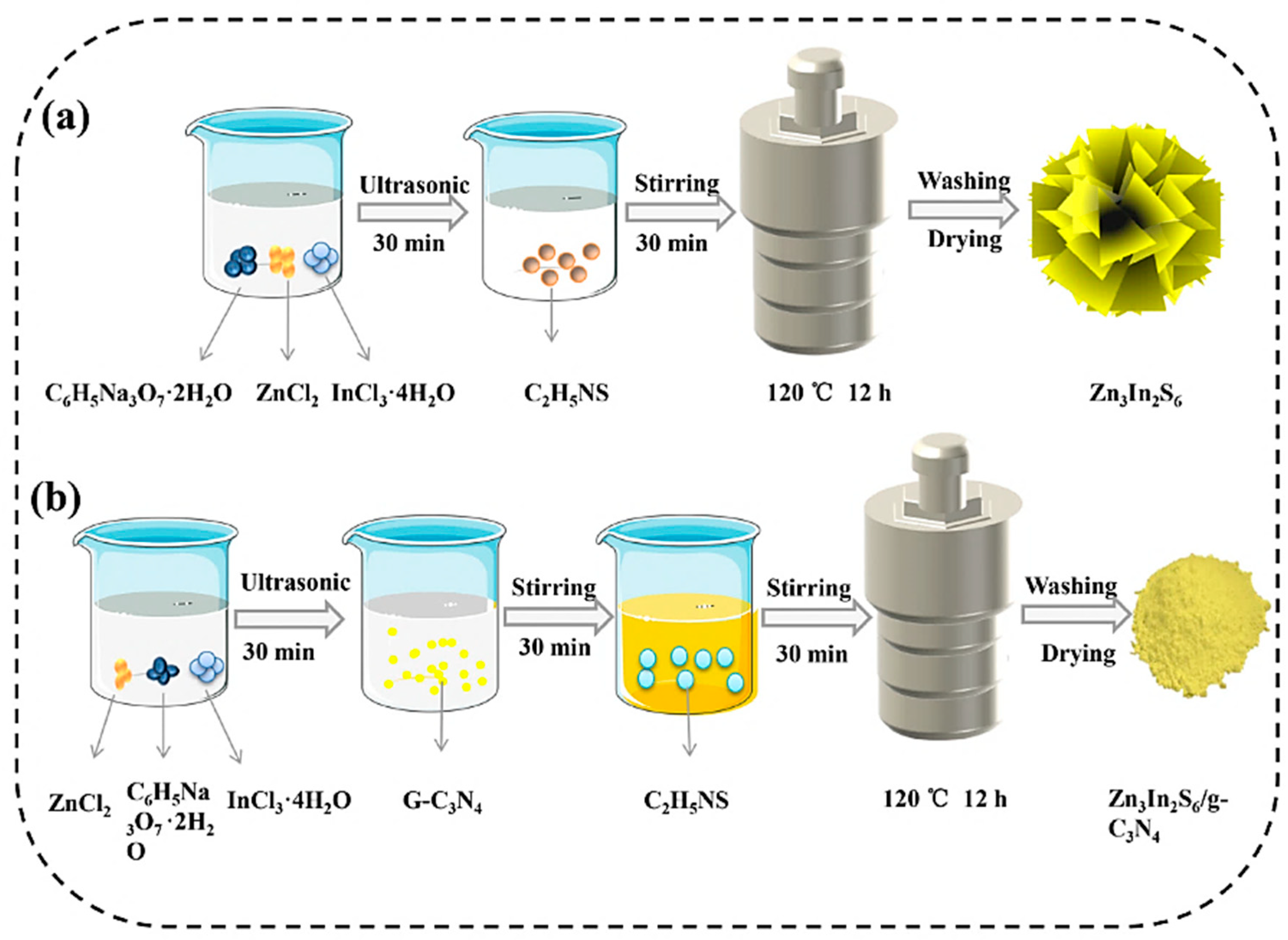
- Huo, H.; Li, Y.; Wang, S.; Tan, S.; Li, X.; Yi, S.; Gao, L. Construction of Highly Active Zn3In2S6 (110)/g-C3N4 System by Low Temperature Solvothermal for Efficient Degradation of Tetracycline under Visible Light. Int. J. Mol. Sci. 2022, 23, 13221. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Kong, X.; Liang, Y.; Li, Z.; Wu, S.; Chang, C.; Luo, S.; Cui, Z. In Situ Synthesis of a Novel Mn3O4/g-C3N4 p-n Heterostructure Photocatalyst for Water Splitting. J. Colloid. Interface Sci. 2021, 586, 778–784. [Google Scholar] [CrossRef]
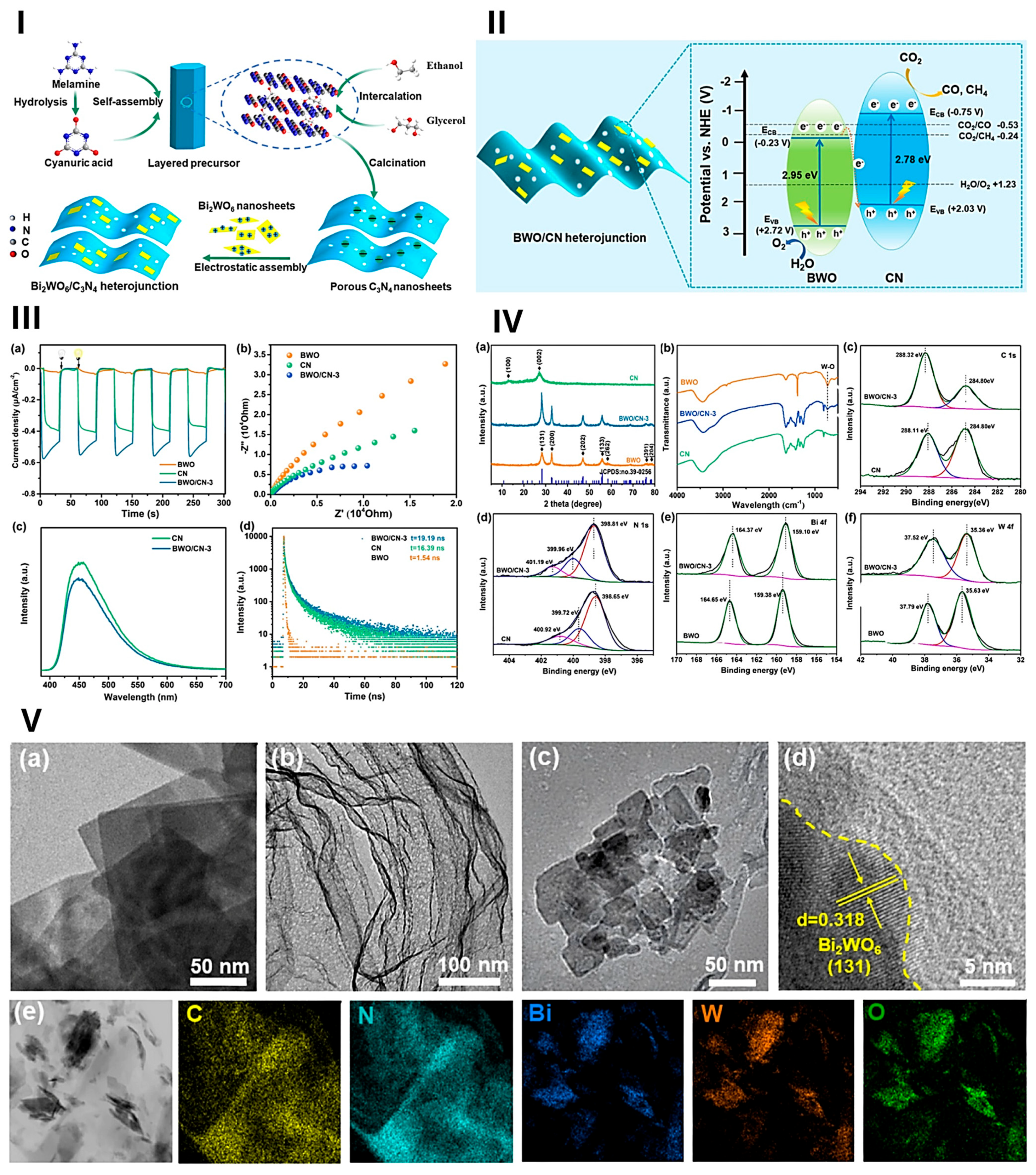
- Qin, F.; Xia, Y.; Yang, D.; Xiao, T.; Zhu, X.; Feng, W.; Qi, Z. Enhanced Photocatalytic Activity of G-C3N4/Bi2WO6 Heterojunction via Z-Scheme Charge-Transfer Mechanism. J. Mol. Struct. 2024, 1316, 139023. [Google Scholar] [CrossRef]
- Reddy, C.V.; Nagar, A.; Shetti, N.P.; Reddy, I.N.; Basu, S.; Shim, J.; Kakarla, R.R. Novel g-C3N4/BiVO4 Heterostructured Nanohybrids for High Efficiency Photocatalytic Degradation of Toxic Chemical Pollutants. Chemosphere 2023, 322, 138146. [Google Scholar] [CrossRef]
- Song, T.; Yu, X.; Tian, N.; Huang, H.-W. Preparation, Structure and Application of g-C3N4/BiOX Composite Photocatalyst. Int. J. Hydrogen Energy 2021, 46, 1857–1878. [Google Scholar] [CrossRef]
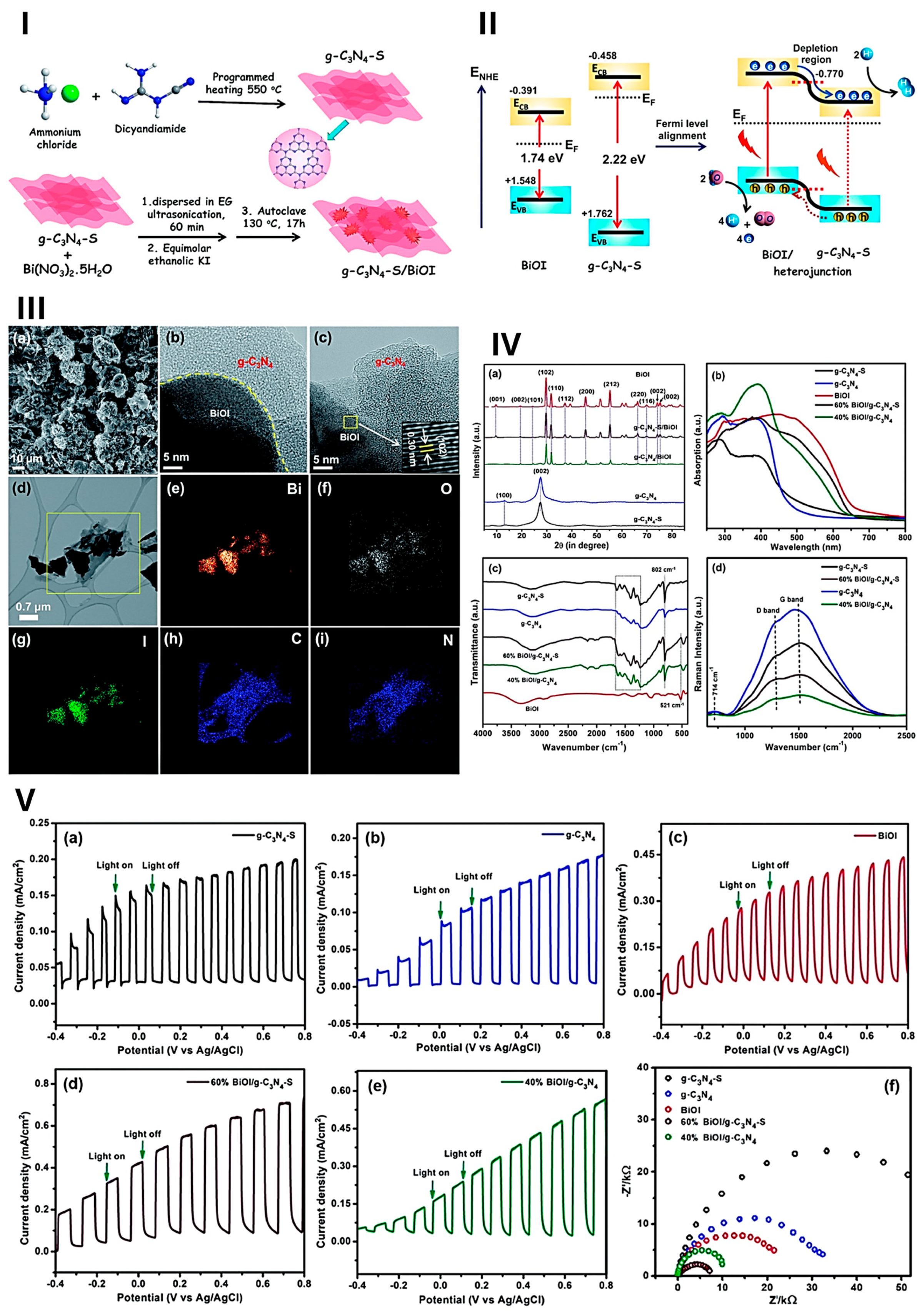
- Liu, H.; Huo, W.; Zhang, T.C.; Ouyang, L.; Yuan, S. Photocatalytic Removal of Tetracycline by a Z-Scheme Heterojunction of Bismuth Oxyiodide/Exfoliated g-C3N4: Performance, Mechanism, and Degradation Pathway. Mater. Today Chem. 2022, 23, 100729. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Shen, Z.; Wu, S.; Su, Y.; Pei, L.; Ji, Z.; Ding, M.; Bai, W.; Chen, Y.; Yu, Z.T.; et al. Liquid Exfoliation of G-C3N4 Nanosheets to Construct 2D-2D MoS2/g-C3N4 Photocatalyst for Enhanced Photocatalytic H2 Production Activity. Appl. Catal. B 2019, 246, 120–128. [Google Scholar] [CrossRef]
- Li, W.; Ma, Q.; Wang, X.; He, S.-A.; Li, M.; Ren, L. Hydrogen Evolution by Catalyzing Water Splitting on Two-Dimensional g-C3N4-Ag/AgBr Heterostructure. Appl. Surf. Sci. 2019, 494, 275–284. [Google Scholar] [CrossRef]
- Patial, B.; Bansal, A.; Gupta, R.; Mittal, S.K. Hydrothermal Synthesis of (m-t)BiVO4/g-C3N4 Heterojunction for Enhancement in Photocatalytic Degradation of Imidacloprid. J. Environ. Chem. Eng. 2023, 11, 111138. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. MXene-Based Photocatalysts in Degradation of Organic and Pharmaceutical Pollutants. Molecules 2022, 27, 6939. [Google Scholar] [CrossRef]
- Su, P.; Zhang, C.; Liu, Y.; Zhang, J.; Djellabi, R.; Wang, R.; Guo, J.; Zhang, R.; Guo, H.; Ding, X.; et al. Boosting PFOA Photocatalytic Removal from Water Using Highly Adsorptive and Sunlight-Responsive ZIF67/MIL-100(Fe) Modified C3N4. J. Environ. Chem. Eng. 2023, 11, 110765. [Google Scholar] [CrossRef]
- Chabib, L.; Nursal; Miskam, M.; Kaus, N.H.M.; Shafie, M.H.; Hamid, M.; Saragi, I.R.; Isnaeni, I.; Amilia, D.; Dalimunthe, I.B.; et al. Optimization of g-C3N4 Nanoparticles on Structural, Morphological, and Optical Properties as Organic Pollutants Adsorbent in Glycerin. Case Stud. Chem. Environ. Eng. 2025, 11, 101057. [Google Scholar] [CrossRef]
- Li, C.; Yu, S.; Dong, H.; Liu, C.; Wu, H.; Che, H.; Chen, G. Z-Scheme Mesoporous Photocatalyst Constructed by Modification of Sn3O4 Nanoclusters on g-C3N4 Nanosheets with Improved Photocatalytic Performance and Mechanism Insight. Appl. Catal. B 2018, 238, 284–293. [Google Scholar] [CrossRef]
- Jiang, F.; Yan, T.; Chen, H.; Sun, A.; Xu, C.; Wang, X. A g-C3N4–CdS Composite Catalyst with High Visible-Light-Driven Catalytic Activity and Photostability for Methylene Blue Degradation. Appl. Surf. Sci. 2014, 295, 164–172. [Google Scholar] [CrossRef]
- Bloh, J.Z. Intensification of Heterogeneous Photocatalytic Reactions Without Efficiency Losses: The Importance of Surface Catalysis. Catal. Lett. 2021, 151, 3105–3113. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mahmoud, S.A.; Mohamed, A.A. Unveiling the Photocatalytic Potential of Graphitic Carbon Nitride (g-C3N4): A State-of-the-Art Review. RSC Adv. 2024, 14, 25629–25662. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Wu, Q.; Du, P.; Zhu, J.; Dai, S.; Yang, S. Incorporating Graphitic Carbon Nitride (g-C3N4) Quantum Dots into Bulk-Heterojunction Polymer Solar Cells Leads to Efficiency Enhancement. Adv. Funct. Mater. 2016, 26, 1719–1728. [Google Scholar] [CrossRef]
- Mamba, F.B.; Mbuli, B.S.; Ramontja, J. Synergistic Effect of ZnO/Ag2O@g-C3N4 Based Nanocomposites Embedded in Carrageenan Matrix for Dye Degradation in Water. Heliyon 2024, 10, e31109. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Z.; Jia, J.; Robertson, J.; Guo, Y. 2D WSe2/MoSi2N4 Type-II Heterojunction with Improved Carrier Separation and Recombination for Photocatalytic Water Splitting. Appl. Surf. Sci. 2023, 611, 155674. [Google Scholar] [CrossRef]
- Resasco, J.; Zhang, H.; Kornienko, N.; Becknell, N.; Lee, H.; Guo, J.; Briseno, A.L.; Yang, P. TiO2/BiVO4 Nanowire Heterostructure Photoanodes Based on Type II Band Alignment. ACS Cent. Sci. 2016, 2, 80–88. [Google Scholar] [CrossRef]
- Kumari, P.; Bahadur, N.; Kong, L.; O’Dell, L.A.; Merenda, A.; Dumée, L.F. Engineering Schottky-like and Heterojunction Materials for Enhanced Photocatalysis Performance—A Review. Mater. Adv. 2022, 3, 2309–2323. [Google Scholar] [CrossRef]
- Alam, K.M.; Kumar, P.; Kar, P.; Thakur, U.K.; Zeng, S.; Cui, K.; Shankar, K. Enhanced Charge Separation in g-C3N4–BiOI Heterostructures for Visible Light Driven Photoelectrochemical Water Splitting. Nanoscale Adv. 2019, 1, 1460–1471. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, B.; Yu, J. A New Understanding of the Photocatalytic Mechanism of the Direct Z-Scheme g-C3N4/TiO2 Heterostructure. Phys. Chem. Chem. Phys. 2016, 18, 31175–31183. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Xue, Y.; Yao, G.; Zheng, S. A Facile Synthesis of g-C3N4/TiO2 Hybrid Photocatalysts by Sol–Gel Method and Its Enhanced Photodegradation towards Methylene Blue under Visible Light. Adv. Powder Technol. 2016, 27, 330–337. [Google Scholar] [CrossRef]
- Chen, H.; Xie, Y.; Sun, X.; Lv, M.; Wu, F.; Zhang, L.; Li, L.; Xu, X. Efficient Charge Separation Based on Type-II g-C3N4/TiO2 -B Nanowire/Tube Heterostructure Photocatalysts. Dalton Trans. 2015, 44, 13030–13039. [Google Scholar] [CrossRef]
- Jiang, P.; Yu, Y.; Wang, K.; Liu, W. Efficient Electron Transfer in g-C3N4/TiO2 Heterojunction for Enhanced Photocatalytic CO2 Reduction. Catalysts 2024, 14, 335. [Google Scholar] [CrossRef]
- Basivi, P.K.; Selvaraj, Y.; Perumal, S.; Bojarajan, A.K.; Lin, X.; Girirajan, M.; Kim, C.W.; Sangaraju, S. Graphitic Carbon Nitride (g–C3N4)–Based Z-Scheme Photocatalysts: Innovations for Energy and Environmental Applications. Mater. Today Sustain. 2025, 29, 101069. [Google Scholar] [CrossRef]
- Fernández-Catalá, J.; Greco, R.; Navlani-García, M.; Cao, W.; Berenguer-Murcia, Á.; Cazorla-Amorós, D. g-C3N4-Based Direct Z-Scheme Photocatalysts for Environmental Applications. Catalysts 2022, 12, 1137. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, Q.; Li, K.; Zhang, Y.; Liu, E.; Li, X. Recent Advances on g–C3N4–Based Z-Scheme Photocatalysts: Structural Design and Photocatalytic Applications. Int. J. Hydrogen Energy 2023, 48, 196–231. [Google Scholar] [CrossRef]
- Mane, P.; Bae, H.; Burungale, V.; Lee, S.W.; Misra, M.; Parbat, H.; Kadam, A.N.; Ha, J.S. Interface-Engineered Z-Scheme of BiVO4/g-C3N4 Photoanode for Boosted Photoelectrochemical Water Splitting and Organic Contaminant Elimination under Solar Light. Chemosphere 2022, 308, 136166. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.; Chen, W.; Huang, M.; Xu, J.; Lv, J. Construction of a Switchable g-C3N4/BiVO4 Heterojunction from the Z-Scheme to the Type II by Incorporation of Pyromellitic Diimide. Cryst. Growth Des. 2022, 22, 1645–1653. [Google Scholar] [CrossRef]
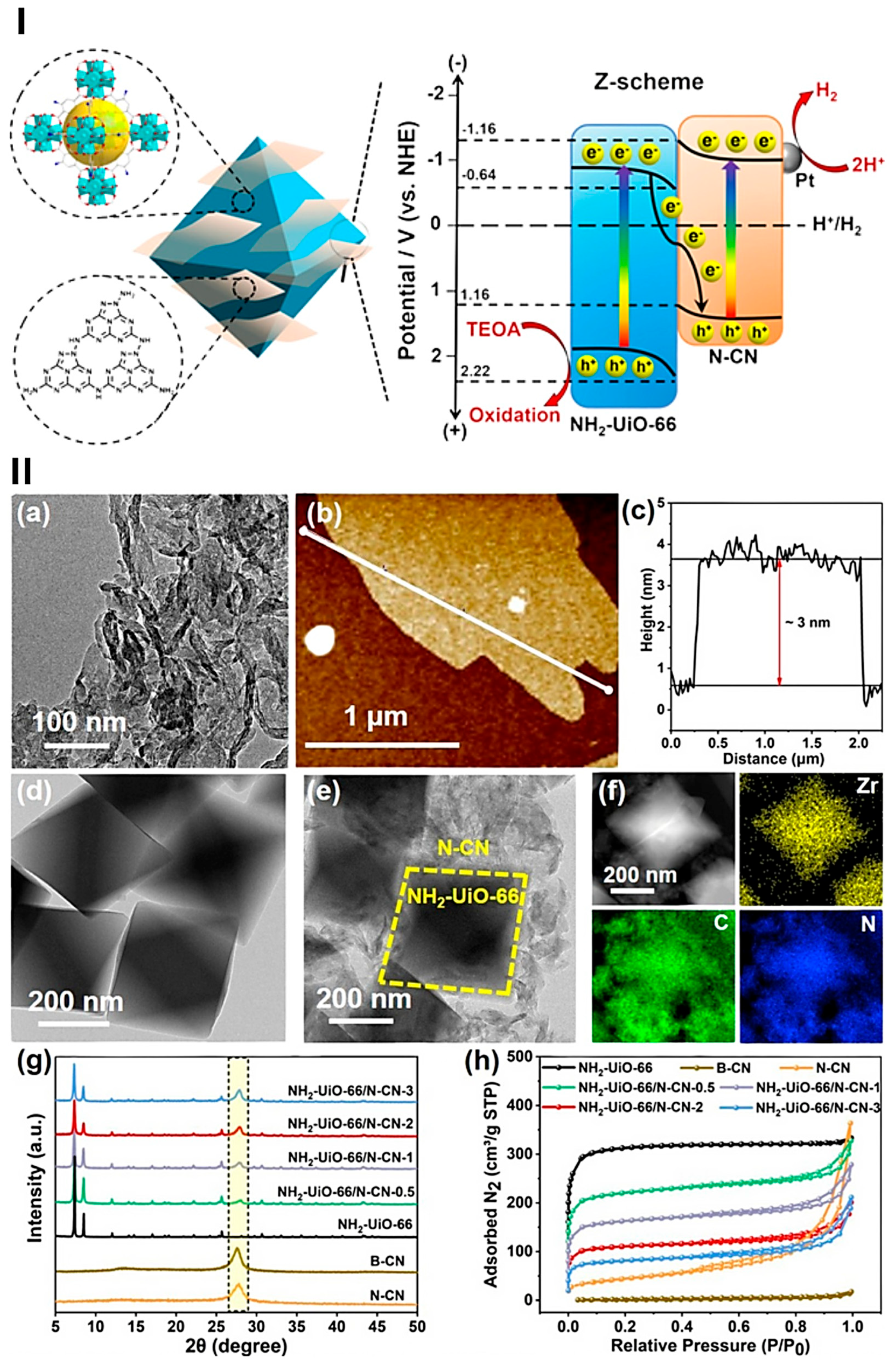
- Wu, B.; Sun, T.; Liu, N.; Lu, L.; Zhang, R.; Shi, W.; Cheng, P. Modulation of Z-Scheme Heterojunction Interface between Ultrathin C3N5 Nanosheets and Metal-Organic Framework for Boosting Photocatalysis. ACS Appl. Mater. Interfaces 2022, 14, 26742–26751. [Google Scholar] [CrossRef]
- Vinesh, V.; Preeyanghaa, M.; Kumar, T.R.N.; Ashokkumar, M.; Bianchi, C.L.; Neppolian, B. Revealing the Stability of CuWO4/g-C3N4 Nanocomposite for Photocatalytic Tetracycline Degradation from the Aqueous Environment and DFT Analysis. Environ. Res. 2022, 207, 112112. [Google Scholar] [CrossRef]
- Li, X.; Qiu, Y.; Zhu, Z.; Chen, T.; Zhang, H.; Yin, D. Construction of Magnetically Separable Dual Z-Scheme g-C3N4/α-Fe2O3/Bi3TaO7 Photocatalyst for Effective Degradation of Ciprofloxacin under Visible Light. Chem. Eng. J. 2022, 440, 135840. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Li, X.; Fang, B. Emerging Polymeric Carbon Nitride Z-Scheme Systems for Photocatalysis. Cell Rep. Phys. Sci. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Schumacher, L.; Marschall, R. Recent Advances in Semiconductor Heterojunctions and Z-Schemes for Photocatalytic Hydrogen Generation. Top Curr Chem 2022, 380, 53. [Google Scholar] [CrossRef]
- Chatterjee, A.; Wang, L.; Voort, P.V.D. Metal–Organic Frameworks in Photocatalytic Z-Scheme Heterojunctions: An Emerging Technology. Chem. Commun. 2023, 59, 3627–3654. [Google Scholar] [CrossRef]
- Łęcki, T.; Zarębska, K.; Wierzyńska, E.; Korona, K.P.; Chyży, P.; Piotrowski, P.; Skompska, M. Z-Scheme BiVO4/g-C3N4 Photocatalyst—With or Without an Electron Mediator. Molecules 2024, 29, 5092. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.; Xu, J.; Wang, Q.; Wang, X.; Lv, G.; Zhou, J. The Synthesis of H-BN-Modified Z-Scheme WO3/g-C3N4 Heterojunctions for Enhancing Visible Light Photocatalytic Degradation of Tetracycline Pollutants. ACS Omega 2022, 7, 6035–6045. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, B.; Luo, G.; Yu, J.; Cao, S. S-Scheme Heterojunction Photocatalysts Based on 2D Materials. Mater. Today 2024, 81, 137–158. [Google Scholar] [CrossRef]
- Tang, Q.; Tao, W.; Hu, J.; Gui, T.; Wang, Z.; Xiao, Y.; Song, R.; Jiang, Y.; Guo, S. Bi2WO6/C3N4 S-Scheme Heterojunction with a Built-In Electric Field for Photocatalytic CO2 Reduction. ACS Appl. Nano Mater. 2023, 6, 17130–17139. [Google Scholar] [CrossRef]
- Ge, H.; Xu, F.; Cheng, B.; Yu, J.; Ho, W. S-Scheme Heterojunction TiO2/CdS Nanocomposite Nanofiber as H2-Production Photocatalyst. ChemCatChem 2019, 11, 6301–6309. [Google Scholar] [CrossRef]
- Zhang, M.; Miao, H.; Fan, J.; Sun, T.; Tang, C.; Liu, E. Internal Electric Field-Modulated S-Scheme Ni3Se4/TiO2 Nanoparticle Heterojunction for Efficient Photocatalytic H2 Evolution. ACS Appl. Nano Mater. 2023, 6, 18284–18294. [Google Scholar] [CrossRef]
- Xi, Y.; Chen, W.; Dong, W.; Fan, Z.; Wang, K.; Shen, Y.; Tu, G.; Zhong, S.; Bai, S. Engineering an Interfacial Facet of S-Scheme Heterojunction for Improved Photocatalytic Hydrogen Evolution by Modulating the Internal Electric Field. ACS Appl. Mater. Interfaces 2021, 13, 39491–39500. [Google Scholar] [CrossRef]
- Guo, T.; Dang, R.; Zheng, A.; Wang, S.; Dong, X.; Xiong, Z.; Chen, Z.; Hu, Z.; Chen, W.; Wang, C. Engineering Charge Transfer in a Two-Dimensional S-Scheme Heterojunction Photocatalyst via Built-in Electric Field for Selective Biomass Valorization. Green. Chem. 2025, 27, 1157–1168. [Google Scholar] [CrossRef]
- Hassan, F.; Backer, S.N.; Almanassra, I.W.; Atieh, M.A.; Elbahri, M.; Shanableh, A. Solar-Matched S-Scheme ZnO/g-C3N4 for Visible Light-Driven Paracetamol Degradation. Sci. Rep. 2024, 14, 12220. [Google Scholar] [CrossRef]
- Li, F.; Zhu, G.; Jiang, J.; Yang, L.; Deng, F.; Arramel; Li, X. A Review of Updated S-Scheme Heterojunction Photocatalysts. J. Mater. Sci. Technol. 2024, 177, 142–180. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Z.; Yang, Q.; Wang, L.; Xing, Y. Review on g-C3N4-Based S-Scheme Heterojunction Photocatalysts. J. Mater. Sci. Technol. 2022, 125, 128–144. [Google Scholar] [CrossRef]
- Huang, B.; Xu, K.; Zhao, Y.; Li, B.; Jiang, S.; Liu, Y.; Huang, S.; Yang, Q.; Gao, T.; Xie, S.; et al. Review of the Versatility and Application Potentials of g-C3N4-Based S-Scheme Heterojunctions in Photocatalytic Antibiotic Degradation. Molecules 2025, 30, 1240. [Google Scholar] [CrossRef]
- Muthuganesh, A.; Rafi, S.M.; Jacob, I.D.; Soundranayagam, J.P.; Surender, S.; Elangovan, P.; Flora, X.H. Efficient Methylene Blue Dye Degradation via Visible Light-Activated g-C3N4/CuO Nanocomposites. Hybrid. Adv. 2025, 9, 100420. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wang, X.; Xia, Y.; Zhang, A.; Shi, J.; Gao, L.; Wei, H.; Chen, W. Z-Scheme BiVO4/g-C3N4 Heterojunction: An Efficient, Stable and Heterogeneous Catalyst with Highly Enhanced Photocatalytic Activity towards Malachite Green Assisted by H2O2 under Visible Light. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126445. [Google Scholar] [CrossRef]
- Qiu, S.; Li, J. High-Efficiency Ag-Modified ZnO/g-C3N4 Photocatalyst with 1D-0D-2D Morphology for Methylene Blue Degradation. Molecules 2024, 29, 2182. [Google Scholar] [CrossRef]
- Hamza, A.M.; Alshamsi, H.A. Design of Novel Z-Scheme g-C3N4/TiO2/CuCo2O4 Heterojunctions for Efficient Visible Light-Driven Photocatalyic Degradation of Rhodamine B. Sci. Rep. 2024, 14, 23596. [Google Scholar] [CrossRef]
- Kocijan, M.; Vukšić, M.; Kurtjak, M.; Ćurković, L.; Vengust, D.; Podlogar, M. TiO2-Based Heterostructure Containing g-C3N4 for an Effective Photocatalytic Treatment of a Textile Dye. Catalysts 2022, 12, 1554. [Google Scholar] [CrossRef]
- Yaqoubi, M.; Salavati-Niasari, M.; Ghanbari, M. S-Scheme CuMn2O4/g-C3N4 Heterojunction: Fabrication, Characterization, and Investigation of Photodegradation Potential of Organic Pollutants. Appl. Water Sci. 2025, 15, 13. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Ali, M.H. Pharmaceutical Wastewater as Emerging Contaminants (EC): Treatment Technologies, Impact on Environment and Human Health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, N.; Sehrawat, P.; Kansal, S.K. Solar-Light-Driven Photocatalytic Degradation of Pharmaceutical Pollutants Utilizing 2D g-C3N4/BiOCl Composite. Environ. Toxicol. Pharmacol. 2023, 99, 104110. [Google Scholar] [CrossRef]
- Marzouqi, F.A.; Farsi, B.A.; Kuvarega, A.T.; Lawati, H.A.J.A.; Kindy, S.M.Z.A.; Kim, Y.; Selvaraj, R. Controlled Microwave-Assisted Synthesis of the 2D-BiOCl/2D-g-C3N4 Heterostructure for the Degradation of Amine-Based Pharmaceuticals under Solar Light Illumination. ACS Omega 2019, 4, 4671–4678. [Google Scholar] [CrossRef]
- Bi, R.; Liu, J.; Zhou, C.; Shen, Y.; Liu, Z.; Wang, Z. In Situ Synthesis of g-C3N4/TiO2 Heterojunction by a Concentrated Absorption Process for Efficient Photocatalytic Degradation of Tetracycline Hydrochloride. Environ. Sci. Pollut. Res. 2023, 30, 55044–55056. [Google Scholar] [CrossRef]
- Zhao, Y.; Zada, A.; Yang, Y.; Pan, J.; Wang, Y.; Yan, Z.; Xu, Z.; Qi, K. Photocatalytic Removal of Antibiotics on g-C3N4 Using Amorphous CuO as Cocatalysts. Front. Chem. 2021, 9, 797738. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Zada, A.; Yu, X.; Liu, F.; Jin, G. NiFe2O4/g-C3N4 Heterostructure with an Enhanced Ability for Photocatalytic Degradation of Tetracycline Hydrochloride and Antibacterial Performance. Chemosphere 2022, 307, 135717. [Google Scholar] [CrossRef]
- Yin, X.; Sun, X.; Li, D.; Xie, W.; Mao, Y.; Liu, Z.; Liu, Z. 2D/2D Phosphorus-Doped g-C3N4/Bi2WO6 Direct Z-Scheme Heterojunction Photocatalytic System for Tetracycline Hydrochloride (TC-HCl) Degradation. Int. J. Environ. Res. Public Health 2022, 19, 14935. [Google Scholar] [CrossRef]
- Duan, X.; Yang, J.; Zhu, J.; Li, H.; Fang, Y.; Liu, R.; Yang, C.; Liu, W.; Ding, C.; Liu, Q.; et al. Activated CdS/Sulfur Doped g-C3N4 Photocatalyst for Dye and Antibiotic Degradation: Experimental and DFT Verification of S-Scheme Heterojunction. Environ. Res. 2025, 266, 120487. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhang, D.; Yao, S.; Dong, S.; Chen, Q.; Fan, F.; Jia, H.; Dong, M. Construction of Bi2WO6/g-C3N4 Z-Scheme Heterojunction and Its Enhanced Photocatalytic Degradation of Tetracycline with Persulfate under Solar Light. Molecules 2024, 29, 1169. [Google Scholar] [CrossRef]
- Kobkeatthawin, T.; Trakulmututa, J.; Amornsakchai, T.; Kajitvichyanukul, P.; Smith, S.M. Identification of Active Species in Photodegradation of Aqueous Imidacloprid over g-C3N4/TiO2 Nanocomposites. Catalysts 2022, 12, 120. [Google Scholar] [CrossRef]
- Taghilou, S.; Nakhjirgan, P.; Esrafili, A.; Dehghanifard, E.; Kermani, M.; Kakavandi, B.; Pelalak, R. Performance, Progress, and Mechanism of g-C3N4-Based Photocatalysts in the Degradation of Pesticides: A Systematic Review. Chemosphere 2024, 368, 143667. [Google Scholar] [CrossRef]
- Feng, C.; Ouyang, X.; Deng, Y.; Wang, J.; Tang, L. A Novel g-C3N4/g-C3N4−x Homojunction with Efficient Interfacial Charge Transfer for Photocatalytic Degradation of Atrazine and Tetracycline. J. Hazard. Mater. 2023, 441, 129845. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Long, Z.; Zhang, C.; Ma, Y.; Hao, X.; Zhang, H.; Pan, C. Photodegradation of Imidacloprid in Aqueous Solution by the Metal-Free Catalyst Graphitic Carbon Nitride Using an Energy-Saving Lamp. J. Agric. Food Chem. 2015, 63, 4754–4760. [Google Scholar] [CrossRef]
- Inoue, T.; Chuaicham, C.; Saito, N.; Ohtani, B.; Sasaki, K. Z-Scheme Heterojunction of Graphitic Carbon Nitride and Calcium Ferrite in Converter Slag for the Photocatalytic Imidacloprid Degradation and Hydrogen Evolution. J. Photochem. Photobiol. A Chem. 2023, 440, 114644. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Y.; Zhou, K.; Ba, D.; Ao, Y.; Wang, P. In-Situ Construction of Z-Scheme g-C3N4/WO3 Composite with Enhanced Visible-Light Responsive Performance for Nitenpyram Degradation. Chin. Chem. Lett. 2021, 32, 2179–2182. [Google Scholar] [CrossRef]
- Truc, N.T.T.; Duc, D.S.; Thuan, D.V.; Tahtamouni, T.A.; Pham, T.D.; Hanh, N.T.; Tran, D.T.; Nguyen, M.V.; Dang, N.M.; Chi, N.T.P.L.; et al. The Advanced Photocatalytic Degradation of Atrazine by Direct Z-Scheme Cu Doped ZnO/g-C3N4. Appl. Surf. Sci. 2019, 489, 875–882. [Google Scholar] [CrossRef]
- An, X.; Xu, X.; Guo, W.; Chen, Z.; Miao, Z.; Wu, Z. Bi-Functional Biochar-g-C3N4-MgO Composites for Simultaneously 2 Minimizing the Pollution: Photocatalytic Degradation of Pesticides 3 and Phosphorus Recovery as Slow-Release Fertilizer. J. Environ. Manage. 2023, 344, 118489. [Google Scholar] [CrossRef]
- Tabasum, S.; Rani, S.; Sharma, A.; Dhupar, N.; Singh, P.P.; Bagri, U.; Kumar, D. Efficient Photocatalytic Degradation of Chlorpyrifos Pesticide from Aquatic Agricultural Waste Using g-C3N4 Decorated Graphene Oxide/V2O5 Nanocomposite. Top. Catal. 2024, 67, 725–736. [Google Scholar] [CrossRef]
- Nekooie, R.; Ghasemi, J.B.; Badiei, A.; Shamspur, T.; Mostafavi, A.; Moradian, S. Design and Synthesis of g-C3N4/(Cu/TiO2) Nanocomposite for the Visible Light Photocatalytic Degradation of Endosulfan in Aqueous Solutions. J. Mol. Struct. 2022, 1258, 132650. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Preethi, J.; Meenakshi, S. Removal of Chlorpyrifos, an Insecticide Using Metal Free Heterogeneous Graphitic Carbon Nitride (g-C3N4) Incorporated Chitosan as Catalyst: Photocatalytic and Adsorption Studies. Int. J. Biol. Macromol. 2019, 132, 289–299. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, W.; Liao, G.; Li, X.; Wang, J.; Tang, Y.; Li, L. Flexible Construct of N Vacancies and Hydrophobic Sites on g-C3N4 by F Doping and Their Contribution to PFOA Degradation in Photocatalytic Ozonation. J. Hazard. Mater. 2022, 428, 128222. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, Y.; Peng, Z.; He, Q.; Zhao, W.; Zhang, W.; Yin, D.; Zhang, Y.; Tang, J. Photocatalytic Degradation of Perfluorooctanoic Acid (PFOA) from Water: A Mini Review. Environ. Pollut. 2024, 343, 123212. [Google Scholar] [CrossRef]
- Navidpour, A.H.; Safaei, J.; Johir, M.A.H.; Ni, B.J.; Dashti, A.; Li, X.; Zhou, J.L. Zinc Oxide@citric Acid-Modified Graphitic Carbon Nitride Nanocomposites for Adsorption and Photocatalytic Degradation of Perfluorooctanoic Acid. Adv. Compos. Hybrid. Mater. 2024, 7, 53. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Song, Y.; Cao, L.; Dou, Y.; Yu, J.; Zhang, Y.; He, J.; Dai, W.; Yao, C.; et al. Enhanced and accelerated degradation of PFOA using visible light—Applying semiconductor carbon nitride as an accelerator. J. Environ. Chem. Eng. 2023, 12, 111653. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Yu, H.; Song, Y.; Gan, X. Accelerating Degradation of Perfluorooctanoic Acid by Iron Doping of Visible Light Catalyst: Experiments Meet Theories. Chem. Eng. J. 2024, 498, 155450. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.; Sun, C.; Liu, Y.; Ma, M.; Chen, F.; Zhou, L.; Zheng, Y. Trace Co Coupled and Tourmaline Doped g-C3N4 for Visible-Light Synergistic Persulfate System for Degradation of Perfluorooctanoic Acid. J. Clean. Prod. 2022, 372, 133745. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Li, X.; Luo, Y.; Zheng, H.; Li, X.; Chen, L.; Li, F. Adsorption–Photocatalysis Synergistic Removal of Perfluoroalkyl/Polyfluoroalkyl Substances by Reusable Chitin/Polyethylene Imine/O-g-C3N4 Sponges in Water Environments. Chem. Eng. J. 2025, 511, 162006. [Google Scholar] [CrossRef]
| Heterostructure | Synthesis Method | Environmental Pollutants | Degradation (%) | Time (min) | Source Light | Ref. |
|---|---|---|---|---|---|---|
| g-C3N4/grafeno | Ensemble solution | Atrazine | 100 | 300 | Visible | [31] |
| g-C3N4/ZnO | Hydrothermal | Crystal violet | 97 | 180 | Visible | [32] |
| BiFeO3/g-C3N4 | Calcination | Rhodamine B | ~94 | - | Visible | [33] |
| g-C3N4/Mg-ZnFe2O4 | Hydrothermal | Methylene blue | High | - | Visible | [34] |
| g-C3N4/ZnO | Calcination | Crystal violet | 95.9 | 120 | UV | [35] |
| g-C3N4/SnO2 | Calcination | nitric oxide (NO) | 32 | 30 | Visible | [61] |
| Zn3In2S6/g-C3N4 | solvothermal | Tetracycline | 80 | 150 | Visible | [62] |
| g-C3N4/BiOCl, BiOBr, BiOI | Impregnation | Dye | High | - | Visible | [66] |
| MIL-100(Fe)@g-C3N4 | Calcination | PFOA | 70 | - | Visible | [72] |
| CuWO4/g-C3N4 | Calcination | Tetracycline | 88 | 120 | Visible | [94] |
| ZnO/g-C3N4 | Hydrothermal calcination | Paracetamol | 95 | 60 | Visible | [107] |
| g-C3N4/BiVO4 | Solvothermal | Malachite green | 98 | 60 | Visible | [112] |
| Ag/ZnO/g-C3N4 | Autoensemble | Methyl blue | 98 | 30 | UV-Visible | [113] |
| g-C3N4/TiO2/CuCo2O4 | Solvothermal | Rhodamine B | 99 | 60 | Solar simulated | [114] |
| g-C3N4@TiO2 | Hydrothermal | Methyl blue | 99 | 60 | UV-A and simulated solar irradiation | [115] |
| CuMn2O4/g-C3N4 | Coprecipitation—ultrasonic | Erythrosine | 91 | 90 | Visible | [116] |
| g-C3N4/TiO2 | Calcination | Tetracycline | 90 | 30 | Solar simulated | [120] |
| NiFe2O4/g-C3N4 | Sol–gel | Tetracycline | 94.5 | 80 | Visible | [122] |
| Bi2WO6/P-g-C3N4 | Solvothermal | Tetracycline | High | - | Visible | [123] |
| CdS/S-g-C3N4 | Difusión en estado sólido (SSD) | Tetracycline | 91 | 60 | Visible | [124] |
| Methyl orange | ~100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada-Movilla, E.; Castillo-Saenz, J.; Valdez-Salas, B.; Ortiz-Pérez, Á.; Beltrán-Partida, E.; Salvador-Carlos, J.; Puello-Polo, E. Challenges and Opportunities for g-C3N4-Based Heterostructures in the Photodegradation of Environmental Pollutants. Catalysts 2025, 15, 653. https://doi.org/10.3390/catal15070653
Estrada-Movilla E, Castillo-Saenz J, Valdez-Salas B, Ortiz-Pérez Á, Beltrán-Partida E, Salvador-Carlos J, Puello-Polo E. Challenges and Opportunities for g-C3N4-Based Heterostructures in the Photodegradation of Environmental Pollutants. Catalysts. 2025; 15(7):653. https://doi.org/10.3390/catal15070653
Chicago/Turabian StyleEstrada-Movilla, Eduardo, Jhonathan Castillo-Saenz, Benjamín Valdez-Salas, Álvaro Ortiz-Pérez, Ernesto Beltrán-Partida, Jorge Salvador-Carlos, and Esneyder Puello-Polo. 2025. "Challenges and Opportunities for g-C3N4-Based Heterostructures in the Photodegradation of Environmental Pollutants" Catalysts 15, no. 7: 653. https://doi.org/10.3390/catal15070653
APA StyleEstrada-Movilla, E., Castillo-Saenz, J., Valdez-Salas, B., Ortiz-Pérez, Á., Beltrán-Partida, E., Salvador-Carlos, J., & Puello-Polo, E. (2025). Challenges and Opportunities for g-C3N4-Based Heterostructures in the Photodegradation of Environmental Pollutants. Catalysts, 15(7), 653. https://doi.org/10.3390/catal15070653






