Direct 2400 h Seawater Electrolysis Catalyzed by Pt-Loaded Nanoarray Sheets
Abstract
1. Introduction
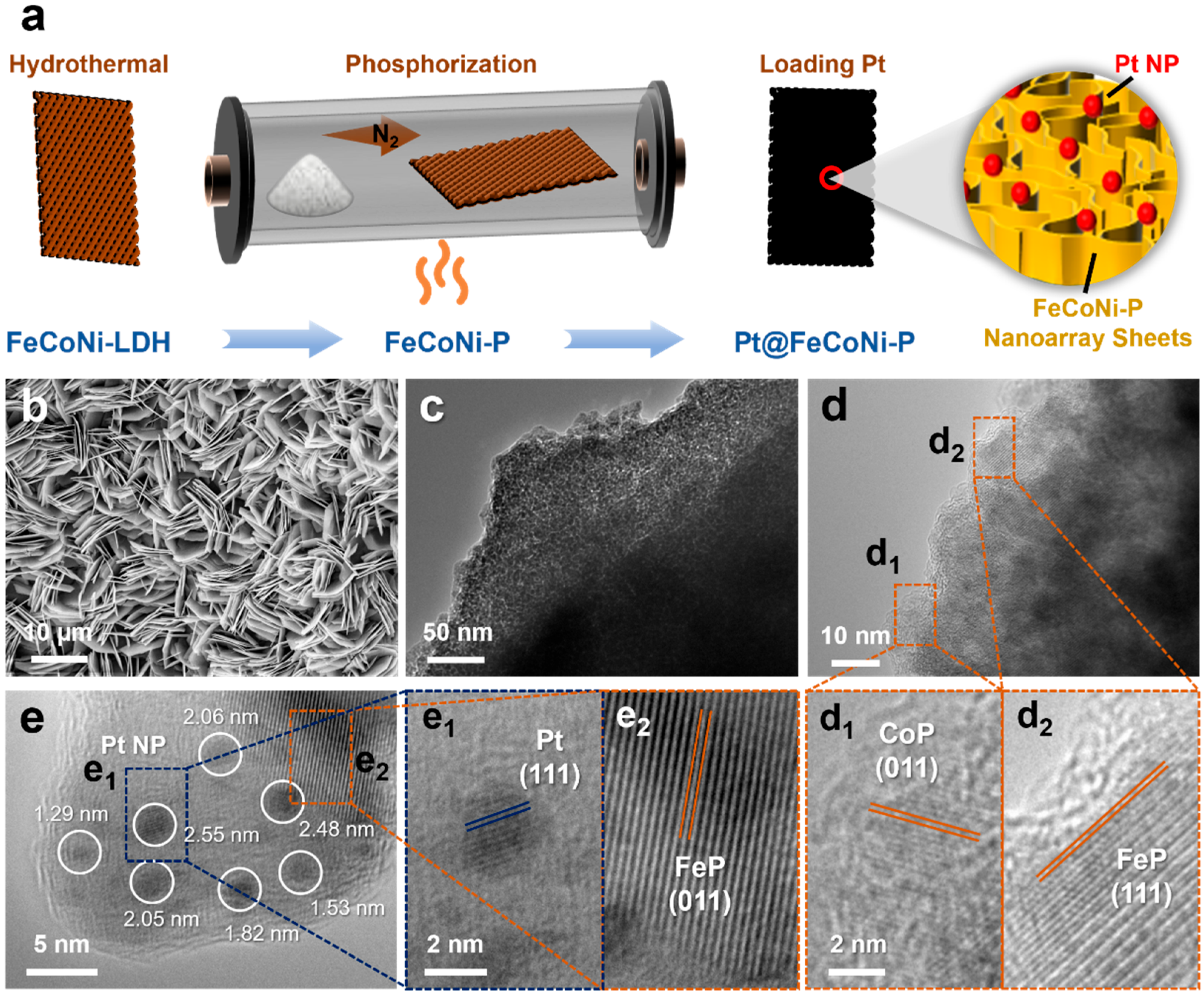
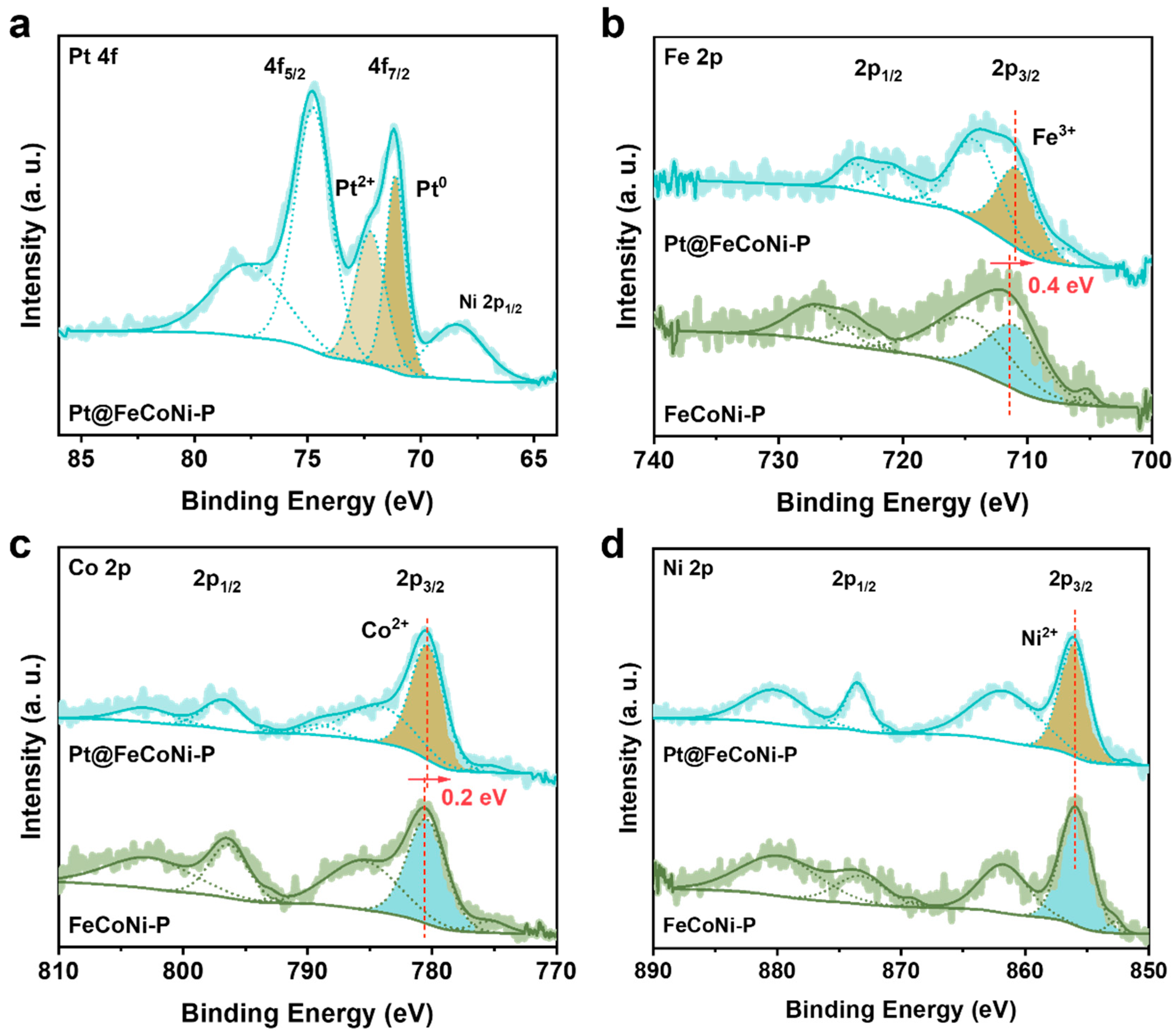
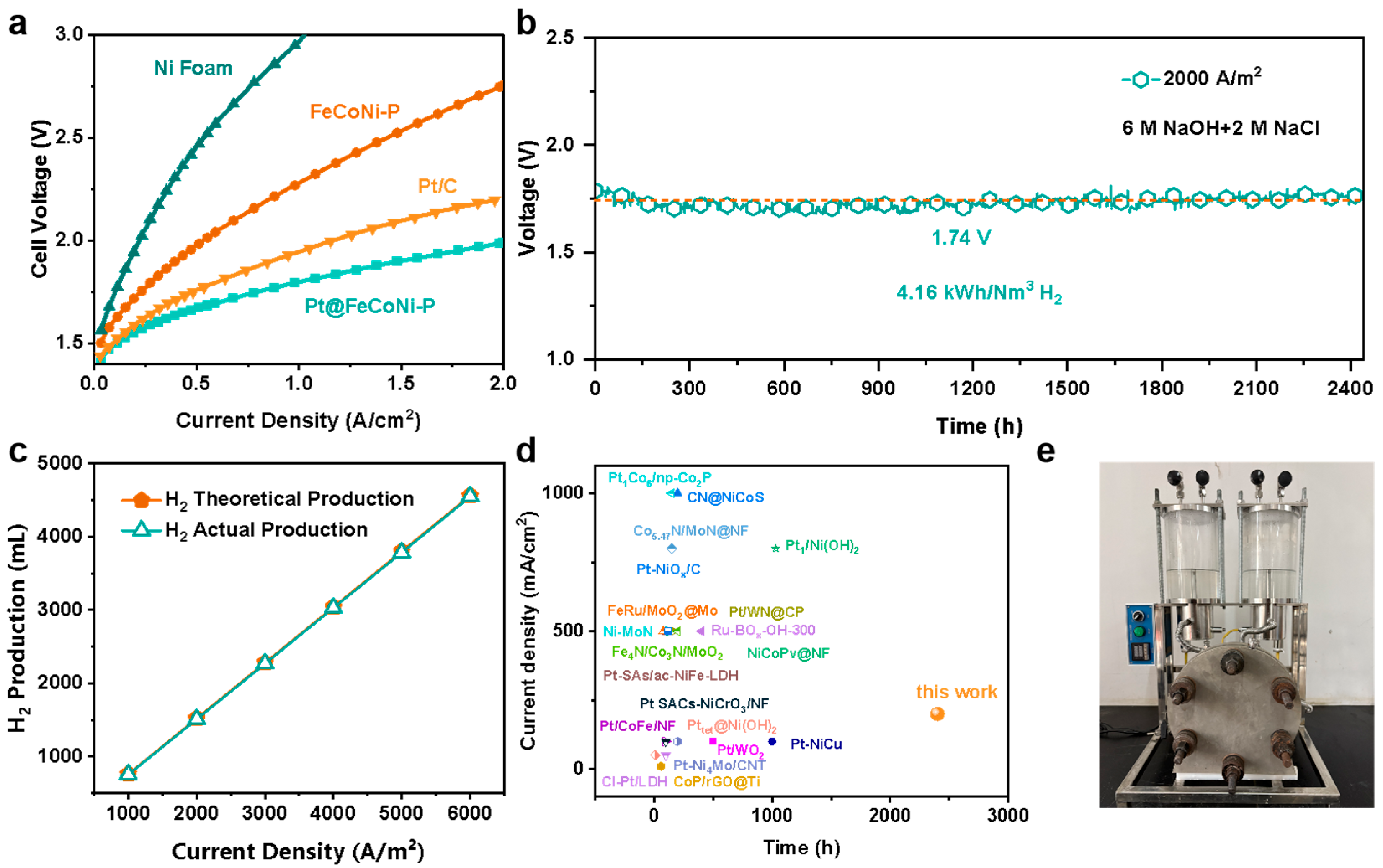
2. Results
3. Material and Methods
3.1. Chemicals
3.2. Synthesis of Pt@FeCoNi-P
- Step 1: Synthesis of NiCoFe-LDH precursor
- Step 2: Phosphidation to form NiCoFe-P
- Step 3: Platinum deposition
3.3. Materials Characterization
3.4. Electrochemical Measurements
3.5. Performance Evaluation of Water Electrolysis Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, G.R.; Kim, J.; Hong, D.; Kim, Y.J.; Jang, H.; Han, H.J.; Hwang, C.-K.; Kim, D.; Kim, J.Y.; Jung, Y.S. Efficient and sustainable water electrolysis achieved by excess electron reservoir enabling charge replenishment to catalysts. Nat. Commun. 2023, 14, 5402. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Y.; Duan, Y.; Feng, X.Y.; Yu, X.; Gao, M.R.; Yu, S.H. Clean and affordable hydrogen fuel from alkaline water splitting: Past, recent progress, and future prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Z.; Liu, T.; Wu, Y.; Lan, C.; Jiang, W.; Zhu, L.; Wang, Y.; Yang, D.; Shao, Z. A membrane-based seawater electrolyser for hydrogen generation. Nature 2022, 612, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Wen, N.; Zhang, D.; Zhao, X.; Jiao, X.; Xia, Y.; Chen, D. Polarization Manipulation of NiO Nanosheets Engineered with Fe/Pt Single Atoms for High-Performance Electrocatalytic Overall Alkaline Seawater Splitting. ACS Catal. 2023, 13, 7868–7878. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Z.; Cheng, S.; Liu, Y.; Deng, B.; Xu, S.; Yu, L.; Yu, Y. Challenges and strategies of chlorine inhibition in anode systems for seawater electrolysis. Sci. China Chem. 2024, 67, 3198–3208. [Google Scholar] [CrossRef]
- Fang, Q.-Y.; Gu, J.-J.; He, R.-X.; Ni, W.-W.; Ran, J.; Deng, N.; He, J.-B. Direct seawater electrolysis through in situ purification using dual ion exchange membranes. J. Solid. State Electrochem. 2025, 1–9. [Google Scholar] [CrossRef]
- Yu, Y.; Li, J.; Luo, J.; Kang, Z.; Jia, C.; Liu, Z.; Huang, W.; Chen, Q.; Deng, P.; Shen, Y.; et al. Mo-decorated cobalt phosphide nanoarrays as bifunctional electrocatalysts for efficient overall water/seawater splitting. Mater. Today Nano 2022, 18, 100216. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J.; Moussab, H. Recent advances on hydrogen production through seawater electrolysis. Mater. Sci. Energy Technol. 2020, 3, 780–807. [Google Scholar] [CrossRef]
- Gao, F.-Y.; Yu, P.-C.; Gao, M.-R. Seawater electrolysis technologies for green hydrogen production: Challenges and opportunities. Curr. Opin. Chem. Eng. 2022, 36, 100827. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, W.; Peng, Y.; Guo, Y.; Zhou, J.; Wang, S.; Yuan, J.; Liao, Y.; Liu, L.; Zhang, Y.; et al. Corrosion-resistant single-atom catalysts for direct seawater electrolysis. Natl. Sci. Rev. 2025, 12, nwaf060. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, Z.; Ge, A.; Liu, L.; Faria, J.L.; Xu, G.; Zhu, M. Direct seawater splitting for hydrogen production: Recent advances in materials synthesis and technological innovation. Green Energy Environ. 2025, 10, 11–33. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Liu, J.; Zhai, Z.; Yang, Z.; Xia, J.; Deng, S.; Qu, X.; Zhang, H.; Wu, D.; et al. Mo-modified band structure and enhanced photocatalytic properties of tin oxide quantum dots for visible-light driven degradation of antibiotic contaminants. J. Environ. Chem. Eng. 2022, 10, 107091. [Google Scholar] [CrossRef]
- Liu, D.; Ai, H.; Chen, M.; Zhou, P.; Li, B.; Liu, D.; Du, X.; Lo, K.H.; Ng, K.-W.; Wang, S.-P.; et al. Multi-Phase Heterostructure of CoNiP/CoxP for Enhanced Hydrogen Evolution Under Alkaline and Seawater Conditions by Promoting H2O Dissociation. Small 2021, 17, 2007557. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-H.; Wang, T.-J.; Sun, B.; Hong, Q.-L.; Ai, X.; Li, S.-N.; Bai, J.; Chen, Y. Recent advances in platinum group metallenes: From synthesis to energy-related electrocatalytic application. Appl. Catal. B Environ. Energy 2025, 366, 125041. [Google Scholar] [CrossRef]
- Wang, J.; Yue, X.; Yang, Y.; Sirisomboonchai, S.; Wang, P.; Ma, X.; Abudula, A.; Guan, G. Earth-abundant transition-metal-based bifunctional catalysts for overall electrochemical water splitting: A review. J. Alloys Compd. 2020, 819, 153346. [Google Scholar] [CrossRef]
- Liang, L.; Jin, H.; Zhou, H.; Liu, B.; Hu, C.; Chen, D.; Zhu, J.; Wang, Z.; Li, H.-W.; Liu, S.; et al. Ultra-small platinum nanoparticles segregated by nickle sites for efficient ORR and HER processes. J. Energy Chem. 2022, 65, 48–54. [Google Scholar] [CrossRef]
- Tian, J.; Xia, M.; Cheng, X.; Mao, C.; Chen, Y.; Zhang, Y.; Zhou, C.; Xu, F.; Yang, L.; Wang, X.-Z.; et al. Understanding Pt Active Sites on Nitrogen-Doped Carbon Nanocages for Industrial Hydrogen Evolution with Ultralow Pt Usage. J. Am. Chem. Soc. 2024, 146, 33640–33650. [Google Scholar] [CrossRef]
- Luo, Y.; Qiao, Y.; He, H.; Chen, M.; Liu, G.; Liu, X.; Pan, L.; Feng, Z.; Tan, R. Rapid synthesis of PtRu nanoparticles on aniline-modified SWNTs in EMSI engineering for enhanced alkaline water electrolysis. J. Power Sources 2025, 631, 236297. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Y.; Zhu, J.; Tian, X.; Liu, G.; Feng, Z.; Pan, L.; Liu, X.; Han, N.; Tan, R. Material Engineering Strategies for Efficient Hydrogen Evolution Reaction Catalysts. Small Methods 2024, 8, e2400158. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, P.; Li, Z.; Lin, Y.; Li, M.; Zi, J.; Shi, H.; Li, G.; Lian, Z.; Li, H. Tuning the Cobalt–Platinum Alloy Regulating Single-Atom Platinum for Highly Efficient Hydrogen Evolution Reaction. Adv. Funct. Mater. 2022, 32, 2205920. [Google Scholar] [CrossRef]
- Fu, L.; Li, M.; Pan, T.; Li, X.; Zhan, X.; Tong, X.; Hu, C.; Tian, J. Enhanced stability and activity of platinum-based catalyst using iron-nitrogen co-doped graphene as support for oxygen reduction reaction. Int. J. Hydrogen Energy 2024, 58, 1204–1213. [Google Scholar] [CrossRef]
- Wang, G.; Zou, H.; Zhang, H.; Xiang, J.; Yang, M.; Liu, J. Carbon-supported FeCoNiP/FeCoNi Mott-Schottky electrocatalyst for alkaline oxygen evolution reaction. Electrochim. Acta 2023, 462, 142723. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Cao, L.; Chen, Y.; Zhu, E.; Lin, Z.; Li, M.; Yan, A.; Zettl, A.; Wang, Y.M. High-performance transition metal–doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Liu, W.; Li, T.; Ding, X.; Xu, W.; Ma, M.; Zhou, D.; Li, Y.; Sun, X. One-step synthesis of ultrathin high-entropy layered double hydroxides for ampere-level water oxidation. Catalysts 2024, 14, 171. [Google Scholar] [CrossRef]
- Yu, T.; Liu, G.; Nie, T.; Wu, Z.; Song, Z.; Sun, X.; Song, Y.-F. Pt-Loaded CoFe-Layered Double Hydroxides for Simultaneously Driving HER and HzOR. ACS Catal. 2024, 14, 14937–14946. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Y.; Liu, J.; Wang, F. Adding refractory 5d transition metal W into PtCo system: An advanced ternary alloy for efficient oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 10700–10709. [Google Scholar] [CrossRef]
- Liu, W.; Guo, X.; Jiang, Z.; Yu, J.; Zhou, L.; Li, T.; Guo, Y.; Li, S.; Ding, B.; Wang, K. Cl−Boosted Active and Stable Seawater Reduction on Pt/CoP Nanoarray Electrocatalysts. Adv. Energy Mater. 2025, 15, 2404978. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Hegazy, M.; Mohamed, I.; Youssef, Y.; Ibrahim, M. Green Hydrogen Gas Production Using an Adapted Electrolysis Method. Egypt. J. Chem. 2023, 66, 241–254. [Google Scholar] [CrossRef]
- Bao, D.; Huang, L.; Gao, Y.; Davey, K.; Zheng, Y.; Qiao, S.-Z. Dynamic Creation of a Local Acid-like Environment for Hydrogen Evolution Reaction in Natural Seawater. J. Am. Chem. Soc. 2024, 146, 34711–34719. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Chen, X.; Wen, Y.; Chen, H.; Zhang, S.; Yang, H.; Li, W.; Zhou, L.; Xu, B.; Xu, W.; et al. Solidophobic Surface for Electrochemical Extraction of High-Valued Mg(OH)2 Coupled with H2 Production from Seawater. Nano Lett. 2024, 24, 5920–5928. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Fan, H.; Liu, Q.; Yan, Z.; Wang, A.; Yang, B.; Wang, E. Engineering Interfacial Sulfur Migration in Transition-Metal Sulfide Enables Low Overpotential for Durable Hydrogen Evolution in Seawater. Nat. Commun. 2024, 15, 6154. [Google Scholar] [CrossRef]
- Guo, L.; Chi, J.; Cui, T.; Zhu, J.; Xia, Y.; Guo, H.; Lai, J.; Wang, L. Phosphorus Defect Mediated Electron Redistribution to Boost Anion Exchange Membrane-Based Alkaline Seawater Electrolysis. Adv. Energy Mater. 2024, 14, 2400975. [Google Scholar] [CrossRef]
- Shen, L.-W.; Wang, Y.; Shen, L.; Chen, J.-B.; Liu, Y.; Hu, M.-X.; Zhao, W.-Y.; Xiong, K.-Y.; Wu, S.-M.; Lu, Y.; et al. Ruthenium nanoparticles decorated with surface hydroxyl and borate species boost overall seawater splitting via increased hydrophilicity. Energy Environ. Sci. 2024, 17, 3888–3897. [Google Scholar] [CrossRef]
- Xu, X.; Lu, Y.; Shi, J.; Hao, X.; Ma, Z.; Yang, K.; Zhang, T.; Li, C.; Zhang, D.; Huang, X.; et al. Corrosion-Resistant Cobalt Phosphide Electrocatalysts for Salinity Tolerance Hydrogen Evolution. Nat. Commun. 2023, 14, 7708. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, F.; Song, S.; Ning, M.; Zhu, Q.; Zhou, J.; Gao, G.; Chen, Z.; Zhou, Q.; Xing, X.; et al. Efficient Alkaline Water/Seawater Hydrogen Evolution by a Nanorod-Nanoparticle-Structured Ni-MoN Catalyst with Fast Water-Dissociation Kinetics. Adv. Mater. 2022, 34, 2201774. [Google Scholar] [CrossRef]
- Wang, B.; Luo, Z.; Sun, H.; Chen, M.; Zhang, Y.; Zhao, X.; Qiu, G.; Xiao, B.; Zhou, T.; Lu, Q.; et al. Electronic Modulation of Amorphous/Crystalline Nife Ldh by Atomic Pt Loading Enabling Industrial Hydrogen Production in Alkaline Water And Seawater. J. Energy Chem. 2025, 107, 427–439. [Google Scholar] [CrossRef]
- Geng, S.; Chen, L.; Wu, Y.; Wang, Y.; Song, S. Lattice-Matched Metal/Wn Catalyst with Highly Oxygenophilic W Sites for Hydrogen Production in Seawater Electrolyzer. J. Energy Chem. 2025, 105, 302–311. [Google Scholar] [CrossRef]
- Huo, M.; Sun, X.; Sun, J.; Li, Q.; Zhang, X.; Gu, X.; Xing, Z.; Chang, J. High-Performance FeRu Alloy Electrocatalyst Integrated with a Mo Substrate for Hydrogen Evolution Reaction in Seawater. Small 2025, 2412729. [Google Scholar] [CrossRef]
- Shen, X.; Li, H.; Ma, T.; Jiao, Q.; Zhao, Y.; Li, H.; Feng, C. Construction of Heterojunction-Rich Metal Nitrides Porous Nanosheets Electrocatalyst for Alkaline Water/Seawater Splitting at Large Current Density. Small 2024, 20, 2310535. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Li, D.; Zhang, Y.; Zhang, Y.; Yu, F.; Yang, L.; Wang, X.; Tang, D.; Zhou, H. Complementary Multisite Turnover Catalysis toward Superefficient Bifunctional Seawater Splitting at Ampere-Level Current Density. Adv. Mater. 2024, 36, 2405852. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zheng, X.; Gao, M.; Liu, Y.; Pan, H.; Sun, W. Platinum-Nickel Oxide Cluster-Cluster Heterostructure Enabling Fast Hydrogen Evolution for Anion Exchange Membrane Water Electrolyzers. Angew. Chem. Int. Ed. 2025, 64, e202422062. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Gao, P.; Shen, Y.; Qiao, L.; Ma, M.; Guo, X.; Cheng, D. Fabricating Lattice-Confined Pt Single Atoms with High Electron-Deficient State for Alkali Hydrogen Evolution Under Industrial-Current Density. Adv. Mater. 2025, 37, 2414138. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, J.; Chen, J.; Fang, Y.; Han, X.; Chen, J.; Li, Y.; Wang, Y.; Liu, J.; Wang, L. Pinpointing the axial ligand effect on platinum single-atom-catalyst towards efficient alkaline hydrogen evolution reaction. Nat. Commun. 2022, 13, 6875. [Google Scholar] [CrossRef]
- Wan, C.; Zhang, Z.; Dong, J.; Xu, M.; Pu, H.; Baumann, D.; Lin, Z.; Wang, S.; Huang, J.; Shah, A.H.; et al. Amorphous nickel hydroxide shell tailors local chemical environment on platinum surface for alkaline hydrogen evolution reaction. Nat. Mater. 2023, 22, 1022–1029. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, Z.; Wang, Z.; Xie, F.; Yuan, X.; Tan, Y. Manipulating Interfacial Water Via Metallic Pt1Co6 Sites on Self-Adaptive Metal Phosphides to Enhance Water Electrolysis. Adv. Mater. 2025, 37, 2419644. [Google Scholar] [CrossRef]
- Meng, F.; Zhu, L.; Li, R.; Jiang, J.; Li, Y.; Wu, Y.; Fan, Y.; Ren, P.; Xu, H.; Wang, D.; Zhang, J.; An, M.; Yang, P. Engineering Metal-Support Interaction for Manipulate Microenvironment: Single-Atom Platinum Decorated on Nickel-Chromium Oxides Toward High-Performance Alkaline Hydrogen Evolution. Adv. Funct. Mater. 2025, 35, 2416678. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Wu, X.; Zhang, D.; Zhang, Y.; Xiong, J.; Wu, Z.; Lai, J.; Wang, L. Pt doping and strong metal–support interaction as a strategy for NiMo-based electrocatalysts to boost the hydrogen evolution reaction in alkaline solution. J. Mater. Chem. A 2022, 10, 15395–15401. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, H.; Shen, Z.; Li, X.; Fang, J.; Sun, H.; Deng, C.; Zhou, L.; Kuang, Y. Direct 2400 h Seawater Electrolysis Catalyzed by Pt-Loaded Nanoarray Sheets. Catalysts 2025, 15, 634. https://doi.org/10.3390/catal15070634
Xin H, Shen Z, Li X, Fang J, Sun H, Deng C, Zhou L, Kuang Y. Direct 2400 h Seawater Electrolysis Catalyzed by Pt-Loaded Nanoarray Sheets. Catalysts. 2025; 15(7):634. https://doi.org/10.3390/catal15070634
Chicago/Turabian StyleXin, Huijun, Zudong Shen, Xiaojie Li, Jinjie Fang, Haoran Sun, Chen Deng, Linlin Zhou, and Yun Kuang. 2025. "Direct 2400 h Seawater Electrolysis Catalyzed by Pt-Loaded Nanoarray Sheets" Catalysts 15, no. 7: 634. https://doi.org/10.3390/catal15070634
APA StyleXin, H., Shen, Z., Li, X., Fang, J., Sun, H., Deng, C., Zhou, L., & Kuang, Y. (2025). Direct 2400 h Seawater Electrolysis Catalyzed by Pt-Loaded Nanoarray Sheets. Catalysts, 15(7), 634. https://doi.org/10.3390/catal15070634





