Synergistic Integration of g-C3N4 with SnS: Unlocking Enhanced Photocatalytic Efficiency and Electrochemical Stability for Dual-Functional Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. X-Ray Diffraction (XRD)
2.2. Optical Properties
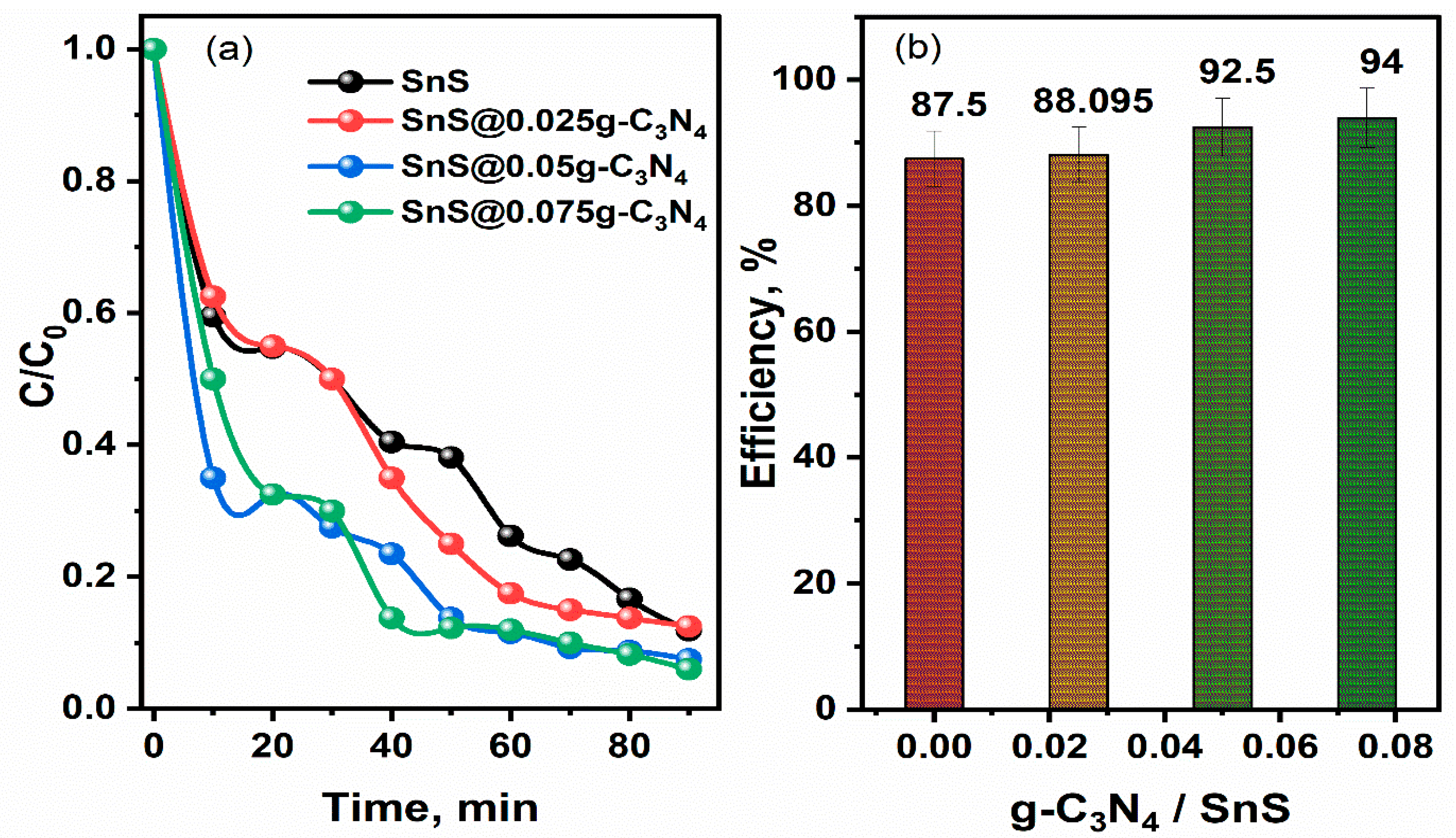
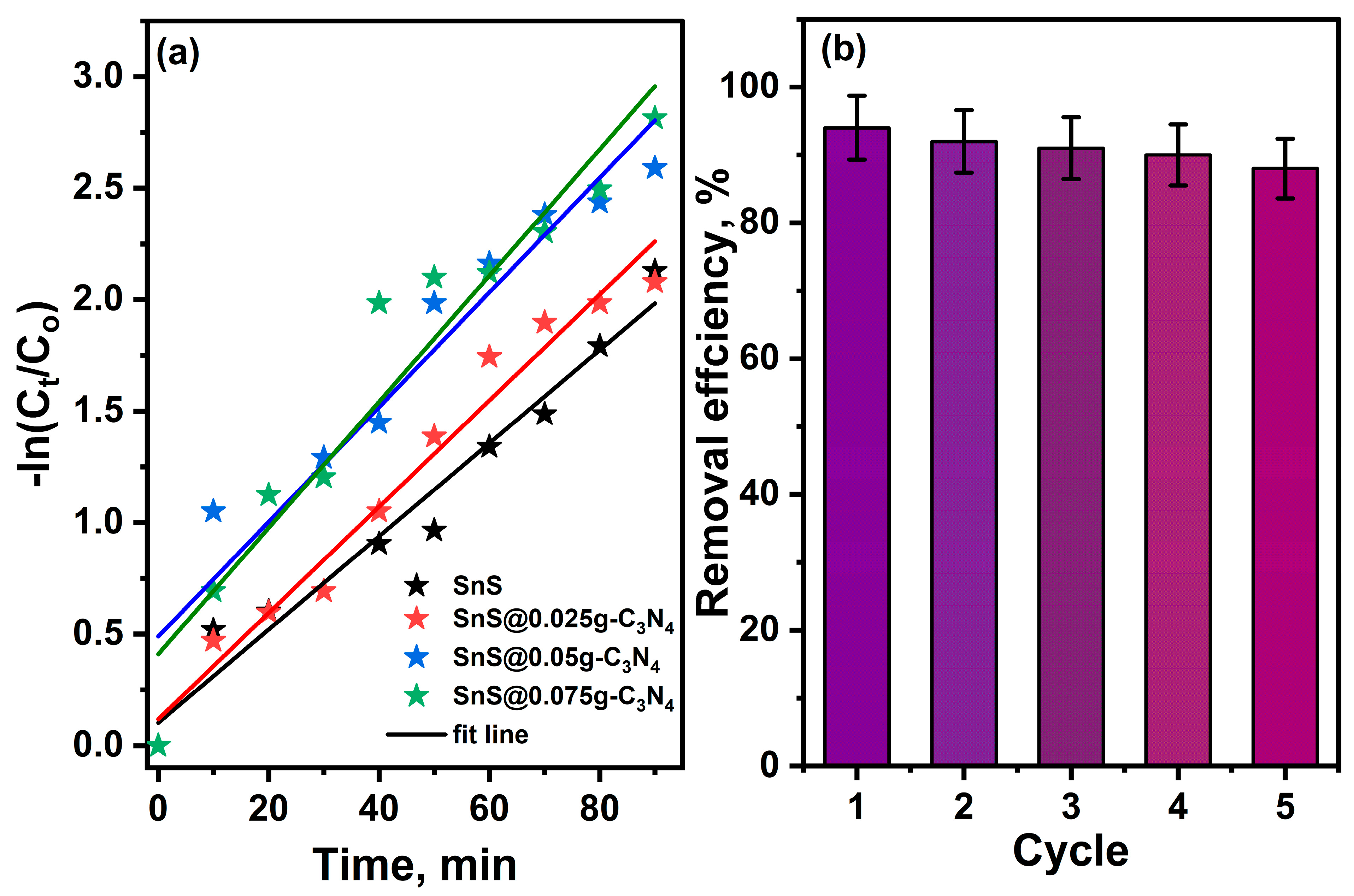
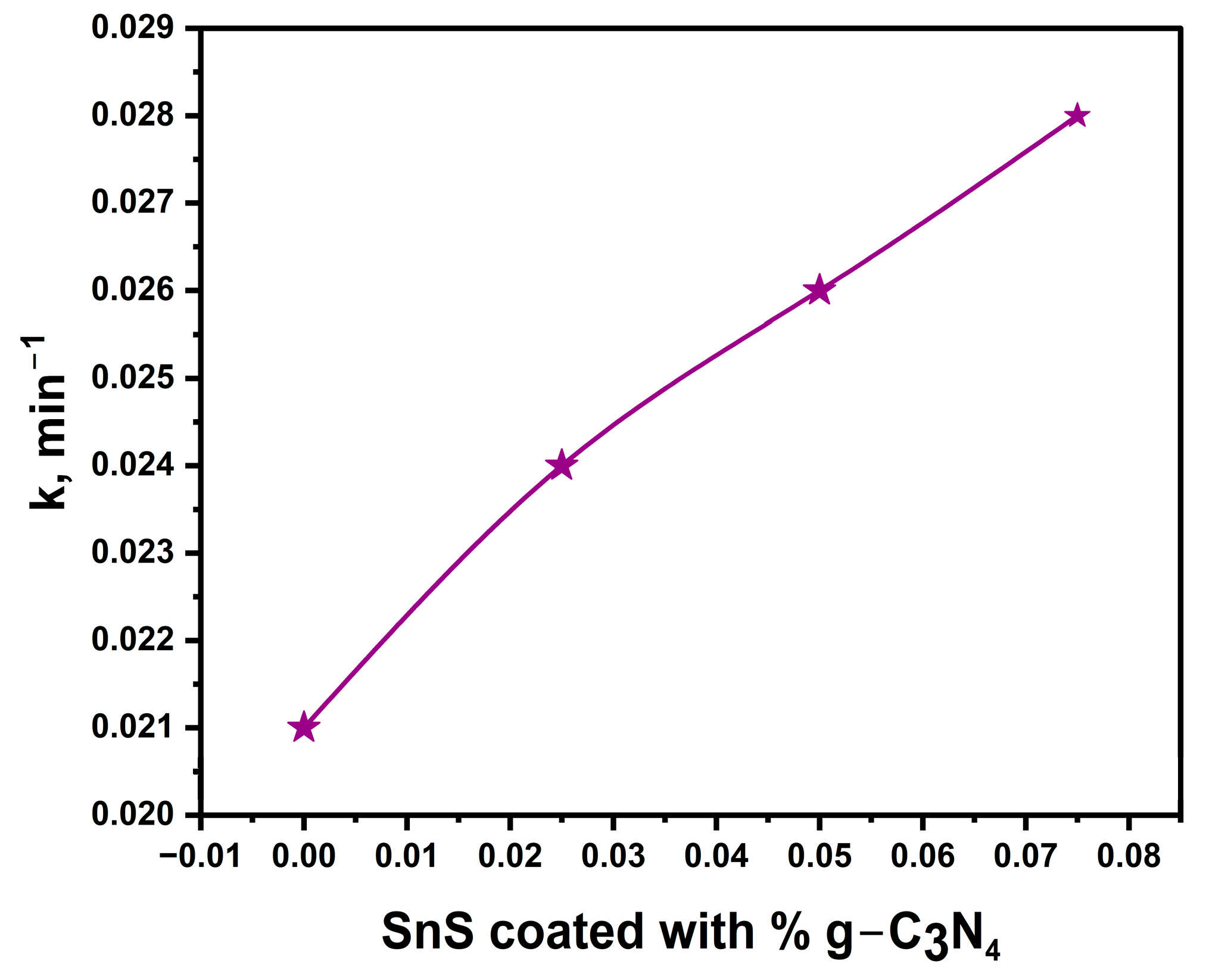
2.3. Photocatalytic Performance
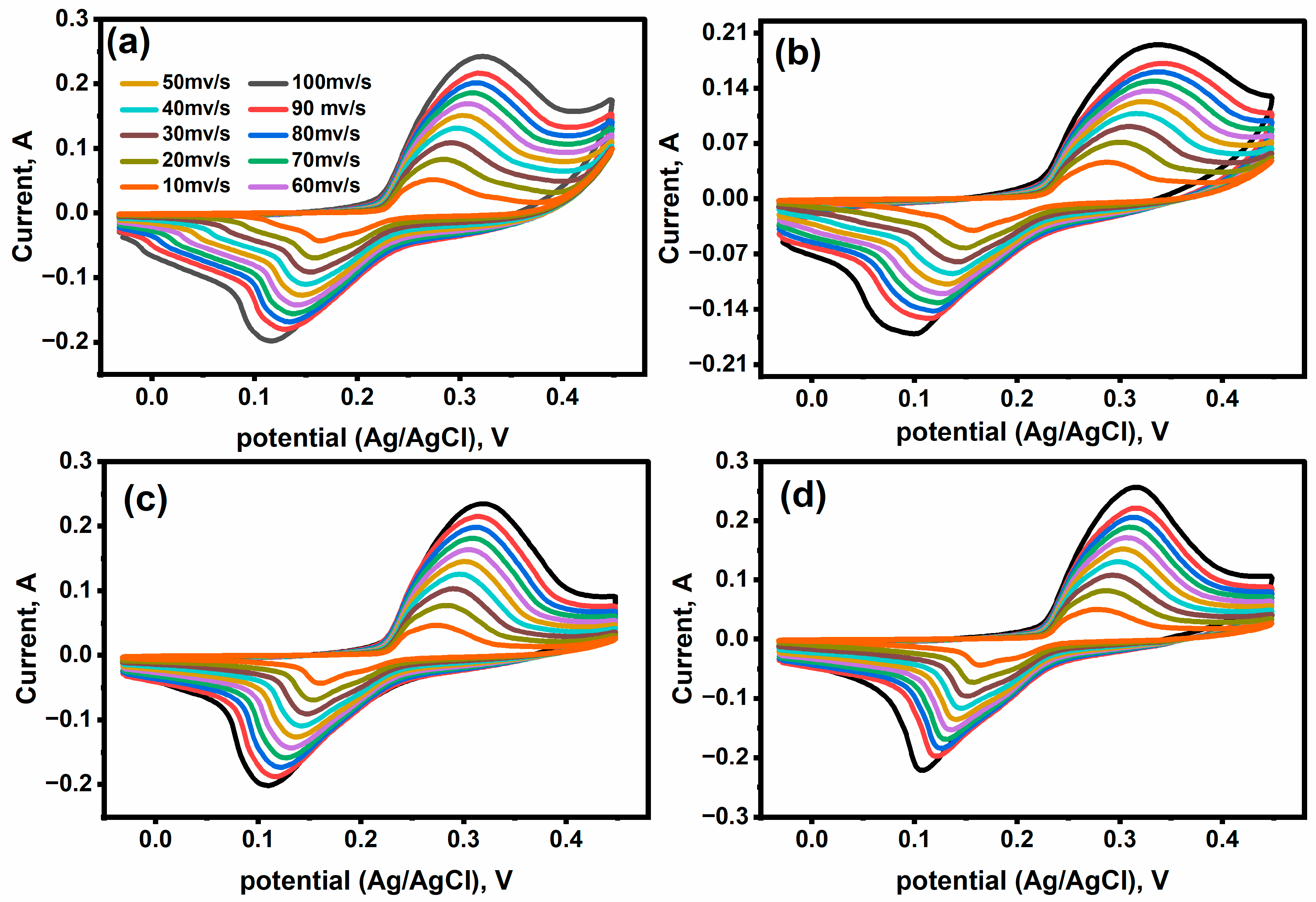
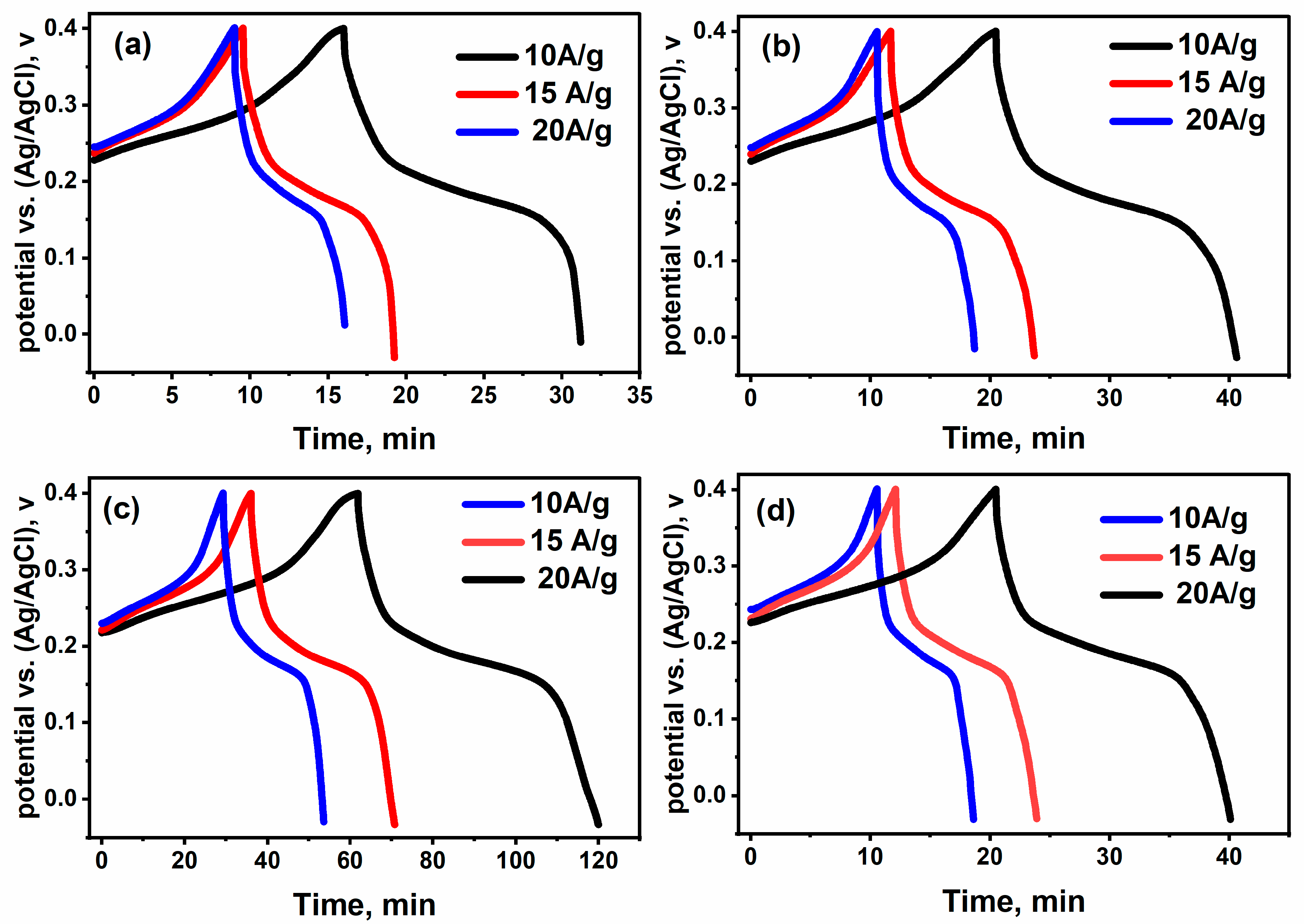
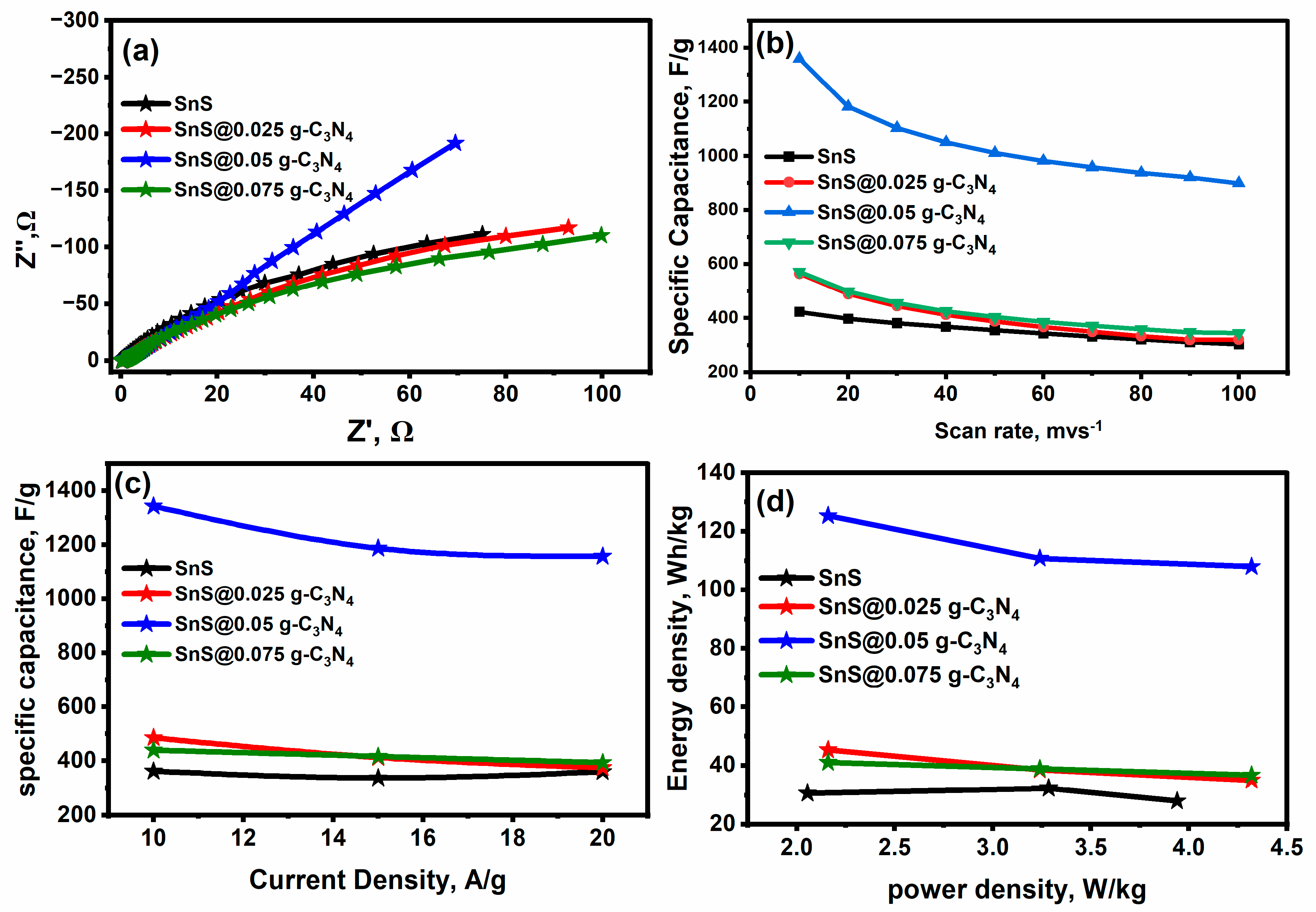
2.4. Electrochemical (Supercapacitor) Performance
3. Experimental Technique
3.1. Materials and Reagents
3.2. Procedure Synthesis
3.3. Characterization
3.4. Evaluation of Photocatalytic Activity in SnS Coated with g-C3N4
3.5. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, J.; Thakur, A.; Goyal, A. Industrial wastewater and its toxic effects. In Chemistry in the Environment: Biological Treatment of Industrial Wastewater; Shah, M.P., Ed.; The Royal Society of Chemistry: London, UK, 2021; pp. 1–14. [Google Scholar]
- Singh, A. A review of wastewater irrigation: Environmental implications. Resour. Conserv. Recycl. 2021, 168, 105454. [Google Scholar] [CrossRef]
- Ethiraj, S.; Samuel, M.S.; Indumathi, S.M. A comprehensive review of the challenges and opportunities in microalgae-based wastewater treatment for eliminating organic, inorganic, and emerging pollutants. Biocatal. Agric. Biotechnol. 2024, 60, 103316. [Google Scholar] [CrossRef]
- Yasasve, M.; Manjusha, M.; Manojj, D.; Hariharan, N.; Preethi, P.S.; Asaithambi, P.; Karmegam, N.; Saravanan, M. Unravelling the emerging carcinogenic contaminants from industrial waste water for prospective remediation by electrocoagulation—A review. Chemosphere 2022, 307, 136017. [Google Scholar] [CrossRef]
- Mahlambi, M.M.; Ngila, C.J.; Mamba, B.B.; Zhang, X. Recent Developments in Environmental Photocatalytic Degradation of Organic Pollutants: The Case of Titanium Dioxide Nanoparticles—A Review. J. Nanomater. 2015, 2015, 790173. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of Titanium Dioxide (TiO2) Modification on Its Application to Pollution Treatment—A Review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Graimed, B.H. Recent developments in industrial organic degradation via semiconductor heterojunctions and the parameters affecting the photocatalytic process: A review study. J. Water Process. Eng. 2022, 47, 102671. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef]
- Di, J.; Xiong, J.; Li, H.; Liu, Z. Ultrathin 2D photocatalysts: Electronic-structure tailoring, hybridization, and applications. Adv. Mater. 2018, 30, 1704548. [Google Scholar] [CrossRef]
- Batool, M.; Sanumi, O.; Jankovic, J. Application of artificial intelligence in the materials science, with a special focus on fuel cells and electrolyzers. Energy AI 2024, 18, 100424. [Google Scholar] [CrossRef]
- Mak, C.H.; Han, X.; Du, M.; Kai, J.-J.; Tsang, K.F.; Jia, G.; Cheng, K.-C.; Shen, H.-H.; Hsu, H.-Y. Heterogenization of homogeneous photocatalysts utilizing synthetic and natural support materials. J. Mater. Chem. A 2021, 9, 4454–4504. [Google Scholar] [CrossRef]
- Carminati, S.A.; Rodríguez-Gutiérrez, I.; de Morais, A.; da Silva, B.L.; Melo, M.A.; Souza, F.L.; Nogueira, A.F. Challenges and prospects about the graphene role in the design of photoelectrodes for sunlight-driven water splitting. RSC Adv. 2021, 11, 14374–14398. [Google Scholar] [CrossRef]
- Lah, N.A.C. Late transition metal nanocomplexes: Applications for renewable energy conversion and storage. Renew. Sustain. Energy Rev. 2021, 145, 111103. [Google Scholar] [CrossRef]
- Norton, K.J.; Alam, F.; Lewis, D.J. A Review of the Synthesis, Properties, and Applications of Bulk and Two-Dimensional Tin (II) Sulfide (SnS). Appl. Sci. 2021, 11, 2062. [Google Scholar] [CrossRef]
- Pejjai, B.; Reddy, V.R.M.; Gedi, S.; Park, C. Status review on earth-abundant and environmentally green Sn-X (X = Se, S) nanoparticle synthesis by solution methods for photovoltaic applications. Int. J. Hydrogen Energy 2017, 42, 2790–2831. [Google Scholar] [CrossRef]
- Zhou, T.; Pang, W.K.; Zhang, C.; Yang, J.; Chen, Z.; Liu, H.K.; Guo, Z. Enhanced Sodium-Ion Battery Performance by Structural Phase Transition from Two-Dimensional Hexagonal-SnS2 to Orthorhombic-SnS. ACS Nano 2014, 8, 8323–8333. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Bhanderi, D.; Lakhani, P.; Modi, C.K. Graphitic carbon nitride (g-C3N4) as an emerging photocatalyst for sustainable environmental applications: A comprehensive review. RSC Sustain. 2023, 2, 265–287. [Google Scholar] [CrossRef]
- Ai, L.; Shi, R.; Yang, J.; Zhang, K.; Zhang, T.; Lu, S. Efficient combination of g-C3N4 and CDs for enhanced photocatalytic performance: A review of synthesis, strategies, and applications. Small 2021, 17, 2007523. [Google Scholar] [CrossRef]
- Wen, Y.; Sun, Y.; Liu, Y.; Zhao, M.; Zhao, L. Green synthesis of 2% g-C3N4/SnS2-V3/CQD1 composite photocatalyst from waste plant soot for efficient U(VI) removal: Mechanistic insights. Chem. Eng. J. 2024, 494, 153247. [Google Scholar] [CrossRef]
- Hegde, S.; Bose, R.S.C.; Surendra, B.; Vinoth, S.; Murahari, P.; Ramesh, K. SnS-Nanocatalyst: Malachite green degradation and electrochemical sensor studies. Mater. Sci. Eng. B 2022, 283, 115818. [Google Scholar] [CrossRef]
- Xie, X.; Ge, P.; Xue, R.; Lv, H.; Xue, W.; Liu, E. Enhanced photocatalytic H2 evolution and anti-photocorrosion of sulfide photocatalyst by improving surface reaction: A review. Int. J. Hydrogen Energy 2023, 48, 24264–24284. [Google Scholar] [CrossRef]
- Ying, H.; Han, W.Q. Metallic Sn-based anode materials: Application in high-performance lithium-ion and sodium-ion batteries. Adv. Sci. 2017, 4, 1700298. [Google Scholar] [CrossRef]
- Mei, S.; An, W.; Fu, J.; Guo, W.; Feng, X.; Li, X.; Gao, B.; Zhang, X.; Huo, K.; Chu, P.K. Hierarchical micro-flowers self-assembled from SnS monolayers and nitrogen-doped graphene lamellar nanosheets as advanced anode for lithium-ion battery. Electrochimica Acta 2020, 331, 135292. [Google Scholar] [CrossRef]
- Jo, W.-K.; Lee, J.Y.; Selvam, N.C.S. Synthesis of MoS2 nanosheets loaded ZnO–g-C3N4 nanocomposites for enhanced photocatalytic applications. Chem. Eng. J. 2016, 289, 306–318. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total. Environ. 2021, 767, 144896. [Google Scholar] [CrossRef]
- Kumar, S.G.; Kavitha, R.; Manjunatha, C. Review and perspective on rational design and interface engineering of g-C3N4/ZnO: From type-II to step-scheme heterojunctions for photocatalytic applications. Energy Fuels 2023, 37, 14421–14472. [Google Scholar] [CrossRef]
- Aboraia, A.M.; Al-Omoush, M.; Solayman, M.; Saad, H.M.H.; Khabiri, G.; Saad, M.; Alsulaim, G.M.; Soldatov, A.V.; Ismail, Y.A.M.; Gomaa, H. A heterostructural MoS2QDs@UiO-66 nanocomposite for the highly efficient photocatalytic degradation of methylene blue under visible light and simulated sunlight. RSC Adv. 2023, 13, 34598–34609. [Google Scholar] [CrossRef]
- El-Maaref, A.A.; Hasaneen, M.F.; Alraddadi, S.; Ismail, Y.A.M.; Aboraia, A.M. The effect of rGO on the enhancement of photocatalytic activity of the CdS nanorods. J. Mater. Sci. Mater. Electron. 2024, 35, 2289. [Google Scholar] [CrossRef]
- Aboraia, A.M.; Almohammedi, A.; Alraddadi, S.; Taha, S.A.; Saad, M.; Sharaf, I.; Ismail, Y.A.M. Comparison study between as-synthesized ZnO and ZnO derived from ZiF-8 metalorganic framework in removing methylene blue. Mod. Phys. Lett. B 2024, 38, 2450221. [Google Scholar] [CrossRef]
- Kumar, G.G.; Reddy, K.; Nahm, K.S.; Angulakshmi, N.; Stephan, A.M. Synthesis and electrochemical properties of SnS as possible anode material for lithium batteries. J. Phys. Chem. Solids 2012, 73, 1187–1190. [Google Scholar] [CrossRef]
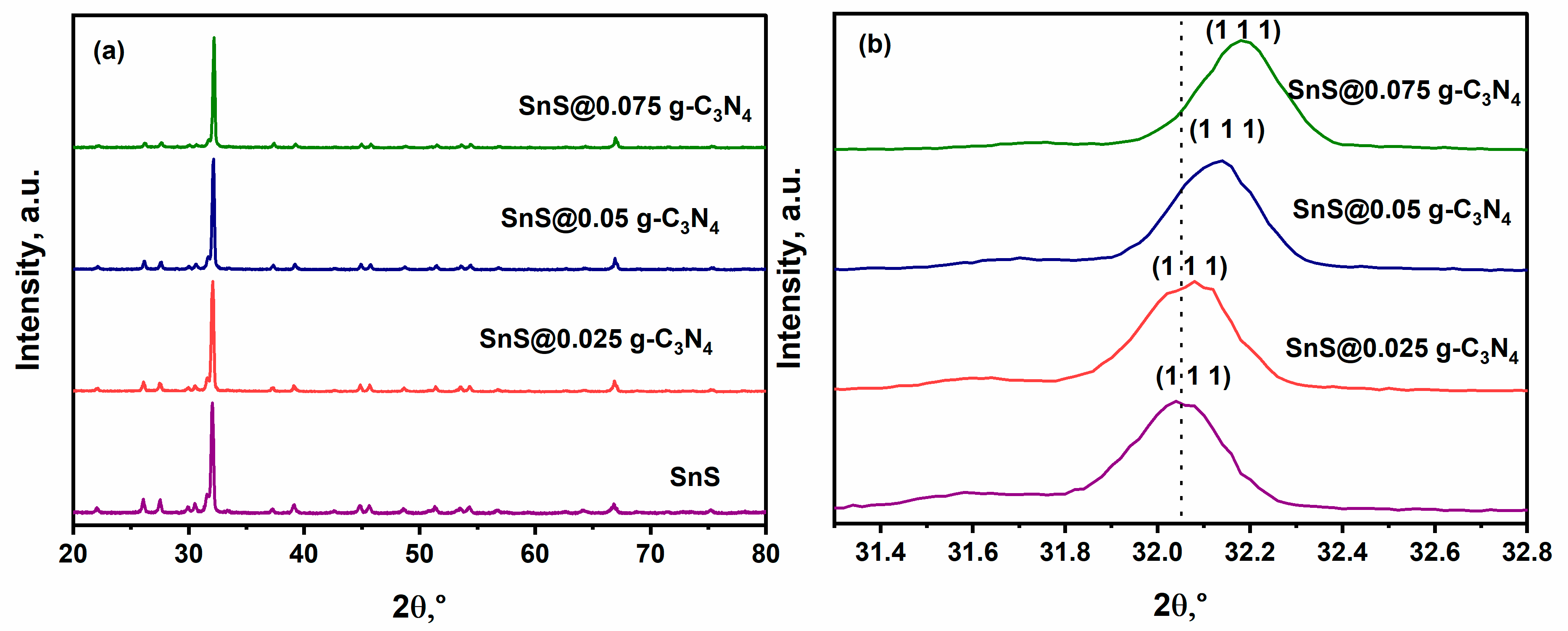
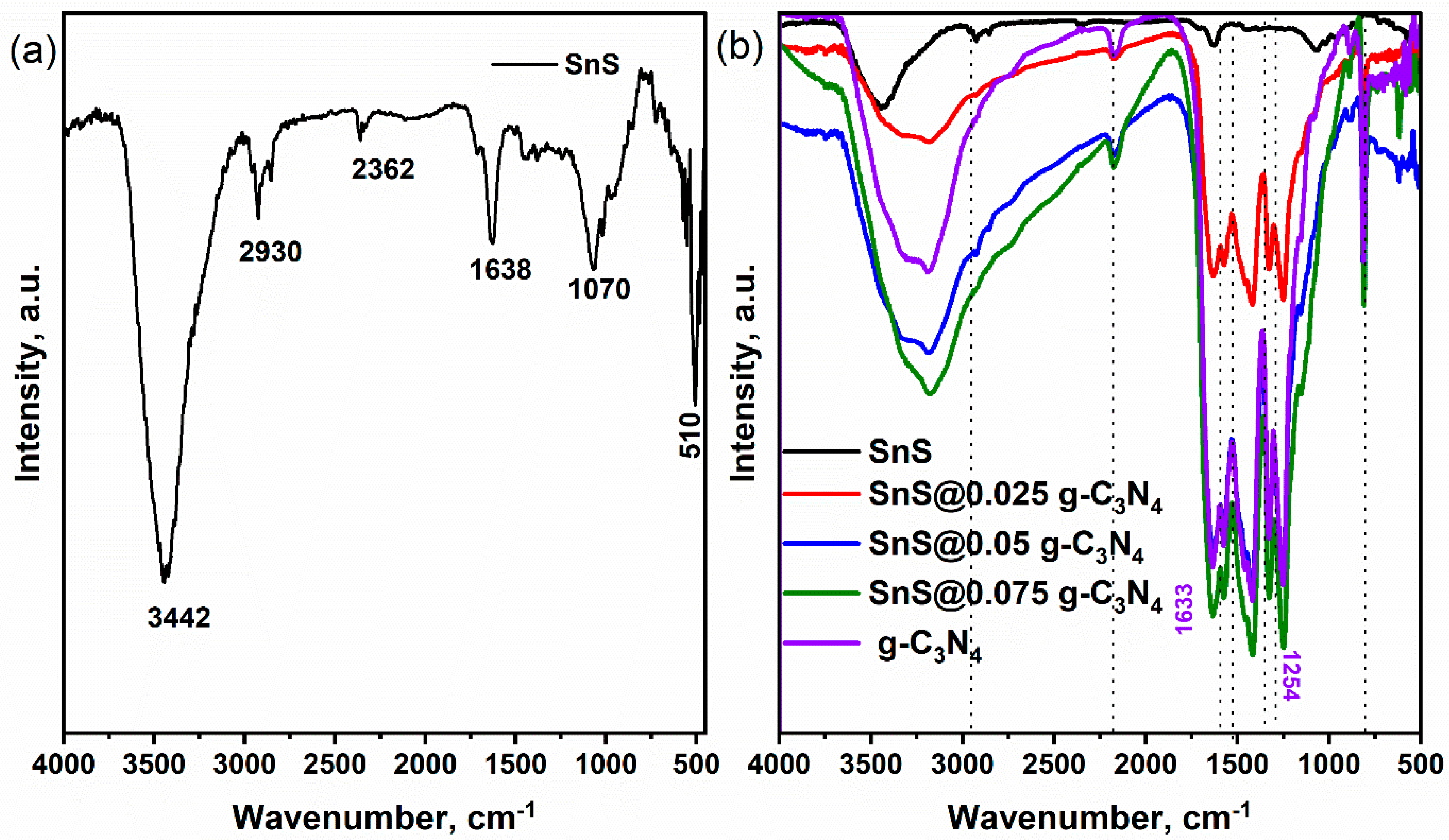

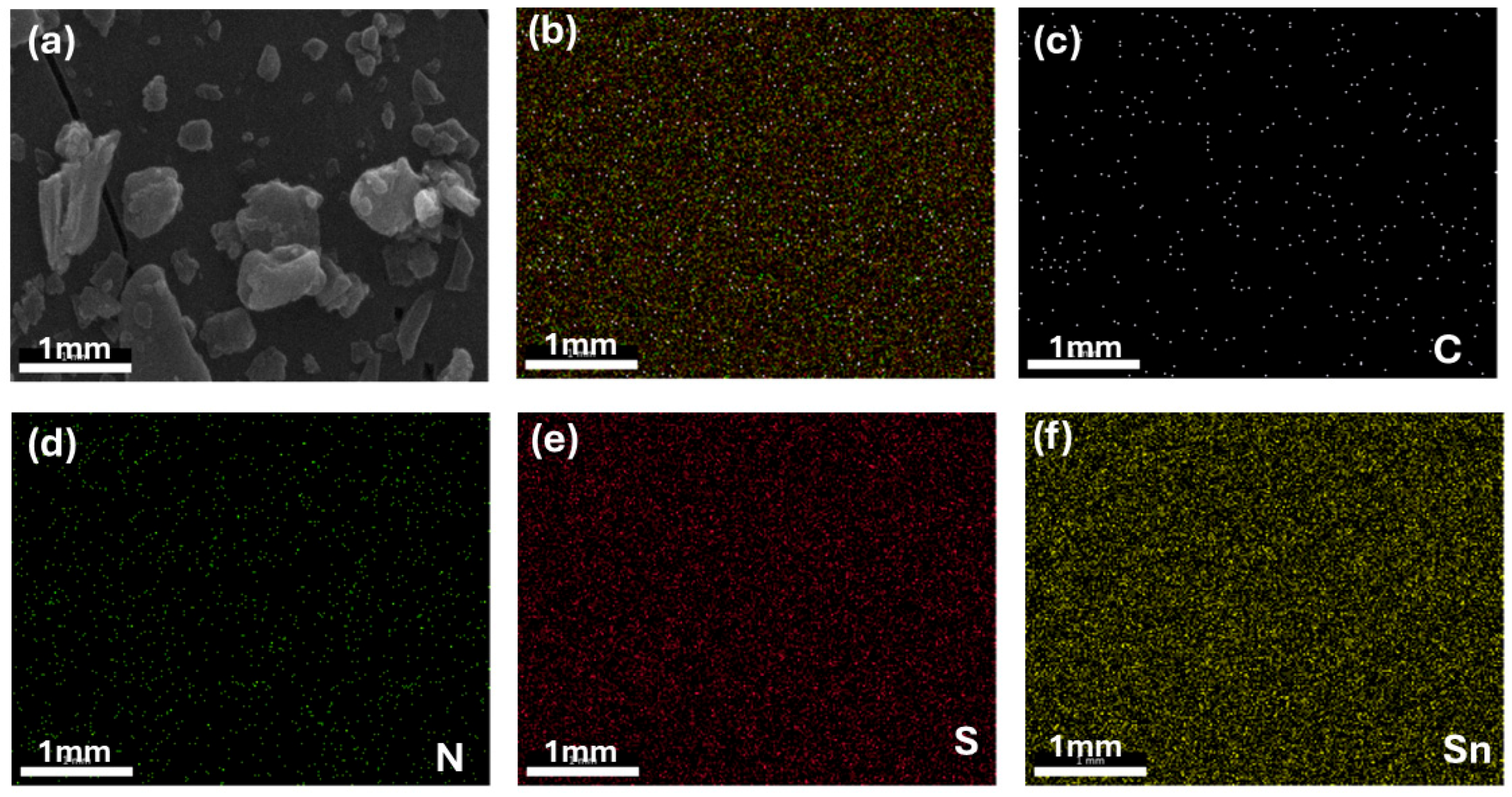
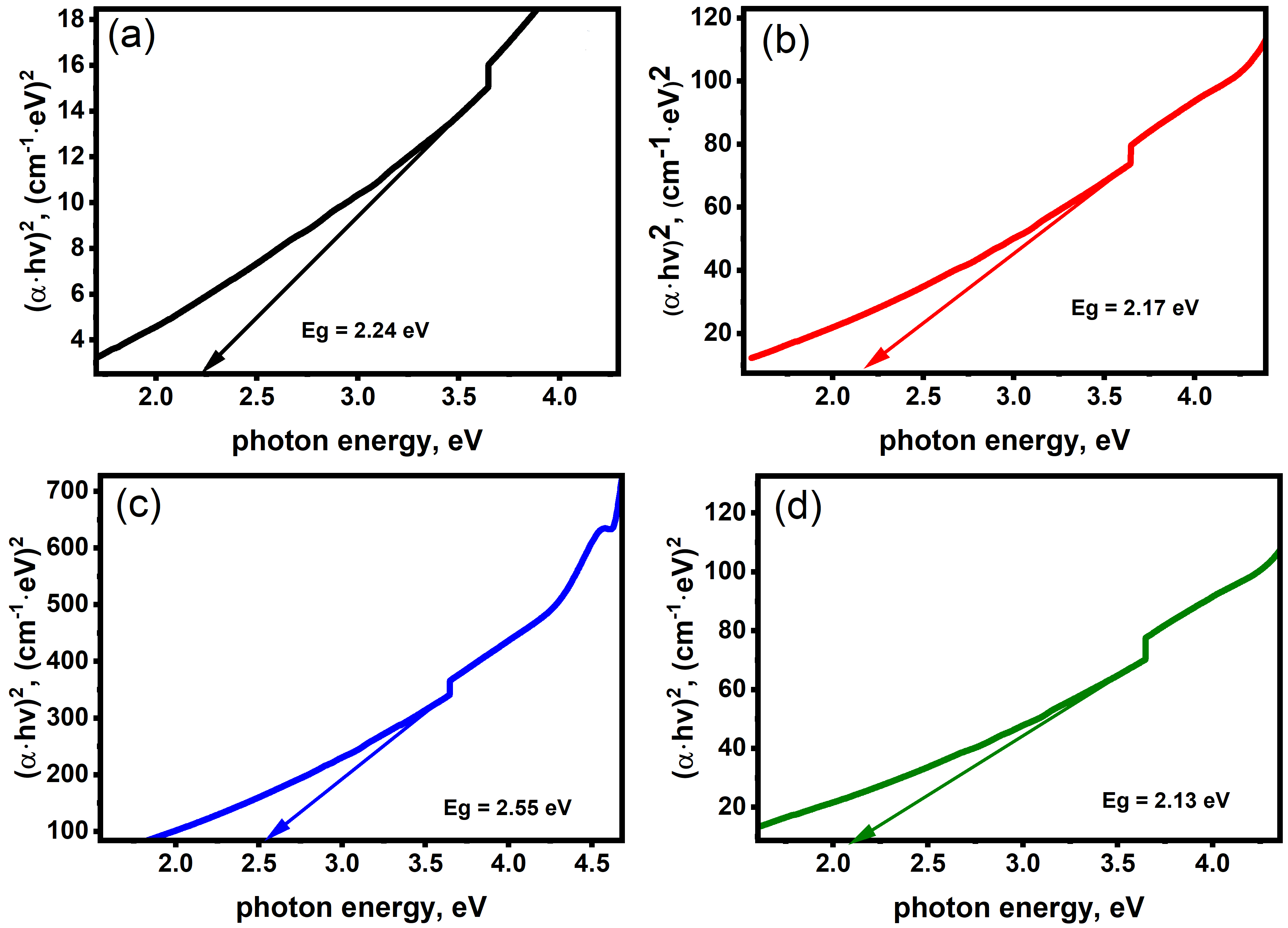
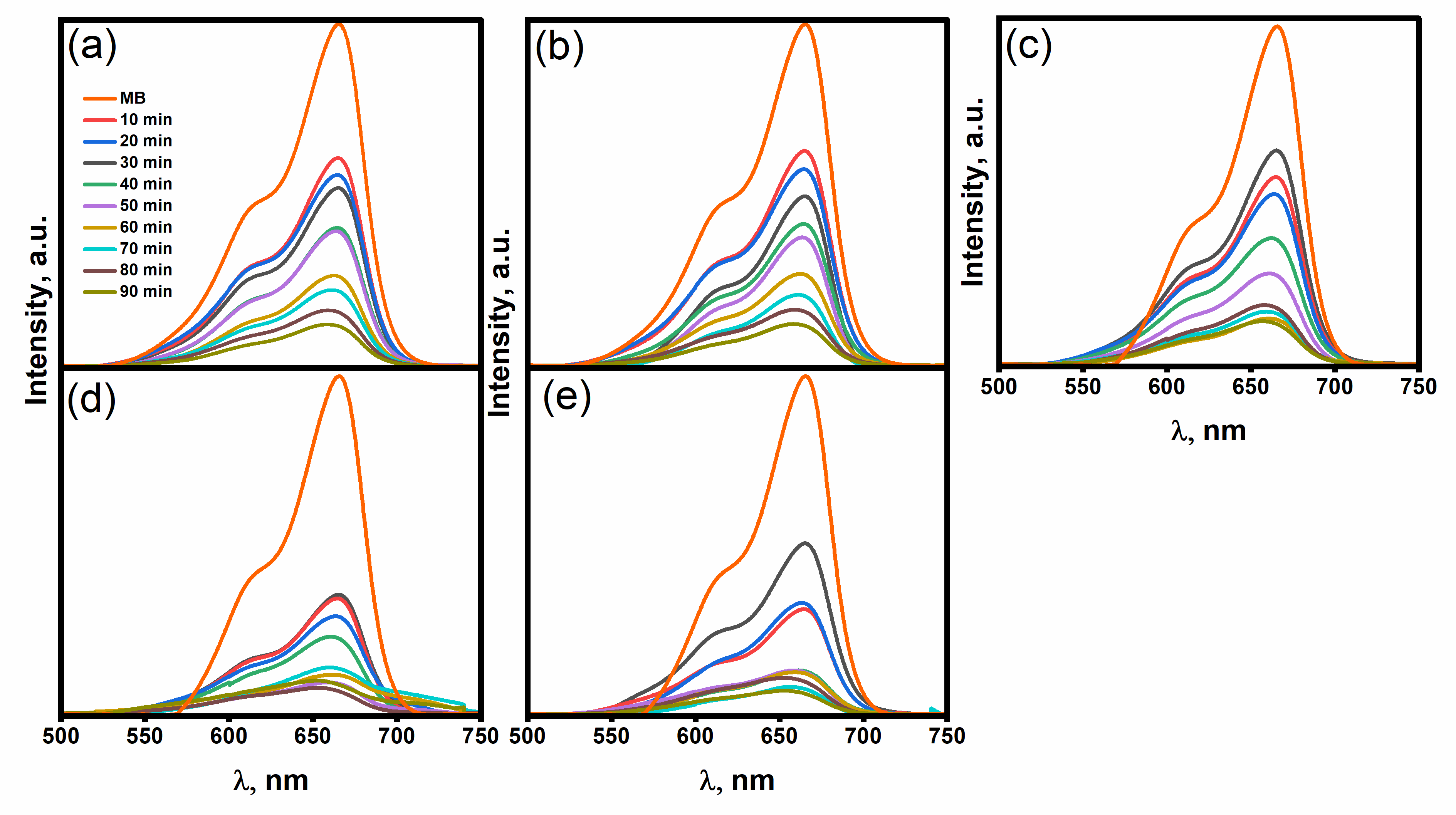
| Sample | Crystalline Size (d) | Strain (ε) × 10−3 | Dislocation Density (δ) × 10−4 |
|---|---|---|---|
| SnS | 44.405 | 1.5525 | 5.07 |
| SnS@0.025wt% g-C3N4 | 46.69 | 1.1575 | 4.58 |
| SnS@0.05wt% g-C3N4 | 37.5 | 0.8275 | 7.11 |
| SnS@0.075wt% g-C3N4 | 28.72 | 0.4975 | 12.1 |
| Sample | Efficiency | K, min−1 | R2 |
|---|---|---|---|
| SnS | 87.5% | 0.021 | 0.98 |
| SnS@0.025 g-C3N4 | 88.1% | 0.024 | 0.97 |
| SnS@0.05 g-C3N4 | 92.5% | 0.026 | 0.92 |
| SnS@0.075 g-C3N4 | 94% | 0.028 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.; Abdel-Rahim, F.M.; Alkallas, F.H.; Trabelsi, A.B.G.; Alraddadi, S.; Aboraia, A.M. Synergistic Integration of g-C3N4 with SnS: Unlocking Enhanced Photocatalytic Efficiency and Electrochemical Stability for Dual-Functional Applications. Catalysts 2025, 15, 629. https://doi.org/10.3390/catal15070629
Ahmed A, Abdel-Rahim FM, Alkallas FH, Trabelsi ABG, Alraddadi S, Aboraia AM. Synergistic Integration of g-C3N4 with SnS: Unlocking Enhanced Photocatalytic Efficiency and Electrochemical Stability for Dual-Functional Applications. Catalysts. 2025; 15(7):629. https://doi.org/10.3390/catal15070629
Chicago/Turabian StyleAhmed, Aya, Farid M. Abdel-Rahim, Fatemah H. Alkallas, Amira Ben Gouider Trabelsi, Shoroog Alraddadi, and Abdelaziz M. Aboraia. 2025. "Synergistic Integration of g-C3N4 with SnS: Unlocking Enhanced Photocatalytic Efficiency and Electrochemical Stability for Dual-Functional Applications" Catalysts 15, no. 7: 629. https://doi.org/10.3390/catal15070629
APA StyleAhmed, A., Abdel-Rahim, F. M., Alkallas, F. H., Trabelsi, A. B. G., Alraddadi, S., & Aboraia, A. M. (2025). Synergistic Integration of g-C3N4 with SnS: Unlocking Enhanced Photocatalytic Efficiency and Electrochemical Stability for Dual-Functional Applications. Catalysts, 15(7), 629. https://doi.org/10.3390/catal15070629










