Abstract
Layered double hydroxide Ti-Zn-CO3 was synthesized by the co-precipitation method with a molar ratio of 2. The synthesized material was characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), thermal analysis (TGA/DTG), UV–vis diffuse reflection spectroscopy (DRS), and Scanning Electron Microscopy (SEM). The photocatalytic degradation of Trypan Blue (TB) and Naphthol Green B (NGB) dyes from aqueous solutions under UV irradiation was investigated. The effects of contact time, photocatalyst dose, dye concentration, solution pH, scavenger effect, and regeneration of catalyst were investigated. The kinetic study showed that the equilibrium was reached within 30 min and 40 min for TB and NGB dyes, respectively, with photodegradation efficiency of around 91% and 83% for TB and NGB dyes, respectively, for dye concentration of 25 mg∙L−1, and the pseudo-first order showed good agreement with the reaction. The optimum photocatalyst dose is 20 mg (1 g∙L−1) and 30 mg (1.5 g∙L−1) for TB and NGB dyes, respectively, and the optimal pH of reaction was found to be 7 for both TB and NGB dyes. This study was established to highlight the photodegradation performance of the prepared catalyst Ti-Zn-CO3 for the degradation of (TB and NGB) dyes chosen as pollutants, and the fact that it can be used many times, which has an economical effect. This mean that the prepared sample is a potential catalyst with good photocatalytic activity, stability, and reusability.
1. Introduction
Various organic contaminants and micropollutants such as dyes, surfactants, excipients, pharmaceuticals, personal care products, industrial chemicals [1], pesticides, and many others are present in the wastewater [2]. Each year, approximately 400 million tons of pollutants are discharged into the aquatic environment after the batch processes followed in Textile industries [3]. There are currently over 10,000 commercially available dyes, and over 700,000 tons of dyes are produced each year [4]. Dyes are widely used in various branches such as the textile industry, cosmetics, and paper production [5]. They affect the aquatic system by reducing sunlight transmission into water [6]. They are carcinogenic substances, toxic to microorganisms, aquatic life, and human beings by constituting a serious concern to the ecosystem [7].
Trypan Blue (TB) and Naphthol Green B (NGB) dyes belong to the azoic group of dyes generally used in various industries [8], including textiles, prescription drugs, and biomedical studies [9].
However, their vast use has led to their presence in industrial effluents and wastewater. Due to insufficient treatment techniques, Trypan Blue (TB) and Naphthol Green B (NGB), like many other synthetic dyes, find their way into wastewater streams [10]. Most dyes, including TB and NGB, have adverse effects on health and the environment, and their natural decompositions take a long time [11,12]; thus, wastewater purification from organic dyes is of great importance [13].
There is therefore an urgent need to develop efficient and economical treatment processes capable of treating large volumes of wastewater containing considerable concentrations of organic dyes [14].
Fortunately, researchers around the world are paying more attention to this and are actively developing techniques to remove pollutants from wastewater [15].
Various techniques, such as electrolysis, oxidation, adsorption, and photocatalysis, are employed to eliminate pollutants. Photodegradation is favored for its high efficiency and environmental friendliness [16].
While oxidation is very important, photocatalysis can also be used for reduction reactions related to heavy metal removal from water and water splitting [17].
In recent years, the use of photocatalytic technology to degrade wastewater has been rapidly developed and has attracted much attention in the field of wastewater purification and other environmental applications [18] due to the simplicity of the process that degrades pollutants into small, harmless molecules [19].
The key to a successful photocatalytic process is the development of a suitable and nontoxic photocatalyst with high stability, photocatalytic activity, and low cost [20]. Current research focuses on the use of photocatalysts, which degrade pollutants in wastewater using environmentally friendly materials [21].
Among the various alternatives to semiconductor photocatalysts that are currently studied, layered double hydroxide (LDH)-based photocatalysts [22] have attracted considerable attention in recent decades and have been widely used in photocatalysis due to their unique features and remarkable physicochemical properties that make them suitable for environmental applications, such as high surface area, tunable optical properties, significant anion exchange ability, strong and broad light absorption range, simple synthesis, low cost, and high recyclability [1].
Hydrotalcite-like compounds (HT) are LDH minerals. They are natural or synthetic anionic clays with positively charged layers balanced by hydrated anions [7,23]. The general formula of these compounds can be represented as follows: where M2+ and M3+ are the divalent and trivalent cations in the octahedral positions within the hydroxide layers with x normally between 0.17 and 0.33. An− is an exchangeable interlayer anion [24]. Many studies depicted the removal of TB and NGB dyes from the wastewater. Shrikant D. Khandare et al., 2023, reported that the decolorization of Trypan Blue dye by marine bacteria Vibrio sp. was 90.33% in 6 days at 500 mg∙L−1 of dye concentration [25], and that nanoadsorbents, such as zinc oxide (ZnO), graphene oxide (GO), and polyvinyl alcohol polymer (PVA), were successfully applied for the degradation of TB dye (99.8%, 100%, and 92.7%, respectively) [3].
The results showed also that with the use of Ag3PO4/G/SiO2 as a photocatalyst (0.03 g) at an initial dye concentration of 20 mg∙L−1, pH = 2, the degradation percent of TB dye was 98.7% within 10 min of light exposure [10].
A total of 97% and 80% of NGB dye was degraded under solar irradiation using Ag-AC composite and AgNPs as a photo catalyst [26].
CdS-ZnWO4 was also used for the degradation of NGB dye under UV-A light, and about 70% of NGB was degraded within 90 min at pH 9 and catalyst loading 3 g∙L−1 [27].
The present research aims to synthesize the Ti-Zn-CO3 photocatalyst via the co-precipitation method, investigate its structural and optical characterization, and to study the photocatalytic degradation of TB and NGB dyes by Ti-Zn-CO3 under UV radiation. The effect of various conditions for the removal of TB and NGB dyes have been investigated, such as the effects of contact time, photocatalyst dose, dye concentration, solution pH, scavenger effect, and regeneration of catalyst.
The samples were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), thermal analysis (TGA/DTG), UV–vis diffuse reflection spectroscopy (DRS), and Scanning Electron Microscopy (SEM).
2. Results
2.1. Catalyst Characterization
The XRD pattern of Ti-Zn-CO3 hydrotalcite is shown in Figure 1. The XRD pattern shows that well-crystallized hydrotalcite-type solids were obtained and that the compound had layered structure [10,28] and was characterized by diffraction peaks (003), (006), (100), (012), (009), (015), (018), (110), and (113) situated at 2θ 11.61°, 23.38°, 31.7°, 34.56°, 36.19°, 39.10°, 46.65°, 60.13°, and 61.45°. The interlayer distance d003 = 7.61 Å was calculated from the peak (003) using Bragg’s law [29,30], and the lattice parameters were calculated from the reticular planes (110) and (003) by the following relation (a = 2 × d110, c = 3 × d003). The crystallite size D = 48.895 nm was determined from the width at mid-height of the characteristic diffraction line using Scherrer’s formula (see Table 1) [31].
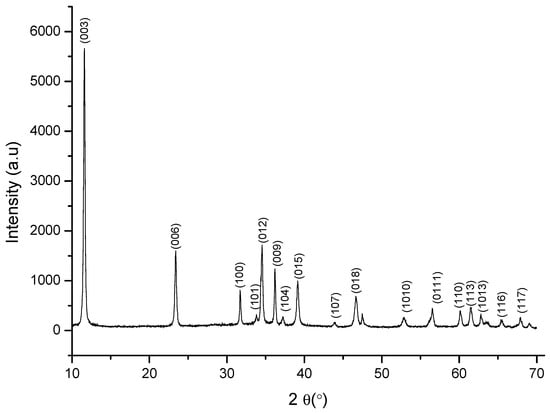
Figure 1.
Powder X-ray diffraction patterns of Ti-Zn-CO3.

Table 1.
The cell parameters and the textural properties of Ti-Zn-CO3.
Likewise, FTIR spectroscopy was recorded for an as-synthesized hydrotalcite sample (Figure 2). The sample showed spectra similar to those appearing in the literature [32]. A wide absorption band centered at 3417 cm−1 could be attributed to the OH stretching vibration mode of the hydroxyl groups, and the water molecules inside the hydrotalcite interlayer are due to the OH stretching vibration [33], both from the surface and interlayer water molecules. The shoulder that is recorded at about 3000 cm−1 is due to the hydrogen bonds of carbonate anions [34]. The band at around 1650 cm−1 is ascribed to OH bending vibration modes of water molecules in the interlayer space [35]. The vibration band at 1495 cm−1 is assigned to the vibration mode of carbonates [33]. The appearance of another band at 1362 cm−1, corresponding to the vibration mode of the carbonate ions, can be observed [36]. Vibration bands at low frequencies (below 1000 cm−1) are assigned to the metal–oxygen–metal bonds forming lamellar layers [37].
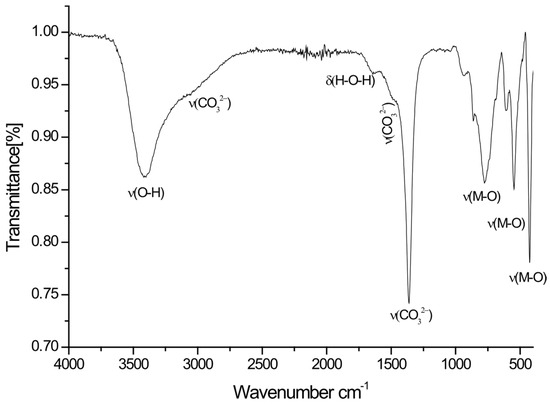
Figure 2.
FTIR spectra of Ti-Zn-CO3.
To investigate the thermal decomposition behavior of Ti-Zn-CO3 LDH during the thermal treatment, thermoanalytical measurements were carried out and results are presented in Figure 3. Each thermogram displays several weight loss stages well-obvious as usually described in the literature.
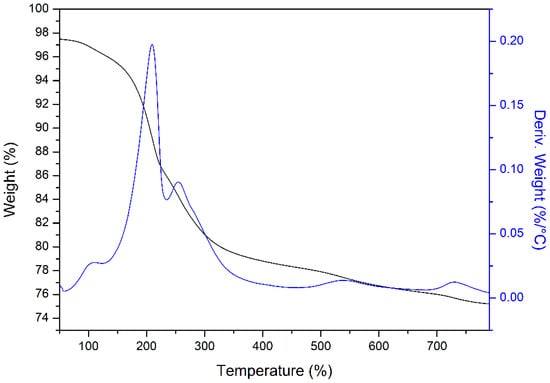
Figure 3.
Thermal analysis (TGA/DTG) of Ti-Zn-CO3.
Thus, a continuous weight loss can be seen between 50 °C and 800 °C, and four major endothermic peaks were observed corresponding to four stages of weight decrease [38]. Firstly, up to 125 °C is due to the physically adsorbed water loss, and their weight loss is 1.32% [39]. Increasing temperature between 125 and 235 °C leads to the loss of the interlayer water molecules and their weight loss is 10.3% [40]. A third weight loss was recorded in the range 235–405 °C, which can be attributed to dihydroxylation due to the loss of OH− in the brucite-like layer as water molecules, and the weight loss at this step is 7.15% [41]. The fourth weight loss, 405–600 °C, is associated with the decarbonation of the compounds (the removal of interlayer anions (CO32−) and releasing of CO2) and their weight loss is 3.55% [42]. The total weight loss is 22.32%.
The optical properties of the semiconductor material were studied using the diffuse reflection spectroscopy (DRS) where the optical band gap was calculated using the Kubelka–Munk function [F(R)] [43] fitted with indirect transition. The band gap energy Eg of the catalyst was determined by the following equation:
where
ν: frequency
K: plank constant
F(r): Kubelka–Munk function
k: molar absorption coefficient
R: reflectance
S: scattering factor
Where was plotted versus energy (Figure 4, and the Eg was determined from the extrapolation of the linear part to the x axe (Figure 4 inset), and herein the point of intersection is the searched band gap Eg = 3.162 eV.
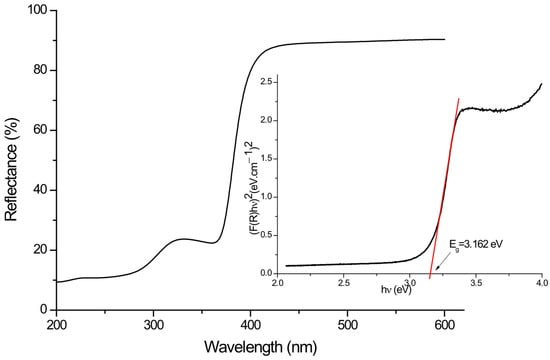
Figure 4.
UV–Visible DRS spectra and Energy band gap (inset) of prepared photocatalyst Ti-Zn-CO3.
The SEM analyses of LDH samples were investigated at two different magnifications as shown in Figure 5. The SEM micrographs show that LDH particles are mainly composed of irregular and porous particles. In general, the LDH has plate-like morphology and hexagonal crystallite forming the so-called morphology “sand roses” aggregations [44].
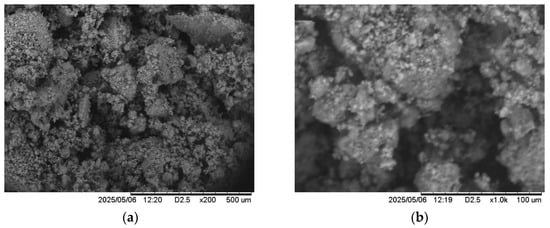
Figure 5.
SEM images of Ti-Zn-CO3, (a) ×500 μm, (b) ×100 μm.
2.2. pH of the Point of Zero Charge pHpzc
The pH at the point of zero charge (pHpzc) of a catalyst is an important parameter for characterizing the solid–solution interface; it corresponds to the pH for which the surface of the solid has zero charge [45]. The graph that represents the ΔpH as a function of the initial pH allows us to determine the pHpzc which corresponds to ΔpH = 0. According to Figure 6, the pHpzc of Ti-Zn-CO3 is 8.07. For pH > pHpzc, the surface is negatively charged; hence, less adsorption of anionic dyes (TB or NGB) occurs, while for pH < pHpzc, the surface is positively charged, which enhances the adsorption of anionic dyes (TB or NGB).
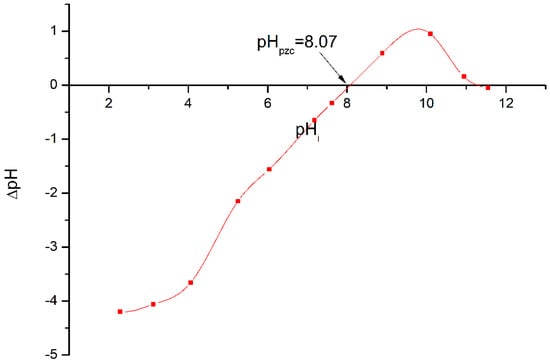
Figure 6.
Determination of the pH of the point of zero charge “pHpzc” of the photocatalyst Ti-Zn-CO3.
3. Discussion
3.1. Photocatalytic Activity of the Synthesized Materials
3.1.1. Effect of Contact Time on Photodegradation
The photocatalytic performances of the prepared catalyst were tested against the TB and NGB dyes with the aim of degradation under UV irradiation (Figure 7a,b). The photolysis of dyes (blank experiment without catalyst) was tested and insignificant photodegradation efficiencies were signaled, about 2.83% and 2.86% of TB and NGB dyes during 180 min of UV irradiation. Adsorption tests in the dark also were checked and only 0.06% and 0.84% of TB and NGB dyes were kept, respectively, during 2 h of UV irradiation.
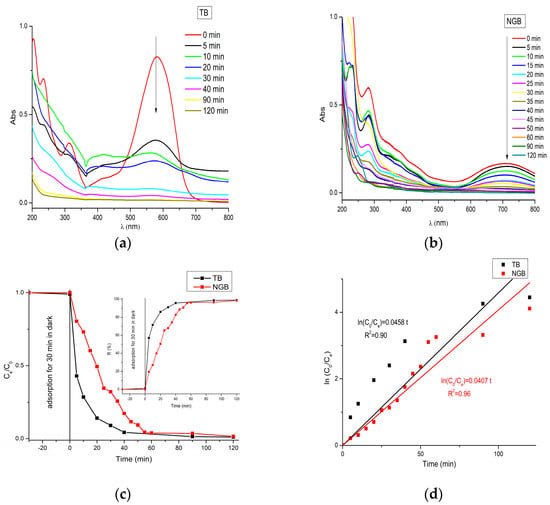
Figure 7.
The UV–vis spectra of Photodegradation of TB (a) and NGB dyes (b), photodegradation of TB and NGB dyes using Zn-Ti-CO3 catalyst (c), and the kinetic plots of the photocatalytic degradation (d).
During the UV irradiation of TB and NGB dyes for 2 h, the photodegradation efficiencies of TB and NGB dyes were 98.83% and 98.36%, respectively (Figure 7c).
Figure 7c shows the photodegradation efficiency (%) of TB and NGB dyes during contact time; it can be seen that during the first 20 min there is a significant change in the slope, which corresponds to a faster degradation, followed by a slight degradation increase over the next few minutes, and finally a steady-state response. On the other hand, the photodegradation of NGB performed well in the first minutes, but with a smaller percentage of degradation than that of the TB photodegradation, so that the photodegradation efficiency of TB dye reached up to 85.91% in the first 20 min while that of the NGB dye reached only 50.55%, and later the catalysts followed an almost asymptotic trend, caused by a very slow change in the degradation process. To understand more of the kinetic study, the data were fitted to the pseudo-first order model written as follows:
where C0 represents the initial concentration of TB and NGB dyes, Ct is the concentration of TB and NGB dyes at t instant, and k is the apparent kinetic constant (min−1). The results were given in Figure 7d and Table 2. From Figure 7d and Table 2, it seems that the catalytic activity of Ti-Zn-CO3 toward TB degradation is slightly higher than that of NGB degradation.

Table 2.
Kinetic parameters of photocatalytic degradation of TB and NGB dyes over Ti-Zn-CO3 catalyst.
This phenomenon was in accordance with the apparent rate constant value, which can also be somewhat higher than that of NGB degradation. That fact led to the generation of more holes on the surface and OH which contribute to the photodegradation process.
3.1.2. Effect of Catalyst Loading on Photodegradation
Photocatalyst loading is one of the crucial operating factors which has an important effect on the photodegradation rate in the photocatalytic processes [46].
Optimization of catalyst loading was carried out to better understand the effect of the catalyst loading on the photodegradation of the TB and NGB dyes (Figure 8) and to avoid a shortage of catalyst and excessive catalyst weight. Several experiments were conducted under 60 min of UV irradiation by varying the catalyst loading from 10 mg/20 mL to 70 mg/20 mL at a dye concentration of 25 mg∙L−1 and the natural pH of the dye solutions. As shown in Figure 8, the degradation of TB and NGB dyes increased as the catalyst loading was increased from 10 to 30 mg. This may be attributed to the increased access of reactants and incident light to the catalyst surface area, which promotes the photocatalytic efficiency. The optimized load was found to be 20 mg (1 g∙L−1) and 30 mg (1.5 g∙L−1) for TB and NGB dyes, respectively. A dose of 30 mg of catalyst was used for further applications for both of the dyes’ degradation. There is no significant change in the photodegradation efficiency when exceeding 20 mg, but a slight decrease in the photodegradation efficiency when exceeding 50 mg of the catalyst was observed; this fact can be caused by light refraction and reduced light penetration into the solution due to the high turbidity of the reaction system [11].
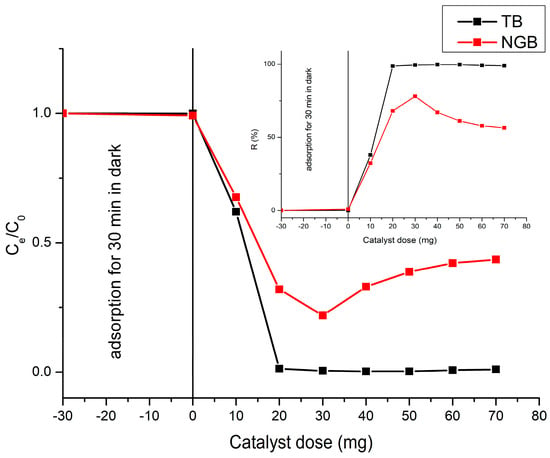
Figure 8.
Effect of catalyst dose on the photodegradation of TB and NGB dyes (Ci = 25 mg∙L−1; V= 20 mL; t = 60 min).
3.1.3. Effect of Dye Concentration
Figure 9 shows the effect of dye concentration on the photocatalytic activity of Ti-Zn-CO3 hydrotalcite.
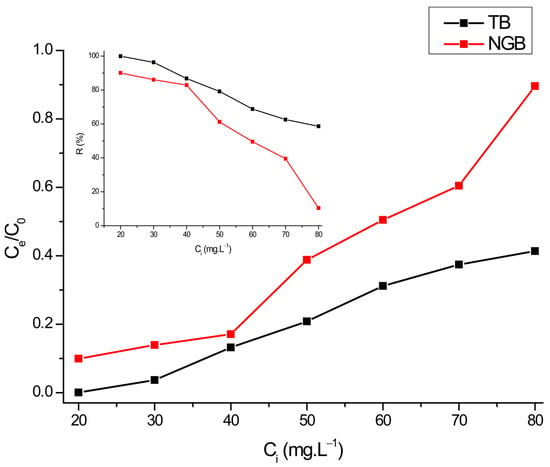
Figure 9.
Effect of dye concentration on the photodegradation of TB and NGB dyes (m = 30 mg, V = 20 mL; t = 60 min).
Several experiments were conducted using the experimental parameters: concentration of dyes (TB and NGB): 20–80 mg∙L−1, catalyst dose: 30 mg/20 mL and at the pH of the dye solution. As the concentration of dye increased, time taken for complete degradation increased. By increasing the dye concentration, the efficiency of degradation decreased, because of the adsorbing of more dye molecules on the catalyst surface leading to a decrease in active sites (lesser number of hydroxyl and superoxide radicals) and thereby reducing light penetration.
After 60 min of illumination, 20 mg∙L−1 of dye solution was completely degraded for TB dye (99.98%) and 90.06% was achieved for NGB dye, while for the concentration of 80 mg∙L−1 dye solution, degradation efficiency was 58.63% and 10.39% for TB and NGB dyes, respectively.
3.1.4. Effect of pH on Photocatalytic Degradation
The pH of a dye solution is one of the main important parameters of photodegradation to study and is as shown in Figure 10 [46]. From the figure it is clear that photocatalytic degradation efficiency was nearly stable in the pH range (5–9), but the highest efficiency was recorded at pH 7 around 99.41% and 87.41% of photodegradation efficiency of TB and NGB dyes. At a lower pH (pH = 3) the photodegradation efficiencies decrease; this result was due to the destruction of the structure of the catalyst in a strong acidic pH medium. At a high pH value, the presence of large amounts of OH− ions in the reaction medium promotes •OH formation which promotes the photodegradation efficiency. In an alkaline medium, the Ti-Zn-CO3 surface is negatively charged (pH > pHpzc = 8.07) (Figure 6); this affects the adsorption of dye molecules due to the repulsion between the catalyst and the dyes (TB or NGB) due to their anionic nature [47], hence, less photodegradation of the TB and NGB dyes occurs. Then, by further increasing pH, the degradation rate begins to decrease; this fact may be due to the increase in solution viscosity at a high pH value reducing the diffusivity of dyes to the catalyst surface and the light absorption by the catalyst [48]. Therefore, the best possible pH for this process is in the range (7–8).
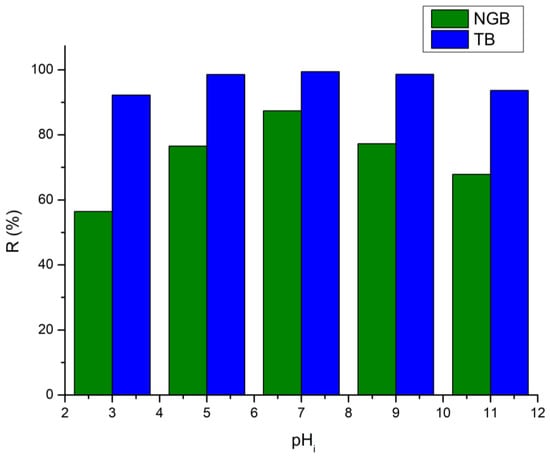
Figure 10.
Effect of initial pH on the photodegradation of TB and NGB dyes (Ci = 25 mg∙L−1; m = 30 mg, V= 20 mL; t = 60 min).
3.1.5. Photocatalyst Stability Study
The regeneration of the catalyst is one of the key aspects of making heterogeneous catalysis technology more economical and suitable for convenient applications [49,50], and to allow repeated use for the degradation of contaminants with almost the same ability, where after several cycles of repeated use a slight decrease in photodegradation efficiency can be noticed.
The experimental recycling of Ti-Zn-CO3 was carried out with the same catalyst loading and dye concentration; after each reaction run, the catalyst was recovered, washed several times with distilled water, dried, and reused.
As can be seen from (Figure 11), after four cycles of use of the catalyst Ti-Zn-CO3 in the photodegradation process of TB and NGB dye, a slight decrease in the photodegradation efficiency can be noticed around 55% and 43.25% of TB and NGB, respectively. This means that the prepared sample is a potential catalyst with high photocatalytic activity, moderate stability, and reusability.
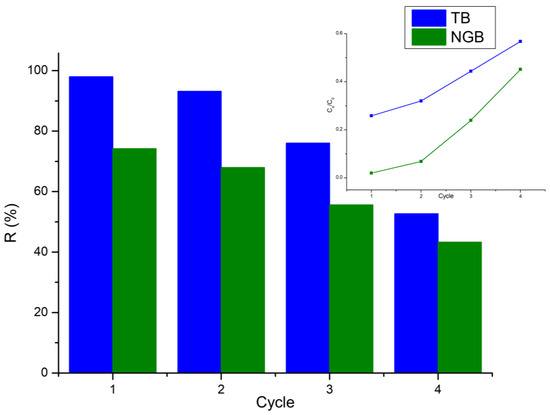
Figure 11.
Cycling runs for the photocatalytic degradation of TB and NGB over Ti-Zn-CO3 photocatalyst.
3.1.6. Effect of Different Scavengers in the Photocatalytic Degradation
Several experiments were carried out with different scavengers to better understand the main active species responsible for photodegradation [51] such as superoxide radicals (−O2•), hydroxide radicals (HO•), holes (h+), and photo-generated electrons (e−). Figure 12a,b depict the role of the used scavengers to identify the active species in photodegradation of TB and NGB dyes by the Ti-Zn-CO3 catalyst under UV irradiation. A total of 40.89% of discoloration of TB was achieved after 60 min of irradiation and addition of ethylenediaminetetraacetic acid (EDTA) (as an h+ hole scavenger), and no remarkable change was observed when using this later with the NGB dye (0.001%). Furthermore, about 75.42% and 42.31% of degradation was noted for the TB and NGB dyes, respectively, when the ethanol was used (as a HO• radical scavenger). In the case of using ascorbic acid as superoxide (−O2•) scavengers, only 13.45% and 19.87% of degradation of TB and NGB dyes, respectively, was achieved, and during the last reaction, with silver nitrate (AgNO3) (as e− scavenger), about 98.03% and 61.90% of TB and NGB dye degradation was achieved during the same irradiation time. The photocatalytic degradation of TB and NGB dyes on the catalyst was postponed by the presence of EDTA, ethanol, and ascorbic acid. From the results, it is obvious that (−O2•) and (h+) were the main active species contributing in the photodegradation of TB and NGB dyes. The hydroxyl HO• was less active in the photodegradation of TB and NGB dyes, and there was no remarkable interaction of e− in the photodegradation process.
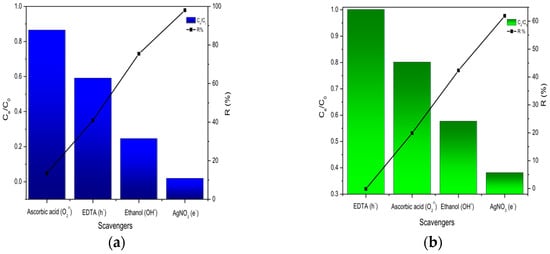
Figure 12.
Evolution of photocatalytic degradation under UV irradiation of (a): TB, (b): NGB in presence of different scavengers.
3.1.7. Photocatalytic Degradation Mechanism of TB and NGB Dyes over the Photocatalyst
The photodegradation of (TB and NGB) dyes is running according to a commonly suggested mechanism [10].
Firstly, the (TB or NGB) dye molecules were adsorbed on the surface of the photocatalyst (Ti-Zn-CO3). The role of adsorption was to condense the TB or NGB dyes on the surface of the photocatalyst, and the photocatalyst could then decompose the TB or NGB dyes into harmless small molecules [52].
Then, under UV irradiation, electrons are excited and transferred from the valence band (VB) to the conduction band (CB), therefore creating holes in the valence band and e− in the conduction band (Equation (3)).
At the same time, oxygen molecules were physically adsorbed on the surface of the photocatalyst Ti-Zn-CO3, and captured the electron from the conduction band, therefore producing ions (Equation (4)) [53].
The holes in the valence band neutralized the hydroxyl groups, which were produced from water molecules, therefore producing hydroxyl radicals (Equation (5)).
The hydroxyl free radicals and superoxide radical anion () are strong oxidizing agents, in addition to their great contribution of holes in the photodegradation process of TB and NGB dyes indicated in the study of the effect of scavengers. Thus TB and NGB dyes could be oxidized by these three agents to H2O and CO2. The possible reaction equations in the photocatalytic process were as follows:
Equation (6) shows that the TB or NGB dyes will be degraded via successive attacks by , , and .
Therefore, the degradation reaction continues as shown in Equations (4)–(6).
4. Materials and Methods
4.1. Materials
4.1.1. Chemicals
All chemicals used during experimental work (ZnCl2⋅6H2O, TiCl3, NaOH, Na2CO3, HCl, TB dye, and NGB dye) were of analytical grade, were used as such without further purification, and were purchased from Biochem Chemopharma. Distilled water was used for the preparation of all types of solutions and dilution when required. The chemical structures of the dyes are shown in Figure 13a,b.
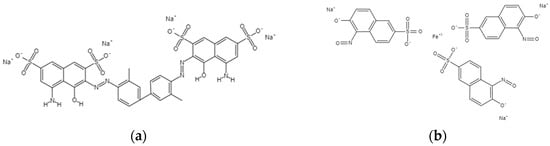
Figure 13.
Chemical structures of (a): Trypan Blue dye (TB), (b): Naphthol green B dye.
4.1.2. Standard Solutions
A total of 1 g of TB or NGB dye was dissolved in 1000 mL of distilled water; this was a 1000 mg∙L−1 stock solution of dye. Standard solutions of dye were prepared by successive dilution of the stock solution.
4.2. Catalyst Synthesis
The sample with carbonate as the interlayer anion was prepared by the co-precipitation method [30] with a molar ratio of 2 at a fixed pH of 10. A solution containing chlorides was dropwise added over a solution containing Na2CO3 (0.5 M) and NaOH (1 M) as the precipitant agent, under continuous stirring. After the addition process, the slurry was continuously stirred during 1 h with the purpose of maintaining the homogeneity of the sample. The suspension was then transferred into the Teflon-lined stainless-steel autoclave and aged at 80 °C overnight.
The obtained precipitate was washed several times with distilled water until a negative test with Silver Nitrate (AgNO3) was obtained and then dried at 70 °C in an oven overnight. Finally, the resulting solid was ground in an agate mortar (denoted Ti-Zn-CO3) and characterized.
4.3. Characterization
The XRD patterns were recorded on a Siemens D-501 diffractometer, using CuKα radiation (λ = 1.5418 Å). XRD patterns were collected over a range of 2θ from 10° to 70°. FTIR spectra were performed within the range of wavenumbers from 4000 to 400 cm−1 on a Perkin Elmer spectrophotometer kind 16PC using KBr pellets.
The thermal analyses were performed with a TA Instruments SDT Q600 with a heating rate of 20 °C/min from 50 to 800 °C.
DRS analyses were performed using a UV–vis spectrometer (JASCO V750) Shimadzu equipped with a 60 mm diffuse reflectance and transmission integrating sphere accessory.
The point of zero charge (PZC) was determined by the Batch equilibrium method [29]. Masses of 30 mg were prepared with a solution of 30 mL of 0.1 M (N) KCl at different pH values from 2 to 12 and shaken for 72 h. Initial pH values were adjusted by adding NaOH or HCl solution. Final pH values (pHf) were measured after the filtration of the solution. The values of (pHf-pHi) versus pHi were plotted and the value of pHpzc corresponds to pHi = pHf.
4.4. Photocatalytic Experiments
The photocatalytic activities of the catalyst were tested for TB and NGB dye degradation (25 mg∙L−1, 20 mL) in a batch system with variable contact time, catalyst loading, dye concentration, scavengers effect, regeneration of the catalyst, and pH of dye solution. A total of 30 mg of catalyst Ti-Zn-CO3 was dispersed in 20 mL of dye solution (TB or NGB) at a pH of solution and room temperature.
The solution was stirred for 30 min in the dark to reach adsorption-desorption equilibrium between the catalyst and the dye, then the degradation process continued with UV irradiation (20 watt with 365 nm) chosen as light source. The distance between the solutions vessels and the UV source was set at 10 cm, the chamber of UV radiation was covered to focus all radiations on the solutions, and the lamp (dispositive from Hoefer scientific instruments UVTM-25) was set perpendicularly on the solution. At regular time intervals, Aliquots were withdrawn and centrifuged to eliminate the catalyst, and the supernatant dye solution was analyzed. The concentration of dyes during the reaction process was measured by UV–vis spectroscopy on Specroscan 80D equipment in the range of 200–800 nm.
The removal rate of the dye degradation is calculated as follows:
where C0 and Ct are the concentrations at time 0 and time t (mg∙L−1).
Additionally, to identify the main active species generated in LDH materials and their role in oxidation-reduction reactions, photocatalytic tests were carried out using different scavengers. The objective was to identify specific reactive species events such as superoxide radicals (−O2•), hydroxide radicals (HO•), holes (h+), and generated electrons (e−), and to determine whether the photocatalyst promotes their presence.
5. Conclusions
In the present study, it was confirmed that Ti-Zn-CO3 hydrotalcite was successfully prepared using a co-precipitation method. The catalytic activity of the catalyst was evaluated by the degradation of TB and NGB dyes under UV irradiation during 60 min. The results showed that the sequential catalytic performance was conducted in the following order: RTB > RNGB under the optimum conditions: the catalyst dose was found to be 20 mg, and 30 mg for TB and NGB, respectively, the dye concentration 25 mg∙L−1, the pH 7. The major active species are superoxide radicals (−O2•) and holes (h+). The regeneration tests of the catalyst over several consecutive uses reveal that the prepared sample is a potential catalyst with good photocatalytic activity, moderate stability, and reusability even after several uses (four cycles).
The results obtained show that the synthesized hydrotalcites can be used as an efficient photocatalyst for the removal of TB and NGB dyes from aqueous solutions, suggesting that the clay appears to have promising applications in environmental and purification areas. Thus, our interest in the results obtained here lies not only in the determination of dye removal potential for the synthesized hydrotalcites samples but also in the potential applications of these hydrotalcites in a full-scale wastewater treatment.
Author Contributions
Conceptualization, S.H.; methodology, S.H.; software, S.H.; validation, S.H.; formal analysis, S.H. and A.B.; investigation, S.H. and N.B.; resources, S.H., N.B., and M.A.; data curation, S.H. and A.B.; writing—original draft preparation, S.H.; writing—review and editing, S.H., A.B., M.S., and S.A.-F.; visualization, S.H., N.B., A.B., and M.S.; supervision, N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by King Saud University: Ongoing Research Funding program (ORF-2025-7), King Saud University, Saudi Arabia.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors gratefully acknowledge the funding support of King Saud University: Ongoing Research Funding program (ORF-2025-7), King Saud University, Saudi Arabia, the authors also gratefully acknowledge the Laboratory of MEB of University of Tlemcen for its SEM. Finally, we thank the Laboratory LCMIA of the University of USTO-MB for its technical support. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TB | Trypan Blue |
| NGB | Naphthol Green B |
| XRD | X-Ray Diffraction |
| FTIR | Fourier transform infrared spectroscopy |
| TGA | Thermogravimetric analysis |
| DTG | Derivative of the TGA |
| DRS | Diffuse Reflection Spectroscopy |
| EDTA | ethylenediaminetetraacetic acid |
| PZC | Point of zero charge |
References
- Despotović, V.; Hadnađev-Kostić, M.; Vulić, T.; Bognár, S.; Karanović, Đ.; Tot, N.; Šojić Merkulov, D. Utilizing Zn(Cu/Cr)Al-Layered Double Hydroxide-Based Photocatalysts for Effective Photodegradation of Environmental Pollutants. Separation 2024, 11, 308. [Google Scholar] [CrossRef]
- Ahmad, B.; Imran, M. Emerging Organic Contaminants, Pharmaceuticals and Personal Care Products (PPCPs): A Threat to Water Quality. In Hazardous Environmental Micro-Pollutants, Health Impacts and Allied Treatment Technologies; Springer: Cham, Switzerland, 2022; pp. 105–141. ISBN 978-3-030-96523-5. [Google Scholar]
- Kathiresan, G.; Vijayakumar, K.; Sundarrajan, A.P.; Kim, H.-S.; Adaikalam, K. Photocatalytic Degradation Efficiency of ZnO, GO and PVA Nanoadsorbents for Crystal Violet, Methylene Blue and Trypan Blue Dyes. Optik 2021, 238, 166671. [Google Scholar] [CrossRef]
- Alsukaibi, A.K.D. Various Approaches for the Detoxification of Toxic Dyes in Wastewater. Processes 2022, 10, 1968. [Google Scholar] [CrossRef]
- Dutta, S.; Adhikary, S.; Bhattacharya, S.; Roy, D.; Chatterjee, S.; Chakraborty, A.; Banerjee, D.; Ganguly, A.; Nanda, S.; Rajak, P. Contamination of Textile Dyes in Aquatic Environment: Adverse Impacts on Aquatic Ecosystem and Human Health, and Its Management Using Bioremediation. J. Environ. Manag. 2024, 353, 120103. [Google Scholar] [CrossRef]
- Sahu, A.; Poler, J.C. Removal and Degradation of Dyes from Textile Industry Wastewater: Benchmarking Recent Advancements, Toxicity Assessment and Cost Analysis of Treatment Processes. J. Environ. Chem. Eng. 2024, 12, 113754. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, S.; Alkhanjaf, A.A.M.; Arora, N.K.; Saxena, B.; Umar, A.; Ibrahim, A.A.; Akhtar, M.S.; Mahajan, A.; Negi, S.; et al. Microbial Fuel Cells for Azo Dye Degradation: A Perspective Review. J. Ind. Eng. Chem. 2025, 142, 45–67. [Google Scholar] [CrossRef]
- Caguiat, J.M.E.; Tiu, E.R.U.; Go, A.D.; dela Rosa, F.M.; Punzalan, E.R. Dataset on the Decolorization of Naphthol Green B Using a UV/Sulfite System: Optimization by Response Surface Methodology. Data Brief 2024, 57, 110924. [Google Scholar] [CrossRef]
- Gunasundari, E.; Kumar, P.S.; Rajamohan, N.; Vellaichamy, P. Feasibility of Naphthol Green-B Dye Adsorption Using Microalgae: Thermodynamic and Kinetic Analysis. Desalin. Water Treat. 2020, 192, 358–370. [Google Scholar] [CrossRef]
- Showman, M.S.; Omara, R.Y.; El-Ashtoukhy, E.-S.Z.; Farag, H.A.; El-Latif, M.M.A. Formulation of Silver Phosphate/Graphene/Silica Nanocomposite for Enhancing the Photocatalytic Degradation of Trypan Blue Dye in Aqueous Solution. Sci. Rep. 2024, 14, 15885. [Google Scholar] [CrossRef]
- Periyasamy, A.P. Recent Advances in the Remediation of Textile-Dye-Containing Wastewater: Prioritizing Human Health and Sustainable Wastewater Treatment. Sustainability 2024, 16, 495. [Google Scholar] [CrossRef]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of Textile Dyes on Health and Ecosystem: A Review of Structure, Causes, and Potential Solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Zhu, H.; Zhang, J.; Li, S.; Chen, Q.; Wang, C.; Wu, T.; Xu, M. Adsorptive Removal of Organic Dyes via Porous Materials for Wastewater Treatment in Recent Decades: A Review on Species, Mechanisms and Perspectives. Chemosphere 2022, 293, 133464. [Google Scholar] [CrossRef] [PubMed]
- Ciğeroğlu, Z.; El Messaoudi, N.; Şenol, Z.M.; Başkan, G.; Georgin, J.; Gubernat, S. Clay-Based Nanomaterials and Their Adsorptive Removal Efficiency for Dyes and Antibiotics: A Review. Mater. Today Sustain. 2024, 26, 100735. [Google Scholar] [CrossRef]
- Younas, F.; Mustafa, A.; Farooqi, Z.U.R.; Wang, X.; Younas, S.; Mohy-Ud-Din, W.; Ashir Hameed, M.; Mohsin Abrar, M.; Maitlo, A.A.; Noreen, S.; et al. Current and Emerging Adsorbent Technologies for Wastewater Treatment: Trends, Limitations, and Environmental Implications. Water 2021, 13, 215. [Google Scholar] [CrossRef]
- Ahmad, I.; Aftab, M.A.; Fatima, A.; Mekkey, S.D.; Melhi, S.; Ikram, S. A Comprehensive Review on the Advancement of Transition Metals Incorporated on Functional Magnetic Nanocomposites for the Catalytic Reduction and Photocatalytic Degradation of Organic Pollutants. Coord. Chem. Rev. 2024, 514, 215904. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.V.; Ahmed, A.; Boedicker, J.R.; Eliwa, E.M.; Moneam, I.A.; Elseman, A.M.; Mohamed, G.G.; Mohamed, M.M.; Salama, T.M. Water Treatment by Perovskite Materials and Their Applications: A Comprehensive Review. J. Ind. Eng. Chem. 2025, 145, 20–32. [Google Scholar] [CrossRef]
- Long, Z.; Li, Q.; Wei, T.; Zhang, G.; Ren, Z. Historical Development and Prospects of Photocatalysts for Pollutant Removal in Water. J. Hazard. Mater. 2020, 395, 122599. [Google Scholar] [CrossRef]
- Pavel, M.; Anastasescu, C.; State, R.-N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic Degradation of Organic and Inorganic Pollutants to Harmless End Products: Assessment of Practical Application Potential for Water and Air Cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mohamed, A.A. Recent Progress in Semiconductor/Graphene Photocatalysts: Synthesis, Photocatalytic Applications, and Challenges. RSC Adv. 2022, 13, 421–439. [Google Scholar] [CrossRef]
- Hussain, R.T.; Hossain, M.S.; Shariffuddin, J.H. Green Synthesis and Photocatalytic Insights: A Review of Zinc Oxide Nanoparticles in Wastewater Treatment—ScienceDirect. Mater. Today Sustain. 2024, 26, 100764. [Google Scholar] [CrossRef]
- Sherryna, A.; Zerga, A.Y.; Zakaria, Z.Y.; Tahir, M.; Jusoh, M. Advances in the Morphological Design and Dimensional Approaches of Layered Double Hydroxides for Photocatalytic Applications: A Critical Review. Energy Fuels 2025, 39, 4565–4609. [Google Scholar] [CrossRef]
- Nemček, L.; Hagarová, I.; Matúš, P. Layered Double Hydroxides as Next-Generation Adsorbents for the Removal of Selenium from Water. Appl. Sci. 2024, 14, 8513. [Google Scholar] [CrossRef]
- Kloprogge, J.T. X-Ray Photoelectron Spectroscopy (XPS) Study of Layered Double Hydroxides with Different Exchangeable Anions. Appl. Sci. 2025, 15, 1318. [Google Scholar] [CrossRef]
- Khandare, S.D.; Teotia, N.; Kumar, M.; Diyora, P.; Chaudhary, D.R. Biodegradation and Decolorization of Trypan Blue Azo Dye by Marine Bacteria Vibrio Sp. JM-17. Biocatal. Agric. Biotechnol. 2023, 51, 102802. [Google Scholar] [CrossRef]
- Devi, T.B.; Ahmaruzzaman, M. AgNPs-AC Composite for Effective Removal (Degradation) of Napthol Green B Dye from Aqueous Solution. ChemistrySelect 2017, 2, 9201–9210. [Google Scholar] [CrossRef]
- Jayamani, G.; Shanthi, M. An Efficient Nanocomposite CdS-ZnWO4 for the Degradation of Naphthol Green B Dye under UV-A Light Illumination. Nano-Struct. Nano-Objects 2020, 22, 100452. [Google Scholar] [CrossRef]
- Castro, L.V.; Alcántar-Vázquez, B.; Manríquez, M.E.; Albiter, E.; Ortiz-Islas, E. Photodegradation of Emerging Pollutants Using a Quaternary Mixed Oxide Catalyst Derived from Its Corresponding Hydrotalcite. Catalysts 2025, 15, 173. [Google Scholar] [CrossRef]
- Ziyat, H.; Bennani, M.N.; Dehmani, Y.; Houssaini, J.; Allaoui, S.; Kacimi, R.; Hajjaj, H. Adsorptive Performance of a Synthesized Mg-Al Hydrotalcite Compound for Removal of Malachite Green: Kinetic, Isotherm, Thermodynamic, and Mechanism Study. Int. J. Environ. Anal. Chem. 2024, 104, 1072–1091. [Google Scholar] [CrossRef]
- Cao, S.; Cao, J.; Zhu, H.; Huang, Y.; Jin, B.; Materazzi, M. Removal of HCl from Gases Using Modified Calcined Mg-Al-CO3 Hydrotalcite: Performance, Mechanism, and Adsorption Kinetics. Fuel 2024, 355, 129445. [Google Scholar] [CrossRef]
- Bouteiba, A.; Elaziouti, A.; Benhadria, N.; Choubane, H.; Laouedj, N.; Bettahar, N. Sunlight-Driven Photocatalytic Degradation of Rhodamine B by BiOCl and TiO2 Deposited on NiCr-LDH. Int. J. Environ. Anal. Chem. 2023, 103, 6722–6741. [Google Scholar] [CrossRef]
- Chouikh, S.; Cheikh, S.; Imessaoudene, A.; Mouni, L.; Amrane, A.; Benahmed, A.; Bettahar, N. Synthesis and Characterization of the Carbonate Hydrotalcites (NiAl-HT, CoAl-HT, and NiCoAl-HT), and Its Application for Removal of the Anionic Azo Dye Titan Yellow from Aqueous Solution. Sustainability 2023, 15, 7948. [Google Scholar] [CrossRef]
- Rezak, N.; Bahmani, A.; Bettahar, N. Adsorptive Removal of P(V) and Cr(VI) by Calcined Zn-Al-Fe Ternary LDHs. Water Sci. Technol. 2021, 83, 2504–2517. [Google Scholar] [CrossRef]
- Saber, O.; Shaalan, N.M.; Ahmed, F.; Kumar, S.; Alshoaibi, A. One-Step Multi-Doping Process for Producing Effective Zinc Oxide Nanofibers to Remove Industrial Pollutants Using Sunlight. Crystals 2021, 11, 1268. [Google Scholar] [CrossRef]
- Bernard, E.; Zucha, W.J.; Lothenbach, B.; Mäder, U. Stability of Hydrotalcite (Mg-Al Layered Double Hydroxide) in Presence of Different Anions. Cem. Concr. Res. 2022, 152, 106674. [Google Scholar] [CrossRef]
- Hubetska, T.S.; Demchenko, V.Y.; Kobylinska, N.G. Chemical Design of Mg(II)/Fe(III)-Layered Double Hydroxides and Their Sorption Properties Toward Ibuprofen. Theor. Exp. Chem. 2024, 60, 64–75. [Google Scholar] [CrossRef]
- Chevinli, A.S.; Rahmatinejad, J.; Hmidi, N.; Rodrigue, D.; Ye, Z. MgFe Layered Double Hydroxide-Graphene Oxide Nanocomposite Adsorbents for Arsenic Removal. J. Water Process Eng. 2024, 59, 105017. [Google Scholar] [CrossRef]
- Lahlahi-Attalhaoui, A.; Cuadra, J.G.; Porcar, S.; Fraga, D.; Nebot-Diaz, I.; Ribeiro, R.A.P.; Paulo, J.G.; Carda, J.B. A High-Speed Method to Obtain Ni-Zn Ferrite Nanoparticles by Microwave Hydrotalcite Decomposition for Magnetic Applications. J. Alloys Compd. 2024, 1004, 175846. [Google Scholar] [CrossRef]
- Xu, M.; Pan, G.; Guo, Y.; Liang, Q.; Yu, Z.; Cao, Y.; Wang, Y. Highly Efficient Oil-Water Separation and Oil Adsorption with Hydrophobic Hydrotalcite/Polyurethane Porous Composite Foam. J. Water Process Eng. 2024, 60, 105211. [Google Scholar] [CrossRef]
- Fortunato, M.; Piccinni, M.; Pastorino, A.; Cardinale, A.M. A Thermal Study on NiAl-Citrate LDH as Catalyst Precursor for Dry Reforming Reaction. J. Therm. Anal. Calorim. 2024. [Google Scholar] [CrossRef]
- Pöyhtäri, S.; Heikkinen, E.-P.; Heikkilä, A. Kinetics of Thermal Decomposition and Hydrogen Reduction of Cobalt Compounds: A Review. Thermochim. Acta 2025, 746, 179952. [Google Scholar] [CrossRef]
- Awan, I.Z.; Ho, P.H.; Beltrami, G.; Fraisse, B.; Cacciaguerra, T.; Gaudin, P.; Tanchoux, N.; Albonetti, S.; Martucci, A.; Cavani, F.; et al. Composition Effect on the Formation of Oxide Phases by Thermal Decomposition of CuNiM(III) Layered Double Hydroxides with M(III) = Al, Fe. Materials 2024, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Piña-Pérez, Y.; Samaniego-Benitez, J.E.; Tzompantzi, F.; Lartundo-Rojas, L.; Garcia-Garcia, A.; Mantilla, A.; Romero-Ortiz, G. Photocatalytic Hydrogen Production Using Bimetallic and Trimetallic Hydrotalcite as Photocatalysts. Mater. Lett. 2023, 330, 133205. [Google Scholar] [CrossRef]
- Michele, A.D.; Boccalon, E.; Costantino, F.; Bastianini, M.; Vivani, R.; Nocchetti, M. Insight into the Synthesis of LDH Using the Urea Method: Morphology and Intercalated Anion Control. Dalton Trans. 2024, 53, 12543–12553. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH Dependent Surface Charging and Points of Zero Charge. X. Update. Adv. Colloid Interface Sci. 2023, 319, 102973. [Google Scholar] [CrossRef]
- Singh, G.; Ubhi, M.K.; Jeet, K.; Singla, C.; Kaur, M. A Review on Impacting Parameters for Photocatalytic Degradation of Organic Effluents by Ferrites and Their Nanocomposites. Processes 2023, 11, 1727. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Al-Absi, R.S. Mechanistic Understanding of the Adsorption and Thermodynamic Aspects of Cationic Methylene Blue Dye onto Cellulosic Olive Stones Biomass from Wastewater. Sci. Rep. 2020, 10, 15928. [Google Scholar] [CrossRef]
- Vu, V.N.; Pham, T.H.T.; Chanthavong, M.; Do, T.H.; Nguyen, T.H.L.; Nguyen, Q.D.; Tran, T.K.N. Enhanced Photocatalytic Degradation of Rhodamine-B under Led Light Using CuZnAl Hydrotalcite Synthesized by Co-Precipitation Technique. Inorganics 2022, 10, 89. [Google Scholar] [CrossRef]
- Miceli, M.; Frontera, P.; Macario, A.; Malara, A. Recovery/Reuse of Heterogeneous Supported Spent Catalysts. Catalysts 2021, 11, 591. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, A.; Kumar, S.; Thakur, A.; Thakur, R.; Bhatia, S.K.; Sharma, A.K. Multifaceted Potential Applicability of Hydrotalcite-Type Anionic Clays from Green Chemistry to Environmental Sustainability. Chemosphere 2022, 306, 135464. [Google Scholar] [CrossRef]
- Bouddouch, A.; Akhsassi, B.; Amaterz, E.; Bakiz, B.; Taoufyq, A.; Villain, S.; Guinneton, F.; El Aamrani, A.; Gavarri, J.-R.; Benlhachemi, A. Photodegradation under UV Light Irradiation of Various Types and Systems of Organic Pollutants in the Presence of a Performant BiPO4 Photocatalyst. Catalysts 2022, 12, 691. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Xu, C.; Yang, P.; Ng, D.H.L.; Song, P.; Zuo, M. Hierarchically Porous MoS2/CoAl-LDH/HCF with Synergistic Adsorption-Photocatalytic Performance under Visible Light Irradiation. J. Alloys Compd. 2017, 698, 852–862. [Google Scholar] [CrossRef]
- Saber, O.; Osama, M.; Alshoaibi, A.; Shaalan, N.M.; Osama, D. Designing inorganic–magnetic–organic nanohybrids for producing effective photocatalysts for the purification of water. RSC Adv. 2022, 12, 18282–18295. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).