Removal of a Mixture of Pollutants in Air Using a Pilot-Scale Planar Reactor: Competition Effect on Mineralization
Abstract
1. Introduction
2. Results and Discussion
2.1. Oxidation of Butyric Acid
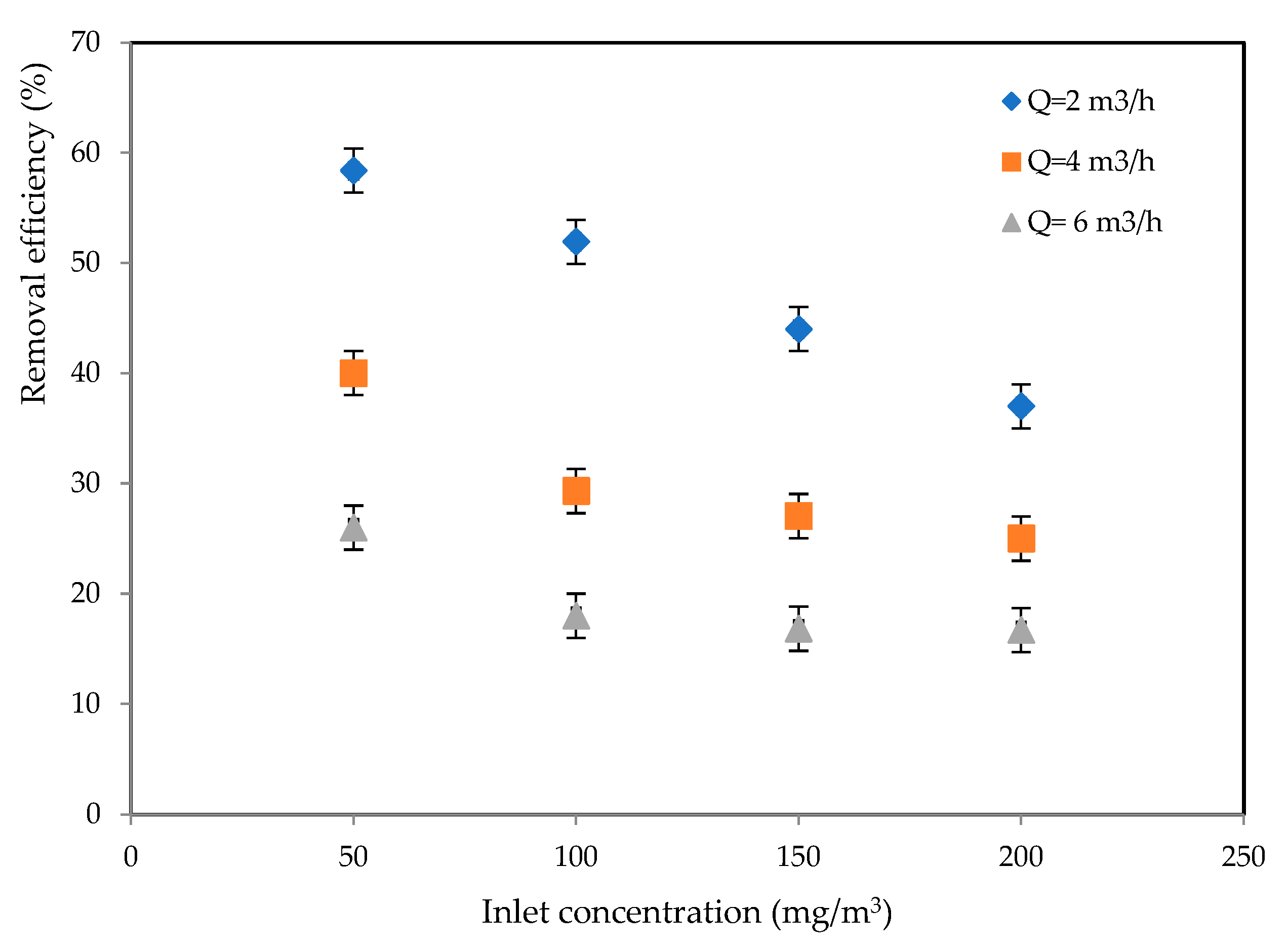
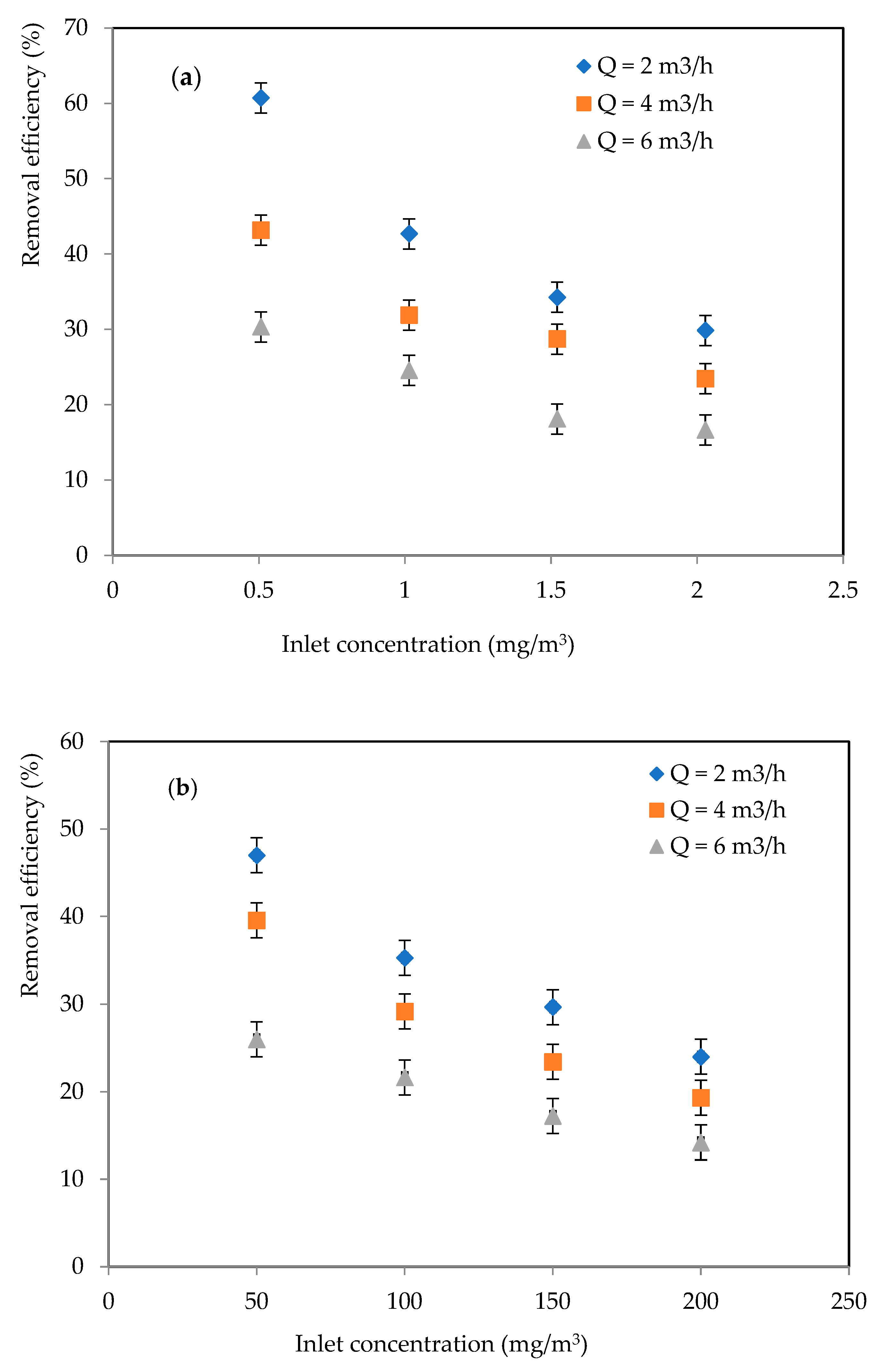
2.1.1. Effects of Inlet Concentration and Flowrate
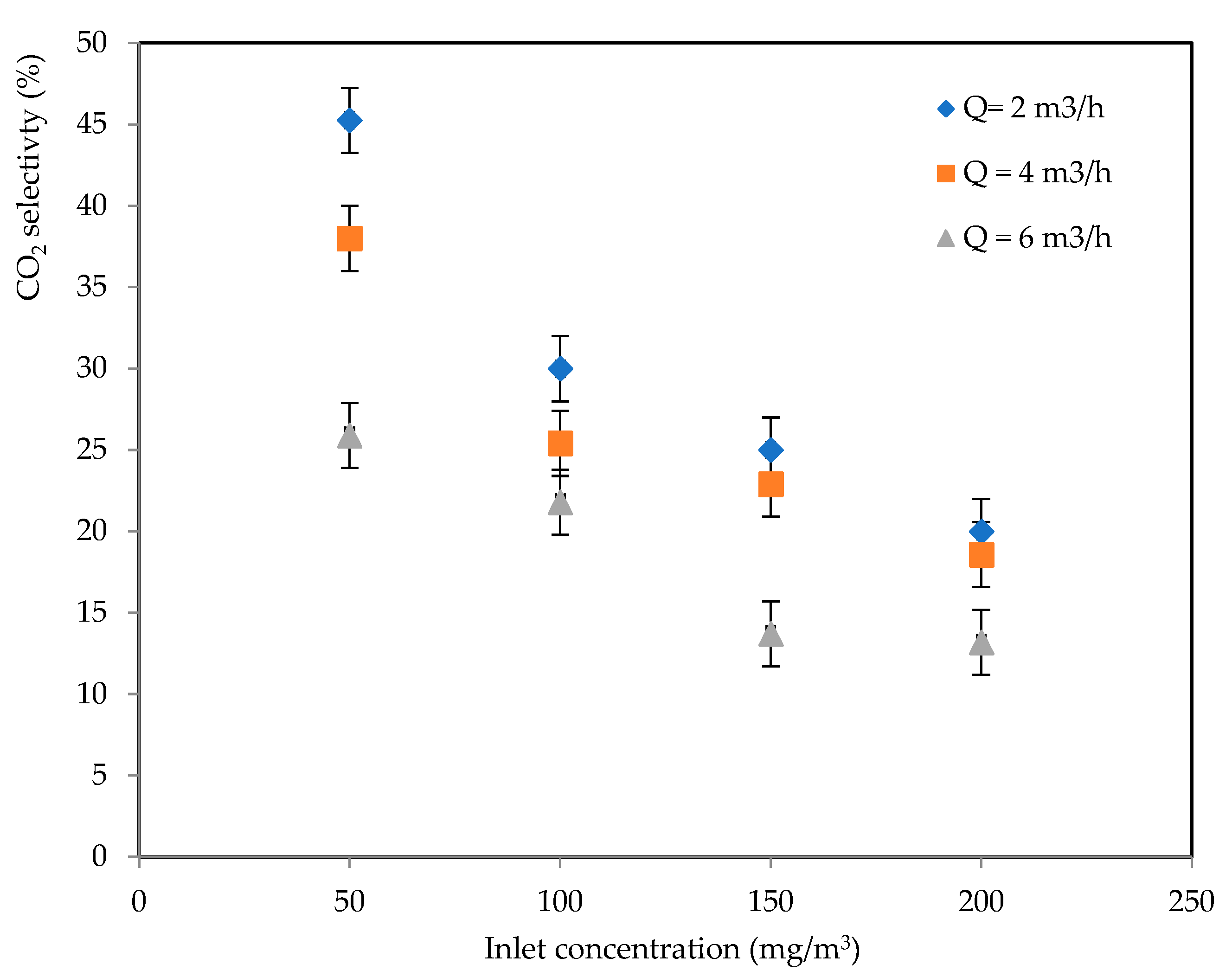
2.1.2. CO2 Selectivity
2.2. Oxidation of Valeric Acid
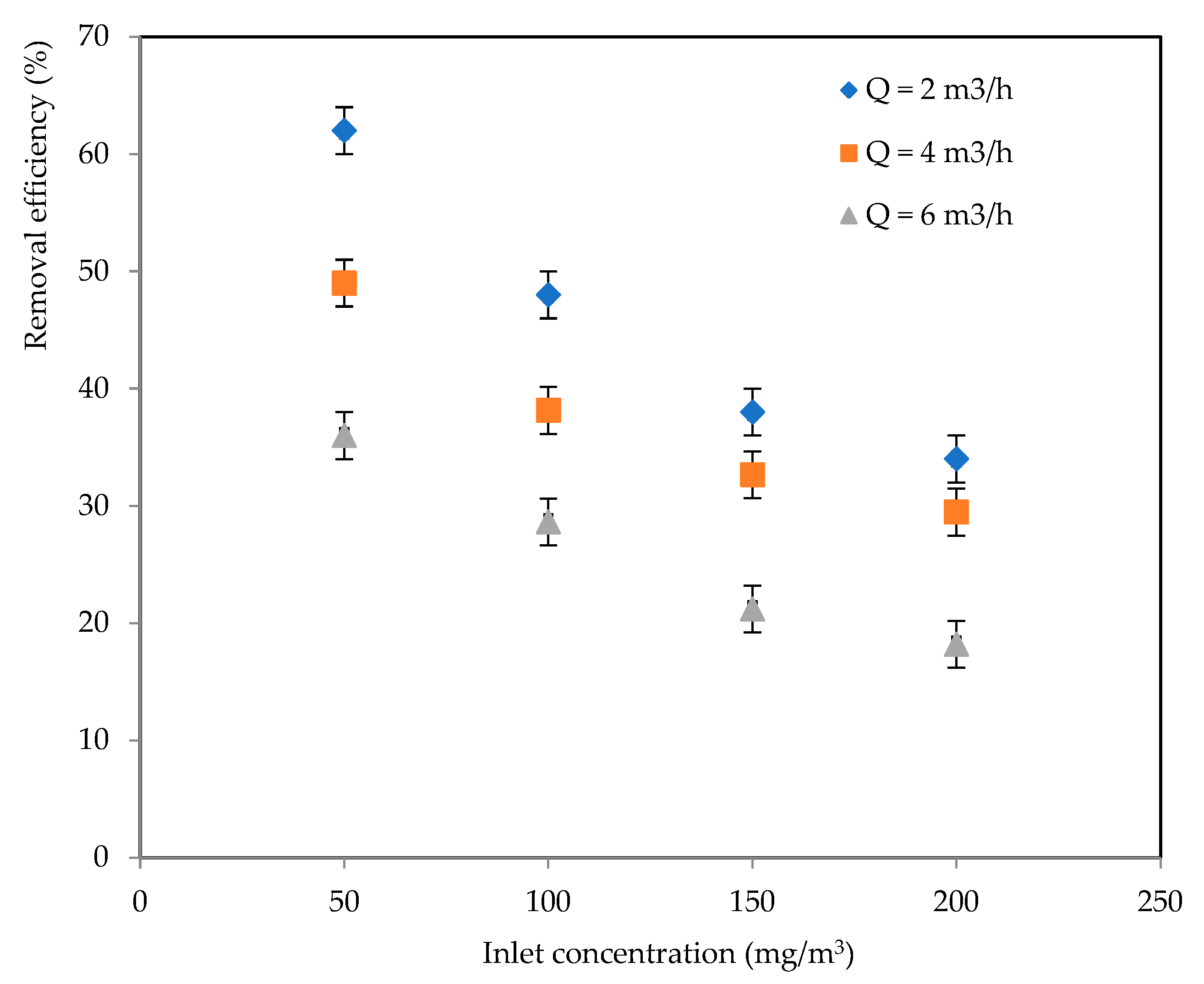
2.2.1. Effects of Inlet Concentration and Flowrate
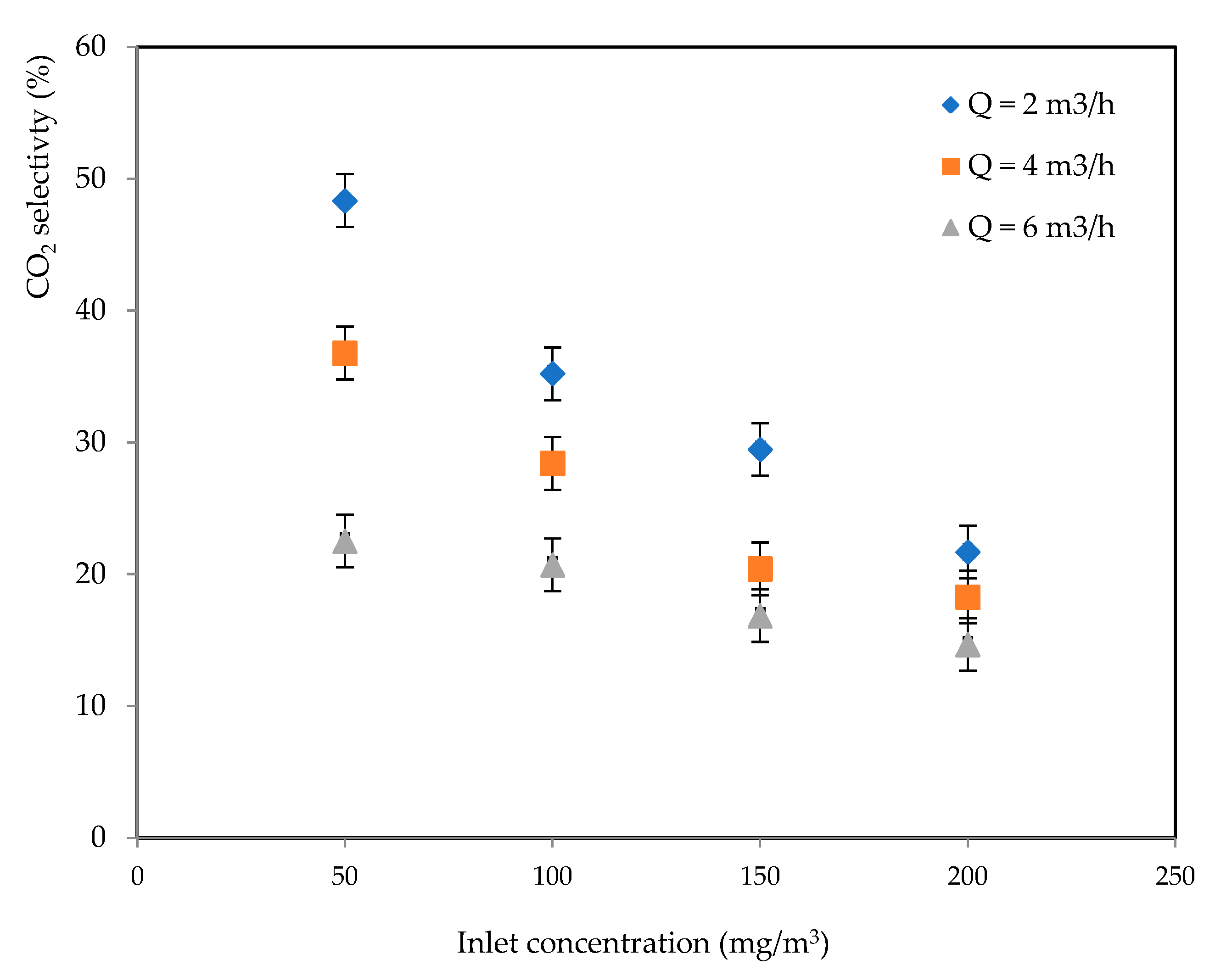
2.2.2. CO2 Selectivity
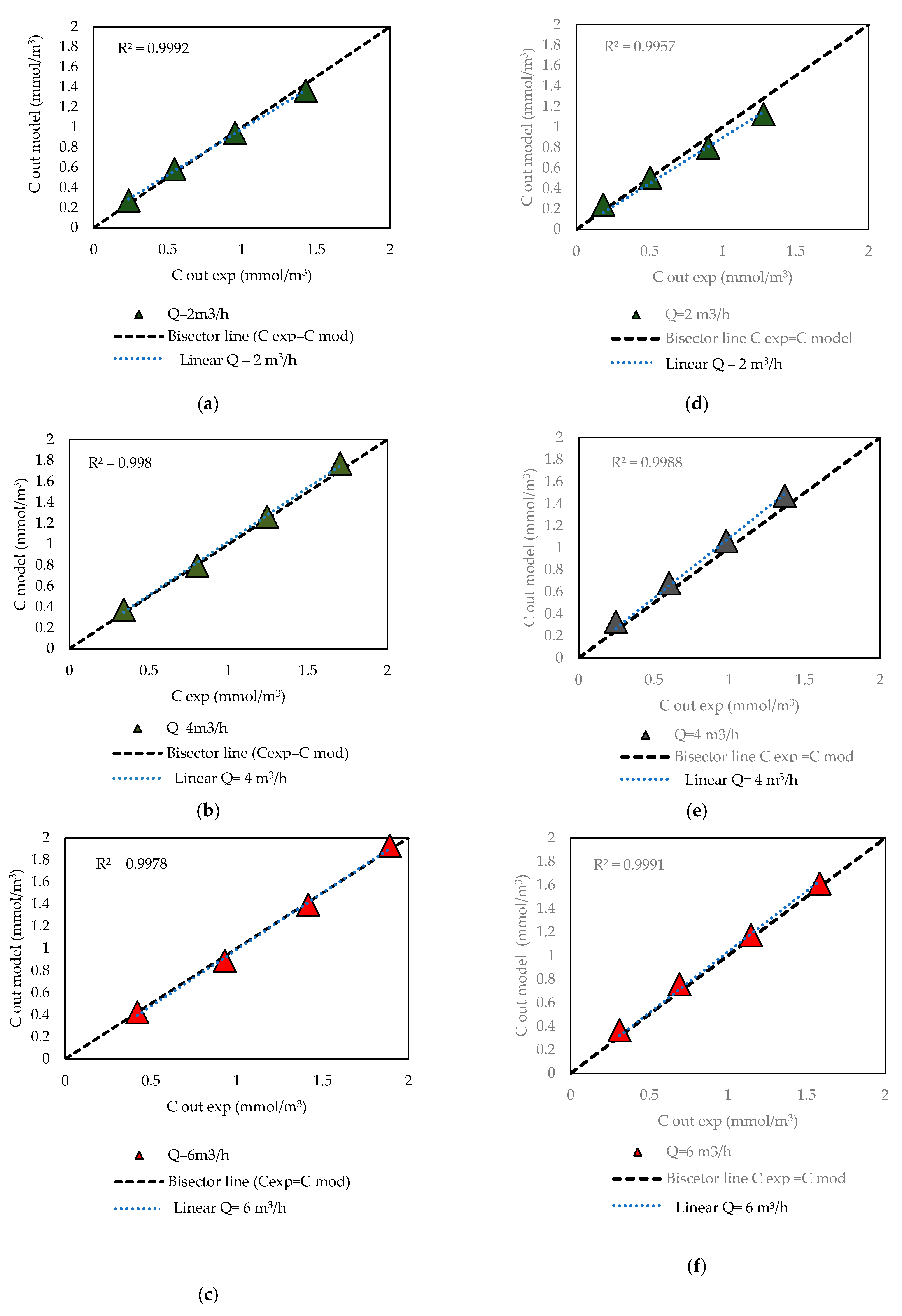
2.3. Kinetic Modeling of Reaction Kinetics with Mass Transfer Consideration
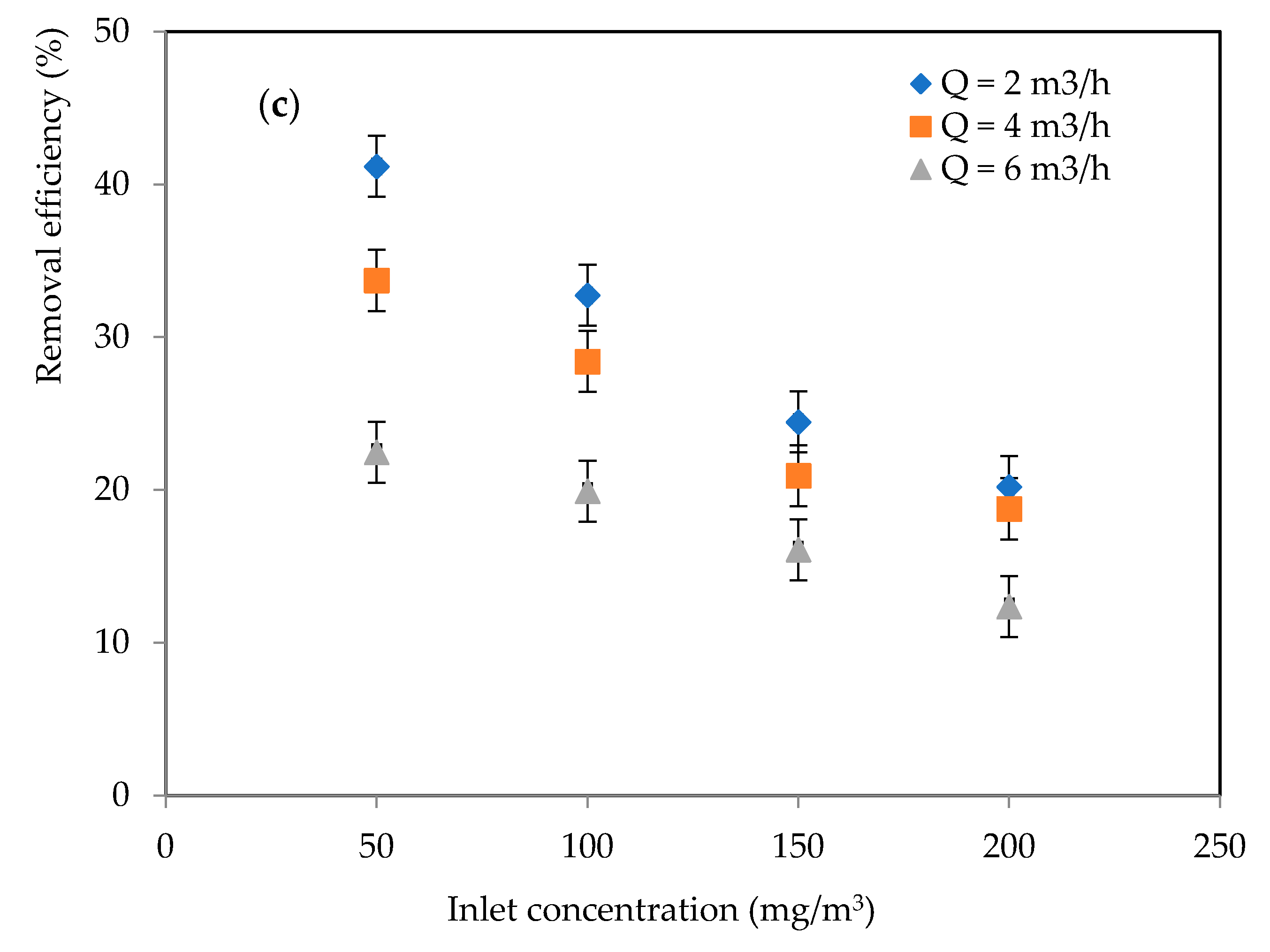
2.4. Oxidation of Binary Mixture Pollutants
2.5. CO2 Selectivity
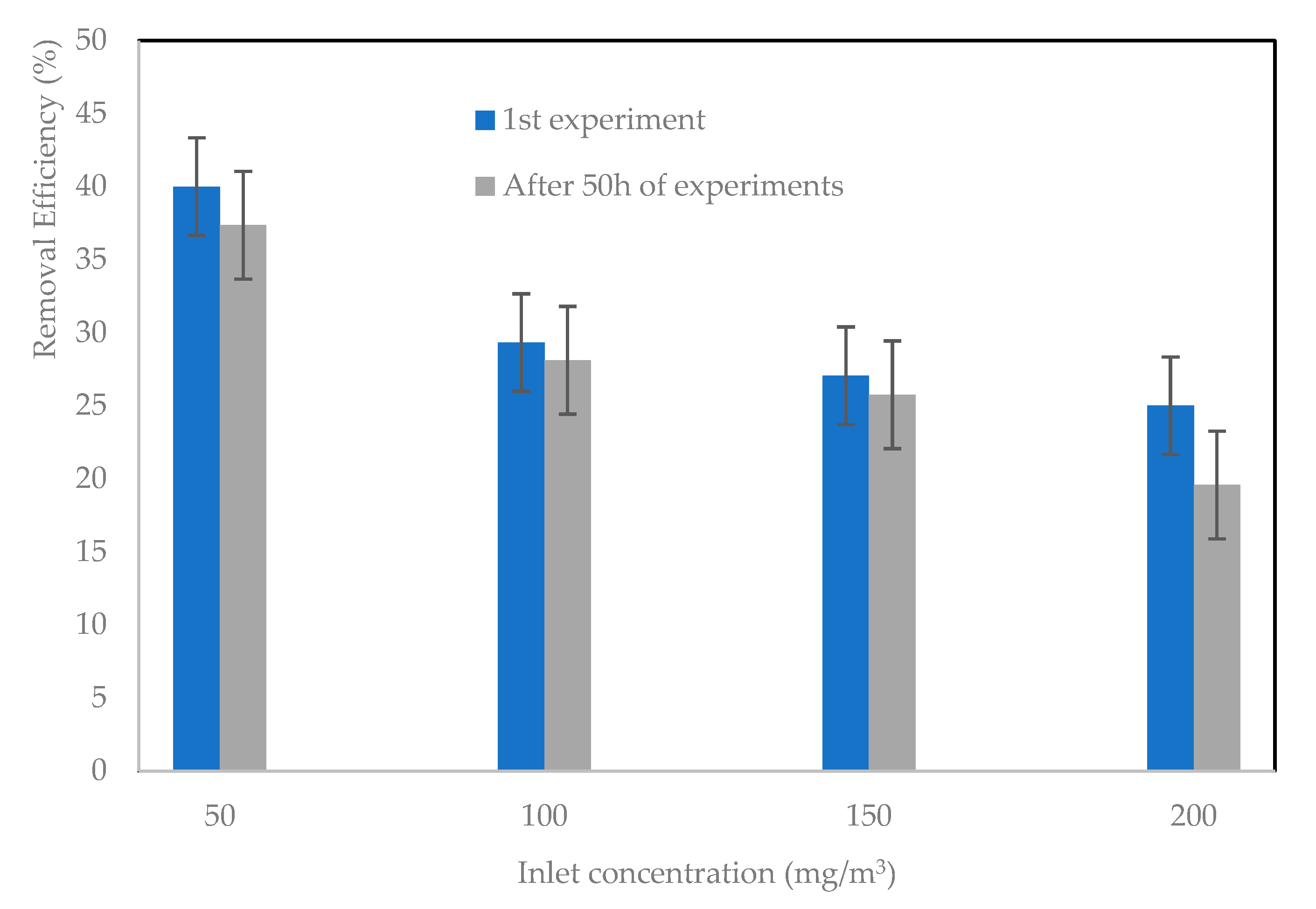
2.6. Reusability and Catalyst Stability
3. Materials and Methods
3.1. Rectangular Reactor
3.2. Catalyst
3.3. Polluted Flow Generation
3.4. Analysis System
3.5. Experimental Protocol
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Binjhade, R.; Mondal, R.; Mondal, S. Continuous photocatalytic reactor: Critical review on the design and performance. J. Environ. Chem. Eng. 2022, 10, 107746. [Google Scholar] [CrossRef]
- Kovalevskiy, N.S.; Lyulyukin, M.N.; Selishchev, D.S.; Kozlov, D.V. Analysis of air photocatalytic purification using a total hazard index: Effect of the composite TiO2/zeolite photocatalyst. J. Hazard. Mater. 2018, 358, 302–309. [Google Scholar] [CrossRef]
- Guo, H.; Su, Y.; Yang, X.; Wang, Y.; Li, Z.; Wu, Y.; Ren, J. Dielectric Barrier Discharge Plasma Coupled with Catalysis for Organic Wastewater Treatment: A Review. Catalysts 2023, 13, 10. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Zhang, X.; Mei, D.; Xu, Y.; Yu, P.; Sun, Y. Nonthermal plasma catalysis enhances simultaneous removal of toluene and ozone over TiO2@ZIF-8. J. Clean. Prod. 2022, 332, 130107. [Google Scholar] [CrossRef]
- Rosa, D.; D’Agostino, F.; Bavasso, I.; Di Palma, L. An Innovative and Easy Method for Iron-Doped Titania Synthesis. Chem. Eng. Trans. 2023, 101, 13–18. [Google Scholar]
- Karoui, S.; Saoud, W.A.; Ghorbal, A.; Fourcade, F.; Amrane, A.; Assadi, A.A. Intensification of non-thermal plasma for aqueous Ciprofloxacin degradation: Optimization study, mechanisms, and combined plasma with photocatalysis. J. Water Process Eng. 2022, 50, 103207. [Google Scholar] [CrossRef]
- Saoud, W.A.; Belkessa, N.; Azzaz, A.A.; Rochas, V.; Mezino, V.; Presset, M.A.; Lechevin, S.; Genouel, A.; Rouxel, S.; Monsimert, D.; et al. Pilot scale investigation of DBD-Plasma photocatalysis for industrial application in livestock building air: Elimination of chemical pollutants and odors. Chem. Eng. J. 2023, 468, 143710. [Google Scholar] [CrossRef]
- Xue, Y.; Sun, W.; Shao, P.; Yuan, Y.; Cui, F.; Shi, W. Degradation of contaminants of PPCPs by photocatalysis for water purification: Kinetics, mechanisms, and cytotoxicity analysis. Chem. Eng. J. 2023, 454, 140505. [Google Scholar] [CrossRef]
- Wang, J.; Huang, D.; Chen, F.; Chen, J.; Jiang, H.; Zhu, Y.; Chen, C.; Zhao, J. Rapid Redox Cycling of Fe(II)/Fe(III) in Microdroplets during Iron-Citric Acid Photochemistry. Environ. Sci. Technol. 2023, 57, 4434–4442. [Google Scholar] [CrossRef]
- Yu, H.; Wang, M.; Yan, J.; Dang, H.; Zhu, H.; Liu, Y.; Wen, M.; Li, G.; Wu, L. Complete mineralization of phenolic compounds in visible-light-driven photocatalytic ozonation with single-crystal WO3 nanosheets: Performance and mechanism investigation. J. Hazard. Mater. 2022, 433, 128811. [Google Scholar] [CrossRef]
- Lyulyukin, M.N.; Kolinko, P.A.; Selishchev, D.S.; Kozlov, D.V. Hygienic aspects of TiO2-mediated photocatalytic oxidation of volatile organic compounds: Air purification analysis using a total hazard index. Appl. Catal. B Environ. 2018, 220, 386–396. [Google Scholar] [CrossRef]
- Mota, H.P.; Quadrado, R.F.N.; Iglesias, B.A.; Fajardo, A.R. Enhanced photocatalytic degradation of organic pollutants mediated by Zn(II)-porphyrin/poly(acrylic acid) hybrid microparticles. Appl. Catal. B Environ. 2020, 277, 119208. [Google Scholar] [CrossRef]
- de Oliveira, R.; Sant’Ana, A.C. Plasmonic photocatalytic degradation of tebuconazole and 2,4-dichlorophenoxyacetic acid by Ag nanoparticles-decorated TiO2 tracked by SERS analysis. Chemosphere 2023, 338, 139490. [Google Scholar] [CrossRef]
- Pavel, M.; Anastasescu, C.; State, R.N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic Degradation of Organic and Inorganic Pollutants to Harmless End Products: Assessment of Practical Application Potential for Water and Air Cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Milani, H.M.; Van Neste, B.; Cosaert, E.; Poelman, D. Assessing the Stability and Photocatalytic Efficiency of a Biodegradable PLA-TiO2 Membrane for Air Purification. Adv. Sustain. Syst. 2024, 9, 2400594. [Google Scholar] [CrossRef]
- Mbrouk, O.; Hafez, H.; Mozia, S.; Othman, A.M.; Mottaleb, M.S.A.A. Stimulated generation of photobiogas by morphologically tuned nanostructured ZnO and ZnO/TiO2. BMC Chem. 2022, 16, 74. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El Jery, A.; Khezami, L.; Assadi, A.A. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Serhane, Y.; Belkessa, N.; Bouzaza, A.; Wolbert, D.; Assadi, A.A. Chemosphere Continuous air purification by front flow photocatalytic reactor: Modelling of the influence of mass transfer step under simulated real conditions. Chemosphere 2022, 295, 133809. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kaushik, R.D.; Purohit, L.P. RGO supported ZnO/SnO2 Z-scheme heterojunctions with enriched ROS production towards enhanced photocatalytic mineralization of phenolic compounds and antibiotics at low temperature. J. Colloid Interface Sci. 2023, 632, 196–215. [Google Scholar] [CrossRef]
- Belkessa, N.; Serhane, Y.; Bouzaza, A.; Khezami, L.; Assadi, A.A. Gaseous ethylbenzene removal by photocatalytic TiO2 nanoparticles immobilized on glass fiber tissue under real conditions: Evaluation of reactive oxygen species contribution to the photocatalytic process. Environ. Sci. Pollut. Res. 2023, 30, 35745–35756. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Tayeb, A.M.; Abdel-Wahab, M.Z.; Ahmed, Z.A.; Bakather, O.Y.; Eldoma, M.A.; Abouatiaa, A.F.F. Degradation of oily effluents using immobilized photocatalyst: Laboratory experimentation and plant design. Alex. Eng. J. 2023, 65, 295–305. [Google Scholar] [CrossRef]
- Huang, W.H.; Lin, C.J.; Huang, T.H.; Chang, C.Y.; Haw, S.C.; Sheu, H.S.; Chen, S.Y.; Dong, C.L.; Kumar, K.; Hwang, B.J.; et al. Mechanistic study for enhanced photocatalytic degradation of acetaminophen by Fe(III) doped TiO2 hollow submicrospheres. Appl. Surf. Sci. 2023, 611, 155634. [Google Scholar] [CrossRef]
- Serhane, Y.; Bouzaza, A.; Wolbert, D.; Assadi, A.A. New UV-LED frontal flow photocatalytic reactor for VOCs treatment: Compactness, intensification and optimization studies. Chem. Eng. J. 2023, 451, 138784. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Lv, X.; Lam, F.L.Y.; Hu, X. Developing SrTiO3/TiO2 heterostructure nanotube array for photocatalytic fuel cells with improved efficiency and elucidating the effects of organic substrates. Chem. Eng. J. 2022, 427, 131602. [Google Scholar] [CrossRef]
- Abutalib, M.M.; Alghamdi, H.M.; Rajeh, A.; Nur, O.; Hezma, A.M.; Mannaa, M.A. Fe3O4/Co3O4-TiO2S-scheme photocatalyst for degradation of organic pollutants and H2production under natural sunlight. J. Mater. Res. Technol. 2022, 20, 1043–1056. [Google Scholar] [CrossRef]
- ZZango, U.; Khoo, K.S.; Garba, A.; Kadir, H.A.; Usman, F.; Zango, M.U.; Da Oh, W.; Lim, J.W. A review on superior advanced oxidation and photocatalytic degradation techniques for perfluorooctanoic acid (PFOA) elimination from wastewater. Environ. Res. 2023, 221, 115326. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Wolbert, D. Photocatalytic oxidation of trimethylamine and isovaleraldehyde in an annular reactor: Influence of the mass transfer and the relative humidity. J. Photochem. Photobiol. A Chem. 2012, 236, 61–69. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.S.H.; Niu, R.; Xu, L.; Lu, Y.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef]
- Bouzaza, A.; Vallet, C.; Laplanche, A. Photocatalytic degradation of some VOCs in the gas phase using an annular flow reactor: Determination of the contribution of mass transfer and chemical reaction steps in the photodegradation process. J. Photochem. Photobiol. A Chem. 2006, 177, 212–217. [Google Scholar] [CrossRef]

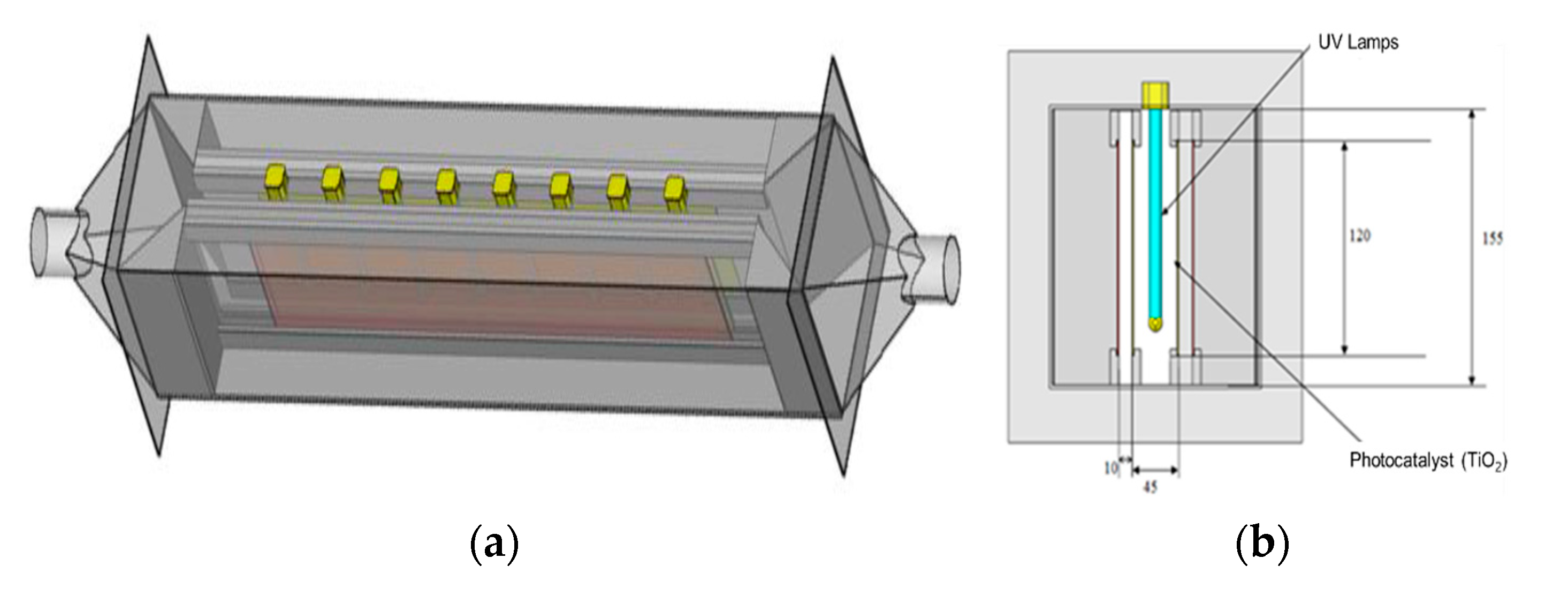
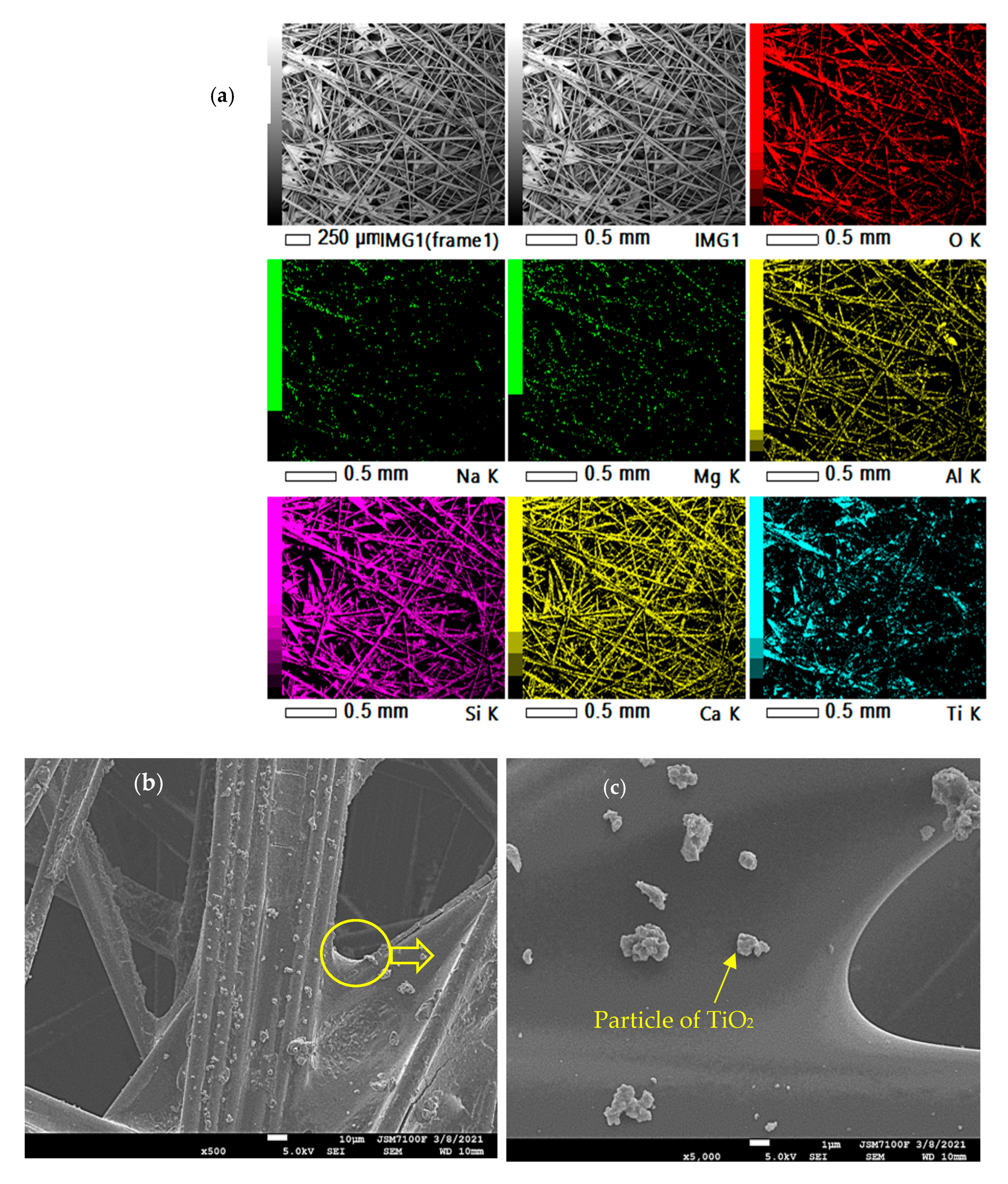
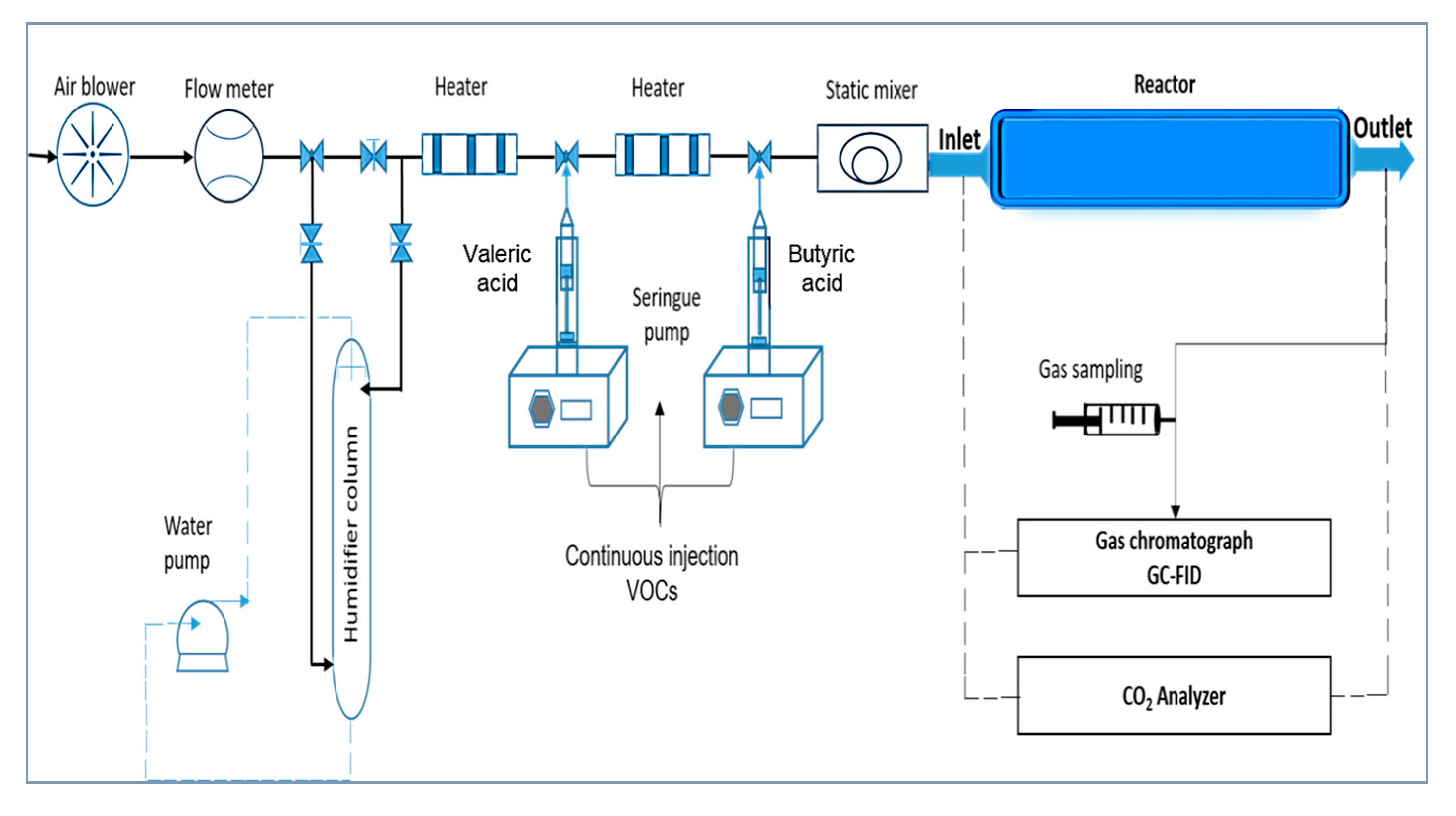
| Compounds | Diffusivity in Air (D, m2/s) |
|---|---|
| Butyric acid | 8.71 × 10−6 |
| Valeric acid | 7.87 × 10−6 |
| Flow Rates (m3/h) | Reynolds | Mass Transfer Coefficient (m/s) | |
|---|---|---|---|
| Butyric Acid | Valeric Acid | ||
| 2 | 434 | 0.00196 | 0.00183 |
| 4 | 868 | 0.00277 | 0.00259 |
| 6 | 1303 | 0.00340 | 0.00318 |
| Butyric Acid | Valeric Acid | ||||
|---|---|---|---|---|---|
| k (mmol/m3.s) | K (m3/mmol) | Correlation (%) | k (mmol/m3.s) | K (m3/mmol) | Correlation (%) |
| 0.13 | 2.60 | 99 | 0.14 | 2.13 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touazi, A.A.; Abidi, M.; Belkessa, N.; Hajjaji, M.-A.; Elfalleh, W.; Assadi, A.A. Removal of a Mixture of Pollutants in Air Using a Pilot-Scale Planar Reactor: Competition Effect on Mineralization. Catalysts 2025, 15, 595. https://doi.org/10.3390/catal15060595
Touazi AA, Abidi M, Belkessa N, Hajjaji M-A, Elfalleh W, Assadi AA. Removal of a Mixture of Pollutants in Air Using a Pilot-Scale Planar Reactor: Competition Effect on Mineralization. Catalysts. 2025; 15(6):595. https://doi.org/10.3390/catal15060595
Chicago/Turabian StyleTouazi, Ahmed Amin, Mabrouk Abidi, Nacer Belkessa, Mohamed-Aziz Hajjaji, Walid Elfalleh, and Amine Aymen Assadi. 2025. "Removal of a Mixture of Pollutants in Air Using a Pilot-Scale Planar Reactor: Competition Effect on Mineralization" Catalysts 15, no. 6: 595. https://doi.org/10.3390/catal15060595
APA StyleTouazi, A. A., Abidi, M., Belkessa, N., Hajjaji, M.-A., Elfalleh, W., & Assadi, A. A. (2025). Removal of a Mixture of Pollutants in Air Using a Pilot-Scale Planar Reactor: Competition Effect on Mineralization. Catalysts, 15(6), 595. https://doi.org/10.3390/catal15060595







