Non-Platinum Group Metal Oxygen Reduction Catalysts for a Hydrogen Fuel Cell Cathode: A Mini-Review
Abstract
1. Introduction
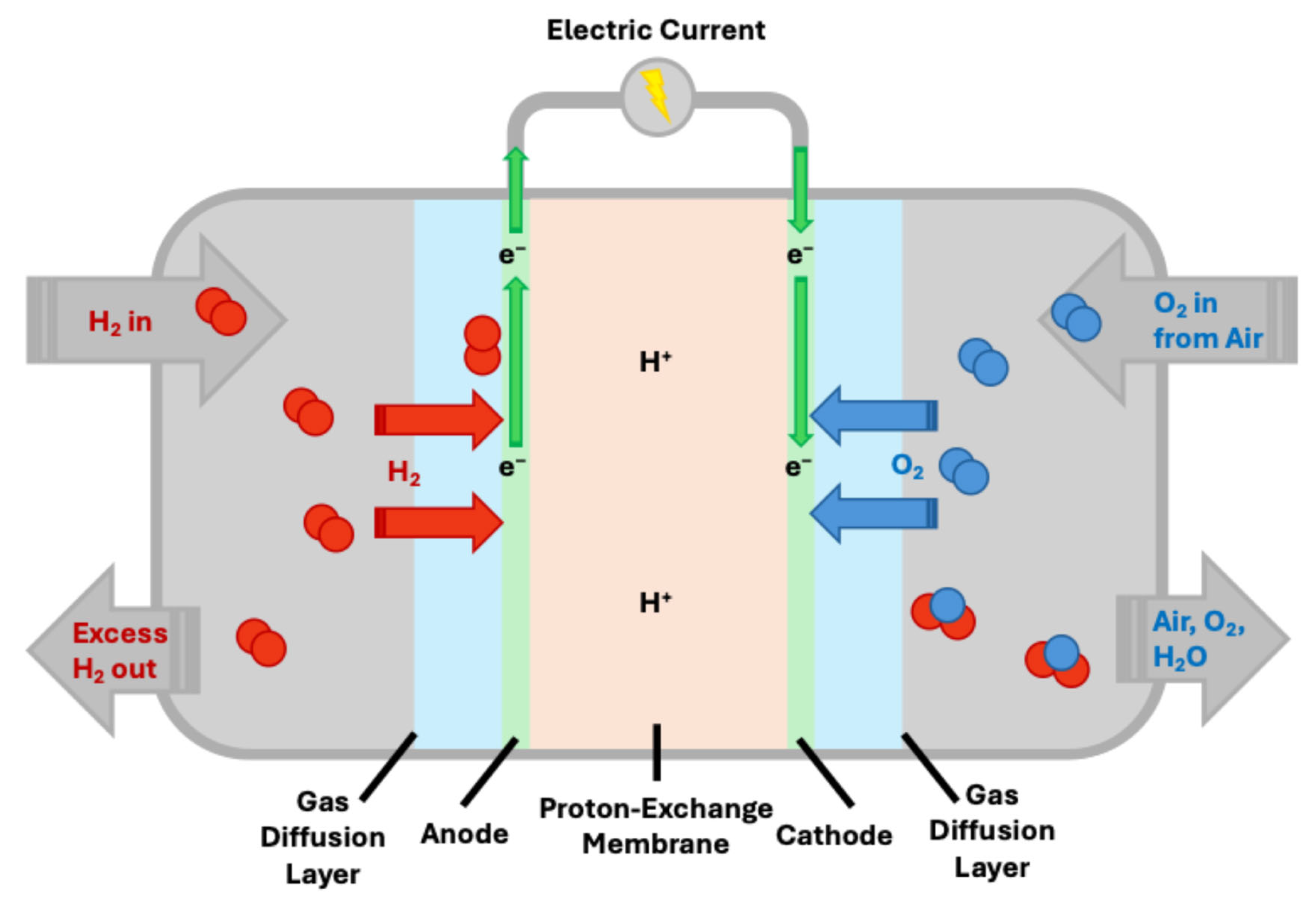
2. Fundamental Aspects of Fuel Cells and ORR
Oxygen Reduction Reaction
3. Synthesis Strategies and Material Design of Non-PGM ORR Catalysts
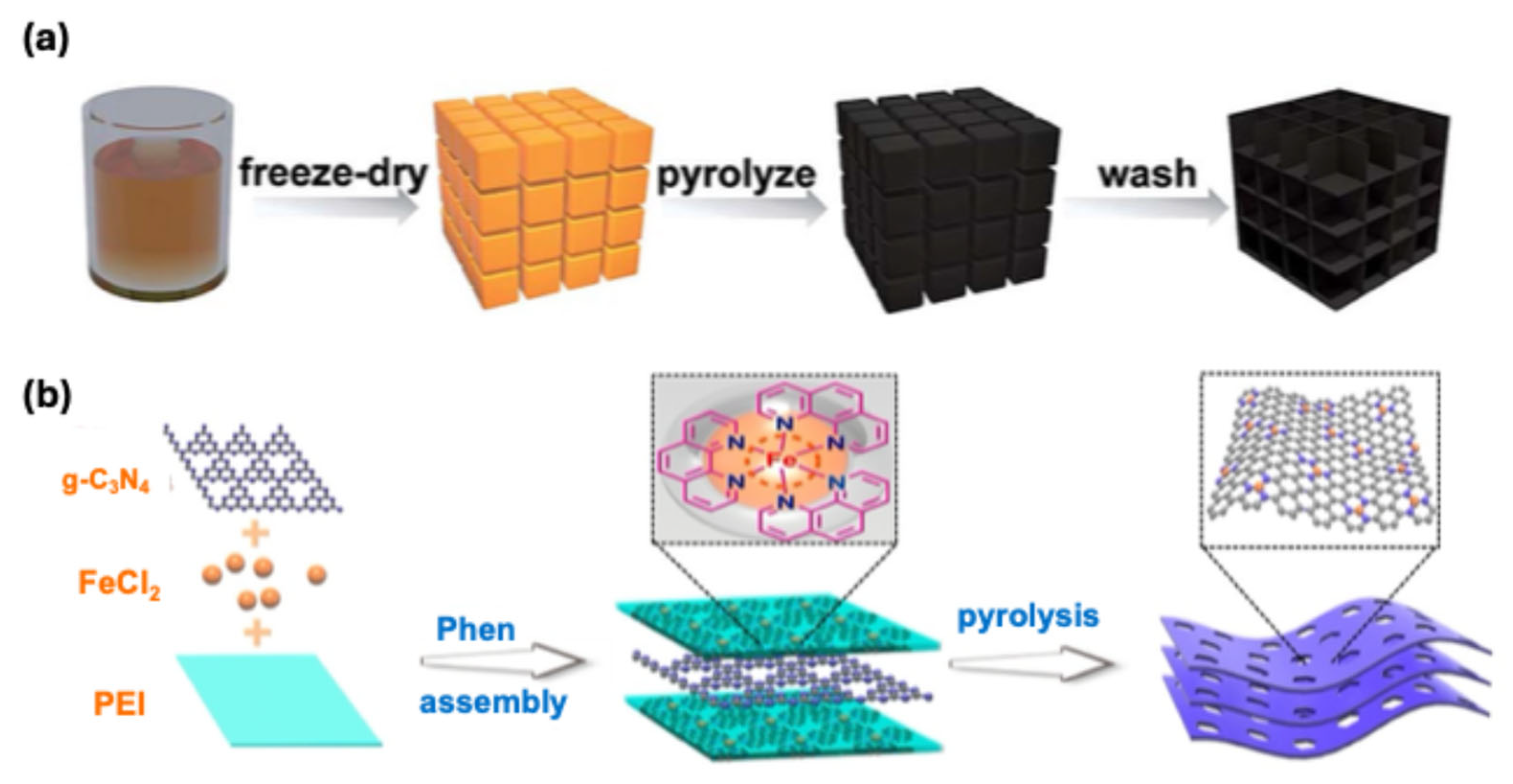
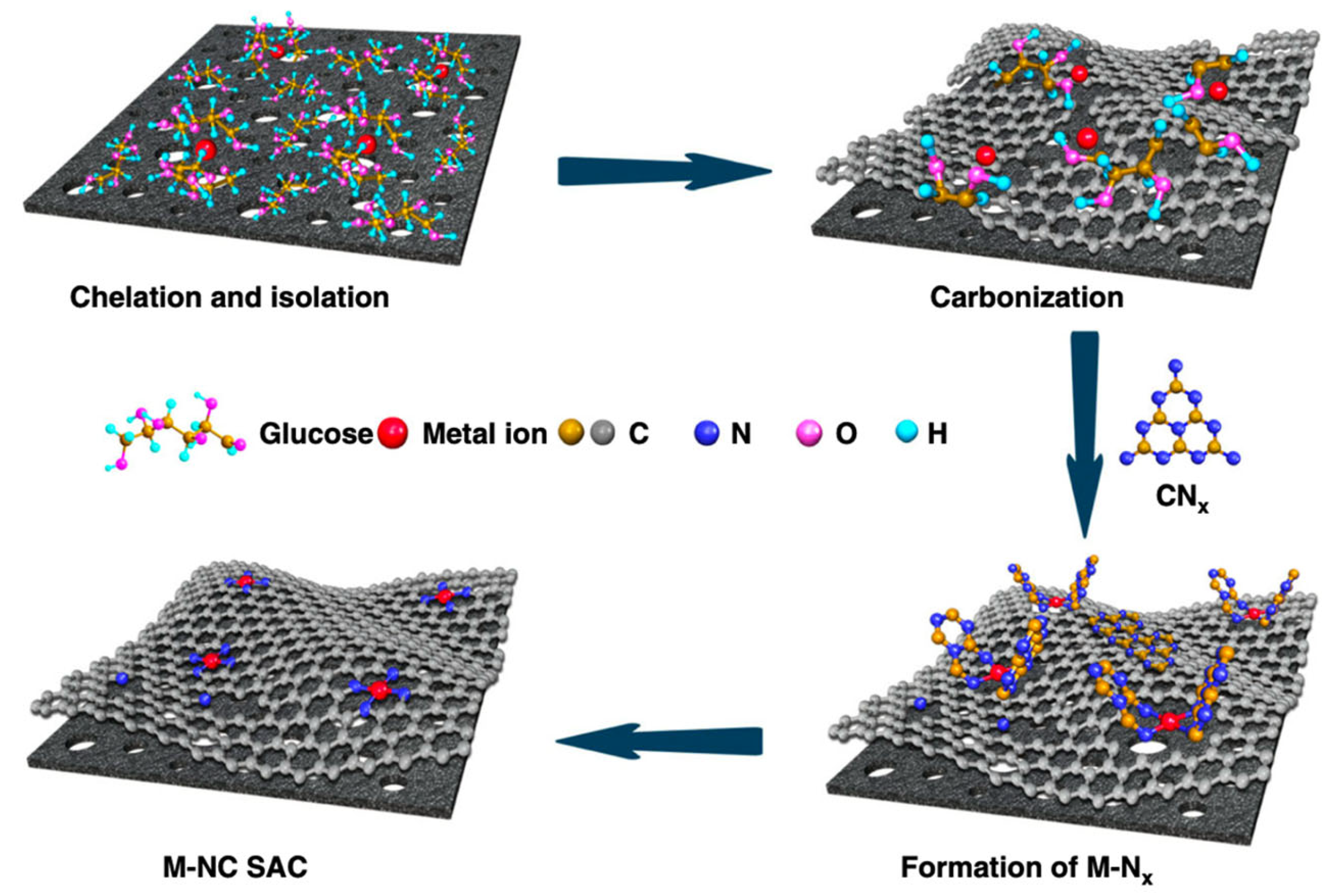
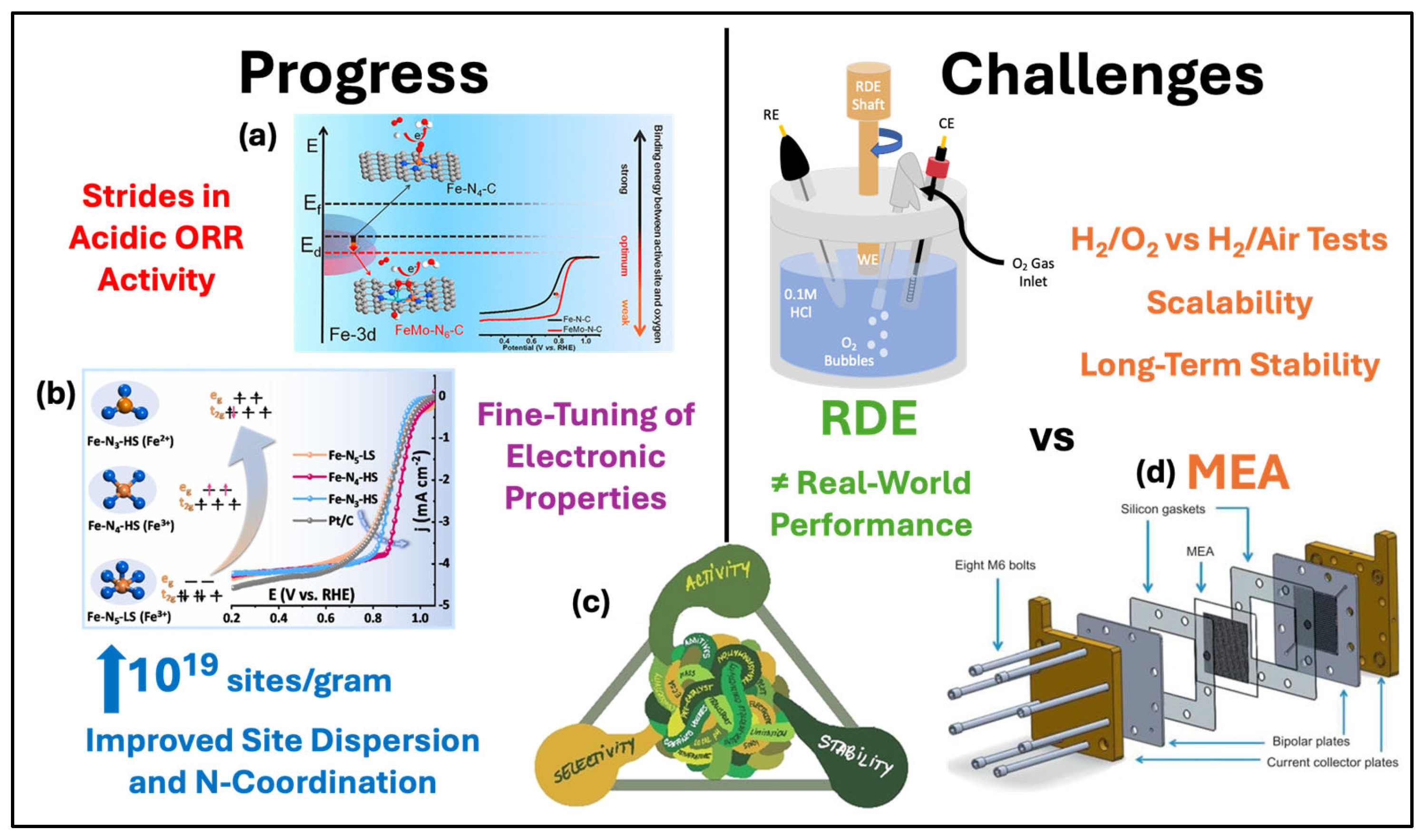
3.1. Iron–Nitrogen–Carbon (Fe-N-C) Catalysts
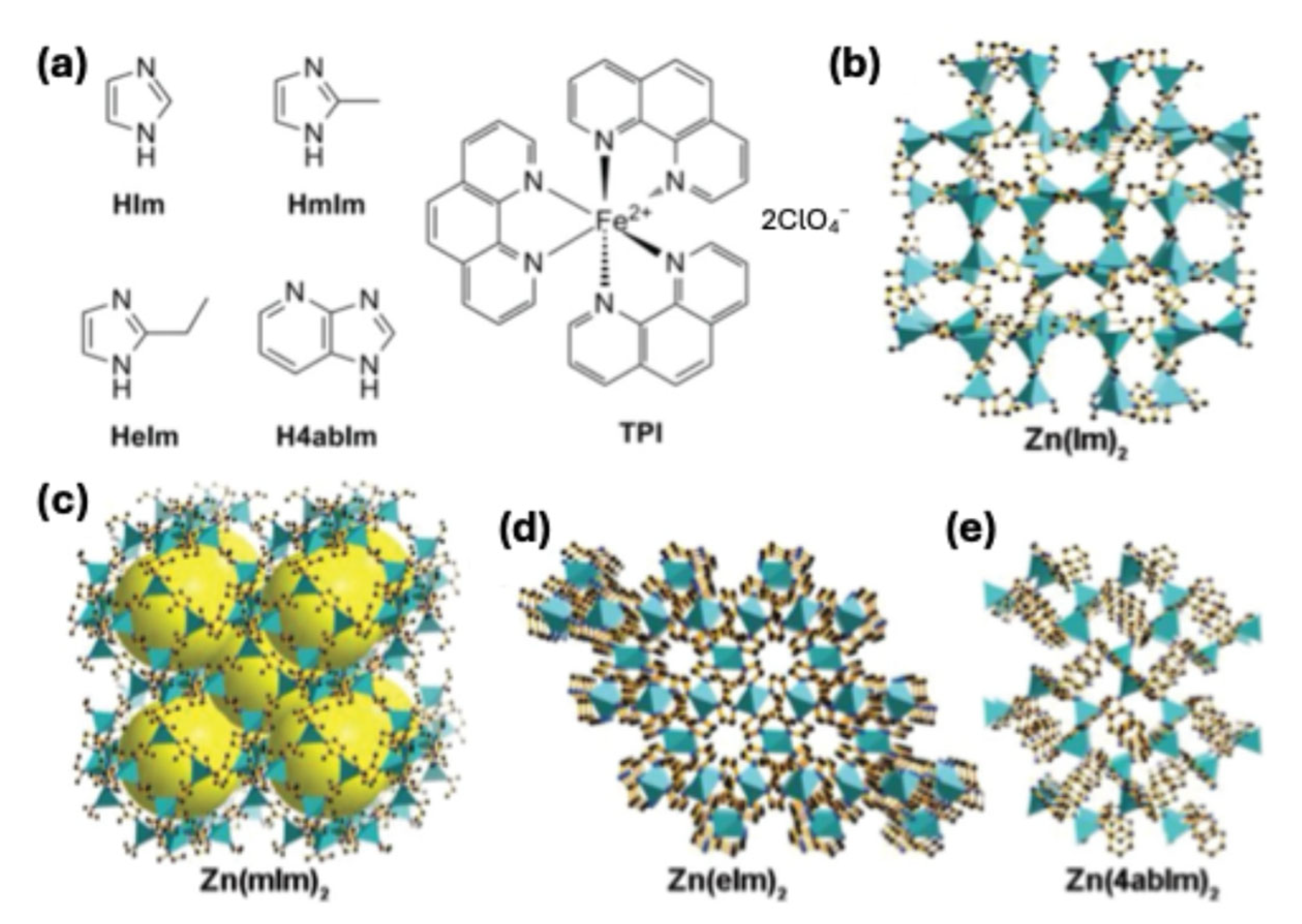
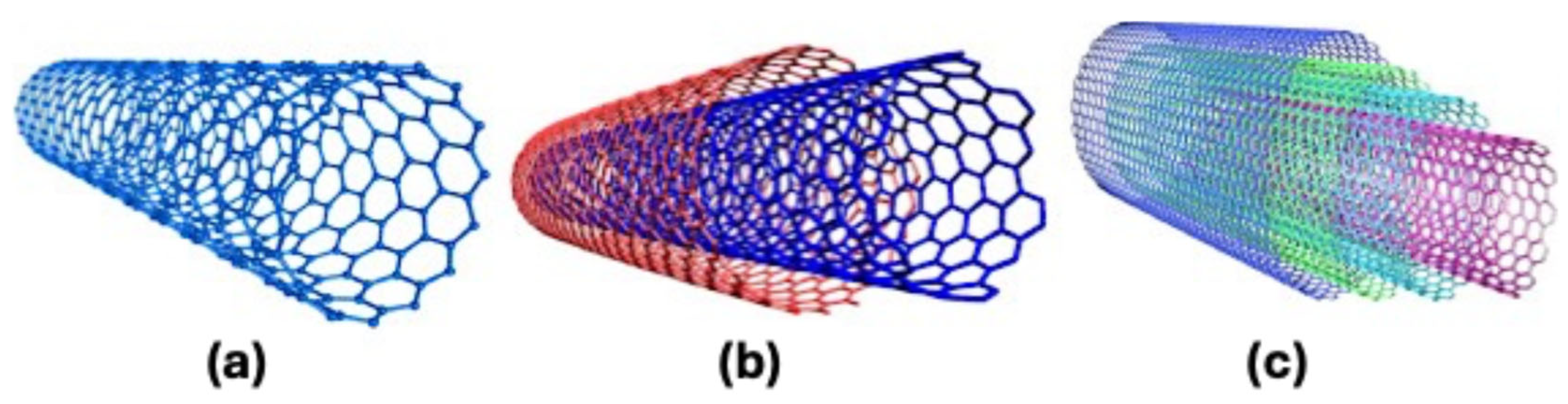
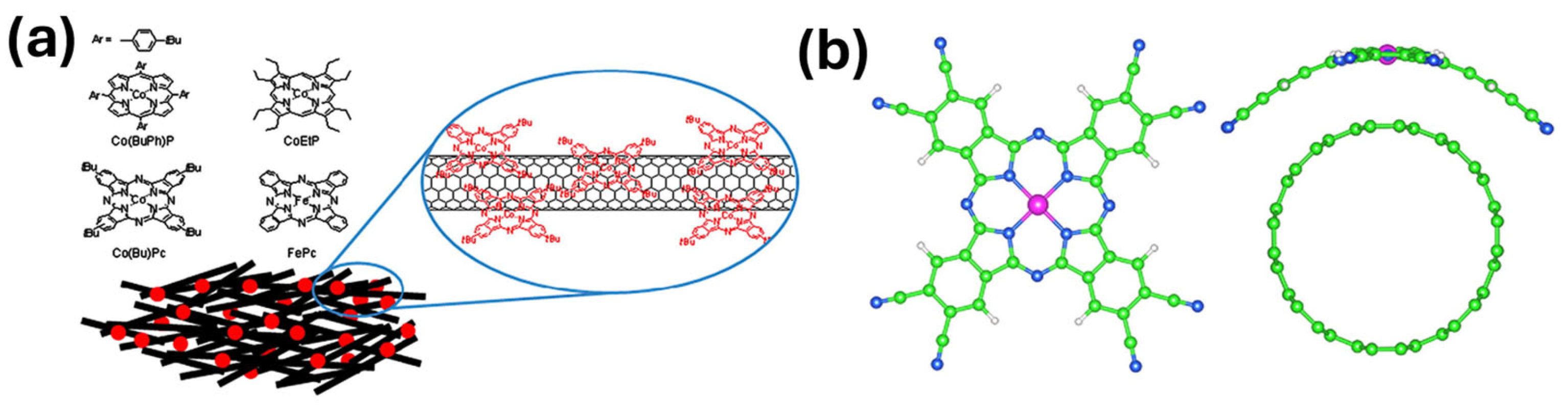
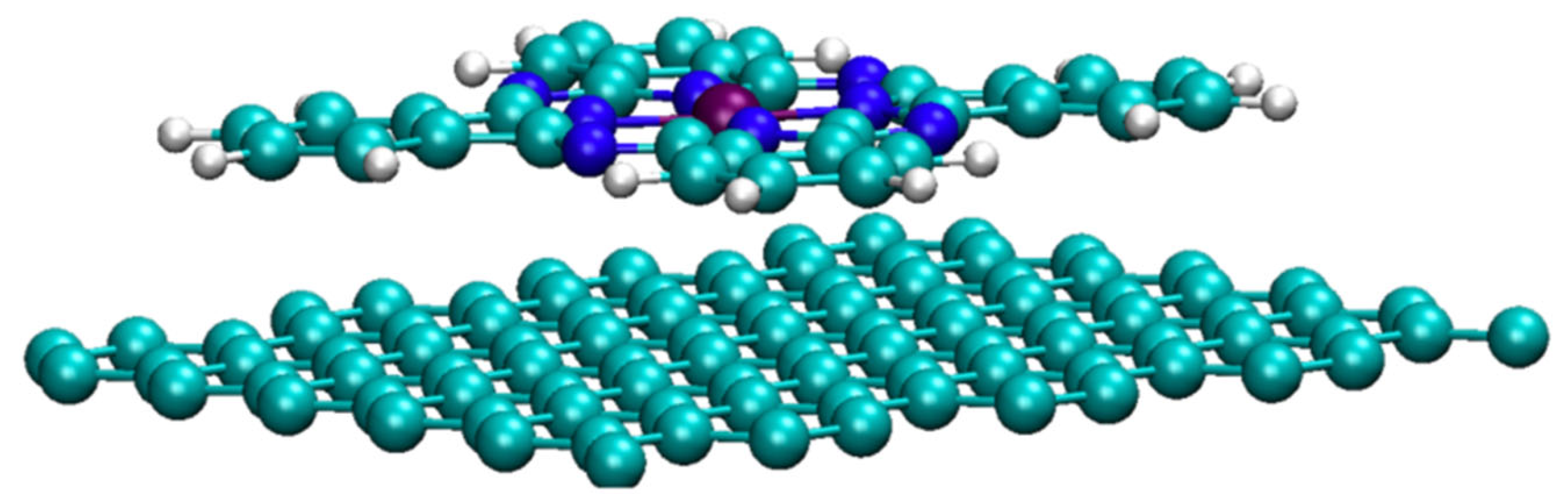
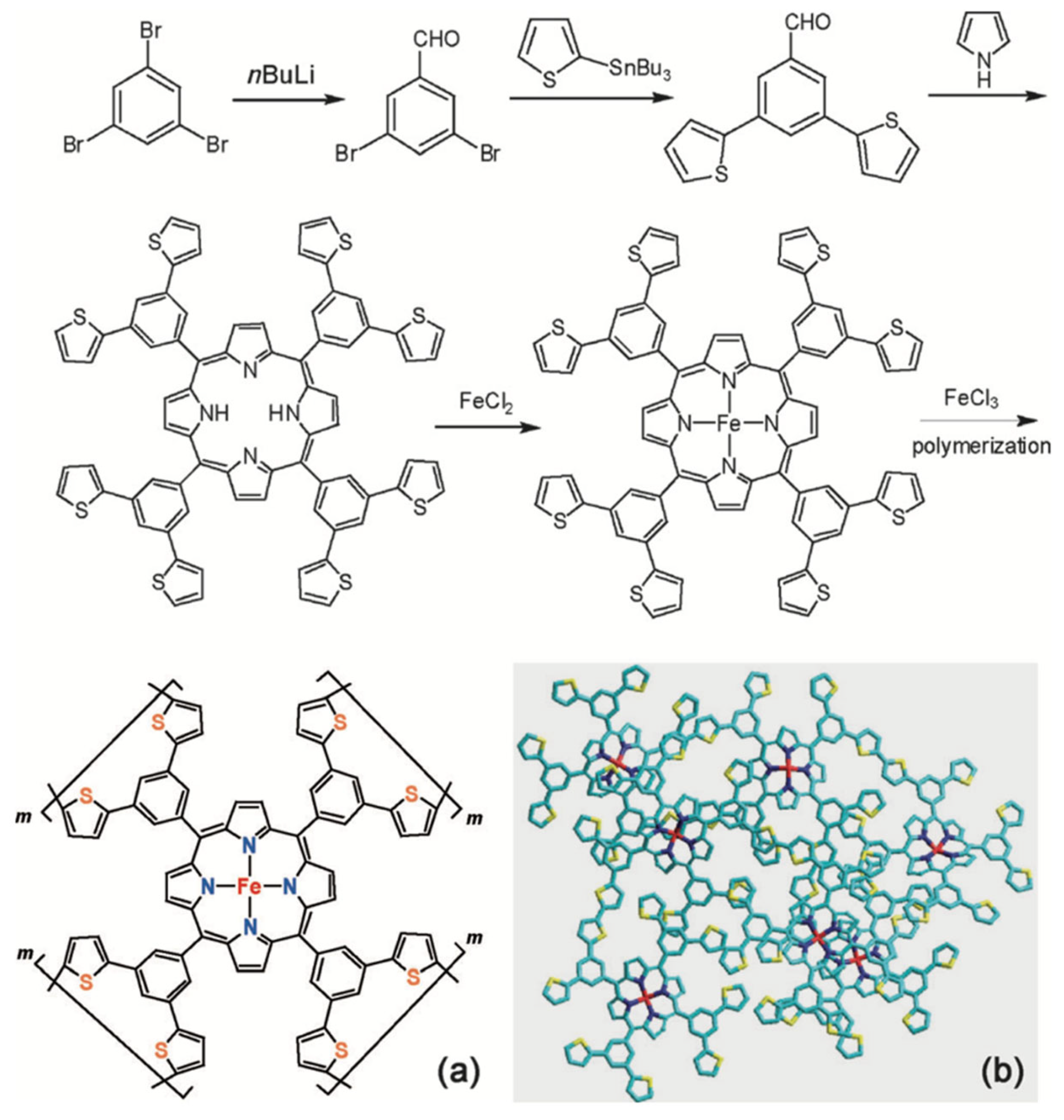
3.2. Molecular Catalysts on Carbon Supports
4. Performance Evaluation of Non-PGM Catalysts
4.1. Electrochemical Characterization Techniques
4.2. Comparison of ORR Activity
4.3. Future Perspectives and Challenges
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alternative Fuels Data Center: Vehicle Registration Counts by State. 2022. Available online: https://afdc.energy.gov (accessed on 7 June 2025).
- EIA (U.S. Energy Information Administration). Gasoline Explained. 2016. Available online: https://www.eia.gov/energyexplained/gasoline/ (accessed on 7 June 2025).
- United States Environmental Protection Agency. Greenhouse Gas Emissions from a Typical Passenger Vehicle; US EPA: Washington, DC, USA, 2022.
- NASA. What Is the Greenhouse Effect? In Global Climate Change: Vital Signs of the Planet; NASA: Washington, DC, USA, 2019. [Google Scholar]
- The Causes of Climate Change. In Climate Change: Vital Signs of the Planet; NASA: Washington, DC, USA, 2019.
- Kuo, G. When Fossil Fuels Run Out, What Then? MAHB: Stanford, CA, USA, 2019. [Google Scholar]
- Lindsey, R. Climate Change: Atmospheric Carbon Dioxide. 2023. Available online: https://Climate.gov (accessed on 7 June 2025).
- Dulău, L.-I. CO2 Emissions of Battery Electric Vehicles and Hydrogen Fuel Cell Vehicles. Clean Technol. 2023, 5, 696–712. [Google Scholar] [CrossRef]
- Kim, J.W.; Boo, K.J.; Cho, J.H.; Moon, I. 1-Key challenges in the development of an infrastructure for hydrogen production, delivery, storage and use. In Advances in Hydrogen Production, Storage and Distribution; Basile, A., Iulianelli, A., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 3–31. [Google Scholar]
- Thompson, S.T.; James, B.D.; Huya-Kouadio, J.M.; Houchins, C.; DeSantis, D.A.; Ahluwalia, R.; Wilson, A.R.; Kleen, G.; Papageorgopoulos, D. Direct hydrogen fuel cell electric vehicle cost analysis: System and high-volume manufacturing description, validation, and outlook. J. Power Sources 2018, 399, 304–313. [Google Scholar] [CrossRef]
- Wang, C.; Spendelow, J.S. Recent developments in Pt–Co catalysts for proton-exchange membrane fuel cells. Curr. Opin. Electrochem. 2021, 28, 100715. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Koestner, R.; Gu, W.; Thompson, L.; Kongkanand, A. Boosting Fuel Cell Performance with Accessible Carbon Mesopores. ACS Energy Lett. 2018, 3, 618–621. [Google Scholar] [CrossRef]
- Müller-Hülstede, J.; Uhlig, L.M.; Schmies, H.; Schonvogel, D.; Meyer, Q.; Nie, Y.; Zhao, C.; Vidakovic, J.; Wagner, P. Towards the Reduction of Pt Loading in High Temperature Proton Exchange Membrane Fuel Cells—Effect of Fe−N−C in Pt-Alloy Cathodes. ChemSusChem 2023, 16, e202202046. [Google Scholar] [CrossRef]
- Chen, L.; Wan, X.; Zhao, X.; Li, W.; Liu, X.; Zheng, L.; Liu, Q.; Yu, R.; Shui, J. Spatial porosity design of Fe-N-C catalysts for high power density PEM fuel cells and detection of water saturation of the catalyst layer by a microwave method. J. Mater. Chem. A 2022, 10, 7764–7772. [Google Scholar] [CrossRef]
- Asset, T.; Atanassov, P. Iron-Nitrogen-Carbon Catalysts for Proton Exchange Membrane Fuel Cells. Joule 2020, 4, 33–44. [Google Scholar] [CrossRef]
- Birry, L.; Zagal, J.H.; Dodelet, J.-P. Does CO poison Fe-based catalysts for ORR? Electrochem. Commun. 2010, 12, 628–631. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, Y.; Liu, W.; Sun, X. The chemistry, recent advancements and activity descriptors for macrocycles based electrocatalysts in oxygen reduction reaction. Coord. Chem. Rev. 2020, 402, 213047. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Wang, X.X.; Swihart, M.T.; Wu, G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat. Catal. 2019, 2, 578–589. [Google Scholar] [CrossRef]
- Rabis, A.; Rodriguez, P.; Schmidt, T.J. Electrocatalysis for Polymer Electrolyte Fuel Cells: Recent Achievements and Future Challenges. ACS Catal. 2012, 2, 864–890. [Google Scholar] [CrossRef]
- Vaca, S. Toyota Introduces Second-Generation Mirai Fuel Cell Electric Vehicle as Design and Technology Flagship Sedan; Toyota USA Newsroom: Plano, TX, USA, 2020. [Google Scholar]
- Bezerra, C.W.B.; Zhang, L.; Lee, K.; Liu, H.; Marques, A.L.B.; Marques, E.P.; Wang, H.; Zhang, J. A review of Fe-N/C and Co–N/C catalysts for the oxygen reduction reaction. Electrochim. Acta 2008, 53, 4937–4951. [Google Scholar] [CrossRef]
- Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.-P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Qu, Y.; Yuan, T.; Wang, W.; Wu, Y.; Li, Y. Review of Metal Catalysts for Oxygen Reduction Reaction: From Nanoscale Engineering to Atomic Design. Chem 2019, 5, 1486–1511. [Google Scholar] [CrossRef]
- Shen, S.; Chen, J.; Yan, X.; Cheng, X.; Zhao, L.; Ren, Z.; Li, L.; Zhang, J. Insights into properties of non-precious metal catalyst (NPMC)-based catalyst layer for proton exchange membrane fuel cells. J. Power Sources 2021, 496, 229817. [Google Scholar] [CrossRef]
- Liu, J.; Li, E.; Ruan, M.; Song, P.; Xu, W. Recent Progress on Fe/N/C Electrocatalysts for the Oxygen Reduction Reaction in Fuel Cells. Catalysts 2015, 5, 1167–1192. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-Doped Graphene-Based Materials for Energy-Relevant Electrocatalytic Processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y.; Lv, X.; Han, D.; Zhang, Q.; Niu, L.; Chen, W. Enhanced Catalytic Performance of Pt-Free Iron Phthalocyanine by Graphene Support for Efficient Oxygen Reduction Reaction. ACS Catal. 2013, 3, 1263–1271. [Google Scholar] [CrossRef]
- Yuan, S.; Shui, J.-L.; Grabstanowicz, L.; Chen, C.; Commet, S.; Reprogle, B.; Xu, T.; Yu, L.; Liu, D.-J. A Highly Active and Support-Free Oxygen Reduction Catalyst Prepared from Ultrahigh-Surface-Area Porous Polyporphyrin. Angew. Chem. 2013, 52, 8349–8353. [Google Scholar] [CrossRef]
- Liu, K.; Fu, J.; Lin, Y.; Luo, T.; Ni, G.; Li, H.; Lin, Z.; Liu, M. Insights into the activity of single-atom Fe-N-C catalysts for oxygen reduction reaction. Nat. Commun. 2022, 13, 2075. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, R.; Tao, X.; Qiu, Z.; Chen, G.; Yang, J.; Zhao, Y.; Feng, X.; Müllen, K. Boosting Oxygen Electrocatalytic Activity of Fe-N-C Catalysts by Phosphorus Incorporation. J. Am. Chem. Soc. 2023, 145, 3647–3655. [Google Scholar] [CrossRef] [PubMed]
- Marshall-Roth, T.; Libretto, N.J.; Wrobel, A.T.; Anderton, K.J.; Pegis, M.L.; Ricke, N.D.; Voorhis, T.V.; Miller, J.T.; Surendranath, Y. A pyridinic Fe-N4 macrocycle models the active sites in Fe/N-doped carbon electrocatalysts. Nat. Commun. 2020, 11, 5283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, Z.-Y.; Lai, Y.-J.; You, Y.; Liu, J.-G.; Wu, X.-L.; Terefe, E.; Chen, C.; Song, L.; Rauf, M.; et al. Phenylenediamine-Based FeNx/C Catalyst with High Activity for Oxygen Reduction in Acid Medium and Its Active-Site Probing. J. Am. Chem. Soc. 2014, 136, 10882–10885. [Google Scholar] [CrossRef]
- Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.-P. Iron-Based Catalysts with Improved Oxygen Reduction Activity in Polymer Electrolyte Fuel Cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef]
- Ao, X.; Zhang, W.; Li, Z.; Lv, L.; Ruan, Y.; Wu, H.-H.; Chiang, W.-H.; Wang, C.; Liu, M.; Xiao Cheng, Z. Unraveling the high-activity nature of Fe-N-C electrocatalysts for the oxygen reduction reaction: The extraordinary synergy between Fe–N4 and Fe4N. J. Mater. Chem. A 2019, 7, 11792–11801. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Zhu, M.; Li, Z.; Chen, W.; Wei, W.-C.; Yuan, T.; Qu, Y.; Xu, Q.; Zhao, C.; et al. Boosting Oxygen Reduction Catalysis with Fe–N4 Sites Decorated Porous Carbons toward Fuel Cells. Acs Catal. 2019, 9, 2158–2163. [Google Scholar] [CrossRef]
- Zhao, D.; Shui, J.-L.; Grabstanowicz, L.R.; Chen, C.; Commet, S.M.; Xu, T.; Lu, J.; Liu, D.-J. Highly Efficient Non-Precious Metal Electrocatalysts Prepared from One-Pot Synthesized Zeolitic Imidazolate Frameworks. Adv. Mater. 2014, 26, 1093–1097. [Google Scholar] [CrossRef]
- Bates, J.S.; Khamespanah, F.; Cullen, D.A.; Al-Omari, A.A.; Hopkins, M.N.; Martinez, J.J.; Root, T.W.; Stahl, S.S. Molecular Catalyst Synthesis Strategies to Prepare Atomically Dispersed Fe-N-C Heterogeneous Catalysts. J. Am. Chem. Soc. 2022, 144, 18797–18802. [Google Scholar] [CrossRef]
- Yin, S.; Yi, H.; Liu, M.; Yang, J.; Yang, S.; Zhang, B.-W.; Chen, L.; Cheng, X.; Huang, H.; Huang, R.; et al. An in situ exploration of how Fe/N/C oxygen reduction catalysts evolve during synthesis under pyrolytic conditions. Nat. Commun. 2024, 15, 6229. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Xu, M.; Ly, A.; Gili, A.; Murphy, E.; Asset, T.; Liu, Y.; De Andrade, V.; Segre, C.U.; et al. Catalysts by pyrolysis: Transforming metal-organic frameworks (MOFs) precursors into metal-nitrogen-carbon (M-N-C) materials. Mater. Today 2023, 69, 66–78. [Google Scholar] [CrossRef]
- Malik, W.; Victoria Tafoya, J.P.; Doszczeczko, S.; Jorge Sobrido, A.B.; Skoulou, V.K.; Boa, A.N.; Zhang, Q.; Ramirez Reina, T.; Volpe, R. Synthesis of a Graphene-Encapsulated Fe3C/Fe Catalyst Supported on Sporopollenin Exine Capsules and Its Use for the Reverse Water–Gas Shift Reaction. ACS Sustain. Chem. Eng. 2023, 11, 15795–15807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Z.; Song, H. Preparation and Application of Fe-N Co-Doped GNR@CNT Cathode Oxygen Reduction Reaction Catalyst in Microbial Fuel Cells. Nanomaterials 2021, 11, 377. [Google Scholar] [CrossRef]
- Ma, Q.; Jin, H.; Zhu, J.; Li, Z.; Xu, H.; Liu, B.; Zhang, Z.; Ma, J.; Mu, S. Stabilizing Fe-N-C Catalysts as Model for Oxygen Reduction Reaction. Adv. Sci. 2021, 8, 2102209. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Huang, L.-B.; Liu, X.-Z.; Zhang, Q.-H.; He, C.; Wu, Z.-Y.; Zhang, L.-J.; Wu, J.; Yang, W.; et al. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts. Nat. Commun. 2019, 10, 1278. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Wang, X.; Osmieri, L.; Peng, J.; Cankur, C.; Park, J.; Myers, D.J.; Hyun Kyu, C.; Neyerlin, K.C. Stability of Atomically Dispersed Fe-N-C ORR Catalyst in Polymer Electrolyte Fuel Cell Environment. J. Electrochem. Soc. 2021, 168, 024513. [Google Scholar] [CrossRef]
- Zhan, Y.; Xie, F.; Zhang, H.; Jin, Y.; Meng, H.; Chen, J.; Sun, X. Highly Dispersed Nonprecious Metal Catalyst for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. ACS Appl. Mater. Interfaces 2020, 12, 17481–17491. [Google Scholar] [CrossRef]
- Gianola, G.; Cosenza, A.; Roiron, C.; Pirri, C.F.; Specchia, S.; Atanassov, P.; Zeng, J. Effect of silica leaching treatment during template-assisted synthesis on the performance of FeNC catalysts for oxygen reduction reaction. Electrochim. Acta 2025, 525, 146085. [Google Scholar] [CrossRef]
- Muhyuddin, M.; Mostoni, S.; Honig, H.C.; Mirizzi, L.; Elbaz, L.; Scotti, R.; D’Arienzo, M.; Santoro, C. Enhancing Electrocatalysis: Engineering the Fe–Nx–C Electrocatalyst for Oxygen Reduction Reaction Using Fe-Functionalized Silica Hard Templates. ACS Appl. Energy Mater. 2024, 7, 11691–11702. [Google Scholar] [CrossRef]
- Radwan, A.; Jin, H.; He, D.; Mu, S. Design Engineering, Synthesis Protocols, and Energy Applications of MOF-Derived Electrocatalysts. Nano-Micro Lett. 2021, 13, 132. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, J.; Yuan, P.; Hu, Y.; Qu, G.; Lu, B.-A.; Xue, X.; Yin, H.; Cheng, W.; Jung An, C.; et al. Regulating Fe-spin state by atomically dispersed Mn-N in Fe-N-C catalysts with high oxygen reduction activity. Nat. Commun. 2021, 12, 1734. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhu, J.; Yu, R.; Li, W.; Ji, P.; Liang, L.; Liu, B.; Hu, C.; He, D.; Mu, S. Tuning the Fe–N4 sites by introducing Bi–O bonds in a Fe-N-C system for promoting the oxygen reduction reaction. J. Mater. Chem. A 2022, 10, 664–671. [Google Scholar] [CrossRef]
- Luo, F.; Roy, A.; Sougrati, M.T.; Khan, A.; Cullen, D.A.; Wang, X.; Primbs, M.; Zitolo, A.; Jaouen, F.; Strasser, P. Structural and Reactivity Effects of Secondary Metal Doping into Iron-Nitrogen-Carbon Catalysts for Oxygen Electroreduction. J. Am. Chem. Soc. 2023, 145, 14737–14747. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Pisula, W.; Müllen, K. Graphenes as Potential Material for Electronics. Chem. Rev. 2007, 107, 718–747. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Pan, Y.; Li, L.; Chan, S.H. Graphene-Based Materials for Energy Conversion. Adv. Mater. 2012, 24, 4203–4210. [Google Scholar] [CrossRef]
- Higgins, D.; Zamani, P.; Yu, A.; Chen, Z. The application of graphene and its composites in oxygen reduction electrocatalysis: A perspective and review of recent progress. Energy Environ. Sci. 2016, 9, 357–390. [Google Scholar] [CrossRef]
- Park, J.S.; Chang, D.W. Iron Phthalocyanine/Graphene Composites as Promising Electrocatalysts for the Oxygen Reduction Reaction. Energies 2020, 13, 4073. [Google Scholar] [CrossRef]
- Koh, K.H.; Noh, S.H.; Kim, T.-H.; Lee, W.J.; Yi, S.-C.; Han, T.H. A graphene quantum dot/phthalocyanine conjugate: A synergistic catalyst for the oxygen reduction reaction. RSC Adv. 2017, 7, 26113–26119. [Google Scholar] [CrossRef]
- Tian, H.; Song, A.; Zhang, P.; Sun, K.; Wang, J.; Sun, B.; Fan, Q.; Shao, G.; Chen, C.; Liu, H.; et al. High Durability of Fe-N-C Single-Atom Catalysts with Carbon Vacancies toward the Oxygen Reduction Reaction in Alkaline Media. Adv. Mater. 2023, 35, 2210714. [Google Scholar] [CrossRef]
- Mahmood, A.; Xie, N.; Zhao, B.; Zhong, L.; Zhang, Y.; Niu, L. Optimizing Surface N-Doping of Fe-N-C Catalysts Derived from Fe/Melamine-Decorated Polyaniline for Oxygen Reduction Electrocatalysis. Adv. Mater. Interfaces 2021, 8, 2100197. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Husic, B.E.; Schebarchov, D.; Wales, D.J. Impurity effects on solid–solid transitions in atomic clusters. Nanoscale 2016, 8, 18326–18340. [Google Scholar] [CrossRef] [PubMed]
- Long, D.; Li, W.; Ling, L.; Miyawaki, J.; Mochida, I.; Yoon, S.-H. Preparation of Nitrogen-Doped Graphene Sheets by a Combined Chemical and Hydrothermal Reduction of Graphene Oxide. Langmuir 2010, 26, 16096–16102. [Google Scholar] [CrossRef]
- Qian, W.; Cui, X.; Hao, R.; Hou, Y.; Zhang, Z. Facile Preparation of Nitrogen-Doped Few-Layer Graphene via Supercritical Reaction. ACS Appl. Mater. Interfaces 2011, 3, 2259–2264. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, S.; Engelhard, M.H.; Li, G.; Shao, G.; Wang, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Nitrogen-doped graphene and its electrochemical applications. J. Mater. Chem. 2010, 20, 7491–7496. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552. [Google Scholar] [CrossRef]
- Tang, Y.-B.; Yin, L.-C.; Yang, Y.; Bo, X.-H.; Cao, Y.-L.; Wang, H.-E.; Zhang, W.-J.; Bello, I.; Lee, S.-T.; Cheng, H.-M.; et al. Tunable Band Gaps and p-Type Transport Properties of Boron-Doped Graphenes by Controllable Ion Doping Using Reactive Microwave Plasma. ACS Nano 2012, 6, 1970–1978. [Google Scholar] [CrossRef]
- Yu, S.; Zheng, W.; Wang, C.; Jiang, Q. Nitrogen/Boron Doping Position Dependence of the Electronic Properties of a Triangular Graphene. ACS Nano 2010, 4, 7619–7629. [Google Scholar] [CrossRef]
- Biddinger, E.J.; von Deak, D.; Ozkan, U.S. Nitrogen-Containing Carbon Nanostructures as Oxygen-Reduction Catalysts. Top. Catal. 2009, 52, 1566–1574. [Google Scholar] [CrossRef]
- Li, X.; Fan, L.; Li, Z.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Zhu, H. Boron Doping of Graphene for Graphene–Silicon p–n Junction Solar Cells. Adv. Energy Mater. 2012, 2, 425–429. [Google Scholar] [CrossRef]
- Palacin, T.; Khanh, H.L.; Jousselme, B.; Jegou, P.; Filoramo, A.; Ehli, C.; Guldi, D.M.; Campidelli, S. Efficient Functionalization of Carbon Nanotubes with Porphyrin Dendrons via Click Chemistry. J. Am. Chem. Soc. 2009, 131, 15394–15402. [Google Scholar] [CrossRef] [PubMed]
- Sobamowo, M.G.; Akanmu, J.O.; Adeleye, O.A.; Akingbade, S.A.; Yinusa, A.A. Coupled effects of magnetic field, number of walls, geometric imperfection, temperature change, and boundary conditions on nonlocal nonlinear vibration of carbon nanotubes resting on elastic foundations. Forces Mech. 2021, 3, 100010. [Google Scholar] [CrossRef]
- Morozan, A.; Campidelli, S.; Filoramo, A.; Jousselme, B.; Palacin, S. Catalytic activity of cobalt and iron phthalocyanines or porphyrins supported on different carbon nanotubes towards oxygen reduction reaction. Carbon 2011, 49, 4839–4847. [Google Scholar] [CrossRef]
- Govan, J.; Abarca, G.; Aliaga, C.; Sanhueza, B.; Orellana, W.; Cárdenas-Jirón, G.; Zagal, J.H.; Tasca, F. Influence of cyano substituents on the electron density and catalytic activity towards the oxygen reduction reaction for iron phthalocyanine. The case for Fe(II) 2,3,9,10,16,17,23,24-octa(cyano)phthalocyanine. Electrochem. Commun. 2020, 118, 106784. [Google Scholar] [CrossRef]
- Helsel, N.; Choudhury, P. Regulating Electronic Descriptors for the Enhanced ORR Activity of FePc-Functionalized Graphene via Substrate Doping and/or Ligand Exchange: A Theoretical Study. J. Phys. Chem. C 2022, 126, 4458–4471. [Google Scholar] [CrossRef]
- Helsel, N.; Choudhury, P. Enhancing the Stability of a Pt-Free ORR Catalyst via Reaction Intermediates. Adv. Mater. Interfaces 2023, 10, 2202132. [Google Scholar] [CrossRef]
- Helsel, N.; Choudhury, P. Investigation of bifunctionality of FePc-functionalized graphene for enhanced ORR/OER activity. Mol. Catal. 2023, 545, 113213. [Google Scholar] [CrossRef]
- Creel, E.B.; Lyu, X.; McCool, G.; Ouimet, R.J.; Serov, A. Protocol for Screening Water Oxidation or Reduction Electrocatalyst Activity in a Three-Electrode Cell for Alkaline Exchange Membrane Electrolysis. Front. Energy Res. 2022, 10, 871604. [Google Scholar] [CrossRef]
- Wang, L.; Lee, C.-Y.; Schmuki, P. Solar water splitting: Preserving the beneficial small feature size in porous α-Fe2O3 photoelectrodes during annealing. J. Mater. Chem. A 2013, 1, 212–215. [Google Scholar] [CrossRef]
- Hu, Y.; Jensen, J.O.; Pan, C.; Cleemann, L.N.; Shypunov, I.; Li, Q. Immunity of the Fe-N-C catalysts to electrolyte adsorption: Phosphate but not perchloric anions. Appl. Catal. B Environ. 2018, 234, 357–364. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Lu, G.; Yang, H.; Zhu, Y.; Huggins, T.; Ren, Z.J.; Liu, Z.; Zhang, W. Synthesis of a conjugated porous Co(ii) porphyrinylene–ethynylene framework through alkyne metathesis and its catalytic activity study. J. Mater. Chem. A 2015, 3, 4954–4959. [Google Scholar] [CrossRef]
- Treimer, S.; Tang, A.; Johnson, D.C. A Consideration of the Application of Koutecký-Levich Plots in the Diagnoses of Charge-Transfer Mechanisms at Rotated Disk Electrodes. Electroanalysis 2002, 14, 165–171. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Riasse, R.; Lafforgue, C.; Vandenberghe, F.; Micoud, F.; Morin, A.; Arenz, M.; Durst, J.; Chatenet, M. Benchmarking proton exchange membrane fuel cell cathode catalyst at high current density: A comparison between the rotating disk electrode, the gas diffusion electrode and differential cell. J. Power Sources 2023, 556, 232491. [Google Scholar] [CrossRef]
- Choi, C.H.; Chung, M.W.; Kwon, H.C.; Park, S.H.; Woo, S.I. B, N- and P, N-doped graphene as highly active catalysts for oxygen reduction reactions in acidic media. J. Mater. Chem. A 2013, 1, 3694. [Google Scholar] [CrossRef]
- Lian, Y.; Xu, J.; Zhou, W.; Lin, Y.; Bai, J. Research Progress on Atomically Dispersed Fe-N-C Catalysts for the Oxygen Reduction Reaction. Molecules 2024, 29, 771. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Peng, Q.; Yang, P.; Fu, Q.; Zhu, X.; Liao, Q. Toward an objective performance evaluation of commercial Pt/C electrocatalysts for oxygen reduction: Effect of catalyst loading. Electrochim. Acta 2022, 429, 140953. [Google Scholar] [CrossRef]
- Yang, G.; Lee, C.; Qiao, X.; Babu, S.K.; Martinez, U.; Spendelow, J.S. Advanced Electrode Structures for Proton Exchange Membrane Fuel Cells: Current Status and Path Forward. Electrochem. Energy Rev. 2024, 7, 9. [Google Scholar] [CrossRef]
- Yin, S.; Chen, L.; Yang, J.; Cheng, X.; Zeng, H.; Hong, Y.; Huang, H.; Kuai, X.; Lin, Y.; Huang, R.; et al. A Fe-NC electrocatalyst boosted by trace bromide ions with high performance in proton exchange membrane fuel cells. Nat. Commun. 2024, 15, 7489. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Xiong, X.; Wang, X.; Ye, C.; Li, J.; Sun, W.; Sun, X.; Jiang, J.; Zhuang, Z.; Wang, D.; et al. Regulating the FeN4 Moiety by Constructing Fe–Mo Dual-Metal Atom Sites for Efficient Electrochemical Oxygen Reduction. Nano Lett. 2022, 22, 9507–9515. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Yuan, P.; Jiang, S.; Wei, Y.; Zhou, Y.; Dong, C.-L.; Yan, W.; Mu, S.; Zhang, J.-N. Altering the spin state of Fe-N-C through ligand field modulation of single-atom sites boosts the oxygen reduction reaction. Nano Energy 2023, 105, 108020. [Google Scholar] [CrossRef]
- Masa, J.; Andronescu, C.; Schuhmann, W. Electrocatalysis as the Nexus for Sustainable Renewable Energy: The Gordian Knot of Activity, Stability, and Selectivity. Angew. Chem. Int. Ed. 2020, 59, 15298–15312. [Google Scholar] [CrossRef]
- Pollet, B.G.; Franco, A.A.; Su, H.; Liang, H.; Pasupathi, S. 1-Proton exchange membrane fuel cells. In Compendium of Hydrogen Energy; Barbir, F., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing: Oxford, UK, 2016; pp. 3–56. [Google Scholar]
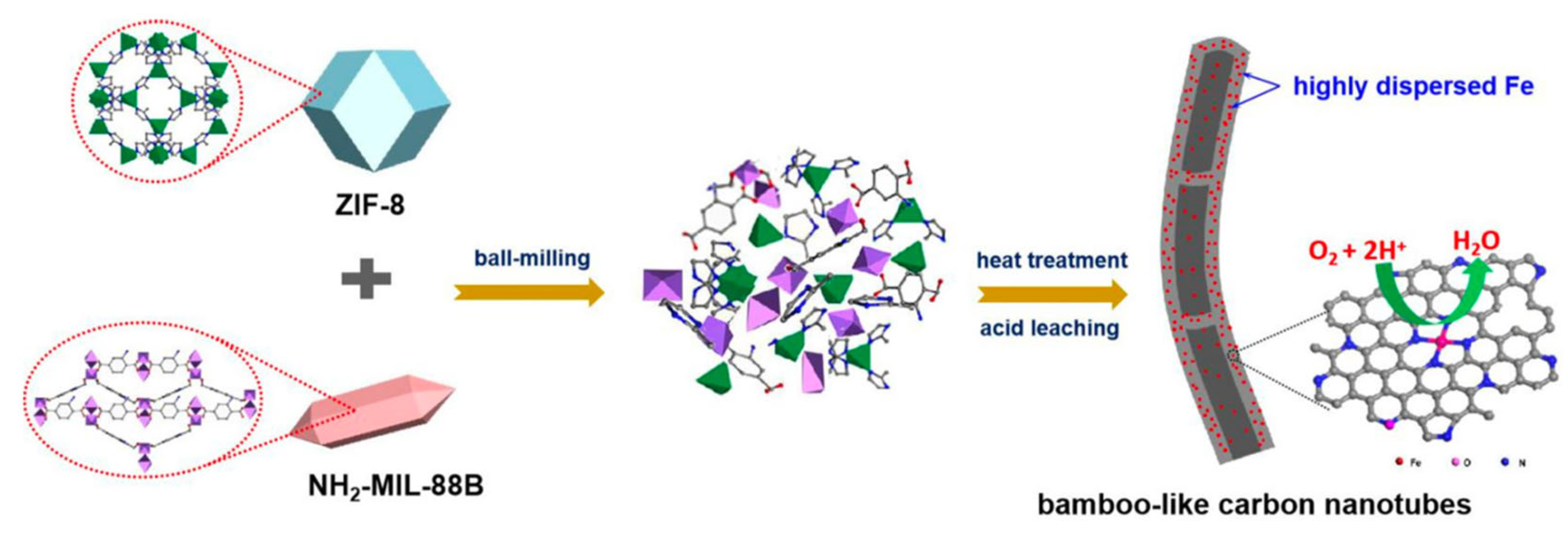
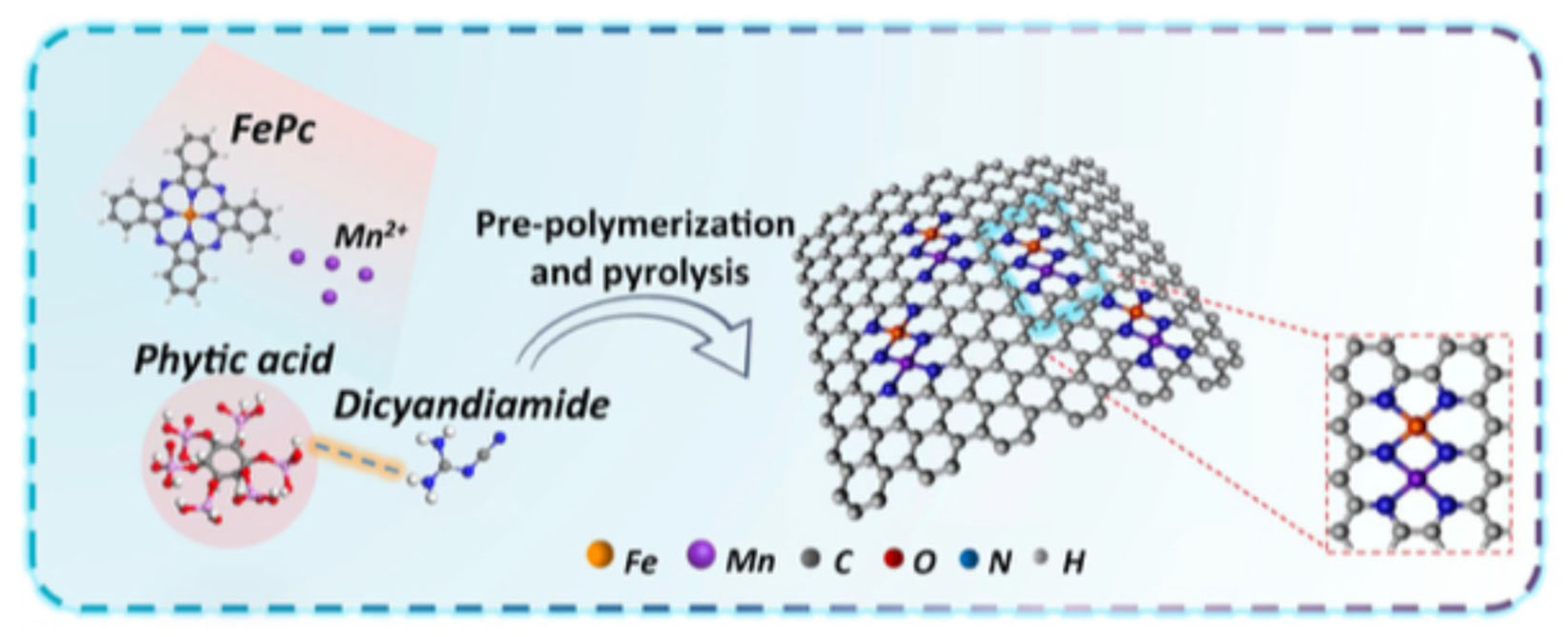
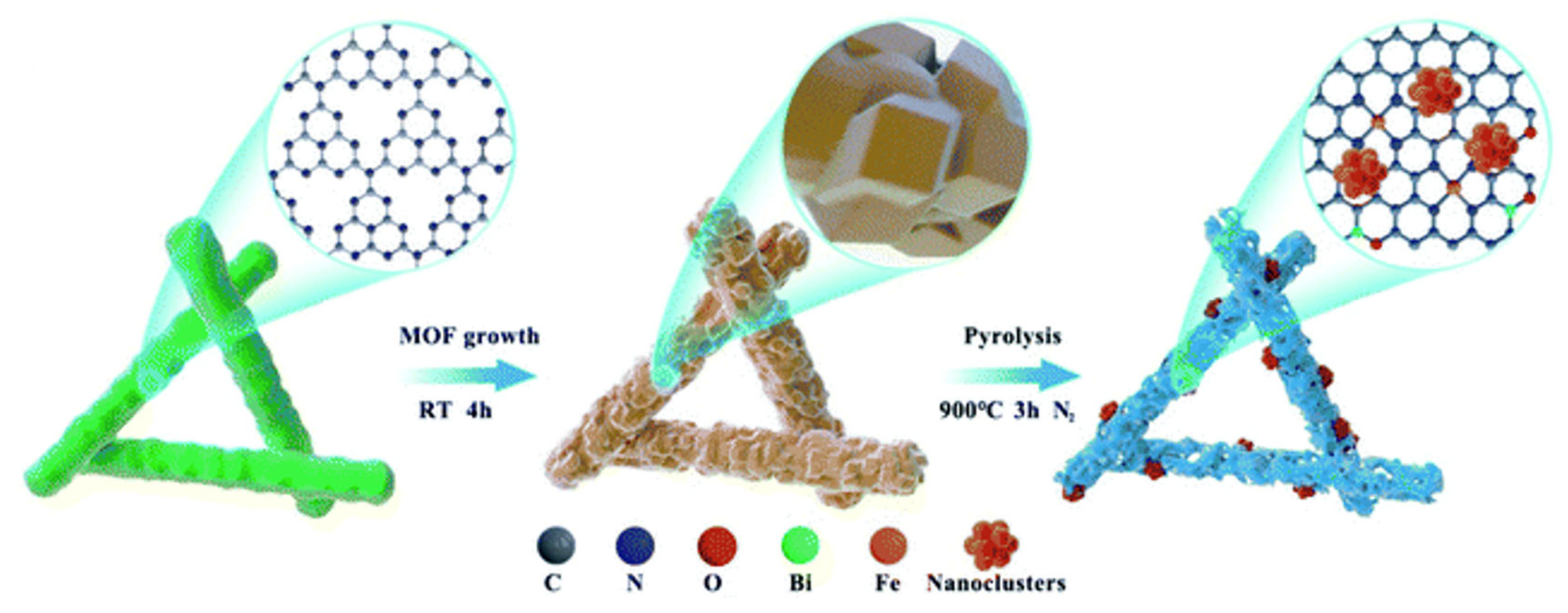
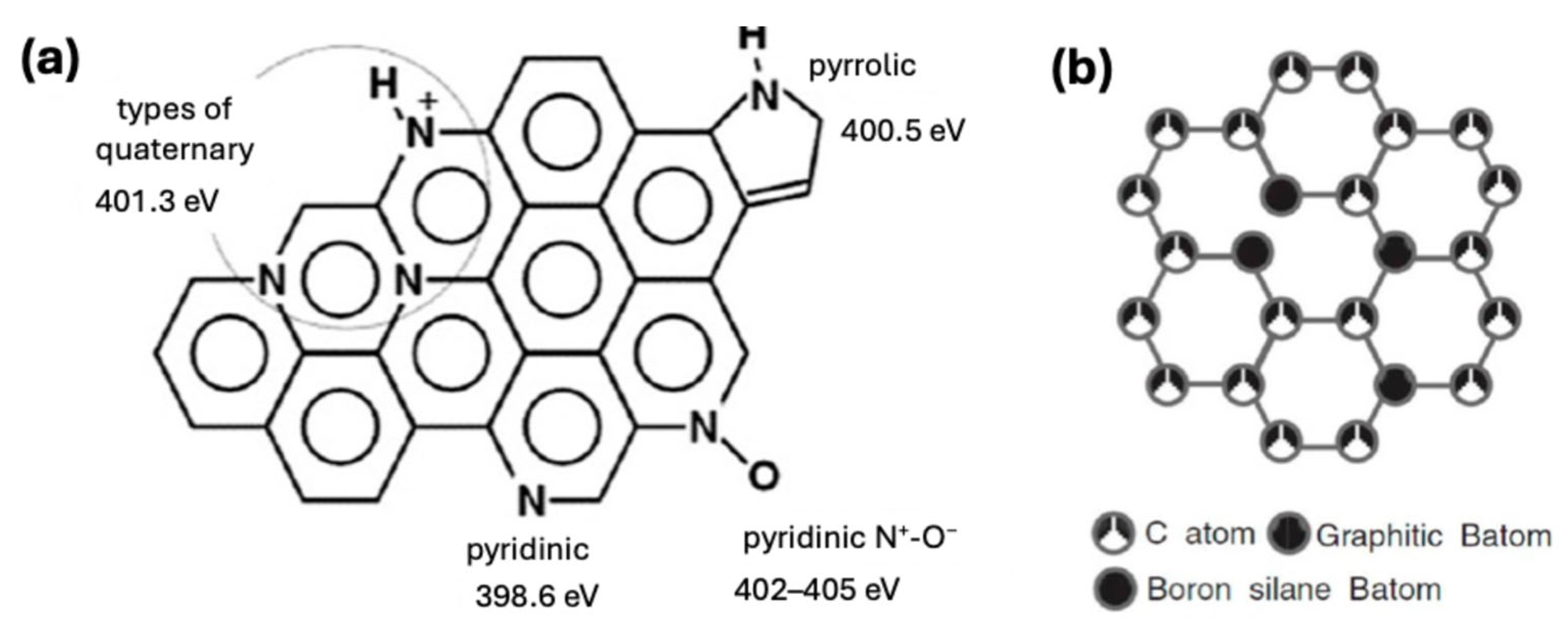
| Environment | Catalyst | Loading (mgcat cm−2) | Electrolyte | Eonset (V) | E1/2 (V) | n | Ref. |
|---|---|---|---|---|---|---|---|
| Acidic | NGr | ^ 0.71 | 0.1 M HClO4 | 0.84 | 0.58 | * (13.4% H2O2) | [86] |
| B, N-Gr | ^ 0.71 | 0.1 M HClO4 | 0.86 | 0.61 | * (1.8% H2O2) | ||
| Fe, Mn/N-C | 0.1 | 0.1 M HClO4 | * | 0.804 | 3.91 | [50] | |
| Fe SAs/N-C | 0.25 | 0.1 M HClO4 | 0.95 | 0.798 | * | [36] | |
| PFeTTPP-700 | 0.4 | 0.1 M HClO4 | 0.93 | 0.73 | 3.96 | [29] | |
| Fe-N-C-10/1-950 | * | 0.1 M HClO4 | 0.96 | 0.78 | ~4 | [46] | |
| Zn(mlm)2TPIP | 0.4 | 0.1 M HClO4 | 0.902 | 0.76 | 3.9 | [37] | |
| Zn(elm)2TPIP | 0.4 | 0.1 M HClO4 | 0.914 | 0.78 | 3.8 | ||
| Fe/Bi-RNC | ^ 1.4 | 0.5 M H2SO4 | 0.899 | 0.97 | 3.93 | [51] | |
| PmPDA-FeNx/C (950 °C) | 0.6 | 0.1 M H2SO4 | 0.94 | 0.82 | * (<1% H2O2) | [33] | |
| Alkaline | Fe SAs/N-C | 0.25 | 0.1 M KOH | 1.02 | 0.91 | 3.91 | [36] |
| Fe, Mn/N-C | 0.1 | 0.1 M KOH | * | 0.928 | * | [50] | |
| FePc/a-MWCNTs | 0.185 | 0.1 M NaOH | 0.917 | 0.817 | 3.81 | [73] | |
| OCNFePc-CNT | ^ 0.2 | 0.1 M NaOH | 0.95 | * | 3.8 | [74] | |
| FeNCNs-800 | 0.36 | 0.1 M KOH | 0.985 | 0.89 | 3.9 | [35] | |
| Fe-NC SAC | 0.6 | 0.1 M KOH | 0.98 | 0.9 | * (<3.5% H2O2) | [44] | |
| g-FePc | ^ 0.113 | 0.1 M KOH | 0.98 | 0.88 | 3.96 | [28] |
| Catalyst | Loading (mgcat cm−2) | Volumetric Current Density (A cm−3) | Peak Power Density (mW cm−2) | Ref. |
|---|---|---|---|---|
| PFeTTPP-700 | 4 | 20.2 @ 0.8 V | 730 @ 0.4 V | [29] |
| Zn(mlm)2TPIP | 2.2 | 67 @ 0.8 V | 620 @ 0.43 V | [37] |
| Zn(elm)2TPIP | 2.2 | 88.1 @ 0.8 V | 500 @ 0.34 V | |
| Fe SAs/N-C | 1.5 | # (^ 1.5 A cm−2 @ 0.89 V) | 750 @ ^ 0.5 V | [36] |
| Fe-N-C-10/1-950 | 4 | # (0.85 A cm−2 @ 0.7 V) | 770 @ ^ 0.48 V | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helsel, N.; Choudhury, P. Non-Platinum Group Metal Oxygen Reduction Catalysts for a Hydrogen Fuel Cell Cathode: A Mini-Review. Catalysts 2025, 15, 588. https://doi.org/10.3390/catal15060588
Helsel N, Choudhury P. Non-Platinum Group Metal Oxygen Reduction Catalysts for a Hydrogen Fuel Cell Cathode: A Mini-Review. Catalysts. 2025; 15(6):588. https://doi.org/10.3390/catal15060588
Chicago/Turabian StyleHelsel, Naomi, and Pabitra Choudhury. 2025. "Non-Platinum Group Metal Oxygen Reduction Catalysts for a Hydrogen Fuel Cell Cathode: A Mini-Review" Catalysts 15, no. 6: 588. https://doi.org/10.3390/catal15060588
APA StyleHelsel, N., & Choudhury, P. (2025). Non-Platinum Group Metal Oxygen Reduction Catalysts for a Hydrogen Fuel Cell Cathode: A Mini-Review. Catalysts, 15(6), 588. https://doi.org/10.3390/catal15060588







