Full-Component Acetylation of Corncob Residue into Acetone-Dissolvable Composite Resin by Titanium Oxysulfate Reagent
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Analysis of Model Compound
2.2. Evaluation of Acetylation Degree
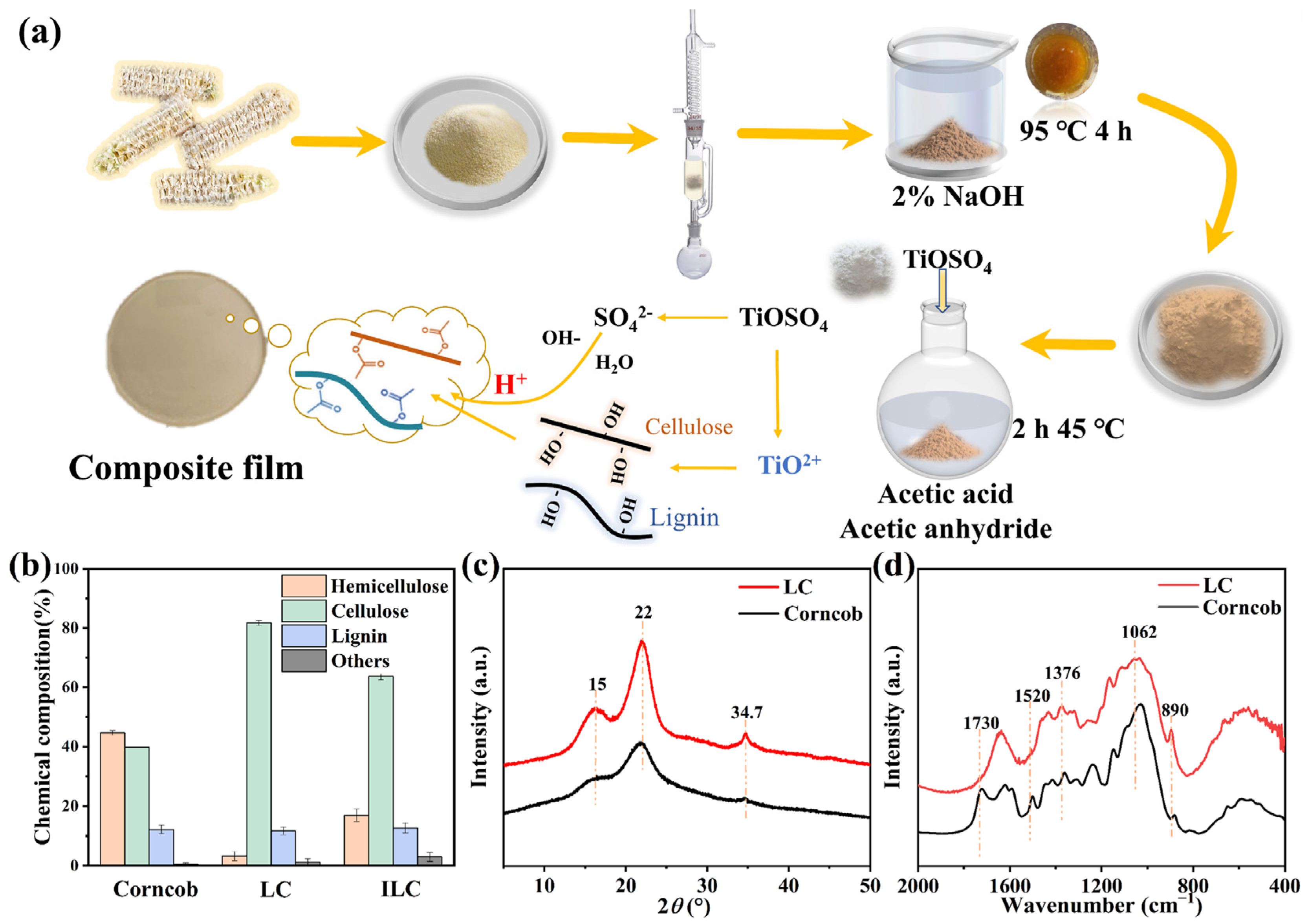
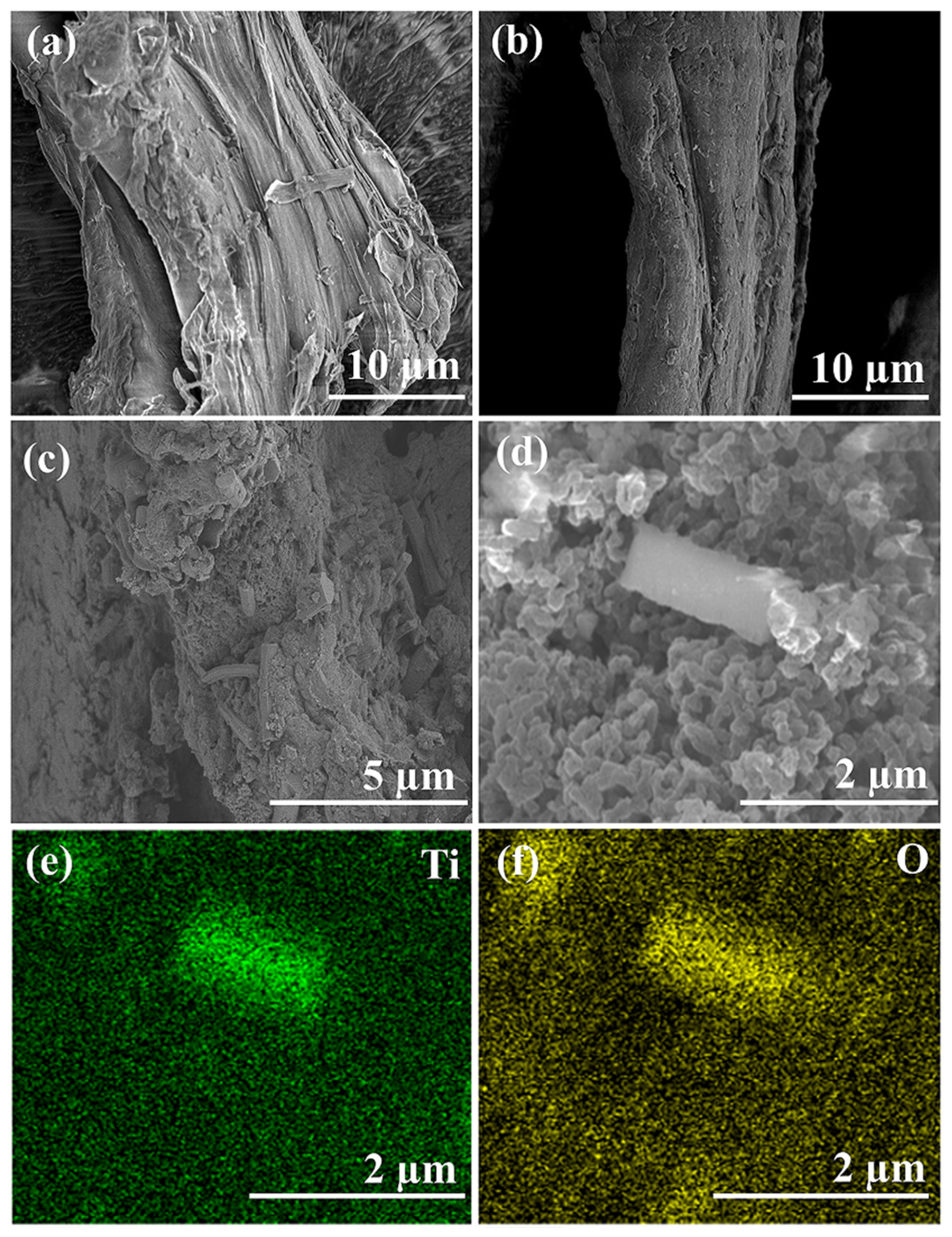
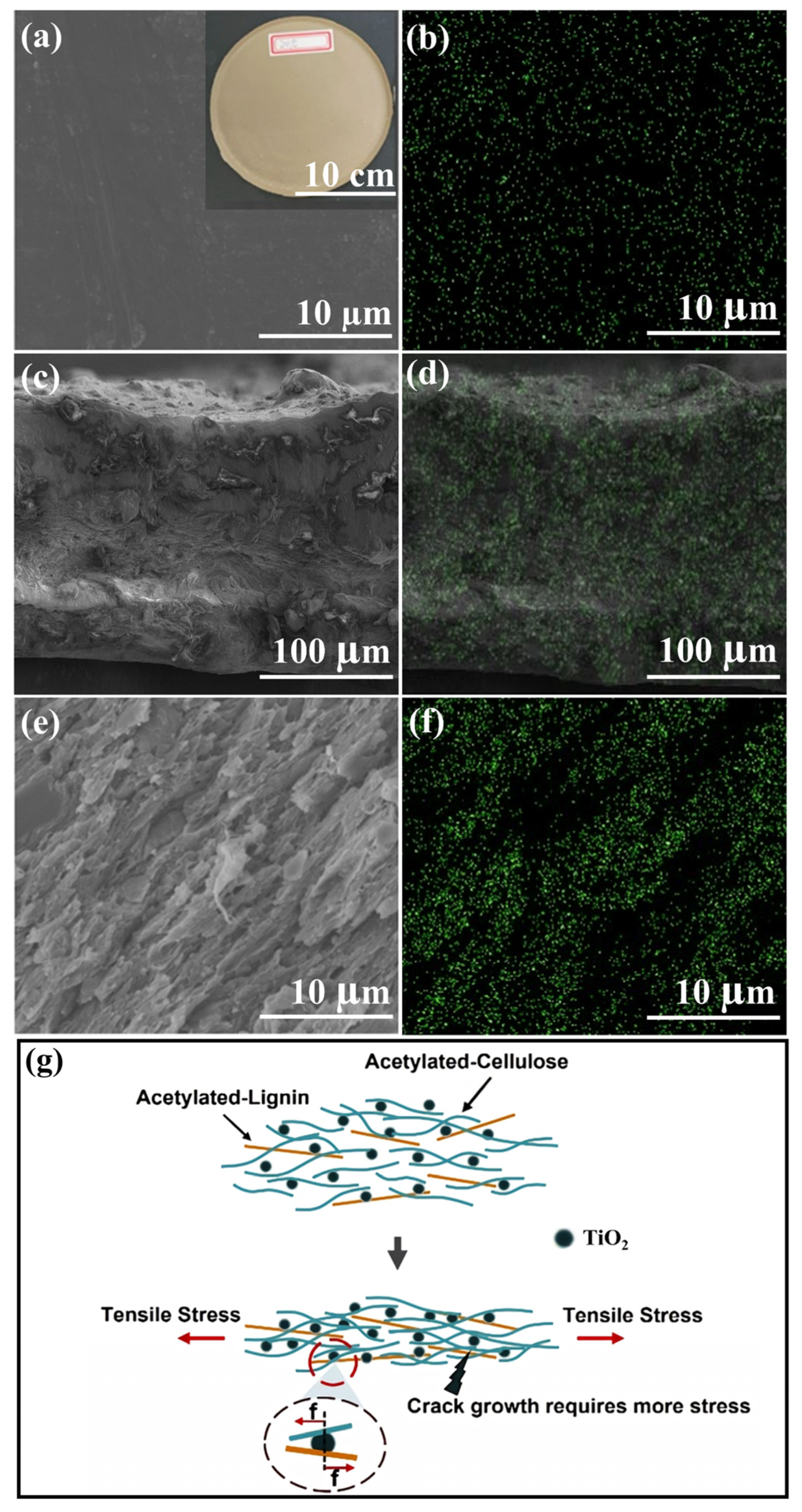
2.3. Structural and Compositional Analyses of ALC-TiO2 Composites
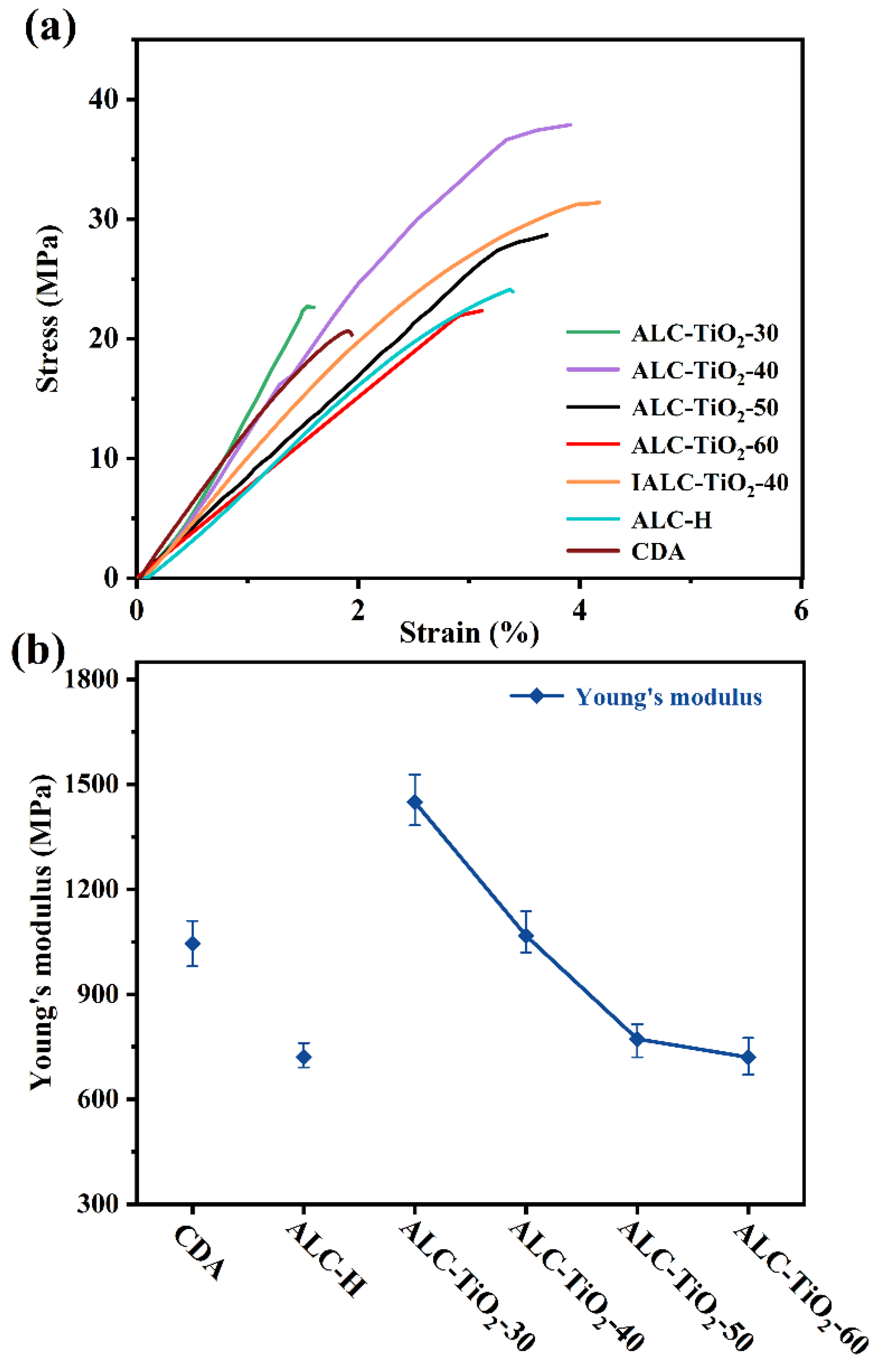
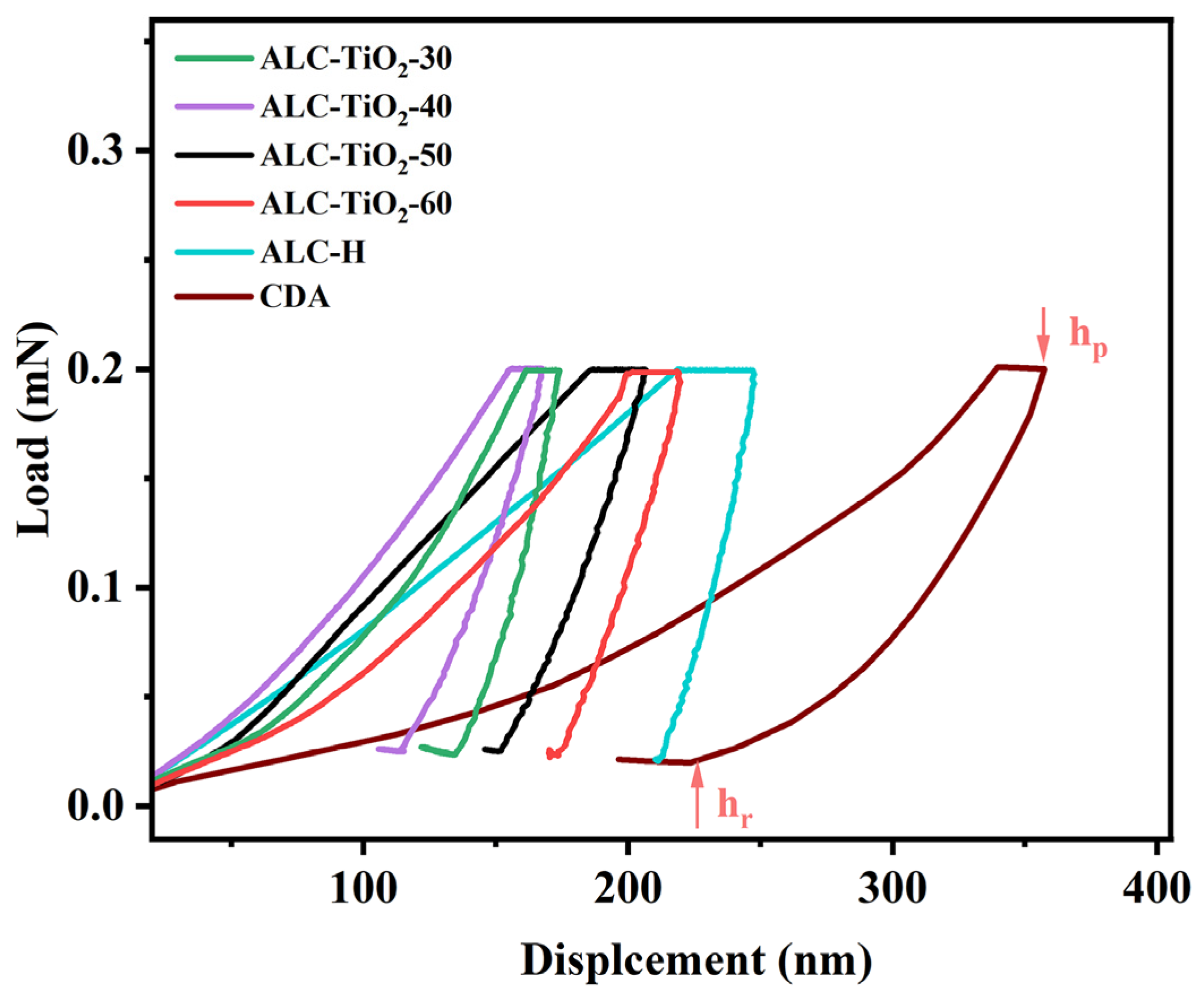
2.4. Analyses of the Mechanical Properties of Acetylated Corncob Residue and TiO2 Composites
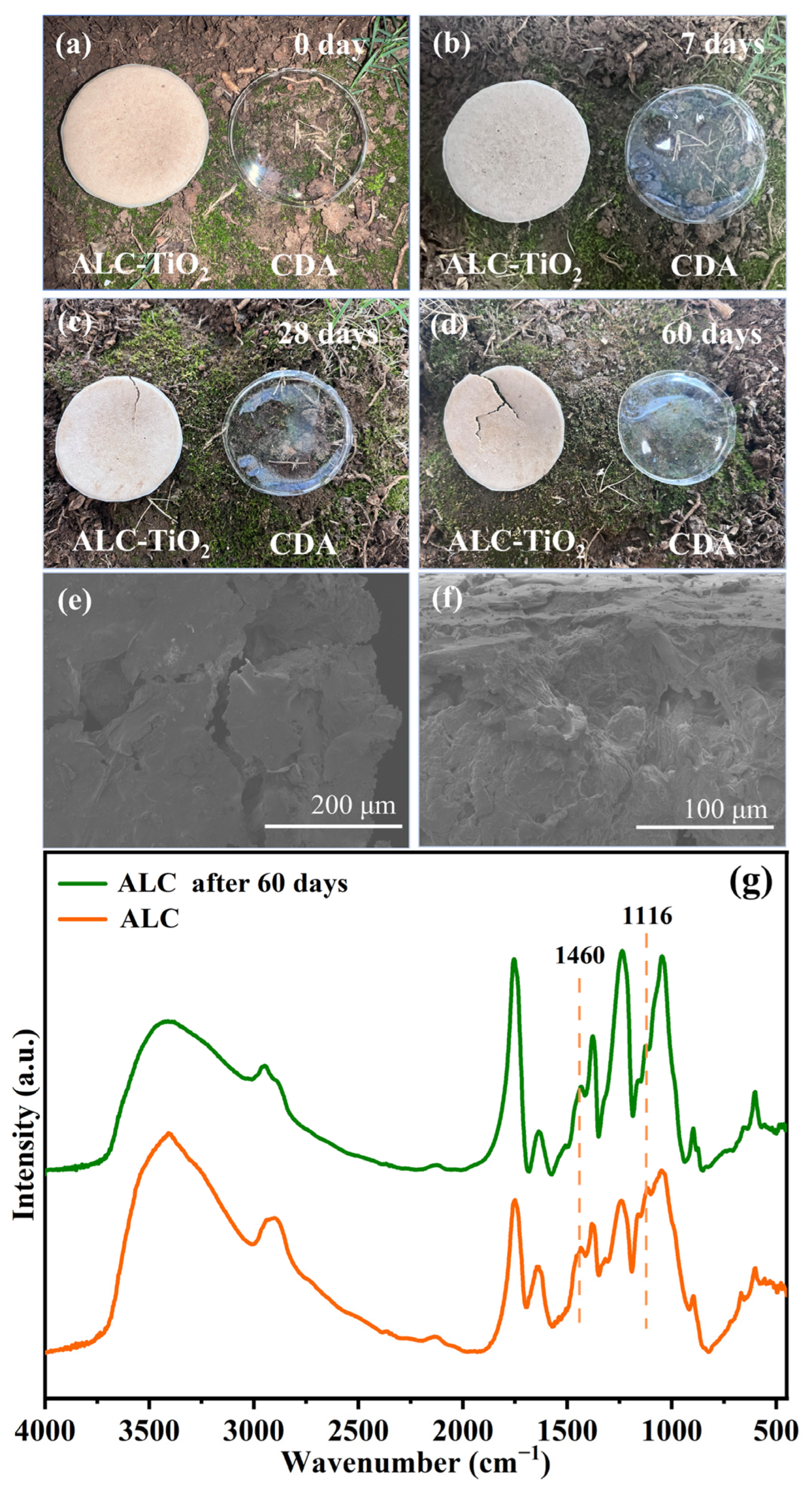
2.5. Environmental Impacts
3. Materials and Methods
3.1. Materials
3.2. Preparation of Corncob Residue Model Compound
3.3. Acetylation of Corncob Residue
3.4. Characterization
3.5. Preparation of Composite Film
3.6. Determination of Acetylation Degree
3.7. Mechanical Property
3.8. Experiments of Environmental Impact
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMAP | (4-dimethylamino) pyridine |
| LC | Model corncob reside |
| ILC | Industrial corncob residue |
| HPLC | High performance liquid chromatography |
| ALC | Corncob residue–TiO2 composite |
| ALC-H | Acetylated corncob residue with H2SO4 |
| EDX | Energy-Dispersive X-Ray Spectroscopy |
| XRD | X-ray diffractometer |
| FTIR | Fourier-transform infrared spectroscopy |
References
- Takada, M.; Niu, R.; Minami, E.; Saka, S. Characterization of three tissue fractions in corn (Zea mays) cob. Biomass Bioenerg. 2018, 115, 130–135. [Google Scholar] [CrossRef]
- Prasad, S.; Singh, A.; Joshi, H.C. Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour. Conserv. Recy. 2007, 50, 1–39. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Castillo-González, E.; De Medina-Salas, L.; Giraldi-Díaz, M.R.; Sánchez-Noguez, C. Vermicomposting: A valorization alternative for corn cob waste. Appl. Sci. 2021, 11, 5692. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, S.; Zhang, L.; Liu, L.; Song, X. The preparation of carboxymethyl cellulose from corncob residue and its effects on paper properties. J. Wood Chem. Technol. 2022, 42, 149–157. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Li, X.; Liu, X.; Fan, J.; Clark, J.H.; Hu, C. The production of furfural directly from hemicellulose in lignocellulosic biomass: A review. Catal. Today 2019, 319, 14–24. [Google Scholar] [CrossRef]
- Gong, L.; Xu, Z.Y.; Dong, J.J.; Li, H.; Han, R.Z.; Xu, G.; Ni, Y. Composite coal fly ash solid acid catalyst in synergy with chloride for biphasic preparation of furfural from corn stover hydrolysate. Bioresour. Technol. 2019, 293, 122065. [Google Scholar] [CrossRef]
- Yang, D.; Yang, Q.; Yang, R.; Zhou, Y.; He, Y. Co-Production of Furfural, Xylo-Oligosaccharides, and Reducing Sugars from Waste Yellow Bamboo Through the Solid Acid-Assisted Hydrothermal Pretreatment. Catalysts 2025, 15, 325. [Google Scholar] [CrossRef]
- Feng, Y.; Jiang, J.; Zhu, L.; Yue, L.; Zhang, J.; Han, S. Effects of tea saponin on glucan conversion and bonding behaviour of cellulolytic enzymes during enzymatic hydrolysis of corncob residue with high lignin content. Biotechnol. Biofuels 2013, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; You, S.; Zhang, M.; Wang, Y.; Qi, W.; Su, R.; He, Z. Improved conversion efficiency of lignin-to-fuel conversion by limiting catalyst deactivation. Chem. Eng. J. 2021, 410, 128270. [Google Scholar] [CrossRef]
- Zhang, X.; Zhan, L.; Yang, Y.; Wu, Y.; Song, X.; Li, R. Investigation on hydrothermal-pyrolysis process to eliminate S-pollution from corncob residue utilization: Experiment and life cycle assessment. Fuel 2024, 362, 130810. [Google Scholar] [CrossRef]
- Araújo, D.; Castro, M.C.R.; Figueiredo, A.; Vilarinho, M.; Machado, A. Green synthesis of cellulose acetate from corncob: Physicochemical properties and assessment of environmental impacts. J. Clean. Prod. 2020, 260, 120865. [Google Scholar] [CrossRef]
- Ramos, R.R.F.; Siqueira, D.D.; Wellen, R.M.R.; Leite, I.F.; Glenn, G.M.; Medeiros, E.S. Development of green composites based on polypropylene and corncob agricultural residue. J. Polym. Environ. 2019, 27, 1677–1685. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Wang, B.; Yang, G.; Lucia, L.A.; Chen, J. Hydrothermal carbonization of corncob residues for hydrochar production. Energy Fuels 2015, 29, 872–876. [Google Scholar] [CrossRef]
- Qu, W.; Xu, Y.; Lu, A.; Zhang, X.; Li, W. Converting biowaste corncob residue into high value added porous carbon for supercapacitor electrodes. Bioresour. Technol. 2015, 189, 285–291. [Google Scholar] [CrossRef]
- Liu, K.; Lin, X.; Yue, J.; Li, X.; Fang, X.; Zhu, M.; Lin, J.; Qu, Y.; Xiao, L. High concentration ethanol production from corncob residues by fed-batch strategy. Bioresour. Technol. 2010, 101, 4952–4958. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Zhang, H.; Ma, L.; Huang, H.; Wu, J.; Zhang, Y. Direct fabrication of hierarchically processed pineapple peel hydrogels for efficient Congo red adsorption. Carbohydr. Polym. 2020, 230, 115599. [Google Scholar] [CrossRef]
- Heise, K.; Rossberg, C.; Strätz, J.; Bäurich, C.; Brendler, E.; Keller, H.; Fischer, S. Impact of pre-treatments on properties of lignocelluloses and their accessibility for a subsequent carboxymethylation. Carbohydr. Polym. 2017, 161, 82–89. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20, 2221–2262. [Google Scholar] [CrossRef]
- Costa, C.; Viana, A.; Silva, C.; Marques, E.F.; Azoia, N.G. Recycling of textile wastes, by acid hydrolysis, into new cellulosic raw materials. Waste Manag. 2022, 153, 99–109. [Google Scholar] [CrossRef]
- Bian, H.; Gao, Y.; Luo, J.; Jiao, L.; Wu, W.; Fang, G.; Dai, H. Lignocellulosic nanofibrils produced using wheat straw and their pulping solid residue: From agricultural waste to cellulose nanomaterials. Waste Manag. 2019, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Buono, P.; Duval, A.; Verge, P.; Averous, L.; Habibi, Y. New insights on the chemical modification of lignin: Acetylation versus silylation. ACS Sustain. Chem. Eng. 2016, 4, 5212–5222. [Google Scholar] [CrossRef]
- de Oliveira, D.R.; Avelino, F.; Mazzetto, S.E.; Lomonaco, D. Microwave-assisted selective acetylation of kraft lignin: Acetic acid as a sustainable reactant for lignin valorization. Int. J. Biol. Macromol. 2020, 164, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huang, Z.; Zhang, Y.; Yang, M.; Chen, D.; Huang, K.; Hu, H.; Huang, A.; Qin, X.; Feng, Z. Efficient solid-phase synthesis of acetylated lignin and a comparison of the properties of different modified lignins. J. Appl. Polym. Sci. 2017, 134, 1–13. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Shanmugam, R.; Basha, A.C.; Sriariyanun, M.; Shanmugam, S.R.; Venkatachalam, P. An Overview of Solid Acid Catalysts in Lignocellulose Biorefineries. Catalysts 2025, 15, 432. [Google Scholar] [CrossRef]
- Fan, G.; Liao, C.; Fang, T.; Luo, S.; Song, G. Amberlyst 15 as a new and reusable catalyst for the conversion of cellulose into cellulose acetate. Carbohydr. Polym. 2014, 112, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-J.; Zhang, J.-F.; Song, F.-E.; Wang, X.-X.; Zhang, T.; Jiang, Q.-K.; Zhang, Q.-D.; Han, Y.-Z.; Tan, Y.-S. Biomass-Based Carbon-Supported Sulfate Catalyst for Efficient Synthesis of Dimethoxymethane from Direct Oxidation of Dimethyl Ether. J. Phys. Chem. Lett. 2021, 12, 11795–11801. [Google Scholar] [CrossRef]
- Yuan, C.Y.; Feng, L.; Qin, X.; Liu, J.X.; Li, X.; Sun, X.C.; Chang, X.X.; Xu, B.J.; Li, W.X.; Ma, D.; et al. Constructing Metal(II)-Sulfate Site Catalysts toward Low Overpotential Carbon Dioxide Electroreduction to Fuel Chemicals. Angew. Chem. Int. Ed. 2024, 63, e202405255. [Google Scholar] [CrossRef]
- Olivito, F.; Jagdale, P.; Oza, G. Direct Production of Furfural from Fructose Catalyzed by Iron(III) Sulfate Using a Simple Distillation Apparatus. ACS Sustain. Chem. Eng. 2023, 11, 17595–17599. [Google Scholar]
- Gong, X.; Li, Q.; Li, T.; Li, C.; Huang, J.; Zhou, N.; Jia, X. Chemical composition and monolignin in alkali and acid treated corncob affect sugar release. Ind. Crops Prod. 2022, 176, 114317. [Google Scholar] [CrossRef]
- Ahvenainen, P.; Kontro, I.; Svedström, K. Comparison of sample crystallinity determination methods by X-ray diffraction for challenging cellulose I materials. Cellulose 2016, 23, 1073–1086. [Google Scholar] [CrossRef]
- Lou, C.; Zhou, Y.; Yan, A.; Liu, Y. Extraction cellulose from corn-stalk taking advantage of pretreatment technology with immobilized enzyme. RSC Adv. 2022, 12, 1208–1215. [Google Scholar] [CrossRef]
- Shao, X.; Wang, J.; Liu, Z.; Hu, N.; Liu, M.; Xu, Y. Preparation and characterization of porous microcrystalline cellulose from corncob. Ind. Crops Prod. 2020, 151, 112457. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, Z.; Jiang, B.; Wei, M.; Zhang, Z.; Zeng, L.; Clark, J.H.; Fan, J. An integrated process for the valorization of corn stover promoted by NaCl in a GVL/H2O system. Green Chem. 2022, 24, 1515–1526. [Google Scholar] [CrossRef]
- Bu, L.; Tang, Y.; Gao, Y.; Jian, H.; Jiang, J. Comparative characterization of milled wood lignin from furfural residues and corncob. Chem. Eng. J. 2011, 175, 176–184. [Google Scholar] [CrossRef]
- Hou, Q.; Bai, C.; Bai, X.; Qian, H.; Nie, Y.; Xia, T.; Lai, R.; Yu, G.; Rehman, M.L.U.; Ju, M. Roles of Ball Milling Pretreatment and Titanyl Sulfate in the Synthesis of 5-Hydroxymethylfurfural from Cellulose. ACS Sustain. Chem. Eng. 2022, 10, 1205–1213. [Google Scholar] [CrossRef]
- Li, L.; Yue, H.; Ji, T.; Li, W.; Zhao, X.; Wang, L.; She, J.; Gu, X.; Li, X. Novel mesoporous TiO2(B) whisker-supported sulfated solid superacid with unique acid characteristics and catalytic performances. Appl. Catal. A Gen. 2019, 574, 25–32. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Wang, K.; Cao, X.; Sun, R. Cellulose acetate fibers prepared from different raw materials with rapid synthesis method. Carbohydr. Polym. 2016, 137, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Wang, L.; Li, L.; Zhao, X.; Chen, Y.; Hua, Z.; Li, X.; Gu, X.; Li, L. Mild Preoxidation Treatment of Pt/TiO2 Catalyst and Its Enhanced Low Temperature Formaldehyde Decomposition. Catalysts 2019, 9, 694. [Google Scholar] [CrossRef]
- Ma, L.; Cui, Y.; Cai, R.; Liu, X.; Zhang, C.; Xiao, D. Optimization and evaluation of alkaline potassium permanganate pretreatment of corncob. Bioresour. Technol. 2015, 180, 1–6. [Google Scholar] [CrossRef]
- Chen, M.; Li, R.-M.; Runge, T.; Feng, J.; Feng, J.; Hu, S.; Shi, Q.-S. Solvent-Free Acetylation of Cellulose by 1-Ethyl-3-methylimidazolium Acetate-Catalyzed Transesterification. ACS Sustain. Chem. Eng. 2019, 7, 16971–16978. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, J.; Meng, T.; Zhang, J.; He, J.; Li, H.; Zhang, Y. Acetone-soluble cellulose acetates prepared by one-step homogeneous acetylation of cornhusk cellulose in an ionic liquid 1-allyl-3-methylimidazolium chloride (AmimCl). Carbohydr. Polym. 2007, 69, 665–672. [Google Scholar] [CrossRef]
- Thygesen, A.; Oddershede, J.; Lilholt, H.; Thomsen, A.B.; Ståhl, K. On the determination of crystallinity and cellulose content in plant fibres. Cellulose 2005, 12, 563–576. [Google Scholar] [CrossRef]
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative analysis of cellulose acetate with a high degree of substitution by FTIR and its application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Hu, C.; Reddy, N.; Yan, K.; Yang, Y. Acetylation of chicken feathers for thermoplastic applications. J. Agric. Food Chem. 2011, 59, 10517–10523. [Google Scholar] [CrossRef] [PubMed]
- Söyler, Z.; Onwukamike, K.N.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M.A.R. Sustainable succinylation of cellulose in a CO2-based switchable solvent and subsequent Passerini 3-CR and Ugi 4-CR modification. Green Chem. 2018, 20, 214–224. [Google Scholar] [CrossRef]
- Morgado, D.L.; Frollini, E. Thermal decomposition of mercerized linter cellulose and its acetates obtained from a homogeneous reaction. Polímeros 2011, 21, 111–117. [Google Scholar] [CrossRef]
- Gao, E.; Li, Q.; Zhao, X.; Zhang, C.; Hua, Z.; Wu, Z.; Huang, C.; Li, L. Reaction behavior of solid acid catalytic cellulose acetylation. Cellulose 2024, 31, 10285–10302. [Google Scholar] [CrossRef]
- Ma, N.; Wang, X.; Zhang, M.; Lu, S.; Hua, Z.; Wu, Z.; An, R.; Li, L. Programmable Interactions of cellulose acetate with octadecyltrichlorosilane-functionalized SiO2 nanoparticles. Langmuir 2023, 39, 5956–5969. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Zhou, L. Nanoscale evaluation of multi-layer interfacial mechanical properties of sisal fiber reinforced composites by nanoindentation technique. Compos. Sci. Technol. 2017, 152, 211–221. [Google Scholar] [CrossRef]
- Huang, Z.; Li, W.; Liu, Z.; Zhang, Y. One pot blending of biopolymer-TiO2 composite membranes with enhanced mechanical strength. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Komarek, R.J.; Gardner, R.M.; Buchanan, C.M.; Gedon, S. Biodegradation of radiolabeled cellulose acetate and cellulose propionate. J. Appl. Polym. Sci. 2003, 50, 1739–1746. [Google Scholar] [CrossRef]
- Cachet, N.; Camy, S.; Benjelloun-Mlayah, B.; Condoret, J.-S.; Delmas, M. Esterification of organosolv lignin under supercritical conditions. Ind. Crops Prod. 2014, 58, 287–297. [Google Scholar] [CrossRef]
- Carmen-Mihaela, P. Structural analysis of photodegraded lime wood by means of FT-IR and 2D IR correlation spectroscopy. Int. J. Biol. Macromol. 2011, 48, 667–675. [Google Scholar]

| Sample | Hydroxyl Value (mg KOH/g) | Acetylation Degree (%) | ||
|---|---|---|---|---|
| 60 min | 120 min | 60 min | 120 min | |
| ALC-TiO2-30 | 101 | 57 | 80.7 | 89.1 |
| ALC-TiO2-40 | 93 | 37 | 82.3 | 92.9 |
| ALC-TiO2-50 | 47 | 50 | 91.0 | 90.5 |
| ALC-TiO2-60 | 41 | 53 | 92.2 | 89.9 |
| ALC-H | 93 | 36 | 82.3 | 93.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhao, X.; Wu, Z.; Ma, N.; Gao, E.; Li, L. Full-Component Acetylation of Corncob Residue into Acetone-Dissolvable Composite Resin by Titanium Oxysulfate Reagent. Catalysts 2025, 15, 587. https://doi.org/10.3390/catal15060587
Zhang C, Zhao X, Wu Z, Ma N, Gao E, Li L. Full-Component Acetylation of Corncob Residue into Acetone-Dissolvable Composite Resin by Titanium Oxysulfate Reagent. Catalysts. 2025; 15(6):587. https://doi.org/10.3390/catal15060587
Chicago/Turabian StyleZhang, Chenhang, Xuejuan Zhao, Zhenyu Wu, Na Ma, Erdong Gao, and Licheng Li. 2025. "Full-Component Acetylation of Corncob Residue into Acetone-Dissolvable Composite Resin by Titanium Oxysulfate Reagent" Catalysts 15, no. 6: 587. https://doi.org/10.3390/catal15060587
APA StyleZhang, C., Zhao, X., Wu, Z., Ma, N., Gao, E., & Li, L. (2025). Full-Component Acetylation of Corncob Residue into Acetone-Dissolvable Composite Resin by Titanium Oxysulfate Reagent. Catalysts, 15(6), 587. https://doi.org/10.3390/catal15060587








