Green Pathways: Enhancing Amine Synthesis Using Deep Eutectic Solvents
Abstract
1. Introduction
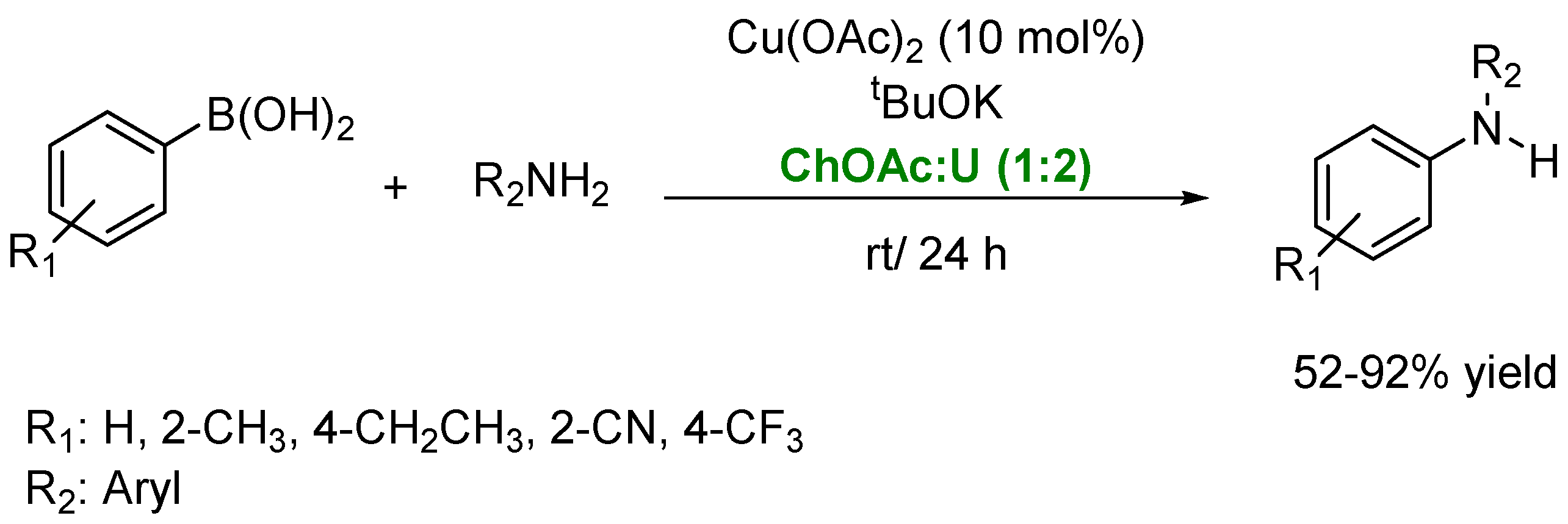
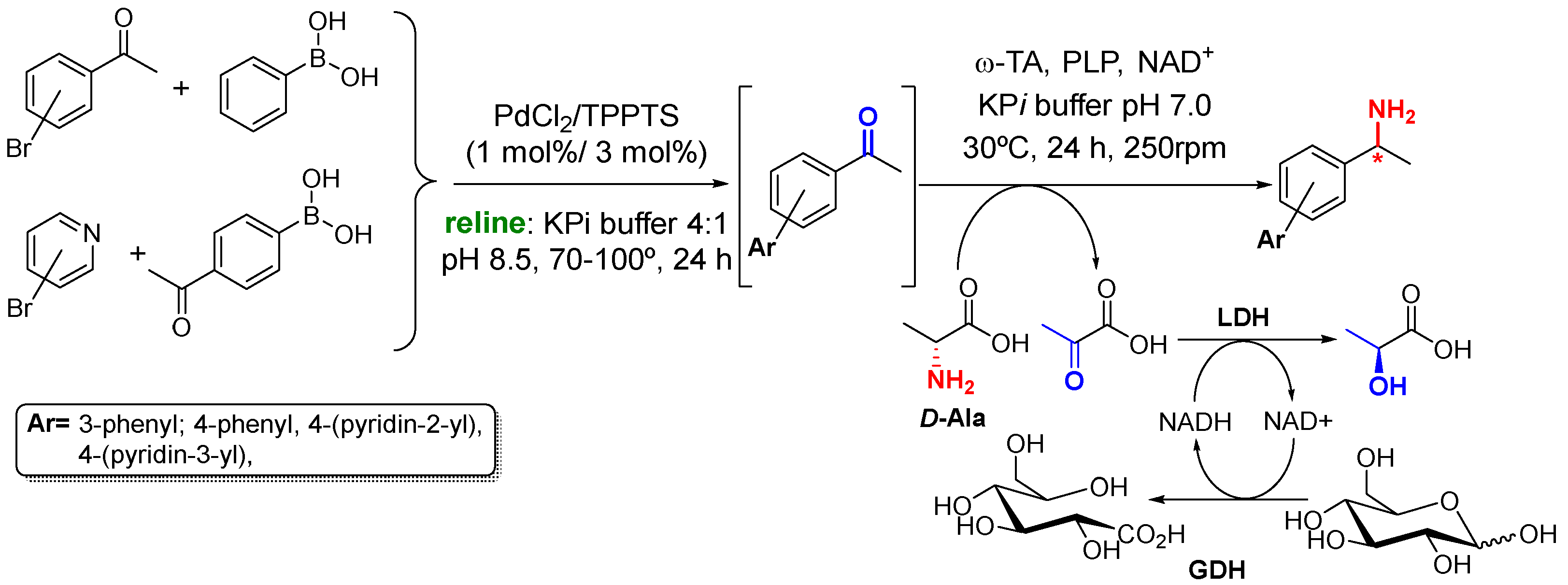
2. Synthesis of Amines in the Presence of Metal-Based Compounds
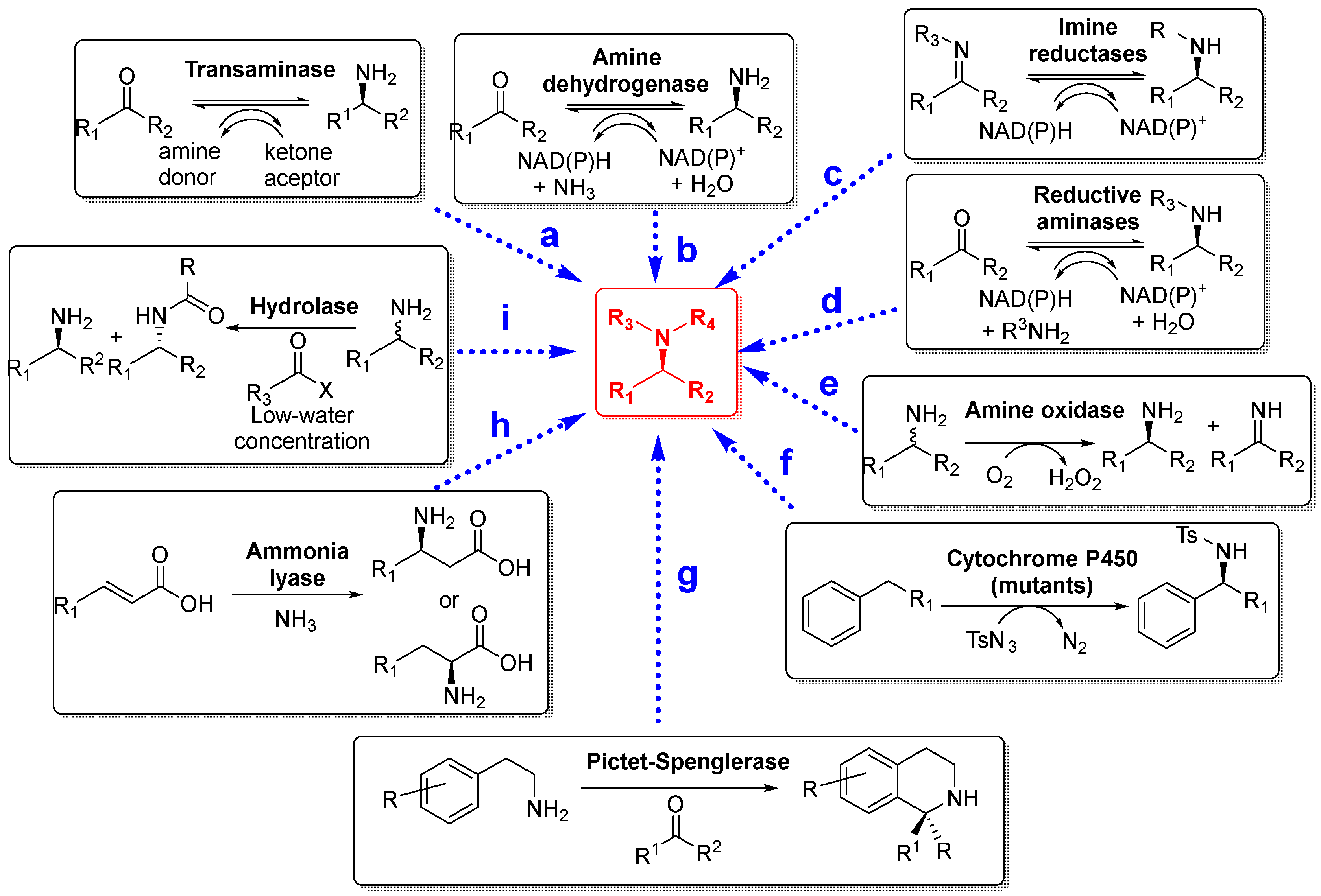
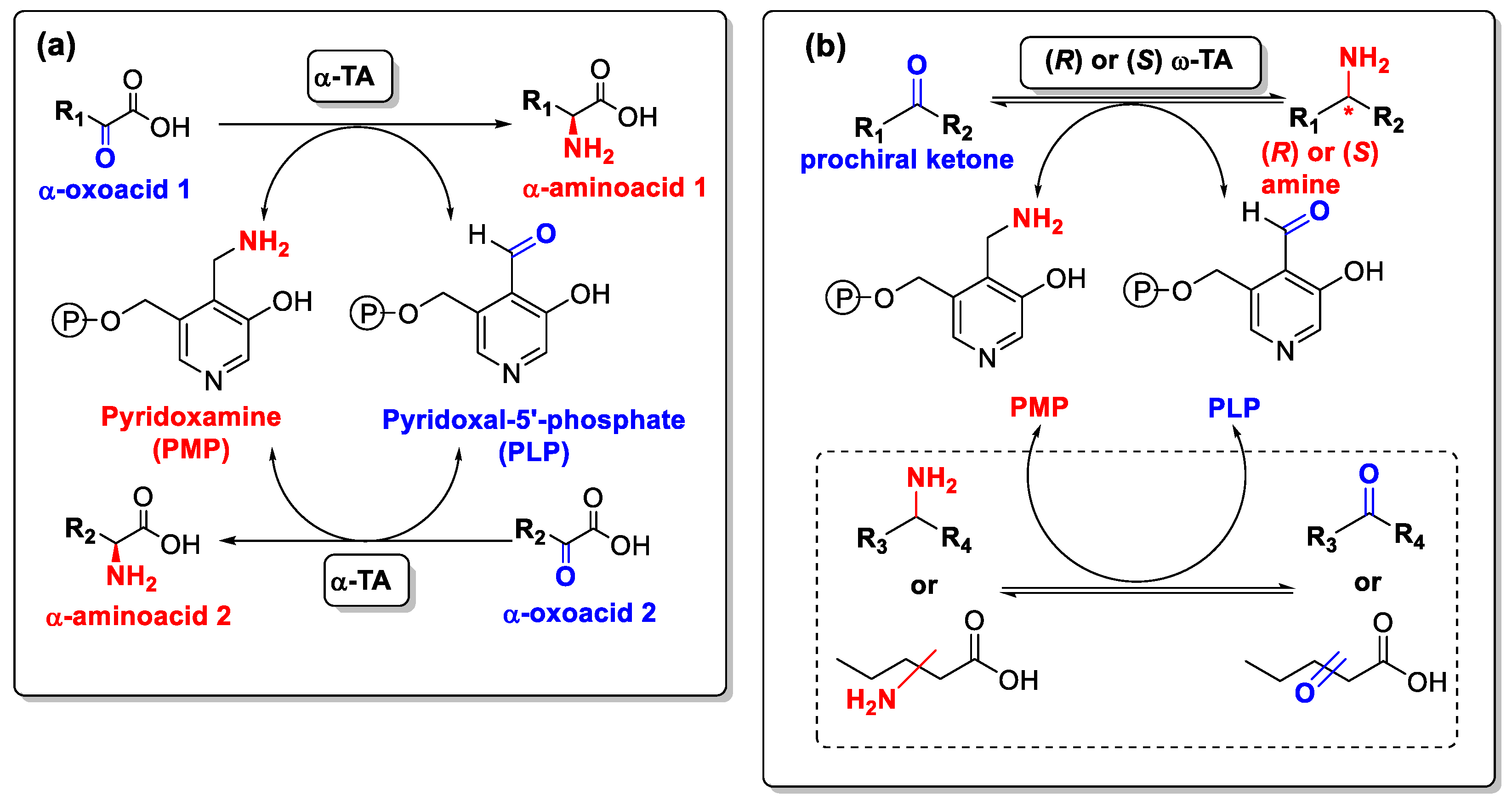
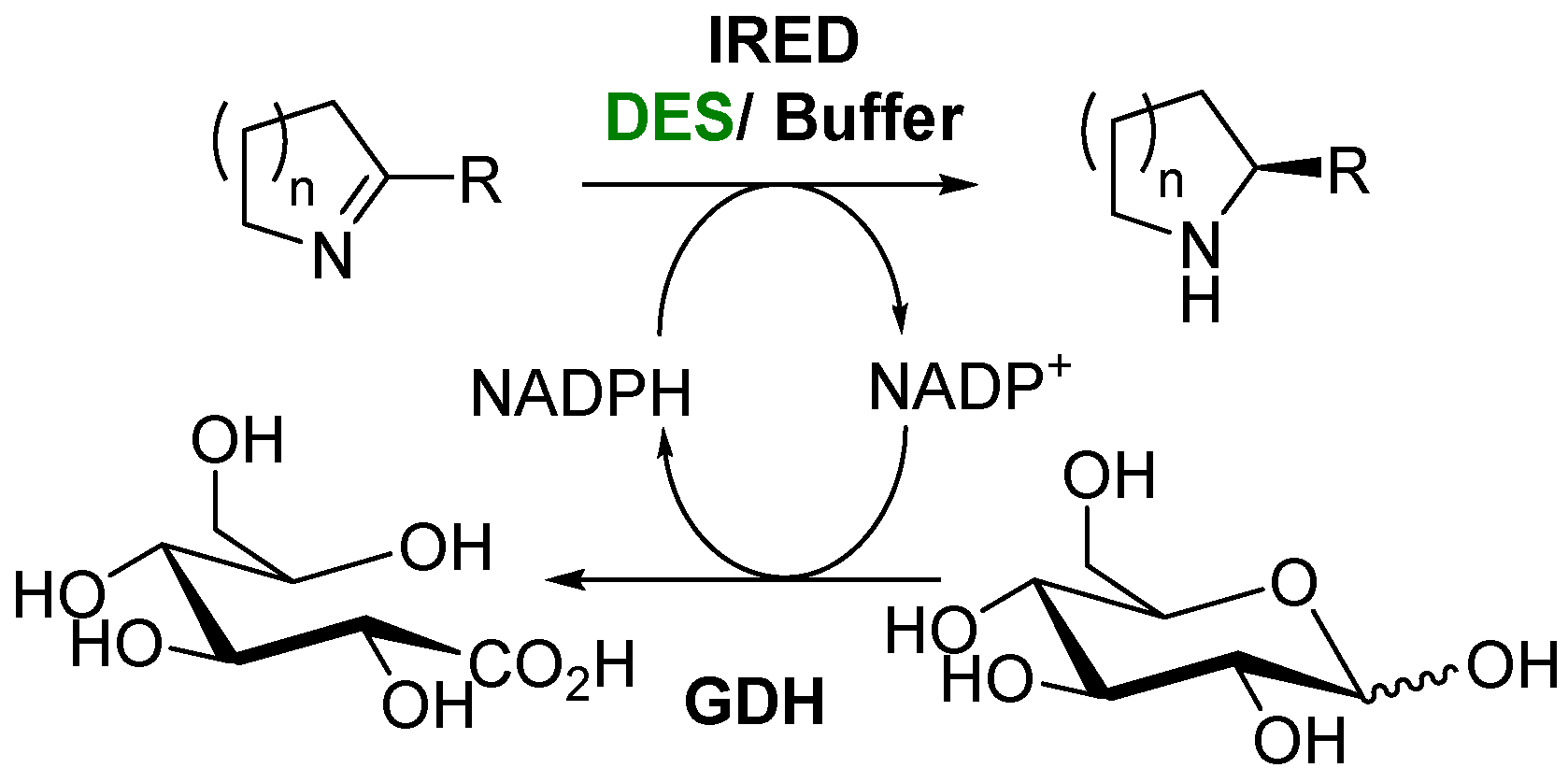
3. Biocatalyzed Preparation of Amines in the Presence of DESs.
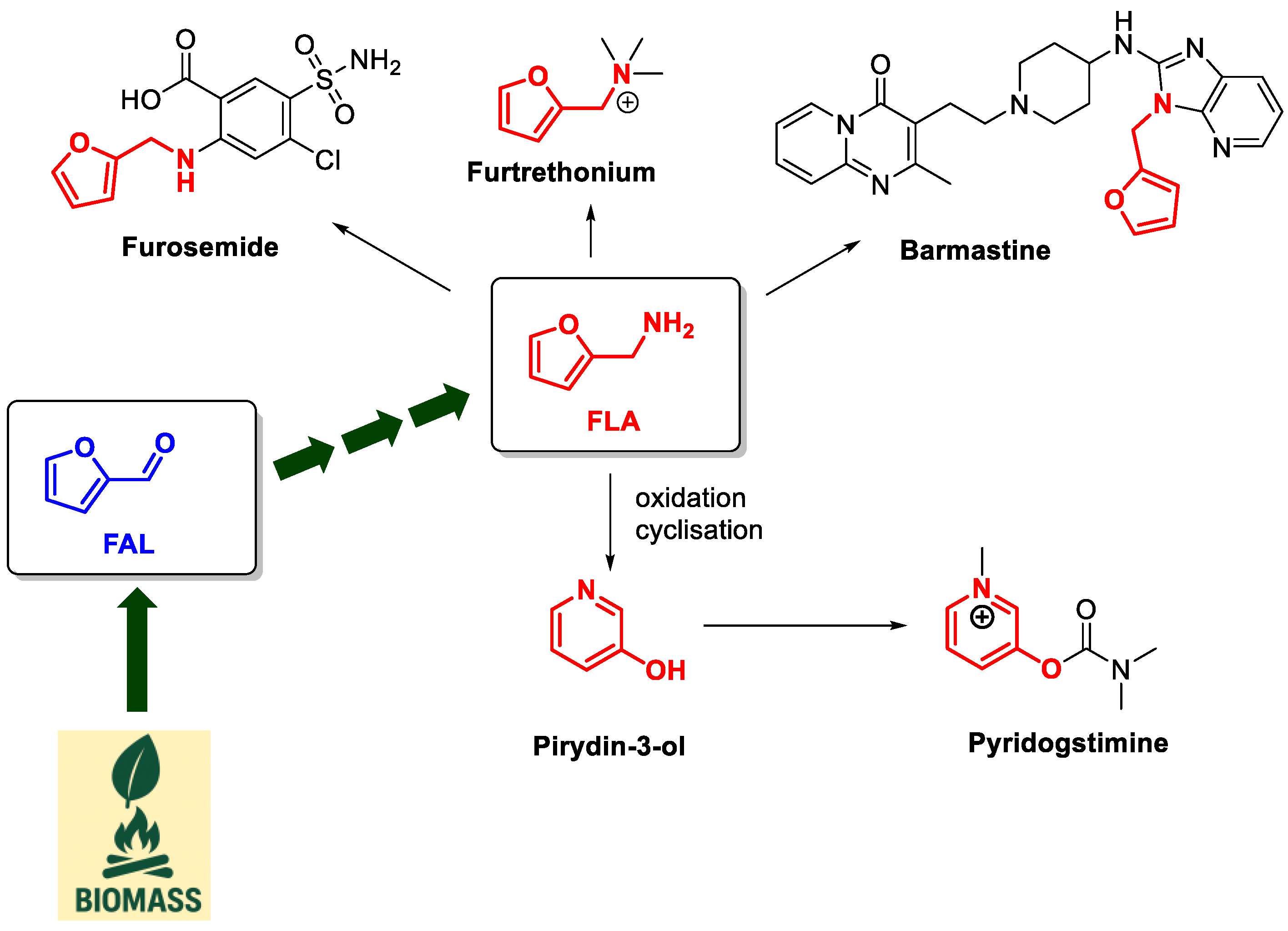
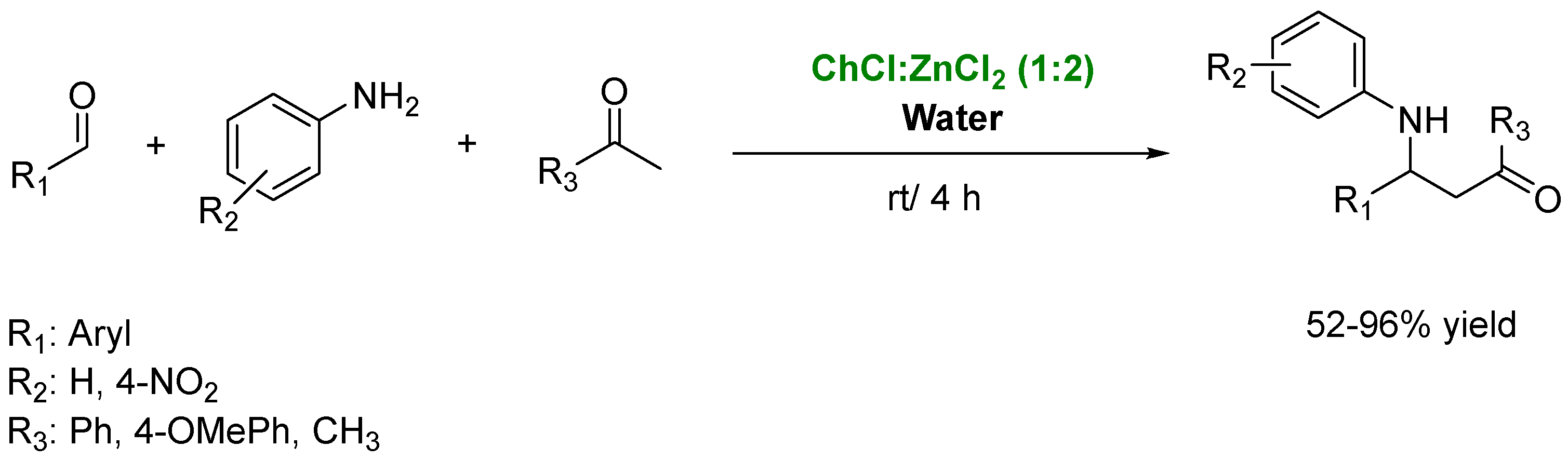
4. Preparation of Amines in the Presence of DES as Solvents/Catalysts
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amines Market Size, Share & Trends Analysis Report by Product (Ethanolamine Fatty Amines, Alkyl Amines), By Application (Crop Protection, Surfactants, Water Treatment, Personal Care, Gas Treatment), by Region, and Segment Forecasts, 2024–2030. Report ID: 978-1-68038-291-4; Grand View Research. 2023. Available online: https://www.grandviewresearch.com/industry-analysis/amines-industry (accessed on 24 April 2025).
- Patil, M.D.; Grogan, G.; Bommarius, A.; Yun, H. Oxidoreductase-Catalyzed Synthesis of Chiral Amines. ACS Catal. 2018, 8, 10985–11015. [Google Scholar] [CrossRef]
- Afanasyev, O.I.; Kuchuk, E.; Usanov, D.L.; Chusov, D. Reductive Amination in the Synthesis of Pharmaceuticals. Chem. Rev. 2019, 119, 11857–11911. [Google Scholar] [CrossRef] [PubMed]
- Vermeeren, B.; Van Praet, S.; Arts, W.; Narmon, T.; Zhang, Y.; Zhou, C.; Steenackers, H.P.; Sels, B.F. From sugars to aliphatic amines: As sweet as it sounds? Production and applications of bio-based aliphatic amines. Chem. Soc. Rev. 2024, 53, 11804–11849. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Patel, I.; Banerjee, S. Importance and Green Synthesis of Amines: A Review. Curr. Org. Chem. 2024, 28, 375–389. [Google Scholar] [CrossRef]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Biobased Amines: From Synthesis to Polymers; Present and Future. Chem. Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Mu, L.; Gao, J.; Zhang, Q.; Kong, F.; Zhang, Y.; Ma, Z.; Sun, C.; Lv, S. Research Progress on Deep Eutectic Solvents and Recent Applications. Processes 2023, 11, 1986. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Database of deep eutectic solvents and their physical properties: A review. J. Mol. Liquids 2023, 384, 121899. [Google Scholar] [CrossRef]
- De Oliveira Vigier, K.; Fracois, J. Synthesis and properties. In Deep Eutectic Solvents: Synthesis, Properties and Applications; Ramón, D.J., Guillena, G., Eds.; Wiley-VCH: Weinheim, Germany, 2020; pp. 1–24. [Google Scholar]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Alvárez-Rivera, G.; Mendiola, J.A. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Molecules 2021, 11, 4897. [Google Scholar] [CrossRef]
- Juliao, D.; Xavier, M.; Mascarehnas, X. Deep eutectic solvents: Viable sustainable electrolytes for supercapacitors. Mat. Today Ener. 2024, 42, 101432. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Ma, Z.; Yan, M. Deep eutectic solvents eutectogels: Progress and challenges. Green. Chem. Eng. 2021, 2, 359–367. [Google Scholar] [CrossRef]
- Tavakol, H.; Shafieyoon, P. Recent advances and new trends in the use of deep eutectic solvents in organic synthesis and other applications. J. Mol. Liq. 2025, 428, 127510. [Google Scholar] [CrossRef]
- Yu, D.; Xue, Z.; Mu, T. Deep eutectic solvents as a green toolbox for synthesis. Cell Rep. Phys. Sci. 2022, 3, 100809. [Google Scholar] [CrossRef]
- Scarpelli, R.; Bence, R.; Herrera Cano, N.C.; Procopio, A.; Wunderlin, D.; Nardi, M. A Review on the Use of Deep Eutectic Solvents in Protection Reactions. Molecules 2024, 29, 818. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Kumar, S.; Bahadur, I.; Singh, T.; Varma, R.S. Deep eutectic solvents as reusable catalysts and promoter for the greener syntheses of small molecules: Recent advances. J. Mol. Liq. 2023, 271, 121013. [Google Scholar] [CrossRef]
- Unlü, A.E.; Arikaya, A.; Takac, S. Use of deep eutectic solvents as catalysts: A mini-review. Green Proc. Synth. 2019, 8, 355–372. [Google Scholar] [CrossRef]
- Shahiri-Haghayegh, M.; Azizi, N. DES as catalyst. In Deep Eutectic Solvents: Synthesis, Properties and Applications; Ramón, D.J., Guillena, G., Eds.; Wiley-VCH: Weinheim, Germany, 2020; pp. 135–170. [Google Scholar]
- Gao, J.; Bhunia, S.; Wang, K.; Gan, L.; Xia, S.; Ma, D. Discovery of N-(naphthalen-1-yl)-N′-alkyl oxalamide ligands enables Cu catalyzed aryl amination with high turnovers. Org. Lett. 2017, 19, 2809–2812. [Google Scholar] [CrossRef]
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: Frommechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef]
- Quivelli, A.F.; Vitale, P.; Perna, F.M.; Capriati, V. Reshaping Ullmann amine synthesis in Deep Eutectic Solvents: A mild approach for Cu-catalyzed C-N coupling reactions with no additional ligands. Front. Chem. 2019, 7, 723. [Google Scholar] [CrossRef]
- Robak, M.T.; Herbage, M.A.; Ellman, J.A. Synthesis and applications of tert-butanesulfinamide. Chem. Rev. 2010, 110, 3600–3740. [Google Scholar] [CrossRef] [PubMed]
- Cicco, L.; Salomone, A.; Vitale, P.; Rios-Lombardia, N.; González-Sabín, J.; García-Alvárez, J.; Perna, F.M.; Capriati, V. Addition of Highly Polarized Organometallic Compounds to N-tert-Butanesulfinyl Imines in Deep Eutectic Solvents under Air: Preparation of Chiral Amines of Pharmaceutical Interest. ChemSusChem 2020, 13, 3583–3588. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Pawar, G.G.; Kumar, S.V.; Jiang, Y.; Ma, D. Selected Copper-Based Reactions for C−N, C−O, C−S, and C−C Bond Formation. Angew. Chem. Int. Ed. 2017, 56, 16136. [Google Scholar] [CrossRef] [PubMed]
- Cicco, L.; Vitale, P.; Perna, F.M.; Capriati, V.; García-Alvárez, J. Cu-catalysed Chan–Evans–Lam reaction meets deep eutectic solvents: Efficient and selective C–N bond formation under aerobic conditions at room temperature. RSC Sustain. 2023, 1, 847–852. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef]
- France, S.P.; Lewis, R.D.; Martínez, C.T. The Evolving Nature of Biocatalysis in Pharmaceutical Research and Development. JACS Au 2023, 3, 715–735. [Google Scholar] [CrossRef]
- Hanefeld, U.; Hollman, F.; Paul, C.E. Biocatalysis making waves in organic chemistry. Chem. Soc. Rev. 2022, 51, 594–627. [Google Scholar] [CrossRef]
- Mutti, F.G.; Knaus, T. Enzymes Applied to the Synthesis of Amines. In Biocatalysis for Practitioners: Techniques, Reactions and Applications; de Gonzalo, G., Lavandera, I., Eds.; Wiley-VCH: Weinheim, Germany, 2021; pp. 143–180. [Google Scholar]
- Cananà, S.; De Nardi, F.; Blangetti, M.; Parisotto, S.; Prandi, C. Biocatalysis in Non-Conventional Media: Unlocking the Potential for Sustainable Chiral Amine Synthesis. Chem. Eur. J. 2024, 30, e202304364. [Google Scholar] [CrossRef]
- Zawodny, W.; Montgomery, S.L. Evolving New Chemistry: Biocatalysis for the Synthesis of Amine-Containing Pharmaceuticals. Catalysts 2022, 12, 595. [Google Scholar] [CrossRef]
- Wu, S.; Xiang, C.; Zhou, Y.; Khan, M.S.H.; Liu, W.; Feiler, C.G.; Wei, R.; Weber, G.; Höhne, M.; Bornscheuer, U.T. A growth selection system for the directed evolution of amine-forming or converting enzymes. Nat. Commun. 2022, 13, 7458. [Google Scholar] [CrossRef]
- D. Patil, M.; Grogan, G.; Bommarius, A.; Yun, H. Recent Advances in ω-Transaminase-Mediated Biocatalysis for the Enantioselective Synthesis of Chiral Amines. Catalysts 2018, 8, 254. [Google Scholar] [CrossRef]
- Kelly, S.A.; Pohle, S.; Wharry, S.; Mix, S.; Allen, C.C.R.; Moody, T.S.; Gilmore, B.F. Application of omega-Transaminases in the Pharmaceutical Industry. Chem. Rev. 2018, 118, 349–367. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.A.; Mix, S.; Moody, T.S.; Gilmore, B.F. Transaminases for industrial biocatalysis: Novel enzyme discovery. Appl. Microbiol. Biotechnol. 2020, 104, 4781–4794. [Google Scholar] [CrossRef]
- Grogan, G. Synthesis of chiral amines using redox biocatalysis. Curr. Opin. Chem. Biol. 2018, 43, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.C.; Brzezniak, A.; France, S.P.; Ramsden, J.I.; Mangas-Sanchez, J.; Montgomery, S.L.; Heath, R.S.; Turner, N.J. Imine reductases, reductive aminases, and amine oxidases for the synthesis of chiral amines: Discovery, characterization, and synthetic applications. Methods Enzymol. 2018, 608, 131. [Google Scholar]
- Yuan, B.; Yang, D.; Qu, G.; Turner, N.J.; Sun, Z. Biocatalytic reductive aminations with NAD(P)H-dependent enzymes: Enzyme discovery, engineering and synthetic applications. Chem. Soc. Rev. 2023, 53, 227–262. [Google Scholar] [CrossRef]
- Tseliou, V.; Masman, M.F.; Knaus, T.; Mutti, F.G. Current Status of Amine Dehydrogenases: From Active Site Architecture to Diverse Applications Across a Broad Substrate Spectrum. ChemCatChem 2024, 16, e202400469. [Google Scholar] [CrossRef]
- Mangas-Sánchez, J. Synthesis of chiral amines employing imine reductases and reductive aminases. In Biocatalysis in Asymmetric Synthesis; de Gonzalo, G., Alcántara, A.R., Eds.; Academic Press: London, UK, 2024; pp. 209–236. [Google Scholar]
- Pollegioni, L.; Molla, G.C. Oxidation with Amine Oxidases and Amino Acid Oxidases. In Biocatalysis in Organic Synthesis 3, 1st ed; Faber, K., Fessner, W.-D., Turner, N.J., Eds.; Georg Thieme Verlag KG: Stuttgart, Germany, 2015. [Google Scholar]
- Prier, C.K.; Zhang, R.K.; Buller, A.R.; Brinkmann-Chen, S.; Arnold, F.H. Enantioselective, intermolecular benzylic C-H amination catalysed by an engineered iron-haem enzyme. Nat. Chem. 2017, 9, 629–634. [Google Scholar] [CrossRef]
- Xu, W.N.; Gao, Y.D.; Su, P.; Huang, L.; He, Z.L.; Yang, L.C. Progress in Enzyme-Catalyzed C(sp3)-H Amination. ACS Catal. 2024, 14, 14139–14160. [Google Scholar] [CrossRef]
- Roddan, R.; Ward, J.M.; Keep, N.H.; Hailes, H.C. Pictet–Spenglerases in alkaloid biosynthesis: Future applications in biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 69–76. [Google Scholar] [CrossRef]
- Parmeggiani, F.; Weise, N.J.; Ahmed, S.T.; Turner, N.J. Synthetic and therapeutic applications of ammonia-lyases and aminomutases. Chem. Rev. 2018, 118, 73–118. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.M.; Hollmann, F.; Mutti, F.G. Synthesis of enantiomerically pure alcohols and amines via biocatalytic deracemisation methods. Catal. Sci. Technol. 2019, 9, 5487–5503. [Google Scholar] [CrossRef]
- Farkas, E.; Oláh, M.; Földi, A.; Kóti, J.; Éles, J.; Nagy, J.; Gal, C.A.; Paizs, C.; Hornyánszky, G.; Poppe, L. Chemoenzymatic Dynamic Kinetic Resolution of Amines in Fully Continuous-Flow Mode. Org. Lett. 2018, 20, 8052–8056. [Google Scholar] [CrossRef]
- Takle, M.J.; Deadman, B.J.; Hellgardt, K.; Dickhaut, J.; Wieja, A.; Hii, K.K.M. A Flash Thermal Racemization Protocol for the Chemoenzymatic Dynamic Kinetic Resolution and Stereoinversion of Chiral Amines. ACS Catal. 2023, 13, 10541–10546. [Google Scholar] [CrossRef]
- Haarr, M.B.; Sriwong, K.T.; O’Reilly, E. Commercial Transaminases for the Asymmetric Synthesis of Bulky Amines. Eur. J. Org. Chem. 2024, 27, e202400257. [Google Scholar] [CrossRef]
- Wang, H.; Tao, Y.; Masuku, M.V.; Cao, J.; Yang, J.; Huang, K.; Ge, Y.; Yu, Y.; Xiao, Z.; Kuang, Y.; et al. Effects of deep eutectic solvents on the biotransformation efficiency of ω-transaminase. J. Mol. Liq. 2023, 377, 121379. [Google Scholar] [CrossRef]
- Wang, H.; Masuku, M.V.; Tao, Y.; Yang, J.; Kuang, Y.; Lyu, C.; Huang, J.; Yang, S. Improved Stability and Catalytic Efficiency of ω-Transaminase in Aqueous Mixture of Deep Eutectic Solvents. Molecules 2023, 28, 3895. [Google Scholar] [CrossRef]
- Kalhor, P.; Ghandi, K. Deep eutectic solvents for pretreatment, extraction, and catalysis of biomass and food waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef]
- Shen, X.J.; Wen, J.L.; Mei, Q.Q.; Chen, X.; Sun, D.; Yuan, T.Q.; Sun, R.C. Facile fractionation of lignocelluloses by biomass-derived deep eutectic solvent (DES) pretreatment for cellulose enzymatic hydrolysis and lignin valorization. Green Chem. 2019, 21, 275–283. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.J. Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: A review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef]
- Mamilla, J.L.K.; Novak, U.; Grilc, M.; Likozar, B. Natural deep eutectic solvents (DES) for fractionation of waste lignocellulosic biomass and its cascade conversion to value-added bio-based chemicals. Biomass Bioener. 2019, 120, 417–425. [Google Scholar] [CrossRef]
- Li, P.; Li, T.; Wu, S. Process parameters and product characterization for efficient extraction of lignin with deep eutectic solvents: A review. Int. J. Biol. Macromol. 2024, 280, 136053. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Pramanik, K. Extraction of lignin from sustainable lignocellulosic food waste resources using a green deep eutectic solvent system and its property characterization. Int. J. Biol. Macromol. 2025, 307, 142094. [Google Scholar] [CrossRef]
- Tong, Z.; Wang, W.; Zeng, S.; Sun, Y.; Meng, J.; Liu, Y.; Xia, Q.; Yu, H. Hydrogen bond reconstruction strategy for eutectic solvents that realizes room-temperature dissolution of cellulose. Green Chem. 2022, 24, 8760–8769. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, G.; Sun, H.; Xu, C.; Wu, B.; Huang, C.; Lei, B. A Novel Strategy to Intensify the Dissolution of Cellulose in Deep Eutectic Solvents by Partial Chemical Bonding. BioResour. 2022, 17, 4167–4185. [Google Scholar] [CrossRef]
- Morán-Aguilar, M.G.; Costa-Trigo, I.; Aguilar-Uscanga, M.G.; Paz, A.; Domínguez, J.M. Development of sustainable sugarcane bagasse biorefinery using a greener deep eutectic solvent from hemicellulose-derived acids. Biomass Bioener. 2024, 186, 107256. [Google Scholar] [CrossRef]
- Xu, G.; Tu, Z.; Hu, X.; Li, M.; Zheng, L.; Zhang, X.; Shi, M.; Wu, Y. New insight into dehydration reaction of xylose and hemic 107256ellulose to furfural over dual-acid deep eutectic solvent catalysts. Chem. Eng. J. 2024, 496, 154112. [Google Scholar] [CrossRef]
- Wang, Z.K.; Li, H.; Lin, X.C.; Tang, L.; Chen, J.J.; Mo, J.W.; Yu, R.S.; Shen, X.J. Novel recyclable deep eutectic solvent boost biomass pretreatment for enzymatic hydrolysis. Bioresour. Technol. 2020, 307, 123237. [Google Scholar] [CrossRef]
- Miao, G.; Wong, J.L.; Chew, J.J.; Khaerudini, D.S.; Sunarso, J.; Xu, F. Deep eutectic solvent pretreatment of oil palm biomass: Promoted lignin pyrolysis and enzymatic digestibility of solid residues. Int. J. Biol. Macromol. 2025, 293, 138847. [Google Scholar] [CrossRef]
- Yong, K.J.; Wu, T.Y.; Lee, C.B.T.L.; Lee, Z.J.; Liu, Q.; Jahim, J.M.; Zhou, Q.; Zhang, L. Furfural production from biomass residues: Current technologies, challenges and future prospects. Biomass Bioenergy 2022, 161, 106458. [Google Scholar] [CrossRef]
- Ji, L.; Tang, Z.; Yang, D.; Ma, C.; He, Y.C. Improved one-pot synthesis of furfural from corn stalk with heterogeneous catalysis using corn stalk as biobased carrier in deep eutectic solvent–water system. Bioresour. Technol. 2021, 340, 125691. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Li, J.; Miao, K.; Song, Y.; Yang, S.; Zhao, S.; Lu, Y.; Hu, J. Atmospheric one-pot fractionation and catalytic conversion of lignocellulose in multifunctional deep eutectic solvent system. Int. J. Biol. Macromol. 2025, 290, 138736. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Peng, Y.; He, Y.C.; Ma, C. Chemobiocatalytic transformation of biomass-derived D-xylose into furfuryl alcohol in a sustainable reaction system. Catal. Commun. 2023, 184, 106783. [Google Scholar] [CrossRef]
- Sharma, R. Furfurylamine Market Segments-by Application (Pharmaceuticals, Agrochemicals, Rubber Additives, Corrosion Inhibitors, and Others), End-User Industry (Chemical, Pharmaceutical, Agriculture, and Others), and Region (Asia Pacific, North America, Latin America, Europe, and Middle East & Africa)-Global Industry Analysis, Growth, Share, Size, Trends, and Forecast 2025-2033; Dataintelo. 2025. Available online: https://dataintelo.com/report/global-furfurylamine-market (accessed on 15 May 2025).
- Huang, X.; Dorhout Mees, E.; Vos, P.; Hamza, S.; Braam, B. Everything we always wanted to know about furosemide but were afraid to ask. Am. J. Physiol. Renal Physiol. 2016, 310, F958–F971. [Google Scholar] [CrossRef]
- Feriani, A.; Gaviraghi, G.; Toson, G.; Mor, M.; Barbieri, A.; Grana, E.; Boselli, C.; Guarneri, M.; Simoni, D.; Manfredini, S. Cholinergic Agents Structurally Related to Furtrethonium. 2. Synthesis and Antimuscarinic Activity of a Series of N-[5-[(1′-Substituted-acetoxy)methyl]-2-furfuryl]dialkylamines. J. Med. Chem. 1994, 37, 4278–4287. [Google Scholar] [CrossRef]
- Awouters, F.; Vermeire, J.; Smeyers, F.; Vermote, P.; van Beek, R.; Niemegeers, C.J.E. Oral antiallergic activity in ascaris hypersensitive dogs: A study of known antihistamines and of the new compounds ramastine (R 57 959) and levocabastine (R 50 547). Drug Dev. Res. 1986, 8, 95–102. [Google Scholar] [CrossRef]
- Richieu, A.; Bertrand, P. Evaluation of Aquivion® as Recyclable Superacid Solid Catalyst in the Oxidation of Furfurylamines with Hydrogen Peroxide to 3-Hydroxypyridines. ChemistrySelect 2023, 8, e202303423. [Google Scholar] [CrossRef]
- Maggi, L.; Mantegazza, R. Treatment of myasthenia gravis: Focus on pyridostigmine. Clin. Drug Investig. 2011, 31, 691–701. [Google Scholar] [CrossRef]
- Goculdas, T.; Ramirez, M.; Crossley, M.; Sadula, S.; Vlachos, D.G. Biomass-Derived, Target Specific, and Ecologically Safer Insecticide Active Ingredients. ChemSusChem 2024, 17, e202400824. [Google Scholar] [CrossRef]
- Shu, H.H.; Shi, L.Q.; Zhang, H.Q.; Liu, Y.; Han, S.L.; Rao, J.Y.; Liu, C.M. Synthesis of furan-based cationic biopolyamides and their removal abilities for perfluoroalkyl and polyfluoroalkyl substances. Chem. Eng. J. 2025, 507, 160306. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, Z.; Wei, J.; Li, Y.; Xiang, D.; Wu, Y.; Que, Y. A Phosphorous-Containing Bio-Based Furfurylamine Type Benzoxazine and Its Application in Bisphenol-A Type Benzoxazine Resins: Preparation, Thermal Properties and Flammability. Polymers 2022, 14, 1597. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, T.A.S.; da Cunha, J.N.; de Oliveira, G.A.; da Silva, T.U.; de Oliveira, S.M.; de Araújo, J.R.; Machado, S.D.P.; D’Elia, E.; Rezende, M.J.C. Nitrogenated derivatives of furfural as green corrosion inhibitors for mild steel in HCl solution. J. Mater. Res. Technol. 2020, 9, 7104–7122. [Google Scholar] [CrossRef]
- Saini, M.K.; Kumar, S.; Li, H.; Babu, S.A.; Saravanamurugan, S. Advances in the Catalytic Reductive Amination of Furfural to Furfural Amine: The Momentous Role of Active Metal Sites. ChemSusChem 2022, 15, e202200107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Chen, B.; Zhou, X.; Kang, S.; Xu, Y.; Wei, J. Selective Synthesis of Furfurylamine by Reductive Amination of Furfural over Raney Cobalt. ChemCatChem 2019, 11, 5562–5569. [Google Scholar] [CrossRef]
- Gould, N.S.; Landfield, H.; Dinkelacker, B.; Brady, C.; Yang, X.; Xu, B. Selectivity Control in Catalytic Reductive Amination of Furfural to Furfurylamine on Supported Catalysts. ChemCatChem 2020, 12, 2106–2115. [Google Scholar] [CrossRef]
- Yang, G.; Li, X.; Ma, W.; Sun, X.; Huang, H. Recent advances in upcycling lignocellulosic biomass through chemoenzymatic processes. Curr. Opin. Green Sustain. Chem. 2025, 53, 101017. [Google Scholar] [CrossRef]
- Giri, P.; Lim, S.; Khobragade, T.P.; Pagar, A.D.; Patil, M.D.; Sarak, S.; Jeon, H.; Joo, S.; Goh, Y.; Jung, S.; et al. Biocatalysis enables the scalable conversion of biobased furans into various furfurylamines. Nat. Commun. 2024, 15, 6371. [Google Scholar] [CrossRef]
- Di, J.H.; Gong, L.; Yang, D.; He, Y.C.; Tang, Z.Y.; Ma, C.L. Enhanced conversion of biomass to furfurylamine with high productivity by tandem catalysis with sulfonated perlite and ω-transaminase whole-cell biocatalyst. J. Biotechnol. 2021, 334, 26–34. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L.; Zhu, X.; Xia, Y.; Ma, C.; Liang, J.; He, Y.C. A Hybrid Catalytic Conversion of Corncob to Furfurylamine in Tandem Reaction with Aluminium-Based Alkaline-Treated Graphite and ω-Transaminase Biocatalyst in γ-Valerolactone–Water. Catal. Lett. 2021, 151, 1834–1841. [Google Scholar] [CrossRef]
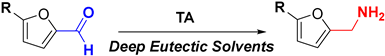
- Li, Q.; Di, J.; Liao, X.; Ni, J.; Li, Q.; He, Y.C.; Ma, C. Exploration of benign deep eutectic solvent-water systems for the highly efficient production of furfurylamine from sugarcane bagasse: Via chemoenzymatic cascade catalysis. Green Chem. 2021, 23, 8154–8168. [Google Scholar] [CrossRef]
- Ni, J.; Li, Q.; Gong, L.; Liao, X.L.; Zhang, Z.J.; Ma, C.; He, Y. Highly Efficient Chemoenzymatic Cascade Catalysis of Biomass into Furfurylamine by a Heterogeneous Shrimp Shell-Based Chemocatalyst and an ω-Transaminase Biocatalyst in Deep Eutectic Solvent-Water. ACS Sustain. Chem. Eng. 2021, 9, 13084–13095. [Google Scholar] [CrossRef]
- Di, J.; Li, Q.; Ma, C.; He, Y.C. An efficient and sustainable furfurylamine production from biomass-derived furfural by a robust mutant ω-transaminase biocatalyst. Bioresour. Technol. 2023, 369, 128425. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Q.; Di, J.; He, Y.; Ma, C. Improved Synthesis of Furfurylamine from a High Titer of Biomass-Derived Furfural by a Thermostable Triple Mutant ω-Transaminase in a Three-Component Deep Eutectic Solvent ChCl/Lactic Acid/Malonic Acid System. ACS Sustain. Chem. Eng. 2023, 11, 7515–7525. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.C.; Ma, C. Elevating biotransamiantion of pineapple peel-derived furfural into furfurylamine by recombinant Escherichia coli carrying pyruvate decarboxylase and mutant ω-transaminase with low loading of amine donor. Food BioSci. 2024, 62, 105164. [Google Scholar] [CrossRef]
- Wang, Z.; Chai, H.; Ren, J.; Tao, Y.; Li, Q.; Ma, C.; Ai, Y.; He, Y. Biocatalytic Valorization of Biobased 5-Hydroxymethylfurfural to 5-Hydroxymethyl-2-furfurylamine in a Three-Constituent Deep Eutectic Solvent-Water System. ACS Sustain. Chem. Eng. 2022, 10, 8452–8463. [Google Scholar] [CrossRef]
- Wu, C.; Li, Q.; Di, J.; He, Y.C.; Ma, C. Enhanced production of 5-hydroxymethyl-2-furfurylamine from biobased HMF by a robust triple mutant ω-transaminase biocatalyst in a betaine:malonic acid—Water medium. Fuel 2023, 343, 127830. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Jiang, S.K.; Shen, X.; Lin, J.C.; Yi, Y.; Ji, X.J. Biocatalytic synthesis of vanillin from biomass-derived compounds: A review. Catal. Today 2025, 445, 115077. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef]
- Sailo, B.L.; Garhwal, A.; Mishra, A.; Hegde, M.; Vishwa, R.; Girisa, S.; Abbas, M.; Alqahtani, M.S.; Abdulhammed, A.; Sethi, G.; et al. Potential of capsaicin as a combinatorial agent to overcome chemoresistance and to improve outcomes of cancer therapy. Biochem. Pharmacol. 2025, 236, 116828. [Google Scholar] [CrossRef]
- Swamy, M.K.; Kumar, A. (Eds.) Capsaicinoids: From Natural Sources to Biosynthesis and their Clinical Applications; Springer Nature: Singapore, 2024. [Google Scholar]
- Maharjan, A.; Vasamsetti, B.M.K.; Park, J.H. A comprehensive review of capsaicin: Biosynthesis, industrial productions, processing to applications, and clinical uses. Heliyon 2024, 10, e39721. [Google Scholar] [CrossRef]
- Heise, N.V.; Quast, J.; Csuk, R. Revisiting capsaicin and nonivamide: Their analogs exert strong inhibitory activity against cholinesterases. Eur. J. Med. Chem. Rep. 2024, 12, 100200. [Google Scholar] [CrossRef]
- Tutka, P.; Wlaź, A.; Florek-Łuszczki, M.; Kołodziejczyk, P.; Bartusik-Aebisher, D.; Łuszczki, J.J. Arvanil, olvanil, AM 1172 and LY 2183240 (various cannabinoid CB1 receptor agonists) increase the threshold for maximal electroshock-induced seizures in mice. Pharmacol. Rep. 2018, 70, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Marzęda, P.; Wróblewska-Łuczka, P.; Florek-Łuszczki, M.; Drozd, M.; Góralczyk, A.; Łuszczki, J.J. Comparison of the Anticancer Effects of Arvanil and Olvanil When Combined with Cisplatin and Mitoxantrone in Various Melanoma Cell Lines—An Isobolographic Analysis. Int. J. Mol. Sci. 2022, 23, 14192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, Y.C.; Ma, C. Efficient synthesis of vanillylamine through bioamination of lignin-derived vanillin by recombinant E. coli containing ω-transaminase from Caulobacter sp. D5 in dimethyl sulfoxide-water. Bioresour. Technol. 2024, 413, 131526. [Google Scholar] [CrossRef]
- Li, Q.; Kong, L.; He, Y.C.; Ma, C. Efficient biosynthesis of vanillylamine via biological transamination of lignin-derived vanillin in a β-cyclodextrin-water medium. Sustain. Chem. Pharm. 2024, 38, 101437. [Google Scholar] [CrossRef]
- Li, Q.; Gao, R.; Li, Y.; Fan, B.; Ma, C.; He, Y.C. Improved biotransformation of lignin-valorized vanillin into vanillylamine in a sustainable bioreaction medium. Bioresour. Technol. 2023, 384, 129292. [Google Scholar] [CrossRef]
- Paris, J.; Telzerow, A.; Ríos-Lombardía, N.; Steiner, K.; Schwab, H.; Morís, F.; Gröger, H.; González-Sabín, J. Enantioselective One-Pot Synthesis of Biaryl-Substituted Amines by Combining Palladium and Enzyme Catalysis in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 5486–5493. [Google Scholar] [CrossRef]
- Liardo, E.; Ríos-Lombardía, N.; Morís, F.; Rebolledo, F.; González-Sabín, J. Hybrid Organo- and Biocatalytic Process for the Asymmetric Transformation of Alcohols into Amines in Aqueous Medium. ACS Catal. 2017, 7, 4768–4774. [Google Scholar] [CrossRef]
- Leipold, L.; Dobrijevic, D.; Jeffries, J.W.E.; Bawn, M.; Moody, T.S.; Ward, J.M.; Hailes, H.C. The identification and use of robust transaminases from a domestic drain metagenome. Green Chem. 2019, 21, 75–86. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution. Angew. Chem. Int. Ed. 2017, 56, 9782–9785. [Google Scholar] [CrossRef]
- Telzerow, A.; Paris, J.; Håkansson, M.; González-Sabín, J.; Ríos-Lombardía, N.; Schürmann, M.; Gröger, H.; Morís, F.; Kourist, R.; Schwab, H.; et al. Amine Transaminase from Exophiala Xenobiotica-Crystal Structure and Engineering of a Fold IV Transaminase that Naturally Converts Biaryl Ketones. ACS Catal. 2019, 9, 1140–1148. [Google Scholar] [CrossRef]
- Farhang, M.; Akbarzadeh, A.R.; Rabbani, M.; Ghadiri, A.M. A retrospective-prospective review of Suzuki–Miyaura reaction: From cross-coupling reaction to pharmaceutical industry applications. Polyhedron 2022, 227, 116124. [Google Scholar] [CrossRef]
- Arnodo, D.; De Nardi, F.; Parisotto, S.; De Nardo, E.; Cananà, S.; Salvatico, F.; De Marchi, E.; Scarpi, D.; Blangetti, M.; Occhiato, E.G.; et al. Asymmetric Reduction of Cyclic Imines by Imine Reductase Enzymes in Non-Conventional Solvents. ChemSusChem 2024, 17, e202301243. [Google Scholar] [CrossRef] [PubMed]
- Falcini, C.; de Gonzalo, G. Deep Eutectic Solvents as Catalysts in the Synthesis of Active Pharmaceutical Ingredients and Precursors. Catalysts 2024, 14, 120. [Google Scholar] [CrossRef]
- Singh, B.; Lobo, H.; Shankarling, G. Selective N-alkylation of aromatic primary amines catalysed by bio-catalyst of Deep Eutectic. Solvent. Catal. Lett. 2011, 141, 178–182. [Google Scholar] [CrossRef]
- Allerberger, F.; Klare, I. In-vitro activity of fosfomycin against vancomycin-resistant enterococci. J. Antimicrob. Chemother. 1999, 43, 211–217. [Google Scholar] [CrossRef]
- Gallardo-Macias, R.; Nakayama, K. Tin(II) Compounds as Catalysts for the Kabachnik-Fields Reaction under Solvent-Free Conditions: Facile Synthesis of α-Aminophosphonates. Synthesis 2010, 2010, 57–62. [Google Scholar] [CrossRef]
- Disale, S.T.; Kale, S.R.; Kahandal, S.S.; Srinivasan, T.G.; Jayaram, R.V. Choline chloride-2ZnCl2 ionic liquid: An efficient and reusable catalysts for the solvent free Kabachnik-Fields reaction. Tetrahedron Lett. 2012, 53, 2277–2279. [Google Scholar] [CrossRef]
- Abranches, D.O.; Coutinho, J.A.P. Everything You Wanted to Know about Deep Eutectic Solvents but Were Afraid to Be Told. Annu. Rev. Chem. Biomol. Eng. 2023, 14, 141–163. [Google Scholar] [CrossRef]
- Carreiro, E.P.; Federsel, H.J.; Hermann, G.J.; Burke, A.J. Stereoselective Catalytic Synthesis of Bioactive Compounds in Natural Deep Eutectic Solvents (NADESs): A Survey across the Catalytic Spectrum. Catalysts 2024, 14, 160. [Google Scholar] [CrossRef]
- Mandal, S.; Narvariya, R.; Sunar, S.L.; Paul, I.; Jain, A.; Panda, T.K. Synthesis of aminophosphorous derivatives using a Deep Eutectic Solvent (DES) in a dual role. Green Chem. 2023, 25, 8266–8272. [Google Scholar] [CrossRef]
- Domínguez de María, P. Biocatalysis, sustainability, and industrial applications: Show me the metrics. Curr. Opin. Green Sustain. Chem. 2021, 31, 100514. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Keshavarzipur, K.; Tavkol, H. Deep Eutectic Solvent as a Recyclable Catalyst for Three-Component Synthesis of b-Amino Carbonyls. Catal. Lett. 2015, 145, 1062–1066. [Google Scholar] [CrossRef]
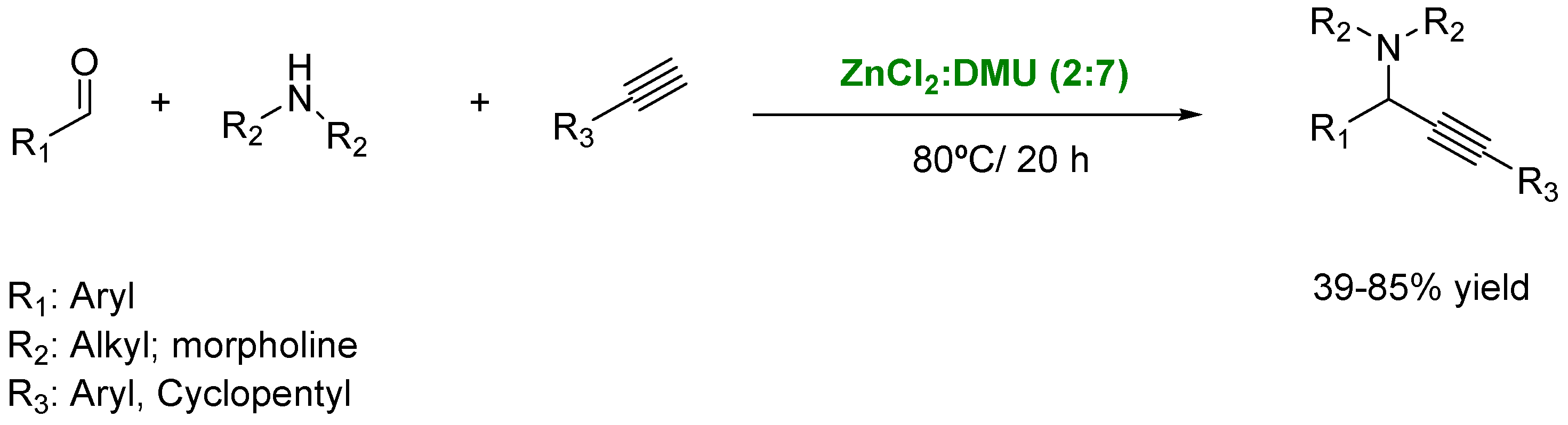
- Obst, M.; Srivastava, A.; Baskaran, S.; König, B. Preparation of Propargyl Amines in a ZnCl2–Dimethylurea Deep-Eutectic Solvent. Synlett 2018, 29, 185–188. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Colonelli, P.; Vásquez, D.; González, M.F.; Rodríguez, J.A.; Theoduloz, C. Studies on quinones Part 44: Novel angucyclinone N-heterocyclic analogues endowed with antitumoral activity. Bioorg. Med. Chem. 2008, 16, 10172–10181. [Google Scholar] [CrossRef]
- Monem, A.; Habibi, D.; Goudarzi, H. An acid-based DES as a novel catalyst for the synthesis of pyranopyrimidines. Sci. Rep. 2023, 12, 18009. [Google Scholar] [CrossRef]
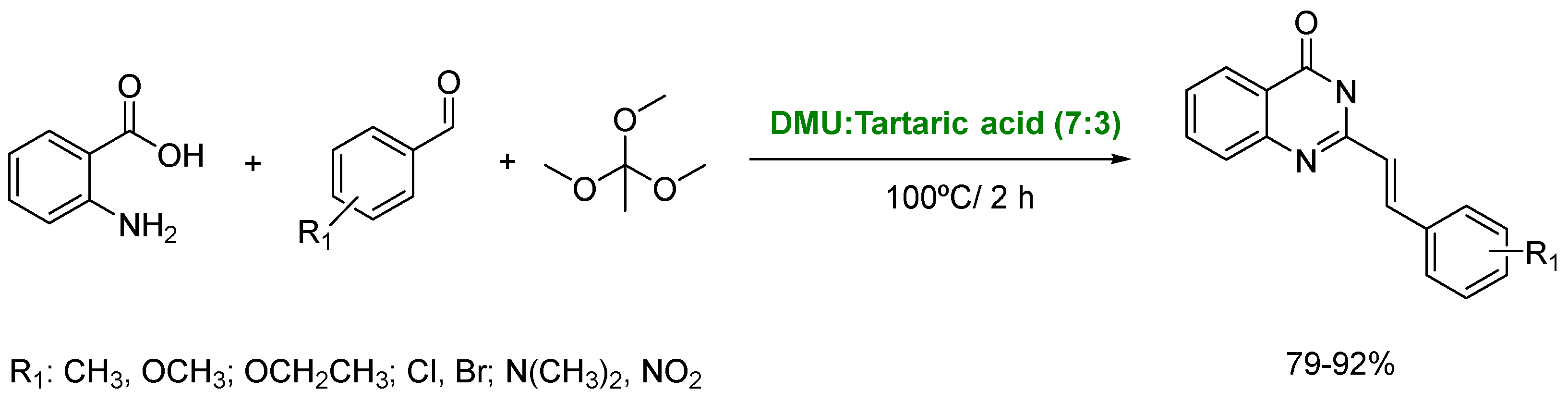
- Chen, X.-W.; Rao, L.; Chen, J.-L.; Zou, Y. Unexpected assembly machinery for 4(3H)-quinazolinone scaffold synthesis. Nat. Commun. 2022, 13, 6522. [Google Scholar] [CrossRef]
- Kotipalli, T.; Kavala, V.; Janreddy, D.; Bandi, V.; Kuo, C.W.; Yao, C.F. Synthesis of 2,3-Disubstituted Quinazolinone Derivatives through Copper Catalyzed C–H Amidation Reactions. Eur. J. Org. Chem. 2016, 2016, 1182–1193. [Google Scholar] [CrossRef]
- Suresh, S.; Nawaz Khan, F.R. Urea-based DES as an amine source to access nitrogen containing heterocycles. Tetrahedron Lett. 2024, 148, 155224. [Google Scholar] [CrossRef]
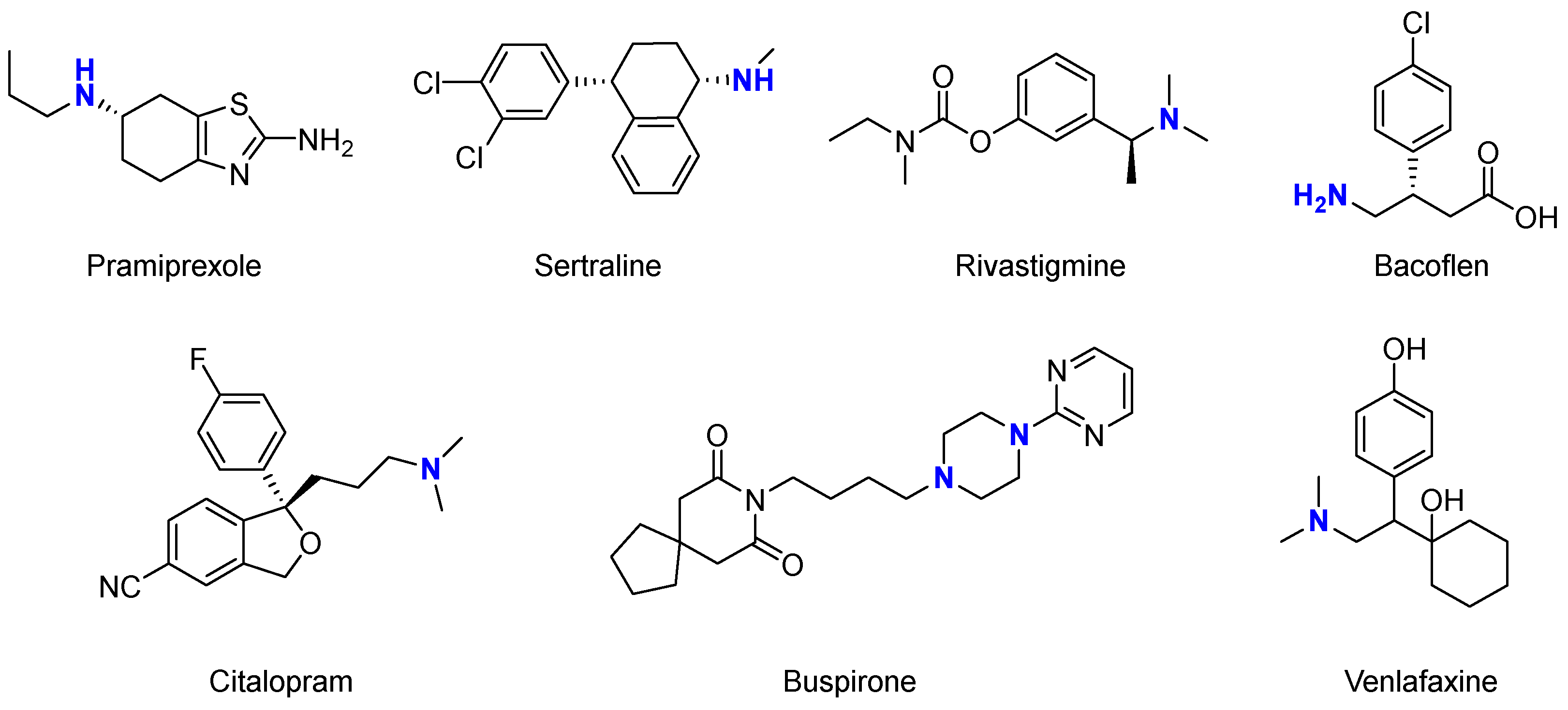
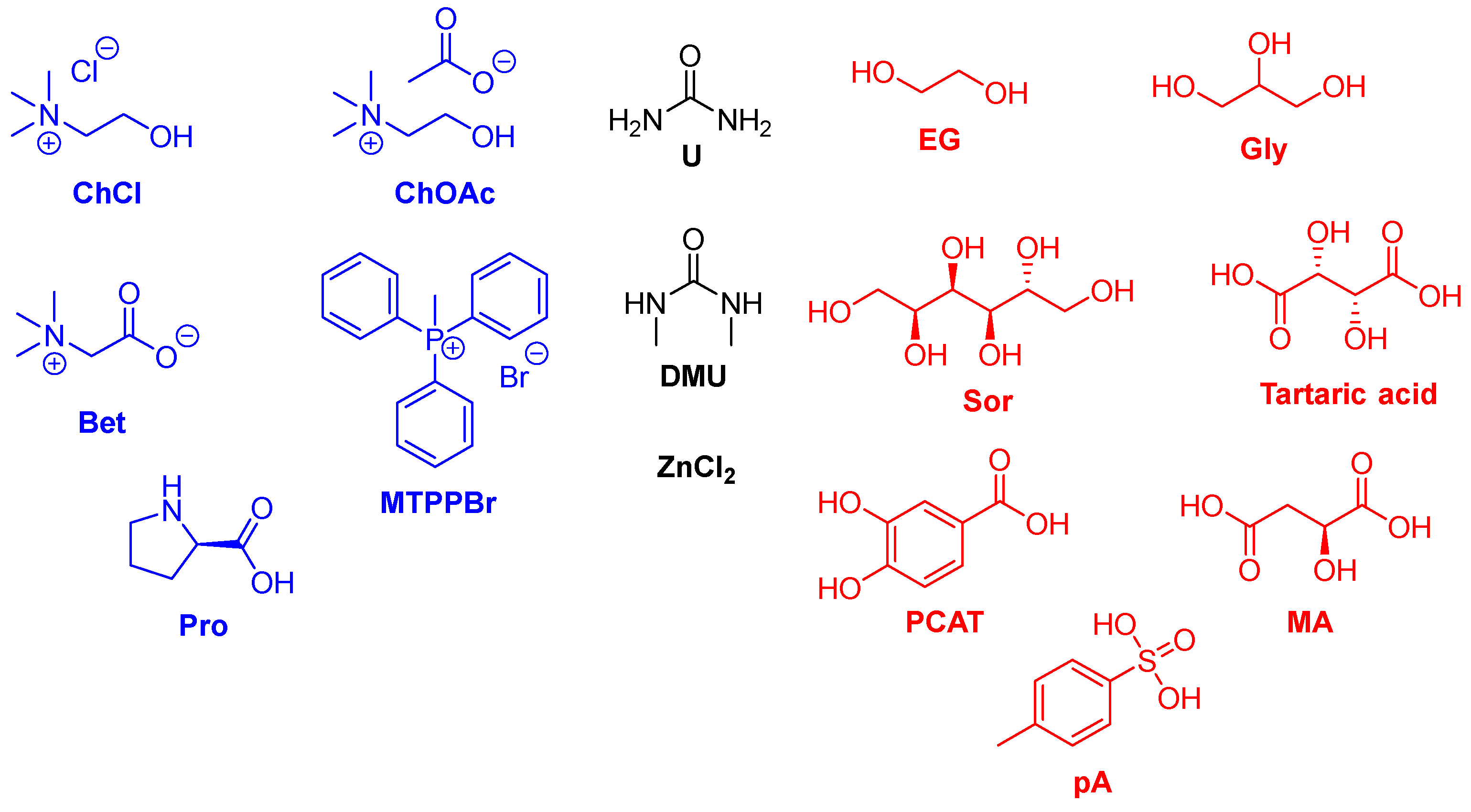
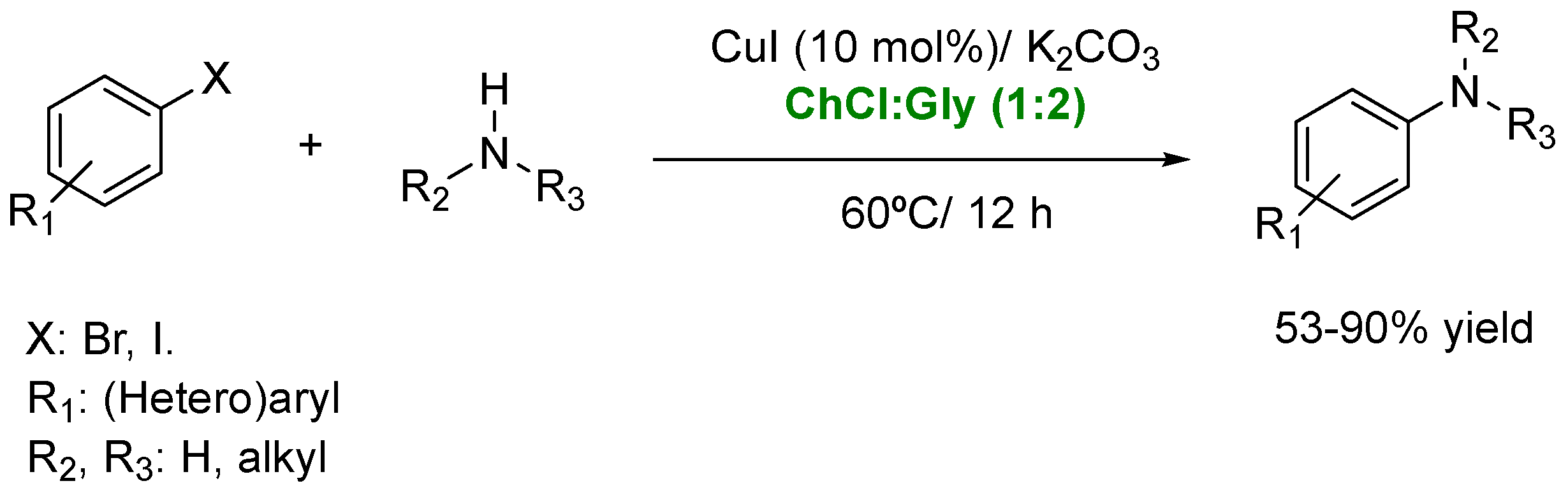
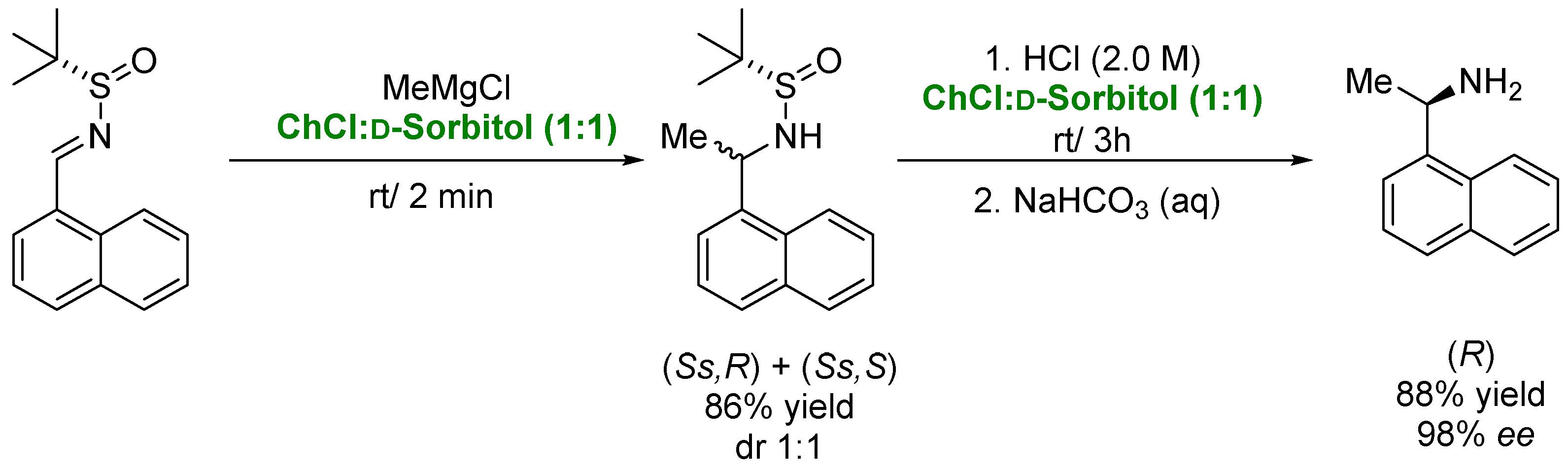



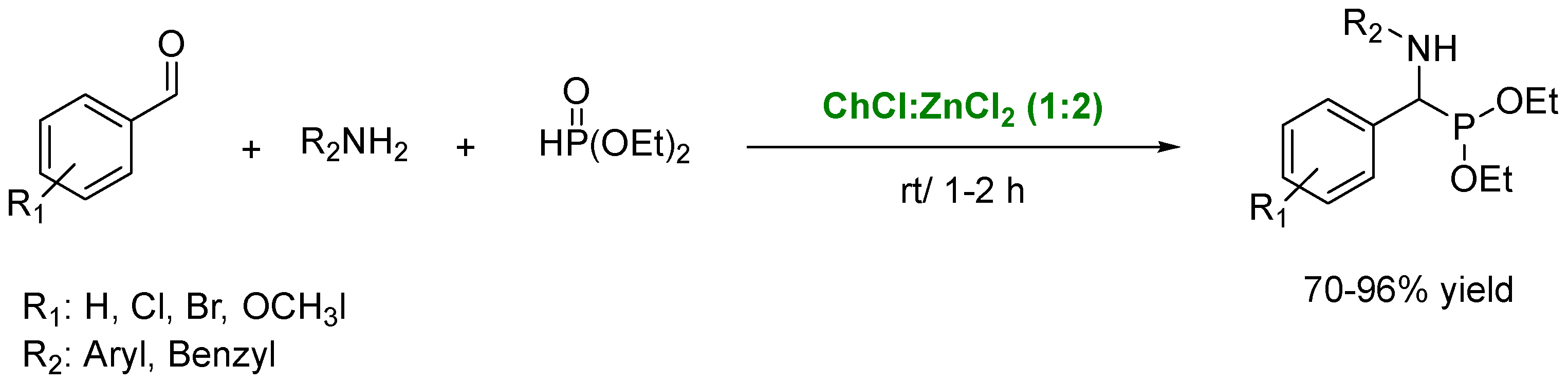

| Solvent | Volatility | Toxicity | Recyclability | Other Properties |
|---|---|---|---|---|
| DMSO | Low | Moderate | Difficult | High polarity |
| Toluene | High | High | Moderate | Flammable, low biodegradability |
| Ethanol | Moderate | Low | Easy | High biodegradability |
| DES | Negligible | Low | Easy | Tailorable properties |
 | |||||
|---|---|---|---|---|---|
| R | Biocatalyst | Amine Donor | Medium | Conv. (%) | Ref. |
| H– | E. coli cells PRSFDuet-CV-AlaDH | NH4Cl | ChCl:EG/water 20:80 (v/v) | ≥99 | [87] |
| H– | E. coli CCZU-XLS160 cells expressing ω-TA and L-alanine dehydrogenase (AlaDH) | NH4Cl | ChCl:EG/water 10:90 (v/v) | ≥99 | [88] |
| H– | E. coli cells expressing a double mutant (AtAT-T130M/E133F) from Aspergillus terreus ω-TA | D-Ala | ChCl:MA/water 30 wt% pH 7.5 | 90.2 | [89] |
| H– | E. coli cells expressing a mutant from (Q97E, H210N, I77L) from A. terreus ω-TA | D-Ala | ChCl:MA:LA/water 5:95 (wt/wt) % | 97.6 | [90] |
| H– | E. coli cells expressing a mutant from A. terreus ω-TA and PDC from Zymomonas mobilis | D-Ala | ChCl:pA (20 wt%, pH 7.5). | 98 | [91] |
| HO–CH2– | E. coli CV cells expressing ω-TA from C. violaceum DSM30191 | L-Ala | MA/Gly/Bet/water pH 8.0 9:91 (wt/wt) | 93.2 | [92] |
| HO–CH2– | E. coli cells expressing a double mutant (AtAT-T130M/E133F) from A. terreus ω-TA | D-Ala | ChCl:MA/water 30 wt% pH 7.5 | ≥99 | [89] |
| HO–CH2– | E. coli strain HNILGD, a triple mutant (G292D, H210N, I77L) of ω-TA from A. terreus | D-Ala | Bet:MA/water 5 wt% pH 8.0 | 97.4 | [93] |
| HO–CH2– | E. coli cells expressing a mutant from A. terreus ω-TA and PDC from Zymomonas mobilis | D-Ala | ChCl:pA (20 wt%, pH 7.5) | ≥99 | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcántara, A.R.; de Gonzalo, G. Green Pathways: Enhancing Amine Synthesis Using Deep Eutectic Solvents. Catalysts 2025, 15, 586. https://doi.org/10.3390/catal15060586
Alcántara AR, de Gonzalo G. Green Pathways: Enhancing Amine Synthesis Using Deep Eutectic Solvents. Catalysts. 2025; 15(6):586. https://doi.org/10.3390/catal15060586
Chicago/Turabian StyleAlcántara, Andrés R., and Gonzalo de Gonzalo. 2025. "Green Pathways: Enhancing Amine Synthesis Using Deep Eutectic Solvents" Catalysts 15, no. 6: 586. https://doi.org/10.3390/catal15060586
APA StyleAlcántara, A. R., & de Gonzalo, G. (2025). Green Pathways: Enhancing Amine Synthesis Using Deep Eutectic Solvents. Catalysts, 15(6), 586. https://doi.org/10.3390/catal15060586








