Research Progress in the Remediation of Arsenic- and Cadmium-Contaminated Groundwater Mediated by Iron and Manganese Biomineralization
Abstract
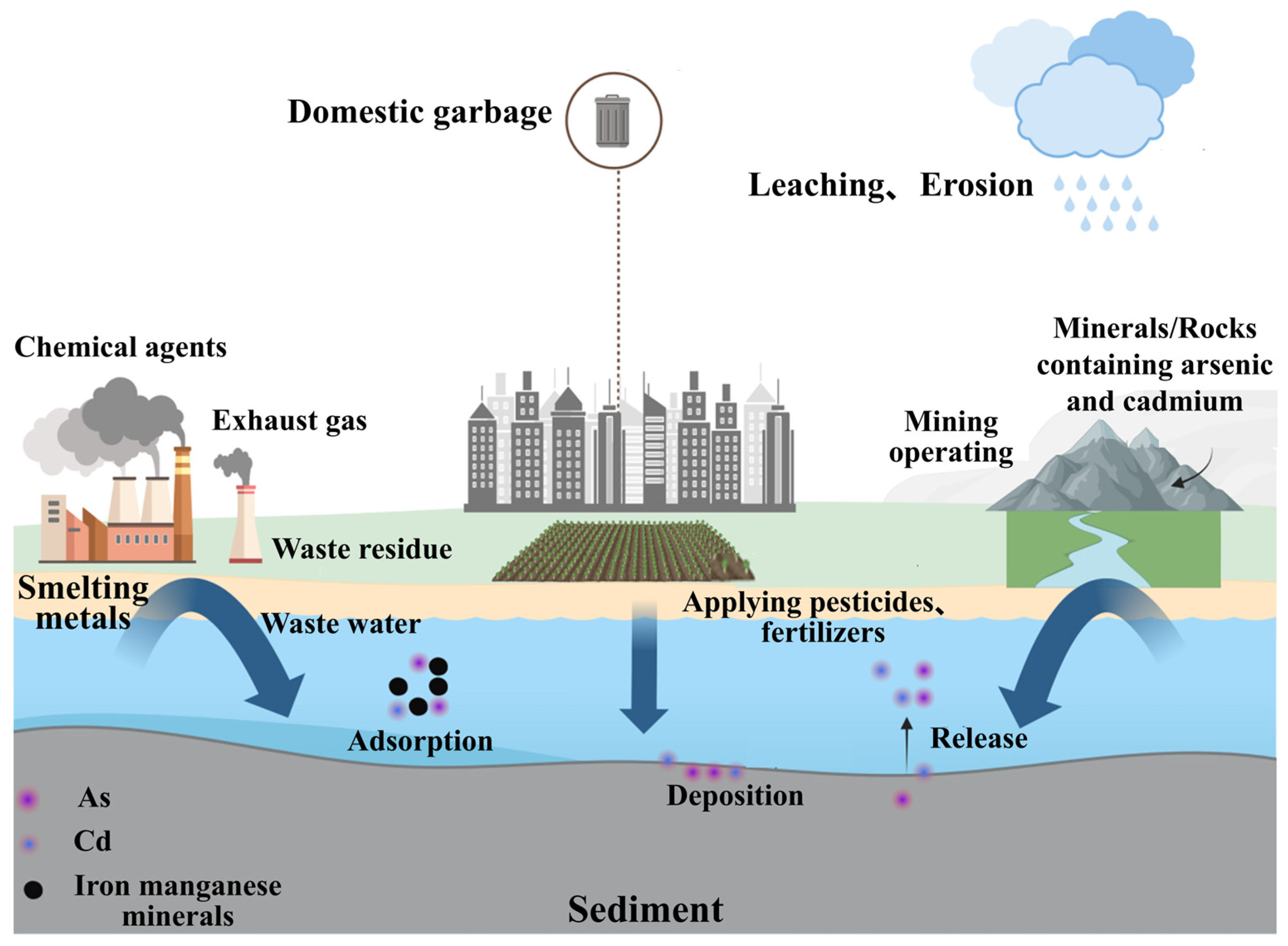
1. Introduction
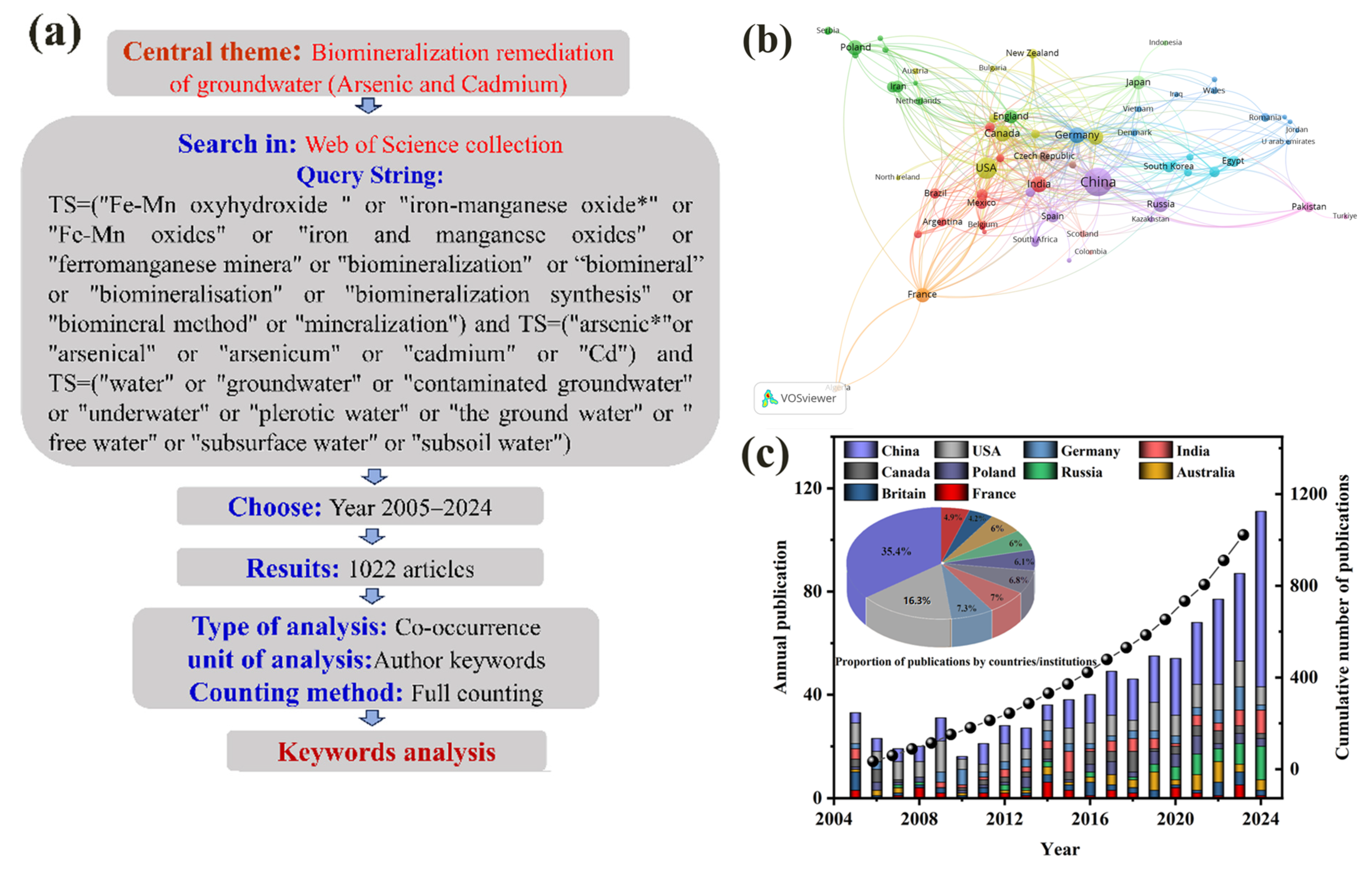
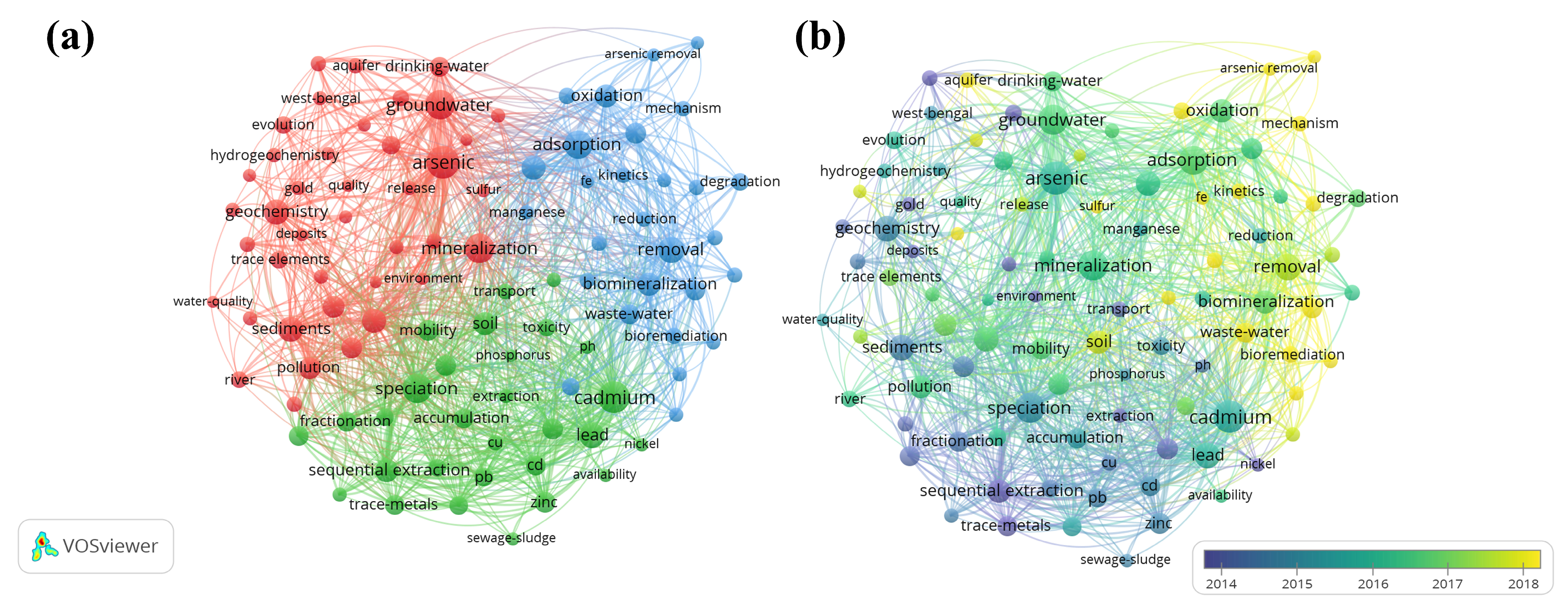
2. Research Methods
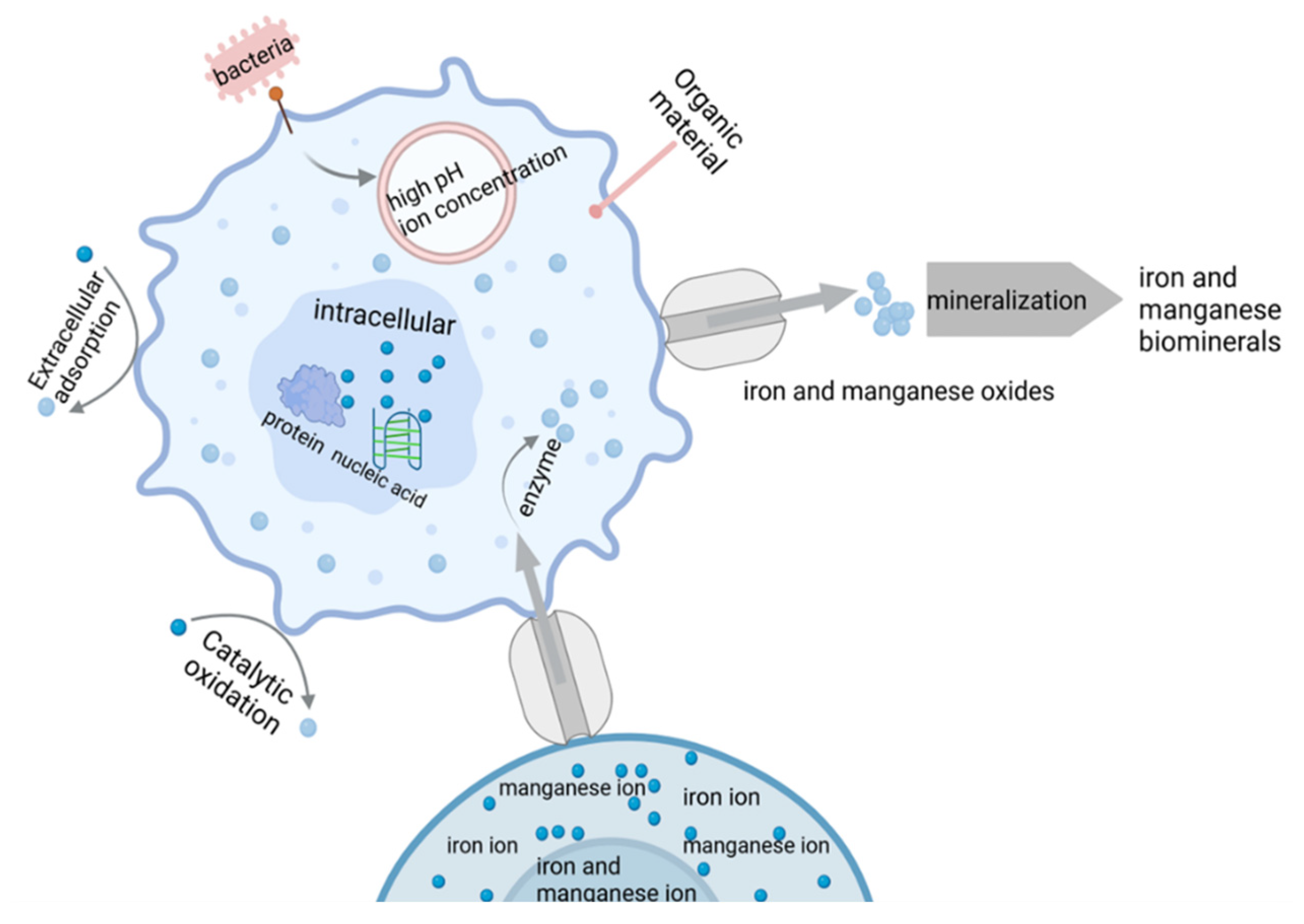
3. Iron and Manganese Biomineralization
3.1. The Role of Functional Microorganisms
3.2. Mineralization Mechanism
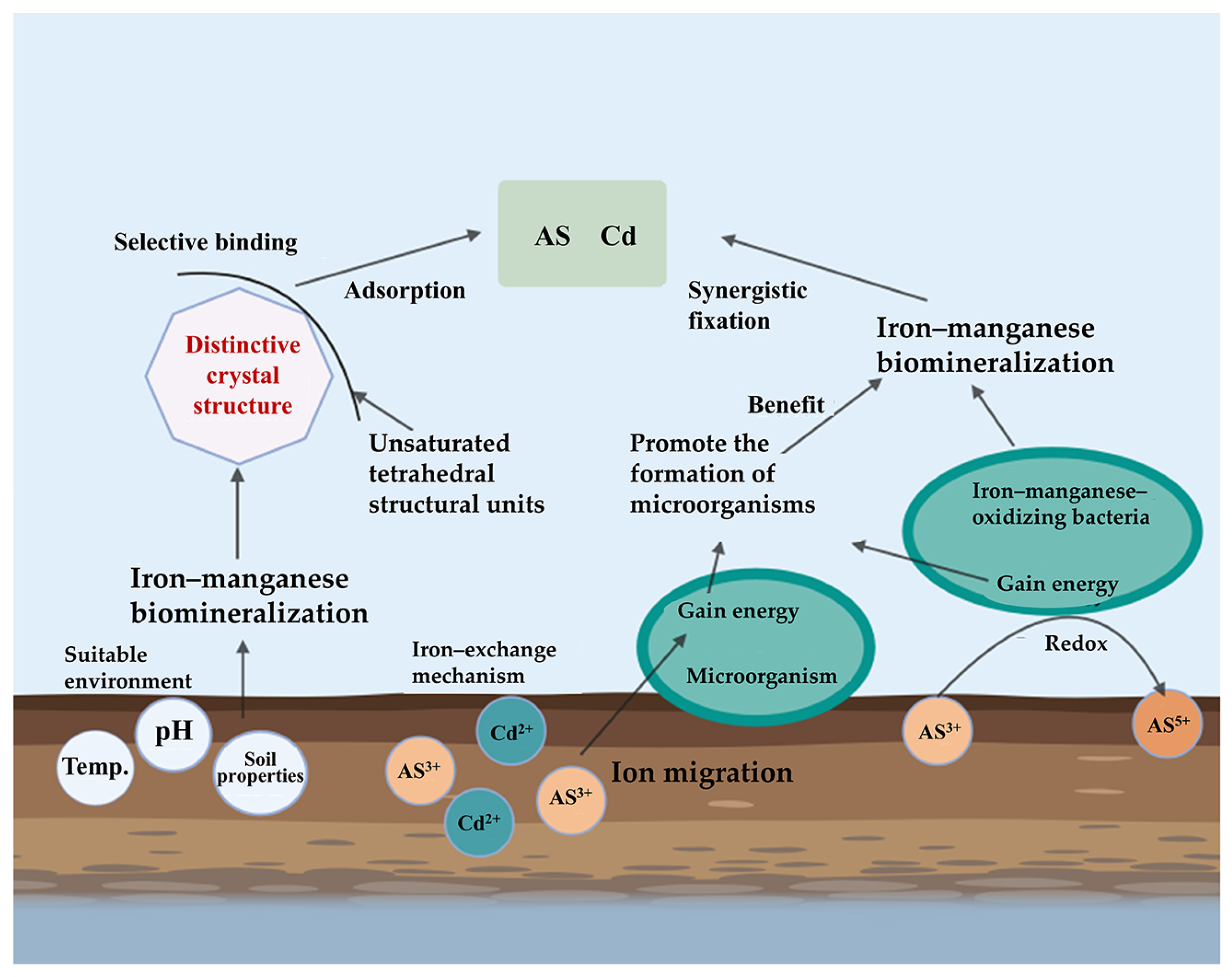
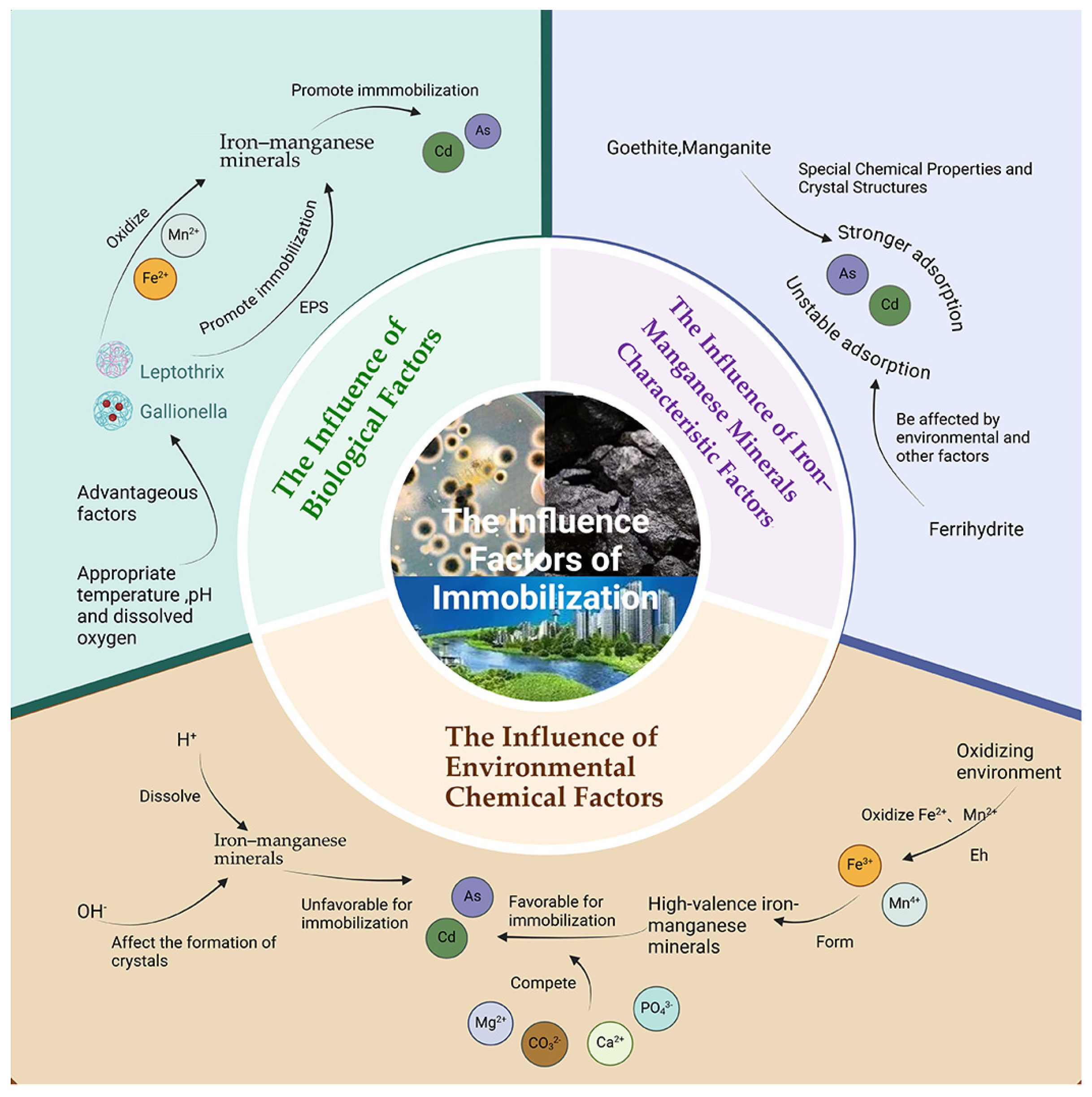
4. Immobilization of As and Cd by Iron and Manganese Biomineralization
4.1. Immobilization Mechanism
4.1.1. Mineral-Specific Binding
4.1.2. EPS-Mediated Chelation
4.1.3. Microbial-Metabolic Coordination
4.2. Influencing Factors
4.3. Application Status
| System/Material | Performance Index | Data | References |
|---|---|---|---|
| Biochar–Bacillus XZM (BCXZM) | Maximum adsorption capacity of As | 42.3 mg/g | [47] |
| EPS (Aqueous solution) | Maximum adsorption capacity of Cd | 45 mg/g | [48] |
| Biogenic Fe–Mn oxides (BFMO) | Oxidation rate of As(III) | 74.1% | [75] |
| BFMO + Morganella morganii MnOx−1 | The 7-day removal efficiency of As(III) | 87.05% | [75] |
| BFMO + Morganella morganii MnOx−1 | The 7-day removal efficiency of As(V) | 94.23% | [75] |
| BFMO + Morganella morganii MnOx−1 | The 28-day removal efficiency of As(III) | 80.26% | [75] |
| BFMO + Morganella morganii MnOx−1 | The 28-day removal efficiency of As(V) | 99.32% | [75] |
| Multi-pollutant system (As/Pb/Cd) | As/Pb/Cd removal efficiency | 22.09–35.33% | [77] |
| Biogoethite and calcium-manganese ore systems | Bioavailable Cd reduction rate | 36.84% | [78] |
| Fe–Mn-reducing bacteria (FMR) | As (III) removal efficiency | Increased by 17% | [13] |
| Fe–Mn-reducing bacteria (FMR) | As (V) removal efficiency | Increased by 16% | [13] |
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Luo, Y. Spatiotemporal Distribution and Probabilistic Health Risk Assessment of Arsenic in Drinking Water and Wheat in Northwest China. Ecotoxicol. Environ. Saf. 2023, 256, 114880. [Google Scholar] [CrossRef]
- Zhang, K.; Mao, K.; Xue, J.; Chen, Z.; Du, W.; Zhang, H. Characteristics and Risk Assessment of Heavy Metals in Groundwater at a Typical Smelter-Contaminated Site in Southwest China. Environ. Pollut. 2024, 357, 124401. [Google Scholar] [CrossRef]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic Contamination of Groundwater: A Global Synopsis with Focus on the Indian Peninsula. Geosci. Front. 2021, 12, 18–35. [Google Scholar] [CrossRef]
- Monika, M.; Gupta, P.K.; Singh, A.; Vaish, B.; Singh, P.; Kothari, R.; Singh, R.P. A Comprehensive Study on Aquatic Chemistry, Health Risk and Remediation Techniques of Cadmium in Groundwater. Sci. Total Environ. 2022, 818, 151784. [Google Scholar] [CrossRef]
- Cui, X.; Cheng, H.; Liu, X.; Giubilato, E.; Critto, A.; Sun, H.; Zhang, L. Cadmium Exposure and Early Renal Effects in the Children and Adults Living in a Tungsten-Molybdenum Mining Areas of South China. Environ. Sci. Pollut. Res. 2018, 25, 15089–15101. [Google Scholar] [CrossRef] [PubMed]
- Fatoki, J.O.; Badmus, J.A. Arsenic as an Environmental and Human Health Antagonist: A Review of its Toxicity and Disease Initiation. J. Hazard. Mater. Adv. 2022, 5, 100052. [Google Scholar] [CrossRef]
- Nogawa, K.; Suwazono, Y.; Nishijo, M.; Sakurai, M.; Ishizaki, M.; Morikawa, Y.; Watanabe, Y.; Kido, T.; Nakagawa, H. Relationship between Mortality and Rice Cadmium Concentration in Inhabitants of the Polluted Jinzu River Basin, Toyama, Japan: A 26 year follow-up. J. Appl. Toxicol. 2018, 38, 855–861. [Google Scholar] [CrossRef]
- Tang, J.; Liao, Y.; Yang, Z.; Chai, L.; Yang, W. Characterization of Arsenic Serious-Contaminated Soils from Shimen Realgar Mine Area, the Asian Largest Realgar Deposit in China. J. Soils Sediments 2016, 16, 1519–1528. [Google Scholar] [CrossRef]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation Technologies for Heavy Metal Contaminated Groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef]
- Chiavola, A.; D’Amato, E.; Gavasci, R.; Sirini, P. Arsenic removal from groundwater by ion exchange and adsorption processes: Comparison of two different materials. Water Supply 2015, 15, 981–989. [Google Scholar] [CrossRef]
- Aliaskari, M.; Schäfer, A.I. Nitrate, arsenic and fluoride removal by electrodialysis from brackish groundwater. Water Res. 2021, 190, 116683. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xie, Z.; Li, P.; Chen, M.; Zhong, Z. A Newly Isolated Indigenous Metal-Reducing Bacterium Induced Fe and Mn Oxides Synergy for Enhanced in situ As(III/V) Immobilization in Groundwater. J. Hydrol. 2022, 608, 127635–127645. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, T.; Liang, J.; Qu, J. The Role of Biogenic Fe-Mn Oxides Formed in situ for Arsenic Oxidation and Adsorption in Aquatic Ecosystems. Water Res. 2016, 98, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Xu, L.; Su, J.; Liu, S.; Ali, A.; Zhang, P.; Cao, S. Denitrification Driven by Additional Ferrous (Fe2+) and Manganous (Mn2+) and Removal Mechanism of Tetracycline and Cadmium (Cd2+) by Biogenic Fe–Mn Oxides. Environ. Res. 2024, 246, 118159. [Google Scholar] [CrossRef]
- Zhao, X.; Xie, Z.; Qiu, R.; Peng, W.; Ju, F.; Zhong, F. Collaborating Iron and Manganese for Enhancing Stability of Natural Arsenic Sinks in Groundwater: Current Knowledge and Future Perspectives. Chem. Geol. 2024, 669, 122369. [Google Scholar] [CrossRef]
- Li, P.; Liu, W.; Gao, K. Effects of Temperature, pH, and UV Radiation on Alkaline Phosphatase Activity in the Terrestrial Cyanobacterium Nostoc flagelliforme. J. Appl. Phycol. 2013, 25, 1031–1038. [Google Scholar] [CrossRef]
- McCutcheon, J.; Southam, G. Advanced Biofilm Staining Techniques for TEM and SEM in Geomicrobiology: Implications for Visualizing EPS Architecture, Mineral Nucleation, and Microfossil Generation. Chem. Geol. 2018, 498, 115–127. [Google Scholar] [CrossRef]
- Perri, E.; Tucker, M.E.; Słowakiewicz, M.; Whitaker, F.; Bowen, L.; Perrotta, I.D. Carbonate and Silicate Biomineralization in a Hypersaline Microbial Mat (Mesaieed sabkha, Qatar): Roles of Bacteria, Extracellular Polymeric Substances and Viruses. Sedimentology 2018, 65, 1213–1245. [Google Scholar] [CrossRef]
- Hoffmann, T.D.; Reeksting, B.J.; Gebhard, S. Bacteria-Induced Mineral Precipitation: A Mechanistic Review. Microbiology 2021, 167, 001049. [Google Scholar] [CrossRef]
- Jimenez-Martinez, J.; Nguyen, J.; Or, D. Controlling Pore-Scale Processes to Tame Subsurface Biomineralization. Rev. Environ. Sci. Bio/Technol. 2022, 21, 27–52. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, Minerals and Microbes: Geomicrobiology and Bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhou, M.; Li, B.; Dong, Y. Mechanisms, Application Advances and Future Perspectives of Microbial-Induced Heavy Metal Precipitation: A review. Int. Biodeterior. Biodegrad. 2023, 178, 105544. [Google Scholar] [CrossRef]
- Perez-Gonzalez, T.; Jimenez-Lopez, C.; Neal, A.L.; Rull-Perez, F.; Rodriguez-Navarro, A.; Fernandez-Vivas, A.; Iañez-Pareja, E. Magnetite Biomineralization Induced by Shewanella oneidensis. Geochim. Cosmochim. Acta 2010, 74, 967–979. [Google Scholar] [CrossRef]
- Bosma, E.F.; Rau, M.H.; van Gijtenbeek, L.A.; Siedler, S. Regulation and Distinct Physiological Roles of Manganese in Bacteria. FEMS Microbiol. Rev. 2021, 45, fuab028. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.J.; Qin, W.; Zhang, C.H.; Li, F.C. Nanoparticle Accumulation in Microbial Induced Carbonate Precipitation: The Crucial Role of Extracellular Polymeric Substance. Geomicrobiol. J. 2020, 37, 837–847. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy Metal–Induced Stress in Eukaryotic Algae—Mechanisms of Heavy Metal Toxicity and Tolerance with Particular Emphasis on Oxidative Stress in Exposed Cells and the Role of Antioxidant Response. Environ. Sci. Pollut. Res. 2022, 29, 16860. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, B.-S.; Chon, C.-M. Characterization of Iron and Manganese Minerals and their Associated Microbiota in Different Mine Sites to Reveal the Potential Interactions of Microbiota with Mineral Formation. Chemosphere 2018, 191, 245–252. [Google Scholar] [CrossRef]
- Pande, V.; Pandey, S.C.; Sati, D.; Bhatt, P.; Samant, M. Microbial Interventions in Bioremediation of Heavy Metal Contaminants in Agroecosystem. Front. Microbiol. 2022, 13, 824084. [Google Scholar] [CrossRef]
- Li, X.; Sun, M.; Zhang, L.; Finlay, R.D.; Liu, R.; Lian, B. Widespread Bacterial Responses and their Mechanism of Bacterial Metallogenic Detoxification Under High Concentrations of Heavy Metals. Ecotoxicol. Environ. Saf. 2022, 246, 114193. [Google Scholar] [CrossRef]
- Dick, G.J.; Torpey, J.W.; Beveridge, T.J.; Tebo, B.M. Direct Identification of a Bacterial Manganese(II) Oxidase, the Multicopper Oxidase MnxG, from Spores of Several Different Marine Bacillus Species. Appl. Environ. Microbiol. 2008, 74, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, G.J.; Tian, N.; Dang, C.C.; Cai, C.; Ding, J.; Liu, B.F.; Xing, D.F.; Ren, N.Q.; Wang, Q.L. Anaerobic Microbial Manganese Oxidation and Reduction: A Critical Review. Sci. Total Environ. 2022, 822, 153513. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, E.J. Microbial Reduction of Geogenic and Synthetic Goethite and Hematite. Minerals 2024, 14, 1086. [Google Scholar] [CrossRef]
- Robles-Fernández, A.; Areias, C.; Daffonchio, D.; Vahrenkamp, V.C.; Sánchez-Román, M. The Role of Microorganisms in the Nucleation of Carbonates, Environmental Implications and Applications. Minerals 2022, 12, 1562. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.R.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tannery Wastewater: A Review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef]
- Lyu, J.; Yu, X.K.; Jiang, M.Y.; Cao, W.R.; Saren, G.; Chang, F.M. The Mechanism of Microbial-Ferromanganese Nodule Interaction and the Contribution of Biomineralization to the Formation of Oceanic Ferromanganese Nodules. Microorganisms 2021, 9, 14–27. [Google Scholar] [CrossRef]
- Staicu, L.C.; van Hullebusch, E.D.; Ackerson, C. Editorial: Microbial Biominerals: Toward New Functions and Resource Recovery. Front. Microbiol. 2021, 12, 796374. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.X.; Owusu, D.C.; Han, Z.Z.; Zhao, H.; Ji, B.; Zhao, Y.Y.; Tucker, M.E.; Zhao, Y.H. Extracellular, Surface, and Intracellular Biomineralization of Bacillus subtilis Daniel-1 Bacteria. Geomicrobiol. J. 2021, 38, 698–708. [Google Scholar] [CrossRef]
- Asta, M.P.; Cama, J.; Martínez, M.; Giménez, J. Arsenic Removal by Goethite and Jarosite in Acidic Conditions and its Environmental Implications. J. Hazard. Mater. 2009, 171, 965–972. [Google Scholar] [CrossRef]
- Amstaetter, K.; Borch, T.; Larese-Casanova, P.; Kappler, A. Redox Transformation of Arsenic by Fe(II)-Activated Goethite (α-FeOOH). Environ. Sci. Technol. 2010, 44, 102–108. [Google Scholar] [CrossRef]
- Peng, L.; Deng, X.; Song, H.; Tan, X.; Gu, J.D.; Luo, S.; Lei, M. Manganese Enhances the Immobilization of Trace Cadmium from Irrigation Water in Biological Soil Crust. Ecotoxicol. Environ. Saf. 2019, 168, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Giménez, J.; Martínez, M.; de Pablo, J.; Rovira, M.; Duro, L. Arsenic Sorption onto Natural Hematite, Magnetite, and Goethite. J. Hazard. Mater. 2007, 141, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Tufano, K.J.; Reyes, C.; Saltikov, C.W.; Fendorf, S. Reductive Processes Controlling Arsenic Retention: Revealing the Relative Importance of Iron and Arsenic Reduction. Environ. Sci. Technol. 2008, 42, 8283–8289. [Google Scholar] [CrossRef]
- Vandana; Priyadarshanee, M.; Das, S. Bacterial Extracellular Polymeric Substances: Biosynthesis and Interaction with Environmental Pollutants. Chemosphere 2023, 332, 138876. [Google Scholar] [CrossRef]
- Huang, L.; Jin, Y.; Zhou, D.; Liu, L.; Huang, S.; Zhao, Y.; Chen, Y. A Review of the Role of Extracellular Polymeric Substances (EPS) in Wastewater Treatment Systems. Int. J. Environ. Res. Public Health 2022, 19, 12191. [Google Scholar] [CrossRef]
- Spanò, A.; Zammuto, V.; Macrì, A.; Agostino, E.; Nicolò, M.S.; Scala, A.; Trombetta, D.; Smeriglio, A.; Ingegneri, M.; Caccamo, M.T.; et al. Arsenic Adsorption and Toxicity Reduction of An Exopolysaccharide Produced by Bacillus licheniformis B3-15 of Shallow Hydrothermal Vent Origin. J. Mar. Sci. Eng. 2023, 11, 325. [Google Scholar] [CrossRef]
- Irshad, S.; Xie, Z.M.; Qing, M.; Nawaz, A.; Mehmood, S.; Alomar, S.Y.; Faheem, M.; Walayat, N. Application of BCXZM Composite for Arsenic Removal: EPS Production, Biotransformation and Immobilization of Bacillus XZM on Corn Cobs Biochar. Biology 2023, 12, 611. [Google Scholar] [CrossRef]
- Lei, Z.; Yu, T.; Ai-Zhong, D.; Jin-Sheng, W. Adsorption of Cd(II), Zn(II) by Extracellular Polymeric Substances Extracted from Waste Activated Sludge. Water Sci. Technol. 2008, 58, 195–200. [Google Scholar] [CrossRef]
- Kuang, X.L.; Shao, J.H.; Chen, A.W.; Luo, S.; Peng, L.; Wu, G.Y.; Gu, J.D. Effects of Bloom-Forming Cyanobacterial Extracellular Polymeric Substances on the Adsorption of Cadmium onto Kaolinite: Behaviors and Possible Mechanisms. SpringerPlus 2016, 5, 12–33. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Krzyszczak, A.; Dobrzyńska, J.; Podkościelna, B.; Zięba, E.; Czemierska, M.; Jarosz-Wilkołazka, A.; Stefaniak, E.A. Extracellular Polymeric Substances Immobilized on Microspheres for Removal of Heavy Metals from Aqueous Environment. Biochem. Eng. J. 2019, 143, 202–211. [Google Scholar] [CrossRef]
- Chen, J.Z.; Qu, C.C.; Lu, M.; Zhang, M.; Wu, Y.C.; Gao, C.H.; Huang, Q.Y.; Cai, P. Extracellular Polymeric Substances and Mineral Interfacial Reactions Control the Simultaneous Immobilization and Reduction of Arsenic (As(V)). J. Hazard. Mater. 2023, 456, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Quan, Q.; Gan, Y.; Dong, J.; Fang, J.; Wang, L.; Liu, J. Effects of Heavy Metals on Microbial Communities in Sediments and Establishment of Bioindicators Based on Microbial Taxa and Function for Environmental Monitoring and Management. Sci. Total Environ. 2020, 749, 141555. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Wasiullah; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of Heavy Metals from Soil and Aquatic Environment: An Overview of Principles and Criteria of Fundamental Processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, K.; Qiu, C.; Bi, Y.; Tian, B.; Bi, X. A Review of Manganese-Oxidizing Bacteria (MnOB): Applications, Future Concerns, and Challenges. Int. J. Environ. Res. Public Health 2023, 20, 1272. [Google Scholar] [CrossRef]
- Dong, H.; Huang, L.; Zhao, L.; Zeng, Q.; Liu, X.; Sheng, Y.; Shi, L.; Wu, G.; Jiang, H.; Li, F.; et al. A Critical Review of Mineral-Microbe Interaction and Co-Evolution: Mechanisms and Applications. Natl. Sci. Rev. 2022, 9, 128–149. [Google Scholar] [CrossRef]
- Piazza, A.; Ciancio Casalini, L.; Pacini, V.A.; Sanguinetti, G.; Ottado, J.; Gottig, N. Environmental Bacteria Involved in Manganese(II) Oxidation and Removal from Groundwater. Front. Microbiol. 2019, 10, 119. [Google Scholar] [CrossRef]
- Guido, A.; Calcagnile, M.; Talà, A.; Tredici, S.M.; Belmonte, G.; Alifano, P. Microbial Consortium Involved in Ferromanganese and Francolite Biomineralization in an Anchialine Environment (Zinzulùsa Cave, Castro, Italy). Sci. Total Environ. 2024, 936, 173423. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, H.; Wang, Z.; Tian, Y.; Tian, W.; Liu, Z. Temperature Alters Bacterial Community Structure in Sediment of Mountain Stream. Sci. Rep. 2024, 14, 31159. [Google Scholar] [CrossRef]
- Jiang, N.; Feng, Y.; Huang, Q.; Liu, X.; Guo, Y.; Yang, Z.; Peng, C.; Li, S.; Hao, L. Effect of Environmental pH on Mineralization of Anaerobic Iron-Oxidizing Bacteria. Front. Microbiol. 2022, 13, 885098. [Google Scholar] [CrossRef]
- Meng, L.; Zuo, R.; Wang, J.-s.; Li, Q.; Du, C.; Liu, X.; Chen, M. Response of the Redox Species and Indigenous Microbial Community to Seasonal Groundwater Fluctuation from a Typical Riverbank Filtration Site in Northeast China. Ecol. Eng. 2021, 159, 106099. [Google Scholar] [CrossRef]
- Ehlert, K.; Mikutta, C.; Kretzschmar, R. Effects of Manganese Oxide on Arsenic Reduction and Leaching from Contaminated Floodplain Soil. Environ. Sci. Technol. 2016, 50, 9251–9261. [Google Scholar] [CrossRef] [PubMed]
- Tuutijärvi, T.; Repo, E.; Vahala, R.; Sillanpää, M.; Chen, G. Effect of Competing Anions on Arsenate Adsorption onto Maghemite Nanoparticles. Chin. J. Chem. Eng. 2012, 20, 505–514. [Google Scholar] [CrossRef]
- Liu, Z.; Ning, X.; Long, S.; Wang, S.; Li, S.; Dong, Y.; Nan, Z. Arsenic and Cadmium Simultaneous Immobilization in Arid Calcareous Soil Amended with Iron-Oxidizing Bacteria and Organic Fertilizer. Sci. Total Environ. 2024, 920, 170959. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wu, S.; Wu, H.; Lin, J.; Cai, Y.; Zhou, D.; Gu, X. Prediction of As and Cd Dissolution in Various Soils Under Flooding Condition. Sci. Total Environ. 2024, 948, 174853–174865. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Sabur, M.A.; Mahamud-Ul-Hoque, M.; Safiullah, S. Competition of Various Ions Present in Shallow Aquifer Water in Respect of Arsenic Removal by Hydrated Ferric Oxide. Asian J. Water Environ. Pollut. 2016, 12, 25–33. [Google Scholar] [CrossRef]
- Meng, D.; Nabi, F.; Kama, R.; Li, S.; Wang, W.; Guo, Y.; Li, Z.; Li, H. The Interaction between Ferrihydrite and Arsenic: A Review of Environmental Behavior, Mechanism and Applied in Remediation. J. Hazard. Mater. Adv. 2024, 13, 100398. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Li, P.; Qin, H.; Liang, J.; Fan, Q. The Adsorption of U(VI) on Magnetite, Ferrihydrite and Goethite. Environ. Technol. Innov. 2021, 23, 101615–101623. [Google Scholar] [CrossRef]
- Perez, J.P.; Tobler, D.; Thomas, A.; Freeman, H.; Dideriksen, K.; Radnik, J.; Benning, L. Adsorption and Reduction of Arsenate during the Fe2+-Induced Transformation of Ferrihydrite. ACS Earth Space Chem. 2019, 3, 884–894. [Google Scholar] [CrossRef]
- Yang, Z.L.; Bai, L.Y.; Su, S.M.; Wang, Y.A.; Wu, C.X.; Zeng, X.B.; Sun, B.H. Stability of Fe-As Composites Formed with As(V) and Aged Ferrihydrite. J. Environ. Sci. 2021, 100, 43–50. [Google Scholar] [CrossRef]
- Hassan, Z.; Westerhoff, H.V. Arsenic Contamination of Groundwater Is Determined by Complex Interactions between Various Chemical and Biological Processes. Toxics 2024, 12, 89. [Google Scholar] [CrossRef]
- McCarty, K.M.; Hanh, H.T.; Kim, K.W. Arsenic Geochemistry and Human Health in South East Asia. Rev. Environ. Health 2011, 26, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Miyata, N.; Tani, Y.; Sakata, M.; Iwahori, K. Microbial Manganese Oxide Formation and Interaction with Toxic Metal Ions. J. Biosci. Bioeng. 2007, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Li, Q.; Sun, Z.; Zhang, W.; Chen, J.; Chen, Z.; Na, S.; Sun, H. Chemical Oxidation of Arsenic in the Environment and its Application in Remediation: A Mini Review. Pedosphere 2023, 33, 185–193. [Google Scholar] [CrossRef]
- Hennebel, T.; De Gusseme, B.; Boon, N.; Verstraete, W. Biogenic Metals in Advanced Water Treatment. Trends Biotechnol. 2009, 27, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Si, M.Y.; Cao, W.; Ou, C.Y.; Tu, G.Y.; Yang, W.C.; Li, Q.Z.; Liao, Q.; Yang, Z.H. Simultaneous Oxidization-Adsorption for Arsenic and Antimony by Biological Fe-Mn Binary Oxides (BFMO): The Efficiencies and Mechanism. Process Saf. Environ. Prot. 2023, 178, 65–73. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in Soils and Groundwater: A Review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Yang, M.; Ning, X.; Nan, Z. Experimental Study on Treatment of Heavy Metal–Contaminated Soil by Manganese-Oxidizing Bacteria. Environ. Sci. Pollut. Res. 2022, 29, 5526–5540. [Google Scholar] [CrossRef]
- Huang, C.; Guo, Z.; Peng, C.; Anaman, R.; Zhang, P. Immobilization of Cd in the Soil of Mining Areas by FeMn Oxidizing Bacteria. Sci. Total Environ. 2023, 873, 162306. [Google Scholar] [CrossRef]
- Qiao, Q.; Yang, X.; Liu, L.; Luo, Y.; Tan, W.; Liu, C.; Dang, Z.; Qiu, G. Electrochemical Adsorption of Cadmium and Arsenic by Natural Fe-Mn Nodules. J. Hazard. Mater. 2020, 390, 122165. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Liang, J.; Yang, X.; Hu, Z.; Li, J.; Xue, Y. The Role of Lysinibacillus Fusiformis S01 in Cadmium Removal from Water and Immobilization in Soil. J. Hazard. Mater. 2025, 485, 136828. [Google Scholar] [CrossRef]
- Saneiyan, S.; Ntarlagiannis, D.; Ohan, J.; Lee, J.; Colwell, F.; Burns, S. Induced Polarization as a Monitoring Tool for in-situ Microbial Induced Carbonate Precipitation (MICP) Processes. Ecol. Eng. 2019, 127, 36–47. [Google Scholar] [CrossRef]
| Characteristic | BCM | BIM |
|---|---|---|
| Regulation Mechanism | Direct cellular templating of mineral nucleation (intracellular/extracellular). | Indirect induction via microbial modification of microenvironmental conditions (pH, redox potential). |
| Mineral Crystallinity | Highly ordered crystals (e.g., intracellular magnetite). | Amorphous or low-ordered oxyhydroxides (e.g., Fe/Mn precipitates formed via pH/redox changes). |
| Driving Force | Active cell-surface adsorption and enzyme-mediated direct nucleation. | Indirect stimulation through enzymatic secretion, acid/base production, or redox shifts. |
| Representative Microbes | Magnetotactic bacteria and fungal hyphae (EPS-mediated templating). | Fe/Mn-oxidizing bacteria and sulfate-reducing bacteria. |
| Role in Groundwater | Rapid metal ion sequestration via surface adsorption or intracellular storage. | Long-term regulation of mineral cycling and contaminant fate through solubility/precipitation dynamics. |
| Factor Category | Performance Index | Data |
|---|---|---|
| Microbial Determinants | Temperature (20–40 °C) | Appropriate temperature maintains the activity of enzymes and promotes mineralization. |
| pH (6–8) | Extreme pH (<5 or >8) significantly inhibits microbial activity, resulting in a decrease in fixation efficiency. | |
| Aerobic condition | The hypoxic environment slows down the oxidation of Fe2+/Mn2+ and hinders the formation of minerals. | |
| Geochemical Controls | pH (<5 or >8) | Acidic conditions promote the dissolution of minerals, whilst alkaline conditions change the structure of minerals. |
| REDOX potential | Low Eh leads to mineral dissolution, while high Eh enhances the adsorption capacity of minerals. | |
| Competing ion | Competing with arsenic and cadmium for binding sites reduces the fixation efficiency. | |
| Mineralogical Properties | Crystalline minerals | It has a stable structure, high adsorption capacity, and good long-term fixation effect. |
| Amorphous minerals | The initial response is fast but the stability is low, and the long-term fixation effect is relatively poor. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Cai, J.; Zhao, X.; Liu, H.; Ju, F.; Li, Y. Research Progress in the Remediation of Arsenic- and Cadmium-Contaminated Groundwater Mediated by Iron and Manganese Biomineralization. Catalysts 2025, 15, 570. https://doi.org/10.3390/catal15060570
Li F, Cai J, Zhao X, Liu H, Ju F, Li Y. Research Progress in the Remediation of Arsenic- and Cadmium-Contaminated Groundwater Mediated by Iron and Manganese Biomineralization. Catalysts. 2025; 15(6):570. https://doi.org/10.3390/catal15060570
Chicago/Turabian StyleLi, Feixing, Jixiang Cai, Xinxin Zhao, Hui Liu, Fanfan Ju, and Youwen Li. 2025. "Research Progress in the Remediation of Arsenic- and Cadmium-Contaminated Groundwater Mediated by Iron and Manganese Biomineralization" Catalysts 15, no. 6: 570. https://doi.org/10.3390/catal15060570
APA StyleLi, F., Cai, J., Zhao, X., Liu, H., Ju, F., & Li, Y. (2025). Research Progress in the Remediation of Arsenic- and Cadmium-Contaminated Groundwater Mediated by Iron and Manganese Biomineralization. Catalysts, 15(6), 570. https://doi.org/10.3390/catal15060570






