Isomeric Anthraquinone-Based Covalent Organic Frameworks for Boosting Photocatalytic Hydrogen Peroxide Generation
Abstract
1. Introduction
2. Results and Discussion
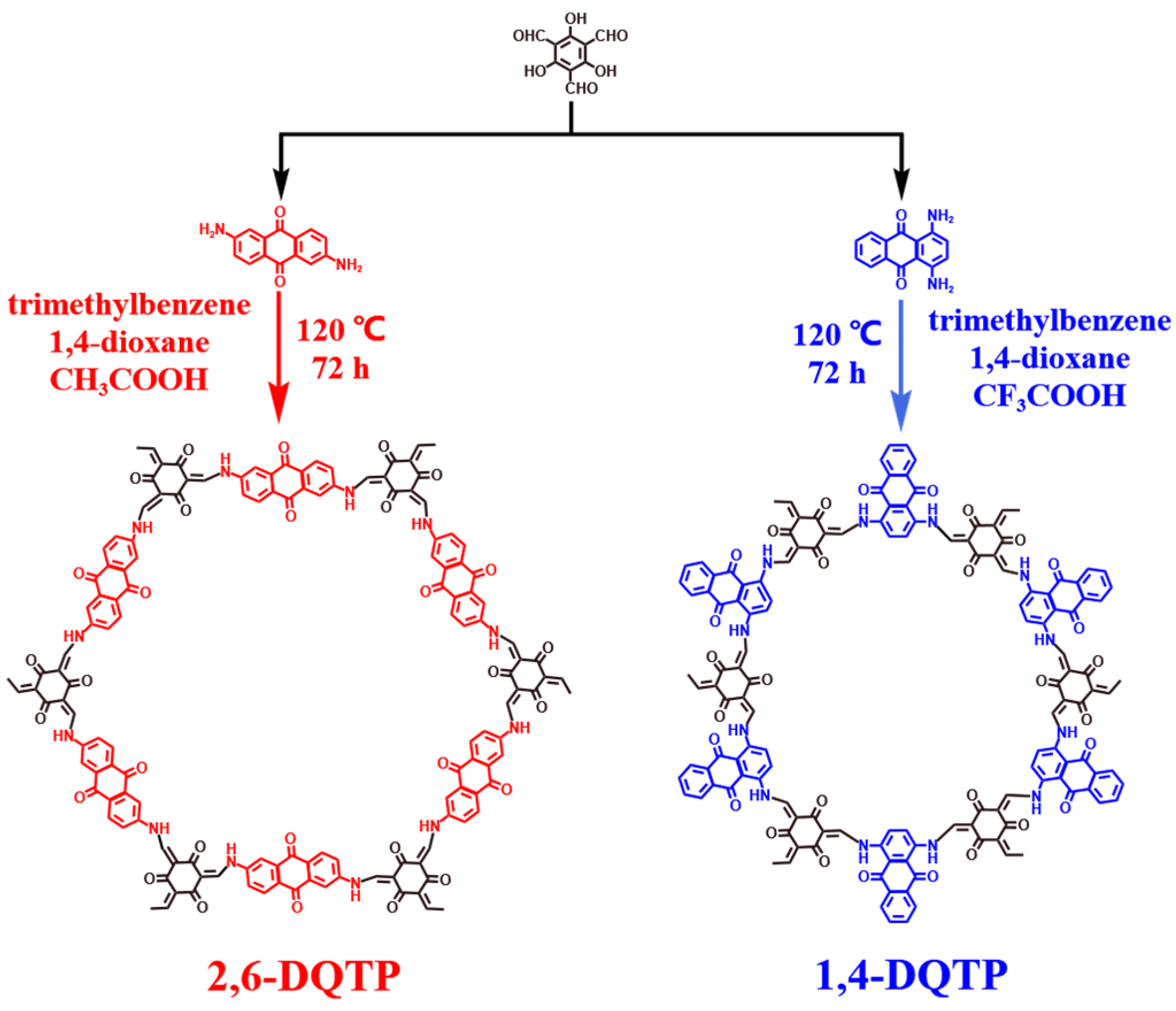
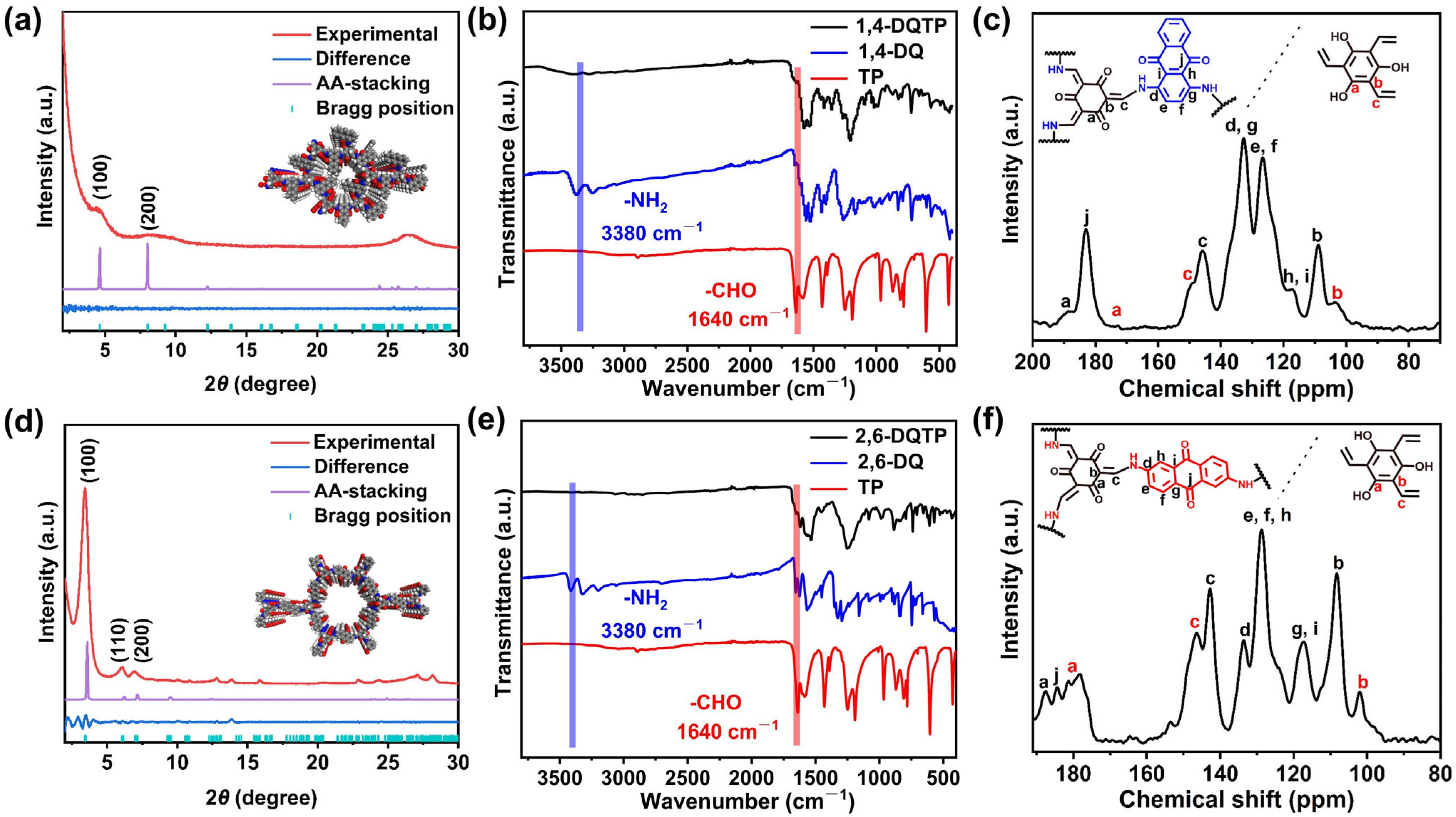
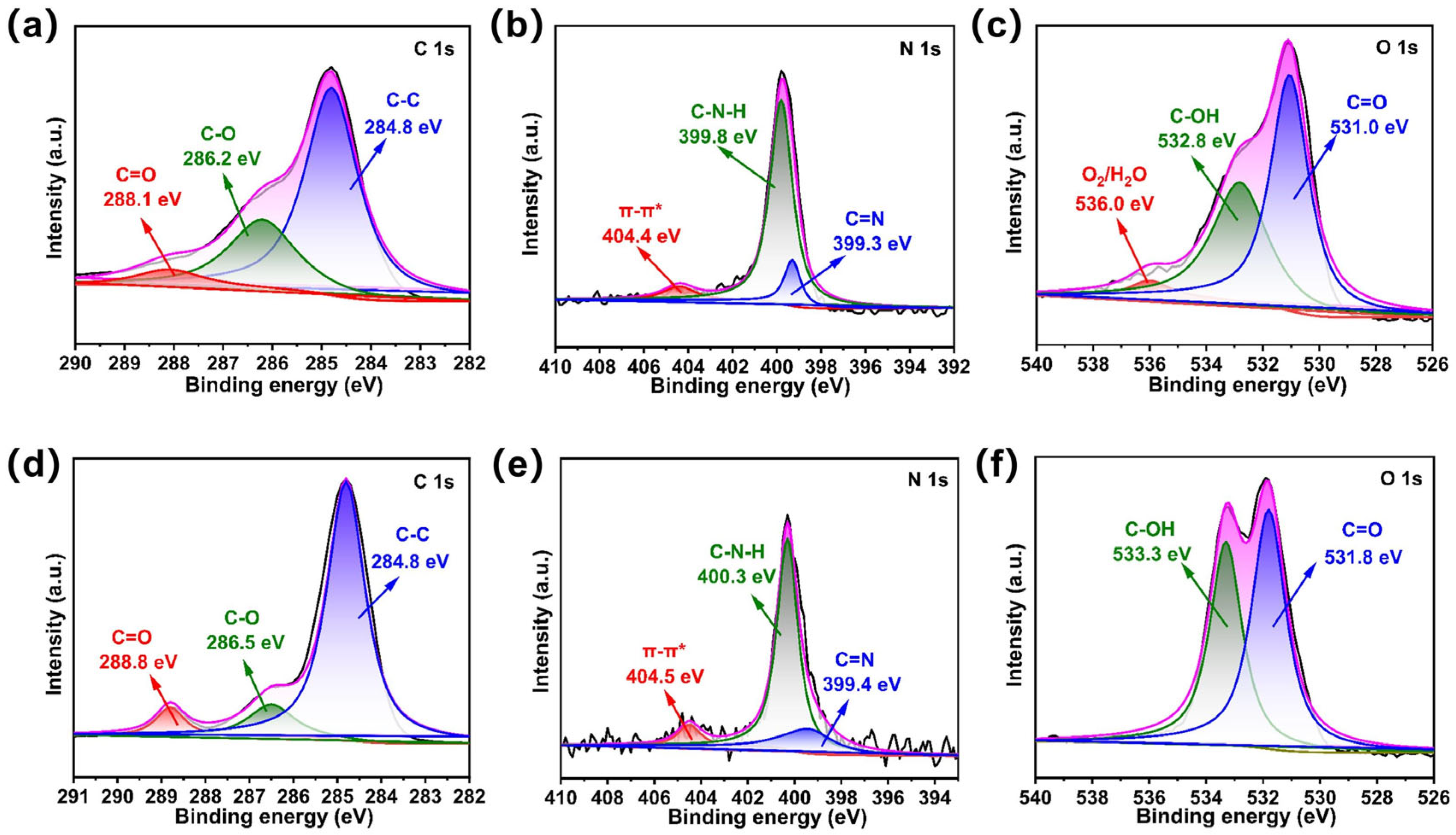
2.1. Structural and Morphological Characterization
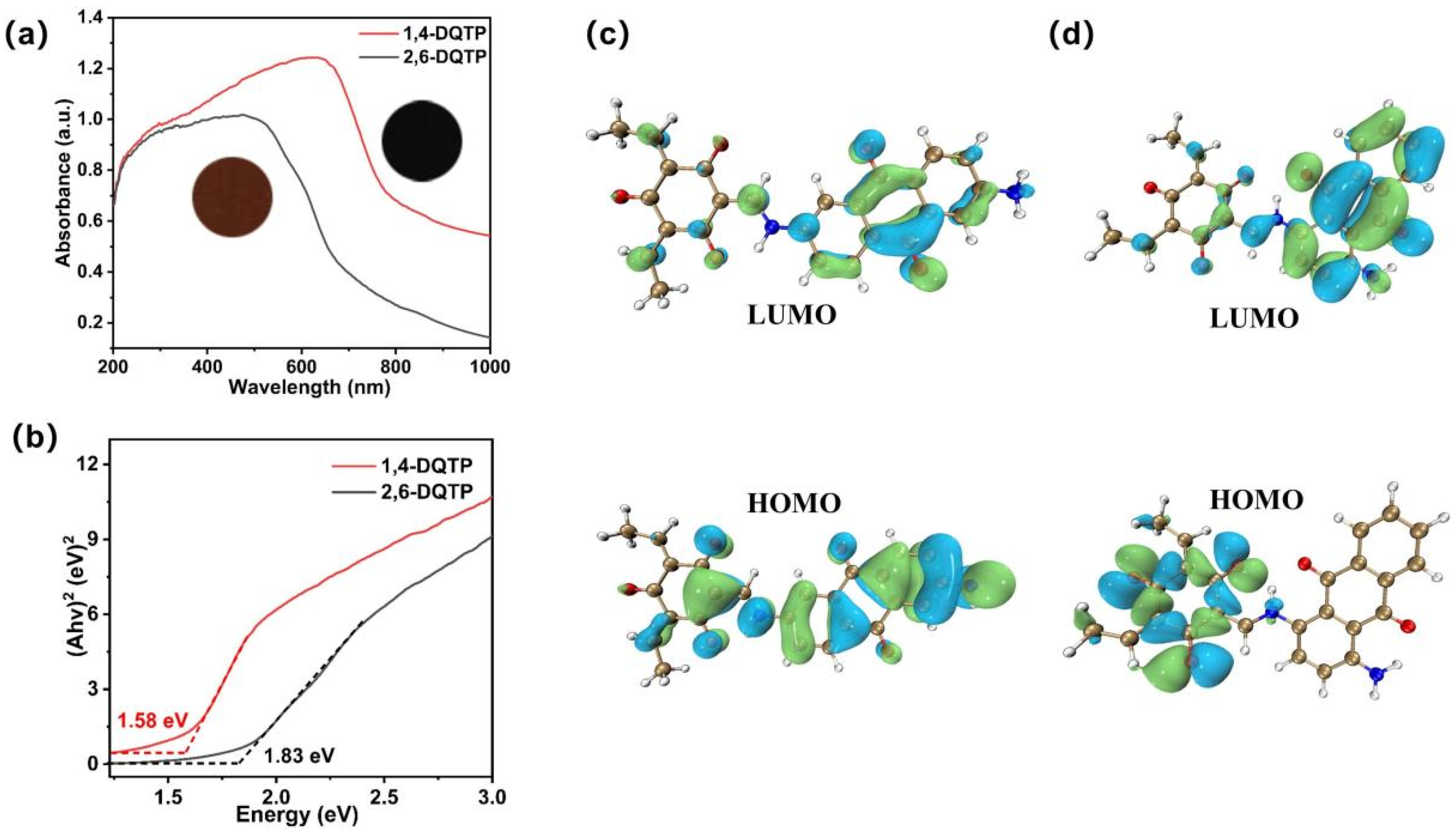
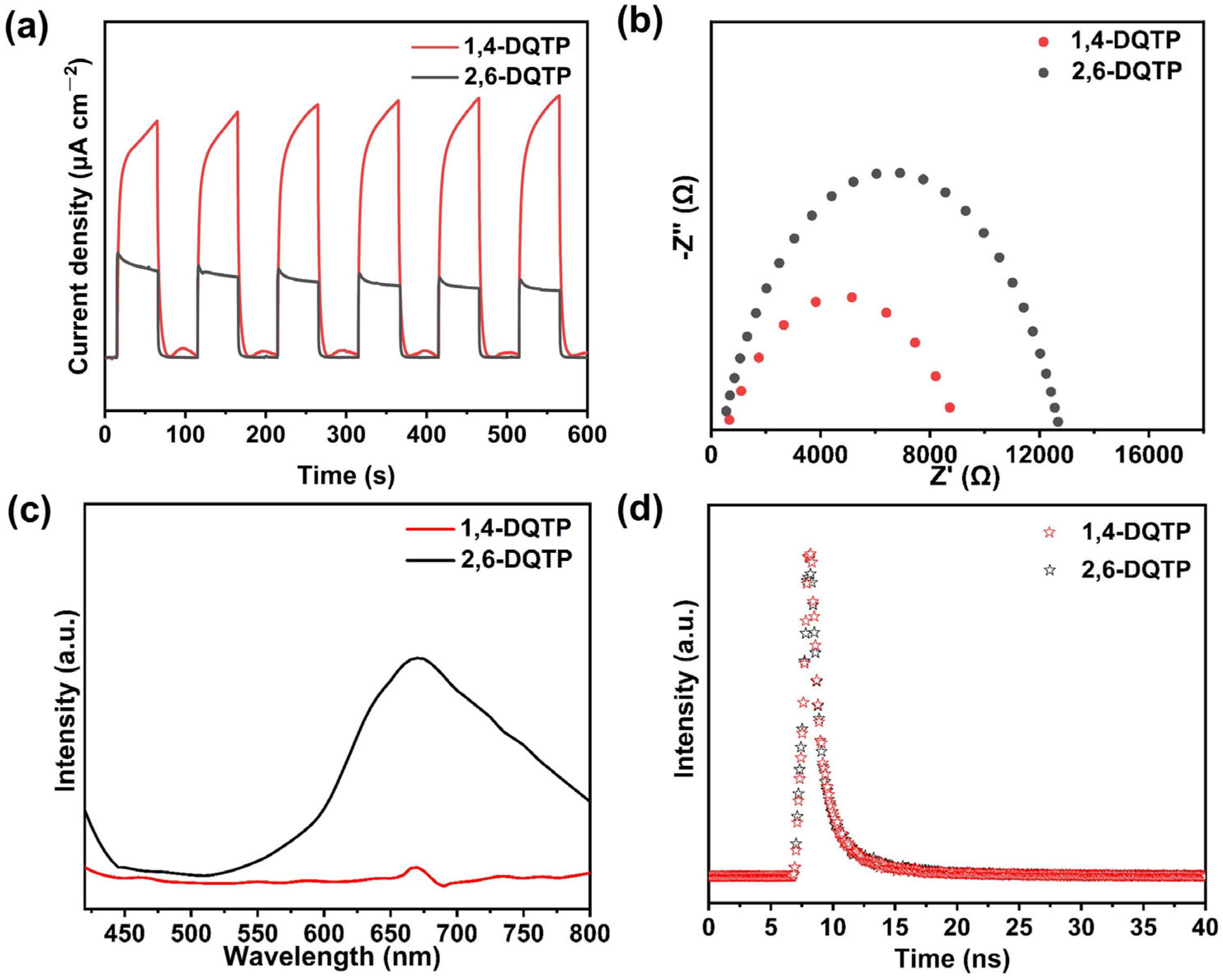
2.2. Optical and Photoelectrochemical Properties
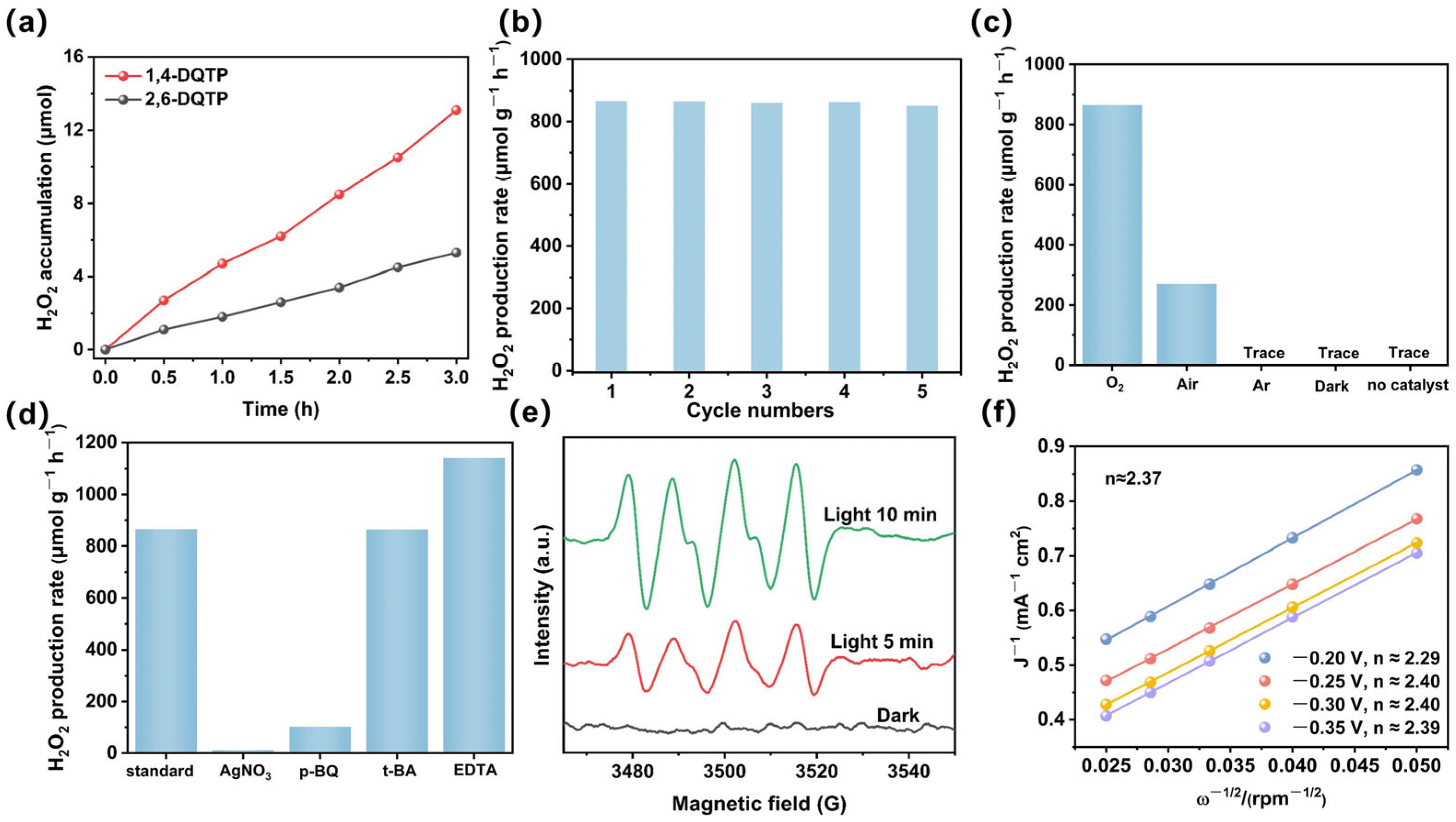
2.3. Photocatalytic H2O2 Production and Its Mechanism
3. Materials and Methods
3.1. Synthesis of COFs
3.2. Photocatalytic H2O2 Production
3.3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, M.; Yan, Y.; Zhang, J.; Xiao, J. Regulating the monomer symmetry of poly-perylene-diimides for photocatalytic H2O2 production. Catalysts 2024, 14, 358. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Li, Y.; Liu, K.; Ju, H.; Wang, J.; Tao, R. Porous organic framework materials for photocatalytic H2O2 production. J. Mater. Sci. Technol. 2024, 200, 185–214. [Google Scholar] [CrossRef]
- Hashimoto, S.; Tatsuno, Y.; Kitagawa, T. Resonance raman evidence for the presence of the FeIV=O bond in horseradish peroxidase compound II. Proc. Jpn. Acad. B Phys. 1984, 60, 345–348. [Google Scholar] [CrossRef]
- Naghmash, M.A.; Saif, M.; Mahmoud, H.R. Transition metal ions doped Bi12SiO20 as novel catalysts for the decomposition of hydrogen peroxide (H2O2). J. Taiwan Inst. Chem. Eng. 2021, 121, 268–275. [Google Scholar] [CrossRef]
- Kou, M.; Wang, Y.; Xu, Y.; Ye, L.; Huang, Y.; Jia, B.; Li, H.; Ren, J.; Deng, Y.; Chen, J.; et al. Molecularly engineered covalent organic frameworks for hydrogen peroxide photosynthesis. Angew. Chem. Int. Ed. 2022, 134, e202200413. [Google Scholar] [CrossRef]
- Ji, R.; Dong, Y.; Sun, X.; Li, P.; Zhang, R.; Pan, C.; Zhao, H.; Zhu, Y. Novel A-D-A type naphthalenediimide supramolecule for H2O2 photosynthesis with solar-to-chemical conversion 1.03%. Adv. Energy Mater. 2024, 14, 2401437. [Google Scholar] [CrossRef]
- Moon, B.C.; Bayarkhuu, B.; Zhang, K.A.I.; Lee, D.K.; Byun, J. Solar-driven H2O2 production via cooperative auto- and photocatalytic oxidation in fine-tuned reaction media. Energy Environ. Sci. 2022, 15, 5082–5092. [Google Scholar] [CrossRef]
- Chu, C.; Zhu, Q.; Pan, Z.; Gupta, S.; Huang, D.; Du, Y.; Weon, S.; Wu, Y.; Muhich, C.; Stavitski, E.; et al. Spatially separating redox centers on 2D carbon nitride with cobalt single atom for photocatalytic H2O2 production. Proc. Natl. Acad. Sci. USA 2020, 117, 6376–6382. [Google Scholar] [CrossRef]
- Ye, Y.-X.; Pan, J.; Shen, Y.; Shen, M.; Yan, H.; He, J.; Yang, X.; Zhu, F.; Xu, J.; He, J.; et al. A solar-to-chemical conversion efficiency up to 0.26% achieved in ambient conditions. Proc. Natl. Acad. Sci. USA 2021, 118, e2115666118. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Yin, Y.; Tian, W.; Zeng, G.; Li, H.; Ye, S.; Wu, L.; Liu, J. Molecular level modulation of anthraquinone-containing resorcinol-formaldehyde resin photocatalysts for H2O2 production with exceeding 1.2% efficiency. Angew. Chem. Int. Ed. 2023, 62, e202218318. [Google Scholar] [CrossRef]
- Xu, X.; Sui, Y.; Chen, W.; Zhou, G.; Li, Y.; Zhong, H.; Wen, H.-R. Anthraquinone-based conjugated organic polymers containing dual oxidation centers for photocatalytic H2O2 production from H2O and O2 under visible-light irradiation. ACS Appl. Polym. Mater. 2023, 5, 7571–7580. [Google Scholar] [CrossRef]
- Xu, X.; Sa, R.; Huang, W.; Sui, Y.; Chen, W.; Zhou, G.; Li, X.; Li, Y.; Zhong, H. Conjugated organic polymers with anthraquinone redox centers for efficient photocatalytic hydrogen peroxide production from water and oxygen under visible light irradiation without any additives. ACS Catal. 2022, 12, 12954–12963. [Google Scholar] [CrossRef]
- Chi, W.; Dong, Y.; Liu, B.; Pan, C.; Zhang, J.; Zhao, H.; Zhu, Y.; Liu, Z. A photocatalytic redox cycle over a polyimide catalyst drives efficient solar-to-H2O2 conversion. Nat. Commun. 2024, 15, 5316. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, H.; Huang, W.; Sui, Y.; Sa, R.; Chen, W.; Zhou, G.; Li, X.; Li, D.; Wen, M.; et al. The construction of conjugated organic polymers containing phenanthrenequinone redox centers for visible-light-driven H2O2 production from H2O and O2 without any additives. Chem. Eng. J. 2023, 454, 139929. [Google Scholar] [CrossRef]
- Sun, X.; Yang, T.; Dong, Y.; Ji, R.; Zhao, H.; Zhang, J.; Zhu, Y. Phenanthrenequinone-modified conjugated polymer enabling photocatalytic H2O2 generation via efficient O2− conversion. Adv. Energy Mater. 2025. [Google Scholar] [CrossRef]
- Sun, H.-H.; Zhou, Z.-B.; Fu, Y.; Qi, Q.-Y.; Wang, Z.-X.; Xu, S.; Zhao, X. Azobenzene-bridged covalent organic frameworks boosting photocatalytic hydrogen peroxide production from alkaline water: One atom makes a significant improvement. Angew. Chem. Int. Ed. 2024, 63, e202409250. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, C.; Liu, J.; Liu, X.; Zhou, Y.; Ou, X.; Weng, B.; Jiang, J.; Han, B. Anthraquinone centers modified covalent organic frameworks for boosted photocatalytic O2-to-H2O2 synthesis: Inhibiting the in-situ decomposition of H2O2. Chem. Eng. J. 2024, 481, 148494. [Google Scholar] [CrossRef]
- Chi, W.; Liu, B.; Dong, Y.; Zhang, J.; Sun, X.; Pan, C.; Zhao, H.; Ling, Y.; Zhu, Y. Boosting H2O2 photosynthesis by accumulating photo-electrons on carbonyl active site of polyimide covalent organic frameworks. Appl. Catal. B Environ. Energy 2024, 355, 124077. [Google Scholar] [CrossRef]
- Chai, E.; Huang, L.; Jiao, L.; Xiao, Z.; Zhang, X.; Wang, Y. A multicomponent triformylphoroglucinol-based covalent organic framework for overall hydrogen peroxide photosynthesis. Chem. Commun. 2024, 60, 3405–3408. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Miao, J.; Wen, X.; Chen, C.; Zhou, B.; Long, M. Keto-enamine-based covalent organic framework with controllable anthraquinone moieties for superior H2O2 photosynthesis from O2 and water. Chem. Eng. J. 2023, 466, 143085. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, S.; Chen, C.; Wen, X.; Miao, J.; Zhou, B.; Long, M.; Zhang, L. Keto-anthraquinone covalent organic framework for H2O2 photosynthesis with oxygen and alkaline water. Nat. Commun. 2024, 15, 2649. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Shen, M.; Shen, Y.; Wang, X.-D.; Lin, W.; Pan, J.; He, J.; Ye, Y.-X.; Yang, X.; Zhu, F.; et al. Spontaneous exciton dissociation in organic photocatalyst under ambient conditions for highly efficient synthesis of hydrogen peroxide. Proc. Natl. Acad. Sci. USA 2022, 119, e2202913119. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shen, M.; Yan, H.; He, Y.; Xu, J.; Zhu, F.; Yang, X.; Ye, Y.-X.; Ouyang, G. Achieving a solar-to-chemical efficiency of 3.6% in ambient conditions by inhibiting interlayer charges transport. Nat. Commun. 2024, 15, 5406. [Google Scholar] [CrossRef]
- Ren, X.; Wang, X.; Song, W.; Bai, F.; Li, Y. Fascinating isomeric covalent organic frameworks. Nanoscale 2023, 15, 4762–4771. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, J.; Zhong, X.; Fang, M.; Hao, J.; Zhao, D.; Wen, X.; Wang, H.; Zhou, Y.; Zhu, Y.; et al. Axial alignment of covalent organic framework membranes for giant osmotic energy harvesting. Nat. Sustain. 2025, 8, 446–455. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Y.; Xie, X.; Song, T. Covalent organic frameworks with tunable bridge positions for photocatalytic CO2 reduction to propylene under visible light illumination. Small 2025, 21, 2408817. [Google Scholar] [CrossRef]
- Li, P.; Zhao, H.; Ji, R.; Chi, W.; Sun, X.; Dong, Y.; Zhu, Y. A diacetylene covalent organic framework through a one-step two-electron O2 reduction pathway for efficient photosynthesis of H2O2. Catal. Sci. Technol. 2024, 14, 2470–2478. [Google Scholar] [CrossRef]
- Li, Q.; Lan, X.; An, G.; Ricardez-Sandoval, L.; Wang, Z.; Bai, G. Visible-light-responsive anthraquinone functionalized covalent organic frameworks for metal-free selective oxidation of sulfides: Effects of morphology and structure. ACS Catal. 2020, 10, 6664–6675. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Shen, J.; Feng, L.; Yao, Y.; Peng, Q. Rational design two- or four-electron reaction pathway covalent organic frameworks for efficient and selective electrocatalytic hydrogen peroxide production. Angew. Chem. Int. Ed. 2025, 64, e202424720. [Google Scholar] [CrossRef]
- Ji, R.; Dong, Y.; Sun, X.; Pan, C.; Yang, Y.; Zhao, H.; Zhu, Y. Efficient H2O2 photocatalysis by a novel organic semiconductor with electron donor-acceptor interface. Appl. Catal. B Environ. Energy 2024, 349, 123884. [Google Scholar] [CrossRef]
- Huang, F.; Wang, Y.; Dong, X.; Lang, X. Imine-linked 2D covalent organic frameworks based on benzotrithiophene for visible-light-driven selective aerobic sulfoxidation. J. Mater. Chem. A 2024, 12, 7036–7046. [Google Scholar] [CrossRef]
- Yue, J.-Y.; Song, L.-P.; Fan, Y.-F.; Pan, Z.-X.; Yang, P.; Ma, Y.; Xu, Q.; Tang, B. Thiophene-containing covalent organic frameworks for overall photocatalytic H2O2 synthesis in water and seawater. Angew. Chem. Int. Ed. 2023, 62, e202309624. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yu, S.; Li, W.; Li, C.; Peng, Y.; Yu, F. Molecular engineering of donor-acceptor sp2-carbon-linked covalent organic frameworks for enhancing photocatalytic hydrogen production. Polym. Chem. 2023, 14, 5133–5139. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Takii, T.; Hagi, T.; Mori, S.; Kofuji, Y.; Kitagawa, Y.; Tanaka, S.; Ichikawa, S.; Hirai, T. Resorcinol-formaldehyde resins as metal-free semiconductor photocatalysts for solar-to-hydrogen peroxide energy conversion. Nat. Mater. 2019, 18, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, D.; Gao, Y.; Li, R.; An, K.; Wang, W.; Zhao, Z.; Xin, X.; Ren, H.; Jiang, Z. On-surface bottom-up construction of COF nanoshells towards photocatalytic H2 production. Research 2021, 2021, 9798564. [Google Scholar] [CrossRef]
- Chen, Z.; Chu, C.; Yao, D.; Li, Q.; Mao, S. Resorcinol-phthalaldehyde resins for photosynthesis of hydrogen peroxide: Modulation of electronic structure and integration of dual channel pathway. Adv. Funct. Mater. 2024, 34, 2400506. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Qi, M.-Y.; Tang, Z.-R.; Xu, Y.-J. Heterostructure-engineered semiconductor quantum dots toward photocatalyzed-redox cooperative coupling reaction. Research 2023, 6, 73. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, L.; Shi, S.; Ren, Y.; Liu, W.; Li, Y.; Duan, F.; Lu, S.; Du, M.; Chen, M. Porphyrin-based covalent organic polymers with bimetallic active sites for boosting photocatalytic CO2 cycloaddition. Chem. Res. Chin. Univ. 2025, 41, 121–130. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, W.; Chi, K.; Zheng, Y.; Kong, X.Y.; Ye, L.; Zhao, Y.; Zhang, K.A.I. Enhanced photocatalytic production of hydrogen peroxide by covalent triazine frameworks with stepwise electron transfer. ACS Catal. 2024, 14, 17654–17663. [Google Scholar] [CrossRef]
- Gu, Y.-Y.; Wang, J.; Tang, Q.; Wei, H.; Ning, J.; Lan, X.; Wang, X.; Li, X.; Jia, Y.; Wang, S.; et al. Insights into substituent effects on the fundamental photocatalytic processes of covalent organic frameworks toward H2 evolution and H2O2 production reactions. ACS Catal. 2024, 14, 11262–11272. [Google Scholar] [CrossRef]
- Liu, Y.; Han, W.-K.; Chi, W.; Mao, Y.; Jiang, Y.; Yan, X.; Gu, Z.-G. Substoichiometric covalent organic frameworks with uncondensed aldehyde for highly efficient hydrogen peroxide photosynthesis in pure water. Appl. Catal. B Environ. 2023, 331, 122691. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Wan, Y.; Zhang, Y.; Qi, Z.; Wu, X.; Xu, H. Acetylene and diacetylene functionalized covalent triazine frameworks as metal-free photocatalysts for hydrogen peroxide production: A new two-electron water oxidation pathway. Adv. Mater. 2020, 32, 1904433. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhou, S.; Zhou, S.; Wang, D.; Zhang, Y.; Hao, X.; Liu, G.; Jin, L.; Liu, P.; Gu, P. Developing three-dimensional hydrophobic photocatalysts to enhance mass transfer for promoting hydrogen peroxide production. Sep. Purif. Technol. 2025, 355, 129774. [Google Scholar] [CrossRef]
- Pan, C.; Bian, G.; Zhang, Y.; Lou, Y.; Zhang, Y.; Dong, Y.; Xu, J.; Zhu, Y. Efficient and stable H2O2 production from H2O and O2 on BiPO4 photocatalyst. Appl. Catal. B Environ. 2022, 316, 121675. [Google Scholar] [CrossRef]
- Qian, Y.; Han, Y.; Zhang, X.; Yang, G.; Zhang, G.; Jiang, H.-L. Computation-based regulation of excitonic effects in donor-acceptor covalent organic frameworks for enhanced photocatalysis. Nat. Commun. 2023, 14, 3083. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Shi, S.; Liu, W.; Duan, F.; Lu, S.; Chen, M. Isomeric Anthraquinone-Based Covalent Organic Frameworks for Boosting Photocatalytic Hydrogen Peroxide Generation. Catalysts 2025, 15, 556. https://doi.org/10.3390/catal15060556
Yan S, Shi S, Liu W, Duan F, Lu S, Chen M. Isomeric Anthraquinone-Based Covalent Organic Frameworks for Boosting Photocatalytic Hydrogen Peroxide Generation. Catalysts. 2025; 15(6):556. https://doi.org/10.3390/catal15060556
Chicago/Turabian StyleYan, Shengrong, Songhu Shi, Wenhao Liu, Fang Duan, Shuanglong Lu, and Mingqing Chen. 2025. "Isomeric Anthraquinone-Based Covalent Organic Frameworks for Boosting Photocatalytic Hydrogen Peroxide Generation" Catalysts 15, no. 6: 556. https://doi.org/10.3390/catal15060556
APA StyleYan, S., Shi, S., Liu, W., Duan, F., Lu, S., & Chen, M. (2025). Isomeric Anthraquinone-Based Covalent Organic Frameworks for Boosting Photocatalytic Hydrogen Peroxide Generation. Catalysts, 15(6), 556. https://doi.org/10.3390/catal15060556






