Abstract
Despite significant advancements in designing tandem catalysts for CO2 hydrogenation to aromatics, the role of zeolite acid property in regulating the selectivity of light aromatics (benzene, toluene, and xylene, abbreviated as BTX) remains unclear. Herein, we report H-ZSM-5 zeolite (denoted as HZ-X, where X represents the Si/Al ratio) integrated with a Na-promoted FeCo-based catalyst (NaFeCo) for CO2 hydrogenation into aromatics via a modified Fischer–Tropsch synthesis pathway. This study systematically modulates the Si/Al ratio of acidic zeolite and examines its critical role in influencing the light aromatics selectivity. The optimized NaFeCo/HZ-50 catalyst achieves a CO2 conversion of 43% with an aromatics selectivity of 41%, including a BTX fraction of 57% in total aromatics. Multiple characterization techniques (NH3-TPD, Py/DTBPy-IR, 27Al NMR, etc.) clarify that acidic zeolite HZ-50 exhibits appropriate acid density and lower external surface acid sites, which synergistically boost the efficient aromatics and BTX synthesis while suppressing the undesirable alkylation and isomerization reactions on the external acid sites. This work develops a highly efficient multifunctional catalyst for CO2 hydrogenation to light aromatics, especially offering guidance for the rational design of acidic zeolite with unique shape-selective functions.
1. Introduction
In recent decades, the extensive utilization of fossil energy sources has substantially contributed to massive CO2 emissions and unprecedented environmental challenges. The utilization of CO2 as a raw material for valuable chemicals synthesis could provide a non-petroleum pathway for basic chemical supplies and alleviate environmental pressure [1,2,3,4]. Aromatics are an important chemical raw material with a wide range of applications. Benzene, toluene, and xylene (BTX), as the most valuable aromatic hydrocarbons, are important raw materials in the polymer industry [5,6]. Compared to the traditional fossil fuel-based aromatics production pathway, the direct transformation of CO2 + H2 into aromatics via thermal/catalytic strategies holds great potential to realize sustainable development [7,8,9].
Thermal/catalytic CO2 hydrogenation to aromatics primarily involves a methanol-mediated route [10,11,12,13] and olefin-mediated route [14,15,16,17,18]. In the methanol-mediated pathway, CO2 first reacts with hydrogen over a reduced metal oxide catalyst to form methanol. Then, the methanol molecules diffuse into the zeolite channels and are converted into aromatics with the aid of acid sites and the shape-selective catalysis function of zeolite. Even though high aromatics selectivity can be achieved from the methanol-mediated pathway, the thermodynamic mismatch between CO2 hydrogenation to methanol and the methanol aromatization reaction results in low CO2 conversion and high undesirable CO selectivity, hindering the high-yield synthesis of aromatics. In the olefin-mediated pathway, the reverse water–gas shift reaction first occurs to generate CO. Subsequently, CO undergoes Fischer–Tropsch synthesis (FTS) with H2 to form olefins. At last, these olefins undergo aromatization in the channels of H-ZSM-5 zeolite to produce aromatics (Scheme 1). The olefin-mediated pathway (also known as the modified FTS pathway) delivers exceptional CO2 conversion efficiency and low CO selectivity, offering promising potential for high-yield synthesis of aromatics [19,20]. From the viewpoint of industrial application, the olefin-mediated route, with the advantage of high-yield aromatics synthesis, could decrease the reactor size and investment cost.
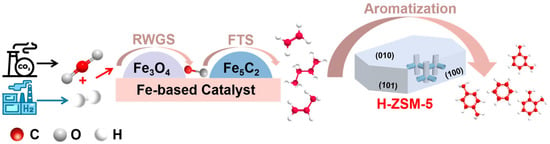
Scheme 1.
Reaction process for the hydrogenation of CO2 to aromatics via the modified FTS pathway.
The design of a multifunctional catalyst that is capable of synergistically catalyzing RWGS, FTS, and aromatization reactions (tandem catalytic system) is the main challenge for the olefin-mediated route. Sun et al. developed a Na-modified ZnFeOx composite catalyst coupled with H-ZSM-5 zeolite for CO2 hydrogenation to aromatics [21]. This study revealed that Zn acts as a structural accelerator to enhance the dispersion of Fe species, significantly facilitating the exposure of the active sites. In addition, the suitable amount of residual sodium (ca. 4.25 wt%), hierarchical pore structure, and the appropriate density of Brønsted acid sites in the ZnFeOx-nNa/H-ZSM-5 composite catalyst significantly enhanced the aromatic selectivity and catalytic stability. Gascon et al. designed a multifunctional catalyst composed of Fe2O3@KO2 and H-ZSM-5 [22]. The KO2-doped Fe2O3 exhibited excellent olefin production activity, while H-ZSM-5 boosted the aromatization process. Under industrial conditions, the catalyst achieved a CO2 conversion of 50% and an aromatic space-time yield (STY) of 9.2 mmol g−1 h−1. Although a tandem catalytic system based on modified FTS pathways for mixed aromatics (containing large amounts of heavy C9+ aromatics) has been extensively studied, the strategy for improving BTX selectivity needs to be further investigated.
The acidic property of zeolite (mainly H-ZSM-5 with MFI topology structure) is the key factor in optimizing the selectivity of BTX [23,24,25]. The charge imbalance caused by substituting Al3+ for Si4+ in the skeleton is the origin of the acidity of zeolite [26,27]. The substitution of Al for Si in the zeolite skeleton results in a negative charge that requires a cation to compensate, and when the cation is H+, a Brønsted acid site is formed [28]. These acid sites are crucial active sites for the aromatization process, promoting C–C bond formation and hydrogen transfer reaction through the proton transfer mechanism [29,30]. Furthermore, the molecular shape-selective effect of microporous in H-ZSM-5 permits only the molecules with kinetic diameters (e.g., BTX) matching the pore dimensions to diffuse through the channels, while bulkier C9+ aromatics are theoretically restricted within the channels. However, the formation of C9+ aromatics in practical application is mainly due to the alkylation and isomerization initiated by the acid sites at the pore mouth of channels and the external surface. These side reactions significantly reduce BTX selectivity. Therefore, by precisely modulating the spatial distribution of acid sites in H-ZSM-5 and rationally suppressing the side reactions on the external surface, the selectivity of BTX from olefin-mediated CO2 hydrogenation can be enhanced effectively [31,32]. The current strategies for erasing external surface acid sites of zeolites are typically through epitaxial growth of Silicalite-1 or SiO2 on the external surface, but these methods involve complicated procedures [13,33]. Recently, a novel H-ZSM-5 zeolite with unique Al distribution characteristics (Al-rich core and Al-deficient surface) has been discovered [34,35,36]. This special acid site distribution could eliminate the side reactions, offering a new approach for designing multifunctional catalysts in olefin-mediated CO2 hydrogenation to BTX. Even though it has been shown that the spatial distribution characteristic of Al sites in this type of H-ZSM-5 zeolite could significantly enhance the BTX selectivity, its application in the modified FTS pathway for CO2 hydrogenation into BTX has not been investigated. In particular, the detailed mechanism by which the Si/Al ratio (Si/Al) affects the product distribution through the modulation of acid strength, acid density, and shape-selective effect has not been systematically revealed.
In this work, we varied the Si/Al ratio of the special H-ZSM-5 zeolite with an Al-rich core and an Al-deficient surface (denoted as HZ-X, where X represents the Si/Al ratio, X = 25, 50, and 150) by adjusting the composition of the synthesized materials. We found a link between zeolite acidity and target product selectivity. NaFeCo/HZ-50 exhibited excellent BTX synthesis performance, which was attributed to its high Bronsted acidity and low external surface acidity. The multifunctional catalyst NaFeCo/HZ-50 achieved 43% CO2 conversion and 41% aromatics selectivity, with BTX accounting for 57% of the total aromatics. At a high Si/Al ratio (HZ-150), the weak acid sites could not powerfully induce the aromatization of olefins toward aromatics. Conversely, under a low Si/Al ratio (HZ-25), the strong acidity at both internal and external surfaces induced the side reactions, resulting in a marked decline in BTX selectivity.
2. Results
2.1. Physicochemical Property of the H-ZSM-5 Zeolite
The preparation process of H-ZSM-5 zeolites (denoted as HZ-X, where X represents the Si/Al ratio) was illustrated in Figure 1a. The X-ray fluorescence (XRF) spectra verified the Si/Al ratio in HZ-X, as shown in Table S1. The morphology of the prepared HZ-X zeolites with different Si/Al ratios was investigated by scanning electron microscopy (SEM). As shown in Figure 1b–d, the HZ-X zeolite presented a hexagonal morphology. The average length of the zeolite was 20 μm.
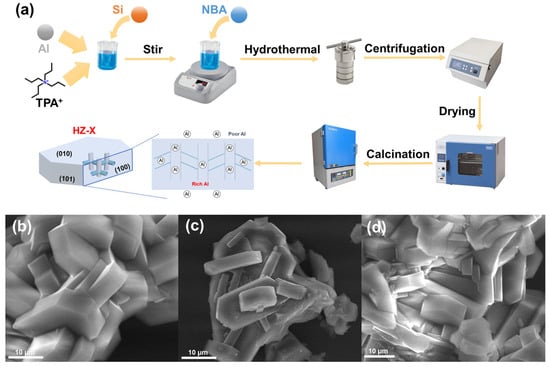
Figure 1.
(a) Schematic illustration of the fabrication process of HZ-X zeolites. (b–d) SEM images of the HZ-X zeolites. (b) HZ-25, (c) HZ-50, and (d) HZ-150.
The crystal phase structure and crystallinity of HZ-X zeolite with different Si/Al ratios were analyzed by X-ray diffractometer (XRD) patterns. The obtained HZ-X zeolites delivered a typical diffraction peak of the zeolite with MFI topology (PDF#47-0638) (Figure 2a), confirming the successful synthesis of H-ZSM-5 zeolite. As shown in Figure 2b and Table S2, with the increase in the Si/Al ratio, the relative crystallinity of zeolite first increased and then decreased. HZ-50 exhibited the highest relative crystallinity, indicating a more regular and stable crystal structure.
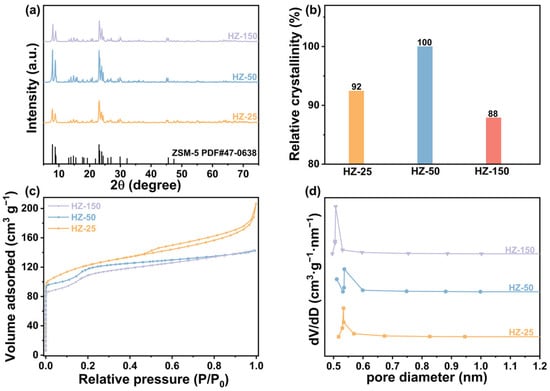
Figure 2.
XRD patterns (a), relative crystallinity (b), nitrogen adsorption-resolution isotherms (c), and pore size distribution (d) of the HZ-X zeolites with different Si/Al ratios.
Nitrogen adsorption and desorption isotherms were employed to investigate the impact of the Si/Al ratio on the architecture property of the acidic zeolites. As demonstrated in Figure 2c, the nitrogen adsorption capacity of all zeolites rapidly increased at low relative pressures (P/P0 = 0–0.2), which is a characteristic of type I isotherms and confirms the microporous-dominated structure. The detailed data are shown in Table S2. As the Si/Al ratio decreased, the specific surface area of the zeolite gradually increased from 378 m2 g−1 to 435 m2 g−1, and the micropore volume progressively rose from 0.161 cm3 g−1 to 0.185 cm3 g−1. The pore size distribution plots indicated that the pore size in zeolites all concentrated in the range of 0.5–0.6 nm, further confirming the microporous structure of the acidic zeolites with different Si/Al ratios (Figure 2d).
2.2. The Catalytic Performance of Aromatics Synthesis from CO2 Hydrogenation
The catalytic performance of NaFeCo/HZ-X catalysts was evaluated in a fixed-bed reactor at 320 °C and 3 MPa for the hydrogenation of CO2 to aromatics. NaFeCo and HZ-X zeolites were pelletized into 20–40 mesh particles, respectively, after which they were physically mixed and loaded into the reactor. The detailed results are shown in Figure 3, with specific data in Tables S3 and S4. Increasing the Si/Al ratio of the zeolitic catalyst led to a continuous reduction in CO2 conversion, dropping from 44.3% to 35.5%. The variation in CO2 conversion can be attributed to the different driving forces stemming from the HZ-X zeolites with different Si/Al ratios and acid strengths. For the product distribution, as the Si/Al ratio increased, the selectivity of CH4 and light olefins was improved, while the selectivity of aromatics exhibited a decreasing trend. These results demonstrated that a lower Si/Al ratio is favorable to enhance the efficiency of olefin transformation and aromatization synthesis. To further compare the aromatization capability of different zeolites, the product distribution of C5+ hydrocarbon compounds is presented in Figure 3c. With the Si/Al ratio increased, the selectivity of olefins and iso-paraffins showed a slight enhancement, while the selectivity of n-paraffins experienced a mild decline. We then analyzed the selectivity of the most important products, light aromatics, BTX. Figure 3b demonstrates the BTX selectivity and space-time yield (STY) obtained from the NaFeCo&HZ-X catalysts. It was not difficult to find that when NaFeCo&HZ-50 had the highest selectivity of BTX and STY, NaFeCo&HZ-25 had the second highest performance, and NaFeCo&HZ-150 had the lowest performance. The above results may be because the excessively strong acidity in HZ-25 resulted in the conversion of the produced light aromatics to C9+ heavy aromatics. In contrast, the excessively weak acidity in HZ-150 was not sufficient to support the aromatization reaction. The NaFeCo&HZ-50 catalyst showed the optimal BTX synthesis performance with a BTX selectivity of 23.7% and a BTX STY of 59.8 mgBTX·gFeCo−1·h−1. The study of the distribution of aromatics fraction (Figure 3d and Table S4) revealed that toluene (T), xylene (X), and C9+ heavy aromatics were dominant in the products. When the Si/Al ratio increased from 25 to 150, the proportion of toluene in total aromatics gradually decreased from 22% to 12.8%, and the proportion of ethylbenzene also declined from 5.3% to 4.2%. In contrast, the proportion of C9+ heavy aromatics first decreased and then increased, first from 40.7% to 37.2% and then to 47.1%. The above-discussed product distribution characteristics indicated that adjusting the Si/Al ratio can effectively optimize the product distribution. The NaFeCo&HZ-50 catalyst achieved high selectivity of aromatics and BTX, with aromatics selectivity of 41.1% and BTX fraction of 57.7% in total aromatics. Furthermore, benefiting from the higher CO2 conversion, NaFeCo&HZ-50 also delivered an excellent STY of light aromatics.
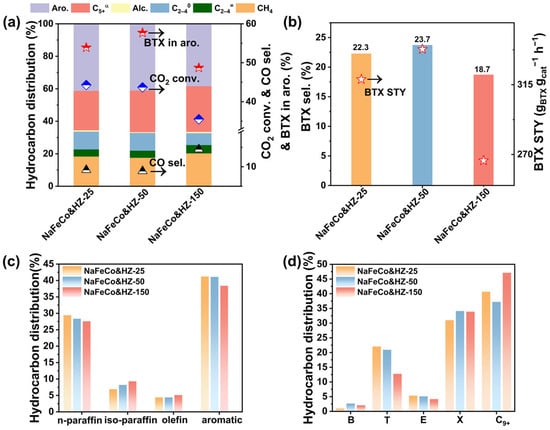
Figure 3.
(a) Catalytic performance of various catalysts for CO2 conversion. (b) BTX selectivity and STY of BTX from NaFeCo/HZ-X catalysts. (c) Product distribution of C5+ hydrocarbons. (d) Product distribution of various aromatics. C5+α denotes the C5+ hydrocarbons excluding aromatics. Alc. denotes the alcohol compounds. C2–C40 and C2–C4= denote the paraffins and olefins of C2–C4 hydrocarbons, respectively. Reaction conditions: 320 °C, 3 MPa, (23.75% CO2, 71.25% H2, and 5% Ar), GHSV = 4500 mL gcat−1 h−1, time on stream (TOS) = 24 h, metal oxide/zeolite mass ratio = 1:3, and catalyst weight of 0.8 g.
2.3. Acidic Property of HZ-X Zeolites
In the CO2 hydrogenation reaction, the synthesis of aromatics, especially BTX, is closely related to the acidic property of zeolites. Optimizing the density of Brønsted acid sites plays a critical role in enhancing aromatics selectivity. Figure 4a presents the ammonia temperature-programmed desorption (NH3-TPD) profiles of different zeolites, where two distinct desorption peaks appeared at 130 °C and 400 °C, respectively. The peak at 130 °C was associated with the weak acid sites, while the peak at 400 °C corresponded to the medium and strong acid sites. As the Si/Al ratio decreased, the desorption peaks shifted to higher temperatures, indicating an increase in acid strength. The specific density of the acid sites is provided in Table S5. HZ-25 exhibited the highest acid density, reaching a total of 144 μmol g−1, while the acid density of HZ-50 decreased to 54 μmol g−1. To further investigate the acidic property of the zeolites, we performed pyridine infrared (Py-IR) spectroscopy analysis (Figure 4b). The three typical peaks at 1445, 1490, and 1545 cm−1 can be assigned to the Lewis acid sites, both Lewis and Brønsted acid sites, and Brønsted acid sites, respectively. In H-ZSM-5 zeolite, the Brønsted acid is associated with the inserted Al3+ in a tetrahedral framework as Si–OH–Al in H-ZSM-5 zeolite, and the Lewis acid is generally derived from the Al3+ located in the extra-framework [37]. As the Si/Al ratio decreased, the amount of Brønsted acid sites increased, which is associated with the increased Al species content. HZ-25 exhibited the highest Brønsted acid concentration of 0.72 mmol g−1, while HZ-50 exhibited a slightly lower value of 0.52 mmol g−1 (Table S6). The variation trends in acid density observed from the Py-IR spectra aligned well with the NH3-TPD results. It should be noted that the difference in the amount of acid sites determined by NH3-TPD and Py-IR was due to the different accessibilities of probe molecules with different molecular sizes (pyridine and NH3) to the acid sites [38]. With a lower Si/Al ratio in zeolite, its acidity gradually enhances, leading to increased CO2 conversion and aromatics selectivity, whereas BTX selectivity first improved and then decreased.
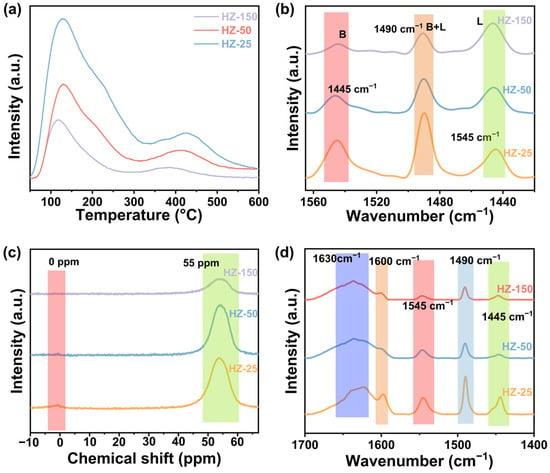
Figure 4.
NH3-TPD profiles (a), Py-IR spectra (b), 27Al MAS NMR spectra (c), and DTBPy-IR spectra (d) of all zeolites with different Si/Al ratios. The Py-IR and DTBPy-IR spectra were recorded after removing the weakly adsorbed Py and DTBPy molecules by evacuation at 150 °C.
It has been proven that the acidic characteristic of zeolite possesses a significant correlation with the spatial distribution of Al sites in the skeletal structure [38]. The zeolite framework contains silanol (Si–OH) and silanol–aluminum (Si–OH–Al) groups [39,40]. Si–OH corresponds to weak acid sites, while Si–OH–Al typically corresponds to stronger Brønsted acid sites associated with tetrahedral coordinated Al3+ in the framework. Therefore, as the amount of tetrahedral Al increases, the acidity strengthens [41]. To characterize the coordination of Al3+ in the zeolite framework and elucidate the association of Al3+ with acidic properties, we conducted an analysis of the coordination states using 27Al solid-state magic angle spinning nuclear magnetic resonance (27Al MAS NMR) spectroscopy (Figure 4c). The spectrum exhibited a prominent peak at 55 ppm, which can be diagnosed as frame Al species. In contrast, the peak at 0 ppm corresponded to the non-framework Al species. All zeolite samples exhibited typical tetrahedral coordination framework Al structure characteristics. It was worth noting that the HZ-25 sample exhibited a weak signal of the non-framework Al species due to the high content of Al3+.
The acid sites on the external surface of the zeolites tend to induce the formation of C9+ heavy aromatics compounds through the alkylation and isomerization of light aromatics [25,42,43]. To investigate the variation in external surface acidity of zeolites with different Si/Al ratios, the 2,6-di-tert-butylpyridine infrared (DTBPy-IR) spectra were obtained. The bulky DTBPy could primarily only interact with the acid sites on the external surface of the zeolite, thereby avoiding interference from acid sites within the pore interiors. Thus, it is an effective probing molecule for studying the external surface acidity of zeolites. As shown in Figure 4d, it had large peaks at 1600, 1630, 1445, 1490, and 1545 cm−1. The large peak at 1600 cm−1 is related to the bending vibration of C=C in the pyridine ring, while the large peak at 1630 cm−1 is related to the hydrogen bonding in the pyridine molecule. Since DTBPy also has a pyridine ring structure, three desorption peaks at 1445, 1490, and 1545 cm−1 also appeared in the spectra. The peak between 1600 and 1700 cm−1 reflected the acidity of the external surface of zeolites. It can be observed that the external surface acidity of the zeolites initially decreased and then increased as the Si/Al ratio increased, with HZ-25 and HZ-150 displaying stronger external surface acidity. In conclusion, moderate Si/Al ratio zeolites featured an Al-rich core and Al-deficient surface, generating spatially distinct acid site distribution (high internal but low surface acid density), which enhances BTX formation efficiency.
Through comprehensive analysis, it was found that the Si/Al ratio of zeolite was significantly and positively correlated with the Brønsted acid strength and framework aluminum density (Figure 4). HZ-50 exhibited moderate Al content, with Al exclusively participating in framework formation, which promotes the synthesis of total aromatics. Meanwhile, its lower external surface acid density facilitated the efficient synthesis of light aromatics BTX by avoiding the undesirable side reactions.
2.4. Relationship Between Zeolite Acidic Property and Light Aromatics Synthesis Performance
The relationship between the acidic property of zeolites with different Si/Al ratios and CO2 hydrogenation performance is shown in Scheme 2. In the modified FTS reaction system, olefin intermediates were first aromatized on acid sites in the pore of the zeolite to form light aromatics. During their diffusion to the external surface, these light aromatics underwent secondary reactions (such as alkylation and isomerization) with the aid of acid sites on the external surface, generating undesirable by-products, such as heavy aromatics or iso-paraffins. The experimental results showed that when the Si/Al ratio was too high, the acid strength was insufficient for the aromatization process. When the Si/Al ratio was too low, a part of the Al might be located on the external surface of the zeolite, which leads to the undesirable side reactions that lower the BTX selectivity. Under the optimal Si/Al ratio, all Al species in the zeolite were involved in the skeleton formation process. It’s appropriate acid site density and distribution optimized the aromatization process and minimized the side reactions, therefore achieving the superior BTX synthesis performance. An increase in the Si/Al ratio of zeolites led to a gradual decrease in acid site density, resulting in slower conversion of intermediates and a decreased CO2 conversion due to hampered driving force in the tandem catalytic system.
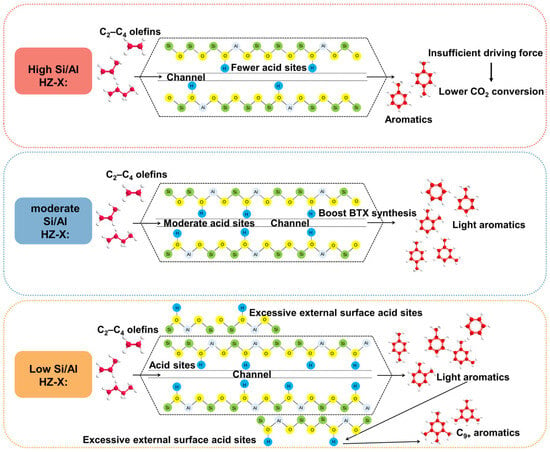
Scheme 2.
Relationship between acidic property and catalytic performance of NaFeCo/HZ-X catalysts.
3. Materials and Methods
3.1. Materials and Chemicals
Ferric nitrate nonahydrate [Fe(NO3)3·9H2O] (Macklin Biochemical Co., Ltd., Shanghai, China), cobalt nitrate hexahydrate [Co(NO3)2·6H2O] (Macklin Biochemical Co., Ltd., Shanghai, China), sodium meta-aluminate (NaAlO2) (Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China), tetrapropylammonium bromide (C12H28BrN) (Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China), n-butylamine (C4H11N) (Macklin Biochemical Co., Ltd., Shanghai, China), Urea (CH4N2O) (Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China), anhydrous sodium carbonate (Na2CO3) (Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China), Colloidal silicon oxide LUDOX® HS-40 (SiO2) (Sigma-Aldrich Life Science & Tech Co., Ltd., Shanghai, China) were of analytical grade and utilized without further purification. The deionized water used for catalyst preparation had a resistivity of 18.2 megohms and was obtained through an ultrapure water system.
3.2. Catalyst Preparation
3.2.1. Synthesis of NaFeCo Catalyst
The FeCo catalyst was synthesized by the urea precipitation method. Typically, Fe(NO3)3·9H2O (12.12 g), Co(NO3)2·6H2O (2.9103 g), and urea (33.033 g) were dissolved in deionized water (330 mL) to form a homogeneous solution. The solution was then placed in an oil bath, heated to 85 °C, and stirred for 2 h. The solution was then kept at 85 °C for 12 h for aging. The product was collected by centrifugation, washed four times with deionized water, and then dried at 70 °C overnight in an oven. The catalyst was then calcined at 350 °C for 4 h under an air atmosphere at a ramp rate of 5 °C min−1 to obtain the FeCo catalyst. Finally, the FeCo catalyst was impregnated with sodium carbonate solution for 3% sodium impregnation, followed by drying in an oven at 70 °C overnight to obtain the final catalyst NaFeCo.
3.2.2. Synthesis of H-ZSM-5 Zeolites
The H-ZSM-5 zeolites with different Si/Al ratios (denoted as HZ-X, where X represents the Si/Al ratio) were synthesized by the hydrothermal method. Typically, deionized water of 91.5 g was added to a 200 mL PTFE bottle. Under the stirring condition, NaAlO2 of 0.08–0.48 g, TPABr of 1.83 g, and C4H11N of 3.92 g were added into the above solution and magnetically stirred for 15 min to make them dissolve thoroughly. Then, SiO2 of 22.53 g was slowly added into the above solution drop by drop, and stirring was continued for 30 min. The molar ratio of all the reagents was SiO2:Al2O3:H2O:TPABr:NBA = 300:1–6:10,160:13.78:107.2. The obtained solution was poured into a stainless steel reactor with PTFE lining and tightly sealed to ensure that the liquid did not leak. The reactor was transferred to a rotary drying oven and fixed on a rotating holder. The hydrothermal temperature was maintained at 180 °C for 48 h, and the rotation speed was 2.0 r min−1. The obtained emulsion was centrifugally washed three times with deionized water, followed by drying overnight at 70 °C to acquire the HZ-X precursor. The precursor was calcinated at 550 °C for 6 h in a muffle furnace with a heating rate of 5 °C min−1 to remove the organic template in HZ-X.
3.3. Catalyst Characterization
X-ray diffraction (XRD) analysis of the obtained catalysts was conducted using Cu Kα radiation and a DX-2700BH X-ray diffractometer (Dandonghaoyuan Instrument Co., Ltd., Dandong, China). The relative crystallinity of the zeolites was obtained by comparing the crystal peak areas in the XRD patterns of the zeolites under the same conditions. The morphology of the catalysts was observed via scanning electron microscopy (SEM) using a Regulus 8100 instrument (Hitachi High-Tech Co., Shanghai, China). Further, the specific surface area and pore size distribution of the samples were determined by the JW-BK200 instrument (JingweiGaoBo Co., Ltd., Beijing, China). The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method. The Horvath–Kawazoe (HK) method was employed to analyze the pore size distribution of microporous. NH3 temperature-programmed desorption (NH3-TPD) experiments were carried out on a DCA-1200 instrument (Builder Electronic Technology Co., Ltd., Beijing, China). Prior to the experiment, the catalyst was pretreated at 150 °C for 1 h to eliminate the H2O present in the microporous zeolite. Subsequently, the sample was saturated with NH3 at 50 °C, and the physisorbed NH3 was removed using He flow. Further, NH3-TPD profiles were recorded under a He flow with a heating rate of 10 °C min−1. The Si/Al ratio of the HZ-X catalyst was evaluated using a PANalytical AXIOS-Petro X-ray fluorescence spectrometer (PANalytical B.V. Co., Netherlands, Beijing, China). Pyridine infrared (Py-IR) and 2,6-di-tert-butylpyridine infrared (DTBPy-IR) spectra were obtained using a Bruker iS50 FTIR spectrometer (Thermo Fisher Scientific Instrument Co., Ltd., Waltham, MA, USA) to distinguish the acid type in HZ-X. Prior to the Py-IR or DTBPy-IR test, the zeolite was pressed into a self-supported wafer and degassed at 400 °C and 10−4 Pa for 1 h. After that, inert gas was saturated with pyridine or 2,6-di-tert-butylpyridine and was introduced into the IR cell for 1 h at room temperature. Finally, the catalyst was heated to 150 °C with a heating rate of 10 °C min−1, then desorbed under a helium atmosphere for 30 min, and finally, Py-IR or DTBPy-IR spectra were collected. The distribution of Al in HZ-X zeolites was analyzed using an AVANCE3-400M (Bruker Co., Beijing, China) wide-cavity solid-state Nuclear Magnetic Resonance (NMR) spectrometer. The acidity data for zeolite was calculated using the following equation:
- (1)
- NH3-TPD:
- (2)
- Py-IR:
The quantitative determination of Bronsted and Lewis acids of the catalysts was based on previous studies [44]. where 1.88 and 1.42 are the extinction coefficients of Brønsted acid and Lewis acid, respectively. The peak areas of the characteristic peaks of Brønsted acid and Lewis acid are labeled as AB/AL. The radius of the zeolite compression tablet is labeled as R. The mass of the compression tablet is labeled as m.
3.4. Catalytic Activity Evaluation
The catalytic performance of the catalysts was measured in a fixed-bed reactor (ZXBLUE Co., Ltd., Beijing, China) with an internal diameter of 12 mm. As shown in Figure 5, 0.2 g of NaFeCo and 0.6 g of HZ-X were made into 20–40 mesh granules and mixed homogeneously. The mixture was mixed with an equal volume of quartz sand and loaded into the reactor. Prior to the reaction, the catalyst was reduced by pure H2 at 400 °C for 4 h. After cooling down to room temperature, the reactant gas (23.75% CO2, 71.25% H2, and 5% Ar) was fed into the reactor until the pressure reached 3 MPa. At the same time, the temperature of the reactor was increased to 320 °C. The exhaust gas was analyzed by an online gas chromatograph (Fuli 9790II, Fuli Analytical Instruments Co., Ltd., Wenling, China) equipped with two detectors (thermal conductivity detector (TCD) and flame ionization detector (FID). The TCD is connected to the TDX-01 (60–80 mesh, 3 mm × 2 m, RuideJingke Instrument Co., Ltd., Zaozhuang, China); Porapak-Q (60–80 mesh, 3 mm × 1 m, Agilent Technologies Co., Ltd., Santa Clara, CA, USA) packed columns for the analysis of Ar, CO, CH4, and CO2; and the FID is connected to the HP-PLOT/Q capillary column (0.53 mm × 30 m, Agilent Technologies Co., Ltd., Santa Clara, CA, USA) to detect gas-phase hydrocarbons. An off-line gas chromatograph (Fuli 9790II, Fuli Analytical Instruments Co., Ltd., Wenling, China) equipped with an FID and a capillary column InerCap-5 (30 m × 0.25 mm, GL Sciences, Tokyo, Japan) was employed to analyze the liquid products.
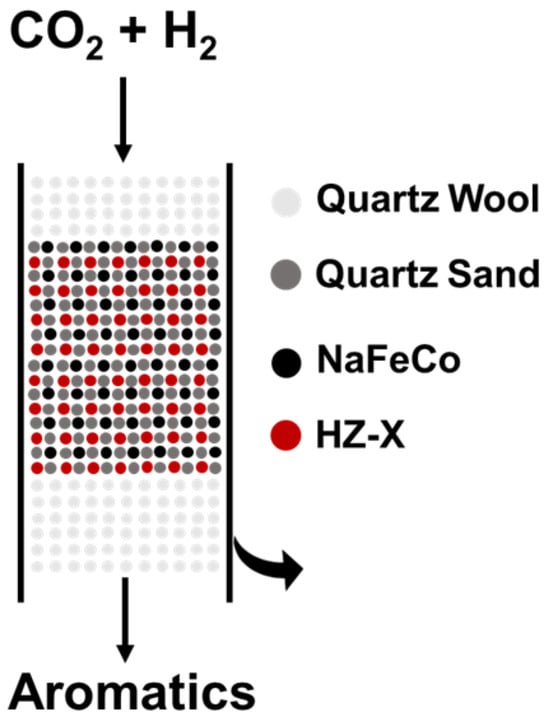
Figure 5.
Schematic diagram of catalyst loading in the reactor.
The specific catalytic performance evaluation method is as follows:
- (1)
- CO2 conversion was calculated according to
- (2)
- Product selectivity was calculated as the percentage of CO2 converted into a given product and according to
- (3)
- CO selectivity for CO2 hydrogenation was calculated according to
4. Conclusions
In summary, we synthesized H-ZSM-5 (denoted as HZ-X, where X represents the Si/Al ratio) zeolites with varying Si/Al ratios and evaluated their catalytic performance in CO2 hydrogenation to aromatics after combining with the NaFeCo catalytic component. The acidic property of the zeolites, particularly the acid density and distribution, plays a crucial role in light aromatics synthesis performance. The structural characteristics of zeolites govern their acid site distribution, and these acid sites serve as the active centers for catalyzing the conversion of CO2 to aromatics. Therefore, tuning the active sites of zeolites can promote the selective synthesis of the target product. The zeolite with a Si/Al ratio of 50 possessed Al-rich core and Al-deficient surface characteristics. This acidic property synergistically boosted the efficient light aromatics synthesis by suppressing the undesirable alkylation and isomerization reactions on the external acid sites. In contrast, the zeolite with a high Si/Al ratio exhibited insufficient acid site density for the aromatization reaction, while the zeolite with a low Si/Al ratio delivered high heavy aromatics selectivity due to the undesirable side reactions, such as alkylation and isomerization. The optimized NaFeCo/HZ-50 catalyst achieved a CO2 conversion of 43% with an aromatics selectivity of 41%, including a BTX fraction of 57% in total aromatics. This work demonstrates that the rational design of zeolite acidic property is critical for efficient CO2 hydrogenation into BTX, offering design guidance for bifunctional catalysts in a targeted synthesis system.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15060557/s1. Table S1: XRF test results of zeolites; Table S2: Texture property of zeolites with different Si/Al ratios; Table S3: Catalytic properties of zeolites with different Si/Al ratios; title; Table S4: Distribution of aromatics in reaction products; Table S5: NH3-TPD data for zeolites with different Si/Al ratios; Table S6: Py-IR data for zeolites with different Si/Al ratios.
Author Contributions
Conceptualization, S.W. and Y.W.; methodology, S.W. and Y.S.; validation, S.W., X.B. and Y.H.; formal analysis, S.W. and Y.S.; investigation, S.W., S.L., Z.B., Z.Z. and X.L.; resources, D.L., Y.W. and M.W.; writing—original draft preparation, S.W.; writing—review and editing, S.W., Y.S. and Y.W.; visualization, S.W., Y.S. and X.L.; supervision, D.L., Y.W. and M.W.; project administration, Y.W. and M.W.; Funding acquisition, Y.W. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key Research and Development Program of China (2023YFB4104500, 2023YFB4104502), the National Natural Science Foundation of China (22478436), the Key Research and Development Program of Shandong Province (2024ZLGX08), and the Science and Technology Innovation Project of the Shandong Energy Group Co., Ltd. (SNKJ2023A03).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that this study received funding from Shandong Energy Group Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
References
- Wang, M.; Luo, J. A coupled electrochemical system for CO2 capture, conversion and product purification. eScience 2023, 3, 100155. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Z.; Liu, L.; Wang, Y.; Kawi, S. Catalytic CO2 conversion to C1 chemicals over single-atom catalysts. Adv. Energ. Mater. 2023, 13, 2301852. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, C.; Tsubaki, N. Recent advancements and perspectives of the CO2 hydrogenation reaction. Green Carbon 2023, 1, 133–145. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Tsubaki, N. Clever Nanomaterials fabrication techniques encounter sustainable C1 catalysis. Acc. Chem. Res. 2023, 56, 2341–2353. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Chen, S.; Wang, X.; Zhou, Z.; Wu, Y.; Zhang, T.; Yang, G.; Han, Y.; Tan, Y. Hydrogenation of CO2 into aromatics over a ZnCrOX-zeolite composite catalyst. Chem. Commun. 2019, 55, 973–976. [Google Scholar] [CrossRef]
- Wei, J.; Yao, R.; Han, Y.; Ge, Q.; Sun, J. Towards the development of the emerging process of CO2 heterogenous hydrogenation into high-value unsaturated heavy hydrocarbons. Chem. Soc. Rev. 2021, 50, 10764–10805. [Google Scholar] [CrossRef]
- Chang, Z.; Qu, Y.; Gu, Z.; Zhou, L.; Li, R.; Sun, Z.; Xu, M.; Chu, M. Production of aromatic hydrocarbons from catalytic pyrolysis of Huadian oil shale using ZSM-5 zeolites as catalyst. J. Anal. Appl. Pyrolysis 2021, 159, 104990. [Google Scholar] [CrossRef]
- Tsubaki, N.; Wang, Y.; Yang, G.; He, Y. Rational design of novel reaction pathways and tailor-made catalysts for value-added chemicals synthesis from CO2 hydrogenation. Bull. Chem. Soc. Jpn. 2023, 96, 291–302. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Wu, M.; Tsubaki, N.J.E. Thermocatalytic hydrogenation of CO2 into aromatics by tailor-made catalysts: Recent advancements and perspectives. EcoMat 2021, 3, e12080. [Google Scholar] [CrossRef]
- Li, W.; Zhan, G.; Liu, X.; Yue, Y.; Tan, K.B.; Wang, J.; Huang, J.; Li, Q. Assembly of ZnZrOX and ZSM-5 on hierarchically porous bio-derived SiO2 platform as bifunctional catalysts for CO2 hydrogenation to aromatics. Appli. Catal. B Environ. 2023, 330, 122575. [Google Scholar] [CrossRef]
- Wang, W.; He, R.; Wang, Y.; Li, M.; Liu, J.; Liang, J.; Yasuda, S.; Liu, Q.; Wu, M.; Tsubaki, N. Boosting methanol-mediated CO2 hydrogenation into aromatics by synergistically tailoring oxygen vacancy and acid site properties of multifunctional catalyst. Chem. Eur. J. 2023, 29, e202301135. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Chen, Z.; Fu, Y.; Liu, Y.; Zhu, W.; Liu, Z. Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 2018, 9, 3457. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, W.; Kazumi, S.; Li, H.; Yang, G.; Tsubaki, N. Direct and oriented conversion of CO2 into value-added aromatics. Chem. Eur. J. 2019, 25, 5149–5153. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guo, L.; Wang, X.; Zhou, W.; Chen, F.; Li, D.; Liu, K.; Ai, P.; Wei, Y.; Cai, M.; et al. Spaced-confined capsule catalysts with tunable micro-environments for efficient CO2 conversion. AIChE J. 2024, 70, e18445. [Google Scholar] [CrossRef]
- Song, G.; Li, M.; Yan, P.; Nawaz, M.A.; Liu, D. High conversion to aromatics via CO2 FT over a CO-Reduced CuFe2O3 catalyst integrated with HZSM5. ACS Catal. 2020, 10, 11268–11279. [Google Scholar] [CrossRef]
- Wen, C.; Jin, K.; Lu, L.; Jiang, Q.; Wu, J.; Zhuang, X.; Zhang, X.; Chen, L.; Wang, C.; Ma, L. Insight into the direct conversion of syngas toward aromatics over the Cu promoter Fe-zeolite tandem catalyst. Fuel 2023, 331, 125855. [Google Scholar] [CrossRef]
- Tian, G.; Li, Z.; Zhang, C.; Liu, X.; Fan, X.; Shen, K.; Meng, H.; Wang, N.; Xiong, H.; Zhao, M.; et al. Upgrading CO2 to sustainable aromatics via perovskite-mediated tandem catalysis. Nat. Commun. 2024, 15, 3037. [Google Scholar] [CrossRef]
- Wang, Y.; Kazumi, S.; Gao, W.; Gao, X.; Li, H.; Guo, X.; Yoneyama, Y.; Yang, G.; Tsubaki, N. Direct conversion of CO2 to aromatics with high yield via a modified Fischer-Tropsch synthesis pathway. Appl. Catal. B Environ. 2020, 269, 118792. [Google Scholar] [CrossRef]
- Xiang, Y.; Kruse, N. Tuning the catalytic CO hydrogenation to straight- and long-chain aldehydes/alcohols and olefins/paraffins. Nat. Commun. 2016, 7, 13058. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; Bitter, J.H.; Khare, C.B.; Ruitenbeek, M.; Dugulan, A.I.; Jong, K.P. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Science 2012, 335, 835–838. [Google Scholar] [CrossRef]
- Cui, X.; Gao, P.; Li, S.; Yang, C.; Liu, Z.; Wang, H.; Zhong, L.; Sun, Y. Selective production of aromatics directly from carbon dioxide hydrogenation. ACS Catal. 2019, 9, 3866–3876. [Google Scholar] [CrossRef]
- Ramirez, A.; Chowdhury, A.; Dokania, A.; Cnudde, P.; Caglayan, M.; Yarulina, I.; Abou-Hamad, E.; Gevers, L.; Ould-Chikh, S.; Wispelaere, K.; et al. Effect of zeolite topology and reactor configuration on the direct conversion of CO2 to light olefins and aromatics. ACS Catal. 2019, 9, 6320–6334. [Google Scholar] [CrossRef]
- Yue, Y.; Tian, J.; Ma, J.; Yang, S.; Li, W.; Huang, J.; Li, Q.; Zhan, G. Regulation of acidity properties of ZSM-5 and proximity between metal oxide and zeolite on bifunctional catalysts for enhanced CO2 hydrogenation to aromatics. Appl. Catal. B Environ. 2024, 355, 124158. [Google Scholar] [CrossRef]
- Wei, J.; Yao, R.; Ge, Q.; Xu, D.; Fang, C.; Zhang, J.; Xu, H.; Sun, J. Precisely regulating Brønsted acid sites to promote the synthesis of light aromatics via CO2 hydrogenation. Appl. Catal. B Environ. 2021, 283, 119648. [Google Scholar] [CrossRef]
- Gu, Y.; Liang, J.; Wang, Y.; Huo, K.; Li, M.; Wang, W.; He, R.; Yasuda, S.; Gao, X.; Yang, G.; et al. Tailoring the product distribution of CO2 hydrogenation via engineering of Al location in zeolite. Appl. Catal. B Environ. 2024, 349, 123842. [Google Scholar] [CrossRef]
- Yang, J.; Gong, K.; Miao, D.; Jiao, F.; Pan, X.; Meng, X.; Xiao, F.; Bao, X. Enhanced aromatic selectivity by the sheet-like ZSM-5 in syngas conversion. J. Energ. Chem. 2019, 35, 44–48. [Google Scholar] [CrossRef]
- Kosari, M.; Lee, K.; Chao, W.; Rimaz, S.; Zhou, S.; Hondo, E.; Xi, S.; Seayad, A.M.; Zeng, H.C.; Borgna, A. Optimizing hollow ZSM-5 spheres (HZSM5) morphology and its intrinsic acidity for hydrogenation of CO2 to DME with copper-aluminum. Chem. Eng. J. 2023, 470, 144196. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Xie, Y.; Zhang, S.; Ning, P.; Wang, X. Tailoring mesoporosity and acid sites for enhanced gaseous As2O3 adsorption by alkaline-etching ZSM-5 with different Si/Al ratios. Sep. Purif. Technol. 2025, 354, 129081. [Google Scholar] [CrossRef]
- Lukyanov, D.B.; Gnep, N.S.; Guisnet, M.R. Kinetic modeling of ethene and propene aromatization over HZSM-5 and GaHZSM-5. Ind. Eng. Chem. Res. 1994, 33, 223–234. [Google Scholar] [CrossRef]
- Yang, X.; Wang, R.; Yang, J.; Qian, W.; Zhang, Y.; Li, X.; Huang, Y.; Zhang, T.; Chen, D. Exploring the reaction paths in the consecutive Fe-Based FT catalyst-zeolite process for syngas conversion. ACS Catal. 2020, 10, 3797–3806. [Google Scholar] [CrossRef]
- Cheng, C.; Li, G.; Ji, D.; Zhao, Y.; Shen, J. Regulating hierarchical structure and acidity of HZSM-5 for methanol to aromatics via protective desiliconization and external surface modification. Microporous Mesoporous Mater. 2021, 312, 110784. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, K.; Chen, X.; Xu, L.; Deng, C.; Wang, Q.; Gao, R.; Jun, K.; Kim, S.; Zhao, T.; et al. Direct hydrogenation of CO2 into valuable aromatics over K/Fe-Cu-Al@HZSM-5 tandem catalysts: Effects of zeolite surface acidity on aromatics formation. Fuel Process. Technol. 2023, 248, 107824. [Google Scholar] [CrossRef]
- Wu, X.; Wang, C.; Zhao, S.; Wang, Y.; Zhang, T.; Yao, J.; Gao, W.; Zhang, B.; Arakawa, T.; He, Y.; et al. Dual-engine-driven realizing high-yield synthesis of para-xylene directly from CO2-containing syngas. Nat. Commun. 2024, 15, 8064. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gao, W.; Liu, G.; Tsubaki, N. A review of CO2 hydrogenation to liquid fuels. ChemSusChem 2025, 18, e202402756. [Google Scholar]
- Lin, S.; He, R.; Wang, W.; Wang, Y.; Gu, Y.; Liu, Q.; Wu, M. Highly selective transformation of CO2 + H2 into para-xylene via a bifunctional catalyst composed of Cr2O3 and twin-structured ZSM-5 zeolite. Catalysts 2023, 13, 1080. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Huang, X.; Zhu, Y.; Li, G.; Gu, Q.; Chen, J.; Ma, L.; Li, X.; He, Q.; et al. Maximizing sinusoidal channels of HZSM-5 for high shape-selectivity to p-xylene. Nat. Commun. 2019, 10, 4348. [Google Scholar] [CrossRef]
- Xin, S.; Wang, Q.; Xu, J.; Chu, Y.; Wang, P.; Feng, N.; Qi, G.; Tébosc, J.; Lafon, O.; Fan, F.; et al. The acidic nature of “NMR-invisible” tri-coordinated framework aluminum species in zeolites. Chem. Sci. 2019, 10, 10159–10169. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Qin, Z.; Chen, Y.; Dong, M.; Li, J.; Zhang, K.; Liu, P.; Wang, J.; Fan, W. Relation of catalytic performance to the aluminum siting of acidic zeolites in the conversion of methanol to olefins, viewed via a comparison between ZSM-5 and ZSM-11. ACS Catal. 2018, 8, 5485–5505. [Google Scholar] [CrossRef]
- Li, H.; Yu, J.; Du, K.; Li, W.; Ding, L.; Chen, W.; Xie, S.; Zhang, Y.; Tang, Y. Synthesis of ZSM-5 zeolite nanosheets with tunable silanol nest contents across an ultra-wide pH range and their catalytic validation. Angew. Chem. Int. Ed. 2024, 63, e202405092. [Google Scholar] [CrossRef]
- Wang, H.; Hou, Y.; Sun, W.; Hu, Q.; Xiong, H.; Wang, T.; Yan, B.; Qian, W. Insight into the effects of water on the ethene to aromatics reaction with HZSM-5. ACS Catal. 2020, 10, 5288–5298. [Google Scholar] [CrossRef]
- Ene, A.B.; Archipov, T.; Roduner, E. Spectroscopic study of the adsorption of benzene on Cu/HZSM5 zeolites. J. Phys. Chem. C 2010, 114, 14571–14578. [Google Scholar] [CrossRef]
- Inagaki, S.; Sato, K.; Hayashi, S.; Tatami, J.; Kubota, Y.; Wakihara, T. Mechanochemical approach for selective deactivation of external surface acidity of ZSM-5 zeolite catalyst. ACS Appli. Mater. Interfaces 2015, 7, 4488–4493. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lyu, H.; Chai, Y.; Liu, B.; Zhao, D.; Liu, C. Selective aromatization of 1-hexene to BTX over core-shell structured Silicalite-1@ZSM-5 catalyst. Sep. Purif. Technol. 2024, 349, 127881. [Google Scholar] [CrossRef]
- Emeis, C. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 147, 347–354. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).