Exploring the Role of Non-Metal Doping in g-C3N4 for CO2 Reduction: A DFT Investigation
Abstract
1. Introduction
2. Results and Discussion
2.1. Geometric Structures of Pristine g-C3N4 and NM-C3N4
2.2. Electronic Properties of Pristine g-C3N4 and NM-C3N4
2.3. CO2 Adsorption on Pristine g-C3N4 and NM-C3N4
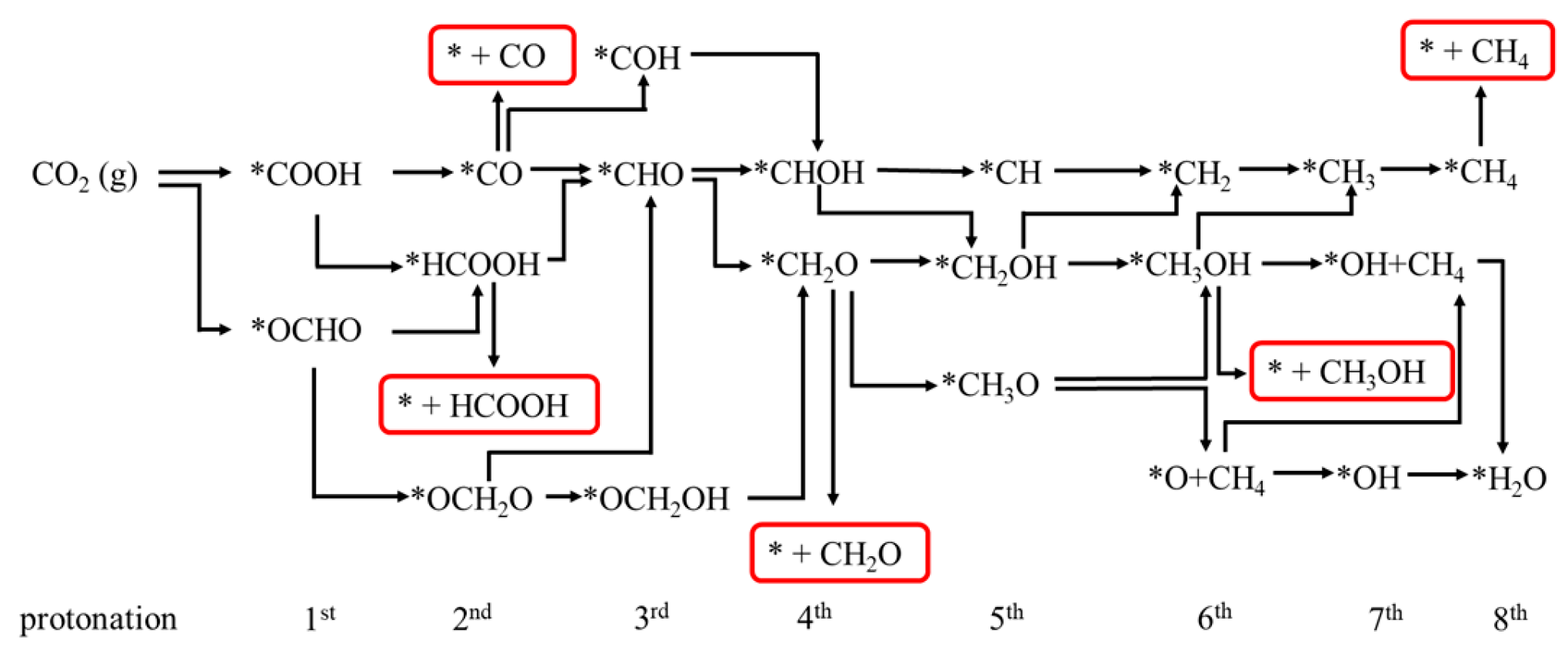
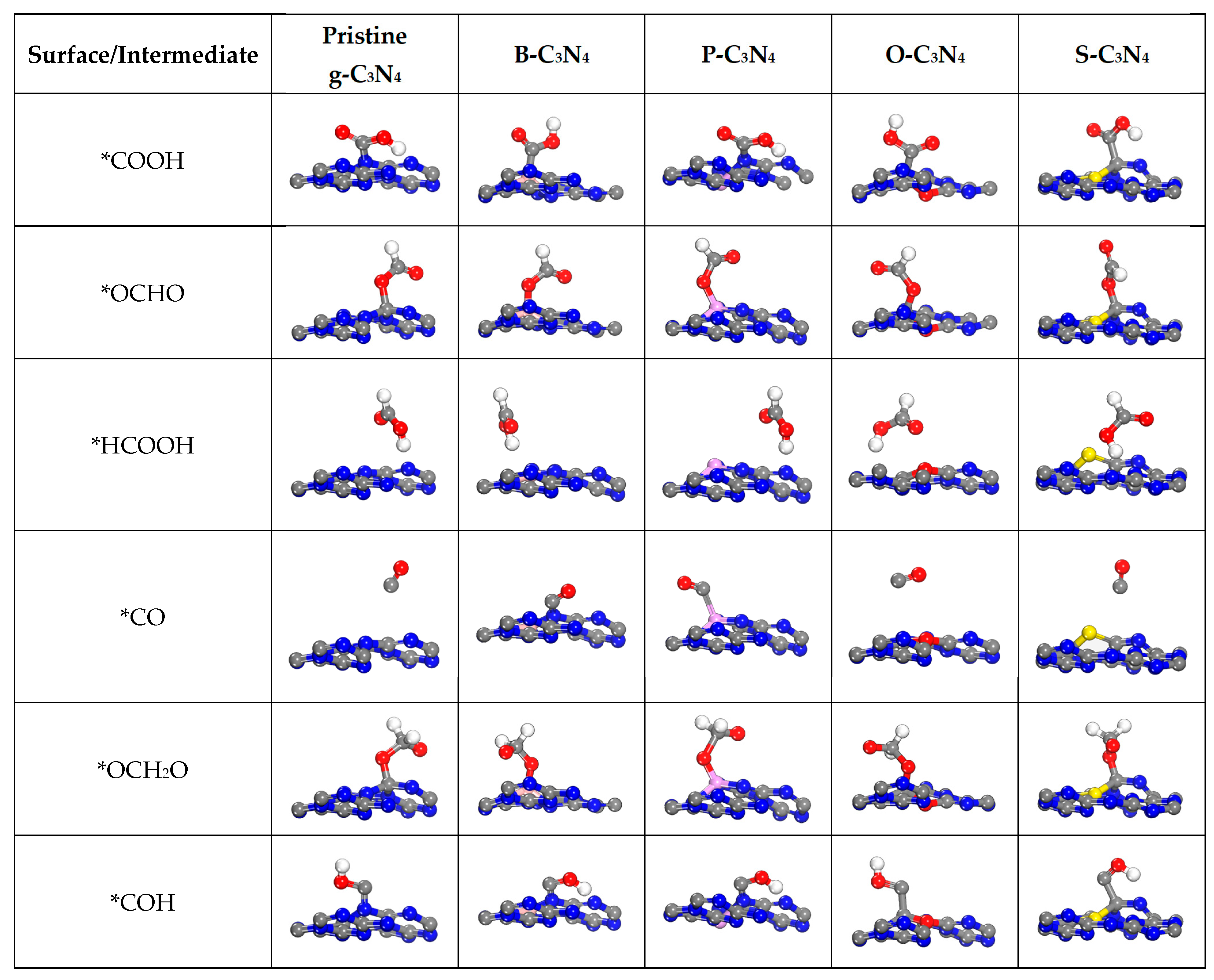
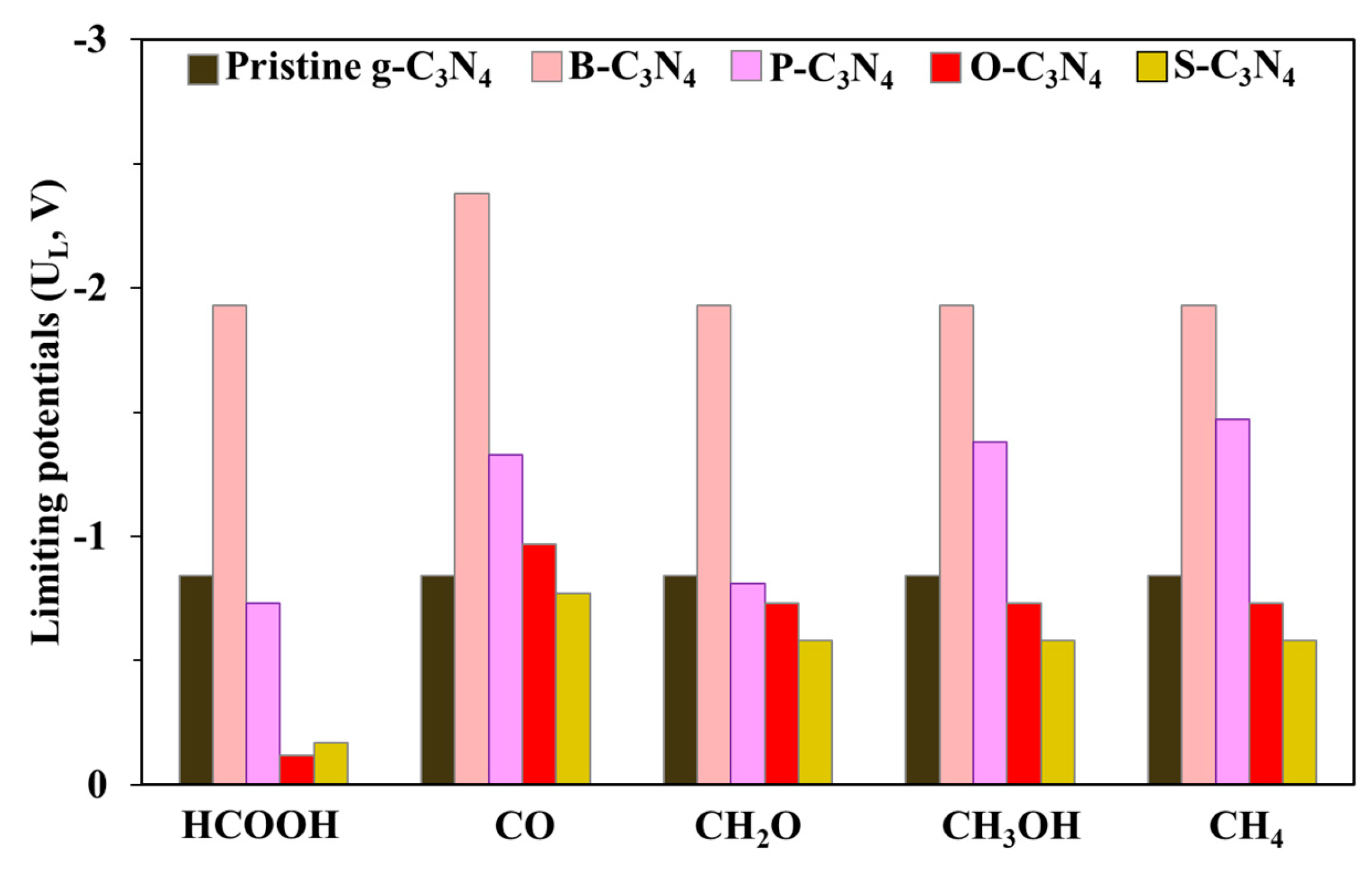
2.4. Electrocatalytic CO2 Reduction to C1 Products
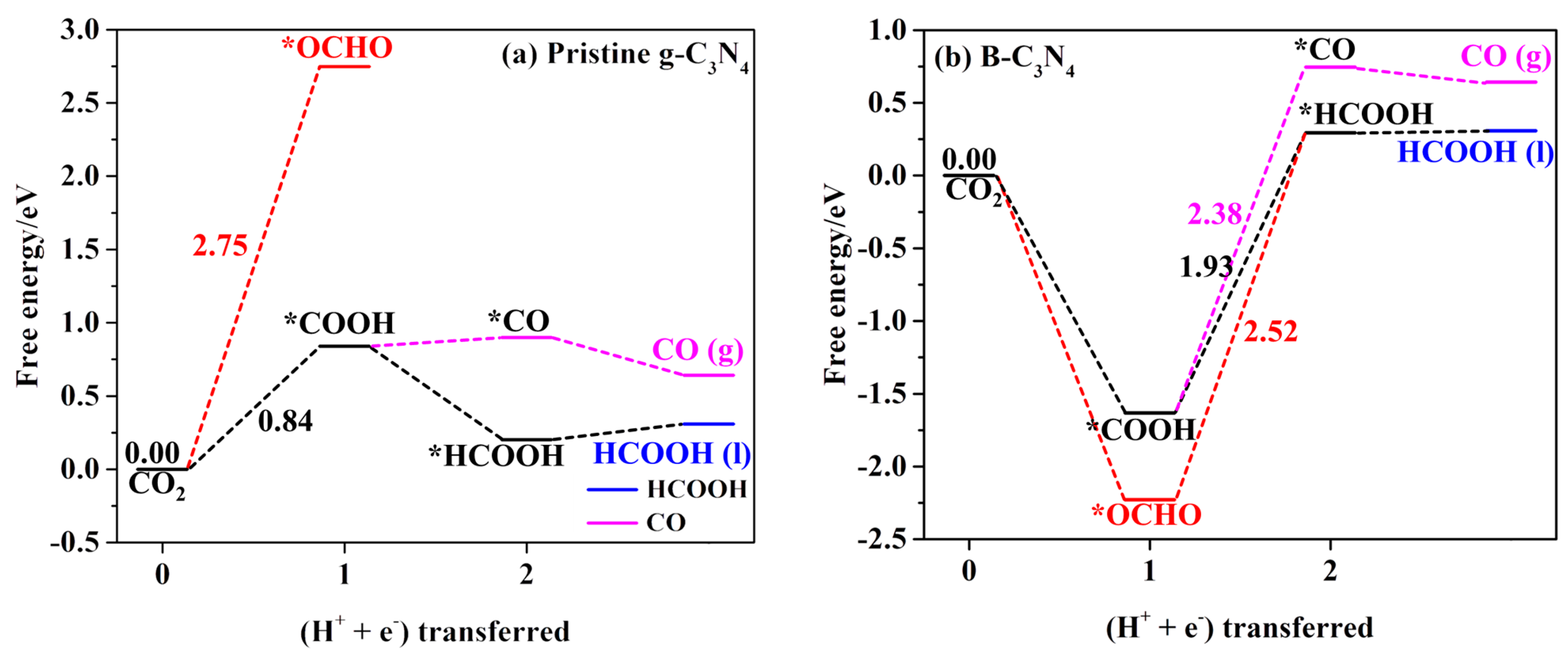
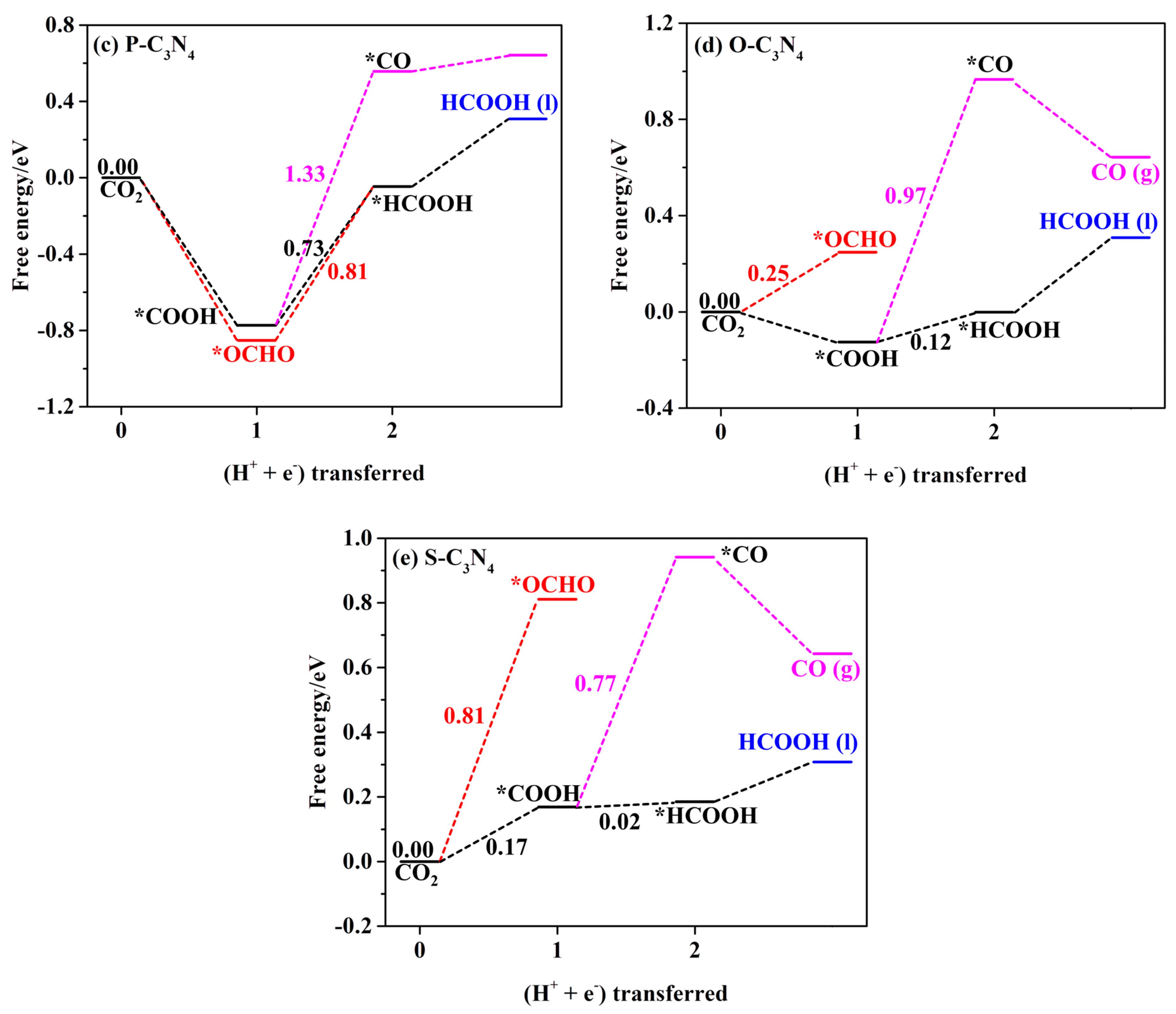
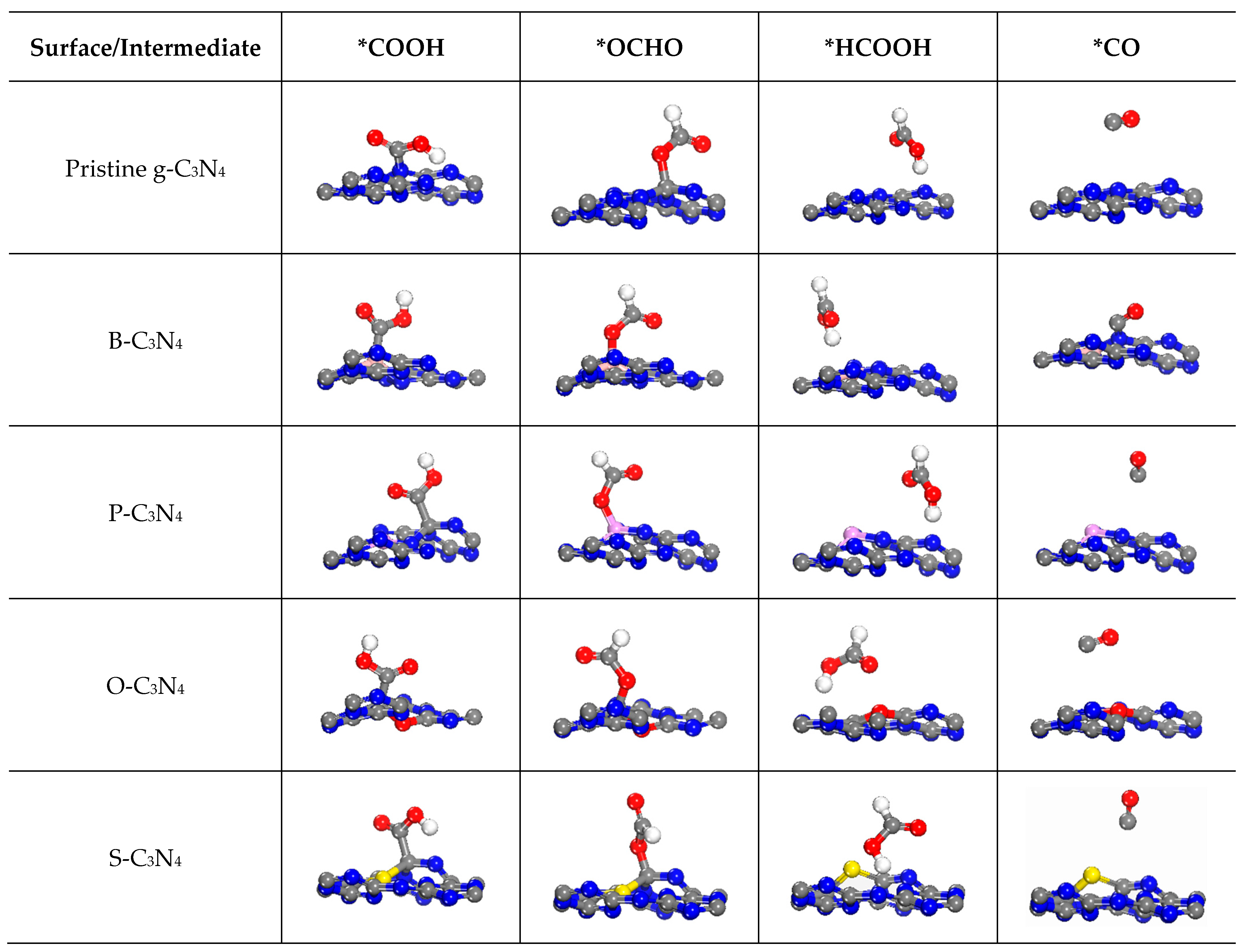
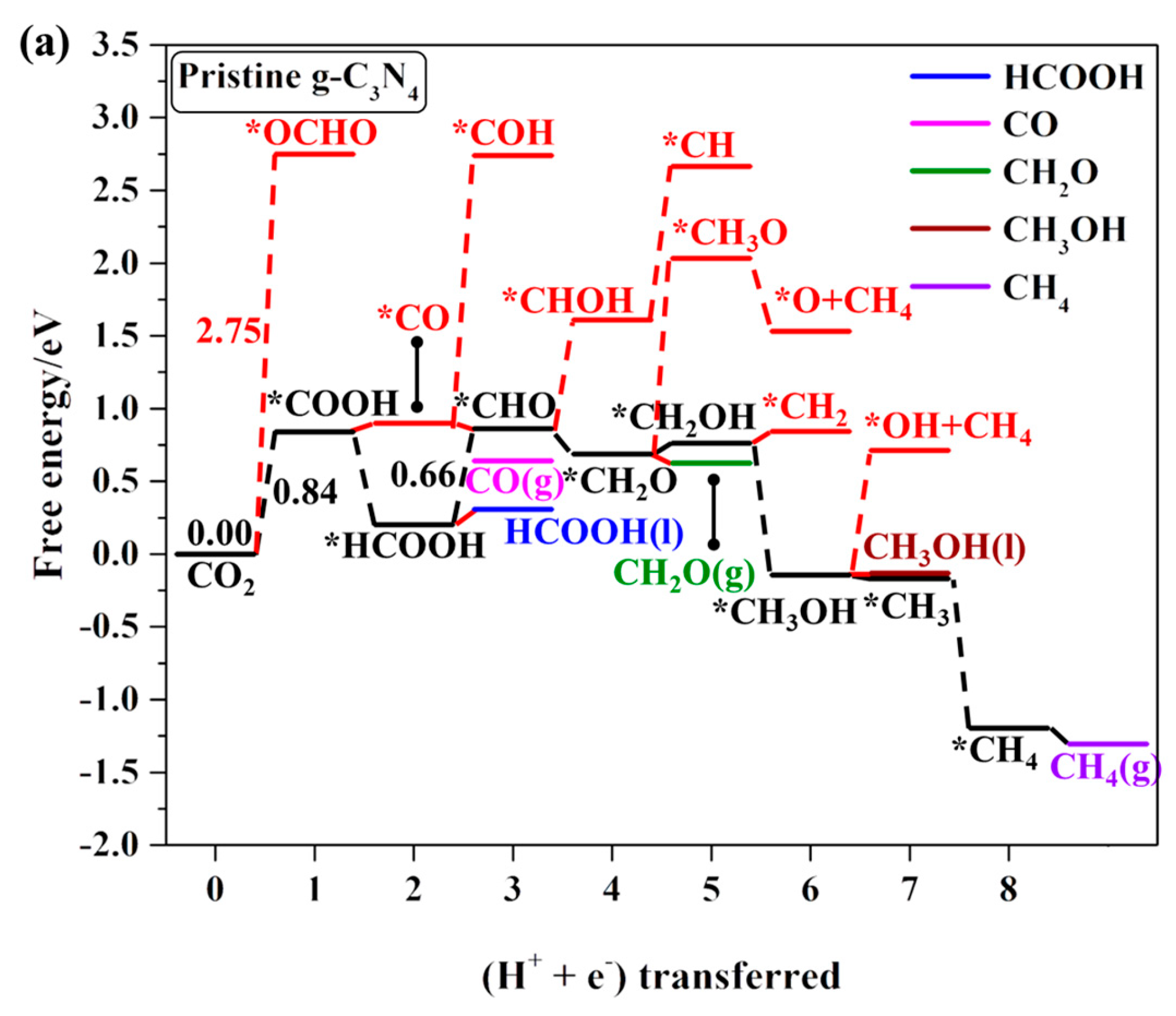
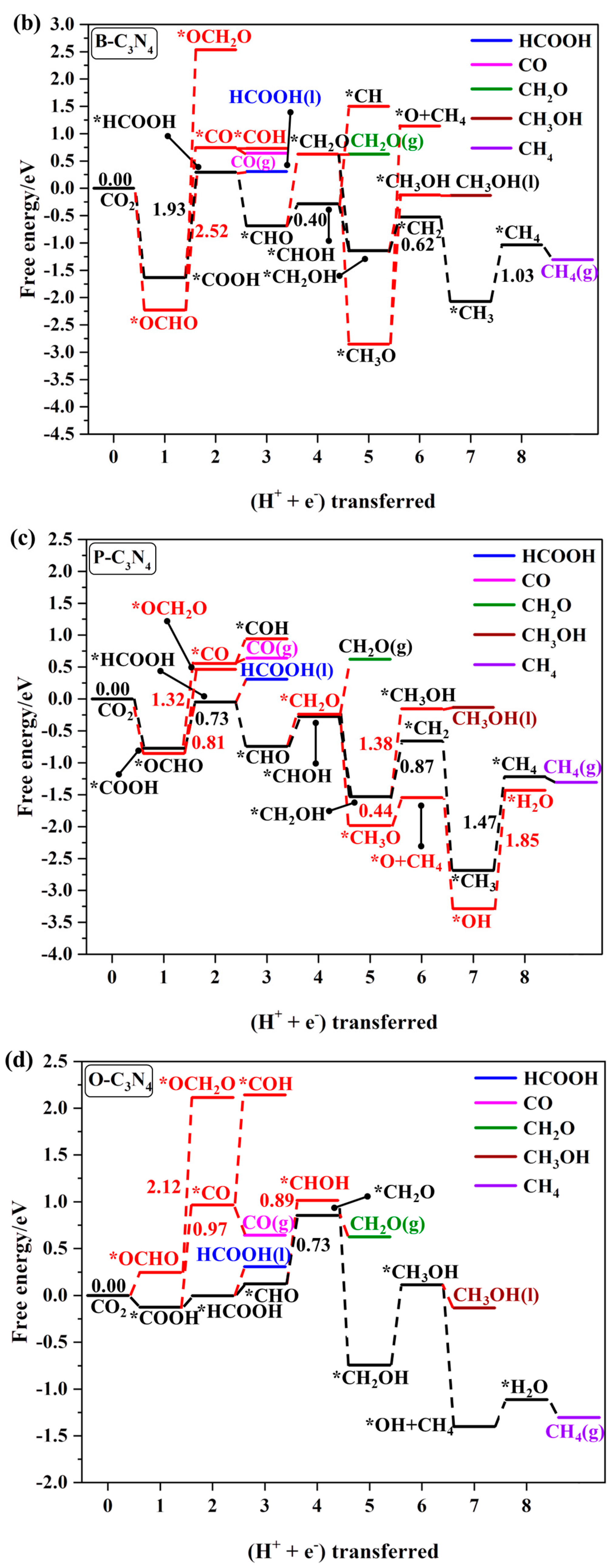
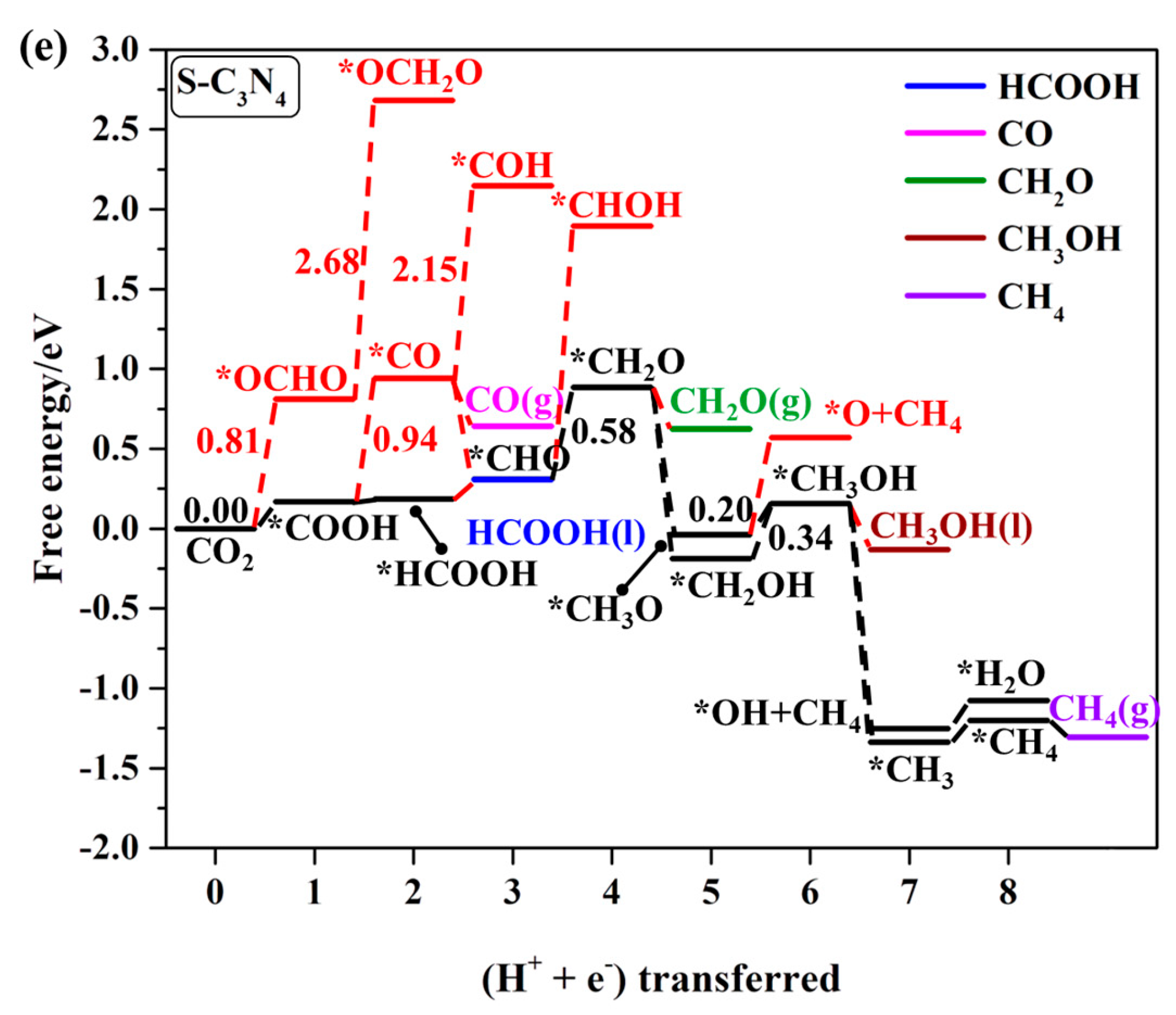
2.4.1. First Protonation to HCOOH and CO
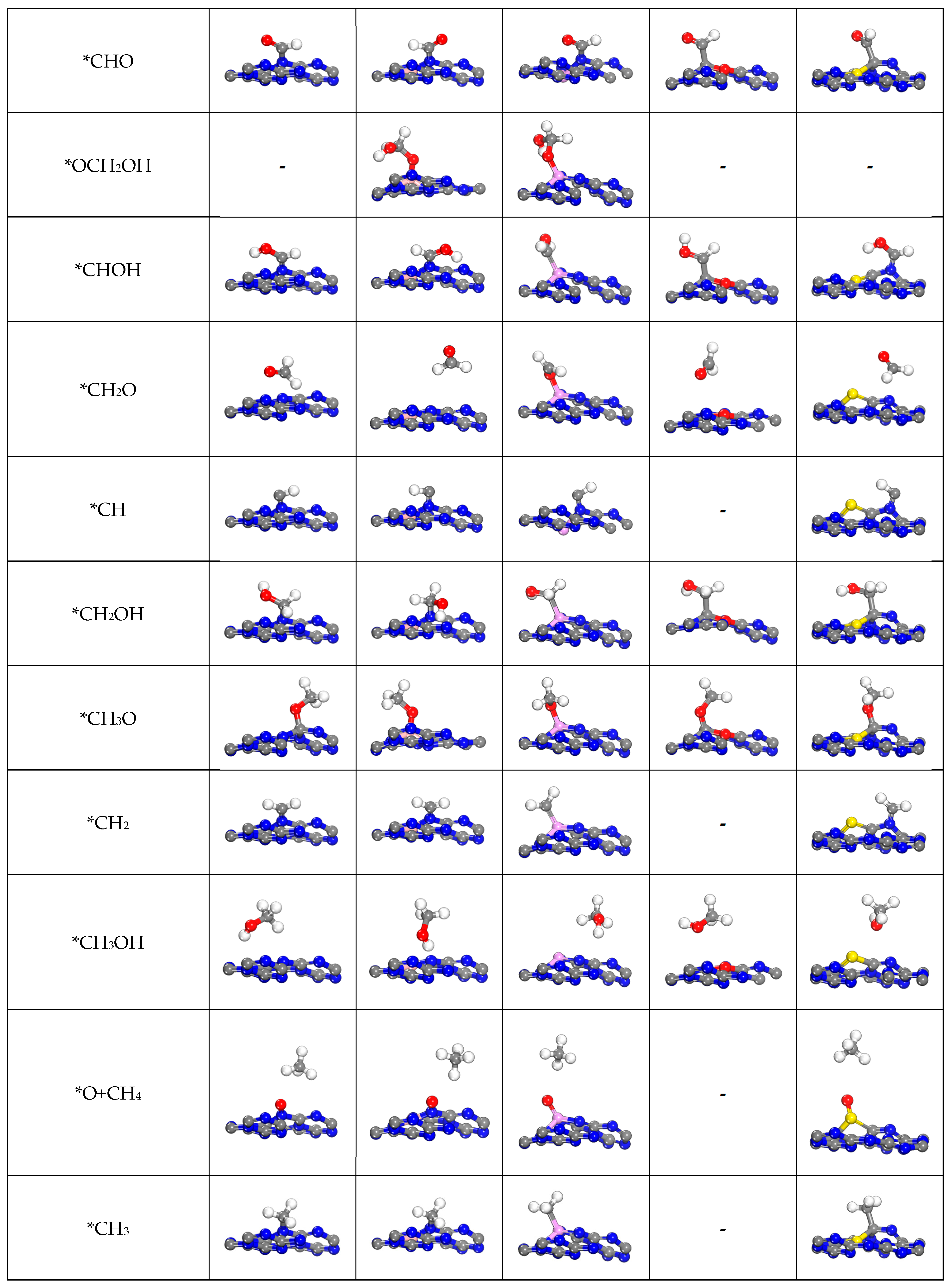
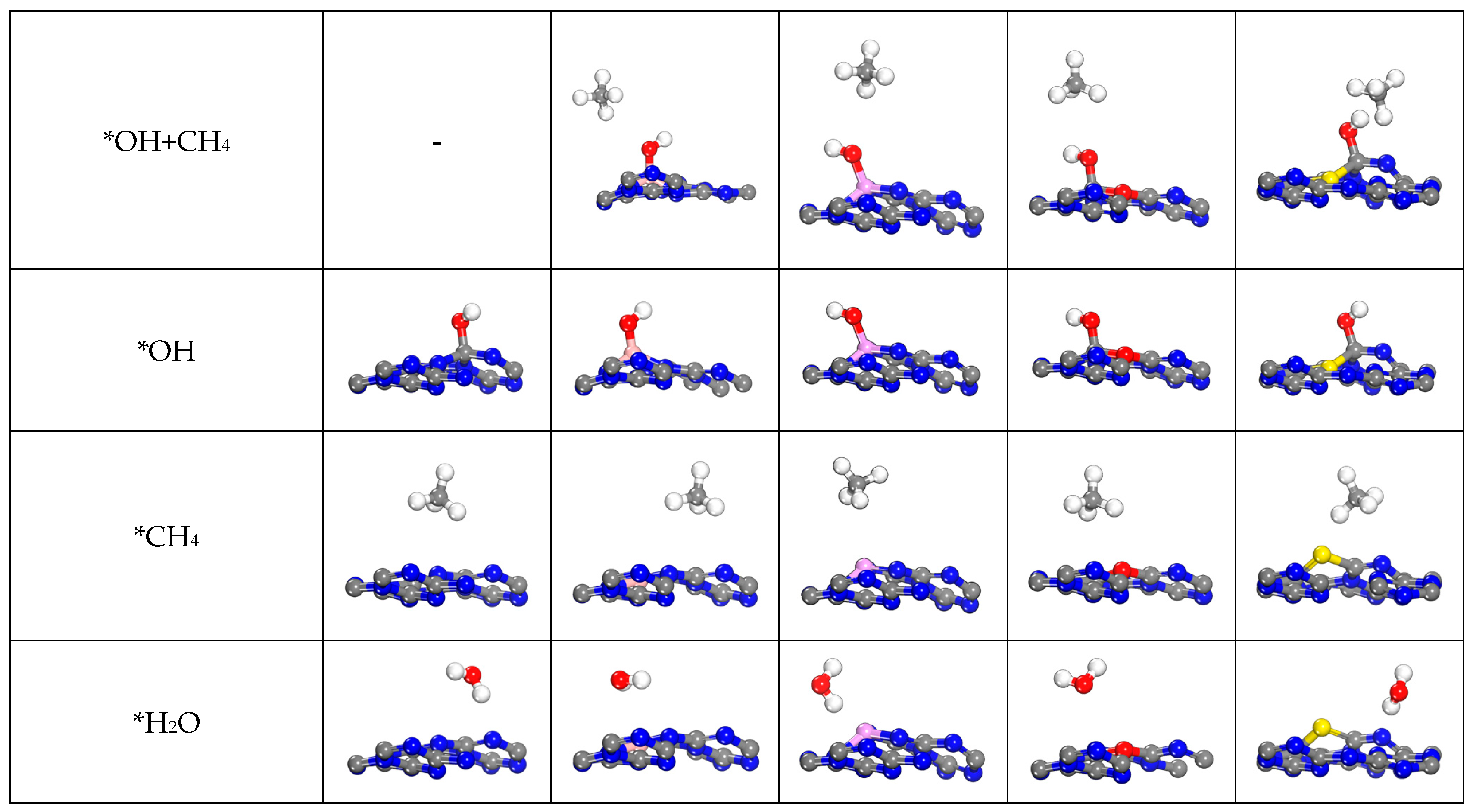
2.4.2. The Other C1 Products for CO2RR
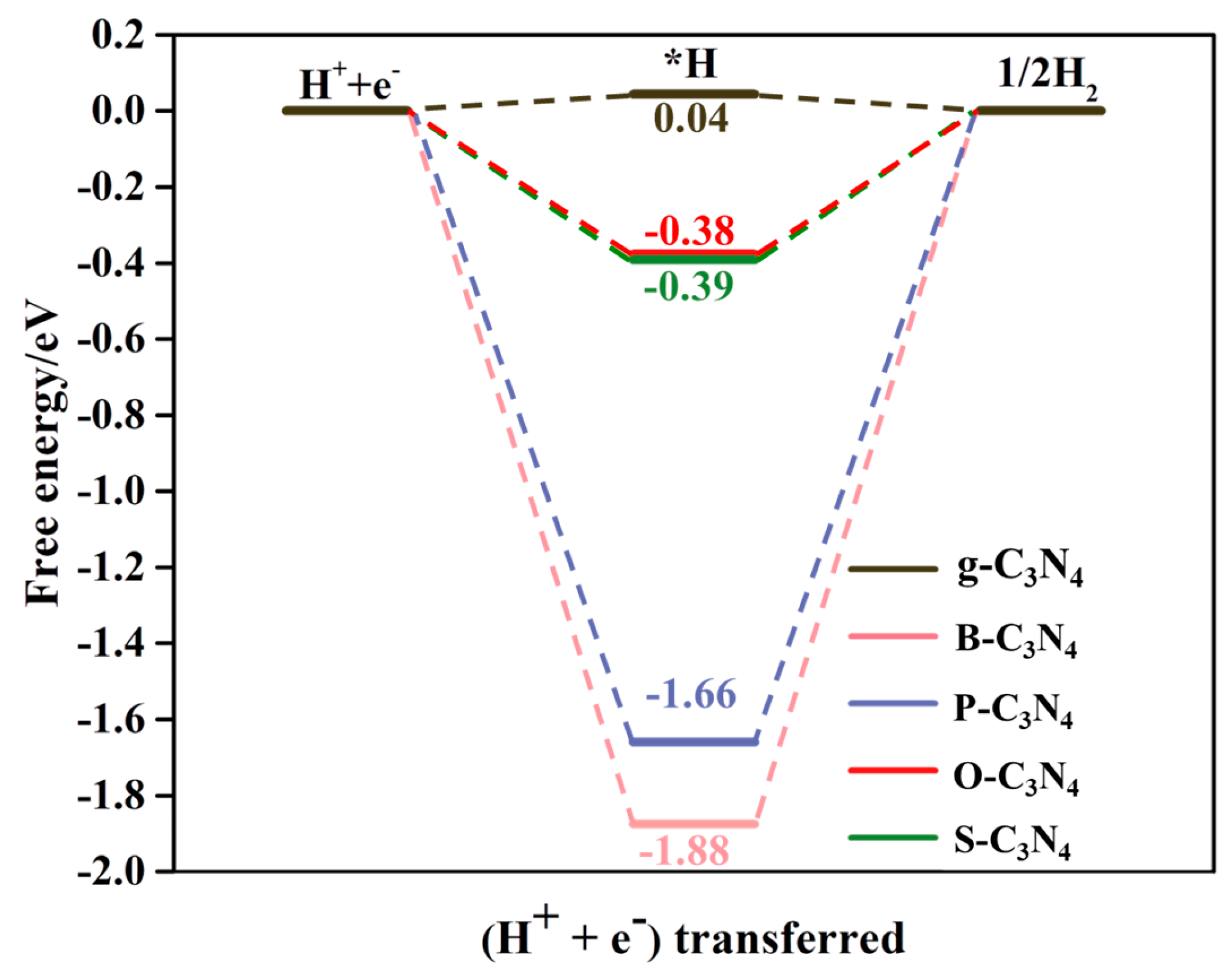
2.5. Competition Between CO2RR and HER on Pristine g-C3N4 and NM-C3N4
3. Methodology
3.1. Computational Details
3.2. Structural Model and Stability Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, F.; Zhao, H.; Deng, W.; Feng, X.; Li, Y. A novel N,Fe-Decorated carbon nanotube/carbon nanosheet architecture for efficient CO2 reduction. Electrochim. Acta 2018, 273, 154–161. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Q. Recent Advances in Breaking Scaling Relations for Effective Electrochemical Conversion of CO2. Adv. Energy Mater. 2016, 6, 1600463. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 2975–2992. [Google Scholar] [CrossRef]
- Liang, S.; Altaf, N.; Huang, L.; Gao, Y.; Wang, Q. Electrolytic cell design for electrochemical CO2 reduction. J. CO2 Util. 2020, 35, 90–105. [Google Scholar] [CrossRef]
- Yang, Z.; Nie, H.; Chen, X.; Chen, X.; Huang, S. Recent progress in doped carbon nanomaterials as effective cathode catalysts for fuel cell oxygen reduction reaction. J. Power Sources 2013, 236, 238–249. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, M.; Zhang, L.; Dai, L.; Xia, Z. Design principles for heteroatom-doped carbon nanomaterials as highly efficient catalysts for fuel cells and metal–air batteries. Adv. Mater. 2015, 27, 6834–6840. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Doping of Carbon Materials for Metal-Free Electrocatalysis. Adv. Mater. 2019, 31, 1804672. [Google Scholar] [CrossRef]
- Meng, N.; Zhou, W.; Yu, Y.; Liu, Y.; Zhang, B. Superficial Hydroxyl and Amino Groups Synergistically Active Polymeric Carbon Nitride for CO2 Electroreduction. ACS Catal. 2019, 9, 10983–10989. [Google Scholar] [CrossRef]
- Liu, C.; Tian, A.; Li, Q.; Wang, T.; Qin, G.; Li, S.; Sun, C. 2D, Metal-Free Electrocatalysts for the Nitrogen Reduction Reaction. Adv. Funct. Mater. 2023, 33, 2210759. [Google Scholar] [CrossRef]
- Wang, X.; Yu, M.; Feng, X. Electronic structure regulation of noble metal-free materials toward alkaline oxygen electrocatalysis. eScience 2023, 3, 100141. [Google Scholar] [CrossRef]
- Lu, X.; Tan, T.H.; Ng, Y.H.; Amal, R. Highly Selective and Stable Reduction of CO2 to CO by a Graphitic Carbon Nitride/Carbon Nanotube Composite Electrocatalyst. Chem.—Eur. J. 2016, 22, 11991–11996. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Zhang, Y.; Lu, L.; Zhang, S.; Chen, Y.; Liu, J.; Jin, H.; Hou, S.; Dai, K.; Song, W. Boosting visible light photocatalytic hydrogen evolution of graphitic carbon nitride via enhancing it interfacial redox activity with cobalt/nitrogen doped tubular graphitic carbon. Appl. Catal. B Environ. 2018, 225, 512–518. [Google Scholar] [CrossRef]
- Al-Ahmed, A. Photocatalytic properties of graphitic carbon nitrides (g-C3N4) for sustainable green hydrogen production: Recent advancement. Fuel 2022, 316, 123381. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Zhang, S.; Cao, J.; Zhang, A.; Zhang, S.; Su, P.; Ai, Y. High-efficiency and selective capture of nitric oxide by fluorine-modified carbon nitride: A DFT investigation. Appl. Surf. Sci. 2022, 593, 153353. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Huang, W.; Kong, Q.; Fan, X.; Wang, M.; Shi, J. N-doped graphitic carbon-incorporated g-C3N4 for remarkably enhanced photocatalytic H2 evolution under visible light. Carbon 2016, 99, 111–117. [Google Scholar] [CrossRef]
- Kumar, S.; Karthikeyan, S.; Lee, A.F. g-C3N4-Based Nanomaterials for Visible Light-Driven Photocatalysis. Catalysts 2018, 8, 74. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Gu, Y.; Li, S.; Li, M.; Wang, X.; Liu, Y.; Shi, K.; Bai, X.; Yao, Q.; Wu, Z.; Yao, H. Recent advances in g-C3N4-based photo-enzyme catalysts for degrading organic pollutants. RSC Adv. 2023, 13, 937–947. [Google Scholar] [CrossRef]
- Mao, X.; Guo, R.; Chen, Q.; Zhu, H.; Li, H.; Yan, Z.; Guo, Z.; Wu, T. Recent Advances in Graphitic Carbon Nitride Based Electro-Catalysts for CO2 Reduction Reactions. Molecules 2023, 28, 3292. [Google Scholar] [CrossRef] [PubMed]
- Yurova, V.Y.; Potapenko, K.O.; Aliev, T.A.; Kozlova, E.A.; Skorb, E.V. Optimization of g-C3N4 synthesis parameters based on machine learning to predict the efficiency of photocatalytic hydrogen production. Int. J. Hydrogen Energy 2024, 81, 193–203. [Google Scholar] [CrossRef]
- Kharina, S.; Kurenkova, A.; Aydakov, E.; Mishchenko, D.; Gerasimov, E.; Saraev, A.; Zhurenok, A.; Lomakina, V.; Kozlova, E. Activation of g-C3N4 by oxidative treatment for enhanced photocatalytic H2 evolution. Appl. Surf. Sci. 2025, 698, 163074. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Zhurenok, A.; Saraev, A.; Gerasimov, E.; Cherepanova, S.; Tkachev, S.; Plusnin, P.; Kozlova, E. Highly efficient hydrogen production under visible light over g-C3N4-based photocatalysts with low platinum content. Chem. Eng. J. 2022, 445, 136721. [Google Scholar] [CrossRef]
- Gao, G.; Jiao, Y.; Waclawik, E.R.; Du, A. Single Atom (Pd/Pt) Supported on Graphitic Carbon Nitride as an Efficient Photocatalyst for Visible-Light Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2016, 138, 6292–6297. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, Y.; Chen, P.; Jaroniec, M.; Qiao, S.-Z. Molecular Scaffolding Strategy with Synergistic Active Centers to Facilitate Electrocatalytic CO2 Reduction to Hydrocarbon/Alcohol. J. Am. Chem. Soc. 2017, 139, 18093–18100. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, F.; Zheng, L.R.; Wang, H.F.; Yang, X.H.; Yang, H.G. Tuning Metal Catalyst with Metal–C3N4 Interaction for Efficient CO2 Electroreduction. ACS Catal. 2018, 8, 11035–11041. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, T.; Deng, X.; Liang, X.; Guo, W.; Lu, X.; Wu, C.-M.L. Electrochemical CO2 Reduction to C1 Products on Single Nickel/Cobalt/Iron-Doped Graphitic Carbon Nitride: A DFT Study. ChemSusChem 2019, 12, 5126–5132. [Google Scholar] [CrossRef]
- Ranjbakhsh, E.; Izadyar, M.; Nakhaeipour, A.; Habibi-Yangjeh, A. P-doped g-C3N4 as an efficient photocatalyst for CO2 conversion into value-added materials: A joint experimental and theoretical study. Int. J. Quantum Chem. 2020, 120, e26388. [Google Scholar] [CrossRef]
- Gao, D.; Liu, Y.; Liu, P.; Si, M.; Xue, D. Atomically Thin B doped g-C3N4 Nanosheets: High-Temperature Ferromagnetism and calculated Half-Metallicity. Sci. Rep. 2016, 6, 35768. [Google Scholar] [CrossRef]
- Li, X.; Zhu, P.; Liu, C.; Pang, H. One step synthesis of boron-doped carbon nitride derived from 4-pyridylboronic acid as biosensing platforms for assessment of food safety. Chem. Commun. 2019, 55, 9160–9163. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhou, Y.; Zhang, L.; Yu, X.; Gao, S.; Sun, X.; Cheng, C.; Liu, Q.; Yang, J. Enhanced n→π* electron transition of porous P-doped g-C3N4 nanosheets for improved photocatalytic H2 evolution performance. Ceram. Int. 2020, 46, 8444–8451. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C.; Smith, S.C.; Chen, Z.; Lu, G.Q.; Cheng, H.-M. Unique Electronic Structure Induced High Photoreactivity of Sulfur-Doped Graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, A.; Chandrasekaran, A.; Jayakumar, S.; Manickam, P.; Alwarappan, S. Sulphur Doped Graphitic Carbon Nitride as an Efficient Electrochemical Platform for the Detection of Acetaminophen. J. Electrochem. Soc. 2019, 166, B1461. [Google Scholar] [CrossRef]
- Qin, H.; Lv, W.; Bai, J.; Zhou, Y.; Wen, Y.; He, Q.; Tang, J.; Wang, L.; Zhou, Q. Sulfur-doped porous graphitic carbon nitride heterojunction hybrids for enhanced photocatalytic H2 evolution. J. Mater. Sci. 2019, 54, 4811–4820. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, N.; Ali, S.S.; Hou, J.; Aslam, I.; Naeem, H.; Boota, M.; Ul-Hussan, M.; Yin, J.; Wang, X. Diverse morphological study for nonmetal-doped g-C3N4 composites with narrow bandgap for improved photocatalytic activity. Res. Chem. Intermed. 2022, 48, 2857–2870. [Google Scholar] [CrossRef]
- Hu, S.; Ma, L.; You, J.; Li, F.; Fan, Z.; Wang, F.; Liu, D.; Gui, J. A simple and efficient method to prepare a phosphorus modified g-C3N4 visible light photocatalyst. RSC Adv. 2014, 4, 21657–21663. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Pan, L.; Wang, Z.; Zhang, X.; Zou, J.-J.; Mi, W.; Zhang, X.; Wang, L. Carbon nitride with simultaneous porous network and O-doping for efficient solar-energy-driven hydrogen evolution. Nano Energy 2015, 12, 646–656. [Google Scholar] [CrossRef]
- Chen, G.; Gao, S.-P. Structure and electronic structure of S-doped graphitic C3N4 investigated by density functional theory. Chin. Phys. B 2012, 21, 107101. [Google Scholar] [CrossRef]
- Cui, J.; Liang, S.; Wang, X.; Zhang, J. First principle modeling of oxygen-doped monolayer graphitic carbon nitride. Mater. Chem. Phys. 2015, 161, 194–200. [Google Scholar] [CrossRef]
- Sun, F.-l.; Fang, Q.-j.; Yu, Y.-f.; Zhang, W.; Pan, J.-k.; Chen, W.-X.; Zhuang, G.-l. Enhancing mechanism of electron-deficient p states on photocatalytic activity of g-C3N4 for CO2 reduction. J. Mater. Chem. A 2022, 10, 9565–9574. [Google Scholar] [CrossRef]
- Wei, B.; Wang, W.; Sun, J.; Mei, Q.; An, Z.; Cao, H.; Han, D.; Xie, J.; Zhan, J.; He, M. Insight into the effect of boron doping on electronic structure, photocatalytic and adsorption performance of g-C3N4 by first-principles study. Appl. Surf. Sci. 2020, 511, 145549. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, D.; Gong, L.; Zhang, L.; Xia, Z. Catalytic Activity Origin and Design Principles of Graphitic Carbon Nitride Electrocatalysts for Hydrogen Evolution. Front. Mater. 2019, 6, 16. [Google Scholar] [CrossRef]
- Lu, S.; Li, C.; Li, H.H.; Zhao, Y.F.; Gong, Y.Y.; Niu, L.Y.; Liu, X.J.; Wang, T. The effects of nonmetal dopants on the electronic, optical and chemical performances of monolayer g–C3N4 by first-principles study. Appl. Surf. Sci. 2017, 392, 966–974. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Y.; Yan, L.; Su, Z. DFT Study on Sulfur-Doped g-C3N4 Nanosheets as a Photocatalyst for CO2 Reduction Reaction. J. Phys. Chem. C 2018, 122, 7712–7719. [Google Scholar] [CrossRef]
- Arumugam, M.; Tahir, M.; Praserthdam, P. Effect of nonmetals (B, O, P, and S) doped with porous g-C3N4 for improved electron transfer towards photocatalytic CO2 reduction with water into CH4. Chemosphere 2022, 286, 131765. [Google Scholar] [CrossRef] [PubMed]
- Aspera, S.M.; David, M.; Kasai, H. First-Principles Study of the Adsorption of Water on Tri-s-triazine-based Graphitic Carbon Nitride. Jpn. J. Appl. Phys. 2010, 49, 115703. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, Z.; Peng, Q.; Zhou, J.; Sun, Z. Novel two-dimensional molybdenum carbides as high capacity anodes for lithium/sodium-ion batteries. J. Mater. Chem. A 2019, 7, 12145–12153. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Back, S.; Jung, Y. TiC- and TiN-Supported Single-Atom Catalysts for Dramatic Improvements in CO2 Electrochemical Reduction to CH4. ACS Energy Lett. 2017, 2, 969–975. [Google Scholar] [CrossRef]
- Back, S.; Lim, J.; Kim, N.-Y.; Kim, Y.-H.; Jung, Y. Single-atom catalysts for CO2 electroreduction with significant activity and selectivity improvements. Chem. Sci. 2017, 8, 1090–1096. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Z.; Tang, X.; Reeti, K.; Huo, P.; Wong, J.W.-C.; Zhao, J. Sulfur-doped g-C3N4 for efficient photocatalytic CO2 reduction: Insights by experiment and first-principles calculations. Catal. Sci. Technol. 2021, 11, 1725–1736. [Google Scholar] [CrossRef]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Springer: New York, NY, USA, 2008; pp. 89–189. [Google Scholar]
- Posada-Pérez, S.; Vidal-López, A.; Solà, M.; Poater, A. 2D carbon nitride as a support with single Cu, Ag, and Au atoms for carbon dioxide reduction reaction. Phys. Chem. Chem. Phys. 2023, 25, 8574–8582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tian, Y.; Yan, L.; Su, Z. Heteroatom-doped C3N as a promising metal-free catalyst for a high-efficiency carbon dioxide reduction reaction. New J. Chem. 2020, 44, 11824–11828. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Cao, X.; Wei, X.; Cao, J.; Lin, K.; Zhou, Y.; Ai, Y. Hydrogenated Si-doped g-C3N4: Promising electrocatalyst for CO2 capture and conversion. Appl. Surf. Sci. 2023, 614, 156195. [Google Scholar] [CrossRef]
- Fu, G.; Zhen, W.; Wang, H.; Zhou, X.; Yang, L.; Zhang, J. Potassium and Boron Co-Doping of g-C3N4 Tuned CO2 Reduction Mechanism for Enhanced Photocatalytic Performance: A First-Principles Investigation. Molecules 2024, 29, 5339. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E. Efficient hybrid density functional calculations in solids: Assessment of the Heyd–Scuseria–Ernzerhof screened Coulomb hybrid functional. J. Chem. Phys. 2004, 121, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Roongcharoen, T.; Mano, P.; Jitwatanasirikul, T.; Sikam, P.; Butburee, T.; Takahashi, K.; Namuangruk, S. Theoretical insight on why N-vacancy promotes the selective CO2 reduction to ethanol on NiMn doped graphitic carbon nitride sheets. Appl. Surf. Sci. 2022, 595, 153527. [Google Scholar] [CrossRef]
- Liu, S.; Chen, L.; Mu, X.; Xu, M.; Yu, J.; Yang, G.; Luo, X.; Zhao, H.; Wu, T. Development of Pdn/g-C3N4 adsorbent for Hg0 removal—DFT study of influences of the support and Pd cluster size. Fuel 2019, 254, 115537. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Injongkol, Y.; Intayot, R.; Yodsin, N.; Montoya, A.; Jungsuttiwong, S. Mechanistic insight into catalytic carbon dioxide hydrogenation to formic acid over Pt-doped boron nitride nanosheets. Mol. Catal. 2021, 510, 111675. [Google Scholar] [CrossRef]
- Hadsadee, S.; Roongcharoen, T.; Takahashi, K.; Jungsuttiwong, S.; Namuangruk, S. Enhanced Electrocatalytic CO2 Reduction Reactivity of S- and N-Doped Fe-Embedded Graphene. ChemPlusChem 2023, 88, e202300306. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Chen, D.; Chen, Z.; Lu, Z.; Tang, J.; Zhang, X.; Singh, C.V. Computational screening of homo and hetero transition metal dimer catalysts for reduction of CO2 to C2 products with high activity and low limiting potential. J. Mater. Chem. A 2020, 8, 21241–21254. [Google Scholar] [CrossRef]
- Xing, G.; Cheng, L.; Li, K.; Gao, Y.; Tang, H.; Wang, Y.; Wu, Z. Theoretical study of two-dimensional bis(iminothiolato)metal monolayers as promising electrocatalysts for carbon dioxide reduction. New J. Chem. 2020, 44, 12299–12306. [Google Scholar] [CrossRef]
- Liu, J.-H.; Yang, L.-M.; Ganz, E. Electrocatalytic reduction of CO2 by two-dimensional transition metal porphyrin sheets. J. Mater. Chem. A 2019, 7, 11944–11952. [Google Scholar] [CrossRef]

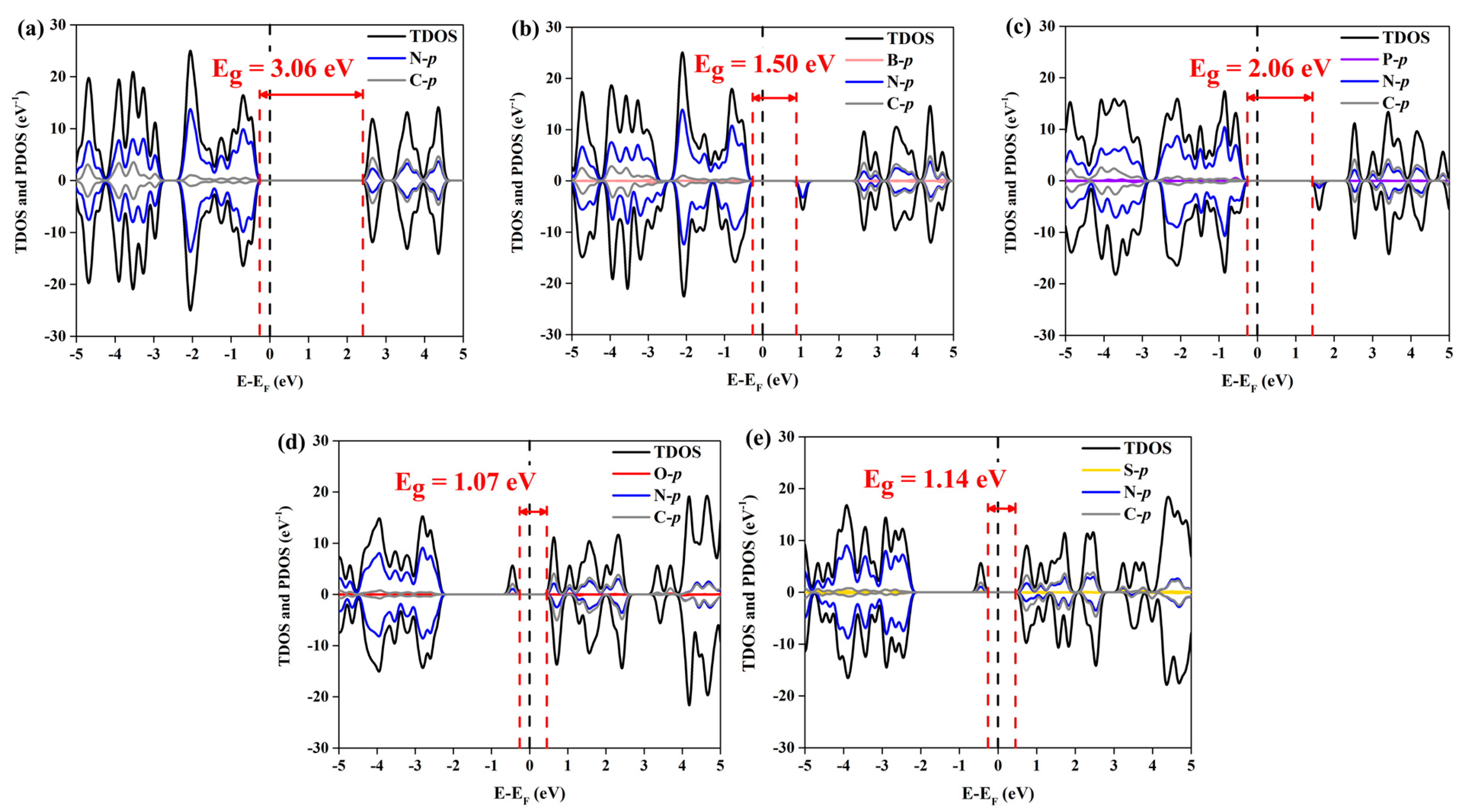




| Surfaces | Q(Non-Metal) | Q(N1/N2) | Q(C1/C2) | Band Gaps |
|---|---|---|---|---|
| g-C3N4 | - | −0.75/−0.89 | 1.22/1.09 | 3.06 |
| B-C3N4 | 1.58 | −0.87/−1.02 | - | 1.50 |
| P-C3N4 | 2.03 | −0.97/−1.22 | - | 2.06 |
| O-C3N4 | −0.76 | - | 1.18/1.24 | 1.07 |
| S-C3N4 | −0.05 | - | 0.86/1.05 | 1.14 |
| Catalysts | PLS | (V) | Product |
|---|---|---|---|
| *Pristine g-C3N4 | CO2 → *COOH | −0.84 | HCOOH, CH2O, CH3OH, and CH4 |
| *B-C3N4 | *COOH → *HCOOH | −1.93 | HCOOH, CH2O, CH3OH, and CH4 |
| *P-C3N4 | *COOH → *HCOOH | −0.73 | HCOOH and CH2O |
| *O-C3N4 | *COOH → *HCOOH | −0.12 | HCOOH |
| *S-C3N4 | CO2 → *COOH | −0.17 | HCOOH |
| *CHO → *CH2O | −0.58 | CH2O, CH3OH, and CH4 | |
| HSi-C3N4 [55] | *OCH2OH → *CH2O | −0.65 | CH4 |
| Ag@g-C3N4 [53] | *OCHO → *HCOOH | −0.37 | HCOOH |
| Cu@g-C3N4 [53] | *COOH → HCOOH | −0.37 | HCOOH |
| Co-C3N4 [26] | *CH2O → *CH2OH | −0.65 | CH3OH |
| Fe-C3N4 [26] | *HCOOH → *CHO | −0.91 | CH3OH and CH4 |
| Ni-C3N4 [26] | *HCOOH → * + HCOOH | −0.74 | HCOOH |
| BN-C3N [54] | CO2 → *HCOO | −0.28 | HCOOH |
| KBCN [56] | *CO2 → *COOH | −0.86 | CH3OH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongkonkan, W.; Takahashi, K.; Injongkol, Y.; Yodsin, N.; Namuangruk, S.; Jungsuttiwong, S. Exploring the Role of Non-Metal Doping in g-C3N4 for CO2 Reduction: A DFT Investigation. Catalysts 2025, 15, 553. https://doi.org/10.3390/catal15060553
Mongkonkan W, Takahashi K, Injongkol Y, Yodsin N, Namuangruk S, Jungsuttiwong S. Exploring the Role of Non-Metal Doping in g-C3N4 for CO2 Reduction: A DFT Investigation. Catalysts. 2025; 15(6):553. https://doi.org/10.3390/catal15060553
Chicago/Turabian StyleMongkonkan, Wassana, Kaito Takahashi, Yuwanda Injongkol, Nuttapon Yodsin, Supawadee Namuangruk, and Siriporn Jungsuttiwong. 2025. "Exploring the Role of Non-Metal Doping in g-C3N4 for CO2 Reduction: A DFT Investigation" Catalysts 15, no. 6: 553. https://doi.org/10.3390/catal15060553
APA StyleMongkonkan, W., Takahashi, K., Injongkol, Y., Yodsin, N., Namuangruk, S., & Jungsuttiwong, S. (2025). Exploring the Role of Non-Metal Doping in g-C3N4 for CO2 Reduction: A DFT Investigation. Catalysts, 15(6), 553. https://doi.org/10.3390/catal15060553







