Development of Cu3P/SnS2 Composite and Its High Efficiency Electrocatalytic Reduction of Carbon Dioxide
Abstract
1. Introduction
2. Results and Discussion
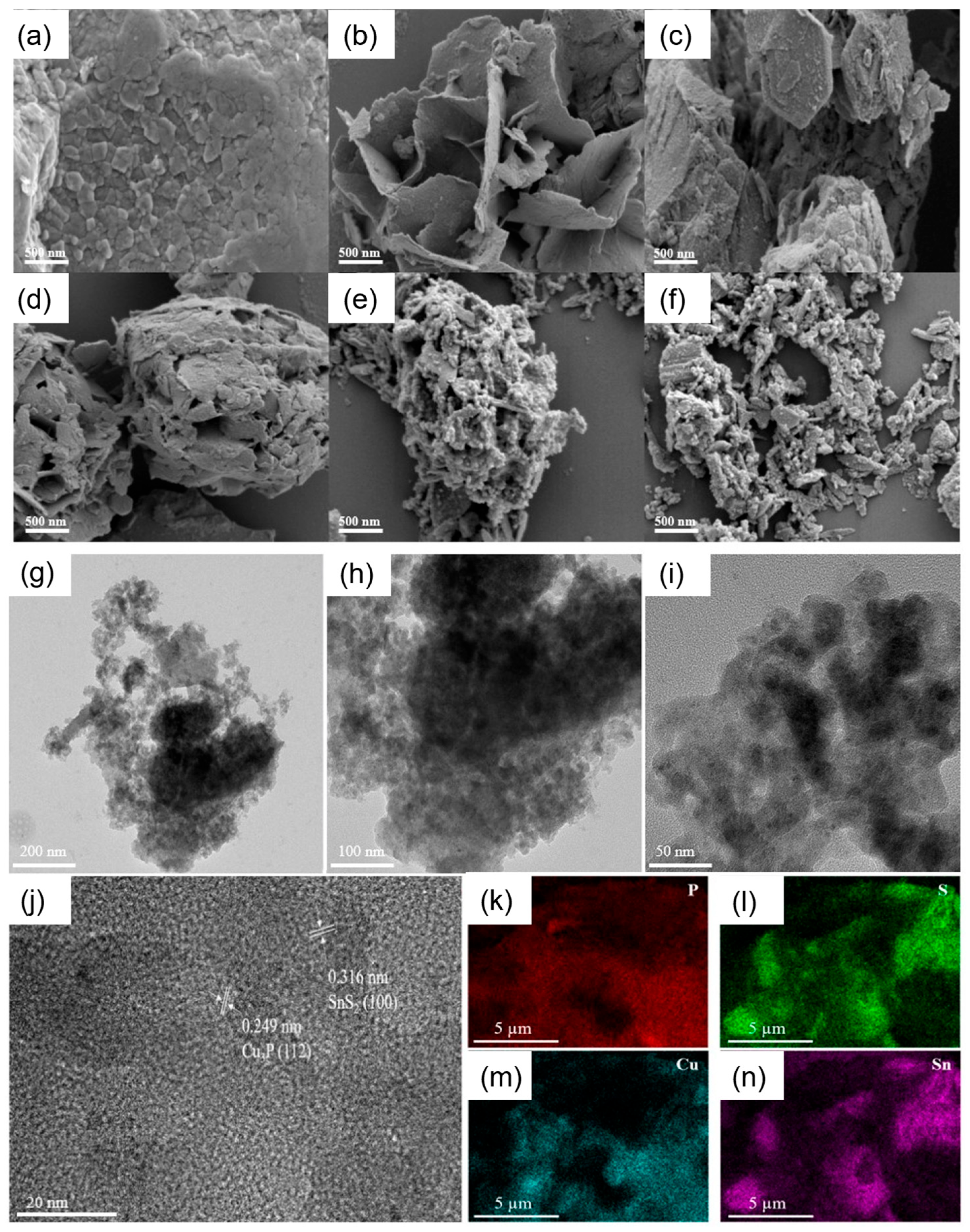
2.1. Synthesis and Characterization of Cu3P/SnS2 Catalysts
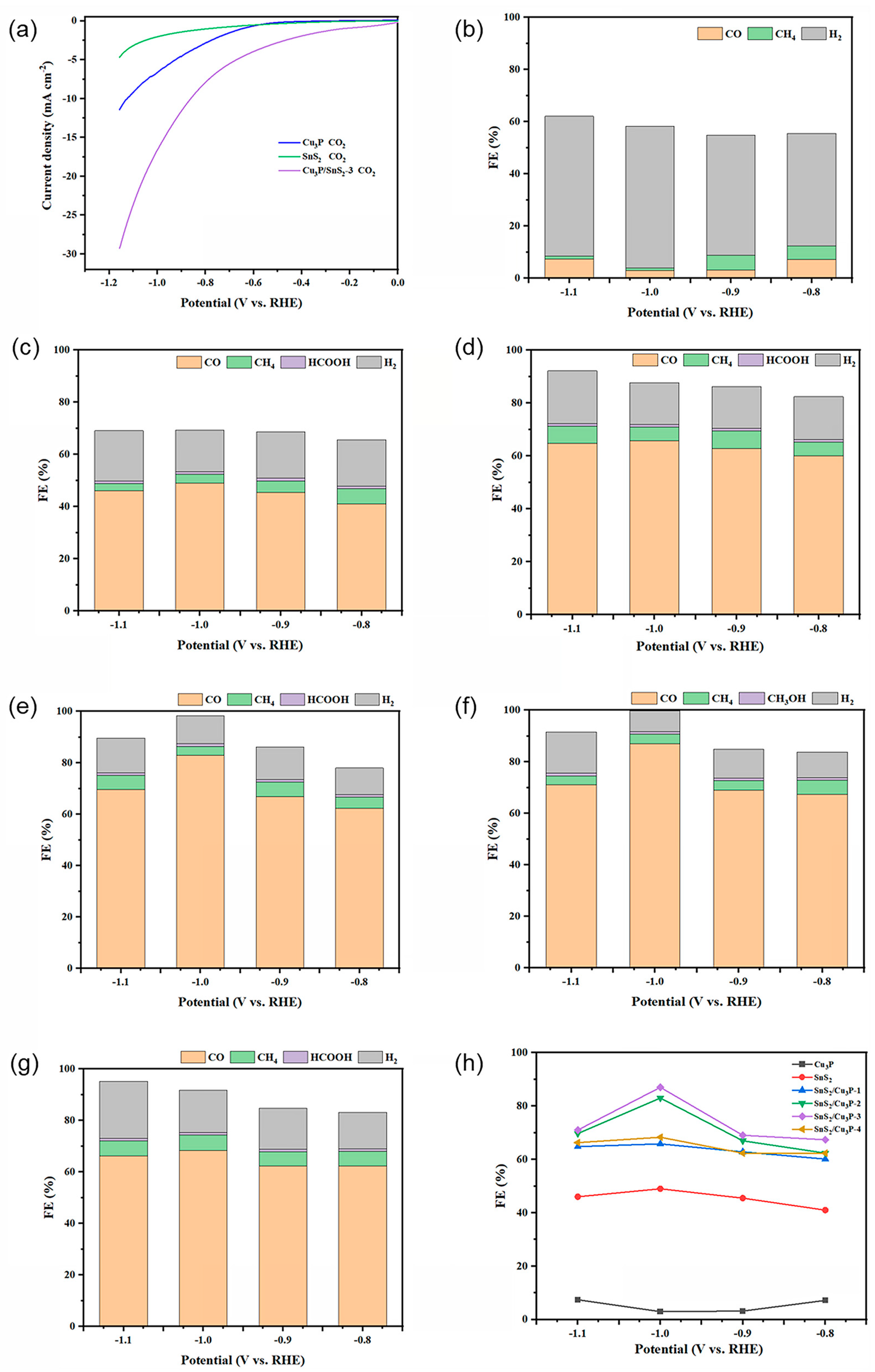
2.2. Electrochemical Performance of Cu3P/SnS2 Catalysts
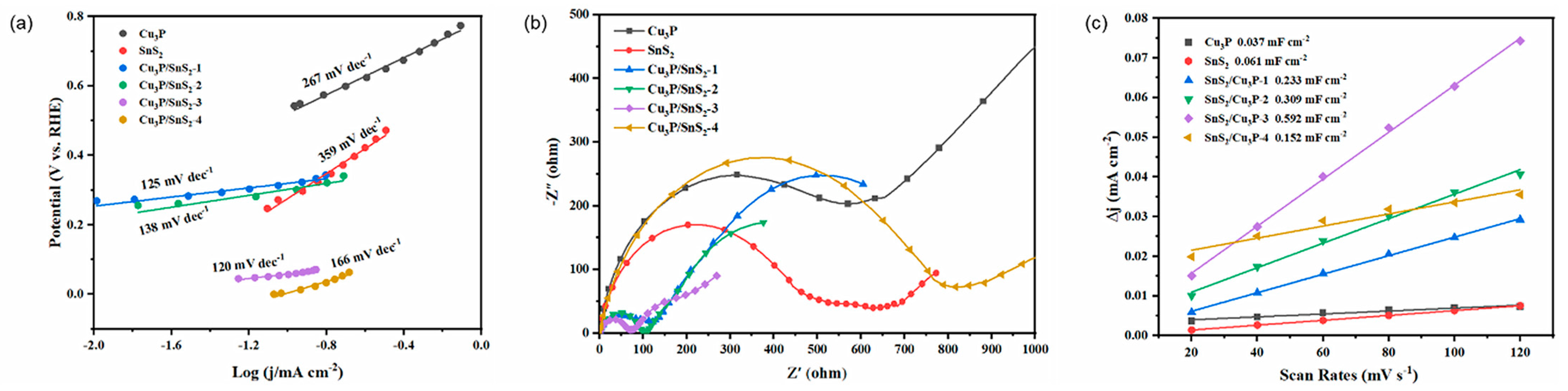
2.3. Mechanism Research of Cu3P/SnS2 Catalysts
3. Experimental Procedures
3.1. Materials
3.2. Synthesis of Cu3P, SnS2, and Cu3P/SnS2
3.3. Characterization
3.4. Electrochemical Measurements
3.5. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- ReferencesAlbo, J.; Alvarez-Guerra, M.; Castaño, P.; Irabien, A. Towards the electrochemical conversion of carbon dioxide into methanol. Green Chem. 2015, 17, 2304–2324. [Google Scholar] [CrossRef]
- Wang, G.X.; Chen, J.X.; Ding, Y.C.; Cai, P.W.; Yi, L.C.; Li, Y.; Tu, C.Y.; Hou, Y.; Wen, Z.H.; Dai, L.M. Electrocatalysis for CO2 conversion: From fundamentals to value-added products. Chem. Soc. Rev. 2021, 50, 4993–5061. [Google Scholar] [CrossRef] [PubMed]
- Kibria, M.G.; Edwards, J.P.; Gabardo, C.M.; Dinh, C.T.; Seifitokaldani, A.; Sinton, D.; Sargent, E.H. Electrochemical CO2 Reduction into Chemical Feedstocks: From Mechanistic Electrocatalysis Models to System Design. Adv. Mater. 2019, 31, 1807166. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Gao, P.; Li, S.G.; Bu, X.N.; Dang, S.S.; Liu, Z.Y.; Wang, H.; Zhong, L.S.; Qiu, M.H.; Yang, C.G.; Cai, J.; et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 2017, 9, 1019–1024. [Google Scholar] [CrossRef]
- Wang, C.L.; Sun, Z.X.; Zheng, Y.; Hu, Y.H. Recent progress in visible light photocatalytic conversion of carbon dioxide. J. Mater. Chem. A 2019, 7, 865–887. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Jung, H.S.; Kang, Y.T. A review: Effect of nanostructures on photocatalytic CO2 conversion over metal oxides and compound semiconductors. J. CO2 Util. 2017, 20, 163–177. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef]
- Kumar, B.; Brian, J.P.; Atla, V.; Kumari, S.; Bertram, K.A.; White, R.T.; Spurgeon, J.M. New trends in the development of heterogeneous catalysts for electrochemical CO2 reduction. Catal. Today 2016, 270, 19–30. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.Q.; Li, Y.F.; Chen, J.; Yu, B.; Wang, J.C.; Zhang, L.; Zhang, J.J. Energy related CO2 conversion and utilization: Advanced materials/nanomaterials, reaction mechanisms and technologies. Nano Energy 2017, 40, 512–539. [Google Scholar] [CrossRef]
- Hu, J.Y.; Liu, H.Z.; Han, B.X. Basic ionic liquids promoted chemical transformation of CO2 to organic carbonates. Sci. China Chem. 2018, 61, 1486–1493. [Google Scholar] [CrossRef]
- White, J.L.; Baruch, M.F.; Pander, J.E.; Hu, Y.; Fortmeyer, I.C.; Park, J.E.; Zhang, T.; Liao, K.; Gu, J.; Yan, Y.; et al. Light-Driven Heterogeneous Reduction of Carbon Dioxide: Photocatalysts and Photoelectrodes. Chem. Rev. 2015, 115, 12888–12935. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Yang, Y.S.; Wei, M. Structural Design and Performance of Electrocatalysts for Carbon Dioxide Reduction: A Review. Acta Chim. Sin. 2022, 80, 199–213. [Google Scholar] [CrossRef]
- Peterson, A.A.; Norskov, J.K. Activity Descriptors for CO2 Electroreduction to Methane on Transition-Metal Catalysts. J. Phys. Chem. Lett. 2012, 3, 251–258. [Google Scholar] [CrossRef]
- Jones, J.P.; Prakash, G.K.S.; Olah, G.A. Electrochemical CO2 Reduction: Recent Advances and Current Trends. Isr. J. Chem. 2014, 54, 1451–1466. [Google Scholar] [CrossRef]
- Ma, W.C.; He, X.Y.; Wang, W.; Xie, S.J.; Zhang, Q.H.; Wang, Y. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chem. Soc. Rev. 2021, 50, 12897–12914. [Google Scholar] [CrossRef]
- Wang, Y.F.; Han, P.; Lv, X.M.; Zhang, L.J.; Zheng, G.F. Defect and Interface Engineering for Aqueous Electrocatalytic CO2 Reduction. Joule 2018, 2, 2551–2582. [Google Scholar] [CrossRef]
- Zhang, W.J.; Hu, Y.; Ma, L.B.; Zhu, G.Y.; Wang, Y.R.; Xue, X.L.; Chen, R.P.; Yang, S.Y.; Jin, Z. Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef]
- Song, Y.F.; Chen, W.; Zhao, C.C.; Li, S.G.; Wei, W.; Sun, Y.H. Metal-Free Nitrogen-Doped Mesoporous Carbon for Electroreduction of CO2 to Ethanol. Angew. Chem. Int. Ed. 2017, 56, 10840–10844. [Google Scholar] [CrossRef]
- Sarfraz, S.; Garcia-Esparza, A.T.; Jedidi, A.; Cavallo, L.; Takanabe, K. Cu-Sn Bimetallic Catalyst for Selective Aqueous Electroreduction of CO2 to CO. ACS Catal. 2016, 6, 2842–2851. [Google Scholar] [CrossRef]
- Huang, J.; Dai, J.W.; Zhu, J.N.; Chen, R.; Fu, X.Q.; Liu, H.F.; Li, G.F. Bimetallic Au-Cu gradient alloy for electrochemical CO2 reduction into C2H4 at low overpotential. J. Catal. 2022, 415, 134–141. [Google Scholar] [CrossRef]
- Fu, Y.; Xie, Q.X.; Wu, L.X.; Luo, J.S. Crystal facet effect induced by different pretreatment of Cu2O nanowire electrode for enhanced electrochemical CO2 reduction to C2+ products. Chin. J. Catal. 2022, 43, 1066–1073. [Google Scholar] [CrossRef]
- Jiang, X.C.; Herricks, T.; Xia, Y.N. CuO nanowires can be synthesized by heating copper substrates in air. Nano Lett. 2002, 2, 1333–1338. [Google Scholar] [CrossRef]
- Ye, J.Q.; Yan, J.P.; Peng, Y.L.; Li, F.W.; Sun, J. Metal-organic framework-based single-atom catalysts for efficient electrocatalytic CO2 reduction reactions. Catal. Today 2023, 410, 68–84. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Sun, T.T.; Zhang, P.P.; Liu, K.L.; Li, F.L.; Xu, L.B. Synthesizing MOF-derived Ni-N-C catalyst via surfactant modified strategy for efficient electrocatalytic CO2 to CO. J. Colloid Interface Sci. 2023, 631, 96–101. [Google Scholar] [CrossRef]
- Sha, Y.F.; Zhang, J.L.; Cheng, X.Y.; Xu, M.Z.; Su, Z.Z.; Wang, Y.Y.; Hu, J.Y.; Han, B.X.; Zheng, L.R. Anchoring Ionic Liquid in Copper Electrocatalyst for Improving CO2 Conversion to Ethylene. Angew. Chem. Int. Ed. 2022, 61, e202200039. [Google Scholar] [CrossRef]
- Feaster, J.T.; Shi, C.; Cave, E.R.; Hatsukade, T.T.; Abram, D.N.; Kuhl, K.P.; Hahn, C.; Norskov, J.K.; Jaramillo, T.F. Understanding Selectivity for the Electrochemical Reduction of Carbon Dioxide to Formic Acid and Carbon Monoxide on Metal Electrodes. ACS Catal. 2017, 7, 4822–4827. [Google Scholar] [CrossRef]
- Yadav, V.S.K.; Noh, Y.; Han, H.; Kim, W.B. Synthesis of Sn catalysts by solar electro-deposition method for electrochemical CO2 reduction reaction to HCOOH. Catal. Today 2018, 303, 276–281. [Google Scholar] [CrossRef]
- He, J.; Liu, X.J.; Liu, H.X.; Zhao, Z.; Ding, Y.; Luo, J. Highly selective electrocatalytic reduction of CO2 to formate over Tin(IV) sulfide monolayers. J. Catal. 2018, 364, 125–130. [Google Scholar] [CrossRef]
- Ren, H.; Wang, X.; Zhou, X.; Wang, T.; Liu, Y.; Wang, C.; Guan, Q.; Li, W. In-situ constructing Cu1Bi1 bimetallic catalyst to promote the electroreduction of CO2 to formate by synergistic electronic and geometric effects. J. Energy Chem. 2023, 79, 263–271. [Google Scholar] [CrossRef]
- Li, M.; Hu, Y.; Dong, G.; Wu, T.C.; Geng, D.S. Achieving Tunable Selectivity and Activity of CO2 Electroreduction to CO via Bimetallic Silver-Copper Electronic Engineering. Small 2023, 19, 2207242. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Z.; An, H.; Arnouts, S.; de Ruiter, J.; Rollier, F.; Bals, S.; Altantzis, T.; Figueiredo, M.C.; Filot, I.A.W.; et al. Near-Unity Electrochemical CO2 to CO Conversion over Sn-Doped Copper Oxide Nanoparticles. ACS Catal. 2022, 12, 15146–15156. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.W.; Huang, M.; Zhao, X.L.; Mou, S.Y.; Dong, F. Interfacial Electrolyte Effects on Electrocatalytic CO2 Reduction. ACS Catal. 2022, 12, 331–362. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Wang, Z.; Shi, H.; Zuo, Y.; Jin, J. Development of Cu3P/SnS2 Composite and Its High Efficiency Electrocatalytic Reduction of Carbon Dioxide. Catalysts 2025, 15, 552. https://doi.org/10.3390/catal15060552
Wei H, Wang Z, Shi H, Zuo Y, Jin J. Development of Cu3P/SnS2 Composite and Its High Efficiency Electrocatalytic Reduction of Carbon Dioxide. Catalysts. 2025; 15(6):552. https://doi.org/10.3390/catal15060552
Chicago/Turabian StyleWei, Haohong, Zhangwei Wang, Huancong Shi, Yuanhui Zuo, and Jing Jin. 2025. "Development of Cu3P/SnS2 Composite and Its High Efficiency Electrocatalytic Reduction of Carbon Dioxide" Catalysts 15, no. 6: 552. https://doi.org/10.3390/catal15060552
APA StyleWei, H., Wang, Z., Shi, H., Zuo, Y., & Jin, J. (2025). Development of Cu3P/SnS2 Composite and Its High Efficiency Electrocatalytic Reduction of Carbon Dioxide. Catalysts, 15(6), 552. https://doi.org/10.3390/catal15060552







