Facile Synthesis of the Single-Atom Decorated Cox-MoS2/RGO Catalysts by Thermal-Annealing Vacancy-Filling Strategy for Highly Efficient Hydrogen Evolution
Abstract
1. Introduction
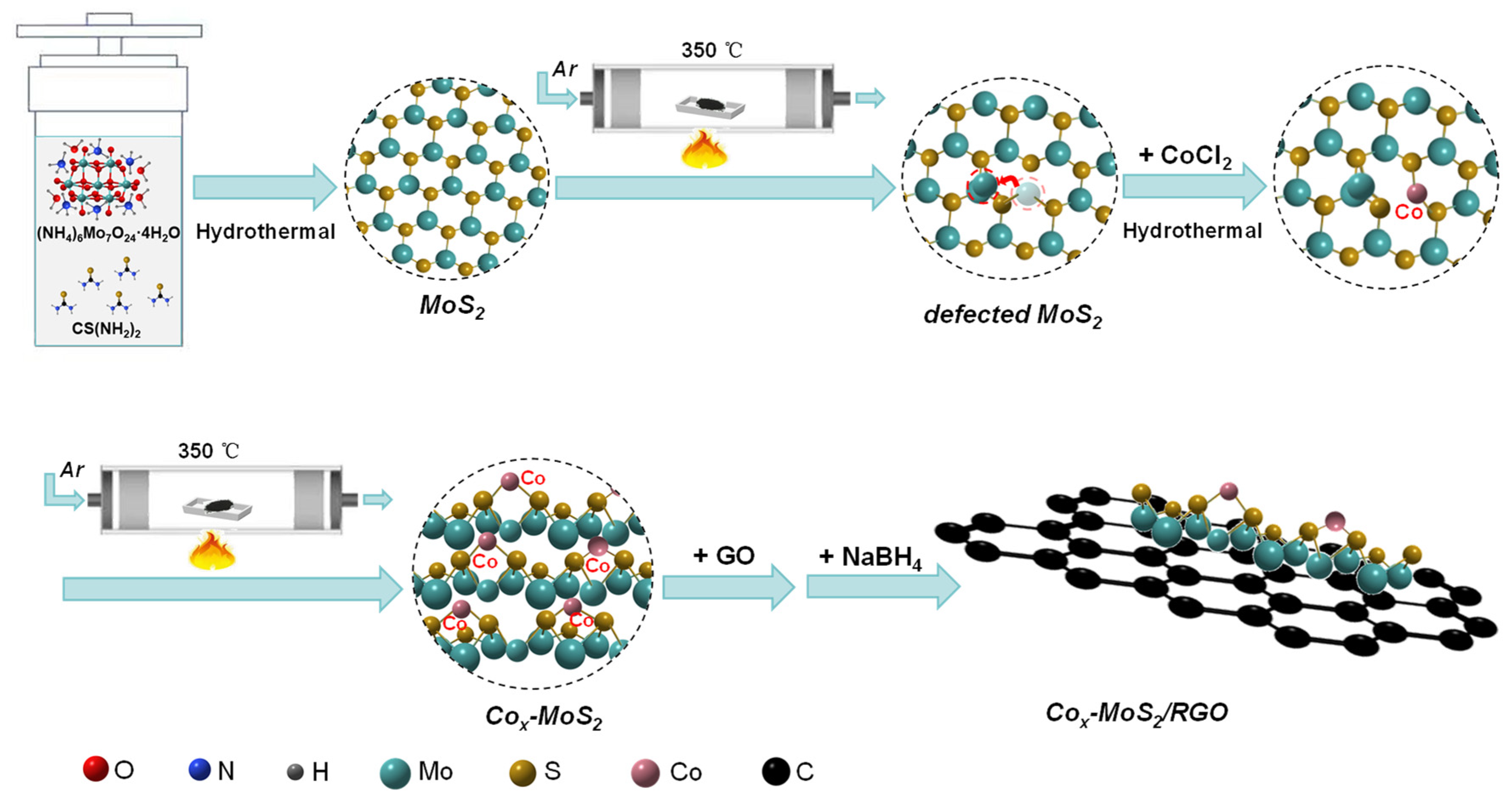
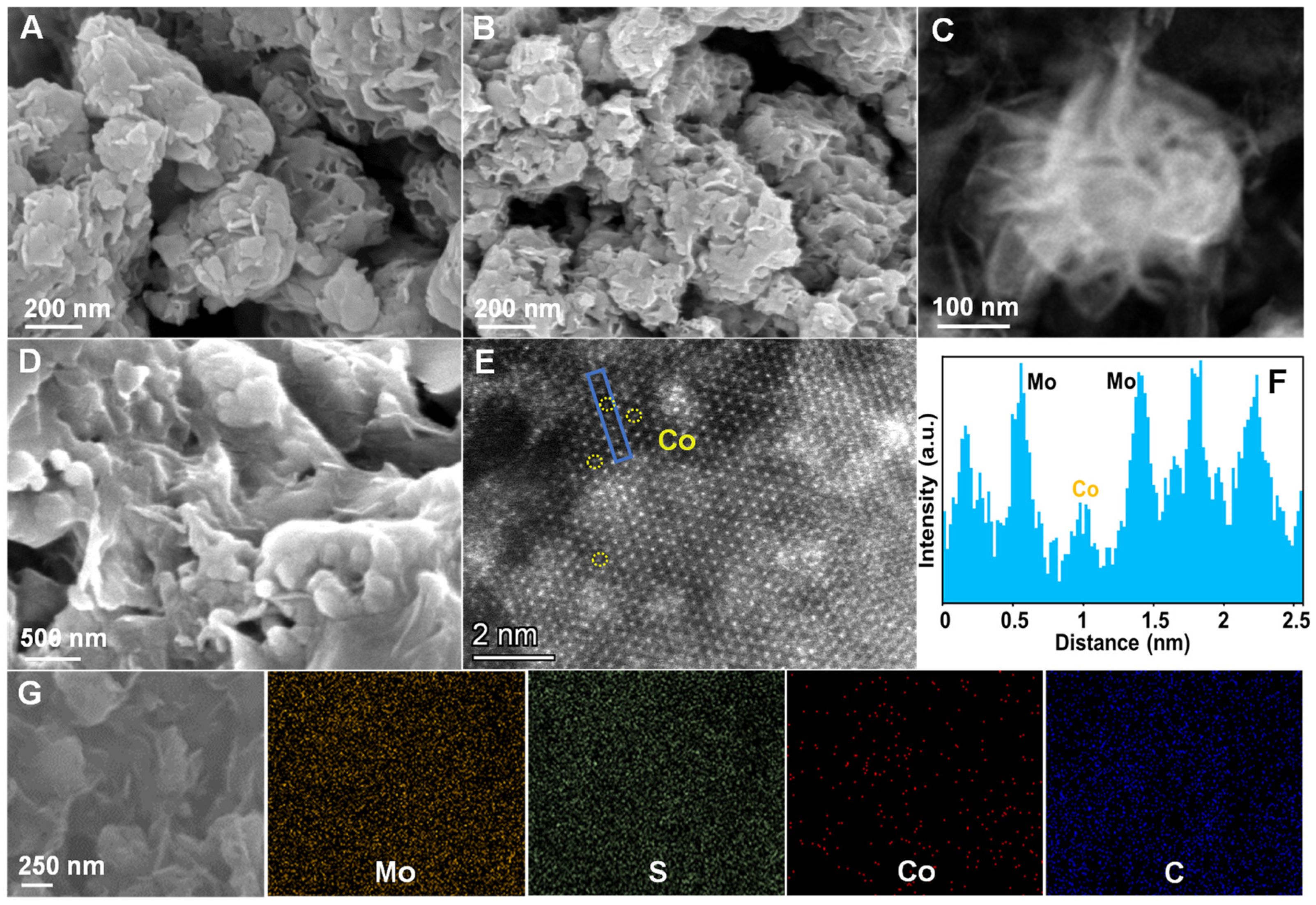
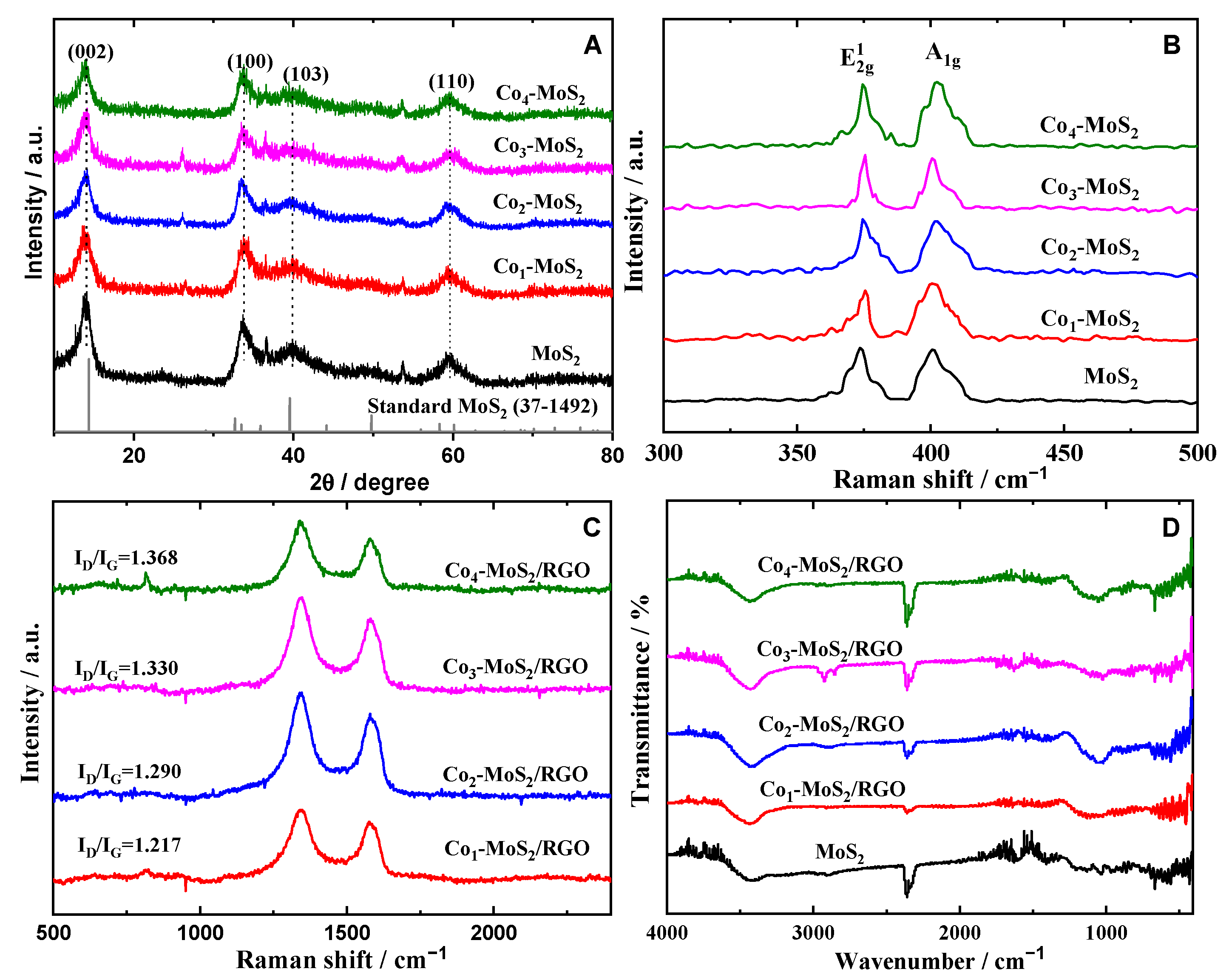
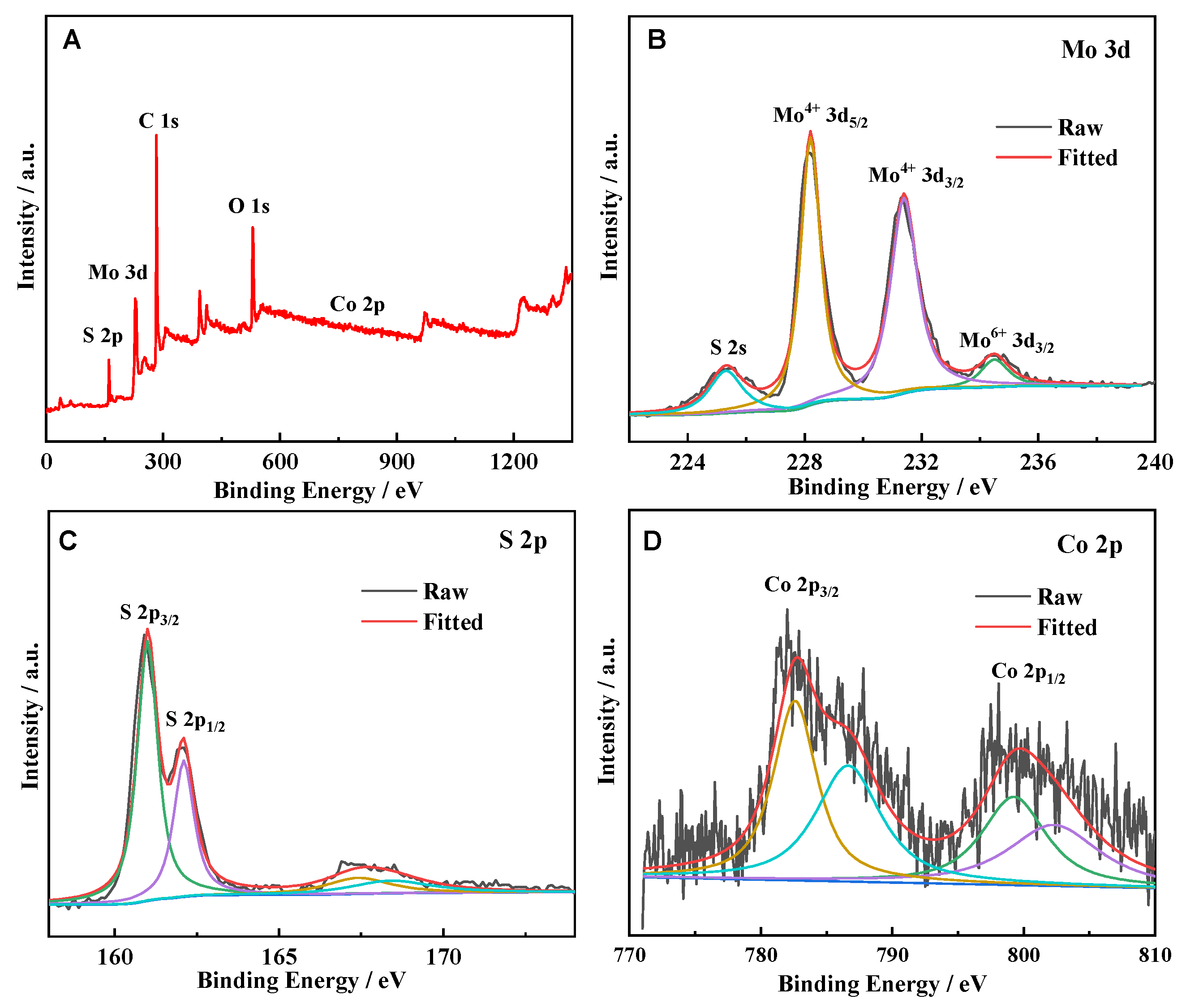
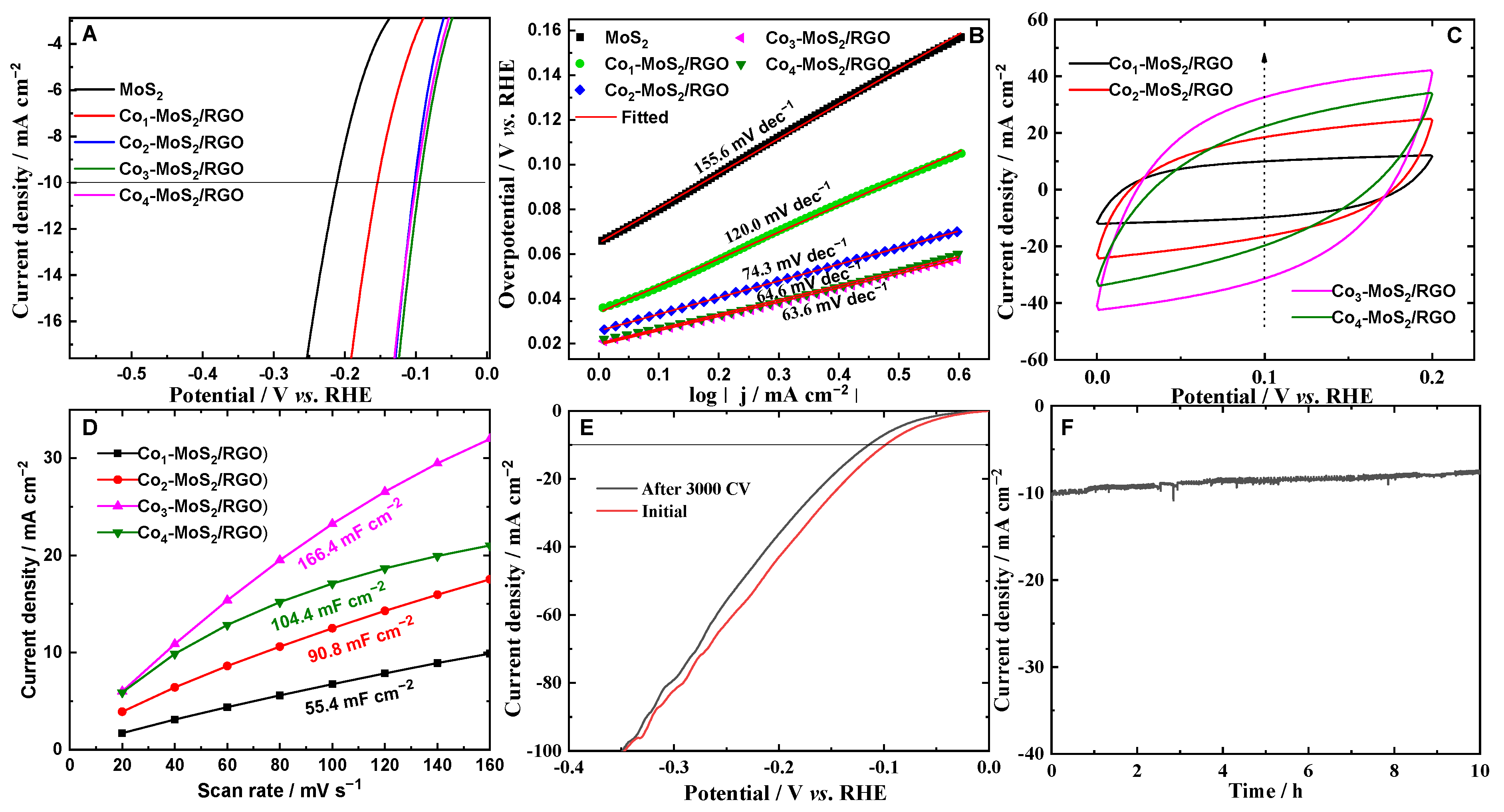
2. Results and Discussion
3. Experimental
3.1. Materials
3.2. Synthesis of Flower-like Spherical MoS2
3.3. Synthesis of Cox-MoS2
3.4. Synthesis of Cox-MoS2/RGO
3.5. Characterization
3.6. Raman Measurements
3.7. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Mi, Z. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Ueckerdt, F.; Verpoort, P.C.; Anantharaman, R.; Bauer, C.; Beck, F.; Longden, T.; Roussanaly, S. On the cost competitiveness of blue and green hydrogen. Joule 2024, 8, 104–128. [Google Scholar] [CrossRef]
- Yu, H.; Díaz, A.; Lu, X.; Sun, B.; Ding, Y.; Koyama, M.; He, J.; Zhou, X.; Oudriss, A.; Feaugas, X.; et al. Hydrogen Embrittlement as a Conspicuous Material Challenge-Comprehensive Review and Future Directions. Chem. Rev. 2024, 124, 6271–6392. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Osterloh, F.E.; Wang, X.; Mallouk, T.E.; Maeda, K. Photocatalytic water splitting. Nat. Rev. Method. Primers 2023, 3, 42. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhou, X.; Jiang, H.; Yang, H.; Li, C. Positively charged Pt-based cocatalysts: An orientation for achieving efficient photocatalytic water splitting. J. Mater. Chem. A 2020, 8, 17–26. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, W.; Xing, L.; Dong, Z.; Yang, Y.; Du, L.; Xie, X.; Ye, S. Keys to Unravel the Stability/Durability Issues of Platinum-Group-Metal Catalysts toward Oxygen Evolution Reaction for Acidic Water Splitting. ACS Cent. Sci. 2024, 10, 2006–2015. [Google Scholar] [CrossRef]
- Yang, R.; Fan, Y.; Zhang, Y.; Mei, L.; Zhu, R.; Qin, J.; Hu, J.; Chen, Z.; Ng, Y.N.; Voiry, D.; et al. 2D Transition Metal Dichalcogenides for Photocatalysis. Angew. Chem. Int. Ed. 2023, 62, 107519. [Google Scholar]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D Transition-Metal-Dichalcogenide-Nanosheet-Based Composites for Photocatalytic and Electrocatalytic Hydrogen Evolution Reactions. Adv. Mater. 2015, 28, 1917–1933. [Google Scholar] [CrossRef]
- Wang, F.; Shifa, T.A.; Zhan, X.; Huang, Y.; Liu, K.; Cheng, Z.; Jiang, C.; He, J. Recent advances in transition-metal dichalcogenide based nanomaterials for water splitting. Nanoscale 2015, 7, 19764–19788. [Google Scholar] [CrossRef]
- He, Y.; Tang, P.; Hu, Z.; He, Q.; Zhu, C.; Wang, L.; Zeng, Q.; Golani, P.; Gao, G.; Fu, W.; et al. Engineering grain boundaries at the 2D limit for the hydrogen evolution reaction. Nat. Commun. 2020, 11, 57. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Chen, L.; Yarley, O.P.N.; Zhou, C. Facile preparation of sugarcane bagasse-derived carbon supported MoS2 nanosheets for hydrogen evolution reaction. Ind. Crops Prod. 2021, 172, 114064. [Google Scholar] [CrossRef]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; El Moutaouakil, A. A Review on MoS2 Properties, Synthesis, Sensing Applications and Challenges. Crystals 2021, 11, 355. [Google Scholar] [CrossRef]
- Liang, N.; Hu, X.; Li, W.; Wang, Y.; Guo, Z.; Huang, X.; Li, Z.; Zhang, X.; Zhang, J.; Xiao, J.; et al. A dual-signal fluorescent sensor based on MoS2 and CdTe quantum dots for tetracycline detection in milk. Food Chem. 2022, 378, 132076. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Zhu, G.; Shen, X.; Kong, L.; Ji, Z.; Xu, K.; Zhou, H.; Zhu, J.; Song, P.; Song, C.; et al. Controllable Sandwiching of Reduced Graphene Oxide in Hierarchical Defect-Rich MoS2 Ultrathin Nanosheets with Expanded Interlayer Spacing for Electrocatalytic Hydrogen Evolution Reaction. Adv. Mater. Interfaces 2018, 5, 1801093. [Google Scholar] [CrossRef]
- He, Q.; Ye, N.; Han, L.; Tao, K. Sulfur Vacancy-Engineered Co3S4/MoS2-Interfaced Nanosheet Array for Enhanced Alkaline Overall Water Splitting. Inorg. Chem. 2023, 62, 21240–21246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wei, S.; Wang, F.; Xu, L.; Liu, Y.; Lin, J.; Pan, K.; Pang, H. Hatted 1T/2H-Phase MoS2 on Ni3S2 Nanorods for Efficient Overall Water Splitting in Alkaline Media. Chem. Eur. J. 2020, 26, 2034–2040. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, B.; Wu, S.; Wang, M.; Zhang, Z.; Cui, B.; He, L.; Du, M. Hierarchical nanocomposite electrocatalyst of bimetallic zeolitic imidazolate framework and MoS2 sheets for non-Pt methanol oxidation and water splitting. Appl. Catal. B 2019, 258, 117970. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Z.; Liu, W.; Zhang, F.; Yan, H. Novel Bifunctional Nitrogen Doped MoS2/COF-C4N Vertical Heterostructures for Electrocatalytic HER and OER. Catalysts 2023, 13, 90. [Google Scholar] [CrossRef]
- Lin, J.; Wang, P.; Wang, H.; Li, C.; Si, X.; Qi, J.; Cao, J.; Zhong, Z.; Fei, W.; Feng, J. Defect-Rich Heterogeneous MoS2/NiS2 Nanosheets Electrocatalysts for Efficient Overall Water Splitting. Adv. Sci. 2019, 6, 1900246. [Google Scholar] [CrossRef]
- Xie, J.; Qu, H.; Xin, J.; Zhang, X.; Cui, G.; Zhang, X.; Bao, J.; Tang, B.; Xie, Y. Defect-rich MoS2 nanowall catalyst for efficient hydrogen evolution reaction. Nano Res. 2017, 10, 1178–1188. [Google Scholar] [CrossRef]
- Tang, T.; Wang, Z.; Guan, J. A review of defect engineering in two-dimensional materials for electrocatalytic hydrogen evolution reaction. Chin. J. Catal. 2022, 43, 636–678. [Google Scholar] [CrossRef]
- Xu, J.; Shao, G.; Tang, X.; Lv, F.; Xiang, H.; Jing, C.; Liu, S.; Dai, S.; Li, Y.; Luo, J.; et al. Frenkel-defected monolayer MoS2 catalysts for efficient hydrogen evolution. Nat. Commun. 2022, 13, 2193. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, S.; Zhang, L.; Huang, H.; Zhang, Q.; Ge, W.; Wang, S.; Tan, T.; Huang, L.; An, Q. Stabilizing and Activating Active Sites: 1T-MoS2 Supported Pd Single Atoms for Efficient Hydrogen Evolution Reaction. Small 2024, 20, 2401537. [Google Scholar] [CrossRef]
- Politano, G.G. Optimizing Graphene Oxide Film Quality: The Role of Solvent and Deposition Technique. C 2024, 10, 90. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, Z.; Xi, S.; Wang, W.; Lu, J.; Chen, P. Graphene quantum dot enabled interlayer spacing and electronic structure regulation of single-atom doped MoS2 for efficient alkaline hydrogen evolution. Chem. Eng. J. 2023, 451, 138951. [Google Scholar] [CrossRef]
- Koudakan, P.A.; Wei, C.; Mosallanezhad, A.; Liu, B.; Fang, Y.; Hao, X.; Qian, Y.; Wang, G. Constructing Reactive Micro-Environment in Basal Plane of MoS2 for pH-Universal Hydrogen Evolution Catalysis. Small 2022, 18, 2107974. [Google Scholar] [CrossRef]
- Politano, G.G.; Castriota, M.; Santo, M.P.D.; Pipita, M.M.; Desiderio, G.; Vena, C.; Versace, C. Variable angle spectroscopic ellipsometry characterization of spin-coated MoS2 films. Vacuum 2021, 189, 110232. [Google Scholar] [CrossRef]
- Liang, J.; Li, H.; Chen, L.; Ren, M.; Fakayode, O.A.; Han, J.; Zhou, C. Efficient hydrogen evolution reaction performance using lignin-assisted chestnut shell carbon-loaded molybdenum disulfide. Ind. Crops Prod. 2022, 193, 116214. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Wang, X.; Guo, Y.; Ni, C.; Wu, J.; Hao, C. High performance hybrid supercapacitors assembled with multi-cavity nickel cobalt sulfide hollow microspheres as cathode and porous typha-derived carbon as anode. Ind. Crops Prod. 2022, 189, 115863. [Google Scholar] [CrossRef]
- Wu, Q.; Zhong, Y.; Chen, R.; Ling, G.; Wang, X. Cu-Ag-C@Ni3S4 with core shell structure and rose derived carbon electrode materials: An environmentally friendly supercapacitor with high energy and power density. Ind. Crops Prod. 2024, 222, 119676. [Google Scholar] [CrossRef]
- Pearson, A.; O’Mullane, A.P. A simple approach to improve the electrocatalytic properties of commercial Pt/C. Chem. Commun. 2015, 51, 11297–11300. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, X.; Sa, R.; Zhang, S.; Wen, Z.; Wang, R. The Electrochemical Overall Water Splitting Promoted by MoS2 in Coupled Nickel–Iron (Oxy)Hydride/Molybdenum Sulfide/Graphene Composite. Chem. Eng. J. 2020, 397, 125454. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Liu, J.; Cao, F.; Cong, H.; Li, H. 1D Core–Shell MOFs Derived CoP Nanoparticles-Embedded N-Doped Porous Carbon Nanotubes Anchored with MoS2 Nanosheets as Efficient Bifunctional Electrocatalysts. Chem. Eng. J. 2021, 419, 129977. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, G.; Yang, T.; Chen, C.; Chang, Z.; Kong, F.; Tian, H.; Cui, X.; Hou, X.; Shi, J. Interfacial engineering of Co-doped 1T-MoS2 coupled with V2C MXene for efficient electrocatalytic hydrogen evolution. Chem. Eng. J. 2022, 450, 138157. [Google Scholar] [CrossRef]
- Kim, M.; Seok, H.; Clament Sagaya Selvam, N.; Cho, J.; Choi, G.H.; Nam, M.G.; Kang, S.; Kim, T.; Yoo, P.J. Kirkendall Effect Induced Bifunctional Hybrid Electrocatalyst (Co9S8@MoS2/N-Doped Hollow Carbon) for High Performance Overall Water Splitting. J. Power Sources 2021, 493, 229688. [Google Scholar] [CrossRef]
- Ma, W.; Li, W.; Zhang, H.; Wang, Y. N-doped carbon wrapped CoFe alloy nanoparticles with MoS2 nanosheets as electrocatalyst for hydrogen and oxygen evolution reactions. Int. J. Hydrogen Energy 2023, 48, 22032–22043. [Google Scholar] [CrossRef]
- Li, D.; Fan, S.; Li, J.; Li, W.; Zhuang, Y.; Li, Y.; Shan, L.; Dong, L.; Yao, J. Preparation and characterization of bifunctional 1T-2H MoS2-Sv/CuS catalyst for electrocatalytic hydrogen and oxygen evolution reaction. Sep. Purif. Technol. 2025, 352, 128176. [Google Scholar] [CrossRef]
- Liu, Q.; Xue, Z.; Jia, B.; Liu, Q.; Liu, K.; Lin, Y.; Liu, M.; Li, Y.; Li, G. Hierarchical Nanorods of MoS2/MoP Heterojunction for Efficient Electrocatalytic Hydrogen Evolution Reaction. Small 2020, 16, 2002482. [Google Scholar] [CrossRef]
- Luo, M.; Liu, S.; Zhu, W.; Ye, G.; Wang, J.; He, Z. An Electrodeposited MoS2-MoO3-x/Ni3S2 Heterostructure Electrocatalyst for Efficient Alkaline Hydrogen Evolution. Chem. Eng. J. 2022, 428, 131055. [Google Scholar] [CrossRef]
- Weng, Z.; Zhu, J.; Lu, L.; Ma, Y.; Cai, J. Regulation of the electric double-layer capacitance of MoS2/ionic liquid by carbon modification. J. Appl. Electrochem. 2023, 53, 689–703. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, G.; Li, Y.; Chu, W.; Wang, L. Transition-metal single atoms in nitrogen-doped graphenes as efficient active centers for water splitting: A theoretical study. Phys. Chem. Chem. Phys. 2019, 21, 3024–3032. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Li, W.; Aimeti, A.-A.; Liu, X.; Nie, J.; Wang, S.; Fu, X. Facile Synthesis of the Single-Atom Decorated Cox-MoS2/RGO Catalysts by Thermal-Annealing Vacancy-Filling Strategy for Highly Efficient Hydrogen Evolution. Catalysts 2025, 15, 524. https://doi.org/10.3390/catal15060524
Yang J, Li W, Aimeti A-A, Liu X, Nie J, Wang S, Fu X. Facile Synthesis of the Single-Atom Decorated Cox-MoS2/RGO Catalysts by Thermal-Annealing Vacancy-Filling Strategy for Highly Efficient Hydrogen Evolution. Catalysts. 2025; 15(6):524. https://doi.org/10.3390/catal15060524
Chicago/Turabian StyleYang, Jiang, Wentao Li, Abdul-Aziz Aimeti, Xinyu Liu, Jiaqi Nie, Shuang Wang, and Xiaoqi Fu. 2025. "Facile Synthesis of the Single-Atom Decorated Cox-MoS2/RGO Catalysts by Thermal-Annealing Vacancy-Filling Strategy for Highly Efficient Hydrogen Evolution" Catalysts 15, no. 6: 524. https://doi.org/10.3390/catal15060524
APA StyleYang, J., Li, W., Aimeti, A.-A., Liu, X., Nie, J., Wang, S., & Fu, X. (2025). Facile Synthesis of the Single-Atom Decorated Cox-MoS2/RGO Catalysts by Thermal-Annealing Vacancy-Filling Strategy for Highly Efficient Hydrogen Evolution. Catalysts, 15(6), 524. https://doi.org/10.3390/catal15060524





