Zinc Indium Sulfide Materials for Photocatalytic Hydrogen Production via Water Splitting: A Short Review
Abstract
1. Introduction
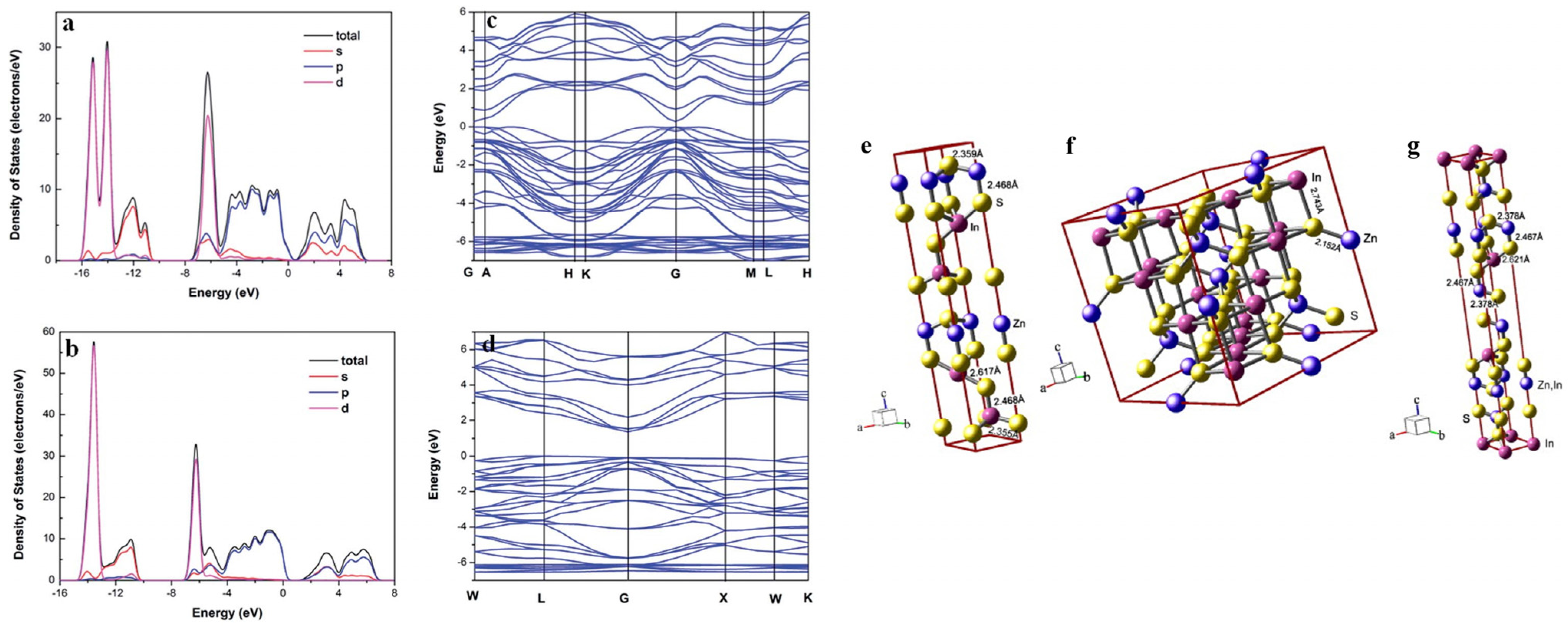
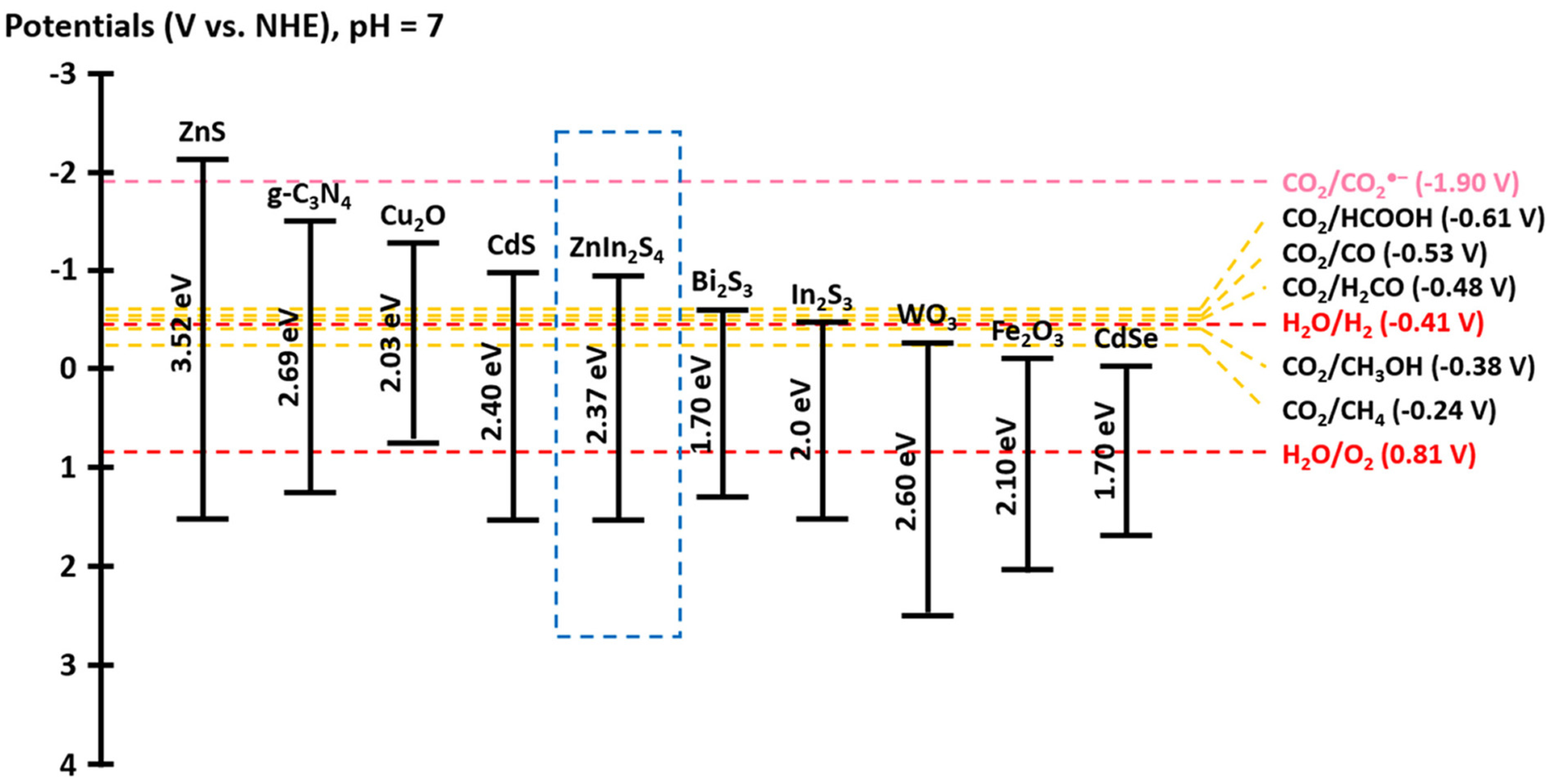
2. Fundamental Properties of ZnIn2S4
3. The Principles of H2 Generation via Water Splitting
4. Hydrogen Generation of ZnIn2S4-Based Photocatalysts
4.1. Modification of ZnIn2S4
4.2. Heterojunctions of Photocatalysis
4.2.1. Type-I Heterojunction
4.2.2. Type-II Heterojunction
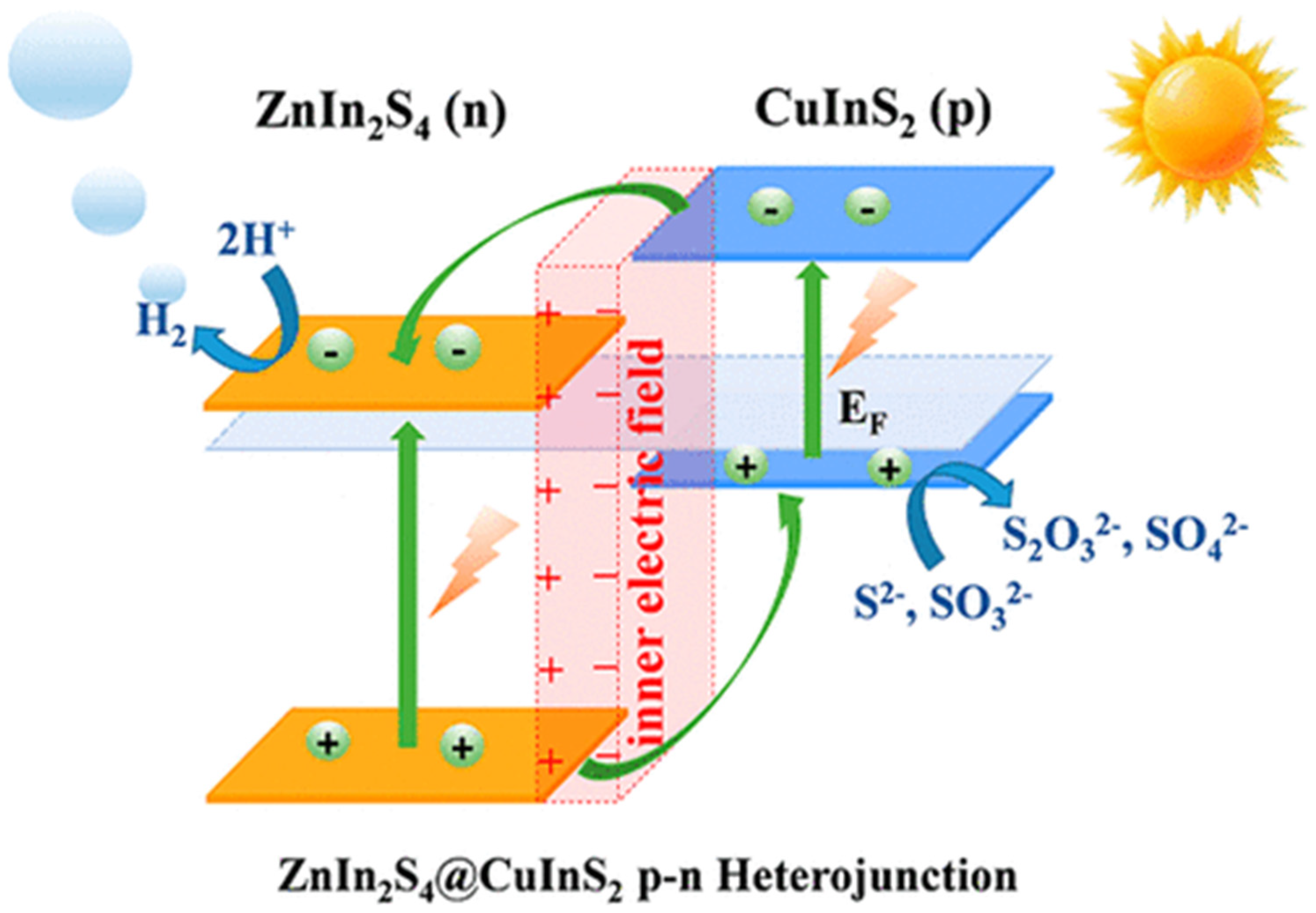
4.2.3. p–n Junction
4.2.4. S-Scheme Heterojunction Photocatalysts
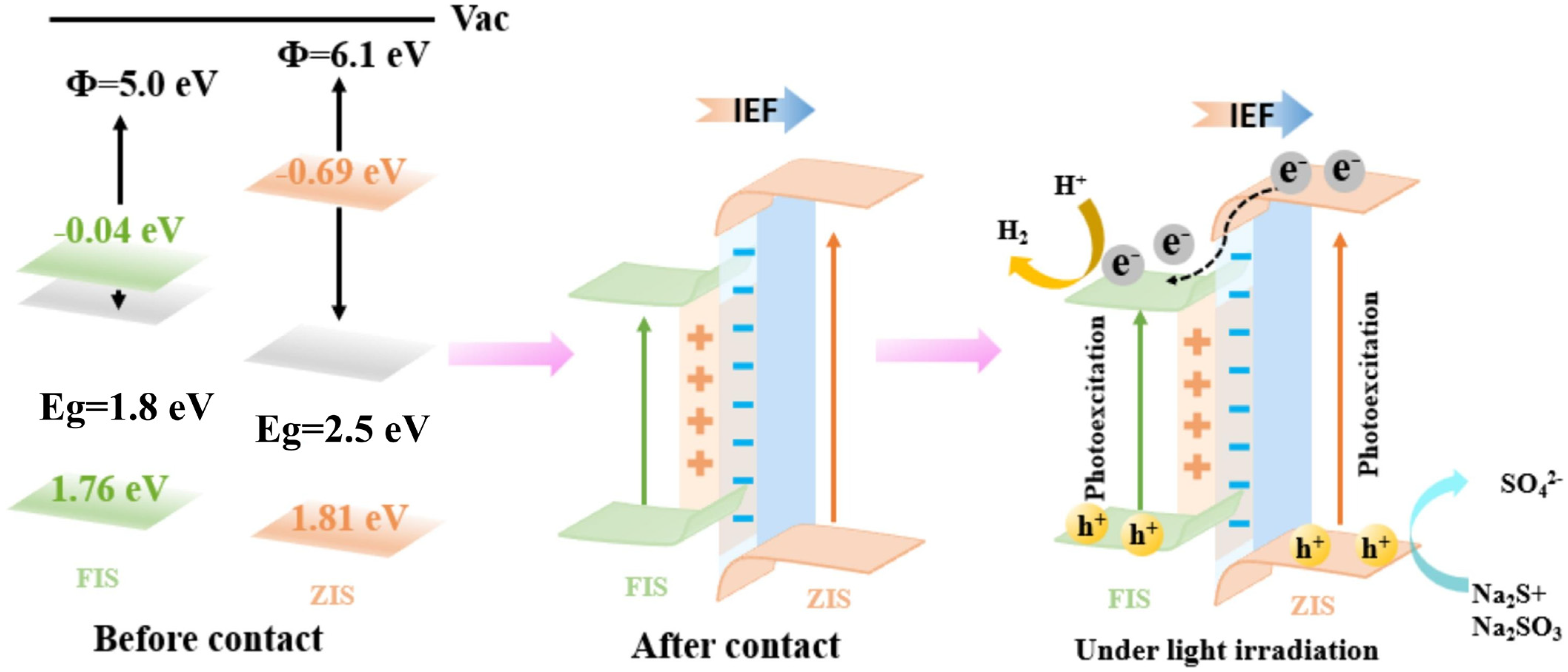
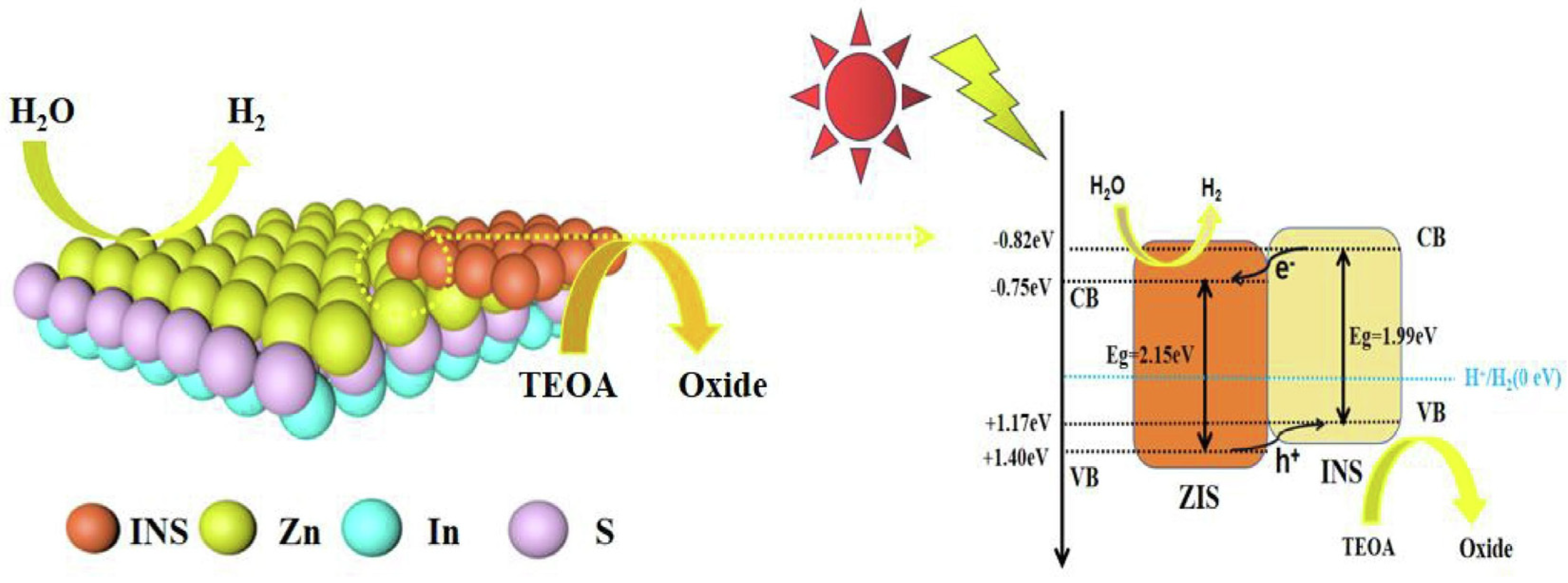
4.2.5. Z-Scheme Heterojunction Photocatalysts
4.3. Metal and Non-Metal-Doped ZnIn2S4
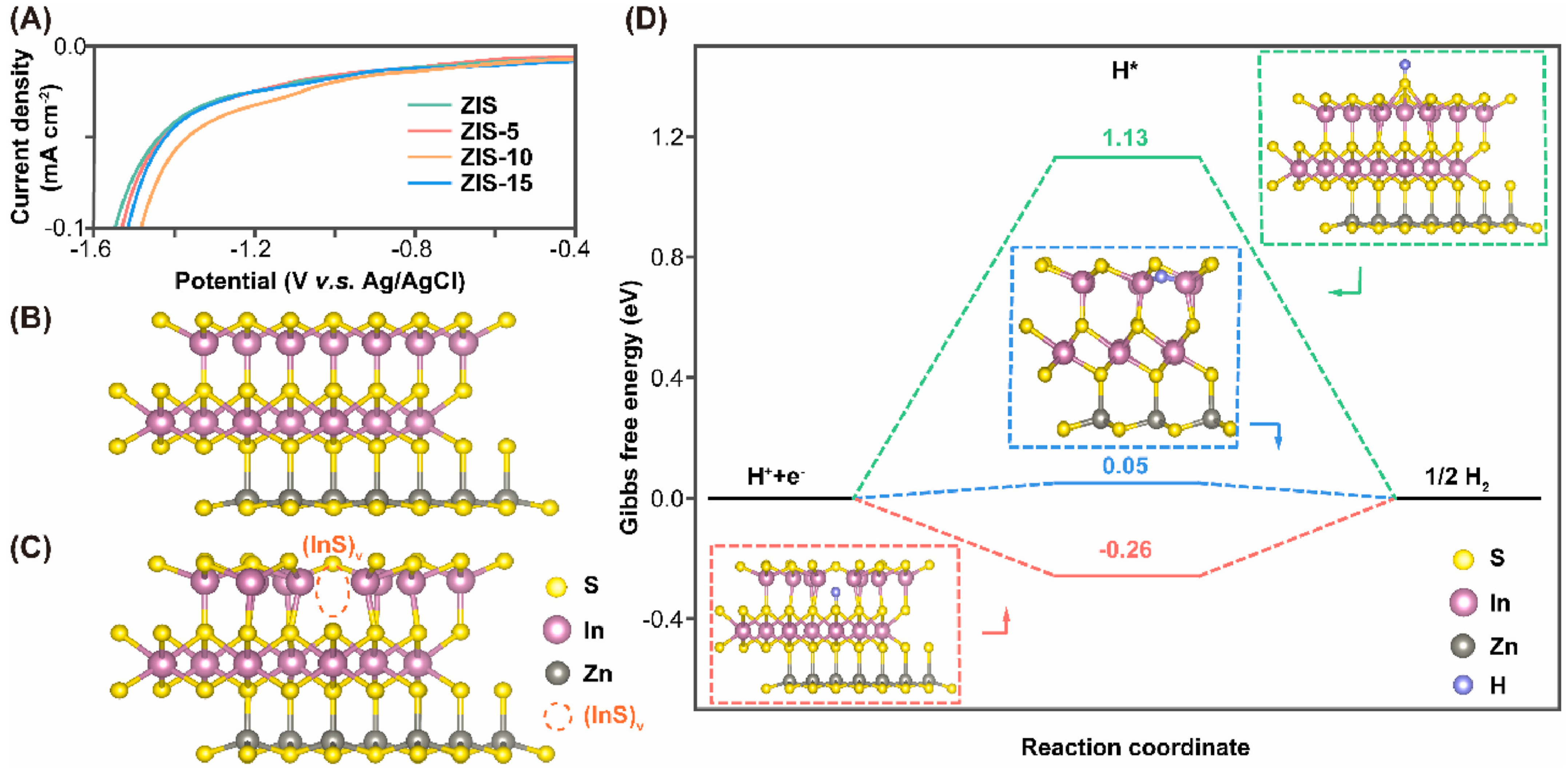
4.4. Vacancy Introducing
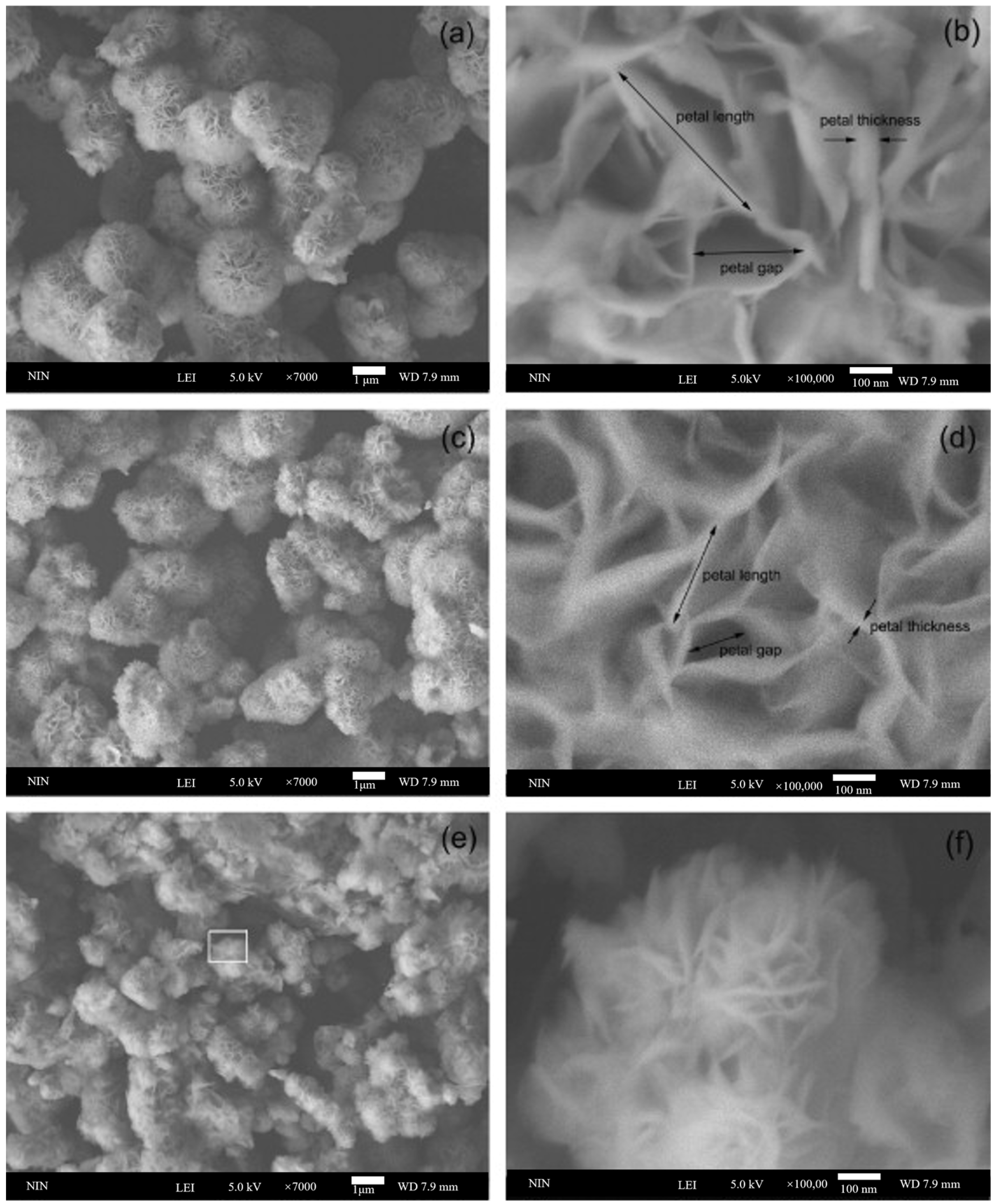
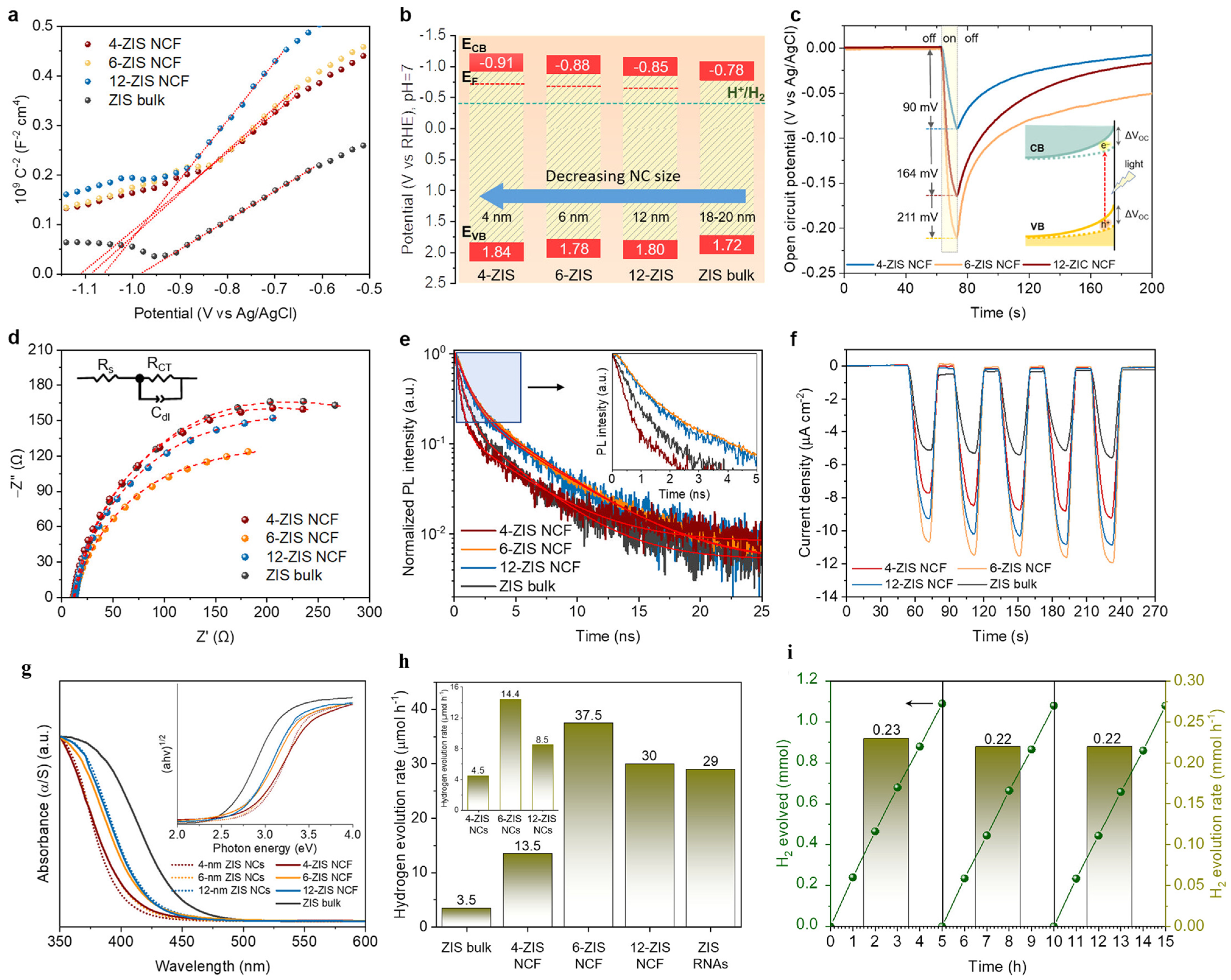
4.5. Size Dependent ZnIn2S4
5. Summary and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kang, Y.; Yang, Y.; Yin, L.-C.; Kang, X.; Liu, G.; Cheng, H.-M. An Amorphous Carbon Nitride Photocatalyst with Greatly Extended Visible-Light-Responsive Range for Photocatalytic Hydrogen Generation. Adv. Mater. 2015, 27, 4572–4577. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Z.; Guo, L.; Zhang, J.; Li, W.; He, H.; Zong, R.; Wang, D.; Jia, Z.; Wen, Y. Experiences and Challenges of Agricultural Development in an Artificial Oasis: A Review. Agric. Syst. 2021, 193, 103220. [Google Scholar] [CrossRef]
- Qi, K.; Zhuang, C.; Zhang, M.; Gholami, P.; Khataee, A. Sonochemical Synthesis of Photocatalysts and Their Applications. J. Mater. Sci. Technol. 2022, 123, 243–256. [Google Scholar] [CrossRef]
- Chen, J.; Dong, C.-L.; Zhao, D.; Huang, Y.-C.; Wang, X.; Samad, L.; Dang, L.; Shearer, M.; Shen, S.; Guo, L. Molecular Design of Polymer Heterojunctions for Efficient Solar–Hydrogen Conversion. Adv. Mater. 2017, 29, 1606198. [Google Scholar] [CrossRef] [PubMed]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Kim, H. Facile Synthesis of Multitasking Composite of Silver Nanoparticle with Zinc Oxide for 4-Nitrophenol Reduction, Photocatalytic Hydrogen Production, and 4-Chlorophenol Degradation. J. Alloys Compd. 2022, 928, 167133. [Google Scholar] [CrossRef]
- Rabbi, M.F.; Popp, J.; Máté, D.; Kovács, S. Energy Security and Energy Transition to Achieve Carbon Neutrality. Energies 2022, 15, 8126. [Google Scholar] [CrossRef]
- Li, C.; Cao, Q.; Wang, F.; Xiao, Y.; Li, Y.; Delaunay, J.-J.; Zhu, H. Engineering Graphene and TMDs Based van Der Waals Heterostructures for Photovoltaic and Photoelectrochemical Solar Energy Conversion. Chem. Soc. Rev. 2018, 47, 4981–5037. [Google Scholar] [CrossRef]
- Kudo, A. Photocatalyst Materials for Water Splitting. Catal. Surv. Asia 2003, 7, 31–38. [Google Scholar] [CrossRef]
- Ho, G.W. Catalysis Science & Technology. Catalysis 2015, 5, 4655–4850. [Google Scholar]
- Montoya, A.T.; Gillan, E.G. Enhanced Photocatalytic Hydrogen Evolution from Transition-Metal Surface-Modified TiO2. ACS Omega 2018, 3, 2947–2955. [Google Scholar] [CrossRef]
- Gupta, B.; Melvin, A.A.; Matthews, T.; Dash, S.; Tyagi, A.K. TiO2 Modification by Gold (Au) for Photocatalytic Hydrogen (H2) Production. Renew. Sustain. Energy Rev. 2016, 58, 1366–1375. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Z.; Dong, C.-L.; Shi, J.; Cheng, C.; Guan, X.; Zong, S.; Luo, B.; Cheng, Z.; Wei, D.; et al. Synergistic Effect of Nitrogen Vacancy on Ultrathin Graphitic Carbon Nitride Porous Nanosheets for Highly Efficient Photocatalytic H2 Evolution. Chem. Eng. J. 2022, 431, 134101. [Google Scholar] [CrossRef]
- Hao, X.; Wang, Y.; Zhou, J.; Cui, Z.; Wang, Y.; Zou, Z. Zinc Vacancy-Promoted Photocatalytic Activity and Photostability of ZnS for Efficient Visible-Light-Driven Hydrogen Evolution. Appl. Catal. B 2018, 221, 302–311. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, Y.; Guo, W.; Wang, L.; Zhang, R.; Liu, Y.; Zhai, Y. Preparation of Double-Vacancy Modified Carbon Nitride to Greatly Improve the Activity of Photocatalytic Hydrogen Generation. Appl. Surf. Sci. 2021, 560, 150029. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Z.; Yang, K.; Yu, Q.; Zhu, X.; Xu, H.; Li, H. Indium-Based Ternary Metal Sulfide for Photocatalytic CO2 Reduction Application. Chin. J. Catal. 2023, 44, 67–95. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Zheng, G.; Mashifana, T.; Dhiman, P.; Sharma, G.; Stadler, F.J. Current Scenario in Ternary Metal Indium Sulfides-Based Heterojunctions for Photocatalytic Energy and Environmental Applications: A Review. Mater. Today Commun. 2023, 36, 106741. [Google Scholar] [CrossRef]
- Chong, W.-K.; Ng, B.-J.; Tan, L.-L.; Chai, S.-P. Recent Advances in Nanoscale Engineering of Ternary Metal Sulfide-Based Heterostructures for Photocatalytic Water Splitting Applications. Energy Fuels 2022, 36, 4250–4267. [Google Scholar] [CrossRef]
- Chai, B.; Liu, C.; Wang, C.; Yan, J.; Ren, Z. Photocatalytic Hydrogen Evolution Activity over MoS2/ZnIn2S4 Microspheres. Chin. J. Catal. 2017, 38, 2067–2075. [Google Scholar] [CrossRef]
- Zheng, X.; Song, Y.; Liu, Y.; Yang, Y.; Wu, D.; Yang, Y.; Feng, S.; Li, J.; Liu, W.; Shen, Y.; et al. ZnIn2S4-Based Photocatalysts for Photocatalytic Hydrogen Evolution via Water Splitting. Coord. Chem. Rev. 2023, 475, 214898. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhu, J.; Li, Y.; Zhao, J.; Li, F. Fabrication of Two-Dimensional Ni2P/ZnIn2S4 Heterostructures for Enhanced Photocatalytic Hydrogen Evolution. Chem. Eng. J. 2018, 353, 15–24. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, R.; Feng, Y.; Wang, J. Photocatalytic Degradation of Antibiotics in Municipal Wastewater over ZnIn2S4. Ionics 2024, 30, 1291–1306. [Google Scholar] [CrossRef]
- Shen, S.; Guo, P.; Zhao, L.; Du, Y.; Guo, L. Insights into Photoluminescence Property and Photocatalytic Activity of Cubic and Rhombohedral ZnIn2S4. J. Solid State Chem. 2011, 184, 2250–2256. [Google Scholar] [CrossRef]
- Sriram, M.A.; McMichael, P.H.; Waghray, A.; Kumta, P.N.; Misture, S.; Wang, X.-L. Chemical Synthesis of the High-Pressure Cubic-Spinel Phase of ZnIn2S4. J. Mater. Sci. 1998, 33, 4333–4339. [Google Scholar] [CrossRef]
- Pan, Y.; Yuan, X.; Jiang, L.; Yu, H.; Zhang, J.; Wang, H.; Guan, R.; Zeng, G. Recent Advances in Synthesis, Modification and Photocatalytic Applications of Micro/Nano-Structured Zinc Indium Sulfide. Chem. Eng. J. 2018, 354, 407–431. [Google Scholar] [CrossRef]
- Oh, V.B.-Y.; Ng, S.-F.; Ong, W.-J. Shining Light on ZnInS Photocatalysts: Promotional Effects of Surface and Heterostructure Engineering toward Artificial Photosynthesis. Ecomat 2022, 4, e12204. [Google Scholar] [CrossRef]
- Ho, G.W.; Chua, K.J.; Siow, D.R. Metal Loaded WO3 Particles for Comparative Studies of Photocatalysis and Electrolysis Solar Hydrogen Production. Chem. Eng. J. 2012, 181–182, 661–666. [Google Scholar] [CrossRef]
- Chang, C.-J.; Wei, Y.-H.; Huang, K.-P. Photocatalytic Hydrogen Production by Flower-like Graphene Supported ZnS Composite Photocatalysts. Int. J. Hydrogen Energy. 2017, 42, 23578–23586. [Google Scholar] [CrossRef]
- Carraro, G.; Maccato, C.; Gasparotto, A.; Montini, T.; Turner, S.; Lebedev, O.I.; Gombac, V.; Adami, G.; Van Tendeloo, G.; Barreca, D.; et al. Enhanced Hydrogen Production by Photoreforming of Renewable Oxygenates through Nanostructured Fe2O3 Polymorphs. Adv. Funct. Mater. 2014, 24, 372–378. [Google Scholar] [CrossRef]
- Zhang, T.; Song, L.; Yang, J.; Wang, J.; Feng, D.; Ma, B. N, O-Doped Surface Modulation of ZnIn2S4 with High Hydrophilicity for Enhanced Photocatalytic Hydrogen Evolution. J. Colloid Interface Sci. 2025, 683, 555–564. [Google Scholar] [CrossRef]
- He, J.; Yang, Z.; Wang, Z.; Gu, L.; Qiu, J.; Ran, J. Sulfur-Vacancy-Enriched ZnIn2S4 Mediates Efficient Charge Transfer for Hydrogen Evolution from Lignocellulose Photoreforming. Chem. Eng. J. 2025, 503, 158433. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, T.; Wang, J.; Wang, W.; Lin, K.; Ma, B.; Zhang, F. Capacitance Effect of ZnIn2S4 with Delicate Morphology Control on Photocatalytic Hydrogen Evolution. Sep. Purif. Technol. 2025, 361, 131283. [Google Scholar] [CrossRef]
- Tahir, M.; Ajiwokewu, B.; Bankole, A.A.; Ismail, O.; Al-Amodi, H.; Kumar, N. MOF Based Composites with Engineering Aspects and Morphological Developments for Photocatalytic CO2 Reduction and Hydrogen Production: A Comprehensive Review. J. Environ. Chem. Eng. 2023, 11, 109408. [Google Scholar] [CrossRef]
- Pan, L.; Wu, G.; Wang, X.; Liu, R.; Yan, P.; Zhu, X.; Mo, Z.; Sun, P.; Miao, Z.; Xu, H. Promotion of Intramolecular Electron Transfer in G-C3N4 Tubes by Introducing Pyridine Structure to Accelerate Photocatalytic Hydrogen Evolution. Colloids Surf., A 2024, 686, 133261. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Guo, L. Morphology, Structure and Photocatalytic Performance of ZnIn2S4 Synthesized via a Solvothermal/Hydrothermal Route in Different Solvents. J. Phys. Chem. Solids 2008, 69, 2426–2432. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Lu, J.; Feng, Y.; Meng, F.; Ma, C.; Yan, Y.; Meng, M. Synergy between van Der Waals Heterojunction and Vacancy in ZnIn2S4/g-C3N4 2D/2D Photocatalysts for Enhanced Photocatalytic Hydrogen Evolution. Appl. Catal. B 2020, 277, 119254. [Google Scholar] [CrossRef]
- Dai, M.; He, Z.; Zhang, P.; Li, X.; Wang, S. ZnWO4-ZnIn2S4 S-Scheme Heterojunction for Enhanced Photocatalytic H2 Evolution. J. Mater. Sci. Technol. 2022, 122, 231–242. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Li, J.; Dong, B.; Zhao, L.; Wang, S. 1D/2D TiO2/ZnIn2S4 S-Scheme Heterojunction Photocatalyst for Efficient Hydrogen Evolution. Chin. J. Catal. 2022, 43, 339–349. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Zou, Z. Recent Advances in Designing ZnIn2S4-Based Heterostructured Photocatalysts for Hydrogen Evolution. J. Mater. Sci. Technol. 2023, 139, 167–188. [Google Scholar] [CrossRef]
- Lin, B.; Li, H.; An, H.; Hao, W.; Wei, J.; Dai, Y.; Ma, C.; Yang, G. Preparation of 2D/2D g-C3N4 nanosheet@ZnIn2S4 Nanoleaf Heterojunctions with Well-Designed High-Speed Charge Transfer Nanochannels towards High-Efficiency Photocatalytic Hydrogen Evolution. Appl. Catal. B 2018, 220, 542–552. [Google Scholar] [CrossRef]
- Jia, X.; Lu, Y.; Du, K.; Zheng, H.; Mao, L.; Li, H.; Ma, Z.; Wang, R.; Zhang, J. Interfacial Mediation by Sn and S Vacancies of P-SnS/n-ZnIn2S4 for Enhancing Photocatalytic Hydrogen Evolution with New Scheme of Type-I Heterojunction. Adv. Funct. Mater. 2023, 33, 2304072. [Google Scholar] [CrossRef]
- Fan, Q.; Yan, Z.; Li, J.; Xiong, X.; Li, K.; Dai, G.; Jin, Y.; Wu, C. Interfacial-Electric-Field Guiding Design of a Type-I FeIn2S4@ZnIn2S4 Heterojunction with Ohmic-like Charge Transfer Mechanism for Highly Efficient Solar H2 Evolution. Appl. Surf. Sci. 2024, 663, 160206. [Google Scholar] [CrossRef]
- Lu, C.; Guo, F.; Yan, Q.; Zhang, Z.; Li, D.; Wang, L.; Zhou, Y. Hydrothermal Synthesis of Type II ZnIn2S4/BiPO4 Heterojunction Photocatalyst with Dandelion-like Microflower Structure for Enhanced Photocatalytic Degradation of Tetracycline under Simulated Solar Light. J. Alloys Compd. 2019, 811, 151976. [Google Scholar] [CrossRef]
- Lin, Y.; Fang, W.; Xv, R.; Fu, L. TiO2 Nanoparticles Modified with ZnIn2S4 Nanosheets and Co-Pi Groups: Type II Heterojunction and Cocatalysts Coexisted Photoanode for Efficient Photoelectrochemical Water Splitting. Int. J. Hydrogen Energy 2022, 47, 33361–33373. [Google Scholar] [CrossRef]
- Ye, J.; Fan, Z.; Wang, Z.; Wang, Y.; Li, J.; Xie, Y.; Ling, Y.; Chen, Y. In2S3-Modified ZnIn2S4 Enhanced Photogenerated Carrier Separation Efficiency and Photocatalytic Hydrogen Evolution under Visible Light. Fuel 2024, 373, 132401. [Google Scholar] [CrossRef]
- Gao, T.; Li, Y.; Tian, J.; Fan, J.; Sun, T.; Liu, E. Facile Fabrication of NiWO4/ZnIn2S4 p-n Heterojunction for Enhanced Photocatalytic H2 Evolution. J. Alloys Compd. 2023, 951, 169939. [Google Scholar] [CrossRef]
- Kong, D.; Hu, X.; Geng, J.; Zhao, Y.; Fan, D.; Lu, Y.; Geng, W.; Zhang, D.; Liu, J.; Li, H.; et al. Growing ZnIn2S4 Nanosheets on FeWO4 Flowers with P-n Heterojunction Structure for Efficient Photocatalytic H2 Production. Appl. Surf. Sci. 2022, 591, 153256. [Google Scholar] [CrossRef]
- Guo, X.; Peng, Y.; Liu, G.; Xie, G.; Guo, Y.; Zhang, Y.; Yu, J. An Efficient ZnIn2S4@CuInS2 Core–Shell p–n Heterojunction to Boost Visible-Light Photocatalytic Hydrogen Evolution. J. Phys. Chem. C 2020, 124, 5934–5943. [Google Scholar] [CrossRef]
- Xie, Z.; Xie, L.; Qi, F.; Liu, H.; Meng, L.; Wang, J.; Xie, Y.; Chen, J.; Lu, C.-Z. Efficient Photocatalytic Hydrogen Production by Space Separation of Photo-Generated Charges from S-Scheme ZnIn2S4/ZnO Heterojunction. J. Colloid Interface Sci. 2023, 650, 784–797. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xian, Q.; Zhao, Y. Direct Z-Scheme TiO2–ZnIn2S4 Nanoflowers for Cocatalyst-Free Photocatalytic Water Splitting. Appl. Catal. B 2021, 291, 120126. [Google Scholar] [CrossRef]
- Shoaib, M.; Qiao, F.; Sun, Q.; Zhao, J. Enhanced Photocatalytic Hydrogen Evolution Properties of Er-Doped ZnIn2S4 Nanostructures via Hydrothermal Synthesis. Catal. Lett. 2025, 155, 90. [Google Scholar] [CrossRef]
- Yang, M.; Zhan, X.-Q.; Ou, D.-L.; Wang, L.; Zhao, L.-L.; Yang, H.-L.; Liao, Z.-Y.; Yang, W.-Y.; Ma, G.-Z.; Hou, H.-L. Efficient Visible-Light-Driven Hydrogen Production with Ag-Doped Flower-like ZnIn2S4 Microspheres. Rare Met. 2024, 44, 1024–1041. [Google Scholar] [CrossRef]
- Sun, L.; Peng, H.; Xue, F.; Liu, S.; Hu, Z.; Geng, H.; Liu, X.; Su, D.; Xu, Y.; Huang, X. Pd Single Atoms Cooperate with S Vacancies in ZnIn2S4 Nanosheets for Photocatalytic Pure-Water Splitting. Sci. China Chem. 2024, 67, 855–861. [Google Scholar] [CrossRef]
- Chong, W.-K.; Ng, B.-J.; Er, C.-C.; Tan, L.-L.; Chai, S.-P. Insights from Density Functional Theory Calculations on Heteroatom P-Doped ZnIn2S4 Bilayer Nanosheets with Atomic-Level Charge Steering for Photocatalytic Water Splitting. Sci. Rep. 2022, 12, 1927. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Shen, T.; Hu, Y.; Zhang, Z.; Liu, Z.; Xu, N.; Song, J.; Guan, R.; Zhou, C. Nitrogen-Doped ZnIn2S4 and TPA Multi-Dimensional Synergistically Enhance Photocatalytic Hydrogen Production. Fuel 2025, 380, 133151. [Google Scholar] [CrossRef]
- Zhang, P.; Lin, J.; Zhao, J.; Lu, C.; Huang, L.; Lin, Z.; Bu, D.; Huang, S. ZnIn2S4 Nanosheets with Geometric Defects for Enhanced Solar-Driven Hydrogen Evolution and Wastewater Treatment. Renew. Energy 2024, 237, 121741. [Google Scholar] [CrossRef]
- Andreou, E.K.; Vamvasakis, I.; Douloumis, A.; Kopidakis, G.; Armatas, G.S. Size Dependent Photocatalytic Activity of Mesoporous ZnIn2S4 Nanocrystal Networks. ACS Catal. 2024, 14, 14251–14262. [Google Scholar] [CrossRef]




| Entry | Type | Mass of Photocatalyst | Sacrificial Agent | Light Source | H2 Generation Rate | Reference |
|---|---|---|---|---|---|---|
| 1 | Aqueous-mediated ZnIn2S4 | 200 mg | Na2SO3/Na2S | 300 W Xenon lamp | 27.3 μmol h−1 | [33] |
| 2 | FIS@ZIS | 25 mg | Na2S/Na2SO3 | 300 W Xenon lamp | 4.21 mmol h−1 g−1 | [41] |
| 3 | In2S3/ZnIn2S4 | 10 mg | 10 vol% TEOA | 300 W Xe lamp, λ > 420 nm | 5.69 mmol h−1 g−1 | [44] |
| 4 | ZnIn2S4@CuInS2 | 50 mg | Na2S/Na2SO3 | 300 W Xenon lamp | 1168 μmol·g−1 | [47] |
| 5 | 2ZnIn2S4/ZnO | 20 mg | None | 300 W Xe lamp | 3.036 mmol h−1 g−1 | [48] |
| 6 | TiO2-ZnIn2S4 | 20 mg | None | 300 W Xe lamp | 214.9 μmol h−1 g−1 | [49] |
| 7 | Pd0.03/ZIS | 20 mg | None | 300 W Xenon lamp, AM 1.5 G | 1037.9 μmol h−1 g−1 | [52] |
| 8 | N-ZIS/TPA | 10 mg | 0.1 M ascorbic acid | 300 W Xe lamp, λ > 420 nm | 17,345.53 μmol h−1 g−1 | [54] |
| 9 | ZIS-10 | 5 mg | 0.05 M ascorbic acid | 300 W Xe lamp (λ > 420 nm) | 10.7 mmol h−1 g−1 | [55] |
| 10 | 6-ZIS NCF | 30 mg | 10% v/v TEA | 300 W Xe lamp | 7.8 mmol h−1 g−1 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Zeng, S.; Yang, S.; Zhang, H.; Ma, Y.; Zhou, G.; Fang, J. Zinc Indium Sulfide Materials for Photocatalytic Hydrogen Production via Water Splitting: A Short Review. Catalysts 2025, 15, 271. https://doi.org/10.3390/catal15030271
Yao L, Zeng S, Yang S, Zhang H, Ma Y, Zhou G, Fang J. Zinc Indium Sulfide Materials for Photocatalytic Hydrogen Production via Water Splitting: A Short Review. Catalysts. 2025; 15(3):271. https://doi.org/10.3390/catal15030271
Chicago/Turabian StyleYao, Lang, Shice Zeng, Shuxiang Yang, Honghua Zhang, Yue Ma, Guangying Zhou, and Jianzhang Fang. 2025. "Zinc Indium Sulfide Materials for Photocatalytic Hydrogen Production via Water Splitting: A Short Review" Catalysts 15, no. 3: 271. https://doi.org/10.3390/catal15030271
APA StyleYao, L., Zeng, S., Yang, S., Zhang, H., Ma, Y., Zhou, G., & Fang, J. (2025). Zinc Indium Sulfide Materials for Photocatalytic Hydrogen Production via Water Splitting: A Short Review. Catalysts, 15(3), 271. https://doi.org/10.3390/catal15030271





