Intracellular Lipases for Enzymatic Synthesis of Phenylalanine Butyramide in a Biphasic Reaction System
Abstract
1. Introduction
2. Results and Discussion
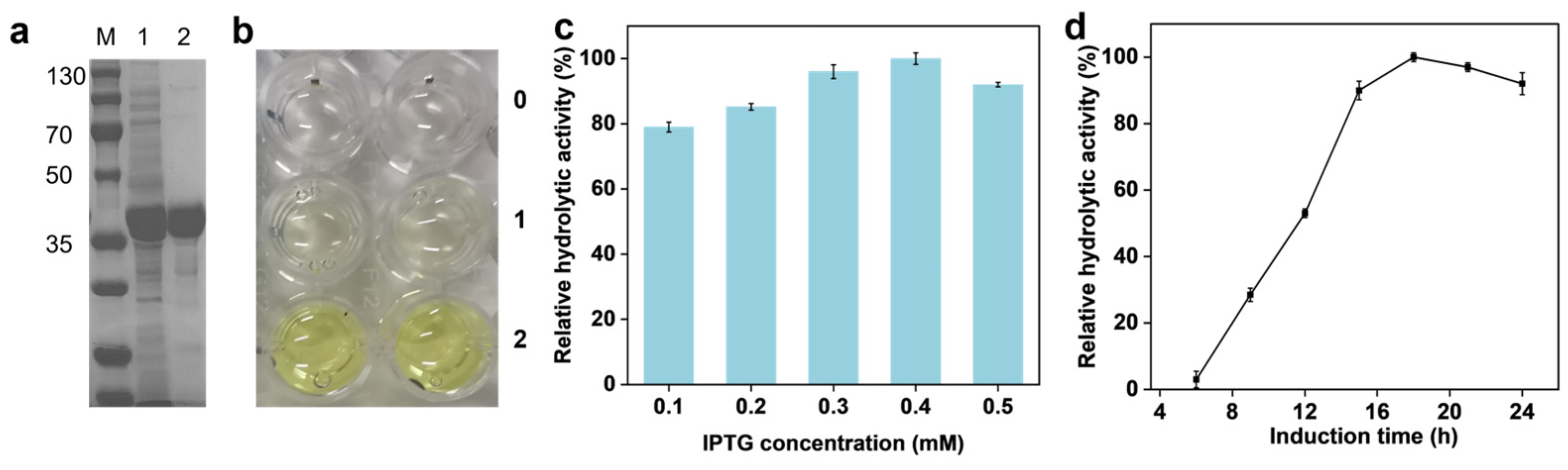
2.1. Expression Purification and Activity Verification of SpL
2.2. SpL-Catalyzed Ammonolysis Reaction for FBA Synthesis
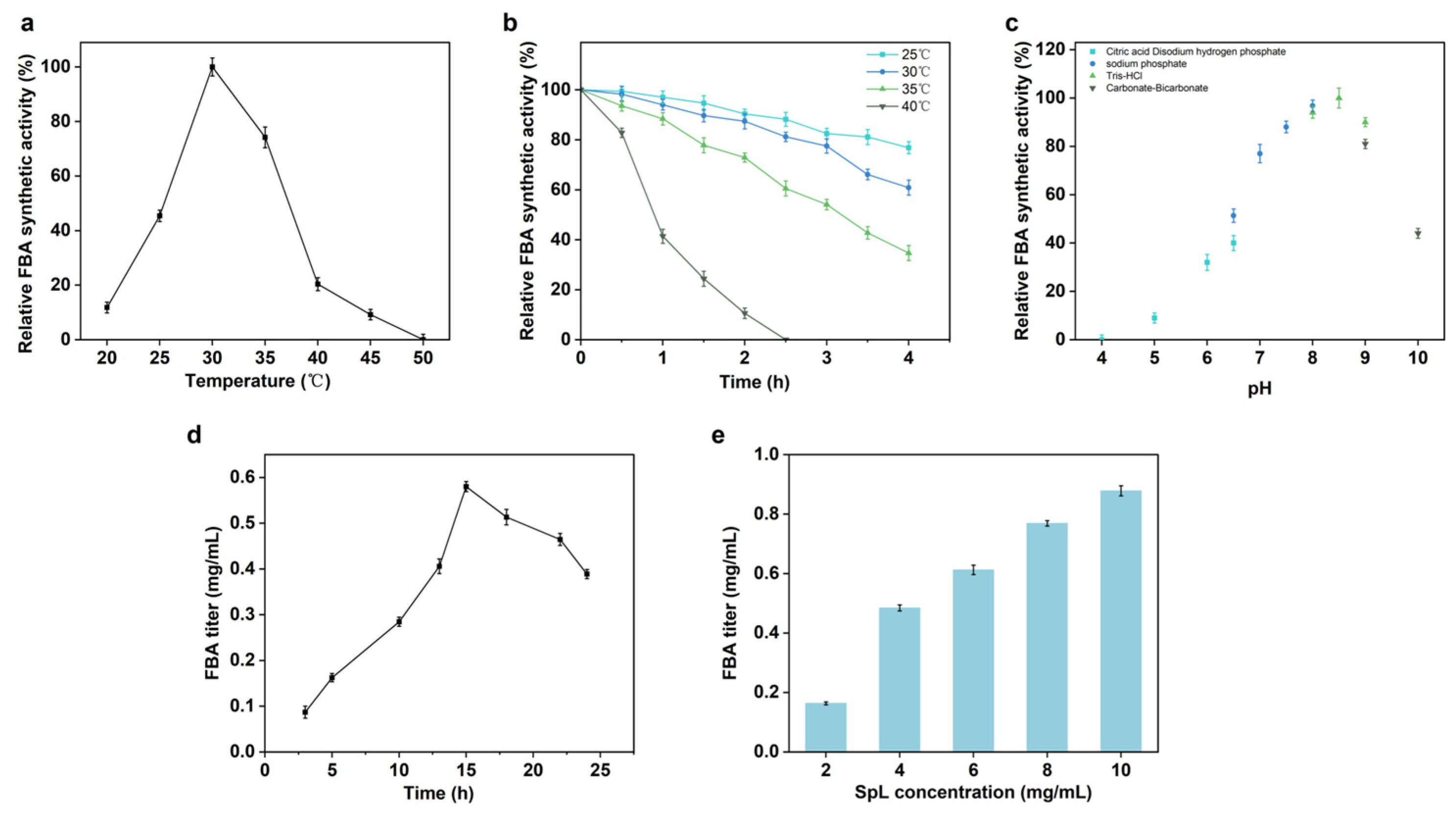
2.3. The Optimization of the Catalytic Conditions of SpL
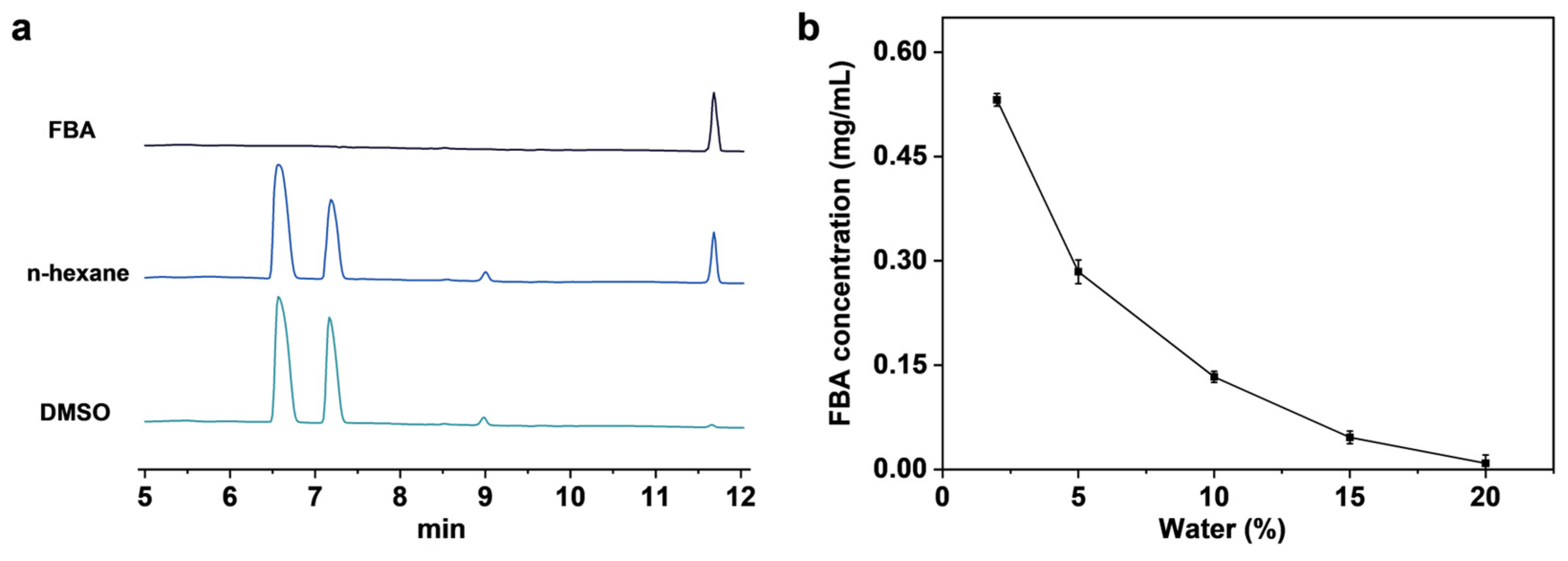
2.4. Analysis of Mechanism of SpL Catalyzed FBA Synthesis
2.5. Analysis of the Hydrolysis Mechanism of SpL
3. Materials and Methods
3.1. Materials
3.2. Cloning and Expression of the SpL
3.3. Purification and Lyophilization of the SpL
3.4. Activity Assay of SpL
3.5. Effect of pH and Temperature on Enzymatic Activity
3.6. Thermal and pH Stability
3.7. The Preparation and Characterization of FBA
3.8. Molecular Docking
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russo, R.; D’agostino, G.; Simeoli, R.; Iacono, A.; Raso, G.M.; Costanzo, M.D.; Leone, L.; Meli, R.; Calignano, A.; Canani, R.B. Pp39 Therapeutic Effects of the New Synthetic Butyrate Derivate N-(1-Carbamoyl-2-Phenyl-Ethyl) Butyramide on Diarrhea and Visceral Pain in Mice. Dig. Liver Dis. 2011, 43, S426. [Google Scholar] [CrossRef]
- Russo, R.; Sasso, O.; D’agostino, G.; Iacono, A.; Mattaceraso, G.; Palomba, M.; Meli, R.; Canani, R.B.; Calignano, A. Pa40 Effects of N-(1-Carbamoyl-2-Phenyl-Ethyl) Butyramide, a New Synthetic Butyrate Prodrug on Gastrointestinal Transit Time Modulation. Dig. Liver Dis. 2010, 42, S357. [Google Scholar] [CrossRef]
- Simeoli, R.; Raso, G.M.; Pirozzi, C.; Lama, A.; Santoro, A.; Russo, R.; Montero-Melendez, T.; Canani, R.B.; Calignano, A.; Perretti, M.; et al. An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis. Br. J. Pharmacol. 2016, 174, 1484–1496. [Google Scholar] [CrossRef]
- Russo, R.; Santarcangelo, C.; Badolati, N.; Sommella, E.; Filippis, A.D.; Dacrema, M.; Campiglia, P.; Stornaiuolo, M.; Daglia, M. In vivo bioavailability and in vitro toxicological evaluation of the new butyric acid releaser N-(1-carbamoyl-2-phenyl-ethyl) butyramide. Biomed. Pharmacother. 2021, 137, 111385. [Google Scholar] [CrossRef]
- Meli, R.; Raso, G.M.; Russo, R.; D’agostino, G.; Simeoli, R.; Iacono, A.; Canani, R.B.; Calignano, A. Co8 a New Synthetic Butyrate Derivate N-(1-Carbamoyl-2-Phenyl-Ethyl) Butyramide Is Effective to Limit Intestinal Inflammation in Dextran Sodium Sulphate-Induced Colitis Animal Model. Dig. Liver Dis. 2012, 44, S244. [Google Scholar] [CrossRef]
- Lama, A.; Annunziata, C.; Coretti, L.; Pirozzi, C.; Guida, F.D.; Izzo, A.N.; Cristiano, C.; Mollica, M.P.; Chiariotti, L.; Pelagalli, A.; et al. N-(1-carbamoyl-2-phenylethyl) butyramide reduces antibiotic-induced intestinal injury, innate immune activation and modulates microbiota composition. Sci. Rep. 2019, 9, 4832. [Google Scholar] [CrossRef]
- Russo, M.; Guida, F.; Paparo, L.; Trinchese, G.; Aitoro, R.; Avagliano, C.; Fiordelisi, A.; Napolitano, F.; Mercurio, V.; Sala, V.; et al. The novel butyrate derivative phenylalanine-butyramide protects from doxorubicin-induced cardiotoxicity. Eur. J. Heart Fail. 2019, 21, 519–528. [Google Scholar] [CrossRef]
- Goswami, A.; Lanen, S.G.V. Enzymatic strategies and biocatalysts for amide bond formation: Tricks of the trade outside of the ribosome. Mol. Biosyst. 2015, 11, 338–353. [Google Scholar] [CrossRef]
- Sawant, D.N.; Bagal, D.B.; Ogawa, S.; Selvam, K.; Saito, S. Diboron-Catalyzed Dehydrative Amidation of Aromatic Carboxylic Acids with Amines. Org. Lett. 2018, 20, 4397–4400. [Google Scholar] [CrossRef]
- Ojeda-Porras, A.; Hernández-Santana, A.; Gamba-Sánchez, D. Direct amidation of carboxylic acids with amines under microwave irradiation using silica gel as a solid support. Green Chem. 2015, 17, 3157–3163. [Google Scholar] [CrossRef]
- Winn, M.; Richardson, S.M.; Campopiano, D.J.; Micklefield, J. Harnessing and engineering amide bond forming ligases for the synthesis of amides. Curr. Opin. Chem. Biol. 2020, 55, 77–85. [Google Scholar] [CrossRef]
- Petchey, M.R.; Grogan, G. Enzyme-Catalysed Synthesis of Secondary and Tertiary Amides. Adv. Synth. Catal. 2019, 361, 3895–3914. [Google Scholar] [CrossRef]
- Fan, X.; Chen, P.; Wu, D.; Zheng, P. Computational Mining of a Novel Acetyl-CoA Synthetase for the Biosynthesis of Unnatural Phenylalanine Butyramide. ChemCatChem 2025, 17, e202401696. [Google Scholar] [CrossRef]
- Rantwijk, F.V.; Hacking, M.A.P.J.; Sheldon, R.A. Lipase-Catalyzed Synthesis of Carboxylic Amides: Nitrogen Nucleophiles as Acyl Acceptor. Monatsh. Chem. 2000, 131, 549–569. [Google Scholar] [CrossRef]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.E.; Miller, R. Lipases at interfaces: A review. Adv. Colloid Interface Sci. 2009, 147–148, 237–250. [Google Scholar] [CrossRef]
- Orsy, G.; Shahmohammadi, S.; Forró, E. A Sustainable Green Enzymatic Method for Amide Bond Formation. Molecules 2023, 28, 5706. [Google Scholar] [CrossRef]
- Lima, R.N.; Anjos, C.S.D.; Orozco, E.V.M.; Porto, A.L.M. Versatility of Candida antarctica lipase in the amide bond formation applied in organic synthesis and biotechnological processes. Mol. Catal. 2019, 466, 75–105. [Google Scholar] [CrossRef]
- Pelt, S.V.; Teeuwen, R.L.M.; Janssen, M.H.A.; Sheldon, R.A.; Dunn, P.J.; Howard, R.M.; Kumar, R.; Martínez, I.; Wong, J.W. Pseudomonas stutzeri lipase: A useful biocatalyst for aminolysis reactions. Green Chem. 2011, 13, 1791–1798. [Google Scholar] [CrossRef]
- Kitaguchi, H.; Fitzpatrick, P.A.; Huber, J.E.; Klibanov, A.M. Enzymatic Resolution of Racemic Amines: Crucial Role of the Solvent. J. Am. Chem. Soc. 1989, 111, 3094–3095. [Google Scholar] [CrossRef]
- Zukic, E.; Mokos, D.; Weber, M.; Stix, N.; Ditrich, K.; Ferrario, V.; Müller, H.; Willrodt, C.; Gruber, K.; Daniel, B.; et al. Biocatalytic Heteroaromatic Amide Formation in Water Enabled by a Catalytic Tetrad and Two Access Tunnels. ACS Catal. 2024, 14, 8913–8921. [Google Scholar] [CrossRef]
- Chong, W.Y.; Qi, Y.; Ji, L.R.; Zhang, Z.H.; Lu, Z.P.; Nian, B.B.; Hu, Y. Computer-Aided Tunnel Engineering: A Promising Strategy for Improving Lipase Applications in Esterification Reactions. ACS Catal. 2023, 14, 67–83. [Google Scholar] [CrossRef]
- Simone, G.D.; Galdiero, S.; Manco, G.; Lang, D.; Rossi, M.; Pedone, C. A snapshot of a transition state analogue of a novel thermophilic esterase belonging to the subfamily of mammalian hormone-sensitive lipase. J. Mol. Biol. 2000, 303, 761–771. [Google Scholar] [CrossRef]
- Palm, G.J.; Fernández-Álvaro, E.; Bogdanović, X.; Bartsch, S.; Sczodrok, J.; Singh, R.K.; Böttcher, D.; Atomi, H.; Bornscheuer, U.T.; Hinrichs, W. The crystal structure of an esterase from the hyperthermophilic microorganism Pyrobaculum calidifontis VA1 explains its enantioselectivity. Appl. Microbiol. 2011, 91, 1061–1072. [Google Scholar] [CrossRef]
- Ding, Y.; Nie, L.; Yang, X.-C.; Li, Y.; Huo, Y.-Y.; Li, Z.; Gao, Y.; Cui, H.-L.; Li, J.; Xu, X.-W. Mechanism and Structural Insights Into a Novel Esterase, E53, Isolated From Erythrobacter longus. Front. Microbiol. 2022, 12, 798194. [Google Scholar] [CrossRef] [PubMed]
- Galmés, M.A.; García-Junceda, E.; Swiderek, K.; Moliner, V. Exploring the Origin of Amidase Substrate Promiscuity in CALB by a Computational Approach. ACS Catal. 2020, 10, 1938–1946. [Google Scholar] [CrossRef]
- Galmés, M.A.; Nödling, A.R.; Luk, L.; Swiderek, K.; Moliner, V. Combined Theoretical and Experimental Study to Unravel the Differences in Promiscuous Amidase Activity of Two Nonhomologous Enzymes. ACS Catal. 2021, 11, 8635–8644. [Google Scholar] [CrossRef]
- Galmés, M.A.; Nödling, A.R.; He, K.N.; Luk, L.Y.P.; Swiderek, K.; Moliner, V. Computational design of an amidase by combining the best electrostatic features of two promiscuous hydrolases. Chem. Sci. 2022, 13, 4779–4787. [Google Scholar] [CrossRef]


| Acyl Substrates | 10% Aqueous | Anhydrous | |
|---|---|---|---|
| n-hexane | Butyric acid | - | - |
| Ethyl butyrate | + | - | |
| DMSO | Butyric acid | - | - |
| Ethyl butyrate | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Chen, P.; Wu, D.; Zheng, P. Intracellular Lipases for Enzymatic Synthesis of Phenylalanine Butyramide in a Biphasic Reaction System. Catalysts 2025, 15, 514. https://doi.org/10.3390/catal15060514
Fan X, Chen P, Wu D, Zheng P. Intracellular Lipases for Enzymatic Synthesis of Phenylalanine Butyramide in a Biphasic Reaction System. Catalysts. 2025; 15(6):514. https://doi.org/10.3390/catal15060514
Chicago/Turabian StyleFan, Xinyu, Pengcheng Chen, Dan Wu, and Pu Zheng. 2025. "Intracellular Lipases for Enzymatic Synthesis of Phenylalanine Butyramide in a Biphasic Reaction System" Catalysts 15, no. 6: 514. https://doi.org/10.3390/catal15060514
APA StyleFan, X., Chen, P., Wu, D., & Zheng, P. (2025). Intracellular Lipases for Enzymatic Synthesis of Phenylalanine Butyramide in a Biphasic Reaction System. Catalysts, 15(6), 514. https://doi.org/10.3390/catal15060514








