Ethanol Dehydration Pathways on NASICON-Type A0.33M2(PO4)3 ((A = Dy, Y, Yb); M = Ti, Zr) Catalysts: The Role of Hydroxyl Group Proton Mobility in Selectivity Control
Abstract
1. Introduction
2. Results
2.1. Phase Purity and Structure Characterization
2.2. Surface and Porosity Characterization
2.3. Acid–Base Properties
2.4. Catalytic Ethanol Dehydration
3. Discussion
4. Materials and Methods
4.1. Synthesis of the Catalysts
4.2. Characterization of the Catalysts
4.3. Catalytic Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, J.; Wang, Y. Recent Advances in Catalytic Conversion of Ethanol to Chemicals. ACS Catal. 2014, 4, 1078–1090. [Google Scholar] [CrossRef]
- Chen, W.H.; Biswas, P.P.; Ong, H.C.; Hoang, A.T.; Nguyen, T.B.; Dong, C.-D. A Critical and Systematic Review of Sustainable Hydrogen Production from Ethanol/Bioethanol: Steam Reforming, Partial Oxidation, and Autothermal Reforming. Fuel 2023, 333, 126526. [Google Scholar] [CrossRef]
- Zhukova, A.; Chuklina, S.; Fionov, Y.; Vakhrushev, N.; Sazonova, A.; Mikhalenko, I.; Zhukov, D.; Isaikina, O.; Fionov, A.; Il’iCheva, A. Enhanced Ethanol Dehydrogenation over Ni—Containing Zirconia—Alumina Catalysts with Microwave—Assisted Synthesis. Res. Chem. Intermed. 2023, 50, 1331–1354. [Google Scholar] [CrossRef]
- Zhukova, A.I.; Chuklina, S.G.; Maslenkova, S.A. Study of Cu Modified Zr and Al Mixed Oxides in Ethanol Conversion: The Structure-Catalytic Activity Relationship. Catal. Today 2021, 379, 159–165. [Google Scholar] [CrossRef]
- Angelici, C.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Chemocatalytic Conversion of Ethanol into Butadiene and Other Bulk Chemicals. ChemSusChem 2013, 6, 1595–1614. [Google Scholar] [CrossRef]
- Anekwe, I.M.S.; Isa, Y.M.; Oboirien, B. Bioethanol as a Potential Eco-Friendlier Feedstock for Catalytic Production of Fuels and Petrochemicals. J. Chem. Technol. Biotechnol. 2023, 98, 2077–2094. [Google Scholar] [CrossRef]
- Chaichana, E.; Boonsinvarothai, N.; Chitpong, N.; Jongsomjit, B. Catalytic Dehydration of Ethanol to Ethylene and Diethyl Ether over Alumina Catalysts Containing Different Phases with Boron Modification. J. Porous Mater. 2019, 26, 599–610. [Google Scholar] [CrossRef]
- Haribal, V.P.; Chen, Y.; Neal, L.; Li, F. Intensification of Ethylene Production from Naphtha via a Redox Oxy-Cracking Scheme: Process Simulations and Analysis. Engineering 2018, 4, 714–721. [Google Scholar] [CrossRef]
- Ngcobo, M.; Makgwane, P.R.; Mathe, M.K. A Minireview on Solid Acid Catalysts for Dehydration of Bioethanol to Renewable Ethylene: An Update on Catalysts Development Progress. Appl. Catal. O Open 2024, 193, 206976. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Yabushita, M.; Tomishige, K. A Perspective on Catalytic Production of Olefinic Compounds from Biomass. RSC Sustain. 2023, 1, 814–837. [Google Scholar] [CrossRef]
- Kostestkyy, P.; Yu, J.; Gorte, R.J.; Mpourmpakis, G. Structure-Activity Relationships on Metal-Oxides: Alcohol Dehydration. Catal. Sci. Technol. 2014, 4, 3861–3869. [Google Scholar] [CrossRef]
- Lv, J.; Wang, D.; Peng, L.; Guo, X.; Ding, W.; Yang, W. Ethanol Dehydration to Ethylene over High-Energy Facets Exposed Gamma Alumina. Catalysts 2023, 13, 994. [Google Scholar] [CrossRef]
- Ward, D.J.; Saccomando, D.J.; Vilela, F.; Walker, G.; Mansell, S.M. Flow Chemistry Enhances Catalytic Alcohol-to-Alkene Dehydration. Catal. Sci. Technol. 2024, 14, 6641–6650. [Google Scholar] [CrossRef]
- Manabe, K.; Iimura, S.; Sun, X.M.; Kobayashi, S. Dehydration Reactions in Water. Brønsted Acid-Surfactant-Combined Catalyst for Ester, Ether, Thioether, and Dithioacetal Formation in Water. J. Am. Chem. Soc. 2002, 124, 11971–11978. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, W.; Brown, E.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Dehydration of Ethanol over Heteropoly Acid Catalysts in the Gas Phase. J. Catal. 2014, 319, 174–181. [Google Scholar] [CrossRef]
- Ohayon Dahan, H.; Landau, M.V.; Herskowitz, M. Effect of Surface Acidity-Basicity Balance in Modified ZnxZryOz Catalyst on Its Performance in the Conversion of Hydrous Ethanol to Hydrocarbons. J. Ind. Eng. Chem. 2021, 95, 156–169. [Google Scholar] [CrossRef]
- Ouayloul, L.; El Doukkali, M.; Jiao, M.; Dumeignil, F.; Agirrezabal-Telleria, I. New Mechanistic Insights into the Role of Water in the Dehydration of Ethanol into Ethylene over ZSM-5 Catalysts at Low Temperature. Green Chem. 2023, 25, 3644–3659. [Google Scholar] [CrossRef]
- Wu, C.Y.; Wu, H.S. Ethylene Formation from Ethanol Dehydration Using ZSM-5 Catalyst. ACS Omega 2017, 2, 4287–4296. [Google Scholar] [CrossRef]
- Pylinina, A.I.; Akhmedova, L.S.; Knyazeva, E.I.; Fionov, Y.A.; Sokolova, E.A. Acid Properties of Cesium-Nickel-Zirconium Complex Phosphates: Effect on Isobutanol Dehydration. Pet. Chem. 2020, 60, 592–596. [Google Scholar] [CrossRef]
- Ermilova, M.M.; Sukhanov, M.V.; Borisov, R.S.; Orekhova, N.V.; Pet’kov, V.I.; Novikova, S.A.; Il’in, A.B.; Yaroslavtsev, A.B. Synthesis of the new framework phosphates and their catalytic activity in ethanol conversion into hydrocarbons. Catal. Today 2012, 193, 37–41. [Google Scholar] [CrossRef]
- Zhukova, A.I.; Asabina, E.A.; Kharlanov, A.N.; Osaulenko, D.A.; Chuklina, S.G.; Zhukov, D.Y.; Pet’kov, V.I.; Deyneko, D.V. Novel Complex Titanium NASICON-Type Phosphates as Acidic Catalysts for Ethanol Dehydration. Catalysts 2023, 13, 185. [Google Scholar] [CrossRef]
- Povarova, E.I.; Pylinina, A.I.; Mikhalenko, I.I. Catalytic Dehydrogenation of Propanol-2 on Na-Zr Phosphates Containing Cu, Co, and Ni. Russ. J. Phys. Chem. A 2012, 86, 935–941. [Google Scholar] [CrossRef]
- Mayorov, P.; Asabina, E.; Zhukova, A.; Osaulenko, D.; Pet’kov, V.; Lavrenov, D.; Kovalskii, A.; Fionov, A. Catalytic Properties of the Framework-Structured Zirconium-Containing Phosphates in Ethanol Conversion. Res. Chem. Intermed. 2021, 47, 3645–3659. [Google Scholar] [CrossRef]
- Novikova, S.A.; Il’in, A.B.; Zhilyaeva, N.A.; Yaroslavtsev, A.B. Catalytic Activity of Li1+XHf2−XInx(PO4)3-Based NASICON-Type Materials for Ethanol Conversion Reactions. Inorg. Mater. 2018, 54, 676–682. [Google Scholar] [CrossRef]
- Ziyad, M.; Rouimi, M.; Portefaix, J. Activity in Hydrotreatment Processes of Ni-Mo Loaded Zirconium Phosphate Zr3(PO4)4. Appl. Catal. A: Gen. 1999, 183, 93–105. [Google Scholar] [CrossRef]
- Mitran, G.; Mieritz, D.G.; Seo, D.K. Highly Selective Solid Acid Catalyst H1−XTi2(PO4)3−x(SO4)x for Non-Oxidative Dehydrogenation of Methanol and Ethanol. Catalysts 2017, 7, 95. [Google Scholar] [CrossRef]
- de Jesús Acosta-Silva, Y.; Lugo-Arredondo, M.I.; Gallardo-Hernández, S.; Garcia-Trejo, J.F.; Matsumoto, Y.; Rivas, S.; Feregrino-Pérez, A.A.; Godínez, L.A.; Méndez-López, A. Comparison of Photocatalytic Activity: Impact of Hydrophilic Properties on TiO2 and ZrO2 Thin Films. Inorganics 2024, 12, 320. [Google Scholar] [CrossRef]
- Ftouni, J.; Muñoz-Murillo, A.; Goryachev, A.; Hofmann, J.P.; Hensen, E.J.M.; Lu, L.; Kiely, C.J.; Bruijnincx, P.C.A.; Weckhuysen, B.M. ZrO2 Is Preferred over TiO2 as Support for the Ru-Catalyzed Hydrogenation of Levulinic Acid to Γ-Valerolactone. ACS Catal. 2016, 6, 5462–5472. [Google Scholar] [CrossRef]
- Phung, T.K.; Hernández, L.P.; Busca, G. Conversion of Ethanol over Transition Metal Oxide Catalysts: Effect of Tungsta Addition on Catalytic Behaviour of Titania and Zirconia. Appl. Catal. A Gen. 2015, 489, 180–187. [Google Scholar] [CrossRef]
- Danilova, I.G.; Dik, P.P.; Sorokina, T.P.; Gabrienko, A.A.; Kazakov, M.O.; Paukshtis, E.A.; Doronin, V.P.; Klimov, O.V.; Noskov, A.S. Effect of Rare Earths on Acidity of High-Silica Ultrastable REY Zeolites and Catalytic Performance of NiMo/REY+Al2O3 Catalysts in Vacuum Gas Oil Hydrocracking. Microporous Mesoporous Mater. 2022, 329, 111547. [Google Scholar] [CrossRef]
- De La Puente, G.; Falabella Souza-Aguiar, E.; María, F.; Zotin, Z.; Lúcia, V.; Camorim, D.; Sedran, U. Influence of Different Rare Earth Ions on Hydrogen Transfer over Y Zeolite. Appl. Catal. A Gen. 2000, 197, 41–46. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Purdy, S.C.; Lin, F.; Unocic, K.A.; Cordon, M.; Wu, Z.; Wang, H.; Hall, J.; Kropf, A.J.; et al. Tailoring Olefin Distribution via Tuning Rare Earth Metals in Bifunctional Cu-RE/Beta-Zeolite Catalysts for Ethanol Upgrading. Appl. Catal. B 2024, 344, 123648. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Zhang, R.; Liu, R. Rare Earth Cation-Modified X Zeolites for Isobutane Alkylation: The Influence of Ionic Radius. Fuel 2024, 363, 130938. [Google Scholar] [CrossRef]
- Chiang, H.; Bhan, A. Catalytic Consequences of Hydroxyl Group Location on the Rate and Mechanism of Parallel Dehydration Reactions of Ethanol over Acidic Zeolites. J. Catal. 2010, 271, 251–261. [Google Scholar] [CrossRef]
- Chuklina, S.; Zhukova, A.; Fionov, Y.; Kadyko, M.; Fionov, A.; Zhukov, D.; Il’icheva, A.; Podzorova, L.; Mikhalenko, I. Selectivity of Ethanol Conversion on Al/Zr/Ce Mixed Oxides: Dehydration and Dehydrogenation Pathways Based on Surface Acidity Properties. ChemistrySelect 2022, 7, e202203031. [Google Scholar] [CrossRef]
- Bykov, D.M.; Gobechiya, E.R.; Kabalov, Y.K.; Orlova, A.I.; Tomilin, S.V. Crystal Structures of Lanthanide and Zirconium Phosphates with General Formula Ln0.33Zr2(PO4)3, Where Ln=Ce, Eu, Yb. J. Solid State Chem. 2006, 179, 3101–3106. [Google Scholar] [CrossRef]
- Asabina, E.A.; Zhukova, A.; Sedov, V.A.; Pet, V.I.; Kovalskii, A.M.; Markova, E.B.; Fukina, D.G.; Koshkin, V.A. Catalytic Characteristics of NASICON-Type Phosphates with Rare Earth Elements in Ethanol Conversion. Solid State Sci. 2025, 162, 107865. [Google Scholar] [CrossRef]
- Asabina, E.; Sedov, V.; Pet’kov, V.; Deyneko, D.; Kovalsky, A. Synthesis, Structure and Luminescence Properties of the Europium–Containing NASICON Type Phosphates. J. Solgel. Sci. Technol. 2023, 105, 547–554. [Google Scholar] [CrossRef]
- Lightfoot, P.; Woodcock, D.A.; Jorgensen, J.D.; Short, S. Low Thermal Expansion Materials: A Comparison of the Structural Behaviour of La0.33Ti2(PO4)3, Sr0.5Ti2(PO4)3 and NaTi2(PO4)3. Int. J. Inorg. Mater. 1999, 1, 53–60. [Google Scholar] [CrossRef]
- Bykov, D.M.; Konings, R.J.M.; Apostolidis, C.; Hen, A.; Colineau, E.; Wiss, T.; Raison, P. Synthesis and Investigation of Neptunium Zirconium Phosphate, a Member of the NZP Family: Crystal Structure, Thermal Behaviour and Mössbauer Spectroscopy Studies. Dalton Trans. 2017, 46, 11626–11635. [Google Scholar] [CrossRef]
- Kurazhkovskaya, V.S.; Bykov, D.M.; Orlova, A.I. Infrared Spectroscopy and Structure of Trigonal Zirconium Orthophosphates with Lanthanides and Actinides. J. Struct. Chem. 2004, 45, 966–973. [Google Scholar] [CrossRef]
- Davydov, A. Molecular Spectroscopy of Oxide Catalyst Surfaces; Sheppard, N.T., Ed.; Wiley: Hoboken, NJ, USA, 2003; ISBN 0-471-9873-X. [Google Scholar]
- Pekounov, Y.; Chakarova, K.; Hadjiivanov, K. Surface Acidity of Calcium Phosphate and Calcium Hydroxyapatite: FTIR Spectroscopic Study of Low-Temperature CO Adsorption. Mater. Sci. Eng. C 2009, 29, 1178–1181. [Google Scholar] [CrossRef]
- Köck, E.M.; Kogler, M.; Bielz, T.; Klötzer, B.; Penner, S. In Situ FT-IR Spectroscopic Study of CO2 and CO Adsorption on Y2O3, ZrO2, and Yttria-Stabilized ZrO2. J. Phys. Chem. C 2013, 117, 17666–17673. [Google Scholar] [CrossRef] [PubMed]
- Paukshtis, E.A.; Yurchenko, E.N. Study of the Acid-Base Properties of Heterogeneous Catalysts by Infrared Spectroscopy. Russ. Chem. Rev. 1983, 52, 242–258. [Google Scholar] [CrossRef]
- Tsyganenko, A.A.; Filimonov, V.N. Infrared spectra of surface hydroxyl groups and crys-talline structure of oxides. J. Mol. Struct. 1973, 19, 579589. [Google Scholar] [CrossRef]
- Phillips, C.B.; Datta, R. Production of Ethylene from Hydrous Ethanol on H-ZSM-5 under Mild Conditions. Ind. Eng. Chem. Res. 1997, 36, 4466–4475. [Google Scholar] [CrossRef]
- Phung, T.K.; Busca, G. Diethyl Ether Cracking and Ethanol Dehydration: Acid Catalysis and Reaction Paths. Chem. Eng. J. 2015, 272, 92–101. [Google Scholar] [CrossRef]
- Inaba, M.; Murata, K.; Takahara, I.; Inoue, K.I. Production of C3+ Olefins and Propylene from Ethanol by Zr-Modified H-ZSM-5 Zeolite Catalysts. Adv. Mater. Sci. Eng. 2012, 2012, 293485. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Tao, L.; Dai, B.; Yang, M.; Chen, Z.; Zhu, X. Dehydration Reaction of Bio-Ethanol to Ethylene over Modified SAPO Catalysts. J. Ind. Eng. Chem. 2010, 16, 717–722. [Google Scholar] [CrossRef]
- Domoroshchina, E.; Kravchenko, G.; Kuz’micheva, G.; Markova, E.; Zhukova, A.; Pirutko, L.; Khramov, E.; Dorokhov, A.; Koroleva, A. The Role of the Compositions of HZSM-5 Zeolites Modified with Nanosized Anatase in Propane and Ethanol Conversion. Catal. Today 2022, 397–399, 511–525. [Google Scholar] [CrossRef]
- Garbarino, G.; Prasath Parameswari Vijayakumar, R.; Riani, P.; Finocchio, E.; Busca, G. Ethanol and Diethyl Ether Catalytic Conversion over Commercial Alumina and Lanthanum-Doped Alumina: Reaction Paths, Catalyst Structure and Coking. Appl. Catal. B 2018, 236, 490–500. [Google Scholar] [CrossRef]
- Phung, T.K.; Lagazzo, A.; Rivero Crespo, M.Á.; Sánchez Escribano, V.; Busca, G. A Study of Commercial Transition Aluminas and of Their Catalytic Activity in the Dehydration of Ethanol. J. Catal. 2014, 311, 102–113. [Google Scholar] [CrossRef]
- Asabina, E.A.; Pet’kov, V.I.; Stenina, I.A.; Yaroslavtsev, A.B. Synthesis and Ionic Conductivity of Na1+2xMxZr2−x(PO4)3 (M–Mg, Mn) NASICON-Type Ceramic Materials. Solid State Sci. 2025, 160, 107786. [Google Scholar] [CrossRef]
- Badmaev, S.D.; Smorygina, A.S.; Paukshtis, E.A.; Belyaev, V.D.; Sobyanin, V.A.; Parmon, V.N. Gas-Phase Carbonylation of Dimethoxymethane to Methyl Methoxyacetate on Solid Acids: The Effect of Acidity on the Catalytic Activity. Kinet. Catal. 2018, 59, 99–103. [Google Scholar] [CrossRef]
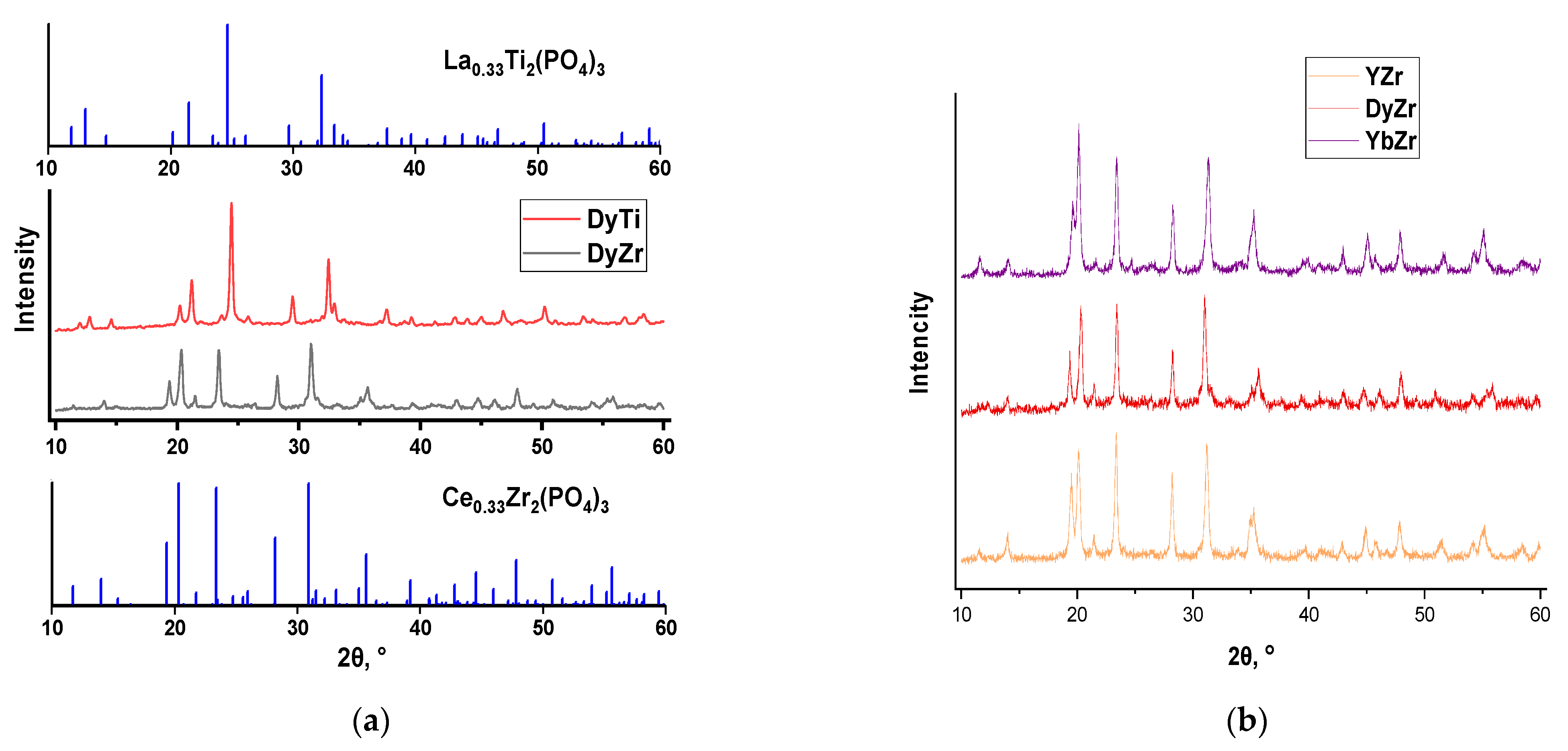
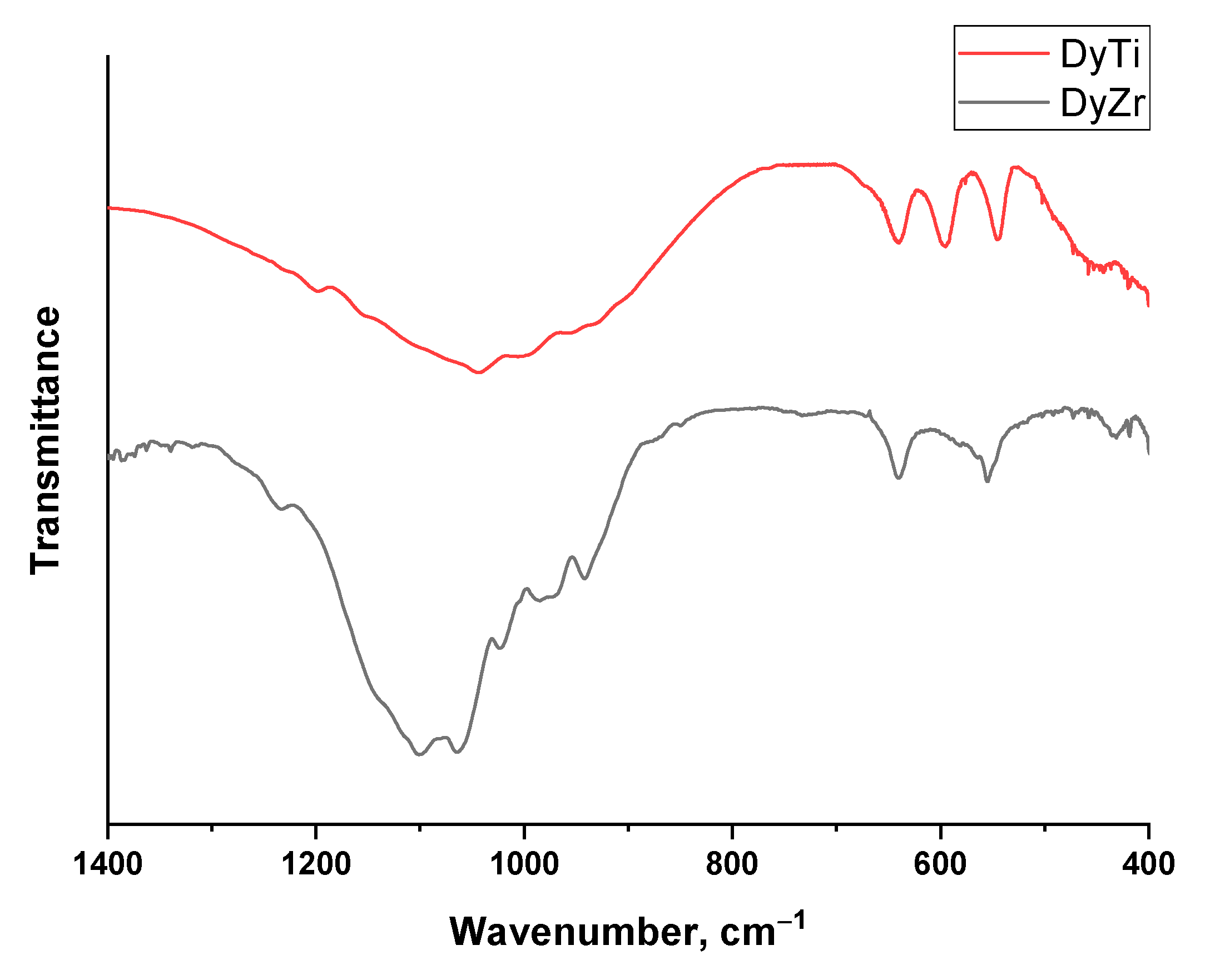

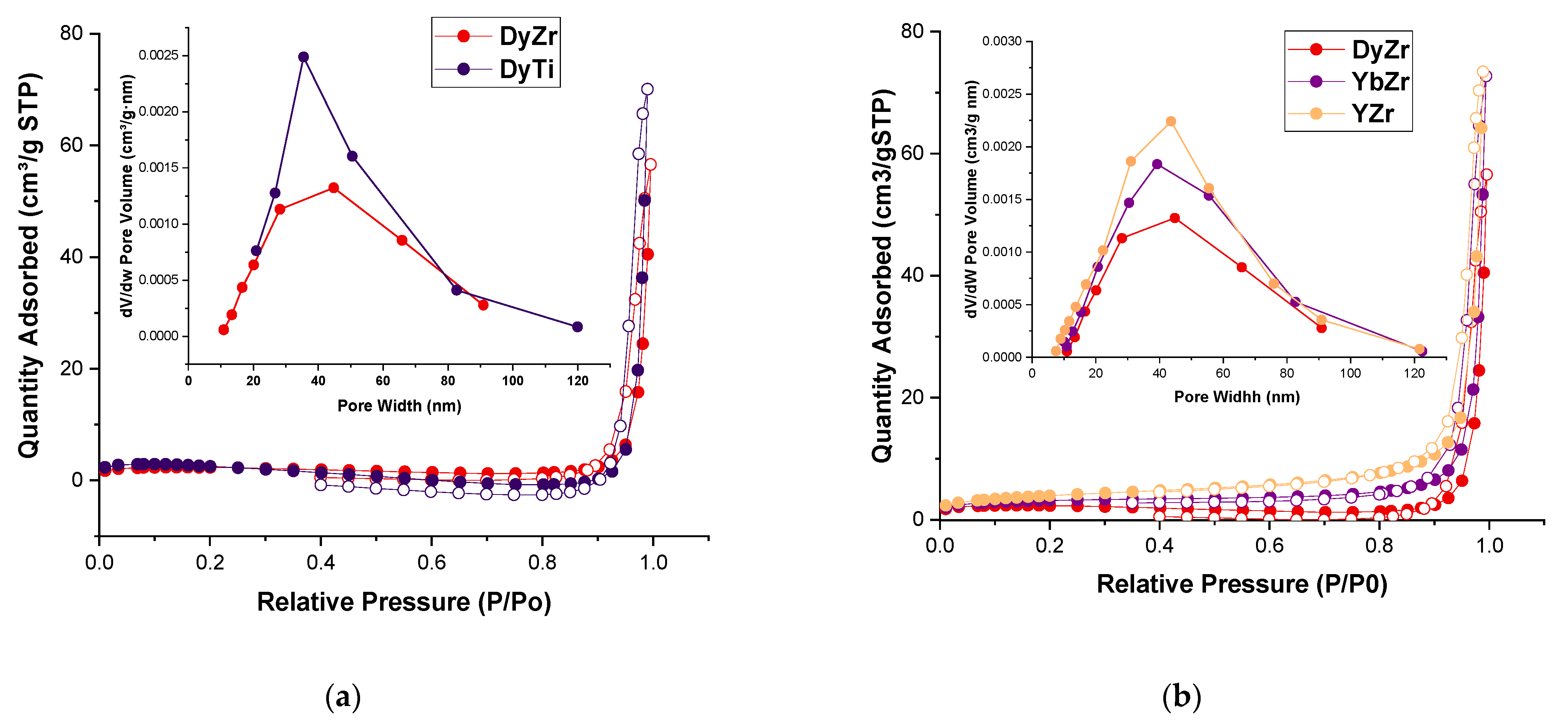
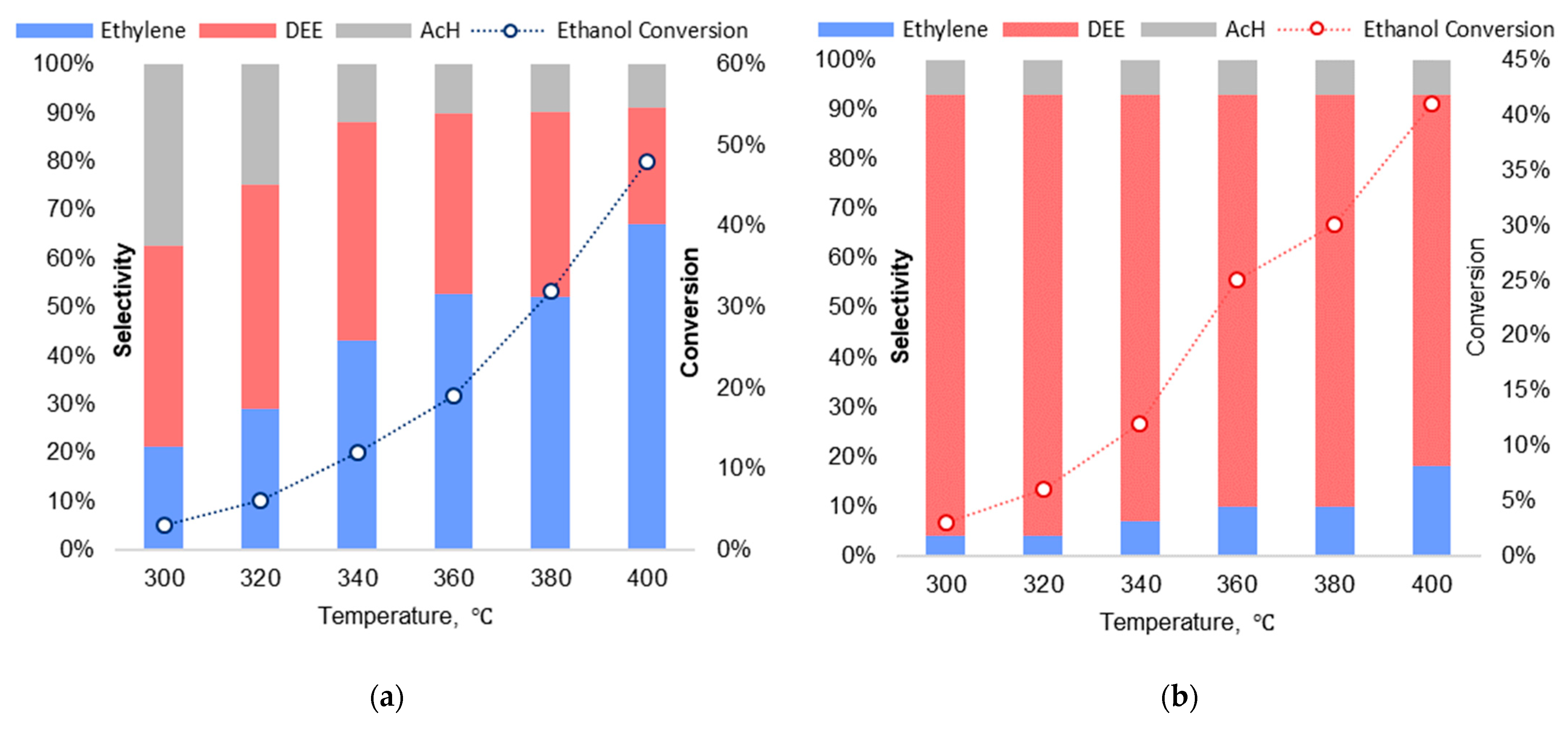

| Composition | r(A3+), Å/r(M4+), Å | a, Å | c, Å | V, Å 3 | r, nm 1 |
|---|---|---|---|---|---|
| DyZr | 0.912/0.72 | 8.823(2) | 22.65(7) | 1527 | 30 |
| YZr | 0.90/0.72 | 8.826(2) | 22.57(2) | 1525 | 32 |
| YbZr | 0.868/0.72 | 8.829(3) | 22.53(1) | 1510 | 27 |
| DyTi | 0.912/0.61 | 8.357(1) | 21.98(1) | 1330 | 28 |
| Composition | BET Surface (m2/g) | BJH Surface (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | Most Probable Pore Diameter (nm) |
|---|---|---|---|---|---|
| DyTi | 7.0 | 10.0 | 0.11 | 44 | 36 |
| DyZr | 7.1 | 7.6 | 0.08 | 41 | 45 |
| YbZr | 10.6 | 10.1 | 0.11 | 45 | 39 |
| YZr | 13.9 | 11.3 | 0.11 | 40 | 44 |
| Sample | ν(OH), cm−1 | Type | ν(OH···H), cm−1 | ∆ν, cm−1 | PA, kJ/mol |
|---|---|---|---|---|---|
| DyTi | 3744 | Ⅰ | 3602 | 142 | 1341 |
| 3702 | Ⅱ | 3555 | 147 | 1334 | |
| 3671 | Ⅲ | 3461 | 210 | 1266 | |
| DyZr | 3764 | Ⅰ | 3602 | 162 | 1316 |
| 3742 | Ⅰ | 3602 | 140 | 1344 | |
| 3698 | Ⅱ | 3550 | 148 | 1333 | |
| 3670 | Ⅲ | 3478 | 192 | 1283 | |
| 3646 | Ⅲ | 3478 | 168 | 1309 | |
| YbZr | 3769 | Ⅰ | 3602 | 167 | 1310 |
| 3743 | Ⅰ | 3602 | 141 | 1342 | |
| 3695 | Ⅱ | 3560 | 135 | 1351 | |
| 3671 | Ⅲ | 3478 | 193 | 1282 | |
| 3644 | Ⅲ | 3478 | 166 | 1311 | |
| YZr | 3768 | Ⅰ | 3602 | 166 | 1311 |
| 3744 | Ⅰ | 3602 | 142 | 1341 | |
| 3696 | Ⅱ | 3566 | 130 | 1358 | |
| 3668 | Ⅲ | 3471 | 197 | 1278 |
| Sample | Ea, kJ∙mol−1 | lnN | ||
|---|---|---|---|---|
| Ethylene | DEE | Ethylene | DEE | |
| DyTi | 119 | 80 | 18.3 | 10.8 |
| DyZr | 139 | 95 | 20.4 | 14.5 |
| YZr | 128 | 100 | 18.8 | 15.4 |
| YbZr | 140 | 104 | 20.0 | 15.8 |
| Catalyst Composition | Conditions | Ethanol Conversion | Selectivity to Ethylene | Selectivity to DEE | Reference(s) |
|---|---|---|---|---|---|
| YbZr | T = 380 °C | 37 | 10 | 81 | This work |
| DyTi | T = 400 °C | 48 | 67 | 24 | This work |
| LaTi | T = 380 °C | 20 | 37 | 45 | [37] |
| MnTi | T = 380 °C | 50 | 40 | 22 | [21] |
| Zr/HZSM-5 | T = 450 °C | 100 | 61 | - | [49] |
| SAPO-34 | T = 400 °C | 92 | 52 | 1 | [50] |
| SAPO-11 | T = 340 °C | 95 | 91 | 9 | |
| Zn-SAPO-11 | T = 340 °C | 85 | 15 | - | |
| WO3-ZrO2 | T = 400 °C | 100 | 100 | - | [29] |
| MoO3-ZrO2 | T = 380 °C | 98 | 69 | - | |
| NA/HZSM-5(12) | T = 200 °C | 10 | 2 | 98 | [51] |
| T = 300 °C | 95 | 98 | 2 | ||
| γ-Al2O3La-γ-Al2O3 | T = 200 °C | 26.9 | 0.6 | 99.4 | [52] |
| T = 400 °C | 100 | 98 | 0 | ||
| T = 200 °C | 18 | 0.5 | 99.5 | ||
| T = 400 °C | 100 | 96.6 | 0.1 | ||
| 5Al2O3-95ZrO2 | T = 340 °C | 12 | 10 | 15 | [35] |
| T = 380 °C | 20 | 45 | 20 |
| Sample | Type of OH Group | Main Product via Ethanol Dehydration | |
|---|---|---|---|
| I | III | ||
| DyTi | P–OH | 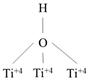 | Ethylene |
| DyZr | P–OH | 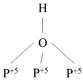 | DEE |
| Zr–OH | 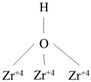 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhukova, A.I.; Sazonova, A.D.; Kharlanov, A.N.; Asabina, E.A.; Pet’kov, V.I.; Sedov, V.A.; Prokhin, V.D.; Osaulenko, D.A.; Fionov, Y.A.; Mikhalenko, I.I.; et al. Ethanol Dehydration Pathways on NASICON-Type A0.33M2(PO4)3 ((A = Dy, Y, Yb); M = Ti, Zr) Catalysts: The Role of Hydroxyl Group Proton Mobility in Selectivity Control. Catalysts 2025, 15, 515. https://doi.org/10.3390/catal15060515
Zhukova AI, Sazonova AD, Kharlanov AN, Asabina EA, Pet’kov VI, Sedov VA, Prokhin VD, Osaulenko DA, Fionov YA, Mikhalenko II, et al. Ethanol Dehydration Pathways on NASICON-Type A0.33M2(PO4)3 ((A = Dy, Y, Yb); M = Ti, Zr) Catalysts: The Role of Hydroxyl Group Proton Mobility in Selectivity Control. Catalysts. 2025; 15(6):515. https://doi.org/10.3390/catal15060515
Chicago/Turabian StyleZhukova, Anna I., Alina D. Sazonova, Andrey N. Kharlanov, Elena A. Asabina, Vladimir I. Pet’kov, Vladislav A. Sedov, Vasiliy D. Prokhin, Diana A. Osaulenko, Yuri A. Fionov, Irina I. Mikhalenko, and et al. 2025. "Ethanol Dehydration Pathways on NASICON-Type A0.33M2(PO4)3 ((A = Dy, Y, Yb); M = Ti, Zr) Catalysts: The Role of Hydroxyl Group Proton Mobility in Selectivity Control" Catalysts 15, no. 6: 515. https://doi.org/10.3390/catal15060515
APA StyleZhukova, A. I., Sazonova, A. D., Kharlanov, A. N., Asabina, E. A., Pet’kov, V. I., Sedov, V. A., Prokhin, V. D., Osaulenko, D. A., Fionov, Y. A., Mikhalenko, I. I., Fionova, E. A., & Zhukov, D. Y. (2025). Ethanol Dehydration Pathways on NASICON-Type A0.33M2(PO4)3 ((A = Dy, Y, Yb); M = Ti, Zr) Catalysts: The Role of Hydroxyl Group Proton Mobility in Selectivity Control. Catalysts, 15(6), 515. https://doi.org/10.3390/catal15060515







